Abstract
Background
Ipilimumab can induce durable disease control and long-term survival in patients with metastatic melanoma. Identification of a biomarker that correlates with clinical benefit and potentially provides an early marker of response is an active area of research.
Patients and methods
Ipilimumab was available upon physician request for patients aged ≥16 years with stage III (unresectable) or IV cutaneous, ocular or mucosal melanoma, who had failed or did not tolerate previous treatments and had no other therapeutic option available. Patients received ipilimumab 3 mg/kg every 3 weeks for four doses. Tumour assessments were conducted at baseline, Week 12 and Week 24 using immune-related response criteria. Patients were monitored continuously for adverse events (AEs), including immune-related AEs. Candidate immunological markers were evaluated in peripheral blood and sera samples collected at baseline and Weeks 4, 7, 10 and 12.
Results
Among 95 patients treated with ipilimumab 3 mg/kg, the immune-related disease control rate at Week 24 was 38 %. With a median follow-up of 24 months, median overall survival was 9.6 months. Both disease control and survival were significantly associated with decreasing levels of lactate dehydrogenase, C-reactive protein and FoxP3/regulatory T cells, and increasing absolute lymphocyte count, between baseline and the end of dosing (Week 12).
Conclusion
Ipilimumab is a feasible treatment option for heavily pretreated patients with metastatic melanoma. Changes in some immunological markers between baseline and the fourth ipilimumab infusion appear to be associated with disease control and survival, but verification in prospective clinical trials is required.
Keywords: Biomarker, Expanded access programme, Immunological, Ipilimumab, Melanoma
Introduction
Ipilimumab, a monoclonal antibody against cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), was the first agent approved for the treatment of unresectable or metastatic (advanced) melanoma that showed an overall survival (OS) benefit in a randomised phase III trial [1]. Clinical trial data have consistently shown that ipilimumab confers meaningful clinical benefit to patients with advanced melanoma, with extended follow-up of phase II trials indicating that ipilimumab can induce durable, potentially curative, tumour regression in some patients [2].
Recently, several other agents have been developed for the treatment of advanced melanoma that target specific molecular mutations. These include vemurafenib and dabrafenib for patients with mutations in the gene encoding BRAF, a key component of the mitogen-activated protein kinase signalling pathway, and trametinib and MEK162 for patients with NRAS or BRAF mutations, which inhibit downstream MEK [3–6]. Although the presence of mutations does not guarantee a positive response to treatment, BRAF mutations facilitate patient selection by excluding those who will not benefit. By contrast, no clinical parameter has consistently been found to be a predictive marker of clinical benefit with ipilimumab, nor have any parameters been validated as a surrogate marker of response or survival benefit.
The identification of potential biomarkers for ipilimumab is an important goal of ongoing research. Predictive biomarkers could potentially be used for estimating treatment outcomes, by identifying those patients who are most likely to benefit from treatment and/or providing an early indication of response to therapy [7, 8]. However, finding biomarkers for immunotherapy is challenging, primarily due to the complexity of the immune system and its interaction with the tumour microenvironment [9]. A number of parameters have previously been explored in patients treated with antibodies against CTLA-4, including gene polymorphisms, the number and function of immune cells, and soluble factors such as lactate dehydrogenase (LDH) or C-reactive protein (CRP), with variable results [10–13].
Here, we explore whether candidate immunological markers can be used to monitor responses in patients with metastatic melanoma treated with ipilimumab 3 mg/kg in an expanded access programme (EAP) in Italy and assess the relationship between each of these parameters, singularly or combined, and survival. Preliminary results from this analysis have been presented previously at the American Society of Clinical Oncology Annual Meeting, Chicago, Illinois, USA, 1–5 June 2012 [14].
Methods
Patients
Adult patients, ≥16 years, with unresectable stage III or IV cutaneous, ocular or mucosal melanoma were eligible for treatment if they had previously failed or were intolerant to ≥1 systemic therapy, had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2, and had no other therapeutic option available. Previous systemic treatment should have been completed ≥28 days before receiving ipilimumab. The EAP protocol was approved by the local independent ethics committees, and all participating patients provided signed informed consent before enrolment.
Treatment
Patients were treated with intravenous ipilimumab 3 mg/kg every 3 weeks, for a total of four doses. In the absence of dose-limiting toxicities, it was recommended that patients with a stable PS received all four doses of ipilimumab. Dose reduction or modification was not allowed, but dose omission or discontinuation was recommended when necessary. Patients with disease progression following either SD of ≥3 months’ duration or an initial objective response (partial response [PR] or complete response [CR]) were eligible for retreatment with ipilimumab 3 mg/kg every 3 weeks for four doses.
Assessments
Tumour assessments were conducted at baseline, after completion of treatment (Week 12) and at Week 24 according to immune-related response criteria (irRC) [15]. Clinical benefit was represented by the immune-related best overall response rate (irBORR) at any time and the immune-related disease control rate (irDCR) at Week 24, defined as the percentage of patients with immune-related CR, PR or SD.
Adverse events were continuously monitored and assessed in all treated patients and were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. AEs were managed using protocol-specific guidelines.
Candidate immunological markers, comprising LDH, CRP, FoxP3/regulatory T cells (Tregs), absolute lymphocyte count (ALC) and white blood cell (WBC) count, were evaluated in peripheral blood and sera samples collected at baseline and Weeks 4, 7, 10 and 12. For Tregs, patients were grouped according to whether they had a ‘low’ result (<0.5 %), an ‘intermediate’ result (0.5–2.6 %) or a ‘high’ result (>2.6 %). These cut-off points were selected based on the range (0.5–2.6 %) observed at diagnosis in a separate cohort of approximately 50 patients with advanced melanoma (data not published).
The number of Tregs was determined by phenotypic evaluation as previously described [16]. Briefly, Treg levels were measured using a flow cytometry assay for peripheral blood mononuclear cells (PBMCs) in CD4+ cells. Cells, stained with antibodies against FoxP3/CD25, were identified according to the expression of CD4+, CD25hi and FoxP3+ by fluorescence-activated cell sorting (FACS) using the FACS ARIA II flow cytometry system and FACS Diva™ software (BD Biosciences; Mountain View, CA, USA). All PBMC samples were analysed within 3 h of collection.
Statistical analysis
Patient and disease characteristics were analysed using descriptive statistics and expressed as either relative frequency (percentages) for discrete variables or median for continuous variables. OS was estimated using Kaplan–Meier analysis and expressed as a median value with corresponding two-sided 95 % confidence intervals (CIs). Differences between survival curves were compared using a log-rank test. Changes in immunological parameters were calculated at Week 12 with respect to basal values and correlated with rates of disease control using the Mann–Whitney test. If Week 12 data were not available, the last determination was considered.
Results
Patients
Among 120 patients enrolled in the EAP at the National Cancer Institute, Naples, Italy, between June 2010 and January 2012, 95 (79 %) received at least one dose of ipilimumab and were eligible for analysis. Baseline characteristics of these patients are provided in Table 1. Most patients had cutaneous melanoma; however, 14 (14 %) had melanoma that was ocular (n = 7) or mucosal (n = 7) in origin. All patients were pretreated, as per protocol, and 48 patients (50 %) had received two or more prior therapies. No patients had received previous treatment with an anti-CTLA-4 antibody although this was not an exclusion criterion in the EAP. Most patients (83 %) had advanced M1c disease, and approximately one-third of patients had asymptomatic brain metastasis at baseline. Among 80 patients who underwent mutational analysis, 21 (26 %) were BRAFV600-mutation positive; 4 patients (5 %) had a mutation in NRAS, including 1 patient who also had the BRAFV600 mutation.
Table 1.
Baseline characteristics of patients who received at least one dose of ipilimumab 10 mg/kg
| Characteristic | N = 95 |
|---|---|
| Median age, years (range) | 58 (17–84) |
| Male/female, n (%) | 54 (57)/41 (43) |
| Time from diagnosis, months (range) | 30 (16–45) |
| Disease stage, n (%) | |
| IIIc | 10 (11) |
| IV M1a | 2 (2) |
| IV M1b | 4 (4) |
| IV M1c | 79 (83) |
| ECOG PS, n (%) | |
| 0 | 63 (66) |
| 1 | 30 (32) |
| 2 | 2 (2) |
| Tumour subtype, n (%) | |
| Cutaneous | 76 (80) |
| Mucosal | 7 (7) |
| Ocular | 7 (7) |
| Unknown | 5 (5) |
| Patients with brain metastases, n (%) | 30 (32) |
| Patients with liver metastases, n (%) | 51 (54) |
| Elevated LDH (>480 IU/L), n (%) | 43 (45) |
| BRAFV600 mutation positive, n/n (%) | 21/80 (26)a |
| NRAS mutation positive, n/n (%) | 4/80 (5)a |
| Number of previous therapies, n (%) | |
| 1 | 47 (50) |
| 2 | 45 (47) |
| ≥3 | 3 (3) |
| Previous therapy, n (%) | |
| Cisplatin plus temozolomide | 55 (58) |
| Dacarbazine | 40 (42) |
| Fotemustine | 15 (16) |
| Vemurafenib | 8 (8) |
| Dabrafenib | 5 (5) |
| MEK162 | 3 (3) |
ECOG PS Eastern Cooperative Oncology Group performance status; LDH lactate dehydrogenase
a1 patient had a BRAF and an NRAS mutation
Of the 95 patients, 76 (80 %) received all four doses of ipilimumab, 11 (12 %) received three doses, 7 (7 %) received two doses and 1 (1 %) received one dose. The most common reason for not completing treatment was rapid disease progression. Six patients (6 %) were retreated with ipilimumab 3 mg/kg upon disease progression.
Efficacy and immunological markers of response
The irDCR at Week 24 was 38 %, comprising 36 patients with an irCR (n = 5), irPR (n = 13) or irSD (n = 18). With median follow-up times of 22 and 24 months for patients with an irCR or irPR, respectively, three irCRs and four irPRs were ongoing. For the remaining patients, median times to progression were 21 months for patients who had achieved an irCR and 18 months for patients who had achieved an irPR. Of the 18 patients with irSD, 13 had progressed after a median of 6.3 months. The irBORR at any time was 26 %, and tumour response appeared independent of BRAF/NRAS mutational status (Table 2). Of the 6 patients retreated with ipilimumab, best response to retreatment was immune-related progressive disease (irPD) in 4 patients (67 %) and irPR in 2 patients (33 %).
Table 2.
Correlation between tumour response and BRAF/NRAS status
| Response according to irRC, n (%) | BRAFV600 mutation | NRAS mutation | ||
|---|---|---|---|---|
| Positivea (n = 20) | Negative (n = 59) | Positive (n = 4) | Negativea (n = 75) | |
| irCR | 2 (10) | 3 (5) | 1 (25) | 4 (5) |
| irPR | 6 (30) | 14 (24) | 1 (25) | 19 (25) |
| irSD | 3 (15) | 19 (32) | 1 (25) | 21 (28) |
| irPD | 9 (45) | 23 (39) | 1 (25) | 31 (41) |
| irBORR | 8 (40) | 17 (29) | 2 (50) | 23 (31) |
| P value | 0.35 | 0.42 | ||
| irDCR | 11 (61) | 36 (55) | 3 (75) | 44 (49) |
| P value | 0.63 | 0.52 | ||
irBORR immune-related best overall response rate; irCR immune-related complete response; irDCR immune-related disease control rate; irPD immune-related progressive disease; irPR immune-related partial response; irRC immune-response criteria; irSD immune-related stable disease
aData missing from 1 patient
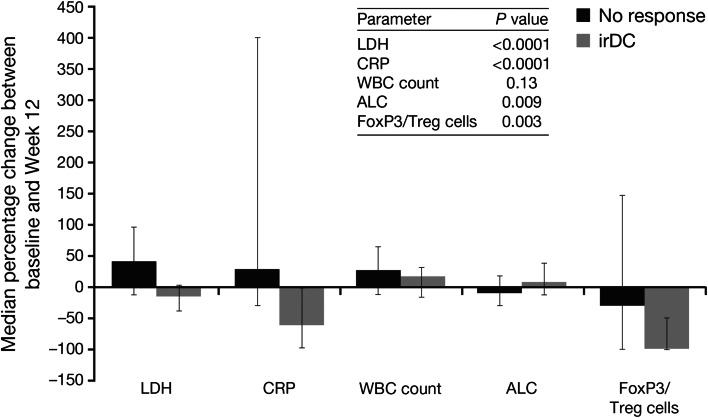
Immunological markers of response were assessed in peripheral blood and sera samples collected from all 95 patients. Categorised levels of LDH, CRP, FoxP3/Treg cells, WBC count and ALC at baseline and Weeks 4, 7, 10 and 12 for patients with and without disease control are shown in Table 3. In general, the proportion of patients with LDH >1.1 × ULN decreased between baseline and Week 12 among patients with disease control and increased in patients without disease control; a similar trend was noted for CRP levels. At Week 12, all of the patients who had disease control had a ‘low’ result (<0.5 %) for Tregs, whereas only 46 % of patients without disease control had <0.5 % Tregs and 45 % had ‘high’ levels (>2.6 %), By contrast, the percentage of patients with a higher ALC increased over time in the group with disease control and decreased in patients without disease control. Between baseline and Week 12, decreasing levels of LDH, CRP and FoxP3/Treg cells, and increasing levels of ALC were all significantly associated with disease control (Fig. 1). The change in absolute numbers of FoxP3/Treg cells showed the same trend as the percentages, i.e. the number of FoxP3/Treg cells in the periphery increased in patients with disease control and decreased in patients without disease control (data not shown). Conversely, the WBC count generally increased between baseline and Week 12 in both patient groups, and there was no significant association between these changes and disease control (P = 0.13).
Table 3.
Levels of immunological markers at baseline and Weeks 4, 7, 10 and 12
| n/n a, % | Baseline | Week 4 | Week 7 | Week 10 | Week 12 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| irDC (n = 36) | No response (n = 59) | irDC (n = 36) | No response (n = 59) | irDC (n = 36) | No response (n = 59) | irDC (n = 36) | No response (n = 59) | irDC (n = 36) | No response (n = 59) | |
| LDH (×ULN) | ||||||||||
| ≤1.1 | 27/36 (75) | 25/59 (42) | 23/35 (66) | 20/58 (34) | 22/33 (67) | 13/53 (25) | 24/29 (83) | 10/46 (22) | 24/25 (96) | 9/32 (28) |
| >1.1 | 9/36 (25) | 34/59 (58) | 12/35 (34) | 38/58 (66) | 11/33 (33) | 40/53 (75) | 5/29 (17) | 36/46 (78) | 1/25 (4) | 23/32 (72) |
| CRP (mg/mL) | ||||||||||
| <5 | 19/34 (56) | 22/59 (37) | 21/30 (70) | 16/47 (34) | 23/30 (77) | 12/41 (29) | 23/29 (79) | 9/32 (28) | 20/27 (74) | 10/35 (29) |
| ≥5 to ≤8 | 5/34 (15) | 17/59 (29) | 2/30 (7) | 8/47 (17) | 4/30 (13) | 9/41 (22) | 5/29 (17) | 6/32 (19) | 5/27 (19) | 10/35 (29) |
| >8 | 10/34 (29) | 20/59 (34) | 7/30 (23) | 23/47 (49) | 3/30 (10) | 20/41 (49) | 1/29 (3) | 17/32 (53) | 2/27 (7) | 15/35 (42) |
| Tregs (%) | ||||||||||
| <0.5 | 13/29 (45) | 13/34 (38) | 8/26 (31) | 10/59 (17) | 13/36 (36) | 5/59 (8) | 20/36 (56) | 0 | 36/36 (100) | 26/56 (46) |
| 0.5 to 2.6 | 16/29 (55) | 19/34 (56) | 18/26 (69) | 40/59 (68) | 23/36 (64) | 45/59 (76) | 16/36 (44) | 35/59 (59) | 0 | 5/56 (9) |
| >2.6 | 0 | 2/34 (6) | 0 | 9/59 (15) | 0 | 9/59 (15) | 0 | 24/59 (41) | 0 | 25/56 (45) |
| WBC (×109/L) | ||||||||||
| <3 | 4/36 (11) | 2/58 (3) | 20/36 (56) | 27/59 (46) | 20/36 (56) | 27/59 (46) | 18/36 (50) | 7/36 (19) | 0 | 0 |
| ≥3 | 32/36 (89) | 56/58 (97) | 16/36 (44) | 32/59 (54) | 16/36 (44) | 32/59 (54) | 18/36 (50) | 29/36 (81) | 34/34 (100) | 54/54 (100) |
| ALC (×109/L) | ||||||||||
| <1 | 2/36 (6) | 13/59 (22) | 3/36 (8) | 10/59 (17) | 2/36 (6) | 20/59 (34) | 0 | 17/59 (29) | 0 | 14/55 (25) |
| ≥1 | 34/36 (94) | 46/59 (78) | 33/36 (92) | 49/59 (83) | 34/36 (94) | 39/59 (66) | 36/36 (100) | 42/59 (71) | 36/36 (100) | 41/55 (75) |
ALC absolute lymphocyte count; CRP C-reactive protein; irDC immune-related disease control; LDH lactate dehydrogenase; Treg regulatory T cells; ULN upper limit of normal; WBC white blood cell
aIndicates the total number of patients within each group for whom data were available at Weeks 4, 7, 10 and 12
Fig. 1.
Median percentage change in immunological markers among patients with and without irDC. Error bars show the interquartile range. ALC absolute lymphocyte count, CRP C-reactive protein, irDC immune-related disease control, LDH lactate dehydrogenase, Treg regulatory T cells, WBC white blood cell
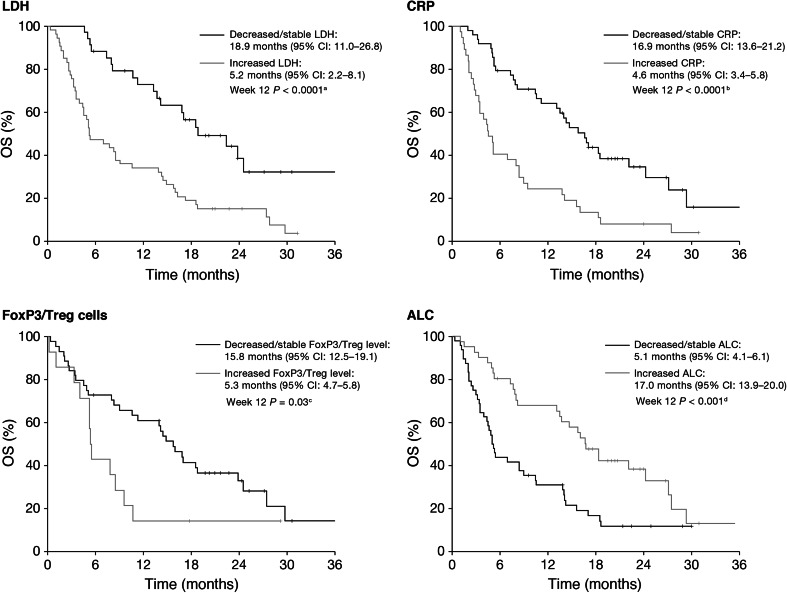
With a median follow-up of 24 months (range 1–36 months), median OS for all patients was 9.6 months (95 % CI 4.7–14.4), with 1- and 2-year survival rates of 46 and 22 %, respectively. A decrease or no change in LDH, CRP and FoxP3/Treg cells between baseline and Week 12 was significantly associated with survival, compared with an increase in these markers; an increase in ALC between baseline and Week 12 was also significantly associated with survival (Fig. 2). No significant association was identified between the change in WBC count from baseline to Weeks 4, 7, 10 or 12 and survival (P = 0.07, P = 0.17, P = 0.90 and P = 0.26, respectively). There were no statistically significant differences in median OS when patients were compared by disease stage (stage III vs. stage IV; P = 0.95) or by the presence/absence of brain metastases (P = 0.85).
Fig. 2.
Relationship between changes in immunological markers, and OS. a Week 4 P = 0.42; Week 7 P = 0.06; Week 10 P = 0.04. b Week 4 P = 0.01; Week 7 P = 0.003; Week 10 P < 0.0001. c Week 4 P = 0.16; Week 7 P = 0.97; Week 10 P = 0.09. d Week 4 P = 0.48; Week 7 P = 0.73; Week 10 P = 0.14. ALC absolute lymphocyte count, CI confidence interval, CRP C-reactive protein, LDH lactate dehydrogenase, OS overall survival, Treg regulatory T cells
Survival appeared independent of BRAF/NRAS mutational status. For patients with and without BRAF/NRAS mutations, median OS was 11.3 months (95 % CI 4.7–17.9) and 10.6 months (95 % CI 3.9–17.3), respectively. One- and two-year survival rates were 50 and 28 % for patients with a BRAF/NRAS mutation, and 48 and 22 % for wild-type patients.
Safety
Among all treated patients, 38 (40 %) reported at least one AE, which were grade 1 or 2 in all cases, with the exception of 2 patients who had grade 3 haematological toxicities (neutropenia and anaemia). Grade 1 or 2 AEs comprised pruritis (n = 27), rash (n = 4), diarrhoea (n = 3), hyper- or hypothyroiditis (n = 3) and increased liver enzymes (n = 2), and no patients required corticosteroid treatment for the resolution of these AEs. In both patients with grade 3 haematological AEs, toxicities appeared after the third dose of ipilimumab and were determined to be ipilimumab-related by autoimmune tests (positive Coombs test and increase of reticulocytes). Consequently, ipilimumab was discontinued and the patients were treated with high-dose corticosteroids until blood cell counts recovered. Median time to resolution of haematological AEs was 4 weeks (range: 2–6 weeks), and corticosteroids were tapered after 8–10 weeks. No patients with lower grade AEs required corticosteroids.
Discussion
Historically, patients with metastatic melanoma had a poor prognosis [17]; however, with the approval of ipilimumab and vemurafenib, and recent Food and Drug Administration-approval of dabrafenib and trametinib, new treatment options with proven survival benefits are now available [1, 3–6]. To date, experience with these agents outside of clinical trials is limited. The EAP provided an opportunity to assess the efficacy and safety of ipilimumab in a setting similar to daily clinical practice. The data show that, similar to the clinical trial setting, ipilimumab was generally well tolerated, and patients had durable clinical benefit, with approximately one-fifth having long-term survival of at least 2 years. Additionally, no association was observed between clinical activity and the presence of BRAF/NRAS mutations, suggesting patients with pretreated advanced melanoma can receive ipilimumab, regardless of mutational status.
Identifying predictive biomarkers and early markers of response with ipilimumab treatment are important goals of current research. At present, no definitive biomarkers have been identified that can be used to predict which patients are most likely to derive clinical benefit. Several preliminary biomarkers have been investigated, but none have been validated in second studies [11, 13, 18–22]. Recently, studies have indicated that early changes in immunological markers may be associated with improved survival. In an analysis of patients treated as part of the EAP in France, increases in ALC and eosinophil count after treatment with ipilimumab 3 mg/kg both correlated with improved survival [23]. Additionally, among 27 patients treated with ipilimumab 10 mg/kg within an EAP, changes in the number of circulating T cells that expressed inducible T cell costimulator during the early stages of treatment, and a low ratio between absolute neutrophil count and ALC, were also associated with better survival [24]. This is consistent with other analyses of patients treated in the EAP, where a high ALC after two doses of ipilimumab or at 6 weeks, was significantly associated with survival [13, 25]. The association of changes in ALC with survival was also recently assessed among approximately 2,000 patients who had received ipilimumab (at various doses, and as either monotherapy or in combination with chemotherapy) as part of their treatment regimen. Consistent with its proposed mechanism of action, treatment with ipilimumab resulted in an increase in mean ALC. However, while a positive association was observed between the rate of increase in ALC and survival, absolute changes in ALC were not found to be specifically predictive of improved survival [26]. By contrast, in the present analysis, an increase in ALC between baseline and Week 12 was significantly associated with disease control and survival. Therefore, further investigations of the potential utility of ALC as an on-treatment pharmacodynamic marker of ipilimumab activity are warranted.
Elevated LDH is known to be prognostic of poor survival in patients with metastatic melanoma [27]. Furthermore, CRP, which is produced as a non-specific acute phase response to most forms of inflammation, is a prognostic factor for patients with metastatic melanoma treated with interleukin-2-based therapies [28]. However, baseline levels of neither of these parameters have been shown to be routinely predictive of treatment benefit with anti-CTLA-4 antibodies in clinical trials [1, 11, 12]. In the registrational phase 3 trial, the survival benefit with ipilimumab was found to be independent of LDH [1]. By comparison, in a subanalysis of patients treated with ipilimumab in a phase II trial, higher baseline CRP levels were associated with improved freedom from relapse and a marginally significant improvement in relapse-free survival. Conversely, among patients treated with the anti-CTLA-4 antibody tremelimumab, low baseline levels of CRP were positively associated with survival [11, 12]. In the ipilimumab EAP in Italy, a decrease in both LDH and CRP levels between baseline and Week 12 was significantly associated with disease control and survival, and three-quarters of patients with disease control had low LDH levels at baseline. These results suggest that assessing LDH and CRP at both baseline and Week 12 may be necessary when considering pharmacodynamic markers of treatment outcome. When 68 patients treated within the UK, EAP were stratified according to baseline LDH ≤upper limit of normal (ULN) or >2xULN, the observed DCR was 48 versus 14 %, and median OS was 13.7 versus 3.1 months, suggesting baseline LDH could potentially be used to determine which patients are less likely to benefit from ipilimumab [29]. However, these data should be interpreted with caution due to the small sample size and lack of a control study arm. Confirmation from well-designed, controlled clinical trials is required before these results can be applied to clinical management.
Naturally occurring Tregs, which express FoxP3, act to suppress antitumour immune responses; therefore, the observed decrease in FoxP3/Tregs in patients with a positive clinical outcome in this analysis supports the idea that an immune-active tumour microenvironment is necessary for a favourable response to ipilimumab. Similarly, in a previous analysis, patients who did not respond to ipilimumab tended to have a greater Treg response in peripheral blood than those who responded [30]. It is possible that a lower frequency of FoxP3/Tregs in the peripheral blood of clinical responders reflects migration to the tumour site. Surprisingly, retrospective studies of patients treated with ipilimumab or tremelimumab have shown that high baseline expression of FoxP3 within the tumour microenvironment is associated with a better prognosis [20]. This paradox, which has also been observed in patients with colorectal cancer and head and neck cancer [31], may occur if FoxP3 is expressed by T cell populations other than Tregs, or if expression is induced by pro-inflammatory cytokines present in the tumour microenvironment [20]. Taking this into consideration, it was hypothesised that Tregs, and thus FoxP3, may be upregulated in the tumours of patients with ongoing, yet suboptimal, antitumour immune responses and that these individuals may be more likely to respond to ipilimumab [20].
The results of this EAP show that ipilimumab is a feasible treatment option for previously treated patients with advanced melanoma, regardless of BRAF or NRAS mutational status. Disease control and survival were significantly associated with decreasing levels of LDH, CRP and FoxP3/Tregs and increasing ALC over the course of treatment. However, further prospective, controlled studies are required to determine whether pharmacodynamic changes in these markers are predictive of treatment effect or simply prognostic for survival.
Acknowledgments
This work was supported in part by the Italian Ministry of Health, via the Ricerca Corrente 2010. The authors would like to thank the patients and investigators who participated in the European EAP. The EAP was sponsored by Bristol-Myers Squibb. Editorial and writing assistance was provided by StemScientific, funded by Bristol-Myers Squibb (BMS).
Abbreviations
- AE
Adverse event
- ALC
Absolute lymphocyte count
- BORR
Best overall response rate
- CI
Confidence interval
- CR
Complete response
- CRP
C-reactive protein
- CTLA-4
Cytotoxic T-lymphocyte-associated antigen-4
- DCR
Disease control rate
- EAP
Expanded access programme
- ECOG
Eastern Cooperative Oncology Group
- FACS
Fluorescence-activated cell sorting
- ir
Immune-related
- irRC
Immune-related response criteria
- LDH
Lactate dehydrogenase
- OS
Overall survival
- PD
Progressive disease
- PBMC
Peripheral blood mononuclear cell
- PR
Partial response
- PS
Performance status
- SD
Stable disease
- Tregs
Regulatory T cells
- ULN
Upper limit of normal
- WBC
White blood cell
References
- 1.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18:2039–2047. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 5.Ascierto PA, Schadendorf D, Berking C, Agarwala SS, van Herpen CM, Queirolo P, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol. 2013;14:249–256. doi: 10.1016/S1470-2045(13)70024-X. [DOI] [PubMed] [Google Scholar]
- 6.Kim KB, Kefford R, Pavlick AC, Infante JR, Ribas A, Sosman JA, et al. Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol. 2013;31:482–489. doi: 10.1200/JCO.2012.43.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atkinson AJ, Magnuson WG, Colbern WA, DeGrutolla VG, DeMets DL, Downing GJ, et al. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 8.Oldenhuis CN, Oosting SF, Gietema JA, de Vries EG. Prognostic versus predictive value of biomarkers in oncology. Eur J Cancer. 2008;44:946–953. doi: 10.1016/j.ejca.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Fox BA, Schendel DJ, Butterfield LH, Aamdal S, Allison JP, Ascierto PA, et al. Defining the critical hurdles in cancer immunotherapy. J Transl Med. 2011;9:214. doi: 10.1186/1479-5876-9-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menard C, Ghiringhelli F, Roux S, Chaput N, Mateus C, Grohmann U, et al. CTLA-4 blockade confers lymphocyte resistance to regulatory T-cells in advanced melanoma: surrogate marker of efficacy of tremelimumab? Clin Cancer Res. 2008;14:5242–5249. doi: 10.1158/1078-0432.CCR-07-4797. [DOI] [PubMed] [Google Scholar]
- 11.Sarnaik AA, Yu B, Yu D, Morelli D, Hall M, Bogle D, et al. Extended dose ipilimumab with a peptide vaccine: immune correlates associated with clinical benefit in patients with resected high-risk stage IIIc/IV melanoma. Clin Cancer Res. 2011;17:896–906. doi: 10.1158/1078-0432.CCR-10-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall MA, Ribas A, Huang B (2010) Evaluation of baseline serum C-reactive protein (CRP) and benefit from tremelimumab compared to chemotherapy in first-line melanoma. J Clin Oncol 28: abstr 2609
- 13.Ku GY, Yuan J, Page DB, Schroeder SE, Panageas KS, Carvajal RD, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767–1775. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simeone E, Gentilcore G, Romano A, Daponte A, Caraco C, Grimaldi AM, et al. Immunological and Biological Changes During Ipilimumab Treatment and their Correlation with Clinical Response and Survival. J Clin Oncol. 2012;30:8573. [Google Scholar]
- 15.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 16.Ascierto PA, Napolitano M, Celentano E, Simeone E, Gentilcore G, Daponte A, et al. Regulatory T cell frequency in patients with melanoma with different disease stage and course, and modulating effects of high-dose interferon-alpha 2b treatment. J Transl Med. 2010;8:76. doi: 10.1186/1479-5876-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korn EL, Liu PY, Lee SJ, Chapman JA, Niedzwiecki D, Suman VJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26:527–534. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 18.Ascierto PA, Kalos M, Schaer DA, Callahan MK, Wolchok JD. Biomarkers for immunostimulatory monoclonal antibodies in combination strategies for melanoma and other tumor types. Clin Cancer Res. 2013;19:1009–1020. doi: 10.1158/1078-0432.CCR-12-2982. [DOI] [PubMed] [Google Scholar]
- 19.Breunis WB, Tarazona-Santos E, Chen R, Kiley M, Rosenberg SA, Chanock SJ. Influence of cytotoxic T lymphocyte-associated antigen 4 (CTLA4) common polymorphisms on outcome in treatment of melanoma patients with CTLA-4 blockade. J Immunother. 2008;31:586–590. doi: 10.1097/CJI.0b013e31817fd8f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamid O, Schmidt H, Nissan A, Ridolfi L, Aamdal S, Hansson J, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011;9:204. doi: 10.1186/1479-5876-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W, Yu D, Sarnaik AA, Yu B, Hall M, Morelli D, et al. Biomarkers on melanoma patient T cells associated with ipilimumab treatment. J Transl Med. 2012;10:146. doi: 10.1186/1479-5876-10-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF, et al. Integrated NY-ESO-1 antibody and CD8 + T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci USA. 2011;108:16723–16728. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delyon J, Mateus C, Lefeuvre D, Lanoy E, Zitvogel L, Chaput N, et al. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol. 2013;24:1697–1703. doi: 10.1093/annonc/mdt027. [DOI] [PubMed] [Google Scholar]
- 24.Di Giacomo AM, Calabro L, Danielli R, Fonsatti E, Bertocci E, Pesce I, et al. Long-term survival and immunological parameters in metastatic melanoma patients who responded to ipilimumab 10 mg/kg within an expanded access programme. Cancer Immunol Immunother. 2013;62:1021–1028. doi: 10.1007/s00262-013-1418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilgenhof S, Du FS, Vandenbroucke F, Everaert H, Salmon I, Liénard D, et al. Single-center experience with ipilimumab in an expanded access program for patients with pretreated advanced melanoma. J Immunother. 2013;36:215–222. doi: 10.1097/CJI.0b013e31828eed39. [DOI] [PubMed] [Google Scholar]
- 26.Postow MA, Chasalow SD, Yuan J, Kuk D, Panageas KS, Cheng M et al (2013) Pharmacodynamic effect of ipilimumab on absolute lymphocyte count (ALC) and association with overall survival in patients with advanced melanoma. J Clin Oncol 31: abstr 9052
- 27.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tartour E, Blay JY, Dorval T, Escudier B, Mosseri V, Douillard JY, et al. Predictors of clinical response to interleukin-2–based immunotherapy in melanoma patients: a French multiinstitutional study. J Clin Oncol. 1996;14:1697–1703. doi: 10.1200/JCO.1996.14.5.1697. [DOI] [PubMed] [Google Scholar]
- 29.Blank CU, Kelderman S, van Tinteren H, Heemskerk B, van den Brom R, Hospers GA et al (2013) Serum lactate dehydrogenase (LDH) as a prognostic selection criterion for ipilimumab treatment in metastatic melanoma. J Clin Oncol 31: abstr 3036 [DOI] [PMC free article] [PubMed]
- 30.Hotson D, Conroy A, Evensen E, Gentilcore G, Simeone E, Esposito A et al (2012) CTLA-4 defines distinct T cell signaling populations in healthy donors and metastatic melanoma patients. J Immunother 11: abstr 760
- 31.Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3 + regulatory T cells infiltrating human carcinomas: the paradox of colorectal cancer. Cancer Immunol Immunother. 2011;60:909–918. doi: 10.1007/s00262-011-1046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]