Abstract
With the National Institutes of Health’s mandate to consider sex as a biological variable (SABV), there has been a significant increase of studies utilizing both sexes. Historically, we have known that biological sex and hormones influence immunological processes and now studies focusing on interactions between the immune, endocrine, and nervous systems are revealing sex differences that influence pain behavior and various molecular and biochemical processes. Neuroendocrine-immune interactions represent a key integrative discipline that will reveal critical processes in each field as it pertains to novel mechanisms in sex differences and necessary therapeutics. Here we appraise preclinical and clinical literature to discuss these interactions and key pathways that drive cell- and sex-specific differences in immunity, pain, and physiology.
Keywords: Neuroimmune, Sex differences, Pain, Physiology, Hormones, Translational
Plain language summary
In the last decade, NIH-funded basic and clinical research studies have been mandated to include sex as a biological variable, and this has resulted in a boom of studies discovering sex differences. We know that females are more likely than males to develop chronic pain conditions and experience higher levels of pain for longer periods of time. Conditions that demonstrate this include migraine, arthritis, and peripheral neuropathy. Furthermore, these sex differences extend to surgical outcomes and are observable at the molecular, cellular, and systemic levels. Although pain perception pathways differ by sex, studies are also focusing on differences in the “conversation” between the immune system and the nervous system while addressing implications of sex hormones to gain a better understanding of the impact on pain, physiology, and behavior. Lifestyle factors such as diet and alcohol consumption also play a role in affecting the perception and impact of pain conditions. As this area of research continues to grow, the development and availability of sex-specific treatment options will grow and lead to improved patient outcomes and more effective pain management strategies.
Highlights
Immune cell types and molecular pathways drive sex specific differences in inflammatory and neuropathic pain.
TLR4 involvement in pain pathways show contextual sex-specific variations and its interaction with TRPV1 channels adds complexity to nociceptor physiology.
Female mice tend to exhibit stronger TRPV1-mediated pain responses compared to males due to estrogen enhancement of TRPV1 expression levels.
The interplay between nuclear receptors (PPARγ, PPARα) and prolactin receptors influences sex-specific neuroimmune interactions. In males, PPARα impacts immune responses and neuroinflammation. In females, PPARγ activity influences pain perception and PRL influences pain sensitivity.
Personalized approaches are needed for pain management in males and females due to sex differences in pain perception and treatment outcomes driven by variations in hormone levels, adiposity, and neuroimmune mechanisms of inflammation.
Neuroimmune interactions, pain, and sex differences: an overview
The study of immune-to-neural interactions, or neuroimmunology, is a burgeoning field that integrates two of the most complex organ systems of the body. With roots in immunology and neuroscience, this integration spans over 45 years of studies with influence from physiology investigators like Ivan Pavlov that hypothesized that immunity could be conditioned, and Hans Selye, who demonstrated that various stressors influence immune and endocrine effects [1–5]. Researchers in the 1970’s coined the term “psychoneuroimmunology” and put this research topic on the map as a pertinent integrative research necessity [6–8]. Over the years, the field has split into defined categories with psychology and molecular neuroscience dictating the major research focus arms [9–11]. A surge in interest and citations in immune-to-neural interactions happened in 2014 and coincided with the national institutes of health’s (NIH) mandate that all funded studies needed to consider sex as a biological variable (SABV) [12]. This is a logical extension because the integrative nature of neuroimmunology allows for the assessment of both behavioral and physiological output that can validate the influence of one system over the other and any apparent sex differences. This is a unique aspect of the field where the inherent experimental design allows for our understanding of these interactions and outputs at various levels (Table 1).
Table 1.
Summary of the described sex differences across various immune-, neuro-, and endocrine-based targets. The denotation of “N/A” highlights interactions and mechanisms that have yet to be elucidated and posits the exploration of whether sex differences exist for these pathways
| Target | Sex Differences | References | |
|---|---|---|---|
| Males | Females | ||
| TLR4 |
• Global deletion of TLR4 attenuates post-inflammatory allodynia. • Intrathecal injection of a TLR4 antagonist reverses pain-like behavior in arthritis. • TLR4 on macrophages drives inflammatory pain. |
• TLR4 on neurons drives neuropathic pain. | Woller et al., [208]; Christianson et al., [209]; Szabo-Pardi et al., [151] |
| Macrophages |
• Pro-inflammatory characterized by M1 states. • Male-biased roles in inflammation and pain. |
• Anti-inflammatory and modulated by estradiol. | Lenert et al., [170]; Yu et al., [182], Rudjito, R., et al., [246] |
| Microglia |
• Pro-inflammatory phenotype characterized by amoeboid morphology and an inflammatory transcript profile. • Male-specific role in mediating the development of chronic neuropathic pain. |
• CSF1 mediates crosstalk between lymphocytes and spinal microglia thus playing a role in sex-specific differences in neuropathic pain. Microglia TLR4 drives female-specific allodynia after short-term alcohol. | Lei et al., [295]; Sorge, [183]; Sorge, [166]; Inyang, [167]; Agalave [207]; Kuhn et al., [174]; Alexander et al., [233] |
| T-cells | • Pain responses characterized by neutrophil recruitment, monocyte infiltration into the CNS, and activation of microglia for neuro-immune crosstalk. |
• Greater involvement in female pain development and outcomes. • In neuropathic injury models, T-cell infiltration into the spinal cord resolved pain in females but not males. • Females lacking T-cells do not experience pregnancy analgesia in neuropathic or inflammatory pain models. |
Sorge et al., [166]; Mifflin et al., [172]; Chernov et al., [173]; Scheff et al., [165]; Kuhn et al. [174]; Rosen et al., [261] |
| Estradiol | • N/A |
• Act on macrophages, which consequently increases the expression of toll-like receptors. • Play a critical role in anti-inflammatory processes, but they can also have pro-inflammatory effects • Low estradiol levels correspond to increased pain perception. ER-α inhibits pain transmission while ER-β promotes pain transmission |
Lenert et al., [248]; Smith et al., [282]; Paredes [286], Tang et al. [278]; Zhong et al. [277]; Li et al. [330] |
| Testosterone |
• Decreases levels of pro-inflammatory cytokines, IL-1β and TNF-α, and an increase in the anti-inflammatory cytokine, IL-10 • Increased concentrations lead to decreased pain outcomes |
• Activated androgen receptors regulate the expression of TRPV1 channels which decreases pain | Malkin et al., [269]; Edinger and Frye [262] & [263]; Lee [264]; Zhou [265] |
| Progesterone | • N/A |
• Decrease inflammation by inhibiting pro-inflammatory cytokine production and increasing anti-inflammatory cytokines • Mediates neuropathic pain. Peaks in progesterone are associated with lower pain severity associated with fibromyalgia |
Klein and Flanagan, [292]; Schertzinger et al., [21] |
| Prolactin | • N/A |
• Increased levels play a role in autoimmune diseases • Prlr in sensory neurons are critical for developing pain from the acute to chronic states in females only |
Shelly et al., [309]; Paige, et al., [312] |
| Fc Receptors | • N/A | • Mediates pain independently of immune cell involvement and inflammation | Qu et al., [159]; Bersellini Farinotti et al., [160]; Goebel et al., [158]; Liu et al., [155]; Jiang [156]; Wang [157] |
| TRPV1 | • Testosterone inhibits TRPV1 expression in inflammatory pain |
• Positively activated by estradiol which suppresses production of cytokines by tissue resident macrophages • Pain outcomes influenced by estradiol concentrations |
Bai et al., [265]; Chen et al., [252]; Wu et al., [284]; Cho and Chaban, [251]; Yamagata et al., [285]; Payrits et al., [249] |
| PPARα |
• Protective and anti-inflammatory • Activation promotes pain relief |
• N/A | Zhang et al., [316]; Sorge et al., [260] |
| PPARγ | • N/A |
• Estradiol promotes anti-inflammatory processes in macrophages • Activation promotes pain relief |
Zhang et al., [316]; Sorge et al., [260] |
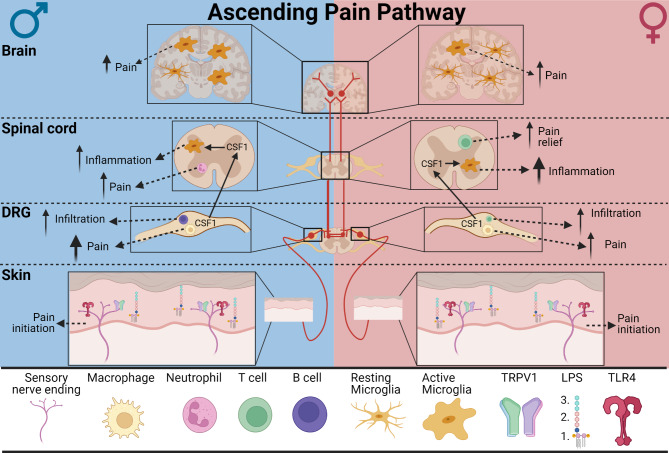
One area that has gained interest is how the nervous system and immune system communicate to modulate pain and how pain differs between sexes. Pain is a multi-faceted, subjective experience that involves the integration of peripheral sensory systems and higher brain processing centers. Within the peripheral nervous system (PNS), the dorsal root ganglia (DRG), which are a heterogeneous population of sensory neurons, innervate peripheral tissues and are the first level of integration of sensory information and mediation of responses to noxious stimuli. These nociceptive neurons, or nociceptors, are characterized by their varying degrees of myelination, size, and signaling capabilities [13]. Recent single-cell RNA-sequencing data indicates that there are at least 10 major populations of sensory neurons in the murine DRG, with numerous subtypes that further distinguish their unique physiological properties [14]. Further, spatial transcriptomics have identified at least twelve sensory neuron subtypes in human DRG [15, 16]. These neurons actively respond to different external stimuli, such as changes in thermal, mechanical, or chemical stimuli. They play a necessary role to alert the individual to potentially harmful situations or environments and initiate defensive behaviors. When activated by a noxious stimulus, DRG nociceptors transduce this signal to the dorsal horn of the spinal cord and then to the brain, including the periaqueductal gray, amygdala, hypothalamus, and other regions of the cerebral cortex, to be processed and interpreted as pain (Fig. 1).
Fig. 1.
The ascending pain pathway (Red). Noxious stimuli, such as thermal, chemical, or mechanical stimuli, or inflammatory stimuli, such as lipopolysaccharide (LPS), are detected by receptors expressed on nociceptors innervating peripheral tissue. Thermal stimuli activate TRP channels, such as TRPV1, while inflammatory stimuli activate other receptors, like activation of TLR4 by LPS. Following stimulation, nociceptors initiate pain transmission by propagating information to sensory neuron cell bodies located in dorsal root ganglia (DRG). These neurons synapse onto second order neurons within the dorsal horn of the spinal cord which in turn transmit nociceptive signals to the brain where it is interpreted as pain. Neuroimmune interactions within different levels of the ascending pain pathway influence the development and maintenance of pain in a sex- and cell-specific manner. Differences in arrow sizes indicate level of contribution, with larger arrows demonstrating greater impact, and are used to highlight sex-specific differences
Short term, or acute pain, can prompt defensive behaviors following injury to facilitate healing. Maladaptive plasticity of the nervous system can translate to various pain states [17]. Long-term, or chronic pain, is characterized by pain that persists beyond a biological benefit and is a pervasive disease state that affects over 20% of the world’s population, with an additional 10% diagnosed with chronic pain each year [18]. In the United States, the Centers for Disease Control and Prevention (CDC) lists chronic pain as the number one cause of long-term disability and loss of productivity [19]. Chronic pain has a higher prevalence in females, which account for 70% of cases; females experience increased severity and duration of pain compared to males [20]. Females are more likely to develop pain disorders that affect the musculoskeletal system such as fibromyalgia [21], osteoarthritis, low back pain [22], and migraines [23, 24]. Moreover, and to a lesser extent, females have reported more pain after invasive procedures than males [25]. Since pain is such a complex physical and emotional state, preclinical or translational studies assess the physical influence of noxious stimuli, or nociception [26].
One mechanism by which chronic pain occurs in the periphery is ectopic activity of peripheral nerves and cell bodies. During ectopic activity, pain is generated as a result of ion channel and receptor upregulation and hypersensitization to neuromodulators [27]. Specific channel subtypes, such as Transient Receptor Potential Channels (TRP) channels TRPA1 and TRPV1, are crucial components of nociceptor signaling as they regulate pain, temperature, and mechanical sensation through Ca2+ influx [28]. Activation of these TRP channels on nociceptors have been implicated in the pathogenesis of chronic pain (Fig. 1). Following injury or inflammation, TRP channels are modulated in 2 ways: (1) their expression in DRG neurons are upregulated and (2) the channels are sensitized through phosphorylation, both of which contribute to enhance neuronal activity and chronic pain [29–31]. This may suggest that TRP channels could be a promising candidate for pain therapeutics [32]. Dysregulation of central nociceptive circuitry can also lead to maladaptive plasticity and chronic pain. Following exposure to certain stimuli, neurons in the central nervous system (CNS) can exhibit increased responsiveness to previously subthreshold inputs from peripheral sensory neurons to enhance pain responses. While intense noxious stimuli elicit pain responses of sensory neurons, it is noteworthy that repeated or prolonged non-noxious stimuli, such as moderate alcohol consumption, short-term high-fat diet, or other dietary components such as ω-6 polyunsaturated fatty acids, also reduce pain thresholds and can contribute to the development of chronic pain [33–35].
The immune system plays a key role in the activation and sensitization of nociceptors. Under inflammatory conditions, such as tissue injury or infection, immune cells release signaling molecules that can stimulate nociceptors to initiate nociceptive signaling or increase expression of receptors for noxious stimuli [36]. Conversely, nociceptive neurons can also regulate immune responses and inflammation [37–39]. The basic mechanisms and dynamics of neuroimmune modulation of pain are under investigation; however, not all is equal between sexes considering cell activity. This review discusses neuroimmune interactions in pain physiology and how these interactions differ between sexes.
Neuroimmune interactions in pain physiology
Considerable evidence shows that immune cells play a significant active role in the onset and persistence of chronic pain conditions. Tissue resident immune cells inhabit dedicated tissue types and adapt to the metabolic function and defense of the tissue [40–45]. Tissue resident immune cells are the main actors in mediating inflammatory responses that precipitate acute and chronic pain in their efforts to preserve tissue integrity and have been demonstrated to be primary contributors to muscle pain [46]. They directly interact with and receive signals from neurons for rapid and stronger immune responses to danger signals, infections, and physiological imbalance within the tissue.
Immune cells are activated by a variety of substances such as damage-associated molecular patterns (DAMPs) released by damaged cells, cytokines, and chemical messengers released from neurons, including norepinephrine and acetylcholine (Ach), substance P and calcitonin gene related peptide (CGRP) [47–51]. The pro-inflammatory cytokine, Tumor Necrosis Factor-α (TNF-α) can activate and induce glutamate release from microglia in cases of oxidative stress to produce neurotoxic effects on neurons [52–54]. Following their activation, immune cells migrate toward damage signals from within the tissue and provide tissue protection by phagocytosis of pathogens and dead cells and by releasing factors to stimulate repair and regeneration of tissue-specific cells such as epithelial cells, fibroblasts, and neurons [55, 56]. Immune cells, such as DRG resident macrophages and natural killer (NK) cells [57–60]can also directly interact with sensory neurons to mediate pain and sensation. For example, injury to the DRG triggers a pro-inflammatory activation state of macrophages [61, 62]. Consequently, this immune response increases phagocytic activity, activates NK cells, induces cytotoxic action, and propagates pro-inflammatory cytokine release culminating in enhanced neuron responsiveness [57, 59]. This enhanced activity leads to maladaptive plasticity and sensitization of neurons to lower pain thresholds and sustain chronic pain states.
In addition to tissue-resident immune cells, circulating immune cells which travel in the blood and lymph, respond to danger signals throughout the entire body, modulate neuronal activity as part of the humoral response and vice versa through neuroimmune crosstalk [37, 63]. For example, neurons can engage circulating monocytes via expression of the chemokine C-C Motif Chemokine Ligand 2 (CCL2) which binds to C-C Motif Chemokine Receptor 2 (CCR2) on monocytes, recruiting them to sites of injury [36, 64–66]. Tissue infiltrated monocytes differentiate into macrophages and modulate neuronal activity directly. Circulating neutrophils and helper T-cells are also recruited to injury sites and contribute to sensory neuron signaling and sensitization by releasing pro-inflammatory cytokines, such as TNF-α, Interleukin-6 (IL-6), and Interleukin-1β (IL-1β), and other factors [67, 68]. These molecules increase neuronal excitation and alter pain sensitivity. For example, IL-1β binds to DRG sensory neurons and promotes nociceptive signaling by relieving the resting slow inactivation of sodium channels to increase sodium currents and nociceptor excitability [69, 70]. Immune cells also release anti-inflammatory cytokines such as Transforming growth factor-β1 (TGF-β1) and Interleukin-10 (IL-10), which promote sensory neuron repair and reduce neuronal signaling to abrogate pain transmission and contribute to pain resolution [71–73]. While these circulating immune cells can be studied via whole blood isolation and plasma analysis, tissue resident immune cells are much more difficult to study due to tissue accessibility issues and overlapping identifying markers to infiltrating circulating cells [74–77].
Other circulating immune components, such as complement proteins C3a and C5a, contribute to direct neuronal stimulation that leads to pain transduction [78–80]. These two complement proteins, generally referred to as anaphylatoxins, are primarily effectors of innate immunity concerned with the defense of neurons by promoting inflammation [81, 82]. C3a and C5a have a neuroprotective role against excitotoxic neuronal injury, promoting neurogenesis, inhibiting caspase-3 activity, regulation of glutamate receptor expression, and astrocyte stimulation [83–87]. Despite these neuroprotective effects, C3a and C5a can trigger pain responses to heat by direct modulation of neurons via activation of neuronal complement-specific receptors and TRPV1 channels [78, 88].
Important to note, this is a two-way conversation, and not only can immune cells influence neuronal activity, but neurons can regulate the activity of immune cells. Sensory neurons transmit electrochemical signals to lymph tissues and immune cells to modulate inflammatory and pain responses through molecular- and receptor-mediated communication [36, 89–91]. Moreover, new research has demonstrated sensory neurons participate in neuroimmune crosstalk with various tissues that were historically considered passive, including the skin, colon, lungs, and adipose tissue to drive physiology and further drive immunological responses [89, 92–95]. Immune cells express receptors for neurotransmitters that modulate their response to noxious signals and activation of these receptors on immune cells regulates the production and release of inflammatory molecules that can in turn stimulate neurons [96, 97]. For example, GABA suppresses T-cell and macrophage cytokine production capabilities, thus dampening inflammatory responses, and GABA bound to receptors on T-cells produces a profound effect by blocking intracellular calcium signaling and MAPK activation [97, 98].
Both neuronal and immune cell types undergo metabolic changes where neuronal synaptic plasticity is modified, and resident immune cells are activated and transformed morphologically [99, 100]. Further, these signals can mediate a variety of genomic and non-genomic effects that modulate pain and homeostasis [101–105]. Genomic effects are coordinated through intracellular receptors that function as transcription factors and influence mRNA levels for specific proteins such as cytokines, prostaglandins, and other inflammatory mediators. Membrane-bound receptors coordinate both genomic and non-genomic effects, inducing rapid signaling via ion channels, G-protein coupled receptors, and growth factor receptors that activate kinase signaling cascades [101, 106]. Both sensory neurons and immune cells express receptors for a variety of signaling molecules that can initiate these cascades, such as purinergic receptors (P2X/P2Y) [107–110], DAMPs [111], and Toll-like receptors (TLR) [111–113].
One example of neuroimmune communication is neuron-microglia crosstalk in the CNS. Microglia, CNS resident immune cells, survey their environments and monitor neuronal conditions. Activated microglia modulate neuronal activity to drive behaviors, like learning and memory [114–118], depression [119–123], sickness [115, 124, 125], and pain [126–130]. Injury can induce the release of various signaling factors from damaged cells [131–133]. These stimuli activate microglia and attract them to damaged areas to clear debris and dead cells. For example, glutamate levels rise under inflammatory conditions, such as a traumatic brain injury, which increases neuronal activity and contributes to the development of central sensitization and persistent pain. In addition to increasing neuronal activity, glutamate induces calcium influx which initiates ATP-ADP release from neurons leading to stimulation of microglial P2Y12 receptors [134]. Microglial P2Y12 stimulation is one of the first steps of microglial activation and induces microglia to migrate toward the site of injury. Further, ATP signaling on microglia via P2X receptors increases intracellular Ca2+ and triggers the release of cytokines and chemokines and is important for phagocytosis responses [135–137]. Microglia also become activated by neurotransmitters, glutamate, and acetylcholine, via NMDA/AMPA and cholinergic receptors, respectively [135, 138–141]. Glutamatergic stimulation of microglia triggers their modulation of neurons by the release of signaling factors to facilitate neuroimmune crosstalk and by directly wrapping around damaged axons [116, 139, 142–145].
The action of microglia in response to neurotransmitters can be different based on their location in the brain. For example, it has been shown that following treatment with the glutamate receptor-agonist, N-methyl-D-aspartic acid, microglia are neuroprotective in the CA3 and DG regions of the hippocampus, maintaining a ramified state in the CA3 region and “hypertrophic” morphology in the DG [146]. In contrast, in the CA1 region, microglia are not neuroprotective, adopt an amoeboid state, and NMDA- excitotoxicity induces neuronal death [146]. It is possible that these different microglial responses to NMDA may be due to different receptor subtype expression in different regions of the brain and differences in age [147].
Immune components expressed by neurons to mediate pain
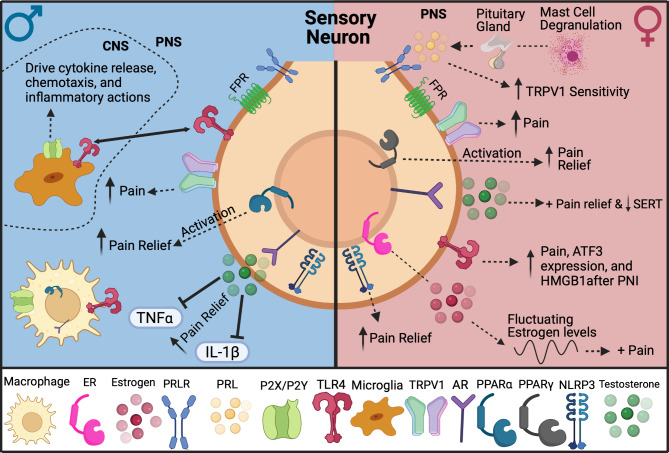
Emerging research shows specific receptors and molecules participate in driving pain states differently depending on sex [101, 148]. Additionally, peripheral sensory neurons release neuropeptides, like Substance P and CGRP, that can directly activate nociceptors and enhance infiltration of circulating immune cells to act on injured nerves, causing sensitization and skewing them toward pain states [148–150]. Activation of Toll-Like Receptor 4 (TLR4) and NOD-Like receptor protein 3 (NLRP3) on neurons in response to pathogen-associated molecular patterns (PAMPs) and DAMPs leads to the expression of molecules that promote maladaptive plasticity of neurons and enhance their sensitization toward pain states. In injury models, sensory neuron signaling via TLR4 and NLRP3 lead to greater pain sensitivity in females compared to males, suggesting activation of these receptors produces different cellular responses [151, 152]. Pathogens release molecules, such as N-formyl peptides, that also circulate and directly activate neurons via N-formyl peptide receptors (FPR) to protect against further damage to infected areas [153] (Fig. 2).
Fig. 2.
Sexual dimorphic mechanisms in neuroimmune signaling driven by interactions with sensory neurons. In females, sensory neurons are thought to be the main drivers in pain signaling as seen by the female-skewed pathways
Immunoglobulin-like receptors (Fc) are expressed on sensory neurons and are a key component in mediating pain independent of immune cell activation or inflammation [154–157]. Fc receptors interface with circulating immunoglobulin subtypes and facilitate increases in intracellular calcium to sensitize sensory neurons or heighten nociception in females [155, 158–160]. In a study investigating the role of Fc receptor subtypes, FcγRII and FcγRIII, using the K/BxN model of serum-transfer arthritis, only FcγRIII-deficient mice had decreased paw inflammation [161]. Intriguingly, not only was there a lack of attenuation of paw inflammation in the FcγRII-deficient mice, but the time to onset was decreased and the severity of arthritis was increased [161]. Understanding the interactions of these immune components and their direct action on neurons to contribute to pain may highlight them as potential targets for prevention of pain.
Sex differences in neuroimmune interactions
Through pre-clinical and clinical studies, neuroimmune interactions have been shown to propagate and maintain pain states differentially in males and females [162, 163] An example of this is the NLRP3 inflammasome in inflammatory pain models, which contributes to female-specific, sensory neuron driven pain [152, 164]. In an oral cancer pain model, male-specific sexual dimorphisms were observed in neutrophil-mediated suppression of pain [165]. Microglia in the CNS are reported to possess a more male-specific role in mediating mechanisms in the development of chronic pain [166, 167], but there is new data that suggests that cell signaling has more influence than specific cell types [168].
In females, T-cells have been shown to have a greater involvement in pain development and outcomes [166, 169–173]. In a neuropathic injury model, T-cell infiltration into the spinal cord was shown to resolve pain in females but not males [166, 174]. Furthermore, female mice lacking T-cells did not experience pregnancy analgesia in neuropathic or inflammatory pain models [175].
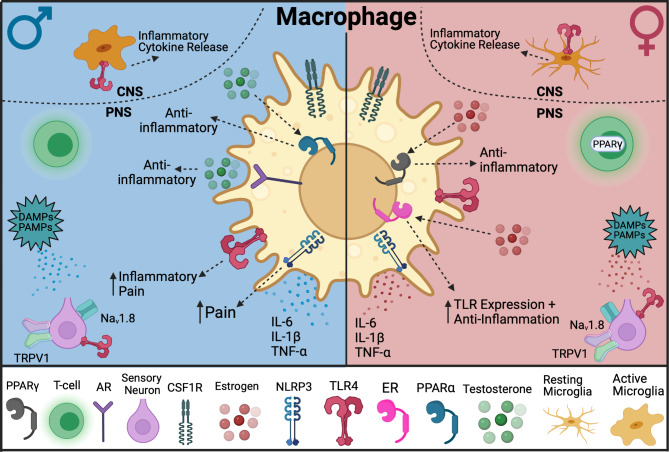
A study that compared central and peripheral immune cell infiltration following nerve injury suggests sex dimorphic processes in the adaptive immune cells that infiltrate the DRG 8 days following injury, with greater B-cell infiltration in male mice and greater T-cell infiltration in female mice [176]. Peripheral nerve injury increases inflammation and pain not only at the site of injury but also in the central nervous system. One mechanism for this spread of pain signals involves colony-stimulating factor 1 (CSF1), a cytokine important for macrophage differentiation. Following peripheral neuropathic injury, CSF1 is expressed de novo in the DRG. CSF1 then travels to the spinal cord where it interacts with microglia in the dorsal horn [177]. Interestingly, research suggests that CSF1 is expressed at higher levels in females than males after neuropathic injury suggesting a sex-dependent regulation of CSF1 signaling during pain development [178]. While studies show male-specific responses to intrathecal CSF1 administration, some lack data on female responses, making direct sex comparison difficult [177, 179]. This raises the possibility that CSF1 signaling on a cellular level may be similar in both sexes, but in females, higher levels of CSF1 may contribute to differences in neuropathic pain. Additionally, if CSF1 expression levels is indeed the key to sex disparity, using the same intrathecal dose of CSF1 may mask sex differences in neuropathic pain models. Intrathecal CSF1 has shown a male-biased outcome due to microglia’s role in regulating pain states in males [127, 180, 181]. CSF1 can mediate crosstalk between lymphocytes and spinal cord microglia in females by acting as a modulator of Treg immunosuppressive activity [174]. Peripheral macrophages also express the receptor for CSF1 (CSF1R) (Fig. 3), suggesting that macrophages in addition to microglia play a role in sex-specific differences in CSF1-mediated neuropathic injury. More evidence for neuroimmune crosstalk, was described when deletion of the CSF1 ligand in sensory neurons reduced tissue resident macrophage proliferation (Ki67+) in the DRG of males but not females following neuropathic injury [182].
Fig. 3.
Sexual dimorphic mechanisms in neuroimmune signaling driven by interactions with macrophages. In males, immune cells are thought to be the main drivers in pain signaling, such as through TLR4 signaling
Additionally, other receptors such as TLR4 on different immune and neuronal populations can lead to differential pain sensitivity in males and females [183]. A study has shown that TLR4 on nociceptors drives early neuropathic pain development in a female-specific manner [151]. These data along with the NLRP3 data imply sensory neuron-derived mechanisms differentiate male and female pain development. The involvement of these receptors and immune cells to facilitate neuroimmune communication can help to determine potential mechanisms underlying pain and immunity.
TLR4: a mediator of sex-specific differences in neuroimmune interactions
Toll receptors are evolutionarily conserved proteins involved in innate immune processes that recognize patterns ranging from lipopolysaccharide (LPS) to nucleic acids such as viral RNA and CpG DNA [184–188]. The subtype TLR4 forms a multi-receptor complex on the surface of a cell with several other proteins and adaptors. LPS, TLR4’s primary agonist, is comprised of 3 primary structure domains: (1) the lipid A tail, (2) the core oligosaccharide, and (3) the O antigen (Fig. 1) [189]. The hydrophobic lipid A tail is a motif that is recapitulated in not only pathogens, but DAMPs produced in vivo, and the backbone of fatty acids consumed in the western diet [34, 190, 191]. TLR4 is expressed on cells in both the CNS and periphery including microglia [192], astrocytes [193], neurons [194], macrophages [195, 196], endothelial cells [197], fibroblasts [198] and more. Since TLR4 is expressed on a variety of cells with specific biological functions, considerations must be made on a per cell basis when interpreting the effects of TLR4 signaling in disease pathology.
Evidence suggests that in the DRG and trigeminal ganglia (TG), TLR4 and TRPV1 are functionally coupled and TLR4 can directly modulate activity of TRPV1 [199, 200]. Infusion of LPS on dissociated TG and DRG neurons potentiated TRPV1 agonist responses such as calcium influx and CGRP release, both of which are not directly linked to TLR4 signaling cascades [201]. In TLR4 knockout mice, TRPV1 agonist-induced pain is significantly reduced [199]. Moreover, TLR4 possesses the ability to modulate sodium channel (Nav) activity—an essential component of nociceptive transmission. Upon activation, TLR4 initiates an intracellular signaling cascade that activates NF-κB which interacts with pain sensitive Navs on nociceptors within minutes of activation to increase Nav currents and decrease recovery time from inactivation, which enhance neuronal excitability and pain signaling [202]. Taken together, these studies reveal a unique interaction between TLR4 and TRPV1 as well as TLR4 and Navs, which enhance the context by which we understand nociceptor physiology.
Specific modulation of pain by TLR4
Evidence has mounted that TLR4 is a critical mediator of the nervous and immune system’s inflammatory response during pain [91, 202–204]. TLR4 gene expression is increased in the spinal cord when rats are injected with intraplantar CFA [205]. Subsequent studies have verified this by showing an increase in microglia reactivity in the dorsal spinal cord following intraplantar CFA injection [206]. Another study also demonstrated the involvement of TLR4 in acute inflammatory pain by utilizing intrathecal injections of high mobility group box 1 (HMGB1), a TLR4 agonist, which enhanced activation of microglia in a TLR4-dependent manner subsequently producing hypersensitivity in mice [207].
TLR4 participates in several inflammatory conditions that have previously been found to differ by sex. For example, rheumatoid arthritis, has a more pronounced effect in women [208]. In a rodent model of arthritis, it was revealed that while both females and males demonstrate allodynia after inflammation, males continue to have tactile allodynia after joint inflammation has resolved [208]. However, global deletion of TLR4 in both sexes attenuated the post-inflammatory allodynia [208]. In a similar model, a global deletion of TLR4 attenuated the post-inflammatory allodynia in males, whereas an intrathecal injection of a TLR4 antagonist given during the inflammatory phase fully reversed the pain-like behavior associated with arthritis in males [209]. These findings suggest that TLR4 signaling alone can sustain the inflammation seen in arthritis and contributes to sex differences in the manifestation of the disorder.
In addition to inflammatory pain, TLR4 has also been shown to be involved in chemotherapy-induced peripheral neuropathy (CIPN). CIPN is one of the major dose-limiting side effects of cancer treatments. Paclitaxel, a taxane-based chemotherapeutic, engages similar inflammatory intracellular signaling pathways as the classical TLR4 ligand, LPS [210]. This is evident by paclitaxel utilizing the same accessory protein required for LPS signal transduction and upregulating inflammatory cytokines such as IL-1β [211]. Paclitaxel treatment upregulates TLR4 expression in the DRG and spinal cord, suggesting its connection with the development of pain [212], and blocking TLR4 signaling reverses this pain phenotype [213].
TLR4 plays a significant role in opioid-induced hyperalgesia [214, 215]. While this seems paradoxical, evidence suggests that µ-opioids can lead to increased pain sensitivity [216]. It is believed that certain opioids can bind to TLR4, which facilitates inflammatory signaling [217]. By acting as TLR4 ligands, traditional opioid receptor agonists induce a pain phenotype or enhance sensitivity [218].
Sex differences in TLR4-mediated pain development and allodynia
Over the last decade, TLR4 has made its way into the spotlight of pain research. However, its exact sex- and cell-specific role in mediating chronic pain development and maintenance have been controversial. One of the first studies to implicate TLR4 signaling in neuropathic pain development sought to characterize the functional role of microglia TLR4 in response to neuropathic injury [219]. They found that TLR4 whole body null mice had an attenuated spinal microglial inflammatory response, but also a reduced nociceptive behavioral phenotype after nerve injury. Subsequent studies have corroborated these findings by pharmacologically inhibiting spinal TLR4 activation [220, 221]. While these studies do provide convincing evidence that suggests immune-mediated TLR4 signaling contributes to neuropathic pain development, sex was not considered as a variable. It was not until a few years later that the sex-specific contribution of TLR4 in both inflammatory and neuropathic pain was addressed [183]. It was found that intrathecal administration of LPS induced a robust pain phenotype in male but not female mice, and antagonism of spinal TLR4 reversed this effect in only males. Moreover, neuronal injury induced neuropathic pain behaviors in both male and female mice, but only male mice showed transient attenuation of pain behaviors following intrathecal TLR4 antagonist injection. Some data suggest that the role of TLR4 in inflammatory and neuropathic pain development is reliant on the timing of TLR4 modulation [209, 222]. While another study demonstrated that intrathecal LPS facilitated mechanical hypersensitivity development in both male and female mice, seemingly contrasting findings of TLR4 inhibition were noticed [183]. Inhibition of spinal TLR4 signaling by intracellular antagonist, TAK-242, prevented development of allodynia, but could not reverse established allodynia in males [223]. In accordance with the previously discussed study, another group demonstrated that TLR4 whole-body null females do not exhibit a reduction in mechanical hypersensitivity like their male counterparts after neuropathic injury [224]. This was one of the first studies to highlight that TLR4 either directly or indirectly influences expression of neuronal injury markers in the periphery. While these results seem contradictory, they may be explained by mouse strain differences used [225–228] and serotype and dose of LPS [229–231].
To address discrepancies in the temporal and cell-specific role of TLR4 in chronic pain, a recent study used microglia-specific TLR4 conditional knockout mice, in which a tamoxifen-dependent Cx3CR1-CreERT2−EYFP mouse line was used [232]. They found that systemic antagonism of TLR4 is effective at improving chronic pain outcomes in a tibial fracture model of complex regional pain syndrome in both sexes when administered at the time of injury as opposed to when pain is already established. Their data showed removing microglial TLR4 after the injury resulted in partial improvement in allodynia of both sexes, suggesting that microglial TLR4 plays less of a role in the maintenance of chronic pain states [232]. Interestingly, their investigation showed a female-specific reliance on peripheral TLR4 expressing myeloid cells to trigger chronic pain. In a different context of pain, recent evidence uses a novel null-reactivatable approach that microglial TLR4 is crucial in mediating female-specific acute mechanical allodynia following ethanol exposure [233]. While microglia-restricted TLR4 expression is sufficient to induce allodynia in females, its role in males is less pronounced, underscoring the potential contributions of TLR4 on tissue macrophages in shaping pain responses in both sexes [233]. Differences in microglial pro-inflammatory signatures and nociceptive signaling demonstrate nuanced neuroimmune interactions of central and peripheral TLR4 signaling. This suggests that TLR4 signaling cascades may be different between sexes and temporal dynamics may influence cell-specific actions of TLR4 in the transition from acute to chronic pain.
Other important considerations in studying the effects of TLR4 in chronic pain are the models and inflammatory mediators used. HMGB1 is a highly dynamic nuclear chromatin-binding protein that orchestrates key biological events such as sterile inflammation and chemoattraction based on redox biology [234]. HMGB1 can signal through multiple receptors including TLR4 [235, 236]. Passive secretion of HMGB1 results from cell death, injury, autophagy, and necrosis [237–239]. Intracellular HMGB1 exists in its all-thiol form (at-HMGB1) and can be oxidized to the disulfide form (ds-HMGB1) when reactive oxygen species present in the extracellular matrix facilitate disulfide bridge formation between cysteine residues 23 and 45 [240]. Ds-HMGB1 functions as an inflammatory cytokine that induces preferential activation of TLR4 [241]. HMGB1 is upregulated in sensory neurons in the DRG, as well as peripheral immune cells and glia in response to neuropathic injury [151]. Upregulation of HMGB1 was also associated with a transition from nuclear to cytosolic localization, which is indicative of active release [242]. Inhibition of HMGB1 signaling by a neutralizing antibody prevents mechanical allodynia development and cytosolic localization of the protein, suggesting that peripheral HMGB1 signaling plays a significant role in the development of neuropathic pain [243]. Moreover, only male rats were used in these experiments. Another study demonstrated that administration of ds-HMGB1 increased excitability of dissociated DRG neurons and treatment with glycyrrhizin, an HMGB1 inhibitor, reversed the HMGB1-dependent increase in neuronal excitability. Moreover, systemic inhibition of HMGB1 signaling reversed mechanical allodynia resulting from nerve injury [244]. A more recent study highlighted the effects of central HMGB1-TLR4 signaling in arthritis-like pain [245]. Intrathecal administration of ds-HMGB1 induced pain-like behaviors in mice of both sexes and is TLR4-dependent. Moreover, pharmacological inhibition of spinal HMGB1 signaling reverses arthritis pain. This study provides additional evidence to support the role of HMGB1 in pain, but it was also one of the first to directly assess sex differences in HMGB1-TLR4 signaling [245]. Although, these findings do not recapitulate the Sorge et al., 2011 study that found robust sex differences in spinal TLR4 signaling suggesting mechanistic differences in the specific ligands, signaling pathways, and cell types responsible for neuropathic pain development between males and females [183]. The involvement of sex- and cell-specific HMGB1 signaling in the periphery has not been extensively studied until recently.
A recent study found that inhibition of TLR4 signaling in peripheral macrophages attenuated ds-HMGB1-induced pain behaviors in male but not female mice, suggesting that the cell and tissue types responsible for mediating peripheral pain mechanisms differ between sexes as well [246]. In the same study, using conditional DRG nociceptor TLR4 knockout animals demonstrated that HMGB1 mediates arthritis pain through TLR4 signaling in both sexes. Another study confirmed these findings by using a similar genetic and pain model [247]. Moreover, by conditional deletion of HMGB1 from the whole DRG neuron population, they confirm that HMGB1 is also responsible for neuropathic pain development during neuronal injury in females. The specific receptors for HMGB1, neuronal subtypes, and male-specific mechanisms were not addressed [247]. Therefore, studies that evaluate the effectiveness of inhibiting both TLR4 and HMGB1 signaling will provide insight on the sex- and cell-specific mechanisms of chronic pain and provides a prime example of a mediator of sex-specific differences in neuroimmune interactions.
Hormonally driven sex differences in neuroimmune communications, pain, and physiology
It is now widely appreciated that the nervous and immune systems communicate with one another to coordinate their dynamic responses. It is also apparent that the endocrine system and hormones also play a role in these actions [248]. The interaction between the nervous, endocrine, and immune systems is bidirectional, indicating that crosstalk between these systems is necessary to not only maintain homeostasis, but also to respond appropriately to tissue injury and infection [36, 248]. While the scientific community has traditionally focused on male-biased research, it is making significant strides to assess differences in nervous and immune system communication between males and females. However, there are still unanswered questions and mechanisms that remain unclear. For instance, TRPV1 activity is well-known for promoting pain and inflammation and can be differentially regulated by different sex steroids. Recent studies found that estradiol levels influence TRPV1 expression in females [249–251], positively modulating its expression to promote pain. However, studies are still inconclusive on whether testosterone modulates TPRV1 expression or activity in humans [252–254]. Studies are still quite sparse regarding sex differences in pain, and thus more studies are needed to unravel the influence of hormones on sex-specific biomarkers driving these dimorphisms.
Accounting for differences between males and females is important as some conditions have a greater prevalence in females compared to males. Moreover, studies confirm that neuroendocrine interactions with the immune system play a role in the pathogenesis of these conditions [255]. For example, males have lower susceptibility to autoimmune disorders compared to females due to testosterone’s protective immunomodulatory function [255, 256]. Studies show that a disproportionate number of females are impacted by autoimmune diseases, chronic pain disorders, and the lack of effective therapeutics [255, 257, 258]. Thus, understanding the sex- and cell-specific differences in neuroimmune modulation is necessary to appropriately design and execute research targeted at effective therapeutic development.
Testosterone
Androgens have been found to mediate sex differences in neuroimmune interactions and within the last decade, numerous studies have highlighted testosterone as a mediator of sex differences in immunity and pain development [259–261]. Studies have shown that testosterone receptors, also called androgen receptors (AR), modulate immune and pain outcomes in gonadectomized mice by promoting µ-opioid and cannabinoid receptor activity in the presence of high levels of testosterone [262–264]. Another study using a model of chronic inflammatory pain in rats demonstrated that testosterone inhibits TRPV1 expression [265]. Although testosterone serves as the primary sex hormone in males, dysregulation in testosterone production has clinical implications in both sexes. In females, hyperandrogenism is associated with polycystic ovary syndrome (PCOS) [266]. PCOS is an endocrine disorder affecting females of reproductive age and has several associated comorbidities including insulin resistance and cardiovascular disease [267]. Further, in females with PCOS, increased levels of androgens correlate with increased cytokine secretion and disrupts the normal balance of the immune system by stimulating some immune cells and inhibiting others, which can produce a state of chronic, low-grade inflammation [268]. It is especially important that normal-range testosterone levels be maintained in both sexes considering the potential risks associated with immune cardiovascular diseases. A previous study found that administration of testosterone in males with androgen deficiency led to decreased levels of pro-inflammatory cytokines IL-1β and TNF-α and an increase in the anti-inflammatory cytokine IL-10 [269]. Testosterone has also been shown to act directly on CD4+ T-cells to increase IL-10 production [256]. Furthermore, research has indicated that testosterone replacement therapy may favorably shift cytokine balance and reduce total cholesterol in males, potentially improving cardiovascular disease risk [269]. Testosterone has also been shown to protect against the development of widespread muscle pain, a hallmark of the female-dominated condition fibromyalgia, in an activity-induced pain model [259]. Hence, more studies unraveling in-depth mechanisms of testosterone in neuroimmune communication are crucial to addressing pain disparities between males and females.
Estrogens and progesterone
Estrogens are produced mainly by the ovaries in females and to a lesser extent, the testes in males [270]. Estrogens are involved in a variety of physiological processes including the resolution of inflammation and may be a promising therapeutic for promoting anti-inflammation [271]. Fluctuations in estrogens and progesterone during the estrus cycle of female rodents alter the circadian rhythm of both physiology and behavior [272]. The two subtypes of estrogen receptors (ER), ER-α and ER-β, bind estrogens and differentially affect inflammation and pain modulation [273]. ER-α has been shown across multiple studies to modulate pain [275–277]. Estradiol (E2), the most prominent estrogen in the human body, signaling increases NMDA receptor activity while ER-α antagonists administered in the spinal cord increase excitatory postsynaptic currents, which may increase pronociceptive signaling [277, 278]. Further, both male and female ER-α knockout mice show small increases in nociceptive behaviors, suggesting that ER-α signaling inhibits pain transmission [276]. There is evidence, however, that ER-α and ER-β signaling may be pronociceptive, as another study found that knockout of either receptor significantly increases elevated responses to mechanical stimuli in female mice [275]. In most neurons with both receptor subtypes co-expressed, high estrogen levels can result in the dimerization of ER-α and ER-β, which suppresses ER-α signaling [280–282]. In a study looking at how estrogens affect µ-opioid receptor levels and activation with a pain stressor in females, low estrogen levels corresponded with increased pain perception and a reduction in the endogenous opioid neurotransmission function [282]. E2 has been found to mitigate the negative effects of glucocorticoids in cognitive processes [283]. Furthermore, E2 positively activates TRPV1 channels at higher concentrations [249, 251, 284, 285]. The interactions between neurotransmitters like serotonin and estrogens have been linked to female-specific differences in immune modulation and pain perception in conditions like irritable bowel syndrome and fibromyalgia [286]. Serotonin is considered an inflammatory mediator and increases pronociceptive signaling. ER activity can increase serotonin’s pronociceptive activity by enhancing postsynaptic responsiveness and increasing serotonin synthesis in the brain [287].
Although both estrogens and progesterone play a role in modulating pain and inflammation, progesterone’s specific mechanisms are not as well-known as that of estrogen. Progesterone is secreted by the corpus luteum where it has downstream implications in reproductive and immune processes, especially during pregnancy [288]. While progesterone has been extensively studied in relation to female reproductive processes, its role in males is not yet fully understood [289]. Immune cells, including macrophages, NK cells, dendritic cells, and T-cells, express progesterone receptors [290]. Progesterone suppresses macrophage and dendritic cell activation, alters T-cell distribution and activity, and causes apoptosis of NK cells expressing progesterone receptors [290, 291]. Via modulation of transcription factors, progesterone decreases inflammation by inhibiting pro-inflammatory cytokine production and increasing the production of anti-inflammatory cytokines [293–295]. A study investigating progesterone’s anti-inflammatory effects in LPS-stimulated microglia found that progesterone inhibits NF-kB activation [295]. Another study reports that peaks in progesterone and testosterone are associated with lower reported pain severity in females with fibromyalgia, suggesting both sex hormones possess a protective role in modulating fibromyalgia pain [21].
Medications containing hormones, such as oral contraceptive pills containing both estrogens and progestins, can also influence pain and contribute to pain states. These contraceptive pills are the most commonly prescribed contraceptive pills in the United States, however, individuals who experience migraines are not recommended to take them due to the increased risk of migraines [296]. Fluctuations in hormone levels during the menstrual cycle is also believed to play a role in the pathogenesis of migraine attacks and signaling of these hormones may explain the greater prevalence of migraine in females [297].
Prolactin
In addition to estrogens and progesterone, prolactin, a hormone produced by the pituitary gland and lymphocytes, is also implicated in driving neuroimmune interactions in pain [248, 298]. Prolactin secretion is regulated by cytokines, but can also function as a cytokine itself, with the prolactin receptor (PRLRs) being included in the cytokine receptor super family [299, 300]. Prolactin stimulates PRLRs of B-cells, macrophages, and T-cells to induce immune responses [301]. Prolactin plays a critical role in the complex sex-specific mechanisms underlying migraine [302, 303]. Reports show a direct correlation between high prolactin levels and the prevalence of chronic migraine, with increased risk of migraines in patients with hyperprolactinemia [306–309]. Additionally, a preclinical study showed dural administration of prolactin resulted in female-specific migraine-like behavior in both cycling and ovariectomized rodents [302, 303, 308]. Overexpression of prolactin and its receptors are causal factors in the development of autoimmunity and pain. For example, hyperprolactinemia plays a role in the clinical manifestations of some autoimmune diseases including multiple sclerosis and Sjogren’s syndrome [309]. Furthermore, using both local and spinal inflammatory pain models, a previous study found that PRLR contributes to hypersensitivity in a female-specific manner [310]. Additionally, PRLR has been found to contribute to sexual dimorphic differences in pain during inflammatory processes in neuronal and non-neuronal cells [310, 311]. In a study that used a mouse line with ablated PRLR in sensory neurons, they found that PRLR in sensory neurons is critical for transitioning pain from the acute to chronic state in females only [312]. Additionally, PRLR expression is higher in neuronal fibers and dural mast cells in female mice while male mice showed little to no expression, which may contribute to increased prevalence of migraines in females [308].
Nuclear receptors: peroxisome proliferator-activated receptors (PPARs)
While hormones and nuclear receptor ligands can contribute to sex differences in neuroimmune communications, they can also serve as modulators of other components that lead to sexual dimorphisms. Peroxisome proliferator-activated receptors (PPARs) are a group of nuclear receptor proteins comprised of three different subtypes (PPARα, PPARγ, and PPARβ/δ). PPARs are involved in glucose metabolism, cell survival, lipid detection, and immunity [313]. While PPARs are expressed in a variety of tissues, the expression of subtypes α and γ in T-cells has been shown to be sexually dimorphic in its contribution to pain and inflammation [314]. PPARα is found in macrophages and lymphocytes where it plays a role in suppressing inflammatory activation [315]. Elevated levels of testosterone in males increase the expression of PPARα, which inhibits IFN-γ, thus suppressing inflammation [316]. A study also found that administration of a PPARα agonist alleviated pain specifically in male mice and this was reversed by eliminating testosterone [166]. Conversely, PPARγ inhibits macrophages and T-cell proliferation and plays a key role in regulatory T-cell activity. Elevated levels of estrogen in females increase PPARγ expression, which inhibits NF-κB and T-cells and suppresses inflammation. Administration of a PPARγ agonist alleviated pain specifically in female mice, and this was reversed by testosterone [166]. Taken together, PPARs and hormones highlight additional mechanisms in which elements of the immune system drive sex differences in pain and inflammation.
Sex differences in pain and peripheral inflammation in the clinic
The neuroimmune mechanisms underlying sex differences and their impact on chronic pain conditions remains a critical research question for clinical translation. Although animal models provide insight into mechanisms underlying pain, neuroimmune communication, and pain resolution, lack of our understanding of nuances between humans and non-human models slows progress. For example, preclinical rodent models suggest females are less responsive to pain therapeutics, such as low efficacy opioids, however, clinical studies indicate the opposite effect in humans, with opioids generally being more effective and potent in females than males [317, 318]. The severity of the ongoing opioid epidemic is evident in the reported 4 and 5.8-fold increase in overdoses since 1999 in males and females respectively [319, 320]. These increases in overdoses make evident the importance of not only advancing pain research, but also better evaluating the differences across sexes.
Additionally, although existing preclinical and clinical research provides some insight into the sex differences in pain in patients, further investigation is needed to fully elucidate the differences in physiology between males and females to develop and prescribe more personalized, effective pain treatments for patients. Given the higher prevalence of chronic pain conditions in females than in males [321], pain research has honed in on efforts to characterize potential sex differences in actual pain sensitivity and mechanisms [12, 322].
Sex differences in endogenous descending pain modulation have been observed in the form of diffuse noxious inhibitory controls (DNIC). DNIC dampens pain signaling in the dorsal horn of the spinal cord via inhibitory neurotransmitters. Research has found that DNIC is more pronounced in males compared to females [323]. However, as with most clinical data, this evidence is inconsistent due to additional psychological factors rendering DNIC sex differences insignificant [324]. In the clinic, females typically have less favorable outcomes regarding therapeutic intervention than males for pain treatment. In the case of musculoskeletal pain, females are more likely to develop chronic knee pain following total knee arthroplasty and twice at risk of experiencing no clinically important improvement in the case of knee osteoarthritis and widespread pain [325].
The sex differences in pain observed clinically are likely driven by many different cell types and their interactions. For example, immune cells can induce sexually dimorphic pain states through inflammation. In patients with periodontitis, females are more likely to have lower pain thresholds and more persistent pain with macrophage activity likely driving these differences [326]. Furthermore, this study found that genes associated with either promotion or inhibition of nociception differed between sexes [326]. Although the exact immune cells that drive many pain states are still under investigation, sex differences in the levels of pro-inflammatory mediators that drive pain and are involved in the recruitment of immune cells have been reported. For example, in inflammatory dental pain, women show a greater expression in the purinergic receptor, P2X 3, and CD39, a marker for chronic ATP release, than men, which was indicative of greater pain in women than men [327].Specific immune cells have also been implicated in osteoarthritis in females and males, with osteoarthritis females having a higher percentage of CD4+ T cells and macrophages, but a lower percentage of monocytes and CD8+ T cells in their synovial fluid compared to osteoarthritis males [328]. These findings indicate that differences in inflammation-related physiology such as pain can be attributed to sex differences.
Macrophage polarization in asthma and headache related pain
Male and female macrophages differ in propensity to polarize towards pro- and anti-inflammatory states [329, 330]. There is a sex-dependent functional state of immune cells in asthmatic patients, with macrophages and monocytes exhibiting a greater anti-inflammatory M2 phenotype in females [331]. Asthma is also a comorbidity of cluster headaches that is more prevalent in females [332]. There may be a bidirectional etiology where cluster headaches cause further alterations to monocytes, and asthma’s underlying alterations of the immune state may be a contributing factor to cluster headache development [332]. For example, asthma-associated inflammation triggers vascular neuroinflammation to induce headaches, and asthma treatment options are also capable of inducing headaches [333]. While females and males both experience retro-orbital pain with cluster headaches, females disproportionately suffer from coexisting jaw, ear, and cheek pain, as well as migraine-like symptoms such as nausea [332]. Furthermore, sex differences exist in treatment for headache pain, as females respond more to lidocaine and CGRP antagonist, whereas males respond more to sumatriptan [332, 336–338].
Implications of adiposity and pain
Adiposity serves as a key consideration in the evaluation of risk for obesity, and its sexually dimorphic character contributes to sex disparities in clinical settings, as males and females have uniquely different adipose distributions [337]. Adiposity influences immune responses and has both a direct physical and physiological role in the development of chronic pain. Female adipose distribution is characterized by higher femoral deposition, along with increased levels of inflammatory C-reactive protein (CRP). Moreover, females exhibit higher levels of IL-6 and leptin, a hormone released by adipose tissue, which is implicated in exacerbating chronic pain severity in female patients [338]. Differential plasma levels of inflammatory markers, such as CRP, in females are specifically due to greater accumulation of subcutaneous fat compared to male counterparts [339]. In the case of diet-induced obesity, males and females express distinct inflammatory responses, with males having higher levels of macrophage accumulation in subcutaneous white adipose tissue. This macrophage accumulation is linked to elevated fatty acid species in these tissues, in which their storage and release causes this male-specific inflammation [340]. Preclinical models have demonstrated higher levels of adiposity directly correlate to increased TLR4 expression to mediate pro-inflammatory responses in tissue highlighting another crucial role for TLR4 in the attribution of pain phenotypes in a sex-dependent manner [343–345]. This translates to the clinic where researchers discovered obese patients have high adipocyte TLR4 expression from subcutaneous abdominal fat [344], suggesting TLR4 may play a role in adiposity-related regulation of immune responses and chronic pain.
Postsurgical recovery and pain
Overall, sex differences in the development of postoperative chronic pain have been identified across many surgery types, with females more frequently reporting severe pain levels one day postoperatively and higher pain scores across the recovery period [345]. Recent studies focused on cardiac surgical outcomes have reported sex differences in postsurgical pain and recovery. In a one year postoperative study, biological females presented with higher levels of postoperative pain than males across time [346], while males had lower mortality rates than females [347, 348]. This indicates that differences in surgical strategy may influence sex differences in recovery outcomes. In total knee arthroplasty procedures, one study found that females experienced more acute postoperative pain, while no sex differences were observed six-weeks postoperatively [349]. Interestingly, this alludes to how biologically, males and females utilize recovery strategies to end up back to homeostasis.
Sex hormone modulation of nociception
As previously stated, sex hormones and their influence are a culprit behind sexually dimorphic nociceptive responses. However, recent preclinical evidence suggests that transient shifts in hormones like estrogen during the estrus cycle in rodents may not be directly responsible for these nociceptive responses, suggesting estrogen level fluctuations during the menstrual cycle may not be directly responsible for nociceptive responses in humans [35, 176, 350]. It is possible that this dichotomy in sex-based pain may be hardwired at or right before birth and modulated to a lesser degree by the fluctuation of hormones during adulthood. As evidence, a recent clinical study in pre-and full-term infants discovered that widespread nociceptive events measured by electroencephalography are different between sexes [351]. Interestingly, no differences were found in response to innocuous touch, indicating that the signaling pathways between the sensory and nociceptive systems are not mutually exclusive.
Sex differences in inflammatory factors in osteoarthritis
Many studies have shown that pain in osteoarthritis influences changes in gait [352], and gait changes contribute to pain in a bidirectional manner. Gait differences exist between sexes both pre- and post-surgically in patients with osteoarthritis, indicating that sex differences exist both in inflammatory osteoarthritis development as well as in recovery from associated surgical intervention [353]. Research in osteoarthritis development has gained traction in recent years, taking into consideration that this condition is more prevalent and painful in females [356–358]. Osteoarthritis has been labeled as a condition associated with less inflammation than other arthritis subtypes [356], however, more recent clinical studies have reported sex-differences in the immune response [356–358]. These sex differences have been observed in both the development and in persistent pain in osteoarthritis, however, the exact mechanisms have yet to be fully elucidated [356–358]. Interestingly, females with osteoarthritis show higher levels of IFN-γ, IL-3, IL-8, IL-18, and TNF-β, and other factors that stimulate macrophages compared to osteoarthritis men [357, 358]. Conversely, others report that IL-8 has been found to be higher in males with osteoarthritis [359]. In addition to sex differences in the levels of inflammatory factors in osteoarthritis, these factors may drive pain differently in females and males. For example, IL-6 levels are correlated with increased pain in females and decreased pain in males, IL-1β and IL-8 levels correlate with increased pain in males and decreased pain in females, which further demonstrates the need for sex specific treatments for osteoarthritis [360]. Collectively, these instances of sexual dimorphisms within the clinic emphasize the importance of understanding the influence of sex on neuroimmune interactions and their relation to pain and medicine.
Conclusions and future perspectives
The neuroimmune “conversation” is implicated in pain physiology, whether through signaling between immune cells and sensory neurons or immune components expressed by neurons. A prime example of bidirectional neuroimmune communication in pain physiology is neuron-microglia crosstalk, in which neurons activate microglia through glutamate or ATP release and activated microglial actions modulate neuronal excitability [142, 144, 145]. Depending on the pain state and the model used, the cell types that drive sexual dimorphisms in pain differ. Sex differences in pain are further influenced by condition and the activity of different cell types, such as microglia in the CNS driving chronic pain development in a sex-specific manner, and by the neuronal expression of prototypic and immune receptors [35]. For example, neuroimmune interactions facilitated by sensory neurons via TLR4 and NLRP3 lead to greater pain sensitivity in females compared to males [151, 152]. The endocrine system and hormones further drive sex differences in neuroimmune communication and pain. Estrogen and progesterone serve as modulators of nuclear hormone receptors, like PPARs, that lead to sexual dimorphisms in immunity and pain. While there is a disproportionate number of females impacted by autoimmune diseases and chronic pain disorders seen clinically, the mechanisms underlying this disparity are understudied. Clinicians and researchers are recognizing the impact of understanding sexual dimorphisms in neuroimmune communication to treat patients more effectively. With this realization, it is paramount that bench-to-bedside approaches are taken with the consideration of the complexities of the individual systems that influence each other, along with the complexities of the human condition. To date, multiple neuroimmune experimental models and clinical findings highlight the influence of sex on physiology and behavior. Females make up ~ 50% of the population, however, until recently, have been excluded from scientific studies and clinical trials that directly impact their health and well-being. It is imperative that studies at all levels include a reasonable representation of sex groups to power statistical analysis to find meaningful results for both sexes. While achieving such numbers, superimposed on the fact that many studies necessitate including several female groups at various stages of the estrus cycle and hormone level determination, makes studies on sex differences expensive and time consuming. However, exclusion of these factors leaves large gaps in our scientific understanding in how pain is driven differently in females and males. Determining the minimum numbers needed for the appropriate statistics will drive research standards and best practices. Elucidating the role of sex differences in neuroimmune communication and pain is essential for the development of efficacious treatments for inflammatory and painful conditions in both sexes.
Acknowledgements
The authors would like to thank current and past Burton lab members for aiding in the development of this manuscript. All graphics were generated by BioRender.com. The content of this manuscript (portions of text) has previously appeared in the thesis of Thomas A. Szabo-Pardi, available online.
Glossary
- At-HMGB1
All-thiol form HMGB1
- CGRP
Calcitonin gene-related peptide
- CIPN
Chemotherapy-induced peripheral neuropathy
- CSF1
Colony-stimulating factor 1
- DAMPs
Damage-associated molecular patterns
- DNIC
Diffuse noxious inhibitory controls
- DRG
Dorsal root ganglion
- Ds-HMGB1
Disulfide HMGB1
- E2
Estradiol
- ER
Estrogen receptor
- Fc
Receptors for immunoglobulins
- HMGB1
High mobility group box 1
- LPS
Lipopolysaccharide
- M1/2 polarization
Pro-inflammatory (M1) and anti-inflammatory (M2) states
- Nav
Voltage-gated sodium channel
- NK
Natural killer cell
- PAMPs
Pathogen-associated molecular patterns
- PCOS
Polycystic ovary syndrome
- PPARs
Peroxisome proliferator-activated receptor
- PRLR
Prolactin receptor
- SABV
Sex as a biological variable
- TG
Trigeminal ganglion
- TLR
Toll-like receptor
- TNF-α
Tumor necrosis factor alpha
- TRPA1
Transient receptor potential ankyrin 1
- TRPV1
Transient receptor potential vanilloid 1
Author contributions
All authors were involved with the conception of the manuscript. S.N.A., A.R.G., E.K.D., & L.R.F. contributed to all phases of writing, research, and bibliography development. A.R.G. developed the initial draft of the figures, H.M.K.A. and T.S.P. contributed to initial drafts and final editing of the manuscript. M.D.B. participated and supervised in all aspects from conception to manuscript preparation, and funding acquisition.
Funding
This research was funded by the NIH/NIDDK, grant number R21DK130015 (M.D.B.), NIH/NIGMS, grant number R35GM147094, the University of Texas System STARS program research support grant (M.D.B.), and the Rita Allen Foundation Grant (M.D.B.).
Data availability
Not Applicable.
Declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors (Alexander, S.N, Green, A.R., Debner, E.K., Abdelhadi, H.M.K., Ramos Freitas, L.E., Szabo-Pardi, T.A., Burton, M.D) have no conflict of interests to declare.
Footnotes
Shevon N. Alexander, Audrey R. Green, Emily K. Debner and Lindsey E. Ramos Freitas are co-first authors.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kourounakis P, Selye H. Influence of steroids and stress on toxicity and disposition of tetraethylammonium bromide. J Pharm Sci. 1976;65:12:1838–40. 10.1002/jps.2600651236. [DOI] [PubMed] [Google Scholar]
- 2.Hadamitzky M, Lückemann L, Pacheco-López G, Schedlowski M. Pavlovian conditioning of immunological and neuroendocrine functions. Physiol Rev. 2020;100:1:357–405. 10.1152/physrev.00033.2018. [DOI] [PubMed] [Google Scholar]
- 3.Kourounakis P, Szabo S, Selye H. Effect of pregnenolone-16alpha-carbonitrile on the rat liver. Arzneimittelforschung. 1976;26(1):74–5. https://www.ncbi.nlm.nih.gov/pubmed/947184. [PubMed] [Google Scholar]
- 4.Du Ruisseau P, Tache Y, Selye H, Ducharme JR, Collu R. Effects of chronic stress on pituitary hormone release induced by combined hemi-extirpation of the thyroid, adrenal and ovary in rats. Neuroendocrinology. 1977;24:3–4. 10.1159/000122707. [DOI] [PubMed] [Google Scholar]
- 5.Salas M, Tuchweber B, Kourounakis P, Selye H. Temperature-dependence of stress-induced hepatic autophagy. Experientia. 1977;33:5:612–4. 10.1007/BF01946531. [DOI] [PubMed] [Google Scholar]
- 6.Cohen N, Ader R. Immunomodulation by classical conditioning. Adv Biochem Psychopharmacol. 1988;44:199–202. https://www.ncbi.nlm.nih.gov/pubmed/3041747. [PubMed] [Google Scholar]
- 7.Ader R, Cohen N. Conditioning of the immune response. Neth J Med. 1991;39:3–4. https://www.ncbi.nlm.nih.gov/pubmed/1791889. :263 – 73. [PubMed] [Google Scholar]
- 8.Cohen N, Moynihan JA, Ader R. Pavlovian conditioning of the immune system. Int Arch Allergy Immunol. 1994;105(2):101–6. 10.1159/000236811. [DOI] [PubMed] [Google Scholar]
- 9.Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. 2011;13:6. 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raison CL, Miller AH. Role of inflammation in depression: implications for phenomenology, pathophysiology and treatment. Mod Trends Pharmacopsychiatry. 2013;28:33–48. 10.1159/000343966. [DOI] [PubMed] [Google Scholar]
- 11.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:1:22–34. 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller LR, Marks C, Becker JB, Hurn PD, Chen WJ, Woodruff T, et al. Considering sex as a biological variable in preclinical research. Faseb J. 2017;31:1:29–34. 10.1096/fj.201600781R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alshawaf AJ, Viventi S, Qiu WZ, D’Abaco G, Nayagam B, Erlichster M, et al. Phenotypic and functional characterization of peripheral sensory neurons derived from human embryonic stem cells. Sci Rep. 2018;8:12. 10.1038/s41598-017-19093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li CL, Li KC, Wu D, Chen Y, Luo H, Zhao JR, et al. Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity (26, pg 83, 2016). Cell Res. 2016;26:8967. 10.1038/cr.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tavares-Ferreira D, Shiers S, Ray PR, Wangzhou A, Jeevakumar V, Sankaranarayanan I, et al. Spatial transcriptomics of dorsal root ganglia identifies molecular signatures of human nociceptors. Sci Transl Med. 2022;14:632eabj8186. 10.1126/scitranslmed.abj8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu H, Usoskin D, Nagi SS, Hu Y, Kupari J, Bouchatta O et al. Single-Soma Deep RNA sequencing of Human DRG Neurons Reveals Novel Molecular and Cellular Mechanisms Underlying Somatosensation. bioRxiv. 2023; 10.1101/2023.03.17.533207
- 17.Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6:7. 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg DS, McGee SJ. Pain as a global public health priority. BMC Public Health. 2011;11:770. 10.1186/1471-2458-11-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, et al. Prevalence of Chronic Pain and High-Impact Chronic Pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:36:1001–6. 10.15585/mmwr.mm6736a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20:3:371 – 80; discussion 435–513; 10.1017/s0140525x97221485 [DOI] [PubMed]
- 21.Schertzinger M, Wesson-Sides K, Parkitny L, Younger J. Daily fluctuations of Progesterone and Testosterone are Associated with Fibromyalgia Pain Severity. J Pain. 2018;19:4:410–7. 10.1016/j.jpain.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruau D, Liu LY, Clark JD, Angst MS, Butte AJ. Sex differences in reported Pain Across 11,000 patients captured in Electronic Medical records. J Pain. 2012;13:3. 10.1016/j.jpain.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell MB, Rasmussen BK, Thorvaldsen P, Olesen J. Prevalence and sex-ratio of the subtypes of migraine. Int J Epidemiol. 1995;24:3:612–8. 10.1093/ije/24.3.612. [DOI] [PubMed] [Google Scholar]
- 24.Faria V, Erpelding N, Lebel A, Johnson A, Wolff R, Fair D, et al. The migraine brain in transition: girls vs boys. Pain. 2015;156:11:2212–21. 10.1097/j.pain.0000000000000292. [DOI] [PubMed] [Google Scholar]
- 25.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL. Sex, gender, and Pain: a review of recent clinical and experimental findings. J Pain. 2009;10:5. 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burrell BD. Comparative biology of pain: what invertebrates can tell us about how nociception works. J Neurophysiol. 2017;117:4:1461–73. 10.1152/jn.00600.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Black JA, Nikolajsen L, Kroner K, Jensen TS, Waxman SG. Multiple sodium channel isoforms and mitogen-activated protein kinases are present in painful human neuromas. Ann Neurol. 2008;64:6644–53. 10.1002/ana.21527. [DOI] [PubMed] [Google Scholar]
- 28.Song MY, Yuan JX. Introduction to TRP channels: structure, function, and regulation. Adv Exp Med Biol. 2010;661:99–108. 10.1007/978-1-60761-500-2_6. [DOI] [PubMed] [Google Scholar]
- 29.Frederick J, Buck ME, Matson DJ, Cortright DN. Increased TRPA1, TRPM8, and TRPV2 expression in dorsal root ganglia by nerve injury. Biochem Biophys Res Commun. 2007;358:4:1058–64. 10.1016/j.bbrc.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 30.Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov. 2009;8(1):55–68. 10.1038/nrd2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu L, Yang F, Luo H, Liu FY, Han JS, Xing GG, et al. The role of TRPV1 in different subtypes of dorsal root ganglion neurons in rat chronic inflammatory nociception induced by complete Freund’s adjuvant. Mol Pain. 2008;4:61. 10.1186/1744-8069-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duitama M, Vargas-López V, Casas Z, Albarracin SL, Sutachan JJ, Torres YP. TRP channels role in Pain Associated with neurodegenerative diseases. Front Neurosci. 2020;14:782. 10.3389/fnins.2020.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyd JT, LoCoco PM, Furr AR, Bendele MR, Tram M, Li Q, et al. Elevated dietary ω-6 polyunsaturated fatty acids induce reversible peripheral nerve dysfunction that exacerbates comorbid pain conditions. Nat Metab. 2021;3:6762–73. 10.1038/s42255-021-00410-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tierney JA, Uong CD, Lenert ME, Williams M, Burton MD. High-fat diet causes mechanical allodynia in the absence of injury or diabetic pathology. Sci Rep. 2022;12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexander SN, Jeong HS, Szabo-Pardi TA, Burton MD. Sex-specific differences in alcohol-induced pain sensitization. Neuropharmacology. 2023;225:109354. 10.1016/j.neuropharm.2022.109354. [DOI] [PubMed] [Google Scholar]
- 36.Pinho-Ribeiro FA, Verri WA, Chiu IM. Nociceptor sensory neuron–Immune interactions in Pain and inflammation. Trends Immunol. 2017;38:1:5–19. 10.1016/j.it.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Udit S, Blake K, Chiu IM. Somatosensory and autonomic neuronal regulation of the immune response. Nat Rev Neurosci. 2022;23. 10.1038/s41583-021-00555-4. :3:157 – 71. [DOI] [PMC free article] [PubMed]
- 38.Wu M, Song G, Li J, Song Z, Zhao B, Liang L, et al. Innervation of nociceptor neurons in the spleen promotes germinal center responses and humoral immunity. Cell. 2024;187:12:2935–e5119. 10.1016/j.cell.2024.04.027. [DOI] [PubMed] [Google Scholar]
- 39.Cohen JA, Edwards TN, Liu AW, Hirai T, Jones MR, Wu J, et al. Cutaneous TRPV1(+) neurons trigger protective innate type 17 anticipatory immunity. Cell. 2019;178. 10.1016/j.cell.2019.06.022. 4:919 – 32 e14. [DOI] [PMC free article] [PubMed]
- 40.Caputa G, Castoldi A, Pearce EJ. Metabolic adaptations of tissue-resident immune cells. Nat Immunol. 2019;20:7. [DOI] [PubMed] [Google Scholar]
- 41.Gray JI, Farber DL. Tissue-Resident Immune cells in humans. Annu Rev Immunol. 2022;40:1:195–220. 10.1146/annurev-immunol-093019-112809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cesta MF. Normal structure, function, and histology of mucosa-associated lymphoid tissue. Toxicol Pathol. 2006;34:5:599–608. 10.1080/01926230600865531. [DOI] [PubMed] [Google Scholar]
- 43.Panda SK, Colonna M. Innate lymphoid cells in mucosal immunity. Front Immunol. 2019;10:861. 10.3389/fimmu.2019.00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Randall TD, Mebius RE. The development and function of mucosal lymphoid tissues: a balancing act with micro-organisms. Mucosal Immunol. 2014;7:3. 10.1038/mi.2014.11. [DOI] [PubMed] [Google Scholar]
- 45.Seo GY, Giles DA, Kronenberg M. The role of innate lymphoid cells in response to microbes at mucosal surfaces. Mucosal Immunol. 2020;13:3:399–412. 10.1038/s41385-020-0265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gong WY, Abdelhamid RE, Carvalho CS, Sluka KA. Resident macrophages in muscle contribute to Development of Hyperalgesia in a mouse model of noninflammatory muscle Pain. J Pain. 2016;17:10:1081–94. 10.1016/j.jpain.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bove D, Lupoli A, Caccavale S, Piccolo V, Ruocco E. Dermatological and immunological conditions due to nerve lesions. Funct Neurol. 2013;28:2:83–91. 10.11138/FNeur/2013.28.2.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, Mucida D. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell. 2016;164:3. 10.1016/j.cell.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riol-Blanco L, Ordovas-Montanes J, Perro M, Naval E, Thiriot A, Alvarez D, et al. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature. 2014;510:7503157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421:6921. [DOI] [PubMed] [Google Scholar]
- 51.Jentho E, Weis S. DAMPs and Innate Immune Training. Front Immunol. 2021;12:699563. 10.3389/fimmu.2021.699563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barger SW, Goodwin ME, Porter MM, Beggs ML. Glutamate release from activated microglia requires the oxidative burst and lipid peroxidation. J Neurochem. 2007;101:5:1205–13. 10.1111/j.1471-4159.2007.04487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R, et al. Tumor necrosis Factor-α induces neurotoxicity via Glutamate Release from hemichannels of activated Microglia in an Autocrine Manner. J Biol Chem. 2006;281:30:21362–8. 10.1074/jbc.m600504200. [DOI] [PubMed] [Google Scholar]
- 54.Takeuchi H, Jin S, Suzuki H, Doi Y, Liang J, Kawanokuchi J, et al. Blockade of microglial glutamate release protects against ischemic brain injury. Exp Neurol. 2008;214(1):144–6. 10.1016/j.expneurol.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Oishi Y, Manabe I. Macrophages in inflammation, repair and regeneration. Int Immunol. 2018;30:11. 10.1093/intimm/dxy054. [DOI] [PubMed] [Google Scholar]
- 56.Rosales C, Uribe-Querol E, Phagocytosis. A fundamental process in immunity. Biomed Res Int. 2017;2017:9042851. 10.1155/2017/9042851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen P, Piao X, Bonaldo P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. 2015;130:605–18. [DOI] [PubMed] [Google Scholar]
- 58.Lim JY, Choi S-I, Choi G, Hwang SW. Atypical sensors for direct and rapid neuronal detection of bacterial pathogens. Mol Brain. 2016;9:1. 10.1186/s13041-016-0202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davies AJ, Kim HW, Gonzalez-Cano R, Choi J, Back SK, Roh SE, et al. Natural killer cells degenerate intact sensory afferents following nerve Injury. Cell. 2019;176. 10.1016/j.cell.2018.12.022. 4:716 – 28.e18. [DOI] [PMC free article] [PubMed]
- 60.Ristoiu V. Contribution of macrophages to peripheral neuropathic pain pathogenesis. Life Sci. 2013;93:23870–81. [DOI] [PubMed] [Google Scholar]
- 61.Zhang F, Miao Y, Liu Q, Li S, He J. Changes of pro-inflammatory and anti-inflammatory macrophages after peripheral nerve injury. RSC Adv. 2020;10:64:38767–73. 10.1039/d0ra06607a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davies AJ, Rinaldi S, Costigan M, Oh SB. Cytotoxic immunity in peripheral nerve injury and pain. Front NeuroSci. 2020;14:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walker FR, Beynon SB, Jones KA, Zhao Z, Kongsui R, Cairns M, et al. Dynamic structural remodelling of microglia in health and disease: a review of the models, the signals and the mechanisms. Brain Behav Immun. 2014;37:1–14. 10.1016/j.bbi.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 64.Tian D-S, Peng J, Murugan M, Feng L-J, Liu J-L, Eyo UB, et al. Chemokine CCL2–CCR2 signaling induces neuronal cell death via STAT3 activation and IL-1β production after Status Epilepticus. J Neurosci. 2017;37:33:7878–92. 10.1523/jneurosci.0315-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Howe CL, LaFrance-Corey RG, Goddery EN, Johnson RK, Mirchia K. Neuronal CCL2 expression drives inflammatory monocyte infiltration into the brain during acute virus infection. J Neuroinflammation. 2017;14:1238. 10.1186/s12974-017-1015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baral P, Udit S, Chiu IM. Pain and immunity: implications for host defence. Nat Rev Immunol. 2019;19:7. 10.1038/s41577-019-0147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45:2:27–37. 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tecchio C, Micheletti A, Cassatella MA. Neutrophil-derived cytokines: facts beyond expression. Front Immunol. 2014;5:508. 10.3389/fimmu.2014.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ozaktay AC, Kallakuri S, Takebayashi T, Cavanaugh JM, Asik I, DeLeo JA, et al. Effects of interleukin-1 beta, interleukin-6, and tumor necrosis factor on sensitivity of dorsal root ganglion and peripheral receptive fields in rats. Eur Spine J. 2006;15:10:1529–37. 10.1007/s00586-005-0058-8. [DOI] [PubMed] [Google Scholar]
- 70.Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, et al. Nociceptors are interleukin-1beta sensors. J Neurosci. 2008;28:52:14062–73. 10.1523/jneurosci.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lynch AM, Walsh C, Delaney A, Nolan Y, Campbell VA, Lynch MA. Lipopolysaccharide-induced increase in signalling in hippocampus is abrogated by IL-10–a role for IL-1 beta? J Neurochem. 2004;88:3. 10.1046/j.1471-4159.2003.02157.x. [DOI] [PubMed] [Google Scholar]
- 72.Vitkovic L, Maeda S, Sternberg E. Anti-inflammatory cytokines: expression and action in the brain. Neuroimmunomodulation. 2001;9:6:295–312. ://000176284300001. [DOI] [PubMed] [Google Scholar]
- 73.Uçeyler N, Schäfers M, Sommer C. Mode of action of cytokines on nociceptive neurons. Exp Brain Res. 2009;196:1:67–78. 10.1007/s00221-009-1755-z. [DOI] [PubMed] [Google Scholar]
- 74.Gray JI, Farber DL. Tissue-Resident Immune cells in humans. Annu Rev Immunol. 2022;40:195–220. 10.1146/annurev-immunol-093019-112809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luoma AM, Suo S, Wang Y, Gunasti L, Porter CBM, Nabilsi N, et al. Tissue-resident memory and circulating T cells are early responders to pre-surgical cancer immunotherapy. Cell. 2022;185:16. 10.1016/j.cell.2022.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Segura E, Kapp E, Gupta N, Wong J, Lim J, Ji H, et al. Differential expression of pathogen-recognition molecules between dendritic cell subsets revealed by plasma membrane proteomic analysis. Mol Immunol. 2010;47:9:1765–73. 10.1016/j.molimm.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 77.Busa R, Bulati M, Badami E, Zito G, Maresca DC, Conaldi PG, et al. Tissue-Resident Innate Immune Cell-based therapy: a cornerstone of immunotherapy strategies for Cancer Treatment. Front Cell Dev Biol. 2022;10:24. 10.3389/fcell.2022.907572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jang JH, Clark DJ, Li X, Yorek MS, Usachev YM, Brennan TJ. Nociceptive sensitization by complement C5a and C3a in mouse. Pain. 2010;148:2. 10.1016/j.pain.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ting E, Guerrero AT, Cunha TM, Verri WA Jr., Taylor SM, Woodruff TM, et al. Role of complement C5a in mechanical inflammatory hypernociception: potential use of C5a receptor antagonists to control inflammatory pain. Br J Pharmacol. 2008;153:5. 10.1038/sj.bjp.0707640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pekna M, Pekny M. The complement system: a powerful modulator and effector of astrocyte function in the healthy and diseased Central Nervous System. Cells. 2021;10:71812. 10.3390/cells10071812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ziabska K, Ziemka-Nalecz M, Pawelec P, Sypecka J, Zalewska T. Aberrant complement System Activation in Neurological disorders. Int J Mol Sci. 2021;22:928. 10.3390/ijms22094675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou W. The new face of anaphylatoxins in immune regulation. Immunobiology. 2012;217:2225–34. [DOI] [PubMed] [Google Scholar]
- 83.Alawieh A, Elvington A, Zhu H, Yu J, Kindy MS, Atkinson C, et al. Modulation of post-stroke degenerative and regenerative processes and subacute protection by site-targeted inhibition of the alternative pathway of complement. J Neuroinflamm. 2015;12:1. 10.1186/s12974-015-0464-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Beek J, Nicole O, Ali C, Ischenko A, MacKenzie ET, Buisson A, et al. Complement anaphylatoxin C3a is selectively protective against NMDA-induced neuronal cell death. NeuroReport. 2001;12:2289–93. 10.1097/00001756-200102120-00022. [DOI] [PubMed] [Google Scholar]
- 85.Osaka H, Mukherjee P, Aisen PS, Pasinetti GM. Complement-derived anaphylatoxin C5a protects against glutamate‐mediated neurotoxicity. J Cell Biochem. 1999;73:3303–11. [PubMed] [Google Scholar]
- 86.Shinjyo N, Stahlberg A, Dragunow M, Pekny M, Pekna M. Complement-derived Anaphylatoxin C3a regulates in Vitro differentiation and Migration of neural progenitor cells. Stem Cells. 2009;27(11):2824–32. 10.1002/stem.225. [DOI] [PubMed] [Google Scholar]
- 87.Mukherjee P, Thomas S, Pasinetti GM. Complement anaphylatoxin C5a neuroprotects through regulation of glutamate receptor subunit 2 in vitro and in vivo. J Neuroinflamm. 2008;5:7. 10.1186/1742-2094-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shutov LP, Warwick CA, Shi X, Gnanasekaran A, Shepherd AJ, Mohapatra DP, et al. The complement system component C5a produces thermal hyperalgesia via macrophage-to-nociceptor signaling that requires NGF and TRPV1. J Neurosci. 2016;36:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chu C, Artis D, Chiu IM. Neuro-immune interactions in the tissues. Immunity. 2020;52:3464–74. 10.1016/j.immuni.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chiu IM, Von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci. 2012;15:8:1063–7. 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10:1:40–52. 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- 92.Eder A, Dobrowolska, Kamhieh-Milz W. The role of adipose tissue in the Pathogenesis and therapeutic outcomes of inflammatory bowel disease. Cells. 2019;8:6628. 10.3390/cells8060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ordovas-Montanes J, Rakoff-Nahoum S, Huang S, Riol-Blanco L, Barreiro O, Von Andrian UH. The regulation of immunological processes by peripheral neurons in Homeostasis and Disease. Trends Immunol. 2015;36:10:578–604. 10.1016/j.it.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Spallanzani RG, Zemmour D, Xiao TL, Jayewickreme T, Li CR, Bryce PJ, et al. Distinct immunocyte-promoting and adipocyte-generating stromal components coordinate adipose tissue immune and metabolic tenors. Sci Immunol. 2019;4:3513. 10.1126/sciimmunol.aaw3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mrozek S, Constantin JM, Geeraerts T. Brain-lung crosstalk: implications for neurocritical care patients. World J Crit Care Med. 2015;4:3163–78. 10.5492/wjccm.v4.i3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chavan SS, Pavlov VA, Tracey KJ. Mechanisms and therapeutic relevance of neuro-immune communication. Immunity. 2017;46:6. 10.1016/j.immuni.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hodo TW, de Aquino MTP, Shimamoto A, Shanker A. Critical neurotransmitters in the Neuroimmune Network. Front Immunol. 2020;11:26. 10.3389/fimmu.2020.01869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prud’homme GJ, Glinka Y, Wang QH. Immunological GABAergic interactions and therapeutic applications in autoimmune diseases. Autoimmun Rev. 2015;14:11:1048–56. 10.1016/j.autrev.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 99.Liu B, Wang K, Gao HM, Mandavilli B, Wang JY, Hong JS. Molecular consequences of activated microglia in the brain: overactivation induces apoptosis. J Neurochem. 2001;77:1:182–9. 10.1046/j.1471-4159.2001.t01-1-00216.x. [DOI] [PubMed] [Google Scholar]
- 100.Gaspar R, Soares-Cunha C, Domingues AV, Coimbra B, Baptista FI, Pinto L, et al. Resilience to stress and sex-specific remodeling of microglia and neuronal morphology in a rat model of anxiety and anhedonia. Neurobiol Stress. 2021;14:100302. 10.1016/j.ynstr.2021.100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mousa A, Bakhiet M. Role of Cytokine Signaling during Nervous System Development. Int J Mol Sci. 2013;14:7:13931–57. 10.3390/ijms140713931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bragazzi NL, Kolahi AA, Nejadghaderi SA, Lochner P, Brigo F, Naldi A, et al. Global, regional, and national burden of Guillain-Barré syndrome and its underlying causes from 1990 to 2019. J Neuroinflammation. 2021;18:1264. 10.1186/s12974-021-02319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kurman RJ, Shih Ie M. The Dualistic Model of ovarian carcinogenesis: revisited, revised, and expanded. Am J Pathol. 2016;186:4733–47. 10.1016/j.ajpath.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Feistritzer C, Clausen J, Sturn DH, Djanani A, Gunsilius E, Wiedermann CJ, et al. Natural killer cell functions mediated by the neuropeptide substance P. Regul Pept. 2003;116:1–3. 10.1016/s0167-0115(03)00193-9. [DOI] [PubMed] [Google Scholar]
- 105.Sabogal-Guáqueta AM, Marmolejo-Garza A, Trombetta-Lima M, Oun A, Hunneman J, Chen T, et al. Species-specific metabolic reprogramming in human and mouse microglia during inflammatory pathway induction. Nat Commun. 2023;14:16454. 10.1038/s41467-023-42096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Salzer I, Ray S, Schicker K, Boehm S. Nociceptor signalling through ion Channel Regulation via GPCRs. Int J Mol Sci. 2019;20:10. 10.3390/ijms20102488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Burnstock G, Boeynaems JM. Purinergic signalling and immune cells. Purinergic Signal. 2014;10:4. 10.1007/s11302-014-9427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:6:752–8. 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 109.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:11:1361–8. 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 110.Zarrinmayeh H, Territo PR. Purinergic receptors of the Central Nervous System: Biology, PET ligands, and their applications. Mol Imaging. 2020;19:26. 10.1177/1536012120927609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Donnelly CR, Chen O, Ji RR. How do sensory neurons sense Danger signals? Trends Neurosci. 2020;43. 10.1016/j.tins.2020.07.008. :10:822 – 38. [DOI] [PMC free article] [PubMed]
- 112.Vijay K. Toll-like receptors in immunity and inflammatory diseases: past, present, and future. Int Immunopharmacol. 2018;59:391–412. 10.1016/j.intimp.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Barajon I, Serrao G, Arnaboldi F, Opizzi E, Ripamonti G, Balsari A, et al. Toll-like receptors 3, 4, and 7 are expressed in the enteric nervous system and dorsal Root Ganglia. J Histochem Cytochemistry. 2009;57(11):1013–23. 10.1369/jhc.2009.953539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:2:181–213. 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 115.Biesmans S, Meert TF, Bouwknecht JA, Acton PD, Davoodi N, De Haes P, et al. Systemic Immune activation leads to Neuroinflammation and Sickness Behavior in mice. Mediat Inflamm. 2013;2013:14. 10.1155/2013/271359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cornell J, Salinas S, Huang HY, Zhou MO. Microglia regulation of synaptic plasticity and learning and memory. Neural Regen Res. 2022;17:4. 10.4103/1673-5374.322423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Delpech JC, Saucisse N, Parkes SL, Lacabanne C, Aubert A, Casenave F, et al. Microglial activation enhances associative taste memory through Purinergic Modulation of glutamatergic neurotransmission. J Neurosci. 2015;35:7:3022–33. 10.1523/jneurosci.3028-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bie B, Wu J, Foss JF, Naguib M. Activation of mGluR1 mediates C1q-Dependent Microglial phagocytosis of glutamatergic synapses in Alzheimer’s Rodent models. Mol Neurobiol. 2019;56:8:5568–85. 10.1007/s12035-019-1467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Afridi R, Suk K. Neuroinflammatory basis of Depression: learning from experimental models. Front Cell Neurosci. 2021;15:9. 10.3389/fncel.2021.691067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang LJ, Zhang JQ, You ZL. Switching of the Microglial activation phenotype is a possible treatment for Depression Disorder. Front Cell Neurosci. 2018;12:13. 10.3389/fncel.2018.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wohleb ES. Neuron-Microglia Interactions in Mental Health Disorders: for better, and for worse. Front Immunol. 2016;7:13. 10.3389/fimmu.2016.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang HX, He Y, Sun ZL, Ren SY, Liu MX, Wang G, et al. Microglia in depression: an overview of microglia in the pathogenesis and treatment of depression. J Neuroinflamm. 2022;19:126. 10.1186/s12974-022-02492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yirmiya R, Rimmerman N, Reshef R. Depression as a Microglial Disease. Trends Neurosci. 2015;38:10637–58. 10.1016/j.tins.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 124.D’Mello C, Ronaghan N, Zaheer R, Dicay M, Le T, MacNaughton WK, et al. Probiotics improve inflammation-Associated Sickness Behavior by Altering Communication between the Peripheral Immune System and the brain. J Neurosci. 2015;35:30:10821–30. 10.1523/jneurosci.0575-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Burton MD, Sparkman NL, Johnson RW. Inhibition of interleukin-6 trans-signaling in the brain facilitates recovery from lipopolysaccharide-induced sickness behavior. J Neuroinflamm. 2011;8:13. 10.1186/1742-2094-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Domercq M, Vazquez-Villoldo N, Matute C. Neurotransmitter signaling in the pathophysiology of microglia. Front Cell Neurosci. 2013;7:17. 10.3389/fncel.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR. Microglia in Pain: detrimental and protective roles in Pathogenesis and Resolution of Pain. Neuron. 2018;100:6. 10.1016/j.neuron.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhuo M, Wu GX, Wu LJ. Neuronal and microglial mechanisms of neuropathic pain. Mol Brain. 2011;4:12. 10.1186/1756-6606-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ward H, West SJ. Microglia: sculptors of neuropathic pain? R Soc Open Sci. 2020;7:623. 10.1098/rsos.200260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kohno K, Tsuda M. Role of microglia and P2X4 receptors in chronic pain. Pain Rep. 2021;6:111. 10.1097/pr9.0000000000000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Simon DW, McGeachy MJ, Bayır H, Clark RS, Loane DJ, Kochanek PM. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Reviews Neurol. 2017;13:3171–91. [DOI] [PubMed] [Google Scholar]
- 132.Mazzitelli JA, Smyth LCD, Cross KA, Dykstra T, Sun J, Du S, et al. Cerebrospinal fluid regulates skull bone marrow niches via direct access through dural channels. Nat Neurosci. 2022;25:5555–60. 10.1038/s41593-022-01029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A, et al. Single-cell RNA sequencing of Microglia throughout the mouse lifespan and in the injured brain reveals Complex Cell-State changes. Immunity. 2019;50:1253–e716. 10.1016/j.immuni.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eyo UB, Peng JY, Swiatkowski P, Mukherjee A, Bispo A, Wu LJ. Neuronal hyperactivity recruits microglial processes via neuronal NMDA receptors and microglial P2Y12 receptors after Status Epilepticus. J Neurosci. 2014;34:32:10528–40. 10.1523/jneurosci.0416-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:2:461–553. 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 136.Farber K, Kettenmann H. Functional role of calcium signals for microglial function. Glia. 2006;54:7656–65. 10.1002/glia.20412. [DOI] [PubMed] [Google Scholar]
- 137.Luo LX, Song SS, Ezenwukwa CC, Jalali S, Sun BS, Sun DD. Ion channels and transporters in microglial function in physiology and brain diseases. Neurochem Int. 2021;142:12. 10.1016/j.neuint.2020.104925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu GJ, Nagarajah R, Banati RB, Bennett MR. Glutamate induces directed chemotaxis of microglia. Eur J Neurosci. 2009;29:6:1108–18. 10.1111/j.1460-9568.2009.06659.x. [DOI] [PubMed] [Google Scholar]
- 139.Liu H, Leak RK, Hu X. Neurotransmitter receptors on microglia. BMJ. 2016;1:2. 10.1136/svn-2016-000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30:10. 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 141.Garaschuk O, Verkhratsky A. Physiology of Microglia. In: Garaschuk O, Verkhratsky A, editors. Microglia: methods and protocols. Methods in Molecular Biology. Totowa: Humana Press Inc; 2019. pp. 27–40. 10.1007/978-1-4939-9658-2_3. [DOI] [PubMed] [Google Scholar]
- 142.York EM, Bernier LP, MacVicar BA. Microglial modulation of neuronal activity in the healthy brain. Dev Neurobiol. 2018;78:6. [DOI] [PubMed] [Google Scholar]
- 143.Kato G, Inada H, Wake H, Akiyoshi A, Miyamoto R, Eto K, et al. Microglial contact prevents excess depolarization and rescues neurons from Excitotoxicity. Eneuro. 2016;3(3):ENEURO0004–162016. 10.1523/Eneuro.0004-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Taylor DL, Diemel LT, Pocock JM. Activation of microglial group III metabotropic glutamate receptors protects neurons against microglial neurotoxicity. J Neurosci. 2003;23:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Szepesi Z, Manouchehrian O, Bachiller S, Deierborg T. Bidirectional microglia-neuron communication in Health and Disease. Front Cell Neurosci. 2018;12:26. 10.3389/fncel.2018.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vinet J, van Weering HRJ, Heinrich A, Kalin RE, Wegner A, Brouwer N, et al. Neuroprotective function for ramified microglia in hippocampal excitotoxicity. J Neuroinflamm. 2012;9:15. 10.1186/1742-2094-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jantzie LL, Talos DM, Jackson MC, Park HK, Graham DA, Lechpammer M, et al. Developmental expression of N-Methyl-D-Aspartate (NMDA) receptor subunits in Human White and Gray Matter: potential mechanism of increased vulnerability in the immature brain. Cereb Cortex. 2015;25:2482–95. 10.1093/cercor/bht246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ellis A, Bennett DLH. Neuroinflammation and the generation of neuropathic pain. Br J Anaesth. 2013;111:1:26–37. 10.1093/bja/aet128. [DOI] [PubMed] [Google Scholar]
- 149.Assas BM, Pennock JI, Miyan JA. Calcitonin gene-related peptide is a key neurotransmitter in the neuro-immune axis. Front NeuroSci. 2014;8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Perner C, Flayer CH, Zhu X, Aderhold PA, Dewan ZNA, Voisin T, et al. Substance P release by sensory neurons triggers Dendritic Cell Migration and initiates the Type-2 Immune response to Allergens. Immunity. 2020;53(5):1063–e777. 10.1016/j.immuni.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Szabo-Pardi TA, Barron LR, Lenert ME, Burton MD. Sensory neuron TLR4 mediates the development of nerve-injury induced mechanical hypersensitivity in female mice. Brain Behav Immun. 2021;97:42–60. 10.1016/j.bbi.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cowie AM, Menzel AD, Oharaa C, Lawlor MW, Stucky CL. NOD-like receptor protein 3 inflammasome drives postoperative mechanical pain in a sex-dependent manner. Pain. 2019;160:8. 10.1097/j.pain.0000000000001555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501:746552–7. 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang T, Ma C. Peripheral nociceptors as Immune sensors in the Development of Pain and Itch. Advances in Experimental Medicine and Biology. Springer Netherlands; 2016. pp. 77–85. 10.1007/978-94-017-7537-3_6. [DOI] [PubMed]
- 155.Liu Y, Caterina MJ, Qu LT. Sensory Neuron expressed fc gamma RI mediates Postinflammatory Arthritis Pain in Female mice. Front Immunol. 2022;13:14. 10.3389/fimmu.2022.889286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jiang HW, Shen XH, Chen ZY, Liu F, Wang T, Xie YK, et al. Nociceptive neuronal Fc-gamma receptor I is involved in IgG immune complex induced pain in the rat. Brain Behav Immun. 2017;62:351–61. 10.1016/j.bbi.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 157.Wang L, Jiang XH, Zheng Q, Jeon SM, Chen T, Liu Y, et al. Neuronal fc gamma RI mediates acute and chronic joint pain. J Clin Invest. 2019;129:9:3754–69. 10.1172/jci128010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Goebel A, Krock E, Gentry C, Israel MR, Jurczak A, Urbina CM, et al. Passive transfer of fibromyalgia symptoms from patients to mice. J Clin Invest. 2021;131:13. 10.1172/jci144201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Qu L, Zhang P, Lamotte RH, Ma C, Brain. Behav Immun. 2011;25:7. 10.1016/j.bbi.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bersellini Farinotti A, Wigerblad G, Nascimento D, Bas DB, Morado Urbina C, Nandakumar KS, et al. Cartilage-binding antibodies induce pain through immune complex–mediated activation of neurons. J Exp Med. 2019;216:8. 10.1084/jem.20181657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Corr M, Crain B. The role of fc gamma R signaling in the K/B x N serum transfer model of arthritis. J Immunol. 2002;169:11:6604–9. 10.4049/jimmunol.169.11.6604. [DOI] [PubMed] [Google Scholar]
- 162.Osborne BF, Turano A, Schwarz JM. Sex differences in the neuroimmune system. Curr Opin Behav Sci. 2018;23:118–23. 10.1016/j.cobeha.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gregus AM, Levine IS, Eddinger KA, Yaksh TL, Buczynski MW. Sex differences in neuroimmune and glial mechanisms of pain. Pain. 2021;162:8. 10.1097/j.pain.0000000000002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cowie AM, Dittel BN, Stucky CL. A novel sex-dependent target for the treatment of Postoperative Pain: the NLRP3 inflammasome. Front Neurol. 2019;10:9. 10.3389/fneur.2019.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Scheff NN, Bhattacharya A, Dowse E, Dang RX, Dolan JC, Wang S, et al. Neutrophil-mediated endogenous Analgesia contributes to sex differences in oral Cancer Pain. Front Integr Neurosci. 2018;12:15. 10.3389/fnint.2018.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sorge RE, Mapplebeck JCS, Rosen S, Beggs S, Taves S, Alexander JK, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18:8. 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Inyang KE, Szabo-Pardi T, Wentworth E, Mcdougal TA, Dussor G, Burton MD, et al. The antidiabetic drug metformin prevents and reverses neuropathic pain and spinal cord microglial activation in male but not female mice. Pharmacol Res. 2019;139:1–16. 10.1016/j.phrs.2018.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yang JX, Wang HF, Chen JZ, Li HY, Hu JC, Yu AA, et al. Potential Neuroimmune Interaction in Chronic Pain: a review on Immune cells in Peripheral and Central Sensitization. Front Pain Res (Lausanne). 2022;3:946846. 10.3389/fpain.2022.946846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Laumet G, Edralin JD, Dantzer R, Heijnen CJ, Kavelaars A. CD3(+) T cells are critical for the resolution of comorbid inflammatory pain and depression-like behavior. Neurobiol Pain. 2020;7:100043. 10.1016/j.ynpai.2020.100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lenert ME, Szabo-Pardi TA, Burton MD. Regulatory T-cells and IL-5 mediate pain outcomes in a preclinical model of chronic muscle pain. Mol Pain. 2023;19:17448069221110691. 10.1177/17448069221110691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rosen SF, Ham B, Haichin M, Walters IC, Tohyama S, Sotocinal SG, et al. Increased pain sensitivity and decreased opioid analgesia in T-cell-deficient mice and implications for sex differences. Pain. 2019;160:2358–66. 10.1097/j.pain.0000000000001420. [DOI] [PubMed] [Google Scholar]
- 172.Mifflin KA, Yousuf MS, Thorburn KC, Huang J, Perez-Munoz ME, Tenorio G, et al. Voluntary wheel running reveals sex-specific nociceptive factors in murine experimental autoimmune encephalomyelitis. Pain. 2019;160:4870–81. 10.1097/j.pain.0000000000001465. [DOI] [PubMed] [Google Scholar]
- 173.Chernov AV, Hullugundi SK, Eddinger KA, Dolkas J, Remacle AG, Angert M, et al. A myelin basic protein fragment induces sexually dimorphic transcriptome signatures of neuropathic pain in mice. J Biol Chem. 2020;295:31:10807–21. 10.1074/jbc.RA120.013696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kuhn JA, Vainchtein ID, Braz J, Hamel K, Bernstein M, Craik V, et al. Regulatory T-cells inhibit microglia-induced pain hypersensitivity in female mice. eLife. 2021;10. 10.7554/elife.69056. [DOI] [PMC free article] [PubMed]
- 175.Rosen SF, Ham B, Drouin S, Boachie N, Chabot-Dore AJ, Austin JS, et al. T-Cell mediation of pregnancy Analgesia Affecting Chronic Pain in mice. J Neurosci. 2017;37:41:9819–27. 10.1523/jneurosci.2053-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lopes DM, Malek N, Edye M, Jager SB, McMurray S, McMahon SB, et al. Sex differences in peripheral not central immune responses to pain-inducing injury. Sci Rep. 2017;7:116460. 10.1038/s41598-017-16664-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Guan Z, Kuhn JA, Wang X, Colquitt B, Solorzano C, Vaman S, et al. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci. 2016;19:1:94–101. 10.1038/nn.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Korczeniewska OA, Husain S, Khan J, Eliav E, Soteropoulos P, Benoliel R. Differential gene expression in trigeminal ganglia of male and female rats following chronic constriction of the infraorbital nerve. Eur J Pain. 2018;22:5875–88. 10.1002/ejp.1174. [DOI] [PubMed] [Google Scholar]
- 179.Kuhn JA, Vainchtein ID, Braz J, Hamel K, Bernstein M, Craik V, et al. Regulatory T-cells inhibit microglia-induced pain hypersensitivity in female mice. Elife. 2021;10:17. 10.7554/eLife.69056.sa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chen G, Luo X, Qadri MY, Berta T, Ji R-R. Sex-dependent Glial Signaling in Pathological Pain: distinct roles of spinal microglia and astrocytes. Neurosci Bull. 2018;34:1:98–108. 10.1007/s12264-017-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mapplebeck JCS, Beggs S, Salter MW. Sex differences in pain: a tale of two immune cells. Pain. 2016;157(2):S2–6. 10.1097/j.pain.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 182.Yu XB, Liu HJ, Hamel KA, Morvan MG, Yu S, Leff J, et al. Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat Commun. 2020;11:112. 10.1038/s41467-019-13839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sorge RE, Lacroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin J-S, Ritchie J, et al. Spinal cord toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci. 2011;31:43:15450–4. 10.1523/jneurosci.3859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gay NJ, Keith FJ. Drosophila toll and IL-1 receptor. Nature. 1991;351:6325:355–6. 10.1038/351355b0. [DOI] [PubMed] [Google Scholar]
- 185.Uematsu S, Akira S. Toll-like receptors (TLRs) and their ligands. Handb Exp Pharmacol. 2008;183:1–20. 10.1007/978-3-540-72167-3_1. [DOI] [PubMed] [Google Scholar]
- 186.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:7:499–511. 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 187.Taguchi T, Mitcham JL, Dower SK, Sims JE, Testa JR. Chromosomal localization of TIL, a gene encoding a protein related to the Drosophila transmembrane receptor toll, to human chromosome 4p14. Genomics. 1996;32(3):486–8. 10.1006/geno.1996.0150. [DOI] [PubMed] [Google Scholar]
- 188.Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosophila toll protein signals activation of adaptive immunity. Nature. 1997;388:6640:394–7. 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 189.Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Huang S, Rutkowsky JM, Snodgrass RG, Ono-Moore KD, Schneider DA, Newman JW, et al. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J Lipid Res. 2012;53:9. 10.1194/jlr.D029546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rocha DM, Caldas AP, Oliveira LL, Bressan J, Hermsdorff HH. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis. 2016;244:211–5. 10.1016/j.atherosclerosis.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 192.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:6:3916–24. 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 193.Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43:3. 10.1002/glia.10256. [DOI] [PubMed] [Google Scholar]
- 194.Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, et al. Pivotal role for neuronal toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104:34. 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:1115–22. 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 196.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, et al. Cutting edge: toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the lps gene product. J Immunol. 1999;162:7. [PubMed] [Google Scholar]
- 197.Lu Z, Li Y, Jin J, Zhang X, Lopes-Virella MF, Huang Y. Toll-like receptor 4 activation in microvascular endothelial cells triggers a robust inflammatory response and cross talk with mononuclear cells via interleukin-6. Arterioscler Thromb Vasc Biol. 2012;32:71696–706. 10.1161/ATVBAHA.112.251181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bhattacharyya S, Wang W, Qin W, Cheng K, Coulup S, Chavez S, et al. TLR4-dependent fibroblast activation drives persistent organ fibrosis in skin and lung. JCI Insight. 2018;3:13. 10.1172/jci.insight.98850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Min H, Cho WH, Lee H, Choi B, Kim YJ, Lee HK, et al. Association of TRPV1 and TLR4 through the TIR domain potentiates TRPV1 activity by blocking activation-induced desensitization. Mol Pain. 2018;14:1744806918812636. 10.1177/1744806918812636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Michot B, Casey SM, Lee CS, Erdogan O, Basu H, Chiu I, et al. Lipopolysaccharide-Induced TRPA1 upregulation in trigeminal neurons is dependent on TLR4 and vesicular exocytosis. J Neurosci. 2023;43:40:6731–44. 10.1523/jneurosci.0162-23.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Diogenes A, Ferraz C, Akopian Aa, Henry M, Hargreaves K. LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J Dent Res. 2011;90:6. [DOI] [PubMed] [Google Scholar]
- 202.Xie MX, Zhang XL, Xu J, Zeng WA, Li D, Xu T, et al. Nuclear factor-kappab gates na(v)1.7 channels in DRG neurons via protein-protein Interaction. iScience. 2019;19:623–33. 10.1016/j.isci.2019.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Guo LH, Schluesener HJ. The innate immunity of the central nervous system in chronic pain: the role of toll-like receptors. Cell Mol Life Sci. 2007;64:9:1128–36. 10.1007/s00018-007-6494-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Heiman A, Pallottie A, Heary RF, Elkabes S. Toll-like receptors in central nervous system injury and disease: a focus on the spinal cord. Brain Behav Immun. 2014;42:232–45. 10.1016/j.bbi.2014.06.203. [DOI] [PubMed] [Google Scholar]
- 205.Raghavendra V, Tanga FY, DeLeo JA. Complete freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20:2. 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- 206.Zhao XH, Zhang T, Li YQ. The up-regulation of spinal toll-like receptor 4 in rats with inflammatory pain induced by complete Freund’s adjuvant. Brain Res Bull. 2015;111:97–103. 10.1016/j.brainresbull.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 207.Agalave NM, Rudjito R, Farinotti AB, Khoonsari PE, Sandor K, Nomura Y, et al. Sex-dependent role of microglia in disulfide high mobility group box 1 protein-mediated mechanical hypersensitivity. Pain. 2021;162:2446–58. 10.1097/j.pain.0000000000002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Woller SA, Ocheltree C, Wong SY, Bui A, Fujita Y, dos Santos GG, et al. Neuraxial TNF and IFN-beta co-modulate persistent allodynia in arthritic mice. Brain Behav Immun. 2019;76:151–8. 10.1016/j.bbi.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Christianson CA, Dumlao DS, Stokes JA, Dennis EA, Svensson CI, Corr M, et al. Spinal TLR4 mediates the transition to a persistent mechanical hypersensitivity after the resolution of inflammation in serum-transferred arthritis. Pain. 2011;152:12:2881–91. 10.1016/j.pain.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zaks-Zilberman M, Zaks TZ, Vogel SN. Induction of proinflammatory and chemokine genes by lipopolysaccharide and paclitaxel (taxol) in murine and human breast cancer cell lines. Cytokine. 2001;15:3156–65. 10.1006/cyto.2001.0935. [DOI] [PubMed] [Google Scholar]
- 211.O’Brien JM, Wewers MD, Moore SA, Allen JN. Taxol and colchicine increase LPS-induced pro-IL-1 beta production, but do not increase IL-1 beta secretion. A role for microtubules in the regulation of IL-1 beta production. J Immunol. 1995;154:8. https://www.ncbi.nlm.nih.gov/pubmed/7706748. [PubMed] [Google Scholar]
- 212.Li Y, Zhang H, Kosturakis AK, Jawad AB, Dougherty PM. Toll-like receptor 4 signaling contributes to Paclitaxel-induced peripheral neuropathy. J Pain. 2014;15:7. 10.1016/j.jpain.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Illias AM, Yu KJ, Hwang SH, Solis J, Zhang H, Velasquez JF, et al. Dorsal root ganglion toll-like receptor 4 signaling contributes to oxaliplatin-induced peripheral neuropathy. Pain. 2022;163:5923–35. 10.1097/j.pain.0000000000002454. [DOI] [PubMed] [Google Scholar]
- 214.Araldi D, Bogen O, Green PG, Levine JD. Role of nociceptor toll-like receptor 4 (TLR4) in opioid-induced hyperalgesia and hyperalgesic priming. J Neurosci. 2019;39:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mercadante S, Arcuri E, Santoni A. Opioid-induced tolerance and hyperalgesia. CNS Drugs. 2019;33:943–55. 10.1007/s40263-019-00660-0. [DOI] [PubMed] [Google Scholar]
- 216.Weber L, Yeomans DC, Tzabazis A. Opioid-induced hyperalgesia in clinical anesthesia practice: what has remained from theoretical concepts and experimental studies? Curr Opin Anaesthesiol. 2017;30:4458–65. 10.1097/ACO.0000000000000485. [DOI] [PubMed] [Google Scholar]
- 217.Li J, Csakai A, Jin J, Zhang F, Yin H. Therapeutic developments targeting toll-like receptor-4-Mediated Neuroinflammation. ChemMedChem. 2016;11:2. 10.1002/cmdc.201500188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ellis A, Grace PM, Wieseler J, Favret J, Springer K, Skarda B, et al. Morphine amplifies mechanical allodynia via TLR4 in a rat model of spinal cord injury. Brain Behav Immun. 2016;58:348–56. 10.1016/j.bbi.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 2005;102:16:5856–61. 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, et al. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4). Eur J Neurosci. 2008;28:120–9. 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bettoni I, Comelli F, Rossini C, Granucci F, Giagnoni G, Peri F, et al. Glial TLR4 receptor as new target to treat neuropathic pain: efficacy of a new receptor antagonist in a model of peripheral nerve injury in mice. Glia. 2008;56:12:1312–9. 10.1002/glia.20699. [DOI] [PubMed] [Google Scholar]
- 222.Bruno K, Woller SA, Miller YI, Yaksh TL, Wallace M, Beaton G, et al. Targeting toll-like receptor-4 (TLR4)-an emerging therapeutic target for persistent pain states. Pain. 2018;159:10. 10.1097/j.pain.0000000000001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Woller SA, Ravula SB, Tucci FC, Beaton G, Corr M, Isseroff RR, et al. Systemic TAK-242 prevents intrathecal LPS evoked hyperalgesia in male, but not female mice and prevents delayed allodynia following intraplantar formalin in both male and female mice: the role of TLR4 in the evolution of a persistent pain state. Brain Behav Immun. 2016;56:271–80. 10.1016/j.bbi.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Stokes JA, Cheung J, Eddinger K, Corr M, Yaksh TL. Toll-like receptor signaling adapter proteins govern spread of neuropathic pain and recovery following nerve injury in male mice. J Neuroinflammation. 2013;10:148. 10.1186/1742-2094-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ghosh C, Bishayi B. Characterization of toll-like Receptor-4 (TLR-4) in the Spleen and Thymus of Swiss albino mice and its modulation in experimental endotoxemia. J Immunol Res. 2015;2015:13. 10.1155/2015/137981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, et al. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999;80(1–2):67–82. 10.1016/s0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]
- 227.Qureshi ST, Larivière L, Leveque G, Clermont S, Moore KJ, Gros P, et al. Endotoxin-tolerant mice have mutations in toll-like receptor 4 (< i > Tlr4). J Exp Med. 1999;189:4615–25. 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, et al. Cutting edge:: toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide:: evidence for TLR4 as the < i > lps gene product. J Immunol. 1999;162:7. ://WOS:000079278000001. [PubMed] [Google Scholar]
- 229.Nedrebo T, Reed RK. Different serotypes of endotoxin (lipopolysaccharide) cause different increases in albumin extravasation in rats. Shock. 2002;18:2. 10.1097/00024382-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 230.Akarsu ES, Mamuk S. Escherichia coli lipopolysaccharides produce serotype-specific hypothermic response in biotelemetered rats. Am J Physiol-Regul Integr Comp Physiol. 2007;292(5):R1846–50. 10.1152/ajpregu.00786.2006. [DOI] [PubMed] [Google Scholar]
- 231.Migale R, Herbert BR, Lee YS, Sykes L, Waddington SN, Peebles D, et al. Specific lipopolysaccharide serotypes induce Differential maternal and neonatal inflammatory responses in a murine model of Preterm Labor. Am J Pathol. 2015;185:92390–401. 10.1016/j.ajpath.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Huck NA, Siliezar-Doyle J, Haight ES, Ishida R, Forman TE, Wu S, et al. Temporal contribution of myeloid-lineage TLR4 to the transition to Chronic Pain: a focus on sex differences. J Neurosci. 2021;41:19:4349–65. 10.1523/JNEUROSCI.1940-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Alexander SN, Reed OA, Burton MD. Spinal cord microglia drive sex differences in ethanol-mediated PGE2-induced allodynia. Brain Behav Immun. 2024;122:399–421. 10.1016/j.bbi.2024.08.026. [DOI] [PubMed] [Google Scholar]
- 234.Kwak MS, Kim HS, Lee B, Kim YH, Son M, Shin JS. Immunological significance of HMGB1 post-translational modification and Redox Biology. Front Immunol. 2020;11:1189. 10.3389/fimmu.2020.01189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995;270:43:25752–61. 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- 236.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:9:7370–7. 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 237.Thorburn J, Horita H, Redzic J, Hansen K, Frankel AE, Thorburn A. Autophagy regulates selective HMGB1 release in tumor cells that are destined to die. Cell Death Differ. 2009;16:1175–83. 10.1038/cdd.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Qin S, Wang H, Yuan R, Li H, Ochani M, Ochani K, et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med. 2006;203:7:1637–42. 10.1084/jem.20052203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:6894:191–5. 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 240.Venereau E, Schiraldi M, Uguccioni M, Bianchi ME. HMGB1 and leukocyte migration during trauma and sterile inflammation. Mol Immunol. 2013;55:1:76–82. 10.1016/j.molimm.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 241.Yamasoba D, Tsubota M, Domoto R, Sekiguchi F, Nishikawa H, Liu K, et al. Peripheral HMGB1-induced hyperalgesia in mice: Redox state-dependent distinct roles of RAGE and TLR4. J Pharmacol Sci. 2016;130:2139–42. 10.1016/j.jphs.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 242.Müller S, Ronfani L, Bianchi ME. Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokine function. J Intern Med. 2004;255. 10.1111/j.1365-2796.2003.01296.x. :3:332 – 43. [DOI] [PubMed]
- 243.Shibasaki M, Sasaki M, Miura M, Mizukoshi K, Ueno H, Hashimoto S, et al. Induction of high mobility group box-1 in dorsal root ganglion contributes to pain hypersensitivity after peripheral nerve injury. Pain. 2010;149:3514–21. 10.1016/j.pain.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 244.Feldman P, Due MR, Ripsch MS, Khanna R, White FA. The persistent release of HMGB1 contributes to tactile hyperalgesia in a rodent model of neuropathic pain. J Neuroinflammation. 2012;9:180. 10.1186/1742-2094-9-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Agalave NM, Larsson M, Abdelmoaty S, Su J, Baharpoor A, Lundback P, et al. Spinal HMGB1 induces TLR4-mediated long-lasting hypersensitivity and glial activation and regulates pain-like behavior in experimental arthritis. Pain. 2014;155:9:1802–13. 10.1016/j.pain.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 246.Rudjito R, Agalave NM, Farinotti AB, Lundback P, Szabo-Pardi TA, Price TJ, et al. Sex- and cell-dependent contribution of peripheral high mobility group box 1 and TLR4 in arthritis-induced pain. Pain. 2021;162:2459–70. 10.1097/j.pain.0000000000002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Yang H, Zeng Q, Silverman HA, Gunasekaran M, George SJ, Devarajan A, et al. HMGB1 released from nociceptors mediates inflammation. Proc Natl Acad Sci U S A. 2021;118:33. 10.1073/pnas.2102034118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lenert ME, Avona A, Garner KM, Barron LR, Burton MD. Sensory neurons, Neuroimmunity, and Pain Modulation by Sex hormones. Endocrinology. 2021;162:817. 10.1210/endocr/bqab109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Payrits M, Sághy É, Csekő K, Pohóczky K, Bölcskei K, Ernszt D, et al. Estradiol sensitizes the Transient Receptor Potential Vanilloid 1 receptor in Pain responses. Endocrinology. 2017;158:10:3249–58. 10.1210/en.2017-00101. [DOI] [PubMed] [Google Scholar]
- 250.Seol SH, Chung G. Review article Estrogen-dependent regulation of transient receptor potential vanilloid 1 (TRPV1) and P2X purinoceptor 3 (P2X3): Implication in burning mouth syndrome. J Dent Sci. 2022;17(1):8–13. 10.1016/j.jds.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Cho T, Chaban VV. Expression of P2X3 and TRPV1 receptors in primary sensory neurons from estrogen receptors-alpha and estrogen receptor-beta knockout mice. NeuroReport. 2012;23:9:530–4. 10.1097/WNR.0b013e328353fabc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Chen SC, Chang TJ, Wu FS. Competitive inhibition of the capsaicin receptor-mediated current by dehydroepiandrosterone in rat dorsal root ganglion neurons. J Pharmacol Exp Ther. 2004;311:2. 10.1124/jpet.104.069096. [DOI] [PubMed] [Google Scholar]
- 253.Mendez-Resendiz KA, Enciso-Pablo O, Gonzalez-Ramirez R, Juarez-Contreras R, Rosenbaum T, Morales-Lazaro SL. Steroids and TRP channels: a close relationship. Int J Mol Sci. 2020;21:1136. 10.3390/ijms21113819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Apkhazava M, Kvachadze I, Tsagareli M, Mzhavanadze D, Chakhnashvili M. The Relationship between Thermal Pain Sensation, Free Testosterone, TRPV1, MOR levels and various degrees of hostility in Young Healthy males. Georgian Med News. 2018;283(109–14). ://MEDLINE:30516504. [PubMed]
- 255.Desai MK, Brinton RD. Autoimmune disease in women: endocrine transition and risk across the Lifespan. Front Endocrinol. 2019;10:19. 10.3389/fendo.2019.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Liva SM, Voskuhl RR. Testosterone acts directly on CD4(+) T lymphocytes to increase IL-10 production. J Immunol. 2001;167:4. 10.4049/jimmunol.167.4.2060. [DOI] [PubMed] [Google Scholar]
- 257.Angum F, Khan T, Kaler J, Siddiqui L, Hussain A. The prevalence of Autoimmune disorders in women: a narrative review. Cureus. 2020. 10.7759/cureus.8094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111:1. 10.1093/bja/aet127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lesnak JB, Inoue S, Lima L, Rasmussen L, Sluka KA. Testosterone protects against the development of widespread muscle pain in mice. Pain. 2020;161:122898–908. 10.1097/j.pain.0000000000001985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sorge RE, Mapplebeck JCS, Rosen S, Beggs S, Taves S, Alexander JK, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18:81081–. 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Rosen S, Ham B, Mogil JS. Sex differences in neuroimmunity and pain. J Neurosci Res. 2017;95(1–2):500–8. 10.1002/jnr.23831. [DOI] [PubMed] [Google Scholar]
- 262.Edinger KL, Frye CA. Testosterone’s analgesic, anxiolytic, and cognitive-enhancing effects may be due in part to actions of its 5 alpha-reduced metabolites in the hippocampus. Behav Neurosci. 2004;118:6:1352–64. 10.1037/0735-7044.118.6.1352. [DOI] [PubMed] [Google Scholar]
- 263.Edinger KL, Frye CA. Testosterone’s anti-anxiety and analgesic effects may be due in part to actions of its 5 alpha-reduced metabolites in the hippocampus. Psychoneuroendocrinology. 2005;30:5. 10.1016/j.psyneuen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 264.Lee KS, Zhang Y, Asgar J, Auh Q-S, Chung M-K, Ro JY. Androgen receptor transcriptionally regulates µ-opioid receptor expression in rat trigeminal ganglia. Neuroscience. 2016;331:52–61. 10.1016/j.neuroscience.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Bai X, Zhang X, Zhou Q. Effect of testosterone on TRPV1 expression in a model of Orofacial Myositis Pain in the rat. J Mol Neurosci. 2018;64:1:93–101. 10.1007/s12031-017-1009-7. [DOI] [PubMed] [Google Scholar]
- 266.Ashraf S, Nabi M, Rasool SU, Rashid F, Amin S. Hyperandrogenism in polycystic ovarian syndrome and role of CYP gene variants: a review. Egypt J Med Hum. 2019;20:1. doi:https://doi.org/ARTN251186/s43042-019-0031-4.
- 267.Williams T, Mortada R, Porter S. Diagnosis and treatment of polycystic ovary syndrome. Am Fam Physician. 2016;94(2):106–13. https://www.ncbi.nlm.nih.gov/pubmed/27419327. [PubMed] [Google Scholar]
- 268.Rudnicka E, Suchta K, Grymowicz M, Calik-Ksepka A, Smolarczyk K, Duszewska AM, et al. Chronic low Grade inflammation in Pathogenesis of PCOS. Int J Mol Sci. 2021;22:7. 10.3390/ijms22073789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:7:3313–8. 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 270.Lee H-R, Kim T-H, Choi K-C. Functions and physiological roles of two types of estrogen receptors, ERalpha and ERbeta, identified by estrogen receptor knockout mouse. Lab Anim Res. 2012;28:2. 10.5625/lar.2012.28.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Villa A, Rizzi N, Vegeto E, Ciana P, Maggi A. Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci Rep. 2015;5:14. 10.1038/srep15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Nakamura TJ, Sellix MT, Menaker M, Block GD. Estrogen directly modulates circadian rhythms of PER2 expression in the uterus. Am J Physiol-Endocrinol Metab. 2008;295(5):E1025–31. 10.1152/ajpendo.90392.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Chen Q, Zhang W, Sadana N, Chen X. Estrogen receptors in pain modulation: cellular signaling. Biology Sex Differences. 2021;12:1. 10.1186/s13293-021-00364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Evrard HC. Estrogen synthesis in the spinal dorsal horn: a new central mechanism for the hormonal regulation of pain. Am J Physiol-Regul Integr Comp Physiol. 2006;291(2):R291–9. 10.1152/ajpregu.00930.2005. [DOI] [PubMed] [Google Scholar]
- 275.Li L, Fan XT, Warner M, Xu XJ, Gustafsson JA, Wiesenfeld-Hallin Z. Ablation of estrogen receptor alpha or beta eliminates sex differences in mechanical pain threshold in normal and inflamed mice. Pain. 2009;143(1–2):37–40. 10.1016/j.pain.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 276.Coulombe MA, Spooner MF, Gaumond I, Carrier JC, Marchand S. Estrogen receptors beta and alpha have specific pro- and anti-nociceptive actions. Neuroscience. 2011;184:172–82. 10.1016/j.neuroscience.2011.02.057. [DOI] [PubMed] [Google Scholar]
- 277.Zhong YQ, Li KC, Zhang X. Potentiation of excitatory transmission in substantia gelatinosa neurons of rat spinal cord by inhibition of estrogen receptor alpha. Mol Pain. 2010;6:5. 10.1186/1744-8069-6-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Tang B, Ji YP, Traub RJ. Estrogen alters spinal NMDA receptor activity via a PKA signaling pathway in a visceral pain model in the rat. Pain. 2008;137:3:540–9. 10.1016/j.pain.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:3905–31. 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 280.Shughrue PJ, Scrimo PJ, Merchenthaler I. Evidence for the colocalization of estrogen receptor-beta mRNA and estrogen receptor-alpha immunoreactivity in neurons of the rat forebrain. Endocrinology. 1998;139:12:5267–70. 10.1210/en.139.12.5267. [DOI] [PubMed] [Google Scholar]
- 281.Vrtačnik P, Ostanek B, Mencej-Bedrač S, Marc J. The many faces of estrogen signaling. Biochemia Med. 2014;24:3329–42. 10.11613/bm.2014.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Smith YR, Stohler CS, Nichols TE, Bueller JA, Koeppe RA, Zubieta JK. Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J Neurosci. 2006;26:21:5777–85. 10.1523/jneurosci.5223-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Herrera AY, Mather M. Actions and interactions of estradiol and glucocorticoids in cognition and the brain: implications for aging women. Neurosci Biobehav Rev. 2015;55:36–52. 10.1016/j.neubiorev.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Wu Y-W, Bi Y-P, Kou X-X, Xu W, Ma L-Q, Wang K-W, et al. 17- -Estradiol enhanced Allodynia of Inflammatory Temporomandibular Joint through Upregulation of hippocampal TRPV1 in Ovariectomized rats. J Neurosci. 2010;30:26:8710–9. 10.1523/jneurosci.6323-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Yamagata K, Sugimura M, Yoshida M, Sekine S, Kawano A, Oyamaguchi A, et al. Estrogens Exacerbate Nociceptive Pain via Up-Regulation of TRPV1 and ANO1 in trigeminal primary neurons of female rats. Endocrinology. 2016;157:11:4309–17. 10.1210/en.2016-1218. [DOI] [PubMed] [Google Scholar]
- 286.Paredes S, Cantillo S, Candido KD, Knezevic NN. An Association of Serotonin with Pain disorders and its modulation by Estrogens. Int J Mol Sci. 2019;20:225729. 10.3390/ijms20225729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Mulak A, Tache Y, Larauche M. Sex hormones in the modulation of irritable bowel syndrome. World J Gastroenterol. 2014;20:10:2433–48. 10.3748/wjg.v20.i10.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Shah NM, Lai PF, Imami N, Johnson MR. Progesterone-related Immune modulation of pregnancy and labor. Front Endocrinol. 2019;10:19. 10.3389/fendo.2019.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Oettel M, Mukhopadhyay AK. Progesterone: the forgotten hormone in men? Aging Male. 2004;7:3236–57. 10.1080/13685530400004199. [DOI] [PubMed] [Google Scholar]
- 290.Hall OJ, Klein SL. Progesterone-based compounds affect immune responses and susceptibility to infections at diverse mucosal sites. Mucosal Immunol. 2017;10:5. 10.1038/mi.2017.35. [DOI] [PubMed] [Google Scholar]
- 291.Arruvito L, Giulianelli S, Flores AC, Paladino N, Barboza M, Lanari C, et al. NK cells expressing a progesterone receptor are susceptible to progesterone-induced apoptosis. J Immunol. 2008;180:8. 10.4049/jimmunol.180.8.5746. [DOI] [PubMed] [Google Scholar]
- 292.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:10. 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 293.Butts CL, Shukair SA, Duncan KM, Bowers E, Horn C, Belyavskaya E, et al. Progesterone inhibits mature rat dendritic cells in a receptor-mediated fashion. Int Immunol. 2007;19:3287–96. 10.1093/intimm/dxl145. [DOI] [PubMed] [Google Scholar]
- 294.Jones LA, Kreem S, Shweash M, Paul A, Alexander J, Roberts CW. Differential modulation of TLR3- and TLR4-mediated dendritic cell maturation and function by progesterone. J Immunol. 2010;185:8:4525–34. 10.4049/jimmunol.0901155. [DOI] [PubMed] [Google Scholar]
- 295.Lei BL, Mace B, Dawson HN, Warner DS, Laskowitz DT, James ML. Anti-inflammatory effects of Progesterone in Lipopolysaccharide-stimulated BV-2 microglia. PLoS ONE. 2014;9:7. ; doi:https://doi.org/ARTNe1039691371/journal.pone.0103969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Shufelt CL, Merz CNB. Contraceptive hormone Use and Cardiovascular Disease. J Am Coll Cardiol. 2009;53:3221–31. 10.1016/j.jacc.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Allais G, Chiarle G, Sinigaglia S, Airola G, Schiapparelli P, Bergandi F, et al. Treating migraine with contraceptives. Neurol Sci. 2017;38:S85–9. 10.1007/s10072-017-2906-9. [DOI] [PubMed] [Google Scholar]
- 298.Matera L. Endocrine, paracrine and autocrine actions of prolactin on immune cells. Life Sci. 1996;59:8:599–614. 10.1016/0024-3205(96)00225-1. [DOI] [PubMed] [Google Scholar]
- 299.De Bellis A, Bizzarro A, Pivonello R, Lombardi G, Bellastella A. Prolactin and autoimmunity. Pituitary. 2005;8:1:25–30. 10.1007/s11102-005-5082-5. [DOI] [PubMed] [Google Scholar]
- 300.Gorvin CM. The prolactin receptor: diverse and emerging roles in pathophysiology. J Clin Transl Endocrinol. 2015;2:3:85–91. 10.1016/j.jcte.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Ben-Jonathan N, Mershon JL, Allen DL, Steinmetz RW. Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocr Rev. 1996;17:6. 10.1210/edrv-17-6-639. [DOI] [PubMed] [Google Scholar]
- 302.Mason BN, Kallianpur R, Price TJ, Akopian AN, Dussor GO. Prolactin signaling modulates stress-induced behavioral responses in a preclinical mouse model of migraine. Headache. 2022;62:1:11–25. 10.1111/head.14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Gazerani P. A link between migraine and prolactin: the way forward. Future Sci OA. 2021;7. 10.2144/fsoa-2021-0047. 9:Fso748. [DOI] [PMC free article] [PubMed]
- 304.Bussone G, Usai S, Moschiano F. How to investigate and treat: Headache and Hyperprolactinemia. Curr Pain Headache Rep. 2012;16:4365–70. 10.1007/s11916-012-0267-x. [DOI] [PubMed] [Google Scholar]
- 305.Strebel PM, Zacur HA, Gold EB, Headache, hyperprolactinemia andprolactinomas. Obstet Gynecol. 1986;68:2:195–9. ://WOS:A1986D432000011. [PubMed] [Google Scholar]
- 306.Bosco D, Belfiore A, Fava A, De Rose M, Plastino M, Ceccotti C, et al. Relationship between high prolactine levels and migraine attacks in patients with microprolactinoma. J Headache Pain. 2008;9:2. 10.1007/s10194-008-0016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Noori-Zadeh A, Karamkhani M, Seidkhani-Nahal A, Khosravi A, Darabi S. Evidence for hyperprolactinemia in migraineurs: a systematic review and meta-analysis. Neurol Sci. 2020;41:1. 10.1007/s10072-019-04035-7. [DOI] [PubMed] [Google Scholar]
- 308.Avona A, Mason BN, Burgos-Vega C, Hovhannisyan AH, Belugin SN, Mecklenburg J, et al. Meningeal CGRP-Prolactin Interaction evokes female-specific migraine behavior. Ann Neurol. 2021;89:6:1129–44. 10.1002/ana.26070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Shelly S, Boaz M, Orbach H. Prolactin and autoimmunity. Autoimmun Rev. 2012;11:6–7. 10.1016/j.autrev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 310.Patil M, Belugin S, Mecklenburg J, Wangzhou A, Paige C, Barba-Escobedo PA, et al. Prolactin regulates Pain responses via a female-selective nociceptor-specific mechanism. iScience. 2019;20:449–65. 10.1016/j.isci.2019.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Mecklenburg J, Wangzhou A, Hovhannisyan AH, Barba-Escobedo P, Shein SA, Zou Y, et al. Sex-dependent pain trajectories induced by prolactin require an inflammatory response for pain resolution. Brain Behav Immun. 2022;101:246–63. 10.1016/j.bbi.2022.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Paige C, Barba-Escobedo PA, Mecklenburg J, Patil M, Goffin V, Grattan DR, et al. Neuroendocrine mechanisms governing sex differences in Hyperalgesic Priming Involve Prolactin Receptor Sensory Neuron Signaling. J Neurosci. 2020;40:37:7080–90. 10.1523/JNEUROSCI.1499-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Lin Y, Wang Y, Li PF, PPARα. An emerging target of metabolic syndrome, neurodegenerative and cardiovascular diseases. Front Endocrinol (Lausanne). 2022;13:1074911. 10.3389/fendo.2022.1074911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Okine BN, Gaspar JC, Finn DP. PPARs and pain. Br J Pharmacol. 2019;176:10:1421–42. 10.1111/bph.14339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Toobian D, Ghosh P, Katkar GD. Parsing the role of PPARs in macrophage processes. Front Immunol. 2021;12:783780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Zhang MA, Rego D, Moshkova M, Kebir H, Chruscinski A, Nguyen H, et al. Peroxisome proliferator-activated receptor (PPAR)alpha and -gamma regulate IFN gamma and IL-17A production by human T cells in a sex-specific way. Proc Natl Acad Sci U S A. 2012;109:24:9505–10. 10.1073/pnas.1118458109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Barrett AC. Low efficacy opioids: implications for sex differences in opioid antinociception. Exp Clin Psychopharmacol. 2006;14:1:1–11. 10.1037/1064-1297.14.1.1. [DOI] [PubMed] [Google Scholar]
- 318.Niesters M, Dahan A, Kest B, Zacny J, Stijnen T, Aarts L, et al. Do sex differences exist in opioid analgesia? A systematic review and meta-analysis of human experimental and clinical studies. Pain. 2010;151:161–8. 10.1016/j.pain.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 319.Goetz TG, Becker JB, Mazure CM. Women, opioid use and addiction. Faseb J. 2021;35:2. 10.1096/fj.202002125R. [DOI] [PubMed] [Google Scholar]
- 320.Mazure CM, Fiellin DA. Women and opioids: something different is happening here. Lancet. 2018;392:10141. [DOI] [PubMed] [Google Scholar]
- 321.Sorge RE, Totsch SK. Sex differences in Pain. J Neurosci Res. 2017;95:6:1271–81. 10.1002/jnr.23841. [DOI] [PubMed] [Google Scholar]
- 322.Mogil JS. OPINION sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13:12859–66. 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- 323.Serrao M, Rossi P, Sandrini G, Amabile GA, Nappi G, Pierelli F. Effects of diffuse noxious inhibitory controls on temporal summation of the RIII reflex in humans. Pain. 2004;112:3. 10.1016/j.pain.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 324.Weissman-Fogel I, Sprecher E, Pud D. Effects of catastrophizing on pain perception and pain modulation. Exp Brain Res. 2008;186:1:79–85. 10.1007/s00221-007-1206-7. [DOI] [PubMed] [Google Scholar]
- 325.Vina ER, Ran D, Ashbeck EL, Kwoh CK. Widespread Pain is Associated with increased risk of no clinical improvement after TKA in women. Clin Orthop Rel Res. 2020;478:7:1453–65. 10.1097/corr.0000000000001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Patel B, Eskander MA, Fang-Mei Chang P, Chapa B, Ruparel SB, Lai Z, et al. Understanding painful versus non-painful dental pain in female and male patients: a transcriptomic analysis of human biopsies. PLoS ONE. 2023;18:9e0291724. 10.1371/journal.pone.0291724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.See LP, Sripinun P, Lu W, Li J, Alboloushi N, Alvarez-Periel E, et al. Increased Purinergic Signaling in Human Dental Pulps with Inflammatory Pain is sex-dependent. J Pain. 2024;25:4:1039–58. 10.1016/j.jpain.2023.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Kriegova E, Manukyan G, Mikulkova Z, Gabcova G, Kudelka M, Gajdos P, et al. Gender-related differences observed among immune cells in synovial fluid in knee osteoarthritis. Osteoarthritis Cartilage. 2018;26:9:1247–56. 10.1016/j.joca.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 329.Keselman A, Fang X, White PB, Heller NM. Estrogen Signaling Contributes to sex differences in macrophage polarization during Asthma. J Immunol. 2017;199:5:1573–83. 10.4049/jimmunol.1601975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Li K, Xu W, Guo Q, Jiang Z, Wang P, Yue Y, et al. Differential macrophage polarization in male and female BALB/c mice infected with coxsackievirus B3 defines susceptibility to viral myocarditis. Circ Res. 2009;105:4353–64. 10.1161/CIRCRESAHA.109.195230. [DOI] [PubMed] [Google Scholar]
- 331.Becerra-Diaz M, Lerner AD, Yu DH, Thiboutot JP, Liu MC, Yarmus LB, et al. Sex differences in M2 polarization, chemokine and IL-4 receptors in monocytes and macrophages from asthmatics. Cell Immunol. 2021;360:10. 10.1016/j.cellimm.2020.104252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Rozen TD, Fishman RS. Female cluster headache in the United States of America: what are the gender differences? Results from the United States Cluster Headache Survey. J Neurol Sci. 2012;317(1–2):17–28. 10.1016/j.jns.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 333.Gutiérrez-Albaladejo N, López-de-Andrés A, Cuadrado-Corrales N, Albaladejo-Vicente R, Villanueva-Orbaiz R, Carricondo F, et al. Asthma is Associated with Back Pain and Migraine-results of Population-based case-control study. J Clin Med. 2023;12:22. 10.3390/jcm12227107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Avona A, Burgos-Vega C, Burton MD, Akopian AN, Price TJ, Dussor G. Dural Calcitonin Gene-related peptide produces female-specific responses in Rodent Migraine models. J Neurosci. 2019;39:22:4323–31. 10.1523/JNEUROSCI.0364-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Wattiez AS, Sowers LP, Russo AF. Calcitonin gene-related peptide (CGRP): role in migraine pathophysiology and therapeutic targeting. Expert Opin Ther Targets. 2020;24:2:91–100. 10.1080/14728222.2020.1724285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Deen M, Correnti E, Kamm K, Kelderman T, Papetti L, Rubio-Beltran E, et al. Blocking CGRP in migraine patients - a review of pros and cons. J Headache Pain. 2017;18:196. 10.1186/s10194-017-0807-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Taylor RW, Grant AM, Williams SM, Goulding A. Sex differences in Regional Body Fat distribution from pre- to Postpuberty. Obesity. 2010;18:7:1410–6. 10.1038/oby.2009.399. [DOI] [PubMed] [Google Scholar]
- 338.Berwal D, Branisteanu DD, Glickman M, Sagar A, Pilitsis JG. The sex-dependent impact of adipose tissue and inflammation on chronic pain - A cross-sectional study from the all of us research program. Cytokine. 2024;179:156614. 10.1016/j.cyto.2024.156614. [DOI] [PubMed] [Google Scholar]
- 339.Cartier A, Cote M, Lemieux I, Perusse L, Tremblay A, Bouchard C, et al. Sex differences in inflammatory markers: what is the contribution of visceral adiposity? Am J Clin Nutr. 2009;89:5:1307–14. 10.3945/ajcn.2008.27030. [DOI] [PubMed] [Google Scholar]
- 340.Varghese M, Griffin C, McKernan K, Eter L, Lanzetta N, Agarwal D, et al. Sex differences in inflammatory responses to adipose tissue lipolysis in Diet-Induced obesity. Endocrinology. 2019;160:2:293–312. 10.1210/en.2018-00797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Kim JJ, Sears DD. TLR4 and insulin resistance. Gastroenterol Res Pract. 2010;2010:1–11. 10.1155/2010/212563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Tramullas M, Finger BC, Dinan TG, Cryan JF. Obesity takes its toll on Visceral Pain: high-Fat Diet induces toll-like receptor 4-Dependent visceral hypersensitivity. PLoS ONE. 2016;11:5e0155367. 10.1371/journal.pone.0155367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:11:3015–25. 10.1172/jci28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Vitseva OI, Tanriverdi K, Tchkonia TT, Kirkland JL, McDonnell ME, Apovian CM, et al. Inducible toll-like receptor and NF-kappaB regulatory pathway expression in human adipose tissue. Obes (Silver Spring). 2008;16:5. 10.1038/oby.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Tighe PJ, Riley JL 3rd, Fillingim RB. Sex differences in the incidence of severe pain events following surgery: a review of 333,000 pain scores. Pain Med. 2014;15:81390–404. 10.1111/pme.12498. [DOI] [PMC free article] [PubMed]
- 346.Bjørnnes AK, Parry M, Lie I, Fagerland MW, Watt-Watson J, Rustøen T, et al. Pain experiences of men and women after cardiac surgery. J Clin Nurs. 2016;25:19–20. 10.1111/jocn.13329. [DOI] [PubMed] [Google Scholar]
- 347.Chung J, Stevens LM, Ouzounian M, El-Hamamsy I, Bouhout I, Dagenais F, et al. Sex-related differences in patients undergoing thoracic aortic surgery. Circulation. 2019;139:9:1177–84. 10.1161/circulationaha.118.035805. [DOI] [PubMed] [Google Scholar]
- 348.Moscarelli M, Lorusso R, Angelini GD, Di Bari N, Paparella D, Fattouch K, et al. Sex-specific differences and postoperative outcomes of minimally invasive and sternotomy valve surgery. Eur J Cardiothorac Surg. 2022;61:3:695–702. 10.1093/ejcts/ezab369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Nandi M, Schreiber KL, Martel MO, Cornelius M, Campbell CM, Haythornthwaite JA, et al. Sex differences in negative affect and postoperative pain in patients undergoing total knee arthroplasty. Biol Sex Differ. 2019;10:123. 10.1186/s13293-019-0237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Dos Santos NL, Lenert ME, Castillo ZW, Mody PH, Thompson LT, Burton MD. Age and sex drive differential behavioral and neuroimmune phenotypes during postoperative pain. Neurobiol Aging. 2023;123:129–44. 10.1016/j.neurobiolaging.2022.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Verriotis M, Jones L, Whitehead K, Laudiano-Dray M, Panayotidis I, Patel H, et al. The distribution of pain activity across the human neonatal brain is sex dependent. NeuroImage. 2018;178:69–77. 10.1016/j.neuroimage.2018.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Tan JS, Tikoft E, O’Sullivan P, Smith A, Campbell A, Caneiro JP, Kent P. The relationship between changes in movement and activity limitation or pain in people with knee osteoarthritis: a systematic review. J Orthop Sports Phys Ther. 2021;1:10. 10.2519/jospt.2021.10418 [DOI] [PubMed]
- 353.Astephen Wilson JL, Dunbar MJ, Hubley-Kozey CL. Knee joint biomechanics and neuromuscular control during gait before and after total knee arthroplasty are sex-specific. J Arthroplasty. 2015;30:1. 10.1016/j.arth.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 354.Valdrighi N, Vago JP, Blom AB, van de Loo FAJ, Blaney Davidson EN. Innate immunity at the core of sex differences in Osteoarthritic Pain? Front Pharmacol. 2022;13:881500. 10.3389/fphar.2022.881500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Mun CJ, Letzen JE, Nance S, Smith MT, Khanuja HS, Sterling RS, et al. Sex differences in Interleukin-6 responses over Time following Laboratory Pain Testing among patients with knee osteoarthritis. J Pain. 2020;21:5–6. 10.1016/j.jpain.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Kim JR, Kim HA. Molecular mechanisms of sex-related differences in arthritis and Associated Pain. Int J Mol Sci. 2020;21:21. 10.3390/ijms21217938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Pan Q, O’Connor MI, Coutts RD, Hyzy SL, Olivares-Navarrete R, Schwartz Z, et al. Characterization of osteoarthritic human knees indicates potential sex differences. Biol Sex Differ. 2016;7:27. 10.1186/s13293-016-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Kosek E, Finn A, Ultenius C, Hugo A, Svensson C, Ahmed AS. Differences in neuroimmune signalling between male and female patients suffering from knee osteoarthritis. J Neuroimmunol. 2018;321:49–60. 10.1016/j.jneuroim.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 359.Solheim N, Östlund S, Gordh T, Rosseland LA. Women report higher pain intensity at a lower level of inflammation after knee surgery compared with men. Pain Rep. 2017;2:3e595. 10.1097/pr9.0000000000000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Perruccio AV, Badley EM, Power JD, Canizares M, Kapoor M, Rockel J, et al. Sex differences in the relationship between individual systemic markers of inflammation and pain in knee osteoarthritis. Osteoarthr Cartil Open. 2019;1(1–2):100004. 10.1016/j.ocarto.2019.100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not Applicable.