Abstract
Globally, age-related diseases represent a significant public health concern among the elderly population. In aging, healthy organs and tissues undergo structural and functional changes that put the aged adults at risk of diseases. Some of the age-related diseases include cancer, atherosclerosis, brain disorders, muscle atrophy (sarcopenia), gastrointestinal (GIT) disorders, etc. In organs, a decline in stem cell function is the starting point of many conditions and is extremely important in GIT disorder development. Many studies have established that aging affects stem cells and their surrounding supportive niche components. Although there is a significant advancement in treating intestinal aging, the rising elderly population coupled with a higher occurrence of chronic gut ailments necessitates more effective therapeutic approaches to preserve gut health. Notable therapeutic strategies such as Western medicine, traditional Chinese medicine, and other health-promotion interventions have been reported in several studies to hold promise in mitigating age-related gut disorders. This review highlights findings across various facets of gut aging with a focus on aging-associated changes of intestinal stem cells and their niche components, thus a deviation from the normal to repercussion, as well as essential therapeutic strategies to mitigate intestinal aging.
Keywords: Aging, Intestine, Stem cell, Niche components and gero-therapies
Graphical abstract
1. Introduction
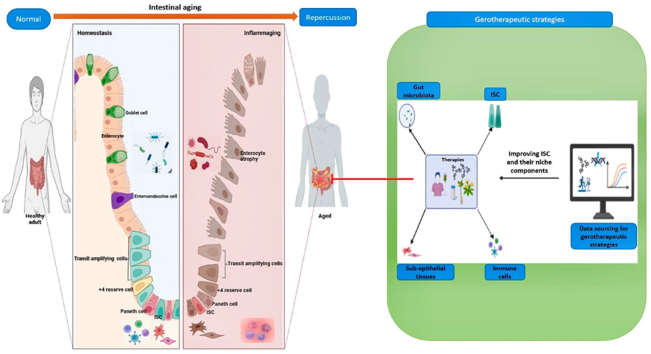
Aging is a natural life process that occurs in living organisms, yet it comes with harmful processes that affect the body's organs including the intestines [1]. As the intestine ages, some essential physiological roles such as digestion, immune secretion, gut-brain communication, nutrient and water absorption, and waste excretion begin to malfunction [2]. In line with this, several studies have reported that aging adversely affects the crucial regenerative intestinal stem cell (ISC) and their niche components (the immune cell component, gut microbiota, epithelium, and sub-epithelial tissues) that ensure intestinal homeostasis, promote its function and prevent gut disorders [3,4]. For this reason, several scientific advances in aging studies have been made in developing gerotherapeutic strategies to improve ISC function which remains the epicenter of gut regeneration and their niche components.
Current gero-therapeutic strategies developed include pharmacological approaches such as Western medicines (Rapamycin, metformin, immunotherapy, senolytics) and traditional Chinese medicines (Bazi bushen capsule, Fufang zhenshu Tiaozhi capsule, and ginsenoside RB1) and non-pharmacological health-promotion interventions such as exercise, fecal microbiota transplantation, diet restriction, gene therapy and stem cell therapy hold much therapeutic promise [5,6]. Of note, stem cell therapy has been used in regenerative medicine to treat different types of diseases [7]. For example, allogenic hematopoietic stem cell transplantation is widely accepted in treating acute lymphoblastic leukemia [8]. Also, cardiac progenitor cells can rescue patients suffering from heart failure and restore cardiac function [9]. Sampazoitis et al. [10] reported that cholangiocyte organoids from stem cells repaired bile ducts in the human liver after transplantation. The success of this treatment strategy in other organs highlights the need to understand the benefits of stem cell therapy in the context of enhancing intestinal function. Furthermore, the concomitant use of niche-targeted treatments with stem cell therapy could maximize benefits associated with niche support to stem cell function and reverse age-associated niche alterations that might impede treatment efficacy [7].
This review will address the role of ISC and niche components in maintaining a normal healthy gut, how aging alters them, resulting in infirmities, and therapeutic strategies to mitigate them. A comprehensive understanding of these will help researchers put more effort into developing appropriate therapeutic approaches to delay gut aging and prevent unwanted outcomes in aged adults.
2. The healthy gut structure
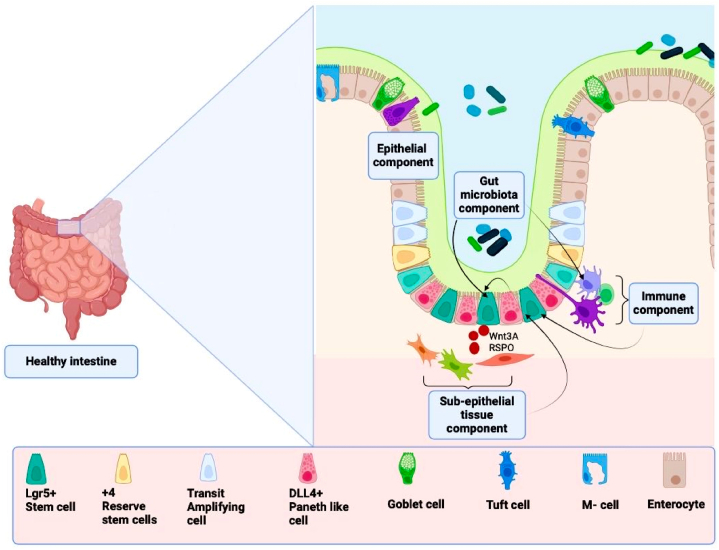
Anatomically, the intestine is segmented into the small intestine (SI) and the large intestine (LI) with segmental ratio length (SI: LI) being approximately (6: 1.5) m for humans and (350: 110) mm for mice, respectively [11]. A transverse cross-section of the intestine shows that the adventitia/serosa depending on whether the tissue is within the peritoneum/retroperitoneal, is seen as the outermost sections followed by the muscular layer, the submucosa, and the mucosa layer [12]. The mucosa layer consists of muscularis mucosa, and lamina propria lined with the vital mucus epithelium which is noted for hosting ISCs, plays many intestinal functional roles, and surrounds the lumen of the intestines [13,14]. The gut is said to be healthy when the morphology and function of the gut epithelium are normal or in homeostasis. Indeed, maintaining gut epithelium homeostasis is driven by the crucial role of ISCs and their niche components composed of microbial components, epithelial cells, immune cells, and sub-epithelial tissues in the gut (Fig. 1).
Fig. 1.
A diagram showing the various ISC niche components supporting crypt-based stem cells in the colon. The figure was adapted with slight modifications from Markandey et al. [15]. (The image was created using Biorender).
2.1. The role of gut microbiota (GM) in maintaining gut health
In the lumen, the gut microbiota is an important ISC niche component implicating microbes including bacteria, enteric viruses, microeukaryotes, protozoa, and fungi species to maintain intestinal homeostasis [16]. Among the bacteria microbes in the human gut, species belonging to the phyla such as Bacteroidetes (Bacteriodes sp, Xylanibacter sp, and Prevotella sp) and Firmicutes (Clostridium, Ruminococcus, Lactobacillus, and bacteria-producing butyrate) are the most dominant whereas other major phyla including Actinobacteria (Bifidobacterium), human disease-causing phyla Proteobacteria (Shigella sp, and Escherichia sp) and Verrucomicrobial (Akkermansia sp) have also been reported [17,18]. Based on their roles in the gut, they are categorized into general bacteria, commensal organisms (Akkermansia muciniphila, Lactobacillus reuteri D8, Bacteroides, etc.), and opportunistic pathobiont (Clostridium difficile, Shigella dysentriae, Vibro cholerae, etc.), with the latter implicated in many gut diseases [19,20]. The commensal gut microbiota produces biologically active metabolites (short-chain fatty acids (SCFA) and bacteriocins) that can further influence host physiological processes [21]. Furthermore, they produce muramyl dipeptide coupled with signaling reactive oxygen species (ROS) which promotes leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5+) ISC proliferation to maintain gut health or homeostasis [21,22]. Recently, A. muciniphila has been reported to promote ISC proliferation, secretes Amuc_1409 to improve intestinal regeneration in organoids, increase metabolites (propionic acid and acetic acid), and contributes to maintaining intestinal homeostasis when administered to mice [23,24]. The secreted succinate and propionic metabolites promoted bowel movement and stimulated mucus production by promoting mucin-2 (Muc2) expression, respectively. In line with this, the protective role offered by commensal bacteria included colonization resistance against pathobionts via nutrient blocking, direct killing of pathobionts to maintain gut epithelium integrity and immune response enhancement by regulating innate lymphoid cells and regulatory T cells (Treg) [20,25]. Ragonnaud and Biragyn [26] reported that GM such as Candida albicans, segmented filamentous bacteria, and Citrobacter rodentium could facilitate pathobiont clearance via recruiting neutrophils and inducing Th-17 cell differentiation. Additionally, Clostridium strains and Bacteriodes fragilis inhibited gut inflammation by inducing Treg differentiation and interleukin (IL) −10 production.
Furthermore, GM plays a critical role in the gut-brain axis, notably in the gut and mental health via neurotransmitters secretion. For instance, acetylcholine that induces GIT smooth muscle contraction is secreted by GM (Bacillus and Lactobacillus), histamine which influences immune response positively or negatively is secreted by GM (Streptococcus, Enterococcus, and Lactobacillus rhanossus), and dopamine which regulates cytokines including IL-4 and Interferon-gamma levels is secreted by GM (Lactobacillus, Escherichia, and Streptococcus) to maintain intestinal homeostasis [27].
2.2. The role of mucosa epithelium in gut health maintenance
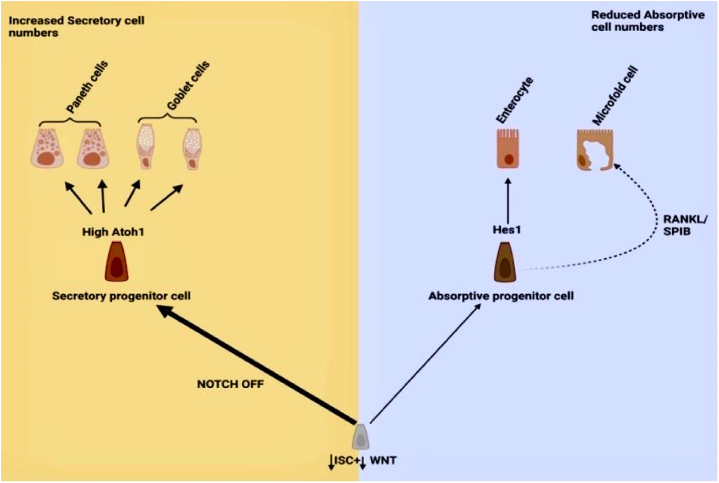
The mucosa epithelium comprises a cocktail of cells with main constituents being absorptive cells (enterocytes (>80 %) and microfold cells (10 % of follicle-associated epithelium)) followed by secretory cells (goblet (10–15 %), paneth (5–15 cells in each SI crypt), enteroendocrine (<1 %) and tuft cells (0.5 %)) which are all located at the villus region while Lgr5 + stem cells, +4 reserve stem cells, fgfbp1+ cells and transit-amplifying ISC progenitor cells are found in crypts [3,28,29].
The enterocytes (absorptive cells) express apolipoprotein C (Apoc3), keratin 20 (krt20), and fatty acid binding protein (Fabp1) markers and played roles such as absorption coupled with offering physical barrier functions [[30], [31], [32]]. Recent reports indicated that enterocytes regulated mucosal immunity by restricting microfold differentiation with the support of the OneCut2 transcription factor in the gut [33].
Microfold cells (absorptive cells) express microfold-related markers (Marcksl1, glycoprotein 2 (GP2), and chemokine CCL9), play a unique role in the gut-associated immune response by engulfing antigens and undergo transcytosis to maintain gut immune homeostasis [34]. They are located in the follicle-associated epithelium and are only associated with the ileum [33]. However, Dillon and Lo [35] reported that they could also be in the large intestine. In exceptional cases, colonic M cells are only likely to occur due to intestinal inflammation and can mitigate colitis in mice [36,37]. Hence, these findings suggest the need to find their precise location in the gut. Nevertheless, they maintained efficient gut immune responses.
Goblet cells belong to the secretory lineage, expressed mucin 2 (Muc2) markers, and are more abundant in the distal colon than other sections along the intestine [38]. They played vital secretory roles including mucus production for gut protection against microbes [39].
The rare tuft cells are cell bearers of double cortin-like kinase 1(DCLK1) markers in murine experiments and cyclooxygenase-1 enzyme expression in humans [40]. In addition, they possess chemosensory characteristics involving succinate and bitter taste receptors [40]. Recently, tuft-2 cells (a subset of tuft cells) were reported to sense bacterial metabolite (N-undecanoylglycine) via the vomeronasal Vmn2r26 to exert antimicrobial immunity [41]. Besides antimicrobial immunity, tuft cells could generate all other epithelial cell types by acting as regenerative stem cells and supplying notch ligands to maintain ISC [[41], [42], [43]].
The enteroendocrine (EE) cells express Claudin-4 markers and are known to produce various hormones that mediate neuronal signaling, digestion, and immunity [44]. They are sources of notch signals required for the maintenance of ISC [43]. Širvinskas et al. [2] reported that EE cells secreted glucagon (Gcg) and Peptide yy (Pyy) for insulin regulation and colonic motility, respectively. This finding indicates the vital role of enteroendocrine in glucose metabolism and gastrointestinal motility.
Paneth cells are secretory cells characterized by lysozyme markers. They are located at the base of the crypt and seen interspersed with high Lgr5+ ISC [45]. They produce mitogenic niche cues (wingless and int-1 (Wnt), Notch, and epidermal growth factor (EGF)), inducible nitric oxide synthase (iNOS), and antimicrobial agents (lysozyme, C-type lectins, phospholipase A2, and α-defensins) to support ISC homeostasis in the small intestines [46]. However, the colonic crypts lacked paneth cells and possessed Notch ligand delta (DLL4+) paneth-like cells that performed close functions [15].
2.3. The role of stem cells in maintaining gut health
Intestinal stem cells (ISC) remain a force to reckon with in gut epithelium regeneration [45]. They are categorized into transit amplifying Lgr5+low ISC progenitor cells (TAC) (located close to crypt-villi axis with low Lgr5+ marker expression), Fgfbp1+ cells (located at the upper part of crypt) +4 Reserve stem cells (+4RSC) (found in +4 position from the crypt base), revival stem cells (revSC) (preceded + 4 cells from the crypt base and expressed high Clusterin levels) and the cycling Lgr5+high stem cells (LGR5+high ISC cells) (located at the base of crypt and marked with high Lgr5+ marker expression) [29,45].
The transit-amplifying ISC progenitor cells (TACs) are daughter ISC cells possessing ki67 markers undergoing a transit phase between ISC and differentiated progeny [47,48]. They are more committed to differentiation into secretory and absorptive lineages by expressing differentially expressed genes that produce six differentiated cell types such as Paneth cell, goblet cells, tuft cells, microfold cells (M cell), enteroendocrine cells and enterocytes in the small intestines whiles in the colon all the differentiated cells were present except paneth cells [49]. Recently, TACs possessing unconventional prefoldin RPB5 interactor (URI) control R-spondin production that guides ISC proliferation and prevents inflammation [47]. Furthermore, they are indispensable during wound healing [50]. Overall, TAC plays a role in maintaining gut health.
The Fgfbp1+cells are multipotent cells found in the transit-amplifying zone marked by the Fgfbp1 marker and distinct from Lgr5+ISCs [29,51]. Following lineage tracing studies with time-resolved fate mapping, the cells generated all crypt-villus cells, sustained epithelial regeneration, and served as a source of Lgr5+ISCs following its ablation in the crypt [51].
The +4 RSC are often quiescent, slow cycling, and reported to replenish the Lgr5+ISC pool following radiation-mediated Lgr5+ ISC ablation [45]. A previous study reported that the appropriate identification of +4 reserve stem cells in crypt remained a challenge since they shared common markers such as leucine-rich repeats and immunoglobulin-like domains 1 (Lrig1), homeodomain-only protein (Hopx), polycomb complex protein Bmi1, and mouse telomerase reverse transcriptase (mTert) with Lgr5+ ISC cells [52]. In finding an appropriate feature for identification, a recent study reported primary cilia on +4 RSC as a distinguishing feature in normal human intestinal crypt (HIEC) and observed them following immunodetection using anti-acetylated-α tubulin [53]. Furthermore, the primary cilia on +4 RSC mediated the Hedgehog pathway which regulated cell cycle events, making them quiescent and resistant to damage. They are only activated to partake in ISC cell plasticity to maintain intestinal health [54].
The revival stem cells (revSC), identified by clusterin (Clu) markers expression using single-cell transcriptomics, have been reported to be rare during homeostasis and often seen after ISC ablation with about 25 % of it located in the +4 position of the crypt [45]. After a gut injury, they are reported to undergo a YAP1-dependent transient expansion to regenerate a functional intestine [55]. This was demonstrated following irradiation (12gy) of Clu cre−ERT2/+; Rosa26 lsl−DTA/+ (Clu ablated) mice. Also, in both acute and chronic DSS colitis models, daughter cells from Clu + revSC contributed immensely to epithelium regeneration following the lineage tracing experiment. Furthermore, Meyer et al. [56] reported that fetal gene signatures were associated with Clu+ revSC, as these cells expressed Hopx and stem cell antigen (SCA-1)/Ly6a markers after injury. Recently, revival stem cells have been reported to express additional markers (Anxa2 and Areg) but lacked proliferative markers (Pcna and Mki67) and are promoted by tumor suppressor p53 to support intestinal regeneration following demonstration using intestinal organoids [57]. Hence, this indicates that the crucial role of revSC during injury is to restore intestinal function by regeneration.
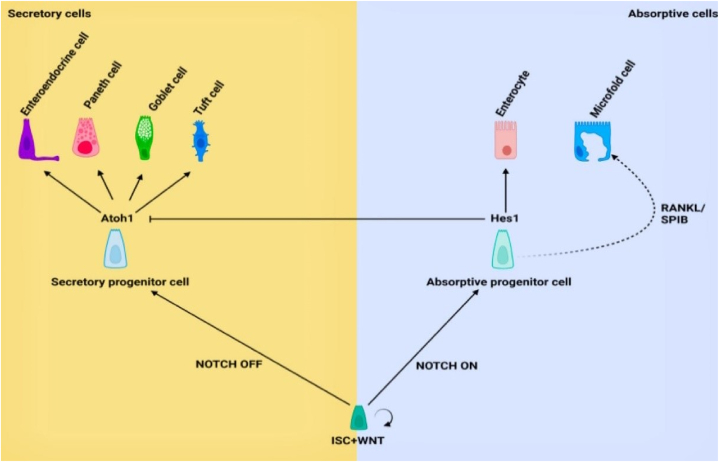
In regeneration, the fast-cycling Lgr5+ high ISC cells with robust expression of Lrig1, Hopx, Bmi1 olfactomedin-4 (Olfm4), achaete scute-like 2 (Ascl2), and mTert markers are geared towards producing daughter cells that move up the crypt [45,58]. These ISC cells can proliferate and differentiate into all crypt-villi cells in the presence of an appropriate repertoire of niche signals within 3–5 days (Fig. 2) [45]. Based on gut demands, they can divide symmetrically to produce daughter cells or asymmetrically to produce one ISC and one TAC to maintain homeostasis [59,60]. Also, ISCs produced daughter cells via symmetrical division more often than asymmetrical division [61]. Hence, this finding suggests that ISCs are constantly needed in crypts. Moreover, there is clonal competition among ISCs described by the neutral competition model between symmetrically dividing ISCs at the clonal edge [62]. Thus, ISCs’ symmetric and asymmetric divisions are properly regulated to maintain normal intestinal homeostasis. Additionally, the capacity of Lgr5+ISCs to differentiate and regenerate depends on the homeostatic metabolic state including lipid metabolism and the oxidative phosphorylation (OXPHOS) state of the mitochondria [63]. During irradiation-mediated injury, the gut epithelium recovered despite ablation of the high cycling ISC. Thus, indicating that other cell populations in the crypt such as +4RSC, revSC, and TAC progenitors can propagate tissue recovery.
Fig. 2.
The homeostatic differentiation and proliferation pattern of ISCs in the presence of niche cues in the small intestine. The figure was adapted with slight modifications from Hohman and Osborne [3]. (The image was created using Biorender).
2.4. Tight junctions and their role in maintaining gut health
A tight junction (TJ) is a structure that links epithelial cells together. They are composed of proteins (Claudins, occludin, TJ protein, and zonula occludin (ZO)) and play a significant protective role against a barrage of external insults to prevent increased permeability but biasedly allow paracellular transport of essential nutrients during absorption coupled with Na+ efflux from the lamina propria [64,65]. Additionally, current reports indicated that Claudin-23 regulated epithelial barrier function by reshaping TJ architecture whereas Claudin-7 was indispensable in intestinal stem cell renewal and differentiation [66,67]. Hence, tight junctions and proteins are vital in maintaining gut health.
2.5. The role of immune cells in maintaining gut health
The intestinal immune cells act as innate or secondary defense against pathogens and modulate inflammation or ISC proliferation [68]. They reside in the lamina propria (neutrophils, plasma cells, dendritic cells), at the Gut Associated Lymphocyte Tissue (macrophages, and innate lymphoid cells (ILC -2/3)) or both (T-helper cells and dendritic cells) [2]. The immune cells interact or crosstalk with epithelial cells (Lgr5+ ISCs) directly via immune receptors or via secreted cytokines to maintain gut health [2,68].
The secreted cytokines from immune cells played several roles in stem cell proliferation and regeneration. For example, type 3 innate lymphoid cells (ILC3) secrete IL-22 to activate ISCs regeneration when cocultured with ISCs in vitro. Likewise, type 2 innate lymphoid cells (ILC2) facilitated ISC renewal by secreting IL-13. In the normal gut, cytokines such as IL-6, IL-13, IL-17A, IL-22, and IL-33 enhanced ISC proliferation and differentiation [69]. Also, IL-22 and IL-10 could promote gut wound healing by modulating Wnt and Notch ligands to expand transit-amplifying ISC progenitor cells [50,70]. Thus, these findings suggest that the immune cells and cytokines maintain gut homeostasis.
2.6. The role of sub-epithelial tissues in gut health maintenance
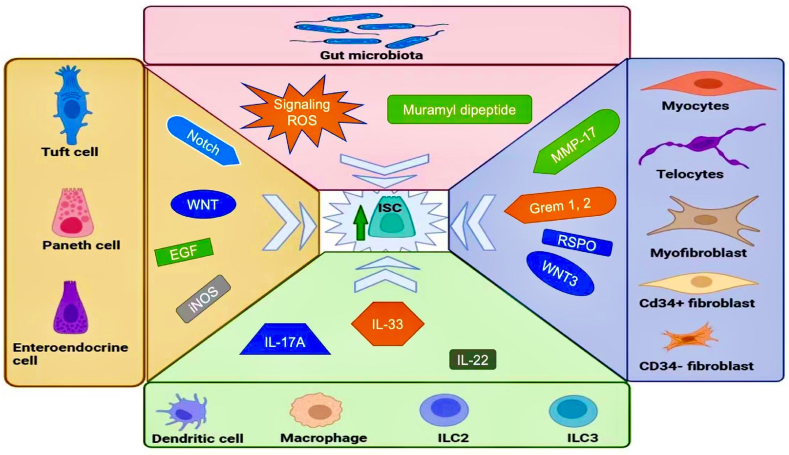
The sub-epithelial tissues or mesenchymal compartments are part of the ISC niche components. They comprise the stromal connective tissue (myocytes, pericytes, stromal stem cells, myofibroblast, Pdgfra low CD34+fibroblast, Cd34-fibroblast, trophocytes, and telocytes) that provide vital chemical cues/mitogens including Wnt3, R-spondin (RSPO), matrix metallopeptidase 17 (MMP-17) and bone morphogenetic protein (BMP) inhibitors to regulate stem cell activities including regeneration, proliferation, and differentiation [71,72]. Recently, sub-epithelial tissues such as mesenchymal cells and smooth muscle cells have been reported to shape the intestinal structures including the canonical villi formation via Wnt production and BMP inhibitors secretion [71,73]. Similarly, Xiang et al. [74] reported that Twist 2 stromal cell population and Acta 2 lineage expression on pericytes and telocytes supplied Wnt to ISC to promote intestinal homeostasis. Furthermore, myofibroblasts below the ISC produced BMP inhibitors such as Chordin-like 1, Germlin 1, and 2 to maintain ISC function. Moreover, RSPO-3+GREM1+ fibroblast and mitogen-activated protein kinase kinase kinase 2 (MAP3K2) stromal cells supplied ISCs with RSPO1 and RSPO3 to maintain regeneration [75]. Hence, the sub-epithelial cell component creates a homeostatic milieu by modulating the BMP and promoting the Wnt coupled with RSPO to maintain ISC function and support intestinal health. Altogether, the ISC niche components supported ISCs to maintain gut health (Fig. 3). Hereafter, we discussed the repercussions of aging on the intestine in the context of the age-associated alteration of the crucial ISC and their niche components that maintain gut homeostasis.
Fig. 3.
An overview of the intestinal stem niche support to Lgr5 stem cell proliferation to maintain gut health. (The image was created using Biorender).
3. Repercussions of aging on the aged intestine
Intestinal aging by chronological aging, unhealthy diets, medications, and sedentary lifestyles could lead to several age-related intestinal malfunctions. Aging repercussions in the gut comprise changes in morphology and functional decline that result in diseases. In aged mammals, the morphology and structure of the intestine changed and altered the normal intestinal function (digestion disorder, malabsorption, alteration of gut-brain communication, and chronic bowel movement disorder) which could increase the risk of diseases [76]. Similarly, alterations in muscular layers, length, and circumference of the intestine were observed in the aged mice and reported to affect intestinal function [77]. Hence, this suggests that aging affects the intestine and could affect the critical players involved in maintaining gut homeostasis.
3.1. Gut microbiota dysbiosis and aging repercussions
The gut of the elderly exhibits a higher abundance of opportunistic pathobionts (C. difficile and H. hepaticus) that are associated with a myriad of gut disorders compared to the intestines of a young individual [4]. In microbial intestinal colonization, aging compromised the adhesion quality and thinned the mucus lining leading to the weak attachment of healthy intestinal flora such as Akkermansia sp and Bifidobacterium while promoting the abundance of pathogenic Proteobacteria, Bacteriodetes, and many other pathobionts [4,26,78]. As a result, this increased the proportion of pathobionts and the prevalence of microorganism-associated molecular patterns (MAMPs) or pathogen-associated molecular patterns (PAMPs) that are associated with severe gut-health complications [79]. Chen et al. [80] have reported that an imbalance of gut microbiota/dysbiosis in the intestine could promote gut aging and induce age-related diseases. Similarly, Yang et al. [81] reported that gut microbiota dysbiosis in the aged crypt could activate Cyclic GMP-AMP synthase-stimulator of Interferon genes (cGAS-STING) in epithelial cells to propagate pathological conditions like cancer and inflammatory bowel disease. Recent reports indicated that bacteria exposure causes immune-senescence in B-cells and further drives gut microbiota aging [82]. Furthermore, aging-related gut microbiota alteration contributes to inflammation, immune-senescence (impaired immune function), and increased gut permeability, and was associated with gut disorders (irritable bowel syndrome (IBS) and inflammatory bowel diseases (IBD)) [76]. Interestingly, neurotransmitters such as histamine secretions have been reported to increase in gut dysbiosis-related disorders such as IBS. Metabolomic and metagenomic data from the Integrative Human Microbiome Project showed that IBS patients suffered high levels of histamine in the gut [83]. Hence, this suggests GM dysbiosis could negatively affect the intestinal health of the aged adult. In addition, alteration in gut microbiota significantly affected gut-brain communication via neurologic, immune, endocrine, and humoral/metabolic pathways [84]. Hence, these findings suggest several mechanisms by which aging adversely affects the host via changes to the gut microbiota. Interestingly, there is an exception to the rule, as centenarians have been reported to undergo healthy aging and possessed diverse microbes including viruses, and beneficial Bacteroides that protected them against diseases while decreasing pathobionts [85,86]. Likewise, Ragonnaud and Biragyn [26] reported that centenarians from Italy and China shared health-related bacteria from the genera including Akkermansia, Oscillospira, Bifidobacterium, and family Christensenellaceae that played a role in gut homeostasis by reducing gut inflammation and protecting the integrity of the gut epithelium. Thus, shedding light on biological pathways that could help increase healthy gut aging.
3.2. Mucosa epithelium impairment and aging repercussions
In the aged mice, the crypt-villus architecture of the epithelium was altered following an increase in the villi length, cell numbers, and crypt size coupled with a decrease in crypt numbers [87]. In addition, the crypts exhibited a reduced capacity to expel dead cells and aggravated local inflammatory responses [88]. Aside from this, recent reports showed that the epithelium cell population changed with metabolism dysregulation, growth rate altered with severe cellular damage, and escalating cell cycle arrest with high senescent protein expression (p16INK4A) [2,89]. In line with this, inflammaging phenotypes were observed in all epithelial cells and were most abundant in enterocytes [90]. Thus, this indicates that an aged gut is primed for gut inflammatory diseases. Also, absorptive cells (enterocytes and microfold cells) were decreased and implicated in age-related malabsorption and immune dysfunction [3,91].
Correspondingly, paneth (with increased notum expression, mammalian target of rapamycin complex 1 (mTORC1), and reduced peroxisome proliferator-activated receptor alpha (PPAR-α) activity) and goblet cell numbers increased coupled with a decline in mitogenic cue production [87]. Conversely, another report suggested a decrease in only goblet cell numbers in the distal part of SI [92,93]. Also, a previous study indicated that there was no impairment in the number and function of paneth cells during aging [94]. Hence, this suggests the need for further studies to address this disparity, nevertheless, aging caused alteration in cell numbers.
Širvinskas et al. [2] reported increased tuft cells and enteroendocrine cells coupled with reduced production of ligands (Gcg and Pyy) which could affect bowel movement in the distal colon during aging. Hence, this indicates resourceful insights into how aging distorts the homeostatic balance of epithelial cells and their role in the gut. The role of aging in the intestines has been thoroughly reviewed by Hohman and Osborne [3].
3.3. Stem cell dysfunction and aging repercussions
Aging affects stem cells (TAC, revSC, +4RSC, and LGR5+high ISC cells) and their function in the intestines [95]. In the aged mice, TAC numbers were reduced in the gut with apparent villus aging and impaired epithelium regeneration [95]. Similarly, the proliferative markers (ki67) on TAC were reduced in the aged gut [96].
In recovery, aging impaired cell cycling, made cells slower in motility, and hampered repair movement [88]. In repair, aging increased cell cycle inhibitors (p53 and p21 expression) that regulated quiescence and negatively affected the role of quiescent +4RSC in maintaining intestinal homeostasis [54]. Additionally, the transient p53 which promoted revSC, however, upon aging increased in expression and affected the number and gut restorative role of revSC [57,97]. In line with this, aging-associated decline in p53 function reduced colonic repair in aged mice following radiation exposure [98]. A recent study has shown that impaired repair could result in epithelium disruption and can drive gastrointestinal syndrome [24]. These findings indicate that aging compromises p53 functions to dysregulate revSC-mediated gut repair.
In the aged, the Lgr5+ high ISC size increased coupled with a reduced lateral surface-volume ratio and decreased non-myosin II expression which further diminished its ability to procure niche signals, hence, impairing regeneration [99]. This supported other studies that reported that aged stem cells could form fewer colonies with impaired regenerative ability and repressed cell cycle pathways [63,87]. Furthermore, there is accelerated clonal drift upon aging due to reduced adhesion of aged ISCs and affected clonal dynamics [62]. Currently, in mammals, whether the number of stem cells increases or decreases in the crypt with aging is still a debate requiring further research [87]. Nonetheless, the stem cell Lgr5+ marker was reduced and the mean expression of Lgr5 mRNA was decreased by 33 % in old mice [63]. Also, the alternative stem cell marker Ascl2 was decreased in the aged crypt. A previous study reported that Olfm4+ ISCs were reduced from 5 cells per crypt to three cells per crypt in the aged gut [94]. In addition, aged ISC aberrantly expressed inflammatory phenotypes such as increased expression of C-C chemokine receptor type 2 (Ccr2) and immune receptors (MHCII) which are implicated in gut disease development including IBD [90,100]. During differentiation, aged stem cells expressed secretory markers and differentiated more towards secretory cell lineage (Fig. 4) [87]. Also, mitochondria dysfunction characterized by upregulated oxidative phosphorylation (OXPHOS) and fatty acid metabolism in aged ISC altered the homeostatic range for proper functioning [63,101]. In addition, mitochondria deoxyribonucleic acid (DNA) mutations in aged ISC impaired its regenerative function and promoted intestinal aging via activating transcription factor 5 (ATF5)-dependent mitochondrial unfolded protein response activation [102]. Igarashi et al. [94] have reported that aged stem cells exhibited regeneration defects despite their interaction with the niche component paneth cell in an ex-vivo experiment. In line with this, aged ISCs that were exposed to radiation exhibited reduced DNA repair responses with mutations in these cells associated with adenomas [103]. In all, aging affects ISCs size, number, expression, and their function.
Fig. 4.
The impaired differentiation pattern of Lgr5+ ISCs skewed toward secretory lineages. The figure was adapted with slight modifications from Hohman and Osborne [3]. (The image was created using Biorender).
However, the impact of aging on other stem cells (Fgfbp1+) remains under study.
3.4. Decline in tight-junction barrier function and aging repercussion
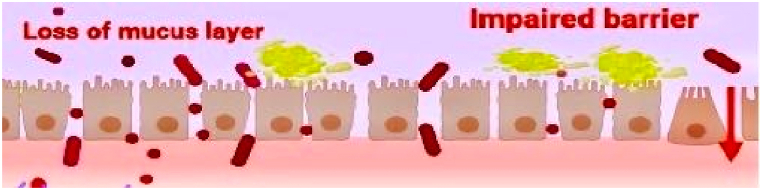
Aside from compromising epithelial cells, aging impaired TJ integrity (reducing occludin, zonula occludens-1 (ZO-1), and junctional adhesion molecules (JAM) expression) in mice, consequently, leading to an overwhelming influx of pathogens and their metabolites that worsen the intestinal barrier damage conditions (Fig. 5) [104]. Similarly, González-Muniesa et al. have reported reduced expression of JAM2, TJ proteins-1 (TJP1), and TJ proteins-2 (TJP2) in aged mice, thus increasing intestinal permeability (“leaky gut”) [76]. A previous report indicated that claudin-1, -3, ZO-1, E-cadherin, and occludin expressions were reduced in the distal colon of aged mice and aged people [96]. In addition, the pore-forming claudin-2 protein was significantly expressed at tight junctions and provided passageways for toxins or pathogens [96]. Thus, these findings present reasonable explanations for gut inflammation correlating positively with aging.
Fig. 5.
Impaired tight junction integrity with a high influx of pathogenic microbes. (The image was created using Biorender).
3.5. Immune cells functional perturbation and aging repercussions
The gut defensive immune cells are mostly triggered by the increase of these age-associated pathobionts traversing compromised epithelial barrier, with their chronic stimulation implicated in intestinal tissue degeneration and dyshomeostasis [20]. In the aged, the regulatory T cells (Treg) became compromised due to the poor induction ability of dendritic cells in response to retinoic acid [104]. Širvinskas et al. [2] reported that naïve lymphocytes tend to decrease in number due to the skewing of lymphocytes toward an activated T-cell state in the aged colon of mice. Also, cytotoxic CD4+ T-cells were significantly increased in the lamina propria of the intestine [105]. Hence, this tends to cause an imbalance of effector T-cells and Treg coupled with increasing susceptibility to immune-related diseases such as inflammation and autoimmune diseases [106]. In immune cell memory, CD8+T and CD45RA-memory cells were high in aged individuals and persisted in great numbers [107]. Furthermore, González-Muniesa et al. [76] reported that Cd4 and Cd72, which play roles in T-cells and B-cells functions, respectively, were reduced in the aged gut. Also, immune receptors (toll-like receptor 4 (Tlr4) and toll-like receptor 7 (Tlr7)) and lipopolysaccharide-binding proteins were significantly downregulated [76]. Besides toll-like receptors, aging increased other gut-immune receptors implicated in inflammation such as the nod-like receptor family pyrin-domain containing 3 (NLRP3) [108]. Of note, mutations in nod-like receptors (NOD2) have been associated with Chron's disease [109].
In addition, elevated pro-inflammatory cytokines (Il-1β, Il-12, and tumor necrosis factor-alpha (TNF-α)) increased epithelial permeability, promoted damage, and compromised gut barrier integrity in the gut of the elderly [110]. Hence, this perturbed immune state could promote gut inflammation diseases such as IBD in the older adult. Likewise, IL-6 and IL-10 were significantly reduced in the aged gut of mice, thus disrupting epithelial cell development, maintenance, and wound healing activities, respectively [76]. Recently, interferon-gamma (IFNγ) has been linked to intestinal aging. Elevation of IFNγ levels can induce signal transducer and activator of transcription 1 (Stat1) activity in stem cells which prime aberrant skewed differentiation [105]. Hence, this suggests that immune cytokines partake in stem cell lineage fate decisions in the aged adult. Other inflammatory gene expressions (Ccl-2 and Ccl-12) in the aged colon of mice have also been reported [76]. Altogether, aging impairs the immune system in the gut to disrupt gut homeostasis and facilitates disorders related to inflammaging. The impact of aging on the gut immune function has been thoroughly reviewed elsewhere by Zheng et al. [104].
3.6. Intestinal sub-epithelial tissue and aging
Aging-associated senescence coupled with high mammalian target of rapamycin (mTOR) activities is ubiquitous in aged stromal cells [111]. In the aged mice, senescent stromal cells are characterized by reduced nuclear localization of transcriptional coactivator protein (YAP/TAZ) that resulted in mitogen production decline, premature aging phenotypes, and led to cellular senescence of other cells including ISCs [112,113,114]. Liu et al. [115] have reported that YAP/TAZ activation required for fibroblast transformation from intestinal smooth muscle cells during wound healing was decreased in the aged intestine. Also, compromised Ca2+ mobilization due to high mTOR activities in the smooth muscle of the aged colon affected gut motility and promoted disorders such as IBS [[116], [117], [118]]. Aging altered mitochondria potential in smooth muscle cells, affected metabolism, and reduced contractility [118]. Moreover, a change in smooth muscle cellular proteins (caveolin-1 expression was reduced) and high apoptosis of smooth muscle cells with defective mitochondria structure has been reported and reviewed elsewhere by Saffrey [119]. Hence, this suggests how aging greatly affects sub-epithelial tissues.
Altogether, aging caused alteration in ISCs and their niche components and led to infirmities that require appropriate therapeutic strategies to mitigate them.
4. Strategies to mitigate the repercussions of gut aging
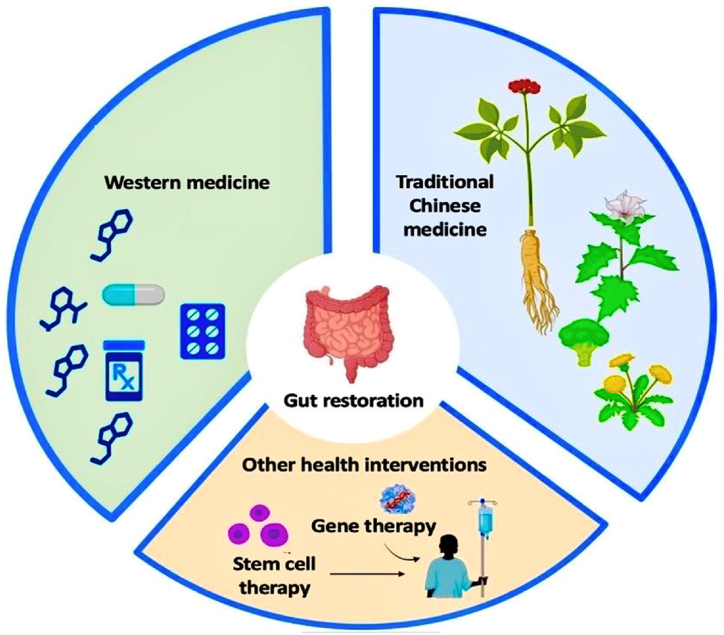
As discussed above, niche components are important and gut problems are closely associated with them. For this reason, therapies aimed to protect, nourish, and restore homeostasis in ISC niche components and ISCs are ideal for preventing unwanted diseases in the aged. Here, we reported available intervention strategies such as Western medicine, traditional Chinese medicine, and other health-promotion interventions used in delaying gut aging by supporting the ISC and their niche components (Fig. 6). We specifically focused on and collected the most cited articles in English journals published in the past and recent years from Google Scholar, PubMed, and ScienceDirect databases.
Fig. 6.
Therapeutic intervention against gut aging: Western medicine, Traditional Chinese Medicine and other health interventions. (The image was created using Biorender).
4.1. Western medicine as a gut aging intervention
Western medicine is a system of treatment developed based on the hypothetical deductions from research work, relies on evidence-based medicine, and uses single molecular drugs such as small molecules for disease treatment [120]. Here, we reviewed some of the notable drugs in Western medicine that may be used in gut-aging interventions. Also, we reported some treatment limitations, side effects, and the category of individuals who may be suited for effective therapeutic outcomes for some of the approaches.
4.1.1. Metformin
Metformin (biguanide with 50% bioavailability via oral route) is the most prescribed drug for the treatment of type-2 diabetes but is currently repurposed to serve as a gerotherapeutic drug [121,122]. A study reported that a higher proportion of metformin when orally administered, accumulates in the gut (primary reservoir) and can directly regulate the gut microbiota/increased levels of Akkermansia [123]. In the intestine, it impeded stem cell aging, modulated mitochondria function, inhibited cellular senescence via restoring nicotinamide adenine dinucleotide (NAD) levels, prevented macromolecular damages, recovered tight junction protein, reduced telomere attrition, ameliorated colonic pathological inflammation via activating AMP-activated protein kinase (AMPK) while inhibiting p53 pathway and improved nutrient sensing [121,124]. In addition, it has been reported to reduce the expression of chemokines (T-cell activation gene 3), pro-inflammatory cytokines (Macrophage colony-stimulating factor, TNF-α and IL-1α), and LPS-induced nuclear factor kappa B (NF-κB) on smooth muscle cells while increasing ISC numbers via the Wnt/β-catenin pathway and promoting glycolysis in the cells [125,126]. Of note, this drug prevented “leaky gut” by suppressing the Wnt pathway to promote the differentiation pattern of ISCs into the goblet cell, modulated the gut microbiome to increase beneficial gut metabolites, increased occludin, and reduced inflammation in old mice [127]. Although it remains effective as a gut-aging intervention, further studies are required to precisely understand its interaction with the Wnt pathway in promoting ISC self-renewal and differentiation. It is noteworthy that prolonged use of metformin has been associated with adverse gastrointestinal effects (nausea, vomiting, and diarrhea) in the gut of nearly 30% of patients, and its usage was discontinued in 5% of patients due to severe adverse effects [128]. Some of the reasons for the adverse effects could be the metformin-induced metabolic remodeling of Akkermansia muciniphila and Escherichia spp that produced H2S and CO2 gases, or the genetic polymorphism contributing factor of human organic cation transporter (OCT1) and serotonin transporter (which promotes serotonin uptake) in intestinal enterocytes [128,129]. The OCT1 is an intestinal transporter of the drug and could prevent metformin-associated increase of serotonin, which is associated with GI disorders. A recent study reported that individuals with a reduced-function allele of OCT1 and low-expressing S∗ allele of the Serotonin reuptake transporter gene (serotonin uptake) suffered metformin intolerance [130]. Hence, metformin treatment might not be ideal for individuals possessing these genotypes. Although supplementing the gut with probiotics could help offset the effects of metabolic remodeling associated with metformin treatment, an extensive pharmacogenetic assessment is needed to delineate individuals for whom this treatment would be most suitable [129].
4.1.2. Rapamycin
Rapamycin treatment as a repurposed gerotherapeutic intervention, targets the mTOR inhibition pathway and rejuvenates ISC in mice by reversing the transcription profile of aged stem cells [63]. The aging of ISCs is reported to be driven by mTORC1 via the p38 mitogen-activated protein kinase (MAPK)-p53 pathway and inhibiting it using rapamycin could be an effective strategy [95]. It is worth noting that rapamycin does not support ISC rejuvenation by Sirtuin-activating drugs such as NAD+ precursors nicotinamide riboside (Sirtuin 1 activator) which implicates mTORC1 activation in its mechanism of gut rejuvenation [94]. These findings suggest that the concomitant use of rapamycin with mTORC1 activating drugs such as NAD+ precursors nicotinamide riboside has to be avoided. Also, based on mTORC activity, rapamycin exerts opposing effects in ISCs and niche component paneth cells [131]. This finding reveals different responses between cells in the gut epithelium.
Also, rapamycin can modulate the gut microbiota, increase autophagy, improve immune function, delay immunosenescence, and exhibit potent immunosuppressive effects via modulating adaptive and innate immune responses [132,133]. Recently, it was reported in one study that it can attenuate age-related decline by increasing lysozyme and intestinal lysosomal alpha-mannosidase V (LManV) to increase the autophagy of enterocytes in Drosophila while maintaining paneth cell structure and barrier function integrity in mice [134]. This effect was sustained even after ten days and six months of drug withdrawal in Drosophila and mice, respectively. Hence, this finding suggests that rapamycin induces a memory effect in the gut cells. Aside from the memory effect on gut cells, rapamycin also prevented inflammation by blocking the aged-related increase of NF-κB in both mice and flies, repressed PGRP-LC inflammaging mediated immune receptors in Drosophila, elevated syntaxin 12/13 levels in mice, and increased lifespan via the endothelial system [135]. Hence, this suggests the role of the rapamycin effect across many species.
Furthermore, a significant increase in mTOR activity affects Ca2+ mobilization in aged smooth muscle cells and could be mitigated using rapamycin. However, Martín-Cano et al. [111] reported a low response of the drug in the aged smooth muscle of guinea pig's colon due to the loss of function of its mTOR complex FK506 binding protein-12 (FKBP12) in calcium release and requires further studies to understand the constitutive activation of mTOR activity in cells. Rapamycin delayed senescence in mesenchymal stromal cells, and blocked mTORC1 and mTORC2, with the latter implicated in side effects [136,137].
Some of the side effects such as immunosuppression, impaired wound healing, hyperlipidemia, and glucose intolerance serve as potential clinical barriers to the use of rapamycin in treating age-related diseases in humans [137,138]. A recent study reported that intermittent administration of rapamycin (1X/5 days) and other Food and Drug Administration (FDA) approved rapamycin analogs (temsirolimus and everolimus) have a reduced impact on glucose tolerance, beta cell function, pyruvate intolerance, and immune system to an extent [139]. Nevertheless, these reduced side effects of rapamycin analogs have been reported to differ between individuals [139]. Also, there are varying individual responses to mTOR inhibition by rapamycin and this approach would not benefit everybody [136]. Although rapamycin and their analogs have no serious adverse effect in healthy individuals, those suffering from age-related diseases were reported to be susceptible to rapamycin-associated adverse effects such as increased triglycerides, low-density lipoprotein (LDL) cholesterol levels, and an increased number of infections [140]. Similarly, bi-daily administration of rapamycin analogs for 28 days was associated with hyperglycemia, nausea, vomiting, fatigue, diarrhea, and thrombocytopenia in both young and aged adults suffering tumor diseases (Clinical trial registration, NCT01353625) [141]. Hence, the health status of the individual and a careful assessment of the benefits against the risks should be considered before drug administration.
4.1.3. Small molecules
Small molecules are molecules developed from leads obtained from rational drug design or isolated from natural origin and characterized by low molecular weight (<900Da) [142]. Due to their beneficial role in the gut and ability to traverse cells easily, small molecules such as Butyrate, Roxadustat (FG-4592), GSK343, Garcinol, Trichostatin A, and curcumin have gained much attention in disease treatment.
Butyrate is a gut microbiota metabolite synthesized from diet fibers in the body [143]. Stimulation of butyrate level or luminal administration of butyrate plays several roles in reducing gut inflammation dose-dependently and ameliorating mucosal inflammation via preventing NF-κB activation. It has also been shown to cause IκBα degradation, and regulate oxidative state while enhancing gut contraction/motility in animal models, promoting gut barrier function and preventing “leaky gut” [144,145]. In a study, butyrate was shown to mitigate aged gut microbiota-induced detrimental effects by decreasing GIT permeability, suppressing inflammation (IL-1β, IL-6, and TNF-α), and protecting the brain via the butyrate-free fatty acid receptor 2/3 (FFAR2/3) pathway [146]. Hence, these findings suggest their beneficial use in improving aged gut health. Recently, Sodium phenylbutyrate 4 (4-PBA) was approved by the FDA in the United States of America for use in patients and has been reported to be safe [147]. However, recent paradoxical reports indicate that SCFA including butyrate could promote CD4+ T-cell effector function in ulcerative colitis patients and could worsen gut barrier disruption [148].
Roxadustat (FG-4592) is a hypoxia-inducible factor prolyl hydroxylase used in the treatment of chronic anemia. Current reports show that FG-4592 promoted ISC proliferation and differentiation by upregulating hypoxia-inducible factor 2 and regulating the Tlr4 pathway in radiated mice [149,150]. The drug is also able to modulate the gut microbiota and promote the production of beneficial metabolites [151]. Hence, this suggests it could be useful in the aged gut. However, care must be taken as several studies have reported side effects including gut disorders, thrombosis, back pain, dizziness, and nasopharyngitis in patients with peritoneal dialysis and non-dialysis chronic kidney disease [152,153].
GSK343 (inhibitor of EZH2) has been reported to protect the intestine against sepsis-induced injury in-vivo by reducing the levels of inflammatory cytokines (IL-6, TNF-α and IL-1β), improving TJ protein expression, restoring paneth cell level and preventing cellular apoptosis [154,155]. Similarly, it could suppress inflammation and increase the MDSC population in the lamina propria [156]. Scuderi et al. [157] reported that GSK343 is cytotoxic, therefore its potential effect on normal cells should be considered. Although the drug has been shown to reverse conditions such as inflammaging, further studies using aging models are yet to be assessed.
Garcinol is a polyisoprenylated benzophenone extracted from the rind of the fruit of Garcinia indica [158]. It possesses anti-neoplastic potential, anticancer activity (by regulating NF-κB and Janus kinase (JAK)/STAT3), anti-inflammatory and antioxidant activity. Recent studies have indicated that it could increase intestinal symbiotic bacteria (Firmicutes, and Rominicoccus torques), improve intestinal morphology, and bolster intestinal barrier function which is beneficial in promoting gut health [[159], [160], [161]]. Khan et al. [162] reported that it promoted intestinal growth at low concentrations (<1 μM) but inhibited it at high concentrations (3.2–21.4 μM) in vitro. In immune-regulatory function, Wang et al. [163] reported that garcinol reduced proinflammatory cytokines (IL-1β, TNF-α, and IL-6), NF-κB p65 protein, and decreased intestinal permeability in weaned piglets by increasing ZO-1 and occludin protein expression. Currently, no serious side effect has been observed in vivo following administration of 40% garcinol (100 mg/kg/day) to rodents [164]. Similarly, Choudhury et al. [165] reported fewer side effects with the use of garcinol. Although this drug has no serious side effects, further studies are required to support its use for improving gut health in the elderly.
Trichostatin A, a derivative of dienohydroxamic acid is a small molecule with several roles including alleviating gut dysbiosis, inhibiting inflammatory mediators (down-regulating NF-κB, cyclooxygenase-2 (COX-2), iNOS expressions, inhibiting prostaglandin E2 (PGE2) and protein nitrosylation levels), protecting the intestine against injury and promoting structural restoration of the irradiated intestine [166,167]. Although its role in the gut has been reported, it is more suited as an anticancer agent with no significant drug-associated toxicities observed in cancer patients [168]. Furthermore, its role in the aged gut is yet to be explored. Nevertheless, the information above indicates that it could promote gut health in the elderly.
Sirtuin activators including NAD+ supplements remain an important small molecule with promising aging intervention activities [169,170]. They play a role in maintaining metabolic homeostasis, DNA damage repair, cell survival, and differentiation which are needed in mitigating intestinal aging [171]. In the gut, the mitochondria sirtuin 4 (Sirt4) was reported to shape the gut microbiota via modulating lysosomal overexpression in flies [172]. Similarly, sirtuin 3 modulated the gut microbiota, maintained expression of tight junction protein (claudin 8, claudin 15, and occludin), and possessed anti-inflammatory properties by decreasing pro-inflammatory cytokine (IL-1β and TNF-α) in mice [173]. Also, sirtuin 1 (Sirt1) activation was reported to increase ISC numbers by increasing translation via deacetylating ribosomal S6 kinase (S6K1) and promoting mTORC1-dependent phosphorylation of S6K1 [131]. Recently, Igarashi et al. [94] reported that Sirt 1 activation by NAD+ precursor nicotinamide riboside could rescue age-associated decline in ISC pool numbers, and function and repair gut damage. Although Sirt activators could prolong lifespan, few side effects have been reported [174,175]. Another study reported that Sirt activators have opposing effects on different targets and are tissue-specific [176]. Also, individuals with single nucleotide polymorphism in their Sirt1 gene could have a compromised Sirt1 activity and may not benefit from Sirtuin activators [177]. However, this information suggests that it could be used in the treatment of aging gut repercussions.
Curcumin (diferuloylmethane) is obtained from Curcuma longa (turmeric plant) and possesses immunomodulatory, antioxidative, antiapoptotic, and anti-inflammatory functions. Recently, Gan et al. [178] reported that curcumin increased intestinal antioxidative capacity and mRNA expression of tight junction proteins. Furthermore, curcumin could positively alter microbial composition by increasing beneficial microbes, improving barrier function, and suppressing inflammation [179]. The use of curcumin in healthy individuals has great benefits and is considered safe for use especially when administered at 180 mg/day [180]. However, minimal side effects such as diarrhea, yellow stool, and headache have been reported in seven individuals administered with varying doses of curcuminoid (500 -12000 mg) [181]. This finding raises concerns about its long-term usage. Also, individuals who received curcumin (0.45-3.6 g/day) for four months suffered adverse effects including an increase in lactate dehydrogenase content, diarrhea, nausea, and many more [182]. Likewise, an increase in urinary frequency and nausea has been associated with the use of curcumin [180]. Nevertheless, its anti-aging role has been thoroughly reviewed by Izadi et al. [183].
4.1.4. Senolytic drugs
Senolytic drugs (Fisetin, glycosides, A1331852, quercetin, FoxO4 peptides, B-cell lymphoma-extra large (BCL-XL) inhibitor A1155463, Navitoclax, and Dasatinib) have been reported to slow aging, and prevent aging-associated diseases including cancer by selectively removing deleterious senescent cells [6]. Recently, Ramesh et al. [184] reported that combining low doses of BCL-XL inhibitor A1155463 with FGFR4 inhibitors could eradicate even the most resistant human colorectal cancer while providing a non-toxic therapeutic opportunity.
Aside from this, Navitoclax (ABT-263) is reported to inhibit anti-apoptotic protein (BCL-XL, B-cell lymphoma-w (BCL-w), B-cell lymphoma-2 family protein resembling Boo (Bcl-b), and the notable B-cell lymphoma-2 (Bcl2)) which tends to be upregulated in ISCs during adenoma formation [185,186]. Thus, informing on its potential role in the gut. Recently, ABT-263 was reported to induce apoptosis, decrease fibrotic gene expression (CTGF genes) in normal human intestinal myofibroblast, and mitigate inflammation (by reducing IL-6, TNF-α, and IL-12) as well as fibrotic gene expression in a dose-dependent manner in CBA/J mice S. typhimurium models [187]. However, side effects including neutropenia and thrombocytopenia were reported in patients who were administered with navitoclax daily for a week (Clinicaltrial.gov (NCT00406809)) [188]. Conversely, sole treatment using navitoclax in some individuals has an acceptable safety profile [186].
Dasatinib is a tyrosine kinase inhibitor that inhibits Src family tyrosine kinases and possesses antitumor activity [189]. Recently, it was reported to have tolerable toxicity in patients suffering from metastatic gastrointestinal stromal tumors who are notable to be resistant to imatinib and sunitinib therapy (Clinicaltrial.gov (NCT02776878)) [190]. Also, dasatinib inhibited ephrin A2-ephrinA1 complex to reduce intestinal inflammation, decreased leukocyte infiltration, reduced barrier leakages, and protected the intestines against radiation-mediated intestinal damage [191]. Although this class of drug improves longevity, the safety profile in the elderly is still under study. Moreover, single therapy of these drugs is less effective than combinational therapy in targeting multiple anti-apoptotic pathways of senescent cells [192]. This finding indicates that single senolytic therapy suffers a therapeutic drawback.
A summary of the recent findings of some senolytics has been presented in Table 1 below.
Table 1.
Summary of Senolytics on therapeutic rejuvenation in mice between mid and old ages.
| Therapeutic intervention |
Mouse age | Treatment | Comparison control | Findings | References |
|---|---|---|---|---|---|
| Ablation of senescent cells | |||||
| Quercetin + Dasatinib | 18 months | Old Balb/c mice were administered with Quercetin and Dasatinib | Young and old Balb/c mice were administered with oral placebo administration | Drug combination reduced intestinal senescence (p16 & p21) inflammation while modulating specific microbiota signatures in aged mice | [193] |
| Navitoclax (ABT263) | 24 months | Navitoclax administered to natural aging mice | Vehicle (10 % ethanol, 30%PEG 400, 60 % Phosal50) | Navitoclax improved mesenchymal senescent cell clearance coupled with limited efficacy in age-related bone loss | [194] |
| Venetoclax + verteporfin (Yap inhibitor) | 4–6 weeks | Administration of Venetoclax and verteporfin in Ras1 and NF2 KO NOD-SCID mice | Vehicle-treated NOD-SCID mice | Combined therapy synergistically suppressed gut cancer stemness and in-vivo metastasis | [195] |
4.1.5. Perindopril
Perindopril is an ACE inhibitor used in hypertension management which could be repurposed as a gerotherapy intervention [196]. It improved exercise capacity and maintained the quality of life in the elderly [197]. Recently, Sayed et al. [198] reported that it could reduce proinflammatory cytokines via suppression of TLR4/NF-κB and c-Fos/c-Jun pathways coupled with the promotion of PPAR-/SIRT1 cytoprotective signals in intestinal injury. Previous studies reported dry cough, diarrhea, erectile dysfunction, headache, and hypotension as some common side effects of the drug [199,200]. Nonetheless, this drug could be used in the management of age-related gut disorders.
In all, Western medicine holds promise for treating gut-related diseases. Although the anti-aging mechanism of some of these drugs in the gut remains unclear, their use in animals as well as humans remains promising and encourages further development to avoid toxicity in the aged.
4.2. Traditional Chinese medicine (TCM) in gut rejuvenation
While Western medicine holds promise, the complexity of the aging process necessitates a multi-faceted approach that employs TCM as complementary medicine. In TCM, aged patients are treated in a “personalized” way using herbal formulations or nutraceuticals according to treatment practices and the experience of the Doctor [201]. Here, we reported some significant nutraceutical components of TCM which may be administered as a pill, capsule, or decoction with the focus on mitigating gut aging-related diseases or dysfunction.
4.2.1. Berberine
Berberine is a natural alkaloid found in Chinese plants, often delivered in capsules and used for the treatment of several ailments including IBD [202]. Luo et al. [203] reported that Berberine activated Lgr5+ ISC and promoted the expression of Wnt genes in the resident stromal cells of the ISC niche. Also, proinflammatory cytokines (IL-1β and TNF-α) were significantly reduced while anti-inflammatory cytokine (IL-4) was increased in colon tissues via JAK/STAT3, TNF, and NF-κB pathways following Berberine treatment [202]. Similarly, other reports confirmed the antioxidant activity of Berberine and suggested it promoted Treg via the mTOR pathway, inhibited T-cell (TH1 and Th17 cells) differentiation, and played an anti-inflammatory role via IL6/STAT3/NF-κB pathway [204]. Hence, this mitigates the perturbed balance of the elevated T-cell proportion over Treg in the aged gut. Recently, this alkaloid has been reported to modulate gut microbiota and improve the intestinal barrier via TLR4/NF-κB/mTORC pathway and autophagy [205]. This suggests the multiple pathways through which Berberine exerts its gut protective effect. Although the use of this drug is considered safe in normal adults, it could result in toxicities in diabetic adults. Furthermore, high doses of the drug are associated with gastric lesions, dyspnea, and cardiac hypotension [206]. In a clinical phase 1 study, Berberine was reported to be safe for use and well tolerated when co-administered daily with mesalamine (ClinicalTrials.gov (NCT02365480)) [207].
4.2.2. Bazi bushen (BZBS)
Bazi bushen capsule is a clinically used and safe anti-aging drug, majorly comprising 14 herbs and possessing several bioactive compounds (1498 compounds) that delay stem cell senescence, improve telomerase activity, reduce β-galactosidase, and support balanced cell differentiation [[208], [209], [210]]. Recently, it has been reported to restore the gut with good microbes by increasing Firmicutes/Bacteroides ratio, reducing inflammatory cytokines (IL-1β and IL-18) secretion, improving intestinal barrier function by inhibiting NLRP inflammasome-mediated pyroptosis, restoring intestinal villi structure, and promoting Lgr5+ ISCs stemness in SAMP8 mice [211]. Similarly, Xu et al. [5] reported that this capsule reduced senescent-related markers (p16, p21, and p53), inhibited inflammation by downregulating proinflammatory cytokines (IL-1β, IL-6, and TNF-α), and suppressed the TLR4/NF-κB pathway. Hence, this suggests that Bazi bushen could be a good anti-aging drug for mitigating gut dysbiosis, preventing immunosenescence, and promoting ISC function. However, the therapeutic benefits of BZBS on smooth muscle cells and individually differentiated progenies are yet to be determined. Also, BZBS improved DNA repair pathways, and reduced cellular oxidative stress by upregulating Sirt3-mediated superoxide dismutase 2 (SOD2) deacetylation and FoxO1 to boost antioxidant activity (catalase and SOD2 activity) in naturally aging C57BL/6J mice [212]. These pieces of evidence suggest that BZBS could mitigate aging-associated cellular errors and boost the health span of the elderly. Yet, a large number of genes related to gut aging remains to be further studied and their discovery could offer avenues for a better understanding of other mechanisms by which BZBS mitigates age-related gut changes and repercussions. Although this drug is well tolerated and considered safe, minor adverse effects such as constipation and nasopharyngeal inflammation have been reported in elderly patients following 12 weeks of drug administration [210].
4.2.3. Fufang zhenshu TiaoZhi
Fufang zhenshu Tiaozhi (FZT) is a capsule made from 8 Chinese herbs and has been prescribed for treating glucose and lipid metabolism disorders [92,213]. Recently, it has been reported to delay intestinal aging by improving gut metabolites and flora, enhancing telomerase activity, and preventing gut inflammation mediators (COX-2, IL-6, TNF-α, and activator protein-1) in aged C57BL/6J mice [92]. Similarly, other studies confirmed that FZT could modulate the gut microbiota, mitigate intestinal inflammation, and prevent barrier disruption [214,215]. Furthermore, FZT improved intraepithelial lymphocytes and goblet cell numbers which tend to decrease during aging according to previous studies [92]. However, their therapeutic role in ISCs is yet to be studied. Recent reports indicate that FZT could decrease the expression of inflammatory genes (IL-1β, IL-6, TAK1-binding protein 1 (TAB-1), Ccl2, Tlr2, and Tlr4) and restore tight junction protein (ZO-1, E-cadherin, Cldn-2, and -4) in HFD-fed mice [215]. This evidence indicates that FZT could be used to treat similar gut changes observed with gut aging. Furthermore, its long-term use with no obvious toxicities warrants its investigation into the elderly suffering from gut diseases.
4.2.4. Ginseng (Ginsenoside Rb1)
Ginsenoside Rb1 (GRb1), a major constituent of ginseng (a famous herb in traditional Chinese medicine) has been shown to remarkably modulate gut microbiota and regulate the sirtuins family (SIRT-1, -3, and -6) to impede intestinal aging in C57BL/6 mice [216]. In line with this, gut tight junction protein (Cldn (2,3,7, and 15)) and ISC-related genes (Lgr5) among other genes such as Tert, proto-oncogene (c-Myc) and marker of proliferation ki67 were significantly increased following GRb1 administration [216]. Similarly, Wan et al. [217] reported that it can regulate microecological balance and reduce inflammatory cytokines. Of note, GRb1 could suppress intestinal inflammatory mediators (malondialdehyde (MDA), Il-6, IL-β, and Tnf-α), reduce intestinal injury by activating the phosphatidylinositol 3′-kinase (PI3K)/protein kinase B (Akt)/nuclear factor-erythroid 2-related factor 2 (Nrf2) pathway and mitigate oxidative stress [218]. The evidence above suggests that ginseng constituents may be beneficial in treating aged gut-associated ailments. However, some studies have reported the teratogenic effect of GRb1 (30–50 μg/ml) in mice and rats [219]. Nonetheless, administering this drug at a clinically accepted dose in humans does not exhibit these detrimental effects.
4.2.5. Liangxue-Guyuan-Yishen decoction
Liangxue-Guyuan-Yishen decoction is a medicinal preparation from 10 Chinese medicinal herbs and has gained much attention due to its effectiveness in protecting the gut against radiation with low toxicity [220]. Liangxue-Guyuan-Yishen decoction has been reported to promote ISC regeneration, improve tight junction protein expression via Wnt and MEK/ERK pathway, and enhance immune response via Tlr4/myeloid differentiation primary response 88 (MyD88)/NF-κB pathway [220,221]. Similarly, a recent study reported that the decoction promoted Akkermansia levels and improved ISC numbers to protect against radiation-induced intestinal injury in mice [222]. Furthermore, there were no apparent side effects associated with the use of the decoction [222]. Although gut aging studies remain limited, more studies are warranted to ascertain the pharmacological effect of this herbal mixture on the sub-epithelial niche components of the intestine.
4.2.6. Other nutraceuticals in TCM used globally with gut rejuvenation properties
Some nutraceuticals/herbal medicines in TCM have been reported to be used in many countries and served as a bridge connecting Western medicine based on a fair share of philosophical thoughts on aging gut repercussions [223]. Here, we focused on some of the worldwide commonly used nutraceuticals in TCM with significant therapeutic effects in the gut.
Vitamin D is a derivative of ergosterol which can be synthesized in the skin during exposure to ultraviolet light or obtained from food such as fish and eggs [224]. In TCM, sunlight and Wenyang herbs which supplemented the body with vitamin D shared treatment pathways with Vitamin D supplements administered in Western medicine [225]. Li et al. [226] reported that vitamin D and its receptor (VDR) complex protected ISCs and progenitors from apoptosis via the Pmaip1-mediated pathway. Recently, vitamin D receptors complexed with herbs containing vitamin D could maintain tight junction integrity, promote intestinal healing capacity, and prevent irritable bowel syndrome as reported by Yao et al. [227]. Vitamin D has been reported to modulate the gut microbiota. A dose-dependent concentration of vitamin D3 was reported to modulate innate and adaptive immune cells, increase beneficial bacteria, and reduce pathogenic pathobionts in healthy vitamin D-deficient adults [228]. However, Yao et al. [229] have reported no changes in gut microbiota composition in the gut of the elderly following monthly oral supplementation of 60000 IU Vitamin D for five years (Australian New Zealand Clinical Trials Registry: ACTRN12613000743763). Although there is no special warning attached to the oral intake of vitamin D supplements, one could risk having vitamin D poisoning or hypervitaminosis D, and adverse effects in the intestine such as constipation and nausea may arise in combination therapy with calcium [230,231].
Proanthocyanidins (PAs) are a major class of phytochemicals in cranberry, cocoa, and grape seeds. They have been studied for their potential health benefits, including their effects on the intestine, and aging. PAs have been shown to exert antioxidant, and anti-inflammatory effects, which may help protect intestinal stem cells and their niche from damage caused by aging processes [232]. Recently, Zhu et al. [233] reported that procyanidin B2 (B-type proanthocyanidins) promoted Lgr5 expression and repressed oxidative stress via Wnt/β-catenin and Nrf2/antioxidant responsive element (ARE) pathways, respectively. Furthermore, Casanova-Martí et al. [234] have reported that long-term exposure to PAs improved epithelial differentiation of both secretory linage cells (paneth, goblet, and enteroendocrine cells (L-cells)) and enterocytes by modulating β-catenin pathway, promoting the early expression of gene transcription factors (Neurod1 and Pax6) and maintaining Notch-mediated lateral inhibition in-vitro. Hence, this suggests they could be used to mitigate the skewed cellular differentiation as seen in the aged gut. Additionally, they modulated the microbiome toward a healthy profile, promoted regeneration, repaired intestinal tissues, and strengthened barrier integrity [235]. These findings indicate that they could mitigate age-associated gut defects. Generally, PAs (2500 mg) are considered safe and well-tolerated in healthy adults, children, patients, and pregnant women [[236], [237], [238]]. Chen et al. [239] have reported that although the use of proanthocyanidins is encouraged, the safety profile and clinical evidence in the elderly must be ascertained.
Quercetin is a polyphenol with a low bioavailability and is normally found in most fruits and vegetables [240]. It is utilized in TCM as well as constitutes a major part of the Western diet (approximately 15 mg) [241,242]. It possesses antioxidant, anti-inflammatory, and antimicrobial activities which are beneficial properties of aging intervention drugs [243]. Yan et al. [244] reported that quercetin could prevent ISC aging by preventing ROS in Drosophila. Furthermore, it is known to significantly impact the intestinal microenvironment with many therapeutic benefits including abrogating intestinal flora disorder to protect the intestine in gut-free mice coupled with intestinal barrier function recovery and the increase in mucosal thickness [245]. Similarly, Lan et al. [243] reported that the antimicrobial activity of quercetin had a direct activity on modulating the gut microbiota, promoting beneficial SCFA production and reversing fecal metabolite anomalies. Also, quercetin has been reported to be administered as a senotherapeutic compound and inhibited BCL2/BCL2L1, PI3K/AKT, and TP53/P21 pathways. Therapeutically, it is administered in senolytic combination therapy with dasatinib to resolve the drawback associated with the single senolytic treatment that is not entirely efficient in targeting multiple anti-apoptotic pathways of senescent cells [192]. Recently, natural senotherapeutic repurposing candidates including piperlongumine found in long pepper and natural senomorphics (phloretin, Parthenolide, and curcumin) have been reported to be natural substitutes for dasatinib in the combination therapy following computational identification [246]. Furthermore, piperlongumine has been reported to inhibit Th17-differentiation without affecting Treg and could revert the aging-related increase in Th17/Treg ratio. Although quercetin is beneficial, some common side effects such as increased weight in rats, nephrotoxicity in animals with damaged kidneys, and reduced bioavailability of other drugs in combinational therapy in human and mice studies [247,248]. Although the reduced bioavailability could be beneficial in reducing the side effects of other drugs, it is advisable to consult the physician before its concomitant use with other drugs [248]. Furthermore, its long-term use in the elderly has not been assessed. Nonetheless, the above information underscores its beneficial use in maintaining gut health in the elderly.
Ginger (Zingiber officinale) is a popular herbal plant with many pharmacological benefits such as anti-inflammatory, antioxidant, and anticancer activity attributed to its rhizome [249]. In gut health maintenance, several studies reported that derived ginger products (Shogaol, ginger root powder, and ginger exosome-like nanoparticles (ELNs)) could modulate the gut microbiota composition, promote intestinal stem cell function, strengthen intestinal barrier function, and mitigate colitis via increasing IL-22 production [[250], [251], [252]]. Recently, 6-Shogaol was reported to promote apoptosis of colon cancer cells, and inhibited their migration via the NF-κB (IKKβ)/NF-κB/Snail signaling pathway [253]. Recent reports indicated that no adverse effects were associated with the use of ginger in breast cancer patients receiving chemotherapy [254]. However, vertigo, heartburn, and headache have been associated with ginger consumption in other studies and need to be monitored [253,254]. Given these gut therapeutic effects, its role in gut aging remains an exciting area yet to be explored.
Cinnamon is a common herb with several bioactive compounds and belongs to the family Lauraceae [255]. It possessed several polyphenolic compounds and volatile phenols with antioxidant, anti-cancer, and anti-inflammatory activities [256]. Other pharmacological effects include its neuroprotective and gastroprotective effects. Due to its therapeutic benefits, they are used in food preparation and as supplements. Several studies reported that products of cinnamon could be used to improve the intestinal flora imbalance following gut injury and the intestinal barrier by modulating claudin-2 expression in the gut [257,258]. The long-term use of cinnamon and high doses of it are associated with adverse effects such as nausea, rash, stomach ache, mucositis, and dermatitis and must be clinically monitored [259]. However, its role in the aged gut remains to be explored.
In summary, TCM has made major strides in academic research, such as being cited in medical journals, and has employed most herbal formulations, with many of the nutraceuticals globally accepted and used for treatment. Although these nutraceuticals exhibited tremendous therapeutic benefits, their efficacy and safety based on long-term clinical trials are needed to give credence to their use on a long-term basis. Thus, offering opportunities for exploration by researchers in their quest to promote healthy intestinal aging, and also bridging the biocultural disparity between Eastern and Western nutraceuticals.
4.3. Other health-associated gerotherapeutic strategies in gut rejuvenation
4.3.1. Dietary intervention
Interestingly, extra dietary components such as seafood as an aging intervention are becoming popular globally. Recently, the consumption of ark clams (seafood) has been reported to alleviate gut barrier damage, reduce oxidative stress, modulate the gut microbiota, increase immunoglobulin A (IgA), and SCFA production to protect the gut in D‐galactose‐induced aging rats [260]. Also, a whey protein diet has been reported to be an effective measure in restoring key nutrients, especially increasing protein intake which is insufficient in the elderly. A systematic review showed that a whey protein diet improved the health span of the elderly while a metabolic analysis showed that whey protein supplementation was ideal for modulating the gut microbiome of the elderly [261,262]. However, adverse effects on the kidneys and liver have been associated with the indiscriminate use of whey protein and these effects are aggravated in people living sedentary lifestyles [263]. Furthermore, a low histamine diet could be a gold standard treatment that keeps excessive histamine secretion in check. Likewise, microbiota dietary targeted interventions such as probiotics, prebiotics, and post-biotics have been encouraged for use in the aged [264]. For example, bacterial species of genus Bifidobacteria could be considered for adequate supplementation as they are beneficial in the degradation of excessive histamine, maintenance of colonic tight junction, inhibition of pro-inflammatory cytokines, production of polyamines to scavenge reactive oxidative species, and promotion of lifespan in mice [26]. A recent meta-analyses study on the long-term use of probiotics including Bifidobacterium and Lactobacillus strains in the elderly showed a significant impact in improving constipation symptoms as well as their cognitive function which improved their quality of life [265]. Although dietary intervention has beneficial impact on the gut and is safer than pharmacological treatments, the side effects associated with it should not be overlooked [266]. Hence, this information strongly gives scientific credence that diet may mitigate gut biological aging and its repercussions.
4.3.2. Dietary restrictions and fasting
Dietary restrictions (such as reduced intake of a high-fat diet) and fasting have been reported to be beneficial in promoting stem cell function in the intestines [267]. Green et al. [268] reported that restricting an isoleucine-containing diet could improve metabolic health, reduce frailty, decrease the risk of cancer, and increase health span in old HET3 mice. In support, religiously practicing dietary or calorie restriction for two years could reduce cellular senescence biomarkers and SenMayo gene set responses in healthy young to middle-aged humans [269]. Currently, the clinical test results of dietary restriction in old adults expressing p16 and p21 markers remain to be reported. Recent reports on dietary restriction show that it is closely related to the risk of malnutrition in susceptible groups and further studies are required to understand their efficacy, and safety in different population groups [266]. Also, Jaime and Mank [270] have reported that dietary restriction could increase the risks of osteoporosis, cause depression, and result in weight loss. Therefore, this treatment will require that the patient understand the risks of the treatment. Furthermore, this treatment will be more suitable for obese individuals [270].
Also, 24-h fasting as a non-pharmacological intervention has been reported to promote ISC function by activating the PPAR-mediated fatty acid oxidation program in aged mice [271]. Furthermore, intermittent fasting including Ramadan 30 consecutive days of fasting was associated with increased microbiome diversity and provoked upregulation of butyric acid-producing Lachnospiraceae which promoted health benefits in middle-aged adults [272]. Similarly, the gut Bacteroides and Firmicutes increased by 21 % and 13 % respectively, after Ramadan fasting [273]. Although fasting may be beneficial, it may aggravate chronic gut disorders such as ulcerative colitis in older men [274]. Recently, a fasting-mimicking diet that mimics the benefits of traditional fasting could reduce the side effects of fasting to an extent [266]. However, there is a need to understand the associated risk of intermittent fasting or fasting-mimicking diets in the aged gut. Nevertheless, fasting remains beneficial, and further studies are required to affirm fasting-associated benefits in the aged gut of the elderly.
4.3.3. Exercise or physical activity
Physical activity or exercise has been one of the strategies reported to promote healthy aging in the elderly. Chae et al. [275] have reported that PA improved intestinal homeostasis by activating the apelin receptor-AMP-activated kinase (APJ-AMPK) pathway in epithelial cells to facilitate renewal. Furthermore, PA improved gut microbiota composition with some favorable intestinal flora including Bifidobacteria slowing the rate of biological aging in elderly people [276,277]. Recently, a systematic review indicated it could modulate the gut microbiota in the aged with no clear indication of which taxa are the most affected by this intervention [277]. However, SCFA metabolites were reported to decrease in elderly women after PA [276]. Also, other small-scale studies have conflicting reports on the impact of exercise on aging-related microbial α-diversity abundance [262,278]. Thus, making this intervention a bit controversial and necessitating further exploration. Recently, a large-scale study showed that regular exercise partially restored the abundance of Firmicutes, Bacteroidetes, and Verrucomicrobia among other microbes and significantly decreased α-microbial diversity in the elderly as confirmed in other studies [278,279].
Furthermore, regular endurance exercise could regulate the immune cells and prevent aging-associated repercussions such as inflammaging, immunosenescence, and maladaptive immune aging via decreasing exhausted T-cells, reducing TLR pathways, and increasing natural killer cell migration as well as naïve T cells [280]. Tarca et al. [281] have reported that dizziness, fatigue, and muscle pain are the side effects associated with PAs. Although these side effects are normal responses in PAs, embedding PAs in clinical treatment has been reported to be beneficial in people receiving peritoneal dialysis.
Furthermore, chronic exercise could reduce the risk of cardiovascular disease [282]. Conversely, PAs can increase the risk of sudden cardiac death in men above 40 years [282]. These shreds of evidence indicate that PAs in individuals suffering from cardiovascular diseases should be closely monitored. Nevertheless, they can be used in the elderly to promote gut health.
4.3.4. Fecal microbiota transplantation
Fecal microbiota transplantation (FMT) has been given credence to prolonging lifespan and reversing intestinal aging [6]. Gut microbiota restoration presents an auspicious strategy for gut health maintenance since it can delay aging along with beneficial effects including stem cell proliferation and immune system regulation [283]. Several studies on FMT in mice remain promising and clinical evidence in humans has to be carried out with a focus on safety and effectiveness [283]. Likewise, a pilot study revealed that FMT mitigated gut microbiota dysbiosis by increasing Clostridiales abundance while decreasing the pathogenic Enterobacteriales in patients suffering steroid-resistant lower gastrointestinal acute graft-versus-host disease for which there are limited treatment options (Trial registration number NCT# 03214289) [284]. Recently, the US FDA approved an oral FMT capsule Vowst (formerly known as SER-109) which is administered one dose daily for three consecutive days and can be used to effectively treat Clostridiodes difficile infections [285]. In the elderly, FMT has been considered safe and effective for treating severe, recurrent, and complicated C. difficile infections [286]. Furthermore, FMT can restore gut microbiota in the crypts of ulcerative patients [287]. Thus, it is of great importance to develop novel approaches that accurately regulate the gut microbiota to repair the disrupted internal equilibrium. Adverse effects of this treatment approach include abdominal pain, nausea, vomiting, and constipation [288].
4.3.5. Cell reprogramming and transplantation intervention
Most cellular intervention often comes as a last resort due to therapeutic failure using drugs and employs induced pluripotent stem cells or adult stem cells (mesenchymal cells or ISCs) [289]. Indeed, the inability of stem cells to repair damage is associated with many age-associated gut diseases and requires stem cell therapy using organoids derived from induced pluripotent stem cells or adult stem cells [290]. In intestinal aging, several studies using organoids derived from mice or rats make us understand the basic science of aging and related diseases. Moreover, the significant differences in genes across different species coupled with high failure in clinical translatability into human subjects make it imperative to employ human organoids derived from stem cells for efficient treatment [289]. The application of human organoids has been of interest as they mirror in-situ tissue characteristics of the gut of their donors and provide a more personalized treatment for intestinal diseases that may occur in them. As discussed above, the organoid derivation efficiency is reduced when the donor is an aged adult. Moreover, to the best of our knowledge, the use of human organoids in mitigating intestinal aging in aged adults without diseases has been understudied. Here, we reported the therapeutic benefits of organoids derived from both mice and humans used in mitigating age-related gut diseases in the context of stem cell potency.
Induced pluripotent stem cells are stem cells (with pluripotent abilities) derived from somatic cells after exposure to cues and simulate embryonic stages of development [291]. It is interesting to note that induced pluripotent stem cells (iPSC) could produce organoids with all the components in the intestines (epithelial cells, mesenchymal cells, fibroblast, immune cells, and smooth muscle cells) [292]. Also, they serve as therapeutic tools in gut rejuvenation, tissue regeneration, and personalized medicine. In tissue regeneration, induced pluripotent stem cells reconstituted the colonic epithelium in mice and humans after transplantation [293]. Although this approach addressed ethical limitations associated with the use of human embryonic stem cells, its clinical use is still limited due to the possibility of cells becoming oncogenic [294]. Furthermore, the dedifferentiation process leading to iPSC from aged somatic cells could have most of the genetic and epigenetic information on aging lost, thus limiting its use in aging preclinical studies to test drug efficacy [295].
Conversely, adult stem cells are limited to multipotent abilities and compared to induced pluripotent stem cells, they have been reported in many ongoing or even closed clinical trials [289,296,297]. Furthermore, they can accumulate and represent age-associated mutations that occur in both the small and large intestines and are very useful in precision therapy [292].
Adult mesenchymal stem cells (MSCs) have been widely used in pre-clinical (in-vitro and in-vivo) and clinical regenerative studies [298,299]. Moussa et al. [298] demonstrated the gut rescue effect following the co-injection of transplanted mesenchymal cells with colonic-derived epithelial cells to mimic clinical translatability. Moreover, they are considered a treatment option for mitigating IBD in patients [294]. In clinical trials, human umbilical cord-derived mesenchymal stem cell therapy is being used in patients with active ulcerative colitis (ClinicalTrials.gov ID, NCT02442037) and advanced colorectal cancer (ClinicalTrials.gov ID, NCT06446050). Furthermore, mesenchymal stem cells have been used in treating inflammatory bowel disease (ClinicalTrials.gov ID, NCT 03299413). However, the current status of the above clinical trials is unknown. Conversely, a Phase 2 clinical trial using adult human MSCs in treating moderate to severe Crohn's disease has been completed (ClinicalTrials.gov ID, NCT00294112). Although this treatment is beneficial, challenges due to the aging microenvironment persist and could hamper the efficacy of MSC transplantation therapy leading to unsatisfied results in some aged adults [300]. Chen et al. [300] reported that niche-targeted therapies such as senolytics and sirtuin activators could enhance MSC transplantation therapy.
The ISCs are multipotent and limited to only the development of epithelial cells on the crypt-villi axis. The ISC delivery in the form of organoids to damaged intestinal epithelial remains a promising strategy for gut restitution as reviewed by Rees et al. [45]. In detail, this strategy involves the long-term three-dimensional (3D) culture of enteroids (stem cells derived from the small intestine)/colonoids (stem cells derived from the colon). These stem cells are obtained from surgical resections or endoscopic biopsies and later transferred into the patient to regenerate the epithelial cells of the intestine [301,302]. In line with this, Jee et al. [303] demonstrated that the Lgr5+ stem cells are pivotal in reconstituting the epithelium of mice suffering radiation proctitis following enema delivery of colon organoids. Also, organoids derived from human ISCs mitigated intestinal failure and short bowel syndrome in rats [304]. Recently, human intestinal organoids generated to contain immune cells led to immune cell activation when transplanted into mice possessing humanized immune systems [305]. The results from the human organoids in mice experiments became the premier basis for clinical trials in rescuing patients suffering from ulcerative colitis using organoid transplantation therapy [306].
Furthermore, human intestinal organoid was used to mitigate colonic injury following delivery in a hydrogel vehicle to the injured intestinal mucosa [307]. Although this regeneration method is limited by scale, a protocol for engineering mucosa graft tissue from decellularizing native human tissue salvaged the limitation and mitigated intestinal failure in patients [308]. Currently, the collection of intestinal organoids from biopsies to establish biobanks and characterize them before therapeutic screening is still ongoing (ClinicalTrials.gov ID, NCT05294107). Of note, human organoids could be used in high throughput screening of drugs to understand an individual's response to therapy and safety whereas its grafting in animals offers insights into the role of organoids in treating gut diseases and could be translated into the elderly. The use of human organoids for clinical and translation therapy is limited due to the cost associated with organoid culture and the long time allocated to generate a comprehensive representative of a patient's organ. To date and to the best of our knowledge, intestinal organoid transplantation in the elderly without gut disease has not been explored yet.
Also, organoids derived from stem cells can be combined with gene therapy or reprogrammed by transient reprogramming, direct lineage reprogramming, and partial reprogramming to enhance them before being introduced into the affected area of the gut.
In gene therapy, modern sophisticated tools such as clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated protein 9 (Cas9) (CRISPR/Cas9) are used to screen for regulators of intestinal cell fate, label colon stem cells, and modify or correct genes associated with diseases in organoids [[309], [310], [311]]. Also, CRISPR/Cas9 is used in lineage tracing system for human ISCs following human colonoid engraftment into the rectum surface of a mouse [312]. However, the translatability of this approach to improve an individual's intestinal function is yet to be reported despite their promise of retaining stemness to promote gut health. In cell reprogramming, Miura and Suzuki [293] have reported that pluripotent stem cells could be guided by direct lineage reprogramming to form human intestinal organoids for biomedical applications. In Table 2, we have summarized the findings of some gerotherapeutic strategies obtained from in-vivo gut studies, especially in mice ranging between mid-age and old age.
Table 2.
Summary of recent gut studies and findings on other therapeutic interventions on mice between mid and old ages.
| Therapeutic intervention | Mouse age | Treatment | Comparison control | Findings | References |
|---|---|---|---|---|---|
| Ketogenic Diet | 3–5 months | Hmgcs2 KO mice fed with a ketogenic diet for 3–4 weeks | Chow-fed mice | The ketogenic diet restored differentiation defects and enhanced crypt organoid forming capacity in Hmgcs2-null intestines | [313] |
| Fasting-mimicking diet | 8 weeks | DSS-treated C57BL/6 mice fed with a fasting-mimicking diet | Naive C57BL/6 mice | Improved microbiota diversity, increased intestinal stem cells, improved immune function and reversed IBD | [314] |
| Transient reprogramming | Middle age | Transgenic mice reprogrammable mouse strain with doxycycline induction of Sox4, Oct2, Klf4, c-Myc | Untreated mice | Long-term in-vivo reprogramming results in intestinal and liver toxicity | [315] |
| Direct lineage reprogramming | 10 Weeks old | Reprogrammed mouse fibroblast via induction of Hnf, Foxa3, Gata6 and Cdx2 | Organoids from Lgr5-EGFP-ires-CreERt2 Mice | Fibroblast-acquired budding organoids restored the colon and intestine | [293] |
| Gene therapy-mediated partial reprogramming | 124 weeks | Adeno-associated Virus gene -mediated therapy + doxycycline | PBS + doxycycline mice | Slight increase in age extension (109 %) and age reversal which will benefit the increasingly large aging population | [316] |
5. Discussion
The intestine is a significant part of the body where any deterioration of the gut epithelium is highly associated with adverse impacts on the gut and overall health. At the core of this are several advances made to understand the functional components of the gut epithelium (ISC and their niche components). Growing evidence suggests that aging adversely impacts ISCs and their niche component leading to ISC senescence, immunosenescence, inflammation, gut microbiota dysbiosis, malabsorption, and others which could increase the risk of systemic diseases. In preventing age-related diseases, gerotherapeutic strategies employ pharmacological approaches (Western and Traditional Chinese medicine) and non-pharmacological approaches (lifestyle modifications, dietary restrictions, cellular programming, and many more) with promising anti-aging results to improve gut health by targeting ISC and their niche components. Although these approaches improve gut health, some treatments remain to be investigated in aged gut models. Moreover, treatments on the recently identified FGFBP1+stem cell could offer insights into novel therapy development and possible transplantation into the gut of aged adults. It must be noted that anti-aging treatment requires long-term systemic treatment, thus making some approaches a concern due to associated side effects [317]. To the best of our knowledge, the safety profiles of some of these drugs in the elderly are yet to be determined. Conversely, combining some of the above approaches in the elderly has been accepted and encouraged to be used. Recent clinical trials in the elderly (>70 years) have shown that combining exercise with protein and vitamin D supplementation can significantly improve the quality of life without any significant adverse effect (ClinicalTrials.gov NCT02873559) [318].
It is worthy to note that, in-vivo and in-vitro models have provided more insights into understanding the basic science of aging and stem cell therapy than human studies that suffer limitations including ethical considerations and limited accessibility of tissues [319,320]. Although aging studies using mice are popular in geroscience, they do not accurately represent human states due to unsurmountable gene differences across species, species-specific gut microbiota differences, and species-specific disease states [302]. Recently, organoids derived from individual's stem cells rather than animal models offer insights into understanding the disease affecting the individual, making drug discovery or development process faster and more effective. Intestinal organoids derived from an individual's cells combined with genomic data could help devise an individualized treatment strategy and identify which individuals would respond better to a particular treatment than others. Altogether, the impact of the aging microenvironment on the efficacy of stem cell therapy and the side effects of some niche targeted therapies, warrants more efforts into developing efficient strategies against aged-gut diseases. Further studies are also required to understand the underlying mechanism of these suitable approaches in mitigating gut aging.
6. Conclusion and outlook
In summary, we provided information on the healthy gut maintained by the crucial ISCs coupled with ISC niche components comprising the epithelial, sub-epithelial tissues, immune and microbial components. However, aging affects stem cells and niche components and alters their function, resulting in repercussions. Currently, information on how aging impacts other differentiated cells and the role of the declining function of aged ISCs including FGFBP1+cells towards disease contribution are understudied and require further research. Some hallmarks of aging including mitochondria dysfunction and senescence that tend to affect some gut component functions were discussed in this review. Also, discordant reports associated with different models or settings could be eschewed by following uniform standards or guidelines for creating an aging gut model or validating a mechanism.
Although studies, using animal models of intestinal disorders are still under review, their significant progress in translating basic biological discoveries could help treat human intestinal disorders to a fair extent. Furthermore, regenerative therapies using programmable adult ISCs and MSCs remain promising in clinical trials for treating disorders in the aged adult. Yet, caution must be exercised when using long-term in-vivo reprogramming treatment due to associated toxicity to the intestine and liver. Furthermore, the use of human intestinal organoids remains promising and could provide personalized “tailored” drugs for better responses. Indeed, the variation in treatment effectiveness, as well as drug toxicities associated with some approaches, indicates that we put more effort into developing efficient treatment strategies against gut aging.
In conclusion, maintenance of gut health by ISC and niche components is affected by aging and a deep understanding of the aging process as well as existing gerotherapies will make researchers develop efficient ISC and niche-targeted therapies to rescue them.
Ethics declaration
Review and/or approval by an ethics committee was not needed for this study because it does not constitute the research conducted.
Funding
The National Natural Science Foundation of China (Nos. 8176140166 and 82104671), the Great Program of the Science Foundation of Tianjin (Nos. 18JCZDJC33200), the Great Program for Postgraduate Science Research Fund of Tianjin University of Traditional Chinese Medicine (Nos. 2020YJSS204 and YJSKC-20201027), and Heilongjiang Province Fund (Nos. LH2020H102).
Data availability statement
No data was used for the research described in the article. This review article has not been deposited in publicly available repository.
CRediT authorship contribution statement
Joseph K. Abankwah: Writing – original draft, Conceptualization. Ying Wang: Writing – review & editing. Jida Wang: Writing – review & editing. Susan Enechojo Ogbe: Writing – review & editing. Lisa Dal Pozzo: Writing – review & editing. XiaoQian Chu: Writing – review & editing, Supervision. YuHong Bian: Writing – review & editing, Supervision, Funding acquisition.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work, the author(s) used [Grammerly TOOL/SERVICE] to [EDIT]. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Declaration of competing interest
All authors declare no conflict of interest.
Acknowledgments
We are grateful to Biorender for providing us with templates for the Figures.
Contributor Information
XiaoQian Chu, Email: chuxiaoqian8@163.com.
YuHong Bian, Email: bianyuhong_2012@163.com.
List of Abbreviation
- BZBS
Bazi bushen
- cGAS-STING
Cyclic GMP-AMP synthase-stimulator of Interferon genes
- CRISPR-Cas9
clustered regularly interspaced short palindromic repeats-CRISPR-associated protein 9
- DNA
Deoxyribonucleic acid
- FMT
Fecal microbiota transplantation
- iPSC
induced pluripotent stem cells
- ISCs
Intestinal stem cells
- JAM
Junction Adhesion molecule
- Nrf2
nuclear factor-erythroid 2-related factor 2
- RNA
Ribonucleic acid
- PAs
proanthocyanidins
- PRRs
Pathogen recognizing receptors
- Treg
Regulatory T-cells
- TNF
Tumour necrosis factor
References
- 1.Le Couteur D.G., Thillainadesan J. What is an aging-related disease? An epidemiological perspective. J Gerontol A Biol Sci Med Sci. Nov. 2022;77(11):2168–2174. doi: 10.1093/GERONA/GLAC039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Širvinskas D., et al. Single-cell atlas of the aging mouse colon. iScience. May 2022;25(5) doi: 10.1016/j.isci.2022.104202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hohman L.S., Osborne L.C. A gut-centric view of aging: do intestinal epithelial cells contribute to age-associated microbiota changes, inflammaging, and immunosenescence? Aging Cell. Sep. 2022;21(9) doi: 10.1111/ACEL.13700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conway J., Duggal N.A. Ageing of the gut microbiome: potential influences on immune senescence and inflammageing. Ageing Res. Rev. Jul. 2021;68 doi: 10.1016/j.arr.2021.101323. [DOI] [PubMed] [Google Scholar]
- 5.Xu Z., et al. Bazi Bushen maintains intestinal homeostasis through inhibiting TLR4/NFκB signaling pathway and regulating gut microbiota in SAMP6 mice. J. Sci. Food Agric. Nov. 2023;103(14):7273–7283. doi: 10.1002/JSFA.12812. [DOI] [PubMed] [Google Scholar]
- 6.Wu Z., Qu J., Zhang W., Liu G.-H. Stress, epigenetics, and aging: unraveling the intricate crosstalk. Mol. Cell. Jan. 2024;84(1):34–54. doi: 10.1016/j.molcel.2023.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Farahzadi R., Valipour B., Montazersaheb S., Fathi E. Targeting the stem cell niche micro-environment as therapeutic strategies in aging. Front. Cell Dev. Biol. 2023;11 doi: 10.3389/FCELL.2023.1162136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fathi E., Farahzadi R., Sheervalilou R., Sanaat Z., Vietor I. A general view of CD33+ leukemic stem cells and CAR-T cells as interesting targets in acute myeloblatsic leukemia therapy. Blood Res. Mar. 2020;55(1):10. doi: 10.5045/BR.2020.55.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fathi E., Valipour B., Vietor I., Farahzadi R. An overview of the myocardial regeneration potential of cardiac c-kit+ progenitor cells via PI3K and mapk signaling pathways. Future Cardiol. May 2020;16(3):199–209. doi: 10.2217/FCA-2018-0049. [DOI] [PubMed] [Google Scholar]
- 10.Sampaziotis F., et al. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science. Feb. 2021;371(6531):839–846. doi: 10.1126/science.aaz6964. 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engevik A.C., Engevik M.A. Exploring the impact of intestinal ion transport on the gut microbiota. Comput. Struct. Biotechnol. J. 2021;19:134–144. doi: 10.1016/j.csbj.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fish E.M., Shumway K.R., Burns B. Physiology, small bowel. StatPearls. Jan. 2024 https://www.ncbi.nlm.nih.gov/books/NBK532263/ [Online]. Available: [PubMed] [Google Scholar]
- 13.Sumigray K.D., Terwilliger M., Lechler T. Morphogenesis and compartmentalization of the intestinal crypt. Dev. Cell. Apr. 2018;45(2):183–197.e5. doi: 10.1016/j.devcel.2018.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maeda K. Quantitative prediction of intestinal absorption of drugs from in vitro study: utilization of differentiated intestinal epithelial cells derived from intestinal stem cells at crypts. Drug Metab. Dispos. Sep. 2023;51(9):1136–1144. doi: 10.1124/dmd.122.000966. [DOI] [PubMed] [Google Scholar]
- 15.Markandey M., et al. Gut microbiota: sculptors of the intestinal stem cell niche in health and inflammatory bowel disease. Gut Microb. 2021;13(1) doi: 10.1080/19490976.2021.1990827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beresford-Jones B.S., et al. The Mouse Gastrointestinal Bacteria Catalogue enables translation between the mouse and human gut microbiotas via functional mapping. Cell Host Microbe. Jan. 2022;30(1):124–138. doi: 10.1016/J.CHOM.2021.12.003. .e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rizzatti G., Lopetuso L.R., Gibiino G., Binda C., Gasbarrini A. Proteobacteria: a common factor in human diseases. BioMed Res. Int. 2017;2017 doi: 10.1155/2017/9351507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ionescu R.F., Enache R.M., Cretoiu S.M., Gaspar B.S. Gut microbiome changes in gestational diabetes. Int. J. Mol. Sci. Oct. 2022;23(21) doi: 10.3390/IJMS232112839. 2022, Vol. 23, Page 12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cresci G.A.M., Izzo K., Microbiome Gut. Adult Short Bowel Syndrome: Nutritional, Medical, and Surgical Management. Jan. 2019:45–54. doi: 10.1016/B978-0-12-814330-8.00004-4. [DOI] [Google Scholar]
- 20.Pickard J.M., Zeng M.Y., Caruso R., Núñez G. Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. Sep. 2017;279(1):70–89. doi: 10.1111/imr.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou Q., Ye L., Huang L., Yu Q. The research progress on intestinal stem cells and its relationship with intestinal microbiota. Front. Immunol. May 2017;8(MAY):599. doi: 10.3389/FIMMU.2017.00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmad Sophien A.N., Jusop A.S., Tye G.J., Tan Y.F., Wan Kamarul Zaman W.S., Nordin F. Intestinal stem cells and gut microbiota therapeutics: hype or hope? Front. Med. Jul. 2023;10 doi: 10.3389/FMED.2023.1195374/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S., et al. Mucin degrader Akkermansia muciniphila accelerates intestinal stem cell-mediated epithelial development. Gut Microb. 2021;13(1):1–20. doi: 10.1080/19490976.2021.1892441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang E.J., et al. The secreted protein Amuc_1409 from Akkermansia muciniphila improves gut health through intestinal stem cell regulation. Nat. Commun. Apr. 2024;15(1):1–20. doi: 10.1038/s41467-024-47275-8. 2024 15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spragge F., et al. Microbiome diversity protects against pathogens by nutrient blocking. Science. Dec. 2023;382(6676) doi: 10.1126/SCIENCE.ADJ3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ragonnaud E., Biragyn A. Gut microbiota as the key controllers of “healthy” aging of elderly people. Immun. Ageing. Jan. 2021;18(1):1–11. doi: 10.1186/S12979-020-00213-W. 2021 18:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashim H.M., Makpol S. A review of the preclinical and clinical studies on the role of the gut microbiome in aging and neurodegenerative diseases and its modulation. Front. Cell. Neurosci. Nov. 2022;16 doi: 10.3389/FNCEL.2022.1007166/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Der Heijden M., Vermeulen L. Stem cells in homeostasis and cancer of the gut. Mol. Cancer. Mar. 2019;18(1) doi: 10.1186/S12943-019-0962-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hindson J. Fgfbp1+ cells: upper crypt intestinal epithelial regeneration. Nat. Rev. Gastroenterol. Hepatol. 2024:1. doi: 10.1038/s41575-024-00965-6. 1, Jul. 2024. [DOI] [PubMed] [Google Scholar]
- 30.Yan K.S., et al. Intestinal enteroendocrine lineage cells possess homeostatic and injury-inducible stem cell activity. Cell Stem Cell. Jul. 2017;21(1):78–90.e6. doi: 10.1016/j.stem.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berková L., et al. Terminal differentiation of villus tip enterocytes is governed by distinct Tgfβ superfamily members. EMBO Rep. Sep. 2023;24(9) doi: 10.15252/embr.202256454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoultz I., Keita Å.V. The intestinal barrier and current techniques for the assessment of gut permeability. Cells. Aug. 2020;9(8):1909. doi: 10.3390/cells9081909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luna Velez M.V., et al. ONECUT2 regulates RANKL-dependent enterocyte and microfold cell differentiation in the small intestine; a multi-omics study. Nucleic Acids Res. Feb. 2023;51(3):1277–1296. doi: 10.1093/NAR/GKAC1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobayashi N., Takahashi D., Takano S., Kimura S., Hase K. The roles of peyer's patches and microfold cells in the gut immune system: relevance to autoimmune diseases. Front. Immunol. Oct. 2019;10 doi: 10.3389/FIMMU.2019.02345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dillon A., Lo D.D. M cells: intelligent engineering of mucosal immune surveillance. Front. Immunol. Jul. 2019;10(JUL) doi: 10.3389/FIMMU.2019.01499/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bennett K.M., et al. Induction of colonic M cells during intestinal inflammation. Am. J. Pathol. May 2016;186(5):1166. doi: 10.1016/J.AJPATH.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura Y., et al. Microfold cell-dependent antigen transport alleviates infectious colitis by inducing antigen-specific cellular immunity. Mucosal Immunol. Jul. 2020;13(4):679–690. doi: 10.1038/s41385-020-0263-0. [DOI] [PubMed] [Google Scholar]
- 38.Beumer J., et al. BMP gradient along the intestinal villus axis controls zonated enterocyte and goblet cell states. Cell Rep. Mar. 2022;38(9) doi: 10.1016/J.CELREP.2022.110438. [DOI] [PubMed] [Google Scholar]
- 39.Cortez V., Schultz-Cherry S. The role of goblet cells in viral pathogenesis. FEBS J. Dec. 2021;288(24):7060–7072. doi: 10.1111/febs.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hendel S.K., Kellermann L., Hausmann A., Bindslev N., Jensen K.B., Nielsen O.H. Tuft cells and their role in intestinal diseases. Front. Immunol. Feb. 2022;13 doi: 10.3389/FIMMU.2022.822867/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiong Z., et al. Intestinal Tuft-2 cells exert antimicrobial immunity via sensing bacterial metabolite N-undecanoylglycine. Immunity. Apr. 2022;55(4):686–700. doi: 10.1016/J.IMMUNI.2022.03.001. .e7. [DOI] [PubMed] [Google Scholar]
- 42.Huang L., et al. Tuft cells act as regenerative stem cells in the human intestine. bioRxiv. Mar. 2024 doi: 10.1101/2024.03.17.585165. 2024.03.17.585165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Es J.H., et al. Enteroendocrine and tuft cells support Lgr5 stem cells on Paneth cell depletion. Proc. Natl. Acad. Sci. U. S. A. Dec. 2019;116(52):26599–26605. doi: 10.1073/PNAS.1801888117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh P.N.P., et al. Transcription factor dynamics, oscillation, and functions in human enteroendocrine cell differentiation. bioRxiv. Jan. 2024 doi: 10.1101/2024.01.09.574746. 2024.01.09.574746. [DOI] [PubMed] [Google Scholar]
- 45.Rees W.D., Tandun R., Yau E., Zachos N.C., Steiner T.S. Regenerative intestinal stem cells induced by acute and chronic injury: the saving grace of the epithelium? Front. Cell Dev. Biol. Nov. 2020;8 doi: 10.3389/FCELL.2020.583919/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang L., et al. Paneth cell-derived iNOS is required to maintain homeostasis in the intestinal stem cell niche. J. Transl. Med. Dec. 2023;21(1):1–14. doi: 10.1186/S12967-023-04744-W/FIGURES/7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaves-Pérez A., Santos-de-Frutos K., de la Rosa S., Herranz-Montoya I., Perna C., Djouder N. Transit-amplifying cells control R-spondins in the mouse crypt to modulate intestinal stem cell proliferation. J. Exp. Med. Nov. 2022;219(11) doi: 10.1084/JEM.20212405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li J.-M., et al. Single-cell transcriptomics identifies a unique entity and signature markers of transit-amplifying cells in human corneal limbus. Investigative Opthalmology & Visual Science. Jul. 2021;62(9):36. doi: 10.1167/iovs.62.9.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koo B.K., Clevers H. Stem cells marked by the R-spondin receptor LGR5. Gastroenterology. 2014;147(2):289–302. doi: 10.1053/J.GASTRO.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 50.Zha J.M., et al. Interleukin 22 expands transit-amplifying cells while depleting Lgr5+ stem cells via inhibition of Wnt and notch signaling. Cell Mol Gastroenterol Hepatol. Jan. 2019;7(2):255–274. doi: 10.1016/J.JCMGH.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Capdevila C., et al. Time-resolved fate mapping identifies the intestinal upper crypt zone as an origin of Lgr5+ crypt base columnar cells. Cell. Jun. 2024;187(12):3039–3055. doi: 10.1016/j.cell.2024.05.001. .e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu D., Yan H., He X.C., Li L. Recent advances in understanding intestinal stem cell regulation. F1000Res. 2019;8 doi: 10.12688/F1000RESEARCH.16793.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sénicourt B., et al. Primary cilium identifies a quiescent cell population in the human intestinal crypt. Cells. Apr. 2023;12(7) doi: 10.3390/CELLS12071059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tümpel S., Rudolph K.L. Quiescence: good and bad of stem cell aging. Trends Cell Biol. Aug. 2019;29(8):672–685. doi: 10.1016/J.TCB.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 55.Ayyaz A., et al. Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature. Apr. 2019;569(7754):121–125. doi: 10.1038/s41586-019-1154-y. 2019 569:7754. [DOI] [PubMed] [Google Scholar]
- 56.Meyer A.R., Brown M.E., McGrath P.S., Dempsey P.J. Injury-induced cellular plasticity drives intestinal regeneration. Cell Mol Gastroenterol Hepatol. Jan. 2022;13(3):843–856. doi: 10.1016/J.JCMGH.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morral C., et al. p53 promotes revival stem cells in the regenerating intestine after severe radiation injury. Nat. Commun. Apr. 2024;15(1):1–14. doi: 10.1038/s41467-024-47124-8. 2024 15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsai Y.H., et al. Acquisition of NOTCH dependence is a hallmark of human intestinal stem cell maturation. Stem Cell Rep. May 2022;17(5):1138. doi: 10.1016/J.STEMCR.2022.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Srinivasan T., et al. Notch signalling regulates asymmetric division and inter-conversion between lgr5 and bmi1 expressing intestinal stem cells. Sci. Rep. May 2016;6 doi: 10.1038/SREP26069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cancedda R., Mastrogiacomo M. Transit Amplifying Cells (TACs): a still not fully understood cell population. Front. Bioeng. Biotechnol. May 2023;11 doi: 10.3389/FBIOE.2023.1189225/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sei Y., Feng J., Chow C.C., Wank S.A. Stem Cells, Tissue Engineering, Development, and Cancer: asymmetric cell division-dominant neutral drift model for normal intestinal stem cell homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. Jan. 2019;316(1):G64. doi: 10.1152/AJPGI.00242.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hageb A., et al. Reduced adhesion of aged intestinal stem cells contributes to an accelerated clonal drift. Life Sci. Alliance. Aug. 2022;5(8) doi: 10.26508/LSA.202201408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi J., et al. Intestinal stem cell aging at single-cell resolution: transcriptional perturbations alter cell developmental trajectory reversed by gerotherapeutics. Aging Cell. May 2023;22(5) doi: 10.1111/ACEL.13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brescia P., Rescigno M. The gut vascular barrier: a new player in the gut-liver-brain axis. Trends Mol. Med. Sep. 2021;27(9):844–855. doi: 10.1016/J.MOLMED.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 65.Horowitz A., Chanez-Paredes S.D., Haest X., Turner J.R. Paracellular permeability and tight junction regulation in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. Apr. 2023;20(7):417–432. doi: 10.1038/s41575-023-00766-3. 2023 20:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xing T., et al. Tight junction protein claudin-7 is essential for intestinal epithelial stem cell self-renewal and differentiation. Cell Mol Gastroenterol Hepatol. Jan. 2020;9(4):641–659. doi: 10.1016/J.JCMGH.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raya-Sandino A., et al. Claudin-23 reshapes epithelial tight junction architecture to regulate barrier function. Nat. Commun. Oct. 2023;14(1):6214. doi: 10.1038/s41467-023-41999-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin X., et al. IL-17RA-signaling in Lgr5+ intestinal stem cells induces expression of transcription factor ATOH1 to promote secretory cell lineage commitment. Immunity. Feb. 2022;55(2):237–253. doi: 10.1016/J.IMMUNI.2021.12.016. .e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mei X., Gu M., Li M. Plasticity of Paneth cells and their ability to regulate intestinal stem cells. Stem Cell Res. Ther. Aug. 2020;11(1):1–13. doi: 10.1186/S13287-020-01857-7/FIGURES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deng F., et al. Interleukin-10 expands transit-amplifying cells while depleting Lgr5+ stem cells via inhibition of Wnt and notch signaling. Biochem. Biophys. Res. Commun. Dec. 2020;533(4):1330–1337. doi: 10.1016/J.BBRC.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 71.McCarthy N., et al. Smooth muscle contributes to the development and function of a layered intestinal stem cell niche. Dev. Cell. Apr. 2023;58(7):550–564. doi: 10.1016/J.DEVCEL.2023.02.012. .e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martín-Alonso M., et al. Smooth muscle-specific MMP17 (MT4-MMP) regulates the intestinal stem cell niche and regeneration after damage. Nat. Commun. Dec. 2021;12(1) doi: 10.1038/S41467-021-26904-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maimets M., et al. Mesenchymal-epithelial crosstalk shapes intestinal regionalisation via Wnt and Shh signalling. Nat. Commun. Feb. 2022;13(1):1–12. doi: 10.1038/s41467-022-28369-7. 2022 13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiang J., et al. A stromal lineage maintains crypt structure and villus homeostasis in the intestinal stem cell niche. BMC Biol. Dec. 2023;21(1):1–15. doi: 10.1186/S12915-023-01667-2/FIGURES/6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Choi J., Augenlicht L.H. Intestinal stem cells: guardians of homeostasis in health and aging amid environmental challenges. Exp. Mol. Med. Mar. 2024;56(3):495–500. doi: 10.1038/s12276-024-01179-1. 2024 56:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.González-Muniesa P., Gámez-Macías Paola Elizabeth, Félix-Soriano E., Samblas M., Sáinz N., Moreno-Aliaga María Jesús. Intestinal permeability, gut inflammation, and gut immune system response are linked to aging-related changes in gut microbiota composition: a study in female mice. J Gerontol A Biol Sci Med Sci. Apr. 2024;79(4) doi: 10.1093/GERONA/GLAE045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nguyen T.T., Baumann P., Tüscher O., Schick S., Endres K. The aging enteric nervous system. Int. J. Mol. Sci. May 2023;24(11):9471. doi: 10.3390/ijms24119471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sovran B., et al. Age-associated impairment of the mucus barrier function is associated with profound changes in microbiota and immunity. Sci. Rep. Dec. 2019;9(1) doi: 10.1038/S41598-018-35228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ghosh T.S., Shanahan F., O'Toole P.W. The gut microbiome as a modulator of healthy ageing. Nat. Rev. Gastroenterol. Hepatol. Apr. 2022;19(9):565–584. doi: 10.1038/s41575-022-00605-x. 2022 19:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen S., et al. Fusobacterium nucleatum reduces METTL3-mediated m6A modification and contributes to colorectal cancer metastasis. Nat. Commun. Mar. 2022;13(1):1–16. doi: 10.1038/s41467-022-28913-5. 2022 13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang Y., Wang L., Peugnet-González I., Parada-Venegas D., Dijkstra G., Faber K.N. cGAS-STING signaling pathway in intestinal homeostasis and diseases. Front. Immunol. Sep. 2023;14 doi: 10.3389/FIMMU.2023.1239142/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Walters H. Bacterial induction of B cell senescence drives gut microbiota aging. Nat Aging. Jun. 2023;3(6):634. doi: 10.1038/S43587-023-00444-8. [DOI] [PubMed] [Google Scholar]
- 83.Chen H., et al. A forward chemical genetic screen reveals gut microbiota metabolites that modulate host physiology. Cell. May 2019;177(5):1217–1231.e18. doi: 10.1016/J.CELL.2019.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Appleton J. The gut-brain Axis: influence of microbiota on mood and mental health. Integr. Med.: A Clinician’s Journal. Aug. 2018;17(4):28. [Online]. Available:/pmc/articles/PMC6469458/ [PMC free article] [PubMed] [Google Scholar]
- 85.Johansen J., et al. Centenarians have a diverse gut virome with the potential to modulate metabolism and promote healthy lifespan. Nat Microbiol. Jun. 2023;8(6):1064–1078. doi: 10.1038/S41564-023-01370-6. [DOI] [PubMed] [Google Scholar]
- 86.Pang S., et al. Longevity of centenarians is reflected by the gut microbiome with youth-associated signatures. Nat Aging. Apr. 2023;3(4):436–449. doi: 10.1038/S43587-023-00389-Y. [DOI] [PubMed] [Google Scholar]
- 87.Nalapareddy K., Zheng Y., Geiger H. Aging of intestinal stem cells. Stem Cell Rep. Apr. 2022;17(4):734–740. doi: 10.1016/J.STEMCR.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choi J., et al. Intestinal crypts recover rapidly from focal damage with coordinated motion of stem cells that is impaired by aging. Sci. Rep. Jul. 2018;8(1):1–12. doi: 10.1038/s41598-018-29230-y. 2018 8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jo M.K., et al. Effect of aging on the formation and growth of colonic epithelial organoids by changes in cell cycle arrest through TGF-β-Smad3 signaling. Inflamm. Regen. Dec. 2023;43(1) doi: 10.1186/S41232-023-00282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Funk M.C., et al. Epithelial cells of the intestine acquire cell-intrinsic inflammation signatures during ageing. bioRxiv. Dec. 2021;(12.19.473357):2021. doi: 10.1101/2021.12.19.473357. [DOI] [Google Scholar]
- 91.Sauls R.S., Taylor B.N. Histology, M cell. StatPearls. Nov. 2022 https://www.ncbi.nlm.nih.gov/books/NBK534232/ [Online]. Available: [Google Scholar]
- 92.Shenghua P., Ziqin Z., Shuyu T., Huixia Z., Xianglu R., Jiao G. An integrated fecal microbiome and metabolome in the aged mice reveal anti-aging effects from the intestines and biochemical mechanism of FuFang zhenshu TiaoZhi(FTZ) Biomed. Pharmacother. 2020;121(Jan) doi: 10.1016/J.BIOPHA.2019.109421. [DOI] [PubMed] [Google Scholar]
- 93.Gebert N., et al. Region-specific proteome changes of the intestinal epithelium during aging and dietary restriction. Cell Rep. Apr. 2020;31(4) doi: 10.1016/J.CELREP.2020.107565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Igarashi M., et al. NAD+ supplementation rejuvenates aged gut adult stem cells. Aging Cell. Jun. 2019;18(3) doi: 10.1111/ACEL.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.He D., et al. Gut stem cell aging is driven by mTORC1 via a p38 MAPK-p53 pathway. Nat. Commun. Jan. 2020;11(1):1–13. doi: 10.1038/s41467-019-13911-x. 2020 11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun T.Y., et al. MiR-1-3p and MiR-124-3p synergistically damage the intestinal barrier in the ageing colon. J Crohns Colitis. Apr. 2022;16(4):656–667. doi: 10.1093/ECCO-JCC/JJAB179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pant V., et al. Transient enhancement of p53 activity protects from radiation-induced gastrointestinal toxicity. Proc. Natl. Acad. Sci. U. S. A. Aug. 2019;116(35):17429–17437. doi: 10.1073/PNAS.1909550116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kohzaki M., Suzuki K., Ootsuyama A., Okazaki R. ‘Spontaneous p53 activation in middle-aged C57BL/6 mice mitigates the lifespan-extending adaptive response induced by low-dose ionizing radiation’. npj Aging. Nov. 2023;9(1):26. doi: 10.1038/s41514-023-00123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pentinmikko N., et al. Cellular shape reinforces niche to stem cell signaling in the small intestine. Sci. Adv. Oct. 2022;8(41) doi: 10.1126/SCIADV.ABM1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kazakevych J., Stoyanova E., Liebert A., Varga-Weisz P. Transcriptome analysis identifies a robust gene expression program in the mouse intestinal epithelium on aging. Sci. Rep. Jul. 2019;9(1):1–8. doi: 10.1038/s41598-019-46966-3. 2019 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wan Y., Finkel T. The mitochondria regulation of stem cell aging. Mech. Ageing Dev. 2020;191(Oct) doi: 10.1016/J.MAD.2020.111334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang L., et al. NAD+ dependent UPRmt activation underlies intestinal aging caused by mitochondrial DNA mutations. Nat. Commun. Jan. 2024;15(1):546. doi: 10.1038/s41467-024-44808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Spehar K., Pan A., Beerman I. Restoring aged stem cell functionality: current progress and future directions. Stem Cell. Sep. 2020;38(9):1060–1077. doi: 10.1002/STEM.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zheng H., Zhang C., Wang Q., Feng S., Fang Y., Zhang S. The impact of aging on intestinal mucosal immune function and clinical applications. Front. Immunol. 2022;13(Nov) doi: 10.3389/FIMMU.2022.1029948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Omrani O., et al. IFNγ-Stat1 axis drives aging-associated loss of intestinal tissue homeostasis and regeneration. Nat. Commun. Sep. 2023;14(1):1–19. doi: 10.1038/s41467-023-41683-y. 2023 14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhuang Z., et al. Imbalance of Th17/Treg cells in pathogenesis of patients with human leukocyte antigen B27 associated acute anterior uveitis. Sci. Rep. Jan. 2017;7(1):1–9. doi: 10.1038/srep40414. 2017 7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Booth J.S., Goldberg E., Patil S.A., Barnes R.S., Greenwald B.D., Sztein M.B. Age-dependency of terminal ileum tissue resident memory T cell responsiveness profiles to S. Typhi following oral Ty21a immunization in humans. Immun. Ageing. Dec. 2021;18(1) doi: 10.1186/S12979-021-00227-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang S., et al. Bazi Bushen capsule improves the deterioration of the intestinal barrier function by inhibiting NLRP3 inflammasome-mediated pyroptosis through microbiota-gut-brain axis. Front. Microbiol. 2024;14 doi: 10.3389/FMICB.2023.1320202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kurashima Y., Kiyono H. Mucosal ecological network of epithelium and immune cells for gut homeostasis and tissue healing. Annu. Rev. Immunol. Apr. 2017;35(1):119–147. doi: 10.1146/annurev-immunol-051116-052424. [DOI] [PubMed] [Google Scholar]
- 110.Walker E.M., et al. Inflammaging phenotype in rhesus macaques is associated with a decline in epithelial barrier-protective functions and increased pro-inflammatory function in CD161-expressing cells. Geroscience. Dec. 2019;41(6):739–757. doi: 10.1007/S11357-019-00099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martín-Cano F.E., Camello-Almaraz C., Hernandez D., Pozo M.J., Camello P.J. mTOR pathway and Ca2+ stores mobilization in aged smooth muscle cells. Aging (Albany NY) 2013;5(5):339. doi: 10.18632/AGING.100555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cao X., Wang W., Zhao B. YAP/TAZ links mechanosensing to aging. Life Medicine. Apr. 2023;2(2):1–3. doi: 10.1093/LIFEMEDI/LNAC039. [DOI] [Google Scholar]
- 113.Sladitschek-Martens H.L., et al. YAP/TAZ activity in stromal cells prevents ageing by controlling cGAS-STING. Nature. Jul. 2022;607(7920):790–798. doi: 10.1038/S41586-022-04924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yun J., et al. Senescent cells perturb intestinal stem cell differentiation through Ptk7 induced noncanonical Wnt and YAP signaling. Nat. Commun. Jan. 2023;14(1):1–19. doi: 10.1038/s41467-022-35487-9. 2023 14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu Y., et al. Reduced smooth muscle-fibroblasts transformation potentially decreases intestinal wound healing and colitis-associated cancer in ageing mice. Signal Transduct. Targeted Ther. Aug. 2023;8(1):1–17. doi: 10.1038/s41392-023-01554-w. 2023 8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hafen B.B., Burns B. Physiology, smooth muscle. StatPearls. Aug. 2023 https://www.ncbi.nlm.nih.gov/books/NBK526125/ [Online]. Available: [PubMed] [Google Scholar]
- 117.Wu S., Yang Z., Liu S., Zhang Q., Zhang S., Zhu S. Frailty status and risk of irritable bowel syndrome in middle-aged and older adults: a large-scale prospective cohort study. EClinicalMedicine. Feb. 2023;56 doi: 10.1016/J.ECLINM.2022.101807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Martin-Cano F.E., Camello-Almaraz C., Acuña-Castroviejo D., Pozo M.J., Camello P.J. Age-related changes in mitochondrial function of mouse colonic smooth muscle: beneficial effects of melatonin. J. Pineal Res. Mar. 2014;56(2):163–174. doi: 10.1111/JPI.12109. [DOI] [PubMed] [Google Scholar]
- 119.Saffrey M.J. Aging of the mammalian gastrointestinal tract: a complex organ system. Age (Dordr) 2014;36(3):1019–1032. doi: 10.1007/S11357-013-9603-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cao C., Brown B. Understanding Chinese medicine and western medicine to reach the maximum treatment benefit. J Transl Sci. 2020;6(2) doi: 10.15761/JTS.1000334. [DOI] [Google Scholar]
- 121.Wang Y., et al. Metformin ameliorates chronic colitis-related intestinal fibrosis via inhibiting TGF-β1/smad3 signaling. Front. Pharmacol. May 2022;13 doi: 10.3389/FPHAR.2022.887497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.De La Cuesta-Zuluaga J., et al. Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care. Jan. 2017;40(1):54–62. doi: 10.2337/DC16-1324. [DOI] [PubMed] [Google Scholar]
- 123.Tobar N., et al. Metformin acts in the gut and induces gut-liver crosstalk. Proc. Natl. Acad. Sci. USA. Jan. 2023;120(4) doi: 10.1073/pnas.2211933120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kulkarni A.S., Gubbi S., Barzilai N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metabol. Jul. 2020;32(1):15–30. doi: 10.1016/J.CMET.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jang H., et al. Metformin protects the intestinal barrier by activating goblet cell maturation and epithelial proliferation in radiation-induced enteropathy. Int. J. Mol. Sci. Jun. 2022;23(11) doi: 10.3390/IJMS23115929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Al-Dwairi A., Alqudah M., Al-Shboul O., Alfaqih M., Alomari D. Metformin exerts anti-inflammatory effects on mouse colon smooth muscle cells in vitro. Exp. Ther. Med. Aug. 2018;16(2):985–992. doi: 10.3892/ETM.2018.6222/HTML. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ahmadi S., et al. Metformin reduces aging-related leaky gut and improves cognitive function by beneficially modulating gut microbiome/goblet cell/mucin Axis. J Gerontol A Biol Sci Med Sci. 2020;75(7):E9–E21. doi: 10.1093/GERONA/GLAA056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rena G., Hardie D.G., Pearson E.R. The mechanisms of action of metformin. Diabetologia. Sep. 2017;60(9):1577–1585. doi: 10.1007/S00125-017-4342-Z/FIGURES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Foretz M., Guigas B., Viollet B. Metformin: update on mechanisms of action and repurposing potential. Nat. Rev. Endocrinol. May 2023;19(8):460–476. doi: 10.1038/s41574-023-00833-4. 2023 19:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dujic T., Zhou K., Tavendale R., Palmer C.N.A., Pearson E.R. Effect of serotonin transporter 5-HTTLPR polymorphism on gastrointestinal intolerance to metformin: a GoDARTS study. Diabetes Care. Nov. 2016;39(11):1896–1901. doi: 10.2337/DC16-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Igarashi M., Guarente L. mTORC1 and SIRT1 cooperate to foster expansion of gut adult stem cells during calorie restriction. Cell. Jul. 2016;166(2):436–450. doi: 10.1016/j.cell.2016.05.044. [DOI] [PubMed] [Google Scholar]
- 132.Guo X., et al. Rapamycin extenuates experimental colitis by modulating the gut microbiota. J. Gastroenterol. Hepatol. Dec. 2023;38(12):2130–2141. doi: 10.1111/JGH.16381. [DOI] [PubMed] [Google Scholar]
- 133.Phillips E.J., Simons M.J.P. Rapamycin not dietary restriction improves resilience against pathogens: a meta-analysis. Geroscience. Apr. 2023;45(2):1263–1270. doi: 10.1007/S11357-022-00691-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Juricic P., et al. Long-lasting geroprotection from brief rapamycin treatment in early adulthood by persistently increased intestinal autophagy. Nature Aging. Aug. 2022;2(9):824–836. doi: 10.1038/s43587-022-00278-w. 2022 2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang P., Catterson J.H., Grönke S., Partridge L. Inhibition of S6K lowers age-related inflammation and increases lifespan through the endolysosomal system. Nature Aging. Feb. 2024:1–19. doi: 10.1038/s43587-024-00578-3. 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Antonioli E., Torres N., Ferretti M., de Azevedo Piccinato C., Sertie A.L. Individual response to mTOR inhibition in delaying replicative senescence of mesenchymal stromal cells. PLoS One. Jan. 2019;14(1) doi: 10.1371/JOURNAL.PONE.0204784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang L., Pitcher L.E., Prahalad V., Niedernhofer L.J., Robbins P.D. Targeting cellular senescence with senotherapeutics: senolytics and senomorphics. FEBS J. Mar. 2023;290(5):1362–1383. doi: 10.1111/FEBS.16350. [DOI] [PubMed] [Google Scholar]
- 138.Salmon A.B. About-face on the metabolic side effects of rapamycin. Oncotarget. 2015;6(5):2585. doi: 10.18632/ONCOTARGET.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Arriola Apelo S.I., et al. Alternative rapamycin treatment regimens mitigate the impact of rapamycin on glucose homeostasis and the immune system. Aging Cell. Feb. 2016;15(1):28. doi: 10.1111/ACEL.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lee D.J.W., Hodzic Kuerec A., Maier A.B. Targeting ageing with rapamycin and its derivatives in humans: a systematic review. Lancet Healthy Longev. Feb. 2024;5(2):e152–e162. doi: 10.1016/S2666-7568(23)00258-1. [DOI] [PubMed] [Google Scholar]
- 141.Munster P., et al. First-in-human phase I study of A dual mTOR kinase and DNA-PK inhibitor (CC-115) in advanced malignancy. Cancer Manag. Res. 2019;11 doi: 10.2147/CMAR.S208720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li Q., Kang C. Mechanisms of action for small molecules revealed by structural biology in drug discovery. Int. J. Mol. Sci. Aug. 2020;21(15):1–18. doi: 10.3390/IJMS21155262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Singh V., et al. Butyrate producers, “The Sentinel of Gut”: their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. Jan. 2023;13 doi: 10.3389/FMICB.2022.1103836/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Recharla N., Geesala R., Shi X.Z. Gut microbial metabolite butyrate and its therapeutic role in inflammatory bowel disease: a literature review. Nutrients. May 2023;15(10) doi: 10.3390/NU15102275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fahed G., et al. Metabolic syndrome: updates on pathophysiology and management in 2021. Int. J. Mol. Sci. Jan. 2022;23(2):786. doi: 10.3390/IJMS23020786. 2022, Vol. 23, Page 786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mishra S.P., et al. Abnormalities in microbiota/butyrate/FFAR3 signaling in aging gut impair brain function. JCI Insight. Feb. 2024;9(3) doi: 10.1172/JCI.INSIGHT.168443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Krokowicz L., et al. Sodium butyrate and short chain fatty acids in prevention of travellers' diarrhoea: a randomized prospective study. Trav. Med. Infect. Dis. Mar. 2014;12(2):183–188. doi: 10.1016/J.TMAID.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 148.Trapecar M., et al. Gut-liver physiomimetics reveal paradoxical modulation of IBD-related inflammation by short-chain fatty acids. Cell Syst. Mar. 2020;10(3):223–239. doi: 10.1016/j.cels.2020.02.008. .e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Feng Z., et al. FG-4592 protects the intestine from irradiation-induced injury by targeting the TLR4 signaling pathway. Stem Cell Res. Ther. Dec. 2022;13(1):1–13. doi: 10.1186/S13287-022-02945-6/FIGURES/5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xia P., et al. FG-4592 alleviates radiation-induced intestinal injury by facilitating recovery of intestinal stem cell and reducing damage of intestinal epithelial. Toxicol. Lett. Mar. 2022;357:1–10. doi: 10.1016/J.TOXLET.2021.12.011. [DOI] [PubMed] [Google Scholar]
- 151.Jiang Y., et al. FG-4592 relieves diabetic kidney disease severity by influencing metabolic profiles via gut microbiota reconstruction in both human and mouse models. Front. Physiol. Aug. 2023;14 doi: 10.3389/FPHYS.2023.1195441/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hirai K., et al. Effects of roxadustat on the anemia and iron metabolism of patients undergoing peritoneal dialysis. Front. Med. Jul. 2021;8 doi: 10.3389/FMED.2021.667117/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hirai K., et al. Effects of roxadustat on anemia, iron metabolism, and lipid metabolism in patients with non-dialysis chronic kidney disease. Front. Med. 2023;10 doi: 10.3389/FMED.2023.1071342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yue D., Wang Z., Yang Y., Hu Z., Luo G., Wang F. EZH2 inhibitor GSK343 inhibits sepsis-induced intestinal disorders. Exp. Ther. Med. Feb. 2021;21(5) doi: 10.3892/ETM.2021.9854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cao Y., Li X. GSK343 inhibits EZH2 to promote MST1 expression and confers neuroprotection in kidney failure-induced hypercalcemia: fr-PO235. J. Am. Soc. Nephrol. Nov. 2022;33(11S):390–391. doi: 10.1681/ASN.20223311S1390D. [DOI] [Google Scholar]
- 156.Zhou J., et al. Targeting EZH2 histone methyltransferase activity alleviates experimental intestinal inflammation. Nat. Commun. Jun. 2019;10(1):1–11. doi: 10.1038/s41467-019-10176-2. 2019 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Scuderi S.A., et al. GSK343, an inhibitor of enhancer of zeste homolog 2, reduces glioblastoma progression through inflammatory process modulation: focus on canonical and non-canonical NF-κB/IκBα pathways. Int. J. Mol. Sci. Nov. 2022;23(22) doi: 10.3390/IJMS232213915/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Islam M.R., et al. Natural small molecules in breast cancer treatment: understandings from a therapeutic viewpoint. Molecules. Mar. 2022;27(7):2165. doi: 10.3390/MOLECULES27072165. 2022, Vol. 27, Page 2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang X., et al. Garcinol and its analogues: synthesis, cytotoxic activity and mechanistic investigation. Bioorg. Chem. Apr. 2023;133 doi: 10.1016/J.BIOORG.2023.106389. [DOI] [PubMed] [Google Scholar]
- 160.Yao W., et al. Effects of garcinol supplementation on the performance, egg quality, and intestinal health of laying hens in the late laying period. Poultry Sci. Oct. 2023;102(10) doi: 10.1016/J.PSJ.2023.102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Aggarwal V., et al. Garcinol exhibits anti-neoplastic effects by targeting diverse oncogenic factors in tumor cells. Biomedicines. May 2020;8(5) doi: 10.3390/BIOMEDICINES8050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Khan T., et al. Anticancer plants: a review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules. Dec. 2019;10(1):47. doi: 10.3390/BIOM10010047. 2020, Vol. 10, Page 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang T., et al. Dietary garcinol supplementation improves diarrhea and intestinal barrier function associated with its modulation of gut microbiota in weaned piglets. J. Anim. Sci. Biotechnol. Mar. 2020;11(1):1–13. doi: 10.1186/S40104-020-0426-6/FIGURES/5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 164.Schobert R., Biersack B. Chemical and biological aspects of garcinol and isogarcinol: recent developments. Chem. Biodivers. Sep. 2019;16(9) doi: 10.1002/cbdv.201900366. [DOI] [PubMed] [Google Scholar]
- 165.Choudhury B., Kandimalla R., Elancheran R., Bharali R., Kotoky J. Garcinia morella fruit, a promising source of antioxidant and anti-inflammatory agents induces breast cancer cell death via triggering apoptotic pathway. Biomed. Pharmacother. Jul. 2018;103:562–573. doi: 10.1016/j.biopha.2018.04.068. [DOI] [PubMed] [Google Scholar]
- 166.Dahiya A., Agrawala P.K., Dutta A. Mitigative and anti-inflammatory effects of Trichostatin A against radiation-induced gastrointestinal toxicity and gut microbiota alteration in mice. Int. J. Radiat. Biol. 2023;99(12):1865–1878. doi: 10.1080/09553002.2023.2242929. [DOI] [PubMed] [Google Scholar]
- 167.Zhang Q., et al. Trichostatin A protects against intestinal injury in rats with acute liver failure. J. Surg. Res. Sep. 2016;205(1):1–10. doi: 10.1016/J.JSS.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 168.Przychodzen B., Mohrman M., Brzezynski J., Truslow S., Polymeropoulos C., Polymeropoulos M. Early transcriptional activity of vtr-297 - results from an ongoing phase I clinical trial. Blood. Nov. 2022;140(Supplement 1):4307. doi: 10.1182/BLOOD-2022-171079. 4307. [DOI] [Google Scholar]
- 169.Hubbard B.P., Sinclair D.A. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol. Sci. Mar. 2014;35(3):146. doi: 10.1016/J.TIPS.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sharma A., Chabloz S., Lapides R.A., Roider E., Ewald C.Y. Potential synergistic supplementation of NAD+ promoting compounds as a strategy for increasing healthspan. Nutrients. Jan. 2023;15(2):445. doi: 10.3390/NU15020445. 2023, Vol. 15, Page 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Colloca A., Balestrieri A., Anastasio C., Balestrieri M.L., D’onofrio N. Mitochondrial sirtuins in chronic degenerative diseases: new metabolic targets in colorectal cancer. Int. J. Mol. Sci. Mar. 2022;23(6) doi: 10.3390/IJMS23063212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Knop M., et al. A mitochondrial sirtuin shapes the intestinal microbiota by controlling lysozyme expression. bioRxiv. Jun. 2023 doi: 10.1101/2023.06.02.543385. 2023.06.02.543385. [DOI] [Google Scholar]
- 173.Zhang Y., et al. Crosstalk between gut microbiota and Sirtuin-3 in colonic inflammation and tumorigenesis. Exp. Mol. Med. Apr. 2018;50(4) doi: 10.1038/S12276-017-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tian Y., et al. Coreopsis tinctoria flowers extract ameliorates D-galactose induced aging in mice via regulation of Sirt1-Nrf2 signaling pathway. J. Funct.Foods. Sep. 2019;60 doi: 10.1016/J.JFF.2019.103464. [DOI] [Google Scholar]
- 175.Wu Q.-J., et al. The sirtuin family in health and disease. Signal Transduct. Targeted Ther. Dec. 2022;7(1):402. doi: 10.1038/s41392-022-01257-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang Y., et al. An overview of Sirtuins as potential therapeutic target: structure, function and modulators. Eur. J. Med. Chem. Jan. 2019;161:48–77. doi: 10.1016/j.ejmech.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 177.Grabowska W., Sikora E., Bielak-Zmijewska A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology. Aug. 2017;18(4):447–476. doi: 10.1007/s10522-017-9685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gan Z., et al. Resveratrol and curcumin improve intestinal mucosal integrity and decrease m6A RNA methylation in the intestine of weaning piglets. ACS Omega. Oct. 2019;4(17):17438–17446. doi: 10.1021/ACSOMEGA.9B02236/SUPPL_FILE/AO9B02236_SI_001.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jabczyk M., Nowak J., Hudzik B., Zubelewicz-Szkodzińska B. Curcumin and its potential impact on microbiota. Nutrients. Jun. 2021;13(6) doi: 10.3390/NU13062004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yang Z.-J., et al. Effects and mechanisms of curcumin for the prevention and management of cancers: an updated review. Antioxidants. Jul. 2022;11(8):1481. doi: 10.3390/antiox11081481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hewlings S.J., Kalman D.S. Curcumin: a review of its effects on human health. Foods. Oct. 2017;6(10):92. doi: 10.3390/FOODS6100092. 2017, Vol. 6, Page 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hewlings S.J., Kalman D.S. Curcumin: a review of its' effects on human health. Foods. Oct. 2017;6(10) doi: 10.3390/FOODS6100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Izadi M., et al. Longevity and anti-aging effects of curcumin supplementation. Geroscience. 2024 doi: 10.1007/S11357-024-01092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ramesh P., et al. BCL-XL inhibition induces an FGFR4-mediated rescue response in colorectal cancer. Cell Rep. Feb. 2022;38(7) doi: 10.1016/J.CELREP.2022.110374. [DOI] [PubMed] [Google Scholar]
- 185.van der Heijden M., et al. Bcl-2 is a critical mediator of intestinal transformation. Nat. Commun. Mar. 2016;7(1) doi: 10.1038/ncomms10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.de Vos S., et al. Safety and efficacy of navitoclax, a BCL-2 and BCL-XL inhibitor, in patients with relapsed or refractory lymphoid malignancies: results from a phase 2a study. Leuk. Lymphoma. 2021;62(4):810. doi: 10.1080/10428194.2020.1845332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Johnson L.A., et al. Effect of ABT-263 on intestinal fibrosis in human myofibroblasts, human intestinal organoids, and the mouse Salmonella typhimurium model. Inflamm. Bowel Dis. Feb. 2022;28(2):161–175. doi: 10.1093/ibd/izab166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lagoumtzi S.M., Chondrogianni N. Senolytics and senomorphics: natural and synthetic therapeutics in the treatment of aging and chronic diseases. Free Radic. Biol. Med. Aug. 2021;171:169–190. doi: 10.1016/j.freeradbiomed.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 189.Sikora E., et al. Cellular senescence in brain aging. Front. Aging Neurosci. Feb. 2021;13 doi: 10.3389/fnagi.2021.646924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhou Y., et al. A prospective multicenter phase II study on the efficacy and safety of dasatinib in the treatment of metastatic gastrointestinal stromal tumors failed by imatinib and sunitinib and analysis of NGS in peripheral blood. Cancer Med. Sep. 2020;9(17):6225–6233. doi: 10.1002/cam4.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kim A., Seong K.M., Choi Y.Y., Shim S., Park S., Lee S.S. Inhibition of EphA2 by dasatinib suppresses radiation-induced intestinal injury. Int. J. Mol. Sci. Nov. 2020;21(23):9096. doi: 10.3390/ijms21239096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mannarino M., et al. Senolytic combination treatment is more potent than single drugs in reducing inflammatory and senescence burden in cells from painful degenerating IVDs. Biomolecules. Aug. 2023;13(8) doi: 10.3390/BIOM13081257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Saccon T.D., et al. Senolytic combination of dasatinib and quercetin alleviates intestinal senescence and inflammation and modulates the gut microbiome in aged mice. J Gerontol A Biol Sci Med Sci. Nov. 2021;76(11):1895–1905. doi: 10.1093/GERONA/GLAB002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sharma A.K., et al. The senolytic drug navitoclax (ABT-263) causes trabecular bone loss and impaired osteoprogenitor function in aged mice. Front. Cell Dev. Biol. May 2020;8 doi: 10.3389/FCELL.2020.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kwon J.W., et al. Combined inhibition of Bcl-2 family members and YAP induces synthetic lethality in metastatic gastric cancer with RASA1 and NF2 deficiency. Mol. Cancer. Dec. 2023;22(1):1–24. doi: 10.1186/S12943-023-01857-0/FIGURES/9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kulkarni A.S., Aleksic S., Berger D.M., Sierra F., Kuchel G.A., Barzilai N. Geroscience-guided repurposing of FDA-approved drugs to target aging: a proposed process and prioritization. Aging Cell. Apr. 2022;21(4) doi: 10.1111/ACEL.13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Liguori I., et al. Sarcopenia: assessment of disease burden and strategies to improve outcomes. Clin. Interv. Aging. May 2018;13:913–927. doi: 10.2147/CIA.S149232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sayed A.M., Abdel-Fattah M.M., Arab H.H., Mohamed W.R., Hassanein E.H.M. Targeting inflammation and redox aberrations by perindopril attenuates methotrexate-induced intestinal injury in rats: role of TLR4/NF-κB and c-Fos/c-Jun pro-inflammatory pathways and PPAR-γ/SIRT1 cytoprotective signals. Chem. Biol. Interact. Jan. 2022;351 doi: 10.1016/J.CBI.2021.109732. [DOI] [PubMed] [Google Scholar]
- 199.Elliott W.J., Bistrika E.A. Perindopril arginine and amlodipine besylate for hypertension: a safety evaluation. Expet Opin. Drug Saf. Feb. 2018;17(2):207–216. doi: 10.1080/14740338.2018.1397129. [DOI] [PubMed] [Google Scholar]
- 200.Bojarska J., Remko M., Breza M., Madura I., Fruziński A., Wolf W.M. A proline-based tectons and supramolecular synthons for drug design 2.0: a case study of acei. Pharmaceuticals. Oct. 2020;13(11):338. doi: 10.3390/ph13110338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang M., Moalin M., Haenen G.R.M.M. Connecting West and east. Int. J. Mol. Sci. May 2019;20(9):2333. doi: 10.3390/IJMS20092333. 2019, Vol. 20, Page 2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jiang Y., Zhao L., Chen Q., Zhou L. Exploring the mechanism of berberine intervention in ulcerative colitis from the perspective of inflammation and immunity based on systemic pharmacology. Evid Based Complement Alternat Med. 2021;2021 doi: 10.1155/2021/9970240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Luo Z., et al. Berberine increases stromal production of Wnt molecules and activates Lgr5+ stem cells to promote epithelial restitution in experimental colitis. BMC Biol. Dec. 2022;20(1) doi: 10.1186/S12915-022-01492-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Li Q., et al. Berberine protects mice against dextran sulfate sodium-induced colitis by activating mTORC1 pathway. Front. Pharmacol. Jul. 2019;10:786. doi: 10.3389/FPHAR.2019.00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cao J.W., Chen M.Y., Xu R., Guo M.Y. Therapeutic mechanisms of berberine to improve the intestinal barrier function via modulating gut microbiota, TLR4/NF-κ B/MTORC pathway and autophagy in cats. Front. Microbiol. Jul. 2022;13 doi: 10.3389/FMICB.2022.961885/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kaginelli S.B., Basalingappa K.M. Berberine and its pharmacology potential: a review. www.ejbps.com [Online]. Available:
- 207.Xu L., et al. A phase I trial of berberine in Chinese with ulcerative colitis. Cancer Prev. Res. 2020;13(1):117–126. doi: 10.1158/1940-6207.CAPR-19-0258. [DOI] [PubMed] [Google Scholar]
- 208.Zhang Y., et al. Mechanism of Bazi Bushen capsule in delaying the senescence of mesenchymal stem cells based on network pharmacology and experimental validation. Heliyon. Mar. 2024;10(6) doi: 10.1016/J.HELIYON.2024.E27646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wang T., et al. Repositioning of clinically approved drug Bazi Bushen capsule for treatment of Aizheimer's disease using network pharmacology approach and in vitro experimental validation. Heliyon. Jul. 2023;9(7) doi: 10.1016/J.HELIYON.2023.E17603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mei J., et al. The efficacy and safety of Bazi Bushen Capsule in treating premature aging: a randomized, double blind, multicenter, placebo-controlled clinical trial. Phytomedicine. Jul. 2024;130 doi: 10.1016/J.PHYMED.2024.155742. [DOI] [PubMed] [Google Scholar]
- 211.Zhang S., et al. Bazi Bushen capsule improves the deterioration of the intestinal barrier function by inhibiting NLRP3 inflammasome-mediated pyroptosis through microbiota-gut-brain axis. Front. Microbiol. Jan. 2023;14 doi: 10.3389/FMICB.2023.1320202/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mao X., et al. Bazi Bushen mitigates epigenetic aging and extends healthspan in naturally aging mice. Biomed. Pharmacother. 2023;160(Apr) doi: 10.1016/J.BIOPHA.2023.114384. [DOI] [PubMed] [Google Scholar]
- 213.Diao H., et al. The Chinese medicine Fufang Zhenzhu Tiaozhi capsule protects against atherosclerosis by suppressing EndMT via modulating Akt1/β-catenin signaling pathway. J. Ethnopharmacol. Jul. 2022;293 doi: 10.1016/J.JEP.2022.115261. [DOI] [PubMed] [Google Scholar]
- 214.Chen Z., Yang B., Wang Z., Rong X., Zhu Q., Guo J. Modulation of the gut microbiota by fufang-zhenzhu-tiaozhi capsule attenuates hypertension induced by a high-fructose and high-salt diet. Front. Cell. Infect. Microbiol. Jun. 2022;12 doi: 10.3389/FCIMB.2022.854849/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lan T., et al. Fufang zhenzhu Tiaozhi capsule prevents intestinal inflammation and barrier disruption in mice with non-alcoholic steatohepatitis. Front. Endocrinol. Jun. 2022;13 doi: 10.3389/FENDO.2022.864703/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lei Z., et al. Ginsenoside Rb1 improves intestinal aging via regulating the expression of sirtuins in the intestinal epithelium and modulating the gut microbiota of mice. Front. Pharmacol. Sep. 2022;13 doi: 10.3389/FPHAR.2022.991597/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wan C., et al. Ginsenoside Rb1 enhanced immunity and altered the gut microflora in mice immunized by H1N1 influenza vaccine. PeerJ. 2023;11 doi: 10.7717/PEERJ.16226/SUPP-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chen S., et al. Ginsenoside Rb1 attenuates intestinal ischemia/reperfusion-induced inflammation and oxidative stress via activation of the PI3K/Akt/Nrf2 signaling pathway. Mol. Med. Rep. May 2019;19(5):3633–3641. doi: 10.3892/MMR.2019.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Cao F., Chen F., Su Z., Li L., Huang A., Xu H. Evaluation of embryotoxicity of radix scutellariae based on embryonic stem test. Trop. J. Pharmaceut. Res. Apr. 2015;14(3):431. doi: 10.4314/tjpr.v14i3.11. [DOI] [Google Scholar]
- 220.Wang Y., Dou Y., Feng J., Xu C., Wang Q. Efficacy of Liangxue Guyuan decoction on radiation-induced intestinal injury in rats via the toll-like receptor 4/myeloid differentiation primary response 88/nuclear factor-kappa B pathway. J. Tradit. Chin. Med. Apr. 2021;41(2):254–261. [PubMed] [Google Scholar]
- 221.Yan Z., et al. Therapeutic mechanism of Liangxue-Guyuan-Yishen decoction on intestinal stem cells and tight junction proteins in gastrointestinal acute radiation syndrome rats. J. Radiat. Res. Nov. 2023;64(6):880–892. doi: 10.1093/JRR/RRAD065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yan Z., et al. Revitalizing gut health: liangxue guyuan yishen decoction promotes -induced intestinal stem cell recovery post-radiation in mice. Phytomedicine. Jul. 2024 doi: 10.1016/j.phymed.2024.155888. [DOI] [PubMed] [Google Scholar]
- 223.Li J., Li D., Chen Y., Chen W., Xu J., Gao L. Gut microbiota and aging: traditional Chinese medicine and modern medicine. Clin. Interv. Aging. 2023;18:963. doi: 10.2147/CIA.S414714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Fraser D.R. Physiological significance of vitamin D produced in skin compared with oral vitamin D. J. Nutr. Sci. Feb. 2022;11:1–5. doi: 10.1017/JNS.2022.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhao F., Yang Z., Wang N., Jin K., Luo Y. Traditional Chinese medicine and western medicine share similar philosophical approaches to fight COVID-19. Aging Dis. Aug. 2021;12(5):1162–1168. doi: 10.14336/AD.2021.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Li W., et al. Vitamin d receptor protects against radiation-induced intestinal injury in mice via inhibition of intestinal crypt stem/progenitor cell apoptosis. Nutrients. Sep. 2021;13(9) doi: 10.3390/NU13092910/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yao M., Oduro P.K., Akintibu A.M., Yan H. Modulation of the vitamin D receptor by traditional Chinese medicines and bioactive compounds: potential therapeutic applications in VDR-dependent diseases. Front. Pharmacol. Jan. 2024;15 doi: 10.3389/FPHAR.2024.1298181/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Charoenngam N., Shirvani A., Kalajian T.A., Song A., Holick M.F. The effect of various doses of oral vitamin D3 supplementation on gut microbiota in healthy adults: a randomized, double-blinded, dose-response study. Anticancer Res. 2020;40(1):551–556. doi: 10.21873/ANTICANRES.13984. [DOI] [PubMed] [Google Scholar]
- 229.Pham H., et al. The effect of vitamin D supplementation on the gut microbiome in older Australians – results from analyses of the D-Health Trial. Gut Microb. 2023;15(1) doi: 10.1080/19490976.2023.2221429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Jiao D., Jiang C. Nutritional therapy of older osteoporotic people with supplemental calcium and vitamin D: side effects, fracture rates, and survival – an internationalised meta-analysis. Asia Pac. J. Clin. Nutr. 2024;33(1):1. doi: 10.6133/APJCN.202403_33(1).0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lim K., Thadhani R. Vitamin D toxicity. Brazilian Journal of Nephrology. Jun. 2020;42(2):238–244. doi: 10.1590/2175-8239-jbn-2019-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.González-Quilen C., et al. Beneficial effects of proanthocyanidins on intestinal permeability and its relationship with inflammation. Weight Management. Feb. 2020 doi: 10.5772/INTECHOPEN.91212. [DOI] [Google Scholar]
- 233.Zhu X., et al. Procyanidin B2 promotes intestinal injury repair and attenuates colitis-associated tumorigenesis via suppression of oxidative stress in mice. Antioxidants Redox Signal. Jul. 2021;35(2):75–92. doi: 10.1089/ARS.2019.7911. [DOI] [PubMed] [Google Scholar]
- 234.Casanova-Martí À., et al. Long term exposure to a grape seed proanthocyanidin extract enhances L-cell differentiation in intestinal organoids. Mol. Nutr. Food Res. Aug. 2020;64(16) doi: 10.1002/MNFR.202000303. [DOI] [PubMed] [Google Scholar]
- 235.Redondo-Castillejo R., et al. Proanthocyanidins: impact on gut microbiota and intestinal action mechanisms in the prevention and treatment of metabolic syndrome. Int. J. Mol. Sci. Mar. 2023;24(6) doi: 10.3390/IJMS24065369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yang L., Xian D., Xiong X., Lai R., Song J., Zhong J. Proanthocyanidins against oxidative stress: from molecular mechanisms to clinical applications. BioMed Res. Int. 2018;2018:1–11. doi: 10.1155/2018/8584136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sano A. Safety assessment of 4-week oral intake of proanthocyanidin-rich grape seed extract in healthy subjects. Food Chem. Toxicol. Oct. 2017;108:519–523. doi: 10.1016/J.FCT.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 238.Tang R., Xian D., Xu J., Peng H., Pan S., Zhong J. Proanthocyanidins as a potential novel way for the treatment of hemangioma. BioMed Res. Int. Jan. 2021;2021:1–10. doi: 10.1155/2021/5695378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Chen Y., Wang J., Zou L., Cao H., Ni X., Xiao J. Dietary proanthocyanidins on gastrointestinal health and the interactions with gut microbiota. Crit. Rev. Food Sci. Nutr. 2023;63(23):6285–6308. doi: 10.1080/10408398.2022.2030296. [DOI] [PubMed] [Google Scholar]
- 240.Etxeberria U., et al. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. Jun. 2015;26(6):651–660. doi: 10.1016/J.JNUTBIO.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 241.Liu S.J., Hu S.Q., Chen Y.C., Guo J. Uncovering the mechanism of quercetin for treating spermatogenesis impairment by a network pharmacology approach. Life. Jan. 2021;14(1):699–708. doi: 10.1080/26895293.2021.1961878. [DOI] [Google Scholar]
- 242.Lesjak M., et al. 2017. Antioxidant and Anti-inflammatory Activities of Quercetin and its Derivatives. [DOI] [Google Scholar]
- 243.Lan H., et al. Quercetin modulates the gut microbiota as well as the metabolome in a rat model of osteoarthritis. Bioengineered. 2021;12(1):6240–6250. doi: 10.1080/21655979.2021.1969194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Yan L., Guo X., Zhou J., Zhu Y., Zhang Z., Chen H. Quercetin prevents intestinal stem cell aging via scavenging ROS and inhibiting insulin signaling in Drosophila. Antioxidants. Jan. 2023;12(1) doi: 10.3390/ANTIOX12010059/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Shi T., Bian X., Yao Z., Wang Y., Gao W., Guo C. Quercetin improves gut dysbiosis in antibiotic-treated mice. Food Funct. Sep. 2020;11(9):8003–8013. doi: 10.1039/D0FO01439G. [DOI] [PubMed] [Google Scholar]
- 246.Meiners F., et al. Computational identification of natural senotherapeutic compounds that mimic dasatinib based on gene expression data. Sci. Rep. Mar. 2024;14(1):6286. doi: 10.1038/s41598-024-55870-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Andres S., et al. Safety aspects of the use of quercetin as a dietary supplement. Mol. Nutr. Food Res. Jan. 2018;62(1) doi: 10.1002/MNFR.201700447. [DOI] [PubMed] [Google Scholar]
- 248.Zou H., Ye H., Kamaraj R., Zhang T., Zhang J., Pavek P. A review on pharmacological activities and synergistic effect of quercetin with small molecule agents. Phytomedicine. Nov. 2021;92 doi: 10.1016/J.PHYMED.2021.153736. [DOI] [PubMed] [Google Scholar]
- 249.Kiyama R. Nutritional implications of ginger: chemistry, biological activities and signaling pathways. J. Nutr. Biochem. Dec. 2020;86 doi: 10.1016/J.JNUTBIO.2020.108486. [DOI] [PubMed] [Google Scholar]
- 250.Teng Y., et al. Plant-derived exosomal MicroRNAs shape the gut microbiota. Cell Host Microbe. Nov. 2018;24(5):637–652.e8. doi: 10.1016/J.CHOM.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Hassan S.M.A., Hassan A.H. Effect of shogaol on the expression of intestinal stem cell markers in experimentally induced colitis in BALB/c mice. Anal. Cell Pathol. 2019;2019 doi: 10.1155/2019/5134156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Crichton M., et al. Effect of ginger root powder on gastrointestinal bacteria composition, gastrointestinal symptoms, mental health, fatigue, and quality of life: a double-blind placebo-controlled trial. J. Nutr. Nov. 2023;153(11):3193–3206. doi: 10.1016/J.TJNUT.2023.09.002. [DOI] [PubMed] [Google Scholar]
- 253.Chen M., et al. 6-Shogaol inhibits the cell migration of colon cancer by suppressing the EMT process through the IKKβ/NF-κB/Snail pathway. Integr. Cancer Ther. Jan. 2023;22 doi: 10.1177/15347354231172732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kim S.-D., et al. Efficacy and safety of ginger on the side effects of chemotherapy in breast cancer patients: systematic review and meta-analysis. Int. J. Mol. Sci. Sep. 2022;23(19) doi: 10.3390/IJMS231911267. 2022, Vol. 23, Page 11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Błaszczyk N., Rosiak A., Kałużna-Czaplińska J. The potential role of cinnamon in human health. Forests. May 2021;12(5):648. doi: 10.3390/F12050648. 2021, Vol. 12, Page 648. [DOI] [Google Scholar]
- 256.Pagliari S., et al. Antioxidant and anti-inflammatory effect of cinnamon (cinnamomum verum J. Presl) bark extract after in vitro digestion simulation. Foods. Feb. 2023;12(3) doi: 10.3390/FOODS12030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.li Li A., et al. Effect of cinnamon essential oil on gut microbiota in the mouse model of dextran sodium sulfate-induced colitis. Microbiol. Immunol. Jan. 2020;64(1):23–32. doi: 10.1111/1348-0421.12749. [DOI] [PubMed] [Google Scholar]
- 258.Lonati E., et al. Digested cinnamon (cinnamomum verum J. Presl) bark extract modulates claudin-2 gene expression and protein levels under tnfα/IL-1β inflammatory stimulus. Int. J. Mol. Sci. May 2023;24(11):9201. doi: 10.3390/IJMS24119201. 2023, Vol. 24, Page 9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Hajimonfarednejad M., Ostovar M., Raee M.J., Hashempur M.H., Mayer J.G., Heydari M. Cinnamon: a systematic review of adverse events. Clin. Nutr. Apr. 2019;38(2):594–602. doi: 10.1016/J.CLNU.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 260.Tong T., et al. Dietary supplementation of ark clams protects gut health and modifies gut microbiota in d-galactose-induced aging rats. J. Sci. Food Agric. Jan. 2024;104(2):675–685. doi: 10.1002/JSFA.12958. [DOI] [PubMed] [Google Scholar]
- 261.Camargo L. da R., Doneda D., Oliveira V.R. Whey protein ingestion in elderly diet and the association with physical, performance and clinical outcomes. Exp. Gerontol. Aug. 2020;137 doi: 10.1016/J.EXGER.2020.110936. [DOI] [PubMed] [Google Scholar]
- 262.Cronin O., et al. A prospective metagenomic and metabolomic analysis of the impact of exercise and/or whey protein supplementation on the gut microbiome of sedentary adults. mSystems. Jun. 2018;3(3) doi: 10.1128/MSYSTEMS.00044-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Vasconcelos Q.D.J.S., Bachur T.P.R., Aragão G.F. Whey protein supplementation and its potentially adverse effects on health: a systematic review. Appl. Physiol. Nutr. Metabol. Jan. 2021;46(1):27–33. doi: 10.1139/apnm-2020-0370. [DOI] [PubMed] [Google Scholar]
- 264.Xiao Y., Feng Y., Zhao J., Chen W., Lu W. Achieving healthy aging through gut microbiota-directed dietary intervention: focusing on microbial biomarkers and host mechanisms. J. Adv. Res. Mar. 2024 doi: 10.1016/J.JARE.2024.03.005. [DOI] [PubMed] [Google Scholar]
- 265.Recharla N., Choi J., Puligundla P., Park S.J., Lee H.J. Impact of probiotics on cognition and constipation in the elderly: a meta-analysis. Heliyon. Jul. 2023;9(7) doi: 10.1016/j.heliyon.2023.e18306. 2405–8440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Xiao Y.L., Gong Y., Qi Y.J., Shao Z.M., Jiang Y.Z. Effects of dietary intervention on human diseases: molecular mechanisms and therapeutic potential. Signal Transduct. Targeted Ther. Mar. 2024;9(1):1–34. doi: 10.1038/s41392-024-01771-x. 2024 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Mana M.D., Kuo E.Y.S., Yilmaz Ö.H. Dietary regulation of adult stem cells. Curr Stem Cell Rep. Mar. 2017;3(1):1. doi: 10.1007/S40778-017-0072-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Green C.L., et al. Dietary restriction of isoleucine increases healthspan and lifespan of genetically heterogeneous mice. Cell Metabol. Nov. 2023;35(11):1976–1995. doi: 10.1016/J.CMET.2023.10.005. .e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Aversa Z., et al. Calorie restriction reduces biomarkers of cellular senescence in humans. Aging Cell. Feb. 2024;23(2) doi: 10.1111/ACEL.14038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Jaime K., Mank V. Risks associated with excessive weight loss. StatPearls. Feb. 2024 https://www.ncbi.nlm.nih.gov/books/NBK603752/ [Online]. Available: [PubMed] [Google Scholar]
- 271.Mihaylova M.M., et al. Fasting activates Fatty Acid Oxidation to enhance intestinal stem cell function during homeostasis and aging. Cell Stem Cell. May 2018;22(5):769. doi: 10.1016/J.STEM.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Su J., et al. Remodeling of the gut microbiome during Ramadan-associated intermittent fasting. Am. J. Clin. Nutr. May 2021;113(5):1332–1342. doi: 10.1093/AJCN/NQAA388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Mohammadzadeh A., et al. The interplay between fasting, gut microbiota, and lipid profile. Int. J. Clin. Pract. Oct. 2021;75(10) doi: 10.1111/IJCP.14591. [DOI] [PubMed] [Google Scholar]
- 274.Tibi S., et al. Implications of ramadan fasting in the setting of gastrointestinal disorders. Cureus. Mar. 2023;15(3) doi: 10.7759/CUREUS.36972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Chae S.A., Du M., Son J.S., Zhu M.J. Exercise improves homeostasis of the intestinal epithelium by activation of apelin receptor-AMP-activated protein kinase signalling. J. Physiol. Jun. 2023;601(12):2371–2389. doi: 10.1113/JP284552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Magzal F., et al. Increased physical activity improves gut microbiota composition and reduces short-chain fatty acid concentrations in older adults with insomnia. Sci. Rep. Feb. 2022;12(1):1–14. doi: 10.1038/s41598-022-05099-w. 2022 12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Ramos C., Gibson G.R., Walton G.E., Magistro D., Kinnear W., Hunter K. Systematic review of the effects of exercise and physical activity on the gut microbiome of older adults. Nutrients. Feb. 2022;14(3) doi: 10.3390/NU14030674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Keohane D.M., et al. Four men in a boat: ultra-endurance exercise alters the gut microbiome. J. Sci. Med. Sport. Sep. 2019;22(9):1059–1064. doi: 10.1016/J.JSAMS.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 279.Zhu Q., Jiang S., Du G. Effects of exercise frequency on the gut microbiota in elderly individuals. Microbiol. Aug. 2020;9(8) doi: 10.1002/MBO3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Weyh C., Krüger K., Strasser B. Physical activity and diet shape the immune system during aging. Nutrients. Mar. 2020;12(3) doi: 10.3390/NU12030622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Tarca B., Jesudason S., Bennett P.N., Wycherley T.P., Ferrar K.E. Characteristics and frequency of physical activity and exercise-related side effects in people receiving peritoneal dialysis. J. Ren. Nutr. Jul. 2024;34(4):359–367. doi: 10.1053/J.JRN.2023.12.003. [DOI] [PubMed] [Google Scholar]
- 282.Goodman J.M., Burr J.F., Banks L., Thomas S.G. The acute risks of exercise in apparently healthy adults and relevance for prevention of cardiovascular events. Can. J. Cardiol. Apr. 2016;32(4):523–532. doi: 10.1016/J.CJCA.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 283.Yan H., Ren J., Liu G.-H. Fecal microbiota transplantation: a new strategy to delay aging. hLife. Nov. 2023;1(1):8–11. doi: 10.1016/J.HLIFE.2023.06.002. [DOI] [Google Scholar]
- 284.Youngster I., et al. Fecal microbiota transplantation in capsules for the treatment of steroid refractory and steroid dependent acute graft vs. host disease: a pilot study. Bone Marrow Transplant. Mar. 2024;59(3):409–416. doi: 10.1038/S41409-024-02198-2. [DOI] [PubMed] [Google Scholar]
- 285.Jain N., Umar T.P., Fahner A.F., Gibietis V. Advancing therapeutics for recurrent clostridioides difficile infections: an overview of vowst's FDA approval and implications. Gut Microb. Dec. 2023;15(1) doi: 10.1080/19490976.2023.2232137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Agrawal M., et al. The long-term efficacy and safety of fecal microbiota transplant for recurrent, severe, and complicated clostridium difficile infection in 146 elderly individuals. J. Clin. Gastroenterol. 2016;50(5):403–407. doi: 10.1097/MCG.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 287.Markandey M., et al. Fecal microbiota transplantation refurbishes the crypt-associated microbiota in ulcerative colitis. iScience. May 2023;26(5) doi: 10.1016/J.ISCI.2023.106738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Rapoport E.A., Baig M., Puli S.R. Adverse events in fecal microbiota transplantation: a systematic review and meta-analysis. Ann. Gastroenterol. Mar. 2022;35(2):150. doi: 10.20524/AOG.2022.0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Wang Q., Guo F., Jin Y., Ma Y. Applications of human organoids in the personalized treatment for digestive diseases. Signal Transduct. Targeted Ther. Sep. 2022;7(1):1–30. doi: 10.1038/s41392-022-01194-6. 2022 7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ahmed A.S.I., Sheng M.H., Wasnik S., Baylink D.J., Lau K.-H.W. Effect of aging on stem cells. World J. Exp. Med. Feb. 2017;7(1):1. doi: 10.5493/WJEM.V7.I1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Cerneckis J., Cai H., Shi Y. Induced pluripotent stem cells (iPSCs): molecular mechanisms of induction and applications. Signal Transduct. Targeted Ther. Apr. 2024;9(1):112. doi: 10.1038/s41392-024-01809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Dotti I., Salas A. Potential use of human stem cell–derived intestinal organoids to study inflammatory bowel diseases. Inflamm. Bowel Dis. Nov. 2018;24(12):2501–2509. doi: 10.1093/IBD/IZY275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Miura S., Suzuki A. Generation of mouse and human organoid-forming intestinal progenitor cells by direct lineage reprogramming. Cell Stem Cell. Oct. 2017;21(4):456–471. doi: 10.1016/J.STEM.2017.08.020. .e5. [DOI] [PubMed] [Google Scholar]
- 294.Eiro N., Fraile M., González-Jubete A., González L.O., Vizoso F.J. Mesenchymal (stem) stromal cells based as new therapeutic alternative in inflammatory bowel disease: basic mechanisms, experimental and clinical evidence, and challenges. Int. J. Mol. Sci. Aug. 2022;23(16):8905. doi: 10.3390/ijms23168905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Torrens-Mas M., et al. Organoids: an emerging tool to study aging signature across human tissues. Modeling aging with patient-derived organoids. Int. J. Mol. Sci. Oct. 2021;22(19) doi: 10.3390/IJMS221910547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.mei Tian C., et al. Stem cell-derived intestinal organoids: a novel modality for IBD. Cell Death Discovery. Jul. 2023;9(1):1–16. doi: 10.1038/s41420-023-01556-1. 2023 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Fernández-Susavila H., Bugallo-Casal A., Castillo J., Campos F. Adult stem cells and induced pluripotent stem cells for stroke treatment. Front. Neurol. 2019;10(AUG):908. doi: 10.3389/FNEUR.2019.00908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Moussa L., Lapière A., Squiban C., Demarquay C., Milliat F., Mathieu N. BMP antagonists secreted by mesenchymal stromal cells improve colonic organoid formation: application for the treatment of radiation-induced injury. Cell Transplant. 2020;29 doi: 10.1177/0963689720929683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Li L.-L., Kofi A.J., Li X.-X., Chang J., Bian Y.-H., Chu X.-Q. A promising strategy for repairing tissue damage: mitochondria transfer from mesenchymal stem cells. Biomedical Engineering Communications. 2023;2(4):18. doi: 10.53388/BMEC2023018. [DOI] [Google Scholar]
- 300.Chen H., Liu O., Chen S., Zhou Y. Aging and mesenchymal stem cells: therapeutic opportunities and challenges in the older group. Gerontology. Mar. 2022;68(3):339. doi: 10.1159/000516668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Yoo J.H., Donowitz M. Intestinal enteroids/organoids: a novel platform for drug discovery in inflammatory bowel diseases. World J. Gastroenterol. Aug. 2019;25(30):4125–4147. doi: 10.3748/WJG.V25.I30.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Caipa Garcia A.L., Arlt V.M., Phillips D.H. Organoids for toxicology and genetic toxicology: applications with drugs and prospects for environmental carcinogenesis. Mutagenesis. May 2022;37(2):143–154. doi: 10.1093/MUTAGE/GEAB023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Jee J.H., et al. Functional recovery by colon organoid transplantation in a mouse model of radiation proctitis. Biomaterials. 2021;275(Aug) doi: 10.1016/J.BIOMATERIALS.2021.120925. [DOI] [PubMed] [Google Scholar]
- 304.Sugimoto S., et al. An organoid-based organ-repurposing approach to treat short bowel syndrome. Nature. Feb. 2021;592(7852):99–104. doi: 10.1038/s41586-021-03247-2. 2021 592:7852. [DOI] [PubMed] [Google Scholar]
- 305.Bouffi C., et al. In vivo development of immune tissue in human intestinal organoids transplanted into humanized mice. Nat. Biotechnol. Jan. 2023;41(6):824–831. doi: 10.1038/s41587-022-01558-x. 2023 41:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Watanabe S., et al. Transplantation of intestinal organoids into a mouse model of colitis. Nat. Protoc. Mar. 2022;17(3):649–671. doi: 10.1038/S41596-021-00658-3. [DOI] [PubMed] [Google Scholar]
- 307.Cruz-Acuña R., et al. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat. Cell Biol. Oct. 2017;19(11):1326–1335. doi: 10.1038/ncb3632. 2017 19:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Meran L., Tullie L., Eaton S., De Coppi P., Li V.S.W. Bioengineering human intestinal mucosal grafts using patient-derived organoids, fibroblasts and scaffolds. Nat. Protoc. Oct. 2022;18(1):108–135. doi: 10.1038/s41596-022-00751-1. 2022 18:1. [DOI] [PubMed] [Google Scholar]
- 309.Hansen S.L., et al. An organoid-based CRISPR-Cas9 screen for regulators of intestinal epithelial maturation and cell fate. Sci. Adv. Jul. 2023;9(28) doi: 10.1126/SCIADV.ADG4055/SUPPL_FILE/SCIADV.ADG4055_SM.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.mei Tian C., et al. Stem cell-derived intestinal organoids: a novel modality for IBD. Cell Death Discovery. Jul. 2023;9(1):1–16. doi: 10.1038/s41420-023-01556-1. 2023 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Fujii M., Clevers H., Sato T. Modeling human digestive diseases with CRISPR-cas9-modified organoids. Gastroenterology. Feb. 2019;156(3):562–576. doi: 10.1053/J.GASTRO.2018.11.048. [DOI] [PubMed] [Google Scholar]
- 312.Sugimoto S., et al. Reconstruction of the human colon epithelium in vivo. Cell Stem Cell. Feb. 2018;22(2):171–176.e5. doi: 10.1016/J.STEM.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 313.Cheng C.W., et al. Ketone body signaling mediates intestinal stem cell homeostasis and adaptation to diet. Cell. Aug. 2019;178(5):1115–1131. doi: 10.1016/J.CELL.2019.07.048. .e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Rangan P., et al. Fasting-mimicking diet modulates microbiota and promotes intestinal regeneration to reduce inflammatory bowel disease pathology. Cell Rep. Mar. 2019;26(10):2704–2719.e6. doi: 10.1016/J.CELREP.2019.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Parras A., et al. In vivo reprogramming leads to premature death linked to hepatic and intestinal failure. Nat Aging. Dec. 2023;3(12):1509–1520. doi: 10.1038/S43587-023-00528-5. [DOI] [PubMed] [Google Scholar]
- 316.Cano Macip C., et al. Gene therapy mediated partial reprogramming extends lifespan and reverses age-related changes in aged mice. bioRxiv. Jan. 2023 doi: 10.1101/2023.01.04.522507. 2023.01.04.522507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Mao X., et al. Bazi Bushen mitigates epigenetic aging and extends healthspan in naturally aging mice. Biomed. Pharmacother. 2023;160(Apr) doi: 10.1016/J.BIOPHA.2023.114384. [DOI] [PubMed] [Google Scholar]
- 318.Midttun M., et al. Beneficial effects of exercise, testosterone, vitamin D, calcium and protein in older men—a randomized clinical trial. J Cachexia Sarcopenia Muscle. 2024 doi: 10.1002/JCSM.13498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Taelman J., Diaz M., Guiu J. Human intestinal organoids: promise and challenge. Front. Cell Dev. Biol. Mar. 2022;10 doi: 10.3389/FCELL.2022.854740/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Jin H., Xue Z., Liu J., Ma B., Yang J., Lei L. Advancing organoid engineering for tissue regeneration and biofunctional reconstruction. Biomater. Res. 2024;28 doi: 10.34133/BMR.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article. This review article has not been deposited in publicly available repository.