Abstract
The nonstructural (ns) proteins nsP1 to -4, the components of Semliki Forest virus (SFV) RNA polymerase, were localized in infected cells by confocal microscopy using double labeling with specific antisera against the individual ns proteins. All ns proteins were associated with large cytoplasmic vacuoles (CPV), the inner surfaces of which were covered by small invaginations, or spherules, typical of alphavirus infection. All ns proteins were localized by immuno-electron microscopy (EM) to the limiting membranes of CPV and to the spherules, together with newly labeled viral RNA. Along with earlier observations by EM-autoradiography (P. M. Grimley, I. K. Berezesky, and R. M. Friedman, J. Virol. 2:326–338, 1968), these results suggest that individual spherules represent template-associated RNA polymerase complexes. Immunoprecipitation of radiolabeled ns proteins showed that each antiserum precipitated the other three ns proteins, implying that they functioned as a complex. Double labeling with organelle-specific and anti-ns-protein antisera showed that CPV were derivatives of late endosomes and lysosomes. Indeed, CPV frequently contained endocytosed bovine serum albumin-coated gold particles, introduced into the medium at different times after infection. With time, increasing numbers of spherules were also observed on the cell surfaces; they were occasionally released into the medium, probably by secretory lysosomes. We suggest that the spherules arise by primary assembly of the RNA replication complexes at the plasma membrane, guided there by nsP1, which has affinity to lipids specific for the cytoplasmic leaflet of the plasma membrane. Endosomal recycling and fusion of CPV with the plasma membrane can circulate spherules between the plasma membrane and the endosomal-lysosomal compartment.
The replication of alphaviruses, such as Semliki Forest virus (SFV) and Sindbis virus, has been studied intensively over more than 30 years. These positive-strand, enveloped RNA viruses replicate in the cytoplasms of a variety of vertebrate and insect cells (20, 52). Early electron microscopic (EM) studies of infected cells (1, 14, 17, 18) identified large vacuoles lined on their inner surfaces with small vesicular invaginations, or spherules, with a diameter of about 50 nm. The spherules each had a single membrane, which was continuous with the limiting membrane of the surrounding vacuole. The spherules showed an irregular dark central spot in an otherwise lightly stained interior. In a brilliant study by Grimley et al. (17) the large vacuoles in SFV-infected chicken embryo fibroblasts were defined as type I cytoplasmic vacuoles (CPV-I). Their diameters ranged from 600 to 2,000 nm, and they appeared early in infection. EM autoradiography of cells pulse-labeled with tritiated uridine revealed that RNA synthesis took place in CPV-I (more specifically, in the spherules, which were often also found on the cell surface). The CPV-I were characterized further by Grimley et al. (18), who found them in several different cell lines infected with other alphaviruses. As an indication of the involvement of lysosomes, the authors reported association of acid phosphatase activity in many CPV-I. The CPV-I were found in an isolated membrane fraction, which was enriched in pulse-labeled viral RNA and had RNA polymerase activity in vitro. However, their origin and relationship with cellular organelles remained unsolved (14, 18).
An important clue to the origin of CPV-I, hereafter referred to as CPV, was supplied by Froshauer et al. (15). They described in more detail the spherules, which had electron-dense plugs, sometimes connected to electron-dense material extending into the cytoplasm. This material often contained granules which looked like ribosomes and nucleocapsids, suggesting that nascent RNA molecules protruding from the spherules were probably utilized for translation and nucleocapsid assembly. The endosomal origin of CPV was demonstrated by using endosomal tracers, such as cationic ferritin and horseradish peroxidase, added to the medium during virus replication. The authors proposed that CPV were derived from endosomes and lysosomes, in which the incoming virus particles had entered the cell. The increased number of CPV during infection was interpreted as a result of superinfection by released virus particles.
Some of the conclusions of Froshauer et al. (15) were challenged by Peränen and Kääriäinen (39). By using a temperature-sensitive ts1 mutant of SFV at the restrictive temperature, it was shown that the time of appearance, but not the number, of CPV was dependent on the multiplicity of infection. CPV were also observed when BHK cells were transfected with purified infectious RNA derived from either wild-type or ts1 mutant SFV. Thus, it seems that newly synthesized virus-specific nonstructural (ns) proteins, rather than endocytosed virus particles, must be responsible for the endosomal-lysosomal targeting of the replication complex.
During recent years, we have studied the properties of individually expressed ns proteins of alphaviruses, primarily with SFV as a model. nsP1 (SFV; 537 amino acids [aa]) proved to be an mRNA-capping enzyme with guanine-7-methyltransferase and guanylyltransferase activities (2, 3, 29); nsP2 (799 aa) is an NTPase (46), an RNA helicase (16), and an RNA 5′ triphosphatase (57), as well as a protease responsible for the autocatalytic cleavage of the ns polyprotein (52). nsP3 (482 aa) is a phosphoprotein with poorly defined functions (42, 52, 58). According to genetic evidence, nsP4 (614 aa) is the catalytic subunit of the alphavirus RNA polymerase. It also has sequence signatures common to RNA and DNA polymerases (52).
Here we have used confocal and immuno-EM to localize RNA replication complexes and individual ns proteins in SFV-infected cells. On the basis of these studies, we propose that the replication complexes consist of spherules, which contain as their virus-specific components the RNA template and nsP1 to -4 proteins. On the basis of morphological criteria, we suggest that the assembly of the complexes takes place at the plasma membrane, whereas the ns proteins of incorrectly assembled complexes dissociate from each other and distribute to the cytoplasm and nucleus.
MATERIALS AND METHODS
Infection, radiolabeling, and fractionation of cells.
The origin and cultivation of the SFV prototype strain and BHK21 cells were done as described previously (22). BHK cells were infected with SFV (50 PFU/cell) or mock infected and grown in the presence of actinomycin D (1 μg/ml) and the proteasome inhibitor MG132 (50 μM/ml; Sigma). For coimmunoprecipitation studies of ns proteins, cells in 100-mm-diameter dishes were labeled with 250 μCi of [35S]methionine (100 Ci/mmol; Amersham) from 2 to 4 h after infection and chased for 1 h in minimal essential medium containing a 20-fold excess of unlabeled methionine. A long chase was chosen to allow the processing of the ns polyprotein and the assembly of ns proteins to form the replication complexes. Cells were fractionated at 15,000 × g into a membrane fraction (P15) and the respective supernatant (S15), as described previously (43).
Antisera.
A rat monoclonal antibody (MAb) (BU1/75 [ICR1]; Harlan Sera-Lab Ltd., Loughborough, United Kingdom) against bromodeoxyuridine (BrdU) was used to detect SFV RNA metabolically labeled with bromo-UTP (Sigma). For characterization of the cellular origin of CPV, antibodies against the following cellular markers were used: human lysosome-associated membrane glycoproteins 1 and 2 (lamp-1 and lamp-2) (21), cation-independent mannose 6-phosphate receptor (CI-MPR; Eeva-Liisa Eskelinen, Institute of Biotechnology, Helsinki, Finland), rab7 (Marino Zerial, European Molecular Biology Laboratory, Heidelberg, Germany), p58 (Sigma), cab45 (Vesa Olkkonen, National Health Institute, Helsinki, Finland), protein disulfide isomerase (PDI; Stephen Fuller, European Molecular Biology Laboratory), early endosome-associated antigen 1 (EEA1; Harald Stenmark, Radium Institute, Oslo, Norway), lysobisphosphatic acid (LBPA; T. Kobayashi, University of Geneva, Geneva, Switzerland), and human transferrin receptor (TfR; MAb B3-25; Boehringer Mannheim). The preparation and characterization of MAbs against nsP2 have been described previously (26). Rabbit antisera against nsP1, nsP2, and nsP3 were raised against His-tagged full-length proteins and against a derivate of nsP4 with an amino-terminal deletion of 61 residues. Proteins were expressed in Escherichia coli and purified by metal affinity chromatography, essentially as described for nsP2 (46). The immunization of rabbits was carried out essentially as described previously (25). Guinea pigs were immunized with nsP1, nsP3, and nsP4 as follows. The first subcutaneous injection was in complete Freund's adjuvant (30 to 50 μg/animal). Similar booster injections were given 2 weeks later, followed by two identical boosters with a 1-month interval. Bleeding was done 2 weeks after the last dose. The potencies of the antisera were tested by indirect immunofluorescence, Western blotting, and immunoprecipitation assays using SFV-infected BHK cells as the antigen source.
Immunoprecipitation.
Radiolabeled samples were used for immunoprecipitations with antisera against ns proteins with or without denaturation (1% sodium dodecyl sulfate [SDS] at 100°C for 2 min) as described previously (25, 42). The precipitates were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) in 10% polyacrylamide gels, using normal (0.8%) and low (0.1%) concentrations of bisacrylamide in parallel to separate nsP1 and nsP3 when required. For recapture experiments, antigens were released from protein A-Sepharose in 1% SDS, 100 mM Tris-HCl (pH 7.4), and 10 mM dithiothreitol, followed by dilution in 1 ml of NET buffer (42) supplemented with 10 mM iodoacetamide and 0.5% bovine serum albumin (BSA). The sample was divided into two 500-μl aliquots, which were precipitated with a mixture of anti-nsP1 plus anti-nsP4 and anti-nsP2 plus anti-nsP3 antisera, respectively. A PhosphorImager (Molecular Dynamics) was used for visualization and quantitation of the labeled proteins.
Confocal microscopy.
Indirect-immunofluorescence microscopy was carried out for SFV-infected BHK cell monolayers on coverslips. After fixation with 3% paraformaldehyde in a buffer consisting of 10 mM MES (morpholineethanesulfonic acid) (pH 6.1), 138 mM KCl, 3 mM MgCl2, 2 mM EGTA, and 0.32 M sucrose, the aldehyde groups were quenched with 50 mM NH4Cl. The cells were permeabilized with 0.05% Triton X-100 and treated with the primary antibody followed by either fluorescein isothiocyanate (FITC)- or rhodamine (TRITC)-conjugated secondary antibody. Fluorescent LysoTracker Red DND-99 and fluorescent transferrin (Tfn) (Molecular Probes, Leiden, The Netherlands) were used according to the manufacturer's instructions. The samples were analyzed with an MRC-1024 confocal microscope (Bio-Rad, Cambridge, Mass.) with an American Laser Corporation (Salt Lake City, Utah) laser as the source for the argon-krypton ion laser beam. FITC-stained samples were imaged by excitation at 488 nm and with a 505- to 540-nm bandpass emission filter, and TRITC-stained samples were imaged by excitation at 568 nm with a 598- to 621-nm bandpass emission filter.
EM.
For conventional EM processing, infected cells grown on coverslips were fixed with 2.5% glutaraldehyde in 0.2 M cacodylate, pH 7.2, for 20 min at room temperature (RT). In some experiments, ruthenium red (500 ppm) was included in the fixing medium. Samples were postfixed in 1% OsO4 in 0.2 M cacodylate for 30 min at RT and then left overnight in 1% uranyl acetate in 0.3 M sucrose in distilled water at 4°C before being ethanol dehydrated and embedded in Epon resin.
For preembedding labeling, cells grown on coverslips were fixed, permeabilized, and treated with primary antibody as for immunofluorescence. For single labeling, protein A conjugated with 10-nm-diameter gold particles was used, and for double staining, 5-nm-diameter gold particles conjugated with goat anti-rabbit immunoglobulin G (IgG) and 10-nm-diameter gold particles conjugated with goat anti-rat IgGs were used as secondary antibodies. The preembedded samples were postfixed with 3% glutaraldehyde in 0.2 M PIPES (piperazine-N,N′-bis(2-ethanesulfonic acid), pH 7.2, before osmium treatment and treated thereafter as described above.
Ultrathin frozen sections were prepared according to the method of Tokuyasu (53, 54). Briefly, cell pellets were fixed in a mixture of 4% paraformaldehyde and 0.25% glutaraldehyde in 0.2 M PIPES, pH 7.2, for 30 min at RT. The pellets were infiltrated with 2.1 M sucrose in 20% polyvinylpyrrolidone for 15 min at RT and frozen in liquid nitrogen, and sections were cut at −110°C. Retrieval of sections from the knife was achieved with a 1:1 mixture of 2% methylcellulose (Sigma) and 2.3 M sucrose according to the method of Liou et al. (33).
Colloidal gold particles (5-nm diameter) were prepared according to the method of Slot and Geuze (49) and coated with BSA. Infected cells were exposed to 1 ml of medium containing 250 μl of BSA-gold for various times, followed by washing and the addition of fresh medium. Newly synthesized viral RNA in SFV-infected BHK cells was labeled for 5 min at 4 h after infection with 20 mM bromo-dUTP (BrdUTP; Sigma) introduced to the cells by lipofectin transfection. The cells were exposed to 5 μg of actinomycin D (Sigma)/ml starting 15 min before the addition of BrdUTP.
RESULTS
Immunofluorescence in SFV-infected cells.
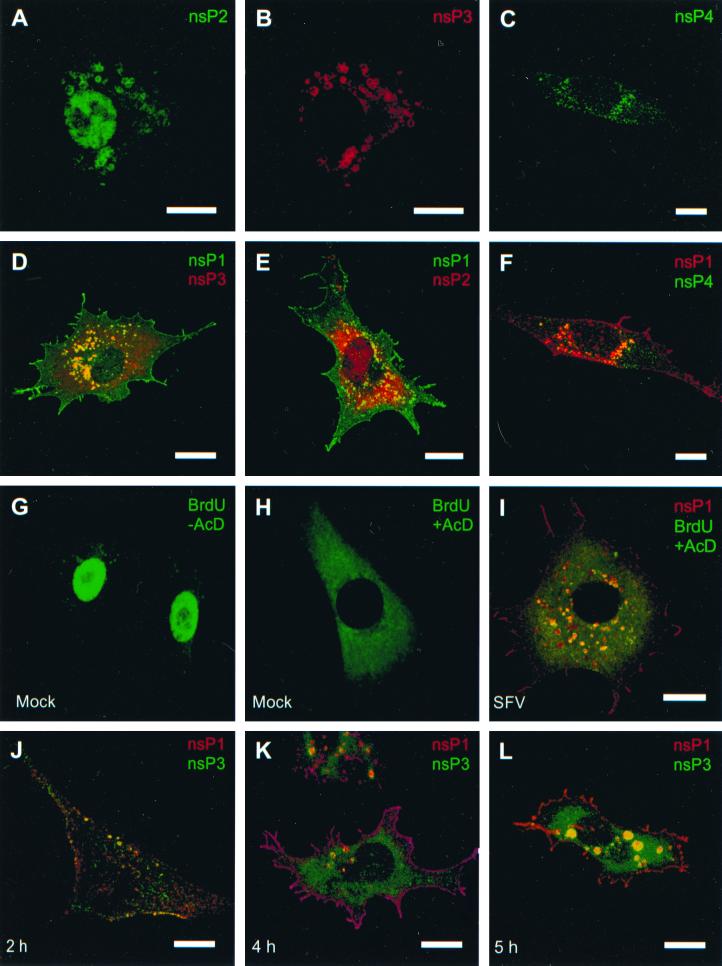
Cells infected with the SFV prototype strain (50 PFU/cell) were examined 4 to 6 h after infection by indirect immunofluorescence with double labeling, using antisera against different ns proteins raised in guinea pig or mouse, together with antibodies made in rabbit. Pairwise double labeling revealed perinuclear vacuoles in all combinations, best recognized in the merged images (Fig. 1D, E, and F). These are typical CPV identified at the light microscopic level (15, 39). In addition to CPV, each antiserum stained other cellular structures as well. nsP1 was regularly found at the plasma membrane and in filopodium-like extensions (Fig. 1D and E) as described previously (27, 28, 40). Anti-nsP2 antisera stained the nucleus, indicating that a substantial amount of nsP2 had been transported to the nucleus as described earlier (41) (Fig. 1A and E), whereas nsP3 appeared in small vesicles distributed throughout the cytoplasm (Fig. 1D and J). Finally, nsP4, the catalytic subunit of SFV RNA replicase, showed a punctate cytoplasmic distribution (Fig. 1C and F). In the presence of actinomycin D, pulse-labeled BrdU-RNA colocalized in CPV-like structures with nsP1, supporting the view that CPV are indeed the synthesis sites of viral RNA (Fig. 1I). Under these conditions, the nuclei of mock-infected cells were devoid of fluorescence (Fig. 1H), whereas in the absence of actinomycin D the nuclei showed bright fluorescence (Fig. 1G).
FIG. 1.
Confocal fluorescence images of SFV-infected BHK cells stained for nsP2 at 6 h p.i. (A), nsP3 at 6 h p.i. (B), and nsP4 at 4 h p.i. (C), double-stained for nsP1 (green) and nsP3 (red) at 4 h p.i. (D), double-stained for nsP1 (green) and nsP2 (red) at 4 h p.i. (E), and stained for nsP1 (red) and nsP4 (green) at 4 h p.i. (F). (G and H) Mock-infected BHK cells treated with BrdUTP for 10 min at 4 h p.i., followed by detection with anti-BrdU antiserum (green) in the absence (G) and presence (H) of 5 μg of actinomycin D/ml. (I) SFV-infected cell labeled with BrdUTP for 10 min in the presence of actinomycin D at 4 h p.i. and stained for BrdU (green) and nsP1 (red). (J, K, and L) Time course of SFV infection of BHK cells double labeled with anti-nsP1 (red) and anti-nsP3 (green) at 2 (J), 4 (K), and 5 (L) h p.i. Bars, 10 μm. Infection and further incubation were done at 37°C.
Next, we studied the localization of replication complexes at different times after infection by using double immunofluorescence with anti-nsP1 and anti-nsP3 antibodies. Early in infection the proteins colocalized in small CPV-like structures in the vicinity of the plasma membrane (Fig. 1J). Later, CPV were larger and were concentrated mostly in the perinuclear area (Fig. 1K and L).
Ultrastructure and immuno-EM of SFV-infected cells.
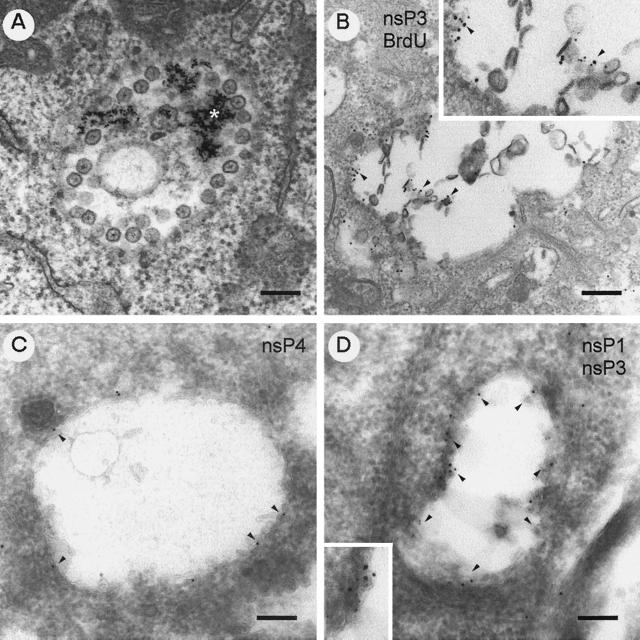
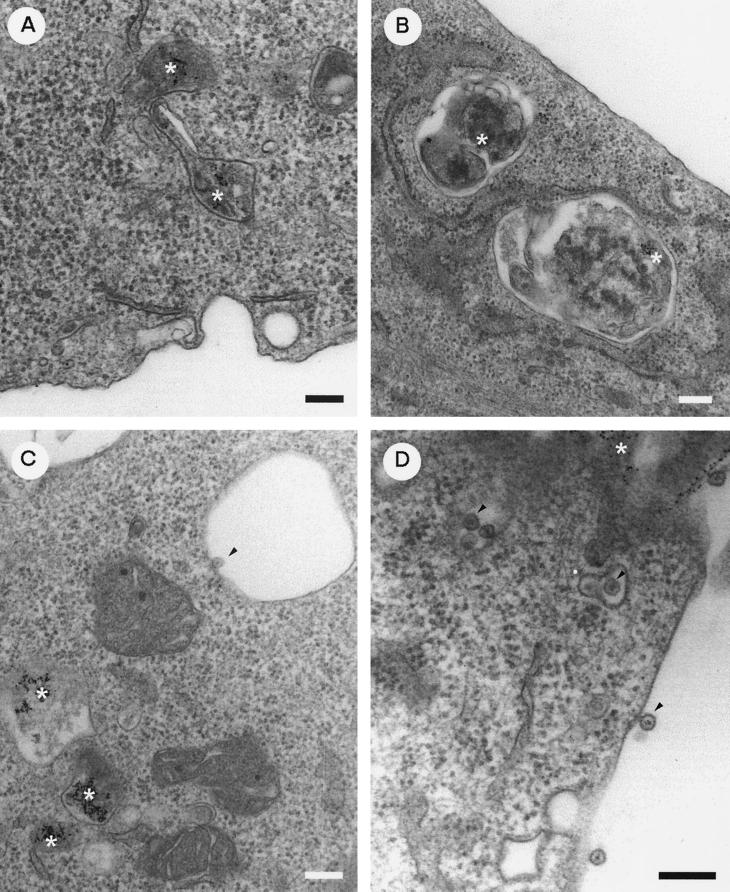
For ultrastructural studies, SFV-infected BHK cells were exposed to colloidal gold particles coated with BSA at the time of infection. BSA-gold served as a marker for the endocytic pathway. For immunolocalization of ns proteins, the infected cells were treated with puromycin (100 μg/ml) for 15 min prior to harvest to remove nascent proteins from mRNAs in the vicinity of CPV. The cells were fixed with glutaraldehyde and embedded in Epon resin. At 5 h after infection, CPV with numerous spherules at their limiting membranes contained endocytosed BSA-gold (Fig. 2A). Inside each spherule was an electron-dense spot with hairy thin spokes radiating towards the periphery of the spherule. For localization of virus-specific RNA synthesis sites in SFV-infected cells, we used preembedding EM technique. The cells were treated with actinomycin D followed by exposure to BrdUTP for 10 min using lipofectin as a carrier, after which the cells were washed and incubated further for 5 min. The cells were permeabilized and fixed before being double labeled with rabbit anti-nsP3 and rat anti-BrdU. As secondary antibodies, we used anti-rabbit IgG-coated 5-nm-diameter gold particles and anti-rat IgG-coated 10-nm-diameter gold particles. Both 5- and 10-nm-diameter particles were seen bound to vacuolar membranes, often in association with spherulelike structures, which were evidently damaged by the detergent treatment (Fig. 2B). Cryoimmuno-EM techniques were used for immunolocalization of the ns proteins in order to better preserve the spherules. To improve the structural preservation of the thawed cryosections, pickup from the knife was performed with a mixture of sucrose-methyl cellulose (33). In cryosections (Fig. 2C and D), the ultrastructure of CPV was fairly well preserved, although the contrast of the images was relatively limited compared to that of conventional osmium-postfixed Epon sections (Fig. 2A). However, CPV were easily recognized as vacuoles with spherulelike invaginations. When sections were treated with antisera against nsP1 to -4, protein A-conjugated gold particles were regularly seen close to the limiting membrane, as shown for nsP4 (Fig. 2C) and by double labeling for nsP1 and nsP3 (Fig. 2D), but also in association with spherulelike structures.
FIG. 2.
Immunolocalization of ns proteins in CPV structures. (A) Epon section of BSA-gold-labeled (asterisk) CPV at 5 h p.i. (B) Preembedded Epon section of double-labeled CPV: 10-nm-diameter gold particles detecting pulse-labeled BrdU-RNA and 5-nm-diameter gold particles detecting nsP3 better visualized in the enlarged inset. Cryoimmuno-EM images of gold-protein A labeling are shown. (C) Anti-nsP4 alone detected by 10-nm-diameter gold particles. (D) Double labeling with anti-nsP1 (5-nm-diameter gold particles) and anti-nsP3 (10-nm-diameter gold particles). The arrowheads point to some antibody-specific gold labeling. Representative detail is shown in the enlarged insets. Bars, 200 nm.
Immunoprecipitation of replication complex.
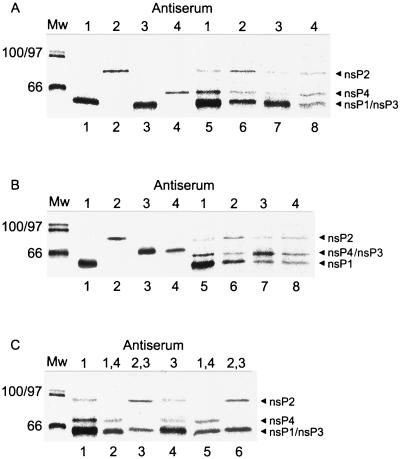
To identify the ns proteins biochemically, [35S]methionine-labeled SFV-infected BHK cells were lysed 5 h postinfection (p.i.) and postnuclear supernatant was separated at 15,000 × g into membrane (P15) and the respective supernatant (S15) fractions. It has been established that essentially all RNA polymerase activity in alphavirus-infected cells is associated with the P15 fraction (8, 43). To control the specificities of our antisera, the labeled proteins were precipitated under both denatured and native conditions. After denaturation of the sample with SDS, each antiserum reacted only with its specific ns protein (Fig. 3A and B, lanes 1 to 4). In normal SDS-PAGE, nsP1 and nsP3 cannot be separated from each other (Fig. 3A, lanes 1 and 3), whereas in the presence of 0.1% bisacrylamide nsP1 and nsP3 have different mobilities (Fig. 3B, lanes 1 and 3). Unfortunately, in that case nsP3 migrates together with nsP4 (Fig. 3B, lanes 3 and 4). Both gel systems were used to analyze the immunoprecipitates of nondenatured samples derived from S15 and P15 preparations of SFV-infected cells, usually at 5 h p.i. All antisera precipitated only their specific target proteins from the S15 fraction, indicating that the ns proteins were not complexed in this “soluble fraction” (data not shown). However, when P15 was used as the antigen source, the precipitation pattern was more complex (Fig. 3A and B, lanes 5 and 8). Anti-nsP1 antiserum precipitated nsP2 and nsP4 in addition to nsP1 (Fig. 3A and B, lanes 5). Anti-nsP2 antiserum precipitated nsP4 and nsP1 (Fig. 3A and B, lanes 6). Similarly, anti-nsP3 also precipitated nsP1, nsP2, and nsP4 (Fig. 3A and B, lanes 7), and anti-nsP4 precipitated nsP1 and nsP2 (Fig. 3A and B, lanes 8). Thus, each ns protein antiserum precipitated, in addition to its specific ns protein, various amounts of other ns proteins. Due to the difficulty of separating nsP1 and nsP3, we used an immunoprecipitation-recapture technique. The complex was isolated from P15 by immunoprecipitation with either nsP1 or nsP3 (Fig. 3C, lanes 1 and 4, respectively). Evidently, more nsP1 (lane 1) and nsP3 (lane 4) than other proteins was precipitated with the respective antisera. To visualize other ns proteins in the precipitate, the immunocomplex was dissociated with SDS before the second immunoprecipitations with combinations of two different antisera (anti-nsP1 plus anti-nsP4 and anti-nsP2 plus anti-nsP3) to resolve the individual ns proteins optimally in SDS-PAGE. When the first precipitation was performed with anti-nsP1 (Fig. 3C, lane 1) followed by the second precipitation with a mixture of anti-nsP1 and anti-nsP4, both proteins were detected unambiguosly (Fig. 3C, lane 2). When the same first precipitate was exposed to a combination of anti-nsP3 and anti-nsP2 antisera, both proteins were again clearly resolved (Fig. 3C, lane 3). When anti-nsP3 was used as the first antiserum (lane 4) followed by second precipitations with anti-nsP1 plus anti-nsP4 (lane 5) and anti-nsP2 plus anti-nsP3 (lane 6), all ns proteins were again complexed together. Quantitations of the bands shown in Fig. 3 and similar analyses were made by PhosphorImager. Molar ratios for nsP4-nsP2 were determined in eight different lanes, giving an average of 1.5 ± 0.7. For nsP1-nsP2, an average of 3.6 ± 1 (four lanes) was determined. Thus, a rough estimate for the composition of the immunoprecipitated complex would be nsP1-nsP2-nsP4 at 4:1:1. A ratio of 2:1 for nsP3-nsP2 was determined from the gel shown in Fig. 3C lane 3. These results, together with the immunofluorescence and immuno-EM data, support the idea that all four ns proteins were associated in the RNA replicase complex in the CPV structures, although not in equimolar ratio.
FIG. 3.
Immunoprecipitation from P15 fraction of [35S]methionine-labeled SFV-infected BHK cells. Shown is an analysis of immunoprecipitates formed by anti-nsP1 to -4 with denatured (A and B, lanes 1 to 4) and nondenatured (A and B, lanes 5 to 8) proteins. Antisera used for immunoprecipitations are indicated by the numbers of the corresponding nsPs above the lanes. SDS-PAGE was performed in a gel with 10% acrylamide and 0.8% bisacrylamide (A) and in a gel with 10% acrylamide and 0.1% bisacrylamide (B). (C) SDS-PAGE (10% acrylamide and 0.8% bisacrylamide) after two successive immunoprecipitations, first with anti-nsP1 (lane 1) and anti-nsP3 (lane 4) antisera, followed by second immunoprecipitations with anti-nsP1 plus anti-nsP4 (lanes 2 and 5) and anti-nsP2 plus anti-nsP3 (lanes 3 and 6). Molecular weight (Mw) markers are shown on the left. The positions of different ns proteins are indicated by arrowheads on the right.
Characterization of CPV with cellular markers.
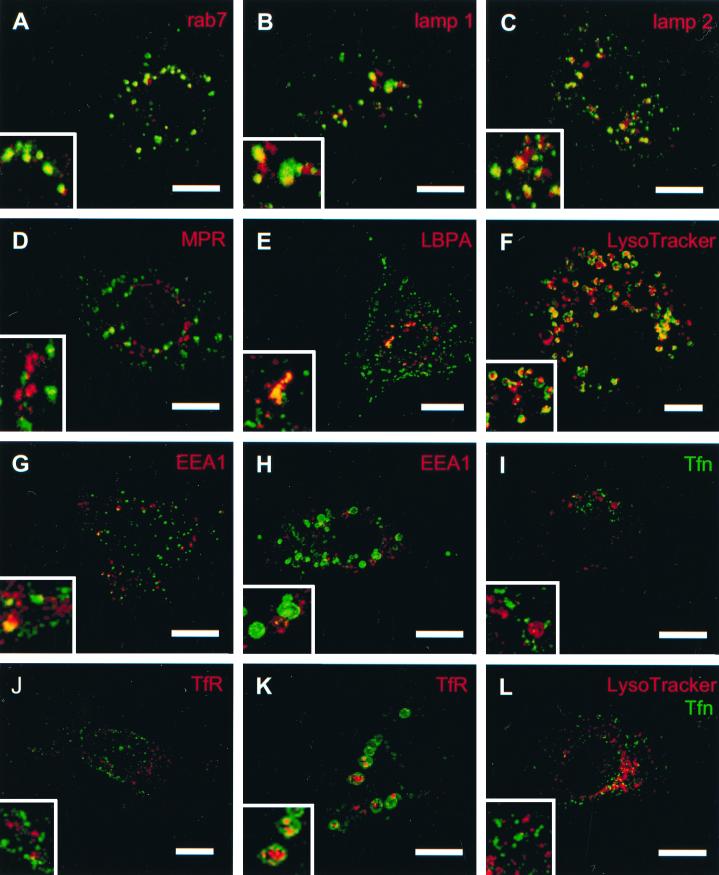
The cellular origin of CPV was investigated by confocal microscopy using organelle-specific markers in double staining with anti-nsP3 antibodies (Fig. 4). The late endosomal markers Rab7 (Fig. 4A), lamp-1 (Fig. 4B), lamp-2 (Fig. 4C), and LBPA (Fig. 4E) colocalized with nsP3 in typical CPV structures. Exposure of SFV-infected BHK cells to LysoTracker for 30 min at 5 h p.i. before fixation and staining with anti-nsP3 antibodies revealed colocalization of both reagents in CPV. LysoTracker accumulates specifically in acidic compartments, such as late endosomes and lysosomes (10). Interestingly, two other late endosomal markers, CI-MPR (Fig. 4D) and Rab9 (not shown), failed to colocalize with nsP3 staining, suggesting selectivity within the late endosome population. We failed to show colocalization of nsP3-stained CPV with TfR (Fig. 4J and K) and Tfn (Fig. 4I). Occasional colocalization with an early endosomal marker (EEA1) was seen 3 but not 6 h p.i. (4G and H, respectively). Thus, virus-specific CPV do not occupy the early endosomal apparatus. As a control for the different distributions of Tfn and lysosomes, LysoTracker reagent was visualized in the same SFV-infected cell at 5 h p.i. (Fig. 4L). Unexpectedly, TfR was found late in infection inside large CPV, indicating that early endosomes had been autophagocytized by them late in infection (Fig. 4K). No consistent colocalization of ns proteins was seen with the markers for the endoplasmic reticulum (ER) (PDI), the ER-Golgi intermediate compartment (p58), or the Golgi complex (cab45), indicating that the organelles of the secretory route were not involved in the RNA synthesis of SFV (data not shown).
FIG. 4.
Confocal immunofluorescence images of SFV-infected BHK cells 3 (G and J), 4 (A to E), 5 (F, I, and L) and 6 (H and K) h p.i. The cells were stained with anti-nsP3 (green, except in panel I, where nsP3 is red) and different cellular markers (red). Merged images and enlarged insets thereof are shown. In panels I and L, SFV-infected BHK cells at 5 h p.i. were exposed for 30 min to fluorescent Tfn (green), and in panels F and L they were exposed for 30 min to LysoTracker (red). Bars, 10 μm.
Biogenesis of CPV.
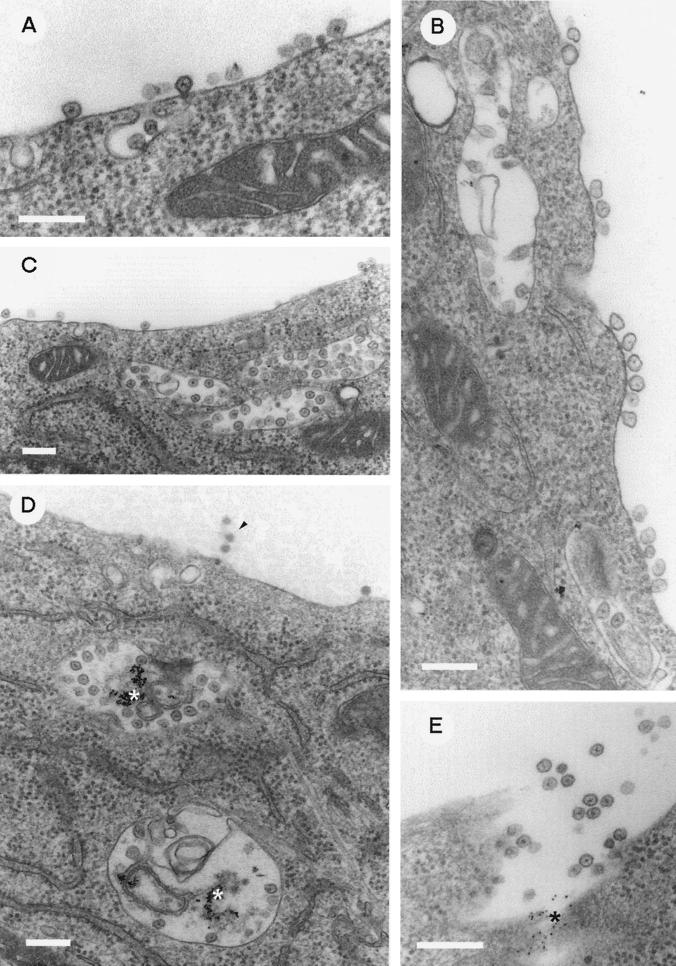
To study the relationship of endocytosis and the formation of CPV during SFV infection (20 PFU/cell), BHK cells were exposed to BSA-gold during virus adsorption, as described above for Fig. 2. After 1 h of adsorption, the cells were washed and supplied with fresh prewarmed medium. Endocytosed gold was found in small tubelike vacuoles, presumably early endosomes. No signs of virus particles or their remnants were observed at the plasma membrane or in the intracellular space (Fig. 5A). Two hours after infection, gold was found in the larger vesicles. Spherules were not detectable either on their limiting membranes or at the plasma membrane (Fig. 5B). The first few spherules were seen at 3 h p.i. on the internal surfaces of intracellular vacuoles devoid of gold (Fig. 5C). At the same time, spherules were also seen on the cell surfaces and in small vesicles (Fig. 5D). At 4 h p.i., spherules were frequently seen at the plasma membrane accompanied by internalization or possibly externalization profiles opening to the exterior of the cell (Fig. 6A). Clusters of spherules were present at the cell surface, often in the close vicinity of CPV beneath the plasma membrane (Fig. 6B and C). CPV with spherules and internalized gold were observed only late in infection (Fig. 6D). Some gold-labeled CPV appeared to have fused with the plasma membrane, releasing their spherules and other contents into the culture medium (Fig. 6E). As suggested by confocal microscopy (Fig. 1 and 4), the sizes of CPV increased while their number decreased during infection. The mean diameters measured from electron micrographs (n = 50 per time point) were about 230, 650, and 870 nm at 4, 5, and 6 h p.i., respectively.
FIG. 5.
Early events in the biogenesis of CPV. BHK cells were exposed to BSA-gold during virus inoculation as described in Materials and Methods. EM images of SFV-infected cells at 1 (A), 2 (B), and 3 (C and D) h after infection are presented. The asterisks denote endocytosed BSA-gold particles, and the arrowheads point to some early spherule structures (C and D). Bars, 200 nm.
FIG. 6.
Late events in the biogenesis of CPV. BSA-gold was administered as for Fig. 5. EM images of SFV-infected BHK cells at 4 (A) and 5 (B to E) h after infection are shown. The asterisks denote endocytosed BSA-gold particles (D and E). In panel E, CPV carrying BSA-gold is exocytosed into the medium. The arrowhead in panel D points to some budding virions at the plasma membrane. Bars, 200 nm.
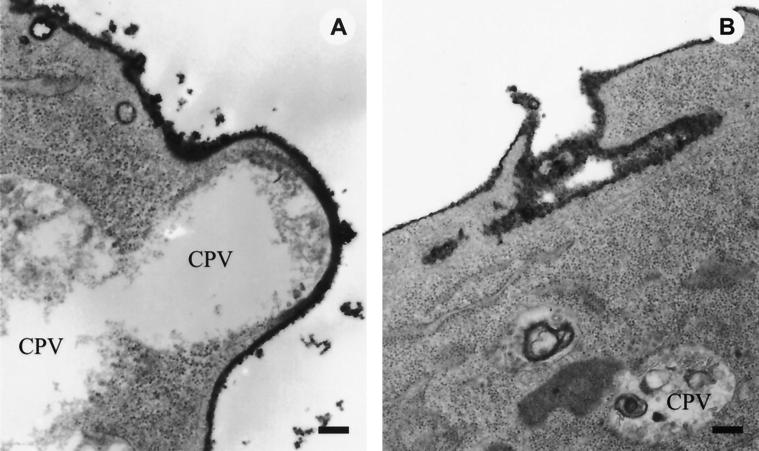
To better understand the direction of flow of the small cytoplasmic vesicles and CPV containing distinguishable spherules, ruthenium red staining was carried out at 5 h p.i. by exposing the cells to the stain during fixation. Ruthenium red reacts with a large number of polyanions, especially with mucopolysaccharides on the cell surface. Thus, all structures stained with this poorly permeable reagent must have been exposed to the medium at or very shortly after the beginning of fixation. Thus, CPV in close proximity to the plasma membrane, which were not stained by ruthenium red, were likely to be moving to the plasma membrane rather than being invaginations of it (Fig. 7A). We assume that the opening of a CPV to the exterior of the cell had taken place on the surface of a cell shown in Fig. 7B.
FIG. 7.
Ruthenium red-stained structures in SFV-infected BHK cells 5 h p.i. (50 PFU/cell). SFV-infected BHK cells at 5 h p.i. were fixed in the presence of ruthenium red for 60 min at RT, as described in Materials and Methods, to stain the cell surfaces. (A) CPV under intact plasma membrane; (B) remnants of a CPV after fusion with plasma membrane. Bars, 200 nm.
In another experiment with a larger virus inoculum (200 PFU/cell), the CPV were observed earlier, starting at 3 h p.i. When the infected cultures were exposed to gold for 10 min at different times after infection, followed by a 10-min incubation before the cells were fixed, the gold particles were observed in CPV for 3 to 6 h p.i. This suggests that active endocytosis continued even during heavy virus infection and that CPV continuously received material from the medium (data not shown).
DISCUSSION
The RNA replication of positive-strand RNA viruses of eukaryotic cells is regularly associated with cytoplasmic membranes. Different viruses utilize different membranes, which serve as matrices for the association of their replication complexes. The replication of poliovirus occurs in vesicular structures with double membranes, which are derived from intracellular membranes by a process resembling autophagy (9). Poliovirus infection results in disorganization of the organelles of the secretory route (11, 12, 48). The RNA synthesis of arteriviruses has been shown to occur on modified intracellular membranes derived from the ER or an intermediate compartment (38, 55). The RNA synthesis of coronaviruses takes place on late endosomal membranes and multivesicular bodies (56), whereas flavivirus RNA replication is associated with intracellular packets of membrane vesicles (60), probably derived from the membranes of the trans-Golgi network and intermediary compartments (34). Several plant viruses replicate on membranes which originate from the ER (36, 44, 47).
Previous morphological studies of alphavirus-infected cells have revealed specific CPV which have been suggested to be the sites of virus-specific RNA replication (15, 17). Here we have used confocal microscopy and immuno-EM as tools in the localization of SFV-specific RNA replicase by pairwise double labeling of the ns proteins in infected cells. Each ns protein had a unique distribution, in addition to overlapping localization in vacuolar structures, which was indistinguishable from the previously described CPV (15, 39). nsP1 was localized beneath the plasma membrane and in filopodiumlike surface extensions, nsP2 was in the nucleus, nsP3 was in association with cytoplasmic vesicle-like structures, and nsP4 was distributed diffusely in the cytoplasm. Thus, the identities of the viral RNA replicase complexes in alphavirus-infected cells cannot be deduced by using antiserum against a single RNA polymerase component.
The role of CPV as sites of the RNA replication complexes was supported by coimmunoprecipitation studies. Each antiserum coprecipitated the other three ns proteins, suggesting that they formed a complex. Similar results have been reported for the isolated RNA replication complex of Sindbis virus (8, 15). The use of a short puromycin pretreatment allowed the localization of all ns proteins to the spherules and limiting membranes of CPV by cryoimmuno-EM. Thus, we are confident that only preexisting replicase components were labeled. In accordance with these results, BrdU-labeled RNA was also localized in spherules and in their close vicinity. Taking together our present data and the EM autoradiography results of Grimley et al. (17), it seems likely that the spherules are the actual sites of viral-RNA synthesis. This means that the 11.5-kb viral RNA is packed within a spherule about 50 nm in diameter. If so, one would expect the nascent RNA molecules to be released from the spherules into the cytoplasm, as suggested by EM images shown by Froshauer et al. (15). Often this material seemed to make a bridge to rough ER (RER) membranes. We assume that the bridge was due to nascent 26S mRNA, which associates with RER when translating the virus structural proteins. In this study, the use of puromycin prevented the immediate translation of the nascent RNAs.
Structures similar to alphavirus CPV have been described for rubella virus-infected cells (32). The CPV double stained with antisera against double-stranded RNA and lysosomal markers, such as anti-lamp1. By immuno-EM, the double-stranded RNA was localized in the immediate vicinity of the limiting membrane of CPV as well as to spherules (35). We have recently shown by immuno-EM that the rubella virus-specific replicase protein P150 and newly synthesized BrdU-labeled RNA colocalize in CPV-associated spherules (25).
Origin and biogenesis of CPV.
Double staining with different organelle-specific markers confirmed that CPV originated from the endosomal-lysosomal compartment. However, no costaining with early endosomal markers (EEA1; TfR or Tfn) could be seen. Several markers (Rab7, LysoTracker, LBPA, lamp-1, and lamp-2) indicated that CPV shared properties of late endosomes and lysosomes (10, 21, 24, 37, 50, 59). Interestingly, no evident colocalization could be seen with ns proteins and CI-MPR or Rab9, which are also markers for late endosomes (19, 50). Although MPR, lamp-1, and lamp-2 are transported from the trans-Golgi network to late endosomes, they are sorted in different vesicles (21). Thus, the ns proteins seem to have some specificity in recognizing subpopulations in the endosomal apparatus.
Circulation of spherules.
Short BSA-gold pulses revealed that CPV were in continuous contact with the medium even late in infection. In some images, discharge of gold particles into the medium was seen from CPV opening to the exterior of the cell. At the same time, spherulelike structures were released into the medium in a process resembling the secretion of exosomes by B lymphocytes (51). Similar phenomena, observed in SFV-infected cells, have been reported for coronavirus-infected cells (56) and late in rubella infection (our unpublished data). Exosomes are small membrane vesicles that are carried to the plasma membrane by a heterogeneous set of endocytic vacuoles designated as “secretory lysosomes” (51). The secretory lysosomes, having a cargo of major histocompatibility complex class II molecules, fuse with the plasma membrane directly, releasing their internal vesicles (i.e., exosomes) into the cell culture. By this means, B lymphocytes present peptide determinants of foreign antigens to the T cells (13, 23). However, when CPV in SFV-infected cells fused with the plasma membrane, most of the spherules seemed to remain associated with the plasma membrane (Fig. 6 and 7).
Spherules were found on the cell surface starting at 3 h p.i. when BHK cells were infected with a multiplicity of infection of 20. They were often in clusters, giving an impression that they had been brought there by a fusion of CPV with the plasma membrane, perhaps by secretory lysosomes, which are induced in fibroblasts of many mammalian species as a response to different exogenous stimuli (7). Grimley et al. (17) showed by EM autoradiography that the spherules at the plasma membrane contained grains from pulse-labeled [3H]uridine, indicating that RNA synthesis was taking place in these structures. At different times after infection, spherules have been seen in coated pits and coated vesicles, evidently in the process of being endocytosed (15). We assume that at least part of the CPV release their spherules to the plasma membrane, from where they may be rapidly internalized by endocytosis. Late in infection, part of the spherules may also be released to the medium, possibly due to enzymatic activities (e.g., lipase and protease) in the secretory lysosomes.
Assembly of replication complexes at the plasma membrane.
Of the SFV ns proteins, only nsP1 showed a clear prevalence for the plasma membrane in infected cells (Fig. 1). When expressed alone by vaccinia virus vector, most of it was associated at the cytoplasmic side of the plasma membrane and in filopodiumlike structures (28). During virus infection and expression of nsP1 alone, it becomes palmitoylated in cysteine residues 418 to 420, causing tight membrane binding (27). To our surprise, a change of C418 to C420 to alanines did not prevent membrane binding of nsP1, nor did this mutation prevent replication of the virus (5). We have recently identified a sequence of 20 aa, starting from glycine 245, which is responsible for the binding of nsP1 to anionic phospholipids, such as phosphatidylserine (PS) (4, 31). PS is greatly enriched in the cytoplasmic leaflet of the plasma membrane (6), which explains why nsP1 is localized there in both infected and transfected cells (Fig. 1D, E, and K) (27, 28, 40). According to these findings, the membrane binding of nsP1 is a two-step process: first, a weaker binding to PS-rich (plasma) membrane takes place, followed by a palmitoylation step, which changes the properties of nsP1 to mimic those of integral membrane proteins (27). To explain the present and previous results, we propose the following hypothesis for the origin of RNA replication complexes, spherules, and CPV.
The primary assembly of the replication complex takes place at the plasma membrane directed by the binding peptide of nsP1 (31). However, only part of the replication complexes assemble correctly. The successfully assembled complexes are internalized via the endosomal pathway, whereas ns proteins which fail to assemble properly are dissociated from each other and distribute to the cytoplasm or nucleus. We propose further that succesfully assembled replication complexes induce the formation of spherules, which most probably are the units of RNA replication, containing a set of all ns proteins and 42S RNA minus-strand template. This spherule unit can assemble at the plasma membrane or at internal membranes rich in anionic phospholipids, possibly at endosomes. At any rate, it will be endocytosed readily, as can be seen in several EM images. It can also return to the plasma membrane as part of the endosomal cycle. However, the half-life of the replication complexes localized in the early endosomes must be extremely short, since we could not colocalize them with EEA1, TfR, or Tfn markers. When infection progresses, more and more CPV become enriched in the perinuclear region in structures carrying Rab7, lamp-1, lamp-2, and LBPA. Rab7 is a known regulator of membrane traffic in the late endosomal compartment (10, 59). Interestingly, late CPV also continuously receive extracellular material, as evidenced by the endocytosis of BSA-gold particles. Thus, we cannot exclude the possibility that late in infection, when the synthesis of ns proteins is shut off (20, 30), recycling of spherules to the plasma membrane continues. These replication complexes may still be active in RNA synthesis, as suggested by EM autoradiography (17), although the cell might try to discharge them through secretory lysosomes, possibly as a cellular response to viral insult.
ACKNOWLEDGMENTS
We thank Tero Ahola and Marja Makarow for critical reading of the manuscript and Airi Sinkko, Arja Strandell, and Tarja Välimäki for excellent technical assistance.
This work was supported by the Technology Development Centre (TEKES) and the Academy of Finland (grant no. 8397). L.K. is a Biocentrum Helsinki Fellow.
REFERENCES
- 1.Acheson N H, Tamm I. Replication of Semliki Forest virus: an electron microscopic study. Virology. 1967;32:128–143. doi: 10.1016/0042-6822(67)90261-9. [DOI] [PubMed] [Google Scholar]
- 2.Ahola T, Kääriäinen L. Reaction in alphavirus mRNA capping: formation of a covalent complex of nonstructural protein nsP1 with 7-methyl-GMP. Proc Natl Acad Sci USA. 1995;92:507–511. doi: 10.1073/pnas.92.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahola T, Laakkonen P, Vihinen H, Kääriäinen L. Critical residues of Semliki Forest virus RNA capping enzyme involved in methyltransferase and guanylyltransferase-like activities. J Virol. 1997;71:392–397. doi: 10.1128/jvi.71.1.392-397.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahola T, Lampio A, Auvinen P, Kääriäinen L. Semliki Forest virus mRNA capping enzyme requires association with anionic membrane phospholipids for activity. EMBO J. 1999;11:3164–3172. doi: 10.1093/emboj/18.11.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahola T, Kujala P, Tuittila M, Blom T, Laakkonen P, Hinkkanen A, Auvinen P. Effects of palmitoylation of replicase protein nsP1 on alphavirus infection. J Virol. 2000;74:6725–6733. doi: 10.1128/jvi.74.15.6725-6733.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen D. Mapping the lipid distribution in the membranes of BHK cells. Mol Membr Biol. 1996;13:81–84. doi: 10.3109/09687689609160580. [DOI] [PubMed] [Google Scholar]
- 7.Andrews N W. Regulated secretion of conventional lysosomes. Trends Cell Biol. 2000;10:316–321. doi: 10.1016/s0962-8924(00)01794-3. [DOI] [PubMed] [Google Scholar]
- 8.Barton D J, Sawicki S G, Sawicki D L. Solubilization and immunoprecipitation of alphavirus replication complexes. J Virol. 1991;65:1496–1506. doi: 10.1128/jvi.65.3.1496-1506.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bienz K, Egger D, Pfister T, Troxler M. Structural and functional characterization of the poliovirus replication complex. J Virol. 1992;66:2740–2747. doi: 10.1128/jvi.66.5.2740-2747.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bucci C, Thompsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell. 2000;11:467–480. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doedens J R, Kirkegaard K. Inhibition of cellular protein secretion by poliovirus proteins 2B and 3A. EMBO J. 1995;14:894–907. doi: 10.1002/j.1460-2075.1995.tb07071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doedens J R, Giddings T H, Kirkegaard K. Inhibition of endoplasmic reticulum-to-Golgi traffic by poliovirus protein 3A: genetic and ultrastructural analysis. J Virol. 1997;71:9054–9064. doi: 10.1128/jvi.71.12.9054-9064.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Escola J-M, Kleijmeer M J, Stoorvogel W, Griffith J, Yoshie O, Geuze H J. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 14.Friedman R M, Levin J G, Grimley P M, Berezesky I K. Membrane-associated replication complex in arbovirus infection. J Virol. 1972;10:504–515. doi: 10.1128/jvi.10.3.504-515.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Froshauer S, Kartenbeck J, Helenius A. Alphavirus RNA replicase is located on the cytoplasmic surface of endosomes and lysosomes. J Cell Biol. 1988;107:2075–2086. doi: 10.1083/jcb.107.6.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez De Cedron M, Ehsani N, Mikkola M L, García J A, Kääriäinen L. RNA helicase activity of Semliki Forest virus replicase protein nsP2. FEBS Lett. 1999;448:19–22. doi: 10.1016/s0014-5793(99)00321-x. [DOI] [PubMed] [Google Scholar]
- 17.Grimley P M, Berezesky I K, Friedman R M. Cytoplasmic structures associated with an arbovirus infection: loci of viral ribonucleic acid synthesis. J Virol. 1968;2:326–338. doi: 10.1128/jvi.2.11.1326-1338.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimley P M, Levin J G, Berezesky I K, Friedman R M. Specific membranous structures associated with the replication of group A arboviruses. J Virol. 1972;10:1326–1338. doi: 10.1128/jvi.10.3.492-503.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu F, Gruenberg J. Biogenesis of transport intermediates in the endocytic pathway. FEBS Lett. 1999;452:61–66. doi: 10.1016/s0014-5793(99)00561-x. [DOI] [PubMed] [Google Scholar]
- 20.Kääriäinen L, Söderlund H. Structure and replication of alphaviruses. Curr Top Microbiol Immunol. 1978;82:15–69. doi: 10.1007/978-3-642-46388-4_2. [DOI] [PubMed] [Google Scholar]
- 21.Karlsson K, Carlsson S R. Sorting of lysosomal membrane glycoproteins lamp-1 and lamp-2 into vesicles distinct from mannose 6-phosphate receptor/g-Adaptin vesicles at the trans-Golgi network. J Biol Chem. 1998;273:18966–18973. doi: 10.1074/jbc.273.30.18966. [DOI] [PubMed] [Google Scholar]
- 22.Keränen S, Kääriäinen L. Isolation and basic characterization of temperature-sensitive mutants from Semliki Forest virus. Acta Pathol Microbiol Scand Sect B. 1974;82:810–820. doi: 10.1111/j.1699-0463.1974.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 23.Kleijmeer M J, Morkowski S, Griffith J M, Rudensky A Y, Geuze H J. Major histocompatibility complex class II compartments in human and mouse B lymphoblasts represent conventional endocytic compartments. J Cell Biol. 1997;139:639–649. doi: 10.1083/jcb.139.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi T, Beuchat M H, Lindsay M, Frias S, Palmiter R D, Sakuraba H, Parton R G, Gruenberg J. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat Cell Biol. 1999;1:113–118. doi: 10.1038/10084. [DOI] [PubMed] [Google Scholar]
- 25.Kujala P, Ahola T, Ehsani N, Auvinen P, Vihinen H, Kääriäinen L. Intracellular distribution of rubella virus nonstructural protein P150. J Virol. 1999;73:7805–7811. doi: 10.1128/jvi.73.9.7805-7811.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kujala P, Rikkonen M, Ahola T, Kelve M, Saarma M, Kääriäinen L. Monoclonal antibodies specific for Semliki Forest virus replicase protein nsP2. J Gen Virol. 1997;78:343–351. doi: 10.1099/0022-1317-78-2-343. [DOI] [PubMed] [Google Scholar]
- 27.Laakkonen P, Ahola T, Kääriäinen L. The effects of palmitoylation on membrane association of Semliki Forest virus RNA capping enzyme. J Biol Chem. 1996;271:28567–28571. doi: 10.1074/jbc.271.45.28567. [DOI] [PubMed] [Google Scholar]
- 28.Laakkonen P, Auvinen P, Kujala P, Kääriäinen L. Alphavirus replicase protein nsP1 induces filopodia and rearrangement of actin filaments. J Virol. 1998;72:10265–10269. doi: 10.1128/jvi.72.12.10265-10269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laakkonen P, Hyvönen M, Peränen J, Kääriäinen L. Expression of Semliki Forest virus nsP1-specific methyltransferase in insect cells and in Escherichia coli. J Virol. 1994;68:7418–7425. doi: 10.1128/jvi.68.11.7418-7425.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lachmi B-E, Kääriäinen L. Control of protein synthesis in Semliki Forest virus-infected cells. J Virol. 1977;22:142–149. doi: 10.1128/jvi.22.1.142-149.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lampio A, Kilpeläinen I, Pesonen S, Karhi K, Auvinen P, Somerharju P, Kääriäinen L. Membrane-binding mechanism of an RNA virus-capping enzyme. J Biol Chem. 2000;275:37853–37859. doi: 10.1074/jbc.M004865200. [DOI] [PubMed] [Google Scholar]
- 32.Lee J-Y, Marshall J A, Bowden D S. Characterization of rubella virus replication complexes using antibodies to double-stranded RNA. Virology. 1994;200:307–312. doi: 10.1006/viro.1994.1192. [DOI] [PubMed] [Google Scholar]
- 33.Liou W, Geuze H J, Slot J W. Improving structural integrity of cryosections for immunogold labeling. J Histochem Cytochem. 1996;106:41–58. doi: 10.1007/BF02473201. [DOI] [PubMed] [Google Scholar]
- 34.Mackenzie J M, Jones M K, Westaway E G. Markers for trans-Golgi membranes and the intermediate compartment localize to induced membranes with distinct replication functions in flavivirus-infected cells. J Virol. 1999;73:9555–9567. doi: 10.1128/jvi.73.11.9555-9567.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magliano D, Marshall J A, Bowden D S, Vardaxis N, Meanger J, Lee J-Y. Rubella virus replication complexes are virus-modified lysosomes. Virology. 1998;240:57–63. doi: 10.1006/viro.1997.8906. [DOI] [PubMed] [Google Scholar]
- 36.Mas P, Beachy R N. Replication of tobacco mosaic virus on endoplasmic reticulum and role of the cytoskeleton and virus movement protein in intracellular distribution of viral RNA. J Cell Biol. 1999;147:945–958. doi: 10.1083/jcb.147.5.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mullock B M, Brigth N A, Fearon C W, Gray S R, Luzio J P. Fusion of lysosomes with late endosomes produces a hybrid organelle of intermediate density and is NSF dependent. J Cell Biol. 1998;140:591–601. doi: 10.1083/jcb.140.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pedersen K W, van der Meer Y, Roos N, Snijder E J. Open reading frame 1a-encoded subunits of the arterivirus replicase induce endoplasmic reticulum-derived double-membrane vesicles which carry the viral replication complex. J Virol. 1999;73:2016–2026. doi: 10.1128/jvi.73.3.2016-2026.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peränen J, Kääriäinen L. Biogenesis of type I cytopathic vacuoles in Semliki Forest virus-infected BHK cells. J Virol. 1991;65:1623–1627. doi: 10.1128/jvi.65.3.1623-1627.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peränen J, Laakkonen P, Hyvönen M, Kääriäinen L. The alphavirus replicase protein nsP1 is membrane-associated and has affinity to endocytic organelles. Virology. 1995;208:610–620. doi: 10.1006/viro.1995.1192. [DOI] [PubMed] [Google Scholar]
- 41.Peränen J, Rikkonen M, Liljeström P, Kääriäinen L. Nuclear localization of Semliki Forest virus-specific nonstructural protein nsP2. J Virol. 1990;64:1888–1896. doi: 10.1128/jvi.64.5.1888-1896.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peränen J, Takkinen K, Kalkkinen N, Kääriäinen L. Semliki Forest virus-specific non-structural protein nsP3 is a phosphoprotein. J Gen Virol. 1988;69:2165–2178. doi: 10.1099/0022-1317-69-9-2165. [DOI] [PubMed] [Google Scholar]
- 43.Ranki M, Kääriäinen L. Solubilized RNA replication complex from Semliki Forest virus-infected cells. Virology. 1979;98:298–307. doi: 10.1016/0042-6822(79)90553-1. [DOI] [PubMed] [Google Scholar]
- 44.Restrepo-Hartwig M A, Ahlquist P. Brome mosaic virus helicase-and polymerase-like proteins colocalize on the endoplasmic reticulum at sites of viral RNA synthesis. J Virol. 1996;70:8908–8916. doi: 10.1128/jvi.70.12.8908-8916.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rikkonen M, Peränen J, Kääriäinen L. Nuclear and nucleolar targeting signals of Semliki Forest virus nonstructural protein nsP2. Virology. 1992;189:462–473. doi: 10.1016/0042-6822(92)90570-f. [DOI] [PubMed] [Google Scholar]
- 46.Rikkonen M, Peränen J, Kääriäinen L. ATPase and GTPase activities associated with Semliki Forest virus nonstructural protein nsP2. J Virol. 1994;68:5804–5810. doi: 10.1128/jvi.68.9.5804-5810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schaad M C, Jensen P E, Carrington J C. Formation of plant RNA virus replication complexes on membranes: role of an endoplasmic reticulum-targeted viral protein. EMBO J. 1997;16:4049–4059. doi: 10.1093/emboj/16.13.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlegel A, Giddings J T H, Ladinsky M S, Kirkegaard K. Cellular origin and ultrastructure of membranes induced during poliovirus infection. J Virol. 1996;70:6576–6588. doi: 10.1128/jvi.70.10.6576-6588.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slot J W, Geuze H J. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol. 1985;38:87–93. [PubMed] [Google Scholar]
- 50.Soldati T, Rancano C, Geissler H, Pfeffer S R. Rab7 and Rab9 are recruited onto late endosomes by biochemically distinguishable processes. J Biol Chem. 1995;270:25541–25548. doi: 10.1074/jbc.270.43.25541. [DOI] [PubMed] [Google Scholar]
- 51.Stinchcombe J C, Griffiths G M. Regulated secretion from hemopoietic cells. J Cell Biol. 1999;147:1–5. doi: 10.1083/jcb.147.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strauss J H, Strauss E G. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tokuyasu K T. A technique for ultracryotomy of cell suspensions and tissues. J Cell Biol. 1973;57:551–565. doi: 10.1083/jcb.57.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tokuyasu K T. Use of polyvinylpyrrolidone and polyvinyl alcohol for cryoultratomy. J Histochem Cytochem. 1989;21:163–171. [Google Scholar]
- 55.van der Meer Y, van Tol H G, Krijnse Locker J, Snijder E J. ORF1a-encoded replicase subunits are involved in the membrane association of the arterivirus replication complex. J Virol. 1998;72:6689–6698. doi: 10.1128/jvi.72.8.6689-6698.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Meer Y, Snijder E J, Dobbe J C, Schleich S, Denison M R, Spaan W J M, Krijnse Locker J. Localization of mouse hepatitis virus nonstructural proteins and RNA synthesis indicates a role for late endosomes in viral replication. J Virol. 1999;73:7641–7657. doi: 10.1128/jvi.73.9.7641-7657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vasiljieva L, Merits A, Auvinen P, Kääriäinen L. Identification of a novel function of the alphavirus capping apparatus—RNA 5′ triphosphatase activity of nsP2. J Biol Chem. 2000;275:17281–17287. doi: 10.1074/jbc.M910340199. [DOI] [PubMed] [Google Scholar]
- 58.Vihinen H, Saarinen J. Phosphorylation site analysis of Semliki Forest virus nonstructural protein 3. J Biol Chem. 2000;275:27775–27783. doi: 10.1074/jbc.M002195200. [DOI] [PubMed] [Google Scholar]
- 59.Vitelli R, Santillo M, Lattero D, Chiariello M, Bifulco M, Bruni C B, Bucci C. Role of the small GTPase rab7 in the late endocytic pathway. J Biol Chem. 1997;272:4391–4397. doi: 10.1074/jbc.272.7.4391. [DOI] [PubMed] [Google Scholar]
- 60.Westaway E G, Mackenzie J M, Kenney M T, Jones M K, Khromykh A A. Ultrastructure of Kunjin virus-infected cells: colocalization of NS1 and NS3 with double-stranded RNA, and NS2B with NS3, in virus-induced membrane structures. J Virol. 1997;71:6650–6661. doi: 10.1128/jvi.71.9.6650-6661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]