Abstract
We have investigated the infectious entry pathway of adeno-associated virus (AAV) and recombinant AAV vectors by assessing AAV-mediated gene transfer and by covalently conjugating fluorophores to AAV and monitoring entry by fluorescence microscopy. We examined AAV entry in HeLa cells and in HeLa cell lines which inducibly expressed a dominant interfering mutant of dynamin. The data demonstrate that AAV internalizes rapidly by standard receptor-mediated endocytosis from clathrin-coated pits (half-time <10 min). The lysosomotropic agents ammonium chloride and bafilomycin A1 prevent AAV-mediated gene transfer when present during the first 30 min after the onset of endocytosis, indicating that AAV escapes from early endosomes yet requires an acidic environment for penetration into the cytosol. Following release from the endosome, AAV rapidly moves to the cell nucleus and accumulates perinuclearly beginning within 30 min after the onset of endocytosis. We present data indicating that escape of AAV from the endosome and trafficking of viral particles to the nucleus are unaffected by the presence of adenovirus, the primary helper virus for a productive AAV infection. Within 2 h, viral particles could be detected within the cell nucleus, suggesting that AAV enters the nucleus prior to uncoating. Interestingly, the majority of the intracellular virus particles remain in a stable perinuclear compartment even though gene expression from nuclear AAV genomes can be detected. This suggests that the process of nuclear entry is rate limiting or that AAV entry involves multiple pathways. Nevertheless, these data establish specific points in the AAV infectious entry process and have allowed the generation of a model for future expansion to specific cell types and AAV vector analysis in vivo.
Gene transfer vectors based on adeno-associated virus type 2 (AAV-2) show great promise for use in human gene therapy. Several clinical trials using these reagents have recently commenced, and a number of patients have already been treated. Although the clinical success of this relatively new vector system has yet to be established, important information has been gained from these as well as a number of earlier, preclinical studies. While AAV can efficiently transfer genes to a number of different cell types (muscle, brain, and liver) (36), it is apparent that there are obstacles which can limit transduction of certain cell types in vivo (2, 17, 31, 44). The further development of AAV-based gene therapy vectors will likely benefit from a more detailed understanding of the interactions between these vectors and target cells and the implications of these interactions to successful gene transfer. Recently, the cellular receptors which define the cell types potentially amenable to AAV-mediated gene therapy have been described (30, 40, 41). However, the mechanisms by which AAV vectors are taken up into cells, translocated to the nucleus, and positioned within the cell such that their transgenes can be expressed are still poorly understood.
Viral receptors are often involved in defining the host range and specific tissue tropism of a virus. Recently, biochemical and genetic evidence has been provided which suggests that cell surface heparan sulfate proteoglycan (HSPG) serves as the primary attachment receptor for AAV (41). Fibroblast growth factor receptor and αvβ5 integrin have also been implicated as coreceptors or facilitators of AAV entry into target cells (30, 40). However, little is known about the process of AAV infection following attachment to the cell surface. In fact, the mechanism of entry for most nonenveloped viruses is not well understood. Several different mechanisms have been suggested based on morphological, ultrastructural, and biochemical studies. Direct penetration upon interaction with the plasma membrane has been suggested for some nonenveloped viruses, such as rotavirus (20), whereas other nonenveloped viruses (e.g., adenovirus, reovirus, poliovirus, and rhinovirus) are believed to follow, at least initially, the same intracellular route as that described for the enveloped viruses (25, 29, 39, 46–48). A common paradigm for these viruses involves binding to a cell surface receptor followed by receptor-mediated endocytosis and endosome release. Although little is known about the entry of AAV, a few studies of the related autonomous parvoviruses, canine parvovirus and minute virus of mice, have been conducted. Minute virus of mice was reported to bind to both specialized (coated pits) and unspecified regions of the cell membrane, and ultrastructural studies have suggested that its internalization occurs via coated pits (24). Similarly, canine parvovirus has been shown to be taken up into cells via small endocytic vesicles and quickly released from these vesicles into the cytoplasm (3, 4). The release of these viruses may take place upon acidification of the vesicle. However, the mechanism and time course of this release from the endosomal compartments remain unclear. Furthermore, the mechanism and time course by which the viral particles, once released into the cytoplasm, travel to the nucleus for expression is unknown. The focus of this study was to evaluate the infectious entry pathway of AAV following attachment of the virus to the cell surface. To accomplish this, we have developed methods of conjugating fluorophores directly to the AAV capsid while preserving the ability of the virus to interact with the cell in a normal manner. Using AAV-2 and recombinant AAV-2-based vectors as a model to interact with and transfer genes into HeLa cells in vitro, the data presented here describe the early steps of AAV entry into human cells. During these analyses we observed that bound AAV particles enter the cell very rapidly via receptor-mediated endocytosis through clathrin-coated pits, that release of the virus into the cytosol occurs within 30 min postinfection and requires endosomal acidification, and that translocation of virus particles results in rapid perinuclear accumulation followed by slower entry into the cell nucleus. These observations begin to define a paradigm for AAV and AAV vector entry in target cells which should impact the utility of these reagents for human gene therapy by providing a molecular understanding of rate-limiting steps required for successful gene transduction.
MATERIALS AND METHODS
Cell culture.
HeLa and 293 cells, which have been described elsewhere (12, 14), were obtained from the American Type Culture Collection, Manassas, Va. and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100 μg/ml) (GIBCO, Grand Island, N.Y.) at 37°C in a 5% CO2 atmosphere. For microscopy, cells were seeded onto two-well chambered tissue culture treated glass slides (Falcon) at a density of 104 cells per chamber or onto four-well chambered cover glasses at a density of 5 × 103 cells per chamber and were used 24 to 48 h later. Chambered cover glasses were coated with poly-d-lysine (Sigma; 0.1 mg/ml; 1 h) prior to use. For confocal microscopy, HeLa S3 cells were grown in modified essential medium (S-MEM; GIBCO) containing 5% horse serum and 5% FBS.
HeLa cells expressing wild-type or the K44A mutant form of dynamin under tetracycline-inducible control (tTA-HeLa) were kind gifts from Sandra Schmid (Scripps Research Institute, La Jolla, Calif.) (9) and were initially maintained in DMEM supplemented with 10% heat-inactivated FBS, 400 μg of gentamicin/ml, 200 ng of puromycin/ml, and 1 μg of tetracycline/ml. For induction of dynamin overexpression, the cells were cultured in the absence of tetracycline for 2 days before being exposed to AAV.
Viruses and vector production and assay.
Adenovirus dl309 (19) has been described previously. Plasmid psub201, used to generate wild-type AAV, and plasmid pAB-11, used to generate AAVlacZ, have been described previously (13, 35). Plasmid pTRUF-5, kindly provided by Sergei Zolotukhin and Nicholas Muzyczka (University of Florida), was used to generate AAVEGFP. Both recombinant AAV vectors express transgenes under the control of the cytomegalovirus immediate-early promoter-enhancer. AAV vectors were produced in the absence of helper virus in 293 cells and purified either by successive bandings on CsCl gradients (23, 38) or by heparan sulfate (HS) affinity chromatography (8, 49). Wild-type AAV was prepared in adenovirus-infected cells by transfection of psub201 plasmid DNA as described previously (34). For preparation of fluorescently labeled AAV, the purity of virus was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to ensure that adenovirus and cellular proteins had been completely removed. Preparations containing material other than the three AAV nonstructural proteins to the limit of detection either were further purified or were discarded because they proved unsatisfactory for fluorescence labeling. Purified virus particles were dialyzed into 10 mM Tris–150 mM NaCl (pH 7.8) containing 10% glycerol and frozen at −80°C until required for use. Particle numbers for wild-type AAV were determined by protein quantitation (7.5 μg of protein is equivalent to 1012 particles). The titer of the recombinant virus, expressed as 293 cell transducing units, was determined by infecting 293 cells in the presence of adenovirus (dl309) and staining for β-galactosidase activity at 36 h (37). Radiolabeled AAV was prepared by adding [3H]methylthymidine (Amersham; 1 μCi/ml final concentration) to 293 cells 8 h after adenovirus (dl309) and wild-type AAV infection. The infected cells were harvested at 48 h postinfection, and the virus was purified as described above. The activity of the labeled virus was approximately 2 × 10−7 cpm per virus particle.
Fluorescent probes.
Purified virus was labeled with the carbocyanine dyes Cy2 and Cy3 (Amersham). Labeled AAV was prepared by adjusting AAV stocks to a concentration of 1 mg/ml (1.33 × 1014 particles/ml) in sodium carbonate-sodium bicarbonate buffer, pH 9.3. Buffer exchange was accomplished by rapid dialysis or by gel filtration on Sephadex G-50 (Pharmacia) spin columns. One milliliter of virus preparation was used to reconstitute the labeling reagent. After 30 min at room temperature, labeled virus was purified by extensive dialysis against 10 mM Tris (pH 7.8)–150 mM NaCl with 10% glycerol, by gel filtration on Sephadex G-50 using the same buffer, or by HS affinity chromatography. The purified virus was aliquoted into single-use vials and stored at −80°C prior to use. Labeled AAV was analyzed by SDS-PAGE to determine the specificity of the labeling reaction and the degree of protein cross-linking. Dye concentration was determined spectrophotometrically, using a molar extinction coefficient of 150,000 M−1 cm−1 at 552 nm for the Cy3 dye and an experimentally determined molar extinction coefficient at 280 nm for purified unlabeled AAV particles. Cy3-labeled adenovirus (Cy3Ad) was prepared as described by Leopold et al. (22).
Virus infections.
HeLa cells were infected with 1.25 × 1011 particles of Cy3-conjugated AAV (Cy3AAV)/ml (approximately 106 particles/cell) in binding buffer (DMEM containing 2 mM glucose, 10 mM HEPES [pH 7.3], and 1% bovine serum albumin) at 37°C unless otherwise noted. Cells were washed three times with binding buffer prior to infection. Lengths of viral exposure are noted but usually consisted of a short, 10-min pulse, after which the cells were washed twice with acid wash buffer [50 mM 2-(N-morpholino)ethanesulfonic acid (MES)–280 mM sucrose, pH 5] and three times with binding buffer. After being washed, the cells were either fixed immediately or maintained at 37°C during additional incubation periods. Prior to fixation, the binding buffer was removed and the cells were washed three times with ice-cold phosphate buffered saline (PBS; GIBCO, Grand Island, N.Y.). Cells were fixed with 4% paraformaldehyde in PBS for 15 min at room temperature and washed three times with PBS. Where indicated, cells were either treated for 5 min at room temperature with 1-μg/ml DAPI (4′,6′-diamidino-2-phenylindole; Molecular Probes, Inc., Eugene, Oreg.) in PBS–0.1% Triton X-100 and washed three times with PBS or mounted in medium containing DAPI (VECTASHIELD; Vector Laboratories, Inc., Burlingame, Calif.). In some experiments, cells were treated with 25 nM ammonium chloride or bafilomycin A1 (Sigma) as indicated.
Fluorescence microscopy.
Images were collected by the use of a Leica DMIRB microscope equipped with 40× NA 0.7 PlanApo DIC, 60× NA 1.4 PlanApo DIC, and 100× NA 1.4 PlanApo DIC objectives and a Hamamatsu intensified cooled charge-coupled device camera. The 16-bit images were digitally enhanced by subtraction of background and by gray-scale adjustment.
RESULTS
Characterization of fluorescently labeled virus.
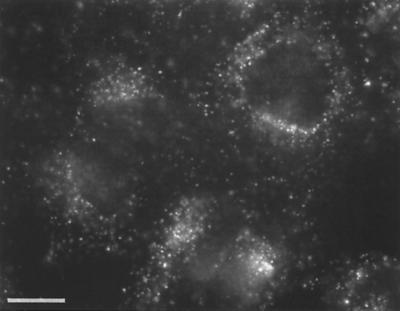
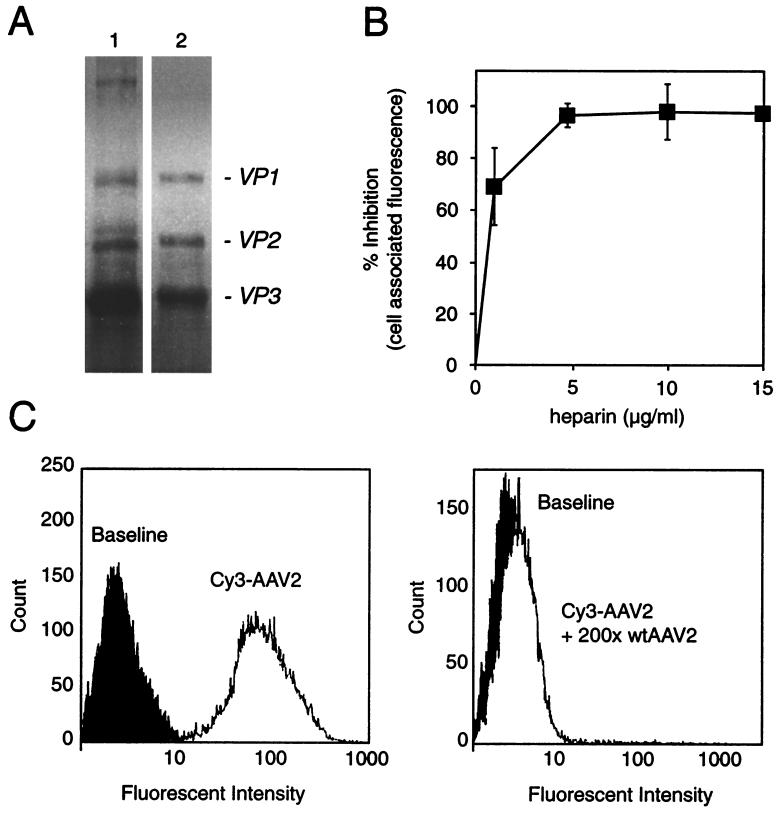
Fluorescently labeled AAV was generated to study the viral entry pathway within the host cell. We characterized this reagent extensively in order to validate wild-type AAV entry steps. Virus labeled with cyanine-based reagents was readily visualized within HeLa cells after viral infection by standard fluorescence microscopy (Fig. 1). Since the fluorescent labeling of the virus involved the covalent modification of the capsid and exposure of virus to harsh conditions (pH 9.3), we were concerned that this process might alter the biological or physical properties of the viral particles. Although the attachment of fluorophore led to a small amount of protein cross-linking (less than 5%, as assessed by SDS-PAGE) (Fig. 2A), specificity for the viral receptor (Fig. 2B), virus attachment (Fig. 1 and 2C), and virus internalization (Fig. 1) were unchanged. Labeled virus particles that maintained normal physical and biological properties had dye-to-viral particle ratios in the range of 1.6 to 2.3 (n = 4). There were no detrimental effects on the physical properties of the viral particles (Fig. 2) or significant biological effects as evidenced by changes in viral titer (∼2 × 1011 infectious units/ml both prior to and following fluorescence labeling). However, at dye-to-particle ratios above the specified range, e.g., >4 (n = 2), titers were severely affected and virus particles were prone to aggregation and precipitation. For this reason, all studies were carried out with AAV labeled at a dye-to-particle ratio of approximately 2. It should also be noted that by SDS-PAGE analysis it was shown that only viral capsid proteins were labeled with fluorophore. Fluorophore was not seen associated with cellular proteins or with any of the nonstructural viral proteins (Fig. 2a). However, it must be stressed that to achieve this level of specificity it was necessary to extensively purify AAV virions. Virus was purified either on three successive CsCl gradients or by a combination of CsCl gradient purification and HS affinity chromatography prior to labeling. Furthermore, all preparations were carefully monitored for the presence of degraded viral proteins or cellular proteins by SDS-PAGE to ensure specificity of the final labeled AAV preparation. The specificity and quality of the labeled virus are demonstrated by the ability to compete cell-associated Cy3AAV-2 fluorescence on HeLa cells with either excess unlabeled virus (Fig. 2C) or soluble HS (Fig. 2B). These results also demonstrate that the labeling reaction has not altered the ability of the virus to interact with its primary attachment receptor on the cell surface. Further evidence that fluorescent AAV has maintained its dependence on HSPG-mediated attachment was demonstrated by our ability to repurify labeled virus via HS affinity chromatography and by the cell binding profile of these reagents for HSPG-deficient cell lines (41). Validation of the labeled virions as described above provided a unique reagent to assay entry mechanisms of AAV infection.
FIG. 1.
Uptake of Cy3AAV-2 by HeLa cells. HeLa cells were incubated with 1.25 × 1011 particles of Cy3AAV-2/ml for 10 min at 37°C, washed to remove virus particles that had not internalized, and maintained at 37°C for 30 min. Labeled virus was visualized by fluorescence microscopy. Bar, 10 μm.
FIG. 2.
Characterization of Cy3-labeled AAV particles: Cy3AAV-2 maintains dependence on HSPG-mediated attachment. (A) SDS-PAGE analysis of labeled capsid proteins (lane 1) and unmodified capsid proteins (lane 2). Protein cross-linking due to the labeling reaction was less than 5%. (B) Quantitative assessment of cell-associated Cy3 fluorescence intensity following coincubation of HeLa cells with Cy3AAV-2 and increasing amounts of heparin. (C) Assessment of cell-associated Cy3 fluorescence by fluorescence-activated cell sorter analysis. HeLa cells were incubated with 2.5 × 1010 particles of Cy3AAV-2/ml for 30 min at 4°C (left panel) or with 2.5 × 1010 particles of Cy3AAV-2/ml in the presence of 5 × 1012 particles of unlabeled AAV-2 (wtAAV2) for 30 min at 4°C (right panel). Both sets of cells were then washed, fixed, and directly subjected to flow-cytometric analysis.
AAV is rapidly internalized via clathrin-coated pits.
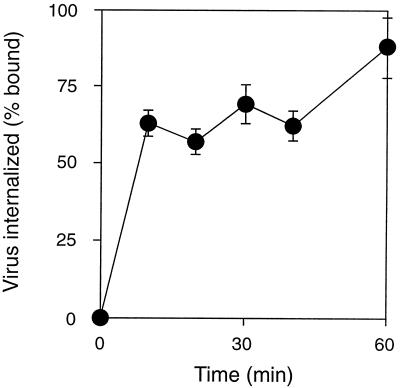
Virus internalization was monitored by using both fluorescently and radioactively labeled AAV-2. However, the use of 3H-labeled virus allowed easier quantification of AAV entry. HeLa cells, which express AAV attachment receptors (HSPG) at high levels, were incubated with [3H]AAV-2 at 4°C for 60 min, washed to eliminate unattached virus, and then incubated at 37°C for different lengths of time (0 to 90 min). Virus particles that had not been internalized were removed from the cell surface by washing with a mildly acidic buffer, and cell-associated radioactivity was determined by scintillation counting. More than 60% of the bound virus particles were taken up into the cells within the first 10 min of incubation at 37°C (Fig. 3). Thus, the internalization half-time for AAV is less than 10 min. Although this rapid entry of AAV into the host cells is indicative of receptor-mediated endocytosis, it was of interest to determine whether productive infection by AAV requires endocytosis from clathrin-coated pits. The recent identification of host cell proteins that regulate clathrin-mediated endocytosis provided an opportunity to more precisely define the mechanism of AAV entry.
FIG. 3.
Internalization of AAV-2 by HeLa cells. HeLa cells were incubated with [3H]AAV-2 at 4°C for 60 min, washed to remove unattached virus, and then incubated at 37°C for various lengths of time (0 to 90 min). Virus that had not internalized was removed from the cell surface by washing with a mildly acidic buffer, and cell-associated radioactivity was determined by scintillation counting. Values shown are means ± standard error and have been adjusted by subtraction of background radioactivity (n = 3).
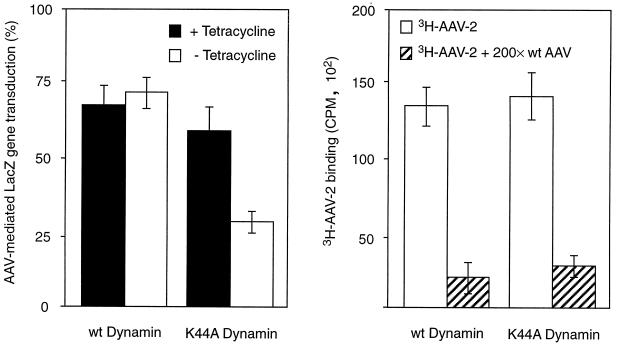
Dynamin is a 100-kDa cytosolic GTPase that selectively regulates clathrin-mediated endocytosis. Dynamin associates with clathrin-coated membrane invaginations and has been proposed to mediate the constriction of coated pits and the budding of coated vesicles from the plasma membrane (18, 43). A dominant-negative mutant form of dynamin containing a point mutation in the GTP binding domain (Lys-44 to Ala-44 [K44A]) has been shown to block clathrin-mediated endocytosis (9). AAV infection of HeLa cells expressing the K44A mutant dynamin was measured by using the AAVlacZ vector. Uninduced tTA-HeLa cells or cells which had been induced by removal of tetracycline for 48 h were infected with AAVlacZ at a virus particle-to-cell ratio of 1,000:1. The number of cells expressing the lacZ reporter gene was then quantitated 48 h postinfection by 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) histochemistry analysis. A significant decrease in AAV-mediated lacZ transduction was observed in HeLa cells induced for K44A expression (minus tetracycline) compared to that evident in tetracycline-regulated HeLa cells (Fig. 4, left panel). The level of AAV-mediated gene delivery to HeLa cells in the presence of tetracycline was the same as that to HeLa cells overexpressing wild-type dynamin under the control of the tetracycline-regulated promoter (Fig. 4, left panel). Furthermore, induction of mutant dynamin 30 min following infection did not inhibit reporter gene expression (data not shown), supporting the involvement of this protein in AAV entry steps prior to gene expression. We also determined that expression of mutant K44A dynamin, or overexpression of wild-type dynamin, did not interfere with virus attachment by analyzing Cy3AAV-2 binding to tetracycline-induced and uninduced HeLa cell lines (Fig. 4, right panel). Therefore, inhibition of AAV-mediated gene delivery was due to decreased virus uptake and not to inhibition of virus attachment or vector gene expression. These findings demonstrate that the predominant route of AAV entry into HeLa cells is via receptor-mediated endocytosis.
FIG. 4.
Entry of AAV-2 is mediated by dynamin-associated uptake pathways. AAV-2-mediated gene transfer (left panel) and AAV-2 attachment (right panel) to HeLa cells overexpressing wild-type (wt) dynamin were compared to those of cells expressing mutant (K44A) dynamin. Cells were exposed to [3H]AAV-2 (1010 particles/ml) or AAVlacZ (108 particles/ml) for 2 h at 4°C, and either attachment (3HAAV-2) was measured immediately or gene transfer (AAVlacZ) was assessed 48 h later. Expression of wild-type or mutant dynamin was induced by removal of tetracycline from the growth medium, as described in Materials and Methods. (Left panel) AAV-2-mediated gene transfer is shown in the presence and absence of tetracycline; (right panel). AAV-2 binding is shown in the absence of tetracycline. The specificity of AAV-2 binding was determined by competition with a 200-fold molar excess of unlabeled virus (wt AAV). Values shown are means ± standard error (n = 6 for each).
AAV requires passage through an acidic compartment for productive infection.
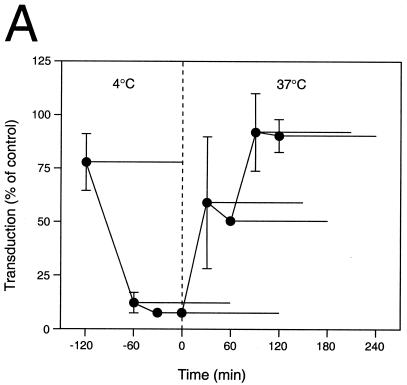
Biochemical studies using the lysosomotropic drug ammonium chloride (28) or the proton pump inhibitor bafilomycin A1 (6) showed that these drugs have a significant inhibitory effect on AAV infectivity and AAV-mediated gene expression (Fig. 5). These drugs essentially block infection of HeLa cells when present during the first 30 min after the onset of endocytosis. Ammonium chloride is known to raise the pH of intracellular organelles within 1 min following addition (28), making it possible to inhibit a low-pH-dependent endosomal escape mechanism at defined time points. The inhibitory effect of ammonium chloride on AAV-mediated gene transfer in HeLa cells is demonstrated in Fig. 5A. This drug did not significantly influence AAV-mediated gene transfer when present exclusively during the adsorption phase at 4°C, a temperature that blocks endocytosis, implying that the presence of ammonium chloride at this time point did not influence binding of AAV to the plasma membrane. We observed half-maximal AAV-mediated gene expression when the drug was added 30 min after the cells were warmed to 37°C, suggesting that endosomal escape had already begun by this time. This observation supports the occurrence of early endosome escape for AAV virions. Furthermore, AAV-mediated gene transfer was completely resistant to ammonium chloride by 90 min after the shift to 37°C, suggesting that penetration of the virus into the cytosol was complete by this time.
FIG. 5.
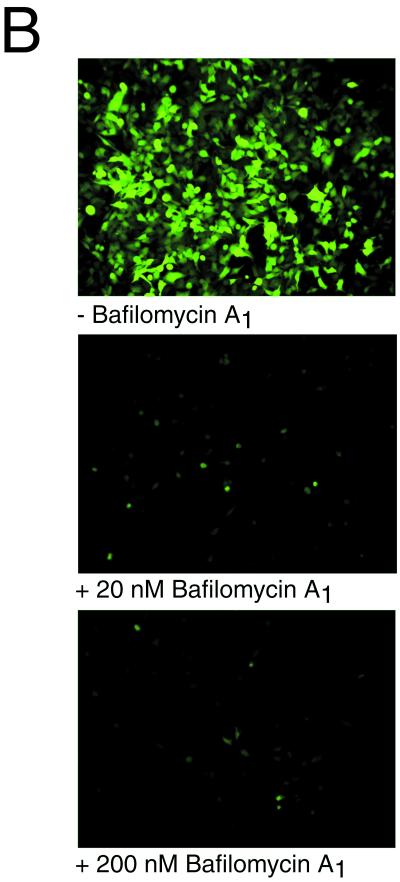
Inhibitory effect of ammonium chloride and bafilomycin A1 on AAV-mediated gene transfer. (A) AAVlacZ vector was bound to HeLa cells at 4°C for 120 min, and unbound virions were washed away. Ammonium chloride (25 mM final concentration) was added at the indicated time points (●) and was present during different 2-h periods as indicated by the horizontal bars. The ammonium chloride was washed out of the cells at the ends of these periods, and gene transfer was assessed at 24 h postinfection by X-Gal histochemistry. Values shown are means ± standard error (n = 3). (B) HeLa cells grown on chambered slides were preincubated without (−) or with bafilomycin A1 (20 or 200 nM) for 30 min at 37°C. AAVEGFP vector (1010 particles/ml) was allowed to bind to the cells for 10 min at 37°C, the cells were washed to remove unbound virus, and vector-mediated green fluorescent protein fluorescence was assessed by fluorescence microscopy 24 h later. Where indicated, bafilomycin A1 was present throughout the experiment. High-level AAV-mediated gene expression was restricted to HeLa cells grown in the absence of bafilomycin A1.
To independently confirm the occurrence of early endosome escape by AAV, we utilized a different drug, bafilomycin A1, during AAV vector infection. Bafilomycin A1 is a potent inhibitor of the vacuolar H+-ATPase responsible for acidification of endosomal vesicles. The influence of different bafilomycin A1 concentrations on AAV infection of HeLa cells was determined. Cells were preincubated with 0, 20, or 200 nM bafilomycin A1 and then infected with the AAVEGFP vector. Infection was monitored 24 h later by measuring green fluorescent protein fluorescence. Both 20 and 200 nM bafilomycin A1 completely inhibited infection of HeLa cells by the AAV vector (Fig. 5B). These findings support the requirement of endosomal acidification and early endosomal escape for efficient AAV vector infection.
Entry of AAV and adenovirus via distinct endosomal pathways.
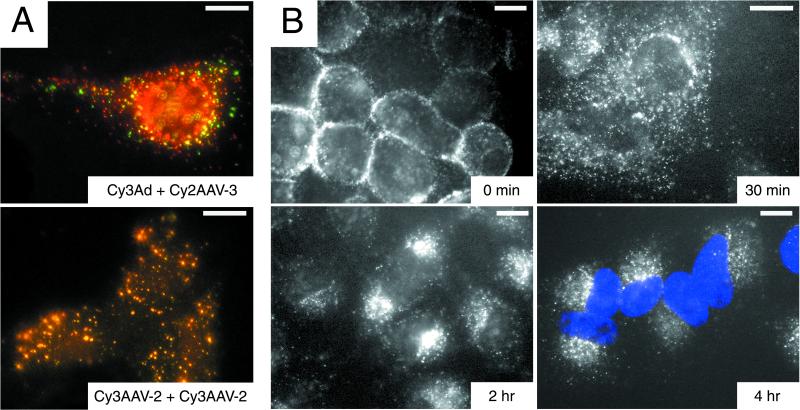
The previous data suggest that AAV is able to penetrate the endosomal membrane fairly quickly following internalization and that this process requires passage through an acidic compartment. Recently, we have shown that αvβ5 integrin is involved in the AAV entry process (40). It is interesting that adenovirus, the AAV helper virus necessary for a productive infection, also requires a slightly acidic pH and binding of the adenovirus penton base protein to αvβ5 integrin in order to efficiently escape from the endosome (7, 29, 47). Although there is substantial evidence demonstrating that AAV genomes can be expressed in host cells in the absence of adenovirus, implying that AAV alone is capable of penetration into the cytosol, it was of interest to determine synergism or competition of endosomal escape following adenovirus and AAV coinfection. Adenovirus has been shown to greatly enhance transduction of some cells by recombinant AAV vectors due to its effect on second-strand DNA synthesis (10, 11). By assessing the fate of fluorescently labeled adenovirus and AAV, we sought to determine whether these viruses ever colocalized within the host cell such that adenovirus might be in a position to physically assist AAV entry into the cytosol. HeLa cells were coinfected with Cy2AAV-3 and Cy3Ad, and the distribution of the viruses was monitored at 10, 20, and 40 min and at 1 h postinfection by fluorescence microscopy. At no time point was there significant colocalization of the AAV (green) and adenovirus (red) labels (Fig. 6A, top panel). In contrast, coinfection with Cy2AAV-3 and Cy3AAV-2 resulted in extensive colocalization of the red and green signals (Fig. 6A, lower panel). While these data do not provide direct evidence of the presence of multiple AAV particles within each endosome, they support it. These results further support the notion that endosomal escape of AAV is efficient and is unaffected by adenovirus. Interestingly, the very early endosomes all contain several AAV particles, as evidenced by label colocalization (yellow signal). This may be indicative of a mechanism of internalization requiring the grouping of multiple receptor-AAV complexes on the surface of the host cell prior to entry.
FIG. 6.
Pulse-labeling evaluation of fluorescent AAV distribution in HeLa cells, demonstrating the lack of intraendosomal colocalization of AAV and adenovirus following endocytosis and time-dependent translocation of AAV from the cell membrane to the perinuclear region. (A) HeLa cells were incubated for 10 min at 37°C with 1.25 × 1011 particles of Cy2AAV-3/ml plus either 1.25 × 1011 particles of Cy3Ad (top panel) or 1.25 × 1011 particles of Cy3AAV-2 (lower panel)/ml and assessed for colocalization by fluorescence microscopy. Colocalization of serotype 2 and 3 AAV, but not of adenovirus and AAV, was evidenced by yellow signal from overlapping red (Cy3) and green (Cy2) signals. Bar, 10 μm. (B) HeLa cells were incubated with 1.25 × 1011 particles of Cy3AAV-2/ml for 2 h at 4°C, washed, then incubated at 37°C for 0, 30, 120, or 240 min and assessed by fluorescence microscopy. The positions of nuclei are evident in the 4-h panel due to DAPI (blue) staining. Bar, 10 μm.
Translocation of AAV to the nucleus.
The efficiency of AAV infection relies to a large extent on the efficient targeting of the AAV genome to the host cell nucleus following infection. This property is reflected in the rapid translocation of virions to the nuclear envelope. Cy3AAV-2 was bound to HeLa cells for 2 h at 4°C. Cells were then washed to remove unbound virus and either fixed immediately or incubated for various periods of time at 37°C to examine redistribution of cell-associated virus (Fig. 6B). Prior to internalization, AAV virions were distributed evenly on the outside of the plasma membrane (Fig. 6B, 0 min). Following 30 min at 37°C, AAV exhibited a disperse, punctate distribution in the cell, likely reflecting virions in both endocytic compartments and free within the cytosol (Fig. 6B, 30 min). The distribution of staining had already started to shift toward the nucleus, with some virus beginning to accumulate at the nuclear envelope; however, the majority of the virions remained widely distributed in the cytoplasm. Later time points show a progressive perinuclear accumulation of AAV virions (Fig. 6B, 2 h and 4 h). By 2 h following internalization, nearly all of the virus particles had accumulated perinuclearly, and they remained at this location throughout the 4-h incubation period.
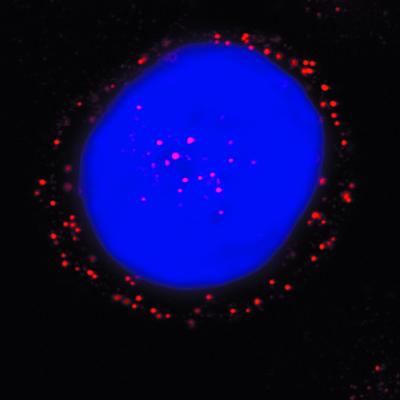
To more accurately access the intracellular distribution of AAV and to determine the potential for nuclear uptake of AAV virions, we used a laser scanning confocal microscope. In this manner, we were able to demonstrate fluorescent AAV particles within the nuclei of host cells within 2 h postinfection (Fig. 7). These observations support a rapid transport of capsid components to the nucleus with transgene expression detected within 3 to 4 h postinfection (data not shown).
FIG. 7.
Distribution of Cy3AAV-2 particles in HeLa cells 2 h postinfection. HeLa S3 cells were pulsed-labeled with 1.25 × 1011 particles of Cy3AAV-2 (red)/ml for 10 min at 37°C, washed to remove uninternalized virus, and incubated at 37°C for 2 h prior to analysis by confocal microscopy. The position of the cell nucleus was assessed by DAPI (blue) staining. A representative image is shown, consisting of a single plane of focus through the center of a cell.
DISCUSSION
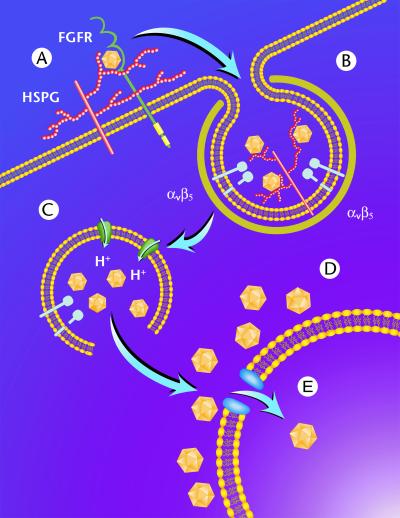
In this study, we established a number of parameters involved in the early steps of AAV entry, including the use of clathrin-coated pits, a requirement for endosome acidification, escape from endosomal vesicles, and perinuclear accumulation and nuclear translocation of virions. These data, combined with recent studies (30, 40, 41) related to AAV receptor usage, have allowed us to propose a model for AAV infection of human cells (Fig. 8). AAV first binds to HSPG on the cell surface (41) (Fig. 8A). This process may be enhanced by the presence of fibroblast growth factor receptor (30). Following binding, AAV is rapidly internalized by clathrin-mediated endocytosis (Fig. 8B) through a process that is aided by the presence of αvβ5 integrin (40). Following internalization into the early endosome, the virus encounters a weakly acidic environment which is sufficient to allow penetration into the cytosol either from this compartment or after transport to another, as-yet-unidentified vesicular compartment (Fig. 8C). Following release into the cytosol, AAV accumulates perinuclearly (Fig. 8D) and slowly penetrates through the nuclear pore complex (NPC) into the nucleus (Fig. 8E). The data generated in our study provide a working template for further confirmation of this model.
FIG. 8.
Schematic representation of AAV entry and endocytic trafficking in HeLa cells. Following binding to cell surface HSPG (A), AAV is rapidly internalized via clathrin-coated pits (B) through a process involving αvβ5 integrin. Once internalized, the virus encounters a weakly acidic environment which is sufficient to allow penetration into the cytosol (C). Following endosome release, AAV accumulates perinuclearly (D) and slowly penetrates through the NPC into the nucleus (E).
Based on the work of others which demonstrated the feasibility of direct fluorophore conjugation for the labeling of adenovirus (15, 16, 22), we sought to use a similar approach for the labeling of AAV in order to monitor viral infection. Critical to the success of this effort was the fact that AAV virions labeled with the carbocyanine dyes Cy2 and Cy3 maintained all viral functions, including binding specificity, internalization, escape from endosomes, translocation to the nucleus, and nuclear entry. Previously, the use of fluorophore-conjugated AAV to evaluate extracellular vector interactions with target cells verified the concept of AAV-2 binding to cell surface HSPG (1, 41) and the role of integrin αvβ5 in viral infection (40). However, more importantly, in this study the use of the fluorescent virus enabled the determination intracellular pathways of viral infection and additional key aspects of virus entry.
The experimental data support AAV internalization from clathrin-coated pits by receptor-mediated endocytosis. After attachment to the cell, the virions were rapidly cleared from the cell surface, and after 7 to 8 min, half of the surface-bound virions had internalized (Fig. 3). This time course of AAV entry is very similar to that established for adenovirus. Since adenovirus is often presented as a model nonenveloped virus which enters cells by receptor-mediated endocytosis, our results are in good agreement with this paradigm (29, 45–47). Furthermore, we present for the first time a molecular view of how these two viruses proceed through a productive coinfection (Fig. 6). Although adenovirus is typically considered to be the primary helper virus of AAV infection, we observed, using double labeling (Cy3Ad and Cy2AAV-3), little if any interaction or cocompartmentalization of these particles after the infection process was initiated, whereas double labeling with two different serotypes of AAV (Cy2AAV-3 and Cy3AAV-2) demonstrated extensive overlap (Fig. 6A), ruling out technical concerns about the inability to observe viral Cy2 and Cy3 colocalization. In addition, these results support the premise that the entry process for serotype 3 AAV is similar to that established here serotype 2 AAV, and the mode of entry of the other AAV serotypes may be similar as well, irrespective of the primary attachment receptor (26). Studies to address this possibility are currently under way. Although numerous studies utilizing recombinant AAV vectors for gene delivery support AAV infection in the absence of adenovirus, these data demonstrate that adenovirus and AAV traffic through the cell separately and that adenovirus is unlikely to be in a position to physically assist AAV entry. Therefore, all aspects of adenovirus helper function must take place after viral infection and passage of the viral particle through the cell and into the nucleus, in agreement with earlier studies (33), and are likely solely related to early gene expression as previously described (10).
To examine the exact mechanism of AAV-2 internalization into cells, we studied AAV vector-mediated gene transfer with normal and mutant HeLa cell lines that have reduced capacity for coated-pit-mediated endocytosis (9). These mutant cells overexpressed a K44A mutant form of dynamin, the cellular protein responsible for pinching off endocytic invaginations formed during endocytosis. Cells expressing the mutant dynamin showed a significant decrease in AAV-mediated gene delivery compared to uninduced cells lacking mutant dynamin. These findings indicate that efficient AAV entry and infection are both regulated by dynamin, an essential component of the clathrin-coated pit endocytic pathway. We have previously shown that entry of AAV into cells is promoted by interaction of the virus with αvβ5 integrin (40). As has been suggested for adenovirus by Wang et al. (46), αv integrin clustering by viral particles could facilitate localization of AAV to coated pits that are destined for internalization. Although direct evidence of this is lacking, the cytoplasmic tail of the β5 subunit of αvβ5 integrin contains the NPXY motif (32, 42), which has been shown to be necessary for the localization of certain receptors to coated pits (27). By direct visualization, we observed clustering of AAV particles before internalization, supporting this concept; however, further studies are needed to determine whether specific internalization sequences in αv integrins mediate AAV uptake into clathrin-coated pits.
While expression of mutant dynamin significantly inhibited virus gene delivery, it did not completely abolish this activity. These findings are consistent with previous reports that the induction of mutant-dynamin expression does not completely block internalization of transferrin, epidermal growth factor, or adenovirus, all of which have been well documented to enter cells via the clathrin-coated pit pathway. Residual AAV-mediated gene delivery may also represent clathrin-independent entry mechanisms such as fluid-phase pinocytosis, which are not affected by expression of mutant dynamin (21). These aspects of alternative pathways of viral uptake may be significant in relation to viral vectors and target cells that are not optimal for wild-type infection. It will be informative to examine alternative pathways of AAV uptake, both in vitro and in vivo, in relation to the specificity of AAV vectors.
Biochemical studies using the lysosomotropic drug ammonium chloride (28) or the proton pump inhibitor bafilomycin A1 (6) showed that these drugs have a significant inhibitory effect on AAV infectivity. These findings are compatible with the view that penetration of AAV from the endosome into the cytosol occurs by a low-pH-dependent mechanism. The finding that neutralization of acidic organelles had to occur within 30 min after the initiation of endocytosis suggests that the early endosomal population is the site of AAV penetration. This notion is further supported by the observation that 20 nM bafilomycin A1 is able to inhibit AAV-mediated gene delivery. Previous studies have shown that 20 nM bafilomycin A1 has no effect on the transport of material from early to late endosomes and only a modest affect on HeLa cell endosome acidification (about 0.2 pH units) (5). The fact that 20 nM bafilomycin A1 blocks AAV infection can be explained by the pH threshold required for infection. From these data, it appears that a slight elevation of the endosomal pH is sufficient to prevent AAV infection.
The efficiency of AAV infection suggests that the AAV capsid proteins alone are able to mediate penetration into the cytosol. Following release of virus from the endosome, AAV is rapidly trafficked to the host cell nucleus. Characteristic perinuclear accumulation of AAV particles is evident within 40 min following the onset of endocytosis. Based on this distribution, it would appear that AAV might be retained in the perinuclear recycling endosome compartment. However, our findings suggest that endosomal escape is complete within this time frame whereas perinuclear distribution of AAV virions persists for several hours. The fact that AAV is no longer within an endosomal compartment is consistent with the observation that AAV particles are not degraded even after several hours of incubation, as if they were partitioned away from the proteases which are normally present in late endosomes or lysosomes.
Earlier studies have suggested that AAV particles can enter the host cell nucleus via the NPC. Since the maximal nuclear pore size is approximately 23 nm, it is likely that translocation of AAV (20 to 25 nm in diameter) through the NPC can take place in the absence of uncoating or major remodeling of the capsid structure. We have provided evidence, attained via confocal microscopy with Cy3-labeled virions, that AAV particles are able to enter the nucleus, suggesting that intact virions enter the nucleus prior to uncoating, although we cannot determine from these studies if the particles are partially uncoated or otherwise modified.
In summary, we have established specific points in the AAV infectious entry pathway which relate to the early steps of viral infection. Binding to a cell surface receptor initiates internalization through clathrin-coated pits followed by release of the viral particle into the cytosol and translocation to the nucleus, where uncoating and gene expression take place. These aspects of AAV entry can now be expanded to specific cell types and to vector analysis in vivo. As we obtain a more detailed molecular picture of the AAV entry process, its implications in terms of modifying AAV vectors with targeting ligands will be significantly enhanced.
ACKNOWLEDGMENTS
The work described here was supported by NIH grants NHLBI 5R01 HL48347-08 and HL51818 (R.J.S.) and Cystic Fibrosis Foundation grants MARZLU96PO and BARTLE99I0 (J.S.B.).
REFERENCES
- 1.Bartlett J S, Samulski R J. Fluorescent viral vectors: a new technique for the pharmacological analysis of gene therapy. Nat Med. 1998;4:635–637. doi: 10.1038/nm0598-635. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett J S, Samulski R J, McCown T J. Selective and rapid uptake of adeno-associated virus type-2 (AAV-2) in brain. Hum Gene Ther. 1998;9:1181–1186. doi: 10.1089/hum.1998.9.8-1181. [DOI] [PubMed] [Google Scholar]
- 3.Basak S, Turner H, Parr S. Identification of a 40- to 42-kDa attachment polypeptide for canine parvovirus in A72 cells. Virology. 1994;205:7–16. doi: 10.1006/viro.1994.1614. [DOI] [PubMed] [Google Scholar]
- 4.Basak S, Turner H. Infectious entry pathway for canine parvovirus. Virology. 1992;186:368–376. doi: 10.1016/0042-6822(92)90002-7. [DOI] [PubMed] [Google Scholar]
- 5.Bayer N, Schober D, Prchla E, Murphy R F, Blaas D, Fuchs R. Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implications for viral uncoating and infection. J Virol. 1998;72:9645–9655. doi: 10.1128/jvi.72.12.9645-9655.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowman E J, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu C Y, Mathias P, Nemerow G R, Stewart P L. Structure of adenovirus complexed with its internalization receptor, αvβ5 integrin. J Virol. 1999;73:6759–6768. doi: 10.1128/jvi.73.8.6759-6768.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark K R, Liu X, McGrath J P, Johnson P R. Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum Gene Ther. 1999;10:1031–1039. doi: 10.1089/10430349950018427. [DOI] [PubMed] [Google Scholar]
- 9.Damke H, Baba T, Warnock D E, Schmid S L. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrari F K, Samulski T, Shenk T, Samulski R J. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher K J, Gao G-P, Weitzman M D, DeMatteo R, Burda J F, Wilson J M. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gey G O, Coffman W D, Kubicek M T. Tissue culture studies of the proliferative capacity of cervical carcinoma and normal epithelium. Cancer Res. 1952;12L:264. [Google Scholar]
- 13.Goodman S, Xiao X, Donahue R E, Moulton A, Miller J, Walsh C, Young N S, Samulski R J, Nienhuis A W. Recombinant adeno-associated virus-mediated gene transfer into hematopoietic progenitor cells. Blood. 1994;84:1492–1500. [PubMed] [Google Scholar]
- 14.Graham F L, Smiley J, Russell W C, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 15.Greber U F, Nakano M Y, Suomalainen M. Adenovirus entry into cells. A quantitative fluorescence microscopy approach. In: Wold W S M, editor. Adenovirus methods and protocols. Vol. 21. Totowa, N.J: Humana Press, Inc.; 1998. pp. 217–230. [Google Scholar]
- 16.Greber U F, Suomalainen M, Stidwill R P, Boucke K, Ebersold M W, Helenius A. The role of the nuclear pore complex in adenovirus DNA entry. EMBO J. 1997;16:5998–6007. doi: 10.1093/emboj/16.19.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halbert C L, Standaert T A, Aitken M L, Alexander I E, Russell D W, Miller A D. Transduction by adeno-associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. J Virol. 1997;71:5932–5941. doi: 10.1128/jvi.71.8.5932-5941.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinshaw J E, Schmid S L. Dynamin self-assembles into rings suggesting a mechanism for coated vesicle budding. Nature. 1995;374:190–192. doi: 10.1038/374190a0. [DOI] [PubMed] [Google Scholar]
- 19.Jones N, Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979;17:683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- 20.Kaljot K T, Shaw R D, Rubin D H, Greenberg H B. Infectious rotavirus enters cells by direct cell membrane penetration, not by endocytosis. J Virol. 1988;62:1136–1144. doi: 10.1128/jvi.62.4.1136-1144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamaze C, Schmid S L. The emergence of clathrin-independent pinocytic pathways. Curr Opin Cell Biol. 1995;7:573–580. doi: 10.1016/0955-0674(95)80015-8. [DOI] [PubMed] [Google Scholar]
- 22.Leopold P L, Ferris B, Grinberg I, Worgall S, Hackett N R, Crystal R G. Fluorescent virions: dynamic trafficking of the pathway of adenoviral gene transfer vectors in living cells. Hum Gene Ther. 1998;9:367–378. doi: 10.1089/hum.1998.9.3-367. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Samulski R J, Xiao X. Role for highly regulated rep gene expression in adeno-associated virus vector production. J Virol. 1997;71:5236–5243. doi: 10.1128/jvi.71.7.5236-5243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linser P, Bruning H, Armentrout R W. Specific binding sites for a parvovirus, minute virus of mice, on cultured mouse cells. J Virol. 1977;24:211–221. doi: 10.1128/jvi.24.1.211-221.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madshus I H, Olsner S, Sandvig K. Mechanism of entry into the cytosol of poliovirus type 1: requirement of low pH. J Cell Biol. 1984;98:1194–1200. doi: 10.1083/jcb.98.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizukami H, Young N S, Brown K E. Adeno-associated virus type 2 binds to a 150-kilodalton cell membrane glycoprotein. Virology. 1996;217:124–130. doi: 10.1006/viro.1996.0099. [DOI] [PubMed] [Google Scholar]
- 27.Mukherjee S, Ghosh R N, Maxfield F R. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 28.Ohkuma S, Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci USA. 1978;75:3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pastan I, Seth P, Fitzgerald D, Willingham M. Adenovirus entry into cells: some new observations on an old problem. In: Notkin L, Oldstone M B A, editors. Concepts in viral pathogenesis II. New York, N.Y: Springer-Verlag; 1986. pp. 141–146. [Google Scholar]
- 30.Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- 31.Qing K, Khuntirat B, Mah C, Kube D M, Wang X-S, Ponnazhagan S, Zhou S, Dwarki V J, Yoder M C, Srivastava A. Adeno-associated virus type 2-mediated gene transfer: correlation of tyrosine phosphorylation of the cellular single-stranded D sequence-binding protein with transgene expression in human cells in vitro and murine tissues in vivo. J Virol. 1998;72:1593–1599. doi: 10.1128/jvi.72.2.1593-1599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramaswamy H, Hemler M E. Cloning, primary structure and properties of a novel human integrin beta subunit. EMBO J. 1990;9:1561–1568. doi: 10.1002/j.1460-2075.1990.tb08275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rose J A, Koczot F. Adenovirus-associated virus multiplication. VII. Helper requirement for viral deoxyribonucleic acid and ribonucleic acid synthesis. J Virol. 1972;10:1–8. doi: 10.1128/jvi.10.1.1-8.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samulski R J, Chang L-S, Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol. 1989;63:3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samulski R J, Chang L-S, Shenk T. A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J Virol. 1987;61:3096–3101. doi: 10.1128/jvi.61.10.3096-3101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samulski R J, Sally M, Muzyczka N. Adeno-associated viral vectors. In: Friedmann T, editor. The development of human gene therapy. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1999. pp. 131–172. [Google Scholar]
- 37.Sanes J R, Rubenstein J L R, Nicolas J F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986;5:3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snyder R O, Xiao X, Samulski R J. Production of recombinant adeno-associated virus vectors. In: Dracopoli N, Haines J, Krof B, Moir D, Seidman C, Seidman J S, editors. Current protocols in human genetics. New York, N.Y: John Wiley & Sons; 1996. pp. 12.1.1–12.2.23. [Google Scholar]
- 39.Sturzenbecker L J, Nibert M, Furlong D, Fields B N. Intracellular digestion of reovirus particles requires a low pH and is an essential step in the viral infectious cycle. J Virol. 1987;61:2351–2361. doi: 10.1128/jvi.61.8.2351-2361.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Summerford C, Bartlett J S, Samulski R J. αVβ5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat Med. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- 41.Summerford C, Samulski R J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki S, Argraves W S, Pytela R, Arai H, Krusius T, Pierschbacher M D, Ruoslahti E. cDNA and amino acid sequences of the cell adhesion protein receptor recognizing vitronectin reveal a transmembrane domain and homologies with other adhesion protein receptors. Proc Natl Acad Sci USA. 1986;83:8614–8618. doi: 10.1073/pnas.83.22.8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takei K, McPherson P S, Schmid S L, Camilli P D. Tubular membrane invaginations coated by dynamin rings are induced by GTP-γS in nerve terminals. Nature. 1995;374:186–190. doi: 10.1038/374186a0. [DOI] [PubMed] [Google Scholar]
- 44.Teramoto S, Bartlett J S, McCarty D, Xiao X, Samulski R J, Boucher R C. Factors influencing adeno-associated virus-mediated gene transfer to human cystic fibrosis airway epithelial cells: comparison with adenovirus vectors. J Virol. 1998;72:8904–8912. doi: 10.1128/jvi.72.11.8904-8912.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Varga M J, Weibull C, Everitt E. Infectious entry pathway of adenovirus type 2. J Virol. 1991;65:6061–6070. doi: 10.1128/jvi.65.11.6061-6070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang K, Huang S, Kapoor-Munshi A, Nemerow G. Adenovirus internalization and infection require dynamin. J Virol. 1998;72:3455–3458. doi: 10.1128/jvi.72.4.3455-3458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wickham T J, Filardo E J, Cheresh D A, Nemerow G R. Integrin αvβ5 selectively promotes adenovirus-mediated cell membrane permeabilization. J Cell Biol. 1994;127:257–264. doi: 10.1083/jcb.127.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeichhardt H, Wertz K, Willingman P, Habermehl K-O. Entry of poliovirus type 1 and mouse Elberfeld (ME) virus into HEp-2 cells: receptor-mediated endocytosis and endosomal or lysosomal uncoating. J Gen Virol. 1985;66:483–492. doi: 10.1099/0022-1317-66-3-483. [DOI] [PubMed] [Google Scholar]
- 49.Zolotukhin S. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]