Abstract
Dendrocalamus brandisii is a high-quality bamboo species that can be used for both bamboo shoots and wood. The nutritional components and flavors of D. brandisii vary from different geographical provenances. However, the unique biological characteristics of bamboo make morphological classification methods unsuitable for distinguishing them. Although the new cultivar ‘Manxie No.1’ has significant differences in the branch characteristics and the color of shoot sheaths compared to the D. brandisii, it still lacks precise genetic information at the molecular level. This study identified 231,789 microsatellite markers based on the whole genome of D. brandisii and analyzed their type composition and distribution on chromosomes in detail. Then, using TP-M13-SSR fluorescence-labeling technology, 34 pairs of polymorphic primers were screened to identify the new cultivar ‘Manxie No.1’ and 11 different geographical provenances of D. brandisii. We also constructed DNA fingerprinting profiles for them. At the same time, we mapped six polymorphic SSRs to the gene of D. brandisii, among which SSR673 was mapped to DhB10G011540, which is related to plant immunity. The specific markers selected in this study can rapidly identify the provenances and the new cultivar of D. brandisii and help explore candidate genes related to some important traits.
Keywords: D. brandisii, ‘Manxie No.1’, microsatellite development, provenances and cultivar identification, DNA fingerprinting, plant genetic markers, bamboo species identification, genetic diversity analysis, molecular characterization of plants
1. Introduction
Bamboo is a monocotyledonous plant in the Bambusoideae of the Poaceae [1]. Its enormous regeneration ability and carbon sequestration efficiency make it an astonishingly effective carbon sink and an indispensable plant for mitigating global warming [2]. The three famous species of sweet bamboo are Dendrocalamus brandisii, Dendrocalamus hamiltonii, and Dendrocalamus asper. The main origins of D. brandisii are in Southern India, Northeastern India, and Myanmar, and it has been introduced to Southeast Asia. It is mainly distributed in Yunnan Province, China, as well as some South and Southeast Asian countries [3,4]. It has excellent characteristics, such as a high survival rate, fast growth, fast emergence, high yield, and good-tasting bamboo shoots. Therefore, it has become a key bamboo species for shoot development in Yunnan Province, China [5]. Previous studies have shown that there are differences in the nutritional composition and flavor of bamboo shoots from different provenances of D. brandisii [6]. Still, they cannot be distinguished by their appearance. The new cultivar ‘Manxie No.1’ is rich in more flavonoids and fewer bitter amino acids, thus having higher nutritional value and better flavor [7]. Although ‘Manxie No.1’ has underdeveloped main branches and significant differences in the color of the shoot sheaths compared to D. brandisii (Figure S1), the identification of new cultivars is still difficult to carry out as the shoot sheaths gradually fall off during the growth process, and the property protection of new cultivars is also threatened to some extent. Therefore, identifying the provenances and new cultivars of D. brandisii has important practical significance and market application value.
Different from crops, the genetic composition of bamboo is basically wild [8]. Most bamboo has a long flowering cycle and dies after flowering, a special biological characteristic that makes traditional morphological classification based on reproductive characteristics unsuitable for bamboo. This has prompted bamboo taxonomists to pay more attention to the classification of vegetative characters [9,10]. However, there are significant differences in the morphology of bamboo’s vegetative organs, making confusion a prominent issue in bamboo classification, especially in species identification. Overall, bamboo classification mainly relies on vegetative organs that are easily affected by the environment, and the process is cumbersome and complex with low accuracy. The unclear species pedigree and obstacles in cultivar identification are important reasons that restrict the development of the bamboo industry [11]. Currently, there is no unified and authoritative classification system, and bamboo classification remains an important issue in bamboo resource research.
With the development of modern molecular biology technology, molecular marker techniques such as RFLP, RAPD, AFLP, SCAR, SSR, ISSR, etc., have been used for germplasm identification and the phylogenetic determination of bamboo plants [12,13,14,15], which greatly improves the accuracy of classification. SSR, also known as a microsatellite, refers to short DNA sequences with variable tandem repeats of 1–6 bp. SSR is one of the most informative and powerful molecular markers in biology. Due to its high inter-species transferability, co-dominant inheritance, multiple alleles, wide genome coverage, high relative abundance, and simple detection, it has been prioritized for many genetic studies [16,17,18,19,20]. The innovation of capillary electrophoresis technology has enhanced the accuracy of SSR [21]; the combination of these two technologies is popular in cultivar identification and provenance identification.
At present, only a few bamboo species in the genera Phyllostachys and Guadua have developed whole-genome SSR markers and conducted related genetic analysis in bamboo, such as Phyllostachys edulis and Guadua chacoensis [15,22]. For the genus Dendrocalamus, there are only reports on the development of whole-genome-based SSR markers for Dendrocalamus strictus [23], genome survey sequencing-based SSR markers for Dendrocalamus longispathus [24], and RNA-seq-based SSR markers for Dendrocalamus latiflorus [25], while there have been no reports on the development of whole-genome SSR markers for other bamboo species in this genus. According to the existing literature, all studies mainly center on different bamboo species. However, reports on the same bamboo species from different geographical provenances are scarce. This study was based on the genome of D. brandisii. SSR markers covering the whole genome were developed and located in different regions of chromosomes and genes. After the specific primers were obtained via agarose gel electrophoresis, 34 polymorphic primers were further screened using capillary electrophoresis to evaluate the polymorphism potential of the new cultivar 'Manxie 1’ and 11 different geographical provenances, and their phylogenetic relationships were explained using cluster analysis. A fluorescent labeled TP-M13-SSR system was established to distinguish the new cultivar ‘Manxie No.1’ and different sources of D. brandisii, and their DNA fingerprintings were constructed, providing a simple method for their identification and protection.
2. Results
2.1. Abundance, Frequency, and Characteristics of Microsatellites
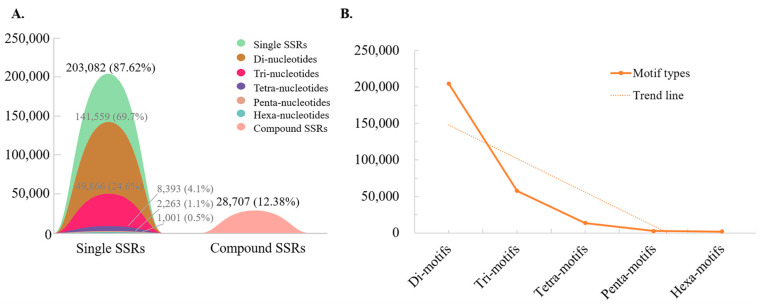
Based on bioinformatics analysis, we detected 231,789 microsatellites in the 2813.5 Mb genome of D. brandisii, of which 231,110 (99.71%) microsatellites were located on chromosomes. The number of perfect SSRs and complex SSRs was 203,082 and 28,707, respectively (Figure 1A), with a microsatellite frequency of 82.4/Mb. In 87.62% of perfect SSRs, the proportion of motifs with different numbers of bases varies. The proportion of microsatellites with dinucleotide repeat motifs is the highest at 69.7%; microsatellites with trinucleotide repeat motifs account for 24.56%, followed by which microsatellites with 4–6 nucleotide repeat motifs account for a smaller proportion, being 4.1%, 1.1%, and 0.5%, respectively (Figure 1A).
Figure 1.
The proportion of different types of microsatellites. (A) The number and proportion of different types of microsatellites. The black numbers and corresponding shapes represent the number and proportion of single and composite SSRs in all SSRs. The gray numbers and corresponding shapes represent the number and proportion of different types of perfect SSRs in all perfect SSRs. (B) Trends in the number of perfect SSRs with different repetitive motifs.
The 28,707 compound SSRs identified in this study were composed of 2–78 perfect SSRs and intermediate sequence connections of less than 100 bp. Among them, the number of composite SSRs containing two perfect SSRs was the highest (21,789), accounting for 75.9%, followed by the number of composite SSRs containing three perfect SSRs (4069), accounting for 14.2%. The number of composite SSRs containing 4–78 perfect SSRs was relatively low (1–1119), accounting for 0.003% to 3.899% (Table S1). For compound SSRs containing two perfect SSRs, the length of the connecting sequence is mostly 0 nt (9124), accounting for 41.87%, followed by 1 nt (1305) and 2 nt (1258), accounting for 5.99% and 5.77%, respectively. There are fewer connecting sequences with a length of 3 nt to 100 nt (41–357), accounting for 0.19% to 1.64% (Table S2).
2.2. Frequency and Characteristics of Motif Repeat
We identified 280,317 motifs, and the number of motifs with different base numbers decreased with increasing motif length (Figure 1B). The main types of motifs with different base numbers are as follows: the di-nucleotide motifs are mainly AT/AT, AG/CT, and GA/TC; the tri-nucleotide motifs are mainly GCC/GGC, CGC/GCG, and CCG/CGG; the tetra-nucleotide motifs are mainly CATA/TATG, ATAC/GTAT, and TACA/TGTA; the penta-nucleotide motifs are mainly GAGCC/GGCTC, CGAGC/GCTCG, and AAAAG/CTTTT; and the hexa-nucleotide motifs are mainly CATATA/TATATG, ATATAC/GTATAT, and TATACA/TGTATA (Table 1).
Table 1.
The number of different types of motifs.
| Types | Motif Types | Number | % |
|---|---|---|---|
| P2 | GA/TC | 27,515 | 19.44% |
| AG/CT | 34,419 | 24.31% | |
| AT/AT | 49,705 | 35.11% | |
| P3 | CCG/CGG | 3992 | 8.01% |
| CGC/GCG | 4451 | 8.93% | |
| GCC/GGC | 5568 | 11.17% | |
| P4 | TACA/TGTA | 506 | 6.03% |
| ATAC/GTAT | 618 | 7.36% | |
| CATA/TATG | 860 | 10.25% | |
| P5 | AAAAG/CTTTT | 94 | 4.15% |
| CGAGC/GCTCG | 94 | 4.15% | |
| GAGCC/GGCTC | 105 | 4.64 | |
| P6 | TATACA/TGTATA | 57 | 5.69% |
| ATATAC/GTATAT | 65 | 6.49% | |
| CATATA/TATATG | 67 | 6.69% |
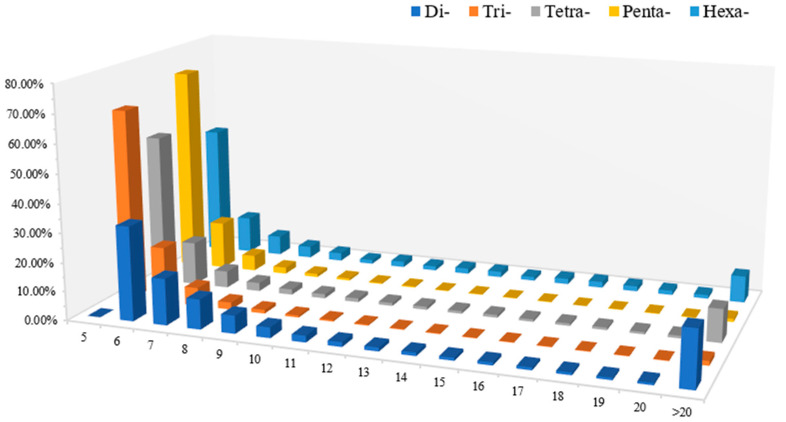
Among the 203,082 perfect SSRs identified in this study, the number of repeats of di-nucleotide motifs ranged from 6 to 187, with those with a repeat frequency of 6 to 8 dominant, accounting for a total of 59.00%. The number of repeats of tri-nucleotide motifs ranged from 5 to 127, with those with a repeat frequency of 5 to 6 dominant, accounting for a total of 85.41%. The number of repeats of tetra-nucleotide motifs ranged from 5 to 571, with those with a repeat frequency of 5 to 6 dominant, accounting for a total of 67.15%, The number of repetitions of the penta-nucleotide motif is 5–72, with those with 5–6 repetitions dominating, accounting for a total of 87.80%. The number of repetitions of the hexa-nucleotide motif is 5–42, with those with 5–6 repetitions dominating, accounting for a total of 58.24% (Figure 2; Table S3).
Figure 2.
Changes in the content of 2–6 nt motifs with different repetitions. The horizontal axis represents the number of repetitions of 2–6 nt motifs; the vertical axis represents the proportion of a certain type of motif with a certain number of repetitions.
2.3. The Distribution of SSRs in the Genome
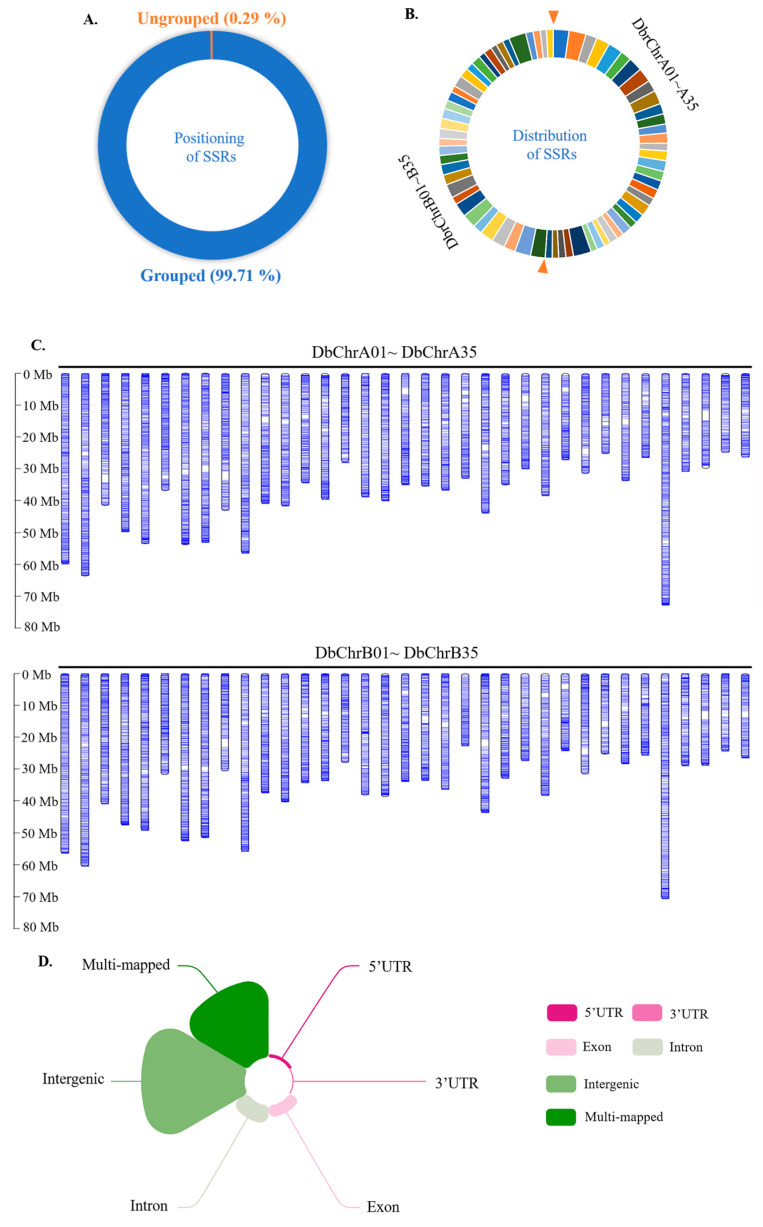
Among the 231,789 identified SSRs, 99.71% were evenly distributed on 70 chromosomes of D. brandisii, and 0.29% were not successfully localized on chromosomes (Figure 3A–C). Based on the location of SSRs on the genome and genome annotation files, we divided these SSRs into six regions, including 5’UTR, 3’UTR, exons, introns, intergene, and multiple mapping regions. SSRs located in multiple mapping regions are defined as SSRs that can be mapped to two or more regions simultaneously. As shown in Figure 3D, through the analysis of SSRs, the results show that SSRs are generally mapped to the intergenic region, and multiple mapping SSRs also account for a large proportion, followed by SSRs mapped to introns and exons, while SSRs mapped to 5’UTR and 3’UTR are relatively few in number.
Figure 3.
Localization of SSR on the Dendrocalamus brandisii chromosome. (A) The proportion of SSRs successfully located on chromosomes. (B) The proportion of SSR on 70 chromosomes. The right side of the two orange arrows represents the number of SSRs on DbrChrA01–A35 in a clockwise direction, while the left side of the two orange arrows represents the number of SSRs on DbrChrB01–B35 in a clockwise direction. (C) Localization of SSRs on the 5’UTR, 3’UTR, exon, intron, intergenic, and multi-mapped D. brandisii. (D) The distribution of SSR on 70 chromosomes of D. brandisii.
2.4. Obtain 34 Pairs of Polymorphic Primers and Perform Gene Mapping on 6 of Them
We utilize the Primer3_core program for Perl language-designed batch primers for all microsatellite flank sequences, resulting in 20,228 pairs of primers. Then, we randomly selected 800 pairs of primers for synthesis, combined PCR and agarose gel electrophoresis to preliminarily screen them, and the results showed that 433 pairs of primers can amplify at least one band (Figure S2). We selected 72 pairs of specific primers that can only amplify one band to conduct TP-M13-SSR PCR amplification on ‘Manxie No.1’ and 11 different geographical provenances of D. brandisii materials. The capillary electrophoresis results showed that the results of the three individuals within each provenance population are consistent, so any one of the samples can be selected as a representative sample for that provenance population. And 34 pairs of primers (Table S4) exhibited polymorphism, with adjacent alleles of each marker differing by multiples of the repeat unit length, indicating that these 34 polymorphic SSR markers are effective and have conservation and transferability across cultivars and provenances.
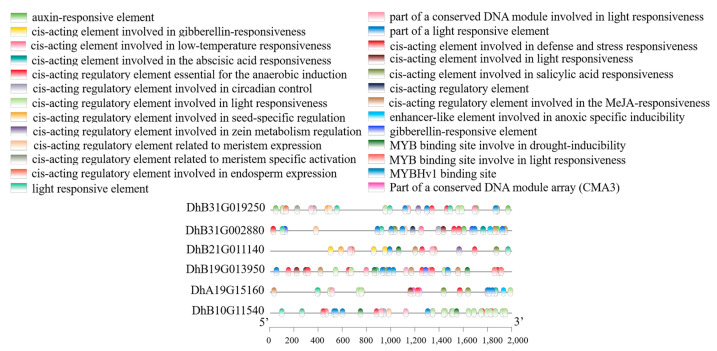
In addition, we performed gene mapping on 6 SSRs (SSR605, SSR243, SSR711, SSR710, SSR673, and SSR182) located on introns from 34 SSR markers, which, respectively, corresponded to DhB21G011140, DhB31G002880, DhA19G015160, DhA19G013950, DhB10G011540, and DhB31G019250. According to the annotation file, DhB21G011140 belongs to the CSN8/PSMD8/EIF3K family and is related to post-translational modifications, protein turnovers, and chaperones. DhB31G002880 is a gene encoding protein tyrosine and serine/threonine kinase and is associated with signal-transduction mechanisms. DhA19G015160 is an aminotransferase class I and II enzyme involved in the process of amino acid transport and metabolism. DhA19G013950 contains the Xrn1 helical domain, which is involved in the nuclear-transcribed mRNA catabolic process. DhB10G011540 contains the caspase domain, which belongs to the MC family of genes and may play an important role in plant immune responses. DhB31G019250 encodes SOS response-associated peptidase (SRAP). The promoters of these six genes all contain multiple light-responsive elements, hormone-responsive elements, and elements necessary for anaerobic induction, indicating that they may participate in the response process to light, hormones, and anaerobic conditions. In addition, some of these genes also contain elements related to low temperature, drought, defense, and meristem expression (Figure 4).
Figure 4.
Diagram of cis-acting elements in the promoters of DhB21G011140, DhB31G002880, DhB31G019250, DhA19G015160, DhA19G013950, and DhB10G011540.
2.5. Genetic Diversity Analysis of ‘Manxie No.1’ and 11 Different Geographical Provenances of D. brandisii Materials Based on 34 Polymorphic SSRs
Genetic diversity analysis was conducted on the ‘Manxie No.1’ and 11 different geographical provenances of D. brandisii representative materials using 34 polymorphic SSR markers. A total of 34 polymorphic SSR markers amplified 97 alleles, with an average of 2.85 alleles per marker. Among them, SSR201 amplified the largest number of alleles (Na = 10). In contrast, 22 markers, such as SSR431 and 711, amplified the fewest alleles (Na = 2). The range of Ne was 1.0868 to 4.1739, with an average of 1.8280 and an average Ho value of 0.6198, which was higher than the average He value of 0.5072. The average value of I is 0.6403 (range of 0.1732–1.7129). The PIC range of polymorphic information is 0.0767–0.7344, with an average value of 0.3152. There are 17 pairs of primers with PIC < 0.25, indicating low polymorphism. The PIC of seven primer pairs is greater than 0.5, which indicates that they possess high polymorphism (Table 2). The correlation graph between Na and PIC values shows that as the number of alleles increases, the polymorphism of specific loci increases (Figure S3). The genetic parameters calculated based on 34 SSR markers indicate that there is a moderate degree of genetic diversity and variation rate between 11 different geographical provenances of D. brandisii and ‘Manxie No.1’.
Table 2.
Polymorphism indicators of 12 important materials were obtained using 34 SSR markers.
| Primer ID | Na 1 | Ne 2 | I 3 | Ho 4 | He 5 | PIC 6 |
|---|---|---|---|---|---|---|
| SSR84 | 8 | 4.1739 | 1.7129 | 0.2065 | 0.7935 | 0.7344 |
| SSR201 | 10 | 3.1304 | 1.6732 | 0.2899 | 0.7101 | 0.6535 |
| SSR18 | 3 | 2.5714 | 1.0114 | 0.3623 | 0.6377 | 0.5355 |
| SSR394 | 4 | 2.2677 | 1.0594 | 0.4167 | 0.5833 | 0.5182 |
| SSR431 | 2 | 1.1803 | 0.2868 | 0.8406 | 0.1594 | 0.1411 |
| SSR605 | 5 | 3.4699 | 1.3776 | 0.2572 | 0.7428 | 0.6057 |
| SSR271 | 3 | 2.6422 | 1.0240 | 0.3514 | 0.6486 | 0.5430 |
| SSR243 | 3 | 2.3415 | 0.9222 | 0.4022 | 0.5978 | 0.4788 |
| SSR599 | 3 | 2.3226 | 0.9184 | 0.4058 | 0.5942 | 0.4768 |
| SSR711 | 2 | 1.0868 | 0.1732 | 0.9167 | 0.0833 | 0.0767 |
| SSR100 | 2 | 1.1803 | 0.2868 | 0.8406 | 0.1594 | 0.1411 |
| SSR603 | 3 | 2.3415 | 0.9222 | 0.4022 | 0.5978 | 0.4788 |
| SSR236 | 5 | 2.9388 | 1.2485 | 0.3116 | 0.6884 | 0.6007 |
| SSR330 | 3 | 2.3415 | 0.9222 | 0.4022 | 0.5978 | 0.4788 |
| SSR602 | 3 | 1.2915 | 0.4563 | 0.7645 | 0.2355 | 0.2124 |
| SSR83 | 2 | 1.1803 | 0.2868 | 0.8406 | 0.1594 | 0.1411 |
| SSR799 | 2 | 1.6000 | 0.5623 | 0.6087 | 0.3913 | 0.3047 |
| SSR171 | 2 | 1.0868 | 0.1732 | 0.9167 | 0.0833 | 0.0767 |
| SSR751 | 2 | 1.9862 | 0.6897 | 0.4819 | 0.5181 | 0.3733 |
| SSR86 | 2 | 1.3846 | 0.4506 | 0.7101 | 0.2899 | 0.2392 |
| SSR122 | 2 | 1.3846 | 0.4506 | 0.7101 | 0.2899 | 0.2392 |
| SSR182 | 2 | 1.1803 | 0.2868 | 0.8406 | 0.1594 | 0.1411 |
| SSR554 | 2 | 1.1803 | 0.2868 | 0.8406 | 0.1594 | 0.1411 |
| SSR417 | 2 | 1.1803 | 0.2868 | 0.8406 | 0.1594 | 0.1411 |
| SSR459 | 2 | 1.1803 | 0.2868 | 0.8406 | 0.1594 | 0.1411 |
| SSR527 | 2 | 1.0868 | 0.1732 | 0.9167 | 0.0833 | 0.0767 |
| SSR11 | 2 | 1.9862 | 0.6897 | 0.4819 | 0.5181 | 0.3733 |
| SSR357 | 2 | 1.1803 | 0.2868 | 0.8406 | 0.1594 | 0.1411 |
| SSR537 | 2 | 1.8000 | 0.6365 | 0.5362 | 0.4638 | 0.3457 |
| SSR710 | 2 | 1.9862 | 0.6897 | 0.4819 | 0.5181 | 0.3733 |
| SSR673 | 2 | 1.1803 | 0.2868 | 0.8406 | 0.1594 | 0.1411 |
| SSR367 | 2 | 1.1803 | 0.2868 | 0.8406 | 0.1594 | 0.1411 |
| SSR205 | 2 | 1.9459 | 0.6792 | 0.4928 | 0.5072 | 0.3680 |
| SSR220 | 2 | 1.1803 | 0.2868 | 0.8406 | 0.1594 | 0.1411 |
| Mean | 2.8529 | 1.8280 | 0.6403 | 0.6198 | 0.5072 | 0.3152 |
| St. | 1.7775 | 0.7912 | 0.4284 | 0.2330 | 0.1594 | 0.1942 |
1 Observed number of alleles. 2 Effective number of alleles. 3 Shannon’s information index. 4 Observed heterozygosity. 5 Expected heterozygosity. 6 Polymorphic information content.
2.6. Cluster Analysis of 1 ‘Manxie No.1’ and 11 Different Geographical Provenances of D. brandisii Materials Based on 34 SSR Markers
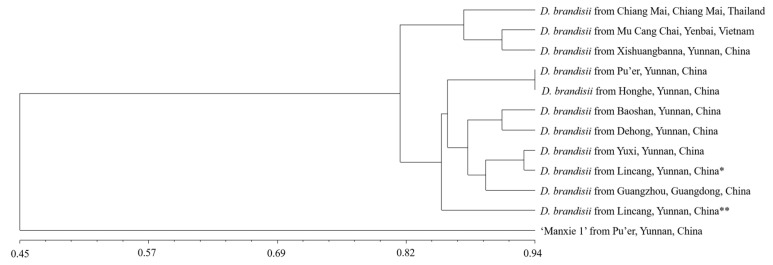
Based on NTSYS v.2.10e software, the genetic similarity coefficient was calculated using the SM coefficient, and a UPGMA tree was constructed based on the genetic similarity coefficient. Similarly, as with result 2.5, we conducted cluster analysis using a representative sample from each population. The analysis showed that 1 ‘Manxie No.1’ and 11 different geographical provenances of D. brandisii could be clustered into 2 main groups at a similarity coefficient of 0.45. The first group also includes two subgroups; the first subgroup is composed of D. brandisii from Chiang Mai, Thailand; Mu Cang Chai County, Vietnam; and Xishuangbanna Dai Autonomous Prefecture, China (the following text is abbreviated as ‘Xishuangbanna’). The genetic similarity coefficients of any two materials range from 0.8454 to 0.9072. Among them, the genetic similarity coefficient of D. brandisii from Mu Cang Chai County, Vietnam, and Xishuangbanna, China, indicates a closer genetic relationship. The second subgroup includes D. brandisii from Pu’er City, Honghe Hani, and Yi Autonomous Prefecture (the following text is abbreviated as ‘Honghe’); Baoshan City, Yunnan Dehong Dai, and Jingpo Autonomous Prefecture (the following text is abbreviated as ‘Dehong’); Yuxi City and Lincang City in Yunnan Province, China; as well as D. brandisii from Guangzhou City in Guangdong Province, China. The genetic similarity coefficient of any two materials ranges from 0.7938 to 0.9381. The genetic similarity coefficient between D. brandisii in Pu’er and Honghe, China, which are geographically adjacent, is the highest at 0.9381, indicating the closest genetic relationship. The genetic similarity coefficient between D. brandisii in Yuxi and Lincang*, China, which are not geographically adjacent, is the second highest, at 0.9278. The genetic similarity coefficient between D. brandisii from the geographically adjacent Baoshan and Dehong, China, is also relatively high, at 0.9072. The independent formation of the second group by ‘Manxie No.1’ proves that ‘Manxie No.1’ has a relatively distant genetic relationship with D. brandisii, which is consistent with the fact that it is an excellent cultivar artificially selected (Figure 5; Table S5). This result may be due to the limited gene exchange between ‘Manxie No.1’ and D. brandisii. The clustering results indicate that there is a certain correlation between the genetic relationships of different geographical provenances of D. brandisii and their geographical distribution. However, it is not completely related to their geographical distribution. Further verification of the accuracy of the UPGMA tree was carried out through the Cophenetic values subroutine in the Clustering program and the Matrix comparison plot subroutine in the Graphics program. The matrix correlation r value of the correlation test was 0.97856, proving that the reliability of the UPGMA tree is very high.
Figure 5.
Cluster analysis of 12 materials based on SSR markers. * represents Cangyuan County, Lincang City, Yunnan Province, China, and ** represents Linxiang District, Lincang City, Yunnan Province, China.
D. brandisii, Dendrocalamus hamiltonii, and Dendrocalamus asper are all part of the Dendrocalamus genus and are known as the world’s three largest bamboo plants with sweet bamboo shoots. We conducted cluster analysis on 2 samples of D. asper, 1 sample of D. hamiltonii, and the aforementioned 12 materials. The results showed that the genetic relationship between D. hamiltonii and D. brandisii was closer than that between D. asper and D. brandisii (Figure S4), and these three types of sweet dragon bamboo could each cluster into one branch. The clustering results were consistent with existing classifications, confirming the applicability and effectiveness of the microsatellite markers identified in this study.
2.7. SSR Core Primers and DNA Fingerprinting of 1 ‘Manxie No.1’ and 11 Different Geographical Prove-Nances of D. brandisii Materials
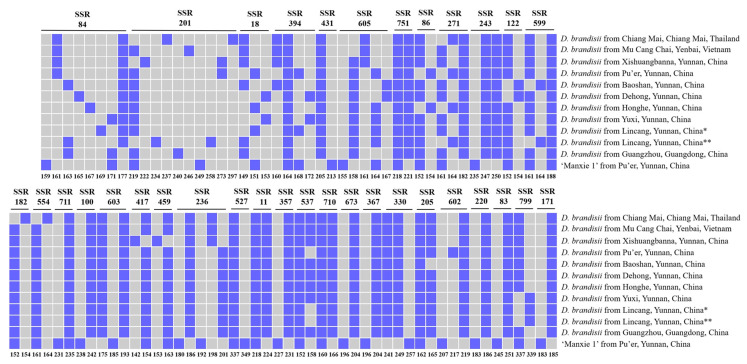
In this study, the primer SSR201 can generate eight band patterns, which can distinguish eight different materials, followed by primer SSR84, which can generate nine band patterns and distinguish nine different materials. These two pairs of primers can distinguish all 12 materials. Therefore, it is determined that this primer combination (SSR84 and SSR201) is the core primer for these 12 materials. By using this primer combination, 12 materials can be distinguished (Figure S5). The overall prediction accuracy of 34 pairs of SSR primers for 12 materials was 100%, and the DNA fingerprinting can be found in Figure 6 and Table S6.
Figure 6.
DNA fingerprinting of 12 materials constructed based on 34 pairs of SSR primers. On the right side of the image is the information of 12 materials, with the SSR name above. The number below the image represents the size of all fragments that the corresponding SSR can amplify. Blue and gray, respectively, represent the presence or absence of fragments. * represents Cangyuan County, Lincang City, Yunnan Province, China, and ** represents Linxiang District, Lincang City, Yunnan Province, China.
3. Discussion
SSRs are considered popular markers for genetic diversity evaluation, germplasm resource identification, purity analysis, and genetic mapping in various organisms due to their high polymorphism, high information content, codominance, and ability to detect alleles at specific loci in a single organism. The rapid development of next-generation sequencing technology has made SSR whole-genome identification easier and more comprehensive [26,27,28,29,30]. There are studies indicating that TP-M13-SSR fluorescence labeling may not be suitable for the cultivar identification of large-genome species [31]. However, the results of this study indicate that TP-M13-SSR fluorescent labeling can be used for DNA fingerprint construction and cultivar and provenances identification of D. brandisii with a large genome, and the system has high throughput, high sensitivity, and high accuracy, making it an effective method for fingerprint identification and the identification of asexual reproduction of D. brandisii.
3.1. SSR Marker Features Developed Based on the Whole Genome of D. brandisii
This study conducted genome-wide SSR identification and analysis on D. brandisii. A total of 231,789 microsatellites were detected from the genome of D. brandisii at 2813.5 Mb, with a microsatellite frequency of 82.4/Mb. The number of microsatellites was much larger than that of smaller genomes such as Arabidopsis thaliana (119.7 Mb) and Oryza sativa (374.5 Mb). However, the microsatellite frequency was much lower than both (135.5/Mb in A. thaliana and 165.5/Mb in O. sativa). Compared with the smaller genomes of Phyllostachys edulis (2051.7 Mb, 127,593, 62.2/Mb) and Zea mays (2066.4 Mb, 107,742, 52.1/Mb), the number of microsatellites is about twice that of the two, and the frequency of microsatellites is also higher [22]. This is inconsistent with the previous research findings that species with larger gene groups have more SSRs but a lower SSR density [32,33]. The number and density of microsatellites may not be related to the genome size.
Generally speaking, there are a large number of di-nucleotide and tri-nucleotide repeats, while the abundance of tetra-nucleotide to hexa-nucleotide repeat sequences is relatively low in the eukaryotic genome [34,35]. Among them, di-nucleotide repeat sequences are considered an indispensable resource due to their highest mutation rate and abundant quantity [36]. In this study, 94.3% of microsatellite repeat motifs were di-nucleotide and tri-nucleotide repeats, of which nearly 70% were di-nucleotide repeats. However, tetra-nucleotide, penta-nucleotide, and hexa-nucleotide repeats only accounted for a very small portion (5.7%). Similar distributions of repeat motifs were also observed in other Poaceous species, such as Ph. edulis, Z. mays, O. sativa, B. distachyon, and S. bicolor, and di-nucleotide and tri-nucleotide repeats still dominate [22]. This is consistent with the usual view that the abundance of microsatellites decreases with increasing repetition length [37,38].
Previous studies have shown that the GC content is higher in animal genomes, while the AT content is higher in plant genomes [39,40]. The data in this study show that the AT content in D. brandisii is also higher than the GC content, which is consistent with other bamboo species such as Guadua chacoensis, Dendrocalamus latiforus, etc. [15,41], and is also consistent with other species such as A. thaliana and S. bicolor [29]. Among many species, AG/CT motifs have the highest proportion, including Poaceae plants such as Ph. edulis, Z. mays, O. sativa, B. distachyon, and S. bicolor and non-Poaceae plants such as Epimedium sagittatum [22,42]. However, in our study, AG/CT motifs (19.44%) were the type with the highest proportion in microsatellites except for AT/AT motifs (35.11%), similar to S. bicolor and A. thaliana [22]. Previous studies have shown that in plants, TC repeat sequences are typically present in the transcriptional region and frequently appear in 5’UTRs. CT microsatellites in 5’UTRs may be involved in antisense transcription and play a role in gene regulation [43]. This suggests that approximately 1/5 of the microsatellite markers developed in this study may be involved in the antisense transcription of D. brandisii, thereby regulating the function of related genes. Monocotyledons have more abundant tri-nucleotide repeat sequences rich in GC than dicotyledonous plants [44]. Monocotyledons such as O. sativa, B. distachyon, and S. bicolor have the highest content of CCG/CGG, while Ph. edulis has the second highest content of CCG/CGG [22]. However, dicotyledonous plants such as A. thaliana and Glycine max rarely exhibit CCG/CGG [44]. In this study, GCC/GGC was the type with the highest proportion of tri-nucleotide repeat motifs in D. brandisii, accounting for 11.17%, followed by CGC/GCG (8.93%) and CCG/CGG (8.01%), which is consistent with this conclusion. Tetra-nucleotide, penta-nucleotide, and hexa-nucleotide repeat sequences, as in most plants, have a lower frequency.
3.2. Genetic Diversity within Bamboo Populations and Determination of Representative Samples for Various Populations of D. brandisii
This study fully considered the typicality and representativeness when selecting samples. We conducted a detailed investigation and analysis of the sample collection site to ensure that the environmental conditions and bamboo growth status of the site can represent the overall situation of the entire study area. At the same time, as bamboo mainly reproduces asexually through rhizomes, in order to avoid duplicate sampling, different samples selected from the same population come from noncontinuous bamboo forests with differences in latitude, longitude, and altitude, ensuring that duplicate samples have relatively independent genetic sources. This precisely proves that the sample we have chosen is more representative. By maintaining a larger sampling distance, the likelihood of duplicate samples being disturbed by the same environment can be reduced, allowing them to reflect the main characteristics of the entire population [45]. The samples we selected showed consistent results under these different conditions, further indicating that they can represent the overall characteristics of geographical provenances to some extent.
Previous studies have shown that there is indeed variation within populations of different provenances of D. brandisii, but this variation is very low [46]. This study showed that the Hs value of allelic marker diversity within populations of different provenance of D. brandisii was 0.0382 ± 0.0032; the mean values of the observed number of alleles (Na), the effective number of alleles (Ne), Shannon’s information index (I), and expected heterozygosity (He) among different individuals within various populations are 1.1338 ± 0.2978, 1.0906 ± 0.2168, 0.0742 ± 0.1704, and 0.0507 ± 0.1184. It is generally believed that genetic diversity is low when the Hs value is less than 0.1, the Na value is less than 2, the Ne value is close to or less than 1, the I value is less than 0.5, and the He value is less than 0.3. Given this, it can be seen that these values are relatively low, indicating limited genetic variation and low levels of genetic diversity among different individuals within the same population of D. brandisii. In our study, we selected three independent samples for each provenance population. Only when the results are consistent can we select one sample as a representative sample. If the results are inconsistent, additional samples need to be added for supplementary confirmation. The repeated individuals selected in the study are located at a certain distance and altitude from each other, and the possibility that they are all variant individuals and that the results of the variation reflected in the 34 polymorphic SSR markers are completely consistent is minimal. Therefore, we believe that consistent results from three replicates are sufficient to support the idea of selecting one as a representative sample for preliminary cluster analysis.
In future research, we will continue to improve our sampling methods to further enhance the typicality and representativeness of samples. Nonetheless, we are also aware that increasing the number of samples for each population would be preferable. We plan to expand the sample size, explore more diverse D. brandisii habitats, and conduct more in-depth genetic analysis to capture a more comprehensive picture of its populations and uncover the factors that contribute to limited genetic diversity. This will help us develop more targeted conservation strategies and management plans for D. brandisii resources. Moreover, we will explore innovative research methods and technologies to better understand the genetic characteristics and evolutionary history of D. brandisii. This could include the use of advanced genomic sequencing techniques and computational models to analyze large-scale genetic data. By doing so, we hope to make contributions to the field of D. brandisii research and conservation and promote the sustainable use and protection of these important resources.
3.3. Genetic Diversity and Clustering of 1 ‘Manxie No.1’ and 11 Different Geographical Provenances of D. brandisii
Due to the unique reproductive characteristics of bamboo, the classification problem has been troubling researchers. Molecular markers are genetic markers based on nucleotide sequence differences between individuals or populations, which intuitively and clearly reflect biological diversity at the DNA level. They are suitable and effective for identifying different plant genera, species, and underspecies. Domestic and foreign scholars have used isoenzyme labeling and molecular marker methods such as the RFLP (Restriction Fragment Length Polymer), RAPD (Random Amplified Polymer DNA), AFLP (Amplified Fragment Length Polymer), ITS (Internal Transcribed Spacer), and SSR (Simple Sequence Repeat) to study the genetic diversity and phylogenetic relationships between bamboo species and genera [30,47,48,49,50]. However, due to the characteristics of asexual reproduction of bamboo plants, there is little research on the genetic diversity of bamboo plants at the subspecies level. We used co-dominant SSR molecular marker technology to successfully identify different provenances of D. brandisii and the new cultivar ‘Manxie No.1’. Moreover, we constructed a DNA fingerprinting of 12 materials based on 34 SSR molecular markers to reflect their differences at the DNA level. This provides a molecular basis for the effective identification of different provenance of D. brandisii and the new cultivar ‘Manxie No.1’.
At present, there is relatively scarce information on the genetic diversity and population structure of bamboo germplasm resources. Due to the asexual reproduction of bamboo through rhizomes, it has low intra-species genetic diversity and genetic differences between populations [51]. Therefore, screening for markers with relatively high abundance and polymorphism for bamboo may be very difficult. Abreu developed and screened 7 polymorphic microsatellite markers for Aulonemia aristulata [52], and Vieira developed and screened 16 polymorphic microsatellite markers for the tropical woody bamboo Tribe Bambusae (Poaceae: Bambusoideae) [53]. Weixin developed and screened 20 polymorphic microsatellite markers for Ph. Edulis [32]. In this study, based on the ability of agarose gel to separate DNA fragments, 72 pairs of specific primers that can amplify only 1 band were initially screened from 800 pairs of primers, thus reducing the complexity and uncertainty in subsequent analysis. Then, based on capillary electrophoresis with higher resolution and sensitivity, 34 pairs of polymorphic primers were further selected from 72 pairs of specific primers that can amplify DNA fragments of different lengths in different samples. The differences in fragment lengths reflect the different number of motif repeats in SSR loci, thereby reflecting genetic differences between samples. Using these 34 pairs of polymorphic SSR primers, genetic diversity analysis was conducted on 12 samples (‘Manxie No.1’ and 11 different geographical provenances of D. brandisii). The PIC value takes into account the frequency and number of alleles and can comprehensively reflect the degree of polymorphism of the locus. For example, at an SSR locus, if there are multiple alleles and their frequency distribution in the sample is relatively uniform, the PIC value of the locus will be higher, indicating that the locus has high genetic diversity. In this study, the average PIC value of these 12 samples was 0.3152, ranging from 0.25 to 0.5, indicating that these samples have a moderate level of genetic diversity. This result provides important information for us to understand the genetic background of these samples. The moderate level of genetic diversity means that the samples have both a certain degree of genetic variability and relative stability. This genetic diversity may be due to the samples coming from different geographical provenances, experiencing different environmental choices, and genetic drift. On average, compared with other bamboo species using SSR molecular markers, the genetic diversity level of sweet dragon bamboo is higher than that of Guadua inermis and Ph. edulis [51,54] but lower than the G. angustifolia in the Colombian coffee eco-region and Kuruna debilis [55,56].
Cluster analysis shows that the genetic diversity of D. brandisii and ‘Manxie No.1’ exhibits extensive variation. ‘Manxie No.1’ can be distinguished from different geographical provenances of D. brandisii and D. hamiltonii, and D. asper can also be distinguished from D. brandisii. The SSR84 + SSR201 primer combination can distinguish 15 sweet dragon bamboo materials. However, clustering analysis shows that the genetic relationship between ‘Manxie No.1’ and D. brandisii is farther than that between D. hamiltonii, D. asper and D. brandisii. The possible reason for this result is that the number of microsatellite molecular markers is insufficient, leading to clustering bias. This situation can be improved by further screening polymorphic markers.
3.4. Identification of Candidate Genes Based on Polymorphic SSRs
Based on the development of polymorphic microsatellite markers, we located six of them on introns of six genes, which are non-coding sequences of broken genes and participate in important biological processes such as transcriptional regulation, variable splicing, and nuclear output [57]. The form and function of introns appearing at different positions in the transcript vary [58], and introns can significantly enhance gene expression [59]; this not only affects the transcription, output, and stability of mRNA to increase its content but also improves the efficiency of mRNA translation [60]. Recent studies have shown that introns promote evolution through exon recombination [61] and achieve multiple protein translations of a single gene through variable splicing [62], playing an important role in gene regulation [63]. Through the annotation file, we speculate that these six polymorphic microsatellite markers may have regulatory effects on their respective six genes, thereby regulating processes such as mRNA breakdown metabolism, amino acid transport and metabolism, protein transport and post-translational modification, signal transduction, plant immunity, and SOS response in D. brandisii. Among them, the homologous gene OsLOL3 of DhB10G011540 corresponding to SSR673 in rice can respond to cold stress, heat stress, and pathogen infection (such as Madurella grisea and Rhizoctonia solani). Yeast two-hybrid experiments have shown that OsMC3 can interact with OsLOL1 and may play a role in programmed cell death (PCD), disease resistance, and growth in rice [64], with PCD being the most typical defense stress response in plants; it plays a crucial role in resisting pathogen infection [65], which is consistent with our prediction that DhB10G011540 may play an important role in plant immune response. The promoters of these six genes contain elements related to light response, hormone response, anaerobic induction, low temperature, drought, defense, and meristem expression, suggesting that they may be involved in related response processes, which provides a reference for future in-depth research.
It should be noted that our discussion here is purely speculative. We do not claim any functional correlation between the SSRs and traits at present. Further research is needed to explore any potential functional correlations between these SSRs and specific traits in D. brandisii. For example, future studies could investigate how the presence of these SSRs might affect the phenotypic characteristics of the plant under different environmental conditions.
4. Materials and Methods
4.1. Plant Materials, Genomic DNA Isolation, and Detection
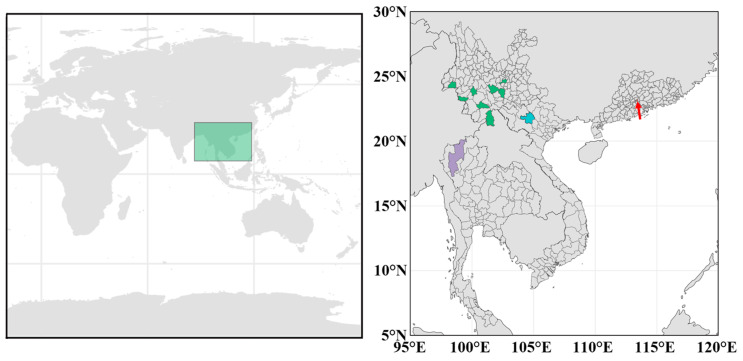
A new cultivar, ‘Manxie No.1’, and 11 different geographical provenances of D. brandisii materials were obtained from the main distribution areas of D. brandisii in China, Thailand, and Vietnam (Figure 7). There is a certain distance between the duplicate individuals of each provenance population, and they are distributed in discontinuous bamboo forests with different latitudes, longitudes, or altitudes to ensure that the duplicate samples have relatively independent genetic sources and can reflect the main characteristics of the entire germplasm population [45]. Finally, these fresh leaves were collected in a sampling bag containing a discolored silicone desiccant and were stored in a −80 °C refrigerator for future use.
Figure 7.
Sampling site labeling diagram for 11 samples of D. brandisii and 1 sample of ‘Manxie No.1’. The figure above shows the location of the sampling sites on a world map. In the figure below, green, red, blue, and purple represent the sampling sites in Yunnan Province, China; Guangdong Province, China; Yenbai Province, Vietnam; and Chiang Mai Province, Thailand.
Genomic DNA was extracted from leaf samples of 3 individuals in each population using a slightly improved cetyltrimethylammonium bromide (CTAB) method [66]. NanoDrop one (Thermo Fisher Scientific, Waltham, MA, USA) was used to determine the DNA concentration, and 1.0% agarose gel electrophoresis was used to detect the DNA quality.
4.2. Microsatellite Identification and Primer Design
All microsatellites in the entire genome sequence of D. brandisii (the genome assembled in reference [7] (https://doi.org/10.1111/jipb.13592 (accessed on 10 December 2023))) were identified using the Perl language platform-based microsatellite identification tool (MISA) (http://pgrc.ipk-gatersleben.de/misa/ (accessed on 6 January 2024)) [67]. The parameter settings are as follows: the di-nucleotide motif should be repeated at least 6 times, and the tri-nucleotide, tetra-nucleotide, penta-nucleotide, and hexa-nucleotide motifs should be repeated at least 5 times. When the sequence length between two simple sequence repeats (SSRs) is less than 100 bp, it is considered a composite SSR [68]. MISA is still used to analyze the number of motifs, repetitions, the number, length, and starting and ending positions of microsatellites with different repeat types on the genome.
The Primer3_core program (https://github.com/primer3-org/primer3 (accessed on 20 January 2024)) suitable for Perl language is used for batch primer design of microsatellite flanking sequences. The main parameter settings are as follows: (i) the primer length is 18–25 bp, (ii) Tm value is 57 °C–62 °C, (iii) the size of the target PCR product is 120–400 bp, and (iv) the GC-content is 40–60%.
4.3. Screening for Specificity and Polymorphism of Primers
The length of SSR is the main factor affecting its polymorphism, and the sequence length is directly proportional to the polymorphism. When the SSR length is ≥ 20 bp, there is a high degree of polymorphism [37,69]. Therefore, the motifs with a length of 2–6 bp are sorted in descending order according to the number of repetitions. We selected 800 pairs of SSR primers that ranked high and a distribution covering 70 chromosomes for synthesis and used ‘Manxie No.1’ genomic DNA as a template for PCR amplification. When the size of the agarose gel electrophoresis band of the PCR amplification product meets the expectation, it can be identified as a specific primer.
Subsequently, TP-M13-SSR PCR was performed using genomic DNA from different geographical provenances of D. brandisii and the new cultivar ‘Manxie No.1’ as templates, and the obtained specific primers were further screened for polymorphism. All PCR and electrophoresis were evaluated for consistency and reproducibility of results through multiple repeated experiments. This method consists of three primers: a forward primer with an 18 bp M13 tail sequence (5’–TGTAAAAACGACGGCCAGT–3’) added at the 5’ end, an M13 universal forward primer with a fluorescent group (6-carboxy-X-rhodamine (ROX, red), hexachloro-6-carboxy-flurrescine (HEX, yellow), tetrachloro-6-carboxy-flurrescine (TET, green) or 6-carboxy-flurrescine (FAM, blue)), and a normal specific reverse primer. The PCR system and reaction program adopt a two-step method, as detailed in Table S7. Fluorescence capillary electrophoresis SSR analyzed PCR end-products on the ABI 3730xl DNA analyzer (Thermo Fisher Scientific, USA), and the results were analyzed using Genemarker v2.2.0 software (https://softgenetics.com/products/genemarker/ (accessed on 22 May 2024)) [70]. Here, due to the relatively limited genetic variation and low level of genetic diversity among different individuals within the same provenance population of D. brandisii [46], we analyzed and read the results of three individuals in each provenance population. If the three results are completely consistent, we selected any one of them as the representative sample of the provenance population. Otherwise, repeat individuals will be added for further validation to determine representative samples for genetic diversity analysis and cluster analysis.
4.4. Data Analyses and Fingerprint Construction
POPGEN32 v1.32 software [71] was used to calculate Nei’s diversity index (Nei’s), Shannon’s information index (I), average observed heterozygosity (HO), and expected heterozygosity (He). PowerMarker v. 3.25 software [72] was used to calculate polymorphism information content (PIC), and NTSYS v. 2.10e software [73] was used to calculate genetic distance and plot the UPGMA cluster tree. Other data analyses and fingerprint construction were completed in Excel 2019.
5. Conclusions
Based on the identification of a large number of SSRs from the genome of D. brandisii, 34 polymorphic microsatellite markers were screened and validated to quickly and accurately distinguish the new variety ‘Manxie No.1’ and D. brandisii from different geographical provenances. These markers help to understand the distribution of genetic diversity and achieve the sustainable use of resources. At the same time, it provides reliable technical means for selecting varieties with excellent traits in horticultural production. Several polymorphic SSRs have been mapped to the genes of D. brandisii. This provides candidate genes for studying its immune response, hormone response, drought stress, and other aspects. The identification technology based on microsatellite markers can be used as a part of product quality standards to standardize the production and circulation of horticultural products and improve the standardization and specialization level of the entire industry.
Acknowledgments
The authors sincerely thank Xingbo Zhang for supplying the D. brandisii and ‘Manxie No.1’ materials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13202910/s1, Figure S1: The difference in shoot sheath color and the number of main branches between ‘Manxie No.1’ and D. brandisii. On the left is D. brandisii, and on the right is ‘Manchier No.1’. (A) The color difference in shoot sheaths between the two. (B) The color difference in the number of branches between the two. Figure S2: Preliminary screening results of agarose gel electrophoresis with some primers. Figure S3: The correlation between alleles and PIC values. Figure S4: Cluster analysis of 15 sweet dragon bamboo germplasms based on SSR markers. Figure S5: Amplification results of core primer of 12 materials. Display of the capillary electrophoresis results of D. brandisii from Chiang Mai, Chiang Mai, Thailand; Mu Cang Chai, Yenbai, Vietnam; Xishuangbanna, Pu’er, Baoshan, Dehong, Honghe, Yuxi, Lincang*, and Lincang**, Yunnan, China; Guangzhou, Guangdong, China; and ‘Manxie No.1’ from Pu’er, Yunnan, China in sequence. Table S1: The number of perfect SSRs contained in compound SSRs and their respective proportions. Table S2: Connection sequence length of compound SSRs containing two perfect SSRs. Table S3: The number of motif repetitions of various types of perfect SSRs. Table S4: Information on 34 pairs of polymorphic primers. Table S5: Genetic similarity coefficients among different materials. Table S6: DNA fingerprint information of the 12 materials. Table S7: The PCR system and PCR program used in the TP-M13-SSR PCR method.
Author Contributions
Conceptualization, R.G. and J.G.; methodology, R.G. and J.X.; software, R.G.; validation, R.G.; formal analysis, R.G.; investigation, R.G. and J.J.; resources, J.J., M.S., and N.X.; data curation, R.G.; writing—original draft preparation, R.G.; writing—review and editing, R.G. and J.G.; visualization, R.G.; supervision, J.G. and Z.C.; project administration, J.G.; funding acquisition, J.G. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
All data are included in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the Fundamental Research Funds of ICBR (1632024023) and the National Key Research and Development Program of China (2023YFD2201901).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Stapleton C., Dransfield S., Widjaja E.A. Plant Resources of South East Asia No. 7 Bamboos. Kew Bull. 1996;51:418. doi: 10.2307/4119344. [DOI] [Google Scholar]
- 2.Kirchhof E. Carbon Sinks of Steel: Exploring Bamboo’s Use to Combat Climate Change. Cons. J. Sustain. Dev. 2021;24:1–8. [Google Scholar]
- 3.Seethalakshmi, Kumar M. Bamboos of India. American Chemical Society; Washington, DC, USA: 1998. [Google Scholar]
- 4.Zhan H., Zhang L.-Y., Deng L., Niu Z.-H., Li M.-B., Wang C.-M., Wang S. Physiological and Anatomical Response of Foliar Silicon Application to Dendrocalamus Brandisii Plantlet Leaves under Chilling. Acta Physiol. Plant. 2018;40:208. doi: 10.1007/s11738-018-2783-8. [DOI] [Google Scholar]
- 5.Bhatt B.P., Singh K., Singh A. Nutritional Values of Some Commercial Edible Bamboo Species of the North Eastern Himalayan Region, India. J. Bamboo Ratt. 2005;4:111–124. doi: 10.1163/1569159054699317. [DOI] [Google Scholar]
- 6.Pei J.L., Li P.C., Wang Q., Wang S.G. Comparision of bamboo shoot nutrients of Dendrocalamus brandisii among different provenances. J. Northwest For. Univ. 2018;33:156–161. [Google Scholar]
- 7.Jiang J., Zhang Z., Bai Y., Wang X., Dou Y., Geng R., Wu C., Zhang H., Lu C., Gu L., et al. Chromosomal-Level Genome and Metabolome Analyses of Highly Heterozygous Allohexaploid Dendrocalamus Brandisii Elucidate Shoot Quality and Developmental Characteristics. J. Integr. Plant Biol. 2024;66:1087–1105. doi: 10.1111/jipb.13592. [DOI] [PubMed] [Google Scholar]
- 8.Ramakrishnan M., Yrjälä K., Vinod K.K., Sharma A., Cho J., Satheesh V., Zhou M. Genetics and Genomics of Moso Bamboo (Phyllostachys Edulis): Current Status, Future Challenges, and Biotechnological Opportunities toward a Sustainable Bamboo Industry. Food Energy Secur. 2020;9:e229. doi: 10.1002/fes3.229. [DOI] [Google Scholar]
- 9.Chatterji R.N., Raizada M.B. Culm-Sheaths as Aid to Identification of Bamboos. Indian For. 1963;89:744–758. [Google Scholar]
- 10.Grosser D., Liese W. Present Status and Problems of Bamboo Classification. J. Arnold Arbor. 1973;54:297–298. doi: 10.5962/p.184524. [DOI] [Google Scholar]
- 11.Singh S.R., Singh R., Kalia S., Dalal S., Dhawan A.K., Kalia R.K. Limitations, Progress and Prospects of Application of Biotechnological Tools in Improvement of Bamboo—A Plant with Extraordinary Qualities. Physiol. Mol. Biol. Plants. 2013;19:21–41. doi: 10.1007/s12298-012-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konzen E.R., Peron R., Ito M.A., Brondani G.E., Tsai S.M. Molecular Identification of Bamboo Genera and Species Based on RAPD-RFLP Markers. Silva Fenn. 2017;51:1691. doi: 10.14214/sf.1691. [DOI] [Google Scholar]
- 13.Ruiz-Sanchez E., Pérez-Alquicira J., de la Luz Perez-Garcia M., García-Martínez M.A. Genetics, Genomics and Breeding of Bamboos. CRC Press; Boca Raton, FL, USA: 2023. Genetic Diversity Assessment and Molecular Markers in Bamboos; pp. 117–153. [Google Scholar]
- 14.Rangsiruji A., Binchai S., Pringsulaka O. Species Identification of Economic Bamboos in the Genus Dendrocalamus Using SCAR and Multiplex PCR. Songklanakarin J. Sci. Technol. 2018;40:640–647. [Google Scholar]
- 15.Rossarolla M.D., Tomazetti T.C., Vieira L.N., Guerra M.P., Klabunde G.H.F., Scherer R.F., Pescador R., Nodari R.O. Identification and Characterization of SSR Markers of Guadua Chacoensis (Rojas) Londoño & P.M. Peterson and Transferability to Other Bamboo Species. 3 Biotech. 2020;10:273. doi: 10.1007/s13205-020-02268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powell W., Machray G.C., Proven J. Polymorphism Revealed by Simple Sequence Repeats. Trends Plant Sci. 1996;1:215–222. doi: 10.1016/1360-1385(96)86898-1. [DOI] [Google Scholar]
- 17.Vieira M.L.C., Santini L., Diniz A.L., Munhoz C.d.F. Microsatellite Markers: What They Mean and Why They Are so Useful. Genet. Mol. Biol. 2016;39:312–328. doi: 10.1590/1678-4685-GMB-2016-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varshney R.K., Graner A., Sorrells M.E. Genic Microsatellite Markers in Plants: Features and Applications. Trends Biotechnol. 2005;23:48–55. doi: 10.1016/j.tibtech.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Marulanda M.L., López A.M., Claroz J.L. Analyzing the Genetic Diversity of Guadua Spp. in Colombia Using Rice and Sugarcane Microsatellites. Crop Breed. Appl. Biotechnol. 2007;7:43–51. doi: 10.12702/1984-7033.v07n01a07. [DOI] [Google Scholar]
- 20.Chen S.Y., Lin Y.T., Lin C.W., Chen W.Y., Yang C.H., Ku H.M. Transferability of Rice SSR Markers to Bamboo. Euphytica. 2010;175:23–33. doi: 10.1007/s10681-010-0159-2. [DOI] [Google Scholar]
- 21.Šalplachta J., Kubesová A., Moravcová D., Duša F. Analysis of Fungi by Capillary Electrophoresis. TrAC-Trends Anal. Chem. 2023;159:116947. doi: 10.1016/j.trac.2023.116947. [DOI] [Google Scholar]
- 22.Zhao H., Yang L., Peng Z., Sun H., Yue X., Lou Y., Dong L., Wang L., Gao Z. Developing Genome-Wide Microsatellite Markers of Bamboo and Their Applications on Molecular Marker Assisted Taxonomy for Accessions in the Genus Phyllostachys. Sci. Rep. 2015;5:8018. doi: 10.1038/srep08018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rohilla S., Ginwal H.S., Rana V., Barthwal S., Meena R.K. Genome-Wide Discovery of Single- and Multi-Locus Simple Sequence Repeat Markers and Their Characterization in Dendrocalamus Strictus: A Commercial Polyploid Bamboo Species of India. Genet. Resour. Crop Evol. 2024;71:2311–2339. doi: 10.1007/s10722-023-01777-4. [DOI] [Google Scholar]
- 24.Meena R.K., Kashyap P., Shamoon A., Dhyani P., Sharma H., Bhandari M.S., Barthwal S., Ginwal H.S. Genome Survey Sequencing-Based SSR Marker Development and Their Validation in Dendrocalamus Longispathus. Funct. Integr. Genom. 2023;23:103. doi: 10.1007/s10142-023-01033-z. [DOI] [PubMed] [Google Scholar]
- 25.Li Q., Su X., Ma H., Du K., Yang M., Chen B., Fu S., Fu T., Xiang C., Zhao Q., et al. Development of Genic SSR Marker Resources from RNA-Seq Data in Camellia Japonica and Their Application in the Genus Camellia. Sci. Rep. 2021;11:9919. doi: 10.1038/s41598-021-89350-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar S.P.J., Susmita C., Sripathy K.V., Agarwal D.K., Pal G., Singh A.N., Kumar S., Rai A.K., Simal-Gandara J. Molecular Characterization and Genetic Diversity Studies of Indian Soybean (Glycine Max (L.) Merr.) Cultivars Using SSR Markers. Mol. Biol. Rep. 2022;49:2129–2140. doi: 10.1007/s11033-021-07030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonthala B., Abdin M.Z., Arya L., Pandey C.D., Sharma V., Yadav P., Verma M. Genome-Wide SSR Markers in Bottle Gourd: Development, Characterization, Utilization in Assessment of Genetic Diversity of National Genebank of India and Synteny with Other Related Cucurbits. J. Appl. Genet. 2022;63:237–263. doi: 10.1007/s13353-022-00684-1. [DOI] [PubMed] [Google Scholar]
- 28.Gower C.M., Gabrielli A.F., Sacko M., Dembelé R., Golan R., Emery A.M., Rollinson D., Webster J.P. Population Genetics of Schistosoma Haematobium: Development of Novel Microsatellite Markers and Their Application to Schistosomiasis Control in Mali. Parasitology. 2011;138:978–994. doi: 10.1017/S0031182011000722. [DOI] [PubMed] [Google Scholar]
- 29.Laoprom N., Sithithaworn P., Ando K., Sithithaworn J., Wongkham S., Laha T., Klinbunga S., Webster J.P., Andrews R.H. Microsatellite Loci in the Carcinogenic Liver Fluke, Opisthorchis Viverrini and Their Application as Population Genetic Markers. Infect. Genet. Evol. 2010;10:146–153. doi: 10.1016/j.meegid.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Nayak S., Rout G.R. Isolation and Characterization of Microsatellites in Bambusa Arundinacea and Cross Species Amplification in Other Bamboos. Plant Breed. 2005;124:559–602. doi: 10.1111/j.1439-0523.2005.01102.x. [DOI] [PubMed] [Google Scholar]
- 31.Li H., Wang T., Li Y., Shi Y., Song Y., Lu P. Application of the TP-M13 Automated Fluorescent-Lablled System of SSR Genotyping in Sorghum. J. Plant Genet. Resour. 2005;6:68–70. [Google Scholar]
- 32.Jiang W., Zhang W., Ding Y. Development of Polymorphic Microsatellite Markers for Phyllostachys Edulis (Poaceae), an Important Bamboo Species in China. Appl. Plant Sci. 2013;1:1200012. doi: 10.3732/apps.1200012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Portis E., Lanteri S., Barchi L., Portis F., Valente L., Toppino L., Rotino G.L., Acquadro A. Comprehensive Characterization of Simple Sequence Repeats in Eggplant (Solanum Melongena L.) Genome and Construction of a Web Resource. Front. Plant Sci. 2018;9:401. doi: 10.3389/fpls.2018.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subramanian S., Mishra R.K., Singh L. Genome-Wide Analysis of Microsatellite Repeats in Humans: Their Abundance and Density in Specific Genomic Regions. Genome Biol. 2003;4:R13. doi: 10.1186/gb-2003-4-2-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Y., Li W., Hu Z., Zeng T., Shen Y., Liu S., Zhang X., Li J., Yue B. Genome-Wide Mining of Perfect Microsatellites and Tetranucleotide Orthologous Microsatellites Estimates in Six Primate Species. Gene. 2018;643:124–132. doi: 10.1016/j.gene.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Chakraborty R., Kimmel M., Stivers D.N., Davison L.J., Deka R. Relative Mutation Rates at Di-, Tri-, and Tetranucleotide Microsatellite Loci. Proc. Natl. Acad. Sci. USA. 1997;94:1041–1046. doi: 10.1073/pnas.94.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Temnykh S., DeClerck G., Lukashova A., Lipovich L., Cartinhour S., McCouch S. Computational and Experimental Analysis of Microsatellites in Rice (Oryza Sativa L.): Frequency, Length Variation, Transposon Associations, and Genetic Marker Potential. Genome Res. 2001;11:1441–1452. doi: 10.1101/gr.184001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grover A., Aishwarya V., Sharma P.C. Biased Distribution of Microsatellite Motifs in the Rice Genome. Mol. Genet. Genom. 2007;277:469–480. doi: 10.1007/s00438-006-0204-y. [DOI] [PubMed] [Google Scholar]
- 39.Yu M., Hon G.C., Szulwach K.E., Song C.-X., Zhang L., Kim A., Li X., Dai Q., Shen Y., Park B., et al. Base-Resolution Analysis of 5-Hydroxymethylcytosine in the Mammalian Genome. Cell. 2012;149:1368–1380. doi: 10.1016/j.cell.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaillon O., Aury J.-M., Brunet F., Petit J.-L., Stange-Thomann N., Mauceli E., Bouneau L., Fischer C., Ozouf-Costaz C., Bernot A., et al. Genome Duplication in the Teleost Fish Tetraodon Nigroviridis Reveals the Early Vertebrate Proto-Karyotype. Nature. 2004;431:946–957. doi: 10.1038/nature03025. [DOI] [PubMed] [Google Scholar]
- 41.Liu M., Qiao G., Jiang J., Yang H., Xie L., Xie J., Zhuo R. Transcriptome Sequencing and De Novo Analysis for Ma Bamboo (Dendrocalamus Latiflorus Munro) Using the Illumina Platform. PLoS ONE. 2012;7:e46766. doi: 10.1371/journal.pone.0046766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng S., Xiao G., Guo J., Fei Z., Xu Y., Roe B.A., Wang Y. Development of a EST Dataset and Characterization of EST-SSRs in a Traditional Chinese Medicinal Plant, Epimedium Sagittatum (Sieb. Et Zucc.) Maxim. BMC Genom. 2010;11:94. doi: 10.1186/1471-2164-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martienssen R.A., Colot V. DNA Methylation and Epigenetic Inheritance in Plants and Filamentous Fungi. Science. 2001;293:1070–1074. doi: 10.1126/science.293.5532.1070. [DOI] [PubMed] [Google Scholar]
- 44.Morgante M., Hanafey M., Powell W. Microsatellites Are Preferentially Associated with Nonrepetitive DNA in Plant Genomes. Nat. Genet. 2002;30:194–200. doi: 10.1038/ng822. [DOI] [PubMed] [Google Scholar]
- 45.Zhao H., Sun S., Ding Y., Wang Y., Yue X., Du X., Wei Q., Fan G., Sun H., Lou Y., et al. Analysis of 427 genomes reveals moso bamboo population structure and genetic basis of property traits. Nat. Commun. 2021;12:5466. doi: 10.1038/s41467-021-25795-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruan Z., Yang H., Tian B., Yang Y., Sun M. Genetic diversity analysis based on ISSR among six populations of Dendrocalamus brandisii in Yunnan Province, China. J. Beijing For. Univ. 2010;32:46–51. [Google Scholar]
- 47.Friar E., Kochert G. Bamboo Germplasm Screening with Nuclear Restriction Fragment Length Polymorphisms. Theor. Appl. Genet. 1991;82:697–703. doi: 10.1007/BF00227313. [DOI] [PubMed] [Google Scholar]
- 48.Das M., Bhattacharya S., Pal A. Generation and Characterization of SCARs by Cloning and Sequencing of RAPD Products: A Strategy for Species-Specific Marker Development in Bamboo. Ann. Bot. 2005;95:835–841. doi: 10.1093/aob/mci088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hodkinson T.R., Renvoize S.A., Chonghaile G.N., Stapleton C.M., Chase M.W. A Comparison of ITS Nuclear RDNA Sequence Data and AFLP Markers for Phylogenetic Studies in Phyllostachys (Bambusoideae, Poaceae) J. Plant Res. 2000;113:259–269. doi: 10.1007/PL00013936. [DOI] [Google Scholar]
- 50.Guo Z.H., Chen Y.Y., Li D.Z., Yang J.B. Genetic Variation and Evolution of the Alpine Bamboos (Poaceae: Bambusoideae) Using DNA Sequence Data. J. Plant Res. 2001;114:315–322. doi: 10.1007/PL00013993. [DOI] [Google Scholar]
- 51.Perez-Alquicira J., Aguilera-Lopez S., Rico Y., Ruiz-Sanchez E. A Population Genetics Study of Three Native Mexican Woody Bamboo Species of Guadua (Poaceae: Bambusoideae: Bambuseae: Guaduinae) Using Nuclear Microsatellite Markers. Bot. Sci. 2021;99:542–559. doi: 10.17129/botsci.2795. [DOI] [Google Scholar]
- 52.Abreu A.G., Grombone-Guaratini M.T., Monteiro M., Pinheiro J.B., Tombolato A.F.C., Zucchi M.I. Development of Microsatellite Markers for Aulonemia Aristulata (Poaceae) and Cross-Amplification in Other Bamboo Species. Am. J. Bot. 2011;98:e90–e92. doi: 10.3732/ajb.1000511. [DOI] [PubMed] [Google Scholar]
- 53.Vieira L.d.N., dos Anjos K.G., Faoro H., Fraga H.P.d.F., Greco T.M., Pedrosa F.d.O., de Souza E.M., Rogalski M., de Souza R.F., Guerra M.P. Phylogenetic Inference and SSR Characterization of Tropical Woody Bamboos Tribe Bambuseae (Poaceae: Bambusoideae) Based on Complete Plastid Genome Sequences. Curr. Genet. 2016;62:443–453. doi: 10.1007/s00294-015-0549-z. [DOI] [PubMed] [Google Scholar]
- 54.Jiang W., Bai T., Dai H., Wei Q., Zhang W., Ding Y. Microsatellite Markers Revealed Moderate Genetic Diversity and Population Differentiation of Moso Bamboo (Phyllostachys Edulis)—A Primarily Asexual Reproduction Species in China. Tree Genet. Genomes. 2017;13:130. doi: 10.1007/s11295-017-1212-2. [DOI] [Google Scholar]
- 55.Muñoz Florez J.E., Londoño X., Rugeles P., Posso A.M., Vallejo F.A. Diversidad y Estructura Genética de Guadua Angustifolia En La Ecorregión Cafetera Colombiana. Biology. 2010;61:45–52. [Google Scholar]
- 56.Attigala L., Gallaher T., Nason J., Clark L.G. Genetic Diversity and Population Structure of the Threatened Temperate Woody Bamboo Kuruna Debilis (Poaceae: Bambusoideae: Arundinarieae) from Sri Lanka Based on Microsatellite Analysis. J. Natl. Sci. Found. 2017;45:53–65. doi: 10.4038/jnsfsr.v45i1.8038. [DOI] [Google Scholar]
- 57.Gao Y., Ge F., Zhang R., Yin D., Zhao Y., Tang H., Zhang L., Yang L. PID: An Integrative and Comprehensive Platform of Plant Intron. Comput. Biol. Chem. 2021;93:107528. doi: 10.1016/j.compbiolchem.2021.107528. [DOI] [PubMed] [Google Scholar]
- 58.Bradnam K.R., Korf I. Longer First Introns Are a General Property of Eukaryotic Gene Structure. PLoS ONE. 2008;3:e3093. doi: 10.1371/journal.pone.0003093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buchman A.R., Berg P. Comparison of Intron-Dependent and Intron-Independent Gene Expression. Mol. Cell. Biol. 1988;8:4395–4405. doi: 10.1128/mcb.8.10.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shaul O. How Introns Enhance Gene Expression. Int. J. Biochem. Cell Biol. 2017;91:145–155. doi: 10.1016/j.biocel.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 61.Long M., de Souza S.J., Gilbert W. Evolution of the Intron-Exon Structure of Eukaryotic Genes. Curr. Opin. Genet. Dev. 1995;5:774–778. doi: 10.1016/0959-437X(95)80010-3. [DOI] [PubMed] [Google Scholar]
- 62.Maniatis T., Tasic B. Alternative Pre-MRNA Splicing and Proteome Expansion in Metazoans. Nature. 2002;418:236–243. doi: 10.1038/418236a. [DOI] [PubMed] [Google Scholar]
- 63.Laxa M. Intron-Mediated Enhancement: A Tool for Heterologous Gene Expression in Plants? Front. Plant Sci. 2017;7:1977. doi: 10.3389/fpls.2016.01977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang L., Zhang H., Hong Y., Liu S., Li D., Song F. Stress-Responsive Expression, Subcellular Localization and Protein–Protein Interactions of the Rice Metacaspase Family. Int. J. Mol. Sci. 2015;16:16216–16241. doi: 10.3390/ijms160716216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cui Y., Peng Y., Zhang Q., Xia S., Ruan B., Xu Q., Yu X., Zhou T., Liu H., Zeng D., et al. Disruption of EARLY LESION LEAF 1, Encoding a Cytochrome P450 Monooxygenase, Induces ROS Accumulation and Cell Death in Rice. Plant J. 2021;105:942–956. doi: 10.1111/tpj.15079. [DOI] [PubMed] [Google Scholar]
- 66.Saghai-Maroof M.A., Soliman K.M., Jorgensen R.A., Allard R.W. Ribosomal DNA Spacer-Length Polymorphisms in Barley: Mendelian Inheritance, Chromosomal Location, and Population Dynamics. Proc. Natl. Acad. Sci. USA. 1984;81:8014–8018. doi: 10.1073/pnas.81.24.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thiel T., Michalek W., Varshney R.K., Graner A. Exploiting EST Databases for the Development and Characterization of Gene-Derived SSR-Markers in Barley (Hordeum Vulgare L.) Theor. Appl. Genet. 2003;106:411–422. doi: 10.1007/s00122-002-1031-0. [DOI] [PubMed] [Google Scholar]
- 68.Tóth G., Gáspári Z., Jurka J. Microsatellites in Different Eukaryotic Genomes: Surveys and Analysis. Genome Res. 2000;10:967–981. doi: 10.1101/gr.10.7.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meglécz E., Nève G., Biffin E., Gardner M.G. Breakdown of Phylogenetic Signal: A Survey of Microsatellite Densities in 454 Shotgun Sequences from 154 Non Model Eukaryote Species. PLoS ONE. 2012;7:e40861. doi: 10.1371/journal.pone.0040861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holland M.M., Parson W. GeneMarker® HID: A Reliable Software Tool for the Analysis of Forensic STR Data. J. Forensic Sci. 2011;56:29–35. doi: 10.1111/j.1556-4029.2010.01565.x. [DOI] [PubMed] [Google Scholar]
- 71.Yeh F.C., Yang R., Boyle T., Ye Z., Xiyan J., Yang R., Boyle T. PopGene32, Microsoft Windows-Based Freeware for Population Genetic Analysis. Version 1.32. Molecular Biology and Biotechnology Centre, University of Alberta; Edmonton, AB, Canada: 2000. [Google Scholar]
- 72.Liu K., Muse S.V. PowerMaker: An Integrated Analysis Environment for Genetic Maker Analysis. Bioinformatics. 2005;21:2128–2129. doi: 10.1093/bioinformatics/bti282. [DOI] [PubMed] [Google Scholar]
- 73.Rohlf F. NTSYS-pc, Numerical Taxonomy and Multivariate Analysis System, Version 2.1. Applied Biostatistics Inc.; Setauket, NY, USA: 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the manuscript.