Abstract
Previous studies have reported that infection of monocytes by viruses such as cytomegalovirus and human immunodeficiency virus weakens host natural immunity. In the present study, we demonstrated the capability of Epstein-Barr virus (EBV) to infect and replicate in freshly isolated human monocytes. Using electron microscopy analysis, we observed the presence of EBV virions in the cytoplasm and nuclei of approximately 20% of monocytes. This was confirmed by Southern blot analysis of EBV genomic DNA sequences in isolated nuclei from monocytes. Infection of monocytes by EBV leads to the activation of the replicative cycle. This was supported by the detection of immediate-early lytic mRNA BZLF-1 transcripts, and by the presence of two early lytic transcripts (BALF-2, which appears to function in DNA replication, and BHRF-1, also associated with the replicative cycle). The late lytic BcLF-1 transcripts, which code for the major nucleocapsid protein, were also detected, as well as EBNA-1 transcripts. However, attempts to detect EBNA-2 transcripts have yielded negative results. Viral replication was also confirmed by the release of newly synthesized infectious viral particles in supernatants of EBV-infected monocytes. EBV-infected monocytes were found to have significantly reduced phagocytic activity, as evaluated by the quantification of ingested carboxylated fluoresceinated latex beads. Taken together, our results suggest that EBV infection of monocytes and alteration of their biological functions might represent a new mechanism to disrupt the immune response and promote viral propagation during the early stages of infection.
Epstein-Barr virus (EBV), a member of the Herpesviridae family, has long demonstrated its capabilities to adapt and evade host defense mechanisms. While it was mainly believed that EBV infects only B cells and epithelial cells of the oropharynx, there is growing evidence that EBV targeted cells are broader than initially believed. In fact, recent studies have demonstrated that EBV can infect thymocytes, as revealed by the detection of BZLF-1 and EBV nuclear antigen (EBNA)-1 transcripts (26). The presence of EBV genome was detected in T lymphocytes and in natural killer cells (21, 24). EBV-infected fibroblasts obtained from the synovial tissue of a rheumatoid arthritis patient were also found to express EBNA-1, EBNA-2, and latent membrane protein 1 (LMP-1) and to spontaneously transform in vitro (29). The presence of EBV genome is also frequently detected in Reed-Sternberg cells found in Hodgkin's disease patients (11). More recently, it was reported that EBV infects human neutrophils in vitro through a CD21 receptor-independent pathway and that such an infection leads to premature cell death by apoptosis (5, 32). The clinical relevance of this study pertains to the observation that neutrophils from infectious mononucleosis patients harbor EBV genome (32).
Mononuclear phagocytes play an active role in the defense of the organism against viral invasion. Rapid recruitment of monocytes/macrophages at the site of infection provides an immediate immune response to limit the spread of the virus during the early stages of infection. Direct elimination of infectious pathogens by monocytes/macrophages mostly occurs by phagocytosis and the generation of degradative enzymes and reactive oxygen metabolites (31). Monocytic cells also contribute to the generation of a specific antiviral immune response by acting as antigen-presenting cells to activate cytotoxic and humoral responses. Impairment in one of these monocytic functions could allow viral agents to evade immune response.
Human immunodeficiency virus type 1 (HIV-1) best illustrates this situation, since several defective monocytic functions such as alteration of cell surface antigen expression, abnormal cytokines synthesis, and impaired accessory cell function were reported as a result of HIV-1 infection of monocytes/macrophages (41, 53). Influenza A virus, which is known to infect human mononuclear phagocytes, selectively induces monocyte-attracting chemokine (46), such as macrophage inflammatory protein 1α and monocyte chemotactic protein 1. In this case, the resulting influx of monocytic cells in infected tissue may therefore represent a viral strategy to recruit new target cells. It was also demonstrated that hepatitis C virus infects peripheral blood monocytes and suppresses secretion of tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β), two important proinflammatory cytokines playing active roles in the regulation of the immune response (37).
Little is known about the interactions of EBV with human monocytes. First, it was reported that EBV specifically binds to monocytes through a receptor distinct from CD21 (19). Second, such interactions were also found to result in the modulation of cytokine gene expression, e.g., induction of IL-1 and IL-6 production (18) and suppression of the synthesis of TNF-α, a pleiotropic cytokine exhibiting antiviral activity (19). Finally, interactions of EBV with monocytes upregulate the formation of important lipid mediators of inflammation, such as leukotrienes, by a mechanism involving the glycoprotein gp350 of the viral envelope (16).
In the present study, we demonstrate that EBV infects and replicates in human monocytes, a process which is accompanied by the suppression of phagocytosis by these cells. This suppressive effects caused by EBV may represent another strategy to affect host defense and promote viral propagation in the early stages of infection.
MATERIALS AND METHODS
Purification of monocytes.
Peripheral blood monocytes were isolated by centrifugation of heparinized venous blood obtained from healthy donors over a Ficoll-Hypaque gradient (Pharmacia, Uppsala, Sweden). Monocytes were then further enriched by Percoll density centrifugation (12) followed by a cell-sorting procedure (Epics Elite ESP, Coulter Electronics Canada) which resulted in 99% pure monocyte suspension, as assessed by flow cytometry with an anti-CD14 monoclonal antibody (Becton Dickinson, Mississauga, Ontario, Canada). Cell viability was >99% as tested by the trypan blue dye exclusion procedure. Monocytes and the EBV-negative lymphoid cell lines YAC-1 and BJAB, which are of murine and human origin, respectively, were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum. The culture medium contained less than 10 pg/ml of endotoxins, as evaluated by the Limulus amoebocyte assay (Sigma, Oakville, Ontario, Canada).
Infection procedure.
Viral preparations of EBV strain B95-8 were produced as previously described (5). Samples of highly purified monocytes (5 × 106 cells) were preincubated with infectious EBV (105 transforming units) in 1 ml of culture medium for 1 h at 37°C in order to favor contacts between viral particles and cells. Cells were then washed three times in Hanks' balanced salt solution (HBSS) (pH 7.4) and further trypsinized with a solution containing 0.05% trypsin–0.5 mM EDTA in order to remove any remaining EBV particles adsorbed to the surface of the cells. Cells were subsequently resuspended (2 × 107 cells/20 ml) in culture medium and cultured in a 75-cm2 tissue culture flask (Falcon, Mississauga, Ontario, Canada) for varying periods of time. When indicated, monocytes were treated with phosphonoacetic acid (PAA) (200 μg/ml), an inhibitor of viral DNA polymerase, for 30 min prior to EBV infection.
Electron microscopy.
Purified monocytes were incubated with EBV for 5 min at 4°C to promote the binding of viral particles to the cell surface and then cultured at 37°C for time periods varying from 5 to 45 min. Cells were washed once in HBSS (pH 7.4) and processed for electron microscopy as described previously (32).
DNA isolation from nuclei of monocytes.
Monocytes (107 cells) were pretreated with 10 μmol of cytochalasin B (Sigma, Oakville, Ontario, Canada) per liter, an inhibitor of phagocytosis, for 10 min prior to infection with EBV. Following infection, cells were washed in HBSS and resuspended in 100 μl of ice-cold buffer containing 0.25 M sucrose, 10 mM HEPES, 1 mM EGTA, and protease inhibitors (1 μM phenylmethylsulfonyl fluoride [PMSF] and 10 μg [each] of leupeptin and aprotinin per ml). Monocytes were then sonicated on ice (20 s, at a power setting of 2 and 60% duty cycle) in a Branson Sonifier 450 (VWR/Canlab, Montreal, Quebec, Canada), sonicates were centrifuged at 12,000 × g for 10 min at 4°C, and the pelleted nuclei were resuspended in HBSS. Genomic DNA was isolated as previously described (43), and any remaining RNA was eliminated by treatment with RNase (Promega, Madison, Wis.). The genomic DNA was digested with the restriction enzyme BamHI, and the presence of EBV genome was evaluated by PCR amplification with BamHI-W primers 5′-GCAGTAACAGGTAATCTCTG-3′ (position 20124 to 20143) and 5′-ACCAGAAATAGCTGCAGGAC-3′ (position 20523 to 20504), as deduced from the viral DNA sequence (3). Two micrograms of DNA was first denatured at 94°C for 2 min and then subjected to 35 amplification cycles as follows: denaturation for 1 min at 94°C, annealing for 1 min at 55°C, extension for 1 min at 72°C in the presence of 0.2 mM deoxynucleoside triphosphates (dNTPs), 2 mM of each primer, 1.7 mM MgCl2, and 2.5 U of Taq DNA polymerase (Promega). PCR products were visualized by ethidium bromide staining on 2% agarose gels and confirmed by hybridization with a specific BamHI W probe (23). DNA integrity was confirmed by amplification and hybridization of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) with primers and probe previously described (13).
Southern blot analysis.
Enriched monocytes (107 cells) were pretreated for 10 min with 10 μmol of cytochalasin B per liter prior to infection with EBV (as described above). Following infection, genomic DNA was isolated (43) and subjected to Southern blot analysis. Briefly, 10 μg of DNA was digested with BamHI, size fractionated by electrophoresis on a 0.8% agarose gel, and then transferred onto a GeneScreen Plus membrane (NEN Life Science Products, Boston, Mass.) for hybridization with the 400-bp BamHI-W PCR fragment (described above) labelled with [32P]dCTP with the Prime-a-Gene labelling system (Promega). The hybridization was performed overnight at 42°C in a solution containing 50% formamide, 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 10% dextran sulfate, 1× Denhardt's solution, and 1% sodium dodecyl sulfate (SDS). After hybridization, membranes were washed at 42°C in 2× SSC for 10 min, followed by two washes in 2× SSC–1% SDS for 20 min each and two stringent washes in 0.2× SSC–1% SDS for 20 min each. The signal was visualized by autoradiography. The YAC-1 cell line, which is nonpermissive to EBV infection, was used as a control.
RNA isolation and amplification of viral transcripts by reverse transcriptase (RT)-PCR.
Unstimulated and EBV-infected monocytes (107 cells) were cultured for various periods of time before RNA extraction. Total RNA from monocytes was isolated with TRIzol reagent (Gibco BRL), according to the manufacturer's instructions. Two micrograms of DNase-treated RNA from unstimulated and EBV-infected monocytes was heated for 5 min at 72°C in the presence of random hexamers, rapidly cooled on ice, and then reverse transcribed to cDNA with 200 U of Moloney murine leukemia virus RT (Promega) in a 25-μl volume containing 0.5 mM dNTPs and 20 U of RNase inhibitor (Boehringer Mannheim, Laval, Quebec, Canada). After 60 min of incubation at 42°C, samples were boiled for 5 min at 94°C, and 5 μl of cDNA samples was subjected to PCR amplification in 50 μl of PCR mixture containing 0.2 mM of the appropriate primers (listed in Table 1), 0.2 mM dNTPs, 2.5 U of Taq polymerase, and 1.7 mM MgCl2. PCR amplification conditions were as described above, and PCR products were separated by electrophoresis on a 2% agarose gel, transferred onto a Hybond-N nylon membrane, and hybridized with γ-32P-5′-end-labeled internal oligonucleotide probes detailed in Table 1. PCR amplification of the GAPDH transcripts was also used as an internal control. To ensure the absence of contaminating genomic DNA, RNA samples were directly amplified under the PCR conditions described above.
TABLE 1.
Primers and probes used in RT-PCR analyses
| Transcript (protein) | Primers and probe | Genome coordinates | Sequence |
|---|---|---|---|
| BKRF1 (EBNA-1) | 5′ Primer (U exon) | 67483–67502 | 5′-TTAGGAAGCGTTTCTTTGAGC-3′ |
| 3′ Primer (K exon) | 107986–107967 | 5′-CATTTCCAGGTCCTGTACCT-3′ | |
| Probe (U exon) | 67544–67563 | 5′-AGAGAGTAGTCTCAGGGCAT-3′ | |
| BZLF-1 (ZEBRA) | 5′ Primer (exon 1) | 102719–102700 | 5′-TTCCACAGCCTGCACCAGTG-3′ |
| 3′ Primer (exon 2/3 splice) | 102330–102341 and 102426–102433 | 5′-GGCAGCAGCCACCTCACGGT-3′ | |
| Probe (exon 2) | 102450–102469 | 5′-CTTAAACTTGGCCCGGCATT-3′ | |
| BHRF-1 (EA) | 5′ Primer (exon 1) | 54461–54480 | 5′-TTCTCTTGCTGCTAGCTCCA-3′ |
| 3′ Primer (exon 1) | 53830–53849 | 5′-GTCAAGGTTTCGTCTGTGTG-3′ | |
| Probe (exon 1) | 54411–54435 | 5′-ATGCACACGACTGTCCCGTATACAC-3′ | |
| BALF-2 (EA) | 5′ Primer (exon 1) | 163094–163072 | 5′-GTCAAGATGTTCAAGGACGTGG-3′ |
| 3′ Primer (exon 1) | 162856–162878 | 5′-CTCATAGCACATACAGATGGGC-3′ | |
| Probe (exon 1) | 162911–162930 | 5′-GCGGTAAAACAGCTGGGTGA-3′ | |
| BcLF-1 (VCA) | 5′ Primer (exon 1) | 136231–136210 | 5′-TATGCCCAATCCCAAGTACACG-3′ |
| 3′ Primer (exon 1) | 135867–135888 | 5′-TGGACGGGTGGAGGAAGTCTTC-3′ | |
| Probe (exon 1) | 136119–136138 | 5′-ACGCGAGGAGGAGGTTATTC-3′ | |
| GAPDH | 5′ Primer (exon 8) | 3070–3089 | 5′-ACCACAGTCCATGCCATCAC-3′ |
| 3′ Primer (exon 9) | 3605–3527 | 5′-TCCACCACCCTGTTGCTGTA-3′ | |
| Probe (exon 8) | 3218–3245 | 5′-CACGGAAGGCCATGCCAGTGAGCTTCCCGT-3′ |
Production of EBV particles by human monocytes.
Production of infectious EBV particles from enriched monocytes was performed by infecting 2 × 107 cells with EBV (4 × 105 transforming units) as described above and kept in culture (75-cm2 flask) for 14 days. The YAC-1 cell line, which is nonpermissive to EBV infection, was used as a mock control. After the appropriate time of culture, cell supernatants containing EBV particles were harvested, and viral particles were purified by ultracentrifugation (25,000 ×g for 3 h at 4°C). Pelleted monocytes were also submitted to freeze-thaw cycles in order to liberate intracellular viral particles. Virus and mock preparations were resuspended in RPMI-1640 and used to infect permissive BJAB cells. The presence of EBNA in BJAB cells was monitored for 4 to 6 days postinfection by indirect immunofluorescence using EBV-positive reference antisera (38).
Phagocytosis assay.
The phagocytic activity of EBV-infected and uninfected purified monocytes was assessed by flow cytometry with carboxylated fluoresceinated microspheres, essentially as described previously (2, 9, 34, 47). For all experiments, 5 × 105 cells were first washed with 1 ml of phosphate-buffered saline (pH 7.4) and resuspended in 350 μl of HBSS supplemented with 5% fetal calf serum in which 6 × 106 carboxylated fluoresceinated microspheres (1.87 μm; Fluoresbrite, Polysciences, Warrington, Pa.) were added to give a ratio of 12 beads/cell. The fluorescent microspheres were examined both microscopically and by flow cytometry to insure that there was no agglomeration and membrane adsorption prior to use in uptake experiments. The phagocytosis proceeded at 37°C for 2 h with constant shaking (150 rpm). After the incubation, the mixture was centrifuged at 4°C (1,000 × g for 3 min) and washed twice with 1 ml of cold phosphate-buffered saline to separate cells from nonphagocytized microspheres. Cells were fixed in 500 μl of 0.5% paraformaldehyde, and 104 cells were analyzed with an EPICS-XL flow cytometer (Coulter Electronics) at an excitation setting of 488 nm and emission setting of 540 nm. Since the microspheres are smaller in diameter than the monocytes, they can be easily discriminated by light scatter. The percentage of fluorescence-positive monocytes was determined by calculating the number of monocytes containing fluorescent beads per 104 cells analyzed × 100. The fluorescence distribution was displayed in a histogram where each peak corresponded to a definite number of microspheres associated per cell. To confirm the results obtained by flow cytometry, each sample was also examined microscopically.
RESULTS
Detection of EBV particles in human monocytes.
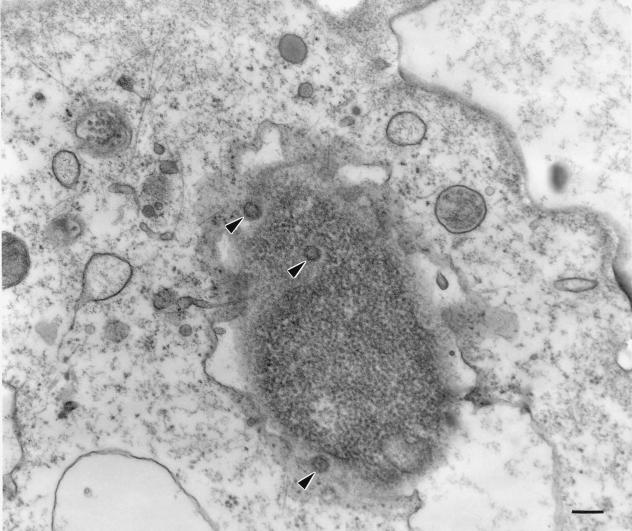
We have previously reported that EBV binds to monocytes, exerts modulatory effects on inflammatory cytokine gene expression (18, 19), and primes monocytes for an increased synthesis of leukotrienes after stimulation with a second agonist (16). In the present study, our objectives were to determine if EBV could penetrate into freshly isolated monocytes and if viral transformation and/or replication would occur. First, we evaluated the presence of EBV particles in monocyte preparations by electron microscopy. Monocytes were incubated with viral particles for 45 min at 37°C and then processed for ultrathin sectioning and examination. As shown in Fig. 1, nucleocapsids of EBV were observed in the cytoplasm and nuclei of approximately 20% of monocytes, suggesting that EBV can indeed penetrate such cells in a phagocytosis-independent manner. EBV nucleocapsids observed were identical to those seen in the cytoplasm of B95-8 cells.
FIG. 1.
EBV infection of human monocytes. Monocytes were incubated on ice in the presence or absence of infectious EBV for 5 min and then cultured at 37°C for 45 min. The preparation and examination of samples was performed as described in Materials and Methods. Virions are indicated by arrows (magnification of ×45,000). Black bar = 200 nm.
Detection of EBV genome in nuclei of monocytes.
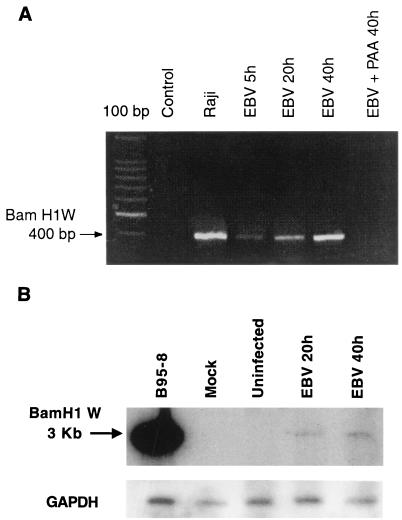
To further confirm the presence of EBV in monocytes, we next attempted to detect EBV DNA by PCR amplification of a fragment located within the BamHI W region of the EBV genome (3). In order to prevent viral internalization by phagocytosis, we first pretreated monocytes with cytochalasin B, an inhibitor of phagocytosis prior to EBV infection, and at different periods of time postinfection, we next extracted viral DNA from isolated nuclei with an equal amount of DNA (1 μg). The presence of EBV genome could be detected at 5 h postinfection and increased from 20 to 40 h postinfection, suggesting that the EBV replicative cycle was initiated (Fig. 2A). The identity of the amplified PCR products was demonstrated by hybridization with an EBV-specific BamHI W probe (data not shown). As controls, nuclei from EBV- and cytochalasin B-treated p815 cells (a cell line capable of phagocytosis) were isolated and submitted to PCR amplification. No EBV DNA could be amplified from such cells (data not shown).
FIG. 2.
Detection of EBV genome in infected monocytes. Monocytes (107 cells) were treated with the phagocytosis inhibitor cytochalasin B (10 μM) for 10 min and infected with EBV for the indicated times. (A) The presence of EBV genome in purified cell nuclei was evaluated by PCR amplification of the BamHI W fragment, and the resulting 400-bp PCR product was visualized by ethidium bromide staining on a 2% agarose gel. In some samples, monocytes were also pretreated with PAA (200 μg/ml) before EBV infection. The Raji cell line was used as a positive control, and noninfected monocytes were used as negative controls. The first lane on the left represents a 100-bp DNA ladder. (B) Genomic DNA isolated from noninfected or EBV-infected monocytes (10 μg) or the Raji cell line (2 μg) was digested with BamHI and subjected to Southern blot analysis with a 32P-labelled BamHI-W probe. The 3-kb hybridization signal (BamHI-W fragment) obtained from the Raji cell line and monocytes previously infected with EBV for 20 and 40 h (EBV 20h, EBV 40h) are shown. Results are representative of three different experiments. YAC-1 cells were used as negative controls.
The presence of EBV DNA in infected monocytes was further confirmed by Southern blot analysis of genomic DNA isolated at indicated times postinfection with the 400-bp PCR fragment contained in the BamHI-W internal repeat region as a probe (Fig. 2B). As with the PCR results (Fig. 2A), the 3-kb BamHI-W signal was detectable at 20 and 40 h postinfection, thereby confirming the presence of viral DNA in monocytes. The decrease in the signal intensity is likely due to the low sensitivity of the Southern blot technique compared to that of PCR. Importantly, the presence of EBV genome was not detected in YAC-1 control cells treated under the same experimental conditions as monocytes.
Detection of immediate-early and early lytic transcripts in EBV-infected monocytes.
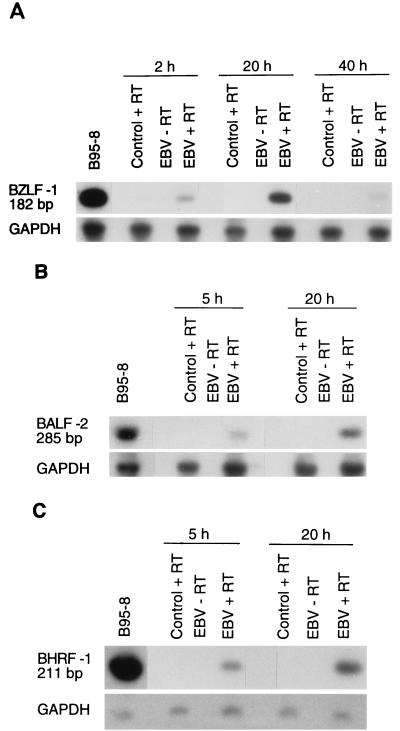
EBV infection may result in the production and release of new virions or may lead to the immortalization and/or transformation of B lymphocytes. As EBV genome was clearly detected in monocytes and as no signs of cellular transformation were observed, we decided to evaluate the presence of specific viral mRNA transcripts associated with the replicative cycle of EBV by RT-PCR analysis. We first looked for the presence of three mRNAs: the BZLF-1 transcript, which is a key immediate-early transactivator of early EBV lytic gene expression, and two early replicative cycle transcripts, BALF-2 and BHRF-1. Monocytes were infected with EBV, and total RNA was extracted at indicated times and submitted to RT-PCR analysis. As shown in Fig. 3A, BZLF-1 transcripts were detectable as early as 2 h postinfection, reached a maximum at 20 h, and declined thereafter. Two other transcripts, BALF-2 (Fig. 3B) and BHRF-1 (Fig. 3C), were detected at 5 h postinfection and also reached a maximum at 20 h. However, the presence of the early antigen (EA) protein could not be detected by immunofluorescence. Taken together, these results suggest that the EBV replicative cycle is initiated in infected monocytes.
FIG. 3.
Detection of immediate-early and early transcripts by RT-PCR analysis. Total RNA was isolated from enriched monocytes (107 cells) exposed to EBV for 2 h and cultured for the indicated time periods. Following treatment with DNase I, RNA was reverse transcribed and amplified with sets of PCR primers specific for each gene (see Table 1). The size of the amplified fragments was 182 bp for BZLF-1 (A), 285 bp for BALF-2 (B), and 211 bp for BHRF-1 (C). PCR products were hybridized by Southern blot analysis using specific probes. GAPDH cDNA was used as an internal control. Tetradecanoyl phorbol acetate-treated B95-8 cells were used as positive controls, and noninfected monocytes were used as negative controls. The results presented are from one experiment and are representative of three separate experiments.
Detection of EBNA-1 and late lytic transcripts in EBV-infected monocytes.
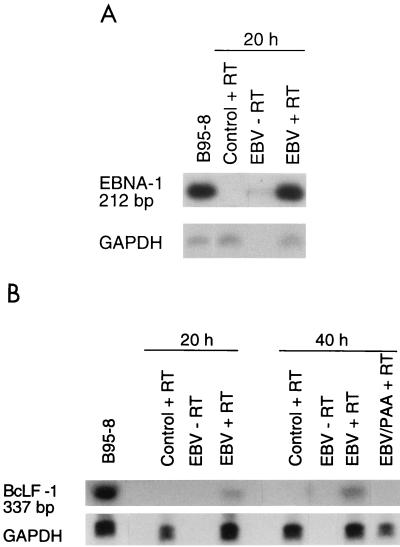
Although EBNA proteins are known to have DNA-binding activity, EBNA-1 and EBNA-2 have very different biological properties. In fact, EBNA-1 is associated with episome persistence, acts as a transactivator of latent genes, activates initiation of DNA replication, and has no effect on tumor cell growth (14, 15, 35, 49). In contrast, EBNA-2 is directly involved in cellular transformation (39, 45, 52). In EBV-infected monocytes, only EBNA-1 transcripts were detected (Fig. 4A) while EBNA-2 transcripts were found to be absent even after 14 days of infection. The presence of EBNA protein was also detected (<5%) in EBV-infected monocytes by immunofluorescence. This is in perfect agreement with the fact that no signs of monocyte transformation were observed following EBV infection.
FIG. 4.
Detection of EBNA-1 and late lytic transcripts by RT-PCR analysis. Total RNA was isolated from enriched monocytes (107 cells), infected with EBV for 2 h, and cultured for the indicated periods of time. Following treatment with DNase I, RNA was reverse transcribed and amplified with sets of PCR primers specific for each gene. The size of the amplified fragments was 212 bp for EBNA-1 (A) and 332 bp for BcLF-1 (B). PCR products were hybridized with specific probes. GAPDH cDNA was used as an internal control. Tetradecanoyl phorbol acetate-treated B95-8 cells were used as positive controls, and noninfected monocytes were used as negative controls. Results are from one experiment and are representative of three separate experiments.
During the replicative cycle, the activation of EBV late lytic genes is also initiated. These genes code mostly for structural viral proteins or for proteins that modify the phenotype of infected cells. Among the late genes, the major nucleocapsid protein is encoded by BcLF-1. The presence of BcLF-1 transcripts was evaluated at different times post-EBV infection. As seen in Fig. 4B, these late transcripts were detected after 20 h of infection and were found to increase by 40 h postinfection. As controls, monocytes treated with the viral DNA polymerase inhibitor PAA were tested for BcLF-1 expression. As expected (Fig. 4B), no BcLF-1 transcripts could be detected in PAA-treated cells. However, the presence of viral capsid antigen (VCA) protein could not be detected by immunofluorescence even after 2 weeks of culture. On the other hand, flow cytometry with anti-gp350 72A1 monoclonal antibodies showed that approximately 1% of EBV-infected monocytes expressed EBV glycoprotein gp350 after 14 days of infection (data not shown), suggesting that some monocytes are fully permissive to EBV infection and replication. EBV glycoprotein gp350 is found on the viral envelope and on the cellular membrane of lytically infected cells (40).
The results reported above strongly suggest that EBV replicates in human monocytes. To further confirm this observation, we performed an additional experiment. Monocytes were infected with EBV and cultured for 14 days to allow viral replication. The YAC-1 mouse cell line, which is not permissive to EBV infection, was also treated with the same culture conditions and used as mock preparation. Cell supernatants were then harvested, and viral particles were isolated by ultracentrifugation. EBV and mock preparations were then used to infect BJAB cells, and after 4 days of culture, the presence of EBNA was evaluated by immunofluorescence. The presence of EBNA-positive cells (≈7%) was only detected in BJAB cell cultures treated with viral preparation produced in monocytes.
Effect of EBV on phagocytic activity of monocytes.
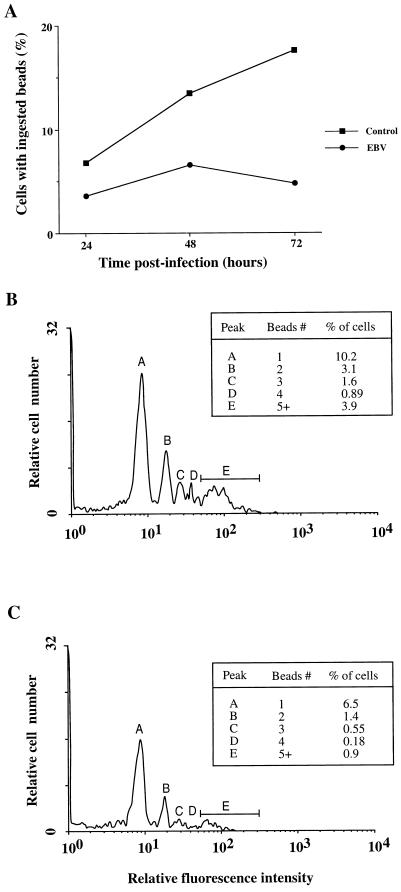
In addition to producing soluble immunoregulatory molecules, monocytes play an active role in the phagocytosis of foreign organisms, which is a crucial step towards the presentation of particulate antigens in the context of major histocompatibility complex class II. Thus, to investigate further the immunosuppressive effects caused by EBV, we tested if the phagocytic activity of monocytes was altered by infection with EBV. The phagocytic activity was evaluated by measuring the uptake of fluoresceinated beads by flow cytometry as described in Materials and Methods. This simple, but highly sensitive, method was used to determine both the percentage of cells with phagocytic activity (fluorescence-positive cells) and the average number of beads associated with each positive cell. Figure 5A shows a kinetic analysis demonstrating that EBV-infected monocytes have a reduced capacity to phagocytose fluorescent beads at 24, 48, and 72 h postinfection with reductions of 40, 52, and 73%, respectively. An average reduction of 50% in phagocytic activity was routinely observed with monocytes isolated from different healthy donors, and the effect was observed for up to 6 days postinfection, after which time both cell viability and their phagocytic ability declined rapidly. Representative histograms obtained with uninfected and EBV-infected monocytes are shown in Fig. 5B and C, respectively. The suppressive effect of EBV on phagocytic activity was seen at all fluorescence levels, each peak corresponding to a definite number of associated fluorescent microspheres. In this particular example, there was a 36, 55, 66, 80, or 77% reduction in fluorescent positive cells associated with 1, 2, 3, 4, or more than 5 beads, respectively (similar results were obtained with a different donor). The probability of coincidence of free beads with cells as they pass through the flow cytometer was kept at a minimum by following these two conditions: (i) a low ratio of beads/cell (12 beads/cells) was used for the assay, and (ii) several washings were performed following the incubation period to remove any nonphagocytosed beads (2). The suppressive effect of EBV was confirmed by another phagocytosis assay which used opsonized zymosan and albumin-fluorescein isothiocyanate. Although less sensitive, there was at least a 50% decrease of fluorescence-positive cells in EBV-treated monocytes (data not shown).
FIG. 5.
Suppression of phagocytosis in EBV-infected monocytes. EBV-infected or uninfected monocytes (5 × 105 cells) were incubated with carboxylated fluoresceinated microspheres (at a ratio of 12 particles/cell), and the uptake of fluorescent particles was measured by flow cytometry, as described in Materials and Methods. (A) The percentage of fluorescence-positive cells (cells associated with at least one fluorescent microsphere) for EBV-infected and uninfected monocytes was measured at 24, 48, and 72 h postinfection. This time course analysis is representative of two experiments performed with two different healthy donors. Panels B and C are typical histograms showing the percentage of fluorescence-positive cells at each level of fluorescence intensity for uninfected and infected monocytes, respectively (this experiment was done at 60 h postinfection). Each peak is related to a definite number of fluorescent microspheres, and the percentage of positive cells contained in each peak is shown in the insert. A total of 104 cells was analyzed for each histogram. Results are representative of four other experiments.
DISCUSSION
Despite the fact that monocytes/macrophages constitute the key elements in nonspecific and specific immune defenses against viral infection, very little is known about the interactions of EBV with these cell types. In the present study, we clearly demonstrated that EBV penetrates and replicates in monocytes. First, by using electron microscopy, nucleocapsids of EBV were observed in the cytoplasm and nuclei of monocytes. Second, the presence of EBV genome in nuclei from cytochalasin B (an inhibitor of phagocytosis)-treated monocytes is also of interest. These results indicate that following viral adsorption, EBV penetrates into monocytes without being phagocytosed. Since CD21 receptor is not expressed on monocytes, these results reinforce our previous observations suggesting that EBV recognizes on monocytes a molecule distinct from this receptor.
It is well known that EBV penetration into human B lymphocytes results in latent or lytic infection in vivo or in cellular transformation in vitro. The fact that no signs of cellular transformation were observed in EBV-enriched monocyte cultures suggests that viral replication may occur. This is in perfect agreement with the presence of immediate-early (BZLF-1) and early lytic (BALF-2 and BHRF-1) transcripts. BZLF-1 transcripts appeared within 2 h after viral infection to reach a maximal level at 20 h postinfection and declined thereafter. Expression of BZLF-1 is transient and encodes the key immediate-early Zebra protein essential to activate the lytic cycle. This is in perfect agreement with other studies with EBV-infected Burkitt's lymphoma tumor cells (Akata cells) in which the lytic cycle was induced with anti-immunoglobulin M treatment and a transient expression of BZLF-1 was observed (50). Later in the lytic cycle, the BALF-2 gene, which encodes for a major DNA-binding protein involved in viral DNA replication, and the BHRF-1 gene, which encodes for an early protein virtually absent in latently infected B lymphocytes, were also detected 5 h postinfection (for a review, see reference 28).
Another interesting result supporting our findings that EBV replicates in human monocytes is the successful detection of late lytic BcLF-1 transcripts which encode for the major nucleocapsid protein and the detection of gp350 expressed on the surface membranes of infected monocytes. These genes are expressed later in the replicative cycle and therefore after the activation of EBV DNA polymerase. This was nicely supported by results from the treatment of monocytes with PAA, an inhibitor of viral DNA polymerase, prior to EBV infection. In PAA-treated cultures, BcLF-1 transcripts were not detected, supporting the idea that EBV initiates a complete lytic cycle in monocytes. While only 1% of EBV-infected monocytes were found to express gp350, we believe that this percentage underestimates the reality. Two main reasons may explain this result: first, viral replication may not occur simultaneously in all EBV-infected monocytes, and second, in contrast to B lymphocytes which express large amount of gp350, infected monocytes could weakly express gp350 on their cellular membrane, thus making it hard to detect gp350 by flow cytometric analysis. The fact that gp350 was never detected in PAA-treated EBV-infected monocytes at any time during the experimental procedures reinforces the validity of the results obtained. Another interesting result is the presence of EBNA-1 transcripts in EBV-infected monocytes. EBNA-1 is known to bind to the oriP domain to initiate viral DNA replication or to govern the EBV episomes in infected cells (14, 15, 35, 49). The absence of EBNA-2 also reinforces the fact that a lytic cycle is activated in EBV-infected monocytes. EBNA-2 is required for the initiation of cell transformation, is readily detectable in the first 24 h of B-cell infection, and reaches maximal levels before EBNA-1 can be detected (1, 22, 32, 42). While EBNA-1 transcripts are present, EBNA-2 transcripts were never detected in EBV-infected monocytes, nor was cellular transformation observed. In addition, LMP-1 and -2 transcripts, or protein expression, also associated with latent infection, were not detected by RT-PCR, flow cytometry, or immunoblotting analysis (data not shown). These results, together with the production and the isolation of infectious viral particles from EBV-infected monocytes, clearly confirm that EBV infects and replicates in human monocytes.
EBV has developed strategies to escape elimination by the immune system, such as induction of latency, capture of host genes, or inhibition of proinflammatory cytokines. One of the main function of monocytes is their ability to internalize foreign organisms. We show here that EBV-infected monocytes are significantly impaired in their ability to phagocytose. The suppressive effect of EBV was observed as early as 24 h postinfection, at which time a decrease of at least 40% in phagocytic activity was noted. In contrast, at 2 h postinfection, monocytes were not functionally compromised in their ability to phagocytose, suggesting that viral genes expressed at later stages of the infection are necessary to cause a defect in phagocytosis. Such impairment of the phagocytosis machinery is expected to be advantageous for the viral outcome. First, it may directly favor the spread of the virus, since phagocytosis is involved in the elimination of foreign organisms. Second, a downregulation of the phagocytic process is likely to interfere with the antigen-presenting capacity of monocytes, which in turn will affect the immune response of the effector T cells. Concomitant alterations of both phagocytosis and antigen-presenting processes have already been reported with monocytes infected with HIV or bovine respiratory syncytial virus (4, 6, 7, 25). In addition, human herpesviruses, including cytomegalovirus, human herpesvirus 6, and human herpesvirus 8, have been shown to infect primary human monocytes/macrophages.
The mechanisms by which EBV affects phagocytosis remain to be elucidated. Possible alterations in the expression of Fcγ and complement receptors, as demonstrated for HIV-1 (27), are currently being investigated. Perhaps deregulation of tyrosine activation motif-mediated phagocytosis by Fcγ receptors is a potential means by which EBV could disrupt phagocytic activity (for a review, see reference 20). As well, there are multiple transmembrane signals aside from protein-tyrosine phosphorylation which could be involved in phagocytosis, including protein kinase C, protein kinase A, casein kinase II, and as-yet-unidentified serine-threonine protein kinases (20, 51). Thomas et al. (51) reported that the impairment of Fc receptor-mediated phagocytosis in HIV-1 infected promonocytic cells was associated with an increased accumulation of cyclic AMP which could be relieved by the addition of an inhibitor of cyclic AMP-dependent protein kinase A. Whether EBV uses similar mechanisms to deregulate phagocytosis remains to be established.
In previous studies, we have demonstrated that EBV interacts with premyelomonocytic cell lines, such as U937 and HL-60, as well as with human monocytes and was able to modulate cytokine synthesis (17, 19). Indeed, upon EBV interaction with these cells, we observed that IL-1 and IL-6 gene transcription was activated, whereas that of TNF-α was inhibited. Since TNF-α is known to exert antiviral activities, we postulated that the suppression of TNF-α release may favor the spread of infection. Knowing that the protein coded by the BARF-1 gene of EBV can neutralize the activity of colony-stimulating factor 1 and block alpha interferon secretion in mononuclear cells (10, 48), we tested for BARF-1 expression in EBV-infected monocytes. No BARF-1 transcripts could be detected in EBV-infected monocytes which might have accounted for the previously reported TNF-α suppression (19). Taken together, we can postulate that targeting monocytes/macrophages may represent an evolutionary advantage for ensuring propagation and persistence of EBV and other herpesviruses within the host (8, 30, 33, 36). This was reinforced by another study which presented evidence of EBV replication in macrophages (44). Cultured macrophages obtained from patients with benign or malignant neoplasms and from healthy donors were kept in culture for several weeks. The authors found EBV genome and detected latent gene expression (EBNA-2 and LMP-1) in those cultures. The presence of EBNA-2 might have facilitated the number of passages performed in vitro and delayed the decrease of cell viability. In our case, EBNA-2 transcripts were always absent in all cultures, which could explain why no cellular growth was observed. The most surprising results from the study by Shimakage et al. (44) is the presence of such latent genes in macrophages from normal tissues and the induction of replicative-associated proteins after treatment with tumor promoter in vitro, indicating that macrophages could be a source for latent infection. Whether macrophages express a higher level of EBV-specific genes than monocytes and whether EBV exists in a different replicative state in macrophages versus monocytes remain to be elucidated. However, this study provides additional indications that monocytes/macrophages may serve as reservoirs of EBV infection.
We have demonstrated that EBV infects, replicates in human monocytes, and significantly reduces the ability of these cells to phagocytose. It was long established that immunosuppression is a key factor for the persistence of EBV within the host. Such effects on monocytes may then contribute to the spread of the virus but also may affect the antigen presentation by reducing incorporation of foreign antigens.
ACKNOWLEDGMENTS
This work was supported by a Medical Research Council of Canada grant to J.G. J.G. and L.F. are recipients of FRSQ and MRC scholarships, respectively.
We thank Pierrette Côté for excellent secretarial assistance.
REFERENCES
- 1.Adams D O, Hamilton T A. Macrophages as destructive cells in host defense. In: Gallin J I, Goldstein I M, Snyderman R, editors. Inflammation: basic principles in clinical correlates. 2nd ed. New York, N.Y: Raven Press; 1992. pp. 637–662. [Google Scholar]
- 2.Andoh A, Fujiyama Y, Kitoh K, Hodohara K, Bamba T, Hosoda S. Flow cytometric assay for phagocytosis of human monocytes mediated via Fcγ-receptors and complement receptor CR1 (CD35) Cytometry. 1991;12:677–686. doi: 10.1002/cyto.990120712. [DOI] [PubMed] [Google Scholar]
- 3.Baer R, Bankier A T, Biggin M D, Deininger P L, Farrell P J, Gibson T J, Hatfull G, Hudson G S, Satchwell S C, Séguin C, Tuffnell P S, Barrell B G. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984;310:207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin G C, Fleischmann J, Chung Y, Koyanagi Y, Chen I S, Golde D W. Human immunodeficiency virus causes mononuclear phagocyte dysfunction. Proc Natl Acad Sci USA. 1990;87:3933–3937. doi: 10.1073/pnas.87.10.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaulieu A D, Paquin R, Gosselin J. Epstein-Barr virus modulates de novo protein synthesis in human neutrophils. Blood. 1995;86:2789–2798. [PubMed] [Google Scholar]
- 6.Bender B S, Davidson B L, Kline R, Brown C, Quinn T C. Role of the mononuclear phagocyte system in the immunopathogenesis of human immunodeficiency virus infection and the acquired immunodeficiency syndrome. Rev Infect Dis. 1988;10:1142–1154. doi: 10.1093/clinids/10.6.1142. [DOI] [PubMed] [Google Scholar]
- 7.Bender B S, Frank M M, Lawley T J, Smith W J, Brickman C M, Quinn T C. Defective reticuloendothelial system Fc-receptor function in patients with acquired immunodeficiency syndrome. J Infect Dis. 1985;152:409–412. doi: 10.1093/infdis/152.2.409. [DOI] [PubMed] [Google Scholar]
- 8.Blasig C, Zietz C, Haar B, Neipel F, Esser S, Brockmeyer N H, Tschachler E, Colombini S, Ensoli B, Sturz M. Monocytes in Kaposi's sarcoma lesions are productively infected by human herpesvirus 8. J Virol. 1997;71:7963–7968. doi: 10.1128/jvi.71.10.7963-7968.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buschmann H, Winter M. Assessment of phagocytic activity of granulocytes using laser flow cytometry. J Immunol Methods. 1989;124:231–234. doi: 10.1016/0022-1759(89)90358-x. [DOI] [PubMed] [Google Scholar]
- 10.Cohen J I, Lekstrom K. Epstein-Barr Virus BARF1 protein is dispensable for B-cell transformation and inhibits alpha interferon secretion from mononuclear cells. J Virol. 1999;73:7627–7632. doi: 10.1128/jvi.73.9.7627-7632.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delsol G, Meggetto F, Brousset P, Cohen-Knafo E, Al Saati T, Rochaix P. Relation of follicular dendritic reticulum cells to Reed-Sternberg cells of Hodgkin's disease with emphasis on the expression of CD21 antigen. Am J Pathol. 1993;142:1729–1739. [PMC free article] [PubMed] [Google Scholar]
- 12.Denholm E M, Wolber F M. A simple method for the purification of human peripheral blood monocytes. J Immunol Methods. 1991;144:247–251. doi: 10.1016/0022-1759(91)90092-t. [DOI] [PubMed] [Google Scholar]
- 13.Ercolani L, Florence B, Denaro M, Alexander M. Isolation and complete sequence of a functional human glyceraldehyde-3-phosphate dehydrogenase gene. J Biol Chem. 1988;263:15335–15341. [PubMed] [Google Scholar]
- 14.Gahn T A, Sugden B. An EBNA-1-dependent enhancer acts from a distance of 10 kilobase pairs to increase expression of the Epstein-Barr virus LMP gene. J Virol. 1995;69:2633–2636. doi: 10.1128/jvi.69.4.2633-2636.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldsmith K, Bendell L, Frappier L. Identification of EBNA1 amino acid sequences required for the interaction of the functional elements of the Epstein-Barr virus latent origin of DNA replication. J Virol. 1993;67:3418–3426. doi: 10.1128/jvi.67.6.3418-3426.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gosselin J, Borgeat P. Epstein-Barr virus modulates 5-lipoxygenase product synthesis in human peripheral blood mononuclear cells. Blood. 1997;89:2122–2130. [PubMed] [Google Scholar]
- 17.Gosselin J, Flamand L, D'Addario M, Hiscott J, Menezes J. Infection of peripheral blood mononuclear cells by herpes simplex and Epstein-Barr viruses: differential induction of interleukin-6 and tumor necrosis factor alpha. J Clin Investig. 1992;89:1849–1856. doi: 10.1172/JCI115789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gosselin J, Flamand L, D'Addario M, Hiscott J, Stephanescu I, Ablashi D V, Gallo R C, Menezes J. Modulatory effects of Epstein-Barr, herpes simplex and human herpes-6 viral infection and co-infection on cytokine synthesis. J Immunol. 1992;149:181–187. [PubMed] [Google Scholar]
- 19.Gosselin J, Menezes J, D'Addario M, Hiscott J, Flamand L, Lamoureux G, Oth D. Inhibition of tumor necrosis factor-α transcription by Epstein-Barr virus. Eur J Immunol. 1991;21:203–208. doi: 10.1002/eji.1830210130. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg S. Signal transduction of phagocytosis. Trends Cell Biol. 1995;5:93–99. doi: 10.1016/s0962-8924(00)88957-6. [DOI] [PubMed] [Google Scholar]
- 21.Guan M, Romano G, Henderson E E. In situ RT-PCR detection of Epstein-Barr virus immediate-early transcripts in CD4+ and CD8+ T lymphocytes. Anticancer Res. 1998;18:3171–3180. [PubMed] [Google Scholar]
- 22.Heller A, Koch T, Schmeck J, van Ackern K. Lipid mediators in inflammatory disorders. Drugs. 1998;55:487–496. doi: 10.2165/00003495-199855040-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herbst H, Dallenbach F, Hummel M, Niedobitek G, Pileri S, Müller-Lantzsch K, Stein H. Epstein-Barr virus latent membrane protein expression in Hodgkin and Reed-Sternberg cells. Proc Natl Acad Sci USA. 1991;88:4766–4770. doi: 10.1073/pnas.88.11.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaneko T, Fukuda J, Yoshihara T, Zheng H, Mori S, Mizoguchi H, Oshimi K. Nasal natural killer (NK) cell lymphoma: report of a case with activated NK cells containing Epstein-Barr virus and expressing CD21 antigen, and comparative studies of their phenotype and cytotoxicity with normal NK cells. Br J Haematol. 1995;91:355–361. doi: 10.1111/j.1365-2141.1995.tb05303.x. [DOI] [PubMed] [Google Scholar]
- 25.Keles I, Woldehiwet Z, Murray R D. The effects of bovine respiratory syncytial virus on the phagocytic and antigen-presenting capacity of peripheral blood monocytes and monocytic cell lines derived from lambs and calves. J Comp Pathol. 1998;118:347–357. doi: 10.1016/s0021-9975(07)80010-7. [DOI] [PubMed] [Google Scholar]
- 26.Kelleher C A, Kaufmann Paterson R, Dreyfus D H, Streib J E, Hu J W, Takase K, Jones J F, Gelfand E W. Epstein-Barr virus replicative-gene transcription during de novo infection of human thymocytes: simultaneous early expression of BZLF-1 and its repressor RAZ. Virology. 1995;208:685–695. doi: 10.1006/viro.1995.1200. [DOI] [PubMed] [Google Scholar]
- 27.Kent S J, Stent G, Sonza S, Hunter S D, Crowe S M. HIV-1 infection of monocyte-derived macrophages reduces Fc and complement receptor expression. Clin Exp Immunol. 1994;95:450–454. doi: 10.1111/j.1365-2249.1994.tb07017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kieff E. Epstein-Barr virus: pathogenesis and pathology. In: Fields B N, Knipe D M, Howley P M, editors. Fields Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2343–2394. [Google Scholar]
- 29.Koide J, Takada K, Sugiura M, Sekine H, Ito T, Saito K, Mori S, Takeuchi T, Uchida S, Abe T. Spontaneous establishment of an Epstein-Barr virus-infected fibroblast line from the synovial tissue of a rheumatoid arthritis patient. J Virol. 1997;71:2478–2481. doi: 10.1128/jvi.71.3.2478-2481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kondo K, Kondo T, Okuno T, Takahashi M, Yamanishi K. Latent human herpesvirus 6 infection of human monocytes/macrophages. J Gen Virol. 1991;72:1401–1408. doi: 10.1099/0022-1317-72-6-1401. [DOI] [PubMed] [Google Scholar]
- 31.Langermans J A, Hazenbos W L, van Furth R. Antimicrobial functions of mononuclear phagocytes. J Immunol Methods. 1994;174:185–194. doi: 10.1016/0022-1759(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 32.Larochelle B, Flamand L, Gourde P, Beauchamp D, Gosselin J. Epstein-Barr virus infects and induces apoptosis in human neutrophils. Blood. 1998;92:291–299. [PubMed] [Google Scholar]
- 33.Larsson S, Soderberg-Naucler C, Moller E. Productive cytomegalovirus (CMV) infection exclusively in CD13-positive peripheral blood mononuclear cells from CMV-infected individuals: implications for prevention of CMV transmission. Transplantation. 1998;65:411–415. doi: 10.1097/00007890-199802150-00021. [DOI] [PubMed] [Google Scholar]
- 34.Lavin D P, Fredrickson A G, Srienc F. Flow cytometric measurement of rates of particle uptake from dilute suspensions by a ciliated protozoan. Cytometry. 1990;11:875–882. doi: 10.1002/cyto.990110804. [DOI] [PubMed] [Google Scholar]
- 35.Lupton S, Levine A J. Mapping of genetic elements of Epstein-Barr virus that facilitate extrachromosomal persistence of Epstein-Barr virus-derived plasmids in human cells. Mol Cell Biol. 1985;5:2533–2542. doi: 10.1128/mcb.5.10.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maciejewski J P, Burening E D, Donahue R E, Sellers S E, Carter C, Young N S, St. Jeor S. Infection of mononucleated phagocytes with human cytomegalovirus. Virology. 1993;195:326–336. doi: 10.1006/viro.1993.1383. [DOI] [PubMed] [Google Scholar]
- 37.Mendoza E C, Paglieroni T G, Zeldis J B. Decreased phorbol myristate acetate-induced release of tumor necrosis factor-alpha and interleukin-1 beta from peripheral blood monocytes of patients chronically infected with hepatitis C virus. J Infect Dis. 1996;174:842–844. doi: 10.1093/infdis/174.4.842. [DOI] [PubMed] [Google Scholar]
- 38.Menezes J, Jondal M, Leibold W, Dorval G. Epstein-Barr virus interactions with human lymphocyte subpopulations: virus adsorption, kinetics of expression of Epstein-Barr virus-associated nuclear antigen, and lymphocyte transformation. Infect Immun. 1976;13:303–310. doi: 10.1128/iai.13.2.303-310.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menezes J, Leibold W, Klein G. Biological differences between Epstein-Barr virus (EBV) strains with regard to lymphocyte transforming ability, superinfection and antigen induction. Exp Cell Res. 1975;92:478–484. doi: 10.1016/0014-4827(75)90404-8. [DOI] [PubMed] [Google Scholar]
- 40.Morgan A J, Douglas Wilson A. Epstein-Barr virus gp350 vaccines. Epstein-Barr Virus Rep. 1997;4:33–39. [Google Scholar]
- 41.Polyak S, Chen H, Hirsch D, George I, Hershberg R, Sperber K. Impaired class II expression and antigen uptake in monocytic cells after HIV-1 infection. J Immunol. 1997;159:2177–2188. [PubMed] [Google Scholar]
- 42.Roper R L, Phipps R P. Prostaglandin E2 regulation of the immune response. Adv Prostaglandin Thromboxane Leukot Res. 1994;22:101–111. [PubMed] [Google Scholar]
- 43.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 44.Shimakage M, Kamura M, Yanoma S, Ibe M, Yokota S, Tsujino G, Kozuka T, Dezawa T, Tamura S, Ohshima A, Yutsudo M, Hakura A. Expression of latent and replicative-infection genes of Epstein-Barr virus in macrophage. Arch Virol. 1999;144:157–166. doi: 10.1007/s007050050492. [DOI] [PubMed] [Google Scholar]
- 45.Skare J, Farley J, Strominger J L, Fresen K O, Cho M S, zur Hausen H. Transformation by Epstein-Barr virus requires DNA sequences in the region of BamHI fragments Y and H. J Virol. 1985;55:286–297. doi: 10.1128/jvi.55.2.286-297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sprenger H, Meyer R G, Kaufmann A, Bussfeld D, Rischkowsky E, Gemsa D. Selective induction of monocyte and not neutrophil-attracting chemokines after influenza A virus infection. J Exp Med. 1996;184:1191–1196. doi: 10.1084/jem.184.3.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steinkamp J A, Wilson J S, Saunders G C, Stewart C C. Phagocytosis: flow cytometric quantitation with fluorescent microspheres. Science. 1982;215:64–66. doi: 10.1126/science.7053559. [DOI] [PubMed] [Google Scholar]
- 48.Strockbine L D, Cohen J I, Farrah T, Lyman S D, Wagener F, DuBose R F, Armitage R J, Spriggs M K. The Epstein-Barr Virus BARF1 gene encodes a novel, soluble colony-stimulating factor-1 receptor. J Virol. 1998;72:4015–4021. doi: 10.1128/jvi.72.5.4015-4021.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sudgen B, Warren N. A promoter of Epstein-Barr virus that can function during latent infection can be transactivated by EBNA-1, a viral protein required for viral DNA replication in latent infection. J Virol. 1989;63:2644–2649. doi: 10.1128/jvi.63.6.2644-2649.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takada K, Ono Y. Synchronous and sequential activation of latently infected Epstein-Barr virus genomes. J Virol. 1989;63:445–449. doi: 10.1128/jvi.63.1.445-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas C A, Weinberger O K, Ziegler B L, Greenberg S, Schieren I, Silverstein S C, El Khoury J. Human immunodeficiency virus-1 env impairs Fc receptor-mediated phagocytosis via a cyclic adenosine monophosphate-dependent mechanism. Blood. 1997;90:3760–3765. [PubMed] [Google Scholar]
- 52.Wang F, Gregory C D, Rowe M, Rickinson A B, Wang D, Birkenbach M, Kikutani H, Kishimoto T, Kieff E. Epstein-Barr virus nuclear antigen 2 specifically induces expression of the B-cell activation antigen CD23. Proc Natl Acad Sci USA. 1987;84:3452–3456. doi: 10.1073/pnas.84.10.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoo J, Chen H, Kraus T, Hirsch D, Polyak S, George I, Sperber K. Altered cytokine production and accessory cell function after HIV-1 infection. J Immunol. 1996;157:1313–1320. [PubMed] [Google Scholar]