Abstract
Purpose: We wanted to study whether an allogeneic melanoma lysate would be a feasible stimulatory antigen source for detection of a peripheral CD4+ T-cell immune response in patients with medically untreated malignant melanoma. The lysate was produced from a melanoma cell line (FM3.29) which expresses high amounts of melanoma antigens. Methods: Fresh peripheral blood was incubated with and without lysate for 6 h in the presence of anti-CD28/anti-CD49d MoAb (for costimulation). After flow cytometric estimation of the frequency of CD69+/IFN-γ+ cells in the CD4+ population, the response to lysate was calculated as the difference between the number of activated IFN-γ-producing CD4+ cells in the lysate-stimulated and the nonstimulated sample. Results: An immune response to lysate was observed in blood samples from 11 of 15 patients (73%) with metastatic melanoma. A weak response was found in 1 of 4 patients radically operated for localized disease, whereas no responders were seen among 7 healthy donors. The fraction of circulating lysate-activated T cells ranged from 0.0037% to 0.080% of the CD4+ population. A negative result of the assay was found occasionally, especially in donors with high background levels of spontaneous IFN-γ production, indicating an inhibitory effect of the lysate. Conclusions: This method for detection of a peripheral T-cell immune response in melanoma patients has several advantages for clinical use. The tumor lysate preparations may contain large numbers of stimulating antigens (known, as well as unknown) and are easily prepared and handled. Potentially, the assay might be useful as a diagnostic tool, a marker of residual or recurrent disease, a prognostic factor, or a predictor or monitor of the effect of antineoplastic therapy including immune-modulating therapy.
Keywords: CD4+ T cells, Flow cytometry, Interferon γ, Malignant melanoma, Tumor lysate
Introduction
There is increasing evidence for a T-cell response against tumor antigens in some cancer patients and that tumor-reactive CD4+ or CD8+ T cells may circulate and be detectable in samples of peripheral blood [12]. Circulating tumor-reactive T cells might be a clinically useful source of information regarding the immunological response to cancer, as a diagnostic or prognostic tool, or as a monitor of treatment, and such T cells could possibly also be isolated, cultured [6], and reinfused for diagnostic or therapeutic purposes [9].
Previous studies have mainly focused on CD8+ T cells which are particularly interesting due to the direct tumor cell killing effect of CTLs [10]. However, it is increasingly recognized that CD4+ T cells may play a major role in tumor immunology. CD4+ T cells provide help during priming to achieve full activation and effector function of tumor-specific CTLs [7], and CD4+ T cells are required for CD8+ T cells to establish long-term specific immunity against tumors [29]. Moreover, in a mouse model, CD4+ effector T cells can suppress tumor completely independent of CD8+ T cells and in the absence of MHC class I and II molecules on the tumor cells [7].
The presence of antigen-specific circulating T cells in patients can be shown directly or indirectly. Direct detection can be done using tetramers of MHC class I and peptide [2, 4]. This approach, however, does require prior knowledge of the immunogenic peptide and its restricting allele [31]. Also, it is important to take into consideration that the tetramer technique in principle detects all T cells which bind the peptide-MHC complex, irrespective of the functional status of the cell [14, 20].
Methods for indirect detection of antigen-specific T cells usually employ culturing of T cells. However, assays involving long-term antigen and/or IL-2 stimulation are not ideal for assessing the in vivo situation. Selectively expanded T cell clones may not be representative [1, 8], and repeated or prolonged in vitro stimulation may result in quantitative and qualitative changes [28], including the possibility of in vitro priming and activation of naïve T cells. Alternatively, assays using short-term in vitro antigen stimulation followed by rapid detection of T-cell cytokine production may be applied. These include the ELISpot assay [16], quantitative PCR analysis for cytokine messenger RNA [11, 20], and flow cytometric detection of intracellular cytokines [19]. The flow cytometric technique is generally considered to be more sensitive than the ELISpot assay and enables the concurrent staining of individual cells with antibodies to multiple cytokines, surface markers, or intracellular proteins [31].
Most previous studies have used well-characterized tumor antigens for T-cell stimulation. However, tumor antigens expressed by human cancers are numerous [30] and probably for a large part unknown, and the significance of finding peripheral T cells reactive to one or a few defined tumor antigens is doubtful with respect to assessment of the individual patients’ polyclonal immune response to the tumor [31]. In the present study we used lysate made from a cultured allogeneic melanoma cell line as tumor antigen source. Allogeneic tumor lysate potentially contains a large number of specific, activating tumor antigens (known, as well as unknown), and is at the same time easily prepared and handled, not requiring access to autologous tumor. For assessment of a T-cell response, we used short-term coculture of lysate and fresh whole blood with costimulatory factors followed by flow cytometric analysis of CD4+ T cells for intracellular IFN-γ production.
Material and methods
Patients and controls
Patients were selected to represent the whole spectrum of melanoma disease stages. At the time of blood test they all showed good performance (WHO Performance Status 0–1); had histologically verified malignant melanoma; had not been treated with chemotherapy, radiotherapy, hormone therapy, or immunotherapy; had not received antihistaminic drugs or blood transfusions within 8 weeks; and had no known autoimmune disease, immune deficiency, or chronic or acute infection. Patients had experienced no other kinds of cancer, except for one (MM7) who had had a basocellular cutaneous carcinoma removed 3 years before the blood test. Clinical staging at the time of blood test was performed according to AJCC [3]. Seven healthy controls (3 males and 4 females) were tested simultaneously with the patients. A written informed consent was obtained for all persons tested. The study was approved by the local ethics committee (registration no. 20000275).
The course of the disease including recurrence or death and response to immunotherapy was assessed by reviewing medical records of all patients after a median follow-up of 600 days (range 201–662 days).
Tumor lysate
Lysate was prepared from the human melanoma cell line FM3.29 [13]. Cells were grown in RPMI 1640 plus 2% pooled human serum in tissue culture flasks. After reaching 70–90% confluence, medium was changed to RPMI 1640 without serum for 2 days. Cells were harvested by trypsinization (0.05% trypsin/EDTA), washed twice in RPMI 1640, resuspended in RPMI 1640, and adjusted to 10×106/ml. The cell suspension was placed in 50-ml tubes, 4–6 ml in each, and subjected to five rounds of freezing (in liquid nitrogen for 10–15 min) and thawing (in water bath, 37°C). The lysate was sonicated 15 min in an ultrasound bath and further centrifuged at 500 g for 15 min. The supernatant was transferred into Eppendorf tubes and centrifuged at 13,000 g for 60 min. Supernatants were collected in one tube and the lysate was filtered through a 0.2-μm filter, transferred to cryotubes, 0.5–1.0 ml/tube, and stored at –70°C.
The FM3.29 clone was chosen as a suitable T-cell-stimulatory agent based on a previous study comparing the in vitro immunization properties of several individual clones from the melanoma cell subline FM3.P, revealing a high expression in FM3.29 of known melanoma differentiation- and progression-associated antigens (including tyrosinase, MART-1/Melan-A, and gp100). Moreover, the cell line expresses MHC class I and class II molecules (including HLA-A2 and HLA-DR), and several adhesion molecules [13].
Flow cytometry
A sample of peripheral blood was drawn in sodium heparin and incubated for a total of 6 h at 37ºC and 5% CO2 with tumor cell lysate (333-μl lysate per ml blood). Serving as unstimulated control, one part of the sample was incubated with HBSS (Gibco, Life Technologies, Paisley, Scotland) instead of tumor cell lysate. Staining for intracellular IFN-γ was achieved using a FastImmune CD4 Intracellular Cytokine Detection kit (Becton Dickinson, San Jose, CA, USA), essentially as per the manufacturer’s instructions. During incubation, both the tumor lysate–exposed sample and the control sample were costimulated by the addition of 1 μg/ml anti-CD28 and anti-CD49d MoAbs. All samples were analyzed using a FACS Calibur flow cytometer (Becton Dickinson) within 24 h of staining. For each sample, 20,000–100,000 gated lymphocytes (50,000–800,000 total events) were collected. Data analysis was performed using FlowJo software (Tree Star, San Carlos, CA, USA).
The T-cell response was assessed by the frequency of CD69+ and IFN-γ+ cells in the CD4+ population. In many samples, false positive events were present. These events, which are a known phenomenon in this kind of analysis [19], were characterized by their fluorescence in both the FL-1 and the FL-2 channel in approximately equal amounts, therefore forming a straight, diagonal line in FL-1/FL-2 dotplots, it being observed whether cells were exposed to the relevant antibodies or to the isotype control mixture. When we backgated the false positive population, the events turned out to have a diffuse distribution in a forward scatter / side scatter dotplot not colocalizing with any of the well-known cell types (data not shown). Nomura et al. [19] reported that most false positive events could be removed by omitting CD62P-positive and CD33-positive cells from the analysis. To this end, these authors included a fourth fluorochrome color for the two additional antibodies, and subsequently used the fourth fluorescence channel to exclude the cell types mentioned. In the present study, we used the characteristic distribution of the false positive events in FL1/FL2 dotplots to simply make an exclusion gate in a cell sample stained with the relevant FITC/PE-conjugated isotype controls. This gate was subsequently applied to the sample from the same patient stained with the anti-CD69 and anti-IFN-γ antibodies.
A gate for enumerating CD69+ and IFN-γ+ cells was set on the FITC/PE dotplot of a cell sample stained with the FITC/PE-conjugated isotype controls (false positive events removed), using a best-fit algorithm to include 0.2% FITC-positive and PE-positive events. To arrive at a corrected value for CD69+ IFN-γ+ cells, the exact percentage of positive events found in the isotype-stained sample was later subtracted from the percentage of positive events found in the sample stained with the anti-CD69 and anti-IFN-γ antibodies. The T-cell response to tumor lysate was calculated as the difference between the corrected percentage of activated CD4+ T cells seen in lysate-stimulated and in nonstimulated samples. Flow cytometric data were analyzed without knowledge of clinical data of patients.
Results
Clinical data and a summary of results of the flow cytometric analyses of 19 melanoma patients and 7 healthy individuals are shown in Table 1. Four patients were in AJCC stage I, eight in stage III, and seven in stage IV. The median age was 57 years (range 22–77 years). Eleven patients had clinically overt disease at the time of blood test. The remaining were clinically disease free and for those, the interval between radical operation and blood test ranged from 16 days to 63 days with an average of 38 days. For patients with metastatic disease (i.e., stages III and IV), the interval from histological diagnosis of melanoma to the blood test ranged from 14 days to 1,364 days with a median of 680 days.
Table 1.
Clinical and flow cytometric data for malignant melanoma patients (MM) and normal controls (N). M male, F female, IL-2 interleukin 2, IFN-α interferon α, WBI low-dose whole-body irradiation, DC vac. dendritic cell vaccination, PD progressive disease, NE not evaluable, PR partial response
| Patient code | AJCC stage of disease | Sex | Age in years | Melanoma-free interval in daysa | Days with diagnosisb | Spontaneous activity (%)c | Response to lysate (%)d | Response to lysate with spontaneous activity subtracted (%)e | Lysate-CD4+ T-cell response | Immunotherapy after blood sample | Best response to immunotherapy | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MM5 | 1 | F | 51 | 19 | 19 | 0.32 | 0.053 | −0.26 | - | Disease free day 642 | ||
| MM2 | I | M | 67 | 16 | 16 | 0.66 | 0.54 | −0.12 | - | Disease free day 662 | ||
| MM17 | I | F | 56 | 48 | 48 | 0.11 | 0.0080 | −0.10 | - | Disease free day 593 | ||
| MM13 | I | F | 63 | 28 | 28 | 0.031 | 0.035 | 0.0037 | + | Disease free day 595 | ||
| MM11 | III | F | 30 | 0 | 25 | 0.054 | −0.014 | −0.054 | - | Disease free day 602 | ||
| MM7 | III | M | 68 | 21 | 1,163 | −0.011 | 0.020 | 0.020 | + | Recurrence day 247, died day 297 | ||
| MM14 | III | M | 34 | 0 | 106 | 0.042 | 0.063 | 0.021 | + | IL-2 | PD | Recurrence day 226, died day 596 |
| MM16 | III | F | 57 | 63 | 1,364 | 0.070 | 0.11 | 0.037 | + | Adjuvant IFN-α | Disease free day 595 | |
| MM10 | III | M | 28 | 0 | 14 | 0.088 | 0.13 | 0.044 | + | Disease free day 602 | ||
| MM3 | III | M | 78 | 0 | 210 | 0.063 | 0.12 | 0.055 | + | Died day 100 | ||
| MM8 | III | F | 72 | 54 | 1,098 | 0.070 | 0.13 | 0.060 | + | Disease free day 635 | ||
| MM1 | III | F | 70 | 58 | 1,243 | 0.66 | 0.74 | 0.080 | + | Recurrence day 137, died day 469 | ||
| MM21 | IV | F | 69 | 0 | 319 | 0.78 | 0.30 | −0.47 | - | IL2+WBI | PD | Alive with disease day 252 |
| MM18 | IV | M | 68 | 0 | 246 | 0.036 | 0.021 | −0.015 | - | IL-2 | NE | Died day 65 |
| MM20 | IV | M | 23 | 0 | 364 | 0.0044 | −0.0011 | −0.0043 | - | IL-2+WBI | PD | Died day 52 |
| MM12 | IV | M | 38 | 0 | 964 | 0.023 | 0.030 | 0.0070 | + | IL-2+ IFN-α, DC vac. | PD | Alive with disease day 600 |
| MM22 | IV | M | 52 | 0 | 1,287 | 0.016 | 0.026 | 0.010 | + | IL-2+WBI, DC vac. | PD | Alive with disease day 201 |
| MM19 | IV | M | 33 | 0 | 1,112 | −0.012 | 0.024 | 0.024 | + | IL-2, DC vac. | PR | Alive with disease day 294 |
| MM4 | IV | M | 63 | 0 | 680 | 0.039 | 0.11 | 0.072 | + | Alive with disease day 656 | ||
| N5 | F | 41 | 0.15 | 0.068 | −0.082 | - | ||||||
| N1 | M | 27 | 0.12 | 0.056 | −0.064 | - | ||||||
| N4 | F | 48 | 0.053 | 0.012 | −0.041 | - | ||||||
| N2 | F | 24 | 0.072 | 0.039 | −0.033 | - | ||||||
| N7 | M | 50 | 0.021 | −0.0050 | −0.021 | - | ||||||
| N3 | F | 46 | 0.047 | 0.027 | −0.020 | - | ||||||
| N6 | F | 52 | 0.0030 | −0.024 | −0.0030 | - |
aInterval between blood test and radical operation. The interval is 0 if patients had clinically overt disease at the time of blood test
bInterval between first histological diagnosis of melanoma and blood test
cThe corrected percentage of IFN-γ+ CD69+ cells among CD4+ lymphocytes upon incubation with control medium
dThe corrected percentage of IFN-γ+ CD69+ cells among CD4+ lymphocytes upon 6-h incubation with FM3.29 tumor lysate
eThe corrected percentage of IFN-γ+ CD69+ cells among CD4+ lymphocytes upon incubation with FM3.29 tumor lysate with the corrected percentage of spontaneously IFN-γ-producing cells subtracted, if above zero
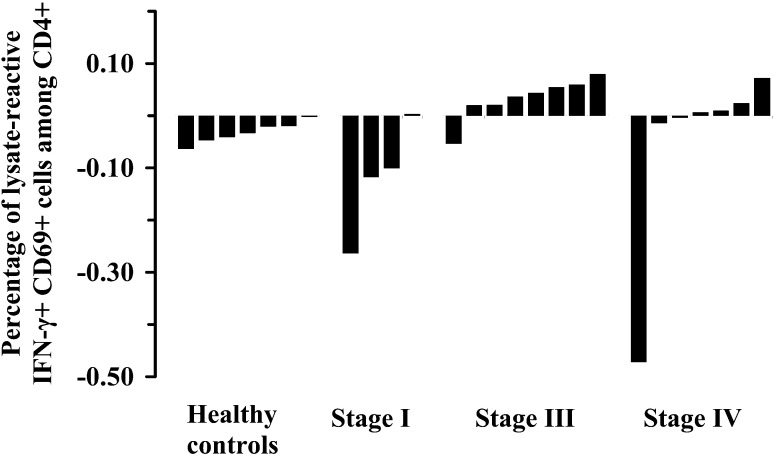
The CD4+ T-cell response to tumor lysate for healthy donors and for melanoma patients grouped according to stage of disease is shown in Fig. 1. For each patient two samples were analyzed: one to which tumor lysate had been added and one control sample without lysate. The patient’s CD4+ T-cell response to the lysate was assessed by subtracting the percentage of IFN-γ+ cells in the control sample from the percentage of IFN-γ+ cells in the lysate-stimulated sample. In general, tumor lysate appeared to have an inhibitory effect, because if no or a few CD4+ cells were induced to produce IFN-γ by tumor lysate in a given sample, the calculated lysate response would most likely become negative. This effect was particularly evident in samples from individuals presenting a substantial frequency of spontaneous IFN-γ-producing cells (see Table 1). On the other hand, the positive responses found in patients, presumably overcame this apparent suppression.
Fig. 1.
T-cell response to melanoma lysate: the fraction of FM3.29-lysate–activated CD4+ T cells in blood samples from healthy controls and malignant melanoma patients, grouped according to stage of disease. Values above zero are interpreted as lysate–T-cell response
The frequency of lysate-reactive CD4+ T cells in responding melanoma patients ranged from 0.0037% to 0.080% with a median of 0.024% of the CD4+ cell population. None of the healthy subjects showed a response, whereas 12 out of 19 melanoma patients (63%) had a response. Response was observed most often in patients with metastatic melanoma—in 11 of 15 (73%)—and especially in stage III patients (88%). One responder was found in four individuals treated by radical excision of stage I melanomas. However, the response was quantitatively weak (approximately half the value of the weakest responder with metastatic disease) and possibly of marginal significance. The responding patients with metastatic malignant melanoma had a significantly longer interval between diagnosis and blood test compared with the nonresponders (mean 840 days versus 239 days). No clear association between CD4+ T-cell response and age or sex was observed.
During follow-up, no recurrences or deaths occurred among stage I patients, whereas six patients with metastatic melanoma expired from their disease. Among stage IV patients, two out of three nonresponders rapidly died, whereas none of four responders has expired. The actuarial 1-year-survival for stage III and IV melanoma patients was 88% among responders and 66% among nonresponders. These differences were not statistically significant, however. The majority of patients with advanced disease received IL-2–based immunotherapy, however, resulting in only one transient partial remission of disease in the present cohort.
Discussion
The present study of a heterogeneous population of malignant melanoma patients and healthy donors demonstrates the feasibility of a simple technique for detecting circulating tumor-reactive T cells, not dependent on access to specific tumor antigens or autologous tumor material.
Most studies aiming at detecting the in vivo presence of tumor-reactive T cells in peripheral blood have employed single, well-characterized, tumor-associated peptides as activating antigen source. The results of these studies suggest that detection of peripheral tumor-reactive T cells might be used as evidence of malignancy and their numbers might be correlated with antitumor immunity [11, 14, 17, 24]. For example, using the ELISpot IFN-γ assay, Nagorsen et al. found that peptide-specific CD8+ T cells against Ep-CAM, HER-2/neu, or CEA could be detected in 32% of 22 patients with colorectal cancer, but not in 8 healthy subjects [17]. Schmittel et al. showed, using a similar technique, that peripheral CD8+ T cells against tyrosinase could be induced in melanoma patients by immunotherapy or chemoimmunotherapy and loss of these T cells was associated with clinical relapse of disease [24]. Circulating tyrosinase-reactive T cells were especially frequent in melanoma patients with clinical response to IL-2–based therapy [23]. Using tetramer staining, Lee et al. detected a circulating population of CD8+ T cells recognizing the HLA-A2–restricted epitopes of Melan-A/MART-1 and tyrosinase in 55% of 11 patients with metastatic malignant melanoma [14]. Similarly, Pittet et al. found high frequencies of Melan-A/MART-1–specific CD8+ T cells in 77% of 13 patients with melanoma, but also in 6 of 10 healthy HLA-A*0201+ individuals [21]. These cells were, however, of the naïve phenotype and do not therefore reflect immune activation by antigen. Recently, Speiser et al. found that a fraction of Melan-A/MART-1–specific T cells in the peripheral blood of patients with advanced melanoma were nonnaïve, revealing tumor-driven immune activation [25].
It should be stressed, however, that if the aim is to assess the polyclonal tumor-specific T-cell response in a patient, the evaluation of T-cell response to one or a few specific peptides is probably insufficient [31]. First, the biological relevance of peripheral T cells being activated upon coincubation with a specific tumor-antigenic peptide in an individual is unknown, in part because no exclusively tumor-restricted antigenic peptide has been found, and in part because some “tumor-associated” peptides activating T cells in vitro might not be presented or processed naturally [27]. Secondly, it is believed that the currently known tumor antigens only represent a minority of antigenic peptides produced by tumors [30], and even when defined antigens are known, there is no guarantee that they will be expressed by a given patient’s tumor or metastases [32].
Hence, for tumors that may not express known, defined tumor-associated antigens and for evaluation of a broader pool of in vivo tumor-reactive T-cell clones, alternative sources of tumor antigens must be employed. These include preparations of whole tumor cells [15], tumor cell apoptotic bodies [5], total tumor peptides [18], or tumor lysate [22]—and may come from autologous tumor [32], from tissue-type–matched allogeneic tumor [15], or from nonmatched allogeneic tumor [5, 18, 22].
In a previous, pioneering study, Letsch et al. used allogeneic HLA-A2- or HLA-A1-matched cultured melanoma cells from several cell lines as antigen source for rapid identification of circulating melanoma-reactive mononuclear cells. In an ELISpot IFN-γ assay, they found a significant immune response in the blood from 11 of 19 patients (58%) with metastatic melanoma, whereas healthy subjects had no or few activated cells [15]. Compared with these results, the findings of the present study seem encouraging as we—using allogeneic tumor cell lysate from a single melanoma cell line—showed a CD4+/IFN-γ T-cell response in 11 out of 15 patients (73%) with metastatic melanoma. It seems likely that careful selection of additional melanoma cell lines would further increase the frequency of responders.
In a few patients with high background levels of spontaneous T-cell IFN-γ production a profound negative effect was obtained, as the T-cell IFN-γ response detected in the lysate-stimulated samples was less than the spontaneous T-cell IFN-γ production. It may be argued that such samples would be less suited for assessment of a tumor lysate–specific response per se and should be excluded from analysis in future studies of the technique. That a sizeable spontaneous T-cell IFN-γ production very often leads to a negative result indicates that addition of the lysate may inhibit the cytokine production, and it is possible that such an effect also reduces the magnitude of any specific response to tumor antigen present in the lysate. The likely inhibitory effect of lysate may be reduced by optimizing the culture conditions including the composition of lysate.
Although the CD4+ T cells of most melanoma patients produced IFN-γ upon short-time coincubation with tumor lysate in vitro, the present study does not provide definite proof that these cells are in fact tumor-reactive in vivo. It is possible that the responses seen could be mediated by alloreactive T cells being activated by polymorphic gene products present in the melanoma cell lysate. Alternatively, the T cells could be reacting with non-tumor-associated peptides which are normally sequestered from the immune system or for other reasons do not give rise to T-cell activation in vivo. We do not find these explanations likely, however, as most stage I patients and all healthy donors showed no response at all. Moreover, the very short incubation time (6 h in total) and the treatment of cell lysate by high-speed centrifugation and sonification should diminish the risk of inducing an alloresponse.
In contrast to the frequent occurrence of response in metastatic melanoma we found only one marginal responder among four patients with stage I melanoma. Similarly, Nagorsen et al. reported no tumor-antigen-specific, circulating T cells in nine early stage colorectal cancer patients of whom eight had been operated on several months prior to the test, whereas more than half of the patients with local or distant metastases showed a response [17]. This apparent correlation between disease extent and presence of circulating tumor antigen–reactive T cells may reflect a scarcity or absence of these cells in localized cancers. Accordingly, it has been suggested that evasion of tumor cells in lymph nodes is a prerequisite for the induction of a tumor antigen–specific T-cell response in patients [17].
An alternative hypothesis linking disease stage with detectable tumor-reactive T cells in the circulation is that in vivo presence of antigen may be required for T cells to become activated in assays using short-term culture. It may follow from this that patients cured by surgery will show negative results after some time. Hence, previous studies suggest that quickly responding T cells are either effector T cells or a special category of memory T cells called “activated” or “cycling” memory T cells [26]. Both effector T cells and activated memory T cells may retain and require T-cell receptor contact with small quantities of specific antigen and disappear within days or weeks, respectively, if antigen is not present [26]. Mathematical modeling suggests that activated memory T cells comprise 95% of the memory pool with persistent, low-dose antigen exposure [4]. If this is valid then the current method may be useful as an “immunological tumor marker”. Indeed, data of the present study showing a correlation between response and disease stage and between response and the period patients might have been exposed to tumor antigens in vivo (roughly estimated by the interval between primary melanoma diagnosis and blood test) fits well with this theory. Together with the suggested more favorable prognosis of metastatic melanoma in responders this may additionally indicate a more indolent course of disease and/or an increased effect of treatment in responders. However, the limited number of patients and short follow-up of the current study do not allow any definite conclusions with respect to prognostic issues.
In the present study, we have shown that a tumor lysate produced from cultured allogeneic melanoma cells could induce a CD4+ T-cell response, easily detectable in peripheral blood samples in the majority of patients with metastatic melanoma, but absent in normal controls. The assay should be methodologically optimized and then thoroughly tested with respect to reliability and reproducibility. Additionally, it must be elucidated in clinical studies, whether the assay might be useful as a diagnostic tool, a marker of residual or recurrent disease, a prognostic factor, or a predictor or monitor of effect of antineoplastic therapy including immune-modulating therapy.
References
- 1.Aebersold Hum Gene Ther. 1990;1:373. doi: 10.1089/hum.1990.1.4-373. [DOI] [PubMed] [Google Scholar]
- 2.Altman Science. 1996;274:94. [PubMed] [Google Scholar]
- 3.American Joint Committee on Cancer (2001) AJCC cancer staging manual, 5th edn. Lippincott-Raven, Philadelphia
- 4.Bucharov Immunol Cell Biol. 2001;79:74. doi: 10.1046/j.1440-1711.2001.00985.x. [DOI] [PubMed] [Google Scholar]
- 5.Chang Anticancer Res. 2000;20:1329. [PubMed] [Google Scholar]
- 6.Dunbar Curr Biol. 1998;8:413. doi: 10.1016/s0960-9822(98)70161-7. [DOI] [PubMed] [Google Scholar]
- 7.Egilmez Cancer Res. 2002;62:2611. [PubMed] [Google Scholar]
- 8.Faure Crit Rev Immunol. 1998;18:77. doi: 10.1615/critrevimmunol.v18.i1-2.90. [DOI] [PubMed] [Google Scholar]
- 9.Griffith, J Natl Cancer Inst. 1989;81:1709. doi: 10.1093/jnci/81.22.1709. [DOI] [PubMed] [Google Scholar]
- 10.Hanson Immunity. 2000;13:265. [Google Scholar]
- 11.Kammula J Natl Cancer Inst. 2001;92:1336. doi: 10.1093/jnci/92.16.1336. [DOI] [PubMed] [Google Scholar]
- 12.Kawakami Immunol Res. 1997;16:313. doi: 10.1007/BF02786397. [DOI] [PubMed] [Google Scholar]
- 13.Kirkin Cancer Immunol Immunother. 1999;48:239. doi: 10.1007/s002620050571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Nat Med. 1999;5:677. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 15.Letsch Int J Cancer. 2000;87:659. doi: 10.1002/1097-0215(20000901)87:5<659::AID-IJC7>3.3.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 16.McCutcheon J Immunol Methods. 1997;210:149. doi: 10.1016/s0022-1759(97)00182-8. [DOI] [PubMed] [Google Scholar]
- 17.Nagorsen Cancer Res. 2000;60:4850. [PubMed] [Google Scholar]
- 18.Nair Eur J Immunol. 1997;27:589. doi: 10.1002/eji.1830270304. [DOI] [PubMed] [Google Scholar]
- 19.Nomura Cytometry. 2000;40:60. doi: 10.1002/(SICI)1097-0320(20000501)40:1<60::AID-CYTO8>3.0.CO;2-J. [DOI] [Google Scholar]
- 20.Panelli Expert Opin Biol Ther. 2002;2:537. doi: 10.1517/14712598.2.5.557. [DOI] [PubMed] [Google Scholar]
- 21.Pittet J Exp Med. 1999;190:705. doi: 10.1084/jem.190.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santin Am J Obstet Gynecol. 2000;183:601. doi: 10.1067/mob.2000.107097. [DOI] [PubMed] [Google Scholar]
- 23.Scheibenbogen Int J Cancer. 2002;98:409. doi: 10.1002/ijc.10205. [DOI] [PubMed] [Google Scholar]
- 24.Schmittel Int J Cancer. 2001;80:39. doi: 10.1002/(SICI)1097-0215(19990105)80:1<39::AID-IJC8>3.3.CO;2-P. [DOI] [Google Scholar]
- 25.Speiser Eur J Immunol. 2002;32:731. doi: 10.1002/1521-4141(200203)32:3<731::AID-IMMU731>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 26.Sprent Curr Opin Immunol. 2001;13:248. doi: 10.1016/S0952-7915(00)00211-9. [DOI] [PubMed] [Google Scholar]
- 27.thor Cancer Immunol Immunother. 1999;34:386. doi: 10.1007/s002620050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.thor Clin Immunol. 2000;96:94. doi: 10.1006/clim.2000.4890. [DOI] [PubMed] [Google Scholar]
- 29.Van J Exp Med. 1986;164:1547. doi: 10.1084/jem.164.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J Mol Med. 1999;77:640. doi: 10.1007/s001099900042. [DOI] [PubMed] [Google Scholar]
- 31.Yee Curr Opin Immunol. 2001;13:141. doi: 10.1016/S0952-7915(00)00196-5. [DOI] [PubMed] [Google Scholar]
- 32.Zier J Immunol Methods. 2000;241:61. doi: 10.1016/S0022-1759(00)00193-9. [DOI] [PubMed] [Google Scholar]