Abstract
Cannabinoid CB2 agonists show therapeutic efficacy without unwanted CB1-mediated side effects. The G protein-biased CB2 receptor agonist LY2828360 attenuates the maintenance of chemotherapy-induced neuropathic nociception in male mice and blocks development of morphine tolerance in this model. However, the cell types involved in this phenomenon are unknown and whether this therapeutic profile is observed in female mice has never been investigated. We used conditional deletion of CB2 receptors to determine the cell population(s) mediating the anti-allodynic and morphine-sparing effects of CB2 agonists. Anti-allodynic effects of structurally distinct CB2 agonists (LY2828360 and AM1710) were present in paclitaxel-treated mice and in mice lacking CB2 receptors in CX3CR1 expressing microglia/macrophages (CX3CR1CRE/+; ), but were absent in mice lacking CB2 receptors in peripheral sensory neurons (AdvillinCRE/+;). The morphine-sparing effect of LY28282360 occurred in a sexually-dimorphic manner, being present in male, but not female, mice. LY2828360 treatment (3 mg/kg per day i.p. x 12 days) blocked the development of morphine tolerance in male and CX3CR1CRE/+; mice with established paclitaxel-induced neuropathy but was absent in male (or female) AdvillinCRE/+; mice. Co-administration of morphine with a low dose of LY2828360 (0.1 mg/kg per day i.p. x 6 days) reversed morphine tolerance in paclitaxel-treated male mice, but not AdvillinCRE/+; mice of either sex. LY2828360 (3 mg/kg per day i.p. x 8 days) delayed, but did not prevent, the development of paclitaxel-induced mechanical or cold allodynia in either or CX3CR1CRE/+; mice of either sex. Our findings have potential clinical implications.
Keywords: CB2, Chemotherapy-induced peripheral neuropathy, Dorsal root ganglion, Opioid tolerance, Cancer
1. Introduction
Morphine is an effective treatment for acute pain management, but unwanted side effects, including physical dependence, respiratory depression, and tolerance, limit its clinical use [e.g.] [1,2]. Recent studies have investigated therapeutic strategies to prevent or block morphine tolerance, with a focus on co-administration of mechanistically distinct analgesics [3–6]. The opioid system can functionally interact with the endogenous cannabinoid system by receptor heterodimerization or signaling cross-talk [7,8]. Indeed, co-administration of opioids with cannabinoid compounds produces synergistic antinociceptive effects in studies examining their analgesic properties [9–11]. Although activation of the cannabinoid 1 (CB1) receptor produces antinociception, unwanted side effects (e.g., motor and memory impairment and dependence) also limit their clinical [12,13]. By contrast, activation of the cannabinoid 2 (CB2) receptor alleviates various types of pathological pain without producing adverse side effects associated with CB1 receptor agonism [14,15]. Additionally, CB2 receptors are involved in the antinociceptive effects of morphine under both normal and inflammatory conditions [16–18]. Previous work from our group has also shown that chronic CB2 receptor activation can prevent or attenuate the development of morphine tolerance in various neuropathic pain models [19–21].
LY2828360 is a potent CB2 receptor agonist with similar affinity for both human and rat CB2 receptors and minimal affinity for the CB1 receptor [22]. Previous studies have shown that LY2828360 suppresses behavioral hypersensitivities in models of neuropathic [19,21,23] and inflammatory [24] pain and can prevent the development of morphine tolerance in mouse models of neuropathic pain [19,21]. LY2828360 was previously evaluated in Phase 2 clinical trial for osteoarthritis pain. Although it was ineffective at treating pain in this specific population of pain patients, it was demonstrated to be safe for use in humans [25]. Given the apparent safety of LY2828360, further investigation of the potential analgesic applications for this compound is important to fully understand its range of therapeutic benefits and to identify which pain states it can best treat. Previously, our lab has identified CB2 receptors in peripheral sensory neurons to be necessary for antiallodynic effects of LY2828360 in both carrageenan-induced inflammatory pain [24] and 2, 3-dideoxycytidine (ddC) anti-retroviral neuropathic pain [21] using cell type specific conditional KO mice. However, the specific cell types involved in the efficacy of LY2828360 in the chemotherapy-induced neuropathic pain model or in its ability to prevent the development of tolerance to morphine’s analgesic effect in this model remain unknown.
We previously reported use of our CB2 EGFP reporter mouse to identify cell types (i.e. Langerhans cells, dendritic cells, keratinocytes) that were dynamically regulated by challenge with the chemotherapeutic agent paclitaxel [23]. Here, we generated conditional knockout (KO) mouse lines with deletion of CB2 receptor from peripheral sensory neurons (AdvillinCRE/+; mice) or microglia/macrophages expressing the protein coding gene C-X3-C Motif Chemokine Receptor 1 (CX3CR1CRE/+; mice) to examine the contribution of these cell types to the development and maintenance of chemotherapy-induced neuropathic pain and the therapeutic effects of CB2 agonists. We also compared effects of LY2828360 with those of AM1710, a CB2 receptor agonist that differs in its ability to penetrate the central nervous system (CNS) [26]. Additionally, we investigated the ability of LY2828360 to prevent or reverse morphine tolerance in neuropathic pain in and our conditional KO mice. Lastly, we examined the impact of prophylactic treatment with LY2828360 in paclitaxel-treated CX3CR1CRE/+; mice. Our studies document a previously unrecognized role for CB2 receptors in primary sensory neurons in anti-allodynic effects of CB2 agonists in chemotherapy-induced neuropathic nociception and document a role for these receptors in a sex-specific sparing of morphine tolerance that we observe in paclitaxel-treated male, but not female, mice.
2. Methods
2.1. Subjects
Adult male C57BL6/J mice were purchased from Jackson labs (Bar Harbor, ME) for qRT-PCR experiment. All conditional KO mice were bred on a C57BL6/J background at Indiana University and included adult male and female , Advillin CRE/+; (deletion of CB2 from peripheral sensory neurons) and CX3CR1CRE/+; mice (deletion of CB2 from microglia/macrophages). To conditionally delete CB2 from the desired cells, female mice carrying two alleles of the floxed CB2 gene [27] were bred with male mice carrying two floxed CB2 alleles and one allele of Cre-recombinase in either the CX3CR1 [28] or Advillin [29,30]. All mice were housed in a temperature-controlled colony room on a 24-hr light-dark cycle. Behavioural testing occurred during the light phase of the cycle. Mice were maintained on ad-libitum food and water. All experiments were approved by the Indiana University Animal Care and Use Committee.
2.2. Drugs
Paclitaxel (Tecoland Corporation, Irvine, CA) was dissolved in a cremophor-based vehicle made of Cremophor EL (Sigma-Aldrich, St. Louis, MO), ethanol (Sigma-Aldrich) and 0.9 % saline (Aqualite System; Hospira, Inc., Lake Forest, IL) at a 1:1:18 ratio as previously published [15]. LY2828360 (8-(2-chlorophenyl)-2-methyl-6-(4-methylpiperazin-1- yl)-9-(tetrahydro-2 H-pyran-4-yl)-9 H- purine) was synthesized by Sai Biotech (Mumbai, India; purity >98 %). AM1710 was synthesized by the lab of Alexandros Makriyannis (Northeastern University, Boston, MA). Morphine (Sigma-Aldrich), AM1710 and LY2828360 were dissolved in a vehicle containing 10 % dimethyl sulfoxide (DMSO; Sigma-Aldrich), and the remaining 90 % consisted of emulphor (Alkamuls EL-620; Rhodia, Cranbury, NJ), ethanol and saline at a 1:1:18 ratio. Naloxone (Sigma-Aldrich) was dissolved in saline. All compounds were administered intraperitoneally (i.p.) at a volume of 10 ml/kg.
2.3. Tissue collection and quantitative real-time polymerase chain reaction (qRT-PCR)
In Experiment 1, real-time (RT) quantitative PCR (qPCR) was used to examine the distribution of GFP mRNA (used as a surrogate marker for CB2 mRNA in mice) and CB2 mRNA in the dorsal root ganglia (DRG), lumbar spinal cord, paw skin and spleen of male and female naïve wild type mice (C57BL/6 J; n = 5 male, 3 female), (n = 5 female) and AdvillinCRE/+; (n = 8 male, 5 female). Mice were perfused with Hank’s balanced Salt solution (HBBS), then DRG, spinal cord, paw skin and spleen were collected and flash frozen in liquid nitrogen then stored at −80 °C until use. DRG and paw skin RNA was extracted using a RLT buffer:2-mercapto ethanol mixture in a 100:1 ratio/RNeasy (Qiagen) RNA mini kit according to the manufacturer’s instructions and spinal cord and spleen RNA was extracted using TRizol (Invitrogen)/RNeasy RNA mini kit. Quantification of total RNA was assessed using NanoDrop 2000 C UV-Vis spectrophotometer at 260 nm.
One-step RT-qPCR was performed using the Luna universal One-Step RT-qPCR kit (New England Biolabs) in a total volume of 10 μl and a template concentration of 50 ng/μl according to manufacturer’s recommendation. Thermal cycle conditions were 55 °C for 10 min, 95 °C for 1 min, followed by 40 cycles of 95 °C for 10 sec and 60 °C for 1 min. A melting curve analysis was performed at 95 °C for 15 sec, 60 °C for 1 min, 95 °C for 15 sec following every run to ensure a single amplified product for each reaction. All reactions were performed in duplicate in 384-well reaction plates (Thermo Fisher Scientific). The mRNA expression levels were quantified by cycle threshold (Ct), where Ct levels are inversely proportional to the amount of target nucleic acid in the sample. Relative gene expression for CB2 and GFP were normalized to the housekeeping gene GAPDH and calculated using the 2−ΔΔCt method [31]. Sequences for qRT-PCR primers were: CB2 sense: 5’-CTCGGTTACAGAAACAGAGGCTGATGTG-3’; CB2 anti-sense: 5’-TCTCTCTTCGAGGGAGTGAACTGAACG-3’; GFP sense: 5’-ACATGGTCCTGCTGGAGTTCGTGAC-3’; GFP anti-sense: 5’-CTCTTCGAGGGAGTGAACTGAACGG-3’; GAPDH sense: 5’-GGGAAGCTCACTGGCATGGC-3’; anti-sense: 5’-GGTCCACCACCCTGTTGCT-3’.
2.4. Behavioral testing
Paw withdrawal thresholds to mechanical stimulation were assessed using an electronic von Frey anesthesiometer (IITC, Woodland Hills, CA) as described in our previously published work [19,23]. Animals were placed on an elevated testing table with a stainless-steel wire mesh platform (0.6 ×0.6 cm gaps in the wire mesh) underneath clear Plexiglass chambers (10.5 ×9×7 cm) for at least 20 minutes prior to testing. Following this habituation period, a force was applied to the midplantar region of the hind paw until a paw withdrawal response was observed. Mechanical stimulation was terminated when the animal withdrew its paw, and the value of the maximum applied force was recorded in grams. Each paw was measured twice at every time point, and values were averaged for each animal.
Sensitivity to cold was assessed immediately following testing for mechanical paw withdrawal thresholds by applying a droplet of acetone (Sigma-Aldrich; approximately 5–6 μL) from the end of a blunt one C.C. syringe hub onto the midplantar region of the hind paw. Total time the mouse spent attending to the acetone-stimulated paw (ie. elevation, shaking or licking) was recorded over a 1-minute period. Each paw was measured three times at every time point, and values were averaged for each animal.
2.5. General in vivo experimental protocol
In all studies, the experimenter was blinded to the treatment conditions and mice were randomly assigned to experimental conditions. Paclitaxel (4 mg/kg i.p.) was administered four times on alternate days (cumulative dose of 16 mg/kg) to induce neuropathic pain as described previously [15]. Control mice received an equal volume of cremophor-based vehicle. Paclitaxel-induced peripheral neuropathy was established prior to pharmacological manipulation, except in the prophylactic study. Responsiveness to mechanical and cold stimulation was measured 30 minutes after drug administration on testing days.
In Experiment 2, we evaluated whether and AdvillinCRE/+; mice develop mechanical and cold hypersensitivities in response to treatment with paclitaxel or its vehicle (n = 4–7 per group, mixed sex ( (n = 4 male, n = 6 female) and AdvillinCRE/+; (n = 5 male, n = 4 female)).
In Experiment 3, we compared the antiallodynic efficacy of two structurally distinct CB2 agonists that differ in their ability to penetrate the central nervous system (CNS) using both and AdvillinCRE/+; male mice (n = 5–6 per group). Mice were given an i.p. injection of either AM1710, a CB2 agonist with limited CNS penetration [26], or LY2828360, a CNS-penetrant CB2 agonist [22], in a dose-response experiment. Escalating doses of AM1710 (1, 3, 5 and 10 mg/kg) or LY2828360 (0.1, 0.3, 1 and 3 mg/kg) were examined for antiallodynic efficacy using a within-subject escalating dosing paradigm. Successive doses were separated by 2–4 days.
In Experiment 4, we examined the antiallodynic efficacy of chronic LY2828360 administration (3 mg/kg per day i.p. x 12 days) or vehicle administered once daily for 12 consecutive days (i.e. phase I) in both male and female and AdvillinCRE/+; mice. This treatment was followed approximately 4 days later by a second phase of once daily dosing for 12 consecutive days (i.e. phase II) of chronic morphine (10 mg/kg per day i.p. x 12 days) or vehicle in the same animals (n = 8–9 per group). Responsiveness to mechanical and cold stimulation were measured on days 1, 4, 8 and 12 during phase I and on days 16, 19, 23 and 27 during phase II (phase II began on day 16).
In Experiment 5, we examined the antiallodynic efficacy of low dose LY2828360 or vehicle in both male and female and AdvillinCRE/+; mice that had previously developed tolerance to morphine (n = 6–8 per group). To induce morphine tolerance, mice received repeated daily injections of morphine in phase I (10 mg/kg per day i.p x 6 days), followed by low dose LY2828360 (0.1 mg/kg per day i.p. x 6 days) or vehicle co-administered with morphine (10 mg/kg per day i.p x 6 days) in phase II. Responsiveness to mechanical and cold stimulation were measured on days 1, 3 and 6 during phase I and on days 7, 9 and 12 during phase II (phase II began on day 7).
In Experiment 6, we evaluated the antiallodynic efficacy of LY2828360 in both and CX3CR1CRE/+; mice as described in experiment 3 (n = 6 for cremophor groups (female only); n = 15–16 for paclitaxel groups, mixed sex ( (n = 8 male, n = 8 female) and CX3CR1CRE/+; ( (n = 8 male, n = 7 female)).
In Experiment 7, we examined the antiallodynic efficacy of chronic LY2828360 or vehicle administered during phase I in both male and female and CX3CR1CRE/+; mice, followed by the assessment of the antiallodynic efficacy of chronic morphine administration during phase II in the same animals, as described in Experiment 4 (n = 6–8 per group).
In Experiment 8, we examined the ability of LY2828360 (3 mg/kg per day i.p. x 8 days) to prevent the development of paclitaxel-induced mechanical allodynia in both and CX3CR1CRE/+; mice (n = 5 per group, mixed sex ( (n = 5 male, n = 5 female) and CX3CR1CRE/+; (n = 5 male, n = 5 female)). Paclitaxel (4 mg/kg i.p.) was injected on days 0, 2, 4 and 6, and LY2828360 was injected on days 0–7. LY2828360 was injected 1-hr prior to paclitaxel on days when both drugs were administered. Responsiveness to mechanical and cold stimulation were measured on days 0, 4, 7, 11, 14 and 18, with behavioral testing performed prior to daily injections.
2.6. Evaluation of opioid receptor-mediated withdrawal symptoms
Mice that received morphine (10 mg/kg per day i.p.) following LY2828360 administration (3 mg/kg per day i.p.) or a combination of morphine and LY2828360 (10 mg/kg per day i.p. morphine co-administered with 0.1 mg/kg per day i.p. LY2828360) in the two-phase studies were challenged with naloxone (5 mg/kg i.p.) to induce opioid withdrawal. Specifically, mice used in Experiments 4, 5, and 7 received a final injection of the test drug and were placed on an elevated glass platform in plastic observation chambers (11.5 wide x 12.5 length x 28 cm high) and were video-recorded for 30 min (i.e. post drug-habituation interval). Then, the same animals were challenged with naloxone (5 mg/kg i.p.) to precipitate opioid withdrawal (i.e. naloxone was injected 30 minutes after the last injection of the test drug) and mice were immediately returned to the observation chamber. Mice were then videorecorded for an additional 30 minutes, after which the experiment was terminated. Our previous studies showed that the dose of naloxone employed here did not precipitate withdrawal jumps in the absence of chronic morphine treatment (e.g., in mice receiving chronic vehicle in lieu of chronic morphine) using an identical protocol [19]. Mice were video recorded, and the number of naloxone-precipitated withdrawal jumps was scored over a 30-minute observation period.
2.7. Statistical analyses
For both paw withdrawal thresholds and time spent attending to acetone-stimulated paw, measurements were averaged across paws for each subject to obtain a single data point per subject per observation. Data from von Frey, acetone and naloxone-precipitated withdrawal testing were analyzed by two-way repeated measures ANOVA followed by Sidak’s multiple comparisons post-hoc test to examine differences between groups. Unpaired two-tailed t-tests were used to analyze qPCR data. Statistical analysis was performed using Graphpad prism 7 (Graphpad prism software, San Diego, CA).
3. Results
3.1. Experiment 1. Both GFP and CB2 mRNAs are reduced in the dorsal root ganglia of AdvillinCRE/+; relative to mice
AdvillinCRE/+; mice showed reduced CB2 mRNA in the DRG compared to both (DRG: ; spinal cord: ; paw skin: ; spleen: ) and wildtype (DRG: ; spinal cord: ; paw skin: ; spleen: ) mice whereas no such changes were observed in lumbar spinal cord, paw skin or spleen. Overall, CB2 mRNA expression levels were similar between and wildtype mice with one exception; a modest but reliable increase in CB2 mRNA expression levels was observed in lumbar spinal cord of mice relative to wild type mice (). Thus, GFP mRNA expression levels tracked CB2 mRNA expression levels in mice in all tissues and were elevated relative to wildtype (background) levels that, as expected, did not express GFP mRNA (DRG: ; spinal cord: ; paw skin: ; spleen: ). In DRG, GFP mRNA expression levels were lower in advillinCRE/+; mice compared to mice (), but not in other tissues (Fig. 1).
Fig. 1.
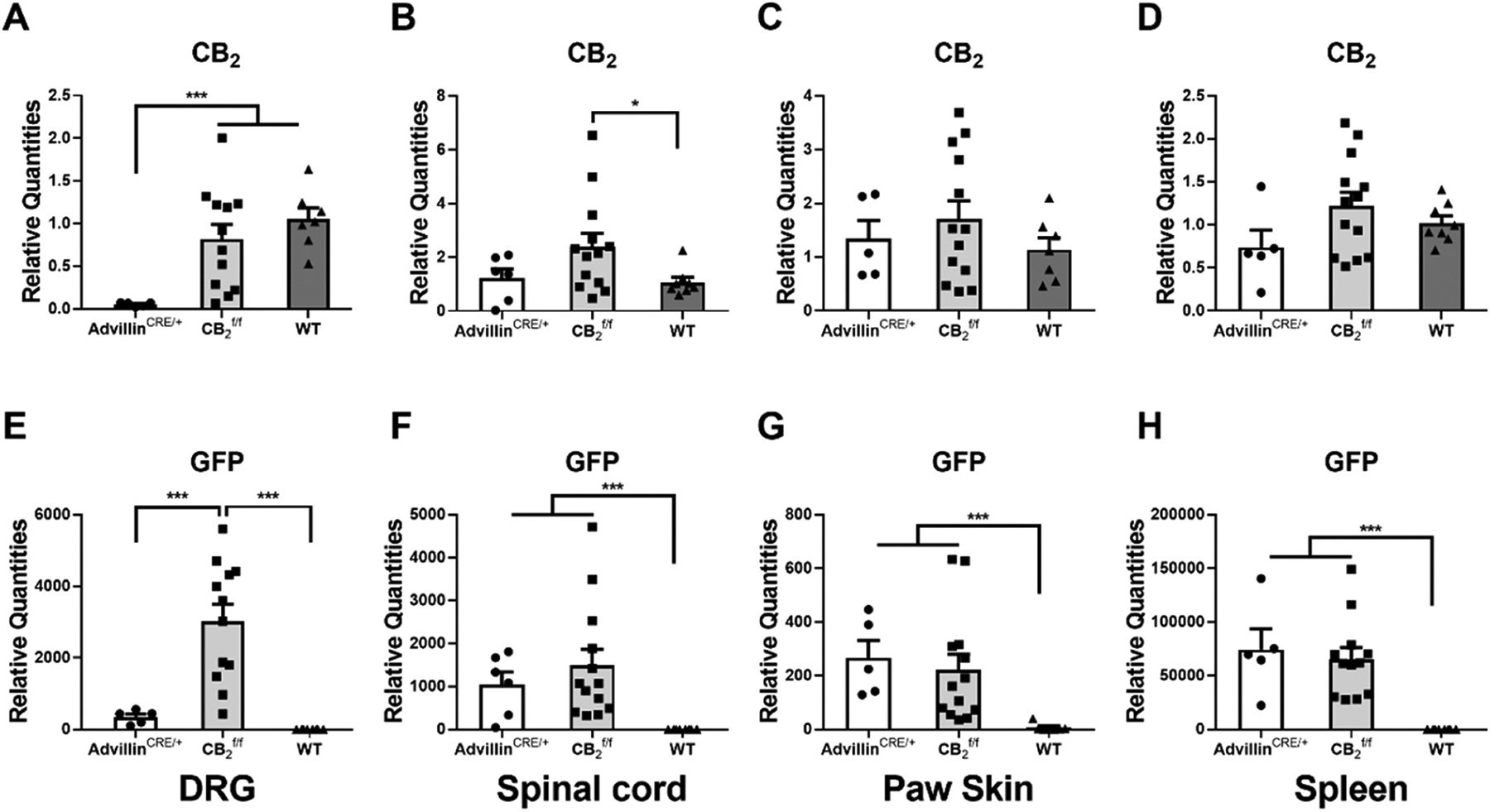
Comparison of CB2 mRNA (A, B, C, D) and GFP mRNA (E, F, G, H) expression in DRG (A, E), spinal cord (B, F), paw skin (C, G) and spleen (D, H) of male and female (n = 8 male, 5 female), AdvillinCRE/+; , (n = 5 female) and wild type (n = 5 male, 3 female) mice. AdvillinCRE/+; mice showed lower levels of CB2 mRNA compared to both and WT mice and lower levels of GFP compared to mice. WT mice did not show any GFP mRNA expression. Mean (±SEM) 2−ΔΔCT values for experiment 1 (unpaired two-tailed t-tests). Asterisk indicates a group difference; *p < 0.05, ***p < 0.001.
3.2. Experiment 2. The chemotherapy agent paclitaxel induced hypersensitivity to mechanical and cold stimulation in both and AdvillinCRE/+; mice
Paclitaxel decreased mechanical paw withdrawal thresholds and increased cold sensitivity as a function of time in both and AdvillinCRE/+; mice. (Mechanical: Time: F4,64 = 24.42, ; Group: F3,16 = 77.53, ; Interaction: F12,64 = 6.71, ; Cold: Time: F4,64 = 21.76, ; Group: F3,16 = 7.78, ; Interaction: F12,64 = 5.696, ). Sidak’s multiple comparisons test revealed decreased mechanical withdrawal threshold ( on day 4, 7, 10 and 14), and increased cold sensitivity ( on day 7, 10 and 14), following paclitaxel (Pac) treatment compared to cremophor (crem) vehicle treatment, in both (Fig. 2A) and AdvillinCRE/+; (Fig. 2B) mice. Responses to mechanical and cold stimulation did not differ between and AdvillinCRE/+; mice in either the paclitaxel or cremophor-based vehicle-treated groups.
Fig. 2.
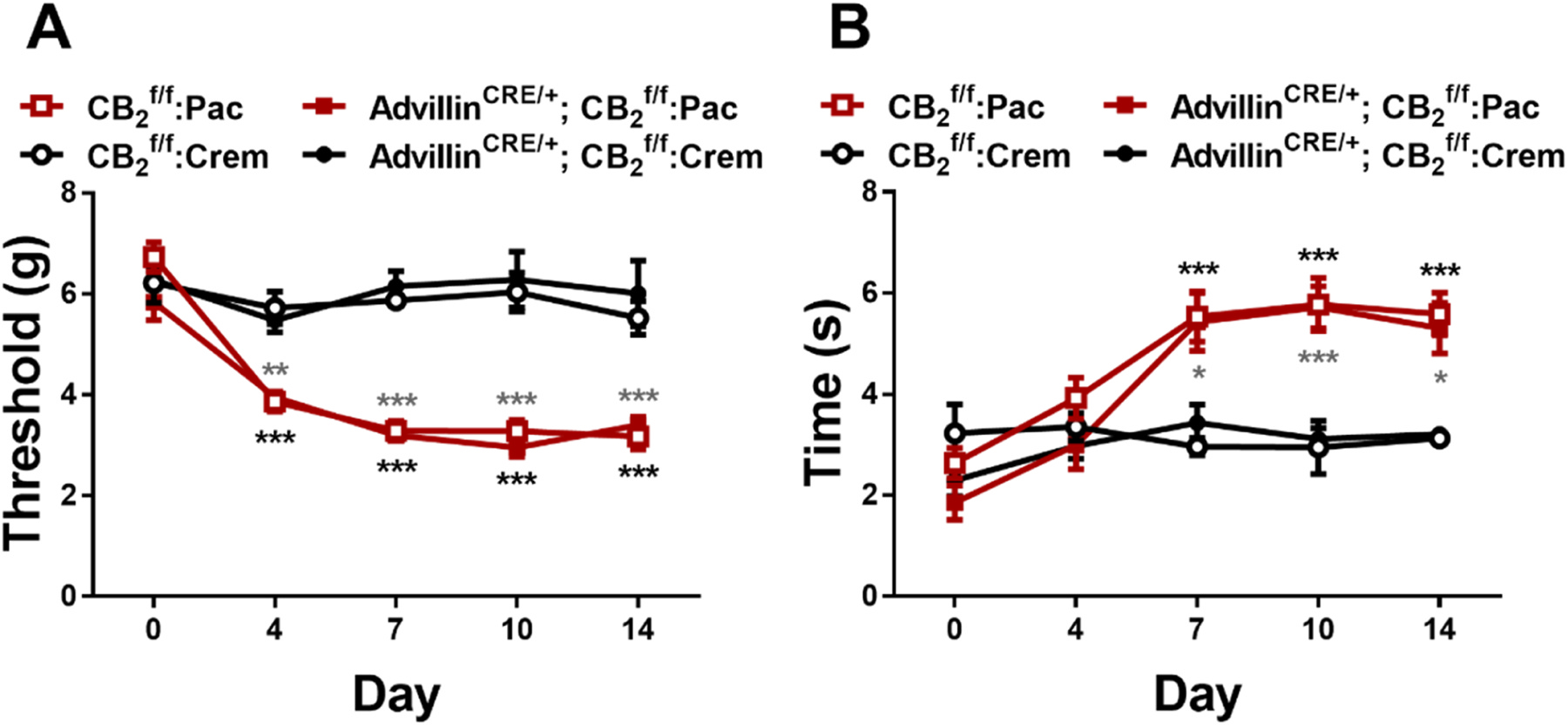
Mechanical (A) and cold (B) allodynia develop normally in and AdvillinCRE/+; mice following paclitaxel treatment (n = 4–7 per group, mixed sex). Data expressed as mean ±SEM, *p < 0.05, **p < 0.005, ***p < 0.001 (two-way ANOVA, Sidak’s multiple comparisons test). Gray asterisk indicate difference between groups; black asterisk indicate difference between AdvillinCRE/+; groups.
3.3. Experiment 3. The CB2 agonists AM1710 and LY2828360 dose dependently reduced mechanical and cold allodynia in , but not AdvillinCRE/+; mice
LY2828360 increased mechanical paw withdrawal thresholds and decreased cold sensitivity as a function of dose and genotype in paclitaxel-treated mice (Mechanical: Dose: F4,40 = 24.21, ; Genotype: F1,10 = 74.71, ; Interaction: F4,40 = 21.81, ; Cold: Dose: F4,40 = 6.441, ; Genotype: F1,10 = 10.05, ; Interaction: F4,40 = 11.13, ). Sidak’s multiple comparison post hoc test revealed that LY2828360 increased mechanical withdrawal thresholds at doses of 0.1 (), 0.3 (, 1 and 3 mg/kg (; Fig. 3A) in mice but not Advillin CRE/+; mice. LY2828360 also decreased cold sensitivity at doses of 1 and 3 mg/kg (; Fig. 3B) in mice but not AdvillinCRE/+; mice. LY2828360 had no effect on mechanical paw withdrawal thresholds (Fig. 3C) in either or AdvillinCRE/+; mice treated with cremophor-based vehicle in lieu of paclitaxel (Mechanical: Dose: F4,32 = 0.3443, p = 0.8460; Genotype: F1,8 = 0.007119, p = 0.9348; Interaction: F4,32 = 0.8399, p = 0.5101). Cold responsiveness changed as a function of dose and was slightly elevated in relative to AdvillinCRE/+; mice treated with cremophor-based vehicle, but the interaction was not significant (Cold: Dose: F4,32 = 3.625, p = 0.0152; Genotype: F1,8 = 5.8, p = 0.0426; Interaction: F4,32 = 0.3422, p = 0.8474; Fig. 3D).
Fig. 3.
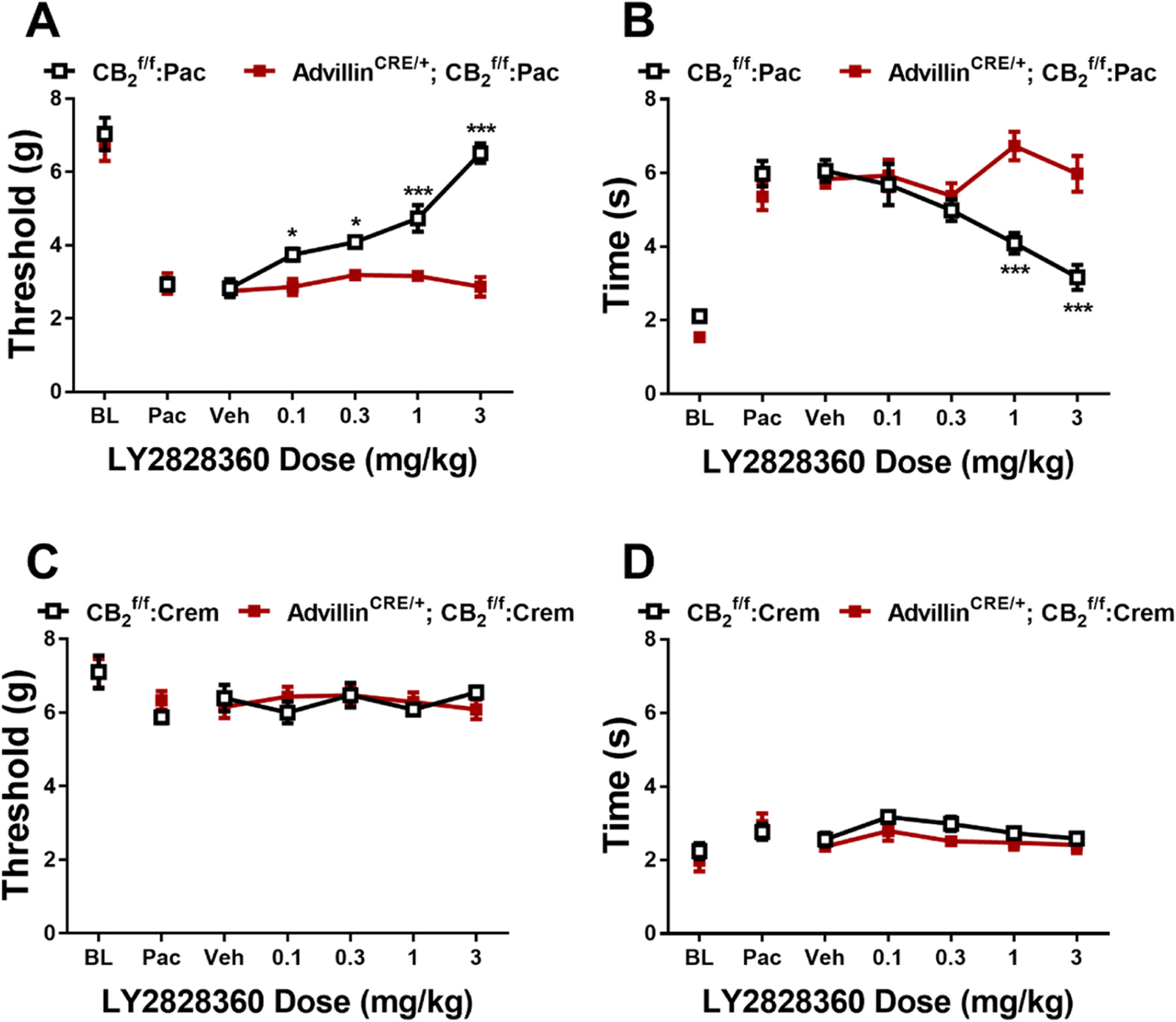
LY2828360 dose dependently alleviated paclitaxel evoked mechanical (A) and cold (B) allodynia in , but not AdvillinCRE/+; male mice. LY2828360 did not alter mechanical (C) or cold (D) sensitivity in or AdvillinCRE/+; control male mice treated with cremophor vehicle (n = 5–6 per group). Data expressed as mean ±SEM, *p < 0.05, ***p < 0.001 (two-way ANOVA, Sidak’s multiple comparisons test).
In paclitaxel-treated mice, AM1710 increased mechanical paw withdrawal thresholds and decreased cold sensitivity as a function of dose and genotype (Mechanical: Dose: F4,40 = 13.29, p < 0.0001; Genotype: F1,10 = 24.48, p = 0.0006; Interaction: F4,40 = 6.385, p = 0.0005; Cold: Dose: F4,40 = 3.782, p = 0.0106; Genotype: F1,10 = 5.3, p = 0.0106; Interaction: F4,40 = 6.386, p = 0.0005). Sidak’s multiple comparisons test revealed that AM1710 increased mechanical withdrawal thresholds at doses of 5 and 10 mg/kg (p < 0.0001; Fig. 4A) and decreased cold sensitivity at doses of 5 (p = 0.0080) and 10 mg/kg (p = 0.0007; Fig. 4B) in but not AdvillinCRE/+; mice. AM1710 did not alter mechanical or cold responsiveness at doses of 1 and 3 mg/kg (p > 0.1331) in either genotype. AM1710 did not alter mechanical (Fig. 4C) or cold (Fig. 4D) responsiveness in either or AdvillinCRE/+; mice treated with cremophor-based vehicle (Mechanical: Dose: F4,32 = 1.486, p = 0.2297; Genotype: F1,8 = 9.052e-005, p = 0.9926; Interaction: F4,32 = 0.338, p = 0.8503; Cold: Dose: F4,32 = 5922, p = 0.6708; Genotype: F1,8 = 5.8, p = 0.8780; Interaction: F4,32 = 0.6736, p = 0.6151).
Fig. 4.
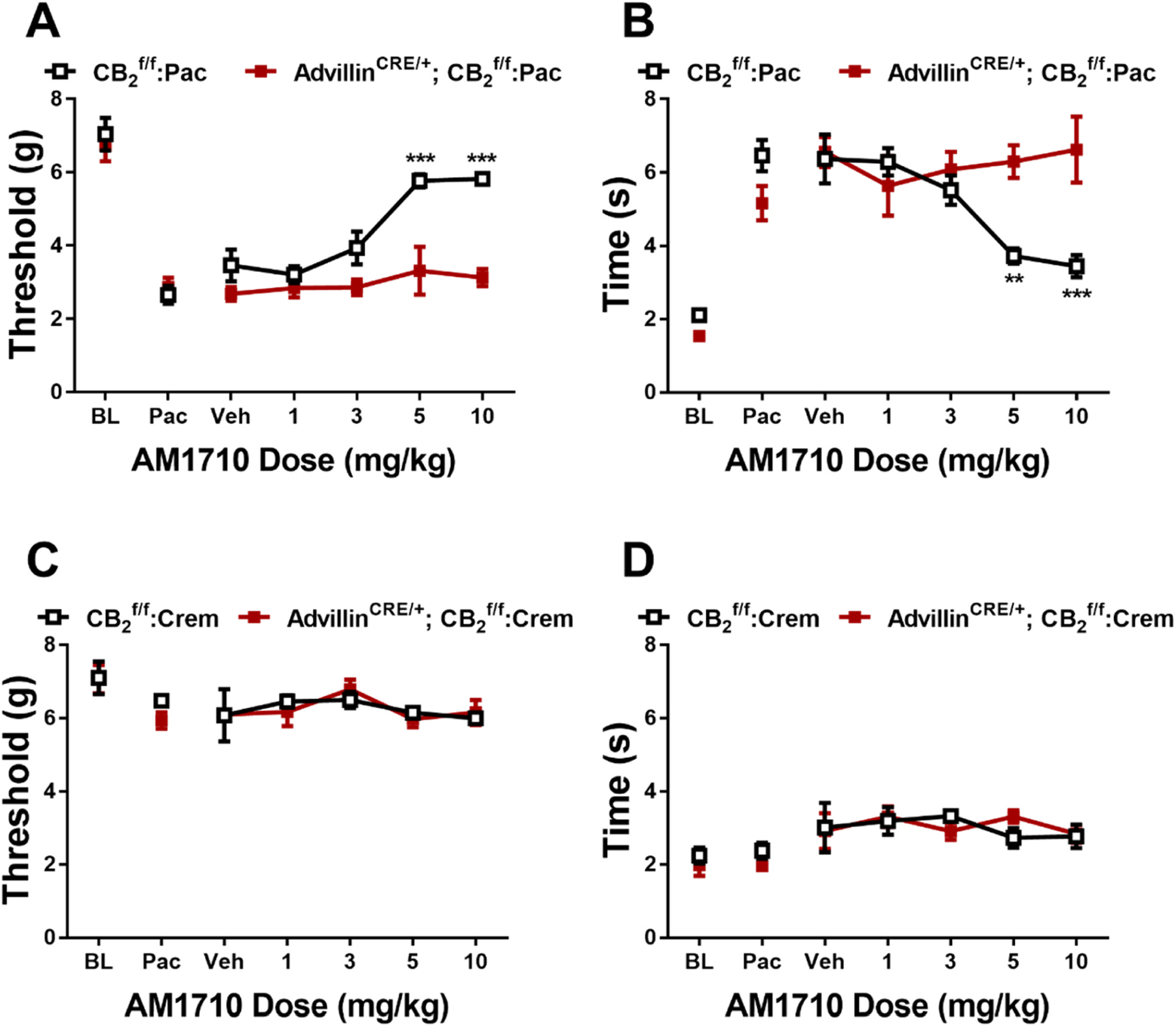
AM1710 dose dependently alleviated paclitaxel-evoked sensitization to mechanical (A) and cold (B) stimulation in , but not AdvillinCRE/+; male mice. AM1710 did not alter mechanical (C) or cold (D) sensitivity in or AdvillinCRE/+; control male mice treated with cremophor vehicle (n = 5–6 per group). Data expressed as mean ±SEM, **p < 0.005, ***p < 0.001 (two-way ANOVA, Sidak’s multiple comparisons test).
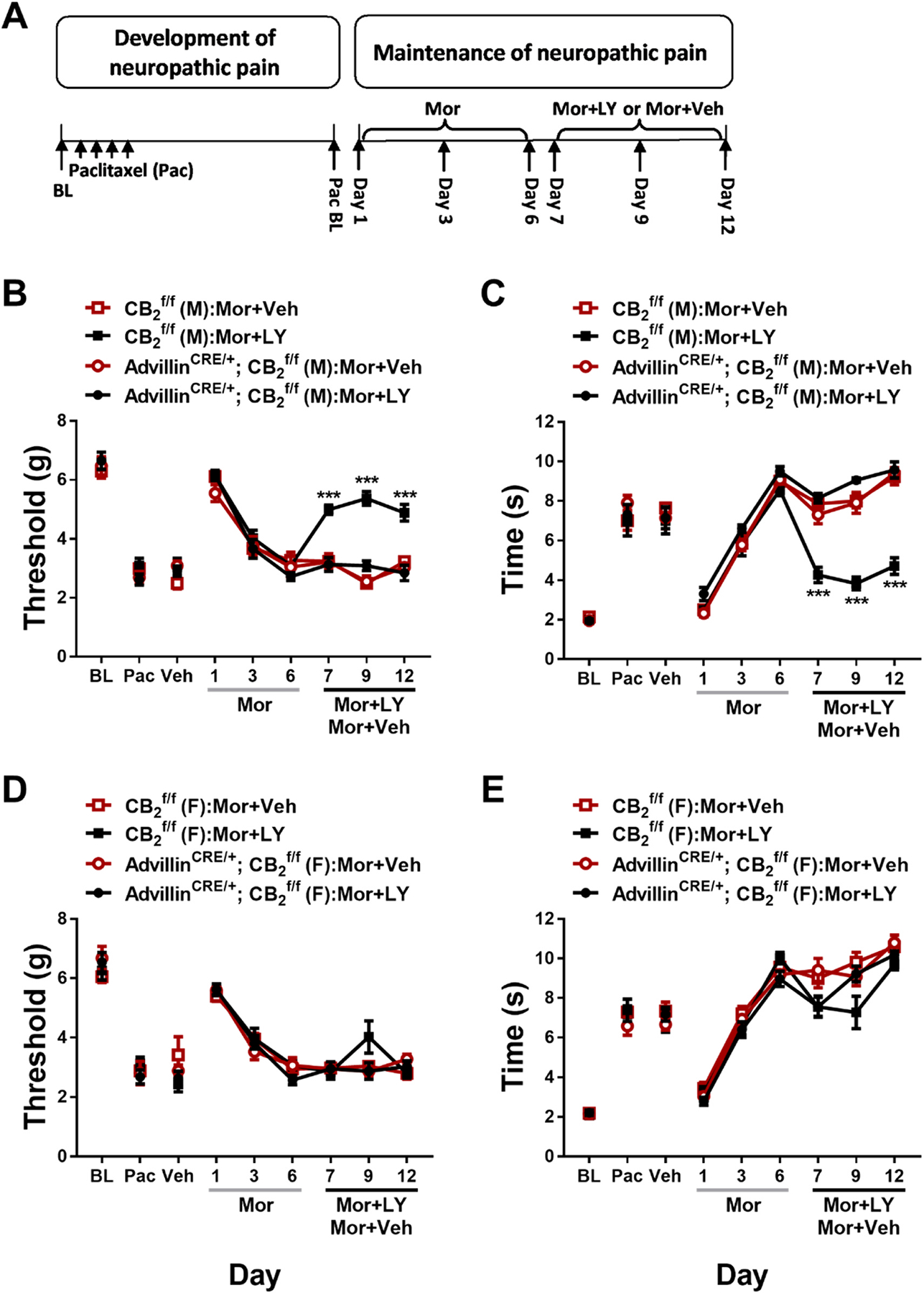
3.4. Experiment 4. Prior chronic dosing with LY2828360 blocked the development of morphine tolerance in male but not female mice and this effect was absent in AdvillinCRE/+; mice
To examine the effects of LY2828360 treatment on the development of morphine tolerance in AdvillinCRE/+; mice, a two-phase pharmacological manipulation was used during the maintenance of neuropathic pain (Fig. 5A). Phase I treatment with LY2828360 (3 mg/kg per day i.p. x 12 days) increased mechanical withdrawal thresholds (Fig. 5B, D) and decreased cold sensitivity (Fig. 5C,E) in but not AdvillinCRE/+; mice. This effect was observed in both male (Mechanical: Time: F5,75 = 35.66, p < 0.0001; Genotype: F1,15 = 191, p < 0.0001; Interaction: F5,75 = 27.37, p < 0.0001; Cold: Time: F5,75 = 73.97, p < 0.0001; Genotype: F1,15 = 144.6, p < 0.0001; Interaction: F5,75 = 39.65, p < 0.0001) and female (Mechanical: Time: F5,70 = 32.81, p < 0.0001; Genotype: F1,14 = 62.4, p < 0.0001; Interaction: F5,70 = 15.67, p < 0.0001; Cold: Time: F5,70 = 100.3, p < 0.0001; Genotype: F1,14 = 75.06, p < 0.0001; Interaction: F5,70 = 43.11, p < 0.0001) mice; Sidak’s multiple comparisons test revealed that LY2828360 increased mechanical paw withdrawal thresholds and decreased cold responsiveness in mice compared to AdvillinCRE/+; mice on all phase I days tested in paclitaxel-treated mice of both sexes (p < 0.0001). Phase II treatment with morphine (10 mg/kg per day i.p. x 12 days) increased mechanical paw withdrawal thresholds (Fig. 5B,D) and decreased cold sensitivity (Fig. 5C,E) without the development of tolerance in male but not male AdvillinCRE/+; mice (Mechanical: Time: F6,90 = 54.69, p < 0.0001; Genotype: F1,15 = 104.8, p < 0.0001; Interaction: F6,90 = 21.81, p < 0.0001; Cold: Time: F6,90 = 90.56, p < 0.0001; Genotype: F1,15 = 72.37, p < 0.0001; Interaction: F6,90 = 42.22, p < 0.0001). This sparing of morphine tolerance was absent in female mice (Fig. 5D,E) of either genotype (Mechanical: Time: F6,84 = 112.5, p < 0.0001; Genotype: F1,14 = 0.007455, p = 0.9324; Interaction: F6,84 = 0.7039, p = 0.6473; Cold: Time: F6,84 = 386.8, p < 0.0001; Genotype: F1,14 = 2.87, p = 0.1124; Interaction: F6,84 = 4.923, p = 0.0002); Sidak’s multiple comparisons test revealed that acute treatment with morphine in Phase II (day 16) increased mechanical paw withdrawal thresholds and decreased cold sensitivity compared to day 15 post-paclitaxel baseline responding in both sexes regardless of genotype (p < 0.0001 for all comparisons). Morphine tolerance developed in male AdvillinCRE/+; and female mice of both genotypes at subsequent time points (p < 0.0001 on days 19–27 for all timepoints compared to day 16), whereas anti-allodynic effects of morphine were preserved in paclitaxel-treated male mice for the duration of phase 2 (p < 0.0001 on days 19–27 compared to male AdvillinCRE/+; mice).
Fig. 5.

History of chronic LY2828360 treatment blocked the development of morphine tolerance in male, but not female, mice, whereas morphine tolerance developed in AdvillinCRE/+; mice of either sex. (A) Schematic shows the injection schedule used to evaluate the two-phase treatment during the maintenance phase of paclitaxel-induced neuropathic pain. Chronic LY2828360 treatment (3 mg/kg per day i.p. x 12 days) alleviated paclitaxel evoked mechanical (B) and cold (C) allodynia in male , but not AdvillinCRE/+; mice and blocked the development of tolerance to the anti-allodynic effects of morphine (10 mg/kg per day i. p. x 12 days). LY2828360 had no effect on mechanical (D) or cold (E) allodynia in female or AdvillinCRE/+; mice and did not block the development of tolerance to the anti-allodynic effects of morphine (n = 8–9 per group). Data expressed as mean ±SEM, ***p < 0.001 (two-way ANOVA, Sidak’s multiple comparisons test).
In paclitaxel-treated and Advillin CRE/+; mice with a history of chronic treatment with LY282860 and morphine, naloxone challenge produced characteristic jumping behavior that was higher in females compared to males but did not differ as a function of genotype and the interaction was not significant (Sex: F1,29 = 6.69, p = 0.0150; Genotype: F1,29 = 1.591, p = 0.2172; Interaction: F1,29 = 0.4572, p = 0.5043; Fig. 6). No withdrawal jumps or signs of abnormal behavior were observed in the 30 min observation interval measured after the final chronic drug injection prior to naloxone challenge in any group.
Fig. 6.
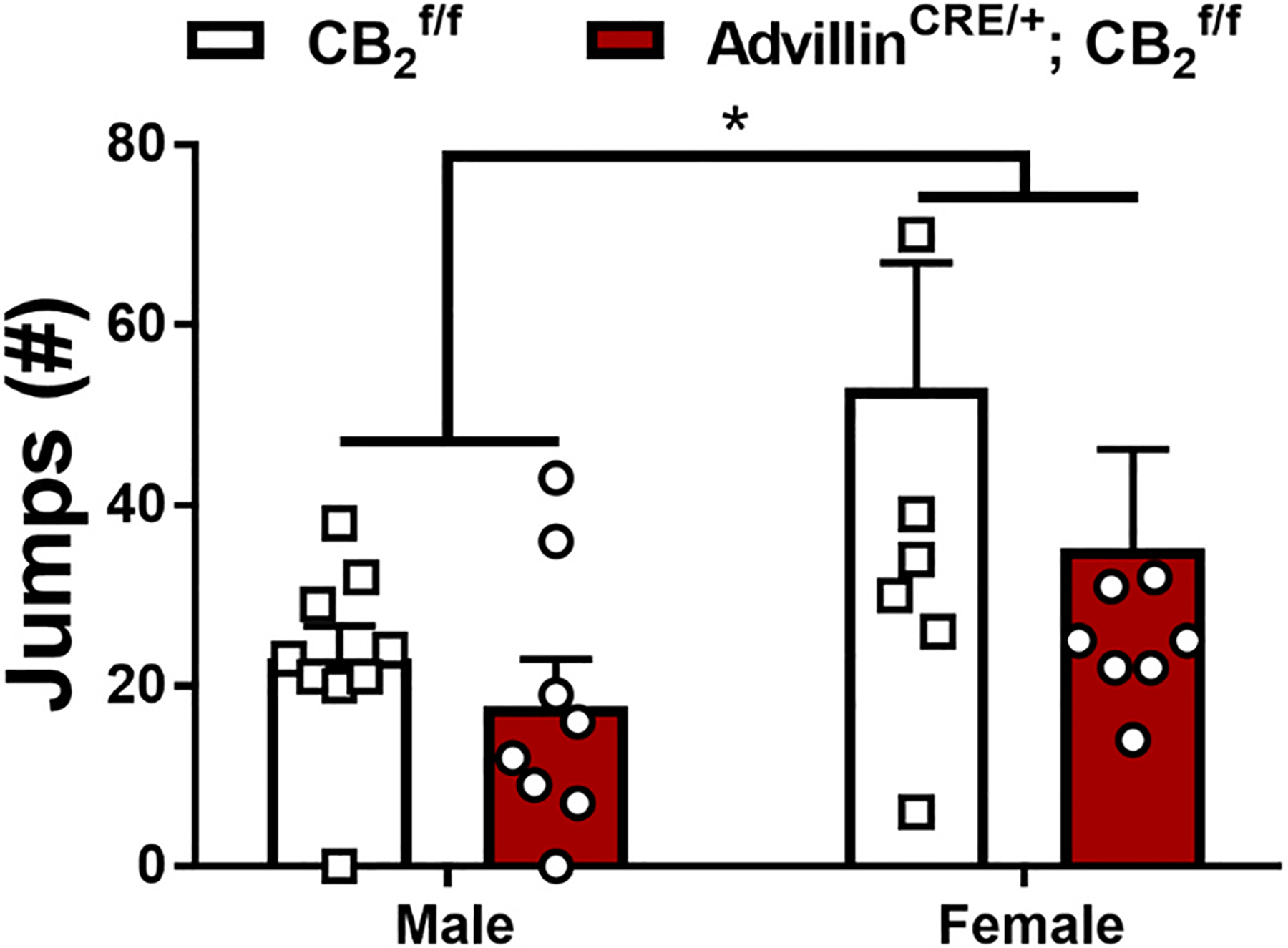
Impact of a history of chronic LY2828360 (3 mg/kg per day i.p. x 12 days) treatment on naloxone-precipitated morphine withdrawal in male and female and AdvillinCRE/+; mice used in Experiment 4. Following chronic treatment with morphine (10 mg/kg per day i.p. x 12 days) male mice show fewer withdrawal jumps compared to female mice regardless of genotype (n = 8–9 per group). Data expressed as mean ±SEM, *p < 0.05 (two-way ANOVA).
3.5. Experiment 5. LY2828360 reversed morphine tolerance in male, but not female, mice when co-administered with morphine and did not block morphine tolerance in either male or female AdvillinCRE/+; mice
We evaluated whether a low dose of LY2828360, administered concurrently with morphine during the maintenance of neuropathic pain would reverse morphine tolerance after it already developed (Fig. 7A). Phase I treatment with morphine (10 mg/kg per day i.p. x 6 days) increased mechanical withdrawal thresholds (Fig. 7B,D) and decreased cold sensitivity (Fig. 7C,E) in both male (Mechanical: Time: F5,120 = 206.5, p < 0.0001; Group: F3,24 = 1.374, p = 0.2748; Interaction: F15,120 = 0.9599, p = 0.5015; Cold: Time: F5120 = 302.9, p < 0.0001; Group: F3,24 = 0.5196, p = 0.6728; Interaction: F15120 = 0.9665, p = 0.4945) and female (Mechanical: Time: F5,125 = 187.5, p < 0.0001; Group: F3,25 = 0.2581, p = 0.8549; Interaction: F15125 = 1.575, p = 0.0899; Cold: Time: F5,120 = 392.8, p < 0.0001; Group: F3,24 = 0.5368, p = 0.6616; Interaction: F15,120 = 1.423, p = 0.1471) and Advillin-CRE/+; mice irrespective of group; Sidak’s multiple comparisons test revealed that morphine increased mechanical paw withdrawal thresholds and decreased cold sensitivity on day 1 of injections compared to paclitaxel baseline for all groups (p < 0.0001). By day 6 of morphine dosing mechanical allodynia was observed in all groups, indicating the development of morphine tolerance (p > 0.9129 compared to paclitaxel baseline). Enhanced cold sensitivity was observed on day 6 of repeated morphine dosing compared to the post-paclitaxel baseline in all groups (p < 0.0369) other than the male AdvillinCRE/+; Mor+VEH group (p = 0.1576 difference from paclitaxel baseline), potentially indicative of opioid-induced hyperalgesia. Co-administration of morphine and LY2828360 (10 and 0.1 mg/kg per day respectively, i.p. x 6 days) in Phase II increased mechanical withdrawal thresholds (Fig. 7B) and decreased cold sensitivity (Fig. 7C) in male but not male AdvillinCRE/+; mice (Mechanical: Time: F5,120 = 165.8, p < 0.0001; Group: F3,24 = 40.39, p < 0.0001; Interaction: F15120 = 7.958, p < 0.0001; Cold: Time: F5120 = 162.8, p < 0.0001; Group: F3,24 = 16.75, p < 0.0001; Interaction: F15120 = 11.82, p < 0.0001). This morphine-sparing effect of LY2828360 treatment was absent in female (Fig. 7D, E) mice (Mechanical: Time: F5,125 = 130, p < 0.0001; Group: F3,25 = 0.2787, p = 0.8403; Interaction: F15125 = 2.225, p = 0.0086; Cold: Time: F5,120 = 208.7, p < 0.0001; Group: F3,24 = 2.456, p = 0.0876; Interaction: F15120 = 2.591, p = 0.0021); Sidak’s multiple comparisons test revealed increased mechanical withdrawal threshold and decreased cold sensitivity on days 7–12 in male: Mor+LY mice (p < 0.0001 compared to paclitaxel baseline), but not male: Mor+VEH or female mice. By day 12, all groups showed enhanced cold sensitivity compared to paclitaxel baseline (p < 0.0006) with the exception of male AdvillinCRE/+; Mor+VEH group that did not differ from paclitaxel baseline (p = 0.0617).
Fig. 7.
Co-administration with LY2828360 reverses morphine tolerance in male, but not female mice, and had no effect on morphine tolerance in AdvillinCRE/+; mice of either sex. (A) Schematic shows the injection schedule used to evaluate the two-phase treatment during the maintenance phase of paclitaxel-induced neuropathic pain. Chronic administration of morphine (10 mg/kg per day i.p. x 6 days) initially alleviated paclitaxel-evoked mechanical (B, D) and cold (C, E) allodynia in both male and female and AdvillinCRE/+; mice, but later resulted in tolerance. Co-administration of morphine with low-dose LY2828360 (0.1 mg/kg per day i.p. x 6 days) caused a reversal of tolerance to morphine’s mechanical (B) and cold (C) anti-allodynic effects in male , but not male AdvillinCRE/+; mice. LY2828360 had no effect on mechanical (D) or cold (E) allodynia in female or female AdvillinCRE/+; mice and did not block the development of tolerance to the anti-allodynic effects of morphine (n = 6–8 per group). Data expressed as mean ±SEM, ***p < 0.001 (two-way ANOVA, Sidak’s multiple comparisons test).
In paclitaxel-treated mice, naloxone challenge produced characteristic jumping behavior that was higher in females compared to males and withdrawal jumps were lower overall in mice receiving concurrent LY2828360 + morphine compared to morphine alone (: Treatment: F1,28 = 4.903, p = 0.0351; Sex: F1,28 = 4.355, p = 0.0461; Interaction: F1,28 = 0.0798, p = 0.7796; Fig. 8A). By contrast, in AdvillinCRE/+; mice receiving the same treatments, no effect of sex or concurrent LY2828360 treatment was observed (AdvillinCRE/+; : Treatment: F1,21 = 0.09588, p = 0.7599; Sex: F1,21 = 0.5371, p = 0.4717; Interaction: F1,21 = 0.2613, p = 0.6146; Fig. 8B). No withdrawal jumps or signs of abnormal behavior were observed in the 30 min observation interval measured after the final chronic drug injection prior to naloxone challenge in any group.
Fig. 8.
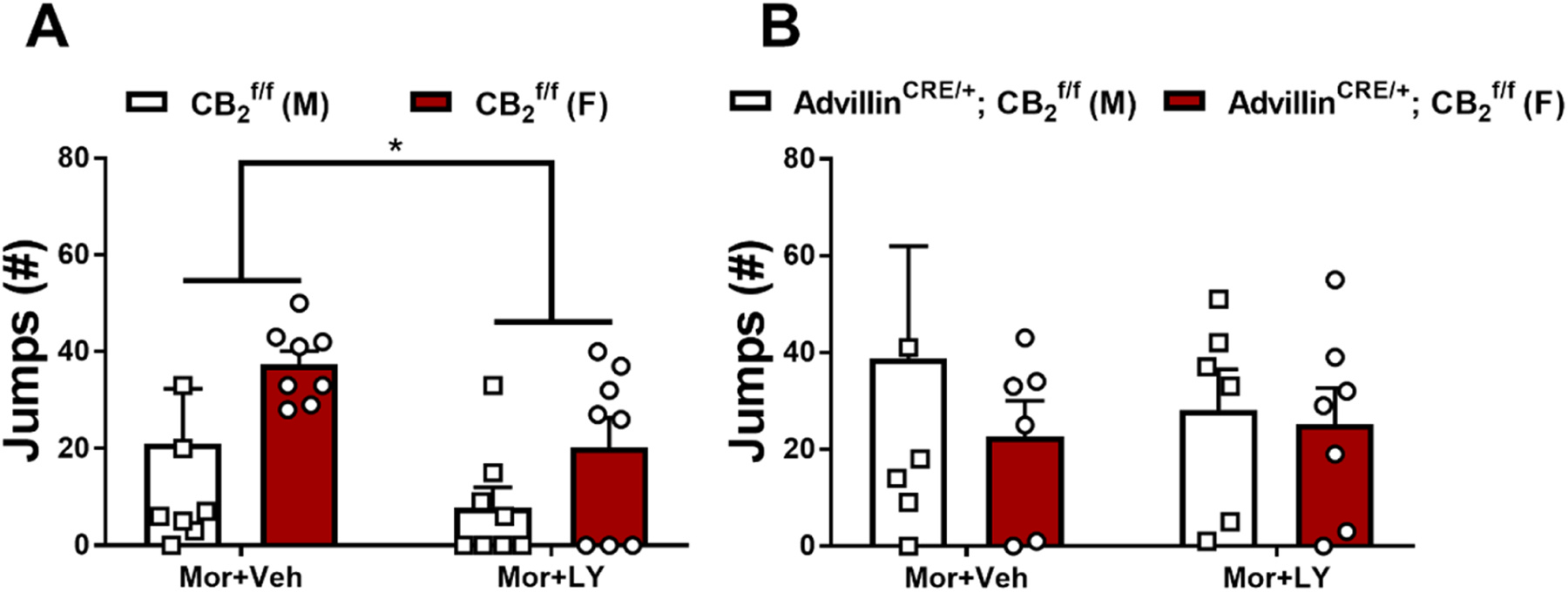
Impact of co-administration of low-dose LY2828360 with morphine (0.1 and 10 mg/kg per day i.p. x 6 days, respectively) on naloxone-precipitated morphine withdrawal in male and female and AdvillinCRE/+; mice used in Experiment 5. mice treated with morphine + vehicle instead of LY2828360 showed an increased number of jumps compared to the LY2828360 + morphine group. No difference in withdrawal jumps was observed between groups in AdvillinCRE/+; mice (n = 6–8 per group). Data expressed as mean ±SEM, *p < 0.05 (two-way ANOVA).
3.6. Experiment 6. The CB2 agonist LY2828360 dose dependently reduced mechanical and cold allodynia in both and CX3CR1CRE/+; mice
LY2828360 dose-dependently increased mechanical paw withdrawal thresholds (Fig. 9A) and decreased cold sensitivity (Fig. 9B) irrespective of genotype in paclitaxel-treated and CX3CR1CRE/+; mice (Mechanical: Dose: F4112 = 121.4, p < 0.0001; Genotype: F1,28 = 0.3664, p = 0.5499; Interaction: F4112 = 0.3888, p = 0.8163; Cold: Dose: F4112 = 91.56, p < 0.0001; Genotype: F1,28 = 0.7823, p = 0.3840; Interaction: F4112 = 2.179, p = 0.0758). LY2828360 did not alter mechanical (Fig. 9C) or cold (Fig. 9D) responsiveness in or CX3CR1CRE/+; mice treated with cremophor-based vehicle in lieu of paclitaxel (Mechanical: Dose: F4,40 = 1.883, p = 0.1322; Genotype: F1,10 = 0.00136, p = 0.9713; Interaction: F4,40 = 1.6, p = 0.1931; Cold: Dose: F4,40 = 10.1, p < 0.0001; Genotype: F1,10 = 0.2299, p = 0.6419; Interaction: F4,40 = 1.016, p = 0.4106).
Fig. 9.
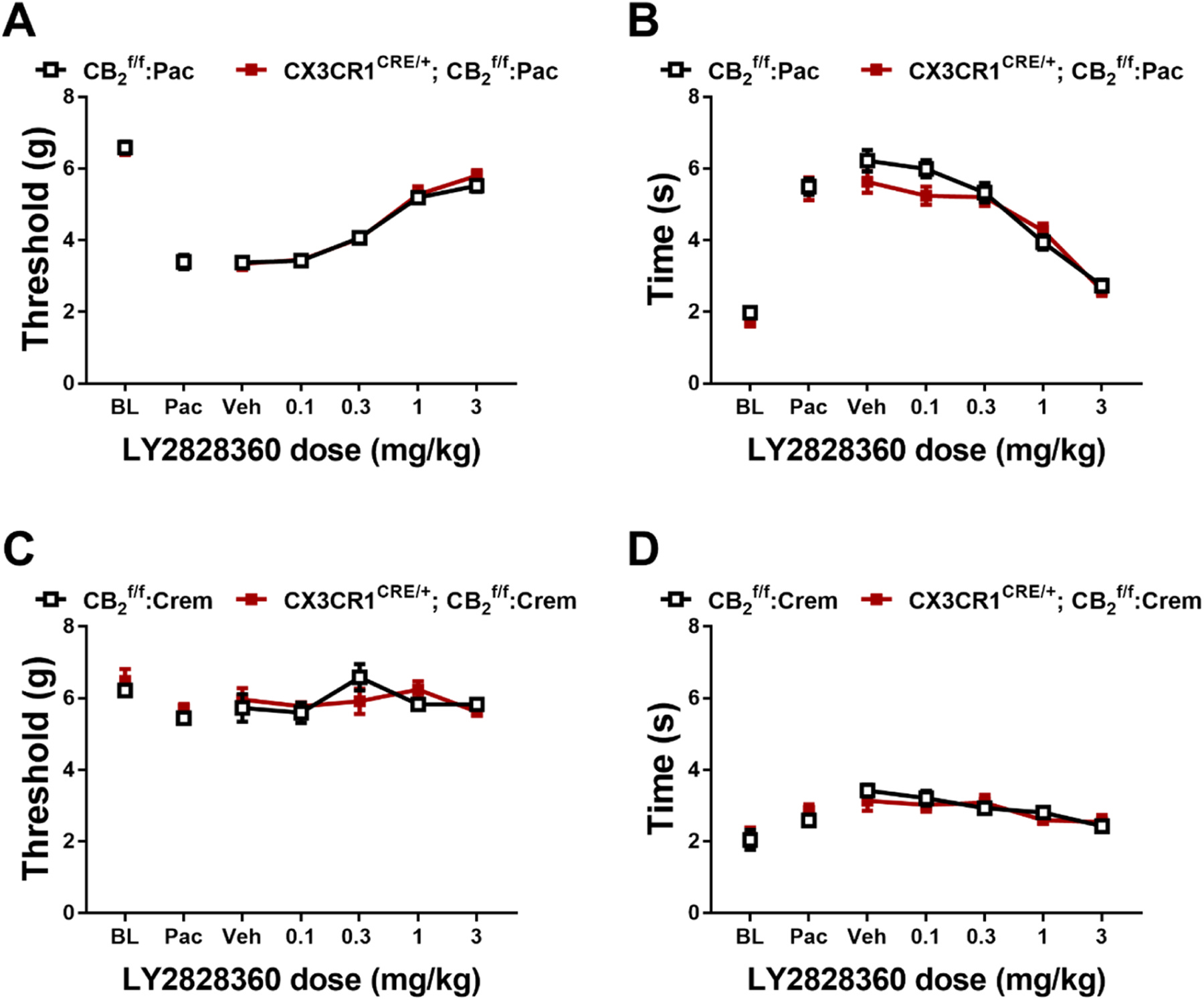
LY2828360 dose-dependently alleviated paclitaxel evoked mechanical (A) and cold (B) allodynia in both and CX3CR1CRE/+; mice. LY2828360 did not alter mechanical (C) or cold (D) sensitivity in and CX3CR1CRE/+; control mice treated with cremophor vehicle (n = 6–16 per group, mixed sex). Data expressed as mean ±SEM (two-way ANOVA).
3.7. Experiment 7. Previous chronic administration of LY2828360 blocked the development of morphine tolerance in male, but not female and CX3CR1CRE/+; mice
To examine the effects of LY2828360 treatment on the development of morphine tolerance in CX3CR1CRE/+; mice, a two-phase pharmacological manipulation was used after neuropathic nociception was established and stable (Fig. 10A). Phase I treatment with LY2828360 (3 mg/kg per day i.p. x 12 days) increased mechanical withdrawal thresholds (Fig. 10B,D) and decreased cold sensitivity (Fig. 10C,E) in both male (Mechanical: Time: F5,70= 54.57, p < 0.0001; Genotype: F1,14 = 0.07038, p = 0.7946; Interaction: F5,70 = 0.279, p = 0.9232; Cold: Time: F5,70 = 87.75, p < 0.0001; Genotype: F1,14 = 0.4061, p = 0.5342; Interaction: F5,70 = 0.2365, p = 0.9451) and female (Mechanical: Time: F5,55 = 52.19, p < 0.0001; Genotype: F1,11 = 0.2598, p = 0.6203; Interaction: F5,55 = 0.9811, p = 0.4376; Cold: Time: F5,55 = 58.25, p < 0.0001; Genotype: F1,11 = 0.1961, p = 0.6665; Interaction: F5,55 = 0.6459, p = 0.6657) mice irrespective of genotype; Sidak’s multiple comparisons test revealed increased mechanical withdrawal threshold and decreased cold sensitivity on all phase I days tested compared to paclitaxel baseline in both sexes (p < 0.0001). Phase II treatment with morphine (10 mg/kg per day i.p. x 12 days) increased mechanical withdrawal thresholds (Fig. 10B,D) and decreased cold sensitivity (Fig. 10C,E) without the development of tolerance in male mice (Mechanical: Time: F6,84 = 102.8, p < 0.0001; Genotype: F1,14 = 0.1651, p = 0.6907; Interaction: F6,84 = 0.4404, p = 0.8498; Cold: Time: F6,84 = 111.3, p < 0.0001; Genotype: F1,14 = 3.658, p = 0.0765; Interaction: F6,84 = 0.9566, p = 0.4597) but this effect was absent in female mice (Mechanical: Time: F6,66 = 65.94, p < 0.0001; Genotype: F1,14 = 0.719, p = 0.4146; Interaction: F6,66 = 1.327, p = 0.2577; Cold: Time: F6,66 = 81.02, p < 0.0001; Genotype: F1,14 = 0.09744, p = 0.7608; Interaction: F6,66 = 1.36, p = 0.2437); Sidak’s multiple comparisons test revealed that acute treatment with morphine in Phase II (day 16) increased mechanical paw withdrawal thresholds and decreased cold sensitivity compared to day 15 post-paclitaxel baseline responding in both sexes regardless of genotype (p < 0.0001 for all comparisons). Morphine tolerance developed in female mice of both genotypes at subsequent time points (p < 0.0218 on days 19–27 for all timepoints compared to day 16), whereas anti-allodynic effects of morphine were preserved in male mice of both genotypes for the duration of phase 2 (p > 0.0233 on days 19–27 compared to day 16).
Fig. 10.
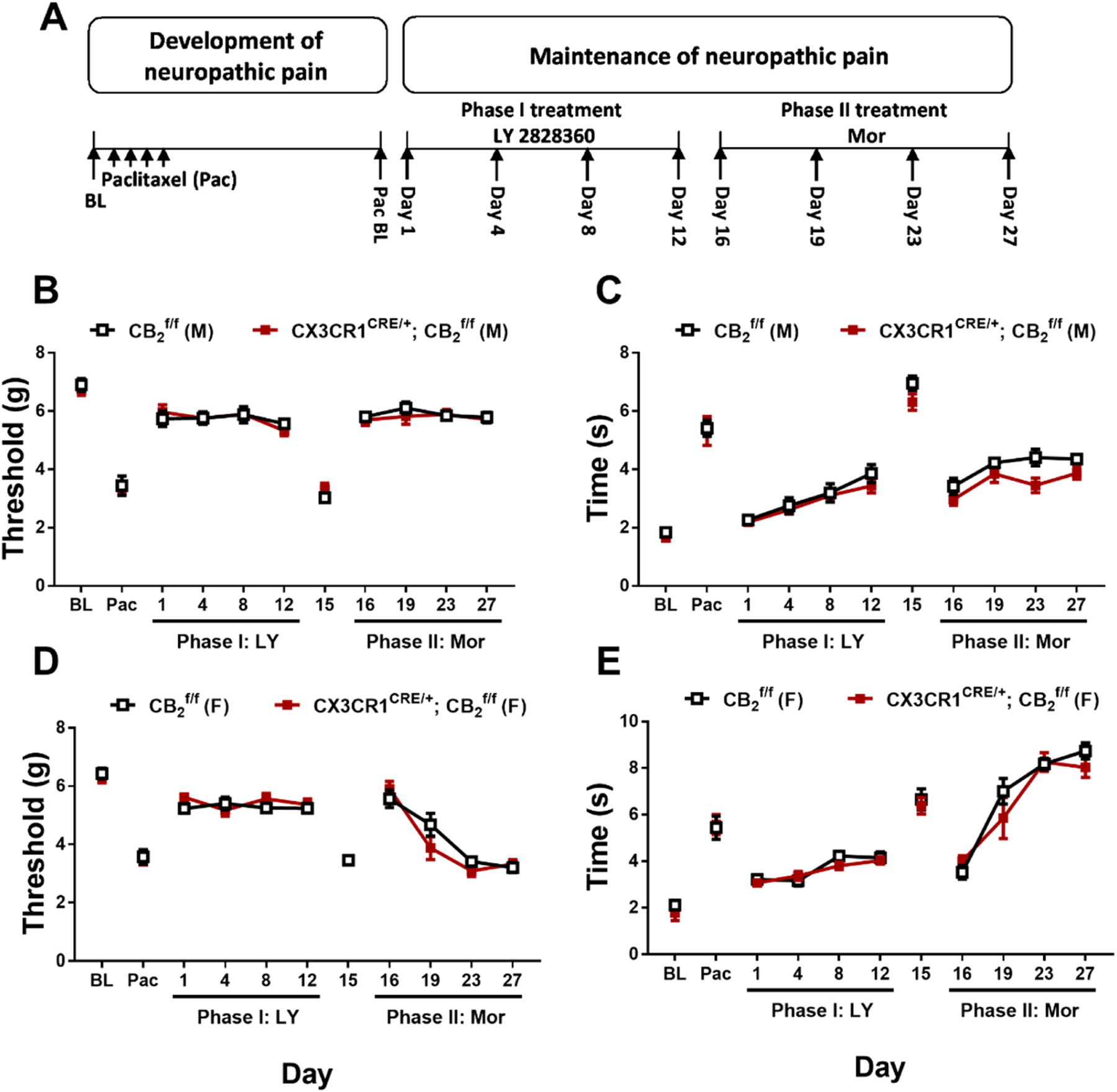
History of chronic LY2828360 treatment blocked the development of morphine tolerance in male, but not female and CX3CR1CRE/+; mice. (A) Schematic shows the injection schedule used to evaluate the two-phase treatment during the maintenance phase of paclitaxel-induced neuropathic pain. Chronic LY2828360 treatment (3 mg/kg per day i.p. x 12 days) alleviated paclitaxel evoked mechanical (B, D) and cold (C, E) allodynia and blocked the development of tolerance to the anti-allodynic effects of morphine (10 mg/kg per day i.p. x 12 days) in male (B, C), but not female (D, E) and CX3CR1CRE/+; mice (n = 6–8 per group). Data expressed as mean ±SEM (two-way ANOVA).
In paclitaxel treated and CX3CR1CRE/+; mice with a history of chronic treatment with LY282860 and morphine, naloxone challenge produced characteristic jumping behavior that did not differ as a function of genotype or sex (Sex: F1,24 = 0.6948, p = 0.4127; Genotype: F1,24 = 0.5811, p = 0.4533; Interaction: F1,24 = 1.904, p = 0.1804; Fig. 11). No withdrawal jumps or signs of abnormal behavior were observed in the 30 min observation interval measured after the final chronic drug injection prior to naloxone challenge in any group.
Fig. 11.
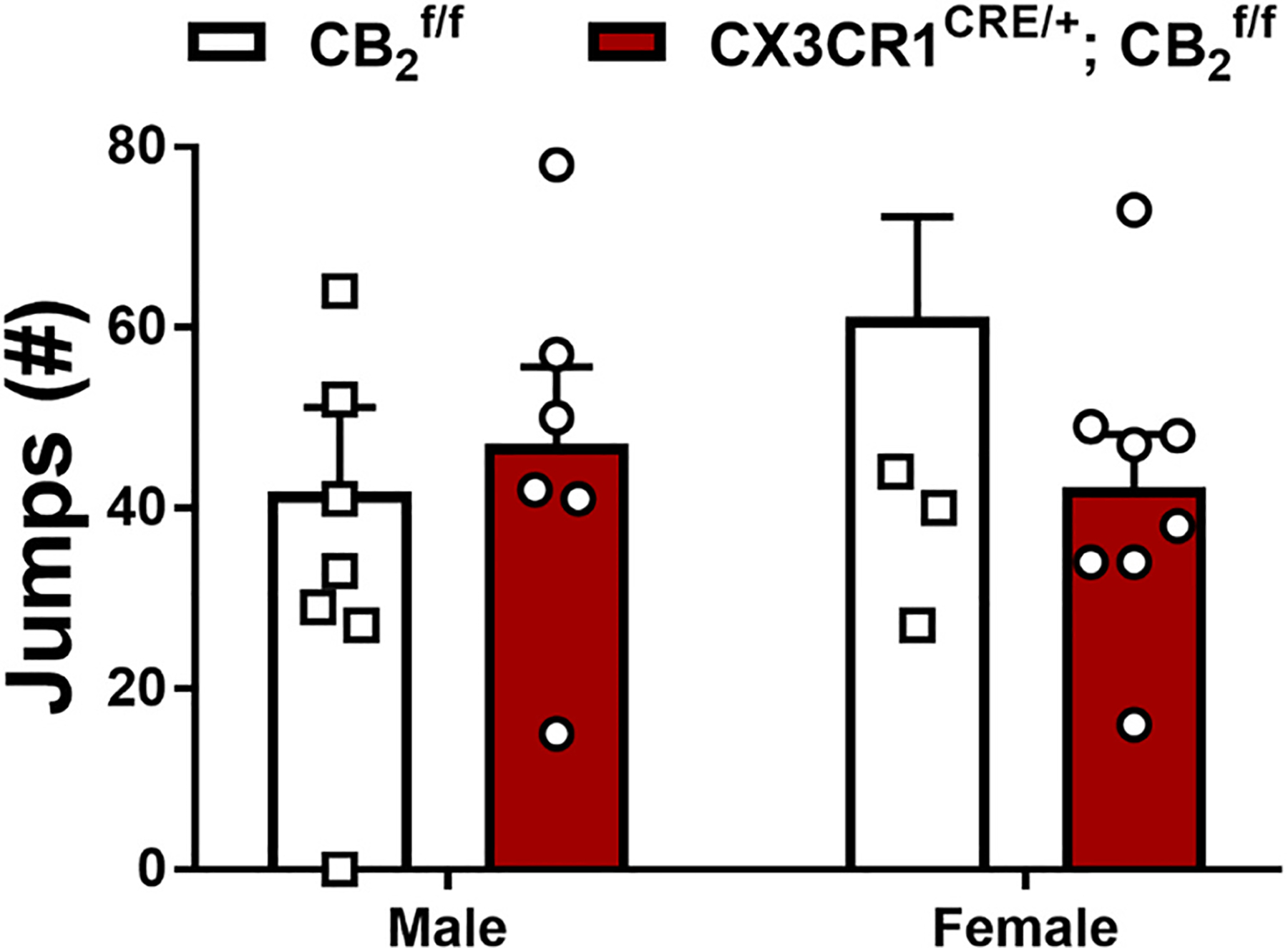
Impact of a history of chronic LY2828360 (3 mg/kg per day i.p. x 12 days) on naloxone-precipitated morphine withdrawal in male and female and CX3CR1CRE/+; mice used in Experiment 7 (n = 6–8 per group). No differences were found between genotype or sex. Data expressed as mean ±SEM, *p < 0.05 (two-way ANOVA).
3.8. Experiment 8. Prophylactic administration of LY2828360 reduced but did not permanently prevent development of mechanical and cold allodynia in both and CX3CR1CRE/+; mice
Prophylactic treatment with LY2828360 for 7 days increased mechanical paw withdrawal thresholds and decreased cold sensitivity during the period of chronic dosing but did not permanently prevent the development of mechanical or cold allodynia in either or CX3CR1CRE/+; mice (Mechanical: Time: F5,80 = 195.1, p < 0.0001; Group: F3,16 = 19.72, p < 0.0001; Interaction: F15,80 = 10.11, p < 0.0001; Cold: Time: F5,80 = 218.7, p < 0.0001; Group: F3,16 = 10.38, p = 0.0005; Interaction: F15,80 = 8.859, p < 0.0001); Sidak’s multiple comparisons test revealed that LY2828360 treatment increased paw withdrawal thresholds in all groups on day 7 of injections, regardless of genotype (p < 0.0001), and this effect of LY2828360 persisted on day 11 in mice compared to vehicle (p = 0.0050). By contrast, no difference in mechanical paw withdrawal threshold was observed between groups at other time points (p > 0.0840), suggesting that LY2828360 delayed, but did not prevent, the development of paclitaxel-induced mechanical allodynia. Similarly, LY2828360 decreased paclitaxel-induced cold responsiveness on day 7 and 11 following initiation of paclitaxel dosing irrespective of genotype. Thus, anti-allodynic efficacy LY282830 outlasted the period of LY2828360 treatment but eventually diminished. Decreased cold sensitivity was observed in mice receiving LY2828360 compared to Vehicle on day 7 and 11 regardless of genotype (p < 0.0051), and this effect still observed on day 18 in mice (p = 0.0167), but not at other timepoints (p > 0.2940; Fig. 12).
Fig. 12.

LY2828360 (3 mg/kg per day i.p. x 8 days) delayed the onset of paclitaxel-induced mechanical (A) and cold (B) allodynia but did not prevent their development. Anti-allodynic effects of LY2828360 were no longer observed 4–7 days following termination of dosing (n = 5 per group, mixed sex). Data expressed as mean ± SEM, **p < 0.005, ***p < 0.001 vs. comparator groups (two-way ANOVA, Sidak’s multiple comparisons test).
4. Discussion
Activation of cannabinoid CB2 receptors suppresses certain types of pathological pain without producing unwanted side effects [e.g.] [14, 32,33]. Our lab has previously examined the ability of the slowly-signaling, G protein-biased CB2 receptor agonist LY2828360 to reduce neuropathic nociception in the paclitaxel model of chemotherapy-induced neuropathic nociception [19,23]. Here we generated mice with a conditional deletion of CB2 receptors from peripheral sensory neurons (AdvillinCRE/+; ) or from CX3CR1-expressing microglia/macrophages (CX3CR1CRE/+; ) to determine the specific population of cells involved in the anti-allodynic effect of LY2828360. First, we validated our AdvillinCRE/+; mouse line using qRT-PCR and showed that both GFP and CB2 mRNA were reduced in the dorsal root ganglia (DRG) derived from AdvillinCRE/+; mice compared to controls, whereas no difference in CB2 mRNA levels were observed in the spinal cord, paw skin or spleen. This finding replicates our previous work showing that AdvillinCRE/+; mice have reduced CB2 and GFP mRNA levels only in the DRG compared to these other tissues [21] and documents that the cKO generated did not result in global spontaneous (germline) deletions. The CX3CR1CRE/+; mouse line used here has been successfully used by our groups to uncover behavioral phenotypes mediated by CB2 agonists in other studies [34] and other models (unpublished).
We used a paclitaxel-induced neuropathy model to compare the anti-allodynic efficacy of LY2828360, which is brain penetrant, with that of AM1710, a CB2 agonist with limited ability to penetrate the CNS [22,26] in paclitaxel-treated mice and two different cKO mouse lines (i.e., AdvillinCRE/+; mice and CX3CR1CRE/+; mice). Both CB2 agonists, LY2828360 and AM1710, dose-dependently reduced mechanical and cold hypersensitivity in mice but lacked anti-allodynic efficacy in AdvillinCRE/+; mice. We also observed anti-allodynic efficacy of LY2828360 in CX3CR1CRE/+; mice. These observations support our hypothesis that CB2 receptors in peripheral sensory neurons are necessary for the anti-allodynic effects of both LY2828360 and AM1710 in paclitaxel-induced peripheral neuropathy. By contrast, CB2 receptors found on CX3CR1-expressing microglia/macrophages do not appear to be involved in the anti-allodynic efficacy of these CB2 agonists. A similar effect was observed in our prior study showing that LY2828360 retains efficacy in CX3CR1CRE/+; but not AdvillinCRE/+; mice in a carrageenan model of inflammatory pain [24]. Additionally, LY2828360 reduced neuropathic nociception produced by the antiretroviral agent 2,3-dideoxycytidine (ddC) in but not in AdvillinCRE/+; mice [21]. Together these studies demonstrate an important role for CB2 receptors in peripheral sensory neurons in CB2-mediated analgesic efficacy in various pain subtypes. By contrast, CB2 receptors in CX3CR1 expressing microglia/macrophages are unlikely to be involved in this phenomenon. Our CX3CR1CRE/+; > mouse model is specific to microglia expressing the C-X3-C Motif Chemokine Receptor 1 gene; therefore our results do not preclude the possibility that other microglia subtypes may contribute to the anti-allodynic effect of CB2 agonists in our studies of inflammatory nociception [24] and toxic neuropathy [21] or in other pain models.
Tolerance limits the therapeutic potential of analgesics such as morphine for treating chronic pain [35]. Our lab has previously shown sustained anti-allodynic efficacy of LY2828360 in the paclitaxel model after repeated dosing in mice [19,21]. We also showed that prior treatment with LY2828360 blocked the development of tolerance to morphine in paclitaxel-treated male mice [19]. However, the cell types underlying these effects were unknown prior to the present report. Here we examined the ability of LY2828360 to prevent or reverse morphine tolerance in paclitaxel-treated and AdvillinCRE/+; mice. In paclitaxel-treated male mice, LY2828360 blocked the development of morphine tolerance; a history of 12-day chronic LY2828360 treatment (3 mg/kg i.p.) prevented subsequent development of tolerance to the anti-allodynic effects of morphine (10 mg/kg i.p. x 12 days) in male and male CX3CR1CRE/+; mice. This effect was sex dependent, as tolerance to the anti-allodynic effects of morphine developed normally in female mice after 3 days in both genotypes, indicating that CB2 receptors only play a critical role in preventing the development of morphine tolerance in male mice. LY2828360 also failed to prevent tolerance to the anti-allodynic effects of morphine in AdvillinCRE/+; mice in either sex, presumably because only male, but not female, mice show the morphine tolerance-sparing effect of LY2828360. These results suggest that CB2 receptors in peripheral sensory neurons but not CX3CR1 expressing microglia/macrophages are necessary for preventing the development of tolerance to the anti-allodynic effects of morphine and that this phenomenon is specific to male mice only. To measure morphine withdrawal in these mice, we challenged mice with naloxone at the end of the 12-day chronic morphine period and measured jumping behavior as a readout of naloxone-precipitated opioid withdrawal. We found that both male and male AdvillinCRE/+; mice showed less jumping behavior in response to naloxone compared to females of both genotypes, whereas no sex differences were observed when comparing mice to CX3CR1CRE/+; mice. Thus, CB2 receptors in peripheral sensory neurons do not appear to be involved in naloxone-precipitated opioid withdrawal behavior as there was no difference in number of withdrawal jumps between male and AdvillinCRE/+ ; mice. Notably, we observed genotypic differences in the development of tolerance to the anti-allodynic effects of morphine in the same mice that received chronic morphine treatment following chronic treatment with LY2828360, with sparing of morphine tolerance observed in male but not male AdvillinCRE/+; mice.
Interestingly, LY2828360 was also able to reverse established morphine tolerance in paclitaxel-treated mice. Tolerance developed in both and AdvillinCRE/+; mice after 3 days of repeated morphine administration and persisted for the entire morphine administration period. When a low dose of LY2828360 (0.1 mg/kg i.p.) was co-administered with morphine after the establishment of morphine tolerance, we observed a restoration of morphine antinociceptive efficacy, consistent with reversal of tolerance to morphine’s anti-allodynic effects. As was seen in the previous morphine tolerance experiment (i.e., in which administration of LY2828360 and morphine never overlapped), this effect was sex dependent as tolerance to morphine’s anti-allodynic effect was maintained in female mice even after co-administration of LY2828360 began. These observations suggest that CB2 receptor activation reduces morphine tolerance in the paclitaxel model in male mice only, a finding which may have clinical implications. Additionally, this reversal of morphine tolerance was not observed in AdvillinCRE/+; mice in either sex, highlighting the importance of CB2 receptors in peripheral sensory neurons in the maintenance of tolerance to morphine’s anti-allodynic effects in males, the only sex in which LY2828360-induced sparing of morphine tolerance was observed [but see 21].
We also compared naloxone-precipitated morphine withdrawal in and AdvillinCRE/+; mice of both sexes. We observed fewer withdrawal jumps in mice that received morphine co-administered with LY2828360 compared to vehicle. These observations suggest that a behaviorally inactive dose of LY2828360, when co-administered with morphine, was sufficient to attenuate withdrawal behavior. By contrast, no protective effect of LY2828360 was observed on withdrawal behavior under the conditions of the two-phase study because LY2828360 was never given concurrently with morphine. In that two-phase study, dosing with LY2828360 terminated 4 days prior to initiation of morphine dosing, and drug effects were likely washed out by the time withdrawal was precipitated with naloxone. By contrast, LY2828360 was on board when co-administered with morphine and was able to impact withdrawal behavior following naloxone challenge, and sex differences in the withdrawal phenotype were not observed. Lastly, in morphine-dependent AdvillinCRE/+; mice we did not observe sex differences or impact of LY2828360 treatment following naloxone-precipitated withdrawal in this experiment. The present results align with previous observations from our lab showing that LY2828360 trended to reduce naloxone-precipitated withdrawal jumps in mice when co-administered with morphine, an effect that was absent in CB2 receptor KO mice [19]. In the present study, we did not evaluate effects of naloxone challenge in mice that receive only vehicle due to breeding limitations; none of the mice used in the present studies are commercially available. However, we previously showed that naloxone does not precipitate withdrawal jumps in mice that receive vehicle in lieu of morphine using an identical protocol to the one used here [19]. Furthermore, no withdrawal jumps (or noteworthy signs of abnormal behavior) were observed in the 30-minute observation interval immediately preceding naloxone challenge (i.e., following the final chronic injection) in any study, indicating that naloxone challenge was necessary to precipitate the observed withdrawal syndrome.
We also investigated whether chronic prophylactic administration of LY2828360 (3 mg/kg i.p.) for 7 days could prevent the development of peripheral neuropathic nociception during the establishment of paclitaxel-induced peripheral neuropathy. LY2828360 delayed, but did not prevent, the development of paclitaxel-induced mechanical and cold allodynia in both and CX3CR1CRE/+; mice. Mechanical and cold hypersensitivity returned following termination of dosing with LY2828360.
Elucidation of therapeutic strategies to prevent or block morphine tolerance has been the focus of significant research efforts [3–6]. The present results show that the CB2 agonist LY2828360 can enhance the anti-allodynic effects of morphine by both preventing and reversing morphine tolerance. However, much of the literature, including our previous report [19] assessed morphine tolerance in male subjects only. The present study replicates findings from Lin et al. [19] showing that chronic pre-treatment with the CB2 agonist LY2828360 can prevent the development of morphine tolerance in a mouse model of paclitaxel-induced neuropathic pain and shows that this phenomenon is preferentially observed in male, but not female, mice. This report also expands upon our initial demonstration of this phenomenon by establishing the cell types that express CB2 that are responsible for this phenomenon. We show that peripheral sensory neuron CB2 receptors are required for the anti-allodynic effects of CB2 agonists in a paclitaxel-induced neuropathy model. We also show that activation of the CB2 receptor can reverse already established morphine tolerance in paclitaxel-treated mice. These results are significant given that many human patients may have pre-existing tolerance to opioids, and, consequently, treatment with LY2828360 may be beneficial in reinstating the anti-allodynic effects of morphine. Overall, LY2828360 may be a useful first line treatment for chemotherapy-induced peripheral neuropathy, as it can supress pathological pain with sustained efficacy, and both prevent and reverse morphine tolerance. Whereas CB2 agonists were effective in suppressing neuropathic nociception in mice of both sexes, the present results suggest that this is not the case for morphine sparing effects, in which a notable sexual dimorphism in sparing of morphine tolerance was observed. Our studies also suggest that a previously underappreciated role of sex may be an important factor to consider in any clinical studies of CB2-opioid interactions.
Acknowledgments
Supported by DA047858 (to AGH and KM), DA009158 (to AM and AGH), an Indiana Addiction Grand Challenge Grant (to AGH), the Research and Education Component of the Advancing a Healthier Wisconsin Endowment at the Medical College of Wisconsin (to CH), and Ministerio de Ciencia e Innovación - Agencia Estatal de Investigación and Fondo Europeo de Desarrollo Regional (Proyecto PID2022-138461OB-I00, supported by MCIN /AEI /10.13039/501100011033 / FEDER, UE (to JR). KG is a Harlan Research Scholar.
Footnotes
CRediT authorship contribution statement
Alexandros Makriyannis: Resources, Funding acquisition. Zhili Xu: Investigation, Formal analysis. Xiaoyan Lin: Investigation, Formal analysis, Conceptualization. Kelsey G. Guenther: Writing – review & editing, Writing – original draft, Visualization, Formal analysis. Andrea Grace Hohmann: Writing – review & editing, Writing – original draft, Visualization, Supervision, Resources, Methodology, Funding acquisition, Conceptualization. Ken Mackie: Writing – review & editing, Resources, Funding acquisition. Cecilia J. Hillard: Resources. Julian Romero: Resources.
Declaration of Competing Interest
The authors have no financial/personal interest or belief that could affect the objectivity of this manuscript. Dr. Hohmann is a consultant for Anagin Inc.
References
- [1].Yekkirala AS, Roberson DP, Bean BP, Woolf CJ, Breaking barriers to novel analgesic drug development, Nat. Rev. Drug Discov 16 (8) (2017) 545–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Coluzzi F, Rullo L, Scerpa MS, Losapio LM, Rocco M, Billeci D, Candeletti S, Romualdi P, Current and Future Therapeutic Options in Pain Management: Multi-mechanistic Opioids Involving Both MOR and NOP Receptor Activation, CNS Drugs 36 (6) (2022) 617–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Habibi-Asl B, Vaez H, Najafi M, Bidaghi A, Ghanbarzadeh S, Attenuation of morphine-induced dependence and tolerance by ceftriaxone and amitriptyline in mice, Acta Anaesthesiol. Taiwan 52 (2014) 163–168. [DOI] [PubMed] [Google Scholar]
- [4].Mansouri MT, Khodayar MJ, Tabatabaee A, Ghorbanzadeh B, Naghizadeh B, Modulation of morphine antinociceptive tolerance and physical dependence by co-administration of simvastatin, Pharmacol. Biochem. Behav 137 (2015) 38–43. [DOI] [PubMed] [Google Scholar]
- [5].Hassanipour M, Amini-Khoei H, Shafaroodi H, Shirzadian A, Rahimi N, Imran-Khan M, Rezayat SM, Dehpour A, Atorvastatin attenuates the antinociceptive tolerance of morphine via nitric oxide dependent pathway in male mice, Brain. Res. Bull 125 (2016) 173–180. [DOI] [PubMed] [Google Scholar]
- [6].Hosseinzadeh H, Parvardeh S, Masoudi A, Moghimi M, Mahboobifard F, Attenuation of morphine tolerance and dependence by thymoquinone in mice, Avicenna J. Phytomed 6 (2016) 55–66. [PMC free article] [PubMed] [Google Scholar]
- [7].Bushlin I, Rozenfeld R, Devi LA, Cannabinoid – opioid interactions during neuropathic pain and analgesia, Curr. Opin. Pharmacol 10 (2010) 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wołyniak M, Małecka-Wojciesko E, Zielińska M, Fabisiak A, A Crosstalk between the Cannabinoid Receptors and Nociceptin Receptors in Colitis—Clinical Implications, J. Clin. Med 11 (22) (2022) 6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Finn DP, Beckett SRG, Roe CH, Madjd A, Fone KCF, Kendall DA, Marsden CA, Chapman V, Effects of coadministration of cannabinoids and morphine on nociceptive behaviour, brain monoamines and HPA axis activity in a rat model of persistent pain, Eur. J. Neurosci 19 (2004) 678–686. [DOI] [PubMed] [Google Scholar]
- [10].Fischer BD, Ward SJ, Henry FE, Dykstra LA, Attenuation of morphine antinociceptive tolerance by a CB(1) receptor agonist and an NMDA receptor antagonist: Interactive effects. Neuropharmacology 58 (2) (2010) 544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kazantzis NP, Casey SL, Seow PW, Mitchell VA, Vaughan CW, Opioid and cannabinoid synergy in a mouse neuropathic pain model, Br. J. Pharmacol 173 (16) (2016) 2521–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rahn EJ, Hohmann AG, Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside, Neurotherapeutics 6 (2009) 713–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cooper ZD, Haney M, Actions of delta-9-tetrahydrocannabinol in cannabis: relation to use, abuse, dependence, Int. Rev. Psychiatr 21 (2009) 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Guindon J, Hohmann AG, Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain, Br. J. Pharmacol 153 (2008) 319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Deng L, Guindon J, Cornett BL, Makriyannis A, Mackie K, Hohmann AG, Chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1-dependent withdrawal, Biol. Psychiatr 77 (2015) 475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lim G, Wang S, Mao J, Central glucocorticoid receptors modulate the expression of spinal cannabinoid receptors induced by chronic morphine exposure, Brain Res. 1059 (2005) 20–27. [DOI] [PubMed] [Google Scholar]
- [17].Merighi S, Gessi S, Varani K, Fazzi D, Mirandola P, Borea PA, Cannabinoid CB (2) receptor attenuates morphine-induced inflammatory responses in activated microglial cells, Br. J. Pharmacol 166 (2012) 2371–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Desroches J, Bouchard JF, Gendron L, Beaulieu P, Involvement of cannabinoid receptors in peripheral and spinal morphine analgesia, Neuroscience 261 (2014) 23–42. [DOI] [PubMed] [Google Scholar]
- [19].Lin X, Dhopeshwarkar AS, Huibregtse M, Mackie K, Hohmann AG, Slowly signaling G-protein biased CB2 cannabinoid receptor agonist LY2828360 supresses neuropathic pain with sustained efficacy and attenuates morphine tolerance and dependence, Mol. Pharmacol 93 (2018) 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li A, Lin X, Dhopeshwarkar AS, Thomaz AC, Carey L, Liu Y, Nikas SP, Makriyannis A, Mackie K, Hohmann AG, Cannabinoid CB2 agonist AM1710 differentially suppresses distinct pathological pain states and attenuates morphine tolerance and withdrawal, Mol. Pharmacol 96 (2019) 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Carey LM, Xu Z, Rajic G, Makriyannis A, Romero J, Hillard C, Mackie K, Hohmann AG, Peripheral sensory neuron CB2 cannabinoid receptors are necessary for both CB2-mediated antinociceptive efficacy and sparing of morphine tolerance in a mouse model of anti-reroviral toxic neuropathy. Pharma. Res 187 (2023) 106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hollinshead SP, Tidwell MW, Palmer J, Guidetti R, Sanderson A, Johnson MP, Chambers MG, Oskins J, Stratford R, Astles PC, Selective cannabinoid receptor type 2 (CB2) agonists: optimization of a series of purines leading to the identification of a clinical candidate for the treatment of osteoarthritic pain, J. Med. Chem 56 (2013) 5722–5733. [DOI] [PubMed] [Google Scholar]
- [23].Lin X, Xu Z, Carey L, Romero J, Makriyannis A, Hillard CJ, Ruggiero E, Dockum M, Houk G, Mackie K, Albrecht PJ, Rice FL, Hohmann AG, A peripheral CB2 cannabinoid receptor mechanism suppresses chemotherapy-induced peripheral neuropathy: evidence from a CB2 reporter mouse, Pain 163 (2022) 834–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Guenther KG, Xu Z, Romero J, Hillard CJ, Mackie K, Hohmann AG, Conditional deletion of CB2 cannabinoid receptors from peripheral sensory neurons eliminates CB2-mediated antinociceptive efficacy in a mouse model of carrageenan-induced inflammatory pain, Neuropharmacology 237 (2023) 109601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pereira A, Chappell A, Dethy J, Hoeck H, Arendt-Nielsen L, Verfaille S, Boulanger B, Jullion A, Johnson M, McNearney T, A proof-of concept (poc) study including experimental pain models (epms) to assess the effecrs of a CB2 agonist (LY2828360) in the treatment of patients with osteoarthritic (oa) knee pain, Clin. Pharmacol. Ther 93 (2013) S56–S57. [Google Scholar]
- [26].Rahn EJ, Thakur GA, Wood JAT, Zvonok AM, Makriyannis A, Hohmann AG, Pharmacological characterization of AM1710, a putative cannabinoid CB2agonist from the cannabilactone class: antinociception without central nervous system side-effects, Pharmacol. Biochem. Behav 98 (2011) 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].López A, Aparicio N, Pazos MR, Grande MT, Barreda-Manso MA, Benito-Cuesta I, Vásquez C, Amores M, Ruiz-Pérez G, García-García E, Beatka M, Tolón RM, Dittel BN, Hilliard CJ, Romero J, Cannabinoid CB2 receptors in the mouse brain: relevance for Alzheimer’s disease, J. Neuroinflamm 15 (2018) 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yona S, Kim K, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S, Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis, Immunity 38 (2013) 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhou X, Wang L, Hasegawa H, Wang F, Deletion of PIK3C3/Vps34 in sensory neurons causes rapid neurodegeneration by disrupting the endosomal but not the autophagic pathway, Proc. Natl. Acad. Sci. USA 17 (2010) 9424–9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Da Silva S, Hasegawa H, Scott A, Zhou X, Wagner AK, Han B, Wang F, Proper formation of whisker barrelettes requires periphery-derived Smad4-dependent TGF-β signaling, Proc. Natl. Acad. Sci. USA 108 (8) (2011) 3395–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Livak KJ, Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method, Methods 25 (2001) 402–408. [DOI] [PubMed] [Google Scholar]
- [32].Murineddu G, Deligia F, Dore A, Pinna G, Asproni B, Pinna GA, Different classes of CB2 ligands potentially useful in the treatment of pain, Recent Pat. CNS Drug. Discov 8 (2013) 42–69. [DOI] [PubMed] [Google Scholar]
- [33].Cabañero D, Martín-García E, Maldonado R, The CB2 cannabinoid receptor as a therapeutic target in the central nervous system, Expert Opin. Ther. Targets 25 (2021) 659–676. [DOI] [PubMed] [Google Scholar]
- [34].Behlke CA, Krause L, Sterrett J, Moe A, Hillard CJ, 2022. Cell specific roles of the CB2R in the acute locomotor response to cocaine [abstract], 2022 June 25–30. In: 32nd Annual Symposium of the International Cannabinoid Research Society. ICRS, Galway, Ireland. Abstract nr 34. [Google Scholar]
- [35].Rosenblum A, Marsch LA, Joseph H, Portenoy RK, Opioids and the treatment of chronic pain: controversies, current status, and future directions, Exp. Clin. Psychopharmacol 16 (2008) 405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]


