Abstract
Most strains of human immunodeficiency virus type 1 (HIV-1) which have only been carried in vitro in peripheral blood mononuclear cells (primary isolates) can be neutralized by antibodies, but their sensitivity to neutralization varies considerably. To study the parameters that contribute to the differential neutralization sensitivity of primary HIV-1 isolates, we developed a neutralization assay with a panel of genetically engineered cell lines (GHOST cells) that express CD4, one of eight chemokine receptors which function as HIV-1 coreceptors, and a Tat-dependent green fluorescent protein reporter cassette which permits the evaluation and quantitation of HIV-1 infection by flow cytometry. All 21 primary isolates from several clades could grow in the various GHOST cell lines, and their use of one or more coreceptors could easily be defined by flow cytometric analysis. Ten of these primary isolates, three that were CXCR4 (X4)-tropic, three that were CCR5 (R5)-tropic, and four that were dual- or polytropic were chosen for study of their sensitivity to neutralization by human monoclonal and polyclonal antibodies. Viruses from the X4-tropic category of viruses were first tested since they have generally been considered to be particularly neutralization sensitive. It was found that the X4-tropic virus group contained both neutralization-sensitive and neutralization-resistant viruses. Similar results were obtained with R5-tropic viruses and with dual- or polytropic viruses. Within each category of viruses, neutralization sensitivity and resistance could be observed. Therefore, sensitivity to neutralization appears to be the consequence of factors that influence the antibody-virus interaction and its sequelae rather than coreceptor usage. Neutralization of various viruses by the V3-specific monoclonal antibody, 447-52D, was shown to be dependent not only on the presence of the relevant epitope but also on its presentation. An epitope within the envelope of a particular virus is not sufficient to render a virus sensitive to neutralization by an antibody that recognizes that epitope. Moreover, conformation-dependent factors may overcome the need for absolute fidelity in the match between an antibody and its core epitope, permitting sufficient affinity between the viral envelope protein and the antibody to neutralize the virus. The studies indicate that the neutralization sensitivity of HIV-1 primary isolates is a consequence of the complex interaction between virus, antibody, and target cell.
The sensitivity of human immunodeficiency virus type 1 (HIV-1) strains to neutralization depends on several factors. For example, the level of intercellular cell adhesion molecule type 1 (ICAM-1) on a virus particle affects the sensitivity with which it can be neutralized by antibody (15, 40). Sawyer et al. (43), using laboratory-adapted and primary isolates, showed that the host cells used for growing the virus stock influenced the sensitivity of the virus to neutralization and that the type of target cells used in the neutralization assay, i.e., T-cell lines or unstimulated or phytohemagglutinin (PHA)-activated peripheral blood mononuclear cells (PBMCs), also contributes to the sensitivities with which neutralization of HIV and other viruses is detected (34, 53, 54).
The isolates that have been adapted to T-cell lines (TCLA strains) have frequently been described as neutralization sensitive. However, data show that there are TCLA strains which are highly sensitive to neutralization, e.g., MN, and TCLA strains that are relatively less so, e.g., RF (28).
A consensus concerning primary isolates suggests that they are difficult to neutralize. However, many reports document that there is a spectrum of neutralization sensitivity among primary isolates just as there is among TCLA strains (19, 22, 38, 49, 52). There has also been a consensus that the neutralization sensitivity of HIV isolates is linked to the phenotype of isolates, that is, that syncytium-inducing (SI) or CXCR4-tropic (X4) viruses (including all laboratory-adapted strains) are more easily neutralized than non-syncytium-inducing (NSI) or CCR5-tropic (R5) viruses (the phenotype of the majority of primary isolates) (50). This is not supported by published data. For instance, Hogervorst et al. (23) made chimeric LAI viruses with the envelopes of an NSI or an SI isolate from the same individual; both chimeric viruses, regardless of NSI or SI phenotype, were neutralized by a heterologous serum pool. With the identification of the HIV coreceptors, CXCR4 and CCR5, coreceptor usage was thought to play a role in the greater sensitivity of TCLA strains to neutralization. However, it was shown recently that whether a strain uses CXCR4 or CCR5, its susceptibility to neutralization remains unchanged (27, 34, 45): Trkola et al. (45) used CD4-blocking reagents and monoclonal antibodies (MAbs) against dualtropic TCLA or primary isolates and showed that neutralization was unaffected by the coreceptor used. La Casse et al. (27) used V3-binding MAbs against a primary isolate and the TCLA clone of the same isolate and came to the same conclusion, as did Montefiori et al., using polyclonal HIV-positive human sera (34).
To quantify the differential neutralization sensitivities of primary isolates, we developed a new assay which is subject to less variability than previously described assays and used it to test a broad panel of primary isolates for sensitivity to Ab-mediated neutralization. “GHOST cells” which were described previously (24, 45), were used as the target cells in this assay. They are human osteosarcoma cells (HOS) that express CD4 and one of several HIV coreceptors. These cells also contain a gene for green fluorescent protein (GFP) under the control of the HIV-2 promoter, which, in the presence of Tat, acts as an indicator of infection, generating a fluorescent cytoplasmic signal which can be detected and enumerated by flow cytometry. The GHOST cells were found to be infectable by all of the primary isolates tested, with the coreceptor preference reflecting the tropism of each isolate. The ease with which infection is detected by flow cytometry was used to advantage in developing a sensitive, reproducible, and convenient neutralization assay which demonstrated that, within each category of viruses defined by phenotype and coreceptor usage, there were neutralization-sensitive and neutralization-resistant strains. Thus, the sensitivity or resistance of a primary isolate to Ab-mediated neutralization is a function of the virus particle and the effects of its interaction with Ab, not a characteristic of any category of viruses defined to date.
MATERIALS AND METHODS
Virus isolates.
A total of 21 primary isolates which had been passaged exclusively in PBMCs were used. These included isolates SF33 and SF2, obtained from J. Levy, University of California at San Francisco, San Francisco, Calif.; isolates CA1, CA5, CA13, CA20, VI191, VI525, VI313, and MAI, obtained from G. van der Groen, Institute of Tropical Medicine, Antwerp, Belgium; isolates 92HT593, 92HT594, 91US056, 92RW021, BK131, SM993, 92TH080, JR-FL, and 89.6, supplied by the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program; and BZ167, supplied originally by J. Mascola, Walter Reed Army Institute of Research, Rockville, Md. MNp, a primary isolate of the MN strain, which had never been passaged in cell lines, was obtained from J. Sullivan, University of Massachusetts Medical School, Worcester, Mass. The clade designation and MT-2-defined phenotype of each primary isolate are shown in Table 1. All virus stocks were prepared by infecting PHA-activated human PBMCs (54). Briefly, frozen PBMCs from HIV-1-negative blood donors were thawed, stimulated with PHA (3 μg/ml; Difco Detroit, Mich.) for 3 days, centrifuged, and infected with 1 ml of virus-infected culture supernatant. After a 1-h exposure of the cells to the virus, the volume of the cell suspension was adjusted to a concentration of 2 × 106 cells/ml and the culture was maintained in RPMI 1640 medium with 10% fetal bovine serum and interleukin-2 (IL-2) (20 U/ml; Boehringer Mannheim Biochemicals, Indianapolis, Ind.) at the same cell concentration. The concentration of p24 in the infected culture supernatant was checked every 3 to 4 days by a noncommercial enzyme-linked immunosorbent assay (26). The infected culture supernatant was collected at 1 to 2 weeks postinfection when the concentration of p24 was at least 100 ng/ml.
TABLE 1.
Syncytium-inducing phenotype of primary isolates from different subtypes
| Isolate | Subtypea | Phenotype in MT-2 cellsa |
|---|---|---|
| 92RW021 | A | NDb |
| CA1 | A | NSI |
| VI191 | A | NSI |
| VI313 | A | NSI |
| SF33 | B | SI |
| SF2 | B | SI |
| 92HT594 | B | SI |
| 92HT593 | B | SI |
| 91US056 | B | NSI |
| JR-FL | B | NSI |
| 93TH080 | B | ND |
| BK131 | B | ND |
| CA5 | B | NSI |
| 89.6 | B | SI |
| MNp | B | SI |
| BZ167 | B | SI |
| MAL | B | SI |
| SM993 | C | NSI |
| CI13 | D | NSI |
| CA20 | F | NSI |
| VI525 | G | SI |
The subtypes and phenotypes of each virus are listed as reported in the AIDS Research and Reference Reagent Program Catalog or in reference 37.
ND, not done.
Cells.
The GHOST cell lines used herein were derived from HOS cells (24). Briefly, HOS cells were transduced with the human CD4 gene encoded by the murine leukemia virus retroviral vector, pMV7neo, to generate a CD4-positive HOS.T4 clone. HOS.T4 cells were subsequently stably cotransfected with a reporter construct consisting of the HIV-2 long terminal repeat directing the expression of humanized green fluorescent protein (GFP) and a selection construct composed of the human cytomegalovirus immediate-early (IE) promoter driving the expression of hygromycin phosphotransferase. Cells stably transfected with the GFP reporter construct were checked for sensitivity to HIV-1 Tat-mediated gene activation. One clone; clone 34, which expressed GFP strongly after Tat transactivation, was designated the parental cell line and chosen for further development. GHOST cl.34 parental cells were transduced with one of the chemokine receptors (CCR1, CCR2, CCR3, CCR5, CXCR4, Bonzo/STRL33, or BOB/gpr15) encoded on the murine leukemia virus vector, pBABEpuro. Cells expressing the CCR5 coreceptor are referred to below as GHOST-R5, those expressing the CXCR4 coreceptor are referred to as GHOST-X4, etc. About 40% of the cells from each of the GHOST cell lines were positive for CD4; 84% of the GHOST-X4 cells were positive for CXCR4; 66% of the GHOST-R5 cells were positive for CCR5. The parent GHOST cells were 3.8% positive for CXCR4 and 0.8% positive for CCR5.
GHOST cells bearing chemokine receptors were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 1% glutamine, 2% penicillin plus streptomycin, Geneticin (200 μg/ml), hygromycin (25 μg/ml), and puromycin (1 μg/ml). The cultures were maintained at 37°C in a 5% CO2 humidified incubator. Cell monolayers, when confluent, were resuspended by using 0.25% trypsin. The cells were maintained for up to 15 passages and then replaced with fresh cells from the cryopreserved stock which had been frozen at the second or third passage.
MAbs and polyclonal Abs.
Five polyclonal serum samples and two MAbs were used in the neutralization tests. Sera F and N are from asymptomatic HIV-positive volunteers from the Veterans Affairs Medical Center (New York, N.Y.) and the University of California Los Angeles Medical Center (Los Angeles, Calif.), respectively. Serum N was provided by S. Miles (University of California Los Angeles Medical Center, Los Angeles, Calif.). Serum FDA-2 is a serum pool derived from four bleeds from an HIV-positive patient obtained from the NIH AIDS Research and Reference Reagent Program. HIVIG-Ug is the immunoglobulin G (IgG) fraction from pooled HIV-positive serum obtained in Uganda (supplied by B. Jackson, Johns Hopkins University, Baltimore, Md.); it was used at a starting concentration of 0.5 mg/ml. Pool 2 is a serum pool from 33 randomly selected HIV-positive subjects at the Veterans Affairs Medical Center, New York. An HIV-negative human serum specimen was used in the experiments as a negative control. All sera were heat inactivated at 56°C for 30 min prior to use.
Two human MAbs were used in this study, 447-52D and IgG1b12. 447-52D, produced in this laboratory, was previously described as a broadly cross-reactive V3-specific MAb (18, 19). IgG1b12, a recombinant antibody which recognizes an epitope overlapping the CD4 binding domain of the HIV-1 envelope (8), was obtained from the NIH AIDS Research and Reference Reagent Program. Stocks of both MAbs were stored in frozen aliquots as purified IgG (1 mg/ml) and used at concentrations ranging from 0.1 to 25 μg/ml.
Infectivity assay.
GHOST cells were seeded in 24-well plates (Falcon; Fisher Scientific, Springfield, N.J.) at 6 × 104 cells/well/0.5 ml. On the following day, the medium was removed and the monolayers, about 70% confluent, were infected with undiluted virus stocks (100 μl/well). To each well was added DEAE-dextran to a final concentration of 8 μg/ml. The virus was allowed to adsorb overnight, after which the virus-containing medium was removed and the cell monolayers were washed once with phosphate-buffered saline. Subsequently, 1 ml of complete medium, as described above, was added per well. The day on which the virus was added was considered day 0. Cells were harvested on day 4 or 5 postinfection (p.i.). On the day of harvest, the cell monolayers were once again washed with phosphate-buffered saline, resuspended in 300 μl of 1 mM EDTA in PBS, and fixed in formaldehyde at a final concentration of 2%. The cells were then analyzed with a FACScan flow cytometer (Becton Dickenson, San Jose, Calif.). The live cells were gated on the basis of forward and side scatter. Because of the autofluorescence of uninfected GHOST cells due to basal expression of the indicator cassette, the gain on the FL 1 channel was set to bring the mean channel fluorescence of uninfected cells to <102. The number of infected cells was determined by using a scattergram of fluorescence versus forward scatter after setting the gates with uninfected cells. A total of 15,000 to 20,000 events was scored. The total number of cells was about 106/well on the day of harvest. Hence the number of cells scored was approximately 1/50 of the total cells in culture. As noted above, 3.8% of the GHOST parental cells are CXCR4 positive, and hence all the coreceptor derivatives express a low but detectable level of CXCR4. To account for this, GHOST-CCR1 cells were used to establish background infectability. The mean number of fluorescent GHOST-CCR1 cells + 2 standard deviations after infection with the viruses used in this study was considered the cutoff. On this basis, >99 fluorescent cells/15,000 cells had to be present for a virus to be considered positive for infectivity in each of the GHOST cell lines tested.
GHOST cell neutralization assay.
The method for the GHOST cell neutralization assay was essentially as described above for infectivity studies except that a fixed dilution of each virus stock was used, based on predetermined infectivity titers; the virus dilution used was chosen to give about 200 to 800 fluorescent cells per 15,000 events in the absence of anti-HIV antibodies. Polyclonal Ab or MAb preparations were diluted serially in fivefold dilutions. Equal volumes of diluted Ab preparations and virus were mixed and incubated for 1 h at 37°C; 100 μl of the virus-Ab mixture was added to duplicate wells and incubated overnight in the presence of DEAE-dextran (8 μg/ml). Subsequent washes, incubations, harvest, and readout procedures were performed as described above. Neutralization assays were typically terminated 3 to 4 days p.i. Care was taken to terminate the assay before cell lysis occurred. Formation of moderate-sized syncytia did not seem to affect the flow analysis, since the forward and side scatter gates included almost all of the viable cells.
RESULTS
Determination of coreceptor preferences by primary isolates from different clades.
A panel of 21 primary isolates belonging to different clades and demonstrating different SI/NSI phenotypes when grown in MT-2 cells was chosen for infectivity studies (Table 1). GHOST cells expressing CCR1, CCR2, CCR3, CCR5, CXCR4, BOB, or Bonzo were infected with virus stocks in the presence of DEAE-dextran since this compound was required for optimal infectivity by some viruses. For example, while isolate SF33 was not significantly affected by the presence of DEAE-dextran, isolate CA5 showed infectivity only in its presence (data not shown). Thus, for all infectivity and neutralization assays, DEAE-dextran was included during virus adsorption.
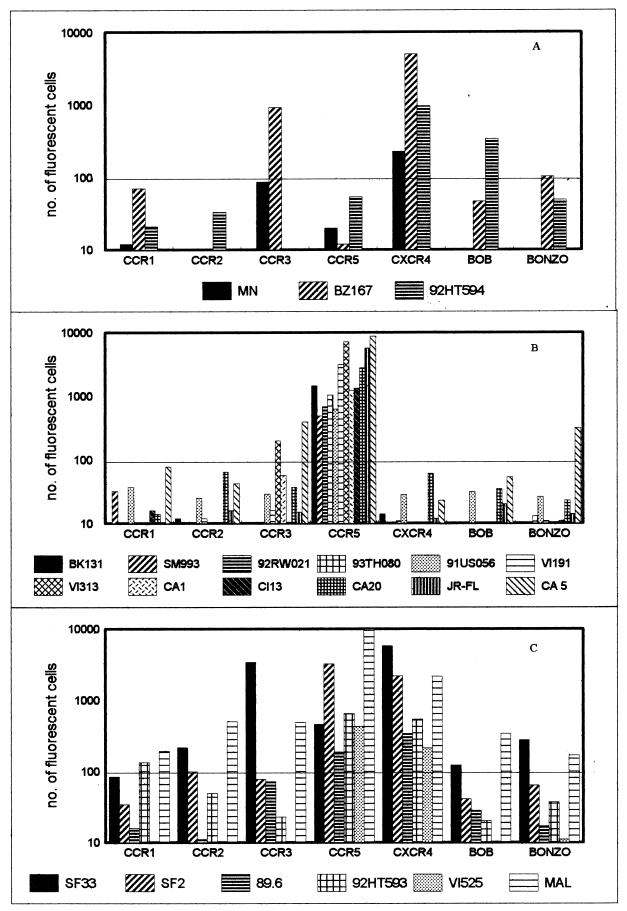
Infection by primary isolates of GHOST cells expressing the various chemokine receptors was readily detected by flow cytometry, as depicted in Fig. 1. Of nine SI viruses tested, only three, BZ167, MNp and 92HT594, were X4, using CXCR4 but not CCR5. However, BZ167 could also use CCR3, while 92HT594 also used BOB (Fig. 1A). The other six SI viruses were dualtropic, using both CXCR4 and CCR5, or polytropic, using both major coreceptors and CCR2, CCR3, and/or Bonzo; these three coreceptors generally mediated infection with lower efficiencies than did CXCR4 and CCR5 (Fig. 1C). The 12 NSI viruses tested were R5, defined as using CCR5 but not CXCR4. Viruses CA5 and VI313 also used CCR3 and Bonzo, respectively (Fig. 1B). Again, these two coreceptors mediated infection with lower efficiency.
FIG. 1.
Twenty-one primary isolates were tested for their coreceptor preference in GHOST cells expressing CCR1, CCR2, CCR3, CCR5, CXCR4, BOB, or Bonzo. The virus isolates were either SI (A), NSI (B), or dual- or polytropic (C). One representative experiment of two or three is shown, with values being the mean of duplicate observations. The mean number of fluorescent cells observed in GHOST-CCR1 cells with all 21 viruses + 2 standard deviations was the cutoff value (horizontal line).
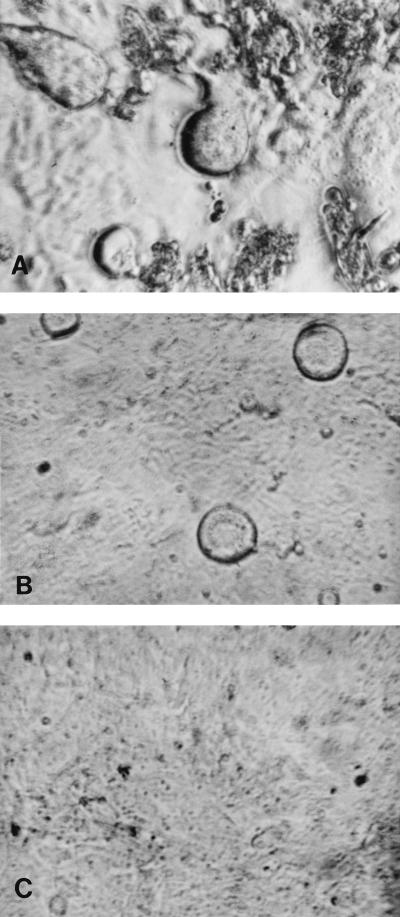
All the isolates, irrespective of coreceptor usage or SI/NSI phenotype, caused syncytium formation in the GHOST cells. For the majority of R5 viruses, the cytopathic effect was apparent early compared to that seen with viruses which used other coreceptors. Figures 2A and B show syncytium formation in GHOST cells by the R5 virus VI313 and by the X4/R5 virus 89.6, respectively, on day 3 p.i. Uninfected cells are shown in Fig. 2C.
FIG. 2.
Syncytia seen in GHOST-C5 cells infected with NSI virus VI313 (A) or with dualtropic virus 89.6 (B). (C) Uninfected cells.
Infection kinetics.
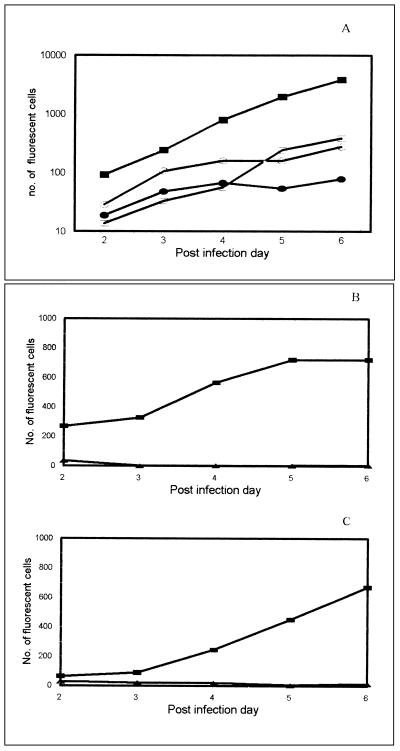
The kinetics of infection for a polytropic virus (SF33) and a dualtropic virus (92HT593), as assessed fluorocytometrically, are shown in Fig. 3A for GHOST-X4 and GHOST-R5 cells. SF33 showed a lower efficiency of infection in GHOST-R5 cells compared to that in GHOST-X4 cells, although the rates of infection in the two cell lines were parallel. Isolate 92HT593 showed similar kinetics in the two cell lines.
FIG. 3.
(A) Kinetics of infection of SF33 in GHOST-X4 (■) and GHOST-R5 (□) cells and of 92HT593 in GHOST-X4 (•) and GHOST-R5 (○) cells. (B and C) Effect of pretreatment with HIV immune serum pool 2 (▴) and normal serum (■) on the infection kinetics of SF33 in GHOST-X4 (B) and GHOST-R5 (C) cells. Sera were tested at a final dilution of 1:40.
Ab-mediated neutralization of primary isolate infection of GHOST cells.
The neutralization assay was first performed with the polytropic clade B SF33 isolate in GHOST-X4 and GHOST-R5 cells. The HIV-positive serum pool 2 and an HIV-negative serum specimen were used at a final dilution of 1:40. The cells were harvested from days 2 to 6 p.i. The number of fluorescing GHOST-X4 or GHOST-R5 cells in wells which were infected with virus treated with normal serum was about 700 on day 6 (Fig. 3B and C). This was reduced to background levels throughout the observation period when virus was pretreated with the anti-HIV pool 2 (Fig. 3B and C). The day of harvest did not affect the degree of neutralization observed.
A checkerboard neutralization assay was also carried out with SF33 and MAb 447-52D or the HIV-positive serum F. Varying the virus input by 1 order of magnitude, resulting in 170 to 1,165 infected cells/15,000 cells in the absence of antibody, did not influence the neutralization titers observed in the assay (data not shown).
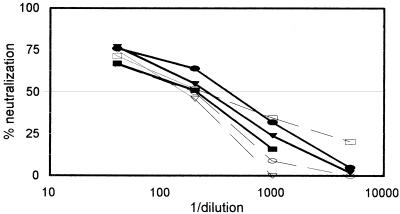
Subsequently, 10 primary isolates from clade B with different coreceptor preferences were tested in the GHOST cell neutralization assay against individual HIV-positive sera, the HIV-positive serum pool, HIVIG-Ug, and two human MAbs. Each Ab-virus combination was tested in duplicate, and each assay was repeated at least twice. There was very little variability in the duplicates, with the variation ranging from 0.3 to 10%. Neutralization curves are shown in Fig. 4 for the polytropic primary isolate SF2 with three individual immune sera in both GHOST-X4 and GHOST-R5 cells. Similar neutralization curves were used to determine the 50% neutralization titers, which are shown in Fig. 5.
FIG. 4.
Neutralization of SF2 by three immune sera (N, F, and FDA-2) in GHOST-X4 (bold lines) and GHOST-R5 (dashed lines) cells. Each serum dilution was tested in duplicate, and the percent neutralization was calculated by using the mean. The dose-response curves obtained are from one representative experiment of two or three carried out with each serum sample.
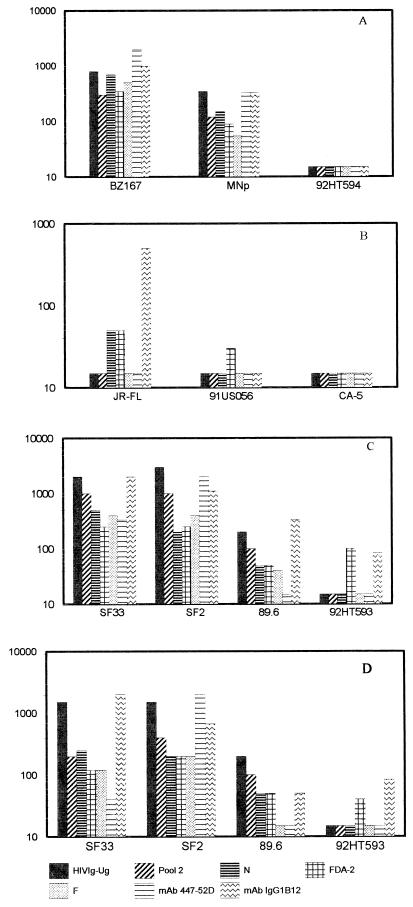
FIG. 5.
The 50% neutralizing titers shown on the y axis were determined for 10 primary isolates with seven antibody preparations. The X4-tropic viruses were tested on GHOST-X4 cells (A), the R5-tropic viruses were tested on GHOST-R5 cells (B), and the polytropic viruses were tested on GHOST-X4 (C) or GHOST-R5 (D) cells. Fivefold dilutions of the Ab preparations were tested for neutralization against a fixed dilution of virus. The stocks of the MAb preparations were adjusted to 1 mg/ml; thus, a titer of 1:1,000 is equivalent to 1 μg/ml. HIVIG-Ug was used at a starting concentration of 0.5 mg/ml; thus, an HIVIG-Ug titer of 1:1,000 corresponds to 0.5 μg/ml. Antibody preparations that did not neutralize an isolate at the lowest dilution tested (1:40) are shown graphically with an arbitrary titer of 1:15, which should be considered negative.
Of the X4 viruses, BZ167 was neutralized best, with 50% serum neutralizing titers of 1:500 to 1:1,000 and 50% neutralizing MAb concentrations of 1 to 5 μg/ml (Fig. 5A). The primary isolate MN was also neutralized by all the Ab preparations but with lower titers. 92HT594 could not be neutralized by any of the Ab preparations tested; the lowest dilution of antiserum tested was 1:40 and the highest concentration of MAb tested was 25 μg/ml for 447-52D and 13.5 μg/ml for IgG1b12 (Fig. 5A).
Of the R5 viruses, JR-FL could be neutralized by sera N and FDA-2 at titers of 1:50 and by MAb IgG1b12 at 2 μg/ml. Only 44% neutralization was achieved with isolate 91USO56 and FDA-2 at a 1:40 dilution. Isolate CA5 could not be neutralized by any of the Ab preparations tested (Fig. 5B).
With dual- and polytropic viruses, the patterns of neutralization were essentially the same whether the viruses were assayed on GHOST-X4 or GHOST-R5 cells (Fig. 5C and D, respectively). Only SF33 showed slightly lower titers when tested on GHOST-R5 (compared to GHOST-X4) with pool 2, the three individual HIV-positive sera, and the anti-V3 MAb, 447-52D. SF33 (in GHOST-X4) and SF2 could be neutralized best, at serum titers and MAb concentrations of ∼1:1,000 and 1 to 2 μg/ml, respectively. Isolate 89.6 showed moderate neutralization, and 92HT593 showed poor neutralization.
Thus, in each of the different virus categories defined by coreceptor usage, there was a spectrum of differential susceptibilities to Ab-mediated neutralization. R5 viruses appeared to be somewhat more resistant than X4, dual-, or polytropic viruses, but this conclusion may be affected by the particular viruses used from the panel available for testing.
As shown in Fig. 5, neither 447-52D nor IgG1b12 neutralized all the isolates. MAb 447-52D neutralized 4 of 10 strains, and IgG1b12 neutralized 7 of 10 strains. When 50% neutralizing concentrations were achieved for MAb 447-52D, they ranged from <1 to 20 μg/ml; the range for IgG1b12 ranged from <0.5 to 10 μg/ml. The core epitope of MAb IgG1b12 has not been identified, and therefore it was not possible to correlate its activity with any particular virus sequence. The core epitope of MAb 447-52D was previously identified as GPXR by Pepscan analysis with overlapping peptides from the V3 sequence of the MN strain (18). Binding to the V3MN peptide, however, accounts for only about 10% of the binding energy of the MAb (18); while neutralizing activity was associated with this V3 sequence (12), it was also correlated with the dissociation rate constant (48), which is affected by both sequence and conformation. This information suggested that the sequence at the crown of the V3 loop might not be sufficient to confer Ab binding that would lead to neutralization and that under certain conditions, conformation might overcome the need for absolute fidelity in the core epitope. To examine this, partial V3 sequences of the 10 isolates used in the neutralization experiments were analyzed; they are shown in Table 2. Of the 10 viruses, 8 contain the GPXR core epitope, but only 3 of these (BZ167, MNp, and SF2) were neutralized by MAb 447-52D. This suggests that the presence of the core epitope is not sufficient to confer neutralization sensitivity. Conversely, one of the two isolates that does not contain the core epitope (SF33) was neutralized by MAb 447-52D, suggesting that conformation-dependent structures can confer sufficient binding energy to effect neutralization.
TABLE 2.
Sequence comparison of the V3 loop of viruses studied for neutralization
| Virus Isolate | V3 loop sequencea | Neutralization by Anti-V3 MAb 447-52D |
|---|---|---|
| Consensus B | SIHI..GPGR..AFYTTGE | |
| BZ167 | R-R-..----..T--.--K | + |
| MNp | R---..----..-----KN | + |
| 92HT594 | R-R-..----..VW----Q | − |
| JR-FL | --TL..----..------D | − |
| 91US056 | G---..---A..---A--- | − |
| CA5 | G---..----..-IF---- | − |
| SF33 | R-TS..---K..VL----- | + |
| SF2 | --Y-..----..--H---R | + |
| 89.6 | RLS-..----..---ARRN | − |
| 92HT593 | R-S-..----..--RA-.K | − |
The sequences were obtained from the database of Myers et al. (35), and the box denotes the core epitope recognized by MAb 447-52d. Dots are for alignment; dashes denote homology.
DISCUSSION
We have shown that within each class of HIV-1 primary isolates categorized by coreceptor usage, there is a spectrum of neutralization sensitivity. The neutralization sensitivity of the isolates was determined by a new assay developed with genetically engineered GHOST cell lines which express CD4 and one of several chemokine receptors known to function as HIV coreceptors. The use of the GHOST cell lines as target cells in this neutralization assay substantially reduces the variability inherent in PBMC-based neutralization assays, which is a result of donor variation. The GHOST cell assay is also advantageous because it measures the number of infected cells directly, in contrast to the measurement of p24 or reverse transcriptase in the PBMC neutralization assay, which provides only an indirect assessment of the level of infection.
The applicability of the GHOST cells to the development of a neutralization assay useful with a broad range of primary HIV-1 isolates was established by determining the susceptibility of the cells to infection with 21 different primary isolates. Simultaneously, the range of coreceptor usage was also determined for these isolates. Coreceptor usage was easily discerned for each virus by simply measuring the induced fluorescence of the GFP reporter gene upon infection of the GHOST cells carrying the coreceptor used by the virus being studied. Until now, identification of coreceptor usage by primary isolates has depended on quantitation of p24 or syncytium formation with cell lines expressing one of the coreceptors (3, 45). In addition to CXCR4 and CCR5, CCR3 was used by several viruses, but it was used at a slightly lower efficiency. CCR3 is present on a wide range of cells (21, 42, 47), and the ability to use CCR3 may be relevant to the progression of disease. Other coreceptors, e.g., BOB and Bonzo, were similarly used by a minority of viruses and at relatively low efficiency.
Conflicting conclusions about the sensitivity of primary isolates to neutralization have been drawn. Many groups have shown that patients’ sera display comparable neutralization titers with laboratory-adapted strains and primary isolates (1, 28, 43–45). Several other studies, however reported a difference in neutralization sensitivities between laboratory-adapted and primary isolates, based on results with sera from vaccinees (29), HIV-positive sera (29, 34), and MAbs (14). Since TCLA isolates are SI and a majority of primary isolates are NSI (50), the data suggesting a greater neutralization sensitivity for laboratory-adapted strains has inaccurately been transformed into a consensus that SI isolates are more sensitive to neutralization than are NSI isolates. Our studies show that there is a spectrum of sensitivities within each virus phenotype to Ab-mediated neutralization and that some SI (X4) primary isolates are difficult to neutralize while some NSI (R5) isolates are neutralization sensitive, indicating that phenotype has little to do with virus sensitivity to neutralization.
Compared to other viruses, serum neutralization titers to HIV appear to be low (32). One of the reasons for this observation could be a lower sensitivity of the neutralization assays currently used for HIV. Multiple approaches to developing a sensitive and convenient neutralization assay have been tried. Back et al. (4) developed a transfection-neutralization assay in which CD4-negative cells transfected with proviral DNA of molecular clones were cocultured with PBMCs. Candotti et al. (9) described an assay in which, instead of quantitation of p24, HIV provirus synthesis was measured by PCR. The sensitivity of detecting neutralization was not improved by these techniques. Several assays that directly measure the reduction in infectivity commonly use T-cell lines, which restricts the assays to SI viruses. Most commonly, PBMCs are used as target cells in measuring the neutralization of primary isolates, and amplification products such as p24 or RT are quantified for the readout. The conditions for these PBMC-based assays vary widely (reviewed in reference 51) and result in broad variations in the neutralizing activity detected. In assays where virus is exposed to unstimulated PBMCs (54), target cells include CXCR4-expressing T cells and CCR5-expressing lymphocytes and monocytes (7), permitting the assessment of neutralization of both SI and NSI viruses. In neutralization assays with PBMCs activated with PHA and maintained in IL-2, PHA down-regulates CCR5 while IL-2 up-regulates CCR5 (7); thus, the status of the target cells varies with respect to coreceptor expression and depends on the particular conditions used for this “conventional” assay system. An alternative system with less variable target cells would therefore be highly desirable. Potential target cells for this purpose include genetically engineered cells expressing CD4, coreceptors, and an indicator gene. Such cell lines, with one of several indicator genes controlled by an HIV promoter, have been used to detect HIV infection. The indicator genes that have been used include chloramphenicol acetyltransferase (11), β-galactosidase (25), and luciferase (13). Recently, secreted alkaline phosphatase was used as the indicator gene in a neutralization assay in which the output was measured as chemiluminescence (33).
In the studies described above, GHOST cells served as target cells and both MAbs and polyclonal Abs were used to mediate neutralization. The MAbs provide more quantitative analyses and provide more refined information for analyzing specific epitopes involved in neutralization. The two MAbs used in this study were against functionally different sites: MAb 447-52D is a V3-specific MAb (18, 20), and IgG1b12 is directed to an epitope that overlaps the CD4 binding domain (8, 41). The 50% neutralizing concentrations ranged from <1 to 20 μg/ml for 447-52D and from <0.5 to 10 μg/ml for IgG1b12. Analysis of the neutralization sensitivities of the 10 isolates tested with MAb 447-52D revealed that effective neutralization did not always correlate with the presence of the core epitope defined for this MAb. Thus, the core epitope could be present in the envelope of a virus, e.g., 92HT593, that the MAb failed to neutralize, or could have a substitution in the core epitope, e.g., SF33, and still be neutralized. Since it is known that MAb 447-52D recognizes both linear and conformational aspects of the virus envelope (18), the data suggest that presentation of the same epitope on the envelopes of different isolates varies, affecting the binding of Ab or the conformational changes that the MAb induces in the virus envelope. This could occur, for example, as the result of a change in the dissociation rate of the Ab from the virion; this is known to profoundly affect the neutralizing capacity of MAbs (48). It could also occur if changes at sites other than the core epitope affect epitope exposure or conformation. For example, changes in the glycosylation of gp120 affect the exposure of epitopes (5) and changes in amino acids affect epitopes at distant sites (36, 39). Other factors, such as the presence of adhesion molecules on the surface of virions, may also play a role in changing the neutralization characteristics of the virus (6, 16, 40).
Loss of neutralization sensitivity is not necessarily accompanied by loss of antibody binding, indicating that changes in the epitope may affect the way in which the MAb interferes with the process of virus infectivity, e.g., fusion and uncoating (31, 36). The finding that a single passage of plasma virus through PHA-stimulated PMBCs changes the neutralization profiles and surface characteristics of primary isolates (6, 17) highlights the mutability of HIV. However, what actually contributes to the neutralization sensitivity of an isolate is not known. Probably several factors contribute, since different Abs function at different steps in virus infection. Thus, an anti-V3 conformation-dependent Ab blocks infection at a postinternalization step (2), the Fab fragment of IgG1b12 neutralizes at a postfusion step, the whole IgG1b12 molecule inhibits virus fusion (30), and receptor blocking has been reported as a mechanism of action for several human anti-HIV MAbs (10, 46). Therefore, the neutralization of an isolate is defined not just by the presence of an epitope or by virus interaction with CXCR4 or CCR5, but also by the presentation of the epitope, the way it interacts with the Ab, and the effect of this interaction on the virus.
ACKNOWLEDGMENTS
This work was supported in part by NIH grants HL 59725, AI 36085, and AI 32424; by NIAID grant AI 27742 supporting the NYU Center for AIDS Research; and by funds from the Research Center for AIDS and HIV Infection of the Department of Veterans Affairs, New York, N.Y. V.N.K. is a postdoctoral fellow of the Damon Runyan-Walter Winchell Foundation, and D.R.L. is an investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Arendrup M, Sonnerborg A, Svennerholm B, Akerblom L, Nielsen C, Clausen H, Olofsson S, Nielsen J O, Hansen J S. Neutralizing antibody response during human immunodeficiency virus type 1 infection: type and group specificity and viral escape. J Gen Virol. 1993;74:855–863. doi: 10.1099/0022-1317-74-5-855. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong S J, McInerney T L, McLain L, Wahren B, Hinkula J, Levi M, Dimmock N J. Two neutralizing anti-V3 monoclonal antibodies act by affecting different functions of human immunodeficiency virus type 1. J Gen Virol. 1996;77:2931–2941. doi: 10.1099/0022-1317-77-12-2931. [DOI] [PubMed] [Google Scholar]
- 3.Auewarakul P, Louisirirotchanakul S, Sutthent R, Taechowisan T, Kanoksinsombat C, Wasi C. Analysis of neutralizing and enhancing antibodies to human immunodeficiency virus type 1 primary isolates in plasma of individuals infected with env genetic subtype B and E viruses in Thailand. Viral Immunol. 1996;9:175–185. doi: 10.1089/vim.1996.9.175. [DOI] [PubMed] [Google Scholar]
- 4.Back N K, Smit L, Hogervorst E, van Wijk A C, Goudsmit J, Tersmette M. Development and evaluation of an HIV-1 transfection-neutralization assay. J Acquired Immune Defic Syndr. 1994;7:531–538. [PubMed] [Google Scholar]
- 5.Bandres J C, Wang Q F, O’Leary J, Baleaux F, Amara A, Hoxie J, Zolla-Pazner S, Gorny M K. Human immunodeficiency virus (HIV) envelope binds to CXCR4 independently of CD4, and binding can be enhanced by interaction with soluble CD4 or by HIV envelope deglycosylation. J Virol. 1998;72:2500–2504. doi: 10.1128/jvi.72.3.2500-2504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bastiani L, Laal S, Zolla-Pazner S, Kim M. Host cell-dependent alterations in envelope components of HIV-1 virions. J Virol. 1997;71:3444–3450. doi: 10.1128/jvi.71.5.3444-3450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bleul C C, Wu L, Hoxie J A, Springer T A, MacKay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton D R, Pyati J, Koduri R, Sharp S J, Thornton G B, Parren P W H I, Sawyer L S W, Hendry R M, Dunlop N, Nara P L, Lamacchia M, Garratty E, Stiehm E R, Bryson Y J, Cao Y, Moore J P, Ho D D, Barbas C F., III Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 9.Candotti D, Rosenheim M, Huraux J M, Agut H. Two PBMC-based neutralization assays depict low reactivity of both anti-V3 monoclonal antibodies and immune sera against HIV-1 primary isolates. J Virol Methods. 1997;64:81–93. doi: 10.1016/s0166-0934(96)02145-3. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y H, Dierich M P. Identification of a second site in HIV-1 gp41 mediating binding to cells. Immunol Lett. 1996;52:153–156. doi: 10.1016/0165-2478(96)02603-x. [DOI] [PubMed] [Google Scholar]
- 11.Ciminale V, Felber B K, Campbell M, Pavlakis G N. A bioassay for HIV-1 based on env-CD4 interaction. AIDS Res Hum Retroviruses. 1990;6:1281. doi: 10.1089/aid.1990.6.1281. [DOI] [PubMed] [Google Scholar]
- 12.Conley A J, Gorny M K, Kessler II J A, Boots L J, Lineberger D, Emini E A, Ossorio M, Koenig S, Williams C, Zolla-Pazner S. Neutralization of primary HIV-1 virus isolates by the broadly-reactive anti-V3 monoclonal antibody, 447-52D. J Virol. 1994;68:6994–7000. doi: 10.1128/jvi.68.11.6994-7000.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng H, Unutmaz D, Kewalramani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 14.D’Souza M P, Livnat D, Bradac J A, Bridges S H The AIDS Clinical Trials Group Antibody Selection Working Group. Evaluation of monoclonal antibodies to human immunodeficiency virus type 1 primary isolates by neutralization assays: performance criteria for selecting candidate antibodies for clinical trials. J Infect Dis. 1997;175:1056–1062. doi: 10.1086/516443. [DOI] [PubMed] [Google Scholar]
- 15.Fortin J, Cantin R, Lamontagne G, Tremblay M. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J Virol. 1997;71:3588–3596. doi: 10.1128/jvi.71.5.3588-3596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank I, Stoiber H, Godar S, Stockinger H, Steindl F, Katinger H W D, Dierich M P. Acquisition of host cell-surface-derived molecules by HIV-1. AIDS. 1996;10:1611–1620. doi: 10.1097/00002030-199612000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Gauduin M, Allaway G P, Maddon P J, Barbas III C F, Burton D R, Koup R A. Effective ex vivo neutralization of human immunodeficiency virus type 1 in plasma by recombinant immunoglobulin molecules. J Virol. 1996;70:2586–2592. doi: 10.1128/jvi.70.4.2586-2592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorny M K, Conley A J, Karwowska S, Buchbinder A, Xu J, Emini E A, Koenig S, Zolla-Pazner S. Neutralization of diverse HIV-1 variants by an anti-V3 human monoclonal antibody. J Virol. 1992;66:7538–7542. doi: 10.1128/jvi.66.12.7538-7542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorny M K, VanCott T C, Hioe C, Israel Z R, Michael N L, Conley A J, Williams C, Kessler II J A, Chigurupati P, Burda S, Zolla-Pazner S. Human monoclonal antibodies to the V3 loop of HIV-1 with intra- and inter-clade cross-reactivity. J Immunol. 1997;159:5114–5122. [PubMed] [Google Scholar]
- 20.Gorny M K, Xu J, Karwowska S, Buchbinder A, Zolla-Pazner S. Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J Immunol. 1993;150:635–643. [PubMed] [Google Scholar]
- 21.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C R, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 22.Hioe C E, Xu S, Chigurupati P, Burda S, Williams C, Gorny M K, Zolla-Pazner S. Neutralization of HIV-1 primary isolates by polyclonal and monoclonal human antibodies. Int Immunol. 1997;9:1281–1290. doi: 10.1093/intimm/9.9.1281. [DOI] [PubMed] [Google Scholar]
- 23.Hogervorst E, De Jong J, Van Wijk A, Bakker M, Valk M, Nara P, Goudsmit J. Insertion of primary syncytium-inducing (SI) and non-SI envelope V3 loops in human immunodeficiency virus type 1 (HIV-1) LAI reduces neutralization sensitivity to autologous, but not heterologous, HIV-1 antibodies. J Virol. 1995;69:6342–6351. doi: 10.1128/jvi.69.10.6342-6351.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.KewalRamani, V. N., B. Volsky, D. Kwon, W.-K. Xiang, J. Gao, D. Unutmaz, C. M. Hill, R. E. Sutton, and D. R. Littman. Unpublished data.
- 25.Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laal S, Burda S, Sharpe S, Zolla-Pazner S. A rapid, automated microplate assay for measuring neutralization of HIV-1. AIDS Res Hum Retroviruses. 1993;9:781–785. doi: 10.1089/aid.1993.9.781. [DOI] [PubMed] [Google Scholar]
- 27.LaCasse R A, Follis K E, Moudgil T, Trahey M, Binley J M, Planellas V, Zolla-Pazner S, Nunberg J H. Coreceptor utilization by human immunodeficiency virus type 1 is not a primary determinant of neutralization sensitivity. J Virol. 1998;72:2491–2495. doi: 10.1128/jvi.72.3.2491-2495.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mascola J R, Louwagie J, McCutchan F E, Fischer C L, Hegerich P A, Wagner K F, Fowler A K, McNeil J G, Burke D S. Two antigenically distinct subtypes of HIV-1: viral genotype predicts neutralization immunotype. J Infect Dis. 1994;169:48–54. doi: 10.1093/infdis/169.1.48. [DOI] [PubMed] [Google Scholar]
- 29.Mascola J R, Snyder S W, Weislow O S, Belay S M, Belshe R B, Schwartz D H, Clements M L, Dolin R, Graham B S, Gorse G J, Keefer M C, McElrath M J, Walker M C, Wagner K F, McNeil J G, McCutchan F E, Burke D S for the NIAID AIDS Vaccine Evaluation Group. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 30.McInerney T L, McLain L, Armstrong S J, Dimmock N J. A human IgG1 (b12) specific for the CD4 binding site of HIV-1 neutralizes by inhibiting the virus fusion entry process, but b12 Fab neutralizes by inhibiting a postfusion event. Virology. 1997;233:313–326. doi: 10.1006/viro.1997.8547. [DOI] [PubMed] [Google Scholar]
- 31.McKeating J A, Bennett J, Zolla-Pazner S, Schutten M, Ashelford S, Brown A L, Balfe P. Resistance of a human serum-selected human immunodeficiency virus type 1 escape mutant to neutralization by CD4 binding site monoclonal antibodies is conferred by a single amino acid change in gp120. J Virol. 1993;67:5216–5225. doi: 10.1128/jvi.67.9.5216-5225.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLain L, Dimmock N J. Single- and multi-hit kinetics of immunoglobulin G neutralization of human immunodeficiency virus type 1 by monoclonal antibodies. J Gen Virol. 1994;75:1457–1460. doi: 10.1099/0022-1317-75-6-1457. [DOI] [PubMed] [Google Scholar]
- 33.Means R E, Greenough T, Desrosiers R C. Neutralization sensitivity of cell culture-passaged simian immunodeficiency virus. J Virol. 1997;71:7895–7902. doi: 10.1128/jvi.71.10.7895-7902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montefiori D C, Collman R G, Fouts T R, Zhou J Y, Bilska M, Hoxie J A, Moore J P, Bolognesi D P. Evidence that antibody-mediated neutralization of human immunodeficiency virus type 1 by sera from infected individuals is independent of coreceptor usage. J Virol. 1998;72:1886–1893. doi: 10.1128/jvi.72.3.1886-1893.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myers G, Korber B, Foley B, Smith R F, Jeang K, Mellors J W, Wain-Hobson A. Human retroviruses and AIDS. Los Alamos, N.M: Theoretical Biology and Biophysics, Los Alamos National Laboratories; 1996. [Google Scholar]
- 36.Nara P L, Smit L, Dunlop N, Hatch W, Waters M D, Kelliher J, Gallo R C, Fischinger P J, Goudsmit J. Emergence of viruses resistant to neutralization by V3-specific antibodies in experimental human immunodeficiency virus type 1 (IIIB) infection of chimpanzees. J Virol. 1990;64:3779–3791. doi: 10.1128/jvi.64.8.3779-3791.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nyambi, P. N., L. Heyndrickx, W. Janssens, F. Daeyaert, P. Lewi, K. Fransen, B. Willems, S. Coppens, K. Vereecken, P. Piot, and G. van der Groen. Identification of specific amino acid motifs and biophenotypic properties among primary HIV-1 group M (subtype A-H) and O isolates belonging to different neutralization clusters. Submitted for publication.
- 38.Nyambi P N, Nkengasong J, Lewi P, Andries K, Janssens W, Fransen K, Heyndrickx L, Piot P, van der Groen G. Multivariate analysis of human immunodeficiency virus type 1 neutralization data. J Virol. 1996;70:6235–6243. doi: 10.1128/jvi.70.9.6235-6243.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reitz M S, Wilson C, Naugle C, Gallo R C, Robert-Guroff M. Generation of a neutralization-resistant variant of HIV-1 is due to selection for a point mutation in the envelope gene. Cell. 1988;54:57–63. doi: 10.1016/0092-8674(88)90179-1. [DOI] [PubMed] [Google Scholar]
- 40.Rizzuti C D, Sodroski J G. Contribution of virion ICAM-1 to human immunodeficiency virus infectivity and sensitivity to neutralization. J Virol. 1997;71:4847–4851. doi: 10.1128/jvi.71.6.4847-4851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roben P, Moore J P, Thali M, Sodroski J, Barbas C F., III Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J Virol. 1994;68:4821–4828. doi: 10.1128/jvi.68.8.4821-4828.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sallusto F, Mackay C R, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;227:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 43.Sawyer L W, Wrin M T, Crawford-Miksza L, Potts B, We Y, Weber P A, Alfonso R D, Hanson C V. Neutralization sensitivity of human immunodeficiency virus type 1 is determined in part by the cell in which the virus is propagated. J Virol. 1994;68:1342–1349. doi: 10.1128/jvi.68.3.1342-1349.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scarlatti G, Albert J, Rossi P, Hodara V, Biraghi P, Muggiasca L, Fenyo E M. Mother-to-child transmission of human immunodeficiency virus type 1: correlation with neutralizing antibodies against primary isolates. J Infect Dis. 1993;168:207–210. doi: 10.1093/infdis/168.1.207. [DOI] [PubMed] [Google Scholar]
- 45.Trkola A, Ketas T, KewalRamani V N, Endorf F, Binley J M, Katinger H, Robinson J, Littman D R, Moore J P. Neutralization sensitivity of human immunodeficiency virus type 1 primary isolates to antibodies and CD4-based reagents is independent of coreceptor usage. J Virol. 1998;72:1876–1885. doi: 10.1128/jvi.72.3.1876-1885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ugolini S, Mondor I, Parren P W H I, Burton D R, Tilley S, Klasse P J, Sattentau Q J. Inhibition of virus attachment to CD4+ target cells is a major mechanism of T cell line-adapted HIV-1 neutralization. J Exp Med. 1997;186:1287–1298. doi: 10.1084/jem.186.8.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uguccioni M, Mackay C R, Ochensberger B, Loetscher P, Rhis S, LaRosa G J, Rao P, Ponath P D, Baggiolini M, Dahinden C A. High expression of the chemokine receptor CCR3 in human blood basophils. Role in activation by eotaxin, MCP-4, and other chemokines. J Clin Invest. 1997;100:1137–1143. doi: 10.1172/JCI119624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.VanCott T C, Bethkes F R, Polonis V R, Gorny M G, Zolla-Pazner S, Redfield R R, Birx D L. Dissociation rate of antibody-gp120 binding interactions is predictive of V3-mediated neutralization of HIV-1. J Immunol. 1994;153:449–459. [PubMed] [Google Scholar]
- 49.Weber J, Fenyo E-M, Beddows S, Kaleebu P, Bjorndal A the WHO Network for HIV Isolation and Characterization. Neutralization serotypes of HIV-1 field isolates are not predicted by genetic subtype. J Virol. 1996;70:7827–7832. doi: 10.1128/jvi.70.11.7827-7832.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang L, He T, Kuntsman K, Wu S, Guo Y, Neumann A U, Ho D D, Wolinsky S M. Abstracts of the 5th Conference on Retroviruses and Opportunistic Infections. 1998. HIV-1 phenotype, co-receptor usage and disease progression, abstr. 281; p. 132. [Google Scholar]
- 51.Zolla-Pazner S. Mechanisms contributing to the neutralization of HIV-1. Immunol Lett. 1996;51:89–93. doi: 10.1016/0165-2478(96)02560-6. [DOI] [PubMed] [Google Scholar]
- 52.Zolla-Pazner S, Alving C, Belshe R, Berman P, Burda S, Chigurupati P, Clements M L, Duliege A-M, Excler J-L, Kahn J, McElrath M J, Sharpe S, Sinangil F, Steimer K, Walker M C, Wassef N, Xu S. Neutralization of a clade B primary isolate by sera from HIV-uninfected recipients of candidate AIDS vaccines. J Infect Dis. 1997;175:764–774. doi: 10.1086/513969. [DOI] [PubMed] [Google Scholar]
- 53.Zolla-Pazner S, Lubeck M, Xu S, Burda S, Natuk R J, Sinangil F, Steimer K, Gallo R C, Eichberg J W, Matthews T, Robert-Guroff M. Induction of neutralizing antibodies in T-cell line-adapted and primary human immunodeficiency virus type 1 isolates with a prime-boost vaccine regimen in chimpanzees. J Virol. 1998;72:1052–1059. doi: 10.1128/jvi.72.2.1052-1059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zolla-Pazner S, Sharpe S. A resting cell assay for improved detection of antibody-mediated neutralization of HIV-1 primary isolates. AIDS Res Hum Retroviruses. 1995;11:1449–1457. doi: 10.1089/aid.1995.11.1449. [DOI] [PubMed] [Google Scholar]