Abstract
Postexposure prophylaxis (PEP) after intravaginal exposure to human immunodeficiency virus (HIV) was investigated using the HIV type 2 (HIV-2)/pig-tailed macaque transmission model. PEP for 28 days with the reverse transcriptase inhibitor (R)-9-(2-phosphonylmethoxypropyl)adenine (PMPA; tenofovir) was initiated 12 to 72 h following HIV-2 exposure. Systemic infection was not evident in the 12- and 36-h groups, as defined by plasma viremia, cell-associated provirus, antibody responses, and lymph node virus. Breakthrough infection in the 72-h group was detected at week 16 post-virus exposure. These results demonstrate for the first time using a vaginal transmission model that early intervention after high-risk sexual exposures may prevent infection.
Reducing exposure to human immunodeficiency virus (HIV) through behavior modification remains the primary and most accepted method for preventing infection. Administering postexposure prophylaxis (PEP) with antiretrovirals following a high-risk sexual exposure to HIV remains controversial (5). Human studies addressing PEP efficacy for sexual exposure remain difficult to implement because of the required sample size for meaningful evaluation as well as ethical considerations regarding placebo control (7, 10). Therefore, only minimal anecdotal clinical evidence of PEP efficacy currently exists.
To date, systematic PEP investigations involving nonhuman primate models have shown promise for preventing infection under certain conditions (1). However, most studies have utilized intravenous (i.v.) exposures with simian retroviruses and, like for studies of human needlestick exposures (3, 4), the validity of extrapolating results to sexual exposures remains unclear. There are important variables related to viral dissemination patterns after a vaginal exposure and the effective window for prophylaxis initiation that may impact PEP efficacy. This investigation represents the first efforts to address the potential efficacy of early antiretroviral prophylaxis following HIV exposure mimicking heterosexual contact, the major mode for worldwide transmission (12).
In this study, 16 naïve female pig-tailed macaques, ages 3 to 5 years and weighing 5.5 to 8.5 kg (Charles Rivers Laboratories, San Antonio, Tex.), were used in accordance with the recommendations of the Centers for Disease Control and Prevention Animal Care and Use Committee. An inoculum of HIV type 2 (HIV-2) strain GB122 (previously shown to infect pig-tailed macaques [18, 19]) was generated by limited propagation of the original AIDS patient isolate onto a mixture of phytohemagglutinin-(p)-activated peripheral blood mononuclear cells (PBMC) from four source macaques. The titer of this challenge stock was determined by standard techniques (8, 9) and consisted of approximately 104 tissue culture infectious doses per ml or at least 102 pig-tailed macaque i.v. infectious doses. All virus exposures involved atraumatic inoculation of cell-free virus into the vaginal pouch via a sterile gastric feeding tube. Anesthetized macaques remained recumbent and were intravaginally inoculated three times over 2 h, with each exposure and absorption period separated by ∼1 h.
Longitudinal blood specimens were collected and processed as described previously (19). Cervicovaginal lavage (CVL) specimens were obtained by instilling 4 ml of sterile phosphate-buffered saline directed at the cervical os. Cervicovaginal cells were separated from CVL supernatants by mild centrifugation (15 min at 400 × g) and whole inguinal lymph node (ILN) biopsy specimens were harvested during the course of study. Routine cell sieving procedures (Cellector tissue sieve; E-C Apparatus, St. Petersburg, Fla.) were used to generate ILN total cell suspensions. Half of each ILN suspension was used to derive a direct cellular lysate for duplicate DNA PCR analysis, while remaining cells were either cocultured for virus isolation or cryopreserved.
Nested DNA PCR was used to detect HIV-2 provirus in PBMC or ILN cells as described previously (19). Virus isolations from ∼107 viable purified PBMC or whole cells recovered from ILN biopsy specimens were attempted using the PM-1 T-cell line (CD4+ X4+ R5+) by standard coculturing techniques (8, 18, 19). Culture supernatants were monitored for core antigen levels through at least 4 weeks. Detection of virus-specific antibody was achieved as described previously (18, 19). Virion-associated HIV-2 RNA (vRNA) was quantitatively measured in plasma or cell-free CVL supernatants by a reverse transcriptase-mediated PCR (RT-PCR) prototype assay system similar in design to that detailed by Mulder et al. (16) and more recently described by Nkengasong et al. (17). Assay sensitivity was determined to be ∼100 copies per ml.
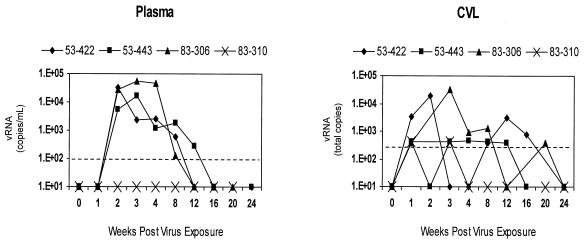
All macaques in this study received three doses (3 ml each) of the HIV-2GB122 stock by the intravaginal route during a 2-h period (total inoculum, ∼105 tissue culture infectious doses), thus producing a high infection rate (75%) in control macaques not receiving PEP (Table 1; Fig. 1). In these animals, rising plasma vRNA levels were observed, with peak viral loads occurring at week 2 or 3 postinoculation (p.i.). Quantifiable levels of vRNA were also observed in cell-free supernatants derived from CVL specimens collected longitudinally from all infected macaques and, in most cases, were the earliest signs of infection. All infected control animals showed a typical acute phase of HIV-2 infection for this species, characterized by early peaks of plasma viremia, early establishment of chronic provirus in PBMC, the ability to readily isolate virus from PBMC (data not shown), and a classic antibody response beginning at 3 or 4 weeks p.i. In most cases, plasma virus became undetectable by week 12 to 16 p.i. Interestingly, macaque 83-310 (classified as exposed, uninfected) had detectable vRNA in CVL specimens only at weeks 1 and 3 p.i. (Fig. 1), with no other indication of productive infection.
TABLE 1.
HIV-2-specific provirus detection in PBMC and serologic status
| Study group and macaque | Detectiona/statusb at wk post-virus exposure
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 8 | 12 | 16 | 20 | 24 | |
| Untreated controls | ||||||||||
| 53-422 | −/− | −/− | +/− | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ |
| 53-443 | −/− | −/− | +/− | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ |
| 83-306 | −/− | −/− | +/− | +/− | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ |
| 83-310 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| 12-h PEP | ||||||||||
| 83-268 | −/− | −/− | −/− | −/− | −c/− | −/− | −/− | −/− | −/− | −/− |
| 83-283 | −/− | −/− | −/− | −/− | −c/− | −/− | −/− | −/− | −/− | −/− |
| 83-308 | −/− | −/− | −/− | −/− | −c/− | −/− | −/− | −/− | −/− | −/− |
| 83-297d | −/− | −/− | −/− | −/− | −c/− | NT | NT | NT | NT | NT |
| 36-h PEP | ||||||||||
| 52-121 | −/− | −/− | −/− | −/− | −c/− | −/− | −/− | −/− | −/− | −/− |
| 83-267 | −/− | −/− | −/− | −/− | −c/− | −/− | −/− | −/− | −/− | −/− |
| 83-269 | −/− | −/− | −/− | −/− | −c/− | −/− | −/− | −/− | −/− | −/− |
| 83-273 | −/− | −/− | −/− | −/− | −c/− | −/− | −/− | −/− | −/− | −/− |
| 72-h PEP | ||||||||||
| 72-34 | −/− | −/− | −/− | −/− | −c/− | −/− | −/− | −/− | −/− | −/− |
| 83-285 | −/− | −/− | −/− | −/− | −c/− | −/− | −/− | −/− | −/− | −/− |
| 83-305 | −/− | −/− | −/− | −/− | −c/− | −/− | −/− | +/+ | +/+ | +/+ |
| 83-303d | −/− | −/− | −/− | NT | NT | NT | NT | NT | NT | NT |
+, nested DNA-PCR amplification and detection of HIV-2 protease gene sequences; −, no HIV-2-specific signal was detected; NT, not tested.
+, confirmed HIV-2-specific seroconversion; −, lack of seroresponse; NT, not tested.
DNA PCRs and virus isolation results were also negative for ILN biopsy specimens.
Longer-term follow-up was not possible due to unanticipated death.
FIG. 1.
HIV-2 load in plasma and CVL specimens through 24 weeks after intravaginal virus exposure in untreated control macaques (n = 4). Plasma vRNA levels are reported as log10 copies per milliliter and virus levels in CVL supernatants are indicated as log10 total copies per lavage specimen. The sensitivity limits are 100 copies/ml for plasma and 400 total copies for CVL supernatants with a 50-μl sample equivalent input into the assay system (dashed line).
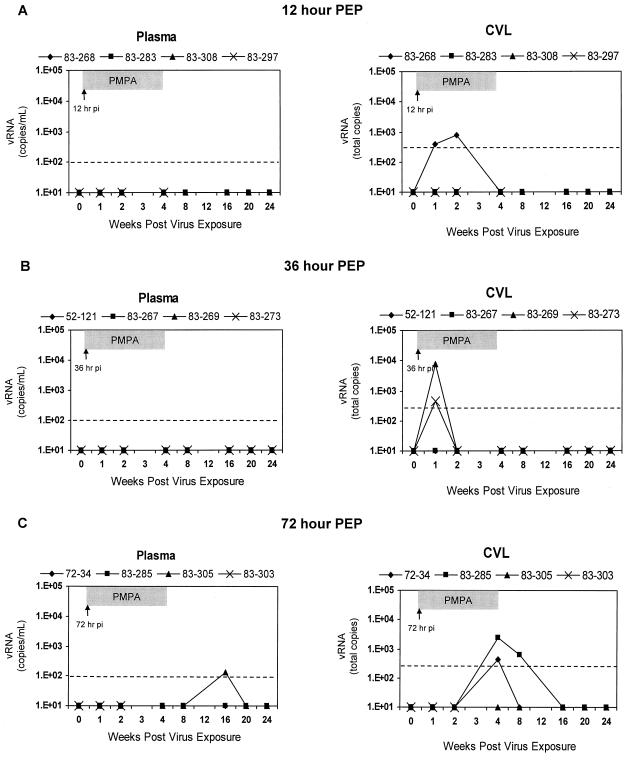
Groups of HIV-2-exposed macaques (n = 4 each) were subsequently treated with (R)-9-(2-phosphonylmethoxypropyl)adenine (PMPA) (tenofovir; Gilead Sciences, Foster City, Calif.) by subcutaneous injection of 30 mg/kg of body weight daily for 28 days, starting 12, 36, or 72 h after the last viral inoculation. None of the four macaques in the 12-h group showed any indication of systemic infection (Fig. 2A). While plasma viremia was not observed, vRNA was observed in early CVL specimens (weeks 1 and 2) from a single macaque in this group. HIV-2 provirus was not detected in PBMC, attempts of virus isolation from PBMC were not successful (data not shown), and virus-specific antibody responses remained negative for each macaque in the 12-h group (Table 1). Also, ILN tissues harvested from each macaque on the final day (day 28) of PMPA treatment were virus negative, as assessed by nested PCR for provirus and virus isolation. One macaque (83-297) in the 12-h group was found dead at week 5 p.i. as a result of circumstances unrelated to any experimental procedure. Postmortem tissues were PCR negative for proviral DNA (not shown).
FIG. 2.
Longitudinal HIV-2 vRNA levels in plasma versus CVL supernatants after intravaginal virus exposure in experimental macaque groups (n = 4 each) receiving PEP with PMPA at the indicated times. Results are reported as in Fig. 1.
Similarly, those macaques (n = 4) receiving PEP at 36 h post-virus exposure were also protected from systemic infection and remained negative by virologic and serologic parameters through 24 weeks p.i. (Table 1; Fig. 2B). Furthermore, ILN specimens collected from all four macaques in the 36-h group were provirus and virus isolation negative. In this group, transient vRNA signals were also observed in early CVL specimens collected from two animals.
Macaques (n = 4) receiving PMPA treatment at 72 h postexposure also showed a lack of systemic infection through the first 12 weeks p.i. by all parameters (Fig. 2C). Once again, vRNA in some CVL specimens was detectable; however, these signals were seen later than in the other treatment groups. One macaque had a low-level signal at week 4 p.i., while another had higher vRNA titers detectable at weeks 4 and 8 p.i. One macaque in the 72-h group (83-303) died at week 2 p.i. from unrelated causes. One confirmed seroconversion in the 72-h group was observed at week 16 p.i., indicating delayed, systemic infection and PEP failure (Table 1). A low level of plasma viremia was detected and circulating PBMC were provirus positive, although vRNA was not observed in CVL specimens. The other two macaques in the 72-h group remained uninfected by all systemic parameters through 6 months post-virus exposure. Further follow-up data, up to 1 year after virus exposure for all macaques receiving PEP, remained identical to those results shown for week 24.
The novel aspects of this experimental design involve (i) the use of intravaginal exposure, (ii) the evaluation of a timing strategy for prophylaxis which would be realistic following a potential high-risk human heterosexual contact, (iii) the use of a human-derived-retrovirus (HIV-2) vaginal transmission model, and (iv) direct assessment of CVL supernatant virus levels. The results demonstrated that early intervention with a potent antiretroviral regimen in response to a vaginal retrovirus exposure significantly reduced the establishment of systemic infection in macaques treated 36 h or sooner after exposure (zero of eight infected) compared with untreated controls (three of four infected; P = 0.018 by Fisher's exact test). However, the regimen did not protect all macaques in which PMPA treatment was initiated 72 h after virus exposure, a finding consistent with previous animal data derived using i.v. exposures to simian immunodeficiency virus (SIV) (1).
Elegant macaque studies (13, 14) have linked the efficiency of vaginal transmission to the extent with which a virus strain replicates in vivo after i.v. inoculation. It still remains unclear as to how much linkage, if any, exists between viral virulence and mucosal transmission efficiencies (20). The intravaginal transmission method detailed in this study served to model high-risk vaginal exposures resulting in an extremely high infection rate (75%) compared with that suggested by human data (0.1 to 0.2% risk per receptive vaginal episode) (11).
The most dramatic protection reported in macaque PEP studies has followed the use of the nucleotide analog RT inhibitor PMPA (23–27). Initiation of PMPA treatment no later than 24 h after i.v. SIV challenge, with continued prophylaxis for 28 days, was the most effective regimen in those studies. Delaying prophylaxis initiation (48 or 72 h postexposure) or shortening treatment to 10 or 3 days reduced PMPA efficacy (23). Different exposure routes and a possible requirement for localized replication prior to systemic dissemination most likely contributed to the differences between the findings of these previous studies and those of our investigation. PMPA treatment following oral SIV exposure in newborns has also been reported (26); however, treatment that was started up to 5 days after inoculation led to alterations in rapid disease course outcomes rather than preventing systemic infection. Furthermore, the optimal effective duration for PEP remains unknown and has not been fully explored (3, 6), even in animal studies. Böttiger et al. (2) demonstrated a dramatic protective effect (12 of 12 macaques) with only a 3-day regimen using another RT inhibitor (BEA-005) when administered 1 to 8 h following i.v. inoculation with SIV.
The lack of evidence for systemic infection until 16 weeks p.i. for the breakthrough animal, even in ILN specimens on the final day of PMPA therapy, is intriguing and perhaps indicates the presence of an early low-level infection, possibly localized to the site of exposure. The observation of this delayed infection in our model (∼3 months later than in untreated controls) and recent human evidence (21) further support the need for adequate follow-up periods after PEP administration to monitor for delayed seroconversions.
Evaluation of CVL supernatant virus levels in this study was highly informative and provided some suggestive evidence for possible localized virus production. The detectable cervicovaginal virus signals noted in some macaques during treatment may be a consequence of PMPA's inability to suppress all virus activity localized to the exposure site. Indeed, successful PEP prevention of a systemic infection may occur at the level of virus dissemination events.
The important issue of localized virus replication at a mucosal exposure site in the absence of an overt systemic infection has also been raised by intriguing investigations into transient infections of drug-naïve macaques resulting from both intrarectal and intravaginal SIV transmissions (15, 22). Although virus detection in genital mucosal compartments was not included, the investigations' results indicated a potential for inducing a transient infection state within a virus-exposed macaque. This phenonemon seems unlikely in our study due to the consistent lack of systemic virus detection up to a full year after virus exposure in those animals protected by PEP; however, tissue reservoirs harboring low levels of virus cannot be ruled out by our present findings.
In summary, our findings indicate that early intervention with a potent antiretroviral regimen may be successful in preventing infection via vaginal exposure to a human-derived retrovirus. The data provide additional insight into the critical timing related to PEP initiation for maximum effectiveness and have generated a proof of concept for the use of antiretroviral agents following a high-risk heterosexual exposure to HIV in humans.
Acknowledgments
We are grateful to Norbert Bischofberger (Gilead Sciences) for supplying the PMPA (tenofovir) required for this study via a materials transfer agreement with CDC; Shirley Kwok, Cindy Christopherson, and Kelly LeGassic of Roche Molecular Systems (Alameda, Calif.) for support with HIV-2 vRNA quantitation and reagents; Gale Galland for serving as the attending veterinarian for this study protocol; J. Rick White and Lucius Brown for monitoring and maintenance of our macaque cohort; Ryan Siemers for assistance with serologic screening assays; and Kevin DeCock for valuable input and discussions of pertinent issues related to immediate PEP for the prevention of HIV infection.
REFERENCES
- 1.Black R J. Animal studies of prophylaxis. Am J Med. 1997;102:39–44. doi: 10.1016/s0002-9343(97)00059-4. [DOI] [PubMed] [Google Scholar]
- 2.Böttiger D, Johansson N-G, Samuelsson B, Zhang H, Putkonen P, Vrang L, Öberg B. Prevention of simian immunodeficiency virus, SIVsm, or HIV-2 infection in cynomolgus monkeys by pre- and postexposure administration of BEA-005. AIDS. 1997;11:157–162. doi: 10.1097/00002030-199702000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Cardo D M, Culver D H, Ciesielski C A, Srivastava P U, Marcus R, Abiteboul D, Heptonstall J, Ippolito G, Lot F, McKibben P S, Bell D M the CDC Needlestick Surveillance Group. A case-control study of HIV seroconversation in health care workers after percutaneous exposure. N Engl J Med. 1997;337:1485–1490. doi: 10.1056/NEJM199711203372101. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Case control study of HIV seroconversation in health-care workers after percutaneous exposure to HIV-infected blood—France, United Kingdom, and United States, January 1988–August 1994. Morb Mortal Wkly Rep. 1995;44:929–933. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Management of possible sexual, injecting-drug-use, or other nonoccupational exposure to HIV, including considerations related to antiretroviral therapy: Public Health Service statement. Morb Mortal Wkly Rep. 1998;47(RR17):1–14. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Public Health Service guidelines for the management of health-care worker exposures to HIV and recommendations for postexposure prophylaxis. Morb Mortal Wkly Rep. 1998;47(RR-7):1–33. [PubMed] [Google Scholar]
- 7.Katz M H, Gerberding J L. Postexposure treatment of people exposed to the human immunodeficiency virus through sexual contact or injection-drug use. N Engl J Med. 1997;336:1097–1099. doi: 10.1056/NEJM199704103361512. [DOI] [PubMed] [Google Scholar]
- 8.Lohman B L, McChesney M B, Miller C J, McGowan E, Joye S M, Van Rompay K K A, Reay E, Antipa L, Pedersen N C, Marthas M L. A partially attenuated simian immunodeficiency virus induces host immunity that correlates with resistance to pathogenic virus challenge. J Virol. 1994;68:7021–7029. doi: 10.1128/jvi.68.11.7021-7029.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Looney D J, McClure J, Kent S J, Radaelli A, Kraus G, Schmidt A, Steffy K, Greenberg P, Hu S L, Morton W R, Wong-Staal F. A minimally replicative HIV-2 live-virus vaccine protects M. nemestrina from disease after HIV-2287 challenge. Virology. 1998;242:150–160. doi: 10.1006/viro.1997.8992. [DOI] [PubMed] [Google Scholar]
- 10.Lurie P, Miller S, Hecht F, Chesney M, Lo B. Postexposure prophylaxis after nonoccupational HIV exposure. Clinical, ethical, and policy considerations. JAMA. 1998;280:1769–1773. doi: 10.1001/jama.280.20.1769. [DOI] [PubMed] [Google Scholar]
- 11.Mastro T D, de Vincenzi I. Probabilities of sexual HIV-1 transmission. AIDS. 1996;10(Suppl. A):S75–S82. doi: 10.1097/00002030-199601001-00011. [DOI] [PubMed] [Google Scholar]
- 12.Mertens T E, Burton A, Stoneburner R, Sato P, Beer D L, Caraël M, Belsey E. Global estimates and epidemiology of HIV-1 infections and AIDS: further heterogeneity in spread and impact. AIDS. 1994;8(Suppl. 1):S361–S372. [PubMed] [Google Scholar]
- 13.Miller C J. Host and viral factors influencing heterosexual HIV transmission. Rev Reprod. 1998;3:42–51. doi: 10.1530/ror.0.0030042. [DOI] [PubMed] [Google Scholar]
- 14.Miller C J, Marthas M, Greenier J, Lu D, Dailey P J, Lu Y. In vivo replication capacity rather than in vitro macrophage tropism predicts efficiency of vaginal transmission of simian immunodeficiency virus or simian/human immunodeficiency virus in rhesus macaques. J Virol. 1998;72:3248–3258. doi: 10.1128/jvi.72.4.3248-3258.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller C J, Marthas M, Torten J, Alexander N J, Moore J P, Doncel G F, Hendrickx A G. Intravaginal inoculation of rhesus macaques with cell-free simian immunodeficiency virus results in persistent or transient viremia. J Virol. 1994;68:6391–6400. doi: 10.1128/jvi.68.10.6391-6400.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulder J, McKinney N, Christopherson C, Sninsky J, Greenfield L, Kwok S. Rapid and simple PCR assay for quantitation of human immunodeficiency virus type 1 RNA in plasma: application to acute retroviral infection. J Clin Microbiol. 1994;32:292–300. doi: 10.1128/jcm.32.2.292-300.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nkengasong, J. N., L. Kestens, P. D. Ghys, S. Koblavi-Deme, R. A. Otten, C. Bile, C. Maurice, M. Kalou, M. Laga, S. Z. Wiktor, and A. E. Greenberg. Dual infection with human immunodeficiency virus type one (HIV-1) and type two (HIV-2): impact on HIV-1 viral load and immune activation markers in HIV-seropositive female sex workers in Abidjan, Cote d'Ivoire. AIDS Res. Hum. Retrovir., in press. [DOI] [PubMed]
- 18.Otten R A, Brown B G, Simon M, Lupo L D, Parekh B S, Lairmore M D, Schable C, Schochetman G, Rayfield M A. Differential replication and pathogenic effects of HIV-1 and HIV-2 in Macaca nemestrina. AIDS. 1994;8:297–306. doi: 10.1097/00002030-199403000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Otten R A, Ellenberger D L, Adams D R, Fridlund C A, Jackson E, Pieniazek D, Rayfield M A. Identification of a window period for susceptibility to dual infection with two distinct human immunodeficiency virus type 2 isolates in a Macaca nemestrina (pig-tailed macaque) model. J Infect Dis. 1999;180:673–684. doi: 10.1086/314968. [DOI] [PubMed] [Google Scholar]
- 20.Pauza C D, Horejsh D, Wallace M. Mucosal transmission of virulent and avirulent lentiviruses in macaques. AIDS Res Hum Retrovir. 1998;14(Suppl. 1):S83–S87. [PubMed] [Google Scholar]
- 21.Ridzon R, Gallagher K, Ciesielski C, Ginsberg M B, Robertson B J, Luo C C, DeMaria A. Simultaneous transmission of human immunodeficiency virus and hepatitis C virus from a needle-stick. N Engl J Med. 1997;336:919–922. doi: 10.1056/NEJM199703273361304. [DOI] [PubMed] [Google Scholar]
- 22.Trivedi P, Horejsh D, Hinds S B, Hinds P W, II, Wu M S, Salvato M S, Pauza C D. Intrarectal transmission of simian immunodeficiency virus in rhesus macaques: selective amplification and host responses to transient or persistent viremia. J Virol. 1996;70:6876–6883. doi: 10.1128/jvi.70.10.6876-6883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai C-C, Emau P, Follis K E, Beck T W, Benveniste R E, Bischofberger N, Lifson J D, Morton W R. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl)adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72:4265–4273. doi: 10.1128/jvi.72.5.4265-4273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai C-C, Follis K E, Sabo A, Beck T W, Grant R F, Bischofberger N, Benveniste R E, Black R. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science. 1995;270:1197–1199. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- 25.Van Rompay K K A, Berardi C J, Aguirre N L, Bischofberger N, Lietman P S, Pedersen N C, Marthas M L. Two doses of PMPA protect newborn macaques against oral simian immunodeficiency virus infection. AIDS. 1998;12:F79–F83. doi: 10.1097/00002030-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Van Rompay K K A, Dailey P J, Tarara R P, Canfield D R, Aguirre N L, Cherrington J M, Lamy P D, Bischofberger N, Pedersen N C, Marthas M L. Early short-term 9-[2-(R)-(phosphonomethoxy)propyl]adenine treatment favorably alters the subsequent disease course in simian immunodeficiency virus-infected newborn rhesus macaques. J Virol. 1999;73:2947–2955. doi: 10.1128/jvi.73.4.2947-2955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Rompay K K A, Marthas M L, Lifson J D, Berardi C J, Vasquez G M, Agatep E, Dehqanzada Z A, Cundy K C, Bischofberger N, Pedersen N C. Administration of (R)-9-[2-(phosphonylmethoxy)propyl]adenine (PMPA) for prevention of perinatal simian immunodeficiency virus infection in rhesus macaques. AIDS Res Hum Retrovir. 1998;14:761–773. doi: 10.1089/aid.1998.14.761. [DOI] [PubMed] [Google Scholar]