Abstract
Spinal cord injury (SCI) is a primary lesion of the spinal cord that results from external forces or diseases, accompanied by a cascade of secondary events. Nitric oxide, an endogenous gas that functions as a signaling molecule in the human body, plays a crucial role in vasodilation of smooth muscles, regulation of blood flow and pressure, and inflammatory response. This article provides a comprehensive overview of the involvement of nitric oxide in SCI and highlights recent advances in basic research on pharmacological agents that inhibit nitric oxide elevation after SCI, offering valuable insights for future therapeutic interventions targeting SCI.
Keywords: nitric oxide; spinal cord injury; inflammation; inducible nitric oxide synthase; endothelial nitric oxide synthase; cyclic guanosine 3′,5′-monophosphate; reactive oxygen species; methylprednisolone
INTRODUCTION
Spinal cord injury (SCI) is a pathological condition that results from damage to the spinal cord and associated tissues, leading to profound consequences.1 It may result in deficiency of motor, sensory, and autonomic functions.2 The prevalence of SCI is higher in males, and its peak incidence occurs within a few minutes after the occurrence of the injury. Following SCI, rapid swelling of the spinal cord occurs, leading to complete occupation of the spinal canal diameter at the level of injury. When cord swelling surpasses venous blood pressure, secondary ischemia is observed. Consequently, autoregulation of blood flow ceases, and neurogenic shock ensues in the spinal cord, which causes systemic hypotension that further exacerbates ischemia. Furthermore, release of toxic chemicals and transfer of electrolytes from damaged nerve membranes can trigger cascading reactions that result in secondary damage, which significantly amplifies the initial mechanical trauma by affecting or killing neighboring cells.3
Functioning as a gaseous chemical messenger, nitric oxide (NO) serves as a prominent endogenous molecule involved in various physiological processes within the central nervous system.4 NO plays a pivotal role in the regulation of interneuron communication, cerebral blood flow, memory formation, synaptic plasticity, intracellular signal transmission, and neurotransmitter release.5 NO is generated through the stereospecific oxidation of the guanidino group of L-arginine by a family of enzymes known as NO synthases (NOS). NOS has been classified into three isoforms based on their location: NOS-I, also known as neuronal or brain NOS (nNOS); NOS-II, also known as inducible NOS (iNOS); and NOS-III, also known as endothelial NOS (eNOS). nNOS and eNOS are expressed constitutively as constitutive NOS, whereas iNOS is induced in response to inflammatory processes.6,7 NO in the human brain has several key effects. First, it serves as a pivotal signaling molecule that triggers the activation of cyclic guanosine 3′,5′-monophosphate (cGMP), thereby inducing vasodilation in vascular smooth muscles. Second, NO mediates the impact of glutamate on N-methyl-D-aspartate receptors to finely regulate both blood flow and pressure, with its functionality being reliant on eNOS within cerebral and coronary vessels.8 Third, short-lived reactive species, such as dinitrogen trioxide, nitroxyl, and peroxynitrite (ONOO−/ONOOH), are generated to serve as sources of free radicals for oxidation–reduction reactions, thereby facilitating the direct oxidation of lipids, proteins, and DNA.9 Furthermore, as a pivotal mediator of diverse inflammatory responses, it exerts regulatory control over the intricate network of inflammation.10
After SCI occurs, the elevation of NO levels leads to the overexpression of iNOS. NO can induce neuronal loss and oxidative damage by reacting with reactive oxygen species (ROS). Furthermore, excessive NO production may result in protein nitrosylation, lipid peroxidation, and functional impairment of associated proteins. The generation of NO, peroxynitrite, and nitrated proteins rapidly increases following acute SCI. Depending on their quantity and microenvironmental conditions, these molecules play a crucial role in both destructive and reparative processes that ensue after acute SCI.11 However, other constitutive forms of NOS, such as eNOS and nNOS, may produce NO, which is neuroprotective in the case of SCI.12 In this study, we investigated the potential mechanisms underlying NO pathways following SCI, with a focus on pathological and biological reactions. Furthermore, this article provides an overview of the recent drugs and methods used to regulate NO in animal SCI models. A comprehensive understanding of these potential mechanisms can offer valuable guidance and assistance for future treatments of SCI. This paper searched PubMed for articles related to NO and SCI in the past 30 years, mainly in the past 3–5 years.
NITRIC OXIDE SYNTHESIS AFTER SPINAL CORD INJURY BASED ON THE TIME COURSE
Although constitutive NOS is constitutively expressed in the central nervous system to maintain cellular homeostasis by producing NO, iNOS is predominantly expressed in various cell types under pathological conditions, such as inflammation, immune response, and trauma. The induction of iNOS requires the presence of several inflammatory cytokines, including interferon-γ, tumor necrosis factor-alpha (TNF-α), and interleukin (IL)-1β.13 However, interpreting reports on changes in NO levels after SCI and their pathophysiological consequences poses challenges. Cherian et al.14 devised a method for categorizing the immediate- (< 30 minutes) and late-stage (> 6 hours) changes following SCI for the analysis of constitutive NOS and iNOS. Thirty minutes after SCI, the level of NO in the injured spinal cord is approximately three-fold higher than that in the uninjured spinal cord. During this time frame, significant metabolic and hemodynamic abnormalities manifest within the spinal cord. SCI triggers widespread depolarization, leading to a substantial increase in the extracellular concentration of potassium and excitatory toxic amino acids, while intracellular calcium accumulates.15 The activity of nNOS exhibits a similar pattern to the concentration of NO in the early stages, with an increase observed at 5 minutes after injury and subsequent return to the baseline level after 1 hour, followed by sustained increases for several consecutive days. The activation of iNOS in the later stages elucidates the delayed NO surge occurring within 24 hours to a few days following injury.15 In contrast, the expression of iNOS mRNA is specifically observed in the injured tissue within 2 hours after SCI, with its levels gradually increasing over consecutive days. The subsequent activation of iNOS during the later stages elucidates the delayed NO surge occurring between 24 hours and a few days following injury.16
NITRIC OXIDE/CYCLIC GUANOSINE 3',5'-MONOPHOSPHATE PATHWAY RELATED TO THE PATHOLOGY OF SPINAL CORD INJURY
The involvement of activated soluble guanylyl cyclases, the generation of cGMP, and the activity of cGMP-dependent protein kinases have been demonstrated to play a pivotal role in the NO signal transduction pathway within the central nervous system.17,18 Morris demonstrated that the superficial layers of the dorsal horn in the spinal cord of neonatal rats represent the primary site of the NO/cGMP pathway (Figure 1).19
Figure 1.
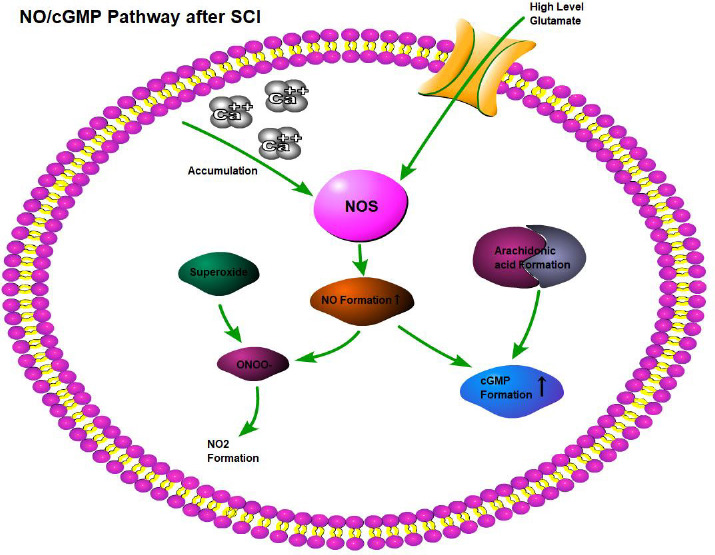
NO is an endogenous gas that acts as a signal molecule in the human body.
Note: NO is produced by L-arginine and oxygen under the action of NOS. Created with BioRender.com. cGMP: Cyclic guanosine 3′,5′-monophosphate; NO: nitric oxide; NOS: nitric oxide synthase; SCI: spinal cord injury.
Elevated cGMP levels in the dorsal horn of the spinal cord appear to be associated with nociceptive sensitization induced by the activation of cGMP-dependent protein kinase Iα.20 Other factors may also impact NO-mediated cGMP formation in SCI. The elevated levels of glutamate generated at the site of spinal cord lesions and excessive accumulation of intracellular Ca2+ can stimulate NOS activity,21 creating suitable conditions for NO synthesis and causing adverse effects on the white matter. The extreme production of NO and superoxide may stimulate the production of nitrite peroxide (ONOO−), which in turn may be decomposed, thus obtaining nitrogen dioxide and a compound with similar hydroxyl reactivity, leading to the oxidation of proteins, DNA, and membranes, damaging lipid peroxidation. Furthermore, free fatty acids released from SCI, particularly arachidonic acid,22 may lead to the overexpression of NOS, accompanied by an increase in cGMP in cells.23
The NO/cGMP pathway also plays a complex and pivotal role in pain modulation following SCI. SCI-induced neuronal cell discharge triggers the generation of NO, which diffuses to satellite glial cells. This, in turn, stimulates the synthesis of cGMP within satellite glial cells, leading to their activation and subsequent alterations that contribute to neuronal hyperexcitability and nociceptive signaling.24 Sensitization of peripheral pain is initiated by the release of inflammatory mediators from the injured tissues. In the context of inflammatory pain, NO can be derived from resident cells or newly migrated cells, such as neutrophils and monocytes, with neutrophil-derived NO potentially contributing to the induction of inflammatory pain and tissue damage. However, Spiller proposed that the activation of the soluble guanylyl cyclase/cGMP/protein kinase G pathway could lead to the activation of the ATP-sensitive potassium channel (KATP). This activation results in intracellular potassium expulsion, leading to hyperpolarization and directly blocking acute and persistent hyperalgesia. Several studies have demonstrated that the NO/cGMP pathway influences the analgesic effects of certain drugs used for neuropathic pain treatment,25,26,27 such as tramadol, clonidine gabapentin, and indomethacin.28 Furthermore, NO activates guanylate cyclase and enhances the synthesis of cGMP, which plays a crucial role in the regulation of muscle relaxation and synaptic transmission. These findings provide valuable insights into enhancing motor function and alleviating bladder spasm following SCI.
FUNCTIONS OF ENDOTHELIAL NITRIC OXIDE SYNTHASE THROUGH THE SER-1177 AND THR-495 PHOSPHORYLATION PATHWAY AFTER SPINAL CORD INJURY
Osuka et al.29 demonstrated that severe SCI leads to the degradation of eNOS and nNOS, whereas mild SCI results in the phosphorylation of eNOS at Ser-1177 for 1–2 days followed by a subsequent decrease in its levels. Phosphorylation and activation of eNOS at Ser-1177, predominantly expressed in the endothelial cells of microvessels within the gray matter, were indicated by an increase in the intensity of nicotinamide adenine dinucleotide phosphate-dependent flavotransferase immunoreactivity within the intact gray matter endothelium 1 day after SCI.30 eNOS expression was significantly elevated 1 and 14 days after spinal cord transection injury, as reported by Varziri et al.31 The phosphorylation of eNOS at Ser-1177 and Thr-495 has been demonstrated to be regulated by multiple protein kinases, including cyclic adenosine monophosphate-dependent kinase and adenosine monophosphate-activated protein kinase. Notably, phosphorylation at Ser-1177 plays a crucial role in activating eNOS.32 In contrast, phosphorylation at Thr-495 inhibits eNOS activity. The phosphorylation of eNOS is a determinant outcome after cerebral ischemia.33 NO from eNOS has neuroprotective effects and can induce dilation of surrounding blood vessels after cerebral ischemia.34 After brain injury, it plays a crucial role in safeguarding the cerebral blood flow. The phosphorylation of eNOS also contributes to the inhibition of platelet aggregation in penumbral microvessels by acting on N-methyl-D-aspartate receptors.35
The infiltration of leukocytes within the parenchyma36 and proliferation of smooth muscle cells.37 Meanwhile, Estévez et al.38 reported that NO produced by NOS can greatly inhibit apoptotic neuronal death. In the subacute stage following SCI, eNOS is generally activated, increasing blood flow, and shows potential involvement in protective and reparative responses via phosphorylation pathways.
RESPONSE OF INFLAMMATION AND INDUCIBLE NITRIC OXIDE SYNTHASE ACTIVATION
The characteristics of inflammatory responses following SCI encompass infiltration of inflammatory cells and release of inflammatory mediators, which enhance blood vessel permeability, resulting in edema and disruption of the blood-brain barrier.39 The inflammatory response following SCI primarily arises from the infiltration of neutrophils at the injury site. Neutrophils can release various substances, including ROS, free radicals of reactive nitrogen species, cytokines, chemokines, and diverse enzymes.40 The activity and accumulation of neutrophils are regulated by various cytokines, including TNF-α, IL-1, and IL-6, which are maintained through the intermediate transcriptional process known as nuclear factor kappa B (NF-κB). NF-κB is typically inactivated in the cytoplasm and is associated with inhibitor of NF-κB (IκB) as an endogenous inhibitor protein. In response to inflammatory stimulation and certain physical factors (e.g., depolarization, bleeding, hyperglycemia, and shear stress) commonly observed in SCI, NF-κB is activated. These biochemical and physical factors may induce the activation of IκB kinase, leading to the phosphorylation of IκB kinase that subsequently triggers the activation of NF-κB.41 NF-κB functions as a transcriptional regulator for the synthesis of iNOS.42 Consequently, iNOS generates substantial amounts of NO, leading to cellular damage. Following SCI, the infiltration of leukocytes increases ROS and NO levels. Central nervous system cells exposed to NO and ROS undergo oxidative stress induced by ROS and nitrogen-responsive species, which can result in pathological alterations in various biological substrates, such as lipid peroxidation. In summary, iNOS is activated within inflamed tissues10 and actively participates in the inflammatory response, and the use of iNOS inhibitors can effectively impede this reaction.
In conclusion, the phosphorylation-mediated involvement of eNOS in SCI may contribute to protective and reparative responses. Extracorporeal shock wave therapy has been reported to enhance angiogenesis and growth factor expression by activating eNOS and vascular endothelial growth factor, thereby promoting revascularization following SCI.43 The same objective can be accomplished through future drug development. The involvement of NO and cGMP in peripheral nociception following SCI remains contentious. Their protective effects during the initial hours after inflammation and their cytotoxic effects during the subsequent progression of inflammatory pain are widely acknowledged, contingent on varying concentrations of NO produced, dosage of NOS modulators, and the location and stage of the pathological process. Extensive clinical trials are required to establish the optimal timing and concentration for usage; iNOS plays a pivotal role in the inflammatory response after SCI, offering insights into the development of novel anti-inflammatory drugs.28
SPINAL CORD INJURY THERAPY
The pathophysiology of SCI encompasses primary injury and a cascade of secondary events. The inflammatory response plays a pivotal role in controlling neuronal damage and regeneration after SCI, making it a crucial component of secondary injury. Therefore, attenuating the early inflammatory response may limit tissue lesions and functional deficits. In terms of treatment modalities, medical intervention is probably the most expeditious approach for reducing the degree of spinal cord damage. Despite numerous studies conducted on this subject, no consensus protocol has been established. Methylprednisolone (MP) remains the sole U.S. Food and Drug Administration-approved drug for SCI treatment, with administration within 8 hours being recommended. However, confidence in the validity of the results has diminished following the adoption of a high-dose MP regimen based on the findings of the National Acute Spinal Cord Injury Study II and III. Note that MP is ineffective in patients with spinal cord rupture, whereas those with mild injuries can recover spontaneously without requiring MP treatment.44 However, caution must be exercised because high-dose MP may lead to pulmonary and gastrointestinal complications; elderly patients are particularly susceptible to respiratory complications and infection. Another steroid agent, dexamethasone, may offer benefits in cases that involves metastatic spinal cord compression by reducing inflammation and preventing further nerve damage; however, significant controversy exists regarding the therapeutic dosage.45
Recently, research on the inhibition of NO production to reduce injury after SCI has emerged as a prominent area of investigation, offering potential avenues for SCI treatment and inflammatory response mitigation. This field encompasses various approaches, including Chinese herbal medicine, clinical drugs, exosomes, hormones, and nerve electrical stimulation. Some interventions act by inhibiting specific targets, such as mitogen-activated protein kinases. Furthermore, these studies have identified novel pathways associated with the pathogenesis of SCI, such as IL-6 receptor/Janus kinase-signal transducer and activator of transcription and miR-146a-5p/G-protein-coupled receptor 17.46,47 Most importantly, many interventions facilitate body recovery and enhance motor function by attenuating NO-mediated oxidative stress.
Herbal medicine
Fu et al.48 observed a reduction in iNOS expression and nitrite levels in resveratrol-treated rats, providing evidence for the antioxidant role of resveratrol in scavenging free radicals and its ability to inhibit the iNOS/p38 mitogen-activated protein kinase signaling pathway. Xu et al.49 reported that the mechanism of action of resveratrol may involve scavenging free radicals through meta-analysis, reducing levels of malondialdehyde, and increasing superoxide dismutase levels. This process could inhibit neuronal apoptosis and enhance neurological function recovery in a rat SCI model.49 Lv et al.50 found that a single administration of resveratrol (20 or 40 mg/kg) could significantly improve the motor function of rats with SCI, inhibit the expression of iNOS, and reduce the production of NO and inflammatory factors (e.g., IL-1β, IL-6, and TNF-α) in the spinal cord tissue. It reduces the activation of NLRP3 (NOD-, LRR- and pyrin domain-containing 3) inflammasome, thus alleviating the inflammatory response of microglia and playing a neuroprotective role.50
SCI increases the activation of mitogen-activated protein kinase at the injury site. Quercetin mitigates oxidative stress by inhibiting mitogen-activated protein kinase and reducing the downstream generation of free radicals following NO-mediated cell damage.51 The level of NO could be reduced by icariin through the inhibition of the iNOS/NO signaling pathway. Furthermore, pretreatment with Ganoderma lucidum polysaccharide effectively mitigated inflammation and oxidative stress, significantly decreasing NO levels compared with those in the control group.52
L-theanine is derived from glutamate and ethylamine found in green tea. This chemical undergoes absorption by the gut before being distributed into the brain through the bloodstream. In rats, it enhances functional recovery following SCI by effectively scavenging excessive free radicals, subsequently inhibiting inflammation and subsequent apoptosis.53 Paeoniflorin, the primary bioactive compound derived from peony root, exhibits neuroprotective properties by modulating the NF-κB signaling pathway. Through the downregulation of iNOS expression, paeoniflorin enhances motor function recovery and promotes cell viability in rats.54 The administration of dihydrotanshinone effectively suppressed the expression of NO in rats following SCI, significantly reducing iNOS levels. These findings highlight the potent antioxidant activity of dihydrotanshinone in SCI-induced rats.55
Tetramethylpyrazine, an alkaloid derived from Ligusticum chuanxiong, inhibits NO production and reduces the inflammatory response in peripheral tissues. Lin et al.56 developed a nanoparticle system modified with a human immunodeficiency virus transcription transactivator that effectively enhances the delivery of tetramethylpyrazine to spinal cord tissue, ensuring optimal safety profiles while promoting neural function recovery in rats. Fisetin exerts its neuroprotective potential by modulating the NF-κB/IκBα pathway, suppressing the release of the inflammatory mediator iNOS, and reducing ectopic pain and hyperalgesia in experimental SCI.57 The hydrophilic bile acid ursodeoxycholic acid, which has been used in traditional Chinese herb medicine for over 3000 years, exerts its anti-inflammatory effects by attenuating the expression of iNOS through the inhibition of phosphorylation signals, including extracellular regulated kinase, JNK, and p38. Consequently, this modulation reduces the proinflammatory response and facilitates functional recovery in rats with SCI. Furthermore, ursodeoxycholic acid exhibits a certain degree of anti-inflammatory activity.58
Micro-RNA
After SCI, the levels of miR-92b-5p, IL-18, and IL-18 binding protein decreased, whereas the release of the classical inflammatory factors iNOS, TNF-α, and IL-1β increased. Following the administration of an miR92b-5p inhibitor in mice with SCI, miR-92b-5p levels were downregulated, which resulted in the alleviation of the inflammatory response through the effective action of IL-18 binding protein. Inhibition of miR-92b-5p effectively suppressed the effects of IL-18, mitigated its inflammatory impact, and preserved neuronal integrity by reducing interference from IL-18 binding protein after SCI.59 Experimental evidence confirms that miR-34a can alleviate SCI by inhibiting the expression of high mobility group box 1 in the Toll-like receptor 4 signaling pathway using an in vitro SCI model. Inhibition of miR-34a expression exacerbates inflammation, suppresses cell proliferation, induces cell apoptosis, and upregulates iNOS protein expression and NO levels. MiR-34a alleviates SCI by reducing the production of inflammatory cytokines by inhibiting high mobility group box 1 expression in the Toll-like receptor 4 signaling pathway.60 Upregulation of miR-223-5p following SCI is implicated in the immune-inflammatory response. Inhibition of miR-223-5p significantly upregulated the expression of the neurotrophic factor neuregulin-1, leading to the suppression of neuronal apoptosis and glial scar formation, thereby promoting neuronal repair and improving neurological function.61
G-protein-coupled receptor 17 is one of the three genes specifically expressed in adult hippocampal neuronal progenitors and plays a crucial role in brain repair processes. It has been demonstrated that decreased expression of miR-146a-5p in the spinal cord reduces neuropathic pain.62 Bioinformatic analysis revealed that G-protein-coupled receptor 17 3′-untranslated region contains a binding site for miR-146a-5p. He et al.47 further confirmed that G-protein-coupled receptor 17 is negatively regulated by miR-146a-5p as its target. By regulating the expression of miR-146a-5p/G-protein-coupled receptor 17, Zhenbao pills can inhibit neuronal apoptosis, significantly reduce iNOS expression, attenuate inflammation levels, and promote spinal cord function recovery.47
After SCI, miR-21 expression significantly increases in vivo. Inhibition of miR-21 effectively suppressed inflammation, reduced iNOS generation, and facilitated recovery from SCI by downregulating the IL-6 receptor/Janus kinase-signal transducer and activator of transcription signaling pathway.46 The application of exosomes derived from human umbilical cord mesenchymal stem cells represents a promising therapeutic strategy for treating SCI. These human umbilical cord mesenchymal stem cell-derived exosomes, characterized by an average particle size of 70 nm, effectively induce the polarization of the M1 to M2 phenotype in bone marrow-derived macrophages. In vivo studies have shown that intravenous administration of human umbilical cord mesenchymal stem cell-derived exosomes following SCI reduces the levels of inflammatory factors, including iNOS, TNF-α, macrophage inflammatory protein-1alpha, IL-6, and interferon-γ, thereby promoting functional recovery.63,64,65 Overall, this approach exhibits anti-inflammatory effects and enhances motor function restoration after SCI.66
Metal-organic framework and exosomes
Metal–organic framework nanomaterials are a network structure formed by coordination bonds between metal ions and organic ligands. Because of their controllable pore size, biodegradability, and adjustable size, metal–organic framework nanomaterials have garnered increasing attention in the field of biomedicine, particularly in sustained drug release. Exosomes have emerged as promising nanocarriers for drug delivery and targeted therapy. As an alternative to stem cell therapy, exosomes are natural membrane vesicles (50–150 nm) derived from various cells, including mesenchymal stem cells. Exosomes carry proteins, lipids, and genetic materials that reflect their cellular origin; they facilitate intercellular communication and induce diverse biological effects both locally and remotely. Guo et al.67 demonstrated that intranasally administered exosomes derived from mesenchymal stem cells can cross the blood-brain barrier and migrate to the injured spinal cord region. When loaded with phosphatase and tensin homologous small interfering RNA, exosomes derived from mesenchymal stem cells can effectively reduce microgliosis and astrogliosis while mitigating the inflammatory response caused by NO, ultimately leading to significant functional recovery in rats with complete SCI.67
Clinical drugs
Trehalose, a nonreducing disaccharide synthesized by various organisms, including bacteria, yeast, fungi, insects, and plants, confers protection against environmental stresses, such as high temperature, freezing, dehydration, and oxidation, to ensure stability. Following injury in rats, trehalose significantly reduced NO levels 4 hours and 1, 3, and 7 days after injury with percentages of 47%, 59%, 70%, and 35%, respectively. Moreover, trehalose inhibited NO synthesis in macrophage-like cells treated with hemolysate. These findings highlight the neuroprotective potential of trehalose through the attenuation of post-traumatic cytokines and reactive substances.68
Topiramate, a widely used antiepileptic and antimigraine medication, decreased eNOS and iNOS levels following oral administration of topiramate at a dosage of 40 mg/kg with four doses administered every 12 hours. This observation suggests that these two enzymes collectively contribute to the pathophysiology of SCI; however, further investigations are required to elucidate the mechanism underlying topiramate-induced reduction in NOS levels.69
Resolvins represent a novel class of endogenous specialized pro-resolving mediators that possess specific properties to facilitate the resolution of inflammatory responses. Resolvin D3, which is derived from DHA, has been identified as an inhibitor of inflammatory reactions. In both RAW 264.7 cells and mice, Resolvin D3 exhibited enhanced immunoreactivity toward M2 macrophage markers, which are known for their beneficial effects, while concurrently suppressing the expression of M1 markers associated with detrimental outcomes in humans and rodents. These findings suggest a significant reduction in NO production within macrophages upon polarization toward the M2 phenotype following treatment with Resolvin D3 after SCI.70
The newly discovered polypeptide Apelin-13 plays a crucial neuroprotective role in the central nervous system. In the Apelin-13 pretreatment group, NO and eNOS levels were significantly higher than those in the sham group but significantly lower than those in the model group. Furthermore, the addition of an Apelin-13 inhibitor increased the levels of NO and eNOS. Pretreatment with Apelin-13 effectively mitigated spinal cord ischemia/reperfusion injury, attenuated oxidative stress, and inhibited autophagy in spinal cord tissue.71
iNOS can function as a receptor for c-fiber afferents, leading to urinary muscle hyperexcitation in mice with SCI. Following SCI, iNOS mRNA levels are upregulated in neurons, which is significantly attenuated by treatment with vibegron. These findings suggest that vibegron effectively reduces the inflammatory response along the bladder afferent pathway and improves storage dysfunction following SCI.72
Nicoredil functions as an opener of NO donor and KATP channels, directly activating KATP channels while indirectly stimulating NO production. When administered 4 hours and 3 days before ischemia, nicoredil exhibits the ability to enhance NO levels, resulting in significant preservation of motor function.73
Neovascularization is crucial for the survival of the neural parenchymal tissue. In the healthy spinal cord, the vasculature plays a vital role in providing nutrients and eliminating metabolic waste products, thereby maintaining normal function. The mechanical forces associated with SCI disrupt the vasculature, which results in local hypoxia at the lesion center. Angiogenesis initiates several days after SCI because of hypoxia but significantly decreases approximately 14 days after injury, thus failing to adequately support the SCI repair process. Zhao et al.74 discovered that metformin activates the adenosine monophosphate-activated protein kinase/eNOS pathway, which promotes angiogenesis and facilitates recovery of neurological function following SCI. This novel therapeutic approach offers potential for enhancing angiogenesis and functional restoration.74
Melatonin promotes the transition of microglia/macrophages from an M1 to an M2 state by attenuating iNOS production, thereby facilitating functional recovery and decreasing neuroinflammation following SCI.75
Neural electrical stimulation
Application of low-frequency pulsed electromagnetic fields is a noninvasive therapeutic approach for diverse medical conditions. This approach significantly reduces the levels of ROS and iNOS within the injured spinal cord, thereby exerting preventive effects on inflammation and oxidative stress.76
Others
Intestinal flora dysbiosis can enhance the expression of inflammatory factors, such as iNOS, by activating the Toll-like receptor 4/myeloid differentiation factor 88 signaling pathway, thereby exacerbating SCI.77
Attempts have been made to use stem cells to treat human diseases for decades. The most typical example is the use of stem cell transplantation to treat various malignant or benign blood disorders. At present, this technology has matured and is widely used in the field of hematology, and it has great clinical value. Recently, stem cell treatments have also been developed for various neurological diseases, such as cerebral hemorrhage, ischemic stroke, traumatic brain injury, and subarachnoid hemorrhage. At present, an increasing number of animal experiments and clinical trials have shown that the use of stem cells can play a beneficial role in treating SCI. Stem cell therapy has great potential to rescue damaged tissues and promote the recovery of neurological function.78
Clinical trial
Inflammatory changes and treatment options following SCI have been validated in animal models; however, clinical studies and drug applications are relatively scarce in this area. Some clinical studies have explored the use of pharmacological interventions to mitigate SCI-induced damage and improve motor function, whereas others have focused on pain management after SCI. Corticosteroids have traditionally been used to treat SCI, with intravenous MP therapy being particularly effective in preventing the inflammatory cascade associated with NO-induced secondary SCI.79 The findings from the National Acute Spinal Cord Injury Study III indicate that 24 hours of MP therapy is sufficient for patients initiating treatment within 3 hours of injury onset. However, if treatment starts between 3 and 8 hours after injury, a longer duration of 48-hour MP therapy has shown improved neurological outcomes. Note that this prolonged administration carries an increased risk of infection, including severe pneumonia and sepsis.80
Two studies81,82 observed that Wharton's jelly mesenchymal stem cell-derived exosomes reduced NO content in vitro in 10 patients with chronic complete SCI by intravaginal infusion of expanded Wharton jelly mesenchymal stromal cells and found that compared with placebo, sensation improved in the adjacent segments of the injury site. However, other clinically relevant effects, such as increased maximum bladder volume and compliance and reduced neurogenic bladder hyperactivity and external sphincter synergy, were observed only at the individual level. No changes were observed in the measures of motor function, spasticity, somatosensory and motor evoked potentials intestinal function, quality of life, and independence.83
Within 72 hours of injury, ependymal stem cells/progenitor cells, a type of adult pluripotent stem cells that can differentiate into nerve and glial cells, are activated. These cells then migrate from the central canal of the spinal cord to the injury site. Most ependymal stem cells/progenitor cells differentiate into astrocytes, and a small percentage of these cells differentiate into oligodendrocytes that produce myelin. Astrocytes recruit macrophages, which then restrict axon growth, mediate the inflammatory response, and limit the extent of SCI. In the glial scars that form, inhibitory molecules released by astrocytes account for the majority. Monocyte chemotactic protein-1 directly promotes the recruitment of M1-proinflammatory macrophages to the injury site via the recombinant chemokine C–C motif receptor. These macrophages act by releasing TNF-α and iNOS, which are associated with the inhibition of axon growth in spinal cord neurons. Human embryonic stem cell-derived OPCs can release neurotrophic factors, including neurite growth promotion factor 2 and hepatocyte growth factor, which promote neurite regeneration and extension.84 Fessler et al.85 experimentally confirmed that oligodendrocyte progenitor cells can be safely used in patients with postsubacute cervical SCI. The low-dose temporary immunosuppressive regimen was well tolerated. At 1-year follow-up, 21 (96%) of the 22 patients in the intentional-treatment group had grade 1 or higher neurological function recovery in at least one limb, and 7 (32%) patients had grade 2 or higher neurological function recovery in at least one limb. However, this method relies on intraoperative injection, which is difficult to perform and requires high operation skills.85 Simultaneously, because they are nonautologous cells, they are prone to immune rejection. Chow et al.86 suggested that plasma phosphorylated neurofilament can be used as a response biomarker of the severity of acute SCI and clinical treatment outcomes and analyzed the pharmacokinetics, pharmacodynamics, and effects of lisuzole on axon degradation in patients with traumatic cervical SCI. The safety of rilozole in patients with SCI has been confirmed; however, ideal pharmacodynamics could not be established because of the low enrollment size.86
Other studies have focused on physical or electrical stimulation to improve motor function and pain after SCI.87,88,89,90,91 Electroacupuncture may be an analgesic mechanism that effectively downregulates the function of the L-arginine /NO/cGMP pathway in the spinal cord of rats in a pathological state and reduces central sensitization.92 In patients with SCI, a single exercise and simultaneous functional electrical stimulation may be more effective in generating muscle strength than exercise alone.88,89
LIMITATION
This paper has many shortcomings. First, due to time, resource, and knowledge limitations, investigators may not be able to cover all relevant literature, which may result in some important studies or ideas being missed in the review. Second, only PubMed articles were searched, and no other biomedical databases were involved. Third, the effect of NO on the related pathways and SCI was not discussed in terms of molecular structure.
CONCLUSION
Taken together, there is still a long way to go before existing basic research can be translated into clinical application. Although improvement in muscle or nerve function after SCI has been observed in animals, safety cannot be guaranteed, and validation of clinical trials is lacking. The clinical treatment direction of SCI is currently focused on the early anti-inflammatory stage and the later rehabilitation stage. The effectiveness of some methods has been fully verified; however, there is still a large gap in the recovery of normal function. It is gratifying that the safety of some new drugs or methods has been verified in humans. In the future, it will be necessary to expand the number of research objects based on safety to promote the recovery of neurological function in patients after SCI.
In this study, we conducted a comprehensive literature review to investigate the pivotal role of NO in SCI and elucidated its potential mechanisms, including temporal dynamics. Further investigations are warranted to unravel the neuroprotective and cytotoxic effects of distinct subtypes of NOS and other mediators involved in the modulation of the NO cascade. By adopting this approach, novel therapeutic targets can be identified and subsequently translated into clinical applications for mitigating secondary damage following SCI while enhancing functional recovery. The complete clinical significance of this article lies in successfully translating basic research findings into practical interventions, such as the development of NO inhibitors specifically tailored for treating SCI.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
Editor note: GC is an Editorial Board member of Medical Gas Research. He is blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer review handled independently of this Editorial Board member and his research group.
Data availability statement
No additional data are available.
REFERENCES
- 1.Eli I, Lerner DP, Ghogawala Z. Acute traumatic spinal cord injury. Neurol Clin. 2021;39:471–488. doi: 10.1016/j.ncl.2021.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Karsy M, Hawryluk G. Modern medical management of spinal cord injury. Curr Neurol Neurosci Rep. 2019;19:65. doi: 10.1007/s11910-019-0984-1. [DOI] [PubMed] [Google Scholar]
- 3.Anjum A, Yazid MD, Fauzi Daud M, et al. Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci. 2020;21:7533. doi: 10.3390/ijms21207533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melis MR, Argiolas A. Erectile function and sexual behavior: a review of the role of nitric oxide in the central nervous system. Biomolecules. 2021;11:1866. doi: 10.3390/biom11121866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Gallagher K, Puledda F, O'Daly O, et al. Neuronal nitric oxide synthase regulates regional brain perfusion in healthy humans. Cardiovasc Res. 2022;118:1321–1329. doi: 10.1093/cvr/cvab155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alagarsamy S, Lonart G, Johnson KM. Regulation of nitric oxide synthase activity in cortical slices by excitatory amino acids and calcium. J Neurosci Res. 1994;38:648–653. doi: 10.1002/jnr.490380607. [DOI] [PubMed] [Google Scholar]
- 7.Kiedrowski L, Costa E, Wroblewski JT. Glutamate receptor agonists stimulate nitric oxide synthase in primary cultures of cerebellar granule cells. J Neurochem. 1992;58:335–341. doi: 10.1111/j.1471-4159.1992.tb09315.x. [DOI] [PubMed] [Google Scholar]
- 8.Soda T, Brunetti V, Berra-Romani R, Moccia F. The emerging role of N-methyl-D-aspartate (NMDA) receptors in the cardiovascular system: physiological implications, pathological consequences, and therapeutic perspectives. Int J Mol Sci. 2023;24:3914. doi: 10.3390/ijms24043914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinert JR, Amal H. The contribution of an imbalanced redox signalling to neurological and neurodegenerative conditions. Free Radic Biol Med. 2023;194:71–83. doi: 10.1016/j.freeradbiomed.2022.11.035. [DOI] [PubMed] [Google Scholar]
- 10.Man MQ, Wakefield JS, Mauro TM, Elias PM. Regulatory role of nitric oxide in cutaneous inflammation. Inflammation. 2022;45:949–964. doi: 10.1007/s10753-021-01615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tardivo V, Crobeddu E, Pilloni G, et al. Say “no” to spinal cord injury: is nitric oxide an option for therapeutic strategies? Int J Neurosci. 2015;125:81–90. doi: 10.3109/00207454.2014.908877. [DOI] [PubMed] [Google Scholar]
- 12.LeBaron TW, Kharman J, McCullough ML. An H2-infused, nitric oxide-producing functional beverage as a neuroprotective agent for TBIs and concussions. J Integr Neurosci. 2021;20:667–676. doi: 10.31083/j.jin2003071. [DOI] [PubMed] [Google Scholar]
- 13.Karki R, Sharma BR, Tuladhar S, et al. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2021;184:149–168.e17. doi: 10.1016/j.cell.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherian L, Hlatky R, Robertson CS. Nitric oxide in traumatic brain injury. Brain Pathol. 2004;14:195–201. doi: 10.1111/j.1750-3639.2004.tb00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amar AP, Levy ML. Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery. 1999;44:1027–1039. doi: 10.1097/00006123-199905000-00052. discussion 1039-1040. [DOI] [PubMed] [Google Scholar]
- 16.Chatzipanteli K, Garcia R, Marcillo AE, Loor KE, Kraydieh S, Dietrich WD. Temporal and segmental distribution of constitutive and inducible nitric oxide synthases after traumatic spinal cord injury: effect of aminoguanidine treatment. J Neurotrauma. 2002;19:639–651. doi: 10.1089/089771502753754109. [DOI] [PubMed] [Google Scholar]
- 17.Bredt DS, Snyder SH. Nitric oxide, a novel neuronal messenger. Neuron. 1992;8:3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Robinson PJ. Cyclic GMP-dependent protein kinase and cellular signaling in the nervous system. J Neurochem. 1997;68:443–456. doi: 10.1046/j.1471-4159.1997.68020443.x. [DOI] [PubMed] [Google Scholar]
- 19.Morris R, Southam E, Gittins SR, de Vente J, Garthwaite J. The NO-cGMP pathway in neonatal rat dorsal horn. Eur J Neurosci. 1994;6:876–879. doi: 10.1111/j.1460-9568.1994.tb00998.x. [DOI] [PubMed] [Google Scholar]
- 20.Cornelison LE, Woodman SE, Durham PL. Inhibition of trigeminal nociception by non-invasive vagus nerve stimulation: investigating the role of GABAergic and serotonergic pathways in a model of episodic migraine. Front Neurol. 2020;11:146. doi: 10.3389/fneur.2020.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAdoo DJ, Hughes MG, Xu GY, Robak G, de Castro R., Jr. Microdialysis studies of the role of chemical agents in secondary damage upon spinal cord injury. J Neurotrauma. 1997;14:507–515. doi: 10.1089/neu.1997.14.507. [DOI] [PubMed] [Google Scholar]
- 22.Rehncrona S, Westerberg E, Akesson B, Siesjö BK. Brain cortical fatty acids and phospholipids during and following complete and severe incomplete ischemia. J Neurochem. 1982;38:84–93. doi: 10.1111/j.1471-4159.1982.tb10857.x. [DOI] [PubMed] [Google Scholar]
- 23.Toborek M, Garrido R, Malecki A, et al. Nicotine attenuates arachidonic acid-induced overexpression of nitric oxide synthase in cultured spinal cord neurons. Exp Neurol. 2000;161:609–620. doi: 10.1006/exnr.1999.7308. [DOI] [PubMed] [Google Scholar]
- 24.Hanani M. How is peripheral injury signaled to satellite glial cells in sensory ganglia? Cells. 2022;11:512. doi: 10.3390/cells11030512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiller F, Oliveira Formiga R, Fernandes da Silva Coimbra J, Alves-Filho JC, Cunha TM, Cunha FQ. Targeting nitric oxide as a key modulator of sepsis, arthritis and pain. Nitric Oxide. 2019;89:32–40. doi: 10.1016/j.niox.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Liu S, Wang Z, Zhang Y, Wang K. Hydrogen sulfide attenuates diabetic neuropathic pain through NO/cGMP/PKG pathway and μ-opioid receptor. Exp Biol Med (Maywood) 2020;245:823–834. doi: 10.1177/1535370220918193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding Y, Yao P, Hong T, Han Z, Zhao B, Chen W. The NO-cGMP-PKG signal transduction pathway is involved in the analgesic effect of early hyperbaric oxygen treatment of neuropathic pain. J Headache Pain. 2017;18:51. doi: 10.1186/s10194-017-0760-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shnayder NA, Petrova MM, Popova TE, et al. Prospects for the personalized multimodal therapy approach to pain management via action on NO and NOS. Molecules. 2021;26:2431. doi: 10.3390/molecules26092431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osuka K, Watanabe Y, Takagi T, et al. Activation of endothelial nitric oxide synthase following spinal cord injury in mice. Neurosci Lett. 2008;436:265–268. doi: 10.1016/j.neulet.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 30.Yang JY, Kim HS, Lee JK. Changes in nitric oxide synthase expression in young and adult rats after spinal cord injury. Spinal Cord. 2007;45:731–738. doi: 10.1038/sj.sc.3102036. [DOI] [PubMed] [Google Scholar]
- 31.Vaziri ND, Lee YS, Lin CY, Lin VW, Sindhu RK. NAD(P)H oxidase, superoxide dismutase, catalase, glutathione peroxidase and nitric oxide synthase expression in subacute spinal cord injury. Brain Res. 2004;995:76–83. doi: 10.1016/j.brainres.2003.09.056. [DOI] [PubMed] [Google Scholar]
- 32.Chen ZP, Mitchelhill KI, Michell BJ, et al. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- 33.Michell BJ, Chen Z, Tiganis T, et al. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem. 2001;276:17625–17628. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- 34.Atochin DN, Wang A, Liu VW, et al. The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo. J Clin Invest. 2007;117:1961–1967. doi: 10.1172/JCI29877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radomski MW, Palmer RM, Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987;92:639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kubes P, Granger DN. Nitric oxide modulates microvascular permeability. Am J Physiol. 1992;262:H611–615. doi: 10.1152/ajpheart.1992.262.2.H611. [DOI] [PubMed] [Google Scholar]
- 37.von der Leyen HE, Gibbons GH, Morishita R, et al. Gene therapy inhibiting neointimal vascular lesion: in vivo transfer of endothelial cell nitric oxide synthase gene. Proc Natl Acad Sci U S A. 1995;92:1137–1141. doi: 10.1073/pnas.92.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Estévez AG, Spear N, Thompson JA, et al. Nitric oxide-dependent production of cGMP supports the survival of rat embryonic motor neurons cultured with brain-derived neurotrophic factor. J Neurosci. 1998;18:3708–3714. doi: 10.1523/JNEUROSCI.18-10-03708.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma HS, Winkler T, Stålberg E, Gordh T, Alm P, Westman J. Topical application of TNF-alpha antiserum attenuates spinal cord trauma induced edema formation, microvascular permeability disturbances and cell injury in the rat. Acta Neurochir Suppl. 2003;86:407–413. doi: 10.1007/978-3-7091-0651-8_85. [DOI] [PubMed] [Google Scholar]
- 40.Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods. 1985;14:157–167. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- 41.Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-kappaB in the resolution of inflammation. Nat Med. 2001;7:1291–1297. doi: 10.1038/nm1201-1291. [DOI] [PubMed] [Google Scholar]
- 42.Togashi H, Sasaki M, Frohman E, et al. Neuronal (type I) nitric oxide synthase regulates nuclear factor kappaB activity and immunologic (type II) nitric oxide synthase expression. Proc Natl Acad Sci U S A. 1997;94:2676–2680. doi: 10.1073/pnas.94.6.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fiani B, Davati C, Griepp DW, Lee J, Pennington E, Moawad CM. Enhanced spinal therapy: extracorporeal shock wave therapy for the spine. Cureus. 2020;12:e11200. doi: 10.7759/cureus.11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas AX, Riviello JJ, Jr., Davila-Williams D, et al. Pharmacologic and acute management of spinal cord injury in adults and children. Curr Treat Options Neurol. 2022;24:285–304. doi: 10.1007/s11940-022-00720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Canseco JA, Karamian BA, Bowles DR, et al. Updated review: the steroid controversy for management of spinal cord injury. World Neurosurg. 2021;150:1–8. doi: 10.1016/j.wneu.2021.02.116. [DOI] [PubMed] [Google Scholar]
- 46.Ning SL, Zhu H, Shao J, Liu YC, Lan J, Miao J. MiR-21 inhibitor improves locomotor function recovery by inhibiting IL-6R/JAK-STAT pathway-mediated inflammation after spinal cord injury in model of rat. Eur Rev Med Pharmacol Sci. 2019;23:433–440. doi: 10.26355/eurrev_201901_16852. [DOI] [PubMed] [Google Scholar]
- 47.He Y, Lv B, Huan Y, et al. Zhenbao pill protects against acute spinal cord injury via miR-146a-5p regulating the expression of GPR17. Biosci Rep. 2018;38:BSR20171132. doi: 10.1042/BSR20171132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu S, Lv R, Wang L, Hou H, Liu H, Shao S. Resveratrol, an antioxidant, protects spinal cord injury in rats by suppressing MAPK pathway. Saudi J Biol Sci. 2018;25:259–266. doi: 10.1016/j.sjbs.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu BP, Yao M, Li ZJ, et al. Neurological recovery and antioxidant effects of resveratrol in rats with spinal cord injury: a meta-analysis. Neural Regen Res. 2020;15:482–490. doi: 10.4103/1673-5374.266064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lv R, Du L, Liu X, Zhou F, Zhang Z, Zhang L. Polydatin alleviates traumatic spinal cord injury by reducing microglial inflammation via regulation of iNOS and NLRP3 inflammasome pathway. Int Immunopharmacol. 2019;70:28–36. doi: 10.1016/j.intimp.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 51.Ocal O, Borcek AO, Pasaoglu O, Gundogdu AC, Kaplanoglu GT, Baykaner MK. Can quercetin be an option for treatment of spinal cord injury? An experimental study. Turk Neurosurg. 2019;29:247–253. doi: 10.5137/1019-5149.JTN.23799-18.1. [DOI] [PubMed] [Google Scholar]
- 52.Jia G, Zhang Y, Li W, Dai H. Neuroprotective role of icariin in experimental spinal cord injury via its antioxidant, anti-neuroinflammatory and anti-apoptotic properties. Mol Med Rep. 2019;20:3433–3439. doi: 10.3892/mmr.2019.10537. [DOI] [PubMed] [Google Scholar]
- 53.Yang CC, Chang KC, Wang MH, et al. l-Theanine improves functional recovery after traumatic spinal cord injury in rats. J Formos Med Assoc. 2020;119:1405–1414. doi: 10.1016/j.jfma.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 54.Wang B, Dai W, Shi L, et al. Neuroprotection by paeoniflorin against nuclear factor kappa B-induced neuroinflammation on spinal cord injury. Biomed Res Int. 2018;2018:9865403. doi: 10.1155/2018/9865403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu L, Qian J. Dihydrotanshinone I Alleviates Spinal Cord Injury via Suppressing Inflammatory Response, Oxidative Stress and Apoptosis in Rats. Med Sci Monit. 2020;26:e920738. doi: 10.12659/MSM.920738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin Y, Wan Y, Du X, et al. TAT-modified serum albumin nanoparticles for sustained-release of tetramethylpyrazine and improved targeting to spinal cord injury. J Nanobiotechnology. 2021;19:28. doi: 10.1186/s12951-020-00766-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cui J, Fan J, Li H, Zhang J, Tong J. Neuroprotective potential of fisetin in an experimental model of spinal cord injury: via modulation of NF-κB/IκBα pathway. Neuroreport. 2021;32:296–305. doi: 10.1097/WNR.0000000000001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ko WK, Kim SJ, Jo MJ, et al. Ursodeoxycholic acid inhibits inflammatory responses and promotes functional recovery after spinal cord injury in rats. Mol Neurobiol. 2019;56:267–277. doi: 10.1007/s12035-018-0994-z. [DOI] [PubMed] [Google Scholar]
- 59.Lin XL, Zhu J, Wang LM, Yan F, Sha WP, Yang HL. MiR-92b-5p inhibitor suppresses IL-18 mediated inflammatory amplification after spinal cord injury via IL-18BP up-regulation. Eur Rev Med Pharmacol Sci. 2019;23:1891–1898. doi: 10.26355/eurrev_201903_17226. [DOI] [PubMed] [Google Scholar]
- 60.Zhou J, Shuang O, Li J, Cai Z, Wu C, Wang W. miR-34a alleviates spinal cord injury via TLR4 signaling by inhibiting HMGB-1. Exp Ther Med. 2019;17:1912–1918. doi: 10.3892/etm.2018.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guan YZ, Sun C, Wang HL, et al. MiR-223-5p inhibitor suppresses microglia inflammation and promotes Nrg-1 in rats of spinal cord injury. Eur Rev Med Pharmacol Sci. 2019;23:9746–9753. doi: 10.26355/eurrev_201911_19537. [DOI] [PubMed] [Google Scholar]
- 62.Khan MZ, He L. Neuro-psychopharmacological perspective of Orphan receptors of Rhodopsin (class A) family of G protein-coupled receptors. Psychopharmacology (Berl) 2017;234:1181–1207. doi: 10.1007/s00213-017-4586-9. [DOI] [PubMed] [Google Scholar]
- 63.Sindhu S, Akhter N, Wilson A, et al. MIP-1α expression induced by co-stimulation of human monocytic cells with palmitate and TNF-α involves the TLR4-IRF3 pathway and is amplified by oxidative stress. Cells. 2020;9:1799. doi: 10.3390/cells9081799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao ZS, Zhang CJ, Xia N, et al. Berberine-loaded M2 macrophage-derived exosomes for spinal cord injury therapy. Acta Biomater. 2021;126:211–223. doi: 10.1016/j.actbio.2021.03.018. [DOI] [PubMed] [Google Scholar]
- 65.Wahlund CJE, Güclüler G, Hiltbrunner S, Veerman RE, Näslund TI, Gabrielsson S. Exosomes from antigen-pulsed dendritic cells induce stronger antigen-specific immune responses than microvesicles in vivo. Sci Rep. 2017;7:17095. doi: 10.1038/s41598-017-16609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun G, Li G, Li D, et al. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater Sci Eng C Mater Biol Appl. 2018;89:194–204. doi: 10.1016/j.msec.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 67.Guo S, Perets N, Betzer O, et al. Intranasal delivery of mesenchymal stem cell derived exosomes loaded with phosphatase and tensin homolog siRNA repairs complete spinal cord injury. ACS Nano. 2019;13:10015–10028. doi: 10.1021/acsnano.9b01892. [DOI] [PubMed] [Google Scholar]
- 68.Nazari-Robati M, Akbari M, Khaksari M, Mirzaee M. Trehalose attenuates spinal cord injury through the regulation of oxidative stress, inflammation and GFAP expression in rats. J Spinal Cord Med. 2019;42:387–394. doi: 10.1080/10790268.2018.1527077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Narin F, Hanalioglu S, Ustun H, Kilinc K, Bilginer B. Topiramate as a neuroprotective agent in a rat model of spinal cord injury. Neural Regen Res. 2017;12:2071–2076. doi: 10.4103/1673-5374.221164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim J, Joshi HP, Sheen SH, et al. Resolvin D3 promotes inflammatory resolution, neuroprotection, and functional recovery after spinal cord injury. Mol Neurobiol. 2021;58:424–438. doi: 10.1007/s12035-020-02118-7. [DOI] [PubMed] [Google Scholar]
- 71.Xu Z, Li Z. Experimental study on the role of Apelin-13 in alleviating spinal cord ischemia reperfusion injury through suppressing autophagy. Drug Des Devel Ther. 2020;14:1571–1581. doi: 10.2147/DDDT.S241066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shimizu N, Gotoh D, Nishimoto M, et al. Efficacy of vibegron, a novel β3-adrenoreceptor agonist, for lower urinary tract dysfunction in mice with spinal cord injury. Int J Urol. 2021;28:1068–1072. doi: 10.1111/iju.14630. [DOI] [PubMed] [Google Scholar]
- 73.Ikeno Y, Ghincea CV, Roda GF, et al. Optimizing nicorandil for spinal cord protection in a murine model of complex aortic intervention. Semin Thorac Cardiovasc Surg. 2022;34:28–38. doi: 10.1053/j.semtcvs.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 74.Zhao JY, Sheng XL, Li CJ, et al. Metformin promotes angiogenesis and functional recovery in aged mice after spinal cord injury by adenosine monophosphate-activated protein kinase/endothelial nitric oxide synthase pathway. Neural Regen Res. 2023;18:1553–1562. doi: 10.4103/1673-5374.360245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y, Liu Z, Zhang W, et al. Melatonin improves functional recovery in female rats after acute spinal cord injury by modulating polarization of spinal microglial/macrophages. J Neurosci Res. 2019;97:733–743. doi: 10.1002/jnr.24409. [DOI] [PubMed] [Google Scholar]
- 76.Wang C, Liu Y, Wang Y, et al. Low-frequency pulsed electromagnetic field promotes functional recovery, reduces inflammation and oxidative stress, and enhances HSP70 expression following spinal cord injury. Mol Med Rep. 2019;19:1687–1693. doi: 10.3892/mmr.2019.9820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang Q, Cao L. Intestinal flora and neurological disorders. Sheng Wu Gong Cheng Xue Bao. 2021;37:3757–3780. doi: 10.13345/j.cjb.210253. [DOI] [PubMed] [Google Scholar]
- 78.Gao L, Peng Y, Xu W, et al. Progress in stem cell therapy for spinal cord injury. Stem Cells Int. 2020;2020:2853650. doi: 10.1155/2020/2853650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Falavigna A, Quadros FW, Teles AR, et al. Worldwide steroid prescription for acute spinal cord injury. Global Spine J. 2018;8:303–310. doi: 10.1177/2192568217735804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ameer MA, Tessler J, Munakomi S, Gillis CC. StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. Central cord syndrome. [PubMed] [Google Scholar]
- 81.Song JY, Kang HJ, Ju HM, et al. Umbilical cord-derived mesenchymal stem cell extracts ameliorate atopic dermatitis in mice by reducing the T cell responses. Sci Rep. 2019;9:6623. doi: 10.1038/s41598-019-42964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hazrati A, Soudi S, Hashemi SM. Wharton’s jelly mesenchymal stem cells-derived exosomes and imipenem in combination reduce apoptosis and inflammatory responses in E.coli-infected HepG2 cells. Iran J Allergy Asthma Immunol. 2022;21:273–286. doi: 10.18502/ijaai.v21i3.9801. [DOI] [PubMed] [Google Scholar]
- 83.Albu S, Kumru H, Coll R, et al. Clinical effects of intrathecal administration of expanded Wharton jelly mesenchymal stromal cells in patients with chronic complete spinal cord injury: a randomized controlled study. Cytotherapy. 2021;23:146–156. doi: 10.1016/j.jcyt.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 84.Antonios JP, Farah GJ, Cleary DR, Martin JR, Ciacci JD, Pham MH. Immunosuppressive mechanisms for stem cell transplant survival in spinal cord injury. Neurosurg Focus. 2019;46:E9. doi: 10.3171/2018.12.FOCUS18589. [DOI] [PubMed] [Google Scholar]
- 85.Fessler RG, Ehsanian R, Liu CY, et al. A phase 1/2a dose-escalation study of oligodendrocyte progenitor cells in individuals with subacute cervical spinal cord injury. J Neurosurg Spine. 2022;37:812–820. doi: 10.3171/2022.5.SPINE22167. [DOI] [PubMed] [Google Scholar]
- 86.Chow DS, Nguyen A, Park J, et al. Riluzole in Spinal Cord Injury Study (RISCIS)-Pharmacokinetic (PK) Sub-Study: an analysis of pharmacokinetics, pharmacodynamics, and impact on axonal degradation of riluzole in patients with traumatic cervical spinal cord injury enrolled in the RISCIS phase III randomized controlled trial. J Neurotrauma. 2023;40:1889–1906. doi: 10.1089/neu.2022.0499. [DOI] [PubMed] [Google Scholar]
- 87.Bergmann M, Zahharova A, Ereline J, Asser T, Gapeyeva H, Vahtrik D. Single session exercises and concurrent functional electrical stimulation are more effective on muscles’ force generation than only exercises in spinal cord injured persons: a feasibility study. J Musculoskelet Neuronal Interact. 2020;20:472–479. [PMC free article] [PubMed] [Google Scholar]
- 88.Biktimirov A, Bryukhovetskiy I, Sharma A, Sharma HS. Neuromodulation and quality of life for patient with spasticity after spinal cord injury. Int Rev Neurobiol. 2023;172:79–99. doi: 10.1016/bs.irn.2023.03.003. [DOI] [PubMed] [Google Scholar]
- 89.Guo Y, Gao F, Li J, et al. Effect of electromyographic biofeedback training on motor function of quadriceps femoris in patients with incomplete spinal cord injury: A randomized controlled trial. NeuroRehabilitation. 2021;48:345–351. doi: 10.3233/NRE-201647. [DOI] [PubMed] [Google Scholar]
- 90.Jo HJ, Kizziar E, Sangari S, et al. Multisite hebbian plasticity restores function in humans with spinal cord injury. Ann Neurol. 2023;93:1198–1213. doi: 10.1002/ana.26622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaur J, Ghosh S, Sahani AK, Sinha JK. Mental imagery as a rehabilitative therapy for neuropathic pain in people with spinal cord injury: a randomized controlled trial. Neurorehabil Neural Repair. 2020;34:1038–1049. doi: 10.1177/1545968320962498. [DOI] [PubMed] [Google Scholar]
- 92.Yan LP, Hou BQ, Li SD, Wang LL, Ma C. Electroacupuncture relieved neuropathic pain by suppressing L-Arg/NO/cGMP pathway in the lumbar spinal cord in rats with spared nerve injury. Zhen Ci Yan Jiu. 2019;44:893–897. doi: 10.13702/j.1000-0607.190096. [DOI] [PubMed] [Google Scholar]


