Abstract
The formulation of the ABC model by a handful of pioneer plant developmental geneticists was a seminal event in the quest to answer a seemingly simple question: how are flowers formed? Fast forward 30 years and this elegant model has generated a vibrant and diverse community, capturing the imagination of developmental and evolutionary biologists, structuralists, biochemists and molecular biologists alike. Together they have managed to solve many floral mysteries, uncovering the regulatory processes that generate the characteristic spatio-temporal expression patterns of floral homeotic genes, elucidating some of the mechanisms allowing ABC genes to specify distinct organ identities, revealing how evolution tinkers with the ABC to generate morphological diversity, and even shining a light on the origins of the floral gene regulatory network itself. Here we retrace the history of the ABC model, from its genesis to its current form, highlighting specific milestones along the way before drawing attention to some of the unsolved riddles still hidden in the floral alphabet.
We retrace the history of the ABC model, highlighting specific milestones along the way before drawing attention to some of the unsolved riddles still hidden in the floral alphabet.
History
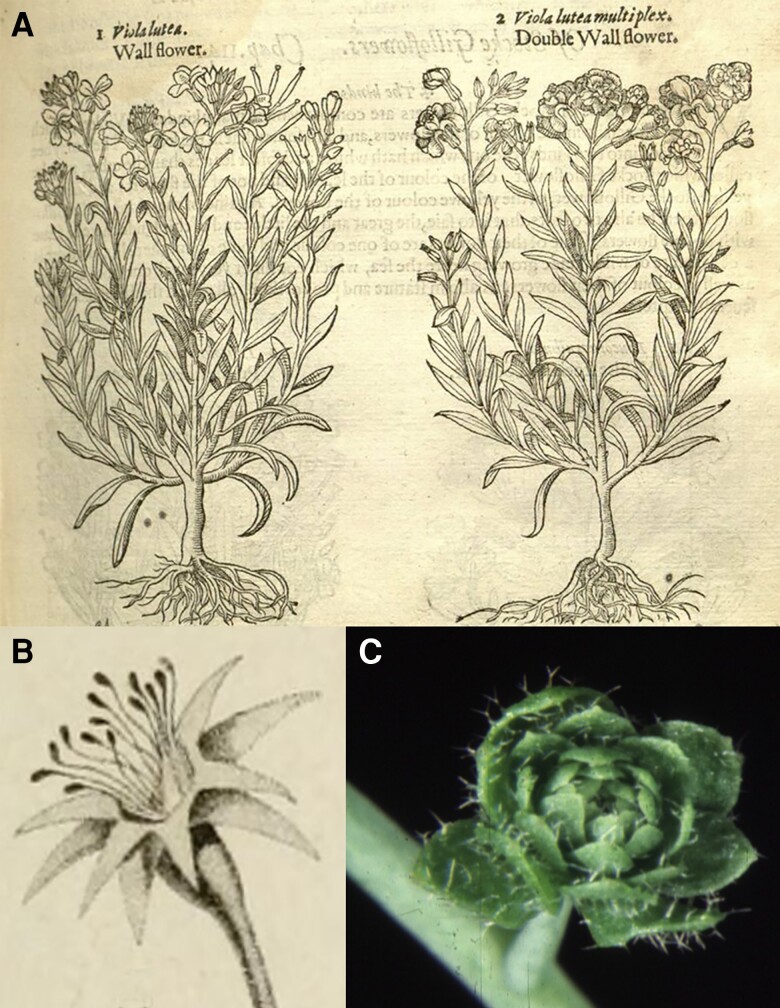
Humans have long been fascinated by flowers, perhaps initially due to their presence as a sign or harbinger of food. Thus, it is not surprising that variations in flower structure are described in the oldest surviving botanical literature. For example, Theophrastus wrote (circa 300 BC), “Among roses there are many differences, in the number of petals, … in beauty of colour, and in sweetness of scent. Most have five petals, but some have twelve or twenty, and some a great many more than these; for there are some, they say, which are even called “hundred-petalled.” [vol II, book VI 3 to 5, p39 (Theophrastus 1916)]. A few hundred years later, Pliny also described (possibly the same) rose variants: “The essential points of difference in the rose are the number of the petals, the comparative number of thorns on the stem, the colour, and the smell. The number of the petals, which is never less than five, goes on increasing in amount, till we find one variety with as many as a hundred, and thence known as the “centifolia” [Book XXI Chap 10 (Plinius the Secondus 1634)]. As China is the center of diversification of the genus Rosa, some variants likely predate the Greek and Roman descriptions (Wang 2007). Following the Renaissance, when herbals describing medicinal plants proliferated during the age of exploration, descriptions and drawings of “double flowers” also multiplied, not only for roses, for example, the double-flowered rose depicted on page 93 in Dodoens’ Florum, et Coronariarum Odoratarumque Nonnullarum Herbarum Historia (Dodoens 1568), but for other species as well. Gerard (Gerard et al. 1597) depicted no fewer than 15 species with double-flowered variants, including in the Brassicaceae (Fig. 1A), the latter being included in some earlier publications (L’Obel 1581; Dodoens 1583). Centuries later, double flowers of Aquilegia vulgaris were examined in greater detail with the finding that some stamens are converted into petals with nectaries, with petals from one whorl neatly fitting into those of another (Moquin-Tandon 1841).
Figure 1.
Early descriptions of flower variants. A) Single- (left) and double-flowered (right) wallflowers, Erysimum cheiri (Gerard et al. 1597). B) An apple mutant in which the petals are transformed to another whorl of sepals and the stamens are transformed into carpels. This tree was used for cross-fertilization, where “the ladies and young girls of Saint-Valery compete to ‘make their apple’ [that's the expression they use]. A hermaphrodite flower, picked in dry weather from any apple tree, is applied to each flower and left there until fertilization is completed and it falls off naturally. Then a ribbon of colour is attached to the fertilized bouquet so that, when autumn comes, each person can recognize the fruit created by their own hand” (Tillette de Clermont-Tonnere 1825). C) An apetala2-1 pistillata-1 agamous-1 flower consisting of an indeterminate number of whorls of leaf-like organs. (A,B, Biodiversity Heritage Library; C, photo, J.L.B.).
While double-flowered blossoms are conspicuous, other less striking flower variants were also described centuries ago (Fig. 1B). For example, an apple tree (Malus) in M. Aux's orchard in Saint-Valery-sur-Somme that was “similar in every way to the common apple tree in terms of leaves and flower arrangement, differs in a very remarkable way in the structure of its flowers and fruits. A tomentose peduncle supports a flower composed of a calyx with ten folioles fused at the base, arranged in two alternating rows, the inner ones slightly shorter. The corolla and stamens are absent; the styles, fourteen in number, slightly hairy at the base, are surmounted by a very vigorous oblique stigma” (Tillette de Clermont-Tonnere 1825). Usually in apple flowers “the calyx is simple, with five leaflets; here, it is clearly double, with the inner part similar to the outer part, persisting like it and cannot be assimilated to a corolla. In the former, there are five styles and stigmas; in ours, there are fourteen. Ordinary apples have only five compartments; these have fourteen in two parallel rows, representing two apples fused end to end” (Tillette de Clermont-Tonnere 1825). This phenotype was maintained after grafting onto a normal apple tree, but unfortunately, the phenotype of progeny derived from crosses between mutant flowers and wild-type stamens was not reported (Tillette de Clermont-Tonnere 1825). A similar, albeit less severe, phenotype was noted in a Rhododendron variant, known as Misome-sho in Japanese and described in 1692, that exhibits a sepaloid corolla and altered stamen development (Cheon et al. 2018).
It is clear from the descriptions of the Malus and Aquilegia flowers that it was recognized that there had been a homeotic transformation of one organ type into another (Tillette de Clermont-Tonnere 1825; Moquin-Tandon 1841), although the term “homeotic” was not coined until later. For example, Moquin-Tandon's (1841, p. 386) interpretation of the mutant Malus flowers, “It is possible that, due to the absence of stamens, the androecium has transformed into a row of carpels that are fused to the ordinary carpels. In that case, the phenomenon in question would fall into the category of organic metamorphoses”. Later, Masters, in his Vegetable Teratology, uses the Malus example to illustrate his concept of metamorphy (Masters 1869). Bateson subsequently replaced Masters’ metamorphy with the term we know today, homeosis (Bateson 1894).
While anatomical and morphological descriptions of “normal” flower development and variations thereof abounded (see Moquin-Tandon 1841; Payer 1857; Masters 1869; Penzig 1890-4; Wordsell 1915-6), the connection to heritability was not often made. Perhaps some of the reluctance stemmed from observations such as made by Darwin: “Such flowers as the carnation, common tulip, and hyacinth, which are believed to be descended, each from a single wild form, present innumerable varieties, differing almost exclusively in the size, form, and colour of the flowers. These and some other anciently cultivated plants which have been long propagated by offsets, pipings, bulbs, & c., become so excessively variable, that almost each new plant raised from seed forms a new variety, ‘all of which to describe particularly,’ as old Gerarde wrote in 1597, ‘were to roll Sisyphus's stone, or to number the sands‘” (Darwin 1876). However, heritability is alluded to occasionally. Darwin himself described a number of heritable floral variants, including a poppy in which the stamens are converted into pistils [de Candolle quoted in (Darwin 1876)], and several authors noted the inheritance of a number of peloric floral varieties, including that of Antirrhinum majus (Naudin 1865; Godron 1874; Darwin 1876). And much earlier, Parkinson noted that while double-flowered plants never bore seed, if double-flowered plants are desired, one should collect plants from single-flowered plants in the vicinity of the double-flowered plant, and sometimes a proportion of these would be double-flowered even though the original single-flowered parents were indistinguishable (Parkinson 1629). Following the rediscovery of Mendel in the early 20th century, it was rapidly determined that a Matthiola double-flower phenotype was recessive with respect to the single-flower phenotype (Bateson et al. 1905), explaining why double flowers, which are entirely male and female sterile, only arise from single-flowered seed stocks, apparently at random, according to breeders in the days before genetics. Furthermore, as noted by some early breeders, single-flowered plants bearing flowers with extra petals may indicate plants that we now know as heterozygotes (Bateson et al. 1905).
For a majority of the 20th century, genetics and developmental biology of plants continued in distinct trajectories despite having merged in the study of animals in the mid-20th century (Meyerowitz et al. 1989; Prunet and Meyerowitz 2016). Thus, early models of flower development typically employed hormonal or biophysical signals or forces, rather than genes, that would influence floral organ identity. Perhaps the earliest model was proposed by Goethe where he viewed floral organs as modified leaves, with their modification from leaves into floral organs directed by ascending sap that became more refined as it made its way to the apex (Goethe 1790). Abnormal organs could be viewed as a failure of refinement of the sap. Other models relied on similar relay mechanisms such that one type of floral organ would induce the next inner set of organs. In some models the mechanism was proposed to be biomechanical (Green 1988), while in others, genetic programs were invoked (Heslop-Harrison 1963). However, these models failed to explain existing floral varieties where organ types were absent or in inappropriate positions. In contrast, models based on concentric rings of hormonal activity, avoiding the inter-whorl communication, were better equipped to explain known floral mutant phenotypes but invoked shifts in the positions of organ primordia rather than changes in the concentric rings of hormonal activity (Brieger 1935; Holder 1979).
Genetics
But I (J.L.B.) did not know most of the above as I peered through the dissecting microscope in a basement annex of the Kerckhoff building at Caltech in early 1990 when I was a PhD student in Elliot Meyerowitz's laboratory. I was searching among Arabidopsis plants for a triple mutant in a family segregating for apetala2-1, agamous-1, and pistillata-1. While its phenotype could be predicted based on already constructed double mutant combinations (Bowman et al. 1989), there was a thrill of observation and contemplating that you have knowledge no one else in the world possessed (Fig. 1C). It was late it the evening, and all members of Elliot's lab had left, and so I sauntered down the hallway to share the discovery with my mate, Joe Minor, who was working on sea urchins in the neighboring laboratory.
These observations were the culmination of experiments begun in the early 1980s, initially by Maarten Koornneef, who was constructing an Arabidopsis genetic map and who isolated several mutants affecting floral architecture (Koornneef et al. 1983). Elliot obtained these stocks from Maarten and, subsequently, Bob Pruitt, a previous PhD student in Elliot's laboratory, and Elliot thought the mutants exhibited homeotic phenotypes. When I joined the laboratory in late 1986, I continued the construction of multiple mutants, a process initiated by Bob and Elliot. At first, we had solitary mutant alleles of each of 5 loci [APETALA1 (AP1), APETALA2 (AP2), APEPALA3 (AP3), PISTILLATA (PI), AGAMOUS (AG)]. These were chosen based on their homeotic phenotypes, although AP1 was initially left aside due to its broader role that we now know as determining floral meristem (FM) identity. The chosen loci reflected an early decision to focus on the roles in organ identity, ignoring the effects of the mutations on organ number (e.g. organ initiation or fusion in the case of carpels) and determinacy, even though it was clear the chosen genes might have functions additional to those directly involved in organ identity. In January 1988, David Smyth joined the laboratory as a sabbatical visitor, and we set about looking at wild-type flower development using scanning electron microscopy (Smyth et al. 1990). Given the size of Arabidopsis flowers, this technique was critical to interpreting mutant phenotypes, although it was not without its challenges (see Bowman 2013). David also undertook a mutant screen to isolate additional mutant alleles of the above genes as well as identify other loci (Smyth 2023). The additional alleles were key to estimating whether alleles were likely to be null or hypomorphic, influencing the construction of the model. One early incarnation was drawn on the laboratory fridge door, where we debated whether the A/C boundary was sharp or fuzzy—and based on strong ap2 alleles (Komaki et al. 1988; Kunst et al. 1989; Bowman et al. 1991), we later drew the boundary sharp. In comparison to older models, the idea of concentric rings of activity was a common theme, only now each concentric ring was defined by specific gene activities (Bowman et al. 1989).
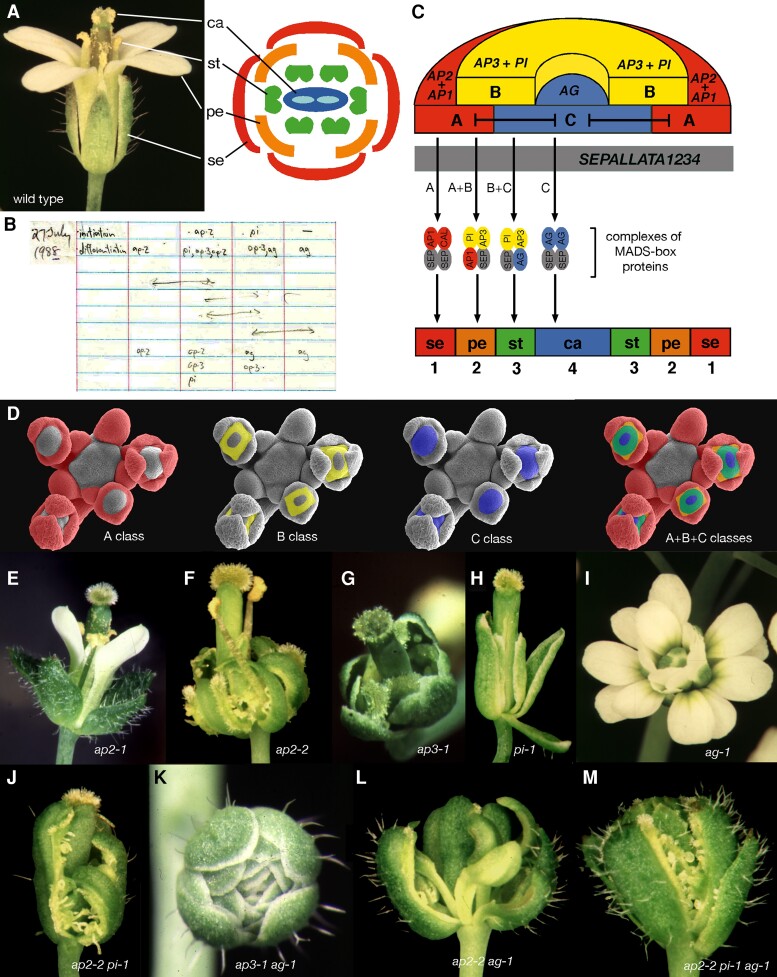
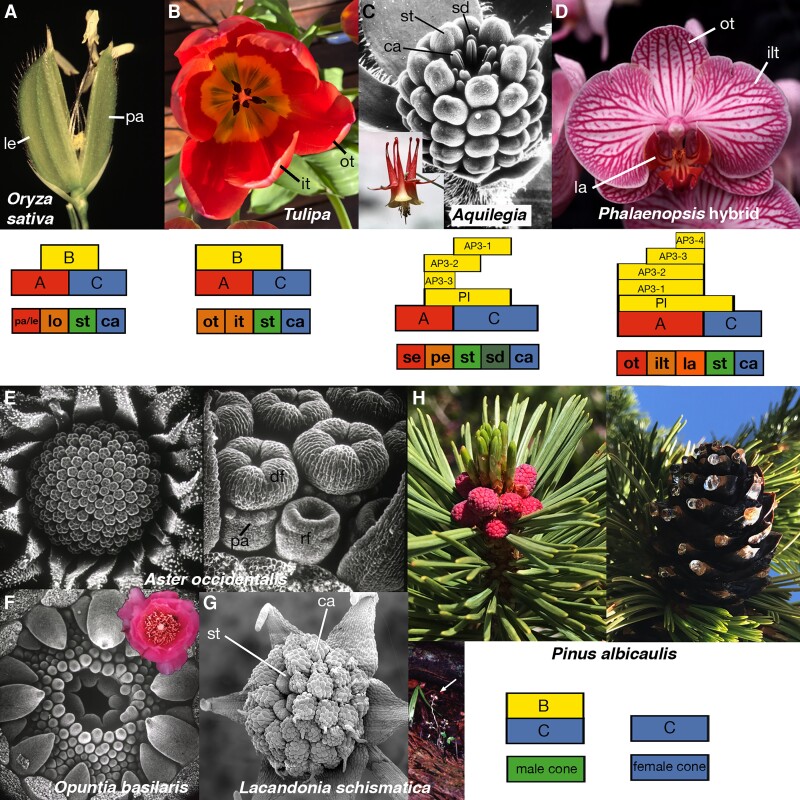
Simply stated, the ABC model postulates 3 distinct gene activities (A, B, and C), each of which are present in 2 adjacent whorls of the Arabidopsis flower, act alone and in combination to specify the 4 types of floral organ (Fig. 2, A to D) (Bowman et al. 1991). A second postulate was that the A and C activities are mutually antagonistic, a prerequisite to be consistent with the single mutant phenotypes (Fig. 2, E to I). The model immediately suggests phenotypes for most double and triple mutants (Fig. 2, J to M). While most double mutant combinations were additive, the key double mutant was ap2 ag, where both class A and C are missing, leaving only B present in whorls 2 and 3, and no homeotic function in whorls 1 and 4, both situations not found in any whorl in wild-type flowers (Fig. 2L). The ap2 ag phenotype could be described as carpelloid sepals in the first and fourth whorls and stamenoid petals in the second and third whorls, both being new organ types not observed in wild type (Bowman et al. 1989, 1991). Two conclusions can be drawn from the observed phenotype. First, agamous mutations have phenotypic effects in the outer whorls in an ap2 background and vice versa, indicating that the 2 gene activities are in fact mutually antagonistic. Second, that the organs in the middle 2 whorls are neither wild-type petals nor wild-type stamens indicates that B class alone in Arabidopsis does promote one or the other. Intriguingly, the cells of these organs are also intermediate between wild-type petals and wild-type stamen cells and thus formed a new cell type. This is in contrast to cases where we observed mosaic organs with distinct boundaries between known cell types in single or multiple mutant combinations (Bowman et al. 1989, 1991), suggesting that floral organ identities were determined largely cell autonomously. One mutant allele of APETALA3, ap3-1, and all alleles of APETALA2 were found to be temperature sensitive, allowing the timing of gene activity to be elucidated. Consistent with a role in organ identity specification, gene function was required during the time of organ initiation and early development, with the sensitive period of stamens being quite abbreviated while that of petals was more prolonged (Bowman et al. 1989).
Figure 2.
The ABC model and mutant phenotypes on which it is based. A) Wild-type Arabidopsis flower (left) and floral diagram (right). se, sepal; pe, petal; st, stamen; ca, carpel; floral structure: se, pe, st, ca. B) Early version of the ABC model from mid-1988. C) ABC model in its current incarnation; the ABC activities are whorl specific while those of SEPALLATA are throughout the flower. “Dimers of dimers,” or quartets, of MIKCC-type MADS box proteins determine organ identity. D) Early expression of the ABC genes mapped onto developing flowers. E)apetala2-1: floral structure: le (leaf), pe, st, ca. F)apetala2-2: floral structure: ca, missing, st, ca. G)apetala3-1: floral structure: se, se, ca, ca. H)pistillata-1. floral structure: se, se, ca (most fused with the central carpels), ca. I)agamous-1: floral structure: (se, pe, pe)n—the flower meristem is indeterminate with the organs repeating. J)apetala2-2 pistillata-1: floral structure: ca, missing, missing, ca—in some cases all organs are fused into a single structure. K)apetala3-1 agamous-1: floral structure: (se, se, se)n. L)apetala2-2 agamous −1: floral structure: (le/ca (carpelloid leaf), st/pe (stamenoid petal), st/pe)n. M)apetala2-2 pistillata-1 agamous-1: floral structure: (le/ca (carpelloid leaf), le/ca, le/ca)n. All photos by J.L.B.; A (Lee et al. 2005b); D, F, H, J, L, M (Bowman 2001; copyright 2001, John Wiley & Sons, Ltd., reproduced with permission); G (Bowman et al. 1989); K (Bowman et al. 1991; copyright 1991, Company of Biologists, reproduced with permission).
Concomitant with our work in Arabidopsis, 2 groups working with Antirrhinum (Enrico Coen's group at the John Innes Centre in Norwich and Suzanna Schwarz-Sommer's group at the Max Planck Institute in Cologne) identified mutants with similar phenotypes and independently came up with a BC model of flower development (Carpenter and Coen 1990; Schwarz-Sommer et al. 1990), the major difference being the lack of Antirrhinum A function genes. While it might be difficult to fathom among the current generation of young geneticists, the ABC/BC models were constructed entirely with genetics (no molecular biology or genomics) and could have been formulated decades or even a century before [see (Bowman et al. 2012)]—we even named one room in Elliot's laboratory where histology was performed “the 19th-century room.” Thus, one question was, why did it take so long? And a second question, why did multiple groups converge on the same methodology and largely similar conclusions almost simultaneously? Such convergence is a common theme in science and must indicate that the time was ripe for discovery, either via a technical or conceptual advance, the latter being key in the present case. I was personally influenced by Ed Lewis, whose laboratory was upstairs and whose seminal work in Drosophila on the identification and characterization of the bithorax complex led to a model whereby multiple genes act in combination to specify the identity of the thoracic and abdominal segments of the fly body (Lewis 1978)—I recall with both fondness and horror that one of my first experiences as a graduate student was to present this paper in a journal club with Ed in the audience, who “gently” corrected me when I went astray. Thus, it did not seem such a large jump to think about a similar model for the concentric whorls of the flower.
Molecules
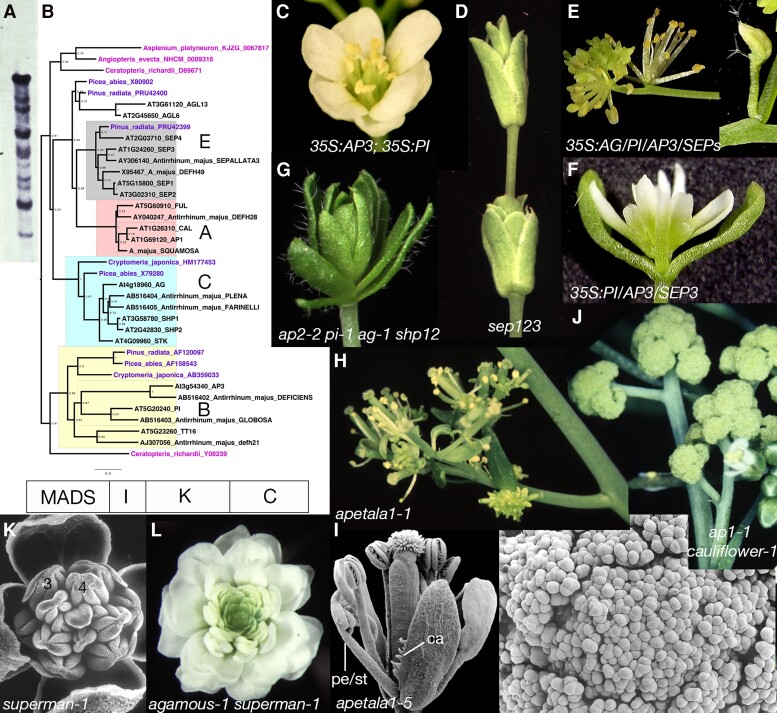
The mid-1980s were a time of revolutionary insight into the molecular bases of animal development, with the discovery that genes of the bithorax complex and Antennapedia encoded paralogous proteins now known as homeobox proteins (Garber et al. 1983; McGinnis et al. 1984b; Scott and Weiner 1984; Karch et al. 1985) and that homologous genes were widespread in animals (McGinnis et al. 1984a). It was immediately speculated that homologs might exist in plants and these might also encode homeotic functions, despite the independent evolution of multicellular plants and animals from a single-celled ancestor. While homeobox genes were subsequently identified in plants (Vollbrecht et al. 1991), the first ABC gene cloned was DEFICIENS, a B class gene in Antirrhinum, and rather than being similar to homeobox proteins, it had sequence similarity with the known transcription factors MCM1 of yeast and SRF of mammals (Sommer et al. 1990). Some months later, the cloning of the Arabidopsis C class gene, AGAMOUS, was reported, and this too was homologous with MCM1, DEF, and SRF (Yanofsky et al. 1990), giving rise to the moniker of “MADS”-box for the putative DNA binding sequence of the gene family (Schwarz-Sommer et al. 1990). Given that 2 of the ABC genes encoded MADS-box genes, it was immediately speculated that other ABC genes may also encode homologous proteins. While the first genes cloned, DEFICIENS and AGAMOUS, were identified via transposon/T-DNA tagging approaches, cloning of subsequent genes took advantage of homology-based approaches. Although one could not scan genome sequences at the time, low stringency Southern blots and library screens provided a fruitful approach to clone paralogs (Fig. 3A). Thus, the remaining B and C class genes in both Arabidopsis and Antirrhinum were quickly identified, either by homology to MADS-box genes in general or via assumption of orthology between the 2 species (Jack et al. 1992; Tröbner et al. 1992; Bradley et al. 1993; Goto and Meyerowitz 1994). However, in retrospect the Antirrhinum C-class gene PLENA turned out to be a ortholog of Arabidopsis SHATTERPROOF1/2 genes, which are sister paralogs of AGAMOUS (Davies et al. 1999). Later, the Arabidopsis A class gene APETALA1 was also cloned based on MADS-box homology (Mandel et al. 1992b). The Antirrhinum AP1 ortholog, SQUAMOSA, was not recognized as an A class gene but rather was proposed to play a role in flower meristem identity (Huijser et al. 1992), a theme we will return to shortly. It was also soon recognized that plant genomes harbor a large contingent of MADS-box genes that extended well beyond the ABC genes [e.g. (Ma et al. 1991; Pnueli et al. 1991)], and these would subsequently be targets of future reverse genetic approaches (Fig. 3B).
Figure 3.
Molecular and genetic extensions of the ABC model. A) Low stringency Southern blot using AGAMOUS as a probe on Arabidopsis DNA cut with EcoRI. B) Simplified MADS-box gene phylogeny: A-class, red; B-class, yellow; C-class, blue; E-class, grey; gymnosperm sequences, purple; fern sequences, pink. In both B and C clades, additional angiosperm paralogs are present and often have functions related to the B and C-class genes. Gymnosperm orthologs exist (or existed) for all classes, and the split between the B-class and A/C/E classes occurred in the common ancestor of euphyllophytes. The MIKCC protein structure is indicated at the bottom; MADS, DNA binding domain; I, intervening domain; K, keratin-related domain; C, carboxyl terminal domain. C)35S:APETALA3; 35S:PISTILLATA: floral structure: pe, pe, st, st. D)sepallata123: floral structure: (se, se, se)n. E)35S:AG/PI/AP3/SEP3: floral structure: (st, st, st)n; leaves are transformed into stamens. F)35S:PI/AP3/SEP3: leaves, but not cotyledons, are transformed into petals. G)apetala2-2 pistillata-1 agamous-1 shatterproof12: floral structure: (le, le, le)n. H)apetala1-1: floral structure: br (bract-like), pe (or missing), st, ca; first whorl bracts may also have flower meristem in their axils, and ectopic flower meristems can arise. The photo depicts a “single” basal flower, with later produced flowers having decreasing indeterminacy. I)apetala1-5: floral structure: br (or br/ca), pe (or pe/st), st, ca. this flower has both a stamenoid petal (pe/st) and carpel (ca) tissue at the margin of a first whorl bract. J)apetala1-1 cauliflower-1: when grown in nonfloral inductive conditions, what should have been a flower is only a collection of inflorescence meristems that reiterate a spiral initiation pattern (lower panel). K) Additional whorls of stamens (4) develop interior to the third whorl (3) in superman flowers; floral structure: se, pe, st, st, (st). L) Flowers of superman agamous double mutants have an outer whorl of sepals and an indeterminate number of whorls of petals; floral structure: se, pe, pe, pe, etc. E-F courtesy of Koji Goto; all other photos by JLB except G by Ji-Young Lee; C (Bowman 2001; copyright 2001, John Wiley & Sons, Ltd., reproduced with permission); D, G (Lee et al. 2005b); I (Bowman et al. 1993; copyright 1993, Company of Biologists, reproduced with permission).
The cloning of the genes enabled the examination of the ABC model from a molecular perspective, with expression patterns of B and C class genes largely congruent with mutant phenotypes (Yanofsky et al. 1990; Jack et al. 1992; Schwarz-Sommer et al. 1992; Tröbner et al. 1992; Bradley et al. 1993; Goto and Meyerowitz 1994). Critically, in Arabidopsis, the expression pattern of AGAMOUS expanded into all floral whorls in the A-class mutant apetala2, supporting the hypothesized antagonism between these 2 classes (Drews et al. 1991). Having the cloned genes in hand also allowed further tests by creating gain-of-function alleles in Arabidopsis (disappointingly, Antirrhinum is still difficult to transform). Thus, it was noted that ectopic expression of the Brassica napus AGAMOUS gene throughout the flower was sufficient to transform the identity of the outer whorls of tobacco flowers into carpels and stamens, respectively, upholding one tenet of the model and also providing additional support for universal ABC gene activity across eudicots (Mandel et al. 1992a). Likewise, ectopic expression of both APETALA3 and PISTILLATA was sufficient to produce homeotic transformations in the first and fourth whorls (Krizek and Meyerowitz 1996) (Fig. 3C). Given that APETALA3 and PISTILLATA were required for B-class activity [they act as an obligate heterodimer (Schwarz-Sommer et al. 1992; Goto and Meyerowitz 1994; Davies et al. 1996; Riechmann et al. 1996)], one surprise was that ectopic expression of PISTILLATA alone partially transformed sepals into petals and ectopic expression of APETALA3 alone partially transformed carpels into stamens. These observations were reconciled by careful examination of gene expression patterns, with APETALA3 expressed during early sepal development and PISTILLATA expression detected in the fourth whorl. Thus, where the 2 gene expression patterns overlap determines B-class activity, and it is possible that the “sloppy” expression patterns could facilitate evolution of perianth phenotypes. Notably, where B- or C-class genes were expressed throughout the entire plant, the leaves were curled and small, but no transformation of leaves into floral organs was observed (Mandel et al. 1992a; Krizek and Meyerowitz 1996).
Converting leaves into floral organs
A major advance at the end of beginning of this century was the discovery of E-class MADS box function. Pivotal was the continued investigation of MADS-box paralogs in Arabidopsis and the discovery that a triple mutant of 3 paralogs resulted in a flower with all sepals (Fig. 3D), implying that this class of gene was required for B- and C-class function (Pelaz et al. 2000). Inclusion of mutations in a fourth paralog resulted in flowers with all leaf-like organs, indicating their requirement for all 4 floral organ types (Ditta et al. 2004). The genes, named SEPALLATA, were expressed through the flower and did not appear to provide any organ identity specification activity on their own because their ectopic expression alone is not sufficient to convert leaves to floral organ identity (Honma and Goto 2001); for this reason, I argued that this should be called F-class, for Flower. Remarkably, ectopic expression of ABC genes along with SEPALLATA genes was sufficient to convert leaves into floral organs (Honma and Goto 2001; Pelaz et al. 2001). Thus, the MADS-box genes are necessary for the specification of floral organ identity and are sufficient (along with E-class) to convert leaves into floral organs, supporting ideas of the evolution of floral organs from leaf-like ancestors (Fig. 3, E and F). It was noted earlier that DEFICIENS, GLOBOSA, and SQUAMOSA proteins could form a ternary complex (Egea-Cortines et al. 1999), and ternary and quaternary complexes of Arabidopsis proteins including ABC and SEPALLATA MADS-box proteins (e.g. B + C + SEP and A + B + SEP) were observed, prompting the hypothesis that these complexes form the molecular basis of the ABC model (Honma and Goto 2001). These observations were summarized in the “quartet” model wherein MADS-box proteins act as “dimers of dimers’ in their binding to regulatory sequences of target genes (Theissen and Saedler 2001) (see “Good things come in fours” section below).
That the ABC triple mutant produced carpelloid leaves while the organs of the sepallata1234 quadruple were more leaf-like suggested that additional MADS-box genes active in the ABC triple mutant background contributed to carpel differentiation. Two AGAMOUS-related paralogs, SHATTERPROOF1/2, were found to be ectopically expressed in the apetala2 agamous background and shown to be responsible for AGAMOUS-independent carpel development (Savidge et al. 1995; Pinyopich et al. 2003). Thus, flowers of the pentuple mutant, apetala2 pistillata agamous shatterproof1/2 (Fig. 3G), consist entirely of leaf-like organs (Lee et al. 2005a).
A molecular explanation for the indeterminacy of agamous flowers was provided by the observation that WUSCHEL, which acts to maintain stem cells in the flower meristem, is repressed directly by AGAMOUS (Sun et al. 2009; Liu et al. 2011). The repression of WUSCHEL may also explain other determinacy effects observed when carpels develop in inappropriate whorls, such as the reduced numbers of organs in genotypes containing apetala2 null alleles. Finally, that carpels promote fusion and determinacy is supported by reduced determinacy in genotypes, where carpel identity is reduced by introduction SPATULA or CRABS CLAW mutant alleles (Alvarez and Smyth 1999).
The curious case of A-class and subfunctionalization of B- and C-classes
When venturing away from Arabidopsis, genetic manipulations did not always yield the phenotypes expected if function was strictly conserved with Arabidopsis ABC orthologs. Diving into the complex histories of plant genomes revealed that gene/genome duplications, followed by neo- and subfunctionalization or loss, are the source of many variations observed.
The conservation, or even the existence, of A-class has been debated, with the most common hypothesis being that A-class is a continuation of FM identity (e.g. Causier et al. 2010), as is also observed in Arabidopsis. In Arabidopsis, A-class was defined as having 2 functions in the perianth—the repression of C-class and an organ identity role (Bowman et al. 1991). That there exists a distinct A-class activity is supported by the observation that organs of the middle whorls of apetala2 agamous flowers are petal/stamen hybrids, and thus, B-class plus SEPALLATA does not specify petals. Furthermore, sepals are not leaves (or bracts), and thus their identity must be specified by some activity, even if it overlaps with the activity that promotes FM identity.
A-class activity was proposed to be composed of the MADS-box gene APETALA1 and APETALA2 (Irish and Sussex 1990; Bowman et al. 1991, 1993), encoding an AP2-type transcription factor (Jofuku et al. 1994). In null apetala1 alleles sepals are converted into bract-like organs (Fig. 3H), and petals are usually missing (Irish and Sussex 1990; Bowman et al. 1993). However, in hypomorphic apetala1 alleles (Fig. 3I) carpelloid tissue can be formed on the first whorl organs and the petals may be partially transformed into stamens (Bowman et al. 1993), a phenotype often neglected in later reviews of the ABC model. A role for APETALA1 in organ identity could also be masked by the action of CAULIFLOWER, a closely related paralog (Kempin et al. 1995): the double mutant ap1 cal produces inflorescence meristems instead of flower ones (Fig. 3J), indicating both genes have a role in FM identity and precluding observation of potential organ identity defects (Bowman et al. 1993). Notably, the sepallata1234 quadruple mutant does not appear to have defects in FM specification, although SEPALLATA4 may have redundant roles with APETALA1 in this regard (Ditta et al. 2004).
Consistent with mutual antagonism and A and C classes in Arabidopsis, AGAMOUS mRNA is detected in all 4 whorls of apetala2 mutants (Drews et al. 1991), and APETALA1 mRNA expands to the inner whorls in agamous flowers (Gustafson-Brown et al. 1994). However, AGAMOUS mRNA also accumulates in the inner whorls of agamous flowers (Gustafson-Brown et al. 1994), and APETALA2 mRNA can be found at some levels in all 4 whorls of wild-type flowers (Jofuku et al. 1994), indicating that their mutual antagonism is complex. The negative regulation of APETALA2 by miR172 provides one mechanism to restrict AP2 activity post-transcriptionally (Aukerman and Sakai 2003; Chen 2004). Expression of miR172 in the central whorls during early flower development appears to provide quantitative regulation of AP2 activity, with the balance of AGAMOUS and APETALA2 activity, especially in the third whorl, proposed to influence organ identity (Wollmann et al. 2010).
Outside of Arabidopsis, findings in asterids demonstrated that floral homeotic phenotypes are incompatible with a straightforward transfer of Arabidopsis A ortholog function (Cartolano et al. 2007; Morel et al. 2017). The AP2 family comprises APETALA2-type and TOE-type clades (Wang et al. 2016). Petunia AP2A/ROB1 and Antirrhinum LIP1/2 genes are APETALA2 orthologs, but unlike APETALA2, these are unable to repress the C-function. Instead, it is another miRNA, called BLIND in Petunia and FIS in Antirrhinum, that tackles this task by targeting NF-YA transcription factors. BLIND is helped by BLIND ENHANCER (BEN), and only the double mutant blind ben mimics the expected A class phenotype (Morel et al. 2017). BEN is a TOE-type AP2 whose members control flowering time, not organ identity, in Arabidopsis. BEN was also shown to work with the 3 Petunia AP2-type genes called ROBs (Repressor Of B function) so that BEN and ROBs together repress B function (but not C-function) in the sepal whorl. To summarize, in Petunia A function relies on a combination of a related AP2 gene and an unrelated microRNA (BEN + BLIND); reciprocally, the APETALA2 orthologs (ROB genes) do not repress C function but rather team up with BEN to repress B function.
Compared to A class, the molecular agents fulfilling B and C functions appear broadly conserved across angiosperms. This difference in extent of conservation may be related to the antiquity of the functions, with B and C evolving at the dawn of seed plants, while A-function appears to be an angiosperm novelty (Wellmer et al. 2014). Nonetheless, variations involving related paralogs exist. For example, the B gene APETALA3 is only 1 of 2 old twins: a duplication created APETALA3 and TM6 somewhere near the base of eudicots (Kramer et al. 1998; Kim et al. 2004), but Arabidopsis and Antirrhinum have both lost their TM6 ortholog. So the original floral development genetic models only tell part of the story, and B function can be more complicated in other species. In Petunia the APETALA3 ortholog (PhDEF) and PhTM6 redundantly control stamen identity, but petals are specified by PhDEF only. Phtm6 mutants have a wild-type phenotype, whereas in a Phdef mutant petals are replaced by sepals but the stamens are normal (Vandenbussche et al. 2004; Rijpkema et al. 2006). A similar logic applies to the PISTILLATA lineage, which represents the other leg of the B function in Arabidopsis: the Petunia genome hosts 2 PI orthologs acting redundantly to specify petal and stamen identity (Vandenbussche et al. 2004), with one paralog having acquired an additional role controlling stamen to petal tube fusion. Hence, the expected B phenotype is only obtained when both paralogs are defective. Such duplications also play important roles for petal elaboration (see the “Beyond eudicots” section below), impacting not only organ identity specification but morphogenesis as well (Kramer et al. 2007; Sharma and Kramer 2013; Hsu et al. 2015, 2021).
Evolution within the MADS-box gene family also affected how C-function is executed. Duplications yielded 3 genes in the “C-lineage”: AGAMOUS, SHATTERPROOF1/2 (PLENA), and SEEDSTICK, with the first duplication producing SEEDSTICK occurring in the ancestral seed plant and the other occurring within eudicots [e.g. (Carvalho et al. 2018)]. In Petunia both AGAMOUS and SHATTERPROOF1/2 orthologs must be inactivated to produce typical C-class mutant phenotype (Kapoor et al. 2002; Heijmans et al. 2012). In Arabidopsis, AGAMOUS fulfils the C function with SHATTERPROOF1/2 controlling aspects of carpel and fruit development (Liljegren et al. 2000). In contrast, PLENA executes the C function in snapdragon, while the AGAMOUS ortholog, FARINELLI, only plays a secondary role during stamen development (Bradley et al. 1993; Davies et al. 1999). The third paralog of the clade, SEEDSTICK, directs ovule development (Pinyopich et al. 2003) and is sometimes referred to as D class that is unrelated to floral organ identity. However, even SEEDSTICK alone can promote aspects of carpel development in the absence AGAMOUS (Favaro et al. 2003), and perhaps its role in ovule development reflects an ancestral function of this clade since ovules predate flowers.
Regarding E-class, in rice (Ohmori et al. 2009; Li et al. 2010), Petunia (Rijpkema et al. 2009), orchid (Hsu et al. 2015, 2021), and maize (Thompson et al. 2009) SEPALLATA orthologs and also their close paralogs, the AGL6 genes, can all deliver E function, adding another layer of redundancy and complexity to the ABC story (for review, see Dreni and Zhang 2016).
To accommodate these variations, it was proposed that the model be referred to as the (A)B(C) model (Causier et al. 2010; Theissen et al. 2016) with (A) accounting for A and E activities and (C) combining C and D roles. However, now that we have a broader evolutionary perspective of gene evolution within the ABCE clades (Fig. 3B) combined with functional analyses (above and later), most described variation within core eudicots is derived from vagaries in subfunctionalization of paralogous genes rather than any fundamental differences. It remains to be seen whether exceptions will occur in species with “noncanonical” perianths, such ANA-grade and magnoliid angiosperms (Wellmer et al. 2014).
Paddling upstream and downstream
As all big discoveries, the formulation of the ABC model immediately raised difficult questions: what regulatory processes generate the spatio-temporal expression patterns central to the homeotic function of ABC genes? How can the combinations uncovered by genetic analyses transpose into molecular mechanisms allowing different protein complexes to activate distinct developmental programs? How do the regulatory activities of TF-encoding ABC genes translate into morphogenic effects that shape the different types of floral organs, specifying a range of often organ-specific cell fates? Although we still lack a high-resolution picture, the last 20 years of floral development research have added many pieces to this incomplete puzzle. Our understanding of the complex networks behind the characteristic ABC expression domains has been synthesized regularly over the last 2 decades (Zahn et al. 2006; Causier et al. 2009; Pelayo et al. 2021). In the interest of brevity, we only summarize here the main messages and highlight recent breakthroughs toward solving long-lasting riddles.
Swimming upstream
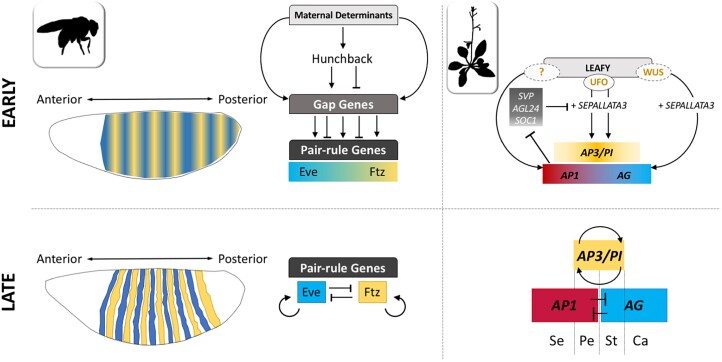
Early work rapidly established that regulation of ABC genes expression is a multi-layered process integrating inputs from hormonal signaling pathways with the activity of transcription factors, histone modifying enzymes, and chromatin remodeling complexes and roles for post-transcriptional mechanisms often involving mobiles noncoding RNAs and RNA processing factors (e.g. Cheng et al. 2003; Chen 2004; Zhao et al. 2007; Yamaguchi et al. 2009; Wu et al. 2012, 2018; Jung et al. 2014; Sacharowski et al. 2015; Poyatos-Pertíñez 2016; Morel et al. 2017; Lee et al. 2022; Lin et al. 2023). These investigations exposed a 3-step waltz regulating ABC genes: upstream factors initiate transcription across broad territories (step 1), followed by refinement (step 2), and then maintenance (step 3) of precise expression domains. Step 2 and 3 rely extensively on cross regulatory interactions and feedback loops between floral homeotic genes in a manner reminiscent of the processes establishing discreet stripes of pair-rule gene expression in flies: combined inputs of maternal determinants and GAP genes first trigger expression of pair-rule genes in broad territories, which then get refined through cross regulations between the pair-rule genes themselves (Edgar et al. 1989; Pankratz and Jäckle 1990) (Fig. 4). Flower patterning follows a similar logic: the broad expression domains of ABC genes in nascent floral meristems largely overlap at first, but cross inhibition between A and C later renders their expression mutually exclusive while positive feedback loops between APETALA3 and PISTILLATA enhance and eventually restrict any significant B genes transcription to the subset of meristematic cells that initially expressed both (Fig. 4). Additionally, B-class genes may act to directly repress genes required for carpel development, providing another layer of cross-regulation (Wuest et al. 2012).
Figure 4.
Parallels of developmental gene network architectures. Patterning of pair-rule genes expression [such as Even-skipped (Eve) and Fushi tarazu (Ftz)] along the antero-posterior axis of the fly body involves the induction of broad overlapping expression domains by various combination of upstream regulators (GAP genes) at early stages, followed by establishment of discreet stripes through cross-regulation between the different pair-rule genes. Similarly, the overlapping expression domains of ABC genes are refined through the action of positive feedback (i.e. between APETALA3 (AP3) and PISTILLATA (PI) B class genes) and cross inhibition (i.e. between A and C class genes). Feedforward loops are also common regulatory motifs to control the timing of events patterning the fly body or the floral meristem of Arabidopsis: in Drosophila, maternal determinants such as Bicoid activate the expression of Hunchback and together they pattern GAP gene expression. In Arabidopsis LFY promotes APETALA1 (AP1) transcription during floral induction, which inhibits expression of SEPALLATA3 repressors (AGL24/SVP/SOC1), preventing precocious expression of B and C class genes. LEAFY and UNUSUAL FLORAL ORGAN (UFO) physically interact (Chae et al. 2008); but whether the partnerships LEAFY—WUSCHEL (WUS) and LEAFY—unknown factor (?) that regulate the expression of A and C class genes respectively also require direct protein-protein interaction remains unclear (Lohmann et al. 2001; Zhao et al. 2018). Se, sepal; Pe, petal, St, stamen; Ca, carpel.
Cadastral genes help define and maintain sharp boundaries between the expression domains of floral homeotic genes: for example, SUPERMAN and RABBIT EARS encode C2H2 zinc finger repressors that turn off APETALA3 in whorl 4 (Sakai et al. 1995; Prunet et al. 2017) and AGAMOUS in whorl 2 (Krizek et al. 2006; Huang et al. 2012), respectively. In superman mutants, it appears as if the flower meristem is “stuck” in the production of third whorls (Fig. 3, K and L) (Bowman et al. 1992; Baum et al. 2001). These boundaries are no-mans-land for ABC gene expression that keep organ identity apart but also physical borders characterized by a lack of growth, allowing morphogenesis to proceed independently in each whorl to produce discreet organ types.
Many regulators of ABC genes are not exclusive agents of floral organs but instead participate in the control of other developmental processes. A key finding was the discovery that molecular players inducing floral transition directly participate in the activation of floral homeotic selectors. One of those elements, LEAFY (LFY), stands out because it can activate the transcription of A, B, C, and E genes by binding directly to their regulatory regions (Parcy et al. 1998; Busch et al. 1999; Wagner et al. 1999; Honma and Goto 2001; Lohmann et al. 2001; Lamb et al. 2002; Benlloch et al. 2011; Moyroud et al. 2011; Winter et al. 2011; Rieu et al. 2023). Because LFY is a central regulator of the reproductive transition, these results elegantly connect induction of flowering to specification of floral organ identity but also yield an initial paradox: how can a regulator expressed throughout the floral buds activate different floral homeotic genes in spatially distinct territories? With its uniform expression across floral meristems, LFY seemed at first ill-suited to such task.
Watch this space
A possible solution lies in LFY's ability to activate transcription of selected genes only when interacting with specific partners confined to distinct subdomains of the floral meristem. The model proposes WUSCHEL as a cofactor of LFY to promote C gene expression in the inner whorls, UNUSUAL FLORAL ORGAN (UFO) to turn on B genes in whorl 2 and 3 (Levin and Meyerowitz 1995; Wilkinson and Haughn 1995; Lee et al. 1997; Parcy et al. 1998; Honma and Goto 2000; Lenhard et al. 2001), and an unknown cofactor to activate A expression (Parcy et al. 1998; Lohmann et al. 2001) (Fig. 4). However, mechanistic evidence supporting such synergies were initially lacking. The UFO-LEAFY partnership was especially puzzling: fusing a strong activation VP16 domain to LEAFY is sufficient to increase APETALA1 and AGAMOUS expression but fails to affect APETALA3 transcription, suggesting that the cofactor is also needed for LFY binding to a B gene promoter (Parcy et al. 1998). However, UFO encodes an F-box protein involved in ubiquitination of proteins heading for degradation and, as such, not obviously connected to protein-DNA interactions.
Results obtained a decade later show that LFY DNA binding domain and UFO interact physically in vivo—not via the F-box that enrolls UFO in the SCF complex but via a C-term Kelch-type β-propeller domain and that this interaction is necessary to recruit UFO to APETALA3 promoter (Chae et al. 2008), but the mystery surrounding LFY-UFO teamwork remained incomplete for the next 15 years. By coupling functional investigation with biochemical approaches, Rieu and colleagues confirmed that the contribution of UFO to the activation of B gene does not require ubiquitination. Instead, the 3D visualization of the LFY-UFO complex bound to DNA inferred from cryoEM data and AlphaFold2 predictions showed that UFO contact both DNA and LFY and thus constitute a bona fide cofactor. Remarkably, UFO tweaks the DNA binding properties of LFY so that it can now stick to DNA sites (named LUBS for LFY-UFO Binding sites) devoid of the canonical LFY cis-elements. In other words, UFO acts as a fairy godmother that turns DNA motifs unable to recruit LFY effectively into strong binding sites. Neither UFO nor LFY can attach to such LUBS by themselves because their affinity is too weak. This elucidates the molecular causes of the LFY-UFO synergy proposed nearly 25 years ago and also explains why previous studies failed to find strong LFY binding sites in the PISTILLATA promoter (Rieu et al. 2023). Beyond solving the mystery of B gene regulation by LFY, this uncovered a novel mechanism where an F-box protein directly regulates transcription by modulating the DNA binding specificity of a central regulator of development. Hence, 30 years on research into the ABC model keeps yielding breakthroughs reaching far beyond a mere understanding of flower formation.
A question of tempo
The timing of ABC expression also needs to be carefully balanced: a risk inherent to being controlled by FM identity factor like LFY is that homeotic genes could be expressed too early, leading to incomplete floral organ differentiation and precocious meristem termination. The structure of the regulatory network orchestrating ABC expression provides a safeguard by embedding a regulatory delay in the form of a feedforward loop between LFY, AP1, and SEP3, once again, not unlike feedforward loops at work during the early stages of drosophila body patterning (Fig. 4). SEP3 and LFY together activate B and C gene transcription (Liu et al. 2009; Winter et al. 2011). LFY and APETALA1 activate each other during FM specification (Goslin et al. 2017), but SEP3 is absent at that stage as its expression is repressed by SVP, AGL24 and SOC1 (Liljegren et al. 1999; Wagner et al. 1999). APETALA1 accumulation later allows repression of SVP/AGL24/SOC1 triggering SEP3 production and eventually B and C gene expression (Liu et al. 2009).
ABC genes must also keep quiet during the vegetative phase at the risk of interfering with correct leaf development; this is not simply due to the absence of LFY but also necessitates active silencing of floral homeotic genes (Goodrich et al. 1997). Recent studies have started to identify specific factors involved in recruiting polycomb complexes to those loci: for instance, 2 Zinc Fingers were shown to recruit PRC2 to induce heterochromatin formation at APETALA1, APETALA3, and AGAMOUS in Arabidopsis (Hu et al. 2023). Those factors are repressed by LFY and APETALA1, allowing release of vegetative inhibition once floral induction is set in motion.
High maintenance
ABC gene expression is initiated and spatially polished in young FM but must also be maintained at later stages. Failure to do so impairs floral morphogenesis, prompting floral organ identity switch and incomplete differentiation of floral organs. The mechanisms responsible for expression maintenance differ from those accounting for ABC initiation, and piecing together findings from the last 20 years builds a clearer picture of those late processes: for instance, AG is eventually switched on even when LFY is absent (Weigel and Meyerowitz 1993), and this late AG expression relies in part on post-transcriptional processes involving HUA and HEN proteins (Western et al. 2002). When those are nonfunctional, stamen become mosaic organs exhibiting characteristics of both petal and sepal tissues, whereas carpels become sepal-like.
Transcription maintenance also engages feedback loops: for example, SEP3 enhances its expression both directly by binding to its own promoter but also indirectly by repressing, together with APETALA1, the transcription of its repressors AGL24, SOC1, and SVP (Kaufmann et al. 2009). Maintenance also involves epigenetic processes—for instance, ARABIDOPSIS TRITHORAX 1, a plant homolog of the fly trithorax, is necessary to sustain high enough expression levels of A, C, and to a lesser extent B genes (Alvarez-Venegas et al. 2003).
A similar logic applies to B genes: if their expression is turned off prematurely, patches of sepaloid tissues appear in petals. Examining this behavior in a group of aquatic early diverging angiosperms uncovered a possible role for environmental control: in water lilies, exposure to light, even late in development, allows sepal-like features to develop within otherwise petal-like organs (Warner et al. 2008, 2009). Such observations led to the formulation of the Mosaic theory, according to which genetic programs that elaborate characteristics of sepal and petal tissues evolved early in angiosperms history but were mostly controlled by environmental inputs such as light or mechanical cues. Later on, those programs were placed under the sole control of the floral organ identity network and lost their sensitivity to environmental inputs. This represents a candidate case to backup the evo-devo scenario arguing that plasticity could lead to the evolution of novelty (here the production of a differentiated perianth with clear sepal and petal identities) when followed by accommodation and assimilation (Sommer 2020).
Floating downstream
Another key question is how combinations of ABC proteins lead to floral organ differentiation. One approach to identifying downstream targets was to isolate mutants affecting only a single organ type, e.g. carpels (Alvarez and Smyth 1999). For example, CRABS CLAW mutants alter carpel development and whereas some effects are independent of AGAMOUS activity, others seem to rely on AGAMOUS (Alvarez and Smyth 1999). Indeed, when molecular approaches were available, CRABS CLAW was found to be a direct downstream target of AGAMOUS (Gómez-Mena et al. 2005; Ó’Maoiléidigh et al. 2013), with requisite dual CArG boxes (MADS-box binding site) for binding of MADS-quartets required for its expression (Lee et al. 2005a). CRABS CLAW's role in carpel development is conserved across angiosperms, with the rice ortholog, DROOPING LEAF, indispensable for carpel specification (Yamaguchi et al. 2004).
CRABS CLAW was also found to be required for nectary development (Bowman and Smyth 1999). Arabidopsis nectaries are normally found at the base of stamens and appear to be a third whorl organ independent of the identity of the floral organ occupying that whorl (Baum et al. 2001; Lee et al. 2005a). Such a role for CRABS CLAW was found to be widespread in eudicots regardless of nectary position within the flower, or even elsewhere in the plant, leading to speculation that nectaries came under CRABS CLAW control when they became associated with reproductive organs within the flower (Lee et al. 2005b). However, my (J.L.B.) foray into flower development was coming to an end as we discovered CRABS CLAW was a member of a small gene family, the YABBY gene family (Bowman and Smyth 1999; Siegfried et al. 1999), and enhancer mutagenesis revealed a role for this family and another, the KANADI gene family, in establishing organ polarity not only in the floral organs but also leaves (Eshed et al. 1999, 2001).
Disappointingly, 20 years later our understanding of what lays downstream of ABC remains surprisingly fragmentary: genome-wide binding approaches (SELEX-seq, ChIP-seq, and Dap-seq) have successfully identified in vivo binding sites of ABC regulators associated with genes controlling cell differentiation, division, and expansion, participating in hormonal signaling, or coding for other TFs and even chromatin remodeling and assembly factors (Kaufmann et al. 2009, 2010b; Yant et al. 2010; Wuest et al. 2012; Ó’Maoiléidigh et al. 2013, Pajoro et al. 2014; Chen et al. 2018; Lai et al. 2020). However, picking out bona fide regulatory cis-elements from inactive binding sites remains challenging and although many ABC direct targets have been predicted, only a relatively small number have been validated. Despite this, remarkable progress has been made uncovering the mode of action of the floral quartets [recently reviewed in (Goslin et al. 2023; Käppel et al. 2023)] and in particular toward understanding how closely related MADS proteins with very similar DNA binding properties identify targets accurately and selectively among thousands possible targets. Teamwork is key and ABC proteins achieve specificity by following the metaphor of the Swiss cheese model: many leaky layers, which individually would confer very imperfect selectivity, can forge a highly specific system when compounded with each other.
Good things come in fours
Nearly all ABC genes encode MIKCC-type MADS-box TFs, which probably originated from a duplication of MEF2-type genes in the ancestral land plant (Qiu et al. 2023). These TFs display 4 characteristic domains: a MADS-box domain (M), followed by an Intervening (I), a Keratin-like (K), and a C-terminal (C) domains (Ma et al. 1991) (Fig. 3B). Over the last 10 years, SEPALLATA3 has established itself as a model MIKCC-MADS protein for structural investigations (Puranik et al. 2014; Lai et al. 2021). The MADS domain is made of alpha helices exposed by beta-structures and constitutes a well-conserved DNA binding domain (DBD) allowing floral regulators to bind CArG box motifs (CC[A/T]6GG) as dimers. Although the I domain does not directly contact DNA, the MADS domain is unable to bind effectively to its target without it (Lai et al. 2021). The I motif stabilizes the weak interaction between 2 MADS domains, affecting the conformation of the dimer and hence tuning the strength and specificity of the binding to a given locus (Lai et al. 2021). As relatively ill-defined motifs, the CArG boxes are found at high frequency across any plant genome: about 15,000 unique sites in the genome of Arabidopsis match (CC[A/T]6GG) and over 300,000 are detected when 1 mismatch is allowed (Folter and Angenent 2006). ABC proteins have come up with an original strategy to deal with this conundrum common to many TFs.
First, ChIP-seq data independently obtained for A- (Kaufmann et al. 2010b; Pajoro et al. 2014), C- (Ó’Maoiléidigh et al. 2013), B- (Wuest et al. 2012), and E-genes (Kaufmann et al. 2009; Pajoro et al. 2014) revealed that the DNA motif bound by MIKCC-MADS proteins is likely longer (16 bp) than the 10 nucleotides of the original CArG-box. Although this partly explains why not all CArG-box are genuine binding sites, it does not account for differences in specificity between the possible quartets. Mapping genome-wide binding sites of ABC proteins also showed that specific CArG-boxes are not unique targets of a given MADS TF dimer (most CArG-boxes can be bound by most dimers and vice versa) but that preference orders for distinct CArG boxes vary between dimers. For example, DAP-seq data show that SEP3-SEP3 and SEP3-AG dimers have different binding specificities (Lai et al. 2020). Quantitative differences exist not only due to CArG-box base content but also to shape readout (e.g. the width of the minor groove) primarily performed by the N-term extension of the MADS- domain (Käppel et al. 2018) that contributes to specificity of target genes (Muiño et al. 2014; Smaczniak et al. 2017; Aerts et al. 2018).
The second part of the solution to the specificity riddle lies in the physical interactions required to generate quartets: the ABC model quickly led to the assumption that complexes involving 4 floral MADS proteins were needed to trigger specific gene expression, a hypothesis known as the quartet model (Honma and Goto 2001; Theissen and Saedler 2001). Several milestones anchoring this model in reality were subsequentially reached. Those include the reconstitution of quartet formation in vitro (Melzer and Theissen 2009), the elucidation of the structure and role of each subdomain in DNA recognition and tetramer formation (Puranik et al. 2014; Lai et al. 2021), and the demonstration that quartets can induce DNA looping with different quartets exhibiting unique preferences for specific spacing and orientation of the 2 CArG boxes (Melzer et al. 2009; Mendes et al. 2013; Jetha et al. 2014). In vitro (Veron et al. 2007; Zhang et al. 2018) but also in vivo data (Abraham-Juárez et al. 2020; Mao et al. 2021) indicate that quartet composition (e.g. BCE) is mostly conserved across flowering plants. The ability to form quartets rests on the coiled-coil domains of the K region (Puranik et al. 2014), which is made of 2 amphiphatic alpha helixes: the first helix along with the N-term portion of the second one strengthens the dimer. The C-term of the second helix serves as interface for interaction between 2 DNA-bound dimers, mediating tetramerization.
Tetramerization enhances specificity but could also be central to quartet transcriptional activity (Mendes et al. 2013; Hugouvieux et al. 2018): tetramer formation facilitates cooperative binding known to trigger sharp on/off transcriptional responses, well-suited to control critical developmental events (Theissen and Melzer 2007; Kaufmann et al. 2010a). Multimer assembly also creates new interfaces, expanding possibilities to recruit partners such as cofactors or epigenetic regulators (reviewed by Goslin et al. 2023)—how floral quartets recruit chromatin modifying factors remains to be fully understood, but examples are emerging: STERILE TASSEL SILKY EAR1 (STS1) and SILKY1 (SI1) are PISTILLATA and APETALA3 maize orthologs, respectively. IP-MS assays in maize revealed STS1 homodimers and STS1-SI1 heterodimers both interact in vivo with CHR126b, a chromatin remodeling complex ATPase. STS1 homodimers can also contact other chromatin remodeling and scaffolding factors such as CHR12, BRAHMA1, and FRIGIDA-LIKE PROTEIN4a, indicating that floral complex composition directly influences recruitment of different partners (Abraham-Juárez et al. 2020).
Tetramerization appears to be necessary for floral meristem determinacy, and correct organogenesis of floral organs in Arabidopsis as development of petal, stamen, and carpels is severely impaired or even abolished when SEPALLATA3 completely lacks the interface required for tetramer formation (Hugouvieux et al. 2023). However, cooperative 2-site binding to DNA by quartets of Arabidopsis SEPALLATA3-AGAMOUS does not impact the regulation of all target genes equally: it is necessary to trigger floral meristem termination but is not essential to floral organogenesis since expression of SEPΔtet, a SEPALLATA3 splice variant that exhibits impaired but not abolished tetramerization, suffices to restore petal and stamen development in a sepallata1/2/3 triple mutant (Hugouvieux et al. 2018). Similarly, AGAMOUS can interact with histone modifiers and chromatin remodelers such as CHR11/CHR17 to activate YUCCA4 and promote carpel development, but this does not require interaction between a quartet and chromatin modifying agents and might instead result from direct interaction between AGAMOUS and CHR11 [reviewed in (Pelayo et al. 2021)]. Thus, ABC proteins can regulate transcription and contribute to the production of floral organs outside of the floral quartet framework.
ABC through the evolutionary lens
When one examines flower architecture across the quarter-million species of angiosperms, a common theme is organ order. Although flowers differ enormously in numbers, shapes, positions and even numbers of whorls of organs, the organ order (sepals, petals, stamens, carpels) is nearly constant (although missing organ types is a common theme). That similar phenotypes were observed in Arabidopsis, a rosid, and Antirrhinum, an asterid, immediately suggested that their common ancestor (that of core eudicots) and perhaps all angiosperms utilized a common genetic program, consistent with a singular origin of flowers (Coen and Meyerowitz 1991). Yet could a universal and relatively simple model underpin the diversity of floral forms seen across the angiosperms? And what is the evolutionary origin of the floral network? To account for various floral morphologies, modifications of ABC model by shifting/sliding or fading boundaries have been proposed (van Tunen et al. 1993; Bowman 1997; Kramer et al. 2003; Buzgo et al. 2004), and we consider only a few variations here.
Variations on a theme
One early line of investigation concerned how the ABC model might apply to grass flowers, which have a highly derived perianth architecture. Maize silky1 flowers exhibited transformations of stamens into carpels and lodicules into palea/lemma-like organs—a canonical B-class mutant phenotype—and subsequent cloning of the gene revealed silky1 to encode an APETALA3 ortholog (Ambrose et al. 2000). Thus, lodicules were interpreted to be petal homologs and the palea/lemma to be sepal homologs (Fig. 5A). C-function was also found to be conserved, although it was split across 2 paralogs (Mena et al. 1996). Similar results were found in rice (Kyozuka et al. 2000), indicating the ABC model applies to grass flowers, despite their divergent perianths. A minor variation of the ABC model was proposed for tulip flowers based on a B-class mutant in which both outer whorls of tepals (perianth organs not differentiated into sepals and petals) were transformed into sepal-like organs not normally found in tulips (Fig. 5B), and stamens were transformed into carpels (van Tunen et al. 1993), with gene expression patterns of ABC orthologs supporting this hypothesis (Kanno et al. 2003).
Figure 5.
Evolutionary conservation of the ABC model and its variants. A) The outermost whorl of grass flowers is occupied by the palea (p) and lemma (l) and the second whorl by lodicules, which swell to facilitate flower opening. B) The outer 2 whorls of tulips are tepals, which are petal-like, and B-class gene expression is in all whorls except the innermost carpels. C) A proliferation of APETALA3 paralogs have sub- and neofunctionalized to produce 3 distinct compositions of B-class, with 1 set promoting the development of staminodes (sd), modified stamen-like organs that may act to deter herbivory (Hodges and Tucker 2005). D) Orchid also possesses a proliferation of APETALA3 paralogs that act in combination to promote differentiation of the outer tepals (ot), inner lateral tepals (ilt), and labellum (la) that comprise the perianth. E) The capitulum of Asteraceae consists of central disc florets (df) and peripheral ray florets (rf), with the fertile disc florets developing a conspicuous pappus ring (p), homologous with sepals, in their outer whorls. F) In some species, organ identity transitions may not be abrupt, such as with petal and stamen identity in Opuntia. G)Lacandonia schismatica is the only angiosperm where the order of organs is transposed, with stamens (st) developing interior to carpels (ca). L. schismatica plants (arrow) grow on the Lacandon jungle floor, are small (10 cm), achlorophyllous, and saprophytic. H) Pines have separate male and female cones, with C-class being expressed in both and B-class limited to male cones. SEM of L. schismatica courtesy of Barbara Ambrose; all other photos by J.L.B.
Transient assays, such as virus-induced gene silencing, facilitated functional investigations in a wider range of systems. Combined with modern -omics approaches, these not only painted a more holistic view of how floral organ identity is specified by the ABC but also started to unveil the molecular bases accounting for morphological variation. Two cases of parallel evolution exemplify sub- and neofunctionalization of B-class genes in the evolution of flower novelty. [I (J.L.B.) once remarked that researchers in the field followed a similar path—they multiplied and diversified via sub- and neofunctionalization…] In the rununculid Aquilegia, diversification of APETALA3 orthologs results in 3 distinct combinations that promote petal, stamen, and staminode identity (Kramer et al. 2007; Sharma and Kramer 2013). In this case a novel organ, the staminode, is produced, whose ecological function is unknown, although they likely play a role in pollination (Fig. 5C). Another ranunculid, Thalictrum, exhibits expansion of B-class activity into the outer whorl that results in petaloid sepals in a parallel evolution of this phenotype with tulips (Martínez-Gómez et al. 2020).
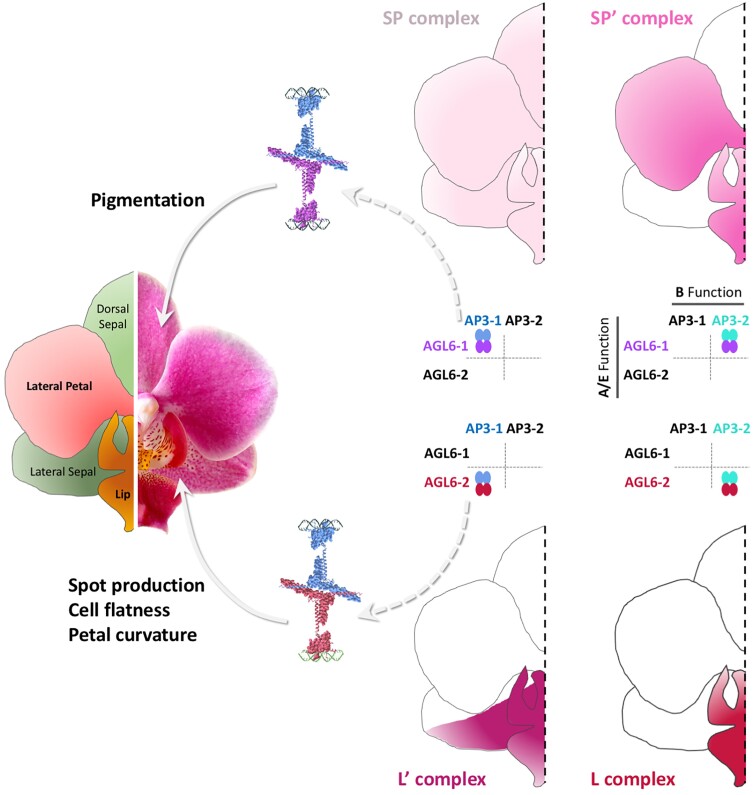
Sub- and neofunctionalization of B-class is also observed in orchids, where there has been a diversification of APETALA3 orthologs into 4 distinct clades (Fig. 5D). In this case, the perianth organs are specified by a “perianth code,” with each distinct organ type having a different qualitative, and sometimes quantitative, suite of B and E activities that compete with each other, making individual tepals morphologically distinct from each other: sepal/petal vs. labellum (Mondragon-Palomino and Theissen 2011; Pan et al. 2011; Hsu et al. 2015). Such complexes also appear to have gained function beyond specifying organ identity: quartet components seem to vary spatially with exquisite precision so that subregions within an organ could harbor different multimers to sculpt and pattern a given organ section (Fig. 6)—for instance, interfering with the formation of specific quartets in moth orchids prevented curved edge formation and red anthocyanin spots production at the base of lateral sepal (Hsu et al. 2021).
Figure 6.
Paralog sub- and neofunctionalization generates diversity. Paralogs of A/E (AGL6-1, AGL6-2) and B (AP3-1, AP3-2) class genes in orchids have acquired distinct expression patterns, allowing the formation of different quartets across the perianth. This “P code” (Hsu et al. 2015) can explain the activation of different targets genes in different organs within a whorl (i.e. lateral petals vs. lip) or even between different part of the same organ (lower and upper region of the lateral petals): the SP and L” complexes comprise a dimer of AP3-1 (light blue) combines with a dimer of AGL6-1 (purple) or AGL6-2 (red) respectively. Those can bind to promoters harboring distinct CArG box shape, spacing, and orientation, allowing the 2 quartets to regulate different morphological features. The SP complex can trigger uniform pigment production across the petal/sepal epidermis while L” induces spot formation and promotes the production of flat epidermis cells associated with a curvature of the lower lateral petal region (Photo, modified from Zygomorf1 by Christer Johansson via Wikimedia Commons https://creativecommons.org/licenses/by-sa/3.0/).
Downregulation of the B-class gene GGLO1 in the Asteraceae family plant Gerbera hybrida resulted in transformation of second whorl organs into ones bearing pappus bristles, indicating that the pappus bristles of wild-type Asteraceae flowers represent sepals modified to facilitate seed dispersal (Yu et al. 1999) (Fig. 5E). Another proposed modification of the ABC model is one where the boundaries between the different classes are indistinct, that is, fading/fuzzy borders (Buzgo et al. 2004; Soltis et al. 2007), and which may apply to species where the distinction between sepals and petals or petals and stamens can be vague (Fig. 5F). Lastly, the only known angiosperm in which the organ order of the reproductive organs is transposed is Lacandonia schismatica (Fig. 5G), a saprophytic plant growing only in the Lacandon jungle of southern Mexico. In this species the B- and C-class gene expression patterns mirror organ identity (Alvarez-Buylla et al. 2010). Closely related to L. schismatica is the dioecious Triuris brevistylus (Espinosa-Matias et al. 2012), whose female flowers resemble those of L. schismatica except for the central stamens, providing a possible evolutionary pathway to the unique floral architecture of L. schismatica.
Another early avenue of excitement was whether the ABC genes would be involved in the evolution of monoecy or dioecy in angiosperms. Now that a number of dioecious angiosperms have been molecularly characterized, it seems clear that the ABC genes are not the sex determining genes [as speculated in (Bowman 1997)] but rather that the sex determination mechanisms may converge on changes in B-class gene activity [reviewed in (Montalvão et al. 2021; Zhang et al. 2022)]. One potential exception may be in a gymnosperm, Cycas, where GGM13 (which resides in the phylogenetic sister group to B-class genes) is located in the male determining region of the Y chromosome (Liu et al. 2022). Finally, it must be stressed that not all floral diversity originates from modification of floral MADS-box genes or their downstream targets. For example, change in floral ground plan, such as number and position of floral organ primordia appears largely independent of ABC gene activity (Smyth 2018).
ANA grade and basal eudicots
ABC homologs tend to exhibit broader expression patterns in early diverging angiosperms (Kim et al. 2005; Yoo et al. 2010; Li et al. 2015; Moschin et al. 2021). In vitro studies indicate those are capable of forming floral quartets, but protein-protein interactions are more promiscuous in ANA grade species and early diverging eudicots compared to core eudicots (Liu et al. 2010; Melzer et al. 2014; Li et al. 2015; Rümpler et al. 2018). Hence, the elaboration of the ABC complexes during flowering plant diversification may be best understood by drawing parallels with the formation of a neural network: during animal brain development a large number of neurons is produced (i.e. extensive duplication of MIKCC-MADS TFs), then as many synapses as possible form between those neurones producing innumerable connections (i.e. almost all combinations of protein-protein interactions between promiscuous MADS-box genes are possible as expression patterns largely overlap). Finally, pruning ensues to selectively eliminate many connections [i.e. paralogous MADS are lost, protein-protein interaction become exclusive between those that remain (Melzer et al. 2014; Li et al. 2015), and expression patterns are sharpened]. Taken together these processes certainly contributed to sculpt the ABC model of eudicots as we know it but also likely introduced the robustness and canalization needed for reliable floral development.
Gymnosperms
While flowers are an angiosperm innovation, ABC-type MADS box genes predate the evolution of flowers (Tandre et al. 1995). Gymnosperm C-class gene expression was detected in both developing female and male cones (Tandre et al. 1998), whereas B-class gene expression is limited to male cones (Mouradov et al. 1999; Sundstrom et al. 1999), suggesting that the interplay between B and C components of the ABC model, key to sex determination, evolved in the ancestral seed plant (Fig. 5H). However, functional investigations in gymnosperms remain difficult, and progress has been limited to heterologous expression, in vitro biochemical assays, and characterization of a handful of natural variants (Carlsbecker et al. 2013). That gymnosperm genomes possess genes similar to A- or E-class angiosperm genes, some of which are expressed in cones (Carlsbecker et al. 2013; Gramzow et al. 2014), suggests that quartets of MADS-box proteins may act in gymnosperms as well (Theißen et al. 2016). Indeed, gymnosperm MIKCC-type proteins can form quartet complexes, with C-homotetramers and BC-heterotetramers able to loop DNA and E proteins (AGL6) not essential to complex formation, at least in vitro (Wang et al. 2010). Whether BC and BCE complexes are both functionally relevant in vivo and/or are capable of activating different sets of genes cannot yet be tested. However, reconstruction and characterization of an ancestral version of BCE complexes suggested that E was necessary for quartet formation specifying male identity in the ancestral seed plant (Ruelens et al. 2017). Some regulatory players accounting for the spatio-temporal expression patterns typical of angiosperms ABC also predate flower evolution: B-gene promoters in Welwitschia can recruit LFY to high-affinity binding sites also present in regulatory regions of other conifer B-genes (Moyroud et al. 2017). LFY may already rely on its cofactor UFO to regulate gymnosperm B gene expression because the LFY-UFO connection is likely ancient, but functional UFO genes remain to be identified in nonflowering plants (Rieu et al. 2023).
Thus, key components required to build a flower were already present in the ancestral seed plant, so that the floral ABC network likely derived from a minimal gene regulatory circuit that controlled reproductive organ identity in the last common ancestor of seed plants. In this view the origin of the flower primarily required the construction of an elaborate perianth surrounding the reproductive organs and the evolution of the carpel enclosing the ovules, with the subsequent development of a fruit enclosing the seeds. In this respect, the continued generation of MADS-box gene paralogs was instrumental in the evolution and diversification of angiosperm fruit morphologies (e.g. Gu et al. 1998; Ferrándiz and Fourquin 2014; Karlova et al. 2014; Schilling et al. 2018; Liu et al. 2020).
Origins
The origin of MIKCC-MADS-box genes lies in a duplication in the ancestral land plant, and while most bryophyte species have effectively only a single MIKCC-MADS-box gene (Bowman et al. 2017; Koshimizu et al. 2018), the seeds of ABCE origins can be seen in the ancestral euphyllophyte (Fig. 3B) (Henschel et al. 2002; Kofuji et al. 2003; Wellmer et al. 2014; Qiu et al. 2023). Land plant MIKCC-MADS proteins can form quartets in vitro, with a duplication of sequence within the K domain critical for forming the interface required for tetramer formation (Rümpler et al. 2023). Hence, quartet emergence predates the MADS-boxes learning their ABCs. The ability to form tetramers combined with expansion and diversification of MADS-box genes in the ancestral seed plant, and more dramatically within the ancestral angiosperm, enhanced the combinatorial power of MIKCC complexes (Rümpler et al. 2023) and may have contributed to the evolution of tissue and organ types during development. In the ancestral land plant, sex was determined during the haploid generation (Bowman 2022), but in seed plants the sporophyte largely controls gametophyte development, including sex expression. In seedless vascular plants, MIKCC-MADS genes are broadly expressed, often in both generations [e.g. (Hasebe et al. 1998; Ambrose et al. 2021)]. One intriguing speculation is that MIKCC-MADS genes were co-opted into a role in determining sporophyte sex during tracheophyte evolution as the sporophyte was assuming control over gametophyte differentiation. And peering deeper in time, given the role of MCM1 in the yeast life cycle, an idea worth exploring is whether land plant MADS-box genes have retained vestiges of a fundamental role in the eukaryotic life cycle (Herskowitz 1989), as have land plant homeodomain genes (Dierschke et al. 2021; Hisanaga et al. 2021).
Conclusion: a short and biased selection of floral riddles
Thirty years ago, we could not have predicted what a cottage industry the characterization of ABC MADS-box genes in various angiosperms would become, contributing to a deeper and broader understanding of evo-devo processes that have shaped floral architecture. Reciprocally, they also accentuate remaining gaps in our knowledge. For instance, a given quartet is expected to activate different sets of genes at different timepoints as organ primordia initiate, grow, and differentiate, but whether this is the case and how it is achieved remain obscure. Equally, to generate the diversity of floral forms genes regulated by the ABC genes is likely to differ somewhat between species. However, a systematic examination of ABC targets conservation between species is still lacking. Even if a full picture of those targets becomes available, this still does not explain how gene activity translates into morphogenetic effects: here, high-resolution live-imaging coupled with computational simulations and theoretical modeling [see e.g. (Refahi et al. 2021)] could be instrumental in understanding how homeotic regulators interact with gene regulatory networks that specify organ polarity, floral symmetry, and tissue layer identity to sculpt undifferentiated primordia into floral organs with characteristic shape, size, patterned cell types, and metabolic properties. Research into the origins of the ABC genes established that the gene regulatory networks controlling flower development stem from recycling of an ancient circuit at work in the reproductive structures of last common ancestor of seed plants. However, what rewiring occurred along the lineage leading to flowering plants so that discreet B and B + C expression domains could be established enclosed by a sterile perianth remains enigmatic.
These puzzles represent only a handful of the remaining questions and by no means constitute an exhaustive list, but we hope they will spark the curiosity of future flower enthusiasts to continue the successful adventure of the ABC.
Acknowledgments
J.L.B. extends special thanks Elliot Meyerowitz and David Smyth as his “flower mentors” from 1986-95, as they provided both the direction and freedom to pursue curiosity driven discovery research, a theme continuing throughout my entire career. E.M. thanks the members of her laboratory for useful discussions.
Contributor Information
John L Bowman, School of Biological Sciences, Monash University, Melbourne, VIC 3800, Australia; ARC Centre of Excellence for Plant Success in Nature and Agriculture, Monash University, Melbourne, VIC 3800, Australia.
Edwige Moyroud, The Sainsbury Laboratory, Cambridge University, Cambridge CB2 1LR, UK; Department of Genetics, University of Cambridge, Cambridge CB2 3EJ, UK.
Author contributions
Both authors contributed to the outline and preparation of this review. JLB was the primary author for the History, Genetics, Molecules and Converting leaves into floral organs sections; EM was the primary author of the Swimming upstream and Floating downstream sections; both authors contributed to the Curious case and A and subfunctionalization of B- and C-classes, and the ABC through the evolutionary lens sections.
Funding
Work in J.L.B.'s laboratory is funded by the Australian Research Council (DP200100225, DP210101423, CE200100015). E.M. thanks the Gatsby Charitable Foundation and the Isaac Newton/Wellcome Trust ISSF for funding.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
References
- Abraham-Juárez MJ, Schrager-Lavelle A, Man J, Whipple C, Handakumbura P, Babbitt C, Bartlett M. Evolutionary variation in MADS box dimerization affects floral development and protein abundance in maize. Plant Cell. 2020:32(11):3408–3424. 10.1105/tpc.20.00300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aerts N, de Bruijn S, van Mourik H, Angenent GC, van Dijk ADJ. Comparative analysis of binding patterns of MADS-domain proteins in Arabidopsis thaliana. BMC Plant Biol. 2018:18(1):131. 10.1186/s12870-018-1348-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla ER, Ambrose BA, Flores-Sandoval E, Vergara-Silva F, Englund M, Garay-Arroyo A, Garcia-Ponce B, de la Torre-Barcena E, Espinosa-Matias S, Martinez E, et al. B-function expression in the flower center underlies the homeotic phenotype of Lacandonia schismatica (Triuridaceae). Plant Cell. 2010:22(11):3543–3559. 10.1105/tpc.109.069153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Venegas R, Pien S, Sadder M, Witmer X, Grossniklaus U, Avramova Z. ATX-1, an Arabidopsis homolog of trithorax, activates flower homeotic genes. Curr Biol. 2003:13(8):627–637. 10.1016/S0960-9822(03)00243-4 [DOI] [PubMed] [Google Scholar]
- Alvarez J, Smyth DR. CRABS CLAW and SPATULA, two Arabidopsis genes that control carpel development in parallel with AGAMOUS. Development. 1999:126(11):2377–2386. 10.1242/dev.126.11.2377 [DOI] [PubMed] [Google Scholar]
- Ambrose BA, Lerner DR, Ciceri P, Padilla CM, Yanofsky MF, Schmidt RJ. Molecular and genetic analyses of the silky1 gene reveal conservation in floral organ specification between eudicots and monocots. Mol Cell. 2000:5(3):569–579. 10.1016/S1097-2765(00)80450-5 [DOI] [PubMed] [Google Scholar]
- Ambrose BA, Smalls TL, Zumajo-Cardona C. All type II classic MADS-box genes in the lycophyte Selaginella moellendorffii are broadly yet discretely expressed in vegetative and reproductive tissues. Evol Dev. 2021:23(3):215–230. 10.1111/ede.12375 [DOI] [PubMed] [Google Scholar]
- Aukerman MJ, Sakai H. Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell. 2003:15(11):2730–2741. 10.1105/tpc.016238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson W. Materials for the study of variation treated with especial regard to discontinuity in the origin of species. London: Macmillan; 1894. [Google Scholar]
- Bateson W, Saunders ER, Punnett RC. Reports to the evolution committee, report II. Experimental studies in the physiology of heredity- matthiola. London: Royal Society; 1905. [Google Scholar]
- Baum SF, Eshed Y, Bowman JL. The Arabidopsis nectary is an ABC-independent floral structure. Development. 2001:128(22):4657–4667. 10.1242/dev.128.22.4657 [DOI] [PubMed] [Google Scholar]
- Benlloch R, Kim MC, Sayou C, Thévenon E, Parcy F, Nilsson O. Integrating long-day flowering signals: a LEAFY binding site is essential for proper photoperiodic activation of APETALA1. Plant J. 2011:67(6):1094–1102. 10.1111/j.1365-313X.2011.04660.x [DOI] [PubMed] [Google Scholar]
- Bowman JL. Evolutionary conservation of angiosperm flower development at the molecular and genetic levels. J Biosci. 1997:22(4):515–527. 10.1007/BF02703197 [DOI] [Google Scholar]
- Bowman JL. eLS encyclopedia of life sciences. John Wiley & Sons, Ltd; 2001. p. A734. [Google Scholar]
- Bowman JL. My favourite flowering image. J Exp Bot. 2013:64(18):5779–5782. 10.1093/jxb/erq044 [DOI] [PubMed] [Google Scholar]
- Bowman JL, Alvarez J, Weigel D, Meyerowitz EM, Smyth DR. Control of flower development in Arabidopsis thaliana by APETALA1 and interacting genes. Development. 1993:119(3):721–743. 10.1242/dev.119.3.721 [DOI] [Google Scholar]
- Bowman JL, Kohchi T, Yamato KT, Jenkins J, Shu S, Ishizaki K, Yamaoka S, Nishihama R, Nakamura Y, Berger F, et al. Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell. 2017:171(2):287–304.e15. 10.1016/j.cell.2017.09.030 [DOI] [PubMed] [Google Scholar]
- Bowman JL, Sakai H, Jack T, Weigel D, Mayer U, Meyerowitz EM. SUPERMAN, a regulator of floral homeotic genes in Arabidopsis. Development. 1992:114(3):599–615. 10.1242/dev.114.3.599 [DOI] [PubMed] [Google Scholar]
- Bowman JL, Smyth DR. CRABS CLAW, a gene that regulates carpel and nectary development in Arabidopsis, encodes a novel protein with zinc finger and helix-loop-helix domains. Development. 1999:126(11):2387–2396. 10.1242/dev.126.11.2387 [DOI] [PubMed] [Google Scholar]
- Bowman JL, Smyth DR, Meyerowitz EM. Genes directing flower development in Arabidopsis. Plant Cell. 1989:1(1):37–52. 10.1105/tpc.1.1.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman JL, Smyth DR, Meyerowitz EM. Genetic interactions among floral homeotic genes. Development. 1991:112(1):1–20. 10.1242/dev.112.1.1 [DOI] [PubMed] [Google Scholar]
- Bowman JL, Smyth DR, Meyerowitz EM. The ABC model of flower development: then and now. Development. 2012:139(22):4095–4098. 10.1242/dev.083972 [DOI] [PubMed] [Google Scholar]
- Bradley D, Carpenter R, Sommer H, Hartley N, Coen E. Complementary floral homeotic phenotypes result from opposite orientations of a transposon at the plena locus of Antirrhinum. Cell. 1993:72(1):85–95. 10.1016/0092-8674(93)90052-R [DOI] [PubMed] [Google Scholar]
- Brieger FG. The developmental mechanics of normal and abnormal flowers in Primula. Proc Linn Soc Lond. 1935:147:126–130. 10.1111/j.1095-8312.1934.tb01264.x [DOI] [Google Scholar]
- Busch MA, Bomblies K, Weigel D. Activation of a floral homeotic gene in Arabidopsis. Science. 1999:285(5427):585–587. 10.1126/science.285.5427.585 [DOI] [PubMed] [Google Scholar]
- Buzgo M, Soltis DE, Soltis PS. Floral developmental morphology of Amborella trichopoda (Amborellaceae). Int J Plant Sci. 2004:165(6):925–947. 10.1086/424024 [DOI] [Google Scholar]
- Carlsbecker A, Sundström JF, Englund M, Uddenberg D, Izquierdo L, Kvarnheden A, Vergara-Silva F, Engström P. Molecular control of normal and acrocona mutant seed cone development in Norway spruce (Picea abies) and the evolution of conifer ovule-bearing organs. New Phytologist. 2013:200(1):261–275. 10.1111/nph.12360 [DOI] [PubMed] [Google Scholar]
- Carpenter R, Coen ES. Floral homeotic mutations produced by transposon-mutagenesis in antirrhinum-majus. Gene Dev. 1990:4(9):1483–1493. 10.1101/gad.4.9.1483 [DOI] [PubMed] [Google Scholar]
- Cartolano M, Castillo R, Efremova N, Kuckenberg M, Zethof J, Gerats T, Schwarz-Sommer Z, Vandenbussche M. A conserved microRNA module exerts homeotic control over Petunia hybrida and Antirrhinum majus floral organ identity. Nat Genet. 2007:39(7):901–905. 10.1038/ng2056 [DOI] [PubMed] [Google Scholar]
- Carvalho DS, Schnable JC, Almeida AMR. Integrating phylogenetic and network approaches to study gene family evolution: the case of the AGAMOUS family of floral genes. Evol Bioinform Online. 2018:14:117693431876468. 10.1177/1176934318764683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causier B, Bradley D, Cook H, Davies B. Conserved intragenic elements were critical for the evolution of the floral C-function. Plant J. 2009:58(1):41–52. 10.1111/j.1365-313X.2008.03759.x [DOI] [PubMed] [Google Scholar]
- Causier B, Schwarz-Sommer Z, Davies B. Floral organ identity: 20 years of ABCs. Semin Cell Dev Biol. 2010:21(1):73–79. 10.1016/j.semcdb.2009.10.005 [DOI] [PubMed] [Google Scholar]
- Chae E, Tan QK-G, Hill TA, Irish VF. An Arabidopsis F-box protein acts as a transcriptional co-factor to regulate floral development. Development. 2008:135(7):1235–1245. 10.1242/dev.015842 [DOI] [PubMed] [Google Scholar]
- Chen D, Yan W, Fu LY, Kaufmann K. Architecture of gene regulatory networks controlling flower development in Arabidopsis thaliana. Nat Commun. 2018:9(1):4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science. 2004:303(5666):2022–2025. 10.1126/science.1088060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Kato N, Wang W, Li J, Chen X. Two RNA binding proteins, HEN4 and HUA1, act in the processing of AGAMOUS Pre-mRNA in Arabidopsis thaliana. Dev Cell. 2003:4(1):53–66. 10.1016/S1534-5807(02)00399-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon K-S, Nakatsuka A, Tasaki K, Kobayashi N. Long-lasting corolla cultivars in Japanese azaleas: a mutant AP3/DEF homolog identified in traditional azalea cultivars from more than 300 years ago. Front Plant Sci. 2018:8:2239. 10.3389/fpls.2017.02239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen E, Meyerowitz EM. The war of the whorls: genetic interactions controlling flower development. Nature. 1991:353(6339):31–37. 10.1038/353031a0 [DOI] [PubMed] [Google Scholar]
- Darwin C. The variation of animals and plants under domestication. 2nd ed. New York: D. Appleton; 1876. [Google Scholar]
- Davies B, Egea-Cortines M, Silva EDA, Saedler H, Sommer H. Multiple interactions amongst floral homeotic MADS box proteins. EMBO J. 1996:15(16):4330–4343. 10.1002/j.1460-2075.1996.tb00807.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B, Motte P, Keck E, Saedler H, Sommer H, Schwarz-Sommer Z. PLENA and FARINELLI: redundancy and regulatory interactions between two antirrhinum MADS-box factors controlling flower development. EMBO J. 1999:18(14):4023–4034. 10.1093/emboj/18.14.4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierschke T, Flores-Sandoval E, Rast-Somssich MI, Altoff F, Zachgo S, Bowman JL. Gamete expression of TALE class HD genes activates the diploid sporophyte program in Marchantia polymorpha. eLife. 2021:10:e57088. 10.7554/eLife.57088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G, Pinyopich A, Robles P, Pelaz S, Yanofsky MF. The SEP4 gene of Arabidopsis thaliana functions in floral organ and meristem identity. Curr Biol. 2004:14(21):1935–1940. 10.1016/j.cub.2004.10.028 [DOI] [PubMed] [Google Scholar]
- Dodoens R. Florum, et coronariarum odoratarumque nonnullarum herbarum historia. Antwerp: Christophori Plantini; 1568. [Google Scholar]
- Dodoens R. Stirpium historiae pemptades sex sive libri XXX. Antwerp: Christophori Plantini; 1583. [Google Scholar]
- Dreni L, Zhang D. Flower development: the evolutionary history and functions of the AGL6 subfamily MADS-box genes. J Exp Bot. 2016:67(6):1625–1638. 10.1093/jxb/erw046 [DOI] [PubMed] [Google Scholar]
- Drews GN, Bowman JL, Meyerowitz EM. Negative regulation of the Arabidopsis homeotic gene AGAMOUS by the APETALA2 product. Cell. 1991:65(6):991–1002. 10.1016/0092-8674(91)90551-9 [DOI] [PubMed] [Google Scholar]
- Edgar BA, Odell GM, Schubiger G. A genetic switch, based on negative regulation, sharpens stripes in Drosophila embryos. Dev Genet. 1989:10(3):124–114. 10.1002/dvg.1020100303 [DOI] [PubMed] [Google Scholar]
- Egea-Cortines M, Saedler H, Sommer H. Ternary complex formation between the MADS-box proteins SQUAMOSA, DEFICIENS and GLOBOSA is involved in the control of floral architecture in Antirrhinum majus. EMBO J. 1999:18(19):5370–5379. 10.1093/emboj/18.19.5370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshed Y, Baum SF, Bowman JL. Distinct mechanisms promote polarity establishment in carpels of Arabidopsis. Cell. 1999:99(2):199–209. 10.1016/S0092-8674(00)81651-7 [DOI] [PubMed] [Google Scholar]
- Eshed Y, Baum SF, Perea JV, Bowman JL. Establishment of polarity in lateral organs of plants. Curr Biol. 2001:11(16):1251–1260. 10.1016/S0960-9822(01)00392-X [DOI] [PubMed] [Google Scholar]
- Espinosa-Matias S, Vergara-Silva F, Vazquez-Santana S, Martinez-Zurita E, Marquez-Guzman J. Complex patterns of morphogenesis, embryology, and reproduction in Triuris brevistylis, a species of Triuridaceae (Pandanales) closely related to Lacandonia schismatica. Botany. 2012:90(11):1133–1151. 10.1139/b2012-060 [DOI] [Google Scholar]
- Favaro R, Pinyopich A, Battaglia R, Kooiker M, Borghi L, Ditta G, Yanofsky MF, Kater MM, Colombo L. MADS-box protein complexes control carpel and ovule development in Arabidopsis. Plant Cell. 2003:15(11):2603–2611. 10.1105/tpc.015123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrándiz C, Fourquin C. Role of the FUL–SHP network in the evolution of fruit morphology and function. J Exp Bot. 2014:65(16):4505–4513. 10.1093/jxb/ert479 [DOI] [PubMed] [Google Scholar]
- Folter SD, Angenent GC. Trans meets cis in MADS science. Trends Plant Sci. 2006:11(5):224–231. 10.1016/j.tplants.2006.03.008 [DOI] [PubMed] [Google Scholar]
- Garber RL, Kuroiwa A, Gehring WJ. Genomic and cDNA clones of the homeotic locus antennapedia in Drosophila. EMBO J. 1983:2(11):2027–2036. 10.1002/j.1460-2075.1983.tb01696.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard J, Dodoens R, Norton J, Priest R. The herball, or, generall historie of plantes/gathered by John Gerarde of London, master in chirurgerie. London: John Norton; 1597. [Google Scholar]
- Godron D-A. Des races végétales qui doivent leur ongine a une monstruosité. Mémoires de l'Académie de Stanislas Académie de Nancy. 1874:6:77–95. [Google Scholar]
- Goethe JWV. Versuch die metamorphose der Pflanzen zu erklaren. Gotha: C. W. Ettinger; 1790. [Google Scholar]
- Gómez-Mena C, Folter SD, Costa MMR, Angenent GC, Sablowski R. Transcriptional program controlled by the floral homeotic gene AGAMOUS during early organogenesis. Development. 2005:132(3):429–438. 10.1242/dev.01600 [DOI] [PubMed] [Google Scholar]
- Goodrich J, Puangsomlee P, Martin M, Long D, Meyerowitz EM, Coupland G. A polycomb-group gene regulates homeotic gene expression in Arabidopsis. Nature. 1997:386(6620):44–51. 10.1038/386044a0 [DOI] [PubMed] [Google Scholar]
- Goslin K, Finocchio A, Wellmer F. Floral homeotic factors: a question of specificity. Plants. 2023:12(5):1128. 10.3390/plants12051128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin K, Zheng B, Serrano-Mislata A, Rae L, Ryan PT, Kwaśniewska K, Thomson B, Ó’Maoiléidigh DS, Madueño F, Wellmer F, et al. Transcription factor interplay between LEAFY and APETALA1/CAULIFLOWER during floral initiation. Plant Physiol. 2017:174(2):1097–1109. 10.1104/pp.17.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto K, Meyerowitz EM. Function and regulation of the Arabidopsis floral homeotic gene PISTILLATA. Genes Dev. 1994:8(13):1548–1560. 10.1101/gad.8.13.1548 [DOI] [PubMed] [Google Scholar]
- Gramzow L, Weilandt L, Theißen G. MADS goes genomic in conifers: towards determining the ancestral set of MADS-box genes in seed plants. Ann Bot. 2014:114(7):1407–1429. 10.1093/aob/mcu066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PB. A theory for inflorescence development and flower formation based on morphological and biophysical analysis in Echeveria. Planta. 1988:175(2):153–169. 10.1007/BF00392424 [DOI] [PubMed] [Google Scholar]
- Gu Q, Ferrándiz C, Yanofsky MF, Martienssen R. The FRUITFULL MADS-box gene mediates cell differentiation during Arabidopsis fruit development. Development. 1998:125(8):1509–1517. 10.1242/dev.125.8.1509 [DOI] [PubMed] [Google Scholar]
- Gustafson-Brown C, Savidge B, Yanofsky MF. Regulation of the Arabidopsis floral homeotic gene APETALA1. Cell. 1994:76(1):131–143. 10.1016/0092-8674(94)90178-3 [DOI] [PubMed] [Google Scholar]
- Hasebe M, Wen CK, Kato M, Banks JA. Characterization of MADS homeotic genes in the fern Ceratopteris richardii. Proc Natl Acad Sci U S A. 1998:95(11):6222–6227. 10.1073/pnas.95.11.6222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijmans K, Ament K, Rijpkema AS, Zethof J, Wolters-Arts M, Gerats T, Vandenbussche M. Redefining C and D in the petunia ABC. Plant Cell. 2012:24(6):2305–2317. 10.1105/tpc.112.097030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henschel K, Kofuji R, Hasebe M, Saedler H, Münster T, Theißen G. Two ancient classes of MIKC-type MADS-box genes are present in the moss Physcomitrella patens. Mol Biol Evol. 2002:19(6):801–814. 10.1093/oxfordjournals.molbev.a004137 [DOI] [PubMed] [Google Scholar]
- Herskowitz I. A regulatory hierarchy for cell specialization in yeast. Nature. 1989:342:749–757. 10.1038/342749a0 [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J. Sex expression in flowering plants. Brookhaven Symp Biol. 1963:16:109–125. [Google Scholar]
- Hisanaga T, Fujimoto S, Cui Y, Sato K, Sano R, Yamaoka S, Kohchi T, Berger F, Nakajima K. Deep evolutionary origin of gamete-directed zygote activation by KNOX/BELL transcription factors in green plants. Elife. 2021:10:e57090. 10.7554/eLife.57090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder N. Positional information and pattern formation in plant morphogenesis and a mechanism for the involvement of plant hormones. J Theor Biol. 1979:77(2):195–212. 10.1016/0022-5193(79)90307-2 [DOI] [PubMed] [Google Scholar]
- Honma T, Goto K. The Arabidopsis floral homeotic gene PISTILLATA is regulated by discrete cis-elements responsive to induction and maintenance signals. Development. 2000:127(10):2021–2030. 10.1242/dev.127.10.2021 [DOI] [PubMed] [Google Scholar]
- Honma T, Goto K. Complexes of MADS-box proteins are sufficient to convert leaves into floral organs. Nature. 2001:409(6819):525–529. 10.1038/35054083 [DOI] [PubMed] [Google Scholar]
- Hsu H-F, Chen W-H, Shen Y-H, Hsu W-H, Mao W-T, Yang C-H. Multifunctional evolution of B and AGL6 MADS box genes in orchids. Nat Commun. 2021:12(1):902. 10.1038/s41467-021-21229-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H-F, Hsu W-H, Lee Y-I, Mao W-T, Yang J-Y, Li J-Y, Yang C-H. Model for perianth formation in orchids. Nat Plants. 2015:1(5):1–8. 10.1038/nplants.2015.46 [DOI] [Google Scholar]
- Hu T, Li X, Du L, Manuela D, Xu M. LEAFY and APETALA1 down-regulate ZINC FINGER PROTEIN 1 and 8 to release their repression on class B and C floral homeotic genes. Proc Natl Acad Sci U S A. 2023:120(22):e2221181120. 10.1073/pnas.2221181120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, López-Giráldez F, Townsend JP, Irish VF. RBE controls microRNA164 expression to effect floral organogenesis. Development. 2012:139(12):2161–2169. 10.1242/dev.075069 [DOI] [PubMed] [Google Scholar]
- Hugouvieux V, Blanc-Mathieu R, Paul M, Janeau A, Xu X, Lucas J, Lai X, Galien A, Yan W, Nanao M, et al. SEPALLATA-driven MADS transcription factor tetramerization is required for inner whorl floral organ development. bioRxiv 541941. 10.1101/2023.05.23.541941, 23 May 2023, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed]
- Hugouvieux V, Silva CS, Jourdain A, Stigliani A, Charras Q, Conn V, Conn SJ, Carles CC, Parcy F, Zubieta C. Tetramerization of MADS family transcription factors SEPALLATA3 and AGAMOUS is required for floral meristem determinacy in Arabidopsis. Nucleic Acids Res. 2018:46(10):4966–4977. 10.1093/nar/gky205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijser P, Klein J, Lonnig W-E, Meijer H, Saedler H, Sommer H. Bracteomania, an inflorescence anomaly, is caused by the loss of function of the MADS-box gene squamosa in Antirrhinum majus. EMBO J. 1992:11(4):1239–1249. 10.1002/j.1460-2075.1992.tb05168.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish VF, Sussex IM. Function of the apetala1-1 gene during Arabidopsis floral development. Plant Cell. 1990:2(8):741–751. 10.1105/tpc.2.8.741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack T, Brockman LL, Meyerowitz EM. The homeotic gene APETALA3 of Arabidopsis thaliana encodes a MADS box and is expressed in petals and stamens. Cell. 1992:68(4):683–697. 10.1016/0092-8674(92)90144-2 [DOI] [PubMed] [Google Scholar]
- Jetha K, Theißen G, Melzer R. Arabidopsis SEPALLATA proteins differ in cooperative DNA-binding during the formation of floral quartet-like complexes. Nucleic Acids Res. 2014:42(17):10927–10942. 10.1093/nar/gku755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofuku KD, den Boer BGW, Van Montagu M, Okamuro JK. Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell. 1994:6(9):1211–1225. 10.1105/tpc.6.9.1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John JL. Origin of a land flora. Nat Plants. 2022:8:1352–1369. 10.1038/s41477-022-01283-y [DOI] [PubMed] [Google Scholar]
- Jung J-H, Lee S, Yun J, Lee M, Park C-M. The miR172 target TOE3 represses AGAMOUS expression during Arabidopsis floral patterning. Plant Sci. 2014:215–216:29–38. 10.1016/j.plantsci.2014.09.010 [DOI] [PubMed] [Google Scholar]
- Kanno A, Saeki H, Kameya T, Saedler H, Theißen G. Heterotopic expression of class B floral homeotic gene supports a modified ABC model for tulip (Tulipa gesneriana). Plant Mol Biol. 2003:52(4):831–841. 10.1023/A:1025070827979 [DOI] [PubMed] [Google Scholar]
- Kapoor M, Tsuda S, Tanaka Y, Mayama T, Okuyama Y, Tsuchimoto S, Takatsuji H. Role of petunia pMADS3 in determination of floral organ and meristem identity, as revealed by its loss of function. Plant J. 2002:32(1):115–127. 10.1046/j.1365-313X.2002.01402.x [DOI] [PubMed] [Google Scholar]
- Käppel S, Melzer R, Rümpler F, Gafert C, Theißen G. The floral homeotic protein SEPALLATA3 recognizes target DNA sequences by shape readout involving a conserved arginine residue in the MADS-domain. Plant J. 2018:95(2):341–357. 10.1111/tpj.13954 [DOI] [PubMed] [Google Scholar]
- Käppel S, Rümpler F, Theißen G. Cracking the floral quartet code: how do multimers of MIKCC-type MADS-domain transcription factors recognize their target genes? Int J Mol Sci. 2023:24(9):8253. 10.3390/ijms24098253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch F, Weiffenbach B, Peifer M, Bender W, Duncan I, Celniker S, Crosby M, Lewis EB, Karch F. The abdominal region of the bithorax complex. Cell. 1985:43(1):81–96. 10.1016/0092-8674(85)90014-5 [DOI] [PubMed] [Google Scholar]
- Karlova R, Chapman N, David K, Angenent GC, Seymour GB, Maagd RAD. Transcriptional control of fleshy fruit development and ripening. J Exp Bot. 2014:65(16):4527–4541. 10.1093/jxb/eru316 [DOI] [PubMed] [Google Scholar]
- Kaufmann K, Muiño JM, Jauregui R, Airoldi CA, Smaczniak C, Krajewski P, Angenent GC. Target genes of the MADS transcription factor SEPALLATA3: integration of developmental and hormonal pathways in the Arabidopsis flower. PLoS Biol. 2009:7(4):e1000090. 10.1371/journal.pbio.1000090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann K, Pajoro A, Angenent GC. Regulation of transcription in plants: mechanisms controlling developmental switches. Nat Rev Genet. 2010a:11(12):830–842. 10.1038/nrg2885 [DOI] [PubMed] [Google Scholar]
- Kaufmann K, Wellmer F, Muiño JM, Ferrier T, Wuest SE, Kumar V, Serrano-Mislata A, Madueño F, Krajewski P, Meyerowitz EM, et al. Orchestration of floral initiation by APETALA1. Science. 2010b:328(5974):85–89. 10.1126/science.1185244 [DOI] [PubMed] [Google Scholar]
- Kempin SA, Savidge B, Yanofsky MF. Molecular basis of the cauliflower phenotype in Arabidopsis. Science. 1995:267(5197):522–525. 10.1126/science.7824951 [DOI] [PubMed] [Google Scholar]
- Kim S, Koh J, Yoo M-J, Kong H, Hu Y, Ma H, Soltis PS, Soltis DE. Expression of floral MADS-box genes in basal angiosperms: implications for the evolution of floral regulators. Plant J. 2005:43(5):724–744. 10.1111/j.1365-313X.2005.02487.x [DOI] [PubMed] [Google Scholar]
- Kim S, Yoo M-J, Albert VA, Farris JS, Soltis PS, Soltis DE. Phylogeny and diversification of B-function MADS-box genes in angiosperms: evolutionary and functional implications of a 260-million-year-old duplication. Am J Bot. 2004:91(12):2102–2118. 10.3732/ajb.91.12.2102 [DOI] [PubMed] [Google Scholar]
- Kofuji R, Sumikawa N, Yamasaki M, Kondo K, Ueda K, Ito M, Hasebe M. Evolution and divergence of the MADS-box gene family based on genome-wide expression analyses. Mol Biol Evol. 2003:20(12):1963–1977. 10.1093/molbev/msg216 [DOI] [PubMed] [Google Scholar]
- Komaki MK, Okada K, Nishino E, Shimura Y. Isolation and characterization of novel mutants of Arabidopsis thaliana defective in flower development. Development. 1988:104(2):195–203. 10.1242/dev.104.2.195 [DOI] [Google Scholar]
- Koornneef M, Eden JV, Hanhart CJ, Stam P, Braaksma FJ, Feenstra WJ. Linkage map of Arabidopsis thaliana. J Hered. 1983:74(4):265–272. 10.1093/oxfordjournals.jhered.a109781 [DOI] [Google Scholar]
- Koshimizu S, Kofuji R, Sasaki-Sekimoto Y, Kikkawa M, Shimojima M, Ohta H, Shigenobu S, Kabeya Y, Hiwatashi Y, Tamada Y, et al. Physcomitrella MADS-box genes regulate water supply and sperm movement for fertilization. Nat Plants. 2018:4(1):36–45. 10.1038/s41477-017-0082-9 [DOI] [PubMed] [Google Scholar]
- Kramer EM, Di Stilio VS, Schluter PM. Complex patterns of gene duplication in the APETALA3 and PISTILLATA lineages of the Ranunculaceae. Int J Plant Sci. 2003:164(1):1–11. 10.1086/344694 [DOI] [Google Scholar]
- Kramer EM, Dorit RL, Irish VF. Molecular evolution of genes controlling petal and stamen development: duplication and divergence within the APETALA3 and PISTILLATA MADS-box gene lineages. Genetics. 1998:149(2):765–783. 10.1093/genetics/149.2.765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer EM, Holappa L, Gould B, Jaramillo MA, Setnikov D, Santiago PM. Elaboration of B gene function to include the identity of novel floral organs in the lower eudicot aquilegia. Plant Cell. 2007:19(3):750–766. 10.1105/tpc.107.050385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizek BA, Lewis MW, Fletcher JC. RABBIT EARS is a second-whorl repressor of AGAMOUS that maintains spatial boundaries in Arabidopsis flowers. Plant J. 2006:45(3):369–383. 10.1111/j.1365-313X.2005.02633.x [DOI] [PubMed] [Google Scholar]
- Krizek BA, Meyerowitz EM. The Arabidopsis homeotic genes APETALA3 and PISTILLATA are sufficient to provide the B class organ identity function. Development. 1996:122(1):11–22. 10.1242/dev.122.1.11 [DOI] [PubMed] [Google Scholar]
- Kunst L, Klenz JE, Martinez-Zapater J, Haughn GW. AP2 gene determines the identity of perianth organs in flowers of Arabidopsis thaliana. Plant Cell. 1989:1(12):1195–1208. 10.2307/3868917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyozuka J, Kobayashi T, Morita M, Shimamoto K. Spatially and temporally regulated expression of rice MADS box genes with similarity to Arabidopsis class A, B and C genes. Plant Cell Physiol. 2000:41(6):710–718. 10.1093/pcp/41.6.710 [DOI] [PubMed] [Google Scholar]
- Lai X, Stigliani A, Lucas J, Hugouvieux V, Parcy F, Zubieta C. Genome-wide binding of SEPALLATA3 and AGAMOUS complexes determined by sequential DNA-affinity purification sequencing. Nucleic Acids Res. 2020:48(17):9637–9648. 10.1093/nar/gkaa729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai X, Vega-Léon R, Hugouvieux V, Blanc-Mathieu R, Wal FVD, Lucas J, Silva CS, Jourdain A, Muino JM, Nanao MH, et al. The intervening domain is required for DNA-binding and functional identity of plant MADS transcription factors. Nat Commun. 2021:12(1):4760. 10.1038/s41467-021-24978-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RS, Hill TA, Tan QK-G, Irish VF. Regulation of APETALA3 floral homeotic gene expression by meristem identity genes. Development. 2002:129(9):2079–2086. 10.1242/dev.129.9.2079 [DOI] [PubMed] [Google Scholar]
- Lee J-Y, Baum SF, Alvarez J, Patel A, Chitwood DH, Bowman JL. Activation of CRABS CLAW in the nectaries and carpels of Arabidopsis. Plant Cell. 2005a:17(1):25–36. 10.1105/tpc.104.026666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-Y, Baum SF, Oh S-H, Jiang CZ, Chen JC, Bowman JL. Recruitment of CRABS CLAW to promote nectary development within the eudicot clade. Development. 2005b:132(22):5021–5032. 10.1242/dev.02067 [DOI] [PubMed] [Google Scholar]
- Lee U-S, Bieluszewski T, Xiao J, Yamaguchi A, Wagner D. H2a.Z contributes to trithorax activity at the AGAMOUS locus. Mol Plant. 2022:15(2):207–210. 10.1016/j.molp.2022.01.005 [DOI] [PubMed] [Google Scholar]
- Lee I, Wolfe DS, Nilsson O, Weigel D. A LEAFY co-regulator encoded by UNUSUAL FLORAL ORGANS. Curr Biol. 1997:7(2):95–104. 10.1016/S0960-9822(06)00053-4 [DOI] [PubMed] [Google Scholar]
- Lenhard M, Bohnert A, Jürgens G, Laux T. Termination of stem cell maintenance in Arabidopsis floral meristems by interactions between WUSCHEL and AGAMOUS. Cell. 2001:105(6):805–814. 10.1016/S0092-8674(01)00390-7 [DOI] [PubMed] [Google Scholar]
- Levin JZ, Meyerowitz EM. UFO: an Arabidopsis gene involved in both floral meristem and floral organ development. Plant Cell. 1995:7(5):529–548. 10.1105/tpc.7.5.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978:276(5688):565–570. 10.1038/276565a0 [DOI] [PubMed] [Google Scholar]
- Li H, Liang W, Jia R, Yin C, Zong J, Kong H, Zhang D. The AGL6-like gene OsMADS6 regulates floral organ and meristem identities in rice. Cell Res. 2010:20(3):299–313. 10.1038/cr.2009.143 [DOI] [PubMed] [Google Scholar]
- Li L, Yu X-X, Guo C-C, Duan X-S, Shan H-Y, Zhang R, Xu G-X, Kong H-Z. Interactions among proteins of floral MADS-box genes in nuphar pumila (Nymphaeaceae) and the most recent common ancestor of extant angiosperms help understand the underlying mechanisms of the origin of the flower. J Syst Evol. 2015:53(4):285–296. 10.1111/jse.12148 [DOI] [Google Scholar]
- Liljegren SJ, Ditta GS, Eshed Y, Savidge B, Bowman JL, Yanofsky MF. Control of fruit dehiscence in Arabidopsis by the SHATTERPROOF MADS-box genes. Nature. 2000:404(6779):766–770. 10.1038/35008089 [DOI] [PubMed] [Google Scholar]
- Liljegren SJ, Gustafson-Brown C, Pinyopich A, Ditta GS, Yanofsky MF. Interactions among APETALA1, LEAFY, and TERMINAL FLOWER1 specify meristem fate. Plant Cell. 1999:11(6):1007–1018. 10.1105/tpc.11.6.1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Yuan T, Guo H, Guo Y, Yamaguchi N, Wang S, Zhang D, Qi D, Li J, Chen Q, et al. The regulation of chromatin configuration at AGAMOUS locus by LFR-SYD-containing complex is critical for reproductive organ development in Arabidopsis. Plant J. 2023:116(2):478–496. 10.1111/tpj.16385 [DOI] [PubMed] [Google Scholar]
- Liu X, Kim YJ, Müller R, Yumul RE, Liu C, Pan Y, Cao X, Goodrich J, Chen X. AGAMOUS terminates floral stem cell maintenance in Arabidopsis by directly repressing WUSCHEL through recruitment of polycomb group proteins. Plant Cell. 2011:23(10):3654–3670. 10.1105/tpc.111.091538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Ma H, Jung S, Main D, Guo L. Developmental mechanisms of fleshy fruit diversity in Rosaceae. Annu Rev Plant Biol. 2020:71(1):547–573. 10.1146/annurev-arplant-111119-021700 [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang S, Li L, Yang T, Dong S, Wei T, Wu S, Liu Y, Gong Y, Feng X, et al. The Cycas genome and the early evolution of seed plants. Nat Plants. 2022:8(4):389–401. 10.1038/s41477-022-01129-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Xi W, Shen L, Tan C, Yu H. Regulation of floral patterning by flowering time genes. Dev Cell. 2009:16(5):711–722. 10.1016/j.devcel.2009.03.011 [DOI] [PubMed] [Google Scholar]
- Liu C, Zhang J, Zhang N, Shan H, Su K, Zhang J, Meng Z, Kong H, Chen Z. Interactions among proteins of floral MADS-box genes in basal eudicots: implications for evolution of the regulatory network for flower development. Mol Biol Evol. 2010:27(7):1598–1611. 10.1093/molbev/msq044 [DOI] [PubMed] [Google Scholar]
- L'Obel MD. Kruydtboeck oft beschrÿuinghe van allerleye ghewassen, kruyderen, hesteren, ende gheboomten. Antwerpen: Christoffel Plantyn; 1581. [Google Scholar]
- Lohmann JU, Hong RL, Hobe M, Busch MA, Parcy F, Simon R, Weigel D. A molecular link between stem cell regulation and floral patterning in Arabidopsis. Cell. 2001:105(6):793–803. 10.1016/S0092-8674(01)00384-1 [DOI] [PubMed] [Google Scholar]
- Ma H, Yanofsky MF, Meyerowitz EM. AGL1-AGL6, an Arabidopsis gene family with similarity to floral homeotic and transcription factor genes. Gene Dev. 1991:5(3):484–495. 10.1101/gad.5.3.484 [DOI] [PubMed] [Google Scholar]
- Mandel MA, Bowman JL, Kempin SA, Ma H, Meyerowitz EM, Yanofsky MF. Manipulationn of flower structure in transgenic tobacco. Cell. 1992a:71(1):133–143. 10.1016/0092-8674(92)90272-E [DOI] [PubMed] [Google Scholar]
- Mandel MA, Gustafson-Brown C, Savidge B, Yanofsky MF. Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature. 1992b:360(6401):273–277. 10.1038/360273a0 [DOI] [PubMed] [Google Scholar]
- Mao W-T, Hsu W-H, Li J-Y, Yang C-H. Distance-based measurement determines the coexistence of B protein hetero- and homodimers in lily tepal and stamen tetrameric complexes. Plant J. 2021:105(5):1357–1373. 10.1111/tpj.15117 [DOI] [PubMed] [Google Scholar]
- Martínez-Gómez J, Galimba KD, Coté EY, Sullivan AM, Stilio VSD. Spontaneous homeotic mutants and genetic control of floral organ identity in a ranunculid. Evol Dev. 2020:23(3):197–214. 10.1111/ede.12357 [DOI] [PubMed] [Google Scholar]
- Masters MT. Vegetable teratology: an account of the principle deviations from the usual construction of plants. London: Ray Society; 1869. [Google Scholar]
- McGinnis W, Garber RL, Wirz J, Kuroiwa A, Gehring WJ. A homologous protein-coding sequence in Drosophila homeotic genes and its conservation in other metazoans. Cell. 1984a:37(2):403–408. 10.1016/0092-8674(84)90370-2 [DOI] [PubMed] [Google Scholar]
- McGinnis W, Levine MS, Hafen E, Kuroiwa A, Gehring WJ. A conserved DNA sequence in homoeotic genes of the Drosophila antennapedia and bithorax complexes. Nature. 1984b:308(5958):428–433. 10.1038/308428a0 [DOI] [PubMed] [Google Scholar]
- Melzer R, Härter A, Rümpler F, Kim S, Soltis PS, Soltis DE, Theißen G. DEF- and GLO-like proteins may have lost most of their interaction partners during angiosperm evolution. Ann Bot. 2014:114(7):1431–1443. 10.1093/aob/mcu094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer R, Theissen G. Reconstitution of ‘floral quartets’ in vitro involving class B and class E floral homeotic proteins. Nucleic Acids Res. 2009:37(8):2723–2736. 10.1093/nar/gkp129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer R, Verelst W, Theißen G. The class E floral homeotic protein SEPALLATA3 is sufficient to loop DNA in ‘floral quartet’-like complexes in vitro. Nucleic Acids Res. 2009:37(1):144–157. 10.1093/nar/gkn900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena M, Ambrose BA, Meeley RB, Briggs SP, Yanofsky MF, Schmidt RJ. Diversification of C-function activity in maize flower development. Science. 1996:274(5292):1537–1540. 10.1126/science.274.5292.1537 [DOI] [PubMed] [Google Scholar]
- Mendes MA, Guerra RF, Berns MC, Manzo C, Masiero S, Finzi L, Kater MM, Colombo L. MADS domain transcription factors mediate short-range DNA looping that is essential for target gene expression in Arabidopsis. Plant Cell. 2013:25(7):2560–2572. 10.1105/tpc.112.108688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz EM, Smyth DR, Bowman JL. Abnormal flowers and pattern formation in floral development. Development. 1989:106(2):209–217. 10.1242/dev.106.2.209 [DOI] [Google Scholar]
- Mondragon-Palomino M, Theissen G. Conserved differential expression of paralogous DEFICIENS- and GLOBOSA-like MADS-box genes in the flowers of Orchidaceae: refining the ‘orchid code’. Plant J. 2011:66(6):1008–1019. 10.1111/j.1365-313X.2011.04560.x [DOI] [PubMed] [Google Scholar]
- Montalvão APL, Kersten B, Fladung M, Müller NA. The diversity and dynamics of sex determination in dioecious plants. Front Plant Sci. 2021:11:580488. 10.3389/fpls.2020.580488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moquin-Tandon A. Elements de tiratologie vigitale, ou histoire abrigie des anomalies de I'Organisation dans les vigitaux. Paris: J.-P. Loss; 1841. [Google Scholar]
- Morel P, Heijmans K, Rozier F, Zethof J, Chamot S, Bento SR, Vialette-Guiraud A, Chambrier P, Trehin C, Vandenbusschea M. Divergence of the floral A-function between an asterid and a rosid species. Plant Cell. 2017:29(7):1605–1621. 10.1105/tpc.17.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschin S, Nigris S, Ezquer I, Masiero S, Cagnin S, Cortese E, Colombo L, Casadoro G, Baldan B. Expression and functional analyses of nymphaea caerulea MADS-box genes contribute to clarify the complex flower patterning of water lilies. Front Plant Sci. 2021:12:730270. 10.3389/fpls.2021.730270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouradov A, Hamdorf B, Teasdale RD, Kim JT, Winter KU, Theissen G. A DEF/GLO-like MADS-box gene from a gymnosperm: pinus radiata contains an ortholog angiosperm B class floral homeotic genes. Dev Genet. 1999:25(3):245–252. [DOI] [PubMed] [Google Scholar]
- Moyroud E, Minguet EG, Ott F, Yant L, Posé D, Monniaux M, Blanchet S, Bastien O, Thévenon E, Weigel D, et al. Prediction of regulatory interactions from genome sequences using a biophysical model for the Arabidopsis LEAFY transcription factor. Plant Cell. 2011:23(4):1293–1306. 10.1105/tpc.111.083329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyroud E, Monniaux M, Thévenon E, Dumas R, Scutt CP, Frohlich MW, Parcy F. A link between LEAFY and B-gene homologues in Welwitschia mirabilis sheds light on ancestral mechanisms prefiguring floral development. New Phytol. 2017:216(2):469–481. 10.1111/nph.14483 [DOI] [PubMed] [Google Scholar]
- Muiño JM, Smaczniak C, Angenent GC, Kaufmann K, van Dijk ADJ. Structural determinants of DNA recognition by plant MADS-domain transcription factors. Nucleic Acids Res. 2014:42(4):2138–2146. 10.1093/nar/gkt1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naudin C. Nouvelles récherches sur l'hybridité dans les végétaux. Nouvelles archives du Muséum d'histoire naturelle. 1865:1:25–176. [Google Scholar]
- Ohmori S, Kimizu M, Sugita M, Miyao A, Hirochika H, Uchida E, Nagato Y, Yoshida H. MOSAIC FLORAL ORGANS1, an AGL6-like MADS box gene, regulates floral organ identity and meristem fate in rice. Plant Cell. 2009:21(10):3008–3025. 10.1105/tpc.109.068742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ó’Maoiléidigh DS, Wuest SE, Rae L, Raganelli A, Ryan PT, Kwaśniewska K, Das P, Lohan AJ, Loftus B, Graciet E, et al. Control of reproductive floral organ identity specification in Arabidopsis by the C function regulator AGAMOUS. Plant Cell. 2013:25(7):2482–2503. 10.1105/tpc.113.113209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajoro A, Madrigal P, Muiño JM, Matus JT, Jin J, Mecchia MA, Debernardi JM, Palatnik JF, Balazadeh S, Arif M, et al. Dynamics of chromatin accessibility and gene regulation by MADS-domain transcription factors in flower development. Genome Biol. 2014:15(3):R41. 10.1186/gb-2014-15-3-r41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z-J, Cheng C-C, Tsai W-C, Chung M-C, Chen W-H, Hu J-M, Chen H-H. The duplicated B-class MADS-box genes display dualistic characters in orchid floral organ identity and growth. Plant Cell Physiol. 2011:52(9):1515–1531. 10.1093/pcp/pcr092 [DOI] [PubMed] [Google Scholar]
- Pankratz MJ, Jäckle H. Making stripes in the Drosophila embryo. Trends Genet. 1990:6:287–292. 10.1016/0168-9525(90)90234-W [DOI] [PubMed] [Google Scholar]
- Parcy F, Nilsson O, Busch MA, Lee I, Weigel D. A genetic framework for floral patterning. Nature. 1998:395(6702):561–566. 10.1038/26903 [DOI] [PubMed] [Google Scholar]
- Parkinson J. Paradisi in sole paradisus terrestris. London: Humfrey Lownes and Robert Young; 1629. [Google Scholar]
- Payer J-B. Traité d’organogénie comparée de la fleur. Paris: Libraire de Victor Masson; 1857. [Google Scholar]
- Pelayo MA, Yamaguchi N, Ito T. One factor, many systems: the floral homeotic protein AGAMOUS and its epigenetic regulatory mechanisms. Curr Opin Plant Biol. 2021:61:102009. 10.1016/j.pbi.2021.102009 [DOI] [PubMed] [Google Scholar]
- Pelaz S, Ditta GS, Baumann E, Wisman E, Yanofsky MF. B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature. 2000:405(6783):200–203. 10.1038/35012103 [DOI] [PubMed] [Google Scholar]
- Pelaz S, Tapia-López R, Alvarez-Buylla ER, Yanofsky MF. Conversion of leaves into petals in Arabidopsis. Curr Biol. 2001:11(3):182–184. 10.1016/S0960-9822(01)00024-0 [DOI] [PubMed] [Google Scholar]
- Penzig O. Pflanzen-teratologie systematisch geordnet. Genua: A. Ciminago; 1890–4. [Google Scholar]
- Pinyopich A, Ditta GS, Savidge B, Liljegren SJ, Baumann E, Wisman E, Yanofsky MF. Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature. 2003:424(6944):85–88. 10.1038/nature01741 [DOI] [PubMed] [Google Scholar]
- Plinius the Secondus TE. The history of the world, commonly called the naturall historie of C. Plinius Secundus. London: A. Islip; 1634. [Google Scholar]
- Pnueli L, Abu-Abeid M, Zamir D, Nacken W, Schwarz-Sommer Z, Lifschitz E. The MADS box gene family in tomato: temporal expression during floral development, conserved secondary structures and homology with homeotic genes from Antirrhinum and Arabidopsis. Plant J. 1991:1(2):255–266. 10.1111/j.1365-313X.1991.00255.x [DOI] [PubMed] [Google Scholar]
- Poyatos-Pertíñez S, Quinet M, Ortíz-Atienza A, Yuste-Lisbona FJ, Pons C, Giménez E, Angosto T, Granell A, Capel J, Lozano R. A factor linking floral organ identity and growth revealed by characterization of the tomato mutant unfinished flower development (ufd). Front Plant Sci. 2016:7:1648. 10.3389/fpls.2016.01648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunet N, Meyerowitz EM. Genetics and plant development. Comptes Rendus Biol. 2016:339(7–8):240–246. 10.1016/j.crvi.2016.05.003 [DOI] [PubMed] [Google Scholar]
- Prunet N, Yang W, Das P, Meyerowitz EM, Jack TP. SUPERMAN prevents class B gene expression and promotes stem cell termination in the fourth whorl of Arabidopsis thaliana flowers. Proc Natl Acad Sci U S A. 2017:114(27):7166–7171. 10.1073/pnas.1705977114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puranik S, Acajjaoui S, Conn S, Costa L, Conn V, Vial A, Marcellin R, Melzer R, Brown E, Hart D, et al. Structural basis for the oligomerization of the MADS domain transcription factor SEPALLATA3 in Arabidopsis. Plant Cell. 2014:26(9):3603–3615. 10.1105/tpc.114.127910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Li Z, Walther D, Köhler C. Updated phylogeny and protein structure predictions revise the hypothesis on the origin of MADS-box transcription factors in land plants. Mol Biol Evol. 2023:40(9):msad194. 10.1101/2023.01.10.523452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refahi Y, Zardilis A, Michelin G, Wightman R, Leggio B, Legrand J, Faure E, Vachez L, Armezzani A, Risson A-E, et al. A multiscale analysis of early flower development in Arabidopsis provides an integrated view of molecular regulation and growth control. Dev Cell. 2021:56(4):540–556. 10.1016/j.devcel.2021.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann JL, Krizek BA, Meyerowitz EM. Dimerization specificity of Arabidopsis MADS domain homeotic proteins APETALA1, APETALA3, PISTILLATA, and AGAMOUS. Proc Natl Acad Sci U S A. 1996:93(10):4793–4798. 10.1073/pnas.93.10.4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieu P, Turchi L, Thévenon E, Zarkadas E, Nanao M, Chahtane H, Tichtinsky G, Lucas J, Blanc-Mathieu R, Zubieta C, et al. The F-box protein UFO controls flower development by redirecting the master transcription factor LEAFY to new cis-elements. Nat Plants. 2023:9(2):315–329. 10.1038/s41477-022-01336-2 [DOI] [PubMed] [Google Scholar]
- Rijpkema AS, Royaert S, Zethof J, van der Weerden G, Gerats T, Vandenbussche M. Analysis of the petunia TM6 mADS box gene reveals functional divergence within the DEF/AP3 lineage. Plant Cell. 2006:18(8):1819–1832. 10.1105/tpc.106.042937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijpkema AS, Zethof J, Gerats T, Vandenbussche M. The petunia AGL6 gene has a SEPALLATA-like function in floral patterning. Plant J. 2009:60(1):1–9. 10.1111/j.1365-313X.2009.03917.x [DOI] [PubMed] [Google Scholar]
- Ruelens P, Zhang Z, van Mourik H, Maere S, Kaufmann K, Geuten K. The origin of floral organ identity quartets. Plant Cell. 2017:29(2):229–242. 10.1105/tpc.16.00366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rümpler F, Tessari C, Gramzow L, Gafert C, Blohs M, Theißen G. The origin of floral quartet formation—ancient exon duplications shaped the evolution of MIKC-type MADS-domain transcription factor interactions. Mol Biol Evol. 2023:40(5):msad088. 10.1093/molbev/msad088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rümpler F, Theißen G, Melzer R. A conserved leucine zipper-like motif accounts for strong tetramerization capabilities of SEPALLATA-like MADS-domain transcription factors. J Exp Bot. 2018:69(8):1943–1954. 10.1093/jxb/ery063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacharowski SP, Gratkowska DM, Sarnowska EA, Kondrak P, Jancewicz I, Porri A, Bucior E, Rolicka AT, Franzen R, Kowalczyk J, et al. SWP73 subunits of Arabidopsis SWI/SNF chromatin remodeling complexes play distinct roles in leaf and flower development. Plant Cell. 2015:27(7):1889–1906. 10.1105/tpc.15.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Medrano LJ, Meyerowitz EM. Role of SUPERMAN in maintaining Arabidopsis floral whorl boundaries. Nature. 1995:378(6553):199–203. 10.1038/378199a0 [DOI] [PubMed] [Google Scholar]
- Savidge B, Rounsley SD, Yanofsky MF. Temporal relationship between the transcription of two Arabidopsis MADS box genes and the floral organ identity genes. Plant Cell. 1995:7(6):721–733. 10.1105/tpc.7.6.721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling S, Pan S, Kennedy A, Melzer R. MADS-box genes and crop domestication: the jack of all traits. J Exp Bot. 2018:69(7):1447–1469. 10.1093/jxb/erx479 [DOI] [PubMed] [Google Scholar]
- Schwarz-Sommer Z, Hue I, Huijser P, Flor PJ, Hansen R, Tetens F, Lönnig WE, Saedler H, Sommer H. Characterization of the antirrhinum floral homeotic MADS-box gene deficiens: evidence for DNA binding and autoregulation of its persistent expression throughout flower development. EMBO J. 1992:11(1):251–263. 10.1002/j.1460-2075.1992.tb05048.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Sommer Z, Huijser P, Nacken W, Saedler H, Sommer H. Genetic-control of flower development by homeotic genes in Antirrhinum-majus. Science. 1990:250(4983):931–936. 10.1126/science.250.4983.931 [DOI] [PubMed] [Google Scholar]
- Scott MP, Weiner AJ. Structural relationships among genes that control development: sequence homology between the antennapedia, ultrabithorax, and fushi tarazu loci of Drosophila. Proc Natl Acad Sci U S A. 1984:81(13):4115–4119. 10.1073/pnas.81.13.4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma B, Kramer E. Sub- and neo-functionalization of APETALA3 paralogs have contributed to the evolution of novel floral organ identity in Aquilegia (columbine, Ranunculaceae). New Phytol. 2013:197(3):949–957. 10.1111/nph.12078 [DOI] [PubMed] [Google Scholar]
- Siegfried KR, Eshed Y, Baum SF, Otsuga D, Drews GN, Bowman JL. Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development. 1999:126(18):4117–4128. 10.1242/dev.126.18.4117 [DOI] [PubMed] [Google Scholar]
- Smaczniak C, Muiño JM, Chen D, Angenent GC, Kaufmann K. Differences in DNA binding specificity of floral homeotic protein complexes predict organ-specific target genes. Plant Cell. 2017:29(8):1822–1835. 10.1105/tpc.17.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth DR. Evolution and genetic control of the floral ground plan. New Phytol. 2018:220(1):70–86. 10.1111/nph.15282 [DOI] [PubMed] [Google Scholar]
- Smyth DR. How flower development genes were identified using forward genetic screens in Arabidopsis thaliana. Genetics. 2023:224(4):iyad102. 10.1093/genetics/iyad102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth DR, Bowman JL, Meyerowitz EM. Early flower development in Arabidopsis. Plant Cell. 1990:2(8):755–768. 10.1105/tpc.2.8.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis DE, Chanderbali AS, Kim S, Buzco T, Soltis PS. The ABC model and its applicability to basal angiosperms. Ann Bot. 2007:100(2):155–163. 10.1093/aob/mcm117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer RJ. Phenotypic plasticity: from theory and genetics to current and future challenges. Genetics. 2020:215(1):1–13. 10.1534/genetics.120.303163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer H, Beltrán J-P, Huijser P, Pape H, Lonnig W-E, Saedler H, Schwarz-Sommer Z. Deficiens, a homeotic gene involved in the control of flower morphogenesis in Antirrhinum majus: the protein shows homology to transcription factors. EMBO J. 1990:9(3):605–613. 10.1002/j.1460-2075.1990.tb08152.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Xu Y, Ng K-H, Ito T. A timing mechanism for stem cell maintenance and differentiation in the Arabidopsis floral meristem. Gene Dev. 2009:23(15):1791–1804. 10.1101/gad.1800409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom J, Carlsbecker A, Svensson ME, Svenson M, Johanson U, Theissen G, Engstrom P. MADS-box genes active in developing pollen cones of Norway spruce (Picea abies) are homologous to the B-class floral homeotic genes in angiosperms. Dev Genet. 1999:25(3):253–266. [DOI] [PubMed] [Google Scholar]
- Tandre K, Albert VA, Sundgås A, Engström P. Conifer homologues to genes that control floral development in angiosperms. Plant Mol Biol. 1995:27(1):69–78. 10.1007/BF00019179 [DOI] [PubMed] [Google Scholar]
- Tandre K, Svenson M, Svensson ME, Engström P. Conservation of gene structure and activity in the regulation of reproductive organ development of conifers and angiosperms. Plant J. 1998:15(5):615–623. 10.1046/j.1365-313x.1998.00236.x [DOI] [PubMed] [Google Scholar]
- Theissen G, Melzer R. Molecular mechanisms underlying origin and diversification of the angiosperm flower. Ann Bot. 2007:100(3):603–619. 10.1093/aob/mcm143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theissen G, Melzer R, Rümpler F. MADS-domain transcription factors and the floral quartet model of flower development: linking plant development and evolution. Development. 2016:143(18):3259–3271. 10.1242/dev.134080 [DOI] [PubMed] [Google Scholar]
- Theissen G, Saedler H. Plant biology. Floral quartets. Nature. 2001:409(6819):469–471. 10.1038/35054172 [DOI] [PubMed] [Google Scholar]
- Theophrastus. Enquiry into plants and minor works on odours and weather signs. London: William Heinemann; 1916. [Google Scholar]
- Thompson BE, Bartling L, Whipple C, Hall DH, Sakai H, Schmidt R, Hake S. bearded-ear encodes a MADS box transcription factor critical for maize floral development. Plant Cell. 2009:21(9):2578–2590. 10.1105/tpc.109.067751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillette de Clermont-Tonnere M. Sur une variété femelle du pommier commun. Mémoires de la Société linnéenne de Paris. 1825:3:164–167. [Google Scholar]
- Tröbner L, Ramirez P, Motte I, Hue P, Huijser W, Lönnig H, Saedler H, Sommer H, Schwarz-Sommer Z. Globosa—a homeotic gene which interacts with deficiens in the control of antirrhinum floral organogenesis. EMBO J. 1992:11(13):4693–4704. 10.1002/j.1460-2075.1992.tb05574.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker SC, Hodges SA. Floral ontogeny of Aquilegia, Semiaquilegia, and Enemion (Ranunculaceae). Int J Plant Sci.. 2005:166(4):557–574. 10.1086/429848 [DOI] [Google Scholar]
- van Tunen AJ, Eikelboom W, Angenent G. Floral organogenesis in tulipa. Flower Newslett. 1993:16:33–38. [Google Scholar]
- Vandenbussche M, Zethof J, Royaert S, Weterings K, Gerats T. The duplicated B-class heterodimer model: whorl-specific effects and complex genetic interactions in petunia hybrida flower development. Plant Cell. 2004:16(3):741–754. 10.1105/tpc.019166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veron AS, Kaufmann K, Bornberg-Bauer E. Evidence of interaction network evolution by whole-genome duplications: a case study in MADS-box proteins. Mol Biol Evol. 2007:24(3):670–678. 10.1093/molbev/msl197 [DOI] [PubMed] [Google Scholar]
- Vollbrecht E, Veit B, Sinha N, Hake S. The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature. 1991:350(6315):241–243. 10.1038/350241a0 [DOI] [PubMed] [Google Scholar]
- Wagner D, Sablowski RWM, Meyerowitz EM. Transcriptional activation of APETALA1 by LEAFY. Science. 1999:285(5427):582–584. 10.1126/science.285.5427.582 [DOI] [PubMed] [Google Scholar]
- Wang GL. A study on the history of Chinese roses from ancient works and images. Acta Hortic. 2007:751(751):347–356. 10.17660/ActaHortic.2007.751.44 [DOI] [Google Scholar]
- Wang P, Cheng T, Lu M, Liu G, Li M, Shi J, Lu Y, Laux T, Chen J. Expansion and functional divergence of AP2 group genes in spermatophytes determined by molecular evolution and Arabidopsis mutant analysis. Front Plant Sci. 2016:7. 10.3389/fpls.2016.01383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-Q, Melzer R, Theißen G. Molecular interactions of orthologues of floral homeotic proteins from the gymnosperm Gnetum gnemon provide a clue to the evolutionary origin of ‘floral quartets’. Plant J. 2010:64(2):177–190. 10.1111/j.1365-313X.2010.04325.x [DOI] [PubMed] [Google Scholar]
- Warner KA, Rudall PJ, Frohlich MW. Differentiation of perianth organs in Nymphaeales. TAXON. 2008:57(4):1096–1109. 10.1002/tax.574006 [DOI] [Google Scholar]
- Warner KA, Rudall PJ, Frohlich MW. Environmental control of sepalness and petalness in perianth organs of waterlilies: a new Mosaic theory for the evolutionary origin of a differentiated perianth. J Exp Bot. 2009:60(12):3559–3574. 10.1093/jxb/erp202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D, Meyerowitz EM. Activation of floral homeotic genes in Arabidopsis. Science. 1993:261(5129):1723–1726. 10.1126/science.261.5129.1723 [DOI] [PubMed] [Google Scholar]
- Wellmer F, Bowman JL, Davies B, Ferrándiz C, Fletcher JC, Franks RG, Graciet E, Gregis V, Ito T, Jack TP, et al. Flower development: open questions and future directions. In: Riechmann JL, Wellmer F, editors. Flower development, methods and protocols. New York: Humana Press; 2014. p. 103–124. [DOI] [PubMed] [Google Scholar]
- Western TL, Cheng Y, Liu J, Chen X. HUA ENHANCER2, a putative DExH-box RNA helicase, maintains homeotic B and C gene expression in Arabidopsis. Development. 2002:129(7):1569–1581. 10.1242/dev.129.7.1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson MD, Haughn GW. UNUSUAL FLORAL ORGANS controls meristem identity and organ primordia fate in Arabidopsis. Plant Cell. 1995:7(9):1485–1499. 10.2307/3870137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter CM, Austin RS, Blanvillain-Baufumé S, Reback MA, Monniaux M, Wu M-F, Sang Y, Yamaguchi A, Yamaguchi N, Parker JE, et al. LEAFY target genes reveal floral regulatory logic, cis motifs, and a link to biotic stimulus response. Dev Cell. 2011:20(4):430–443. 10.1016/j.devcel.2011.03.019 [DOI] [PubMed] [Google Scholar]
- Wollmann H, Mica E, Todesco M, Long JA, Weigel D. On reconciling the interactions between APETALA2, miR172 and AGAMOUS with the ABC model of flower development. Development. 2010:137(21):3633–3642. 10.1242/dev.036673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wordsell WC. The principles of plant teratology. London: Ray Society; 1915–6. [Google Scholar]
- Wu H-W, Deng S, Xu H, Mao H-Z, Liu J, Niu Q-W, Wang H, Chua N-H. A noncoding RNA transcribed from the AGAMOUS (AG) second intron binds to CURLY LEAF and represses AG expression in leaves. New Phytol. 2018:219(4):1480–1491. 10.1111/nph.15231 [DOI] [PubMed] [Google Scholar]
- Wu M-F, Sang Y, Bezhani S, Yamaguchi N, Han S-K, Li Z, Su Y, Slewinski TL, Wagner D. SWI2/SNF2 chromatin remodeling ATPases overcome polycomb repression and control floral organ identity with the LEAFY and SEPALLATA3 transcription factors. Proc Natl Acad Sci U S A. 2012:109(9):3576–3581. 10.1073/pnas.1113409109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuest SE, O’Maoileidigh DS, Rae L, Kwasniewska K, Raganelli A, Hanczaryk K, Lohan AJ, Loftus B, Graciet E, Wellmer F. Molecular basis for the specification of floral organs by APETALA3 and PISTILLATA. Proc Natl Acad Sci U S A. 2012:109(33):13452–13457. 10.1073/pnas.1207075109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Nagasawa N, Kawasaki S, Matsuoka M, Nagato Y, Hirano HY. The YABBY gene DROOPING LEAF regulates carpel specification and midrib development in Oryza sativa. Plant Cell. 2004:16(2):500–509. 10.1105/tpc.018044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A, Wu M-F, Yang L, Wu G, Poethig RS, Wagner D. The microRNA-regulated SBP-box transcription factor SPL3 is a direct upstream activator of LEAFY, FRUITFULL, and APETALA1. Dev Cell. 2009:17(2):268–278. 10.1016/j.devcel.2009.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky MF, Ma H, Bowman JL, Drews GN, Feldmann KA, Meyerowitz EM. The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature. 1990:346(6279):35–40. 10.1038/346035a0 [DOI] [PubMed] [Google Scholar]
- Yant L, Mathieu J, Dinh TT, Ott F, Lanz C, Wollmann H, Chen X, Schmid M. Orchestration of the floral transition and floral development in Arabidopsis by the bifunctional transcription factor APETALA2. Plant Cell. 2010:22(7):2156–2170. 10.1105/tpc.110.075606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo M, Soltis PS, Soltis DE. Expression of floral MADS-box genes in two divergent water lilies: nymphaeales and nelumbo. Int J Plant Sci. 2010:171(2):121–146. 10.1086/648986 [DOI] [Google Scholar]
- Yu D, Kotilainen M, Pöllänen E, Mehto M, Elomaa P, Helariutta Y, Albert VA, Teeri TH. Organ identity genes and modified patterns of flower development in Gerbera hybrida (Asteraceae). Plant J. 1999:17(1):51–62. 10.1046/j.1365-313X.1999.00351.x [DOI] [PubMed] [Google Scholar]
- Zahn LM, Feng B, Ma H. Beyond the ABC-model: regulation of floral homeotic genes. In: Soltis DE, Leebens-Mack JH and Soltis PS eds., Advances in botanical research, developmental genetics of the flower. London: Academic Press; 2006. p. 163–207. [Google Scholar]
- Zhang Z, Coenen H, Ruelens P, Hazarika RR, Al Hindi T, Oguis GK, Vandeperre A, van Noort V, Geuten K. Resurrected protein interaction networks reveal the innovation potential of ancient whole-genome duplication. Plant Cell. 2018:30(11):2741–2760. 10.1105/tpc.18.00409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Pan L, Guo W, Li Y, Wang W. A convergent mechanism of sex determination in dioecious plants: distinct sex-determining genes display converged regulation on floral B-class genes. Front Plant Sci. 2022:13:953445. 10.3389/fpls.2022.953445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Chen Z, Liu X, Che G, Gu R, Zhao J, Wang Z, Hou Y, Zhang X. CsLFY is required for shoot meristem maintenance via interaction with WUSCHEL in cucumber (Cucumis sativus). New Phytol. 2018:218(1):344–356. 10.1111/nph.14954 [DOI] [PubMed] [Google Scholar]
- Zhao L, Kim Y, Dinh TT, Chen X. Mir172 regulates stem cell fate and defines the inner boundary of APETALA3 and PISTILLATA expression domain in Arabidopsis floral meristems. Plant J. 2007:51(5):840–849. 10.1111/j.1365-313X.2007.03181.x [DOI] [PMC free article] [PubMed] [Google Scholar]