Abstract
Carboxypeptidase D (gp180), one of many candidate receptors proposed for hepatitis B viruses (HBVs), was examined and found to be the actual cellular receptor for avian HBVs. This conclusion was based on the following observations: (i) gp180 was the only host protein that bound with high affinity to the pre-S ectodomain of the large duck hepatitis B virus (DHBV) envelope protein, which is known to be essential for virus infection; (ii) a pre-S subdomain which determines physical binding to gp180 was found to coincide with a domain functionally defined in infection competition experiments as a receptor binding domain; (iii) soluble gp180, lacking the membrane anchor, efficiently inhibited DHBV infection; (iv) efficient interspecies gp180–pre-S interaction was limited to the natural hosts of avian hepadnaviruses; and (v) expression of gp180 in a heterologous hepatoma cell line mediated cellular attachment and subsequent internalization of fluorescently labeled viral particles into vesicular structures. However, gp180 expression did not render transfected heterologous cells permissive for productive infection, suggesting that a species-specific coreceptor is required for fusion to complete viral entry. In contrast to the case for known virus receptors, gp180 was not detected on the hepatocyte cell surface but was found to be concentrated in the Golgi apparatus, from where it functions by cycling to and from the plasma membrane.
Human hepatitis B virus (HBV) is the prototype member of the Hepadnaviridae family, which is comprised of members found in woodchucks, squirrels, and some birds, such as Pekin duck and grey heron. These viruses all have the same genomic organization and virus structure, a distinct tissue tropism, and an extremely narrow host range, restricting them to their natural host and some closely related species (9). It is commonly assumed that the interaction of these viruses with their cell surface receptors during virus uptake is, at least partially, responsible for this narrow host range. The players participating in and the molecular mechanisms underlying these early events are, despite major efforts, still poorly understood. For the medically relevant HBV, this is mainly due to the lack of an efficient in vitro infection system; liver-derived cell lines are not infectable, and furthermore, in addition to primary human hepatocytes not being readily available, the ability to infect these cells is poor and varies drastically with the preparation and donor (19). Hence, although many receptor candidates for human HBV have been postulated (reviewed in reference 4), none have been proven, in infection experiments, to be involved in hepadnavirus entry.
In contrast, the animal model duck hepatitis B virus (DHBV) provides readily available cultures of primary duck hepatocytes (PDHs), which can be efficiently infected (29). Thus, this model enables the investigation of the early events in the hepadnavirus infectious cycle on the molecular level. As possible ligands for binding to cellular receptor molecules, the avian hepadnaviruses encode two related envelope proteins by differential translation initiation within the same open reading frame: the major S protein, providing 80% of the surface protein content, and the larger L protein, in which the S protein is N-terminally extended by the hydrophilic pre-S domain of 161 amino acids. The two envelope proteins are also present in the abundantly secreted, noninfectious subviral particles (SVPs), making these particles indistinguishable from DNA-containing virions in terms of their initial interaction with the host cells. By using these SVPs, it has been shown that DHBV binds a cellular receptor on the hepatocyte surface via the pre-S domain of the viral L protein (13). A reported candidate receptor that binds to this region of the DHBV L protein is gp180, a 180-kDa glycoprotein found in duck liver (15). This protein was isolated, cloned, and characterized as the prototype of a new class of membrane-bound carboxypeptidases (16), later designated carboxypeptidase D (24). Homolog proteins from other species have since been cloned and characterized (27, 36), confirming that carboxypeptidase D is a type I transmembrane protein which consists of a large luminal/extracellular portion, a transmembrane domain, and a C-terminal cytoplasmic tail of about 60 amino acids. Consistent with receptor function, gp180 was previously reported to be located at both internal and plasma membranes (15). However, gp180 (like carboxypeptidases D from other species) was found not only in duck liver but also in a variety of other tissues which are known to be not susceptible to DHBV infection. Moreover, expression of gp180 following transfection of LMH cells (a chicken hepatoma cell line capable of generating and secreting infectious virus) did not render these cells permissive to DHBV infection (16). Finally, antibodies to gp180 have not been reported to neutralize DHBV infection of PDHs. Thus, despite the experimental advantages of the avian HBV system, there is still a need to clarify the mechanisms by which these hepadnaviruses enter the cell, the nature of the cellular components involved, and in particular the role of gp180 in these processes.
Using recombinant pre-S polypeptides in an infection competition assay, we mapped a subdomain within the pre-S region of the DHBV L protein which is required for efficient binding to the cellular receptor (30). The results obtained led us to conclude that DHBV uptake involves two discrete steps, with gp180 possibly functioning as a primary virus attachment site. In complementary work, we present here direct gp180 binding data obtained with recombinant pre-S and viral particles in vitro as well as in a cellular context. Our studies demonstrate that gp180, a Golgi-resident protein, is the cellular receptor for avian hepadnaviruses which mediates virus attachment and internalization into the host cell. However, although virus-gp180 binding contributes to host discrimination of avian hepadnaviruses, this interaction does not appear to be the major determinant of their pronounced host specificity.
MATERIALS AND METHODS
gp180-expressing plasmids.
The gp180-coding sequence (16), kindly provided by Kazuyuki Kuroki, was subcloned via NcoI into a minimal pUC plasmid containing the cytomegalovirus promoter and the bovine growth hormone poly(A) site to create plasmid pC-gp180. pC-gp180Tm was derived from a similar construct, pC-myc-gp180, containing additionally a Myc epitope tag inserted into the first KpnI site in the coding region. Mutant pC-gp180Tm terminates after the predicted transmembrane region at Ile-1334 (...CVCSI.).
Fluorescent conjugates.
Recombinant DHBV pre-S was conjugated to fluorescein by using 5(6)-carboxyfluorescein N-hydroxysuccinimide ester (Boehringer Mannheim). Ten microliters of a freshly prepared stock solution of the dye (10 mg/ml in dimethyl sulfoxide) was added to 0.5 mg of pre-S in 1 ml of buffer C (200 mM Na-borate, pH 8.5). Following incubation for 1 h at room temperature, labeled pre-S was separated from the unreacted dye and transferred to phosphate-buffered saline (PBS) by using a PD-10 column (Pharmacia). DHBV particles were enriched from high-titer duck serum by one-step centrifugation (13). The peak fraction (500 μl), containing approximately 1014 particles/ml, diluted with 500 μl of 2× buffer C was labeled as described above.
Biochemical binding assay.
All of the His6-tagged pre-S polypeptides were prepared and handled as reported elsewhere (30). Coupling of pre-S to CH-activated Sepharose (Pharmacia) was carried out according to the manufacturer’s instructions (2 h, room temperature; 100 mM NaPi–100 mM NaCl [pH 6.5]–4 mg of DHBV or HHBV pre-S per mg of dry resin). Liver extracts were prepared by Dounce homogenization in buffer H (150 mM NaCl, 10 mM Tris-HCl [pH 7.4], 2 g of aprotinin/liter, 2 g of leupeptin/liter, 1 mM phenylmethylsulfonyl fluoride, 1% Triton X-100) (5 ml/g of liver) and removal of the nuclei and debris by centrifugation. The pre-S–Sepharose (500 pmol of pre-S) was incubated for 4 h (4°C) in the absence or presence of uncoupled ligand with 200 μl of extract and 600 μl of buffer H at 4°C. The Sepharose was washed four times with buffer W (buffer H without protease inhibitors). Specifically bound proteins were eluted for 1 h with excess free pre-S (360 nmol in 50 μl; 37°C), loaded on a sodium dodecyl sulfate (SDS) gel, and detected by silver staining; alternatively, the washed Sepharose was resuspended in 50 μl of Laemmli buffer and boiled for 10 min, and gp180 was detected by Western blotting (ECL system; Amersham). The binding of gp180 to 500 pmol of pre-S coupled to Sepharose was determined to be inhibited by 50% with approximately 5 pmol of free pre-S; 250 pmol of the free polypeptides was therefore used for competition experiments (see Fig. 2B).
FIG. 2.
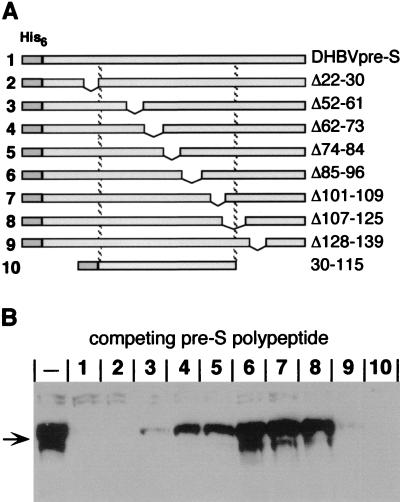
Mapping of the region within the DHBV pre-S sequence required for gp180 interaction. (A) Schematic representation of a series of His-tagged pre-S polypeptides used to map the gp180 binding domain. The numbers on the left correspond to the lanes in panel B. The borders of each deletion are given at the right. The dashed lines reflect the borders of the minimal domain determined to be required for receptor interaction on PDHs competing DHBV infection (30). (B) Western blot probed with anti-gp180 antiserum, detecting gp180 bound to immobilized DHBV pre-S with or without (−) the presence of the respective competing pre-S polypeptide (1 to 10 in panel A).
Infection inhibition.
PDHs were prepared and cultured as previously described (23). Soluble gp180 (sgp180) was purified from culture supernatants of high five insect cells infected with a recombinant baculovirus encoding the extracellular domain of gp180 (amino acids 1 to 1308). Construction of the recombinant virus and purification of sgp180 have been described elsewhere (6, 31). sgp180 and DHBV-containing duck serum (100 DNA-containing particles/cell) were mixed and immediately applied in 500 μl of maintenance medium to 1 well of a 12-well culture dish containing about 8 × 105 cells. After 14 h of incubation, the cells were washed and further cultivated. After 6 days, cells were harvested and intracellular viral DNA was prepared by using the QIAamp blood kit (Qiagen) and subjected to DNA dot blot analysis as previously described (23). The radioactive signals were quantified with a Molecular Dynamics PhosphorImager.
Internalization assay.
HuH7 cells were cultivated in six-well culture dishes or (for confocal microscopy) on coverglass chamber slides (Nunc) and transfected with gp180-expressing plasmids by using a standard Ca phosphate protocol or Superfect (Qiagen). At 1 to 2 days after transfection, the cells were incubated with labeled substrates (50 to 100 μl/ml of culture medium) for 4 h at 37°C, washed with PBS, and fixed with 3% paraformaldehyde for 20 min. The fixed cells were permeabilized with 0.25% Triton X-100 in PBS and immunostained either with a gp180 polyclonal antibody or, in the case of gp180Tm, with a Myc monoclonal antibody (9E10) and then with a tetramethylrhodamine-conjugated secondary antibody. Fluorescence was analyzed with a Leica DM IRB inverted fluorescence microscope (20×/0.40 objective) equipped with an automatic photo camera and the Leica TCS NT confocal laser scanning microscope (63×/1.2 objective).
gp180 localization.
PDHs cultured on coverglass chamber slides were fixed and reacted with a gp180 antiserum, followed by a secondary fluorescein-conjugated antibody. HuH7 cells transfected with pC-myc-gp180 were fixed at 1 day posttransfection and costained with a polyclonal antiserum against TGN46 and monoclonal antibody 9E10. The secondary antibodies were conjugated with fluorescein and tetramethylrhodamine, respectively. Immunofluorescence was analyzed by confocal microscopy as described above.
RESULTS
gp180 is the dominant pre-S binding protein in duck hepatocytes.
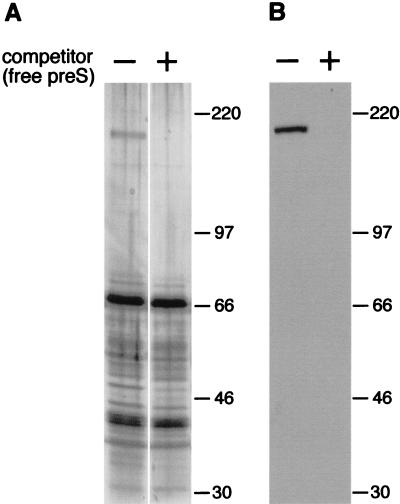
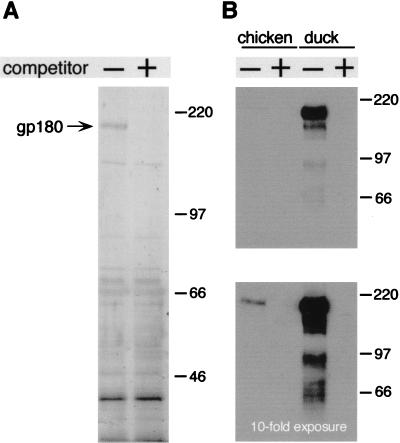
DHBV infection of cultured PDHs can be efficiently inhibited by addition of recombinant DHBV pre-S polypeptide from Escherichia coli (30). Hence, recombinant DHBV pre-S is able to bind to the viral receptor on hepatocytes and to prevent virus binding and subsequent infection. We have used this recombinant protein to assess whether cellular pre-S binding proteins, other than the previously reported gp180, are involved in virus attachment and might be targeted by pre-S during infection inhibition. For this purpose, we immobilized DHBV pre-S covalently to Sepharose (see Materials and Methods) and then incubated this affinity matrix with Triton X-100 extracts of either duck liver or PDHs or with solubilized liver membranes. Bound proteins were eluted with an excess of the free ligand. Although SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining revealed that many proteins were eluted from the pre-S matrix (Fig. 1A, left lane), gp180 was the only protein consistently present in the eluate, regardless of the source of the input, and whose binding to the affinity matrix could be inhibited by the addition of free competing pre-S (Fig. 1A, right lane) or viral particles (not shown). The identity of gp180 was verified by Western blotting (Fig. 1B). The rate constants observed for the binding to the pre-S–Sepharose confirmed the specificity of this interaction: by measuring the disappearance of gp180 from the supernatant (detected by radioimmunoblotting) and its reappearance in the presence of free ligand, we estimated the on rate of this reaction to be approximately 104 M−1 s−1; an off reaction was not detectable after 24 h at 4°C and therefore was assumed to be slower than 3 × 10−6 s−1 (data not shown). These preliminary kinetic data are supported by a detailed BIAcore analysis with recombinant gp180 and pre-S polypeptide (31).
FIG. 1.
gp180 is the dominant DHBV pre-S binding protein in duck liver. (A) Duck liver proteins eluted from a DHBV pre-S affinity matrix were analyzed by SDS-PAGE and silver staining. Binding had been carried out in the absence (−) or the presence (+) of 100 pmol of free DHBV pre-S polypeptide as a competitor. Molecular masses (in kilodaltons) are indicated on the right. (B) An immunoblot from a similar experiment with an anti-gp180 antiserum as the probe.
The domains within the DHBV L protein determining functional binding to a cellular receptor and physical binding to gp180 overlap extensively.
With a set of terminally and internally deleted pre-S polypeptides, infection inhibition studies mapped the essential region for efficient receptor binding to amino acids 30 to 115 of the viral L protein (30). To confirm that the cellular receptor interacting with this region was gp180, we used the identical deletion mutants (Fig. 2A) to map the domain required for gp180 binding. Mirroring the infection competition mentioned above, we performed a binding competition assay, where an excess of the free pre-S polypeptides was present during binding; uncompeted, matrix-bound gp180 was then detected by Western blotting (Fig. 2B).
With this procedure, mutants Δ22-30, Δ128-139, and 30-115 (Fig. 2B, lanes 2, 9, and 10) efficiently inhibited gp180 binding to the DHBV pre-S matrix, indicating that the gp180 binding domain maps between amino acids 30 and 115. Deletions between amino acids 30 and 115 all affected binding efficiency; some residual gp180 competition competence was observed with pre-S mutants Δ52-61, Δ62-73, and Δ74-84 (lanes 3, 4, and 5), defining an inner domain (amino acids 85 to 115) where deletions completely abolished gp180 interaction (lanes 6, 7, and 8).
These gp180 binding data strikingly correlate with complementary receptor interaction data obtained with the same mutants and synthetic peptides (30). A correlation between functional receptor interaction and gp180 binding was also seen with L protein from grey heron HBV (HHBV), another avian hepadnavirus; an HHBV pre-S polypeptide bound duck gp180 (1, 11) and blocked DHBV infection (30). Since all pre-S–gp180 binding data are in good accordance with corresponding cellular receptor binding data from infection competition experiments (30), we conclude that gp180 is the receptor for DHBV on the liver cell surface.
sgp180 efficiently inhibits DHBV infection.
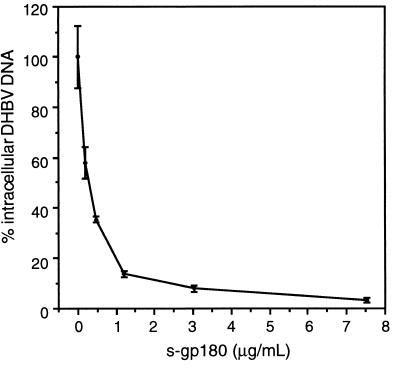
To further corroborate that gp180 is the cellular DHBV receptor, we examined whether a recombinant gp180 which lacked the transmembrane region and the cytoplasmic domain (sgp180) could block DHBV infection of PDHs, as was shown to be the case for other virus-receptor systems, such as human immunodeficiency virus (HIV) and CD4 (34). sgp180, obtained from insect cells infected with recombinant baculovirus (31), was used in an infection inhibition assay similar to the one described for the recombinant pre-S polypeptides (30). With increasing amounts of sgp180 present during DHBV infection of PDH cultures, inhibition of infection occurred in a concentration-dependent manner (Fig. 3). An almost complete loss of infection (>97% inhibition) was observed at sgp180 concentrations of 7.5 μg/ml; 50% inhibition was achieved at about 0.13 μg/ml, a concentration which corresponds to seven molecules of sgp180 per DHBV particle, assuming that, in the serum used for infection, DHBV virions were accompanied by a 1,000-fold excess of noninfectious but receptor binding-competent SVPs.
FIG. 3.
sgp180 efficiently inhibits DHBV infection. PDHs were incubated with a DHBV-containing duck serum (102 DNA-containing particles/cell; 14 h, 37°C) in the presence of increasing amounts of sgp180. On day 6 postinfection, the cells were harvested and assayed by DNA dot blotting for intracellular DHBV DNA. Values from two independent measurements were normalized to the value obtained in the absence of sgp180; bars indicate standard deviations. One microgram of sgp180 per milliliter corresponds to approximately 50 molecules of sgp180 per viral particle, assuming 103 SVPs per DNA-containing virion.
gp180 is a Golgi-resident protein.
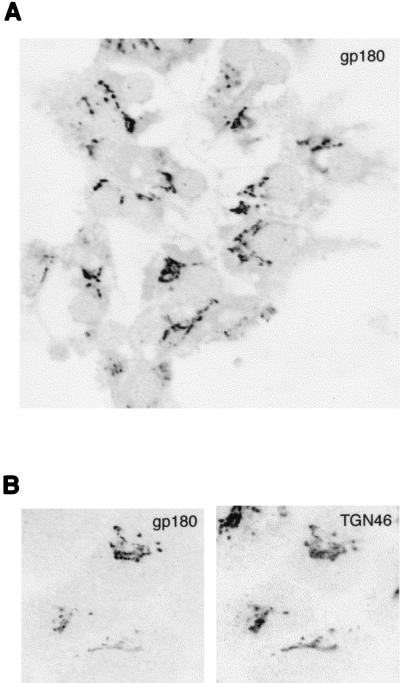
gp180 was formerly reported to be cell surface protein (15). Readdressing the question of the subcellular localization of gp180, we used confocal microscopy to perform immunofluorescence analysis of endogenous gp180 in cultured PDHs. The cells were fixed at 2 days postplating, immunostained with an anti-gp180 antiserum, and analyzed. By this method, gp180 was detected exclusively in tubular, perinuclear structures intracellularly (Fig. 4A) and was not found to reside at the plasma membrane to a detectable degree.
FIG. 4.
gp180 is concentrated in the Golgi apparatus. (A) PDHs prepared from a duckling were immunostained with an anti-gp180 antiserum and analyzed by confocal microscopy. (B) HuH7 cells were transfected with Myc epitope-tagged gp180. At 1 day posttransfection, the cells were fixed and coimmunostained for gp180 (tetramethylrhodamine) (left panel) and for endogenous TGN46 (fluorescein) (right panel). Fluorescence was analyzed by confocal microscopy (sequential scanning of the same cells).
To examine the identity of the gp180-containing intracellular compartment, we transfected pC-myc-gp180, a plasmid expressing a Myc epitope-tagged gp180 under control of the cytomegalovirus promoter (see Materials and Methods), into HuH7 cells, a human liver-derived cell line. At 1 day posttransfection, the cells were coimunostained with monoclonal antibody 9E10 for gp180 and with an antiserum against TGN46, a Golgi marker protein (21). Analysis by confocal microscopy revealed an accurate colocalization of expressed gp180 and endogenous TGN46 (Fig. 4B), suggesting that gp180 localizes to the Golgi apparatus rather than to the cell surface.
gp180 transfection mediates uptake of DHBV particles in nonpermissive cells.
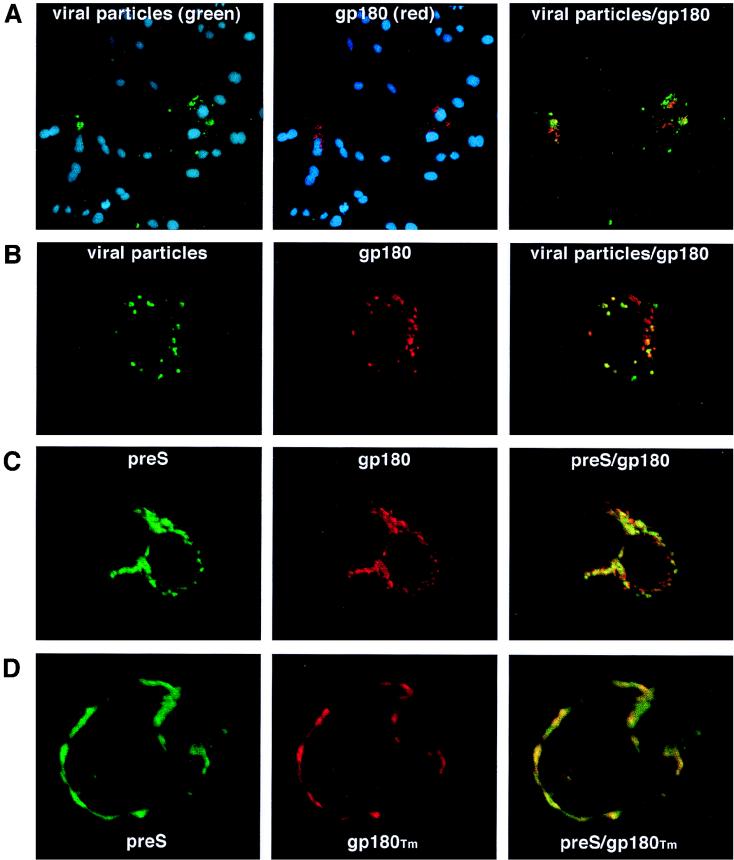
If gp180 is the cellular DHBV receptor, one would assume that transiently expressed gp180 could function as a receptor for DHBV, mediating virus uptake in nonpermissive cells. To examine this, HuH7 cells (nonpermissive for DHBV infection) were transfected with pC-gp180, incubated with fluorescein-labeled DHBV particles or recombinant DHBV pre-S polypeptide, washed, fixed, counterimmunostained for gp180, and examined for the presence and the localization of fluorescent viral proteins and gp180 (Fig. 5). Expression of gp180 was also verified by Western blotting (not shown).
FIG. 5.
gp180 mediates uptake of DHBV particles or pre-S polypeptide in nonpermissive cells. HuH7 cells were transfected with gp180 (A, B, and C) or with gp180Tm, a mutant lacking the cytoplasmic tail (D). After incubation with fluorescein-labeled DHBV particles (A and B) or pre-S (C and D), the cells were fixed and immunostained for the transfected gp180 (red fluorescence). Nuclei were stained with DAPI (A) (blue fluorescence). Fluorescence was analyzed either by conventional fluorescence microscopy, using sequential multiple exposures of the same cells (A), or by confocal fluorescence microscopy (B, C, and D) (simultaneous scans).
In these experiments, the fluorescently labeled viral substrates not only bound to cells that expressed the receptor but also were selectively internalized. In Fig. 5A this is shown for viral particles by conventional fluorescence microscopy: the nuclei of total cells are stained with DAPI (4′,6-diamidino-2-phenylindole) and seen as blue fluorescence. From these, only the transfected cells (gp180, stained red) took up the labeled particles (green fluorescence). Confocal microscopy revealed that the internalized viral particles colocalized with gp180 in a vesicular, mostly perinuclear compartment (Fig. 5B). Another fraction of gp180 was localized to tubular structures and was not found to be associated with viral particles. Fluorescently labeled pre-S polypeptide was internalized with a similar efficiency (Fig. 5C).
In addition, we used a construct (pC-gp180Tm) which expresses a C-terminally truncated form of gp180 (gp180Tm) lacking the cytosolic domain. In this case, the ligand was found to be arrested on the cell surface along with the exposed mutant receptor, apparently being anchored in the plasma membrane but unable to undergo endocytosis (Fig. 5D). Taken together, these findings demonstrate that gp180 binds the virus at the cell surface and is capable of cointernalizing bound particles. However, gp180-expressing HuH7 cells were not infected, as judged from the results of immunofluorescence analysis, while intracellular core antigen was not detected at day 5 postincubation with DHBV-containing duck serum (not shown).
Virus-gp180 interaction is conserved and restricted to hosts of avian hepadnaviruses.
In the DHBV infection competition assay (30) and the gp180 binding competition assay mentioned above, the avian pre-S proteins from DHBV and HHBV were found to be functionally interchangeable, suggesting that initial gp180 binding may be a common step in the uptake mechanisms of these two avian hepadnaviruses. To substantiate this hypothesis, we investigated whether the gp180 homolog from grey heron liver extract was a potent binding protein for the HHBV L protein. Using an immobilized HHBV pre-S polypeptide and conditions similar to those used in the previous experiment with duck liver extract (Fig. 1A), we found an analogous 180-kDa binding protein (Fig. 6A). In addition, as already observed with duck liver extracts (Fig. 1A), many nonspecifically bound proteins, whose binding was unaffected by an excess of free HHBV pre-S, were eluted from the matrix. The heron gp180 was found to bind DHBV pre-S with an efficiency similar to that of duck gp180 (not shown). In contrast, when we analyzed chicken liver extracts, which contain similar levels of a serologically cross-reacting gp180 homolog, we could not detect, by silver staining, specific binding of this or any other protein to the DHBV pre-S matrix (not shown). A very weak binding of the chicken homolog was detected only by the more sensitive Western blotting technique (Fig. 6B, lower panel). In the absence of a chicken hepadnavirus, these observations suggest that an efficient pre-S–gp180 interaction is a common feature of hepadnavirus infection of avian hosts.
FIG. 6.
Efficient gp180–pre-S interaction is restricted to the hosts of avian hepadnaviruses. (A) Proteins from heron liver, bound to HHBV pre-S–Sepharose in the absence (−) or the presence (+) of 250 pmol of free HHBV pre-S as a competitor, were eluted and analyzed by SDS-PAGE and silver staining. (B) Anti-gp180 immunoblot from a similar experiment comparing the affinities of binding of duck liver gp180 and the homologous protein from chicken liver to a DHBV pre-S–matrix. Free DHBV pre-S was used as a competitor to demonstrate specific binding (+). Similar levels of protein from either species were present during the binding reaction, and the antibody used detected the proteins from duck and chicken equally well. Molecular masses (in kilodaltons) are indicated on the right.
DISCUSSION
The results presented here provide convincing evidence that carboxypeptidase D, or gp180, is the receptor that is recognized by avian hepadnaviruses on the surface of the host hepatocytes. This conclusion is based on several observations, which have various implications that are of general interest with regard to the hepadnavirus entry mechanism. First, gp180 was found to be the only pre-S-specific binding protein detected in extracts from duck liver and also from fractions enriched for plasma membrane proteins. Our data do not rule out the presence of other DHBV binding proteins at much lower levels or which only weakly interact; however, considering the high affinity and apparent abundance of gp180, it seems unlikely that such molecules would serve as virus receptors.
Second, using a binding competition assay, we mapped a pre-S subdomain required for efficient gp180 interaction, which strikingly correlated with the receptor-interacting domain defined previously in infection competition experiments (30). From both experimental approaches, the interaction domain responsible appears to be divided into a core domain essential for binding, ranging from amino acids 85 to 115, and a more N-terminally located region, including sequences up to amino acid 30, which enhances receptor affinity. This observation clarifies earlier apparently conflicting gp180–pre-S binding data, which mapped the binding domain to amino acids 43 to 108 (11) or amino acids 87 to 102 (28) of the DHBV L protein. Bruns et al. (2) recently reported the enhancement of DHBV infection with noninfectious SVPs. By using recombinant SVPs which carried deletions in the L protein, the region required for causing this enhancement was mapped to the same amino acids that are defined here as the core interaction domain for gp180 binding. This strongly suggests that the infection enhancement requires the SVPs to bind the cellular receptor gp180.
Third, sgp180 efficiently blocked DHBV infection in a dose-dependent manner. About seven molecules of sgp180 per viral particle (assumed to contain about 10 surface-exposed pre-S domains) were estimated to be required for 50% inhibition (Fig. 3). If so, this result suggests that a viral particle, once it has been complexed by sgp180, is no longer able to enter the cell; this implies that cellular gp180, as well as other putative binding proteins at the cell surface, cannot readily displace a virus-bound soluble receptor molecule. Moreover, it appears that a potential uptake of virus particles mediated through soluble forms of carboxypeptidase D (25) is not an alternative option for the initiation of hepadnavirus infection.
Finally, using fluorescently labeled DHBV particles and nonpermissive HuH7 cells, we have demonstrated that gp180 mediated the uptake of these particles, indicating that gp180 was capable of binding the virus at the cell surface and causing its internalization. This internalization appeared to be independent of a virus-triggered receptor oligomerization, since monomeric pre-S polypeptides were taken up similarly well.
Immunofluorescence studies with PDHs show that gp180 is detected exclusively in an internal, Golgi-like compartment. Indeed, in HuH7 cells, transiently expressed gp180 colocalized with TGN46, a Golgi marker protein (21), in an internal tubular compartment; it was also detected at the cell surface at very high expression levels, as observed in the initial analysis of gp180 localization (15). The cytoplasmic domain of gp180 contains several motifs indicative of intracellular sorting of the protein, e.g., a dileucine motif similar to that shown to be responsible for the endocytosis of CD3 chains (17). Accordingly, gp180 lacking the cytoplasmic tail (gp180Tm) still migrated to the plasma membrane, but it could no longer be recycled and therefore accumulated at the cell surface (Fig. 5D). These observations indicate that gp180 is a Golgi-resident protein that maintains its subcellular location by being continuously recycled from the plasma membrane in a similar fashion to that for TGN38 or furin (3, 20). This model is also supported by experiments detecting the recycling of mouse carboxypeptidase D to the trans-Golgi network in the pituitary cell line AtT-20 (33). To our knowledge, gp180 is the first instance of a Golgi-resident protein acting as a virus receptor by cycling to the plasma membrane.
The internalization of fluorescently labeled DHBV particles (Fig. 5A and B) and the cellular trafficking of gp180 suggest that DHBV uptake involves endocytosis. This is in accordance with data suggesting that energy-dependent endocytosis is required for productive DHBV infection (14). However, our data do not exclude the possibility that fusion occurs at the cell surface, as is known to be the case for HIV despite the CD4 receptor being internalized (20a).
It has been proposed (18) that the carboxypeptidase activity of gp180 could be involved in the uptake mechanism, processing internally cleaved L protein. So far, we have not found any evidence supporting this notion, as a potent carboxypeptidase D inhibitor (2-mercaptomethyl-3-guanidinoethylthiopropanoic acid; 50% inhibitory concentration = 150 nM) (24) did not interfere with DHBV infection even at concentrations of up to 100 μM (32).
Carboxypeptidase D appears to be the common receptor of avian hepadnaviruses; HHBV and DHBV, the phylogenetically most distantly related viruses within this subfamily, show nonrestricted interspecies interaction between the viral ligands and the cellular receptor molecules. No such interaction was observed between DHBV pre-S and chicken carboxypeptidase D, suggesting that interspecies interaction at the receptor level may have been the prerequisite for an evolutionary spread of DHBV-related hepadnaviruses to other avian hosts.
Infectibility could not, to a reproducibly measurable degree, be conferred to primary chicken hepatocytes in transfection experiments with gp180-expressing plasmids (1). In addition, there is no efficient cross-infection of ducklings or PDH cultures with HHBV (7, 10, 26); however, phenotypically mixed HHBVs presenting DHBV pre-S surface domains efficiently infect PDHs (7, 10). Together, these observations add further support to the notion that second host-specific factors (coreceptors) are required at a late step during entry of these viruses (30). Coreceptors involved in host and/or tissue restriction have been proposed and/or identified for other viruses, such as adenovirus (35), echovirus (22), or, most prominently, HIV, where different strains dock to CD4 but use distinct chemokine receptors as cofactors for fusion (5, 8). In the present study, we show that DHBV particles are internalized by HuH7 cells expressing gp180 independently of other duck-specific factors; we therefore assume that a presently unknown host-specific coreceptor participates in an essential step following endocytosis, most likely in triggering fusion. Since we and others (15, 28) could not find another pre-S binding protein, we assume that a putative coreceptor is not characterized by abundance and high affinity to the pre-S ectodomain of these viruses.
While our model answers most questions satisfactorily, a contradiction that we currently cannot resolve is the extremely high efficiency of liver-specific DHBV infection in ducklings (12), despite the ubiquitous expression of gp180 (15) and the high affinity of the virus-receptor interaction.
So far, no evidence has been put forward to indicate that proteins homologous to gp180 could also serve as a receptor for HBV and the other mammalian hepadnaviruses. While we cannot exclude this possibility until studies are extended to the mammalian situation, it appears likely that the mechanisms of entry for the mammalian viruses and the characteristics of the cellular and viral components involved are similar to the ones discussed here, even though the receptor molecules may not be identical.
ACKNOWLEDGMENTS
We are especially grateful to Kazuyuki Kuroki for supplying some gp180 expression constructs, gp180 antibodies, and advice in the initial experiments. We thank Bärbel Glass for preparation of PDHs and for providing duck sera; Vas Ponnambalam for providing the anti-TGN46 antiserum; Hans Will for grey heron liver tissue; Christa Kuhn and Stefan Seitz for chicken liver tissue; Claudia Kruse for contributing work to obtain pure sgp180; Lloyd Fricker, his group, and Matthew Hannah for helpful discussions; Elizabeth Grgacic for critical reading and constructive comments; and Karin Coutinho for expert editorial assistance.
K.M.B. thanks the Boehringer Ingelheim Fonds for a predoctoral fellowship. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 229) and by the Fonds der Chemischen Industrie.
REFERENCES
- 1.Breiner, K. B., and H. Schaller. Unpublished data.
- 2.Bruns M, Miska S, Chassot S, Will H. Enhancement of hepatitis B virus infection by noninfectious subviral particles. J Virol. 1998;72:1462–1468. doi: 10.1128/jvi.72.2.1462-1468.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman R E, Munro S. Retrieval of TGN proteins from the cell surface requires endosomal acidification. EMBO J. 1994;13:2305–2312. doi: 10.1002/j.1460-2075.1994.tb06514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeMeyer S, Gong J Z, Suwandhi W, van Pelt J, Soumillon A, Yap S H. Organ and species specificity of hepatitis B virus (HBV) infection: a review of literature with a special reference to preferential attachment of HBV to human hepatocytes. J Viral Hepat. 1997;4:145–153. doi: 10.1046/j.1365-2893.1997.00126.x. [DOI] [PubMed] [Google Scholar]
- 5.Doms R, W, Peiper S C. Unwelcomed guests with master keys: how HIV uses chemokine receptors for cellular entry. Virology. 1997;235:179–190. doi: 10.1006/viro.1997.8703. [DOI] [PubMed] [Google Scholar]
- 6.Eng F J, Novikova E G, Kuroki K, Ganem D, Fricker L D. gp180, a protein that binds duck hepatitis B virus particles, has metallocarboxypeptidase D-like enzymatic activity. J Biol Chem. 1998;273:8382–8388. doi: 10.1074/jbc.273.14.8382. [DOI] [PubMed] [Google Scholar]
- 7.Fehler, F., S. Seitz, and H. Schaller. Unpublished data.
- 8.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 9.Ganem D. Hepadnaviridae. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2703–2737. [Google Scholar]
- 10.Ishikawa T, Ganem D. The pre-S domain of the large viral envelope protein determines host range in avian hepatitis B viruses. Proc Natl Acad Sci USA. 1995;92:6259–6263. doi: 10.1073/pnas.92.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishikawa T, Kuroki K, Lenhoff R, Summers J, Ganem D. Analysis of the binding of a host cell surface glycoprotein to the pre-S protein of duck hepatitis B virus. Virology. 1994;202:1061–1064. doi: 10.1006/viro.1994.1440. [DOI] [PubMed] [Google Scholar]
- 12.Jilbert A R, Miller D S, Scougall C A, Turnbull H, Burrell C J. Kinetics of duck hepatitis B virus infection following low dose virus inoculation: one virus genome is infectious in neonatal ducks. Virology. 1996;226:338–345. doi: 10.1006/viro.1996.0661. [DOI] [PubMed] [Google Scholar]
- 13.Klingmüller U, Schaller H. Hepadnavirus infection requires interaction between the viral pre-S domain and a specific hepatocellular receptor. J Virol. 1993;67:7414–7422. doi: 10.1128/jvi.67.12.7414-7422.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Köck J, Borst E M, Schlicht H J. Uptake of duck hepatitis B virus into hepatocytes occurs by endocytosis but does not require passage of the virus through an acidic intracellular compartment. J Virol. 1996;70:5827–5831. doi: 10.1128/jvi.70.9.5827-5831.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuroki K, Cheung R, Marion P L, Ganem D. A cell surface protein that binds avian hepatitis B virus particles. J Virol. 1994;68:2091–2096. doi: 10.1128/jvi.68.4.2091-2096.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuroki K, Eng F, Ishikawa T, Turck C, Harada F, Ganem D. gp180, a host cell glycoprotein that binds duck hepatitis B virus particles, is encoded by a member of the carboxypeptidase gene family. J Biol Chem. 1995;270:15022–15028. doi: 10.1074/jbc.270.25.15022. [DOI] [PubMed] [Google Scholar]
- 17.Letourneur F, Klausner R D. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting. Cell. 1992;69:1143–1157. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- 18.Li J S, Tong S P, Wands J R. Characterization of a 120-kilodalton pre-S-binding protein as a candidate duck hepatitis B virus receptor. J Virol. 1996;70:6029–6035. doi: 10.1128/jvi.70.9.6029-6035.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mabit H, Vons C, Dubanchet S, Capel F, Franco D, Petit M A. Primary cultured normal human hepatocytes for hepatitis B virus receptor studies. J Hepatol. 1996;24:403–412. doi: 10.1016/s0168-8278(96)80160-7. [DOI] [PubMed] [Google Scholar]
- 20.Molloy S S, Thomas L, VanSlyke J K, Stenberg P E, Thomas G. Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface. EMBO J. 1994;13:18–33. doi: 10.1002/j.1460-2075.1994.tb06231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Pelchen-Matthews A, Armes J E, Griffiths G, Marsh M. Differential endocytosis of CD4 in lymphocytic and non-lymphocytic cells. J Exp Med. 1991;173:575–587. doi: 10.1084/jem.173.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ponnambalam S, Girotti M, Yaspo M L, Owen C E, Perry A C, Suganuma T, Nilsson T, Fried M, Banting G, Warren G. Primate homologues of rat TGN38: primary structure, expression and functional implications. J Cell Sci. 1996;109:675–685. doi: 10.1242/jcs.109.3.675. [DOI] [PubMed] [Google Scholar]
- 22.Powell R M, Ward T, Evans D J, Almond J W. Interaction between echovirus 7 and its receptor, decay-accelerating factor (Cd55): evidence for a secondary cellular factor in A particle formation. J Virol. 1997;71:9306–9312. doi: 10.1128/jvi.71.12.9306-9312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rigg R J, Schaller H. Duck hepatitis B virus infection of hepatocytes is not dependent on low pH. J Virol. 1992;66:2829–2836. doi: 10.1128/jvi.66.5.2829-2836.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song L, Fricker L D. Purification and characterization of carboxypeptidase D, a novel carboxypeptidase E-like enzyme, from bovine pituitary. J Biol Chem. 1995;270:25007–25013. doi: 10.1074/jbc.270.42.25007. [DOI] [PubMed] [Google Scholar]
- 25.Song L, Fricker L D. Tissue distribution and characterization of soluble and membrane-bound forms of metallocarboxypeptidase D. J Biol Chem. 1996;271:28884–28889. doi: 10.1074/jbc.271.46.28884. [DOI] [PubMed] [Google Scholar]
- 26.Sprengel R, Kaleta E F, Will H. Isolation and characterization of a hepatitis B virus endemic in herons. J Virol. 1988;62:3832–3839. doi: 10.1128/jvi.62.10.3832-3839.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan F, Rehli M, Krause S W, Skidgel R A. Sequence of human carboxypeptidase D reveals it to be a member of the regulatory carboxypeptidase family with three tandem active site domains. Biochem J. 1997;327:81–87. doi: 10.1042/bj3270081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong S, Li J, Wands J R. Interaction between duck hepatitis B virus and a 170-kilodalton cellular protein is mediated through a neutralizing epitope of the pre-S region and occurs during viral infection. J Virol. 1995;69:7106–7112. doi: 10.1128/jvi.69.11.7106-7112.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuttleman J S, Pugh J C, Summers J W. In vitro experimental infection of primary duck hepatocyte cultures with duck hepatitis B virus. J Virol. 1986;58:17–25. doi: 10.1128/jvi.58.1.17-25.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urban S, Breiner K M, Fehler F, Klingmüller U, Schaller H. Avian hepatitis B virus infection is initiated by the interaction of a distinct pre-S subdomain with the cellular receptor gp180. J Virol. 1998;72:8089–8097. doi: 10.1128/jvi.72.10.8089-8097.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urban S, Kruse C, Multhaup G. A soluble form of the avian hepatitis B virus receptor: biochemical characterization and functional analysis of the receptor ligand complex. 1998. Submitted for publication. [DOI] [PubMed] [Google Scholar]
- 32.Urban, S. Unpublished data.
- 33.Varlamov O, Fricker L D. Intracellular trafficking of metallocarboxypeptidase D in AtT-20 cells: localization to the trans-Golgi network and recycling from the cell surface. J Cell Sci. 1998;111:877–885. doi: 10.1242/jcs.111.7.877. [DOI] [PubMed] [Google Scholar]
- 34.Weiss R A. Receptor molecule blocks HIV. Nature. 1988;331:15. doi: 10.1038/331015a0. [DOI] [PubMed] [Google Scholar]
- 35.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 36.Xin X, Varlamov O, Day R, Dong W, Bridgett M M, Leiter E H, Fricker L D. Cloning and sequence analysis of cDNA encoding rat carboxypeptidase D. DNA Cell Biol. 1997;16:897–909. doi: 10.1089/dna.1997.16.897. [DOI] [PubMed] [Google Scholar]