Abstract
Neurocognitive impairment is a prevalent and important co-morbidity in virologically suppressed people living with HIV (PLWH), yet the underlying mechanisms remain elusive and treatments lacking. Here, we explored for the first time, use of participant-derived directly induced neurons (iNs) to model neuronal biology and injury in PLWH. iNs retain age- and disease-related features of the donors, providing unique opportunities to reveal novel aspects of neurological disorders. We obtained primary dermal fibroblasts from six virologically suppressed PLWH (range: 27 – 64 years, median: 53); 83% Male; 50% White) and seven matched people without HIV (PWOH) (range: 27 – 66, median: 55); 71% Male; 57% White). iNs were generated using transcription factors NGN2 and ASCL1, and validated by immunocytochemistry and single-cell-RNAseq. Transcriptomic analysis using bulk-RNAseq identified 29 significantly differentially expressed genes between iNs from PLWH and PWOH. Of these, 16 genes were downregulated and 13 upregulated in PLWH iNs. Protein-protein interaction network mapping indicates that iNs from PLWH exhibit differences in extracellular matrix organization and synaptic transmission. IFI27 was upregulated in iNs from PLWH, which complements independent post-mortem studies demonstrating elevated IFI27 expression in PLWH-derived brain tissue, indicating that iN generation reconstitutes this pathway. Finally, we observed that expression of the FOXL2NB-FOXL2-LINC01391 genome locus is reduced in iNs from PLWH and negatively correlates with neurocognitive impairment. Thus, we have identified an iN gene signature of HIV through direct reprogramming of skin fibroblasts into neurons revealing novel mechanisms of neurocognitive impairment in PLWH.
Keywords: HIV, neurodegenerative disease, aging, induced neurons, iNs, HAND, HIV-related neurocognitive impairment, IFI27, directly converted neurons, transdifferentiation
One sentence summary
Direct reprogramming of skin fibroblasts into neurons reveals unique gene signatures indicative of HIV infection in the context of viral suppression.
Introduction
Neurocognitive impairment remains an important co-morbidity of HIV-1 infection in virologically suppressed people living with HIV (PLWH). The introduction of combined antiretroviral therapy (cART) has reduced the prevalence of the most severe forms including HIV-associated dementia. Nonetheless, though milder forms of cognitive impairment are more prominent, the overall burden remains substantial in PLWH with adverse consequences for daily living activities (1–5). A 2020 meta-analysis determined the prevalence of HIV-1-related neurocognitive impairment to be 43.9 % (6).
The cellular mechanisms responsible for the observed neurocognitive impairment among virologically suppressed PLWH are not well understood but suggested to be multifactorial. It has been shown that HIV-1 can enter the brain as early as two weeks after infection where it infects multiple cell types including T-cells, microglia, brain-resident macrophages, and astrocytes (7–10). Neurons are not noticeably infected by HIV-1, yet, the resulting neurotoxic environment impairs neuronal functions driving neurocognitive impairment in PLWH (10).
Transcriptomic analysis of post-mortem brain samples derived from PLWH has shown that HIV-1 infection is associated with a differential neural gene expression indicating the involvement of multiple pathways (e.g., axon guidance, endocytosis, synaptic transmission) in the cognitive decline of PLWH (11). However, as Ojeda-Juárez and Kaul recently pointed out, most of these studies lacked suitable non-HIV-1 controls i.e. brain tissue samples derived from people living without HIV (PWOH), which hampers our understanding of direct HIV-1-associated differential neuronal gene expression (12). Moreover, the analyzed gene expression may have been affected by different co-morbidities and living situations before death as well as sample preparation after death.
In attempts to overcome these issues, differential gene expression has also been analyzed in neurons from a transgenic HIV-1 gp120 expressing mouse model, which reconstitutes a certain HIV-1-induced neuropathology observed in humans (13–15). However, HIV-1 does not naturally infect rodents, cognitive decline in PLWH is influenced by factors beyond gp120, and mouse neuronal biology differs from that of humans. As a result, we are still lacking a neuronal cell system that reflects the multifactorial nature of HIV-1 infection and that allows transcriptional as well as functional analyses of neurons derived from virologically suppressed PLWH.
Recent protocols to generate induced neurons (iNs) by transdifferentiation of participant-derived fibroblasts have made it possible to capture disease- and age-related features of neurons in vitro (16–20). This made it possible to recapitulate known aspects of neurodegenerative diseases as well as to reveal previously unrecognized underlying disease pathomechanisms in cell culture (16–18). Importantly, it has been shown that inducing the pluripotent stem cell state prior to neuronal differentiation of participant-derived cells, as is necessary for the generation of iPSC-derived neurons, erases most age and disease-related characteristics, unlike the iN protocol (21). This appears to be particularly important for the study of diseases in which age is implicated in the pathogenesis like Alzheimer’s or HIV-1-related neurocognitive impairment (17, 22).
Thus, using a previously published iNs protocol that retains donor-specific disease- and age-related characteristics in vitro (17, 21, 23), we investigated whether iNs derived from virologically suppressed PLWH show a differential gene expression compared to iNs derived from demographically matched PWOH.
Results
Generation of participant-derived induced neurons from people living with or without HIV
We generated iNs from six clinically well-characterized people chronically infected with HIV-1 and virologically suppressed on cART (HIV RNA <50 copies/mL), as well as from seven age- and sex-matched people without HIV-1 (PWOH) as control participants following a recently published protocol (Fig. 1A, 1B, 1C, Table S1) (21, 23, 24). PLWH were without neuropsychiatric confounds and underwent comprehensive neurocognitive performance testing (25).
Fig. 1. Transdifferentiation of skin fibroblasts derived from people living with HIV and controls generates induced neurons.
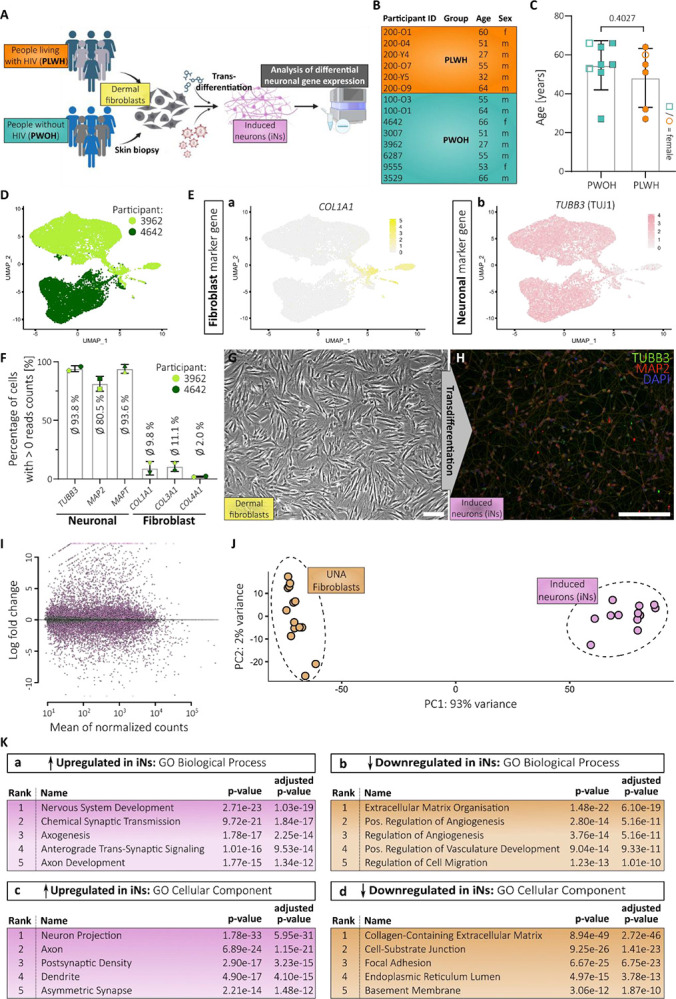
(A) Scheme illustrating study outline. (B) Important participant information divided into people living with HIV-1 (PLWH) and without HIV-1 (PWOH). (C) Age distribution of the two groups of the study cohort. Statistical significance tested with unpaired, two-tailed t-test. Data presented as individual data points with mean ± SD and p-value. (D) UMAP plot showing the sample origin of each data point during scRNA analysis. (E) Gene expression patterns of fibroblast marker gene COL1A1 (a) and neuronal marker gene TUBB3 (b). (F) Percentage of cells from scRNA analysis depicted in (D-E) that express the annotated neuronal and fibroblast marker genes. Data presented as individual data points with mean ± SD. (G) Microscopic image of dermal fibroblasts before transdifferentiation. (H) Microscopic image after immunocytochemistry of induced neurons (iNs) 3-days post-FACS and stained for TUBB3 (TUJ1), MAP2 and nuclei (DAPI). Single channel images are provided in Fig. S1F (G-H) Scale bars are 20 μm. (I) MA plot based on bulk-RNA sequencing showing differential gene expression of all iNs samples compared to UNA fibroblasts. (J) PCA plot showing clustering of iNs- and UNA fibroblast-derived RNA samples after bulk-analysis. (K) Top 5 ranked gene ontology (GO) terms of Biological Processes (a-b) and Cellular Component (c-d) associated with the significantly up- (a and c) and downregulated genes (b and d) in iNs compared to UNA fibroblasts.
Participant age significantly correlated with the estimated duration of HIV-1 infection in our cohort. The Global Deficit Score (GDS) as measurement for the degree of neurocognitive impairment (NCI) did not correlate with age nor the duration of HIV-1 infection reflecting the fact that multiple, complex factors drive NCI in PLWH (Fig. S1A, S1B, S1C).
To generate iNs, participant-derived dermal fibroblasts were transduced with a lentiviral vector (UNA vector (23)) for doxycycline-dependent expression of neuronal transcription factors NGN2 (also NEUROG2), and ASCL1. Transduced fibroblasts were referred to as UNA fibroblasts prior to initiating the transdifferentiation to account for the lentiviral transduction-mediated genetic modification (Fig S1D). Treatment of UNA fibroblasts with doxycycline and a cocktail of differentiation factors for 21 days resulted in a mixed population of neurons and non-converted cells as observed by light microscopy and as previously described (Fig S1E) (24).
To isolate the bona fide iNs from this mixed population, we performed fluorescence-activated cell sorting (FACS) targeting polysialylated-neural cell adhesion molecule (PSA-NCAM) on live (DAPI−) cells (Fig. S1D). To check the purity of the obtained cell population and obtain first insights into the neuronal gene expression, single-cell RNA (scRNA) analysis was conducted with iNs derived from two PWOH (Fig. 1D). Gene expression analysis indicated a small subset of cells with a putative fibroblast-associated transcriptome as indicated by expression of COL1A1, COL3A1, and COL4A1 that is still contained within the isolated cell population (Fig. 1E, a, 1F). Nevertheless, the majority of cells expressed neuronal marker genes TUJ1 (also TUBB3), MAP2, and MAPT (Tau) while lacking fibroblast marker gene expression (Fig. 1E, b, 1F). Importantly, immunocytochemistry at 3-days post-FACS confirmed TUJ1 and MAP2 protein expression and clearly showed the neuronal morphology of iNs (Fig. 1G, 1H, S1F). Overall, this result was in concordance with a prior study from an independent research group following the same protocol. During their study, a population of approx. 90% TUJ1-positive cells was obtained as analyzed by immunocytochemistry (17).
In addition to revealing the expression of typically used pan-neuronal marker genes among our iNs, our scRNA data analysis has confirmed the previous finding of two subpopulations within the iNs: A larger subset of potentially glutamatergic (SLC17A7+) and smaller subset of potentially GABAergic (GAD1+) neurons, with virtually no overlap of expression (Fig. S1G, S1H). Our analysis has further confirmed a lack of choline O-acetyltransferase (CHAT) and tryptophan hydroxylase 2 (TPH2) expression which would indicate the presence of cholinergic or serotonergic neurons, respectively (Fig. S1G).
To expand our validation of the performed transdifferentiation protocol with regard to the entire cohort, we next conducted differential gene expression analysis with bulk-RNA isolated from iNs and their matched UNA fibroblasts derived from all donors (PLWH and PWOH). We harvested RNA while the two matching samples were cultured at the same passage to limit long-term cell culture-mediated effects between the UNA fibroblasts and their corresponding iNs. As a result, the only difference between the UNA fibroblasts and their matched iNs has been the 21 days of transdifferentiation and subsequent cell sorting. The differential gene expression analysis showed that the morphological transition into the neuronal phenotype was accompanied by drastic transcriptional changes with over 10,000 genes being differentially expressed in the iNs compared to the UNA fibroblasts (p-adj. < 0.05, log2fc > +/− 0.5) (Fig. 1I, S2). This finding is supported by principal component analysis (PCA), which depicted a clear separation of the clustered iN and UNA fibroblast samples (Fig. 1J).
The two fibroblast cultures derived from PWOH participants 100-O1 and 100-O3 were generated in our laboratories together with the PLWH fibroblast cultures following in-house protocols (Table S1). Importantly, the respective UNA fibroblast samples derived from participant 100-O1 and 100-O3 showed no association with the PLWH samples that were likewise generated but instead clustered with the rest of the PWOH samples that were obtained elsewhere (Fig. S1I). This indicated that the method of fibroblast culture generation did not affect our downstream analysis and corroborated the validity of our samples for the described analyses.
To verify the neuronal biological state of the iNs, gene set enrichment analysis was performed determining Gene ontology (GO) terms associated with UNA fibroblast to iN transdifferentiation (Fig. 1K). As expected, GO terms of biological processes associated with the upregulated genes after transdifferentiation were linked to neuronal development and function (Fig. 1K, a). This was corroborated by the top GO terms of cellular components, which corresponded to neuron-specific compartments (Fig. 1K, c). Further, downregulated genes following transdifferentiation into iNs were associated with GO terms corresponding to fibroblasts function and related cellular compartments, respectively, underlining the loss of fibroblast-associated characteristics over the 21 days of transdifferentiation (Fig. 1K, b – d). Together, the gene set enrichment analysis on bulk-RNA from all donors strongly support the transdifferentiation of our participant-derived fibroblasts into neurons.
In summary, these analyses demonstrate successful execution of the previously published protocol for the generation of participant-derived iNs from our cohort of six PLWH and seven matched controls of PWOH. Hence, we obtained neurons via a protocol, which preserves age- and disease state-associated biological changes and that allows us to now reveal biologically plausible neuronal differences between virologically suppressed PLWH and PWOH.
PLWH-derived iNs exhibit statistically significant differentially expressed genes compared to iNs from PWOH
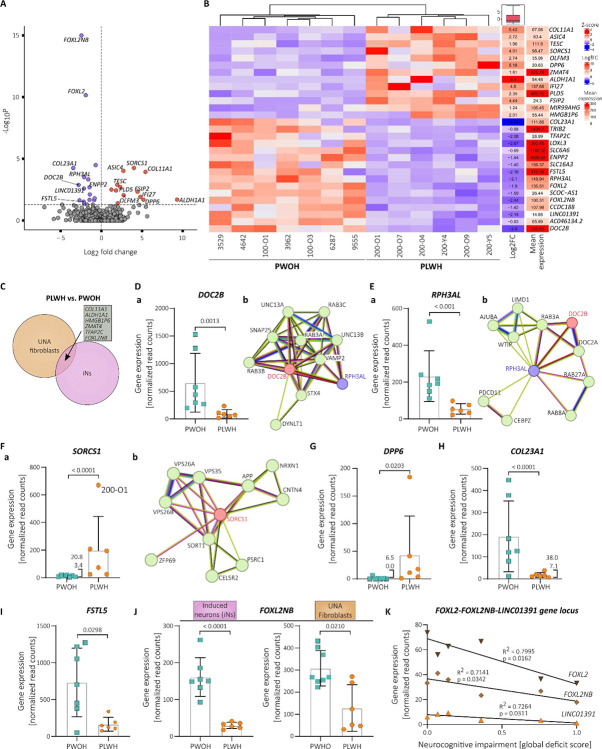
After confirming our ability to successfully transdifferentiate participant-derived fibroblasts from PLWH and PWOH into iNs, we aimed to investigate if the presence of chronic HIV-1 infection in the context of concurrent treatment with combination antiretroviral therapy (cART) affects the gene expression signature of the iNs. For this, we used the obtained bulk-RNA sequencing data to compare gene expression profiles of the iNs derived from PLWH to those derived from PWOH. This transcriptome analysis identified 29 differentially expressed genes (DEGs) between PLWH- and PWOH-derived iNs (p-adj. < 0.05, log2fc > +/− 0.5) (Fig. 2A, 2B, Table S2). Of these, 13 genes were upregulated, and 16 genes downregulated in the iNs derived from PLWH (Fig. 2B). Six of these 29 genes were likewise differentially expressed between the matched UNA fibroblast samples of PLWH vs. PWOH indicating a broader, perhaps cell-type independent effect in PLWH on these genes (Fig. 2C).
Fig. 2. PLWH-derived iNs exhibit statistically significant differentially expressed genes compared to iNs from PWOH.
(A) Volcano plot showing the 29 statistically significant (p-adj. < 0.05, log2fc > +/− 0.5) differentially expressed genes (DEGs) in PLWH-derived iNs compared to PWOH-derived iNs following bulk-RNA analysis iNs. Y-axis plots the p-adj. values and the dotted line indicates the selected cut-off of p-adj. < 0.05. (B) Heatmap showing the clustering of PLWH- vs. PWOH-derived iNs RNA samples based on expression levels of the 29 DEGs while displaying the log2 fold change and mean expression. (C) Venn diagram showing the numbers of DEGs between the PLWH- and PWOH-derived samples for the UNA fibroblasts and iNs. The gene symbols of the six genes that are differentially expressed in both cell types are displayed in the grey box. (D-F) Gene expression levels in PLWH- vs. PWOH-derived iNs (a) and STRING protein association networks (b) of DOC2B (D), RPH3AL (E), and SORCS1 (F)(33, 34). (G-I) Gene expression levels in PLWH- vs. PWOH-derived iNs of DPP6 (G), COL23A1 (H), and FSTL5 (I). (J) Gene expression levels in PLWH- vs. PWOH-derived iNs and UNA fibroblasts of FOXL2NB. (D-J). Data presented as individual data points with mean ± SD and p-adj-value derived from the conducted Wald test corrected for false discovery rates (FDR) using the Benjamini-Hochberg method (see methods section). (K) Correlation of gene expression levels of FOXL2, FOXL2NB, and LINC01391 with neurocognitive impairment in the PLWH study group (n =6). Data points are individual values and lines depict linear regression functions.
We considered the remaining 23 DEGs to be iN-specific in our setting. This list of iN-specific DEGs contained several genes including DOC2B, RPH3AL, SORCS1, and DPP6 that are associated with either known or presumed neuronal functions as well as dysfunctions (26–30). These candidates may present novel pathways relating to neuronal function in virologically suppressed PLWH.
Differential expression of genes related to synaptic transmission pathways in PLWH iNs compared to PWOH iNs
Expression of the iN-specific DEG double C2 domain beta (DOC2B) was reduced by 6.95-fold in PLWH compared to PWOH on average (Fig. 2D, a). DOC2B is readily expressed in the human brain and important for neuronal activity (31). DOC2B has been identified as a cytosolic Ca2+ sensor that mediates spontaneous neurotransmitter release (26). It was shown that a double knockdown of the double C2 domain proteins DOC2A and DOC2B results in a decrease in spontaneous transmitter release from hippocampal neurons, which could be rescued by expressing DOC2B (26). A subsequent study later described its role in hippocampal synaptic plasticity (32). DOC2B interacts with several proteins that are important for neurotransmitter release including components of the SNARE complex as shown by pulldown analysis (26) as well as revealed by STRING network analysis (Fig. 2D, b) (33, 34). Interestingly, the resulting graph of the network analysis also depicts a link to rabphilin 3A like (without C2 domains) (RPH3AL, previously Noc2), another gene that was significantly downregulated in the PLWH- compared to the PWOH-derived iNs (Fig. 2E, a – b). This co-reduction may be due to shared gene regulatory elements that supposedly control DOC2B and RPH3AL transcription on chromosome 17 (35, 36). RPH3AL is a Rab effector protein associated with release of secretory vesicles (27). It is expressed presumably at low levels in the brain (28), and a recent GWAS study showed that the RPH3AL missense mutation rs117190076 increases the risk for late-onset Alzheimer’s disease (37). Hence, downregulation of the genomic locus containing DOC2B as well as RPH3AL may affect synaptic vesicle release in two different ways.
In contrast to DOC2B and RPH3AL, olfactomedin 3 (OLFM3) showed increased expression in iNs derived from PLWH compared to PWOH (Fig. 2A, 2B). OLFM3 protein is expressed in neurons of the cortex, and hippocampus (38). It has been shown to bind different subunits of the postsynaptic AMPA receptor (α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor), namely GRIA1, and GRIA2 and its overexpression in the mouse hippocampus affects their membrane expression (38). In this context, increased OLFM3 expression has been linked to epilepsy because it was suggested to alter AMPA receptor activity (38).
We conclude that iNs derived from PLWH exhibit differential expression of genes directly involved in pre- as well as postsynaptic processes when compared to their counterparts with PWOH.
Expression of Alzheimer’s disease-associated SORCS1 is increased in PLWH-derived iNs
An important pathway in the development of Alzheimer’s disease is the dysregulated processing of amyloid precursor protein (APP), which leads to intracellular accumulation of Aβ. In addition to apolipoprotein E (ApoE), amyloid-beta (Aβ), and Tau protein (hTau) (39, 40), sortilin related VPS10 domain containing receptor 1 (SORCS1) expression is amongst the best known risk factors in Alzheimer’s disease. SORCS1 is associated with different components of the Aβ pathway and has been specifically shown to play a role in aberrant APP trafficking (Fig. 2F, b) (29, 41). In addition to its role in Alzheimer’s, SORCS1 is a general regulator for intracellular trafficking and is important for maintaining neuronal functionality by, for instance, sorting the AMPA glutamate receptor (AMPAR) and synaptic adhesion molecule neurexin (NRXN), which ensures proper glutamatergic transmission (42). We observed significantly elevated SORCS1 expression levels in the iNs of PLWH when compared to the PWOH iNs in our study (Fig. 2F, a). To our knowledge, this is the first reported association of SORCS1 gene expression with HIV-1 infection in the published literature.
Interestingly, the association between SORCS1 SNPs and Alzheimer’s disease appears to exhibit a sexual dimorphism, with a stronger correlation observed in women (40, 43). In our cohort of PLWH, SORCS1 expression was found to be 3-fold higher in the iNs from the participant of female sex (200-O1) compared the iNs from the participant with the second highest SORCS1 expression (Fig. 2F, a). However, the limited sample size prevents us from drawing conclusions regarding the effect of sex on SORCS1 expression.
Expression of the potassium ion channel auxiliary factor DPP6 is increased in iNs derived from PLWH
Dipeptidyl peptidase like 6 (DPP6, previously DPPX) is another iN-specific DEG with a known role in neuronal function. DPP6 exhibited increased expression levels in the iNs derived from PLWH compared to PWOH (Fig. 2G). DPP6 RNA and protein expression throughout the human body is predominantly found within the brain with low region specificity (available from v23.0proteinatlas.org; https://www.proteinatlas.org/ENSG00000130226-DPP6) (44, 45). It is an important auxiliary factor of potassium ion (K+) channels, and its expression is associated with synaptic function and impairments in learning and memory (30, 46–48). In addition, the NHGRI-EBI GWAS catalog (49) includes DPP6 SNPs associated with cognitive decline (GCST009443; GCST90308745) (50, 51), and hippocampal volume (GCST90104700) (52). Despite obtaining a low average expression of DPP6 in our assay (Fig. 2G), it is notable that the performed bulk-RNA sequencing resulted in zero reads for DPP6 RNA in 4 out of 7 iNs samples derived from PWOH whereas DPP6 expression was detected in all PLWH iNs samples (Fig. 2G). Studies investigating DPP6 function in the past have typically used DPP6 knockdown or knockout experiments, which enables only the analysis of lack of DPP6 expression (46–48). Thus, it is difficult to draw any conclusions based on their results about a potential pathophysiological effect of increased DPP6 levels as it is the case in the PLWH iNs. However, a single study on schizophrenia that generated participant-derived induced pluripotent stem cell (iPSC)-derived neurons found increased DPP6 transcript levels in neurons from schizophrenia patients compared to healthy controls (53). In that study, multi-electrode-array recordings and calcium imaging demonstrated decreased neuronal activity in the neuronal cultures of schizophrenia patient-derived cells. Moreover, shRNA-mediated reduction of DPP6 levels as well as pharmacological inhibition of the K+ channel Kv4.2 reversed this observed hypoexcitability, and hypoactivity suggesting a causal relationship between increased DPP6 levels and decreased neuronal activity. We therefore hypothesize that the iNs of PLWH, which showed increased DPP6 expression compared to matched controls, may exhibit the same aberrant excitability - a link that warrants further investigation.
Differential expression of extracellular matrix-associated genes in iNs from PLWH compared to those from PWOH
The extracellular matrix (ECM) plays an important role in neuronal development, and function (54) and the dysregulation of ECM-associated proteins including members of the collagen family has been linked to neurodegeneration (55). Notably, neurons and non-neuronal glial cells actively shape their surroundings by expressing and secreting various ECM proteins, which is required for important processes, e.g., synaptic plasticity (54, 55).
In this study, iNs derived from PLWH exhibited significantly differential gene expression of several ECM-associated proteins when compared to iNs derived from PWOH. In the majority of cases, similar changes in gene expression patterns were not noted in the matched UNA fibroblasts samples indicating neuron-specific effects.
Collagen type XXIII alpha 1 chain (COL23A1) is an ECM-associated protein that may reveal mechanic insights into HIV-1-related neurocognitive decline. Its expression was more than 8-fold reduced in iNs derived from PLWH compared to PWOH (Fig. 2H). Further, we obtained raw read counts for COL23A1 RNA via bulk-RNA sequencing from only four UNA fibroblast samples ranging from 1–12 raw reads, which substantiates the iN-specific COL23A1 expression in our assay (data not shown). Indeed, COL23A1 expression is found across the human brain in multiple cell types including neurons, and astrocytes (available from v23.0proteinatlas.org; https://www.proteinatlas.org/ENSG00000050767-COL23A1/brain) (44, 45). COL23A1 is a type II membrane protein belonging to the transmembrane collagen family (56) and despite a general lack of knowledge concerning its role in neural functions, SNPs of its gene are associated with the rate of cognitive decline in Alzheimer’s disease (GCST010567) (57) and memory performance (GCST90104696) (52).
In addition to COL23A1, PLWH-derived iNs exhibited differential expression of the collagen family member COL11A1 (collagen type XI alpha 1 chain) (Fig. 2B). While potentially of interest, unlike findings with COL23A1, the increased expression of COL11A1 in PLWH samples when compared to PWOH controls was not restricted to iNs and was also observed in the UNA fibroblasts (Fig. 2C). Of note, COL11A1 is expressed in the brain as well as the skin (available from v23.0proteinatlas.org; https://www.proteinatlas.org/ENSG00000060718-COL11A1) (44, 45).
Besides structural proteins like the collagen family members, different secreted proteins are also associated with the ECM. Our iNs from PLWH showed decreased expression levels of ENPP2 (Fig. 2B). The ENPP2 gene encodes ectonucleotide pyrophosphatase/phosphodiesterase 2, which is better known as autotaxin. Autotaxin is an enzyme secreted into the ECM by different cell types throughout the human body including neural cells (58). Autotaxin exerts biological functions by processing lysophosphatidylcholine into lysophosphatidic acid (LPA), which then binds to one of its several G-protein coupled receptors (LPA1-LPA6) (58). Interestingly, LPA signalling is involved in numerous physiological processes including neurogenesis, neuronal differentiation, synapse formation, migration, and cortical development (reviewed in (58)). Hence, decreased expression of the ENPP2 gene in neural cells may affect brain functioning via dysregulated LPA signalling.
Follistatin like 5 is also a secreted protein, which is expressed throughout the human brain, predominantly in the cerebellum, in inhibitory as well as excitatory neurons (available from v23.0proteinatlas.org; https://www.proteinatlas.org/ENSG00000168843-FSTL5) (44, 45). Although its role in physiological brain processes is not well described, single nucleotide polymorphisms (SNPs) found within the FSTL5 gene are associated with general cognitive ability (GCST006269) (59), dementia and Alzheimer’s disease in non-APOE ε4 allele carriers (GCST90244035; GCST90244033) (60), and working memory (GCST006930) (61) as annotated in the NHGRI-EBI Catalog of human genome-wide association studies (GWAS) (downloaded 10/03/2024) (49). We found that expression levels of FSTL5 were on average 4.4-fold decreased in PLWH-derived iNs compared to PWOH iNs (Fig. 2B, 2I).
Protein-protein interaction network mapping supports differential ECM organization, and synaptic transmission in PLWH iNs and indicates neuronal apoptosis as another affected pathway
Since the dysregulation of a single gene product likely affects several other gene products, protein-protein-interaction (PPI) network mapping has become a powerful tool to analyze complex pathomechanisms. Furthermore, different PPI databases (e.g, IntAct, HuRI) that allow network mapping based on curated experimental data sets have made it possible to bridge the gap between RNA expression and protein interactions without the necessity for additional co-precipitation or proximity ligation assays (62, 63).
After identifying 29 DEGs that distinguish PLWH- and PWOH-derived iNs by transcriptomic analysis, we sought to investigate whether PPI network mapping reveals insights into the affected cellular pathways. To this end, we determined the 1st-order interaction partners for the gene products of the 29 DEGs between PLWH- and PWOH-derived iNs using the IntAct Molecular Interaction Database (62) and The Human Reference Interactome (HuRI) (Fig. S3A, Table S3).
Gene set enrichment analysis revealed that biological processes associated with the resulting PPI network were related to neuronal apoptosis (Fig. S3B, a, Table S4). Further, this analysis supported the idea that the organization of the ECM is affected in iNs derived from PLWH compared to PWOH (Fig. S3B, b). In addition to the ECM, the PPI network analysis substantiated an influence of the 29 DEG on synaptic transmission (Fig. S3B, a and c).
When analyzing the obtained PPI network with regard to associated diseases, it was therefore not surprising to find associations with terms like neurodegenerative disease, dementia, or Alzheimer’s disease (Fig. S3B, d – e) (64, 65).
We conclude that the DEGs in iNs from virologically suppressed PLWH may drive various disease-associated pathways in the CNS by direct protein-protein interactions of the respective gene products.
Expression of the FOXL2NB-FOXL2-LINC01391 genome locus is reduced in PLWH-derived iNs and associated with the degree of neurocognitive impairment
Expression of the FOXL2 neighbour gene (FOXL2NB, previously C3orf72) is significantly downregulated in PLWH- when compared to PWOH-derived iNs but also in the UNA fibroblasts (Fig. 2C). However, we found this effect to be more pronounced in the iN samples (Fig. 2J). This suggests that the differential FOXL2NB expression between PLWH and PWOH in the iN samples is not an experimental artifact mediated by the choice of our original cell type, the participant-derived primary dermal fibroblasts, but rather a cell type-independent effect that appears to be more prominent in neurons than in fibroblasts.
Furthermore, expression levels of the transcription factor forkhead box L2 gene (FOXL2), and the long non-coding RNA LINC01391 were significantly reduced only in the PLWH iNs samples and not the PLWH UNA fibroblasts (Fig. 2B, 2C). We found this of particular interest because the three genes, FOXL2NB, FOXL2, and LINC01391 are located in close proximity to each other on human chromosome 3 and their expression is controlled by shared gene regulatory elements as annotated in the GeneHancer database (Fig. S4) (35, 36). Thus, the transcription rate at this genomic locus may be decreased in neurons of PLWH. Interestingly, little is known about the function of LINC01391, and FOXL2NB in the brain but FOXL2 has been very recently associated with Alzheimer’s disease (66). Kavoosi et al. have used published microarray expression data on tissue from different brain regions (e.g., frontal, temporal, and entorhinal cortex) obtained from healthy controls, asymptomatic and symptomatic Alzheimer’s patients to identify FOXL2 via microRNA-mRNA regulatory networks (66, 67).
Moreover, expression levels of FOXL2NB, FOXL2, and LINC01391 showed a negative correlation with the global deficit score in our cohort, i.e., the more pronounced the NCI the lower their expression levels (Fig. 2K). Overall, this data set may indicate a novel pathway in neurocognitive decline among PLWH and give rise to novel marker genes for future experimental studies.
Autopsy-tissue samples from the NeuroAIDS Tissue Consortium confirm increased levels of the iN-specific DEG IFI27 in the brains of PLWH
Sustained inflammation of the CNS is considered a major factor in the development of HIV-1-related neurocognitive impairment. Several genes of the inflammatory signaling cascade have been identified so far that may contribute to this including ISG15, IFIT1, IF44, and IFITM1 (11, 68–70).
Interestingly, our differential gene expression analysis revealed interferon alpha-inducible protein 27 (IFI27) to be upregulated in iNs derived from virologically suppressed PLWH when compared to PWOH (Fig. 3A, a). This IFI27 upregulation was not observed in the UNA fibroblasts and was therefore suggested to be iN-specific (data not shown). STRING network analysis clearly showed that IFI27 is closely associated with ISG15, IFIT1, IF44, IFITM1, and many additional genes of the inflammatory pathway (Fig. 3B). Hence, we concluded that based on our approach to investigate differential neuronal gene expression in virologically suppressed PLWH by generating iNs, that IFI27 may also play a role in HIV-1-associated neuroinflammation.
Fig. 3. IFI27 expression levels are increased in PLWH-derived iNs and post-mortem brain tissue samples compared to PWOH-derived samples.
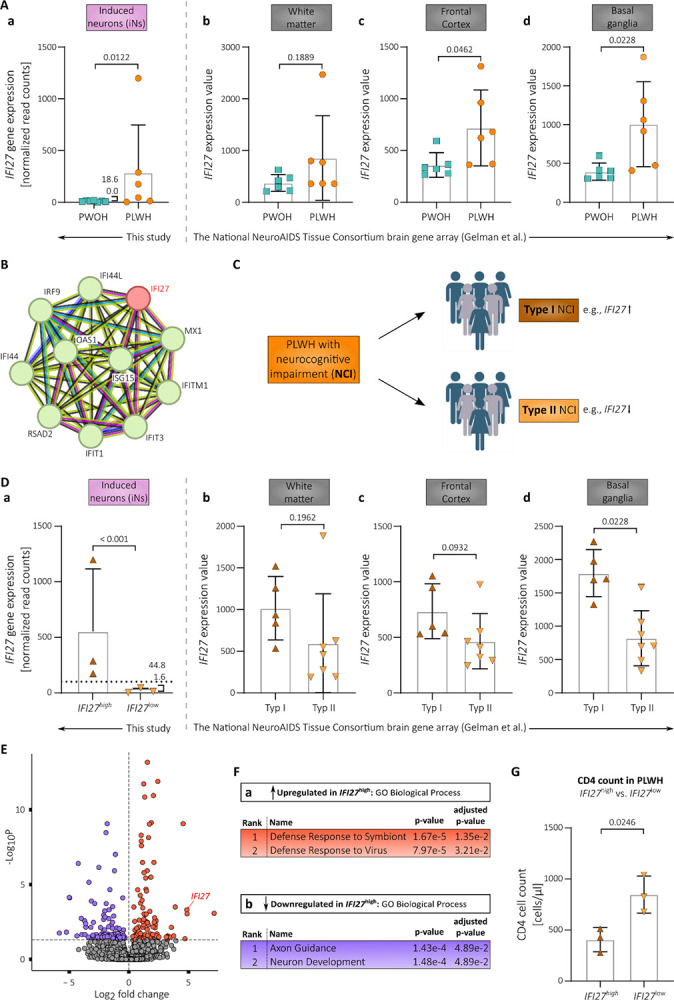
(A) IFI27 gene expression levels in PLWH- vs. PWOH-derived iNs (a) and post-mortem brain tissue samples (b-d). (B) STRING protein association network of IFI27(33, 34). (C) Scheme illustrating the concept of type I and II neurocognitive impairment in PLWH according to Gelman et al. together with the associated IFI27 expression (11) (D) IFI27 gene expression levels in PLWH IFI27high vs. IFI27low iNs (a) and type I vs. type II PLWH-derived post-mortem brain tissue samples (b-d). (E) Volcano plot showing the statistically significant (p-adj. < 0.05, log2fc > +/− 0.5) differentially expressed genes (DEGs) in PLWH IFI27high vs. IFI27low iNs based on our bulk-RNA analysis. (F) Top 2 ranked gene ontology (GO) terms of Biological Processes associated with the significantly up- (a) and downregulated genes (b) in PLWH IFI27high vs. IFI27low iNs. (G) CD4+ T cell counts in PLWH divided into IFI27high vs. IFI27low participants. Statistical significance tested with unpaired, two-tailed t-test (A b-d, D b-d, G) or derived from the conducted Wald test corrected for false discovery rates (FDR) using the Benjamini-Hochberg method on whole-transcriptome data (A a, D a). Data presented as individual data points with mean ± SD. IFI27 expression values in post-mortem brain tissue samples derived from Gelman et al.(11).
Given this strong connection to the inflammatory signals that have been previously linked to HIV-1-related neurocognitive impairment outlined above (Fig. 3B), we mined the literature to find pre-existing evidence supporting our conclusion that IFI27 may be involved as well. Importantly, we found three independent studies comparing gene expression in post-mortem brain samples between PLWH and PWOH that found significantly increased IFI27 expression in brain tissue derived from PLWH (11, 69, 71).
Solomon et al. compared gene expression in frontal white matter tissue between 34 PLWH (≥ 45 years old) on cART and 24 age-matched PLWH via gene expression profiling and showed a more than 2-fold increase in IFI27 expression in the PLWH-derived brain tissue samples (69).
Gabuzda et al. very recently likewise performed gene expression profiling and found an 1.73-fold increased IFI27 expression in the frontal lobe white matter samples derived from 28 PLWH (≥ 40 years old) on cART when compared to samples derived from 20 age- and sex-matched PWOH (71).
Lastly, Gelman et al. performed a gene expression array with post-mortem tissue from the frontal cortex (neocortex), white matter, and basal ganglia (neostriatum) obtained from the National NeuroAIDS Tissue Consortium in 2012 (11). By analyzing their publicly available gene array data set on post-mortem brain tissue, we observed that IFI27 expression levels are significantly elevated in the basal ganglia, and frontal cortex of PLWH without NCI when compared to PWOH (Fig. 3A, c – d). The increase in IFI27 expression observed for the analyzed white matter samples was not significant but nevertheless showed a trend towards upregulation (Fig. 3A, b). Together, this shows that IFI27 expression is increased in the brains of PLWH and that our here generated iNs derived from virologically suppressed PLWH reconstitute this upregulation.
Based on a comparison of DEGs between PWOH and PLWH with NCI that either developed HIV encephalitis (HIVE) or not, Gelman and colleagues have suggested the presence of two distinct pathomechanisms that lead to NCI in PLWH irrespective of cART: With inflammatory changes (Type I NCI) and without inflammatory changes (Type II NCI) (Fig. 3C). Type I and II NCI in PLWH supposedly underlie different biological pathways and several marker genes including IFI27 were identified whose expression levels together could distinguish between the two types (11, 72).
In this regard, expression of IFI27 alone may not be enough to reliably distinguish between the two types because its expression differed significantly only in the basal ganglia samples (Fig 3D, b – c). However, to nevertheless test whether the distinction of type I and II NCI may be preserved after transdifferentiation of dermal fibroblasts into iNs, we first divided our PLWH study group into IFI27 low and high expressing participants (IFI27low vs. IFI27high) (Fig. 3D, a). Based on the average foldchange between IFI27 expression in PWOH vs. PLWH with type I NCI in the post-mortem samples of the basal ganglia (~ 5-fold), we chose a cut-off that was 5-fold the average IFI27 expression in iNs derived from PWOH (= 51.50 normalized read counts).
Next, we performed differential gene expression analysis to compare the transcriptional profile of the IFI27high with the IFI27low PLWH iNs, the first now serving as putative surrogate model for type I NCI (IFI27high), and the latter for type II NCI neurons (IFI27low). We identified 215 DEGs (p-adj. < 0.05, log2fc > +/− 0.5), of which 106 were downregulated and 109 upregulated in the IFI27high PLWH iNs (Fig. 3E, S5A Table S5). The performed PCA did not reveal any obvious clustering between the two groups but on the other hand did not provide evidence against it (Fig. S5B). This might have been due to the low sample size in this context.
Gene set enrichment analysis showed that a significant number of the upregulated genes were associated with antiviral defense (Fig. 3F, a). This is in line with the Ingenuity Pathway Analysis performed by Gelman and colleagues, who found canonical pathways of antiviral defense mechanisms upregulated in their type I NCI samples as well (11). Importantly, GO terms associated with the downregulated genes in the IFI27high PLWH iNs were related to neuronal development and the formation of neuronal processes with the GO term ranked as number 1 being Axon Guidance, a term that has been likewise found to be associated with the downregulated genes in type I NCI samples in the study by Gelman et al. (Fig. 3F, b) (11). Hence, our analysis indicated that studying biological pathways underlying the distinction of type I and II NCI may be possible by transdifferentiation of participant-derived dermal fibroblasts into iNs.
Lastly, to investigate whether the made distinction between IFI27high and IFI27low PLWH iNs can be associated with any biological parameters indicative of disease state or donor characteristics, we analyzed possible associations with the age, duration of HIV-1 infection, neurocognitive impairment, and CD4+ cell count of the respective PLWH donors. Here, we observed a significant association of the CD4+ T- cell count at the day of the skin bunch biopsy on IFI27 expression in the iNs (Fig. 3G, S5C, S5D, S5E).
We conclude that post-mortem sampling of brain tissue from PLWH confirms that the elevated IFI27 expression levels we observed in the here generated PLWH-derived iNs also occur in the brains of PLWH. Further, the identified DEGs between IFI27high and IFI27low PLWH iNs indicate that the distinct mechanisms responsible for type I and II NCI are conserved in iNs and thus support iNs as a novel model system to study cognitive decline in PLWH.
Overall, differential gene expression analysis on participant-derived iNs revealed 29 DEGs between PLWH- and PWOH-derived iNs potentially revealing novel mechanisms and supporting previous concepts of HIV-1-related neuroinflammation and neurocognitive decline.
Discussion
In this study, using a recently described protocol to generate iNs that retains donor-specific disease- and age-related characteristics in vitro, we set out to investigate whether iNs derived from virologically suppressed PLWH exhibit differential gene expression compared to iNs from matched PWOH (24). We identified an iN gene signature of HIV-1 comprising 29 DEGs including genes associated with neuronal functions implicated in cognitive decline.
The performed transdifferentiation to generate iNs resulted in around 90 % cells expressing pan-neuronal marker gene TUBB3 (TUJ1). This is in line with the rate of iNs obtained by the research group that originally established this protocol and applied it to the study of Alzheimer’s disease (17). Further, the same protocol resulted in about 15 – 20% cells expressing the glutamatergic neuronal marker SLC17A7 and about 5 % cells expressing the GABAergic neuronal marker GAD1 in a previous study (16). Consistently, we have observed rates of 32.8 % SLC17A7+ and 7.7 % GAD1+ cells among our iNs underlining the feasibility and comparability of the published protocol among different labs.
Subsequent transcriptomic analysis of the participant-derived iNs revealed 29 DEGs between virologically suppressed PLWH and PWOH. Although this number appears low in comparison to work that applied the iNs system to the study of Alzheimer’s and which found >700 DEGs in iNs derived from participants with Alzheimer’s compared to controls, it is in line with a post-mortem study that conducted microarray-based transcriptome analysis on post-mortem brain samples derived from PLWH and PWOH (11, 17). In this post-mortem study, around 90 probes were significantly regulated in PLWH with no or only slight neurocognitive impairment compared with PWOH while analyzing gross brain tissue (i.e., not only neurons) across the neostriatum, neocortex, and white matter (11).
Interestingly, the authors found the majority of DEGs in the neostriatum (>80 regulated probes) and less than ten in the neocortex and white matter, respectively (11). Given the profound differences in numbers and genes of the different brain compartments, it would be interesting to apply different iNs protocols to our cohort of virologically suppressed PLWH and PWOH to identify putative DEGs in iNs of distinct neuronal subtypes in a future work (19).
We also observed increased expression of SORCS1 in PLWH iNs. SORCS1 is a general regulator for intracellular trafficking, important for maintaining neuronal functionality, and it is associated with Alzheimer’s disease) (29, 41). Notably, the SORCS1 gene encodes multiple protein isoforms (variant A, B, and C) with different trafficking and interacting properties (29, 73). Thus, to elucidate a putative role in HIV-1-related neurocognitive decline based on our observation, more detailed analyses with respect to these isoforms must be performed.
Finally, we observed increased expression levels of the inflammatory gene IFI27 in iNs derived from PLWH compared to PWOH, which is in line with previously conducted post-mortem studies (11, 69, 71). Interestingly, this gene in particular has been associated with HIV-1 in several very recent studies (74–77). Mackelprang et al. found IFI27 to become upregulated in blood samples of PLWH during acute infection and that it remained upregulated in the chronic state (74). The authors concluded that persistent elevation of a narrow set of interferon-stimulated genes including IFI27 underlies chronic immune activation during HIV-1 infection (74). In this regard, our results suggest neuron-derived IFI27 in this model as well. Liu et al. specifically searched for genes associated with immunological non-responders to HIV-1 infection in blood samples and found that IFI27 expression levels are negatively correlated with CD4+ T cell count in PLWH (76). Moreover, they showed that the predictive power of IFI27 expression levels in distinguishing PLWH with poor immune recovery was significant in their study and thus concluded that IFI27 exhibits promising properties as biomarker for CD4+ T cell recovery. In our cohort, IFI27 expression levels in PLWH iNs were likewise negatively associated with CD4+ T cell counts, which supports their findings in the context of neurons. In yet another study, Huang et al. recommended IFI27 as a novel therapeutic target for HIV infection, which was based on differential expression analysis, and PPI network analysis using publicly available data sets on blood samples derived from PLWH and controls (77). Altogether, our findings in the context of PLWH-derived iNs support this recent association of IFI27 with HIV-1 and suggest that its expression may also play a role in HIV-1-related neurocognitive impairment. Of note, in future studies, it would be interesting to investigate a potential link between the classification of NCI type I and NCI type II as introduced by Gelman et al. and the model of immunological non-responder following HIV-1 infection (11).
Besides IFI27, none of the other DEGs revealed here has been recognized in the few studies that compared differential genes expression in PLWH- and PWOH-derived post-mortem brain tissue samples (11, 78, 79). This is perhaps not surprising as our findings, focused on the comparison of iNs including a subset of glutamatergic and GABAergic neurons from PLWH and PWOH, have no direct comparator in the literature. In addition, we have performed unbiased whole-transcriptome bulk-RNA sequencing whereas prior studies on post-mortem brain tissue, e.g. those that identified IFI27 as an upregulated gene in PLWH-derived samples, tended to conduct targeted gene expression profiling on a subset of previously selected genes (e.g., inflammatory genes) (11, 69, 71). Furthermore, our inclusion in this study of PLWH, carefully screened as per our methods to exclude those with significant neuropsychiatric confounds increases the sensitivity of our findings for HIV-1 related effects.
Concerning possible limitations of our study, we think that a comparison of six PLWH iNs samples to seven PWOH iNs has been sufficient to address the question whether gene expression of iNs differentiates virologically suppressed PLWH from PWOW. This is also consistent with previous studies on post-mortem brain samples in this context, which applied similar or even smaller cohort sizes (11, 12, 79, 80). Moreover, our two groups of PLWH and PWOH are very well-matched and the PLWH participants underwent extensive neurocognitive assessment and evaluation of HIV-1-related clinical parameters.
Taken together, we have been the first to study the effects of HIV-1 infection, and infectious disease in general, on neuronal gene expression using the model system of participant-derived iNs. We have found in our cohort that the resulting gene expression of iNs differentiates virologically suppressed PLWH from PWOH by identifying 29 DEGs between the two groups. From here on, subsequent studies should follow by focusing on the single genes identified by us while also including different neuronal subtype iNs protocols, and assays to assess neuronal functionality.
Methods
Study participants
The study was approved by the Rockefeller University Institutional Review Board. Written informed consent was obtained from all participants prior to their entering the study. Enrolled participants underwent a medical history, physical and neurological examination and psychiatric and substance use history at the screening visit. For PLWH and PWOH exclusion criteria adapted from Rippeth et al. (81) included severe neurological or diagnostic and Statistical Manual Fourth Edition-Text Revision (DSM-IV-TR) (82) psychiatric illness that affects cognitive functioning (e.g. schizophrenia, bipolar affective disorder), current diagnosis of major depressive disorder as assessed by the patient health questionnaire nine item depression scale (PHQ-9) (83) and not on stable antidepressant medication greater than 30 days, a history of head injury with loss of consciousness more than 30 min, DSM-IV-TR diagnostic criteria for alcohol or illicit substance abuse or dependence, not in remission, within 1 year of the screening visit (excluding marijuana), moderate or higher efavirenz attributable central nervous system (CNS)-related toxicity or serologic evidence of untreated syphilis or positive hepatitis C serology. All PLWH had documented treatment for at least 1 year with cART and plasma HIV-1 RNA levels below 50 copies/ml for a minimum of 6 months prior to study entry.
Neuropsychological evaluation
The neuropsychological evaluation of the six PLWH recruited at the Rockefeller University was performed as described previously (25). Comprehensive neuropsychological evaluation assessing seven cognitive domains associated with HAND (attention/working memory; processing speed; learning; recall; abstraction/executive functioning; verbal fluency; and motor skills) was adapted from Rippeth et al. (81) and performed at the study visit by a study psychometrist. Using methods that correct for age, education, sex and ethnicity where appropriate, raw scores for all tests were transformed into T-scores (81). T-scores were then converted to deficit scores, which range from a minimum of 0 in the case of no impairment, to a maximum of 5 (3, 84). Calculating the sum of all deficit scores in the testing battery and then dividing by the number of administered tests allowed for determination of the global deficit score (GDS) for each participant, which provides a continuous measure of neurocognitive impairment (NCI).
Dermal fibroblast isolation and propagation
Skin samples from all six PLWH and two of seven PWOH as detailed in the manuscript were collected via skin punch biopsy under Rockefeller University IRB-approved protocol. Dermal fibroblasts were isolated from skin biopsy samples and expanded by the MSK Stem Cell Research Facility. Briefly, a 6 mm diameter skin biopsy was dissected into 10 – 15 smaller pieces, which were then plated on a 10 cm dish. Two to three pieces of samples were transferred into each well of a 6-well plate coated with 0.1 % gelatin and containing 500 μl fibroblast culture medium. The culture medium consisted of DMEM high glucose (ThermoFisher) supplemented with 10 % fetal bovine serum (HyClone), 1X NEAA (ThermoFisher), and 1X L-glutamine (ThermoFisher). A circular coverslip (FisherScientific) was carefully placed on top of the biopsy samples, and 1.5 ml of fibroblast culture medium was added onto the coverslip. Fibroblasts were observed approximately two weeks after plating and were passaged after three weeks onto gelatin-coated plates using trypsin-EDTA (0.05 % EDTA) for expansion. Dermal fibroblasts from five of seven PWOH control participants, chosen to match the demographics of the PLWH, were obtained via MTA from the Coriell Institute for Medical Research (NJ, USA).
Generation of induced neurons
The performed protocol to generate induced neurons (iNs) was adapted from (17, 24). For the generation of lentiviral vectors, six million HEK293T cells were seeded into a 0.1 % gelatine-coated T150 cell culture flask in DMEM (Gibco) supplemented with 10 % fetal calf serum (FCS). The next day, cells were transfected with 6 μg of the packaging plasmid psPAX2 (Addgene #12260), 6 μg of the envelope plasmid pMD2.G (Addgene #12259), and 6 μg the transfer plasmid pLVX-UbC-rtTA-Ngn2:2A:Ascl1 (Addgene #127289) (23). The transfection mix containing polyethylenimine (60 μg/ml) (Polysciences) in 1 ml DMEM, and the three plasmids was added to the cells after an incubation period of 30 min. At 24 h post-transfection, the culture medium was exchanged to 15 ml fresh DMEM supplemented with 10 % FCS. At 48 h post-transfection, lentiviral vectors were harvested by pelleting any cells and cell debris at 400 × g for 5 min, aliquoting the resulting supernatant and storing at −80°C. UNA fibroblasts were generated by transducing 500,000 dermal fibroblasts with 500 μl of the lentiviral vector stock in a T25 cell culture flask while adding 4 μg/ml polybrene (Tocris) to improve transduction efficiency. At 24 h post-transduction, the medium was exchanged to fresh fibroblast medium. Selection with puromycin (1 μ/ml) (Sigma) started 72 h post-transduction. Transduced and selected fibroblasts (UNA fibroblasts) were passaged once and then frozen in liquid nitrogen. To generate iNs, all UNA fibroblasts were thawed the same day and processed in parallel to reduce batch effects during RNA sequencing. UNA fibroblasts were passaged several times after thawing and the neuronal conversion was performed as previously described (24). At day 21 of neuronal conversion, successfully converted iNs were isolated by FACS via staining for the neuronal marker PSA-NCAM. For this, cells were detached from the cell culture flasks with trypsin-EDTA and collected in FACS buffer consisting of 5 % FCS in PBS. Cells were pelleted (400 × g, 5 min) and incubated in a total of 200 μl FACS buffer containing the PE-conjugated PSA-NCAM antibody (Myltenyi Biotec) at a 1:100 dilution. After an incubation for 30 min at 4°C in the dark, the cells were washed twice with 500 μl FACS buffer and resuspended in 300 μl FACS buffer containing 1x DAPI (Thermo Scientific) used as live/dead stain. Cell sorting was performed on a BD FACSymphony™ S6 Cell Sorter at the WCM CLC Flow Cytometry Core Facility. Sorted cells were pelleted and then either lysed according to the respective downstream protocol or cultured in BrainPhys medium (StemCell) supplemented with B27 (1x) (Thermo), N2 (1 %) (StemCell), GDNF (20 ng/ml) (StemCell), BDNF (20 ng/ml) (StemCell), db-cAMP (500 μg/ml) (StemCell), and Laminin (1 μg/ml) (Thermo). For the first 24 hours post-FACS, we supplemented the medium with 10 μM ROCK inhibitor (MedChemExpress).
Microscopic analysis and immunocytochemistry
Medium was removed, and the cells incubated in 4 % PFA for 20 min at room temperature (RT). After washing with PBS, cells were permeabilized with 0.1 % Triton-X-100 in PBS for 10 min at RT. After two additional washing steps with PBS, a blocking solution (2 % BSA in PBS) was applied for 1 h at RT. Primary antibodies were diluted in blocking solution and the cells were incubated with this antibody solution overnight at 4°C. Cells were washed twice with PBS and incubated with the secondary antibodies and Hoechst to stain nuclei for 2 hours at RT in the dark. Microscopic images were taken with the Olympus IX81 microscope (Olympus) using the Slidebook (version 6) software (3i). Image analysis has been performed with Fiji (85). The following antibodies were used in this study: Mouse-anti-Tubulin beta-3 (TUBB3) antibody (BioLegend), chicken-anti-MAP2 antibody (ThermoFisher), Alexa 488-conjugated goat anti-mouse IgG (ThermoFisher) and Alexa 568-conjugated anti-chicken IgG (ThermoFisher).
Single-cell RNA sequencing and analysis
Cells were pelleted after FACS by centrifugation at 1,200 × g for 10 min. Medium was removed until only about 1 ml was left on top of the cells. The cells were resuspended and transferred into 1.5 ml tubes. After another centrifugation step (1,200 × g for 5 min), the complete supernatant was removed, and cells resuspended in 50 μl PBS containing 0.04 % BSA. Single-cell (sc)RNA sequencing has been performed at the Genomics Resources Core Facility (GRCF) at Weill Cornell Medicine. In brief, the 10X Libraries were sequenced on the Illumina NovaSeq6000 platform with pair-end reads (28 bp for read 1 and 90 bp for read 2). Sequencing data were analyzed by the 10X Cell Ranger pipeline (v7.1.0) in two steps. In the first step, Cell Ranger mkfastq demultiplexed samples and generated FASTQ files and in the second step, Cell Ranger count aligned FASTQ files to the 10X pre-built human reference genome (refdata-gex-GRCh38–2020-A) with standard parameters as described on 10X Genomics (https://www.10xgenomics.com/support/software/cell-ranger/latest/analysis/running-pipelines/cr-gex-count) and extracted gene expression UMI counts matrix. Count matrices were processed in RStudio using the Seurat package (86–88). Cells were filtered as previously described (>300/<10,000 unique feature counts and < 30 % mitochondrial reads), which resulted in 5994 (Participant 4642) and 8368 cells (Participant 3962) for downstream analysis (17). Data was normalized using the global-scaling normalization method with LogNormalize (scale factor 10,000). A subset of 2,000 features with high cell-to-cell variation was identified and scaled for downstream analysis. UMAP plots were generated using the identified dimensionality during principal component analysis. Percentages of cells expressing certain genes were determined using the scCustomize package (89).
Bulk-RNA sequencing and analysis
Total RNA was extracted using the RNeasy kit (Qiagen) including the 15 min on-column DNase treatment. RNA integrity and quantity has been determined using the TapeStation instrument (Agilent). All RNA samples exhibited an RNA integrity number (RIN) above 9.0. Libraries were sequenced with paired-end 50 bps on a NovaSeqXplus sequencer. Raw sequencing reads in BCL format were processed through bcl2fastq 2.20 (Illumina) for FASTQ conversion and demultiplexing. After trimming the adaptors with cutadapt (1.18), RNA reads were aligned and mapped to the GRCh38 human reference genome by STAR (2.5.2) and transcriptome reconstruction was performed with Cufflinks (2.1.1) (90, 91). Raw read counts per gene were extracted using HTSeq-count v0.11.2 (92). Read count matrices were important into RStudio and differential gene expression analysis was performed using the DESeq2 package (93). Low count genes (>10 reads) were pre-filtered and effect sizes were shrinked for visualization in MA plots using the apeglm method (94). Statistical significance was tested via the in DESeq2 implemented Wald test (p-value) and corrected for false discovery rates (FDR) using the Benjamini-Hochberg method (p-adj.) DESeq2 median ratios count normalization was used. For visualization and cluster analysis, count data transformation was performed using the vst function (95). Gene set enrichment analysis was conducted with EnrichR, which uses Fisher’s exact test or the hypergeometric test (p-value) and FDR correction via the Benjamini-Hochberg method (p-adj.) (96–98). Gene sets used for this study were derived from Gene Ontology (99, 100), Jensen DISEASES (64), SynGO (101), Reactome (102, 103), and DisGeNet (65) databases.
Protein-protein interaction (PPI) network analysis
We determined 1st-order interaction partners using the free open-source IntAct Molecular Interaction Database system (EMBL-EBI) (62), and the human reference interactome (HuRI) map (Center for Cancer Systems Biology at Dana-Farber Cancer Institute) (63). We generated and retrieved lists of the 1st-order interaction partners of the here identified DEGs based on the underlying literature curation and direct user submission (IntAct) as well as the un-biased, systematic, yeast two-hybrid screen for PPIs (HuRI).
Statistics and software
Besides the beforementioned software, GraphPad was used for a subset of statistical analysis and plots. Inkscape was used for illustrations and finalization of figures. BioRender was used to generate a subset of schemes. Statistical analyses were run with either RStudio using the DESeq2 or Seurat package, GraphPad, or EnrichR and has been depicted throughout the manuscript where applied.
Supplementary Material
Acknowledgements
Many thanks to Fred Gage, Jerome Mertens, and especially Larissa Traxler for their support and help in setting up the iN protocol. We want to thank all members of the Nixon and Furler group for thoughtful discussions and help. We thank Aaron Zhong for helping with the lab work and Johannes Ptok for helping with the bioinformatic analysis. We thank Anand Ramani for sharing his expertise in neuronal cell culture. We thank the members of the WCM CLC Flow Cytometry Core Facility for their services and help in fluorescence-activated cell sorting of the iNs. We thank the Genomics Resources Core Facility (GRCF) at Weill Cornell Medicine for their services and great help regarding the transcriptomic analyses.
Funding
National Institute on Aging (NIA) grant R21AG071433 (THE)
National Institute of Neurological Disorders and Stroke (NINDS) grant R21NS126094 (THE)
German Research Foundation (DFG) grant HU 1636/13-1 (PNO)
National Institute on Aging (NIA) grant R56AG078970 (DFN)
National Institute of Neurological Disorders and Stroke grant R01NS117458 (LCN)
National Institute of Allergy and Infectious Diseases (NIAID) grant UM1AI164559 (LCN)
National Institute of Mental Health (NIMH) grant R01MH130197 (LCN)
National Institute on Drug Abuse (NIDA) grant U01DA058527 (LCN)
National Institute on Drug Abuse (NIDA) grant R01DA052027 (LCN)
National Institute of Allergy and Infectious Diseases (NIAID) grant R56AI125128 (MY)
Competing Interest
THE is a paid consultant for Tonix Pharmaceuticals. LCN reports grants from the NIH and has received consulting fees from work as a scientific advisor for AbbVie, ViiV Healthcare, and Cytodyn and also serves on the Board of Directors of CytoDyn and has financial interests in Ledidi AS, all for work outside of the submitted work. LCN’s interests were reviewed and are managed by Weill Cornell Medicine in accordance with their conflict-of-interest policies. The other authors declare that they have no competing interests in relation to this work.
Funding Statement
National Institute on Aging (NIA) grant R21AG071433 (THE)
National Institute of Neurological Disorders and Stroke (NINDS) grant R21NS126094 (THE)
German Research Foundation (DFG) grant HU 1636/13-1 (PNO)
National Institute on Aging (NIA) grant R56AG078970 (DFN)
National Institute of Neurological Disorders and Stroke grant R01NS117458 (LCN)
National Institute of Allergy and Infectious Diseases (NIAID) grant UM1AI164559 (LCN)
National Institute of Mental Health (NIMH) grant R01MH130197 (LCN)
National Institute on Drug Abuse (NIDA) grant U01DA058527 (LCN)
National Institute on Drug Abuse (NIDA) grant R01DA052027 (LCN)
National Institute of Allergy and Infectious Diseases (NIAID) grant R56AI125128 (MY)
Data and materials availability
Please contact the corresponding author Teresa H. Evering (evering@med.cornell.edu) for any inquiries regarding the used material and uploaded data. Raw data derived from the bulk-RNAseq and scRNAseq experiment will be made publicly available through the Gene Expression Omnibus genomics data repository (https://www.ncbi.nlm.nih.gov/geo/) upon acceptance of the article. Complete lists of differentially expressed genes between our groups are available as supplementary material.
References
- 1.Hammer S. M. et al. , A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med 337, 725–733 (1997). [DOI] [PubMed] [Google Scholar]
- 2.Heaton R. K. et al. , HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75, 2087–2096 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heaton R. K. et al. , The HNRC 500--neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc 1, 231–251 (1995). [DOI] [PubMed] [Google Scholar]
- 4.Sacktor N. et al. , Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology 86, 334–340 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostermann P. N., Evering T. H., The Impact of Aging on HIV-1-related Neurocognitive Impairment. Ageing Res Rev, 102513 (2024). [DOI] [PubMed] [Google Scholar]
- 6.Wei J. et al. , The Prevalence of Frascati-Criteria-Based HIV-Associated Neurocognitive Disorder (HAND) in HIV-Infected Adults: A Systematic Review and Meta-Analysis. Front Neurol 11, 581346 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valcour V. et al. , Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis 206, 275–282 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D S. et al. , HIV-associated neurocognitive disorder--pathogenesis and prospects for treatment. Nature reviews. Neurology 12, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaul M., Lipton S. A., Mechanisms of neuronal injury and death in HIV-1 associated dementia. Curr HIV Res 4, 307–318 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Ellis R. J., Marquine M. J., Kaul M., Fields J. A., Schlachetzki J. C. M., Mechanisms underlying HIV-associated cognitive impairment and emerging therapies for its management. Nat Rev Neurol 19, 668–687 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gelman B. B. et al. , The National NeuroAIDS Tissue Consortium brain gene array: two types of HIV-associated neurocognitive impairment. PLoS One 7, e46178 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ojeda-Juárez D., Kaul M., Transcriptomic and Genetic Profiling of HIV-Associated Neurocognitive Disorders. Front Mol Biosci 8, 721954 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’hooge R., Franck F., Mucke L., De Deyn P. P., Age-related behavioural deficits in transgenic mice expressing the HIV-1 coat protein gp120. Eur J Neurosci 11, 4398–4402 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Maung R. et al. , CCR5 knockout prevents neuronal injury and behavioral impairment induced in a transgenic mouse model by a CXCR4-using HIV-1 glycoprotein 120. J Immunol 193, 1895–1910 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toggas S. M. et al. , Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature 367, 188–193 (1994). [DOI] [PubMed] [Google Scholar]
- 16.Herdy J. R. et al. , Increased post-mitotic senescence in aged human neurons is a pathological feature of Alzheimer’s disease. Cell Stem Cell 29, 1637–1652.e1636 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mertens J. et al. , Age-dependent instability of mature neuronal fate in induced neurons from Alzheimer’s patients. Cell Stem Cell 28, 1533–1548.e1536 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh Y. M., Lee S. W., Yoo A. S., Modeling Huntington disease through microRNA-mediated neuronal reprogramming identifies age-associated autophagy dysfunction driving the onset of neurodegeneration. Autophagy, 1–3 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Church V. A. et al. , Generation of Human Neurons by microRNA-Mediated Direct Conversion of Dermal Fibroblasts. Methods Mol Biol 2239, 77–100 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Mertens J., Reid D., Lau S., Kim Y., Gage F. H., Aging in a Dish: iPSC-Derived and Directly Induced Neurons for Studying Brain Aging and Age-Related Neurodegenerative Diseases. Annu Rev Genet 52, 271–293 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mertens J. et al. , Directly Reprogrammed Human Neurons Retain Aging-Associated Transcriptomic Signatures and Reveal Age-Related Nucleocytoplasmic Defects. Cell Stem Cell 17, 705–718 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aung H. L. et al. , Is There Any Evidence of Premature, Accentuated and Accelerated Aging Effects on Neurocognition in People Living with HIV? A Systematic Review. AIDS Behav 25, 917–960 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herdy J. et al. , Chemical modulation of transcriptionally enriched signaling pathways to optimize the conversion of fibroblasts into neurons. Elife 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou-Yang L. et al. , Direct Conversion of Human Fibroblasts to Induced Neurons. Methods Mol Biol 2352, 73–96 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Evering T. H. et al. , Rates of non-confounded HIV-associated neurocognitive disorders in men initiating combination antiretroviral therapy during primary infection. AIDS 30, 203–210 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groffen A. J. et al. , Doc2b is a high-affinity Ca2+ sensor for spontaneous neurotransmitter release. Science 327, 1614–1618 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukuda M., Kanno E., Yamamoto A., Rabphilin and Noc2 are recruited to dense-core vesicles through specific interaction with Rab27A in PC12 cells. J Biol Chem 279, 13065–13075 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Haynes L. P., Evans G. J., Morgan A., Burgoyne R. D., A direct inhibitory role for the Rab3-specific effector, Noc2, in Ca2+−regulated exocytosis in neuroendocrine cells. J Biol Chem 276, 9726–9732 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Hermey G. et al. , SorCS1 variants and amyloid precursor protein (APP) are co-transported in neurons but only SorCS1c modulates anterograde APP transport. J Neurochem 135, 60–75 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Nadal M. S. et al. , The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron 37, 449–461 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Yao J., Gaffaney J. D., Kwon S. E., Chapman E. R., Doc2 is a Ca2+ sensor required for asynchronous neurotransmitter release. Cell 147, 666–677 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xue R. et al. , Doc2-mediated superpriming supports synaptic augmentation. Proc Natl Acad Sci U S A 115, E5605–E5613 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snel B., Lehmann G., Bork P., Huynen M. A., STRING: a web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res 28, 3442–3444 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szklarczyk D. et al. , The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res 51, D638–D646 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fishilevich S. et al. , GeneHancer: genome-wide integration of enhancers and target genes in GeneCards. Database (Oxford) 2017, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stelzer G. et al. , The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr Protoc Bioinformatics 54, 1.30.31–31.30.33 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Napolioni V., Scelsi M. A., Khan R. R., Altmann A., Greicius M. D., Recent Consanguinity and Outbred Autozygosity Are Associated With Increased Risk of Late-Onset Alzheimer’s Disease. Front Genet 11, 629373 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang S. et al. , Olfactomedin-3 Enhances Seizure Activity by Interacting With AMPA Receptors in Epilepsy Models. Front Cell Dev Biol 8, 722 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reitz C. et al. , SORCS1 alters amyloid precursor protein processing and variants may increase Alzheimer’s disease risk. Ann Neurol 69, 47–64 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang X. et al. , Genomic convergence to identify candidate genes for Alzheimer disease on chromosome 10. Hum Mutat 30, 463–471 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eggert S., Thomas C., Kins S., Hermey G., Trafficking in Alzheimer’s Disease: Modulation of APP Transport and Processing by the Transmembrane Proteins LRP1, SorLA, SorCS1c, Sortilin, and Calsyntenin. Mol Neurobiol 55, 5809–5829 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Savas J. N. et al. , The Sorting Receptor SorCS1 Regulates Trafficking of Neurexin and AMPA Receptors. Neuron 87, 764–780 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He Y., Fang Z., Yu G., Sortilin-related VPS10 domain containing receptor 1 and Alzheimer’s disease-associated allelic variations preferentially exist in female or type 2 diabetes mellitus patients in southern Han Chinese. Psychogeriatrics 12, 215–225 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Sjöstedt E. et al. , An atlas of the protein-coding genes in the human, pig, and mouse brain. Science 367, (2020). [DOI] [PubMed] [Google Scholar]
- 45.Uhlén M. et al. , Proteomics. Tissue-based map of the human proteome. Science 347, 1260419 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Lin L. et al. , DPP6 Loss Impacts Hippocampal Synaptic Development and Induces Behavioral Impairments in Recognition, Learning and Memory. Front Cell Neurosci 12, 84 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaulin Y. A. et al. , The dipeptidyl-peptidase-like protein DPP6 determines the unitary conductance of neuronal Kv4.2 channels. J Neurosci 29, 3242–3251 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin L. et al. , DPP6 regulation of dendritic morphogenesis impacts hippocampal synaptic development. Nat Commun 4, 2270 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sollis E. et al. , The NHGRI-EBI GWAS Catalog: knowledgebase and deposition resource. Nucleic Acids Res 51, D977–D985 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamboh M. I. et al. , Population-based genome-wide association study of cognitive decline in older adults free of dementia: identification of a novel locus for the attention domain. Neurobiol Aging 84, 239.e215–239.e224 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang M. et al. , A genome-wide search for pleiotropy in more than 100,000 harmonized longitudinal cognitive domain scores. Mol Neurodegener 18, 40 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Homann J. et al. , Genome-Wide Association Study of Alzheimer’s Disease Brain Imaging Biomarkers and Neuropsychological Phenotypes in the European Medical Information Framework for Alzheimer’s Disease Multimodal Biomarker Discovery Dataset. Front Aging Neurosci 14, 840651 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naujock M. et al. , Neuronal Differentiation of Induced Pluripotent Stem Cells from Schizophrenia Patients in Two-Dimensional and in Three-Dimensional Cultures Reveals Increased Expression of the Kv4.2 Subunit DPP6 That Contributes to Decreased Neuronal Activity. Stem Cells Dev 29, 1577–1587 (2020). [DOI] [PubMed] [Google Scholar]
- 54.Dityatev A., Schachner M., Extracellular matrix molecules and synaptic plasticity. Nat Rev Neurosci 4, 456–468 (2003). [DOI] [PubMed] [Google Scholar]
- 55.Wareham L. K., Baratta R. O., Del Buono B. J., Schlumpf E., Calkins D. J., Collagen in the central nervous system: contributions to neurodegeneration and promise as a therapeutic target. Mol Neurodegener 19, 11 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Banyard J., Bao L., Zetter B. R., Type XXIII collagen, a new transmembrane collagen identified in metastatic tumor cells. J Biol Chem 278, 20989–20994 (2003). [DOI] [PubMed] [Google Scholar]
- 57.Sherva R. et al. , Genome-wide association study of rate of cognitive decline in Alzheimer’s disease patients identifies novel genes and pathways. Alzheimers Dement 16, 1134–1145 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramesh S., Govindarajulu M., Suppiramaniam V., Moore T., Dhanasekaran M., Autotaxin−Lysophosphatidic Acid Signaling in Alzheimer’s Disease. Int J Mol Sci 19, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davies G. et al. , Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat Commun 9, 2098 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harper J. D. et al. , Genome-Wide Association Study of Incident Dementia in a Community-Based Sample of Older Subjects. J Alzheimers Dis 88, 787–798 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakahara S. et al. , Polygenic risk score, genome-wide association, and gene set analyses of cognitive domain deficits in schizophrenia. Schizophr Res 201, 393–399 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Del Toro N. et al. , The IntAct database: efficient access to fine-grained molecular interaction data. Nucleic Acids Res 50, D648–D653 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luck K. et al. , A reference map of the human binary protein interactome. Nature 580, 402–408 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grissa D., Junge A., Oprea T. I., Jensen L. J., Diseases 2.0: a weekly updated database of disease-gene associations from text mining and data integration. Database (Oxford) 2022, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Piñero J. et al. , The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res 48, D845–D855 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kavoosi S., Shahraki A., Sheervalilou R., Identification of microRNA-mRNA Regulatory Networks with Therapeutic Values in Alzheimer’s Disease by Bioinformatics Analysis. J Alzheimers Dis, (2024). [DOI] [PubMed] [Google Scholar]
- 67.Patel H. et al. , Transcriptomic analysis of probable asymptomatic and symptomatic alzheimer brains. Brain Behav Immun 80, 644–656 (2019). [DOI] [PubMed] [Google Scholar]
- 68.Wang S. et al. , Comprehensive analyses identify potential biomarkers for encephalitis in HIV infection. Sci Rep 13, 18418 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Solomon I. H. et al. , White Matter Abnormalities Linked to Interferon, Stress Response, and Energy Metabolism Gene Expression Changes in Older HIV-Positive Patients on Antiretroviral Therapy. Mol Neurobiol 57, 1115–1130 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Solomon I. H. et al. , Brain and liver pathology, amyloid deposition, and interferon responses among older HIV-positive patients in the late HAART era. BMC Infect Dis 17, 151 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gabuzda D., Yin J., Misra V., Chettimada S., Gelman B. B., Intact Proviral DNA Analysis of the Brain Viral Reservoir and Relationship to Neuroinflammation in People with HIV on Suppressive Antiretroviral Therapy. Viruses 15, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Masliah E., DeTeresa R. M., Mallory M. E., Hansen L. A., Changes in pathological findings at autopsy in AIDS cases for the last 15 years. AIDS 14, 69–74 (2000). [DOI] [PubMed] [Google Scholar]
- 73.Coulson E. J., Andersen O. M., The A-B-C for SORting APP. J Neurochem 135, 1–3 (2015). [DOI] [PubMed] [Google Scholar]
- 74.Mackelprang R. D. et al. , Upregulation of IFN-stimulated genes persists beyond the transitory broad immunologic changes of acute HIV-1 infection. iScience 26, 106454 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parker E. et al. , Gene dysregulation in acute HIV-1 infection - early transcriptomic analysis reveals the crucial biological functions affected. Front Cell Infect Microbiol 13, 1074847 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu X. et al. , Comparative Transcriptional Analysis Identified Characteristic Genes and Patterns in HIV-Infected Immunological Non-Responders. Front Immunol 13, 807890 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang H. et al. , IFI27 is a potential therapeutic target for HIV infection. Ann Med 54, 314–325 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Canchi S., Swinton M. K., Rissman R. A., Fields J. A., Transcriptomic analysis of brain tissues identifies a role for CCAAT enhancer binding protein β in HIV-associated neurocognitive disorder. J Neuroinflammation 17, 112 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Borjabad A. et al. , Significant effects of antiretroviral therapy on global gene expression in brain tissues of patients with HIV-1-associated neurocognitive disorders. PLoS Pathog 7, e1002213 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shapshak P. et al. , Analytic approaches to differential gene expression in AIDS versus control brains. Front Biosci 9, 2935–2946 (2004). [DOI] [PubMed] [Google Scholar]
- 81.Rippeth J. D. et al. , Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc 10, 1–14 (2004). [DOI] [PubMed] [Google Scholar]
- 82.American Psychiatric Association., American Psychiatric Association. Task Force on DSM-IV., Diagnostic and statistical manual of mental disorders : DSM-IV-TR. (American Psychiatric Association, Washington, DC, ed. 4th, 2000), pp. xxxvii, 943 p. [Google Scholar]
- 83.Kroenke K., Spitzer R. L., Williams J. B., The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 16, 606–613 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carey C. L. et al. , Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol 26, 307–319 (2004). [DOI] [PubMed] [Google Scholar]
- 85.Schindelin J. et al. , Fiji: an open-source platform for biological-image analysis. Nat Methods 9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hao Y. et al. , Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat Biotechnol 42, 293–304 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stuart T. et al. , Comprehensive Integration of Single-Cell Data. Cell 177, 1888–1902.e1821 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Satija R., Farrell J. A., Gennert D., Schier A. F., Regev A., Spatial reconstruction of single-cell gene expression data. Nat Biotechnol 33, 495–502 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marsh S., Maelle S., Hoffman P.. (2021).
- 90.Schneider V. A. et al. , Evaluation of GRCh38 and de novo haploid genome assemblies demonstrates the enduring quality of the reference assembly. Genome Res 27, 849–864 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dobin A. et al. , STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Anders S., Pyl P. T., Huber W., HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Love M. I., Huber W., Anders S., Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu A., Ibrahim J. G., Love M. I., Heavy-tailed prior distributions for sequence count data: removing the noise and preserving large differences. Bioinformatics 35, 2084–2092 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Anders S., Huber W., Differential expression analysis for sequence count data. Genome Biol 11, R106 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen E. Y. et al. , Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14, 128 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kuleshov M. V. et al. , Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res 44, W90–97 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xie Z. et al. , Gene Set Knowledge Discovery with Enrichr. Curr Protoc 1, e90 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ashburner M. et al. , Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25, 25–29 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aleksander S. A. et al. , The Gene Ontology knowledgebase in 2023. Genetics 224, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Koopmans F. et al. , SynGO: An Evidence-Based, Expert-Curated Knowledge Base for the Synapse. Neuron 103, 217–234.e214 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu G., Haw R., Functional Interaction Network Construction and Analysis for Disease Discovery. Methods Mol Biol 1558, 235–253 (2017). [DOI] [PubMed] [Google Scholar]
- 103.Milacic M. et al. , The Reactome Pathway Knowledgebase 2024. Nucleic Acids Res 52, D672–D678 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harrison P. W. et al. , Ensembl 2024. Nucleic Acids Res 52, D891–D899 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Please contact the corresponding author Teresa H. Evering (evering@med.cornell.edu) for any inquiries regarding the used material and uploaded data. Raw data derived from the bulk-RNAseq and scRNAseq experiment will be made publicly available through the Gene Expression Omnibus genomics data repository (https://www.ncbi.nlm.nih.gov/geo/) upon acceptance of the article. Complete lists of differentially expressed genes between our groups are available as supplementary material.