Abstract
During human cytomegalovirus (HCMV) infection, the periphery of promyelocytic leukemia protein (PML)-associated nuclear bodies (also known as PML oncogenic domains [PODs] or ND10) are sites for both input viral genome deposition and immediate-early (IE) gene transcription. At very early times after infection, the IE1 protein localizes to and subsequently disrupts PODs, whereas the IE2 protein localizes within or adjacent to PODs. This process appears to be required for efficient viral gene expression and DNA replication. We have investigated the initiation of viral DNA replication compartment formation by studying the localization of viral IE proteins, DNA replication proteins, and the PML protein during productive infection. Localization of IE2 adjacent to PODs between 2 and 6 h after infection was confirmed by confocal microscopy of human fibroblasts (HF cells) infected with both wild-type HCMV(Towne) and with an IE1-deletion mutant HCMV(CR208) that fails to disrupt PODs. In HCMV(Towne)-infected HF cells at 24 to 48 h, IE2 also accumulated in newly formed viral DNA replication compartments containing the polymerase processivity factor (UL44), the single-stranded DNA binding protein (SSB; UL57), the UL112-113 accessory protein, and newly incorporated bromodeoxyuridine (BrdU). Double labeling of the HCMV(CR208)-infected HF cells demonstrated that formation of viral DNA replication compartments initiates within granular structures that bud from the periphery of some of the PODs and subsequently coalesce into larger structures that are flanked by PODs. In transient DNA transfection assays, both the N terminus (codons 136 to 290) and the C terminus (codons 379 to 579) of IE2 exon 5, but not the central region between them, were found to be necessary for both the punctate distribution of IE2 and its association with PODs. Like IE2, the UL112-113 accessory replication protein was also distributed in a POD-associated pattern in both DNA-transfected and virus-infected cells beginning at 6 h. Furthermore, when all six replication core machinery proteins (polymerase complex, SSB, and helicase-primase complex) were expressed together in the presence of UL112-113, they also accumulated at POD-associated sites, suggesting that the UL112-113 protein (but not IE2) may play a role in recruitment of viral replication fork proteins into the periphery of PODs. These results show that (i) subsequent to accumulating at the periphery of PODs, IE2 is incorporated together with the core proteins into viral DNA replication compartments that initiate from the periphery of PODs and then grow to fill the space between groups of PODs, and (ii) the UL112-113 protein appears to have a key role in assembling and recruiting the core replication machinery proteins in the initial stages of viral replication compartment formation.
Human cytomegalovirus (HCMV) typically causes asymptomatic infection in immunocompetent individuals. However, infections of newborns and of immunocompromised individuals, such as organ transplant recipients and patients with AIDS, as well as reactivation from latent infection can lead to severe disease complications and pathogenesis (6, 34).
During HCMV DNA replication in permissive cells, the 230-kb viral genome is first transferred to the nucleus and circularized by joining of the genomic termini; then progeny genomes are generated as large multicopy concatemeric structures (25, 33). Like replication of alpha- and gammaherpesviruses, replication of HCMV, a member of the betaherpesvirus subfamily, requires a conserved set of six core DNA replication proteins (7, 12, 35, 47, 54): the DNA polymerase (UL54) and the associated polymerase processivity factor (UL44), a single-stranded DNA binding protein (SSB; UL57), and the triplex containing DNA helicase (UL105), primase (UL70), and primase-associated factor (UL102) subunits. In addition to these six core machinery proteins, five additional viral proteins (auxiliary components) are required for transient in vitro DNA replication assays that depend on the presence of HCMV oriLyt DNA (35, 36, 48). Among them, UL36-38, TRS1 and IRS1, and immediate-early (IE) proteins IE1 and IE2 appear to have a role primarily in transcriptional transactivation of the replication protein genes (21), whereas only UL84 is essential for promoting oriLyt-dependent DNA replication and the formation of large viral DNA replication compartments in cotransfection assays (47). The UL112-113 protein is needed for efficient replication in the cotransfection assays only in the absence of the viral transactivators (47). A role for UL112-113 in orchestrating viral replication proteins for assembly of replication compartments has been suggested based on its earlier expression compared to other replication proteins and its localization within discrete subnuclear structures in virus-infected cells (37). Recently, UL112-113 was also shown to colocalize with viral DNA both before and during DNA replication in infected cell nuclei (55).
Replication of HCMV DNA has several characteristics. First, it initiates between 24 to 72 h after infection, compared to between 4 and 8 h in herpes simplex virus (HSV) infection. Second, the origin of replication, oriLyt, is a more complex structure than that in HSV and spans an approximately 2-kb region (5, 17, 31). Third, unlike the case for other herpesviruses, a small RNA transcript and persistent RNA-DNA hybrid structures are detected within the origin (18, 42, 47a). Finally, a DNA binding replication initiation protein analogous to the HSV type 1 (HSV-1) origin binding protein UL9 (35, 36) has not been found. Instead, UL84 appears to perform this function by an unknown mechanism (47). Based on these characteristics, a unique mode of DNA replication for HCMV is postulated, and the functions of auxiliary components in the initiation of replication remain to be understood.
Small subnuclear punctate structures known as promyelocytic leukemia protein (PML)-associated nuclear bodies, PML oncogenic domains (PODs) or nuclear domain 10 (ND10), have been shown to be sites for input viral DNA accumulation in adenovirus, simian virus 40, HSV-1, and HCMV infection as well as for IE transcription in HCMV (19, 20). PODs are spherical 0.3- to 0.5-μm structures that are present in most cells, with an average number of 10 to 20 per cell. The PML protein surrounds an electron-dense core that is associated with the nuclear matrix.
The idea that the peripheries of PODs are also associated with viral DNA replication was first proposed for HSV-1. In HSV-infected cells, small viral prereplication sites or foci containing the virus-encoded SSB (ICP8) protein were originally demonstrated by Quinlan et al. (44) when DNA synthesis was blocked by the presence of the viral DNA polymerase inhibitor phosphonoacetic acid (PAA). SSB also forms numerous small punctate structures at early times in the absence of PAA (8), and studies with mutant viruses showed that these required the presence of SSB, the helicase-primase complex (UL5, UL8, and UL52), and the origin binding protein (UL9) (27, 29). In DNA-transfected cells, SSB also forms micropunctate structures in the presence of just the helicase-primase complex, and these colocalize with cellular bromodeoxyuridine (BrdU)-containing replisomes in S-phase cells (57). In contrast, in cotransfected cells receiving the HSV origin DNA plasmid, all seven essential HSV DNA replication proteins are required to form a much smaller number of spherical prereplication foci that can develop into large globular and kidney-shaped DNA replication compartments that are actively engaged in viral DNA synthesis as assayed by PAA-sensitive incorporation of BrdU in non-S-phase cells (57). Both types of punctate foci were also confirmed to be formed in infected cells in the presence of PAA, but only the second type are associated with PODs and are presumed to be the true replication intermediates (28, 30, 52, 57).
Although some of the HCMV viral DNA replication proteins, including SSB (UL57), UL44 (UL44), and UL112-113, have been also shown to be localized in large nuclear structures that resemble HSV-1 replication compartments (9, 14, 22, 23, 37, 41, 47), the cellular location and mechanism for initiation and assembly of HCMV DNA replication proteins are not understood. We previously showed that the IE1 protein localizes to and subsequently disrupts PODs, whereas the IE2 protein localizes within or adjacent to PODs at very early times (1, 3). This process appears to be required for efficient viral gene expression and replication (4, 20). However, the association of HCMV replication proteins with PODs and a possible role in initiation of viral DNA replication compartment formation have not been evaluated previously.
In this study, we have investigated the initiation of HCMV viral DNA replication compartment formation, including the colocalization of the PML, IE, and viral DNA replication proteins, and newly synthesized DNA (BrdU incorporation) during productive infection. We show that both the IE2 and UL112-113 proteins localize adjacent to PODs at very early times after infection and that they are incorporated into viral DNA replication compartments that initiate and grow from the periphery of PODs. We also suggest that UL112-113 may recruit viral replication fork proteins to the POD-associated assembly sites.
MATERIALS AND METHODS
Cell cultures and virus infection.
Permissive human diploid fibroblasts (HF cells) and Vero cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum. HF cells at passages 7 to 13 were used for virus infection. Some samples as indicated were incubated in the presence of PAA at 200 μg/ml after infection.
The IE1-defective mutant HCMV(CR208) and its parent virus, HCMV(Towne), were provided by Edward Mocarski (Stanford University, Stanford, Calif.). The virus stocks used were prepared as described by LaFemina et al. (26). For experiments using indirect immunofluorescence assays (IFA), cells were seeded into four-well chamber slides (0.4 × 105/well), and the subconfluent cells were infected with HCMV(Towne) or HCMV(CR208) at various multiplicities of infection (MOI; PFU per cell). In all cases, input supernatant virus was diluted with serum-free medium and was adsorbed for 1.5 h at 37°C, and then the inoculum was replaced with fresh warmed medium at time zero.
Expression plasmids and transient DNA transfection.
All replication machinery expression plasmids used in this work except pMP18 (IE2) and pJHA309(Flag/UL112-113) were described previously (47). For the six replication fork core machinery proteins, pRTS22 (UL44; processivity factor), pRTS6 (UL54; DNA polymerase), pRTS7 (UL57; SSB), pRTS9 (UL70; primase subunit), pRTS29 (UL102; primase-associated factor), and pRTS10 (UL105; DNA helicase subunit) were used. For the auxiliary components and stimulatory factor, p302 (UL36-38), pRTS18 (IRS1), pMP18 (IE2) (40), pRTS26 (UL112-113), pRTS5 (UL84), and pRTS28 (UL69) were used.
To generate pJHA309 expressing 5′ flag epitope (F)-tagged UL112-113 protein products, the 2.2-kb XbaI-BglII fragment was isolated from pRTS26, converted to a blunt-end form, and inserted into the BglII site of pJH272, an F-tag expression vector derived from pSG5 (16).
Plasmid pRL45, expressing both IE1 and IE2 under their natural transcriptional and splicing signals, plasmids pRL62, pMP88, pMP14, pMP83, and pRL94, expressing both wild-type IE1 and mutant IE2, and plasmids pRL72 and pRL84, expressing in-frame fusions between IE1 and IE2, were all described previously (26, 40).
All IE2 mutant expression plasmids containing deletions between codons 290 and 542 were generated in the pMP18 background for mammalian expression purposes. To do so, the XhoI site upstream of the major IE (MIE) enhancer/promoter region in pMP18 was first destroyed by Klenow fill-in (pJHA100), and the XhoI-StuI sites of IE2 exon 5 in pJHA100 were subsequently used to replace a 760-bp wild-type fragment (containing codons 290 to 542) with the equivalent XhoI-StuI fragments containing deletion mutations as described previously (2). This approach generated pCJC110(IE2Δ290-313), pCJC108(IE2Δ313-346), pCJC109(IE2Δ346-358), pJHA104(IE2Δ358-379), pJHA106(IE2Δ376-404), pJHA105(IE2Δ403-419), and pJHA107(IE2Δ487-518).
For DNA transfection experiments, Vero cells were seeded into two well-slide chambers (0.8 × 105/well) and DNA (3 μg/well) was introduced for transient expression assays in subconfluent cells, using the N,N-bis-(2-hydroxyethyl)-2-aminoethanesulfonic acid-buffered saline version of the calcium phosphate procedure described previously (38).
Antibodies.
Mouse monoclonal antibodies (MAbs) 6E1 and 12E2 against HCMV IE1 (UL123; exon 4) and IE2 (UL122; exon 5), respectively, were obtained from Vancouver Biotech (Vancouver, British Columbia, Canada). MAb CH810, which detects epitopes presented in both IE1 and IE2 (exons 2 and 3), was purchased from Chemicon (Temecula, Calif.). MAbs against ppUL44 (UL44) and BrdU were obtained from Advanced Biotech Inc. (Gaithersburg, Md.) and Becton Dickinson (San Jose, Calif.), respectively. A MAb against the F epitope was purchased from Eastman Kodak Company (New Haven, Conn.). The rabbit antipeptide polyclonal antibody (PAb) referred to as anti-PML(C), directed against amino acids at positions 484 to 498 of PML, was described previously (1, 3). Rabbit antipeptide PAb UL112-113(C), directed against the C terminus of HCMV UL112-113, and a rat antipeptide PAb against HCMV SSB (UL57) were prepared as described previously (39). The epitopes for the UL112-113(C) and SSB antibodies were NH2-AGNGRRRGPRFLEDGL-COOH and NH2-RTRLPVVPKQPKKEPS-COOH, respectively.
IFA.
For IFA, both virus-infected and DNA-transfected cells were fixed by either the methanol or paraformaldehyde procedure. For the methanol procedure, the cells were washed in Tris-buffered saline (TBS), then permeabilized with absolute methanol at 20°C for 10 min and rehydrated in ice-cold TBS for 5 min. For the paraformaldehyde procedure, the cells were washed in phosphate-buffered saline (PBS), fixed with 1% paraformaldehyde solution in PBS at 20°C for 5 min, and then permeabilized in ice-cold 0.2% Triton X-100 solution in PBS for 20 min before incubation with primary antibodies. Mouse MAbs 6E1 (for IE1), 12E2 (for IE2), CH810 (for both IE1 and IE2), and ABI44 (for ppUL44) were used at 1:200 dilutions. Mouse MAb for BrdU was used at a 1:100 dilution, and MAb for the F epitope was used at a 1:600 dilution. Rabbit PAb anti-PML(C) was used at a 1:1,000 dilution, and PAb anti-UL112-113(C) was used at a 1:800 dilution. Rat PAb against SSB was used at a 1:50 dilution. The antibody incubations were carried out in TBS at 30°C for 1 h, followed by incubation with fluorescein isothiocyanate (FITC)-labeled donkey anti-mouse immunoglobulin G (IgG) or rhodamine-coupled donkey anti-rabbit (or anti-rat) IgG antibody at a 1:100 dilution at 37°C for 45 min. For double labeling, mouse monoclonal and rat or rabbit polyclonal antibodies were incubated together. Slides were screened and photographed on a Leitz Dialux 20EB epifluorescence microscope with Image-Pro software (Media Cybernetics, Silver Spring, Md.). For confocal microscopy, a Noran OZ CLSM confocal microscope system with Intervision software (Noran Inc., Madison, Wis.) was used.
For BrdU labeling, the cells were pulse-labeled with 10 μM BrdU for 30 min at 37°C and were fixed and permeabilized as described above. The cells were then treated with 4 N HCl for 10 min at 20°C to expose the incorporated BrdU residues and washed three times for 5 min each with PBS to neutralize the acid before incubation with mouse anti-BrdU MAb.
Western blot analysis.
Cells were washed with PBS and lysed with 0.2 ml of ice-cold lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1.0% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]). Equal amounts of clarified cell extracts were separated by electrophoresis on SDS–8% polyacrylamide gels followed by electroblotting onto nitrocellulose. The blots were blocked by incubation for 1 h at 20°C in PBS plus 0.1% Tween 20 containing 5% nonfat dry milk. The blots were then washed twice with PBS-Tween 20 for 15 min and incubated with appropriate antibodies at a dilution of 1:3,000 for 1 h at room temperature. After three 10-min washes with PBS-Tween 20, the blots were incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (Bio-Rad) for 1 h at 20°C. The blots were washed three times, and reacting protein bands were detected with an enhanced chemiluminescence system (Amersham ECL RP2106) using Kodak XAR film.
RESULTS
Both IE2 and UL112-113 localize adjacent to PODs at very early times after infection.
When HF cells were infected with wild-type HCMV(Towne), the localization of IE2 adjacent to PODs was observed at 2 h after infection by confocal double-label IFA for IE2 and PML (Fig. 1a). This association is very transient and was detected in a only a few infected cells because IE1 normally rapidly displaces PML from the PODs (3). However, in HF cells infected with mutant HCMV(CR208), which lacks all of IE1 exon 4 but still has the ability to replicate efficiently at high MOI (15), the PODs remain intact because of the lack of functional IE1 protein (1). Therefore, HF cells were also infected with HCMV(CR208) at an MOI of 2.0 and observed by confocal double-label IFA for IE2 and PML. Under these conditions, most of IE2 was localized as an overlapping pattern in close proximity to but not identical to PODs in almost all infected cells even at 6 h after infection (Fig. 1b).
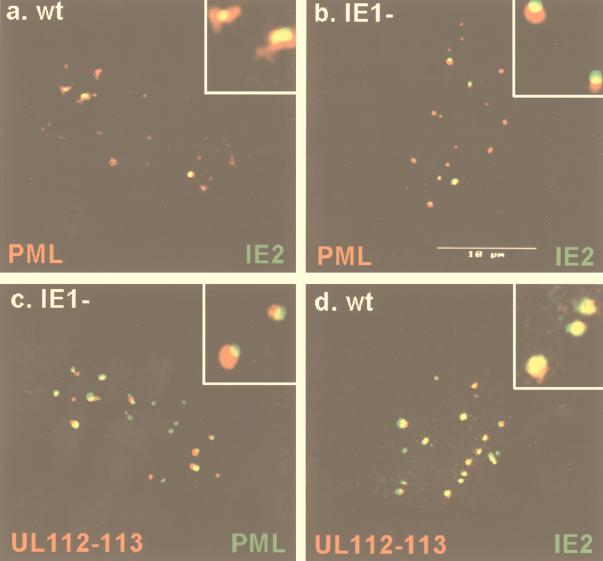
FIG. 1.
Both IE2 and UL112-113 localize adjacent to PODs at very early times after infection. HF cells were infected with HCMV(Towne) at a low MOI (<1.0 PFU per cell) and fixed in methanol at 2 h (a) or 6 h (d) after infection, or they were infected with HCMV(CR208) at an MOI of 2.0 and fixed with paraformaldehyde at 6 h after infection (b and c). Confocal double-label IFA was carried out to detect IE2 and PML (a and b), UL112-113 and PML (c), or UL112-113 and IE2 (d). IE2 was detected with mouse MAb 12E2 and FITC-labeled donkey anti-mouse IgG, and UL112-113 was detected with rabbit PAb UL112-113(C) and rhodamine-coupled donkey anti-rabbit IgG. For PML, either mouse MAb 5E10 and FITC-labeled donkey anti-mouse IgG or rabbit PAb PML(C) and rhodamine-coupled donkey anti-rabbit IgG were used. Confocal images from each fluorochrome were recorded, and only the superimposed merged images are shown. Inserts show high-power magnification of some PODs. Note that association of IE2 and PML in PODs in wild-type virus-infected cells (a) is very transient because of IE1-induced displacement of PML from PODs.
We also raised a rabbit anti-UL112-113 PAb that detects the 84-kDa form of the UL112-113 protein (see Fig. 8) and investigated the distribution pattern of UL112-113 in virus-infected HF cells. In both wild-type and ΔIE1 mutant virus-infected cells, UL112-113 gave a small nuclear punctate pattern at 6 h (Fig. 1c and d; see also Fig. 4) but was not detected at 2 h (data not shown). Because this punctate pattern of UL112-113 resembled PODs, confocal double-label IFA for both UL112-113 and PML was performed. The result showed that like IE2, at least the 84-kDa form of UL112-113 was also distributed adjacent to or touching PODs at 6 h in ΔIE1-infected cells (Fig. 1c). In contrast, when confocal double-label IFA was performed for both IE2 and UL112-113 in wild-type virus-infected cells at 6 h, the two proteins often colocalized and were much more closely associated with each other than either was with PML (Fig. 1d). These results clearly show that both IE2 and UL112-113 initially localize together and adjacent to or touching PODs at very early times after infection, although apparently IE2 targets to PODs at earlier times than UL112-113. They also demonstrate that these localization patterns for both IE2 and UL112-113 are not dependent on the expression of IE1 or on the loss of PML from the PODs.
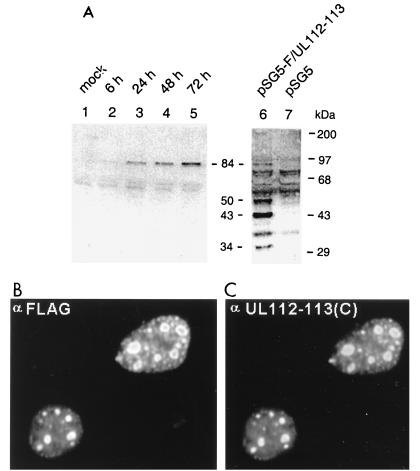
FIG. 8.
Specificity of the antipeptide PAb generated against UL112-113. (A) Immunoblot assay of extracts prepared from virus-infected HF cells and plasmid-transfected Vero cells. For lanes 1 to 5, HF cells were mock infected or infected with HCMV(Towne) at an MOI of 5.0, and total cell extracts were prepared at 6, 24, 48, and 72 h. For lanes 6 and 7, Vero cells were transfected with either a plasmid expressing 5′ F-tagged UL112-113 (pSG5-F/UL112-113, pJHA309) or the empty DNA vector (pSG5), and total extracts were prepared at 48 h after transfection. Equal amounts of each extract were subjected to SDS-PAGE (8% gel) for Western blotting with anti-UL112-113(C) PAb (lanes 1 to 5) or with anti-F MAb (lanes 6 and 7). The positions of the molecular size markers and the four F-tagged UL112-113 protein products (34, 43, 50, and 84 kDa) are indicated. (B and C) IFA images of UL112-113 in DNA-transfected cells. Vero cells were transiently transfected with plasmid pJHA309 expressing the 5′ F-tagged UL112-113 protein. Cells were fixed by the paraformaldehyde procedure at 48 h, and double-label IFA was carried out with mouse MAb directed against the F epitope and rabbit PAb UL112-113(C). (B) F-tagged UL112-113 protein products detected with mouse anti-F MAb and FITC-labeled donkey anti-mouse IgG. (C) The 84-kDa form of UL112-113 detected with rabbit anti-UL112-113(C) PAb and rhodamine-coupled anti-rabbit IgG.
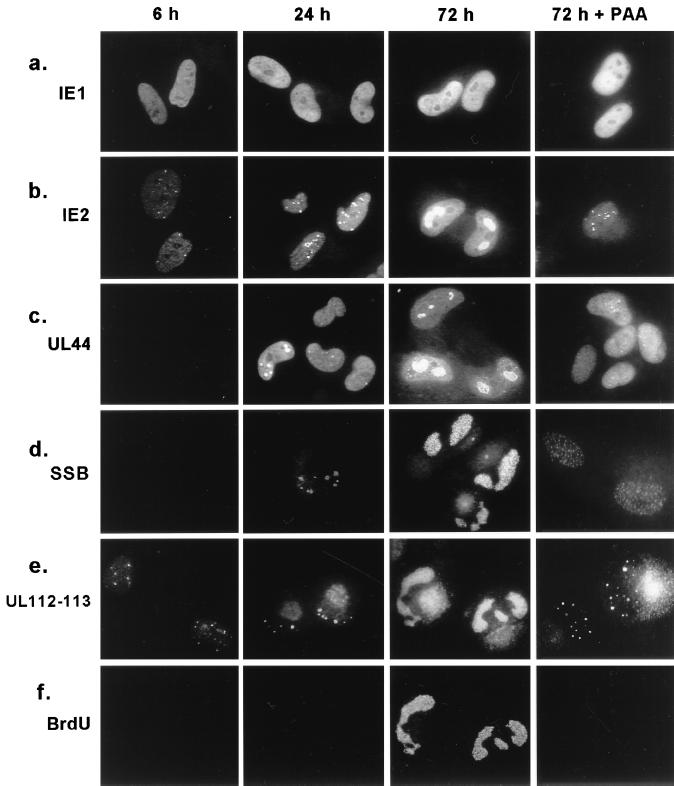
FIG. 4.
Comparison of distribution patterns of five viral proteins at different time points after infection. HF cells were infected with HCMV(Towne) at a low MOI (<1.0) in the absence or presence of PAA (200 μg/ml). Cells were BrdU pulse-labeled and fixed in methanol at 6, 24, and 72 h after infection, and single labeling IFA for IE1, IE2, UL44, and SSB (a to e) or double labeling for both UL112-113 and BrdU (e and f) was carried out as described in Materials and Methods.
Evaluation of the IE2 protein domains required for punctate POD association.
In DNA-transfected HF or Vero cells, the IE2 protein is distributed as a mixed nuclear pattern combining a background diffuse distribution with punctate bodies which exactly colocalize with PML in the PODs (ND/P pattern) (3). In contrast, a fully punctate distribution that only touches PODs is found at early times during HCMV infection of HF cells. To determine which regions of IE2 are required for the nuclear punctate distribution and for colocalization with PML in the absence of other viral proteins, we transfected Vero cells with a variety of IE2 deletion mutants followed by double-label IFA. The results are summarized in Fig. 2A and B. Two independent nuclear localization signals of IE2 have been mapped previously to regions between codons 145 and 151 and codons 321 and 328 (39), and all deletion mutants tested retained at least one of these motifs. When cells were transfected with plasmids expressing either IE2 alone (pMP18) or both IE1 and IE2 (pRL45), the typical wild-type mixed ND/P pattern for IE2 was detected as described previously (3). When cells were transfected with plasmids expressing IE2(Δ86-290) (pRL84) or IE2(Δ136-290) (pMP88), IE2 was distributed as a uniform nuclear diffuse form only, whereas cells transfected with pRL72, expressing IE2(Δ86-135) as a fusion protein (with an insertion of IE1 amino acids 87 to 131), showed a novel modified micropunctate pattern with numerous tiny punctate bodies (MP/P pattern). These IFA results demonstrate that several segments from the region between codons 87 and 290 are required for the normal punctate distribution of IE2. In addition, two mutant proteins, IE2(Δ542-579) lacking the C terminus in pMP14 and IE2(Δ290-492) lacking a large internal region in pMP83, both gave a totally diffuse nuclear distribution without showing any association with punctate bodies.
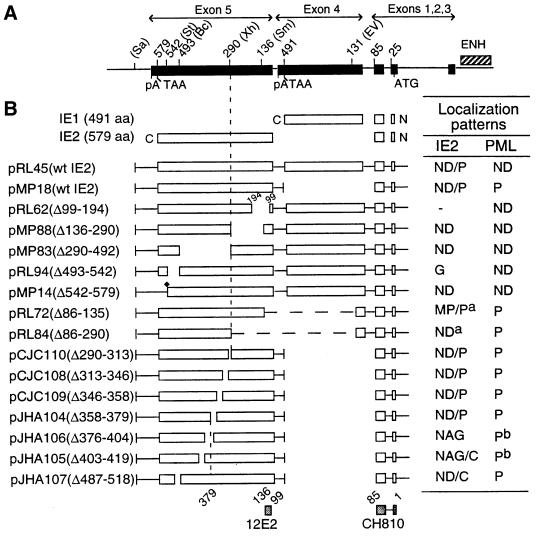
FIG. 2.
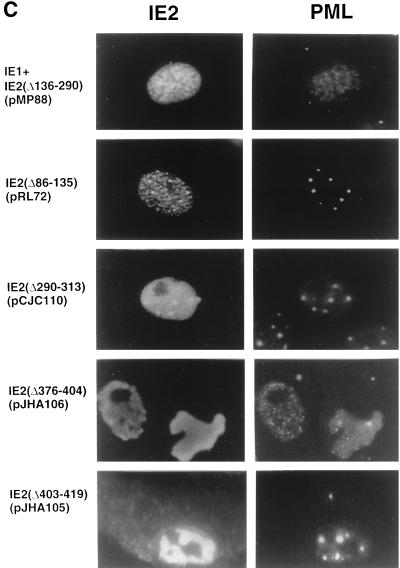
Domain requirements within the IE2 protein for POD association. (A and B) Summary of the localization patterns of both IE2 and PML in Vero cells transiently transfected with genomic plasmids expressing deleted versions of IE2. (A) The overlapping five-exon structure (solid bar) of the MIE gene transcription unit in the inverted (i.e., viral) genomic orientation is illustrated at the top. Positions of key restriction sites used to generate the deleted or truncated versions of IE2 (Bc, BclI; Ev, EcoRV; Sa, SalI; Sm, SmaI; St, StuI; Xh, XhoI) are indicated. The enhancer/promoter region of the MIE locus (ENH; hatched bar) and the translation start (ATG) and termination (TAA) sites as well as polyadenylation sites (pA) are also indicated. (B) Open bars represent coding regions with gaps denoting in-frame deletions; the diamond indicates inserted triple-terminator oligonucleotides; horizontal dashed lines indicate in-frame IE1-IE2 fusion proteins (pRL72 and pRL84). The estimated map locations for the epitopes recognized by the 12E2 and CH810 Mab are shown at the bottom (hatched bars). To detect IE2, mouse MAb 12E2 or CH810 and FITC-labeled donkey anti-mouse IgG were used. PML was detected as described for Fig. 1. IFA patterns: ND, nuclear diffuse; P, punctate; ND/P, a mixture of nuclear diffuse and punctate patterns; MP/P, nuclear micropunctate with concentrated punctate bodies; NAG, nuclear aggregation; NAG/C, NAG pattern with cytoplasmic diffuse; ND/C, nuclear and cytoplasmic diffuse pattern. a, IE2 was detected with mouse MAb CH810; b, reduced number of punctate bodies or numerous micropunctate bodies. aa, amino acids. (C) Double-label IFA images of Vero cells transfected with pMP88 encoding IE2(Δ136-290), pRL72 encoding IE2(Δ86-135), pCJC110 encoding IE2(Δ290-313), pJHA106 encoding IE2(Δ376-404), or pJHA105 encoding IE2(Δ403-419).
When mutant IE2 proteins containing smaller deletions within the internal region between codons 290 and 542 were investigated, four distinct deletions that removed codons 290 and 313, 313 and 346, 346 and 358, or 358 and 379 (pCJC110, pCJC108, pCJC109, and pJHA104) all still showed the typical wild-type punctate (ND/P) staining pattern for IE2 and retained the association with PML. However, three different internal deletions located at further C-terminal positions between codons 376 to 404 (pJHA106), 403 to 419 (pJHA105), and 487 to 518 (pJHA107) all lost the typical punctate pattern but remained positive by IFA. Interestingly, the PML distribution pattern in cells expressing the IE2(Δ376-404) and IE2(Δ403-419) mutants showed a large nuclear aggregated form of IE2 with either only a few remaining PODs or numerous micropunctate spots of PML, whereas the IE2(Δ487-518) mutant gave a nuclear and cytoplasmic diffuse pattern without affecting the punctate PML pattern. Representative patterns for the IE2(Δ136-290), IE2(Δ86-135), IE2(Δ290-313), IE2(Δ376-404), and IE2(Δ403-419) mutant proteins and the PML patterns in the same fields are shown in Fig. 2C.
Therefore, regions from both the nonconserved N terminus (codons 87 to 290) and the conserved DNA binding/dimerization domain in the C terminus (codons 379 to 579) of IE2 exon 5 contribute to both the punctate characteristics of IE2 and its association with PODs, although interestingly the central portion from codons 290 to 379 does not. This association is believed to be indirect because unlike IE1, IE2 does not interact with PML in yeast two-hybrid assays (1).
IE2 is incorporated into viral DNA replication compartments at late times after infection.
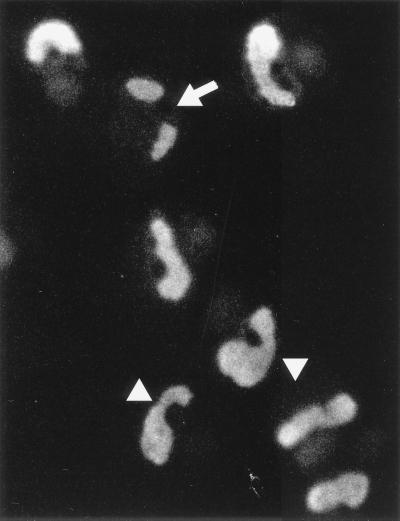
The IE2 protein is required for maximal efficiency of viral DNA replication in transient DNA cotransfection assays (35, 47). To further study the role of IE2 in viral DNA replication, the localization pattern of IE2 was also investigated at later times after infection with wild-type HCMV. Interestingly, the IE2 staining pattern at 96 h after infection of HF cells at high MOI showed large intranuclear structures that resembled viral DNA replication compartments (Fig. 3). Some of the infected cells showed two separate structures within the nucleus (arrow), but in most cells these had become enlarged and combined to form the fully developed irregular oval or kidney-shaped viral DNA replication compartments that nearly completely fill the nucleus (but sparing the nucleolus). These patterns observed by IE2 staining are very similar to those detected by staining for the SSB (UL57), UL44, and UL112-113 proteins at late times after infection (37) as well as for UL44 in functionally active HCMV replication compartments assembled in the cotransfection assay system (47).
FIG. 3.
Localization pattern of IE2 at late times after infection. HF cells were infected with HCMV(Towne) at an MOI of 2.0, fixed in methanol at 96 h, and stained with mouse MAb 12E2 as described for Fig. 1. In most cells, IE2 was stained as large irregular oval structures that occupy most of the nucleus. In some cells, IE2 appeared as two distinct nuclear globular structures (arrow), but these may be connected to each other around the outside of the nucleolus (arrowheads).
To confirm the specificity of incorporation of IE2 into viral DNA replication compartments, we investigated the localization patterns of IE1, IE2, the polymerase processivity factor (UL44), UL112-113, and SSB (UL57), as well as newly incorporated pulse-labeled BrdU, at 6, 24, and 72 h in HF cells infected with HCMV(Towne) at a low MOI (<1.0). At 6 h, IE1 was distributed as a uniform nuclear diffuse pattern (Fig. 4a) and IE2 showed a nuclear punctate pattern (Fig. 4b) as previously described (3). Neither UL44 nor SSB was detected at 6 h (Fig. 4c and d), but when rabbit PAb UL112-113(C) directed against the C terminus of UL112-113 was used, a punctate staining pattern was obtained as described above (Fig. 4e). At 24 h after infection, IE1 remained as a uniform nuclear diffuse pattern, whereas IE2, UL112-113, and SSB all showed punctate structures larger than those at 6 h. UL44 was detected as both a punctate pattern in some cells and a nuclear diffuse pattern in most cells at 24 h, although it gave only a uniform nuclear diffuse form at 12 h after infection (not shown). At 72 h after infection, IE2 as well as UL44, UL112-113, and SSB all accumulated into large globular nuclear structures, including some that resembled complete viral DNA replication compartments, while IE1 still remained in a nuclear diffuse pattern. The cytoplasmic signals detected in virus-infected cells at late times (24 h and 72 h) by the rabbit PAb raised against UL112-113 were also detected with preimmune serum (not shown) and appear to result from the nonspecific binding of rabbit antibodies to virus-induced immunoglobulin Fc receptor-like proteins. This has also been seen for most other rabbit PAbs and has been described by other investigators (14). When double-label IFA for IE2 and UL44 was performed with rabbit antipeptide PAb against IE2 (P3) (38) and mouse MAb for UL44, the proteins were colocalized to the same structures (not shown).
To localize the sites for viral DNA synthesis in infected cells, BrdU pulse-labeled DNA was stained with anti-BrdU MAb at the indicated time points and shown as double labeled for both UL112-113 and BrdU by IFA (Fig. 4e and f). Incorporation of BrdU into large nuclear structures that contain UL112-113 and also resemble those detected by both IE2, UL44, and SSB staining was observed only at and after 72 h, whereas BrdU was not incorporated significantly into virus-infected cells (judged by positive UL112-113 staining) at either 6 or 24 h. This result clearly demonstrates that the globular structures containing IE2, UL44, SSB, and UL112-113 detected at 72 h represent viral DNA replication compartments. Importantly, formation of both the globular and larger kidney-shaped structures containing IE2, UL44, SSB, UL112-113, and BrdU was not detected at 72 h after infection in the presence of PAA. Instead, IE2 and UL112-113 gave small punctate bodies similar to those seen at 6 h, and UL44 was stained as a nuclear diffuse pattern with only very tiny punctate bodies in the presence of PAA. No BrdU signals were detected in these residual UL112-113-positive prereplication foci in either virus-infected S-phase or non-S-phase cells at 72 h in the presence of PAA, although the S-phase cells still showed BrdU incorporation into cellular replisomes (data not shown). These results demonstrate that IE2, but not IE1, is incorporated efficiently into viral DNA replication compartments at late times after infection and that the larger punctate structures containing IE2, UL44, SSB, and UL112-113 detected at 24 h appear to represent the initial stages of formation of viral DNA replication compartments, although viral DNA synthesis does not occur yet at this time point.
Viral DNA replication compartments develop from the periphery of PODs.
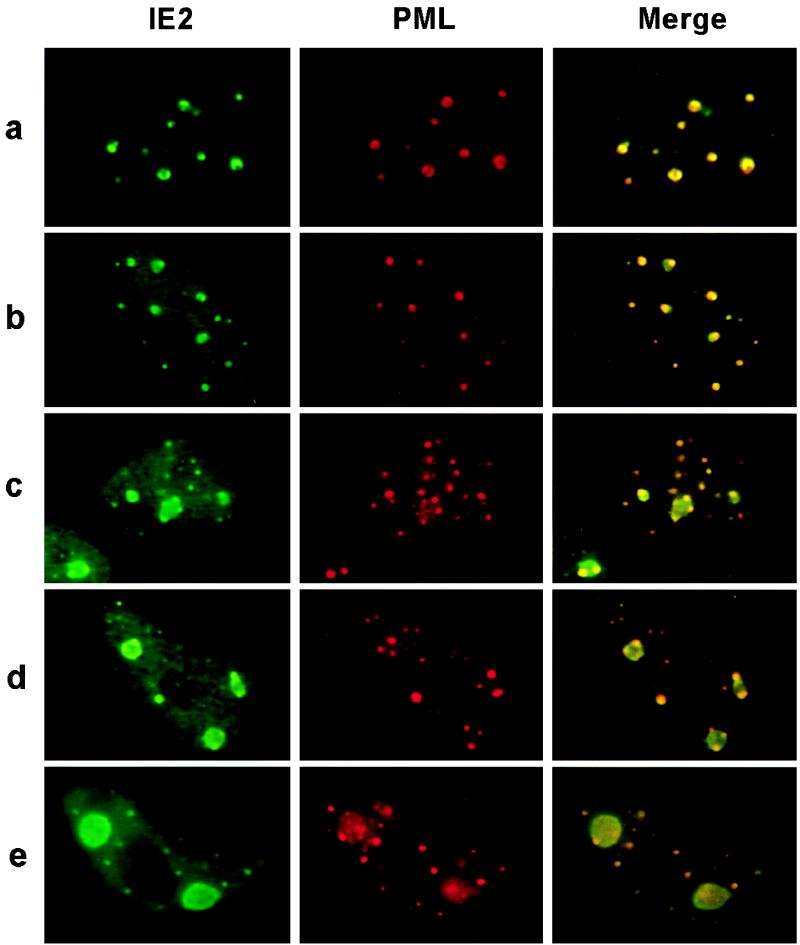
Our observations that (i) both the IE2 and UL112-113 proteins localize adjacent to PODs in small punctate domains at very early times after infection, (ii) these punctate domains become larger at later time points, and (iii) both IE2 and UL112-113 are ultimately incorporated very efficiently into viral DNA replication compartments led us to ask whether the peripheries of PODs where both IE2 and UL112-113 initially accumulate are also sites for the initiation of viral DNA replication compartment formation. Therefore, the localization pattern of IE2 and its association with PODs were further investigated in HF cells infected with HCMV(CR208/ΔIE1) at an MOI of 5.0 (Fig. 5). When the cells were fixed at 48 h after infection, double-label IFA for IE2 and PML showed that IE2 accumulated adjacent to PODs in some infected cells (Fig. 5a) and that budding structures containing IE2 were present at the periphery of PODs in some other infected cells (Fig. 5b). Furthermore, in some cells, larger structures bounded or flanked by several PODs were detected at the same time points (Fig. 5c and d). Therefore, the IE2-containing budding structures that initiate from the periphery of PODs appear to coalesce to make larger structures that are themselves still flanked by PODs. It should be noted that budding structures were not initiated from all IE2 POD-associated domains, suggesting that localization of IE2 at the periphery of PODs is not itself sufficient for initiation of the budding structures. At 72 h after infection, the bulk of the IE2 protein in most cells had accumulated into usually just two separate nuclear globular structures (Fig. 5e) that were presumably in the process of enlarging and assembling into the fully developed kidney-shaped viral DNA replication compartments that nearly completely fill the nucleoplasm by 96 h (Fig. 3).
FIG. 5.
Double-label IFA images demonstrating that formation of viral DNA replication compartments initiates from the periphery of PODs. Double-label IFA images for IE2 and PML representing sequential intermediate stages in formation of early replication compartments are shown. HF cells were infected with IE1-defective mutant HCMV(CR208) at an MOI of 5.0 and fixed in methanol at 48 h (a to d) or 72 h (e) after infection. IE2 was detected with MAb 12E2 (FITC; green), and PODs were detected with PAb PML(C) (rhodamine; red). To produce merge images, each fluorochrome was recorded and the superimposed images were generated with Image-Pro software.
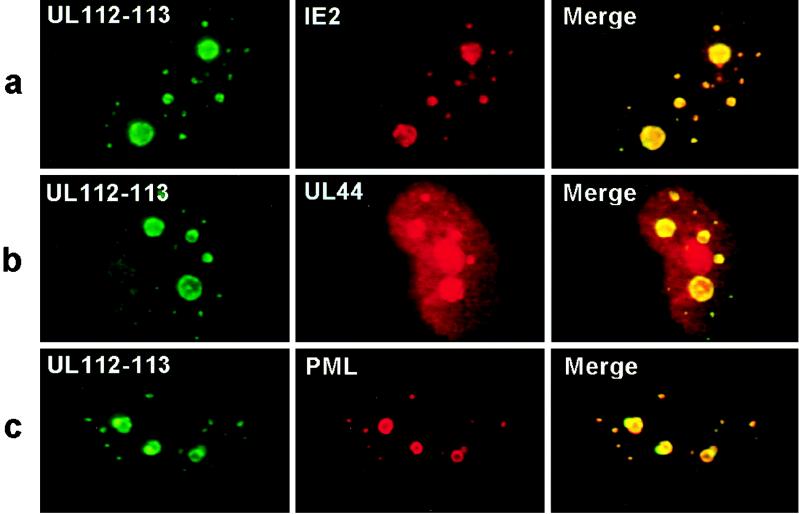
To investigate whether these intermediate-stage nuclear budding structures containing IE2 also contain other viral replication or accessory proteins, double-label IFA for UL112-113 and IE2, for UL112-113 and UL44, or for UL112-113 and PML was performed on ΔIE1-infected HF cells fixed at 48 h (Fig. 6). Again both IE2 and UL112-113 proved to be colocalized either in small punctate bodies or within the larger granular structures (Fig. 6a). UL44 was distributed as a mixture of nuclear diffuse and punctate forms, and the punctate forms were colocalized with UL112-113 (Fig. 6b). Importantly, both the UL112-113 nuclear structures budding from the periphery of PODs and POD-associated small punctate bodies that were detected by IE2 staining (Fig. 5) were also detected by double-label staining for UL112-113 and PML (Fig. 6c). These results demonstrate that the nuclear budding structures containing IE2 that initiate from the periphery of PODs also contain viral DNA replication proteins, including UL44 and UL112-113, and represent initial stages in assembly of viral DNA replication compartments.
FIG. 6.
Double-label IFA images for UL112-113 with either IE2, UL44, or PML at intermediate stages of replication compartment formation. HF cells were infected with HCMV(CR208) and fixed at 48 h after infection as described for Fig. 5. For detection of UL112-113 (a to c), rabbit PAb and FITC-labeled donkey anti-rabbit IgG were used; for detection of IE2 (a), UL44 (b), and PML (c), mouse MAbs 12E2 (for IE2), ABI44 (for UL44), and 5E10 (for PML) and rhodamine-coupled donkey anti-mouse IgG were used. Merge images were obtained as described for Fig. 5.
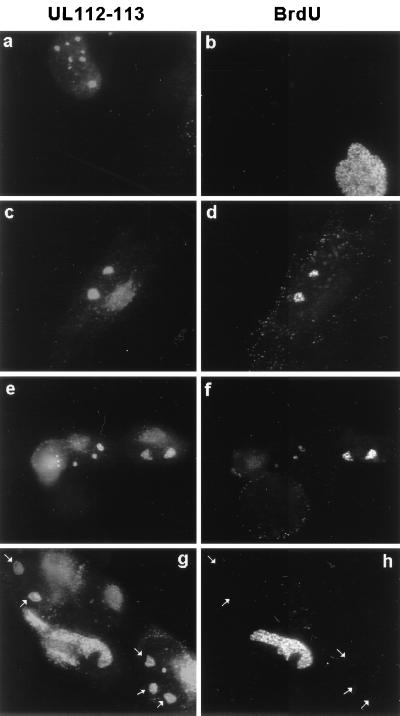
To confirm that the large globular structures containing viral proteins that were detected at 48 h in HCMV(CR208/ΔIE1)-infected cells (Fig. 5 and 6) are also observed in wild-type virus-infected cells, and to show that viral DNA synthesis occurs in these structures, HCMV(Towne)-infected cells were pulse-labeled with BrdU and double-label IFA for both UL112-113 and BrdU was performed at 48 h. In most cells showing two or three large globular structures containing UL112-113, the newly incorporated BrdU signals were also colocalized in the same structures (Fig. 7c to f), although BrdU was not incorporated into cells with several smaller punctate UL112-113 domains, which probably represent earlier stages of infection (Fig. 7a and b). However, interestingly, in some cells showing two or three large globular structures containing UL112-113, BrdU incorporation was not detected in the same structures (Fig. 7g and h). These latter cells may reflect an intermediate stage in which all of the viral replication machinery proteins are not yet assembled sufficiently to allow viral DNA synthesis, or possibly they contain complete assembled pre-replication compartments that lack viral DNA templates.
FIG. 7.
Localization of newly synthesized DNA in intermediate-stage viral DNA replication compartments. HF cells were infected with HCMV(Towne) at a low MOI (<1.0), pulse-labeled with BrdU, and fixed in methanol at 48 h after infection. Double-label IFA for both UL112-113 and BrdU was carried out as described for Fig. 4. Four independent microscopic images are shown. The large granular structures containing UL112-113 but not BrdU are indicated by arrows.
The antipeptide PAb UL112-113(C) detects the intact 84-kDa form of the UL112-113 protein.
The UL112-113 locus expresses four differently spliced polypeptides of 34, 43, 50, and 84 kDa, as shown by immunoblot assays of extracts prepared from HCMV-infected HF cells (53). However, because the antipeptide UL112-113(C) PAb used in our study was raised against the C-terminal 16 amino acids of the UL112-113 transcription unit, it was expected to be specific for only the intact 84-kDa form of UL112-113. To confirm the specificity of the UL112-113(C) PAb, extracts of HF cells infected with HCMV(Towne) were prepared at 6, 24, 48, and 72 h after infection and the proteins were fractionated by SDS-polyacrylamide gel electrophoresis (PAGE) for immunoblot assay. As controls, extracts of Vero cells transfected with either a plasmid expressing 5′ F-tagged UL112-113 or the empty vector alone (pSG5) were also used for immunoblot assay with mouse MAb against the F epitope. The 5′ F tag in the UL112-113 expression vector is expected to be present in all four UL112-113-derived proteins. The Western blot results showed that only the 84-kDa band was specifically detectable with the UL112-113(C) antibody in virus-infected HF cells, whereas all four (34-, 43-, 50-, and 84-kDa) forms of UL112-113 were detected with anti-F antibody in DNA-transfected Vero cells (Fig. 8A). Consistent with previous observations (53), the 84-kDa UL112-113 protein was synthesized within 6 h and increased from early to late times during infection, and the 43-kDa UL112-113 protein was the most abundant among the four related protein products even in DNA-transfected cells.
To compare the distribution pattern of the 84-kDa version of UL112-113 to those of the smaller proteins encoded by the UL112-113 locus by IFA, Vero cells were transfected with plasmid DNA expressing the 5′ F-tagged version of UL112-113. In this case, the UL112-113 products detected by anti-F antibody were distributed as a mixture of both nuclear diffuse and typical small punctate patterns (Fig. 8B) as well as some punctate structures that were larger than those detected with the anti-UL112-113(C) PAb in infected cells. However, double-label IFA revealed that the staining pattern of the 84-kDa form of UL112-113 detected here with UL112-113(C) PAb was exactly the same as that detected by the MAb for the F epitope (Fig. 8C). Therefore, although the pattern in overexpressed transient assays contrasts somewhat with the small punctate pattern only as observed for the 84-kDa form in HF cells infected at a low MOI (Fig. 1c and d; Fig. 4d), the results suggest that all four forms may have the property of targeting to the PODs.
UL112-113 recruits the UL44 polymerase processivity factor into POD-associated sites in DNA transfection assays in the presence of core replication complex components.
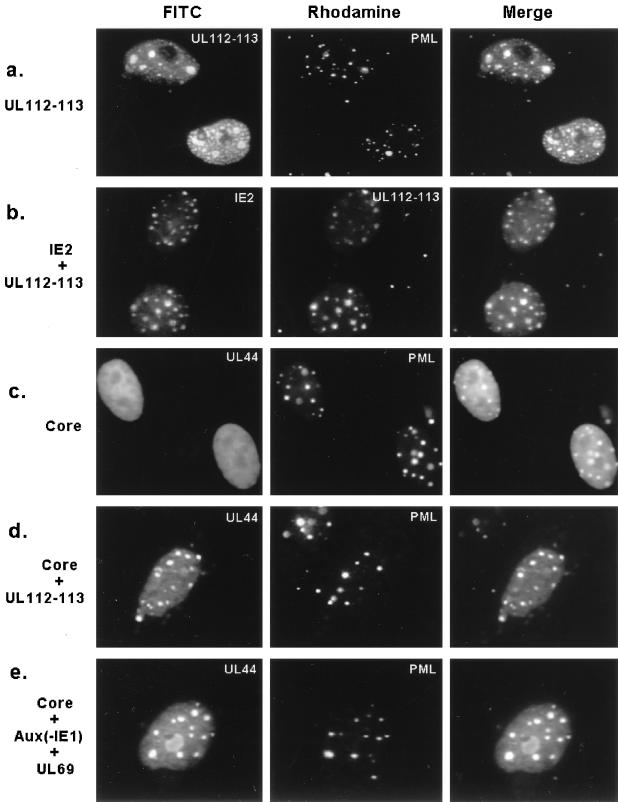
The data presented above demonstrated that at least the 84-kDa form of UL112-113 associates with PODs at 6 h in virus-infected cells and later accumulates at the periphery of PODs together with IE2. To further investigate the association of the UL112-113 protein with PODs, Vero cells were transiently transfected with a plasmid expressing the 5′ F-tagged version of UL112-113 and double labeled for the F epitope and endogenous PML. The results confirmed that both the small punctate and larger globular patterns of UL112-113 were associated with PODs (Fig. 9a) and also demonstrated that even overexpressed UL112-113 product associated with PODs in the absence of other viral proteins. Because IE2 also colocalizes with PODs in transient DNA transfection assays (3), and both IE2 and UL112-113 are colocalized adjacent to PODs at 6 h as well as incorporated into viral DNA replication compartments at 72 h in virus-infected cells as described above, we also examined the distribution of the two proteins when transfected together in Vero cells. As expected, both proteins proved to colocalize within the same punctate and globular structures that presumably correspond to PODs (Fig. 9b).
FIG. 9.
Role of UL112-113 in recruiting core replication proteins to the PODs. Double-label IFA images were obtained after the following combinations of plasmids were cotransfected into Vero cells: (a) F-tagged UL112-113 encoded by pJHA309; (b) IE2 in pMP18 and untagged UL112-113 in pRTS26; (c) the six HCMV core replication machinery proteins UL44 in pRTS22, UL54 in pRTS6, UL57 in pRTS7, UL70 in pRTS9, UL102 in pRTS29, and UL105 in pRTS10; (d) the six core machinery proteins and UL112-113 in pRTS26; and (e) the six core machinery proteins, five auxiliary replication-promoting factors UL36-38 in p302, IRS1 in pRTS18, IE2 in pMP18, UL112-113 in pRTS26, and UL84 in pRTS5, as well as the stimulatory factor UL69 in pRTS28. At 48 h after transfection, the cells were fixed with paraformaldehyde and double labeled as indicated in each panel. To detect F-tagged UL112-113 (a), IE2 (b), and UL44 (c to e), mouse MAbs and FITC-labeled donkey anti-mouse IgG were used. To detect PML (a, c, d, and e) and UL112-113 (b), rabbit PAbs and rhodamine-coupled anti-rabbit IgG were used. Merge images were obtained as described for Fig. 5.
Both IE2 and UL112-113 are expressed at very early times in virus-infected cells and are targeted to POD or to POD-associated domains before the replication fork proteins are synthesized. Therefore, we also examined whether expression of either IE2 or UL112-113 might affect the localization patterns of core replication machinery proteins in transient cotransfection assays. A mouse MAb against the HCMV polymerase processivity factor UL44 was used to track the localization and assembly of the replication proteins. As expected, UL44 was distributed as a uniform nuclear diffuse pattern in Vero cells when transfected alone, and this diffuse pattern was not affected in cells cotransfected with a plasmid expressing IE2 alone (not shown). Similarly, when all six viral core replication machinery proteins (polymerase, UL44, SSB, and the triplex helicase-primase complex) were coexpressed in Vero cells in the absence of the oriLyt plasmid or any accessory proteins, UL44 was still distributed as a typical nuclear diffuse form in all positive cells (Fig. 9c). However, when the same six viral core replication machinery proteins were expressed together with UL112-113, the distribution of UL44 was changed to include typical punctate patterns in a majority of the cells, and double labeling revealed that the reorganized punctate forms of UL44 were colocalized with endogenous PML in the PODs (Fig. 9d). Importantly, this alteration in the distribution of the replication proteins did not occur when they were all cotransfected together with IE2 (not shown). Similarly, even when all six core replication machinery proteins plus UL112-113 and all of the other auxiliary components including UL36-38, IRS1, IE2, UL84 and UL69 (but omitting IE1) were cotransfected together, UL44 was still very efficiently accumulated into POD-associated sites (Fig. 9e). Obviously, not all of these proteins are expected to be expressed in all cotransfected cells, but the results demonstrated that in the context of at least some of the other core replication machinery proteins, UL44 (and presumably the other replication fork complex proteins also) are recruited efficiently into POD-associated sites in the presence of UL112-113.
To evaluate whether all or only some of the core replication complex proteins are required for this altered localization pattern, UL44 and UL112-113 were cotransfected together with various subsets of the six core genes (not shown). The results revealed that although UL112-113 had a small effect on UL44 alone (12% of the doubly expressing cells showing punctate POD-associated UL44 patterns), the effect was greatly enhanced in the presence of either SSB (UL57) alone, polymerase (UL54) alone, or the three helicase-primase complex proteins together, with in each case more than 80% of the UL112-113- and UL44-coexpressing cells showing colocalization in the PODs. Although appropriate antibodies are not available for determination of whether only some or all of the other core replication proteins are similarly recruited to the PODs in infected or cotransfected cells, we conclude that UL112-113 plays an important role in viral DNA replication compartment formation at early stages during virus infection by at least recruiting the UL44 component of the viral DNA replication fork complex into the IE2-containing domains at the periphery of PODs.
DISCUSSION
We have demonstrated here that there is a close physical association in HCMV-infected cells between the subnuclear compartmentalization of viral DNA replication proteins, the virus-encoded early nuclear proteins IE2 and UL112-113, and the punctate nuclear domains containing the cellular tumor suppressor protein PML. As an extension of the concept developed by Ishov and Maul of PODs being deposition sites for foreign DNA (19), they now also appear to be sites for selective accumulation of specific viral proteins involved in both IE transcription and the assembly of functionally active viral DNA synthesis machinery. Considering that both the IE2 and UL112-113 proteins independently accumulate at or adjacent to PODs and that the viral core replication protein complex can also be recruited to POD-associated sites in the presence of UL112-113 but in the absence of viral DNA, it seems clear that there is much more involved in the targeting to PODs than just the deposition of foreign DNA. In fact, these results raise questions about whether indeed the viral DNA goes to PODs in the absence of viral proteins and whether viral proteins might also be involved in specifically recruiting the viral DNA to the PODs. Overall, there now appears to be little doubt that the PODs serve as a framework for formation and assembly of the protein complexes involved in initiation of both IE transcription and viral DNA synthesis. While it is also known that one of the two types of early prereplication foci in HSV is associated with PODs (28, 30, 52, 57), only the connection between IE RNA synthesis and PODs has been addressed previously in HCMV infection systems (20).
Both of the major lytic cycle DNA binding transcription factors IE175 (ICP4) of HSV and Zta of Epstein-Barr virus are known to be efficiently incorporated into viral DNA replication compartments (24, 45, 51). Therefore, it is not surprising that IE2 of HCMV also behaves in this way. However, whether IE2 does so only because of its presumed role in late transcription, as is expected to be the case for IE175, or whether it has dual direct roles in both transcription and DNA replication as for Zta (13) is not known. Nevertheless, there are some clear differences in the behavior of IE2 and IE175. First, IE2 but not IE175 is partially recruited to PODs even in the absence of other viral components in DNA-transfected cells. Second, IE175 associates with functionally active HSV DNA replication compartments but not with prereplication foci in infected cells, and even though it is still recruited to assembled replication compartments in the absence of viral origin sequences in the cotransfection system, IE175 is excluded from them in the presence of PAA (24, 45, 57). In contrast, IE2 is closely associated with early pre-replication structures in HCMV-infected cells that do not yet incorporate BrdU. Even the role of IE2 in IE transcription domains that lie adjacent to PODs at very early times in infected cells is not known to be mimicked by IE175, although this point has not been addressed directly in HSV-infected cells.
Because both HSV and HCMV utilize a second viral IE protein (i.e., IE110/ICP0 or IE1) to target to and disrupt the association of PML with PODs, which occurs before IE2 targets there and before the prereplication foci form (3, 11, 32), many questions arise about the possible functional connections between the disruption of PODs and the assembly of replication complexes. Again the two systems differ in that in the presence of HSV IE110 the SUMO-1 modification of PML and SP100 is abolished and PML is degraded (10), whereas in the presence of HCMV IE1, PML and SP100 are merely displaced from the PODs and there is no effect on amounts or modified forms of PML in wild-type virus-infected cells (3). A recent report has suggested that transfection with IE1 leads to SUMO-1 removal from PML (34a). However, we have found that SUMO-1 is also merely displaced into a nuclear diffuse form together with PML in both HCMV-infected HF cells and in most IE1-transfected Vero cells, although a loss of both the PML and SUMO-1 IFA signals does occur in a subset of highly overexpressing transfected cells (4a). In both cases, infection in the absence of either IE110 or IE1 still allows progression of the lytic cycle, including formation of viral DNA replication compartments (especially at a high MOI) (15, 46, 50). This point was exploited in our studies using an IE1 deletion mutant virus to allow detection of the colocalization or close association of several HCMV proteins with PODs that still contained PML, which obviously cannot be followed with wild-type virus. Reasonable conclusions from all of this are that (i) it is the underlying cellular structures that contain PML and not PML itself (or SP100) that provides the initial structural framework for assembly of both the HCMV IE complexes and the HCMV early DNA replication foci; (ii) the presence or absence of PML in the PODs is not itself essential for either of these processes, although it may profoundly affect their efficiency; and (iii) in the case of HCMV but not of HSV, PML and SP100 may still be available outside of the PODs for some other functional purpose.
Most of our experiments (especially those with wild-type virus) were carried out at a low MOI and therefore also raise an interesting question: when a particular single growing replication compartment is flanked by three or four distinct PODs, could each have contained an associated original input parental viral DNA molecule, or do some early progeny DNA molecules also take up stations at secondary PODs? Alternatively, some PODs may become active participants in these processes without having any associated viral DNA molecules. The latter seems most likely because although the number of early globular pre-replication structures at 24 to 48 h is usually reduced to only 2 or 3 per cell, the number of IE2- and UL112-113-positive PODs (usually more than 10 per cell) at earlier times before DNA synthesis commences probably exceeds the number of input parental viral DNA molecules present. This interpretation assumes that only those DNA molecules from infectious virions enter the nucleus, although this point is unproven and the fate and functional competence of input DNA molecules from the large excess of noninfectious particles present in all herpesvirus virion preparations are currently unknown. Certainly, it is also clear that not all PODs accumulate IE2 or UL112-113 proteins and not all IE2-positive PODs participate in the apparent budding out of assembling pre-replication machinery protein complexes.
Obviously, although segments of both the N- and C-terminal domains of IE2 are needed for its association with PODs, we do not know yet what causes IE2 first to move into adjacent sites next to PODs in infected cells (compared to colocalizing with PODs in transfected cells) and then to assemble with viral DNA replication compartments. Certainly we can state from analysis of the deletion mutants that the DNA binding activity of IE2 is not required for POD association in transfection experiments. These results also seem to imply that the truncated 40-kDa form of IE2 that is synthesized at late times after infection (39, 43, 49) should not be able to target to PODs, although the status of its association with viral DNA replication compartments is unknown. We should also point out in this regard that we have mapped the epitope recognized by the 12E2 IE2-specific MAb to between IE2 amino acids 99 to 136, and therefore only the intact 86-kDa IE form of IE2 and not the 40-kDa form was being detected in these experiments.
The colocalization experiments also clearly show that at 6 h in infected cells the IE2 and UL112-113 proteins both exactly colocalize with each other but that both lie adjacent to or surrounding PODs rather than exactly colocalizing with them. Therefore, what do these adjacent structures represent? Ishov et al. called the IE2-positive POD-associated structures IE transcription domains, because IE mRNA accumulated in some of them and then extended into larger adjacent spliceosome domains (20). However, as described above, most of these structures in our experiments at low MOI probably do not contain the input parental viral DNA templates that would be needed for IE transcription. Recall that in transient single transfections, both viral proteins on their own exactly colocalize with PML in PODs. We believe that these sites adjacent to the PODs actually represent a separate set of distinct subnuclear domains that have not previously been characterized but that touch PODs and may also bridge the PODs to the splicesome domains. Furthermore, these are not specific for infected cells but must preexist in uninfected cells. Our evidence for this comes from observations with a third HSV-encoded IE nuclear protein known as IE68 (or ICP22). In contrast to IE1, IE2, IE110, and UL112-113, this viral protein forms another set of discrete punctate domains in both HSV-infected and DNA-transfected cells that do not colocalize with SC35 spliceosomes or PODs but often touch the PODs (56). Furthermore, when HCMV IE2 and HSV IE68 are transfected together, most IE2- and IE68-specific domains are found to be touching each other (data not shown).
Overall, we conclude that in both HSV and HCMV, the assembly of viral DNA replication compartments consists of complex, stage-specific processes that are intimately connected with specific subnuclear domains and compartments, especially those that are defined by the presence of PML. Some of the detailed events, including the pattern and timing of assembly as well as the nature of the accessory viral proteins involved, differ dramatically in the two systems, but there are also many close parallels, including separate recruitment of the input viral DNA genomes and the viral core replication machinery proteins to POD-associated sites and the involvement of the principal viral IE DNA binding transcription factors. Obviously, many details of the specific protein-protein and DNA-protein interactions between viral and cellular components involved in recruitment and assembly remain to be defined, but studies of intranuclear compartmentalization at the single cell level have now clearly linked three previously disparate areas of HCMV research involving targeting to and disruption of PODs, IE transcription events, and the initiation and assembly of viral DNA replication compartments.
ACKNOWLEDGMENTS
This study was funded by Public Health Service research grant RO1 AI24576 to G.S.H. from the National Institute for Allergy and Infectious Diseases.
We thank Dolores Ciufo for rabbit PAbs against PML and IE68, and we thank Robert Sarisky for plasmids expressing HCMV replication proteins. Generous gifts of plasmids for PML from Ronald M. Evans (The Salk Institute, San Diego, Calif.) and MAb 5E10 from K. van der Krann (Universiteit Van Amsterdam) are greatly acknowledged. We are also grateful to Edward S. Mocarski (Stanford University, Stanford, Calif.) for a gift of samples of the IE1-deleted CR208 virus and its parent, HCMV(Towne). We also thank Mike Delannoy (Department of Cell Biology, Johns Hopkins School of Medicine) for assistance with the confocal microscopy analysis and Sarah Heaggans for help in preparation of the manuscript.
REFERENCES
- 1.Ahn J-H, Brignole III E J, Hayward G S. Disruption of PML subnuclear domains by the acidic IE1 protein of human cytomegalovirus is mediated through interaction with PML and may modulate a RING finger-dependent cryptic transactivator function of PML. Mol Cell Biol. 1998;18:4899–4913. doi: 10.1128/mcb.18.8.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn J-H, Chiou C-J, Hayward G S. Evaluation and mapping of the DNA binding and dimerization domains of the IE2 regulatory protein of human cytomegalovirus using yeast one and two hybrid interaction assays. Gene. 1997;210:25–36. doi: 10.1016/s0378-1119(98)00056-0. [DOI] [PubMed] [Google Scholar]
- 3.Ahn J-H, Hayward G S. The major immediate-early proteins IE1 and IE2 of human cytomegalovirus colocalize with and disrupt PML-associated nuclear bodies at very early times in infected permissive cells. J Virol. 1997;71:4599–4613. doi: 10.1128/jvi.71.6.4599-4613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn, J.-H., and G. S. Hayward. Disruption of PML-associated nuclear bodies by IE1 is required for efficient early stages of viral gene expression and replication in human cytomegalovirus infection. Submitted for publication. [DOI] [PubMed]
- 4a.Ahn, J.-H., and G. S. Hayward. Unpublished data.
- 5.Anders D G, Kacica M A, Pari G, Punturieri S M. Boundaries and structure of human cytomegalovirus oriLyt, a complex origin for lytic-phase DNA replication. J Virol. 1992;66:3373–3384. doi: 10.1128/jvi.66.6.3373-3384.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Britt W J, Alford C A. Cytomegalovirus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2493–2523. [Google Scholar]
- 7.Challberg M. A method for identifying the viral genes required for herpesvirus DNA replication. Proc Natl Acad Sci USA. 1986;83:9094–9098. doi: 10.1073/pnas.83.23.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Bruyn Kops A, Knipe D M. Formation of DNA replication structures in herpes virus-infected cells requires a viral DNA binding protein. Cell. 1988;65:857–868. doi: 10.1016/0092-8674(88)90141-9. [DOI] [PubMed] [Google Scholar]
- 9.Ertl P F, Powell K L. Physical and functional interaction of human cytomegalovirus DNA polymerase and its accessory protein (ICP36) expressed in insect cells. J Virol. 1992;66:4126–4133. doi: 10.1128/jvi.66.7.4126-4133.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everett R D, Freemont P, Saitoh H, Dasso M, Orr A, Kathoria M, Parkinson J. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J Virol. 1998;72:6581–6591. doi: 10.1128/jvi.72.8.6581-6591.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everett R D, Maul G G. HSV-1 IE protein Vmw110 causes redistribution of PML. EMBO J. 1994;13:5062–5069. doi: 10.1002/j.1460-2075.1994.tb06835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fixman E D, Hayward G S, Hayward S D. trans-acting requirements for replication of Epstein-Barr virus ori-Lyt. J Virol. 1992;66:5030–5039. doi: 10.1128/jvi.66.8.5030-5039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fixman E D, Hayward G S, Hayward S D. Replication of Epstein-Barr virus oriLyt: lack of a dedicated virally encoded origin-binding protein and dependence on Zta in cotransfection assays. J Virol. 1995;69:2998–3006. doi: 10.1128/jvi.69.5.2998-3006.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fortunato E A, Spector D H. p53 and RPA are sequestered in viral replication centers in the nuclei of cells infected with human cytomegalovirus. J Virol. 1998;72:2033–2039. doi: 10.1128/jvi.72.3.2033-2039.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greaves R F, Mocarski E S. Defective growth correlates with reduced accumulation of a viral DNA replication protein after low-multiplicity infection by a human cytomegalovirus ie1 mutant. J Virol. 1998;72:366–379. doi: 10.1128/jvi.72.1.366-379.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green S, Issemann I, Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988;16:369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamzeh F, Lietman P, Gibson W, Hayward G S. Identification of the replication origin of human cytomegalovirus by a novel approach utilizing ganciclovir-induced chain termination. J Virol. 1990;64:6184–6195. doi: 10.1128/jvi.64.12.6184-6195.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang L L, Zhu Y, Anders D G. The variable 3′ ends of a human cytomegalovirus oriLyt transcript (SRT) overlap an essential, conserved replicator element. J Virol. 1996;70:5272–5281. doi: 10.1128/jvi.70.8.5272-5281.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishov A M, Maul G G. The periphery of nuclear domain 10 (ND10) as site of DNA virus deposition. J Cell Biol. 1996;134:815–826. doi: 10.1083/jcb.134.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishov A M, Stenberg R M, Maul G G. Human cytomegalovirus immediate early interaction with host nuclear structures: definition of an immediate early transcript environment. J Cell Biol. 1997;138:5–16. doi: 10.1083/jcb.138.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iskenderian A C, Huang L, Reilly A, Stenberg R M, Anders D G. Four of eleven loci required for transient complementation of human cytomegalovirus DNA replication cooperate to activate expression of replication genes. J Virol. 1996;70:383–392. doi: 10.1128/jvi.70.1.383-392.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwayama S, Yamamoto T, Furuya T, Kobayashi R, Ikuta K, Hirai K. Intracellular localization and DNA-binding activity of a class of viral early phosphoproteins in human fibroblasts infected with human cytomegalovirus (Towne strain) J Gen Virol. 1994;75:3309–3318. doi: 10.1099/0022-1317-75-12-3309. [DOI] [PubMed] [Google Scholar]
- 23.Kemble G W, McCormick A L, Pereira L, Mocarski E S. A cytomegalovirus protein with properties of herpes simplex virus ICP8: partial purification of the polypeptide and map position of the gene. J Virol. 1987;61:3143–3151. doi: 10.1128/jvi.61.10.3143-3151.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knipe D M, Senechek D, Rice S A, Smith J L. Stages in the nuclear association of the herpes simplex virus transcriptional activator protein ICP4. J Virol. 1987;61:276–284. doi: 10.1128/jvi.61.2.276-284.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LaFemina R L, Hayward G S. Replicative forms of human cytomegalovirus DNA with joined termini are found in permissively infected human cells but not in nonpermissive Balb/c-3T3 mouse cells. J Gen Virol. 1983;64:373–389. doi: 10.1099/0022-1317-64-2-373. [DOI] [PubMed] [Google Scholar]
- 26.LaFemina R L, Pizzorno M C, Mosca J D, Hayward G S. Expression of the acidic nuclear immediate-early protein (IE1) of human cytomegalovirus in stable cell lines and its preferential association with metaphase chromosomes. Virology. 1989;172:584–600. doi: 10.1016/0042-6822(89)90201-8. [DOI] [PubMed] [Google Scholar]
- 27.Liptak L M, Uprichard S L, Knipe D M. Functional order of assembly of herpes simplex virus DNA replication proteins into prereplication site structures. J Virol. 1996;70:1759–1767. doi: 10.1128/jvi.70.3.1759-1767.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lukonis C J, Burkham J, Weller S K. Herpes simplex virus type 1 prereplication sites are a heterogeneous population: only a subset are likely to be precursor to replication compartments. J Virol. 1997;71:4771–4781. doi: 10.1128/jvi.71.6.4771-4781.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lukonis C J, Weller S K. Characterization of nuclear structures in cells infected with herpes simplex virus type 1 in the absence of viral DNA replication. J Virol. 1996;70:1751–1758. doi: 10.1128/jvi.70.3.1751-1758.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lukonis C J, Weller S K. Formation of herpes simplex virus type 1 replication compartments by transfection: requirements and localization to nuclear domain 10. J Virol. 1997;71:2390–2399. doi: 10.1128/jvi.71.3.2390-2399.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masse M J O, Karlin S, Schachtel G A, Mocarski E. Human cytomegalovirus origin of DNA replication (oriLyt) resides within a highly complex repetitive region. Proc Natl Acad Sci USA. 1992;89:5246–5250. doi: 10.1073/pnas.89.12.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maul G G, Everett R D. The nuclear location of PML, a cellular member of the C3HC4 zinc-binding domain protein family, is rearranged during herpes simplex virus infection by the C3HC4 viral protein ICP0. J Gen Virol. 1994;75:1223–1233. doi: 10.1099/0022-1317-75-6-1223. [DOI] [PubMed] [Google Scholar]
- 33.McVoy M A, Adler S P. Human cytomegalovirus DNA replicates after early circulization by concatemer formation, and inversion occurs within the concatemer. J Virol. 1994;68:1040–1051. doi: 10.1128/jvi.68.2.1040-1051.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mocarski E S., Jr . Cytomegaloviruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2447–2492. [Google Scholar]
- 34a.Müller S, Dejean A. Viral immediate-early proteins abrogate the modification by SUMO-1 of PML and Sp100 proteins, correlating with nuclear body disruption. J Virol. 1999;73:5137–5143. doi: 10.1128/jvi.73.6.5137-5143.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pari G S, Anders D G. Eleven loci encoding trans-acting factors are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA replication. J Virol. 1993;67:6979–6988. doi: 10.1128/jvi.67.12.6979-6988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pari G S, Kacica M A, Anders D G. Open reading frames UL44, IRS1/TRS1, and UL36-38 are required for complementation of human cytomegalovirus oriLyt-dependent DNA synthesis. J Virol. 1993;67:2575–2582. doi: 10.1128/jvi.67.5.2575-2582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Penfold M E T, Mocarski E S. Formation of cytomegalovirus DNA replication compartment defined by localization of viral proteins and DNA synthesis. Virology. 1997;239:46–61. doi: 10.1006/viro.1997.8848. [DOI] [PubMed] [Google Scholar]
- 38.Pizzorno M C, Hayward G S. The IE2 gene products of human cytomegalovirus specifically down-regulate expression from the major immediate-early promoter through a target sequence located near the cap site. J Virol. 1990;64:6154–6165. doi: 10.1128/jvi.64.12.6154-6165.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pizzorno M C, Mullen M-A, Chang Y-N, Hayward G S. The functionally active IE2 immediate-early regulatory protein of human cytomegalovirus is an 80-kilodalton polypeptide that contains two distinct activator domains and a duplicated nuclear localization signal. J Virol. 1991;65:3839–3852. doi: 10.1128/jvi.65.7.3839-3852.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pizzorno M C, O’Hare P, Sha L, LaFemina R L, Hayward G S. trans-activation and autoregulation of gene expression by the immediate-early region 2 gene products of human cytomegalovirus. J Virol. 1988;62:1167–1179. doi: 10.1128/jvi.62.4.1167-1179.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plachter B, Nordin M, Wirgart B Z, Mach M, Stein H, Grillner L, Jahn G. The DNA-binding protein P52 of the human cytomegalovirus reacts with monoclonal antibody CCH2 and associates with the nuclear membrane at late times after infection. Virus Res. 1992;24:265–276. doi: 10.1016/0168-1702(92)90123-q. [DOI] [PubMed] [Google Scholar]
- 42.Prichard M N, Jairath S, Penfold M E T, St. Jeor S, Bohlman M C, Pari G S. Identification of persistent RNA-DNA hybrid structures within the origin of replication of human cytomegalovirus. J Virol. 1998;72:6997–7004. doi: 10.1128/jvi.72.9.6997-7004.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puchtler E, Stamminger T. An inducible promoter mediates abundant expression from the immediate-early 2 gene region of human cytomegalovirus at late times after infection. J Virol. 1991;65:6301–6306. doi: 10.1128/jvi.65.11.6301-6306.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quinlan M, Chen L B, Knipe D M. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell. 1984;36:857–868. doi: 10.1016/0092-8674(84)90035-7. [DOI] [PubMed] [Google Scholar]
- 45.Randall R E, Dinwoodie N. Intranuclear localization of herpes simplex virus immediate-early and delayed-early proteins: evidence that ICP4 is associated with progeny virus DNA. J Gen Virol. 1986;67:2163–2177. doi: 10.1099/0022-1317-67-10-2163. [DOI] [PubMed] [Google Scholar]
- 46.Sacks W R, Shaffer P A. Deletion mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein ICP0 exhibit impaired growth in cell culture. J Virol. 1987;61:829–839. doi: 10.1128/jvi.61.3.829-839.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarisky R T, Hayward G S. Evidence that the UL84 gene product of human cytomegalovirus is essential for initiating oriLyt-dependent DNA replication and formation of replication compartments in cotransfection assays. J Virol. 1996;70:7398–7413. doi: 10.1128/jvi.70.11.7398-7413.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47a.Sarisky, R. T., and G. S. Hayward. Unpublished data.
- 48.Smith J A, Pari G S. Expression of human cytomegalovirus UL36 and UL37 genes is required for viral DNA replication. J Virol. 1995;69:1925–1931. doi: 10.1128/jvi.69.3.1925-1931.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stenberg R M, Depto A S, Fortney J, Nelson J A. Regulated expression of early and late RNAs and proteins from the human cytomegalovirus immediate-early gene region. J Virol. 1989;63:2699–2708. doi: 10.1128/jvi.63.6.2699-2708.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stow N D, Stow E C. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J Gen Virol. 1986;67:2751–2785. doi: 10.1099/0022-1317-67-12-2571. [DOI] [PubMed] [Google Scholar]
- 51.Takagi S, Takada K, Sairenji T. Formation of intranuclear replication compartments of Epstein-Barr virus with redistribution of BZLF1 and BMRF1 gene products. Virology. 1991;185:309–315. doi: 10.1016/0042-6822(91)90778-a. [DOI] [PubMed] [Google Scholar]
- 52.Uprichard S, Knipe D M. Assembly of herpes simplex virus replication proteins at two distinct intranuclear sites. Virology. 1997;229:113–125. doi: 10.1006/viro.1996.8430. [DOI] [PubMed] [Google Scholar]
- 53.Wright D A, Spector D H. Posttranscriptional regulation of a class of human cytomegalovirus phosphoproteins encoded by an early transcription unit. J Virol. 1989;63:3117–3127. doi: 10.1128/jvi.63.7.3117-3127.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu C A, Nelson N J, McGeoch D J, Challberg M D. Identification of herpes simplex virus type 1 genes required for origin-dependent DNA synthesis. J Virol. 1988;62:435–443. doi: 10.1128/jvi.62.2.435-443.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamamoto T, Suzuki S, Radsak H, Hirai K. The UL112/113 gene products of human cytomegalovirus which colocalize with viral DNA in infected cell nuclei are related to efficient viral DNA replication. Virus Res. 1998;56:107–114. doi: 10.1016/s0168-1702(98)00032-x. [DOI] [PubMed] [Google Scholar]
- 56.Zhong, L., D. Ciufo, C. M. J. ap Rhys, J.-H. Ahn, and G. S. Hayward. The herpes simplex virus IE68 (ICP22) immediate-early protein is associated with novel nuclear punctate structures and causes the disruption of both cellular PML oncogenic domains and spliceosome domains. Submitted for publication.
- 57.Zhong L, Hayward G S. Assembly of complete, functionally active herpes simplex virus DNA replication compartments and recruitment of associated viral and cellular proteins in transient cotransfection assays. J Virol. 1997;71:3146–3160. doi: 10.1128/jvi.71.4.3146-3160.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]