Abstract
Functional dendritic cells (DC) are professional antigen presenting cells (APC) and can be generated in vitro from leukemic cells from acute myeloid leukemia AML patients, giving rise to APC of leukemic origin presenting leukemic antigens (DCleu). We have already shown that DC can be successfully generated from AML and myeloplastic syndromes (MDS) cells in serum-free ‘standard’ medium (X-vivo + GM-CSF + IL-4 +TNFα + FL) in 10–14 days. In this study, we present that DC counts generated from mononuclear cells (MNC) varied between 20% (from 55 MDS samples), 34% (from 100 AML samples) and 25% (from 38 healthy MNC samples) medium. Between 53% and 58% of DC are mature CD83+ DC. DC harvests were highest in monocytoid FAB types (AML-M4/M5, MDS-CMML) and independent from cytogenetic risk groups, demonstrating that DC-based strategies can be applied for patients with all cytogenetic risk groups. Proof of the clonal derivation of DC generated was obtained in five AML and four MDS cases with a combined FISH/immunophenotype analysis (FISH-IPA): The clonal numerical chromosome aberrations of the diseases were regularly codetectable with DC markers; however, not with all clonal cells being convertible to leukemia-derived DCleu (on average, 53% of blasts in AML or MDS). To the contrary, not all DC generated carried the clonal aberration (on average, 51% of DC). In 41 AML and 13 MDS cases with a suitable antigen expression, we could confirm FISH-IPA data by Flow cytometry: although DCleu are regularly detectable, on average only 57% of blasts in AML and 64% of blasts in MDS were converted to DCleu. After coculture with DC in mixed lymphocyte reactions (MLR), autologous T cells from AML and MDS patients proliferate and upregulate costimulatory receptors. The specific lysis of leukemic cells by autologous T cells could be demonstrated in three cases with AML in a Fluorolysis assay. In six cases with only few DCleu or few vital T cells available after the DC/MLR procedure, no lysis of allogeneic or autologous leukemic cells was seen, pointing to the crucial role of both partners in the lysis process. We conclude: (1) the generation of DC is regularly possible in AML and also in MDS under serum-free conditions. (2) Clonal/leukemia-derived DCleu can be regularly generated from MDS and AML-MNC; however, not with all blasts being converted to DCleu and not all DC generated carrying leukemic markers. We recommend to select DCleu for vaccinations or ex vivo T-cell activations to avoid contaminations with non-converted blasts and non-leukemia-derived DC and to improve the harvest of specific, anti-leukemic T cells. DC and DC-primed T cells could provide a practical strategy for the immunotherapy of AML and MDS.
Keywords: Dendritic cells, Myelodysplastic syndromes, Acute myeloid leukemia, Serum free culture, Leukemia Derived DC
Introduction
Acute myeloid leukemia (AML) as well as myeloplastic syndromes (MDS) are clonal disorders of hematopoietic stem cells characterized by an impaired normal cell differentiation [24, 30]. About 70% of successfully treated AML patients relapse soon and about 20–50% of intermediate or high-risk MDS patients respond only transiently to chemotherapies [2, 10]. Treatment options for MDS patients are limited due to the, on average, high age of the patients. There is a need for less intensive (post-remission) immunotherapies to maintain stable remissions in AML patients and at least a stable disease in MDS patients. Leukemic blasts are characterized by the expression of myeloid antigens, for instance CD33, CD13 and CD117, often together with lymphoid antigens (e.g. CD56 and CD2) in AML [12] or CD34, CD117 and CD36 in MDS [20, 40]. An insufficient expression of costimulatory antigens, MHC molecules and tumor-associated antigens (TAA) on the surface of cancer cells and disturbed mechanisms of apoptosis are the main reasons for an ineffective immune response in oncological diseases [46]. As professional APC-presenting cells, DC specifically stimulate T-effector cells, especially tumor-cytotoxic T cells [7, 14]. Therefore, they are regarded as interesting candidates for anti-tumor or anti-leukemic vaccination strategies [42, 17]. Special homing antigens for instance CD62L and CD197 were recently detected as important antigens mediating the migration of DC and T cells to lymphoid organs: only differentiated, mature DC are finally able to trigger potent cytotoxic T-cell responses [23, 35, 48]. DC can be generated in vitro from CD14+ monocytes or from CD34+ progenitor cells in the presence of GM-CSF, TNFα and IL-4 and be pulsed with tumor peptides, tumor lysates, tumor-DNA/ RNA or fused with tumor cells [23]. For therapeutical purposes, a great amount of mature DC—the most effective DC—have to be generated under foetal calf serum (FCS)-free culture conditions to avoid anaphylactic complications [38]. In contrast to solid tumors, DCleu can be generated without antigen pulsing by differentiating leukemic cells from AML patients in vitro directly to mature APC, giving rise to leukemic cells co-presenting DC-typical antigens (e.g. CD40, CD86, CD80, CD1a and CD83) [54, 13, 16]. We have already shown that DC can be generated from AML and MDS patients’ mononuclear cell (MNC) samples under serum-free culture conditions using ‘standard’ cytokines (GM-CSF, IL-4, TNFα) and in addition, FL to increase the DC harvest [34, 38, 63].
The aim of our study was to generate, characterize and quantify leukemia-derived DC from AML and in addition from MDS-MNC. The influence of FAB types or cytogenetic risk groups on DC harvests as well as the ability of DC to activate autologous (anti-leukemia-directed) T cells and in addition the influence of PGE2-containing ‘MCM-Mimic’ medium to increase proportions of CCR7 (CD197+) DC were evaluated.
Materials and methods
Patients’ characteristics, sample collection and diagnosis
Mononuclear cells (MNC) from heparinized blood (PB) or bone marrow (BM) (PB-MNC, BM-MNC) were isolated from the interphase by density-gradient centrifugation (Ficoll-Hypaque, Biochrom, Germany), washed and suspended in PBS without Ca2+ and Mg2+ (Biochrom). Diagnosis of AML and MDS cases was based on the French-American-British (FAB) classification [4, 5]. Samples were collected in active stages of the disease from 100 AML patients (83 at first diagnosis, 7 in persisting disease and 10 at relapse), from 55 MDS patients as well as from 38 healthy donors after obtaining informed consent (Table 1). The median age of the AML Patients was 56 years (range: 20–76 years), the female:male ratio was 1:1.8. The median age of the MDS patients was 65 years, the female:male ratio 1:1. Cytogenetic analyses were performed according to the standard protocols and criteria defined by the International System for Human Cytogenetic Nomenclature [41]. Patients were categorized in risk groups as described [26, 27]: ‘Favorable risk’ AML patients had presented with a t(8;21), t(15;17), inv(16), t(16;16); ‘poor risk’ AML patients with −5/5q-, −7/7q-, t(11q23), inv(3), t(3;3), 17p abnormalities or a complex aberrant karyotype (=three abnormalities); ‘intermediate risk’ AML patients had presented with a normal karyotype or with any of the remaining aberrations. ‘Favorable risk’ MDS patients had presented with a normal karyotype, a del(5q) only, a del(20q) only or a – Y only; ‘poor risk’ MDS patients had presented with −7/7q aberrations or a complex aberrant karyotype; ‘intermediate risk’ patients were MDS patients with any of the remaining aberrations.
Table 1.
Characteristics of patients with AML/MDS (a) and of leukemic cell lines (b)
| Diagnosis | FAB type | n | Cytogenetic risk | NA | |||
|---|---|---|---|---|---|---|---|
| Favorable (n) | Interm. (n) | Bad (n) | |||||
| (a) | AML | sM | 2 | 1 | 1 | ||
| (n=100) | pM0 | 12 | 6 | 1 | 5 | ||
| sM0 | 1 | 1 | |||||
| pM1 | 17 | 13 | 4 | ||||
| sM1 | 4 | 2 | 2 | ||||
| pM2 | 19 | 3 | 13 | 3 | |||
| sM2 | 10 | 4 | 1 | 5 | |||
| pM3v | 2 | 2 | |||||
| pM4 | 11 | 7 | 2 | ||||
| sM4 | 3 | 3 | |||||
| pM4eo | 3 | 3 | 2 | ||||
| pM5 | 12 | 4 | 3 | 5 | |||
| sM5 | 2 | 1 | 1 | ||||
| sM6 | 2 | 2 | |||||
| MDS | MDS | 3 | 1 | 3 | |||
| (n=55) | RA | 8 | 7 | 1 | |||
| RAS | 2 | 1 | 1 | ||||
| RAEB | 22 | 9 | 4 | 2 | 7 | ||
| RAEBt | 18 | 9 | 1 | 2 | 6 | ||
| CMML | 2 | 1 | 1 | ||||
| Diagnosis | Name | FAB type | Clonal marker | Blast phenotype (CD) | |
|---|---|---|---|---|---|
| (b) | AML | HL60 | p M2 | -X,−8,−16,−17,+18,+22,ins(1;8)(p?;q), t(5;17)(q;q),del(9)p(13)t(9;14) (q;q),t(9;14)(q;q),t(16;17)(q;q),sideline with:−2,−5,−15,del11(q;q) | 13, 33, 15, 4 |
| Kazumi | p M2 | −9,−13,−16,t(8;21)(q;q),t(9;?)(p22;?) t(?9)(p22;?)t(?9;15)(p;q) | 13, 33, 34, 15, 4 | ||
| Mutz-3 | p M4 | t(1;3)(q;q),inv(3)(q;q),t(2;7)(q;q),inv(7)(p;q),t(12;22)(p;q) | 13, 34, 15, 65,68 | ||
| MDS | Mutz-1 | pRAEB | ,add(1p),(2;?11)(qq),t(3;5)(q;q),t(5;22)(q;q), del(3p),del(3q),del(5q),t(3;6)(p;p),t(7;11)(q;q),t(1;8)(q;q),add(9q),del(9p),add(14p);t(5;15)(p;p),t(7;16)(q;q),add(18p),add(21p) | Dr, 56, 19 |
NA not available
In addition, DC were generated from four different AML or MDS cell lines: Kazumi and HL60 (AML-M2), Mutz-3 (AML-M4) and Mutz-1 (MDS-RAEB).
DC generation
MNC were incubated in 12-well multiwell tissue culture plates in Xvivo 15 (BioWhittaker, Europe) FCS-free medium at a concentration of 2.5×106 cells/ml for 7 (healthy samples) −14 days (AML/MDS samples), containing 800 U/ml GM-CSF (Sandoz, Germany), 500 U/ml IL-4 (CellConcepts, Germany), 40 ng/ml FL (FLT3-Ligand, PromoCell, Germany) and for the last 2 days 200 U/ml TNFα (CellConcepts). In parallel experiments, DC were generated in ‘MCM-Mimic’ medium containing 800 U/ml GM-CSF, 500 U/ml IL-4, 10 ng/ml IL-1β (CellConcepts), 1,000 U/ml IL-6 (CellConcepts), 200 U/ml TNFα, 1 μg/ml PGE2 (Pharmacia biotech, Germany) and in addition 40 ng/ml FL. Half medium exchange was performed every 4–7 days with fresh cytokine-supplemented medium. After 7–14 days in culture, DC were harvested for subsequent experiments. If available, autologous CD3+ cells were positively selected using anti-CD3 antibodies and immunomagnetic MACS separation according to the manufacturer’s instructions (Miltenyi Biotec, Germany) and used for mixed lymphocyte reactions. The purity of CD3+ cells was 98%.
Flow cytometry
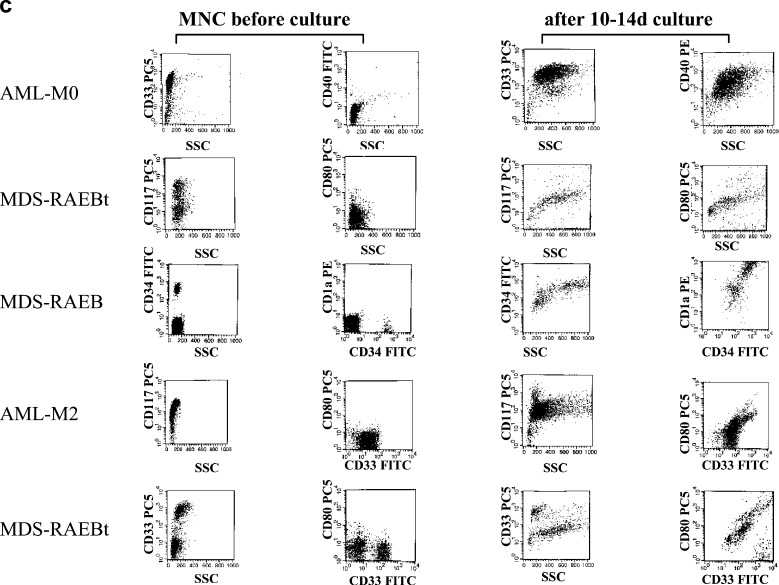
Flow cytometric analyses with a panel of mouse monoclonal antibodies (moAbs) directly conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE) or with tandem Cy5-PE conjugation (PC5) were performed to evaluate and quantify amounts and phenotypes of leukemic cells, DC, B cells, T cells and NK cells in the PB/BM samples analyzed. Antibodies were purchased from Beckman Coulter*, Becton Dickinson**, R&D***, Serotec**** and Biozol***** (CD1a* ; CD3* [T3]; CD14* [LPS-R]; CD15* [Lewis X]; CD19* [B4]; CD28* [CD80/86-ligand]; CD33* ; CD34* ; CD40* ; CD56* [N-CAM]; CD71* [Transferrin receptor]; CD80**** [B7.1]; CD83* [HB15]; CD86**** [B7.2]; CD117* [c-kit]; CD135* [FLT3]; CD137** [4-1BB]; CD137L***** [4-1BBL]; CD152* [CTLA-4]; CD154** [CD40L]; CD197*** [CCR7]; CD209*** [DC-sign]; HLA-Dr* [MHC class II]; 7.1* [NG2-antigen]). In MDS cases, amounts of CD34+ cells were regularly determined and analyzed to evaluate the amounts of undifferentiated cells/blasts [12, 10]. The MNC or cultured cells were suspended in PBS with 20% FCS (Biochrome) and incubated with moAbs according to the manufacturer’s instructions. Appropriate isotype controls were used and at least 5,000 events were evaluated on a FACS Calibur Flow Cytometer (Becton Dickinson, Heidelberg, Germany) using the Cell Quest data acquisition and analysis software (Becton Dickinson). For analysis and quantification of leukemic cells and DC, the total MNC/DC fractions were gated. A leukemic BM sample was considered as ‘positive’ for a surface marker if the percentage of positive events in a gate surrounding blasts, lymphocytes and monocytes was more than 20%, as described [12]. For T-cell analyses a ‘lympho gate’ was created, including lymphocytes and activated lymphocytes (Fig. 1c). Proportions of positive events in defined gates compared with the isotype controls were calculated using the CellQuest Software (Becton Dickinson).
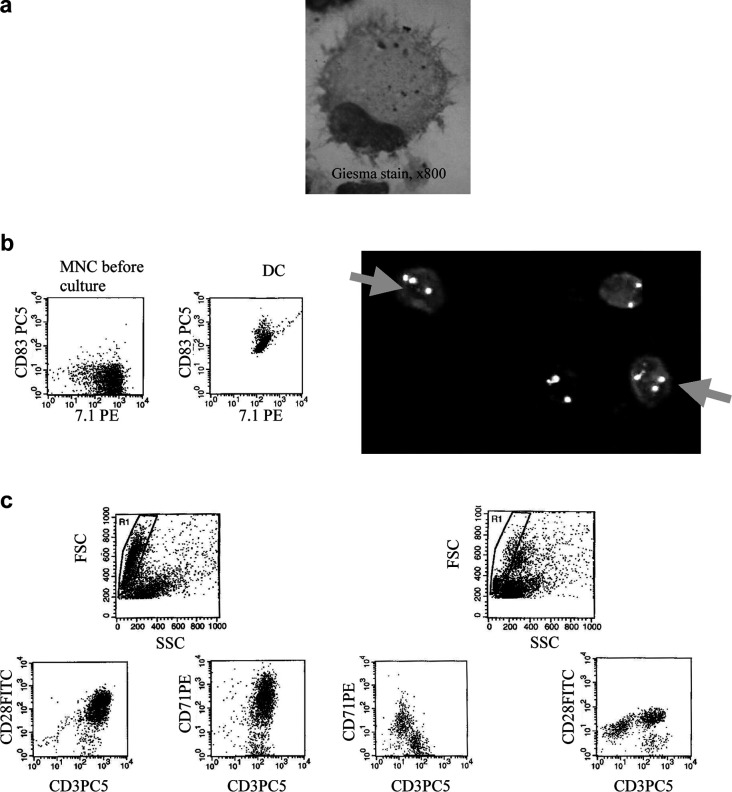
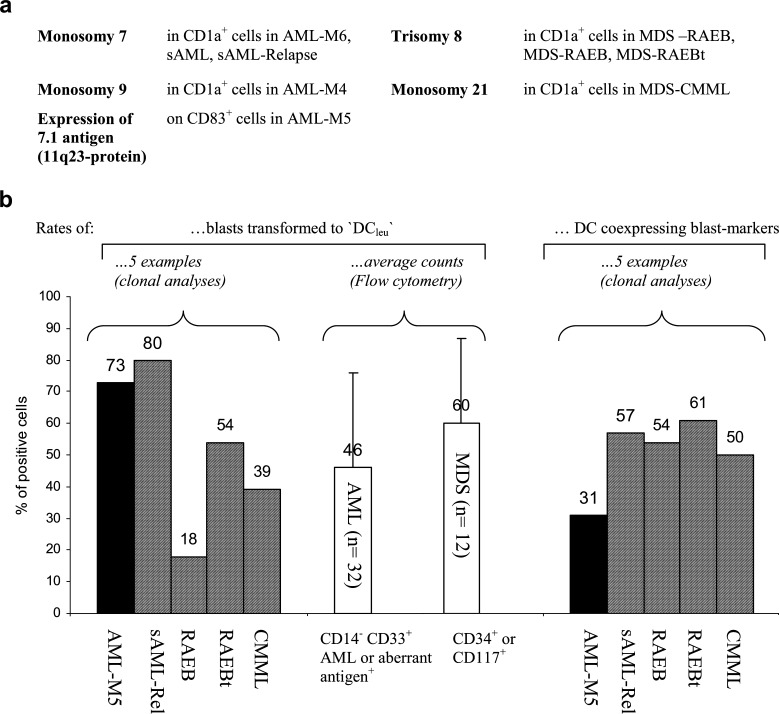
Fig. 1.
DC from AML- and MDS-MNC were grown in GM-CSF, IL-4, TNFα and FL containing Xvivo media for 10–14 days and characterized (a, b) or used to stimulate autologous T cells in MLR in RPMI 1640 media and 10% FCS for 5 days (c). a Morphology of cytospinned DC in a case of AML-M4 after Giesma stain and photograph (x800 fold magnification) shows characteristics of DC: irregular shape and cytoplasmic projections. b left side: Flow-cytometric analyses of AML-M5-MNC before and after the culture procedure demonstrate high blast (7.1+) counts and low CD83 counts in the MNC fraction and high proportions of double stained cells in the DC fraction, thereby proving the leukemic origin of these DC. right side: FISH-IPA analysis, a combined FISH and surface marker assay, enables a distinction of cells coexpressing DC-marker CD1a and the clonal trisomy 8 from non-clonal DC and clonal CD1a-negative cells in the case of MDS-RAEB, thereby proving the leukemic origin of these DC. c Flow cytometric analysis of T cells after (left side) and before (right side) coculture with autologous DC in a case of AML-M5 demonstrate a T-cell activation with respect to proliferation (coexpression of CD71 on CD3+ T-cells) and expression of costimulary ligands (e.g. CD28): After 5 days, an increased Forward Scatter of T cells as well as an expansion of proliferating T cells coexpressing CD28 can be seen
Simultaneous fluorescence in situ hybridization (FISH) and immunophenotyping analysis (‘FISH-IPA’)
AML or MDS samples known to exhibit numerical cytogenetic aberrations were selected for FISH-IPA analyses. Aliquots of MNC or DC created were centrifuged onto slides, air-dried and frozen. Surface-antigen staining of aceton fixated slides was performed using FITC or PE labeled (CD1a) DC markers [29]. Detection of chromosome aberrations was performed according to the manufacturer’s instructions after methanol/acetic acid fixation and denaturation of chromosomes, using centromere-specific probes for chromosome 7, 8 (Oncor, Germany) 9 or 21 (Abbot/Vysis). Hybridizations were carried out overnight at 37°C. Cy3-conjugated streptavidin and AMCA-conjugated antibodies were used for the detection of hybridized DNA probes and cells were counterstained with DAPI. Slides were analyzed using a Zeiss microscope and attached CCD camera with an Image analysis software (MetaSystems). CD1a+ DC carrying the clonal chromosomal markers were finally differentiated from CD1a+ DC without clonal markers and from clonal, cytogenetically aberrant cells not expressing DC antigens (Fig. 1b, right side): proportions of blasts converted to DCleu as well as proportions of DC of leukemic origin were quantified in cases with more than 20 clonal and/or CD1a+ cells.
Viability analysis
To evaluate the amounts of viable cells or DC after the culture procedure, MNC or DC were gated and viable (Annexin− PI−) cells quantified [33]. The test was carried out according to the manufacturer’s instructions (BioVision, BioCat): 1–3×105 cells were incubated in 300 μl binding buffer for 5 min with 3 μl Annexin V-FITC and 3 μl PI in the dark. Without washing, the cells were measured in an FACS Calibur flow cytometer (Becton Dickinson), using the Cell Quest data acquisition and analysis software (Becton Dickinson).
Quantification and characterization of DC
The DC were generated as described, harvested, counted and DC quantified by Flow cytometry: cells which displayed the typical scatter of DC and expressed typical DC antigens (e.g. CD86, CD80, CD40, CD1a and CD83) were counted in a gate surrounding the total MNC/DC fraction analyzed. For quantification, those DC markers were selected that were not expressed on naïve blasts. The DC coexpressing CD83 were defined as ‘mature DC’. Other maturation criteria were the loss of CD14 or CD209 positivity. The DC coexpressing, e.g. CD86 or CD137L were defined as DC coexpressing costimulatory antigens. In AML cases with an immunophenotypically detectable blast population in MNC fractions, amounts of blasts being converted to DC coexpressing specific blast antigens (e.g. 7.1, CD56 and CD117) were quantified (Fig. 1b, left side). In cases with no available specific blast marker combination, but less than 5% CD14+ cells in the MNC-fraction, CD33 or CD13 antibodies were used to estimate proportions of blast cells converted to DCleu. In MDS cases with >10% CD34+ or CD117+ cells, those markers were used to evaluate proportions of cells being converted to DCleu. Microscopical controls were regularly performed and revealed the DC-typical morphology of large cells with irregular shapes and cytoplasmic projections (Fig. 1a).
Mixed lymphocyte cultures (MLR)
Positively selected CD3+ T cells (Milteney Biotech, 1×106 cells/well) from MNC and AML or MDS patients were cocultured with autologous leukemia-derived DC, MNC or without DC or MNC as a control (1×105) in 1 ml RPMI 1640 medium (Biochrom) containing 10% FCS, as described. After 5 days of coculture (DC-induced), T-cell activation with respect to proliferation and expression of DC-contact relevant antigens was measured by Flow cytometry.
T-cell stimulatory, proliferation-inducing function of leukemia-derived DC
The T-cell activating capacity of DC was evaluated by FACS analyses comparing the expression of costimulatory receptors on T cells before and after autologous MNC or DC contact and by evaluating the proliferation activity of T cells. T cells coexpressing, e.g. CD28, CD137 and CD154 were defined as ‘T cells coexpressing costimulatory receptors’. T-cell proliferation was evaluated by the quantification of T cells coexpressing HLA-Dr, CD71 or CD25 before and after DC contact as described [43] (Fig. 1c).
Generation of cytotoxic autologous T cells
Positively selected CD3+ T cells (2×106) were cocultured with 5×104 DC or MNC in a 24-well plate in a final volume of 2 ml RPMI+ 10% FCS, supplemented with IL-7 (day 0–21) (10 μg/ml; CellConcepts) and IL-2 (23 IU/ml (day 7–21) Proleukin R5, Chiron). T cells were restimulated with 5×104 DC or MNC on day 7 and on day 14. Half medium exchange was carried out every 3–4 days. After six days the last restimulation, cells were harvested and the cytotoxicity assay was carried out.
Cytotoxicity assay (Fluorolysis)
The lytic activity of effector T cells was measured by a Fluorolysis assay by counting viable target cells, labeled with specific fluorochrome-labeled antibodies, before and after effector cell (E) contact. T cells (E) from AML or MDS patients or healthy donors were stimulated with DC or MNC, harvested and an aliquot cocultured in 1.5-ml Eppendorf tubes with thawed autologous MNC or DC or other target cells (T) at E:T ratios of 20:1, 10:1, 6:1 and 2:1 for 3 h at 37°C and 5% CO2. Before culture, MNC-target cells were stained for 15 min with two FITC- and/ or PE-conjugated ‘blast’-specific antibodies and cocultured for 3 h with effector cells (T cells or DC as target cells were stained with T-/ DC-specific antibodies). As a control target effector cells were cultured separately and afterwards mingled with T cells. To evaluate the amounts of viable (7AAD−) target cells and to quantify the cell loss after the 3-h incubation time, cells were harvested, washed in PBS and resuspended in a FACS flow solution containing 7AAD (Becton Dickinson, Biosciences Pharmingen) and a defined number of Calibrate APC-labeled beads (Becton Dickinson). Viable cells were gated in a SSC/7AAD gate and afterwards viable cells coexpressing specific blast marker (combinations) were quantified taking into account defined counts of APC-labeled calibration beads as described. Cells were analyzed in a FACS Calibur Flow Cytometer using the CELL Quest software (Becton Dickinson). The percentage of lysis was the difference between proportions of viable blasts before and after the effector cell contact [32].
Statistical methods
Mean and standard deviation, median and range and two-tailed t-tests were performed with a personal computer using Excel 97 (Microsoft). Differences were considered as significant, if the P-value was <0.05.
Results
Characterization of mononuclear cell fractions obtained from AML, MDS and healthy probands
Before culture, we analyzed the mononuclear cells from 100 AML cases in active stages of the disease, 55 MDS and 38 healthy samples by Flow cytometry to quantify monocytes, lymphocytes and blasts and to evaluate or to confirm the blast phenotype. On average, AML samples presented with 11% B cells, 9% T cells, 14% monocytes (5% in non-monocytoid differentiated leukemias) and 59% leukemic cells; in MDS samples, 6% B cells, 15% T cells, 9% NK cells, 12% monocytes and 6% CD34+ cells could be detected. In MNC samples from healthy probands, on average, 10% B cells, 39% T cells and 28% monocytes were found.
Mature and vital DC can be generated from AML and MDS mononuclear cell fractions
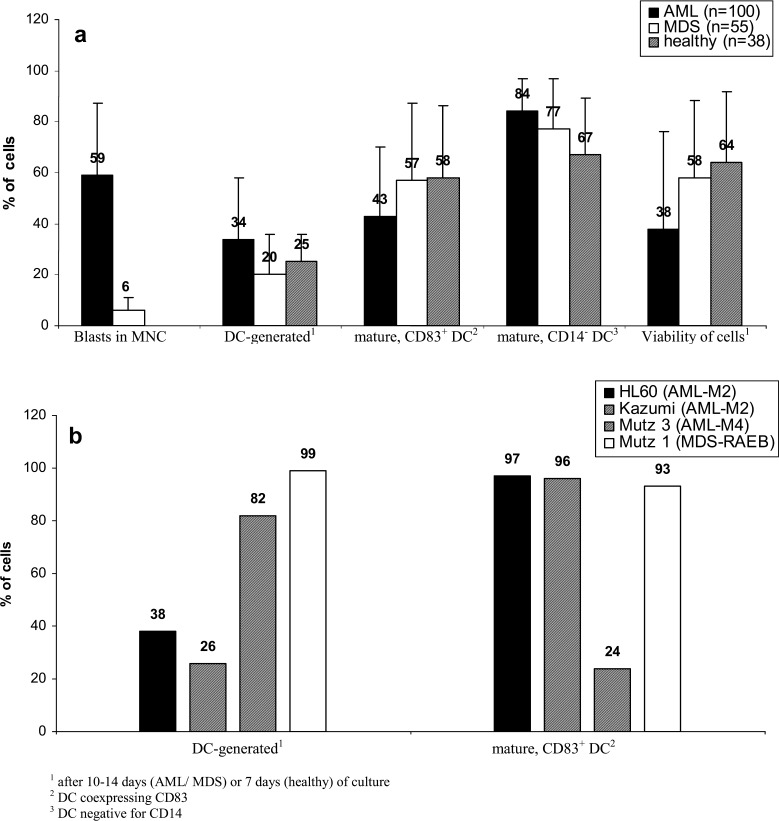
On average, 34% DC from AML samples, 20% from MDS samples and 25% from healthy samples could be generated. Quantification of DC was performed as described (Fig. 2). Between 43 and 58% of these DC coexpressed CD83, a marker for mature DC. Even more of these DC were negative for monocyte-marker CD14 (84% of DC in AML, 77% in MDS and 67% in healthy samples) or were negative for CD209− (76% of DC in AML, 56% in MDS and 50% in healthy samples). Viability of cells analyzed after 7–14 days of culture varied between 38% and 64% with fresh or frozen/thawed samples used as cell sources. The highest DC counts from cell lines were obtained with the Mutz-3 (AML-M4) line. The maturity of these DC was even higher in cell lines (except from Mutz-3) than that in patients’ DC.
Fig. 2.
Viability and degree of maturation of DC from AML and MDS-MNC or leukemic cell lines grown in GM-CSF, IL-4, TNFα and FL containing Xvivo media for 10–14 days (AML/MDS/cell lines) or 7 days (healthy donors). Proportions of immunocytologically detectable blasts in AML or MDS (CD34+ or CD117+ cells in MDS)—MNC and proportions of DC generated from the total MNC-fraction as well as proportions of mature (CD83+ or CD14− DC) or viable DC in DC fractions from healthy, AML or MDS samples (a) or from cell lines (b) are given
We can conclude that mature and vital DC can be generated both from AML and MDS-MNC samples.
DC harvest is highest in monocytoid subtypes, but independent of the cytogenetic risk
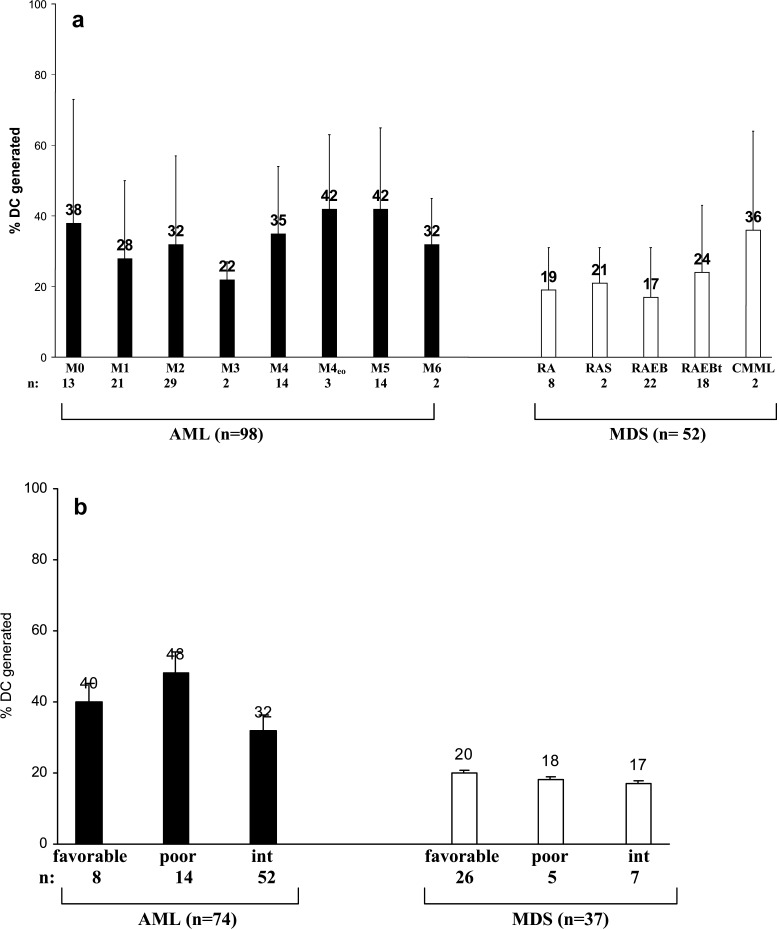
Quantification of DC was performed by Flow cytometry as described. The DC harvests were similar in non-monocytoid AML- or MDS-FAB types with, on average, 30% DC in AML or 20% DC in MDS. The Highest DC-counts were obtained from the (myelo)-monocytoid subtypes of AML (on average 40% DC) or CMML (36% DC, Fig. 3a) and also from the AML-M4 line Mutz3 (Fig. 2b). No differences in DC harvests were seen in AML and MDS cases subdivided into cytogenetic risk groups (Fig. 3b).
Fig. 3.
DC from AML- and MDS-MNC at first diagnosis were grown in GM-CSF, IL-4, TNFα and FL containing Xvivo media for 10–14 days and separated according to FAB types (a) or cytogenetic risk groups (b). Proportions of DC generated are given: DC harvests were highest in monocytoid FAB types, but independent of the cytogenetic risk group
In 6% of AML cases, 31% of MDS cases and in 8% of healthy donors, less than 10% could be generated. With cases subdivided into different FAB types, we found that in 9% of cases with undifferentiated AML (M0-M2), but in none of the remaining AML-FAB types and in 31% of cases with RAEB or RAEBt, less than 10% DC could be generated (data not shown).
This means that the DC harvests are independent of cytogenetic risk groups and highest in monocytoid FAB types in AML and MDS and cannot be generated from all MNC samples.
Autologous T-cell activation is mediated by expression of costimulatory ligands on DC and costimulatory receptors on T cells
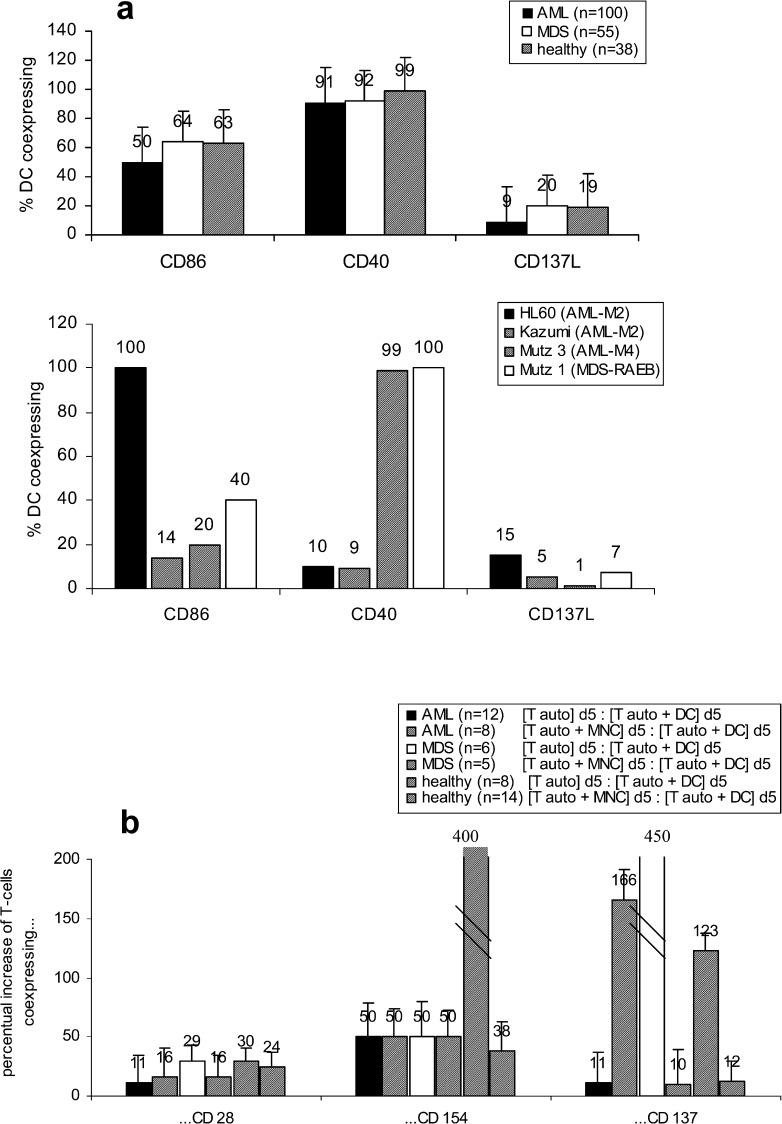
DC obtained from 38 healthy as well as from 100 AML and 55 MDS samples coexpress comparable amounts of costimulatory ligands (e.g. CD137L+, CD40+, CD86+, CD80+ and CD1a, Fig. 4a, left side). These costimulatory ligands were also expressed on DC generated from AML or MDS-cell lines (Fig. 4a, right side). Costimulatory receptors (e.g. CD28, CD154 and CD137) on T cells were upregulated after contact with autologous DC in MLR compared to controls (autologous T cells, cultured in parallel for 5 days). Compared to cocultures of autologous T cells with MNC, a coculture with DC increased amounts of T cells coexpressing costimulatory receptors (Fig. 4b).
Fig. 4.
DC from AML- and MDS-MNC or cell lines were grown in GM-CSF, IL-4, TNFα and FL containing Xvivo media for 10–14 days (healthy donors for 7 days), characterized (a) and used to stimulate autologous T cells in an MLR in RPMI 1640 media and 10% FCS for 5 days (b). a Proportions of DC coexpressing costimulatory ligands are given b Percentual changes of autologous T cells coexpressing costimulatory receptors after the 5-day coculture MLR procedure (‘[Tauto] d5’) compared to non-DC-stimulated controls (‘[Tauto +DC] d5’) are given. Moreover, a comparison of the T-cell stimulatory effect of MNC (‘[Tauto + MNC] d5’) compared to DC after a 5-day coculture with autologous T cells (‘[Tauto +DC] d5’) is given
This means that DC/T-cell contact-mediating antigens are expressed and upregulated on DC and autologous T cells from AML/MDS-MNC in comparable amounts as from healthy PB-MNC, and a conversion of MNC to DC in addition increases the expression of these antigens.
Leukemic origin of DC generated from AML and MDS samples can be proven by FISH-IPA or Flow cytometry
To prove the leukemic/clonal derivation of DC generated from AML or MDS-MNC, ‘FISH-IPA’ analyses were performed in cases with a clonal numerical aberration. The codetection of the clonal marker in CD1a+ cells was interpreted as proof for ‘leukemia-derived DC’ (Fig. 5a). We could demonstrate the clonal monosomy 7 in CD1a+ DC obtained from a case with AML-M6 and in two cases with sAML and the monosomy 9 in CD1a+ cells in a case with AML-M4. The clonal trisomias of chromosomes 8 could be detected in CD1a+ cells in two cases with MDS-RAEB and in a case with MDS-RAEBt. In another case of CMML, the monosomy 21 was demonstrated in CD1a+ cells. Figure 1b (right side) gives an example for the codetection of the clonal trisomy 8 in CD1a+ DC from a patient with MDS-RAEB. Moreover, a coexpression of the 7.1 blast-characterizing antigen, the cell surface protein product of 11q23 aberrations, on CD83+ mature DC derived from a patient with an AML-M5 (characterized by an 11q23 aberration at first diagnosis) is given (Fig. 1b, left side). Most of the leukemic cells in AML are CD33+, thus characterizing the myeloid blast phenotype. In addition, blasts regularly express ‘stem cell’ antigens like CD117, CD34 or non-myeloid lineage antigens like CD56; in MDS, CD34+ or CD117+ cell populations can be detected. In cases with a gain of ‘DC markers’ on the blast population after a 10 to 14-day culture-period, resulting in cells coexpressing blast (e.g. 7.1, CD56) with DC markers, proof for the leukemic derivation was given. In cases with a CD33+ blast phenotype, only cases with less than 5% CD14+ cells in the MNC fraction were included for analyses to exclude normal monocyte-derived DC. In 32 cases with AML and in 12 cases with MDS, a meaningful evaluation was possible and in all/of those cases, a coexpression of blasts and DC markers was seen, proving the leukemic derivation of DC.
Fig. 5.
DC from AML- and MDS-MNC were grown in GM-CSF, IL-4, TNFα and FL containing Xvivo media for 10–14 days and the leukemic/clonal derivation proven by FISH-IPA or Flow cytometry. a Clonal derivation of DC generated from five cases with AML (left side) and four cases with MDS (right side) was proven by codetection of the clonal numerical chromosome aberration with DC (FISH-IPA-analysis). b Quantitative analyses by FISH-IPA or Flow cytometry show that 18–80% of clonal cells in the five cases given are converted to DC carrying the clonal marker (left side); that on average, 46% of leukemic cells in AML (n=32) and 60% of CD34 or CD117+ cells in MDS (n=12) are converted to DCleu (middle). In contrast, 31–61% of DC created from AML and MDS are of clonal origin in the five cases given (right side). c Dot-plot analyses generated in five cases with AML or MDS show that distinct blast populations can be detected in MNC in the ‘SSC/blast-marker’ plot (first row), and that those blast populations do not express DC-markers (second row). After 10–14 days of culture in GM-CSF, IL-4, TNFα and FL containing Xvivo medium, the blast populations gain an increased SSC and an expression of DC markers (third and fourth row). Statistical evaluations allow the exact quantification of blasts which were converted to DCleu (third and fourth row).
FISH-IPA and Flow cytometry allow quantification of blasts converted to DC and of DC of non-leukemic origin
An important question was whether clonal/leukemic cells in AML and MDS were quantitatively converted to leukemia-derived DC (‘DCleu’). In cases with more than 20 evaluable CD1a+ and/ or FISH-evaluable cases, we quantified clonal cells with/without coexpression of CD1a after conversion to DC. We could demonstrate that on average, 53% of clonal cells in AML/MDS (77% of leukemic cells in AML-DC cultures and 37% of clonal cells in MDS-DC cultures (range 18–54)) coexpressed CD1a, giving rise to leukemia-derived DC (Fig. 5b, left side). Quantitative evaluations by Flow cytometry were performed by including 32 AML cases with a CD117+, CD56+ and CD34+ blast phenotype or with a CD33+ AML-blast phenotype and less than 5% CD14+ cells in the MNC fraction. We could demonstrate that on average, 57% of those blasts coexpressed DC antigens after 10–14 days of culture, thus confirming data described above. In 12 MDS samples with >10% immunocytologically detectable blasts (CD34+ or CD117+), we found that 64% of these cells coexpressed DC antigens after 10–14 days of culture (Fig. 5b, middle).
Figure 5c gives some examples of Flow cytometry of MNC/DC in cases with AML and MDS: in cases 1–3, we demonstrate that the leukemic cell population in the MNC fraction, positive for CD33 (AML-M0), CD117 (MDS-RAEBt) or CD34 (MDS-RAEB) can be redetected in the DC culture after 10–14 days of culture, with those cells still being positive for these markers, but in addition characterized by an increased sideward scatter (SSC), typical for DC and an expression of DC markers. The coexpression of these ‘leukemic markers’ on DC demonstrate the conversion of these cells to leukemia-derived DC. In cases 4 and 5, we demonstrate that the leukemic population does not quantitatively gain the DC-typical increased sideward scatter or the coexpression of DC antigens on all blasts, but leukemic cell populations are still detectable at the same ‘MNC-Scatter position’.
In cases with clonal aberrations, we could demonstrate that beside clonal DC also CD1a+ cells without the clonal aberration of the leukemic cells were generated (Fig. 5b, right side).
We can conclude and prove that we can regularly create leukemia-derived DC in AML/MDS cases with clonal or leukemia-specific markers being copresented with DC markers. Moreover, we can summarize that clonal/leukemic cells as well as DC of non-leukemic origin are detectable in the DC culture: about 60% of clonal/leukemic cells in AML or MDS are convertible to DC and vice versa, with about 50% of DC created being of leukemic/clonal origin.
Conversion of MNC to DC increases autologous T cell activation with respect to proliferation, migration and production of leukemia-cytotoxic T cells
We studied the proliferation characteristics of T cells’ healthy AML and MDS probands without DC contact compared to that of T cells mixed with autologous DC for 5 days in an MLR setting. The results are given in Fig. 6a: compared to non-DC-stimulated T-cell controls, DC-stimulated T cells showed an increased stimulation of proliferation. Compared to cocultures of autologous T cells with MNC, a coculture with DC increased amounts of proliferating T cells (Fig. 6a). Figure 1c shows an example of activated T cells after a 5-day stimulation of T cells with autologous DC in a case of AML-M5: amounts of T cells coexpressing CD71 (signaling proliferation) and CD28 (signaling DC contact) are increased.
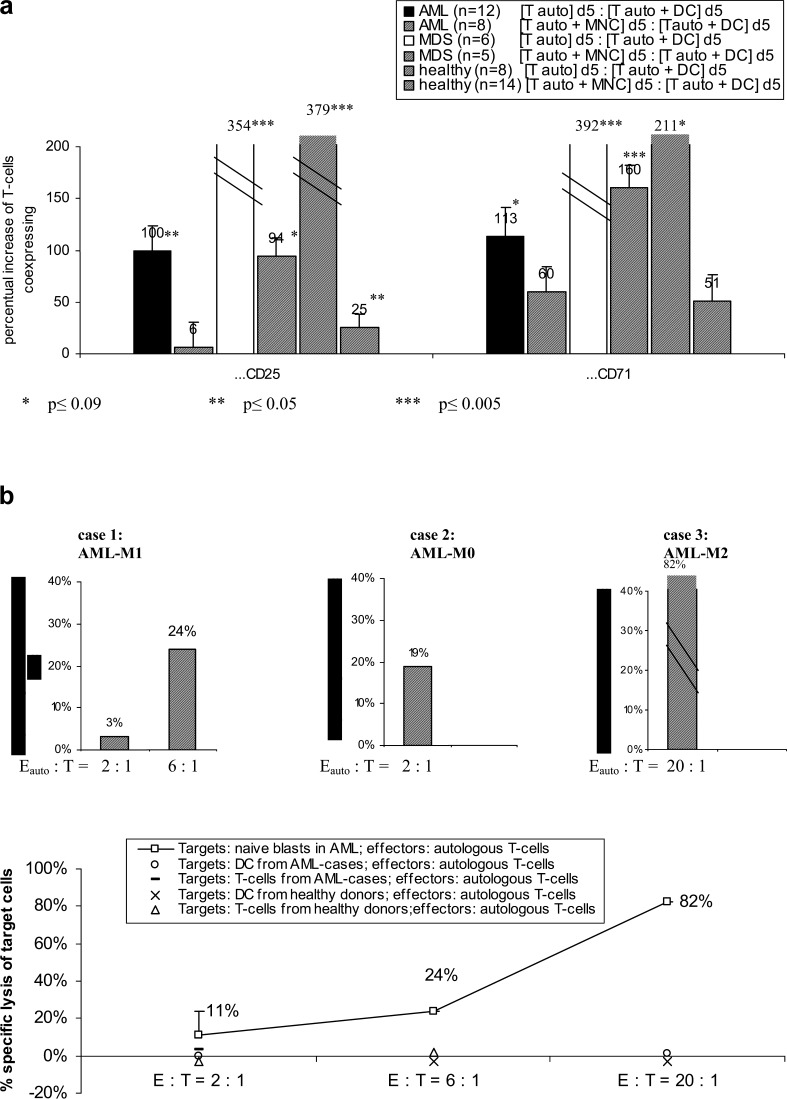
Fig. 6.
DC from AML- and MDS-MNC or healthy donors were grown in GM-CSF, IL-4, TNFα and FL containing Xvivo media for 10–14 days (healthy donors for 7 days), characterized (a) and used to stimulate autologous or allogeneic T cells in an MLR in RPMI 1640 media and 10% FCS for 5 days. a Percentual changes of autologous T cells coexpressing proliferation markers after a 5-day coculture in MLR cultures (‘[Tauto] d5’) compared to non-DC-stimulated controls (‘[Tauto +DC] d5’) are given. Moreover, a comparison of the T-cell stimulatory effect of MNC (‘[Tauto +MNC] d5’) compared to DC after 5-day coculture with autologous T cells (‘[Tauto +DC] d5’) is given. b DC-stimulated autologous T cells from three AML-patients grown in an MLR (RPMI medium, 10% FCS) were cocultured with naïve autologous blasts for 3 h. Specific lyses of blasts after coculture with T cells compared to controls, evaluated by a ‘Fluorolysis assay’, are given (upper part). E: Effector cells (activated autologous (auto) or allogeneic (allo) T-cells); T: Target cells (naïve blasts). In parallel, the specific lysis of other autologous target cells from AML patients (open square, open diamond, –) or healthy donors (x, open triangle) by autologous T cells from AML patients (open square, open diamond, –) or healthy donors (x, open triangle) evaluated by a ‘Fluorolysis assay’ are given (lower part)
CD62L is expressed on CCR7+ or CCR7− T cells. Both the expressions of CD62L and CCR7 on T cells mediate their homing to lymph nodes. We could demonstrate that the expression of these migration antigens is upregulated (6–23) on T cells after autologous DC contact in cases with AML as well as in healthy donors compared to T cells after autologous MNC contact (data not shown).
The specific lytic activity of T cells prepared from AML patients after an autologous DC-activation step was studied in a Fluorolysis assay. In three cases with AML, a specific lysis of naïve blasts expressing patient-specific blast antigens was shown, but no lysis of autologous T cells or DC (Fig. 6 b):
Case 1: MNC from a patient with AML-M1 containing 53% CD33+ /CD117+ blasts were cocultured with autologous DC-primed T cells in the ratio of 2:1 and 6:1. After only 3 h, a specific lysis of 3%/24% CD33+ /CD117+ % blasts was seen (Fig. 6b, upper part, left side). These T cells, however, did not lyse autologous DC after a 3-h coculture in a ratio of 2:1 (Fig. 6b, lower part).
Case 2: MNC from a patient with AML-M0 containing 23% CD34+ blasts were cocultured with autologous DC-primed T cells in the ratio of 2:1. After 3 h, a specific lysis of 19% of CD34+ blasts was seen (Fig. 6b, upper part).
Case 3: MNC from a patient with AML-M2 containing 85% CD34+ blasts were cocultured with autologous DC-primed T cells in the ratio of 20:1. After 3 h, a specific lysis of 82% of CD34+ blasts was seen (Fig. 6b, upper part, right side). These T cells did not lyse autologous DC after a 3-h incubation in the ratio of 20:1(Fig. 6b, lower part).
DC-activated T cells from two healthy donors did not lyse autologous T cells or DC after 3 h, thereby representing another negative control (Fig. 6b, lower part). Using DC-primed or non-DC-primed T cells from three healthy donors, a lysis of 30–90% of allogeneic AML-blasts expressing a patient-specific blast phenotype was seen, thereby representing a positive control (data not shown). In three other cases with AML, no lysis of blasts by autologous or allogeneic DC-activated T cells was seen. In these three cases, less than 5% viable T cells were available. In another AML case, only 4% DC could be generated. Autologous T cells from this patient could not lyse naïve blasts, thereby demonstrating that T cells are necessary to lyse allogeneic or autologous blasts and DC are necessary to activate autologous T cells.
We can conclude that T cells proliferate after autologous contact with DC generated from AML- and MDS-MNC fractions. Moreover, they give rise to cytotoxic subtypes, which are able to specifically lyse (autologous) leukemic cells without lysing autologous (unstimulated) T cells or DC—supposing that enough DC and T cells are available.
Taken together, these data point to the crucial role of stimulated T cells and DCleu to enable a specific lysis of naïve autologous blasts.
Expression of migration antigens on DC can be improved by the cultivation of DC in PGE2 containing ‘MCM-Mimic’ medium
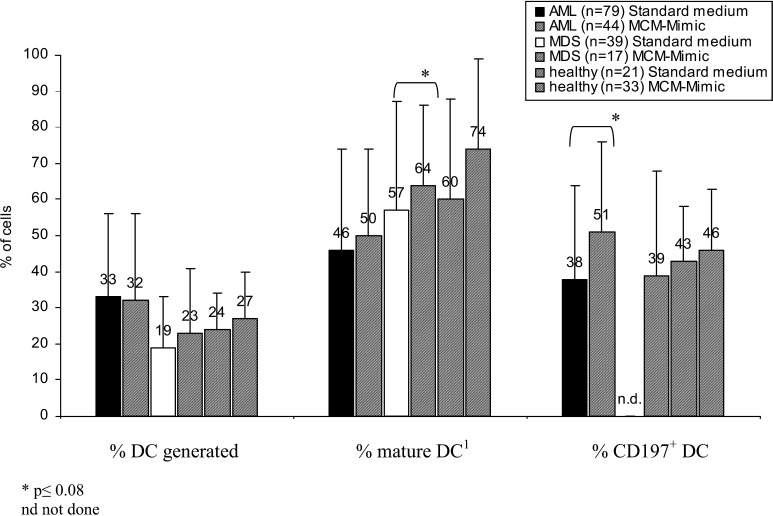
Only DC coexpressing migration markers like CCR7 (CD197) are able to migrate to lymph nodes and to present antigens to naïve T cells. Therefore we studied and compared the expression of migration antigens on DC created in GM-CSF, IL-4, TNFα and FL-standard medium (as described) with DC created in ‘MCM-Mimic’ medium containing GM-CSF, IL-4, TNFα, FL, IL-1â, IL-6 and PGE2. We could show that MCM-Mimic not only increases the percent harvest of mature DC, but also especially proportions of CCR7+ DC compared to DC cultured in ‘standard’ medium (Fig. 7). Proportions of DC- coexpressing costimulatory antigens were not different in the groups compared (data not shown). These data prompted us to change DC-standard medium to ‘MCM-Mimic’.
Fig. 7.
DC from AML- and MDS-MNC or healthy donors were grown in GM-CSF, IL-4, TNFα and FL containing Xvivo media or in ‘MCM-Mimic’ (Xvivo+GM-CSF, IL-4, TNFα, FL, IL-6, IL-1β, PGE2) for 10–14 days (healthy donors for 7 days). Percent of mature, CD83+ DC as well as CCR7+ DC are given
Discussion
In AML and MDS, malignant cells fail to undergo normal differentiation, resulting in the expansion of clonal leukemic cells [30]. Antibody-based immunotherapies with CD33 antibody-radio immunoconjugates, immunotoxins, bispecific and trispecific antibodies are most promising in cases with a high expression of tumor-associated antigens on leukemic cells [1, 57, 11, 56, 3, 52]. Besides DC-based therapies are thought to be one of the most promising tools in the immunotherapy of cancer: DC are distributed all over the body, they can be generated from leukemic cells (giving rise to leukemia-antigen-presenting cells), can gain migration probabilities and can specifically activate T cells [8, 51].
We and others have already shown that DC can be generated in vitro from healthy and leukemic PB or BM-monocytes, or MNC, using media containing FCS, human serum pools or FCS-free media [13, 55, 8, 34, 63, 16, 28, 9]. Due to incompatibility or anaphylactic reactions or inhibitory effects and with respect to clinical applications, serum-free media (e.g. Xvivo, CellGro and AimV) should be preferred [34, 38, 28]. Usually DC media contain GM-CSF, IL-4 and TNFα [34, 62, 44, 33]. We and others have already shown that the addition of FL to DC-media increases the DC harvest [63, 34]. Using this ‘standard’ medium, we could not only generate DC from AML-MNC, but also regularly from MDS-MNC [34]. Now, we show that DC can regularly be created from 100 AML, 55 MDS-MNC or cell lines and 34 healthy DC samples with comparable expression profiles of costimulatory ligands, relevant for a DC/T-cell contact [37, 18, 15, 6, 13, 34] and with comparable amounts of mature DC. Autologous T cells from AML, MDS or healthy donors cocultured with DC in MLRs upregulate costimulatory receptors, those DC costimulatory ligands.
A conversion of MNC to DC increases the expression rate of those antigens on T cells, indicating a ‘DC-mediated’ T-cell activation.
Subdividing AML and MDS cases into FAB-types, we could confirm that the highest DC harvests can be achieved in monocytoid differentiated AML (M4/M5) patients or cell lines as described [58]. In addition, we can add that in MDS the highest DC counts could be obtained in monocytoid MDS-FAB type CMML. To the contrary, most of these AML cases in which less than 10% DC could be generated belonged to ‘non-monocytoid’ differentiated (especially undifferentiated AML-M0-M2) FAB types. In contrast, 25% DC from healthy donors could be grown with, on average, 28% of monocytes in the MNC fractions. This means that the described impaired differentiation of leukemic samples to DC is not a particular problem of the culture system, but a blast-specific problem. Possibly other DC-differentiating methods could yield higher DC counts in those patient groups (see below). DC yields are independent of cytogenetic risk groups. This could mean that a DC-based immunotherapeutic approach could be suitable for all cytogenetic AML or MDS subtypes.
In 1994, Santiago-Schwarz et al. [49] observed that monocyte-derived DC obtained from an AML patient expressed the leukemic blast phenotype. We and others showed by Western blotting that DC generated from an AML sample carrying inv(16) continue to express the leukemia-associated CBFB-MYH11 protein or by demonstrating a coexpression of DC antigens with the protein-product ‘7.1’ of 11q23-aberrant AMLs [34, 16, 54]. The leukemic origin of DC can be proved in AML cases with a clonal chromosome aberration by detection of the clonal marker in sorted DC [28, 16, 63, 53]. We could confirm these data by detecting the numerical chromosome abnormality in CD1a+ DC generated in five cases with AML and in four cases with MDS, proving that regularly MDS-clone-derived DC can be generated under serum-free culture conditions presenting leukemic and DC characteristics. To our knowledge, we are the first to prove the generability of MDS-derived DC from clonal myelodysplastic progenitors under serum-free culture conditions: Rigolin et al. [47] generated DC from only eight MDS patients (in FCS-containing media) and studied the antigen uptake of those DC. In two of these cases, he proved the clonal derivation of the DC.
FISH-IPA analyses allow qualitative and quantitative evaluations of cells coexpressing DC and/or clonal markers. Quantitative evaluations can also be performed in AML- or MDS cases expressing specific blast markers by studying the coexpression with DC antigens. An interesting finding was that clonal/leukemic cells in AML or MDS are not quantitatively converted to leukemia-derived DC, but still clonal/leukemic cells remain in the DC culture. In contrast, we could demonstrate the generability of CD1a+ DC without clonal/leukemic derivation in cases with MDS or AML. With respect to clinical applications, we recommend a detailed Flow-cytometric analysis and quantifications of blasts and DCleu in all individual AML and MDS cases to estimate the efficacy of the DC procedure, amounts of contaminating blasts and to enable decisions whether DC could be applied in an unselected manner or whether they should possibly be generated with other DC-differentiation methods.
We are the first to prove the generability of MDS-derived DC from (clonal) MDS progenitors under serum-free culture conditions and to present such a detailed, qualitative and quantitative analysis of leukemia-derived DC from AML and MDS samples.
Costimulatory antigens are often downregulated, but can also be expressed in AML (what is often correlated with a worse prognosis [25, 39, 60]), thereby indicating that the expression of costimulatory markers on naive leukemic cells alone is not sufficient to induce an anti-leukemic T-cell reaction. These data support the view that the simultaneous presentation of (leukemic) antigens together with costimulatory ligands by professional antigen-presenting cells like DC are necessary to activate T cells. We could demonstrate a DC-mediated, autologous T-cell activation with respect to the expression of ‘DC-contact-relevant’ molecules and an upregulation of migration markers (CCR7 and CD62L), necessary for a migration of naïve or memory T cells to lymph nodes or Peyers’ patches [60, 35], although functional details of migratory functions are still missing. These are crucial steps for the T-cell-activation and differentiation in vivo. After completion of the differentiation program, T cells exert effector functions [35]. Using leukemia-derived DC from AML-MNC, we could detect a significant proliferation of T cells in MLR, as already shown [63, 28]. We can add the information that in addition, MDS-derived DC induce a significant T-cell proliferation compared to controls. Moreover, conversion of MNC to DC further increases this T-cell activation. Autologous T-cell proliferation assays have already been used to support cytotoxicity data, indicating that stimulated T cells give rise to cytotoxic T cells, although proof for the cytotoxic capacity has to be brought [28]. Our data show that T cells generated in MLR with autologous leukemia-derived DC are able to specifically lyse blasts expressing the original blast phenotype after only 3 h without lysing other autologous cells, proving the principle of the effect and function of DCleu in specifically stimulating autologous T cells. An overnight incubation of blasts with activated T cells could possibly increase the rate of lysed autologous/allogeneic cells compared to controls [32]. In contrast, MLR with cell suspensions with low DCleu but high blast counts or with non-viable T cells did not give rise to (enough) cytotoxic autologous T cells, pointing to the crucial role of DCleu in activating leukemia-cytotoxic T cells. In one of these cases, our data could be supported by a ‘Delta-assay’ carried out in parallel by demonstrating that the in vitro proliferation of naïve blasts could not be inhibited by autologous T cells added, due to inactive T cells (data not presented [59]).
The Fluorolysis assay allows a quantitative evaluation of viable/lysed cells expressing a defined (blast) phenotype in an allogeneic or autologous setting without the problem of a high spontaneous lysis rates as described for the Chrom-release assay. The applicability of the assay could be improved by a detailed knowledge of T-cell counts, blast counts and the blast phenotype in every single case. The generability and harvest of specific T cells for clinical applications could be possibly improved by the use of sorted DCleu. Based on the observations of others and ours concerning T-cell activations, it seems that by using a DC-based activation strategy, T cells can be stimulated to undergo a differentiation program necessary for an anti-leukemic as well as a memory effect [22, 60, 35]. These advances could enable clinicians to select, modulate and apply specifically designed immunotherapies for AML or MDS patients, e.g. by vaccinating with DC, by generating allogeneic or autologous T cells for clinical applications using DC-based culture systems or even generating and selecting T-cell subsets (e.g. cytotoxic, memory T cells [61, 64]). However, it has been shown that an AML-derived cell line (CS-1) with DC differentiation does not induce an immune stimulation but in contrast an immune suppression [21]. It is not clear whether ex vivo created DCleu from leukemic patients’ samples could also induce an immunosuppression. Therefore, we recommend to test the individual specific T-cell-activating capacity before an in vivo vaccination of patients with DC. The adoptive transfer of specific CTLs generated with the help of DC seems to be a clinically effective method, especially after non-myeloablative chemotherapy [19].
Results from clinical trials suggest that DC vaccinations have the potential to induce immune responses. MCM and ‘MCM-Mimic’ media currently represent a standard: These media produce homogeneous, viable DC in cancer patients able to migrate and to induce T-helper cell and cytotoxic responses in vivo and in vitro [51, 36]. Little information (and no information for MDS patients) is available about the serum-free generation of mature DC of leukemic patients. Only mature DC are able to migrate in vivo to lymph nodes and to stimulate T cells. PGE2 was shown to be a stimulus for CCR7 expression on DC, with CCR7 guiding DC to lymph nodes [51]. We can confirm that DC generated in PGE2-containing ‘MCM-Mimic’ from AML and MDS-MNC are more mature and CCR7+, although functional migration assays are still missing. PGE2 combined with the biological response modifier OK-432 could possibly give rise to CCR7+ mature DC after a 7-day-culture [50]. Although ‘standard’ media or ‘MCM-Mimic’ media, both used by us, are potent DC stimulatory methods, DC could not be generated from every patient. Westers and Houtenbos et al. [31]; recommend a DC-generation method using Calcium Ionophores plus IL-4, giving rise to mature leukemia-derived DC, other groups recommend cytokine-based short-time culture methods [45].
Those methods might help in generating DC also in AML or MDS cases with low DC harvests, thus yielding a vaccination strategy for most of the patients.
Acknowledgements
The authors gratefully acknowledge Oliver Schiekl, Elke Konhaeuser, Tatjana Heller and Sabine Kaiser and other co-workers of Prof. Kolbs hemopoietic transplantation group for technical assistance and advices, PD Dr. Claudia Schoch (MED III) and PD Dr. C. Doehner (University of Ulm) for disposal of cytogenetic reports, Dr. Schwartz (University of Berlin) and PD Dr. Kern (University of Munich) for disposal of immunophenotypes and Prof. Haferlach (MED III) for disposal of morphological reports. Parts of the results presented were worked out in the course of the thesis of Stefanie Kufner.
References
- 1.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343(14):1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum FR. New targets for therapy in acute myeloid leukemia. Leukemia. 2003;17(3):492–495. doi: 10.1038/sj.leu.2402810. [DOI] [PubMed] [Google Scholar]
- 3.Aul C, Giagounidis A, Germing U, Ganser A. Myelodysplastic syndromes. Diagnosis and therapeutic strategies. Med Klin. 2002;97(11):666–676. doi: 10.1007/s00063-002-1210-4. [DOI] [PubMed] [Google Scholar]
- 4.Balaian L, Ball ED. Direct effect of bispecific anti-CD33×anti-CD64 antibody on proliferation and signaling in myeloid cells. Leuk Res. 2001;25(12):1115–1125. doi: 10.1016/s0145-2126(01)00084-4. [DOI] [PubMed] [Google Scholar]
- 5.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976;33(4):451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 6.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51(2):189–199. [PubMed] [Google Scholar]
- 7.Blazar BR, Kwon BS, Panoskaltsis-Mortari A, Kwak KB, Peschon JJ, Taylor PA. Ligation of 4-1BB (CDw137) regulates graft-versus-host disease, graft-versus-leukemia, and graft rejection in allogeneic bone marrow transplant recipients. J Immunol. 2001;166(5):3174–3183. doi: 10.4049/jimmunol.166.5.3174. [DOI] [PubMed] [Google Scholar]
- 8.Brossart P. Dendritic cells in vaccination therapies of malignant diseases. Transfus Apher Sci. 2002;27:183–186. doi: 10.1016/s1473-0502(02)00041-1. [DOI] [PubMed] [Google Scholar]
- 9.Brossart P, Grunebach F, Stuhler G, Reichardt VL, Mohle R, Kanz L, et al. Generation of functional human dendritic cells from adherent peripheral blood monocytes by CD40 ligation in the absence of granulocyte-macrophage colony-stimulating factor. Blood. 1998;92(11):4238–4247. [PubMed] [Google Scholar]
- 10.Bruserud O, Gjertsen BT, von Volkman HL. In vitro culture of human acute myelogenous leukemia (AML) cells in serum-free media: studies of native AML blasts and AML cell lines. J Hematother Stem Cell Res. 2000;9(6):923–932. doi: 10.1089/152581600750062372. [DOI] [PubMed] [Google Scholar]
- 11.Buechner T, Hiddemann W, Berdel WE, Wormann B, Schoch C, Fonatsch C, et al. 6-Thioguanine, cytarabine, and daunorubicin (TAD) and high-dose cytarabine and mitoxantrone (HAM) for induction, TAD for consolidation, and either prolonged maintenance by reduced monthly TAD or TAD-HAM-TAD and one course of intensive consolidation by sequential HAM in adult patients at all ages with de novo acute myeloid leukemia (AML): a randomized trial of the German AML Cooperative Group. J Clin Oncol. 2003;21(24):4496–4504. doi: 10.1200/JCO.2003.02.133. [DOI] [PubMed] [Google Scholar]
- 12.Bunjes D. 188Re-labeled anti-CD66 monoclonal antibody in stem cell transplantation for patients with high-risk acute myeloid leukemia. Leukemia Lymphoma. 2002;43(11):2125–2131. doi: 10.1080/1042819021000033015. [DOI] [PubMed] [Google Scholar]
- 13.Campana D, Behm FG. Immunophenotyping of leukemia. J Immunol Methods. 2000;243:59–75. doi: 10.1016/s0022-1759(00)00228-3. [DOI] [PubMed] [Google Scholar]
- 14.Caux C, Massacrier C, Vanbervliet B, Dubois B, Durand I, Cella M, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor á: II functional analysis. Blood. 1997;4:1458–1470. [PubMed] [Google Scholar]
- 15.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 16.Charbonnier A, Gaugler B, Sainty D, Lafage-Pochitaloff M, Olive D. Human acute myeloblastic leukemia cells differentiate in vitro into mature dendritic cells and induce the differentiation of cytotoxic T cells against autologous leukemias. Eur J Immunol. 1999;29(8):2567–2578. doi: 10.1002/(SICI)1521-4141(199908)29:08<2567::AID-IMMU2567>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 17.Choudhury A, Liang JC, Thomas EK, Flores-Romo L, Xie QS, Agusala K, et al. Dendritic cells derived in vitro from acute myelogenous leukemia cells stimulate autologous, antileukemic T-cell responses. Blood. 1999;93(3):780–786. [PubMed] [Google Scholar]
- 18.Claxton DF, McMannis J, Champlin R, Choudhury A. Therapeutic potential of leukemia-derived dendritic cells: preclinical and clinical progress. Crit Rev Immunol. 2001;21:147–155. [PubMed] [Google Scholar]
- 19.De Vries IJ, Eggert AA, Scharenborg NM, Vissers JL, Lesterhuis WJ, Boerman OC, Punt CJ, Adema GJ, Figdor CG. Phenotypical and functional characterization of clinical grade dendritic cells. J Immunother. 2002;25(5):429–438. doi: 10.1097/00002371-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elghetany MT. Surface marker abnormalities in myelodysplastic syndromes. Haematologica. 1998;83(12):1104–1115. [PubMed] [Google Scholar]
- 22.Erben U, Thiel E, Bittroff-Leben A, Schoch C, Fichtner I, Durkop H, Notter M. CS-1, a novel c-kithi+ acute myeloid leukemia cell line with dendritic cell differentiation capacity and absent immunogenicity. Int J Cancer. 2003;105(2):232–240. doi: 10.1002/ijc.11053. [DOI] [PubMed] [Google Scholar]
- 23.Falkenburg JH, Smit WM, Willemze R. Cytotoxic T-lymphocyte (CTL) responses against acute or chronic myeloid leukemia. Immunol Rev. 1997;157:223–230. doi: 10.1111/j.1600-065x.1997.tb00985.x. [DOI] [PubMed] [Google Scholar]
- 24.Galea-Lauri J, Darling D, Mufti G, Harrison P, Farzaneh F. Eliciting cytotoxic T lymphocytes against acute myeloid leukemia-derived antigens: evaluation of dendritic cell-leukemia cell hybrids and other antigen-loading strategies for dendritic cell-based vaccination. Cancer Immunol Immunother. 2002;51(6):299–310. doi: 10.1007/s00262-002-0284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giles FJ, Keating A, Goldstone AH, Avivi I, Willmann CL, Kantarjian HM. Acute myeloid leukemia. Hematology. 2002;1:73–110. doi: 10.1182/asheducation-2002.1.73. [DOI] [PubMed] [Google Scholar]
- 26.Graf M , Reif S, Hecht K, Pelka-Fleischer R, Kroell T, Pfister K, Schmetzer H (2004) High expression of costimulatory molecules correlates with low relapse free-survival-probability in acute myeloid leukemia (AML). Ann Hemat, in press [DOI] [PubMed]
- 27.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, Ohyashiki K, Toyama K, Aul C, Mufti G, Bennett J. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–2088. [PubMed] [Google Scholar]
- 28.Haferlach T, Schoch C, Loffler H, Gassmann W, Kern W, Schnittger S, Fonatsch C, Ludwig WD, Wuchter C, Schlegelberger B, Staib P, Reichle A, Kubica U, Eimermacher H, Balleisen L, Gruneisen A, Haase D, Aul C, Karow J, Lengfelder E, Wormann B, Heinecke A, Sauerland MC, Buchner T, Hiddemann W. Morphologic dysplasia in de novo acute myeloid leukemia (AML) is related to unfavorable cytogenetics but has no independent prognostic relevance under the conditions of intensive induction therapy: results of a multiparameter analysis from the German AML Cooperative Group studies. J Clin Oncol. 2003;21(2):256–265. doi: 10.1200/JCO.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Harrison BD, Adams JA, Briggs M, Brereton ML, Liu Yin JA. Stimulation of autologous proliferative and cytotoxic T-cell responses by “leukemic dendritic cells” derived from blasts cells in acute myeloid leukemia. Blood. 2001;97:2764–2771. doi: 10.1182/blood.V97.9.2764. [DOI] [PubMed] [Google Scholar]
- 30.Hessel H, Mittermuller J, Zitzelsberger H, Weier HU, Bauchinger M. Combined immunophenotyping and FISH with sex chromosome-specific DNA probes for the detection of chimerism in epidermal Langerhans cells after sex-mismatched bone marrow transplantation. Histochem Cell Biol. 1996;106(5):481–485. doi: 10.1007/BF02473310. [DOI] [PubMed] [Google Scholar]
- 31.Hirai H. Molecular pathogenesis of MDS. Int J Hematol. 2002;2(Suppl 76):213–221. doi: 10.1007/BF03165120. [DOI] [PubMed] [Google Scholar]
- 32.van der Hoorn MA, van Luxemburg-Heijs SA, van Bergen CA, Bongaerts R, Willemze R, Falkenburg JH. The progenitor cell inhibition assay to measure the anti-leukemic reactivity of T cell clones against acute and chronic myeloid leukemia. Methods. 2003;31(2):113–119. doi: 10.1016/s1046-2023(03)00120-8. [DOI] [PubMed] [Google Scholar]
- 33.Houtenbos I, Westers TM, Stam AG, de Gruijl TD, Scheper RJ, Ossenkoppele GJ, et al. Serum-free generation of antigen presenting cells from acute myeloid leukaemic blasts for active specific immunisation. Cancer Immunol Immunother. 2003;52(7):455–462. doi: 10.1007/s00262-003-0389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kienzle N, Olver S, Buttigieg K, Kelso A. The fluorolysis assay, a highly sensitive method for measuring the cytolytic activity of T cells at very low numbers. J Immunol Methods. 2002;267(2):99–108. doi: 10.1016/s0022-1759(02)00150-3. [DOI] [PubMed] [Google Scholar]
- 35.Koehler T, Plettig R, Wetzstein W, Schmitz M, Ritter M, Mohr B, et al. Cytokine-driven differentiation of blasts from patients with acute myelogenous and lymphoblastic leukemia into dendritic cells. Stem Cells. 2000;18(2):139–147. doi: 10.1634/stemcells.18-2-139. [DOI] [PubMed] [Google Scholar]
- 36.Kufner S, Kroell T, Pelka- Fleischer R, Salem A, Zitzelsberger H, de Valle F, Zirpel I, Nuessler V, Schmetzer H (2004) Leukemia-derived dendritic cells (DC) can be generated from blood or bone marrow cells from patients with acute myeloid leukemia (AML) or myelodysplasia (MDS): a methodological approach under serum-free culture conditions (submitted for publication) [DOI] [PubMed]
- 37.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2(12):982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 38.Lee AW, Truong T, Bickham K, Fonteneau JF, Larsson M, Da Silva I, et al. A clinical grade cocktail of cytokines and PGE2 results in uniform maturation of human monocyte-derived dendritic cells: implications for immunotherapy. Vaccine. 2002;20(Suppl 4):A8–A22. doi: 10.1016/s0264-410x(02)00382-1. [DOI] [PubMed] [Google Scholar]
- 39.Li L, Schmitt A, Reinhardt P, Greiner J, Ringhoffer M, Vaida B, Bommer M, Vollmer M, Wiesneth M, Dohner H, Schmitt M. Reconstruction of CD40 and CD80 in dendritic cells generated from blasts of patients with acute myeloid leukemia. Cancer Immunol. 2003;3:8. [PubMed] [Google Scholar]
- 40.Mackensen A, Drager R, Schlesier M, Mertelsmann R, Lindemann A. Presence of IgE antibodies to bovine serum albumin in a patient developing anaphylaxis after vaccination with human peptide-pulsed dendritic cells. Cancer Immunol Immunother. 2000;49(3):152–156. doi: 10.1007/s002620050614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maeda A, Yamamoto K, Yamashita K, Asagoe K, Nohgawa M, Kita K, Iwasaki K, Ueda T, Takahashi A, Sasada M. The expression of costimulatory molecules and their relationship to the prognosis of human acute myeloid leukemia: Poor prognosis of B7.2-positive leukemia. Br J Haematol. 1998;102:1257–1262. doi: 10.1046/j.1365-2141.1998.00901.x. [DOI] [PubMed] [Google Scholar]
- 42.Maynadie M, Picard F, Husson B, Chatelain B, Cornet Y, Le Roux G, et al. Immunophenotypic clustering of myelodysplastic syndromes. Blood. 2002;100(7):2349–2356. doi: 10.1182/blood-2002-01-0230. [DOI] [PubMed] [Google Scholar]
- 43.Mitelman Supplement. 1995;to:An. [Google Scholar]
- 44.Nestle FO, Banchereau J, Hart D. Dendritic cells: on the move from the bench to bedside. Nat Med. 2001;7:761–765. doi: 10.1038/89863. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen XD, Eichler H, Dugrillon A, Piechaczek C, Braun M, Klueter H. Flow cytometric analysis of T cell proliferation in a mixed lymphocyte reaction with dendritic cells. J Immunol Methods. 2003;9281:1–12. doi: 10.1016/s0022-1759(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 46.Oehler L, Berer A, Kollars M, Keil F, Konig M, Waclavicek M, et al. Culture requirements for induction of dendritic cell differentiation in acute myeloid leukemia. Ann Hematol. 2000;79(7):355–362. doi: 10.1007/s002770000159. [DOI] [PubMed] [Google Scholar]
- 47.Panoskaltsis N, Belanger TJ, Liesveld JL, Abboud CN. Optimal cytokine stimulation for the enhanced generation of leukemic dendritic cells in short-term culture. Leuk Res. 2002;26(2):191–201. doi: 10.1016/s0145-2126(01)00104-7. [DOI] [PubMed] [Google Scholar]
- 48.Restifo NP, Esquivel F, Kawakami Y, et al. Identification of human cancers deficient in antigen processing. J Exp Med. 1993;177:265–272. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rigolin GM, Howard J, Buggins A, Sneddon C, Castoldi G, Hirst WJR, et al. Phenotypic and functional characteristics of monocyte-derived dendritic cells from patients with myelodyplastic syndromes. Br J Haematol. 1999;107:844–850. doi: 10.1046/j.1365-2141.1999.01781.x. [DOI] [PubMed] [Google Scholar]
- 50.Sallusto F, Lanzavecchia A. The instructive role of dendritic cells on T-cell responses. Arthritis Res. 2002;4(Suppl 3):127–132. doi: 10.1186/ar567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santiago-Schwarz F, Coppock DL, Hindenburg AA, Kern J. Identification of a malignant counterpart of the monocyte-dendritic cell progenitor in an acute myeloid leukemia. Blood. 1994;84(9):3054–3062. [PubMed] [Google Scholar]
- 52.Sato M, Takayama T, Tanaka H, Konishi J, Suzuki T, Kaiga T, Tahara H. Generation of mature dendritic cells fully capable of T helper type 1 polarization using OK-432 combined with prostaglandin E(2) Cancer Sci. 2003;94(12):1091–1098. doi: 10.1111/j.1349-7006.2003.tb01405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15(2):138–147. doi: 10.1016/S0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 54.Song LP, Cheng JL, Wang XB, Zhang Z, Fang M, Zhou ZY, et al. A new model of trispecific antibody resulting the cytotoxicity directed against tumor cells. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 2003;35(6):503–510. [PubMed] [Google Scholar]
- 55.Stripecke R, Levine AM, Pullarkat V, Cardoso AA. Immunotherapy with acute leukemia cells modified into antigen-presenting cells: ex vivo culture and gene transfer methods. Leukemia. 2002;16:1974–1983. doi: 10.1038/sj.leu.2402701. [DOI] [PubMed] [Google Scholar]
- 56.Strobl H, Bello-Fernandez C, Riedl E, Pickl WF, Majdic O, Lyman SD, et al. flt3 ligand in cooperation with transforming growth factor-beta1 potentiates in vitro development of Langerhans-type dendritic cells and allows single-cell dendritic cell cluster formation under serum-free conditions. Blood. 1997;90(4):1425–1434. [PubMed] [Google Scholar]
- 57.Suen Y, Lee SM, Aono F, Hou S, Loudovaris M, Ofstein G, et al. Comparison of monocyte enrichment by immuno-magnetic depletion or adherence for the clinical-scale generation of DC. Cytotherapy. 2001;3(5):365–375. doi: 10.1080/146532401753277184. [DOI] [PubMed] [Google Scholar]
- 58.Trail PA, King HD, Dubowchik GM. Monoclonal antibody drug immunoconjugates for targeted treatment of cancer. Cancer Immunol Immunother. 2003;52(5):328–337. doi: 10.1007/s00262-002-0352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsimberidou A, Estey E, Cortes J, Thomas D, Faderl S, Verstovsek S, et al. Gemtuzumab, fludarabine, Cytarabine, and cyclosporine in patients with newly diagnosed acute myelogenous leukemia or high-risk myelodysplastic syndromes. Cancer. 2003;97(6):1481–1487. doi: 10.1002/cncr.11239. [DOI] [PubMed] [Google Scholar]
- 60.Tsuchiya T, Hagihara M, Shimakura Y, Ueda Y, Gansuvd B, Munkhbat B, Inoue H, Tazume K, Kato S, Hotta T. The generation of immunocompetent dendritic cells from CD34+ acute myeloid or lymphoid leukemia cells. Int J Hematol. 2002;75(1):55–62. doi: 10.1007/BF02981980. [DOI] [PubMed] [Google Scholar]
- 61.Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, Riddell SR. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333(16):1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 62.Wang L, Chen S, Liu Y, Fu J, Yu H, Li J, et al. The function of dendritic cells derived from chronic myeloid leukemia. Zhongguo Shi Yan Xue Za Zhi. 2000;8(3):161–165. [PubMed] [Google Scholar]
- 63.Woiciechowsky A, Regn S, Kolb H-I, Roskrow M. Leukemic dendritic cells generated in the presence of FLT3 ligand have the capacity to stimulate an autologous leukemia-specific cytotoxic T cell response from patients with acute myeloid leukemia. Leukemia. 2001;15:246–255. doi: 10.1038/sj.leu.2402013. [DOI] [PubMed] [Google Scholar]
- 64.Zhong RK, Rassenti LZ, Kipps TJ, Chen J, Law P, Yu JF, Ball ED. Sequential modulation of growth factors: a novel strategy for adoptive immunotherapy of acute myeloid leukemia. Biol Blood Marrow Tr. 2002;8(10):557–568. doi: 10.1053/bbmt.2002.v8.pm12434951. [DOI] [PubMed] [Google Scholar]