Abstract
Study Objectives
To investigate the sex-specific association between habitual snoring and overall cancer prevalence and subtypes, and to examine the influence of age, body mass index (BMI), and sleep duration on this association.
Methods
This study utilized data from the National Health and Nutrition Examination Survey cycles between 2005 and 2020 and included 15 892 participants aged 18 and over. We employed inverse probability of treatment weighting based on propensity scores to adjust for confounders when comparing the prevalence of cancer between habitual snorers and non-habitual snorers for each sex and cancer type. Subgroup analyses were conducted based on sleep duration, age, and BMI categories.
Results
The cohort (mean age 48.2 years, 50.4% female, and 30.5% habitual snorers) reported 1385 cancer cases. In men, habitual snoring was linked to 26% lower odds of any cancer (OR 0.74, 95% CI: 0.66 to 0.83), while in women, it showed no significant difference except lower odds of breast cancer (OR 0.77, 95% CI: 0.63 to 0.94) and higher odds of cervix cancer (OR 1.54, 95% CI: 1.18 to 2.01). Age and sleep duration significantly influenced the snoring-cancer relationship, with notable variations by cancer type and sex.
Conclusions
Habitual snoring exhibits sex-specific associations with cancer prevalence, showing lower prevalence in men and varied results in women. These findings emphasize the critical need for further research to uncover the biological mechanisms involved. Future investigations should consider integrating sleep characteristics with cancer prevention and screening strategies, focusing on longitudinal research and the integration of genetic and biomarker analyses to fully understand these complex relationships.
Keywords: cancer prevalence, sex differences, snoring, propensity score, inverse probability of treatment weighting, sleep duration, age, population-based
Statement of Significance.
With cancer continuing to challenge global health, the elucidation of contributing factors, including those from common conditions like habitual snoring, remains a crucial yet underexplored area. While the snoring-cancer link is hypothesized to involve obstructive sleep apnea and its accompanying intermittent hypoxia, the current understanding of snoring’s independent role, absent sleep apnea, is still limited. This gap is particularly pronounced when examining sex-specific cancer risks. This research contributes to the understanding of how habitual snoring could reflect broader health implications beyond known sleep disorders, specifically indicating differential cancer associations across genders. These insights reinforce the need for future research to unravel these connections. They could inform sex-specific novel preventive and screening initiatives tailored to individual risk profiles based on sleep patterns.
Cancer is the leading cause of mortality worldwide, responsible for nearly one in six deaths in 2020 [1]. The burden of cancer incidence and mortality is not only expanding across the world but also exhibits a disproportionate distribution, with countries with higher human development levels having a greater share of the overall cancer incidence burden [2, 3]. In the United States, there were an estimated 1.9 million new cancer cases and 609 360 cancer-related deaths in 2022 [4]. Lung cancer remains the most lethal, with prostate and breast cancers following in male and female populations, respectively [4].
Sleep, as a critical determinant of health, has garnered attention for its potential role in cancer etiology. Habitual snoring, which may affect up to 45% of adult males and 28% of adult females [5], is increasingly recognized as a significant risk factor for cancer [6, 7]. The proposed mechanism linking snoring to cancer remains largely unknown but is hypothesized to involve obstructive sleep apnea (OSA) [8–10]—a condition characterized by disrupted sleep and intermittent hypoxia [10]—and other carcinogenic risk factors such as aging, obesity, cardiometabolic disease, and lifestyle factors [11, 12]. However, the specific relationship between non-apneic snoring and cancer or its subtypes is less understood.
Habitual snoring results from the passage of air through the upper airway during sleep, leading to tissue vibration and noise [13]. It is essential to distinguish this from the cyclical snoring seen in OSA, which includes alternating periods of snoring and silence and may trigger concern in observers due to its severity [14]. Past research on the association between snoring and cancer risk has often neglected to adjust for the presence of sleep apnea or other sleep disorders such as insomnia [6, 7], casting uncertainty on whether it is snoring per se or its common association with OSA that elevates cancer risk.
Sex differences in cancer susceptibility and mortality are among the most consistent observations in cancer epidemiology [15]. For example, males have a higher incidence of colorectal, stomach, and liver cancers and a higher mortality rate from cancers such as lung, colorectal, and stomach compared to females [16]. Yet it is unknown that habitual snoring contributes to the risk of cancer equally for men and women.
To bridge these gaps in knowledge, our study investigated the sex-specific associations of habitual snoring with overall cancer prevalence and cancer subtypes, within a diverse US general adult population. Additionally, we explored whether these associations are influenced by age, body mass index (BMI), and sleep duration, thereby providing a more nuanced understanding of the interplay between habitual snoring and cancer risk across different life stages, BMI subgroups, and sleep duration.
Methods
Data sources and study population
This study utilized participant data derived from the National Health and Nutrition Examination Survey (NHANES), specifically from 2005 to 2008, 2007 to 2008, and 2015 to 2020 cycles. These cycles were selected for their extensive sleep assessment protocols, which offered a more robust dataset for our analysis. NHANES, a nationally representative cross-sectional survey conducted by the Centers for Disease Control and Prevention [17], employed a stratified, multistage, probability cluster sampling method [18]. Detailed descriptions of NHANES design and methods have been documented in prior publications [17]. Participants in NHANES undergo a two-stage evaluation process: an initial household interview followed by a physical examination and further interviews conducted at a mobile examination center [17]. Ethical approval for the NHANES protocol was obtained from the National Center for Health Statistics Research ethics review board, and written informed consent was obtained from participants. As the NHANES data is de-identified and publicly available, the Purdue University Committee on Human Research did not deem additional IRB review necessary for our study. For the purposes of this analysis, we excluded any data pertaining to pregnant individuals and minors under the age of 18 years.
Definition of habitual snoring and other sleep characteristics
In the NHANES interview, participants reported snoring frequency over the past 12 months using a scale ranging from “never” to “frequently” (5–7 nights per week) [19]. Habitual snoring was defined as “frequent” snoring. Participants who reported “never,” “rarely,” or “occasionally” snoring were categorized as the non-habitual snoring comparison group. Sleep duration was self-reported as the average number of hours slept per night on weekdays or workdays, categorized as short (<7 hours), long (>9 hours), or average (7 to 9 hours) [20]. Additionally, participants were asked if a healthcare professional had diagnosed them with a sleep disorder, with affirmative responses prompting further inquiry into specific disorders, such as sleep apnea, insomnia, restless legs syndrome, or other sleep-related conditions.
Assessment of cancer status
Cancer status was determined by participants’ affirmative response to whether a doctor or other health professional had ever diagnosed them with cancer or a malignancy. Those who answered “yes” to this question were then asked to specify the type(s) of cancer diagnosed, with the provision to list up to three distinct cancer types.
Covariates
Covariates in this study included sociodemographic factors, behavioral determinants, and clinical characteristics. Sociodemographic factors included age, sex, race/ethnicity (categorized as non-Hispanic whites, non-Hispanic blacks, Hispanic, and non-Hispanic others), marital status, family income (quantified using the poverty-income ratio, PIR), education level, and health insurance status (categorized as uninsured, private insurance, government insurance, or a combination thereof).
Behavioral determinants included alcohol use and smoking status; the latter was ascertained by serum cotinine levels, with levels≥10 ng/mL indicating active smoking [21]. Clinical characteristics included BMI, presence of cardiometabolic syndrome, and history of hypertension and diabetes. The presence of cardiometabolic syndrome was defined using the National Cholesterol Education Program Adult Treatment Panel III criteria with the presence of three or more metabolic abnormalities: waist circumference >102 cm for men and >88 cm for women; blood pressure ≥130 mmHg systolic and or ≥85 mm Hg diastolic; fasting glucose ≥100 mg/dL; triglycerides ≥150 mg/dL; HDL cholesterol <40 mg/dL for men and <50 mg/dL for women [22]. The presence of diagnosed sleep disorders including sleep apnea, insomnia, restless leg syndrome, and other sleep disorders were also included.
Statistical Analysis
In our observational cross-sectional analysis, participants were categorized by snoring status, and subject characteristics were compared using standardized mean differences. We summarized rates of cancer, any and by subtypes, sex, and snoring frequency.
To balance demographic, clinical, and behavioral risk factors, separate propensity score models were constructed for men and women, with snoring status as the outcome and participant characteristics as predictors. To account for missing covariates, we used multiple imputations with 20 imputations, constructing a separate logistic regression model on each imputed dataset. The propensity model included all demographic and clinical factors in Table 1. Recognizing OSA as a potential confounder due to its association with increased cancer risk and hypoxic burden [23–25], we included OSA as a variable in our propensity model to help balance this confounder across snorers and non-snorers. Predicted probabilities were then used as the inverse probability of treatment weights (IPTW) in subsequent analyses, with analyses performed separately on each imputation set and the results pooled.
Table 1.
Participants Characteristics of Cohorts by the Presence of Habitual Snoring (N = 15 892)
| Characteristics | No Snoring n (%) or mean (sd) |
Habitual Snoring n (%) or mean (sd) |
Standardized mean difference (SMD) | Propensity score weighted SMD |
|---|---|---|---|---|
| N | 11 048 (69.5%) | 4844 (30.5%) | ||
| Demographics | ||||
| Female | 6006 (54.4) | 2002 (41.3) | 0.263 | 0.033 |
| Age, mean, years | 46.1 (19.9) | 50.8 (16.8) | 0.253 | 0.026 |
| Poverty | 0.016 | 0.027 | ||
| Below poverty threshold | 2093 (18.9) | 948 (19.6) | ||
| Average poverty threshold | 964 (8.7) | 459 (9.5) | ||
| Above poverty threshold | 4120 (37.3) | 1912 (39.5) | ||
| Race | 0.091 | 0.044 | ||
| Non-Hispanic white | 4713 (42.7) | 2049 (42.3) | ||
| Non-Hispanic black | 2563 (23.2) | 1030 (21.3) | ||
| Mexican American |
1825 (16.5) | 886 (18.3) | ||
| Other Hispanics | 840 (7.6) | 449 (9.3) | ||
| Other race | 1107 (10.0) | 430 (8.9) | ||
| Education | 0.130 | 0.018 | ||
| Less than high school | 2743 (24.8) | 1433 (29.6) | ||
| High school | 2746 (24.9) | 1242 (25.6) | ||
| Some College | 3218 (29.1) | 1318 (27.2) | ||
| College & above | 2329 (21.1) | 845 (17.4) | ||
| Married or with partner | 3183 (28.8) | 1376 (28.4) | 0.001 | 0.001 |
| With health insurance | 8752 (79.2) | 3835 (79.2) | 0.004 | 0.016 |
| Clinical factors | ||||
| Hypertension | 3279 (29.7) | 1407 (29) | 0.053 | 0.020 |
| Asthma | 1460 (13.2) | 767 (15.8) | 0.075 | 0.001 |
| Diabetes | 853 (7.7) | 701 (14.5) | 0.216 | |
| Cardiometabolic disease | 1162 (10.5) | 998 (20.6) | 0.290 | 0.011 |
| BMI | 27.6 (6.3) | 31.4 (7.5) | 0.538 | 0.060 |
| Lifestyle factors | ||||
| Alcohol use | 5566 (50.4) | 2784 (57.5) | 0.080 | 0.010 |
| Smoking status | 4223 (38.2) | 2490 (51.4) | 0.240 | 0.022 |
| Sleep-related characteristics | ||||
| Restless leg syndrome | 23 (0.2) | 14 (0.3) | 0.017 | 0.035 |
| Insomnia | 101 (0.9) | 55 (1.1) | 0.022 | 0.056 |
| Sleep apnea | 207 (1.9) | 248 (5.1) | 0.216 | 0.013 |
| Sleep duration categories | 0.146 | 0.014 | ||
| Short | 3479 (31.5) | 1858 (38.4) | ||
| Average | 5963 (54.0) | 2368 (48.9) | ||
| Long | 1573 (14.2) | 600 (12.4) | ||
Numbers in the table do not add up due to missing values.
Abbreviations: BMI, body mass index; N, number of participants; SMD, standardized mean difference.
For hypothesis testing, IPTW logistic regression models were employed to evaluate cancer risk by snoring status, calculating odds ratios (ORs) with 95% confidence intervals (95% CIs) for each sex and cancer type. Subgroup analyses were conducted based on sleep duration (short, long, and average), age (<50, 50–64, and 65 years and above), and BMI categories (underweight, healthy, overweight, and obese) to assess the consistency of associations. Additionally, dose–response relationships were explored by analyzing cancer risk across frequencies of snoring (never, 1–2, 3–4, or 5–7 nights a week). All analyses were performed using Stata version 18 (StataCorp, College Station TX).
Results
Participants characteristics
A total of 15 892 individuals, with or without habitual snoring, were analyzed. The mean (standard deviation) age was 48.2 (19.4) years, and 50.4% were female. Cohort characteristics before multivariable adjustment by snoring status are summarized in Table 1. Individuals with habitual snoring were more likely to have income below poverty levels and were less likely to be married or cohabiting. Additionally, they were less likely to have hypertension compared to those without habitual snoring. Differences in the presence of sleep disorders were noted, with individuals reporting habitual snoring showing a higher likelihood of restless leg syndrome and insomnia than their counterparts without habitual snoring (p < .05). Stratified analysis by sex revealed a consistent pattern in most characteristics differentiating habitual snorers from non-snorers (Supplementary Tables 1 and 2). Notably, among women, those with habitual snoring had a higher incidence of alcohol consumption compared to non-snorers (44.7% vs. 43.6%, standardized mean difference [SMD]: 0.023).
Unadjusted relationship between habitual snoring and cancer
Within the analytical cohort, a total of 1385 cancer cases were reported (Supplementary Table 3). Men with habitual snoring exhibited a lower prevalence of any type of cancer compared to non-habitual snorers (non-snoring vs. snoring: 9.1% vs. 7.5%, p < .001), while women with habitual snoring showed a higher prevalence (8.0% vs. 11.4%, p < .001). Additionally, the prevalence of cervical cancer differed significantly between women with and without habitual snoring, with the former reporting higher prevalence (1.9% vs. 0.9%, p < .001).
Sex-specific IPTW-weighted associations of habitual snoring with cancer
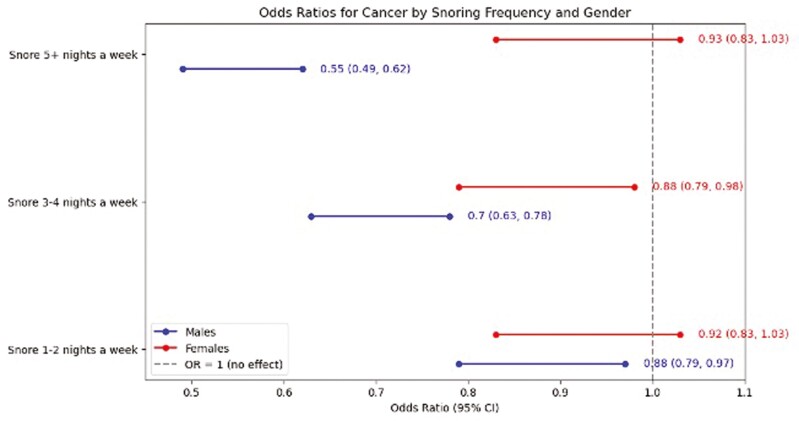
In the IPTW-weighted analysis of the entire cohort, we observed significant variations in cancer risks associated with habitual snoring. The adjusted odds of overall cancer, prostate cancer, breast cancer, and liver cancer were notably lower in habitual snorers compared to non-snorers, with odds ratios of 0.88 (95% CI: 0.81 to 0.95), 0.80 (95% CI: 0.67 to 0.97), 0.80 (95% CI: 0.66 to 0.98), and 0.22 (95% CI: 0.06 to 0.90), respectively. Conversely, the risk for cervix cancer was higher among habitual snorers, with an odds ratio of 1.49 (95% CI: 1.14 to 1.94). These findings provide a foundational context for our sex-stratified analyses, illustrating differential cancer risks associated with snoring across different types (Table 2). For men, habitual snoring was associated with a 26% reduction in the odds of overall cancer compared to non-habitual snorers (OR 0.74, 95% CI: 0.66 to 0.83). In men, the association between habitual snoring and cancer varied across cancer subtypes, showing lower odds for lung cancer, colon and rectum cancer, skin cancer, melanoma, and leukemia. In contrast, among women, no significant difference was observed in the odds of overall cancer prevalence between habitual and non-habitual snorers (OR 1.03, 95% 0.92 to 1.15), except for breast cancer and cervix cancer. Habitual snoring in women was associated with 23% lower odds of breast cancer (OR 0.77, 95% CI: 0.63, 0.94), while the odds of cervix cancer increased by 54% among women with habitual snoring (OR 1.54, 95% CI: 1.18, 2.01). Exploring the dose–response relationship between snoring frequency and overall cancer prevalence in men and women, we found that increasing snoring frequency nights per week reduced the likelihood of overall cancer in men, while such gradation in the association was not evident in women (Figure 1).
Table 2.
IPTW-Weighted Association Between Habitual Snoring and Prevalent Cancer, Stratified by Sex
| Cancer types | Odds Ratios (95% CI) | P value |
|---|---|---|
| All participants | ||
| Cancer of any types | 0.88 (0.81, 0.95) | .002 |
| Lung cancer | 0.65 (0.41, 1.04) | .072 |
| Prostate cancer | 0.80 (0.67, 0.97) | .020 |
| Breast cancer | 0.80 (0.66, 0.98) | .030 |
| Liver cancer | 0.22 (0.06, 0.90) | .034 |
| Colon and rectum cancer | 0.92 (0.71, 1.18) | .501 |
| Non-melanoma skin cancer | 0.93 (0.79, 1.09) | .342 |
| Melanoma | 0.76 (0.56, 1.03) | .077 |
| Uterine cancer | 1.26 (0.87, 1.82) | .226 |
| Pancreas cancer | 2.21 (0.37, 13.27) | .386 |
| Leukemia | 0.46 (0.20, 1.05) | .066 |
| Lymphoma | 0.61 (0.36, 1.03) | .065 |
| Cancer of the brain and nervous systems | 1.55 (0.57, 4.21) | .390 |
| Cancer of the mouth/tongue/lips/Larynx | 0.55 (0.24, 1.29) | .169 |
| Kidney cancer | 0.76 (0.46, 1.25) | .278 |
| Stomach cancer | 0.84 (0.34, 2.09) | .710 |
| Cervix cancer | 1.49 (1.14, 1.94) | .003 |
| Ovary cancer | 0.95 (0.61, 1.47) | .802 |
| Testis cancer | 3.38 (0.65, 17.72) | .149 |
| Males | ||
| Cancer of any types | 0.74 (0.66, 0.83) | <.001 |
| Lung cancer | 0.35 (0.18, 0.70) | .003 |
| Prostate cancer | 0.84 (0.70, 1.01) | .066 |
| Liver cancer | 0.50 (0.12, 2.05) | .338 |
| Colon and rectum cancer | 0.58 (0.39, 0.85) | .005 |
| Non-melanoma skin cancer | 0.81 (0.66, 1.00) | .050 |
| Melanoma | 0.50 (0.32, 0.79) | .003 |
| Leukemia | 0.17 (0.04, 0.73) | .017 |
| Lymphoma | 0.76 (0.39, 1.51) | .439 |
| Cancer of the brain and nervous systems | 4.58 (0.85, 24.74) | .077 |
| Cancer of the mouth/tongue/lips/Larynx | 0.58 (0.22, 1.51) | .263 |
| Kidney cancer | 0.51 (0.26, 0.99) | .046 |
| Stomach cancer | 1.12 (0.36, 3.50) | .840 |
| Testis cancer | 3.38 (0.65, 17.72) | .149 |
| Females | ||
| Cancer of any types | 1.03 (0.92, 1.15) | .583 |
| Lung cancer | 1.38 (0.64, 2.94) | .410 |
| Breast cancer | 0.77 (0.63, 0.94) | .011 |
| Colon and rectum cancer | 1.33 (0.93, 1.90) | .118 |
| Non-melanoma skin cancer | 1.10 (0.85, 1.41) | .471 |
| Melanoma | 1.25 (0.80, 1.94) | .327 |
| Uterine cancer | 1.26 (0.87, 1.82) | .222 |
| Leukemia | 1.32 (0.41, 4.26) | .637 |
| Lymphoma | 0.47 (0.20, 1.10) | .081 |
| Thyroid cancer | 0.86 (0.50, 1.51) | .608 |
| Cancer of the brain and nervous systems | 0.43 (0.08, 2.30) | .323 |
| Cancer of the mouth/tongue/lips/Larynx | 0.67 (0.12, 3.76) | .652 |
| kidney cancer | 1.41 (0.58, 3.40) | .444 |
| stomach cancer | 0.50 (0.10, 2.56) | .404 |
| Cervix cancer | 1.54 (1.18, 2.01) | .002 |
| Ovary cancer | 0.96 (0.61, 1.50) | .852 |
Variables included in propensity model: demographics-Sex, age, poverty levels, race/ethnicity, education, marital status, health insurance status; clinical factors-hypertension, asthma, diabetes, cardiometabolic disease, BMI; lifestyle factors-alcohol use, smoking status; sleep-related characteristics: restless leg syndrome, sleep apnea. Significant P values are denoted by bold type.
Figure 1.
IPTW-weighted relationship between snoring frequency and cancer prevalence, stratified by sex. Variables included in the propensity model: demographics-sex, age, poverty levels, race/ethnicity, education, marital status, health insurance status; clinical factors-hypertension, asthma, diabetes, cardiometabolic disease, BMI; lifestyle factors-alcohol use, smoking status; sleep-related characteristics: restless leg syndrome, sleep apnea.
Roles of age and BMI in sex-specific associations between habitual snoring and cancer
As shown in Tables 3 and 4, the results indicate that age and BMI play important roles in the sex-specific associations between habitual snoring and cancer prevalence. In our IPTW-weighted subgroup analysis by age, we observed different associations of habitual snoring with overall cancer risk across age groups in men. An inverse association was observed in men aged ≤49 years (OR 0.62, 95% CI [0.41, 0.95]) and those aged 65 and above (0.70, 95% CI [0.60, 0.82]; Table 3). However, this association was not statistically significant for men aged 50 to 64 years, although still inverse (OR 0.79, 95% CI[0.62, 1.00]). In terms of specific cancer types, lower odds of colon and rectum cancer, skin cancer, and melanoma associated with habitual snoring were significantly noted only in older males (≥65 years). Age also modified the association of habitual snoring and cancer in women, with increased odds of overall cancer, ovary cancer, melanoma, uterine cancer, and cervical cancer observed predominantly in the younger age groups (≤49 years). Conversely, the inverse associations between habitual snoring and breast and ovary cancer were significant for those aged 65 years and above.
Table 3.
IPTW-Weighted Sex-Specific Relationship Between Habitual Snoring and Cancer, Stratified by Age Groups
| Age groups | ≤49 years | 50–64 years | 65 years and above | |||
|---|---|---|---|---|---|---|
| Males | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Lung cancer | na | 0.10 (0.02,0.54) | .007 | 0.70 (0.33,1.46) | .340 | |
| Prostate cancer | na | 0.77 (0.50,1.18) | .230 | 0.83 (0.67,1.03) | .083 | |
| Liver cancer | na | na | 0.46 (0.11,1.97) | .292 | ||
| Colon and rectum cancer | 0.93 (0.37,2.34) | .881 | 0.78 (0.32,1.89) | .582 | 0.52 (0.32,0.84) | .008 |
| Non-melanoma skin cancer | 1.27 (0.63,2.57) | .500 | 0.88 (0.55,1.39) | .573 | 0.71 (0.55,0.92) | .010 |
| Melanoma | 0.36 (0.06,2.17) | .266 | 1.92 (0.76,4.83) | .166 | 0.30 (0.16,0.55) | <.001 |
| Leukemia | 1.00 (1.00,1.00) | . | 1.00 (1.00,1.00) | . | 1.00 (1.00,1.00) | . |
| Lymphoma | 1.00 (1.00,1.00) | . | 0.65 (0.19,2.15) | .478 | 1.47 (0.55,3.98) | .445 |
| Cancer of the brain and nervous systems | na | na | 4.54 (0.92,22.48) | .064 | ||
| Cancer of the mouth/tongue/lips/Larynx | 1.06 (0.10,11.76) | .961 | 1.00 (1.00,1.00) | . | 0.57 (0.18,1.77) | .332 |
| Kidney cancer | 0.64 (0.09,4.51) | .652 | 0.50 (0.15,1.62) | .247 | 0.44 (0.18,1.07) | .071 |
| Stomach cancer | 1.22 (0.12,12.67) | .865 | 1.00 (1.00,1.00) | . | 3.12 (0.47,20.53) | .237 |
| Testis cancer | na | 1.08 (0.13,8.80) | .942 | 1.00 (1.00,1.00) | . | |
| Cancer of any type | 0.62 (0.41,0.95) | .027 | 0.79 (0.62,1.00) | .053 | 0.70 (0.60,0.82) | <.001 |
| Females | ||||||
| Lung cancer | 6.25 (0.85,46.03) | .072 | 0.35 (0.06,2.02) | .238 | 0.90 (0.27,2.97) | .857 |
| Breast cancer | 0.50 (0.24,1.04) | .064 | 0.86 (0.60,1.22) | .400 | 0.69 (0.53,0.91) | .007 |
| Liver cancer | 1.00 (1.00,1.00) | . | na | 1.00 (1.00,1.00) | . | |
| Colon and rectum cancer | 2.44 (1.00,5.95) | .049 | 0.68 (0.31,1.50) | .338 | 1.54 (0.98,2.41) | .060 |
| Non-melanoma skin cancer | 1.18 (0.62,2.26) | .617 | 1.35 (0.76,2.40) | .304 | 0.94 (0.68,1.29) | .693 |
| Melanoma | 1.00 (1.00,1.00) | . | 3.95 (1.53,10.21) | .005 | 1.01 (0.57,1.79) | .978 |
| Uterine cancer | 2.39 (0.90,6.31) | .080 | 1.98 (1.14,3.45) | .016 | 0.55 (0.29,1.06) | .073 |
| Leukemia | 1.00 (1.00,1.00) | . | 1.00 (1.00,1.00) | . | 1.00 (1.00,1.00) | . |
| Lymphoma | 1.00 (1.00,1.00) | . | 1.00 (1.00,1.00) | . | 0.86 (0.33,2.25) | .761 |
| Thyroid cancer | 1.65 (0.68,3.99) | .270 | 0.45 (0.17,1.17) | .101 | 1.04 (0.33,3.29) | .951 |
| Cancer of the brain and nervous systems | 1.00 (1.00,1.00) | . | 1.66 (0.16,16.90) | .667 | na | |
| Cancer of the mouth/tongue/lips/Larynx | 2.28 (0.23,22.15) | .478 | 1.00 (1.00,1.00) | . | na | |
| Kidney cancer | na | 1.20 (0.25,5.72) | .822 | 1.72 (0.61,4.84) | .302 | |
| Stomach cancer | na | 1.00 (1.00,1.00) | . | 1.51 (0.19,12.05) | .696 | |
| Cervix cancer | 2.34 (1.67,3.28) | <.001 | 1.09 (0.64,1.85) | .756 | 0.54 (0.21,1.39) | .203 |
| Ovary cancer | 2.18 (1.02,4.63) | .043 | 1.00 (0.50,2.01) | .989 | 0.31 (0.11,0.89) | .029 |
| Cancer of any type | 1.34 (1.08,1.68) | .009 | 0.99 (0.80,1.21) | .892 | 0.88 (0.75,1.05) | .152 |
Abbreviations: CI, confidence intervals; IPTW, inverse probability of treatment weighting; N, number of participants; na, information not available due to insufficient sample size to calculate the odds ratios; ORs, odds ratios.
Variables included in propensity model: variables included in the propensity model: demographics-sex, age, poverty levels, race/ethnicity, education, marital status, health insurance status; clinical factors-hypertension, asthma, diabetes, cardiometabolic disease, BMI; lifestyle factors-alcohol use, smoking status; sleep-related characteristics: restless leg syndrome, sleep apnea.
Table 4.
IPTW-Weighted Sex-Specific Relationship Between Habitual Snoring and Cancer, Stratified by BMI Categories
| BMI categories | Underweight (BMI < 18.5) |
Healthy (BMI: 18.24.9) |
Overweight (BMI: 25-29.9) |
Obese (BMI ≥ 30) | ||||
|---|---|---|---|---|---|---|---|---|
| Males | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Lung cancer | na | na | 0.68 (0.28,1.70) | .413 | 0.22 (0.05,1.02) | .054 | 0.44 (0.12,1.57) | .206 |
| Prostate cancer | 1.48 (0.71,3.08) | .296 | 0.69 (0.45,1.06) | .090 | 0.74 (0.55,1.00) | .052 | 0.77 (0.56,1.06) | .114 |
| Colon and rectum cancer | 1.14 (0.42,3.08) | .797 | 1.23 (0.53,2.82) | .627 | 0.67 (0.36,1.26) | .214 | 0.21 (0.09,0.49) | <.001 |
| Mon-melanoma skin cancer | 1.54 (0.73,3.22) | .257 | 0.88 (0.54,1.43) | .596 | 0.64 (0.45,0.91) | .014 | 0.71 (0.50,0.99) | .045 |
| Melanoma | na | na | 0.37 (0.12,1.13) | .082 | 0.35 (0.17,0.75) | .007 | 0.75 (0.38,1.47) | .400 |
| Lymphoma | na | na | 0.65 (0.16,2.58) | .542 | 0.90 (0.25,3.27) | .879 | 0.26 (0.06,1.09) | .065 |
| Kidney cancer | na | na | na | 0.91 (0.36,2.28) | .845 | 0.24 (0.08,0.72) | .011 | |
| Cancer of any type | 1.42 (0.95,2.14) | .091 | 0.62 (0.48,0.81) | <.001 | 0.63 (0.52,0.77) | <.001 | 0.67 (0.55,0.81) | <.001 |
| Female | ||||||||
| Lung cancer | 0.60 (0.23,1.56) | .294 | na | na | na | na | na | na |
| Breast cancer | 0.79 (0.64,0.97) | .026 | na | na | 0.40 (0.10,1.59) | .195 | 0.78 (0.30,2.04) | .617 |
| Colon and Rectum cancer | 1.37 (0.94,2.00) | .098 | Na | na | na | na | na | na |
| Non-melanoma skin cancer | 1.04 (0.78,1.37) | .808 | 2.71 (0.89,8.23) | .078 | na | na | 0.98 (0.40,2.38) | .963 |
| Melanoma | 1.12 (0.67,1.88) | .660 | 0.88 (0.18,4.36) | .880 | na | na | 0.93 (0.24,3.64) | .912 |
| Uterine cancer | 1.21 (0.81,1.79) | .350 | 7.90 (1.11,56.25) | .039 | na | na | na | na |
| Thyroid cancer | 1.39 (0.70,2.77) | .352 | na | na | na | na | 1.58 (0.42,6.01) | .502 |
| Cancer of the brain and nervous systems | 0.87 (0.13,5.93) | .886 | na | na | 1.00 (1.00,1.00) | na | na | na |
| Kidney cancer | 1.60 (0.68,3.78) | .283 | na | na | na | na | na | na |
| Cervix cancer | 0.75 (0.49,1.16) | .201 | 3.90 (2.00,7.61) | <.001 | 2.86 (1.46,5.59) | .002 | 2.17 (1.21,3.90) | .009 |
| Ovary cancer | 1.01 (0.59,1.73) | .978 | 1.00 (1.00,1.00) | . | 2.04 (0.82,5.08) | .127 | 0.39 (0.06,2.39) | .309 |
| Cancer of any type | 0.95 (0.84,1.08) | .456 | 1.25 (0.81,1.95) | .312 | 1.14 (0.73,1.77) | .569 | 1.39 (0.98,1.97) | .067 |
Abbreviations:.CI, confidence intervals; IPTW, inverse probability of treatment weighting; N, number of participants; na, information not available due to insufficient sample size to calculate the odds ratios; OR, odds ratios.
Variables included in the propensity model: demographics-sex, age, poverty levels, race/ethnicity, education, marital status, health insurance status; clinical factors-hypertension, asthma, diabetes, cardiometabolic disease, BMI; lifestyle factors-alcohol use, smoking status; Sleep-related characteristics: restless leg syndrome, sleep apnea.
In men, lower odds of overall cancer were consistent across subgroups stratified by BMI but were not significant among those underweight (Table 4). In women, BMI did not modify the association between habitual snoring and the prevalence of most cancers, except for cervix cancer. The positive association of snoring and cervix cancer was observed only for women in the healthy, overweight, and obese groups, with the strength declining with increasing BMI (OR 3.90 [2.00, 7.61] for healthy; 2.86 [1.46, 5.59] for overweight; and 2.17 [1.21, 3.90] for obese).
Roles of short and long sleep duration in sex-specific association between habitual snoring and cancer
To delineate the roles of short and long sleep duration in the sex-specific association between habitual snoring and cancer prevalence, we stratified the unadjusted and IPTW-weighted analyses by sleep duration. In the unadjusted analysis, males sleeping less than 7 hours per night showed significant differences in overall cancer and lung cancer prevalence between those with and without habitual snoring, with lower prevalence among snorers (Supplementary Table 4). Conversely, among females sleeping more than 9 hours per night, significant differences in overall cancer, uterine, and ovary cancer prevalence were noted, with snorers exhibiting higher cancer prevalence.
In weighted analyses, inverse associations between habitual snoring and overall cancer and lung cancer were observed only in males sleeping less than 7 hours (Supplementary Table 5). Conversely, among females sleeping more than 9 hours per night, increased odds of uterine cancer and overall cancer prevalence were significant, while lower odds of breast cancer prevalence associated with habitual snoring were observed among those females sleeping less than 7 hours per night.
Discussion
Our retrospective cohort study reveals significant sex disparities in the relationship between habitual snoring and cancer prevalence. Using IPTW-weighted analysis to balance demographic, clinical, lifestyle, and sleep-related characteristics, we observed a 26% reduction in overall cancer odds among men with habitual snoring, particularly in lung, colon and rectum, skin, melanoma, and leukemia subtypes. Conversely, among women, no significant difference was observed in overall cancer prevalence except for breast and cervix cancers. Drawing from a dataset of 15 892 nationally representative adults, we also found age played a crucial role in habitual snoring-cancer relationships, with inverse associations noted in men aged ≤49 years and ≥65 years, and positive associations in young and middle-aged women. Sleep duration further influenced these associations, with shorter duration (<7 hours) linked to lower overall and lung cancer prevalence among snoring men, and longer duration (>9 hours) associated with higher overall and uterine cancer prevalence among snoring women. These nuanced findings underscore the complex interplay between habitual snoring and cancer risk across different life stages, sleep durations, and BMI subgroups, emphasizing the need for individualized evaluation and consideration of sex, age, and sleep-related factors in future mechanistic research.
Our study represents the first to document a significant inverse association between habitual snoring and cancer prevalence in men, particularly among males aged ≥65 years or with short sleep duration. While we adjusted for OSA diagnosis, our study, akin to prior epidemiologic research, cannot discern the impact of simple snoring versus snoring with OSA on cancer prevalence. Moreover, underdiagnosis of OSA is prevalent in the general population, with an estimated 93% of women and 82% of men with moderate-to-severe OSA being unaware of their condition [26, 27]. Existing studies on the potential link between OSA and cancer exhibit inconsistency, and sex-specific aspects of this association remain poorly studied [28]. For instance, a study of 267 849 individuals from the Korea National Health Insurance Service registry indicated a lower risk of lung cancer development in males with OSA but not in females, especially those aged>65 years [29]. However, the study’s generalizability was limited by its failure to account for major behavioral and clinical lung cancer risk factors and its homogenous sample [29]. Our findings extend these insights by incorporating demographic, lifestyle, clinical, and sleep-related factors into the analyses, exploring snoring-related cancer prevalence across diverse patient populations. Additionally, an analysis involving 4580 individuals aged 65 and above from the Cardiovascular Health Study highlighted an inverse association between snoring and incident overall cancer risk, considering gender, age, BMI, diabetes, physical activity, and alcohol use [30]. This analysis suggests that the type of cancer and age might influence the epidemiological association with sleep problems and that older age could confer protection against intermittent hypoxia-induced carcinoma growth [30, 31]. These assumptions align with our observed inverse association between habitual snoring and overall cancer, colon and rectum, skin, melanoma cancer in males aged 65 and above, and breast cancer in women aged 65 and above.
A surprising finding of this study was the lower cancer prevalence among habitual snorers compared to non-habitual snorers, particularly in men. While snoring is widely recognized as a primary symptom of OSA [32, 33]—a condition linked to increased cancer risk [8, 23–25, 34]—our findings suggest a more complex relationship. Previous studies have indicated that the association between OSA and cancer typically emerges in more severe cases of apnea, as evidenced by elevated cancer risks in patients with moderate to severe OSA compared to those with mild OSA [25]. Hypoxia and sleep fragmentation are considered to be the main pathologic link in the OSA-cancer incidence relationship [35, 36]. In murine-based reports implicating sleep fragmentation and intermittent hypoxia components of OSA, fragmented sleep accelerates tumor growth and progression through tumor-associated macrophage recruitment and TLR4 signaling [35]. On the other hand, hypoxia increases angiogenesis into the tumoral tissue and induces overexpression of transcription factors, such as hypoxia-inducible factor-1, which are known to trigger upregulation of proangiogenic mediators, such as vascular endothelial growth factor in tumor cells, and enhance tumor progression [36, 37].
Notably, snoring alone does not necessarily indicate the presence of OSA [33]. Advanced signal processing algorithms-based studies have demonstrated only a weak positive correlation between snoring frequency and the apnea–hypopnea index (AHI), a standard measure of OSA severity [33]. This suggests that snoring without accompanying severe apnea may not significantly elevate cancer risk, a hypothesis supported by our data and other population-based studies [38]. For instance, Gozal et al. identified selective cancer risks associated with OSA using a nationwide employee-sponsored health insurance database, where certain cancers like pancreatic and kidney cancers showed increased risk, whereas others like colorectal and breast cancers demonstrated lower risks among those diagnosed with OSA [38]. This aligns with our findings of reduced colorectal and breast cancer prevalence in men and female habitual snorers, respectively, potentially highlighting a complex interplay of factors beyond simple hypoxia-induced carcinogenesis.
Our study refines this perspective by incorporating IPTW to control for important confounders such as smoking, BMI, race/ethnicity, and other demographic characteristics, unlike previous studies that might not have fully accounted for these variables [38]. Despite this, the pathophysiological mechanisms underlying the reduced risk of certain cancers in snorers remain speculative. It is hypothesized that varying cancer types may interact differently with hypoxia-inducible factors, which play critical roles in tumor progression and response to hypoxia [38–41]. Given these complexities, the findings necessitate cautious interpretation, particularly due to the cross-sectional design of our study which limits causal inferences. Future research should aim to replicate these results in larger, prospective cohorts with objective measures of snoring and OSA severity, alongside detailed cancer diagnoses, to better delineate the relationships and underlying mechanisms at play.
Sex differences in the association between habitual snoring and cancer may stem from distinct effects of carcinogenic risk factors in men and women. Genetic studies suggest that snoring correlates differently with lifestyle and clinical traits in each gender. For instance, while snoring prevalence is typically higher in males, tobacco use appears to have a stronger association with snoring in females, whereas alcohol consumption exerts a greater influence on snoring in males [13, 42]. Additionally, population-based research indicates higher cancer rates among heavy-drinking females compared to males [43, 44]. Moreover, several studies have shown a higher incidence of lung cancer in men than in women with similar tobacco smoking exposure levels [45, 46]. Although genetic evidence supports a causal relationship between BMI or whole-body fat mass and snoring, a prior review suggests a higher overall incidence of obesity-related cancer in females compared to males [13, 47]. Although no clinical studies have directly explored the sex-specific pathologic interaction between habitual snoring, carcinogenic risk factors, and cancer, these previous findings imply that varying carcinogenic potencies may contribute to specific cancer risk in individuals with habitual snoring, depending on gender.
Our findings contribute to the existing literature on the gender-specific influence of sleep duration on cancer risk. For instance, a recent study of 14 851 participants from the China Health and Retirement Longitudinal Study revealed elevated overall cancer risk among women sleeping less than 6 hours per night, but not in men [48]. Similarly, an analysis of 469 691 individuals in the UK BioBank identified both insufficient and excess sleep duration as independent risk factors for lung cancer, yet did not find an increased risk associated with snoring [49]. However, these studies did not separately investigate the interaction between sleep duration and snoring in cancer risk for men and women. Our weighted analysis, stratified by sleep duration, revealed that the inverse associations between habitual snoring and overall and lung cancer prevalence were observed only in males sleeping less than 7 hours. Conversely, in females, sleeping more than 9 hours per night was associated with increased odds of uterine and overall cancer prevalence. Furthermore, a systematic review and meta-analysis of cohort studies suggest that long sleep duration and sleep disturbance disrupt the immune-inflammation balance, leading to increases in systemic inflammation markers, which are associated with tumorigenesis [50, 51]. While long sleep might be a marker of chronic conditions [52], these findings support the potential role of chronic inflammation in cancer development, and suggest the possibility of using long sleep duration and habitual snoring as screening markers for cancer risk in women. While further research is warranted to explore the exact biological mechanisms, our study provides valuable insights into potential screening strategies for cancer risk.
There is ongoing debate about the impact of sleep duration on cancer risk. Recent analyses, including Mendelian randomization studies and systematic reviews, have further nuanced our understanding of the relationship between sleep duration and cancer risk [53–58]. For instance, Titova et al. (2021), using Mendelian randomization, found suggestive links between genetic predispositions to shorter sleep durations and increased risks of gastrointestinal cancers, although these findings did not withstand multiple testing corrections [53]. Similarly, a pooled analysis of Japanese cohorts indicated a non-linear association, where both very short and long durations of sleep correlated with an increased risk of certain cancers in men and women of certain age and BMI categories [54]. These findings align with our observations that both short and long sleep durations can modulate cancer risks differently across genders and cancer types, potentially through mechanisms involving systemic inflammation and immune function disruption [53, 54]. Notably, while some studies found significant associations for site-specific cancers, comprehensive reviews suggest that the overall cancer risk might not be significantly associated with sleep duration, highlighting the complexity of these relationships and the potential influence of unmeasured confounders [58]. Given these mixed outcomes, our study contributes to the ongoing discourse, suggesting that sleep duration, much like other lifestyle factors, potentially interacts with biological processes in a manner that may increase or decrease cancer risk. Our findings highlight the need to consider habitual snoring as a potential co-factor in these analyses. Future studies should aim to clarify these pathways and consider the impact of circadian rhythm disruptions, providing a more detailed landscape of how habitual behaviors like snoring and variations in sleep duration intersect with cancer epidemiology.
Previous studies have implicated intermittent hypoxia and sleep fragmentation as key mechanisms linking OSA to cancer incidence [28, 29, 59]. Intermittent hypoxia, characterized by repeated episodes of oxygen desaturation followed by reoxygenation, may lead to the generation of reactive oxygen species, similar to reperfusion injury in ischemic stress [60]. This process could predispose individuals to carcinogenesis by promoting reactive oxygen production in vascular endothelial cells [61]. Additionally, intermittent hypoxia may up-regulate hypoxia-inducible factors, altering substrate metabolism, angiogenesis, and cell differentiation, thus promoting cancer development [62]. Sleep fragmentation, on the other hand, has been associated with sympathetic activation, chronic systemic inflammation, and altered immune cell function, all of which may contribute to carcinogenesis in various organs [63, 64]. However, data on sex differences in the association between OSA and cancer are limited [28], and further research is needed to elucidate the potential role of cancer subtype, hormonal influences, and duration of OSA or habitual snoring on sex-specific mechanisms of carcinogenesis.
Limitations
Despite utilizing a large, diverse population sample and employing IPTW-weighted analysis were used in this study, several limitations must be acknowledged. First, sleep-related characteristics and cancer prevalence were self-reported rather than objectively measured, potentially introducing participant-specific recall bias and misclassification of exposure and response variables. While the definition of habitual snoring may misclassify cases where participants lack a bed partner into control, prior studies suggest high accuracy (up to 96%) of self-reported cancer diagnosis [65]. Secondly, our study design was observational, precluding the establishment of causality. Thirdly, the use of simple measures of sleep characteristics in a cross-sectional survey did not account for changes in sleep patterns over time, necessitating longitudinal cohort studies with repeated measures to validate findings. Additionally, our results may be susceptible to residual confounding from unaccounted covariates, such as medication use (e.g. chemotherapy), and genetic factors, which are not included in our dataset. Future studies should consider incorporating these clinical variables to more thoroughly explore the relationship between habitual snoring and cancer prevalence in men and women. Despite efforts to balance demographic, clinical, lifestyle, and sleep-related characteristics through weighted analyses in our main and subgroup analyses, residual confounding remains a possibility.
Conclusions
Our study elucidates sex-specific associations between habitual snoring and cancer prevalence, revealing inverse associations in males, particularly among males aged over 65 years, and varied effects in females, including lower odds of breast cancer but increased odds of cervix cancer. Additionally, we highlight the modifying role of sleep duration, with shorter duration (<7 hours) linked to decreased overall and lung cancer prevalence in snoring men, and longer duration (>9 hours) associated with elevated overall and uterine cancer prevalence in snoring women. Future research exploring the interactions between different sleep characteristics and cancer could have profound implications. Initiating long-term clinical trials to investigate cancer subtype screening based on objective sleep assessment would refine risk delineation, potentially altering prevention and screening strategies in a more accurate and individualized manner. Integration of genetic, circulating biomarkers, and other clinical and behavioral risk factors in future studies could provide insights into shared mechanisms underlying habitual snoring and cancer.
Supplementary Material
Acknowledgments
We are grateful to the participants and staff of the NHANES study.
Contributor Information
Qinglan Ding, College of Health and Human Sciences, Purdue University, West Lafayette, IN, USA.
Jeph Herrin, Division of Cardiology, Yale School of Medicine, New Haven, CT, USA.
Meir Kryger, Division of Pulmonary, Critical Care & Sleep Medicine, Yale School of Medicine, New Haven, CT, USA.
Author Contributions
Qinglan Ding (Conceptualization [equal], Data curation [lead], Investigation [lead], Methodology [equal], Project administration [lead], Resources [lead], Supervision [lead], Validation [equal], Writing—original draft [lead], Writing—review & editing [lead]), Jeph Herrin (Formal analysis [lead], Methodology [lead], Validation [lead], Visualization [lead], Writing—review & editing [equal]), and Meir Kryger (Conceptualization [lead], Investigation [equal], Methodology [equal], Resources [equal], Supervision [equal], Writing—review & editing [equal])
Data Availability Statement
The data for this study were sourced from NHANES, which is publicly available and can be accessed through the website https://wwwn.cdc.gov/nchs/nhanes/default.aspx.
References
- 1. World Health Organization. Cancer World Health Organization. https://www.who.int/news-room/fact-sheets/detail/cancerAccessed March 02, 2024. [Google Scholar]
- 2. Zheng R, Wang S, Zhang S, et al. Global, regional, and national lifetime probabilities of developing cancer in 2020. Sci Bull (Beijing). 2023;68(21):2620–2628. doi: 10.1016/j.scib.2023.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A.. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 4. American Cancer Society. Cancer facts and figures 2022. American Cancer Society. 2024. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.htmlAccessed March 2, 2024. [Google Scholar]
- 5. Main C, Liu Z, Welch K, Weiner G, Jones S, Stein K.. Surgical procedures and non-surgical devices for the management of non-apnoeic snoring: a systematic review of clinical effects and associated treatment costs. Southampton (UK): NIHR Health Technology Assessment programme: Executive Summaries; 2009. [DOI] [PubMed] [Google Scholar]
- 6. Zhang X, Giovannucci EL, Wu K, et al. Associations of self-reported sleep duration and snoring with colorectal cancer risk in men and women. Sleep. 2013;36(5):681–688. doi: 10.5665/sleep.2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen P, Wang C, Song Q, et al. Impacts of sleep duration and snoring on the risk of esophageal squamous cell carcinoma. J Cancer. 2019;10(9):1968–1974. doi: 10.7150/jca.30172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, et al.; Spanish Sleep Network. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med. 2013;187(1):99–105. doi: 10.1164/rccm.201209-1671OC [DOI] [PubMed] [Google Scholar]
- 9. Chang W-P, Liu M-E, Chang W-C, et al. Sleep apnea and the subsequent risk of breast cancer in women: a nationwide population-based cohort study. Sleep Med. 2014;15(9):1016–1020. doi: 10.1016/j.sleep.2014.05.026 [DOI] [PubMed] [Google Scholar]
- 10. Gozal D, Farré R, Nieto FJ.. Obstructive sleep apnea and cancer: epidemiologic links and theoretical biological constructs. Sleep Med Rev. 2016;27:43–55. doi: 10.1016/j.smrv.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu EE, Suthahar N, Paniagua SM, et al. Association of Cardiometabolic disease with cancer in the community. JACC CardioOncol. 2022;4(1):69–81. doi: 10.1016/j.jaccao.2022.01.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ryu B, Lee S, Heo E, Yoo S, Kim J-W.. Snoring-related polygenic risk and its relationship with lifestyle factors in a Korean population: KoGES study. Sci Rep. 2023;13(1):14212. doi: 10.1038/s41598-023-41369-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Campos AI, García-Marín LM, Byrne EM, Martin NG, Cuéllar-Partida G, Rentería ME.. Insights into the aetiology of snoring from observational and genetic investigations in the UK Biobank. Nat Commun. 2020;11(1):817. doi: 10.1038/s41467-020-14625-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kryger MH. Snoring: a public health hazard? Chest. 1993;104(1):2–3. doi: 10.1378/chest.104.1.2 [DOI] [PubMed] [Google Scholar]
- 15. Dorak MT, Karpuzoglu E.. Gender differences in cancer susceptibility: an inadequately addressed issue. Front Genet. 2012;3:268. doi: 10.3389/fgene.2012.00268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siegel RL, Miller KD, Fuchs HE, Jemal A.. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Accessed January 10, 2022. https://wwwn.cdc.gov/nchs/nhanes/default.aspx [Google Scholar]
- 18. NHANES—About the National Health and Nutrition Examination Survey. https://www.cdc.gov/nchs/nhanes/about_nhanes.htmAccessed February 27, 2023. [Google Scholar]
- 19. Centers for Disease Control and Prevention (CDC). Data from: National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2023. [Google Scholar]
- 20. Krittanawong C, Kumar A, Wang Z, et al. Sleep duration and cardiovascular health in a representative community population (from NHANES, 2005 to 2016). Am J Cardiol. 2020;127:149–155. doi: 10.1016/j.amjcard.2020.04.012 [DOI] [PubMed] [Google Scholar]
- 21. Hukkanen J, JacobP, 3rd, Benowitz NL.. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1):79–115. doi: 10.1124/pr.57.1.3 [DOI] [PubMed] [Google Scholar]
- 22. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the third report of The National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486 [DOI] [PubMed] [Google Scholar]
- 23. Palamaner Subash Shantha G, Kumar AA, Cheskin LJ, Pancholy SB.. Association between sleep-disordered breathing, obstructive sleep apnea, and cancer incidence: a systematic review and meta-analysis. Sleep Med. 2015;16(10):1289–1294. doi: 10.1016/j.sleep.2015.04.014 [DOI] [PubMed] [Google Scholar]
- 24. Wu D, Zhao Z, Chen C, et al. Impact of obstructive sleep apnea on cancer risk: a systematic review and meta-analysis. Sleep Breath. 2023;27(3):843–852. doi: 10.1007/s11325-022-02695-y [DOI] [PubMed] [Google Scholar]
- 25. Cheng H, Li D.. Investigation into the association between obstructive sleep apnea and incidence of all-type cancers: a systematic review and meta-analysis. Sleep Med. 2021;88:274–281. doi: 10.1016/j.sleep.2021.05.031 [DOI] [PubMed] [Google Scholar]
- 26. Young T, Evans L, Finn L, Palta M.. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20(9):705–706. doi: 10.1093/sleep/20.9.705 [DOI] [PubMed] [Google Scholar]
- 27. Balagny P, Vidal-Petiot E, Renuy A, et al. Prevalence, treatment and determinants of obstructive sleep apnoea and its symptoms in a population-based French cohort. ERJ Open Res. 2023;9(3):00053–02023. doi: 10.1183/23120541.00053-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pataka A, Bonsignore MR, Ryan S, et al. Cancer prevalence is increased in females with sleep apnoea: data from the ESADA study. Eur Respir J. 2019;53(6):1900091. doi: 10.1183/13993003.00091-2019 [DOI] [PubMed] [Google Scholar]
- 29. Park MJ, Han KD, Cho JH, Choi JH.. Incidence disparities of obstructive sleep apnea-associated lung cancer by gender; Korean National Health Insurance data analysis. Front Oncol. 2023;13:1214279. doi: 10.3389/fonc.2023.1214279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sillah A, Watson NF, Peters U, et al. Sleep problems and risk of cancer incidence and mortality in an older cohort: The Cardiovascular Health Study (CHS). Cancer Epidemiology. 2022;76:102057. doi: 10.1016/j.canep.2021.102057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Torres M, Campillo N, Nonaka PN, et al. Aging reduces intermittent hypoxia-induced lung carcinoma growth in a mouse model of sleep apnea. Am J Respir Crit Care Med. 2018;198(9):1234–1236. doi: 10.1164/rccm.201805-0892LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maimon N, Hanly PJ.. Does snoring intensity correlate with the severity of obstructive sleep apnea? J Clin Sleep Med. 2010;6(5):475–478. [PMC free article] [PubMed] [Google Scholar]
- 33. Alshaer H, Hummel R, Mendelson M, Marshal T, Bradley TD.. Objective relationship between sleep apnea and frequency of snoring assessed by machine learning. J Clin Sleep Med. 2019;15(3):463–470. doi: 10.5664/jcsm.7676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farré R.. Sleep-disordered breathing and cancer mortality: results from the wisconsin sleep cohort study. Am J Respir Crit Care Med. 2012;186(2):190–194. doi: 10.1164/rccm.201201-0130oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hakim F, Wang Y, Zhang SX, et al. Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling. Cancer Res. 2014;74(5):1329–1337. doi: 10.1158/0008-5472.CAN-13-3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Almendros I, Montserrat JM, Ramírez J, et al. Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. Eur Respir J. 2012;39(1):215–217. doi: 10.1183/09031936.00185110 [DOI] [PubMed] [Google Scholar]
- 37. Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2(1):38–47. doi: 10.1038/nrc704 [DOI] [PubMed] [Google Scholar]
- 38. Gozal D, Ham SA, Mokhlesi B.. Sleep apnea and cancer: analysis of a nationwide population sample. Sleep. 2016;39(8):1493–1500. doi: 10.5665/sleep.6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ren W, Mi D, Yang K, et al. The expression of hypoxia-inducible factor-1α and its clinical significance in lung cancer: a systematic review and meta-analysis. Swiss Med Wkly. 2013;143:w13855. doi: 10.4414/smw.2013.13855 [DOI] [PubMed] [Google Scholar]
- 40. Zhou W, Dosey TL, Biechele T, Moon RT, Horwitz MS, Ruohola-Baker H.. Assessment of hypoxia inducible factor levels in cancer cell lines upon hypoxic induction using a novel reporter construct. PLoS One. 2011;6(11):e27460. doi: 10.1371/journal.pone.0027460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Choi H, Gillespie DL, Berg S, et al. Intermittent induction of HIF-1α produces lasting effects on malignant progression independent of its continued expression. PLoS One. 2015;10(4):e0125125. doi: 10.1371/journal.pone.0125125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Franklin KA, Gíslason T, Omenaas E, et al. The influence of active and passive smoking on habitual snoring. Am J Respir Crit Care Med. 2004;170(7):799–803. doi: 10.1164/rccm.200404-474OC [DOI] [PubMed] [Google Scholar]
- 43. Verplaetse TL, Roberts W, Peltier MR, et al. Risk drinking levels and sex are associated with cancer and liver, respiratory, and other medical conditions. Drug Alcohol dependence reports 2021;1:100007. doi: 10.1016/j.dadr.2021.100007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Verplaetse TL, Peltier MR, Roberts W, et al. Sex and alcohol use disorder predict the presence of cancer, respiratory, and other medical conditions: findings from the national epidemiologic survey on alcohol and related conditions-III. Addict Behav. 2021;123:107055. doi: 10.1016/j.addbeh.2021.107055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stapelfeld C, Dammann C, Maser E.. Sex-specificity in lung cancer risk. Int J Cancer. 2020;146(9):2376–2382. doi: 10.1002/ijc.32716 [DOI] [PubMed] [Google Scholar]
- 46. Kreuzer M, Boffetta P, Whitley E, et al. Gender differences in lung cancer risk by smoking: a multicentre case-control study in Germany and Italy. Br J Cancer. 2000;82(1):227–233. doi: 10.1054/bjoc.1999.0904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Argyrakopoulou G, Dalamaga M, Spyrou N, Kokkinos A.. gender differences in obesity-related cancers. Curr Obes Rep. 2021;10(2):100–115. doi: 10.1007/s13679-021-00426-0 [DOI] [PubMed] [Google Scholar]
- 48. Ning D, Fang Y, Zhang W.. Association of habitual sleep duration and its trajectory with the risk of cancer according to sex and body mass index in a population-based cohort. Cancer. 2023;129(22):3582–3594. doi: 10.1002/cncr.34951 [DOI] [PubMed] [Google Scholar]
- 49. Xie J, Zhu M, Ji M, et al. Relationships between sleep traits and lung cancer risk: a prospective cohort study in UK Biobank. Sleep. 2021;44(9). doi: 10.1093/sleep/zsab089 [DOI] [PubMed] [Google Scholar]
- 50. Irwin MR, Olmstead R, Carroll JE.. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80(1):40–52. doi: 10.1016/j.biopsych.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Grivennikov SI, Greten FR, Karin M.. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Grandner MA, Drummond SP.. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med Rev. 2007;11(5):341–360. doi: 10.1016/j.smrv.2007.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Titova OE, Michaëlsson K, Vithayathil M, et al. Sleep duration and risk of overall and 22 site-specific cancers: a Mendelian randomization study. Int J Cancer. 2021;148(4):914–920. doi: 10.1002/ijc.33286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wilunda C, Abe SK, Svensson T, et al.; Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan. Sleep duration and risk of cancer incidence and mortality: a pooled analysis of six population-based cohorts in Japan. Int J Cancer. 2022;151(7):1068–1080. doi: 10.1002/ijc.34133 [DOI] [PubMed] [Google Scholar]
- 55. Zhao H, Yin JY, Yang WS, et al. Sleep duration and cancer risk: a systematic review and meta-analysis of prospective studies. Asian Pac J Cancer Prev. 2013;14(12):7509–7515. doi: 10.7314/apjcp.2013.14.12.7509 [DOI] [PubMed] [Google Scholar]
- 56. Lu Y, Tian N, Yin J, Shi Y, Huang Z.. Association between sleep duration and cancer risk: a meta-analysis of prospective cohort studies. PLoS One. 2013;8(9):e74723. doi: 10.1371/journal.pone.0074723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Erren TC, Morfeld P, Foster RG, Reiter RJ, Groß JV, Westermann IK.. Sleep and cancer: Synthesis of experimental data and meta-analyses of cancer incidence among some 1,500,000 study individuals in 13 countries. Chronobiol Int. 2016;33(4):325–350. doi: 10.3109/07420528.2016.1149486 [DOI] [PubMed] [Google Scholar]
- 58. Chen Y, Tan F, Wei L, et al. Sleep duration and the risk of cancer: a systematic review and meta-analysis including dose-response relationship. BMC Cancer. 2018;18(1):1149. doi: 10.1186/s12885-018-5025-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Porcacchia AS, Pires GN, Andersen ML, Tufik S.. A cross-sectional analysis of the association between sleep disorders and cancer using data from the National Health and Nutrition Examination Survey (NHANES) 2005-2014. J Clin Sleep Med. 2024;20(4):515-520. doi: 10.5664/jcsm.10932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Suzuki YJ, Jain V, Park A-M, Day RM.. Oxidative stress and oxidant signaling in obstructive sleep apnea and associated cardiovascular diseases. Free Radic Biol Med. 2006;40(10):1683–1692. doi: 10.1016/j.freeradbiomed.2006.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kondoh M, Ohga N, Akiyama K, et al. Hypoxia-induced reactive oxygen species cause chromosomal abnormalities in endothelial cells in the tumor microenvironment. PLoS One. 2013;8(11):e80349. doi: 10.1371/journal.pone.0080349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jun JC, Rathore A, Younas H, Gilkes D, Polotsky VY.. Hypoxia-inducible factors and cancer. Curr Sleep Med Rep. 2017;3(1):1–10. doi: 10.1007/s40675-017-0062-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Castro-Diehl C, Diez Roux AV, Redline S, et al. Sleep duration and quality in relation to autonomic nervous system measures: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep. 2016;39(11):1927–1940. doi: 10.5665/sleep.6218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lee DB, An SY, Pyo SS, Kim J, Kim SW, Yoon DW.. Sleep fragmentation accelerates carcinogenesis in a chemical-induced colon cancer model. Int J Mol Sci . 2023;24(5):4547. doi: 10.3390/ijms24054547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cortés-Ibáñez FO, van Pinxteren B, Sijtsma A, et al. The validity of self-reported cancer in a population-based cohort compared to that in formally registered sources. Cancer epidemiology. 2022;81:102268. doi: 10.1016/j.canep.2022.102268 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data for this study were sourced from NHANES, which is publicly available and can be accessed through the website https://wwwn.cdc.gov/nchs/nhanes/default.aspx.