Abstract
Infection with attenuated simian immunodeficiency virus (SIV) in rhesus macaques has been shown to raise antibodies capable of neutralizing an animal challenge stock of primary SIVmac251 in CEMx174 cells that correlate with resistance to infection after experimental challenge with this virulent virus (M. S. Wyand, K. H. Manson, M. Garcia-Moll, D. C. Montefiori, and R. C. Desrosiers, J. Virol. 70:3724–3733, 1996). Here we show that these neutralizing antibodies are not detected in human and rhesus peripheral blood mononuclear cells (PBMC). In addition, neutralization of primary SIVmac251 in human and rhesus PBMC was rarely detected with plasma samples from a similar group of animals that had been infected either with SIVmac239Δnef for 1.5 years or with SIVmac239Δ3 for 3.2 years, although low-level neutralization was detected in CEMx174 cells. Potent neutralization was detected in CEMx174 cells when the latter plasma samples were assessed with laboratory-adapted SIVmac251. In contrast to primary SIVmac251, laboratory-adapted SIVmac251 did not replicate in human and rhesus PBMC despite its ability to utilize CCR5, Bonzo/STRL33, and BOB/gpr15 as coreceptors for virus entry. These results illustrate the importance of virus passage history and the choice of indicator cells for making assessments of neutralizing antibodies to lentiviruses such as SIV. They also demonstrate that primary SIVmac251 is less sensitive to neutralization in human and rhesus PBMC than it is in established cell lines. Results obtained in PBMC did not support a role for neutralizing antibodies as a mechanism of protection in animals immunized with attenuated SIV and challenged with primary SIVmac251.
Inoculation with live, attenuated strains of virus is a safe and effective means to vaccinate against a number of human viral diseases. A similar vaccine strategy for human immunodeficiency virus type 1 (HIV-1) is being explored in the macaque model of simian immunodeficiency virus (SIV) infection. Attenuated variants of SIV often protect macaques against experimental challenge with virulent virus (1, 6, 9, 26, 45). The nature of this protective immunity is uncertain and appears to be dependent on the level of attenuation and length of time of infection (6, 8, 26, 45). Although this approach faces formidable safety issues which must be resolved before it can gain acceptance for HIV-1 (15, 39), infection with attenuated SIV in macaques represents a practical model in which to investigate in vitro correlates of protective immunity to primate lentiviruses that cause AIDS.
Attenuated variants of SIV have been created by introducing deletions that inactivate one or more genes of molecularly cloned SIVmac239 (20). The capacity for this molecularly cloned virus to cause immunodeficiency and AIDS in rhesus monkeys is markedly reduced by deletion of portions of nef (21, 38). Attempts at greater attenuation led to the introduction of multiple gene deletions to yield several variants that remain infectious in macaques, where they replicate at lower levels than wild-type virus (11, 12, 45). SIVmac239Δnef and SIVmac239Δ3 (containing deletions in nef, vpr, and upstream sequences in U3) have attenuated phenotypes in juvenile and adult macaques and are able to protect against experimental challenge with virulent virus (8, 9, 45). In one study (45), neutralizing antibodies to the animal challenge stock of primary SIVmac251 were detected more often in plasma from animals that resisted infection than in plasma from animals that did not resist infection. Both groups of animals had nearly equal titers of neutralizing antibodies to a laboratory-adapted variant of this virus, indicating that assessments made with the primary virus were more relevant as a correlate of immunity. It has been suggested that neutralizing antibodies to primary SIVmac251 require a lengthy period of antigen exposure for affinity maturation and for developing strong reactivity to native viral envelope glycoprotein structures (7).
In the study mentioned above (45), antibodies that neutralized both a low-passage stock of primary SIVmac251 made in rhesus peripheral-blood mononuclear cells (PBMC) and a stock of laboratory-adapted SIVmac251 made in H9 cells and subjected to multiple passages in H9 cells were detected in CEMx174 cells. Repeated passage of HIV-1 in CD4+ T-cell lines such as H9 can increase the virus’s sensitivity to neutralization in vitro (33, 41). It comes as no surprise, therefore, that laboratory-adapted SIVmac251 is more sensitive to neutralization than primary SIVmac251 in CEMx174 cells and C8166-45 cells (28). This dichotomy in neutralization sensitivity between two stocks of SIVmac251 is reminiscent of a similar dichotomy in neutralization sensitivity between T-cell line-adapted variants and primary isolates of HIV-1, which is thought to result from structural differences in the viral envelope glycoproteins (4, 33). Although most measurements of neutralizing antibodies to T-cell line-adapted variants and primary isolates of HIV-1 are made in T-cell lines and PBMC, respectively, the dichotomy in neutralization sensitivity is not always dependent on indicator cell type (23, 31, 43).
We assessed the neutralization sensitivity of primary SIVmac251 in human and rhesus PBMC by using the same methodology that has been used for primary isolates of HIV-1. PBMC were isolated from human and rhesus macaque peripheral blood as described elsewhere (30, 35) and were stored in liquid nitrogen at a density of 2.5 × 107 cells/ml in RPMI 1640 containing 50% heat-inactivated fetal bovine serum and 10% dimethyl sulfoxide. PBMC were thawed, diluted 1:30 in growth medium containing 20% fetal bovine serum, and stimulated for 2 days with phytohemagglutinin P (5 μg/ml). After additional washing, the cells were resuspended in fresh growth medium containing human interleukin 2 (5%) and were used in neutralization assays. Virus stocks were prepared in H9 cells and human and rhesus PBMC as was done previously (30, 45). Laboratory-adapted SIVmac251 had been passaged repeatedly in H9 cells, and the final preparation of the stock used here was made in the same cells. Stocks of uncloned primary SIVmac251 were derived from a low-passage, rhesus PBMC-grown animal challenge stock (24) by a single expansion in either H9 cells, human PBMC, or rhesus PBMC.
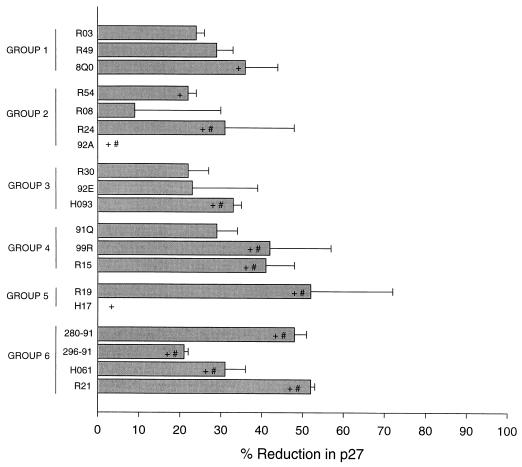
We first assessed a group of plasma samples from animals immunized with either SIVmac316Δnef or SIVmac239Δ3. These were the same samples for which the ability to reduce p27 production by >80% in CEMx174 cells correlated with protection of animals from infection after challenge with primary SIVmac251 (45). These plasma samples were reassessed exactly as was done previously (45), with the exception that human PBMC were used in place of CEMx174 cells. Rhesus PBMC-grown primary SIVmac251 (the same dose as was used in the study reported in reference 45) was incubated with a 1:4 dilution of plasma samples at 37°C for 1 h in triplicate wells of 96-well microdilution plates. Human PBMC were added, and the incubation was continued another 4 h. Cells were washed four times with growth medium to remove residual virus and anti-p27 antibodies that could interfere with p27 measurements. After the washes, the cells were resuspended in interleukin 2-containing growth medium and were incubated until p27 production in virus control wells (no plasma sample) was in a linear phase of increase (7 days, in this case). Viral p27 concentrations were determined by an enzyme-linked immunosorbent assay (Organon Teknika/Akzo, Durham, N.C.). Percent reduction in p27 was assessed relative to the concentration of p27 produced in the presence of plasma from an SIV-naïve macaque (9.27 ng of p27/ml). As shown in Fig. 1, we were unable to detect a reduction in p27 synthesis of >80% with any samples. Minor reductions that did not exceed 52% were seen with some samples, and although the magnitude of reductions tracked with the correlation seen previously, we do not consider them to be significant. For example, reductions of less than 50% in p27 synthesis correspond to minor reductions in infectious virus that often fall within the normal range of variation in this assay. By comparison, an 80% reduction in p27 corresponds to an approximate 5- to 10-fold reduction in infectious virus and is rarely seen with plasma samples from SIV-naïve macaques (unpublished data).
FIG. 1.
Neutralization of primary SIVmac251 in human PBMC by plasma samples from macaques infected with either SIVmac316Δnef or SIVmac239Δ3. Each value is the average percent reduction in p27 in triplicate wells; error bars represent standard deviations. Animals in groups 1, 3, and 5 were infected with SIVmac316Δnef for 8, 20, and 79 weeks, respectively. Animals in groups 2, 4, and 6 were infected with SIVmac239Δ3 for 8, 20, and 79 weeks, respectively. +, samples positive for neutralization in CEMx174 cells; #, animals protected from challenge (7, 45).
We next determined whether we could detect virus neutralization in rhesus PBMC. For this we used plasma samples from a new group of rhesus macaques that had been infected with either SIVmac239Δnef or SIVmac239Δ3 for 1.5 and 3.2 years, respectively. These periods of infection are sufficient to provide potent protection from experimental challenge with primary SIVmac251 in a majority of animals (8, 9, 45). Because these were new groups of animals, we also planned to assess neutralization in CEMx174 cells and human PBMC so that comparisons with our previous results could be made.
We discovered in preliminary neutralization assays that only the primary virus was capable of replicating efficiently in human and rhesus PBMC. To confirm this observation, CEMx174 cells and human and rhesus PBMC were inoculated with an equal volume of each stock of virus, incubated for 4 h, and then washed three times with growth medium to remove residual virus. Fresh growth medium was added, and viral p27 antigen production in culture supernatants was monitored on days 4, 9, and 11 of incubation by using an enzyme-linked immunosorbent assay as described by the supplier (Organon Teknika/Akzo). As shown in Table 1, p27 antigen was detected in CEMx174 cells on day 4 of incubation for all virus stocks, and the concentrations rose to very high levels by days 9 and 11 in each case. Efficient replication of primary SIVmac251 was also detected in human and rhesus PBMC by day 4 of incubation and increased to very high levels by days 9 and 11. Little or no p27 was detected in human and rhesus PBMC for the laboratory-adapted stock during the entire observation period, suggesting that repeated passage in H9 cells gave rise to a virus variant that was no longer capable of replicating in PBMC. Of note, a single passage of primary SIVmac251 in H9 cells had only a minor effect on the ability of the virus to replicate in PBMC (Table 1).
TABLE 1.
Abilities of laboratory-adapted and primary SIVmac251 to replicate in PBMC and CEMx174 cellsa
| Virus (host cell) | Day | Replicationb in:
|
||
|---|---|---|---|---|
| rPBMC | hPBMC | CEMx174 | ||
| Laboratory-adapted (H9) | 4 | <30 | <30 | 953 |
| 9 | <30 | 39 | >3,000 | |
| 11 | <30 | <30 | >30,000 | |
| Primary (H9) | 4 | 35 | 75 | 621 |
| 9 | >3,000 | 1,507 | >3,000 | |
| 11 | 10,979 | 1,980 | >30,000 | |
| Primary (rPBMC) | 4 | 313 | 297 | 2,088 |
| 9 | >3,000 | >3,000 | >3,000 | |
| 11 | 11,396 | 16,248 | >30,000 | |
| Primary (hPBMC) | 4 | >3,000 | 569 | >3,000 |
| 9 | >3,000 | >3,000 | >3,000 | |
| 11 | 13,261 | 19,609 | >30,000 | |
Stocks of laboratory-adapted and primary SIVmac251 were grown in the indicated host cells and were used to inoculate rhesus PBMC (rPBMC), human PBMC (hPBMC), and CEMx174 cells; an equal volume of virus was used for inoculation of each cell type.
Viral p27 concentrations in culture supernatants were quantified on days 4, 9, and 11 of incubation and are given in picograms per milliliter.
The results described above led us to speculate that primary and laboratory-adapted SIVmac251 differed in their coreceptor usage. In addition to CD4, HIV-1 and SIV utilize a variety of coreceptors to gain entry into cells, and cellular tropism is determined in part by which coreceptors are used (for a review, see reference 2). Major coreceptors used by SIV include the chemokine receptor CCR5 (5, 14, 17, 27, 37) and two orphan receptors designated Bonzo/STRL33 and BOB/gpr15 (10, 16, 25). CCR8 is another chemokine receptor used as a coreceptor by some strains of SIV (37). CXCR4, a major coreceptor used by syncytium-inducing variants of HIV-1, does not appear to be used by SIV (5, 14, 17, 27). Minor coreceptors used by HIV-1 but not SIV include CCR3 and CCR2b (17, 37).
Of the coreceptors used by SIV, human PBMC express mRNA transcripts for CCR5, CCR8, and Bonzo/STRL33 but not for BOB/gpr15 (5, 10, 16, 40). CEMx174 cells differ from PBMC by expressing transcripts for BOB/gpr15 but not for CCR5 or Bonzo/STRL33 (5, 10, 16). Coreceptor usage by our stocks of primary and laboratory-adapted SIVmac251 was evaluated in HOS-CD4 cells stably transfected to express different coreceptors (43). As shown in Table 2, both viruses were capable of using BOB/gpr15, which is consistent with their abilities to infect CEMx174 cells efficiently. In agreement with the observations of others (37), neither stock of virus was capable of using CCR3, CCR8, or CXCR4. Finally, both viruses were capable of using CCR5, while only laboratory-adapted SIVmac251 was capable of using Bonzo/STRL33 (Table 2).
TABLE 2.
Coreceptor usage of primary and laboratory-adapted stocks of SIVmac251
| SIVmac251a | Usage of coreceptorb:
|
|||||
|---|---|---|---|---|---|---|
| CXCR4 | CCR3 | CCR5 | CCR8 | Bonzo/ STRL33 | BOB/ gpr15 | |
| Primary | − | − | +++ | − | − | ++ |
| Laboratory adapted | − | − | ++ | − | + | + |
Primary SIVmac251 was grown in rhesus PBMC and is a single passage of the animal challenge stock of this virus. Laboratory-adapted SIVmac251 was passaged repeatedly in H9 cells.
Coreceptor usage was determined in HOS-CD4 cells transfected to express each coreceptor separately. Results are expressed as relative fluorescence intensities generated by Tat-activated green fluorescence protein expression. +++, ++, +, and −, strong, moderate, weak, and negative fluorescence, respectively.
Because PBMC expressed both CCR5 and Bonzo/STRL33, and both stocks of virus were able to use CCR5, our results offered no clear explanation for why only primary SIVmac251 was able to replicate in these cells. We indirectly confirmed that our human PBMC expressed CCR5 on their surfaces by showing that they could support the efficient replication of primary isolates of HIV-1 that use CCR5 as their sole coreceptor (data not shown). Perhaps the differential ability to replicate in PBMC was due to less efficient utilization of CCR5 by laboratory-adapted SIVmac251 compared to primary SIVmac251 (Table 2). Use of Bonzo/STRL33 by laboratory-adapted SIVmac251 was even less efficient (Table 2). It is also possible that the differential replication of these two stocks of virus in PBMC is explained by factors other than coreceptor usage. For example, the inability of SIVmac239 to replicate in macaque alveolar macrophages, although determined by gp120, was not due to restricted virus entry (34). Additional studies will be needed to clarify why only primary SIVmac251 was capable of replicating in PBMC.
Due to the inability of laboratory-adapted SIVmac251 to replicate in human and rhesus PBMC, neutralization of this virus was assessed in CEMx174 cells while neutralization of primary SIVmac251 was assessed in all three cell types. Plasma samples from 12 macaques, 6 infected with SIVmac239Δnef and 6 infected with SIVmac239Δ3, were evaluated (Table 3). High titers of neutralizing antibodies were detected in all plasma samples from both groups of animals when laboratory-adapted SIVmac251 was used in a CEMx174 cell-killing assay as described previously (30). Titers of neutralizing antibodies to primary SIVmac251 in CEMx174 cells were 65 to 2,200 times lower, although still detectable with most samples, in an assay using 80% reduction in p27 synthesis as a cutoff for neutralization as described elsewhere (45). This greater detection of neutralization with laboratory-adapted SIVmac251 is in agreement with the results of a previous study (28).
TABLE 3.
Antibody-mediated neutralization of primary SIVmac251 in CEMx174 cells and human and rhesus PBMC
| Virus and macaque no.a | Neutralization titer for SIVmac251
|
|||
|---|---|---|---|---|
| Laboratory adapted, in CEMx174 cellkilling assayb | Primary, in p27 reduction assayc in:
|
|||
| CEMx174 cells | Human PBMC | Rhesus PBMC | ||
| SIVmac239Δnef | ||||
| H313 | 1:5,239 | 1:10 | <1:5 | <1:5 |
| H323 | 1:5,205 | 1:80 | ≥1:5 | <1:5 |
| H324 | 1:30,319 | 1:50 | <1:5 | <1:5 |
| H343 | 1:11,551 | 1:10 | <1:5 | <1:5 |
| H345 | 1:11,729 | 1:80 | <1:5 | <1:5 |
| H348 | 1:28,883 | 1:20 | ≥1:5 | <1:5 |
| SIVmac239Δ3 | ||||
| 17155 | 1:13,894 | 1:20 | <1:5 | <1:5 |
| 17158 | 1:11,295 | 1:5 | <1:5 | <1:5 |
| 17159 | 1:1,479 | <1:5 | <1:5 | <1:5 |
| 63-88 | 1:2,539 | 1:20 | <1:5 | <1:5 |
| 99-88 | 1:11,764 | 1:10 | <1:5 | <1:5 |
| 120-87 | 1:3,722 | <1:5 | <1:5 | <1:5 |
| SIVmac251 | ||||
| 145-86 | 1:5,850 | 1:50 | <1:5 | <1:5 |
| 244-86 | 1:3,813 | 1:20 | 1:20 | NT |
| 354-95 | 1:13,917 | <1:5 | 1:5 | NT |
| 578-91 | 1:4,025 | <1:5 | <1:5 | NT |
| 579-91 | 1:4,853 | 1:5 | <1:5 | NT |
Macaques were infected with either SIVmac239Δ3 or SIVmac239Δnef for 1.5 and 3.2 years, respectively. Macaques 145-86 and 244-86 had been infected with SIVmac251 for approximately 4 years and remained healthy. The remaining macaques had been infected with SIVmac251 for 2 months (354-95) and 6 months (578-91 and 579-91).
Neutralization titers are the serum dilutions at which 50% of CEMx174 cells were protected from virus-induced cell killing. Laboratory-adapted SIVmac251 was grown in H9 cells.
Viruses were grown in rhesus PBMC and assayed in CEMx174 cells, human PBMC, and rhesus PBMC as described in the text for the p27 reduction assay for which results are shown in Fig. 1. The same volume of virus was used for all assays. Due to differences in infectivity in the different cell types, the 50% tissue culture infectious doses used were 10,000, 1,600, and 800 for CEMx174 cells, human PBMC, and rhesus PBMC, respectively. Neutralization titers are the plasma dilutions at which p27 production was reduced >80% relative to that in serum from an SIV-naïve macaque. Plasma samples were assessed in human and rhesus PBMC at a 1:5 dilution only. Primary SIVmac251 was grown in rhesus PBMC and was a single passage of the animal challenge stock. NT, not tested.
Our results obtained in human and rhesus PBMC indicated that primary SIVmac251 is highly resistant to neutralization in these cells compared to CEMx174 cells. For example, in contrast to the low-level neutralization often detected in CEMx174 cells, neutralization of primary SIVmac251 in human and rhesus PBMC was undetectable (titer, <1:5) for plasma samples from most animals (Table 3). The virus was not entirely resistant, however, since plasma from two animals infected with SIVmac239Δnef (H323 and H348) and two infected with SIVmac251 (244-86 and 354-95) neutralized the virus in human PBMC (Table 3). This is not unlike findings for primary isolates of HIV-1, which are occasionally neutralized in PBMC by serum samples from infected individuals (22, 32, 35, 44) and are broadly sensitive to neutralization by certain human monoclonal antibodies (4).
Cases where neutralization was detected in CEMx174 cells but not in human or rhesus PBMC could not be explained by assay conditions other than the source of cells used as targets for infection. For example, both assays were based on a reduction in p27 synthesis in the presence of antibodies. We were careful to quantify p27 at a time when virus replication in the absence of antibodies was in a linear phase of increase, which improves the reproducibility of the assay (46). Virus dose is another important factor to consider, since lower doses can improve the detection of neutralization (46). Although we used the same volume of virus for all assays, infectivity was approximately 6 to 12 times lower in human and rhesus PBMC than in CEMx174 cells, as determined by a 50% tissue culture infectious dose assay (19), which should have favored the detection of neutralization in PBMC.
The results we obtained in human and rhesus PBMC do not support a role for neutralizing antibodies as a mechanism of protection in animals immunized with attenuated SIV and challenged with primary SIVmac251. In support of this conclusion, several groups have reported protective immunity elicited by attenuated SIV vaccines that cannot be attributed to neutralizing antibodies. Specifically, attenuated SIV has been shown to protect rhesus monkeys against experimental challenge with chimeric simian-human immunodeficiency virus (SHIV) variants containing the envelope glycoproteins of HIV-1 (3, 29, 42). In a reciprocal experiment, infection with a nonpathogenic SHIV was shown to protect against challenge with SIVsm (36). Since neutralizing antibodies are directed to the viral envelope glycoproteins, antibodies raised in response to infection with SIV would not be expected to neutralize SHIV, and vice versa. Protection in these cases was most likely mediated by immune responses to the SIV backbone of SHIV. Infection with attenuated SIV, including the two strains used here, has been shown to induce the production of CD8+ cytotoxic T lymphocytes targeted to SIV Gag (also part of SHIV) and Env (13, 18, 26), and protection in one study correlated with the presence of SIV Gag-specific cytotoxic T lymphocytes (29).
Antibodies raised by attenuated SIV might have greater potential to protect against viruses that are not as resistant to neutralization as primary SIVmac251. For example, immunization with an attenuated variant of SIVmac239, designated SIV/17E-Cl, was shown to protect a subset of animals challenged with SIV/DeltaB670 (6). Antibodies that neutralized the challenge virus correlated with protection, and passive transfer of sera from protected animals conferred protection in two of four SIV-naïve recipients (6). In our laboratory, the animal challenge stock of rhesus PBMC-grown SIV/DeltaB670 is highly sensitive to neutralization in CEMx174 cells by sera from animals infected with SIVmac239Δnef (titers of 1:271 to 1:4,195 [unpublished data]). This observation is in agreement with that of others (7), who also detected potent neutralization of the virus in rhesus macrophages (5a). Primary SIVmac251, being less sensitive to neutralization than SIV/DeltaB670 in vitro, might also be less sensitive to neutralization in vivo. Thus, assessments made in established cell lines, such as CEMx174, identify disparities in neutralization sensitivity between different strains of SIV that might affect the ability to predict antibody efficacy in vivo. Similar disparities exist when assessments of neutralization of a single virus stock are made in CEMx174 cells compared to that in PBMC. Passive immunization experiments with primary SIVmac251 as the challenge virus may provide information to determine which assay, CEMx174 or PBMC, is more predictive of antibody efficacy in vivo.
Acknowledgments
We thank Norman L. Letvin, Keith A. Reimann, and Joern Schmitz for providing serum samples from SIVmac251-infected rhesus macaques, Mickey Murphy-Corb for virus and serum samples used in the assessment of SIV/DeltaB670 neutralization sensitivity, and Janice Clements for helpful discussions and for sharing preliminary results.
This work was supported by NIH grants AI-35166, 6S-1649, and AI28662. V.N.K. is supported by a postdoctoral fellowship from the Damon Runyon–Walter Winchell Foundation. D.R.L. is an investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Almond N, Kent K, Cranage M, Rud E, Clarke B, Stott E J. Protection by attenuated simian immunodeficiency virus in macaques against challenge with virus-infected cells. Lancet. 1995;345:1342–1344. doi: 10.1016/s0140-6736(95)92540-6. [DOI] [PubMed] [Google Scholar]
- 2.Berger, E. A. 1997. HIV entry and tropism: the chemokine receptor connection. AIDS 11(Suppl. A):S3–S16. [PubMed]
- 3.Bogers W M J M, Niphuis H, ten Haaft P, Laman J D, Koornstra W, Heeney J H. Protection from HIV-1 envelope-bearing chimeric simian immunodeficiency virus (SHIV) in rhesus macaques infected with attenuated SIV: consequences of challenge. AIDS. 1995;9:F13–F18. [PubMed] [Google Scholar]
- 4.Burton, D. R., and D. C. Montefiori. 1997. The antibody response in HIV-1 infection. AIDS 11(Suppl. A):S87–S98. [PubMed]
- 5.Chen Z, Zhou P, Ho D D, Landau N R, Marx P A. Genetically divergent strains of simian immunodeficiency virus use CCR5 as a coreceptor for entry. J Virol. 1997;71:2705–2714. doi: 10.1128/jvi.71.4.2705-2714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Clements, J. Personal communication.
- 6.Clements J E, Montelaro R C, Zink M C, Amedee A M, Miller S, Trichel A M, Jagerski B, Hauer D, Martin L N, Bohm R P, Murphey-Corb M. Cross-protective immune responses induced in rhesus macaques by immunization with attenuated macrophage-tropic simian immunodeficiency virus. J Virol. 1995;69:2737–2744. doi: 10.1128/jvi.69.5.2737-2744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole K S, Rowles J L, Jagerski B A, Murphey-Corb M, Unangst T, Clements J E, Robinson J, Wyand M S, Desrosiers R C, Montelaro R C. Evolution of envelope-specific antibody responses in monkeys experimentally infected or immunized with simian immunodeficiency virus and its association with the development of protective immunity. J Virol. 1997;71:5069–5079. doi: 10.1128/jvi.71.7.5069-5079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connor, R. I., D. C. Montefiori, J. M. Binley, J. P. Moore, S. Bonhoeffer, A. Gettie, E. A. Fenamore, K. E. Sheridan, D. D. Ho, P. J. Dailey, and P. A. Marx. Temporal analyses of virus replication, immune responses, and efficacy in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 9.Daniel M D, Kirchhoff F, Czajak C, Sehgal P K, Desrosiers R C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 10.Deng H, Unutmaz D, KewalRamani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 11.Desrosiers R C. HIV with multiple gene deletions as a live attenuated vaccine for AIDS. AIDS Res Hum Retroviruses. 1992;8:411–420. doi: 10.1089/aid.1992.8.411. [DOI] [PubMed] [Google Scholar]
- 12.Desrosiers R C, Lifson J D, Gibbs J S, Czajak S C, Howe A Y M, Arthur L O, Johnson R P. Identification of highly attenuated mutants of simian immunodeficiency virus. J Virol. 1998;72:1431–1437. doi: 10.1128/jvi.72.2.1431-1437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dittmer U, Nisslein T, Bodemer W, Petry H, Sauermann U, Stahl-Hennig C, Hunsmann G. Cellular immune response of rhesus monkeys infected with a partially attenuated nef deletion mutant of the simian immunodeficiency virus. Virology. 1995;212:392–397. doi: 10.1006/viro.1995.1496. [DOI] [PubMed] [Google Scholar]
- 14.Edinger A L, Amadee A, Miller K, Doranz B J, Endres M, Sharron M, Samson M, Lu Z-H, Clements J E, Murphey-Corb M, Peiper S C, Parmentier M, Broder C C, Doms R W. Differential utilization of CCR5 by macrophage and T-cell tropic SIV strains. Proc Natl Acad Sci USA. 1997;94:4005–4010. doi: 10.1073/pnas.94.8.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esparza J the World Health Organization Group. Feasibility of developing live attenuated HIV vaccines: conclusions and recommendations. AIDS Res Hum Retroviruses. 1994;10:221–222. doi: 10.1089/aid.1994.10.221. [DOI] [PubMed] [Google Scholar]
- 16.Farzan M, Choe H, Martin K, Marcon L, Hofmann W, Karlsson G, Sun Y, Barrett P, Marchand N, Sullivan N, Gerard N, Gerard C, Sodroski J. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186:405–411. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill C M, Deng H, Unutmaz D, KewalRamani V N, Bastiani L, Gorny M K, Zolla-Pazner S, Littman D R. Envelope glycoproteins from human immunodeficiency virus types 1 and 2 and simian immunodeficiency virus can use human CCR5 as a coreceptor for viral entry and make direct CD4-dependent interactions with this chemokine receptor. J Virol. 1997;71:6296–6304. doi: 10.1128/jvi.71.9.6296-6304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson R P, Glickman R L, Yang J Q, Kaur A, Dion J T, Mulligan M J, Desrosiers R C. Induction of vigorous cytotoxic T-lymphocyte responses by live attenuated simian immunodeficiency virus. J Virol. 1997;71:7711–7718. doi: 10.1128/jvi.71.10.7711-7718.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson V A, Byington R E. Infectivity assay (virus yield assay) In: Aldovani A, Walker B D, editors. Techniques in HIV research. New York, N.Y: Stockton Press; 1990. pp. 71–76. [Google Scholar]
- 20.Kestler H, Kodama T, Ringler D, Marthas M, Pedersen N, Lackner A, Regier D, Sehgal P, Daniel M, King N, Desrosiers R. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990;248:1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- 21.Kestler H W, III, Ringler D J, Mori K, Panacali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 22.Kostrikis L G, Cao Y, Ngai H, Moore J P, Ho D D. Quantitative analysis of serum neutralization of human immunodeficiency virus type 1 from subtypes A, B, C, D, E, F, and I: lack of direct correlation between neutralization serotypes and genetic subtypes and evidence for prevalent serum-dependent infectivity enhancement. J Virol. 1996;70:445–458. doi: 10.1128/jvi.70.1.445-458.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaCasse R A, Follis K E, Moudgil T, Trahey M, Binley J M, Planelles V, Zolla-Pazner S, Nunberg J H. Coreceptor utilization by human immunodeficiency virus type 1 is not a primary determinant of neutralization sensitivity. J Virol. 1998;72:2491–2495. doi: 10.1128/jvi.72.3.2491-2495.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis M G, Bellah S, McKinnon K, Yalley-Ogunro J, Zack P M, Elkins W R, Desrosiers R C, Eddy G A. Titration and characterization of two rhesus-derived SIVmacchallenge stocks. AIDS Res Hum Retroviruses. 1994;10:213–220. doi: 10.1089/aid.1994.10.213. [DOI] [PubMed] [Google Scholar]
- 25.Liao F, Alkhatib G, Peden K W C, Sharma G, Berger E A, Farber J M. STRL33, a novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–2023. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohman B L, McChesney M B, Miller C J, McGowan E, Joye S M, Van Rompay K K A, Reay E, Antipa L, Pedersen N C, Marthas M L. A partially attenuated simian immunodeficiency virus induces host immunity that correlates with resistance to pathogenic virus challenge. J Virol. 1994;68:7021–7029. doi: 10.1128/jvi.68.11.7021-7029.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcon L, Choe H, Martin K A, Farzan M, Ponath P D, Wu L, Newman W, Gerard N, Gerard C, Sodroski J. Utilization of C-C chemokine receptor 5 by the envelope glycoproteins of a pathogenic simian immunodeficiency virus, SIVmac239. J Virol. 1997;71:2522–2527. doi: 10.1128/jvi.71.3.2522-2527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Means R E, Greenough T, Desrosiers R C. Neutralization sensitivity of cell culture-passaged simian immunodeficiency virus. J Virol. 1997;71:7895–7902. doi: 10.1128/jvi.71.10.7895-7902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller C J, McChesney M B, Lü X, Dailey P J, Chutkowski C, Lu D, Brosio P, Roberts B, Lu Y. Rhesus macaques previously infected with simian/human immunodeficiency virus are protected from vaginal challenge with pathogenic SIVmac239. J Virol. 1997;71:1911–1921. doi: 10.1128/jvi.71.3.1911-1921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montefiori D C, Baba T W, Li A, Bilska M, Ruprecht R M. Neutralizing and infection-enhancing antibody responses do not correlate with the differential pathogenicity of SIVmac239Δ3 in adult and infant rhesus monkeys. J Immunol. 1996;157:5528–5535. [PubMed] [Google Scholar]
- 31.Montefiori D C, Collman R G, Fouts T R, Zhou J Y, Bilska M, Hoxie J A, Moore J P, Bolognesi D P. Evidence that antibody-mediated neutralization of human immunodeficiency virus type 1 by sera from infected individuals is independent of coreceptor usage. J Virol. 1998;72:1886–1893. doi: 10.1128/jvi.72.3.1886-1893.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore J P, Cao Y, Leu J, Qin L, Korber B, Ho D D. Inter- and intraclade neutralization of human immunodeficiency virus type 1: genetic clades do not correspond to neutralization serotypes but partially correspond to gp120 antigenic serotypes. J Virol. 1996;70:427–444. doi: 10.1128/jvi.70.1.427-444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore, J. P., and D. D. Ho. 1995. HIV-1 neutralization: the consequences of viral adaptation to growth on transformed T cells. AIDS 9(Suppl. A):S117–S136. [PubMed]
- 34.Mori K, Ringler D J, Desrosiers R C. Restricted replication of simian immunodeficiency virus strain 239 in macrophages is determined by env but is not due to restricted entry. J Virol. 1993;67:2807–2814. doi: 10.1128/jvi.67.5.2807-2814.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pilgrim A K, Pantaleo G, Cohen O J, Fink L M, Zhou J Y, Zhou J T, Bolognesi D P, Fauci A S, Montefiori D C. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term nonprogressive infection. J Infect Dis. 1997;176:924–932. doi: 10.1086/516508. [DOI] [PubMed] [Google Scholar]
- 36.Quesada-Rolander M, Mäkitalo B, Thorstensson R, Zhang Y-J, Castaños-Velez E, Biberfeld G, Putkonen P. Protection against mucosal SIVsm challenge in macaques infected with a chimeric SIV that expresses HIV type 1 envelope. AIDS Res Hum Retroviruses. 1996;12:993–999. doi: 10.1089/aid.1996.12.993. [DOI] [PubMed] [Google Scholar]
- 37.Rucker J, Edinger A L, Sharron M, Samson M, Lee B, Berson J F, Yi Y, Margulies B, Collman R G, Doranz B J, Parmentier M, Doms R W. Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J Virol. 1997;71:8999–9007. doi: 10.1128/jvi.71.12.8999-9007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rud E W, Cranage M, Yon J, Quirk J, Ogilvie L, Cook N, Webster S, Dennis M, Clark B E. Molecular and biological characterisation of simian immunodeficiency virus strain 32H proviral clones containing nef size variants. J Gen Virol. 1994;75:529–543. doi: 10.1099/0022-1317-75-3-529. [DOI] [PubMed] [Google Scholar]
- 39.Ruprecht R M, Baba T W, Li A, Atehunie S, Hu Y, Liska V, Rasmussen R, Sharma P L. Live-attenuated HIV as a vaccine for AIDS: pros and cons. Semin Virol. 1996;7:147–155. [Google Scholar]
- 40.Samson M, Stordeur P, Labbe O, Soularue P, Vassart G, Parmentier M. Molecular cloning and chromosomal mapping of a novel human gene, ChemR1, expressed in T lymphocytes and polymorphonuclear cells and encoding a putative chemokine receptor. Eur J Immunol. 1996;26:3021–3028. doi: 10.1002/eji.1830261230. [DOI] [PubMed] [Google Scholar]
- 41.Sawyer L S W, Wrin M T, Crawford-Miksza L, Potts B, Wu Y, Weber P A, Alfonso R D, Hanson C V. Neutralization sensitivity of human immunodeficiency virus type 1 is determined in part by the cell in which the virus is propagated. J Virol. 1994;68:1342–1349. doi: 10.1128/jvi.68.3.1342-1349.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibata R, Siemon C, Czajak S C, Desrosiers R C, Martin M A. Live, attenuated simian immunodeficiency virus vaccines elicit potent resistance against a challenge with a human immunodeficiency virus type 1 chimeric virus. J Virol. 1997;71:8141–8148. doi: 10.1128/jvi.71.11.8141-8148.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trkola A, Ketas T, KewalRamani V N, Endorf F, Binley J M, Katinger H, Robinson J, Littman D R, Moore J P. Neutralization sensitivity of human immunodeficiency virus type 1 primary isolates to antibodies and CD4-based reagents is independent of their coreceptor usage. J Virol. 1998;72:1876–1885. doi: 10.1128/jvi.72.3.1876-1885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vancott T C, Polonis V R, Loomis L D, Michael N L, Nara P L, Birx D L. Differential role of V3-specific antibodies in neutralization assays involving primary and laboratory-adapted isolates of HIV type 1. AIDS Res Hum Retroviruses. 1995;11:1379–1390. doi: 10.1089/aid.1995.11.1379. [DOI] [PubMed] [Google Scholar]
- 45.Wyand M S, Manson K H, Garcia-Moll M, Montefiori D C, Desrosiers R C. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou J Y, Montefiori D C. Antibody-mediated neutralization of primary isolates of human immunodeficiency virus type 1 in peripheral blood mononuclear cells is not affected by the initial activation state of the cells. J Virol. 1997;71:2512–2517. doi: 10.1128/jvi.71.3.2512-2517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]