Abstract
Background
Castor is an important industrial raw material. Drought-induced oxidative stress leads to slow growth and decreased yields in castor. However, the mechanisms of drought-induced oxidative stress in castor remain unclear. Therefore, in this study, physiological, biochemical, and RNA-seq analyses were conducted on the roots of castor plants under PEG-6000 stress for 3 d and 7 d followed by 4 d of hydration.
Results
The photosynthetic rate of castor leaves was inhibited under PEG-6000 stress for 3 and 7 d. Biochemical analysis of castor roots stressed for 3 d and 7 d, and rehydrated for 4 d revealed that the activities of APX and CAT were highest after only 3 d of stress, whereas the activities of POD, GR, and SOD peaked after 7 d of stress. RNA-seq analysis revealed 2926, 1507, and 111 differentially expressed genes (DEGs) in the roots of castor plants under PEG-6000 stress for 3 d and 7 d and after 4 d of rehydration, respectively. GO analysis of the DEGs indicated significant enrichment in antioxidant activity. Furthermore, KEGG enrichment analysis of the DEGs revealed significantly enriched metabolic pathways, including glutathione metabolism, fatty acid metabolism, and plant hormone signal transduction. WGCNA identified the core genes PP2C39 and GA2ox4 in the navajowhite1 module, which was upregulated under PEG-6000 stress. On the basis of these results, we propose a model for the response to drought-induced oxidative stress in castor.
Conclusions
This study provides valuable antioxidant gene resources, deepening our understanding of antioxidant regulation and paving the way for further molecular breeding of castor plants.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-05691-4.
Keywords: Castor, PEG stress, RNA-seq
Background
Castor is an annual or perennial herbaceous plant in the Euphorbiaceae family that is widely distributed in tropical and subtropical regions worldwide [1]. The seeds are the most important part of the castor plant, with a broad range of industrial, agricultural, and medical applications. The castor oil produced from the seeds of castor plants is an important industrial raw material. The proportion of ricinoleic acid, a rare hydroxyl fatty acid, in castor oil is more than 90% [2]. Ricinoleic acid enables the use of castor oil in a wide range of industrial applications, including the production of lubricants, coatings, paints, plastics, and biodiesel [3]. The unique chemical properties of ricinoleic acid allow the production of lubricants with good stability under high-temperature conditions, and it can be used in lubricating oils for aircraft and high-performance engines. The ricinoleic acid in castor oil is considered to have anti-inflammatory and antibacterial properties and has been applied in research on and the production of several drugs [4]. Castor plants grow rapidly, exhibit strong adaptability, and can survive in relatively poor soil; thus, they also play a role in agriculture. However, drought leads to low plant survival in castor seedlings, which in turn affects castor yield.
In recent years, with increased frequency of extreme weather events, the number of drought disasters has increased annually. One-third of the Earth’s land area exhibits low plant productivity due to low rainfall. With global warming, arid and semiarid areas are becoming more drought prone, posing a serious threat to food production. The cultivation of castor in China is concentrated mainly in the eastern regions of Inner Mongolia and the western regions of Jilin, Shanxi, and Xinjiang. However, these planting areas are mostly arid and semiarid, and the low average rainfall affects the yield of castor. Therefore, breeding new high-yield and stress-resistant varieties is highly important for meeting industrial demand and increasing farmer incomes.
The photosynthetic efficiency of plants is decreased in arid environments, leading to the accumulation of electrons in the electron transfer chain, which can react with oxygen to form reactive oxygen species (ROS). Moreover, drought stress can also cause peroxidation of cell membrane lipids, which in turn leads to the generation of additional ROS. Notably, plant cells have an effective antioxidant system specifically designed to clear these ROS. Although their high concentrations can cause damage to cells, at low concentrations, they can act as signaling molecules and participate in regulating plant stress responses to drought. Therefore, the dynamic balance of intracellular ROS is crucial for plants to respond effectively to drought stress. Research has shown that under drought conditions, plants maintain cell membrane stability by preventing lipid peroxidation of the cell membrane, and the synthesis of unsaturated fatty acids plays a key role in maintaining cell membrane stability [5–7]. In addition, hormone signal transduction also plays an important role in the plant response to drought stress. For example, type 2 C protein phosphatase (PP2C) can negatively regulate the synthesis of the hormone abscisic acid (ABA), which can increase the activity of antioxidant enzymes, promote stomatal closure, and reduce CO2 fixation, helping inhibit the accumulation of ROS [8]. In addition, ethylene-responsive factors (ERFs), an important class of transcription factors, participate in ethylene signal transduction and can activate the expression of certain antioxidant enzyme-encoding genes, helping to eliminate ROS produced under drought stress. For example, in poplar, the overexpression of ERF194 can increase the activity of antioxidant enzymes (superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT)) and promote the clearance of ROS [9]. In rice, OsERF101 can increase the proline content and CAT activity of plants under drought stress, helping them cope with drought [10].
As a rapid research tool, RNA-seq can reveal new genes involved in stress resistance, which can serve as candidate genes for generating new varieties for use in plant molecular breeding. RNA-seq has been used to study gene expression under drought stress in soybean, potato, foxtail millet, and sweet potato [11–14]. However, the mechanism of drought resistance in castor seedlings is still unclear. We investigated the transcriptional response of castor seedlings under drought stress via RNA-seq technology and identified important drought-responsive genes and regulatory mechanisms. This study provides detailed genetic resources for the molecular breeding of drought-resistant castor plants.
Methods
Experimental sample collection
The castor CSR•181 seeds were provided by the Tongliao Agricultural Science Research Institute. Castor seeds were sown in 7 × 7 cm square nursery pots with vermiculite as the germination substrate and watered every three days with Hoagland’s nutrient solution. After the castor seedlings emerged, they were transferred to a hydroponic system containing Hoagland’s nutrient solution and cultured for three weeks. The Hoagland’s nutrient solution was supplemented with 15% PEG-6000 to stress the castor seedlings for 3 and 7 d, followed by rehydration in hydroponic Hoagland’s nutrient solution for 4 d (for a total of 11 d). The control group seedlings were cultured in hydroponic Hoagland’s nutrient solution throughout. The nutrient solution was refreshed twice weekly. All castor seedlings were cultured in a cultivation room with a dark/light cycle of 8/16 hours, a temperature of 25 °C, and 60% humidity. Root samples from both the treatment and control groups were collected at each time point, with six biological replicates per time point. The samples were frozen in liquid nitrogen and stored at -80 °C.
Determination of physiological and biochemical indicators of castor root systems
In this study, six plants were randomly selected from each treatment group for analysis. The castor growth parameters examined included plant height, stem diameter, and leaf area. Both plant height and stem diameter were measured using a caliper. The leaf area was measured using a leaf area meter (Li-3100 C Area Meter). The Li-3100 C Area Meter was turned on, after which zero-point calibration and full-scale calibration were performed, and the leaf area of the fourth true leaf of the plant was measured.
The respiration rate was measured via an oxygen electrode (Hansatech). A 5 cm segment of castor root was finely chopped and placed into the reaction chamber, followed by the addition of 2 ml of buffer solution (10 mM HEPES, 10 mM MES, 2 mM CaCl2; pH 7.5). Then, 50 µmol of salicylhydroxamic acid (SHAM) was added after 1 min, and 100 µmol of NaN3 solution was added after 2 min. The measurement was completed at 3 min.
Photosynthetic parameters were measured via a Li-6400 portable photosynthesis system. On the day of the experiment, at 10:00 AM, the youngest fourth true leaf was selected, and the photosynthetic rate was measured at the middle part of the leaf using the Li-6400 instrument, where the distance between chambers was < 10 cm. The Li-6400 in-chamber conditions were as follows: LED light intensity of 1,200 µmol m− 2 s− 1, reference CO2 concentration of 500 ppm, flow rate of 400 µmol s− 1, and VPD controlled at 2.1 ± 0.2 kPa.
Hydrogen peroxide (H2O2) was detected spectrophotometrically as described by Willekens et al. [15]. The 100 µmol/L H2O2 acetone reagent was prepared by diluting a 10 mmol/L H2O2 solution to 100 µmol/L with acetone. The standard curve for H2O2 concentration determination was performed as follows: the reaction buffer was prepared by mixing precooled acetone (mL), 5% titanium sulfate (mL), and concentrated ammonia (mL) in a 10:1:2 ratio. A total of 1.3 mL of the reaction buffer was added to each of seven centrifuge tubes. Next, 0 mL, 0.1 mL, 0.2 mL, 0.4 mL, 0.6 mL, 0.8 mL, or 1.0 mL of the prepared 100 µmol/L H2O2 acetone reagent was added to the tubes. The mixture was allowed to stand for 10 min and then centrifuged at 12,000 × g for 10 min. The supernatant was discarded, and the precipitate was retained. Five milliliters of 2 mol/L sulfuric acid was added to the precipitate. After the precipitate dissolved, the volume was increased to 10 mL with ddH2O. The absorbance at 415 nm was measured via a Bio-Rad Microplate Reader 550, and the standard curve was plotted. To prepare the sample extraction liquid, 3 g of castor roots was mixed with 3 mL of precooled acetone and a small amount of quartz sand, the mixture was ground to a homogeneous slurry, and the slurry was transferred to a centrifuge tube. The mixture was centrifuged at 12,000 × g for 10 min, and the supernatant was collected. Then, 0.1 mL of 5% titanium sulfate and 0.2 mL of concentrated ammonia were added to 1 mL of the sample extraction liquid. The mixture was allowed to stand for 10 min and then centrifuged at 12,000 × g for 10 min. The supernatant was discarded, and the precipitate was retained. Five milliliters of 2 mol/L sulfuric acid was added to the precipitate. Once dissolved, the absorbance at 415 nm was measured via a microplate reader, and the H2O2 content in the roots was calculated on the basis of the standard curve.
The MDA content was estimated via the method developed by Wang et al. [16]. Four milliliters of PBS (136.9 mmol NaCl, 2.7 mmol KCl, 10.1 mmol Na₂HPO₄, 1.8 mmol KH₂PO₄; pH 7.4) and a small amount of quartz sand were added to 0.5 g of castor root, the mixture was ground to a homogeneous mixture, and the mixture was transferred to a centrifuge tube. The mixture was centrifuged at 12,000 × g for 10 min, after which the supernatant was collected. One milliliter of the supernatant was mixed with 2 mL of 0.5% thiobarbituric acid, heated at 100 °C for 15 min, and immediately cooled on ice to stop the reaction. The mixture was centrifuged again at 12,000 × g for 10 min, and the supernatant was collected. The MDA content was quantified by measuring the absorbance at 450, 532, and 600 nm. The MDA content is expressed as µmol g− 1 FW.
SOD (EC1.15.1.1) activity was measured according to the methods of Beauchamp and Fridovich [17]. Four milliliters of PBS (136.9 mmol NaCl, 2.7 mmol KCl, 10.1 mmol Na₂HPO₄, 1.8 mmol KH₂PO₄; pH 7.4) and a small amount of quartz sand were added to 0.5 g of castor root, the mixture was ground to a homogeneous mixture, and the mixture was transferred to a centrifuge tube. The mixture was centrifuged at 12,000 × g for 10 min, after which the supernatant was collected. The reaction mixture included 200 µl of enzyme extract, 400 µl of KH₂PO₄ (100 mM, pH 7.0), 50 µl of methionine (260 mM), 80 µl of NBT (4.2 mM), 10 µl of EDTA (10 mM), and 170 µl of riboflavin (130 µM). The mixture was illuminated for 15 min with a cool white fluorescent lamp providing light at 60 µmol m− 2 s− 1. The absorbance of each sample was recorded at a wavelength of 560 nm via a microplate reader. SOD activity is expressed as units of SOD activity per mg of protein per minute (U mg− 1 protein min− 1).
POD (EC 1.11.1.7) activity was measured via the method developed by Ashraf et al. [18]. Preparation of 0.01 mol of phosphate buffer was performed by mixing 61.0 mL of Na₂HPO₄ (0.1 mol) and 39.0 mL of KH₂PO₄ (0.1 mol). Then, ddH2O was added, and the volume was increased to 1000 mL. A total of 0.5 g of castor root was added to 4 mL of PBS (0.01 mol) along with a small amount of quartz sand, and the mixture was ground to a homogeneous paste. The mixture was transferred to a centrifuge tube and centrifuged at 12,000 × g for 10 min, after which the supernatant was collected. The reaction mixture included 100 µl of enzyme extract, 50 µl of guaiacol (0.02 mol), 2.9 mL of phosphate buffer (0.01 mol), and 10 µl of H2O2 (0.04 mol). The absorbance of each sample was measured at a wavelength of 470 nm via a microplate reader. POD activity is expressed in units of POD activity per mg of protein per min (U mg− 1 protein min− 1).
CAT (EC 1.11.1.6) activity was assayed according to the methods of Havir and Mchale with some modifications [19]. First, 0.5 g of castor root was added to 4 mL of PBS (0.2 mol, pH 7.8) along with a small amount of quartz sand. The mixture was ground to a homogeneous paste and then transferred to a centrifuge tube. The mixture was then centrifuged at 12,000 × g for 10 min, and the supernatant was collected. The enzyme extract (2.5 mL) was added to 2.5 mL of H2O2 (0.1 mol) and incubated in a 30 °C water bath for 10 min. Then, 2.5 mL of H2SO4 (10%) was added, and the mixture was titrated with 0.1 mol of KMnO4 until a pink color appeared. The absorbance of each sample was recorded at a wavelength of 240 nm via a spectrophotometer. CAT activity is expressed as the amount of H2O2 decomposed per mg of protein per min (µmol min⁻¹ pro mg⁻¹).
Glutathione reductase (GR, EC 1.6.4.2) activity was determined by NADPH oxidation [20]. A total of 0.5 g of castor root was added to 4 mL of PBS (50 mM, pH 7.8) along with a small amount of quartz sand. The mixture was ground to a homogeneous paste and then transferred to a centrifuge tube. The mixture was then centrifuged at 12,000 × g for 10 min, and the supernatant was collected. The reaction mixture included 200 µL of enzyme extract, 1.7 mL of HEPES, 0.1 mL of NADPH (2.4 mM), and 0.1 mL of GSSG (10 mM). The absorbance of each sample was measured at 340 nm via a microplate reader. GR activity is expressed as the amount of NADPH decomposed per mg of protein per min (µmol min⁻¹ pro mg⁻¹).
An ascorbate peroxidase (APX, EC1.11.1.11) activity assay was performed according to the methods of Nakano and Asada with some modifications [21]. Four milliliters of PBS (50 mM) and a small amount of quartz sand were added to 0.5 g of castor root. The mixture was ground to a homogenate and transferred to a centrifuge tube. The mixture was then centrifuged at 12,000 × g for 10 min, after which the supernatant was collected. The reaction mixture included 100 µL of enzyme extract, 1.7 mL of EDTA-Na2 (0.1 mM), 100 µL of ASA (5 mM), and 100 µL of H2O2 (20 mM). The absorbance of each sample was recorded via a microplate reader at a wavelength of 290 nm. APX activity is expressed as the amount of ASA decomposed per mg of protein per min (µmol min⁻¹ pro mg⁻¹).
The physiological and biochemical data for castor roots were subjected to one-way analysis of variance (ANOVA), followed by Duncan’s multiple range test to evaluate the mean differences at a significance level of 0.05. The significance levels (p) were used to evaluate the significance of the parameters estimated.
RNA extraction, Illumina sequencing, and data quality control
Total RNA from castor roots was extracted via the TRIzol method [22]. A Dynabeads™ mRNA Purification Kit (Thermo Fisher) was used to isolate and purify mRNA from the total RNA of castor roots. The enriched mRNA was fragmented in fragmentation buffer and reverse transcribed to cDNA with random primers. Second-strand cDNA was synthesized by DNA polymerase I, RNase H, dNTPs, and buffer. After the cDNA fragments were purified with a QIAquick PCR Purification Kit, the ends were repaired, poly(A) tails were added, and the fragments were ligated to Illumina sequencing adapters. The ligation products were size-selected via agarose gel electrophoresis, amplified by PCR, and sequenced with an Illumina HiSeq™ 2500 system by Gene Denovo Biotechnology Co. (Guangzhou, China).
Gene annotation and functional characterization
The sequencing reads were subjected to quality control analysis for the removal of short and low-quality reads (those containing adapters, more than 10% unknown nucleotides, or more than 50% low-quality (Q value ≤ 20) bases). All raw data were deposited in the NCBI Gene Expression Omnibus (GEO) with the accession number GSE252706. To identify gene transcripts, all of the reconstructed transcripts were aligned to the reference genome (NCBI: ASM1957865v1) and divided into twelve categories via Cuffcompare. The genes with the class code “u” were unknown transcripts or transcripts from intergenic regions. The edge R package (http://www.rproject.org/) was used to identify differentially expressed genes (DEGs) across samples or groups. Significant DEGs were defined as genes with a |log2FC|>1 and an FDR < 0.05. GO enrichment analysis was performed on the Gene Ontology Consortium website (http://geneontology.org/). KEGG pathway enrichment analyses of DEGs were performed using the KEGG pathway database (https://www.kegg.jp/kegg/pathway.html).
qRT‒PCR (real-time PCR) analysis
The qRT‒PCR primers used were designed via NCBI (https://www.ncbi.nlm.nih.gov/) Primer-BLAST (Table S1). The settings used for primer design in Primer-BLAST were as follows: product length: 100–200 bp, spanning exons and introns; organism: castor bean (taxid: 3988); Tm: 58–62 °C. qRT‒PCR was performed with SYBR Premix TB Green™ Premix Ex Taq™ II (TaKaRa) via an ABI PRISM 7500 Sequence Detection System (Applied Biosystems). The qRT‒PCR mixture contained the following: 100 ng of cDNA, 0.25 µM forward and reverse primers, 10 µL of SYBR, and ddH2O to a total volume of 20 µL. The reaction program was as follows: 95 °C for 5 min; 40 amplification cycles of 95 °C for 10 s, 60 °C for 30 s, and 72 °C for 15 s. The relative expression levels of the DEGs were measured via the ΔΔCT method [23], and the 18 S rRNA gene was used as a reference for data normalization.
Results
Phenotypic changes in castor seedlings under PEG-6000 stress
Under PEG stress, the growth of castor plants was inhibited, and the plant height, leaf area, and stem diameter were lower than those in the control group (Fig. S1). Additionally, as the duration of PEG-6000 stress increased, the respiration rate of castor roots continuously increased (Fig. 1H). To examine the effects of PEG stress on photosynthesis (Pn, Gs, Ci, and Tr) in castor plants, we measured the dynamic parameters Pn, Gs, Ci, and Tr (Table 1). Photosynthesis (Pn, Gs, Ci, and Tr) under PEG stress on day 7 was significantly lower than that on day 3, and recovery of photosynthesis (Pn, Gs, Ci, and Tr) was observed after PEG was removed (11 d).
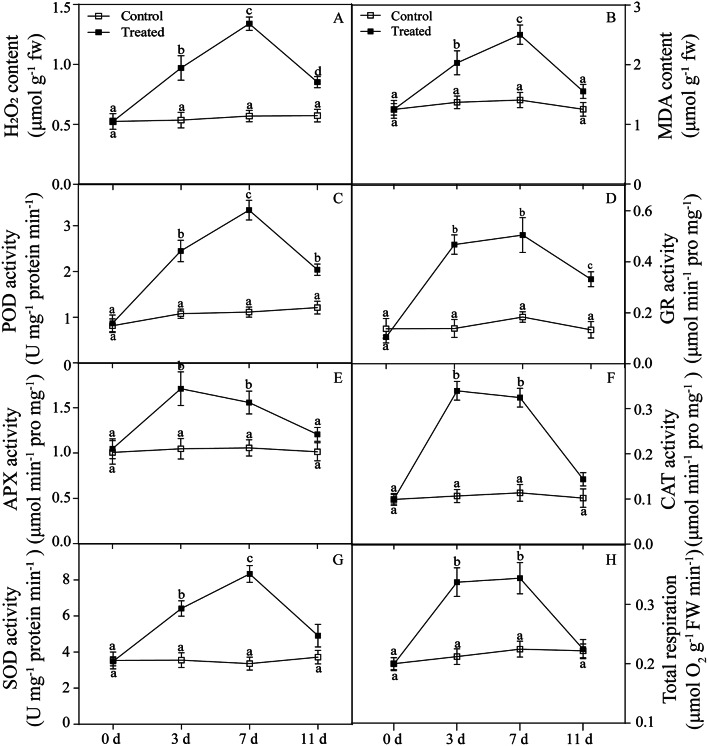
Fig. 1.
Determination of biochemical indices in castor roots under PEG-6000 stress. Different letters above the bars indicate statistically significant differences between different days at P < 0.05 according to Duncan’s multiple range test
Table 1.
The effect of PEG-6000 stress on the photosynthesis of castor
|
Pn (µmol m− 2 s− 1) |
Gs (nmol m− 2 s− 1) |
Ci (µmol mol− 1) |
Tr (µmol m− 2 s− 1) |
|
|---|---|---|---|---|
| CK 0d | 2.13 ± 0.47a | 24.11 ± 1.12b | 465.33 ± 32.19d | 0.59 ± 0.03ab |
| CK 3d | 4.97 ± 1.75b | 50.22 ± 16.53c | 262.67 ± 48.38b | 1.67 ± 0.7c |
| T 3d | 1.72 ± 1.13a | 7.05 ± 3.28a | 163.08 ± 57.95a | 0.21 ± 0.09a |
| CK 7d | 6.46 ± 1.33b | 52.46 ± 9.64c | 291.69 ± 14.26b | 1.17 ± 0.19bc |
| T 7d | 1.24 ± 0.15a | 5.13 ± 1.87a | 145.82 ± 44.31a | 0.20 ± 0.14a |
| CK 11d | 9.49 ± 0.96c | 61.32 ± 9.57c | 321.44 ± 9.12ab | 1.7 ± 0.22c |
| T 11d | 5.43 ± 0.02b | 40.05 ± 2.87 c | 259.57 ± 18.85b | 1.08 ± 0.05bc |
Photosynthetic rate (Pn), stomatal conductance (Gs), intercellular CO2 concentration (Ci), and transpiration (Tr) values are presented as the means ± SEs. According to Duncan’s multiple range test, values indicated by different letters within a column are significantly different (P < 0.05). CK represents the experimental control group, and T represents the 15% PEG-6000 stress group
The contents of H2O2 and MDA in the roots of castor plants significantly increased under PEG-6000 stress for 3 and 7 d compared with those in the control and decreased after 4 d of rehydration (11 d) (Fig. 1A and B). The contents of POD, GR, and SOD in the roots of castor plants under PEG-6000 stress for 3 and 7 d gradually increased (Fig. 1C and D, and Fig. 1G). However, under PEG-6000 stress, the enzymatic activities of APX and CAT in the castor roots of castor initially increased but then decreased. Although the difference between 3 d and 7 d was not significant, the changes during these two periods were very significant compared with those at 0 d (Fig. 1E and F).
Transcriptomic analysis of castor seedlings under PEG-600 stress
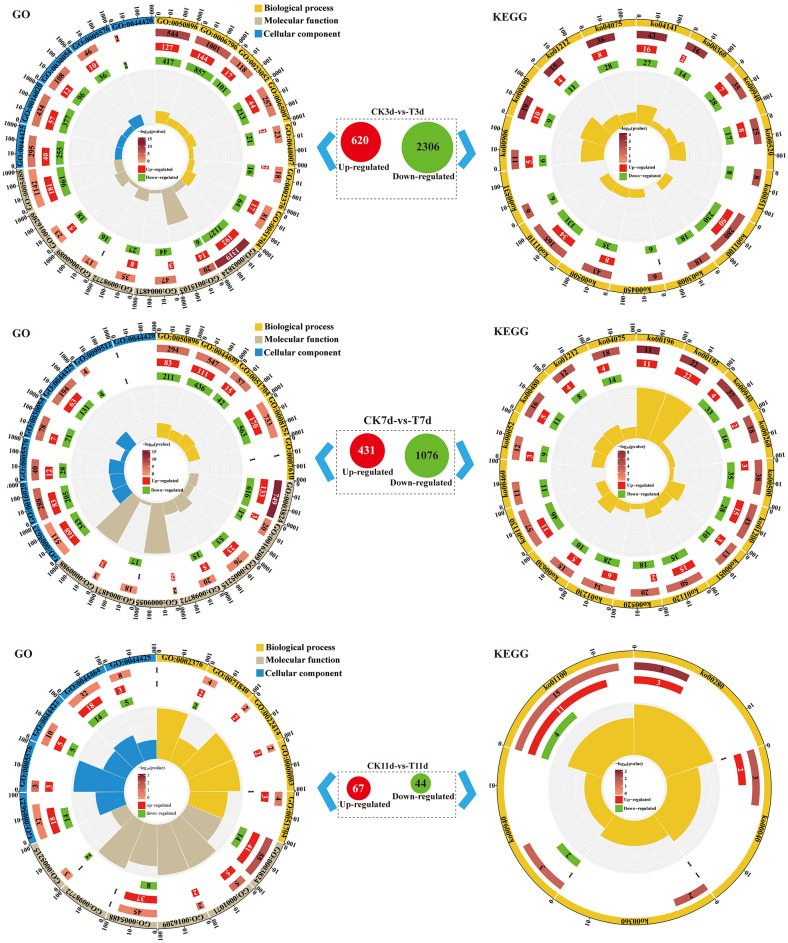
To evaluate the changes in gene expression in castor roots under PEG-6000 stress, we conducted RNA-seq analysis on roots subjected to PEG-6000 stress for 3 and 7 d and rehydrated for 4 d (for a total of 11 d). We used TRIzol™ to extract total RNA from each sample and sequenced the RNA on an Illumina HiSeq™ 2500 platform. Initially, transcriptome sequencing generated ~ 64.29 million raw reads and ~ 49.34 million clean reads for all the samples (Table S2). To identify genes related to drought resistance, we used edgeR (FDR < 0.05 and |log2FC|>1) to perform differential gene expression analysis between the treatment and control groups (Table S3). By comparing Ck3d with T3d, we identified 2,926 DEGs in castor roots, of which 620 were upregulated and 2,306 were downregulated. By comparing CK7d and T7d, we identified 1,507 DEGs, of which 431 were upregulated and 1076 were downregulated. A total of 111 DEGs were identified between Ck11d and T11d, of which 67 were upregulated and 44 were downregulated (Fig. 2).
Fig. 2.
GO and KEGG pathway analyses of the DEGs. GO:0050896, response to stimulus; GO:0006796, single-organism process; GO:0023052, signaling; GO:0065007, biological regulation; GO:0040007, growth; GO:0002376, immune system process; GO:0051704, multiorganism process; GO:0003824, catalytic activity; GO:0015103, transporter activity; GO:0004871, signal transducer activity; GO:0098772, molecular function regulator; GO:0060089, molecular transducer activity; GO:0016209, antioxidant activity; GO:0005488, binding; GO:0044425, membrane part; GO:0016020, membrane; GO:0030054, cell junction; GO:0005576, extracellular region; GO:0044420, extracellular matrix component; GO:0044699, single-organism process; GO:0008152, metabolic process; GO:0007610, behavior; GO:0005215, transporter activity; GO:0009055, electron carrier activity; GO:0000988, transcription factor activity, protein binding; GO:0005623, cell; GO:0099512, supramolecular fiber; GO:0071840, cellular component organization or biogenesis; GO:0022414, reproductive process; GO:0000003, reproduction; GO:0001071, nucleic acid binding transcription factor activity; GO:0044422, organelle part; GO:0044464, cell part. ko00360, phenylalanine metabolism; ko00450, selenocompound metabolism; ko00480, glutathione metabolism; ko00500, starch and sucrose metabolism; ko00511, other glycan degradation; ko00520, amino sugar and nucleotide sugar metabolism; ko00531, glycosaminoglycan degradation; ko00906, carotenoid biosynthesis; ko00940, phenylpropanoid biosynthesis; ko01100, metabolic pathways; ko01110, biosynthesis of secondary metabolites; ko01212, fatty acid metabolism; ko03008, ribosome biogenesis in eukaryotes; ko04075, plant hormone signal transduction; ko04141, protein processing in the endoplasmic reticulum; ko00196, photosynthesis - antenna proteins; ko00195, photosynthesis; ko00260, glycine, serine and threonine metabolism; ko01200, carbon metabolism; ko00051, fructose and mannose metabolism; ko01120, microbial metabolism in diverse environments; ko01230, biosynthesis of amino acids; ko00630, glyoxylate and dicarboxylate metabolism; ko01130, biosynthesis of antibiotics; ko00460, cyanoamino acid metabolism; ko00052, galactose metabolism; ko00280, valine, leucine and isoleucine degradation; ko00040, pentose and glucuronate interconversions
GO enrichment and KEGG pathway analysis of DEGs
We performed GO functional enrichment analyses of the DEGs in the castor root systems (Fig. 2 and Table S4-1). GO analyses of the 3, 7, and 11 d DEGs revealed 43, 44, and 28 enriched GO terms, respectively. The GO terms significantly enriched (P < 0.05) in the roots under PEG-6000 stress for 3 and 7 d included response to stimulus (GO:0050896), signaling (GO:0023052), biological regulation (GO:0065007), antioxidant activity (GO:0016209), and nucleic acid binding transcription factor activity (GO:0001071). The significant increase in antioxidant activity indicates that antioxidants play an important role in the response of castor plants to drought stress.
The metabolic pathways related to drought stress were identified via the KEGG database (Fig. 2 and Table S4-2). In castor roots subjected to PEG-6000 stress for 3 or 7 d, the metabolic pathways significantly enriched (P < 0.05) with DEGs included glutathione metabolism (ko:00480), fatty acid metabolism (ko:01212), and plant hormone signal transduction (ko:04075). The main metabolic pathway-enriched DEGs in the root system of castor after 4 d (11 d) of rehydration included valine, leucine, and isoleucine degradation (ko:00280); pentose and glucuronate conversion (ko:0040); phenoline metabolism (ko:00360); and phenylpropanoid biosynthesis (ko:00940).
Weighted gene coexpression network analysis
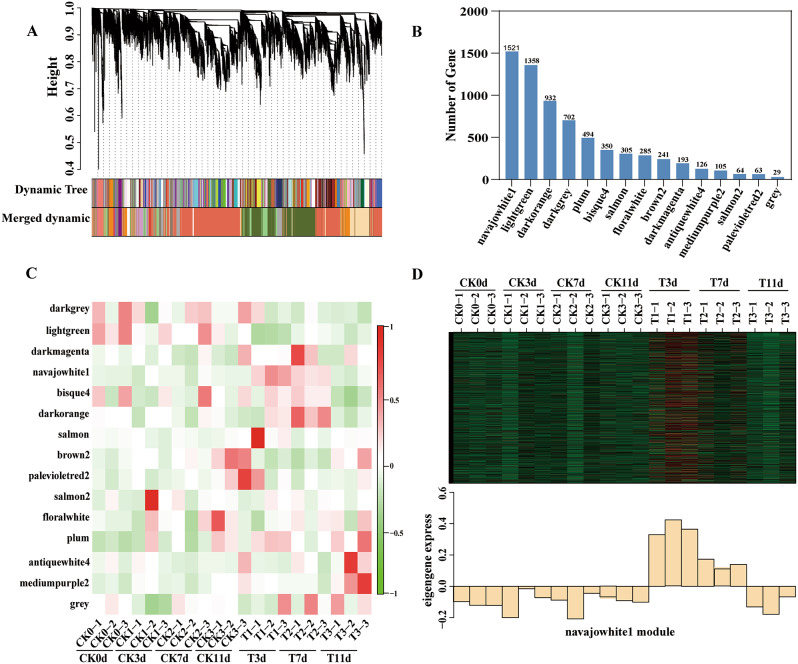
We utilized the R package to perform weighted gene coexpression network analysis (WGCNA) on the DEGs to identify different coexpression modules in castor roots under PEG stress. All the DEGs were assigned to fifteen modules, of which the navajowhite1 module contained the greatest number of genes (1521) (Fig. 3A and B). The navajowhite1 module genes were positively correlated with drought stress and are expected to be upregulated during drought stress to achieve drought tolerance (Fig. 3C and D).
Fig. 3.
WGCNA of DEGs in castor roots. A: Grouping of differential gene expression modules. B: DEGs enriched in each module. C: Matrix showing the module–trait relationships (MTRs) of different modules under control conditions and PEG stress. Positive correlation is represented in red, negative correlation in green, and no correlation in white. D: Expression pattern of the navajowhite1 module. The heatmap was plotted using the log10(FPKM) values
We plotted the log10(FPKM) (fragments per kilobase of exon model per million mapped fragments) values of the genes belonging to the navajowhite1 coexpressed module along with the eigengene expression values to further understand the specificity of the navajowhite1 coexpressed module with respect to the corresponding expression patterns in the different datasets (Fig. 3D). We observed that, in the navajowhite1 module, the gene expression level under drought stress was greater than that in the control, and there was no difference in the gene expression level between the rehydrated group and the control; these results confirmed the positive correlation observed earlier. The genes in the navajowhite1 module were more active early in the response to drought.
GO enrichment and pathway analysis of navajowhite1 module genes
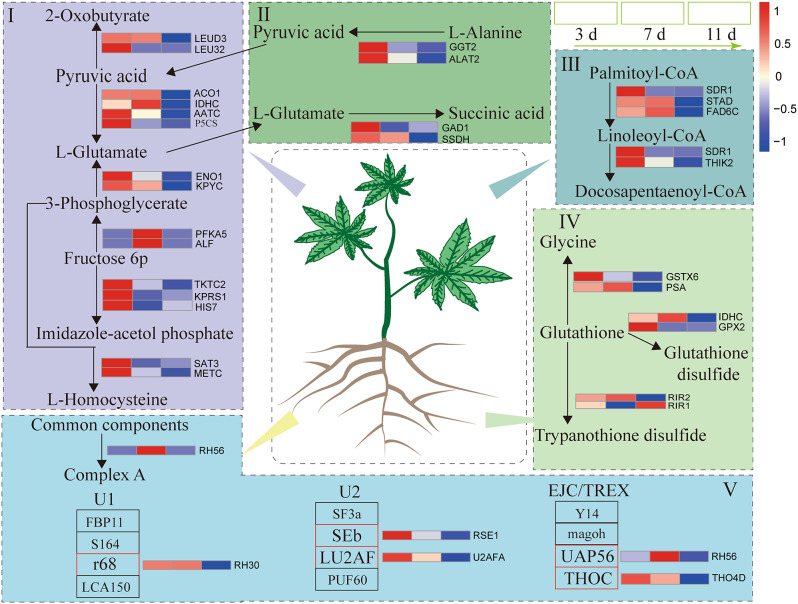
GO term and KEGG pathway enrichment analyses were also conducted to determine the biological functions of the coexpressed DEGs in the navajowhite1 module. As shown in Table S5, the DEGs were significantly enriched in the terms “antioxidants”, “signal transduction”, and “molecular function modulators”. These findings clearly show that the redox process in castor is highly active under PEG-6000 stress. We conducted KEGG pathway enrichment analysis on the coexpressed DEGs in the navajowhite1 module and identified several significantly enriched metabolic pathways, including alanine, aspartate, and glutamate metabolism; amino acid biosynthesis; unsaturated fatty acid biosynthesis; glutathione metabolism; and the spliceosome (Fig. 4). As shown in Fig. 4, DEGs associated with these pathways were upregulated under stress induced by PEG-6000.
Fig. 4.
Coexpressed DEGs in the navajowhite1 module are involved in metabolic pathways. I: biosynthesis of amino acids (ko01230); II: alanine, aspartate, and glutamate metabolism (ko00250); III: biosynthesis of unsaturated fatty acids (ko01040); IV: glutathione metabolism (ko00480); V: spliceosome (ko03040). Relative expression levels are shown by a color gradient from low (blue) to high (red). LEUD3 (ncbi_8267727), aconitase/3-isopropyl malate dehydratase protein; LEU32 (ncbi_8283093), 3-isopropyl malate dehydrogenase 2; ACO1 (ncbi_8267700), aconitate hydratase 1; IDHC (ncbi_8258744), isocitrate dehydrogenase; AATC (ncbi_8268349), aspartate aminotransferase; P5CS (ncbi_8287478), delta-1-pyrroline-5-carboxylate synthase; ENO1 (ncbi_8264565), enolase 1; KPYC (ncbi_8274900), pyruvate kinase; PFKA5 (ncbi_8267060), phosphofructokinase; ALF (ncbi_8288389), fructose-bisphosphate aldolase; TKTC2 (ncbi_8270608), transketolase; KPRS1 (ncbi_8266684), ribose-phosphate pyrophosphokinase 1; HIS7 (ncbi_8280924), imidazole glycerol-phosphate dehydratase; SAT3 (ncbi_8266938), serine acetyltransferase 1; METC (ncbi_8273083), chloroplast cystathionine beta lyase family protein; GGT2 (ncbi_8273496), glutamate-glyoxylate aminotransferase 2; ALAT2 (ncbi_8276551), alanine aminotransferase 2-like; GAD1 (ncbi_ 8286428), glutamate decarboxylase 4; SSDH (ncbi_8279668), succinate-semialdehyde dehydrogenase, mitochondrial; SDR1 (ncbi_8261843), short-chain dehydrogenase/reductase family protein; STAD (ncbi_8263458), stearoyl-acyl-carrier protein desaturase; FAD6C (ncbi_8276565), omega-6 fatty acid desaturase; SDR1 (ncbi_8261843), short-chain dehydrogenase; THIK2 (ncbi_8265629), peroxisomal 3-keto-acyl-CoA thiolase; PSA (ncbi_8287399), puromycin-sensitive aminopeptidase; GSTX6 (ncbi_8262580), glutathione S-transferase X6; IDHC (ncbi_8258744), isocitrate dehydrogenase; GPX2 (ncbi_8264294), glutathione peroxidase; RIR2 (ncbi_8271445), ribonucleoside-diphosphate reductase small chain family protein; RIR1 (ncbi_8283227), ribonucleoside-diphosphate reductase large subunit-like; RH56 (ncbi_8259463), ATP-dependent RNA helicase 56; RH30 (ncbi_8280293), ATP-dependent RNA helicase 30; RSE1 (ncbi_8258409), pre-mRNA-splicing factor; U2AFA (ncbi_8267025), splicing factor U2af small subunit ; THO4D (ncbi_8272182), THO complex subunit 4D
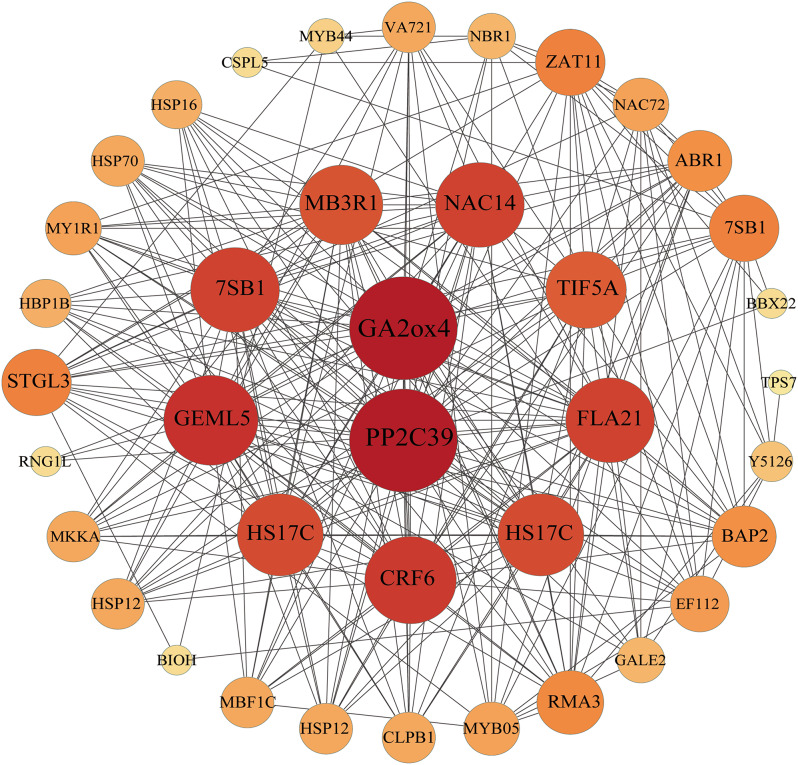
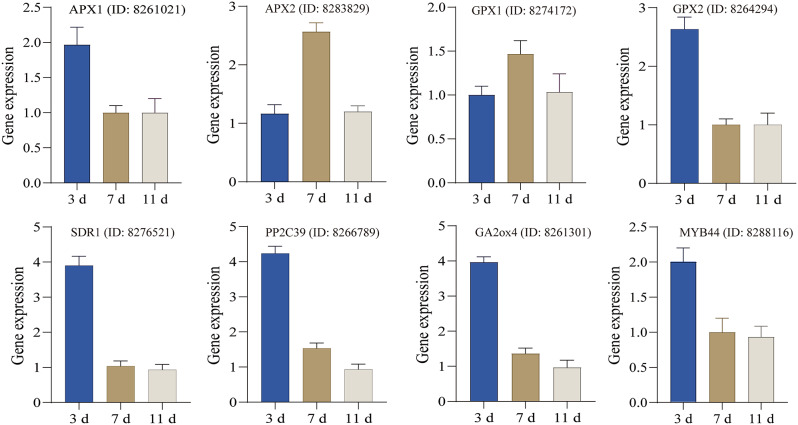
We constructed a navajowhite1 modular network and identified two core genes: GA2ox4 and PP2C39 (Fig. 5). To experimentally confirm our results, we selected eight genes from the navajowhite1 module and performed qRT‒PCR to measure their expression levels after 15% PEG-6000 stress for 3, 7, and 11 d (Fig. 6). The results revealed that the gene expression trend was consistent with the transcriptomic results.
Fig. 5.
Coexpression network analysis of genes in the navajowhite1 module. The larger the circle is and the deeper the red color is, the more genes that gene interacts with
Fig. 6.
qRT‒PCR analysis of DEGs in castor roots. The gene IDs (8261021, 8283829, 8274172, 8264294, 8276521, 8266789, 8261301, and 8288116) were all obtained from the NCBI database of the castor genome. 3d: Comparison of gene expression in the roots of castor plants between the treatment group (subjected to stress for 3 d in 15% PEG-containing Hoagland’s nutrient solution) and the control group (cultivated in Hoagland’s nutrient solution for 3 d). 7d: Comparison of gene expression in the roots of castor plants between the treatment group (subjected to stress for 7 d in 15% PEG Hoagland’s nutrient solution) and the control group (cultivated in Hoagland’s nutrient solution for 7 d). 11d: Comparison of gene expression in the roots of castor plants between the rewatered treatment group (after 4 d of rewatering) and the control group (cultivated in Hoagland’s nutrient solution for 11 d)
Discussion
Physiological and biochemical responses of castor under PEG-6000 stress
The yield and productivity of castor plants are severely impaired after drought stress. Drought causes lipid peroxidation of the cell membrane and the production of many ROS that damage membranes and increase membrane permeability [24]. In this study, the levels of H2O2 and MDA in the root system of castor plants stressed with PEG-6000 for 3 and 7 d were significantly greater than those in the control, indicating that the balance between the production and scavenging of ROS within the cells was disrupted. Studies on crops such as cotton [25], barley [26], corn [27], and rice [28] have shown that drought resistance is related to the ability to clear ROS, but this ability is regulated by enzyme systems such as SOD, POD, CAT, APX, GR, and DHAR [29]. Wang et al. reported that the activities of the SOD, POD, CAT, and GR enzymes in Triarrhena sacchariflora tended to increase under mild drought, whereas under moderate and severe drought, they tended to increase, followed by a downward trend, whereas APX activity tended to increase [30]. However, Yin reported that the SOD, GR, and APX activities in Cerasus humilis leaves tended to decrease after drought stress [31]. In the present study, the APX and CAT activities in castor roots first increased but then decreased after drought stress, and the POD, GR, and SOD activities continued to increase. The results of this study revealed that the response of plant antioxidant enzymes to drought stress varies by species. Drought can damage chloroplasts and reduce photosynthesis rates [32]. Stomata are important channels via which plants exchange gas and water with the external environment and play an important role in helping plants cope with drought. The stomatal conductance (Cs) and intercellular CO2 concentration (Ci) exhibited the same trend, which suggested that the decrease in the photosynthetic rate was caused by stomatal factors [33]. This study revealed that as the duration of PEG stress in castor plants increased, the Pn, Ci, and Cs decreased. Therefore, it is speculated that the decrease in the Pn of castor under PEG stress is caused by stomatal factors.
DEGs involved in cellular antioxidant activity
Drought stress significantly affects the growth and metabolism of plants, leading to damage to their normal homeostasis. In particular, under water deficit conditions, plants experience oxidative stress and the accumulation of various ROS, such as H2O2 and hydroxyl radicals [34]. The regulation of ROS is a key process for plants to adapt to drought, wherein APX and GPX are key enzymes responsible for clearing H2O2. Through GO enrichment analysis of the DEGs, we found significant enrichment in “antioxidant activity”. The genes involved in the antioxidant process included APX1 (ID: 8261021), APX2 (ID: 8283829), GPX1 (ID: 8274172), and GPX2 (ID: 8264294). In Arabidopsis, there are eight APXs, three of which are cytAPXs (AtAPX1, AtAPX2, AtAPX6) [34]. APX1 responds to various abiotic stresses, such as selenium, lead, heat, oxidation, and water stress [35–37]. For example, overexpression of APX1 in a cpr5 mutant could clear excess ROS and restore plant growth [36]. Zandalinas et al.. reported that the response of ABA to APX1 is indispensable during water stress [37]. However, research on APX2 has focused mainly on antioxidant activity and light stress, and the response of APX2 to water stress has not been fully elucidated. In this study, APX1 was significantly upregulated in the roots after 3 d of PEG stress, whereas there was no significant difference after 7 d of PEG stress. Additionally, APX2 showed no significant difference in expression in the roots after 3 d of PEG stress but was significantly upregulated after 7 d. Our qRT‒PCR analysis of the APX1 and APX2 genes revealed that, in castor roots under PEG stress, the expression trends of these genes in the treatment group compared with those in the control group aligned with the transcriptomic data (Fig. 6). The changes in the expression levels of APX1 and APX2 in castor roots under PEG stress may be more conducive to maintaining the homeostasis of ROS within the cells. However, the molecular mechanisms of APX1 and APX2 under drought stress still need further investigation. The GPX gene family plays an important role in different stress responses in plants. For example, the upregulation of bZIP68 oxidation mediated by rice GPX1 plays a role in ABA-independent osmotic stress signaling [38]. Arabidopsis plants transformed with the TaGPX1-D gene exhibit increased tolerance to salt and drought stress [39]. Two wheat GPX genes (W69 and W106) endow Arabidopsis plants with tolerance to salt, H2O2, and ABA [40]. In this study, transcriptomic analysis revealed that GPX2 was significantly upregulated in roots after 3 d of PEG stress, whereas GPX1 was significantly upregulated in roots after 7 d of PEG stress. We conducted qRT‒PCR analysis of GPX1 and GPX2 and found that the changes in the expression levels of these two genes in the treatment group compared with those in the control group were consistent with the transcriptomic results (Fig. 6). These findings suggest that GPX2 is more sensitive to early PEG stress. These findings help to elucidate how plants respond to drought stress by regulating APX and GPX genes, providing new directions for further research.
DEGs involved in glutathione metabolism
Growing evidence suggests that glutathione metabolism is closely related to the drought stress response [41, 42]. Vuković reported that the level of glutathione disulfide (GSSG) significantly increased and that glutathione (GSH) was strongly depleted but recovered to a lesser extent under drought stress in winter wheat [43]. Previous studies have shown that GSH helps eliminate excess ROS in plants under oxidative stress [44, 45]. Therefore, the GSH content in plants is an important indicator of plant antioxidant activity. In this study, 6 DEGs were involved in glutathione metabolism (Fig. 4). Isocitrate dehydrogenase (IDHC) and GPX2 are involved in the generation of GSSG from GSH. RIR1 and RIR2 are involved in the generation of trypanothione disulfide from GSH. Glutathione S-transferase X6 (GSTX6) and puromycin-sensitive aminopeptidase (PSA) are involved in the production of glycine from GSH. The higher the GSH/GSSG ratio is, the stronger the ability of a plant to clear ROS in the body its [46, 47]. In the present study, GPX2 was upregulated in the root system of castor plants under PEG-6000 stress for 3 d but downregulated at 7 d, indicating that the generation of GSSG by GSH was inhibited under severe drought in these plants, which may have increased the GSH/GSSG ratio, allowing the plants to cope with drought stress. The specific GSH/GSSG ratio may also depend on the consumption of GSH through the other two pathways. Therefore, further research is needed on the ability of GPX2 to clear ROS by regulating the GSH/GSSG ratio.
DEGs involved in fatty acid metabolism
Fatty acid metabolism plays an important role in the response of plants to drought stress [13, 48, 49]. Previous studies have shown that Arabidopsis, Salvia officinalis, Vitis vinifera L., and young Cocos nucifera L. plants present increased levels of unsaturated fatty acids under drought stress [50–53]. An increase in unsaturated fatty acid levels helps maintain the fluidity and integrity of cell membranes [54, 55]. In addition, the double bonds in unsaturated fatty acids can react with free radicals, thereby terminating the free radical chain reaction and preventing cellular oxidative damage [56]. In this study, four of the DEGs were involved in fatty acid metabolism: SDR1 (short-chain dihydrogenase), THIK2 (peroxisomal 3-ketoacyl CoA thiolase), PSA (puromycin-sensitive aminopeptidase), and FAD6C (omega-6 fatty acid deficiency) (Fig. 4). SDR1, STAD, and FAD6C are involved in linoleoyl-CoA formation. Linoleoyl-CoA is a long-chain unsaturated fatty acid that contains two double bonds and can be further converted into other types of unsaturated fatty acids through specific enzymatic reactions, such as fatty acid desaturase and fatty acid elongation enzymes [57]. SDR1 and THIK2 are involved in docosapentaenoyl-CoA formation. Although there is currently no direct evidence to suggest that docosapentaenoyl-CoA is a substrate for the formation of unsaturated fatty acids, it is an intermediate in the formation of unsaturated fatty acids [58]. In this study, the expression of SDR1, STAD, FAD6C, and THIK2 was upregulated in castor roots stressed by PEG-6000, which may promote the synthesis of unsaturated fatty acids, thereby playing a crucial role in maintaining cell membrane stability and preventing oxidative damage to cells.
DEGs involved in plant hormone signaling
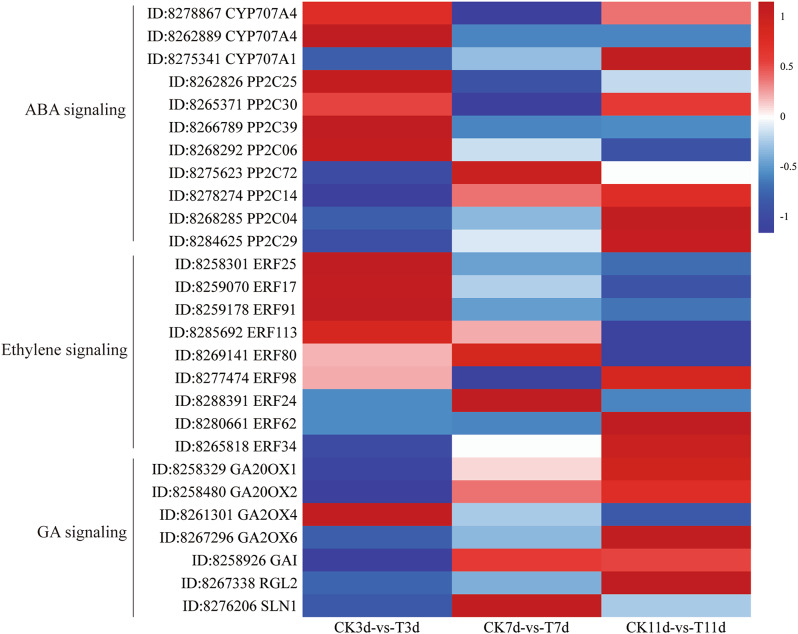
Studies have shown that certain components of phytohormone signaling pathways are involved in drought stress. For example, water deficit stress in plants triggers the synthesis and accumulation of ABA in roots and the transport of ABA to aboveground parts and regulates stomatal closure. There exists a dynamic balance between the synthesis and degradation of endogenous ABA in plants. The degradation of ABA is achieved through ABA 8´‑hydroxylase (CYP707A) [59]. Previous studies have shown that inhibition of the CYP707A gene can increase plant drought and salinity tolerance [60–63]. Inhibition of the CYP707A gene in apple seedlings and grapes enhances plant drought resistance [60–62]. In this study, three CYP707A genes, namely, CYP707A4 (ID: 8278867), CYP707A4 (ID: 8262889), and CYP707A1 (ID: 8275341), were differentially expressed (Fig. 7). CYP707A1 is downregulated in castor roots at both 3 and 7 d of PEG stress. However, the two CYP707A4 genes were upregulated in the roots at 3 d of PEG stress and downregulated at 7 d. The changes in the expression of CYP707A1 and CYP707A4 may help maintain the dynamic balance of ABA. CYP707A1 and CYP707A4 were both downregulated in the castor root system under PEG stress for 7 d, promoting the accumulation of ABA and aiding in the clearance of cellular ROS [64, 65]. Type 2 C protein phosphatase (PP2C) is one of the three main components of the ABA signaling pathway and is responsible for regulating various physiological processes, including stomatal movement and the drought response [66]. In the absence of stress, PP2C inhibits the PYR/PYL/RCAR components in the ABA signaling pathway, placing plants in a nonstressed state. However, under stress conditions, the accumulation of ABA inhibits the activity of PP2C, allowing the transmission of ABA signals and activating stress responses [67–69]. In this study, 8 PPC2 genes were differentially expressed, among which 4 (PP2C25, PP2C30, PP2C39, and PP2C06) were downregulated in the root system of castor plants after 7 d of stress. Nevertheless, the expression of PP2C72, PP2C14, PP2C04, and PP2C29 was downregulated in castor root system after 3 d of stress. WGCNA indicated that PP2C39 is one of the core genes of the navajowhite1 module (Fig. 5). The downregulation of PP2C39 expression in the root system of castor plants after 7 d of stress (Fig. 6) may promote the accumulation of ABA and improve the antioxidant capacity of the plant.
Fig. 7.
Expression patterns of abscisic acid (ABA), ethylene, and gibberellin (GA) signal-related DEGs in castor bean under PEG-6000 stress. CK 3d, CK 7d, and CK 11d: castor plants grown in Hoagland’s solution without PEG for 3, 7, and 11 d. T 3d, T 7d, and T 11d: castor plants stressed with 15% PEG in Hoagland’s solution for 3 d and 7 d and rehydrated for 4 d. The gene IDs were obtained from the NCBI castor genome database
Ethylene signaling plays an important role in the response of plants to drought stress. ERFs are members of the AP2/ERF transcription factor superfamily in plants and play a crucial role in the response to drought stress. Under drought stress conditions, ERFs usually positively regulate plant drought resistance, but they also occasionally negatively regulate plant drought resistance [70–72]. ERF109, ERF114, and ERF115 are ROS-responsive factors in Arabidopsis that mediate ROS signaling and control the recognition of the root stem cell niche (SCN) [73]. In quinoa, CqERF24 can increase antioxidant enzyme activity and activate stress-related genes, increasing plant drought resistance [74]. In corn, ZmEREBP60 is a positive regulatory factor in the drought response that regulates H2O2 decomposition [75]. A total of nine DEGs were identified in this study; seven genes (ERF25, ERF17, ERF91, ERF113, ERF80, ERF98, and ERF24) were upregulated and two genes (ERF62 and ERF34) were downregulated under PEG-6000 stress (Fig. 7). These differentially expressed ERF genes may respond to drought stress by regulating ROS, but the specific regulatory mechanism requires further in-depth research.
Gibberellin signal transduction plays an important regulatory role in plants, especially in the response to environmental stress. The genes involved in gibberellin signal transduction include gibberellin synthesis genes (GA20ox), gibberellin degradation genes (GA2ox), and gibberellin signaling DELLA protein family genes (GAI, RGA). GA20ox (gibberellin 20 oxidase) is one of the key enzymes in the gibberellin biosynthetic pathway. However, GA2ox (gibberellin 2 oxidase) is a key enzyme in the gibberellin degradation pathway. A functional deficiency in maize ZmGA20ox3 can increase the drought resistance of maize seedlings [76]. Eucalyptus plants overexpressing the EguGA20ox1 and EguGA20ox2 genes exhibit increased sensitivity to abiotic stress, whereas those overexpressing the EguGA2ox1 gene exhibit increased stress resistance [77]. At present, the mechanisms by which GA2ox and GA20ox respond to drought stress are unclear, but there is evidence suggesting that gibberellin signaling responds to abiotic stress by regulating antioxidant defense ability and osmoregulatory substances [78]. GA20ox1 and GA20ox2 were downregulated and GA2ox4 was upregulated in PEG-6000-stressed castor roots in the present study (Fig. 7). The upregulation of GA2ox4 expression (Figs. 6 and 7) contributes to the degradation of endogenous GA in cells, which may increase the antioxidant defense ability of plants through GA signal transduction.
Navajowhite1 module genes involved in the response to drought
We performed WGCNA on the DEGs, and the navajowhite1 module was positively correlated with drought stress; moreover, we constructed a gene network map of navajowhite1. In addition to PP2C39 and GA2ox4, NCA14 is one of the core genes (Fig. 7). Many studies have shown that NAC transcription factors positively regulate the response of plants to drought stress [79–82]. The TwNAC01 gene enhances the antioxidant capacity of Arabidopsis plants under drought stress [83]. In addition, overexpression of ThNAC4, VvNAC17, OsNAC006, and AfNAC1 increased the antioxidant capacity of the plants [79, 84–86]. In the present study, the upregulation of NCA14 (ncbi_8270777) expression in the castor root system under PEG-6000 stress may have contributed to the antioxidant capacity of the plant. The MYB transcription factor family is a class of gene regulatory proteins that are widely found in plants; these transcription factors play important roles in plant growth and development and in the response to environmental stresses. MYB44, a member of the MYB family, has different functions in the response to drought stress. For example, GhMYB44 responds to drought stress by regulating plant antioxidant capacity and stomatal aperture [87]. However, BcMYB44 responds to drought stress by inhibiting the accumulation of anthocyanins and regulating stomatal movement and the ROS balance [88]. In the navajowhite1 module in this study, the MYB family members MYB44 (ncbi_8273467), MB3R1 (ncbi_8265448), and MYB05 (ncbi_8267898) were included. MYB44 is upregulated under PEG stress, which helps improve the antioxidant capacity of plants.
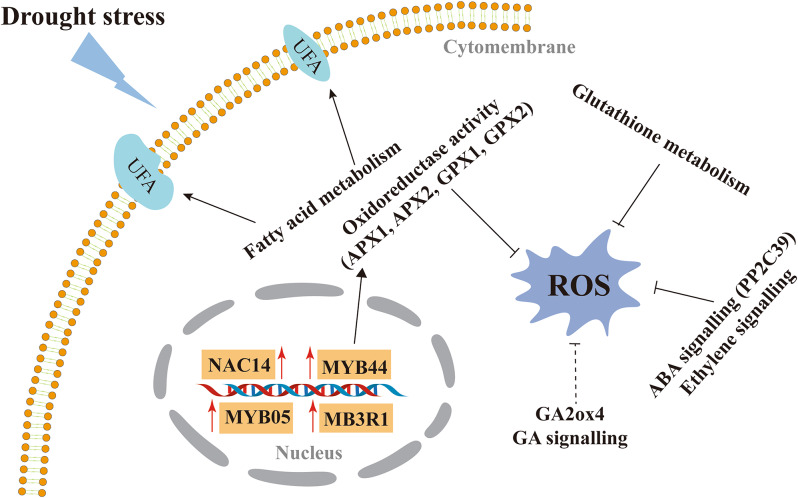
Oxidative stress model of the response of castor plants to drought stress
In this study, an antioxidant stress model of the response of the castor root system to drought stress was constructed on the basis of GO functional enrichment analysis, KEGG pathway analysis, and WGCNA of the DEGs (Fig. 8). First, fatty acid metabolism plays a protective role in maintaining the membrane of castor plants under drought stress and preventing oxidative damage. In addition, antioxidant enzymes, glutathione metabolism, plant hormone signal transduction, NAC transcription factors, and MYB transcription factors play important roles in maintaining the ROS balance in castor root cells under drought stress.
Fig. 8.
Response of castor to oxidative stress under drought stress. The solid arrows represent positive regulation, whereas the lines with blunted ends represent negative regulation. The dashed lines represent a negatively regulated mechanism that might exist in castor, but this warrants further investigation
Conclusions
In this study, we conducted a transcriptomic analysis of the response of castor seedlings to drought stress. On the basis of the GO functional enrichment, KEGG pathway, and WGCNA results for the DEGs, the mechanism underlying maintenance of the ROS balance in castor roots under drought stress was identified, which is an important finding of this study. The ROS balance may be maintained mainly by antioxidant enzymes, glutathione metabolism, hormone signal transduction, NAC transcription factors, and MYB transcription factors. WGNCA analysis revealed that PP2C39, which is involved in ABA signal transduction, and GA2ox4, which is involved in gibberellin signal transduction, are core genes. Therefore, it is worth studying the response of castor plants to drought stress, along with that of PP2C39 and GA2ox4. This study provides excellent genetic resources for the molecular breeding of drought-resistant castor.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank AJE for English language editing services.
Author contributions
Z. Y. processed the experimental materials and wrote the paper. L. P., Z. H. , L.R., L.G., D.J., and W.L. measured physiological and biochemical indicators. Z.Y., L.P., H.Z., and T.D. RNA-Seq data analysis. Z.Y., M.F., and H.F. provided experimental design and funding for this experiment.
Funding
This work was supported by the following agencies: the Natural Science Foundation of Jilin Province (YDZJ202201ZYTS453), the Scientific Research Project of the Jilin Provincial Department of Education (JJKH20210003KJ), the Program for Innovative Research Team of Baicheng Normal University, the Inner Mongolia Autonomous Region Grassland Talent Innovation Team (2022), the 2022 Basic Scientific Research Business Cost Project of Universities Directly Under the Autonomous Region (237), the Open Fund Project of Inner Mongolia Castor Industry Collaborative Innovation Center (MDK2021008, MDK2023001, MDK2023003, MDK2023004), Construction Project of Key Laboratory of Castor Breeding and Comprehensive Utilization in Inner Mongolia Autonomous Region (2023, 2024), and Inner Mongolia Autonomous Region Castor Industry Collaborative Innovation Center Open Fund Project (MDK2022014, MDK2021011).
Data availability
All raw data were deposited at the NCBI gene expression omnibus (GEO) with the accession number GSE252706. Additional analytical data during this study are included in the supplemental information.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors have agreed to the publication of this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yong Zhao and Pei Lei contributed equally to this work.
Contributor Information
Fanjuan Meng, Email: mfj19751@163.com.
Fenglan Huang, Email: huangfenglan@imun.edu.cn.
References
- 1.Tan M, Xue J, Wang L, Huang J, Fu C, Yan X. Transcriptomic analysis for different sex types of Ricinus communis L. during development from apical buds to inflorescences by Digital Gene expression profiling. Front Plant Sci. 2016;6(1664–462X):1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neeharika TSVR, Rani KNP, Rao KVSA, Kumar TP, Prasad RBN. Application of factorial design of experiments for the continuous hydrogenation of enriched castor oil methyl esters. Bull Chem Reaction Eng Catal. 2013;8(1978–2993):154–9. [Google Scholar]
- 3.Nejeliski DM, Duarte LC. Waterproofing of bottle gourd (Lagenaria siceraria) with castor oil polyurethane resin. Materia-rio De Janeiro 2020, 25:2020.
- 4.Yamamoto Y, Harada K, Kasuga S, Hosokawa M. Phospholipase A2-Mediated preparation of phosphatidylcholine containing ricinoleic acid and its anti-inflammatory effect on murine macrophage-like RAW264.7 cells. Biocatal Agric Biotechnol. 2019;19(1878–8181):101141–101141. [Google Scholar]
- 5.Kantakhoo J, Imahori Y. Antioxidative responses to pre-storage hot water treatment of red sweet pepper (Capsicum annuum L.) fruit during cold storage. Foods. 2021;10(2304–8158):3031–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadak MS, Sękara A, Al-Ashkar I, Habib-ur-Rahman M, Skalicky M, Brestic M, Kumar A, Sabagh AE, Abdelhamid MT. Exogenous aspartic acid alleviates salt stress-induced decline in growth by enhancing antioxidants and compatible solutes while reducing reactive oxygen species in wheat. Front Plant Sci. 2022;13:987641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian J, Tian L, Chen M, Chen Y, Wei A. Low temperature affects fatty acids profiling and key synthesis genes expression patterns in Zanthoxylum Bungeanum Maxim. Int J Mol Sci. 2022;23(1422–0067):2319–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim CW, Baek W, Jung J, Kim J-H, Lee SC. Function of ABA in Stomatal Defense against Biotic and Drought stresses. Int J Mol Sci. 2015;16(1422–0067):15251–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huan X, Wang X, Zou S, Zhao K, Han Y, Wang S. Transcription factor ERF194 modulates the stress-related physiology to Enhance Drought Tolerance of Poplar. Int J Mol Sci. 2023;24(1422–0067):788–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin Y, Pan W, Zheng X, Cheng X, Liu M, Ma H, Ge X. OsERF101, an ERF family transcription factor, regulates drought stress response in reproductive tissues. Plant Mol Biol. 2018;98(0167–4412):51–65. [DOI] [PubMed] [Google Scholar]
- 11.Arisha MH, Ahmad MQ, Tang W, Liu Y, Yan H, Kou M, Wang X, Zhang Y, Li Q. RNA-sequencing analysis revealed genes associated drought stress responses of different durations in hexaploid sweet potato. Sci Rep. 2020;10(2045–2322):12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song L, Pan Z, Chen L, Dai Y, Wan J, Ye H, Nguyen HT, Zhang G, Chen H. Analysis of whole transcriptome RNA-seq data reveals many alternative splicing events in soybean roots under Drought stress conditions. Genes. 2020;11(2073–4425):1520–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jian H, Sun H, Liu R, Zhang W, Shang L, Wang J, Khassanov V, Lyu D. Construction of drought stress regulation networks in potato based on SMRT and RNA sequencing data. BMC Plant Biol. 2022;22(1471–2229):381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Sun Z-H, Wang X, Tang Y, Li X, Ren C, Ren J, Wang X, Jiang C, Zhong C, et al. Transcriptome-based analysis of key pathways relating to yield formation stage of foxtail millet under different drought stress conditions. Front Plant Sci. 2023;13:1110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Montagu M, Van, Inzé D, Camp W, Van: Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J. 2014;16(16):4806–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Wang M, Liu L, Meng F. Physiological and proteomic responses of diploid and tetraploid black Locust (Robinia pseudoacacia L.) subjected to salt stress. Int J Mol Sci. 2013;14(10):20299–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdolinejad R, Shekafandeh A. Tetraploidy confers superior in vitro water-stress tolerance to the fig tree (Ficus carica) by reinforcing hormonal, physiological, and biochemical defensive systems. Front Plant Sci. 2022;12:796215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Civello PM, Martinez GA, Chaves AR, Anon MC. Peroxidase from strawberry fruit (Fragaria Ananassa Duch.): partial purification and determination of some properties. J Agric Food Chem. 1995;43(10):2596–601. [Google Scholar]
- 19.Mchale NA. Biochemical and developmental characterization of multiple forms of catalase in Tobacco leaves. Plant Physiol. 1987;84(2):450–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biernacki M, Baranowska-Kuczko M, Niklinska GN, Skrzydlewska E. The FAAH inhibitor URB597 modulates lipid mediators in the brain of rats with spontaneous hypertension. Biomolecules. 2020;10(7):1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22(5):867–80. [Google Scholar]
- 22.Du X-Q, Wang F-L, Li H, Jing S, Yu M, Li J, Wu W-H, Kudla J, Wang Y. The transcription factor MYB59 regulates K+/NO3 – translocation in the Arabidopsis response to low K + stress. Plant Cell. 2019;31(3):699–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C-Y, Lee K-C, Tung S-Y, Huang W-S, Teng C-C, Lee K-F, Hsieh M-C, Kuo H-C. : 2D-DIGE-MS proteomics approaches for identification of gelsolin and peroxiredoxin 4 with lymph node metastasis in colorectal cancer. Cancers. 2022;14(13):3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J, Zhai YJ, Monikh FA, Arenas-Lago D, Grillo R, Vijver MG, Peijnenburg W. The differences between the effects of a Nanoformulation and a conventional form of atrazine to Lettuce: physiological responses, Defense mechanisms, and nutrient displacement. J Agric Food Chem. 2021;69(42):12527–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang PD, Zhang XQ, Zhu YG, Zhu W, Xie HY, Wang XD. Metabolism of reactive oxygen species in cotton cytoplasmic male sterility and its restoration. Plant Cell Rep. 2007;26(9):1627–34. [DOI] [PubMed] [Google Scholar]
- 26.Ma Z, Marsolais F, Bykova NV, Igamberdievi AU. Nitric oxide and reactive oxygen species mediate metabolic changes in barley seed embryo during germination. Front Plant Sci. 2016;7:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao HJ, Cui JJ, Liu SX, Wang SH, Lian YY, Bai YT, Zhu TF, Wu HH, Wang YJ, Yang SP, et al. Natural variations of ZmSRO1d modulate the trade-off between drought resistance and yield by affecting ZmRBOHC-mediated stomatal ROS production in maize. Mol Plant. 2022;15(10):1558–74. [DOI] [PubMed] [Google Scholar]
- 28.Kim SG, Kim ST, Kang SY, Wang Y, Kim W, Kang KY. Proteomic analysis of reactive oxygen species (ROS)-related proteins in rice roots. Plant Cell Rep. 2008;27(2):363–75. [DOI] [PubMed] [Google Scholar]
- 29.Cao Y, Luo QX, Tian Y, Meng FJ. Physiological and proteomic analyses of the drought stress response in Amygdalus Mira (Koehne) Yu et Lu roots. BMC Plant Biol. 2017;17:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Sun H, Sheng JJ, Jin SR, Zhou FS, Hu ZL, Diao Y. Transcriptome, physiological and biochemical analysis of Triarrhena sacchariflora in response to flooding stress. BMC Genet. 2019;20(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin ZP, Ren J, Zhou LJ, Sun LN, Wang JW, Liu YL, Song XS. Water deficit mechanisms in perennial shrubs Cerasus Humilis leaves revealed by physiological and proteomic analyses. Proteome Sci. 2017;15:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang BR, Liu XZ, Xu QZ. Supraoptimal soil temperatures induced oxidative stress in leaves of creeping bentgrass cultivars differing in heat tolerance. Crop Sci. 2001;41(2):430–5. [Google Scholar]
- 33.Liu YM, Zhang XZ, Tran H, Shan L, Kim J, Childs K, Ervin EH, Frazier T, Zhao BY. Assessment of drought tolerance of 49 switchgrass (Panicum virgatum) genotypes using physiological and morphological parameters. Biotechnol Biofuels. 2015;8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan-Lei Dusup/sup Z-YWs, Jing-Wei Fansup/sup, Neil C. Turnersup/sup, Tao Wangsup/sup, Feng-Min Lisup/sup ss: β-Aminobutyric acid increases abscisic acid accumulation and desiccation tolerance and decreases water use but fails to improve grain yield in two spring wheat cultivars under soil drying. J Exp Bot 2012;63(13):4849–4860. [DOI] [PMC free article] [PubMed]
- 35.Suzuki N, Miller G, Sejima H, Harper JF, Mittler R. Enhanced seed production under prolonged heat stress conditions inArabidopsis thalianaplants deficient in cytosolic ascorbate peroxidase 2. J Exp Bot. 2012;64(0022–0957):253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qi F, Li J, Hong X, Jia Z, Wu B, Lin F-C, Liang Y. Overexpression of an antioxidant enzyme APX1 in cpr5 mutant restores its pleiotropic growth phenotype. Antioxidants. 2023;12(2076–3921):301–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zandalinas SI, Balfagón D, Arbona V, Inupakutika MA, Mittler R. ABA is required for the accumulation of APX1 and MBF1c during a combination of water deficit and heat stress. J Exp Bot. 2016;67(0022–0957):5381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou H, Zhang F, Zhai F, Su Y, Zhou Y, Ge Z, Tilak P, Eirich J, Finkemeier I, Fu L, et al. Rice GLUTATHIONE PEROXIDASE1-mediated oxidation of bZIP68 positively regulates ABA-independent osmotic stress signaling. Mol Plant. 2022;15(1674–2052):651–70. [DOI] [PubMed] [Google Scholar]
- 39.Shumayla ST, Madhu YS, K ASAP. TaGPX1-D overexpression provides salinity and osmotic stress tolerance in Arabidopsis. Plant Science: Int J Exp Plant Biol. 2023;337:111881. [DOI] [PubMed] [Google Scholar]
- 40.Chao-Zeng Z, Lei Z, Li-Juan Y, Ming C, Qing-Yu W, Lian-Cheng L, Zhao-Shi X, You-Zhi M, Malcolm B. Two wheat glutathione peroxidase genes whose products are located in chloroplasts improve salt and H2O2 tolerances in Arabidopsis. PLoS ONE. 2013;8(10):e73989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L, Shen Y, Yang W, Pan Q, Li C, Sun Q, Zeng Q, Li B, Zhang L. Comparative metabolic study of two contrasting Chinese Cabbage genotypes under mild and severe Drought stress. Int J Mol Sci. 2022;23(1422–0067):5947–5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wojciechowska N, Alipour S, Stolarska E, Bilska K, Rey P, Kalemba EM. Peptide-bound methionine sulfoxide (MetO) levels and MsrB2 abundance are differentially regulated during the Desiccation Phase in Contrasted Acer seeds. Antioxidants. 2020;9(2076–3921):391–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vuković R, Čamagajevac IŠ, Vuković A, Šunić K, Begović L, Mlinarić S, Sekulić R, Sabo N, Španić V. Physiological, Biochemical and molecular response of different winter wheat varieties under drought stress at germination and seedling growth stage. Antioxidants. 2022;11(2076–3921):693–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Q-S, Xie Y, Rahman MM, Hashem A, Abd_Allah EF, Wu Q-S. Arbuscular Mycorrhizal Fungi and Endophytic Fungi Activate Leaf antioxidant Defense System of Lane Late Navel Orange. J Fungi. 2022;8(2309–608X):282–282. [DOI] [PMC free article] [PubMed]
- 45.Liu X, Xiao K, Zhang A, Zhu W, Zhang H, Tan F, Huang Q, Wu X, Zha D. Metabolomic Analysis, combined with enzymatic and transcriptome assays, to reveal the Browning Resistance mechanism of fresh-cut eggplant. Foods. 2022;11(2304–8158):1174–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park JY, Han S, Ka HI, Joo HJ, Soh S, Yoo KH, Yang Y. Silent mating-type information regulation 2 homolog 1 overexpression is an important strategy for the survival of adapted suspension tumor cells. Cancer Sci. 2019;110(1347–9032):2773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bao-cheng Z, Wang K, Liang Z, Zhu Z, Yang J. Transcriptome Analysis of Glutathione Response: RNA-Seq provides insights into balance between antioxidant response and glucosinolate metabolism. Antioxidants. 2022;11(2076–3921):1322–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Z, Bai C, Wang P, Fu W, Wang L, Song Z, Xi X, Wu H, Zhang G, Wu J. Sandbur Drought Tolerance reflects phenotypic plasticity based on the Accumulation of sugars, lipids, and Flavonoid intermediates and the scavenging of reactive oxygen species in the Root. Int J Mol Sci. 2021;22(1422–0067):12615–12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao M, Ren Y, Wei W, Yang J, Zhong Q, Zheng L. Metabolite Analysis of Jerusalem Artichoke (Helianthus tuberosus L.) seedlings in response to polyethylene glycol-simulated Drought stress. Int J Mol Sci. 2021;22(1422–0067):3294–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gigon A, Matos AR, Laffray D, Zuily-Fodil Y, Pham-Thi A-T. Effect of Drought stress on lipid metabolism in the leaves of Arabidopsis thaliana (Ecotype Columbia). Ann Botany. 2004;94(0305–7364):345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bettaieb I, Zakhama N, Wannes WA, Kchouk ME, Marzouk B. Water deficit effects on Salvia officinalis fatty acids and essential oils composition. Sci Hort. 2009;120(0304–4238):271–5. [Google Scholar]
- 52.Toumi I, Gargouri M, Nouairi I, Moschou PN, Salem-Fnayou AB, Mliki A, Zarrouk M, Ghorbel A. Water stress induced changes in the leaf lipid composition of four grapevine genotypes with different drought tolerance. Biol Plant. 2008;52(0006–3134):161–4. [Google Scholar]
- 53.Repellin A, Thi ATP, Tashakorie A, Sahsah Y, Deroanne C, Zuily-Fodil Y. Leaf membrane lipids and drought tolerance in young coconut palms (Cocos nucifera L). Eur J Agron. 1997;6(1161–0301):25–33. [Google Scholar]
- 54.Zhong D, Du H, Wang Z, Huang B. Genotypic variation in fatty acid composition and Unsaturation Levels in Bermudagrass Associated with Leaf Dehydration Tolerance. J Am Soc Hortic Sci. 2011;136(0003–1062):35–40. [Google Scholar]
- 55.Xu L, Han L, Huang B. Membrane fatty acid composition and saturation levels Associated with Leaf Dehydration Tolerance and Post-drought Rehydration in Kentucky Bluegrass. Crop Sci. 2011;51(0011–183X):273–81. [Google Scholar]
- 56.Ferreri C, Panagiotaki M, Chatgilialoglu C. Trans fatty acids in membranes:: the free radical path. Mol Biotechnol. 2007;37(1):19–25. [DOI] [PubMed] [Google Scholar]
- 57.Li Y, Hu CB, Zheng Y, Xia X-a, Xu WJ, Wang S, Chen W, Sun Z, Huang J. The effects of dietary fatty acids on liver fatty acid composition and ∆6-desaturase expression differ with ambient salinities in Siganus canaliculatus. Comp Biochem Physiol B. 2008;151(1096–4959):183–90. [DOI] [PubMed] [Google Scholar]
- 58.Drouin G, Rioux V, Legrand P. The n-3 docosapentaenoic acid (DPA): a new player in the n-3 long chain polyunsaturated fatty acid family. Biochimie. 2019;159(0300–9084):36–48. [DOI] [PubMed] [Google Scholar]
- 59.Kushiro T, Okamoto M, Nakabayashi K, Yamagishi K, Kitamura S, Asami T, Hirai N, Koshiba T, Kamiya Y, Nambara E. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: key enzymes in ABA catabolism. EMBO J. 2004;23(0261–4189):1647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tomiyama H, Sato M, Opio P, Saito T, Ohkawa K, Ohara H, Todoroki Y, Kondo S. Inhibition of Abscisic Acid 8′-Hydroxylase affects Dehydration Tolerance and Root formation in cuttings of grapes (Vitis labrusca L. × Vitis vinifera L. Cv. Kyoho) under Drought stress conditions. J Plant Growth Regul. 2020;39(0721–7595):1577–86. [Google Scholar]
- 61.Kondo S, Sugaya S, Sugawa S, Ninomiya M, Kittikorn M, Okawa K, Ohara H, Ueno K, Todoroki Y, Mizutani M, et al. Dehydration tolerance in apple seedlings is affected by an inhibitor of ABA 8′-hydroxylase CYP707A. J Plant Physiol. 2012;169(0176–1617):234–41. [DOI] [PubMed] [Google Scholar]
- 62.Kondo S, Kittikorn M, Sugaya S, Todoroki Y, Mizutani M, Hirai N. Dehydration tolerance in Apple Seedlings is advanced by retarding ABA 8′-Hydroxylase CYP707A. Acta Hort. 2014;1042:151–7. [Google Scholar]
- 63.Sales LS, Ohara H, Ohkawa K, Saito T, Todoroki Y, Srilaong V, Kondo S. Salt Tolerance in Apple Seedlings is affected by an inhibitor of ABA 8′-Hydroxylase CYP707A. J Plant Growth Regul. 2017;36(0721–7595):643–50. [Google Scholar]
- 64.Ke Y, Bian T, He W, Han G, Lv M, Guo M, Lu M. Root Abscisic Acid contributes to defending Photoinibition in Jerusalem Artichoke (Helianthus tuberosus L.) under salt stress. Int J Mol Sci. 2018;19(1422–0067):3934–3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Q, Dong G-R, Ma Y, Zhao S, Liu X, Li X-k, Li Y, Hou B-K. Rice Glycosyltransferase Gene UGT85E1 is involved in Drought stress tolerance through enhancing Abscisic Acid Response. Front Plant Sci. 2021;12:790195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuhn J, Boisson-Dernier A, Dizon MB, Maktabi MH, Schroeder JI. The protein phosphatase AtPP2CA negatively regulates Abscisic Acid Signal Transduction in Arabidopsis, and effects of abh1 on AtPP2CA mRNA. Plant Physiol. 2005;140(0032–0889):127–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Umezawa T, Sugiyama N, Mizoguchi M, Hayashi S, Myouga F, Yamaguchi-Shinozaki K, Ishihama Y, Hirayama T, Shinozaki K. Type 2 C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc Natl Acad Sci USA. 2009;106(0027–8424):17588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nishimura N, Sarkeshik A, Nito K, Park S-Y, Wang A, Carvalho PCMd, Lee SS, Caddell D, Cutler SR, Chory J, et al. PYR/PYL/RCAR family members are majorin-vivoABI1 protein phosphatase 2 C‐interacting proteins in Arabidopsis. Plant J. 2010;61(0960–7412):290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moreno-Alvero M, Yunta C, González-Guzmán M, Lozano-Juste J, Benavente JL, Arbona V, Menéndez M, Martinez-Ripoll M, Infantes L, Rodriguez PL, et al. Structure of Ligand-Bound Intermediates of Crop ABA receptors highlights PP2C as necessary ABA co-receptor. Mol Plant. 2017;10(1674–2052):1250–3. [DOI] [PubMed] [Google Scholar]
- 70.Chen N, Qin J, Tong S, Wang W, Jiang Y. One AP2/ERF transcription factor positively regulates Pi Uptake and Drought Tolerance in Poplar. Int J Mol Sci. 2022;23(1422–0067):5241–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dong CY, Liu J. The Arabidopsis EAR-motif-containing protein RAP2.1 functions as an active transcriptional repressor to keep stress responses under tight control. BMC Plant Biol. 2010;10(1471–2229):47–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xing L, Di Z, Yang W, Liu J, Li M, Wang X, Cui C, Wang X, Wang X, Zhang R, et al. Overexpression of ERF1-V from Haynaldia villosa can enhance the resistance of wheat to Powdery Mildew and increase the tolerance to Salt and Drought stresses. Front Plant Sci. 2017;8:1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kong X, Tian H, Yu Q, Zhang F, Wang R, Gao S, Xu W, Li J, Shani E, Fu C, et al. PHB3 maintains Root Stem Cell Niche Identity through ROS-Responsive AP2/ERF transcription factors in Arabidopsis. Cell Rep. 2018;22(2211–1247):1350–63. [DOI] [PubMed] [Google Scholar]
- 74.Zhu X, Wang B, Liu W, Wei X, Wang X, Du X, Liu H. Genome-wide analysis of AP2/ERF gene and functional analysis of CqERF24 gene in drought stress in quinoa. Int J Biol Macromol. 2023;253:127582. [DOI] [PubMed] [Google Scholar]
- 75.Zhu Y, Liu Y, Zhou K, Tian C, Aslam M, Zhang B, Liu W, Zou H. Overexpression of ZmEREBP60 enhances drought tolerance in maize. J Plant Physiol. 2022;275:153763. [DOI] [PubMed] [Google Scholar]
- 76.Liu X, Liu Y, Chen Z, Zhang C, Guo J, Liu Q, Yin Y, Yang H, Xia H, Li B, et al. Gene editing of ZmGA20ox3 improves plant architecture and drought tolerance in maize. Res Square (Research Square). 2023;43(1):18. [DOI] [PubMed] [Google Scholar]
- 77.Wu W, Zhu L, Wang P, Liao Y, Duan L, Lin K, Chen X, Li L, Xu J, Hu H. Transcriptome-based construction of the Gibberellin metabolism and signaling pathways in Eucalyptus grandis× E. Urophylla, and functional characterization of GA20ox and GA2ox in regulating Plant Development and Abiotic stress adaptations. Int J Mol Sci. 2023;24(8):7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Agharkar M, Lomba P, Altpeter F, Zhang H, Kenworthy KE, Lange T. Stable expression of AtGA2ox1 in a low-input turfgrass (Paspalum notatum Flugge) reduces bioactive gibberellin levels and improves turf quality under field conditions. Plant Biotechnol J. 2007;5(1467–7644):791–801. [DOI] [PubMed] [Google Scholar]
- 79.Mijiti M, Wang Y, Rui W, Habuding X. Tamarix Hispida NAC transcription factor ThNAC4 confers Salt and Drought stress tolerance to Transgenic Tamarix and Arabidopsis. Plants. 2022;11(2223–7747):2647–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Y, Zhang T, Xing W, Wang J, Yu W, Zhou Y. Comprehensive genomic characterization of the NAC Transcription Factors and their response to Drought stress in Dendrobium catenatum. Agronomy. 2022;12(2073–4395):2753–2753. [Google Scholar]
- 81.Pang X, Xue M, Ren M, Nan D, Wu Y. Ammopiptanthus mongolicus stress-responsive NAC gene enhances the tolerance of transgenic Arabidopsis thaliana to drought and cold stresses. Genet Mol Biology. 2019;42(1415–4757):624–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hou D, Zhao Z, Hu Q, Li L, Vasupalli N, Zhuo J, Zeng W, Wu A-M, Lin X. PeSNAC-1 a NAC transcription factor from moso bamboo (Phyllostachys edulis) confers tolerance to salinity and drought stress in transgenic rice. Tree Physiol. 2020;40(0829–318X):1792–806. [DOI] [PubMed] [Google Scholar]
- 83.Wang M, Ren L-T, Wei X-Y, Ling Y-M, Gu H-T, Wang S-S, Ma X-F, Kong G-C. NAC Transcription Factor TwNAC01 positively regulates Drought stress responses in Arabidopsis and Triticale. Front Plant Sci. 2022;13:877016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ju Y-T, Yue X, Zhuo M, Wang X, Fang Y, Zhang J. VvNAC17, a novel stress-responsive grapevine (Vitis vinifera L.) NAC transcription factor, increases sensitivity to abscisic acid and enhances salinity, freezing, and drought tolerance in transgenic Arabidopsis. Plant Physiol Biochem. 2020;146(0981–9428):98–111. [DOI] [PubMed] [Google Scholar]
- 85.Wang B, Zhong Z, Wang X, Han X, Yu D-Q, Wang C, Song W, Zheng X, Chen C, Zhang Y. Knockout of the OsNAC006 transcription factor causes Drought and Heat Sensitivity in Rice. Int J Mol Sci. 2020;21(1422–0067):2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li M, Liu Z, Liu C, Zhu F, Wang K, Wang Z, Li X, Lan X, Guan Q. Drought resistance of tobacco overexpressing the AfNAC1 gene of Amorpha fruticosa Linn. Front Plant Sci. 2022;13:980171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Duan B, Xie X, Jiang Y, Zhu N, Zheng H, Liu Y, Hua X, Zhao Y, Sun Y. GhMYB44 enhances stomatal closure to confer drought stress tolerance in cotton and Arabidopsis. Plant Physiol Biochem. 2023;198:107692. [DOI] [PubMed] [Google Scholar]
- 88.Hao Y, Wang J, Hu C, Zhou Q, Mubeen HM, Hou X. Regulation of BcMYB44 on anthocyanin synthesis and drought tolerance in non-heading Chinese cabbage (Brassica campestris ssp. chinensis Makino). Horticulturae. 2022;8(5):351. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw data were deposited at the NCBI gene expression omnibus (GEO) with the accession number GSE252706. Additional analytical data during this study are included in the supplemental information.