Abstract
Objective
We compared the osteoblastogenesis by serially administrating recombinant human bone morphogenetic protein-2 (rhBMP-2) and osteoprotegerin-immunoglobulin Fc segment complex (OPG-Fc).
Methods
The MC3T3-E1 preosteoblast cell line was differentiated for 1, 3, and 7 days with a treatment of OPG-Fc in 10~200 ng/mL concentration and the cell viability was evaluated by Cell Counting Kit-8 analysis. The level of differentiation from MC3T3-E1 cells to osteoblasts was determined by alkaline phosphatase activity. The level of runt domain-containing transcription factor 2 (Runx2) and osteopontin (OPN) manifestation, involved in osteoblast differentiation, was examined by real-time polymerase chain reaction and western blotting.
Results
During MC3T3-E1 cell differentiation, the differentiation level was high with 1-day treatment using 100 ng/mL OPG-Fc. The treatment with 50 ng/mL rhBMP-2 for 7 days, followed by 1-day treatment with 100 ng/mL OPG-Fc produced the highest differentiation level, which was approximately 5.3 times that of the control group (p<0.05). The expression of Runx2 mRNA significantly increased, reaching 2.5 times the level of the control group under the condition of 7-day treatment with rhBMP-2 and 1-day treatment with OPG-Fc (p<0.001). The expression of Runx2 protein significantly increased to approximately 5.7 times that of the control group under the condition of 7-day treatment with rhBMP-2, followed by 1-day treatment with OPG-Fc (p<0.01). The expression of OPN protein showed no change from that of the control group under various conditions of rhBMP-2 and OPG-Fc combinations.
Conclusion
These results imply that the treating preosteoblasts with rhBMP-2 first and then with OPG-Fc increased osteoblast differentiation efficacy.
Keywords: Recombinant human bone morphogenetic protein-2, Osteoprotegerin-Fc, Osteoblast, Differentiation
INTRODUCTION
In bone formation and regeneration mechanisms, maintaining the biomechanical integrity of bones is a complicated process that is controlled by numerous cell lineages, transcriptional regulation, cytokine networks, and growth factors [1]. Research aimed at improving strategies for bone tissue regeneration through the control of various osteogenic factors is constantly progressing. Among osteogenic factors, recombinant human bone morphogenetic protein-2 (rhBMP-2) is a primary growth factor involved in the regeneration of bone tissues. It induces excellent effects in promoting bone formation by involving the input of multifunctional stem cells in the osteogenic lineage [2]. Previous studies have demonstrated that combining BMP-2 with additional drugs increases the effect of bone formation and regeneration. Interleukin-1 receptor antagonist was proven to have a greater effectiveness in murine fermoral defect models when it was used in combination with rhBMP-2 compared with a single treatment of low-dose rhBMP-2 [3]. Another study showed a mouse ectopic bone formation model that simultaneously administrates selective retinoic acid receptor γ antagonist 7C compound and found that BMP-2 increased the volume of newly formed bone after increased cartilaginous tissue formation [4].
The activation of osteoclasts is largely controlled by the receptor activator of nuclear factors κB ligand (RANKL) and receptor activator of nuclear factors κB (RANK) system [5]. In the RANKL/RANK system, RANKL is provided to the receptor RANK through cell-to-cell contact, as a solute. Osteoprotegerin (OPG) is an intrinsic antagonist of the RANKL. A previous study analyzing in vitro data showed that the combined use of bone resorption inhibitors, such as OPG and alendronate (ALN), produced a stronger suppressive effect on osteoclast formation than using OPG or ALN alone [6]. Furthermore, a study with ovariectomized mice reported that the combined use of OPG and ALN more effectively reversed bone loss in the ilium and improved bony tissue recovery compared to using ALN or OPG alone [7]. OPG is known to suppress differentiation of osteoclast, but its effect on osteoblasts has not been proven.
According to the previous study by our lab that compared differentiation conditions of preosteoblasts, osteoblastogenesis increased under a condition of serial treatment with rhBMP-2, which is involved in bone formation and regeneration mechanisms, followed by ALN that restrains osteoclast activation [8]. Based on previous studies, the present study examined the concentration and time that causes the most effective increase in bone formation and regeneration by OPG and rhBMP-2. OPG has a very short circulatory half-life partially because of its heparin-binding domain [9]. The circulatory half-life becomes longer when this domain is deleted and the cut molecule is converged to the Fc part. Hence, the present study employed OPG-immunoglobulin Fc segment complex (OPG-Fc).
MATERIALS AND METHODS
Cell culture and differentiation
Preosteoblastic MC3T3-E1 cells used in this study were purchased from American Type Culture Collection (Manassas, VA, USA). Culture solutions were created by adding 10% fetal bovine serum (Gibco, Grand Island, NY, USA) to 1% penicillin/streptomycin α-minimum essential medium (without ascorbic acid; Gibco). With replacement in 2 to 3 day intervals, the solution was cultivated at 37°C, in a 5% CO2 incubator.
To induce differentiation from MC3T3-E1 cells to osteoblasts, 50 μg/mL of ascorbic acid, and 10 mM of β-glycerophosphate (both from Sigma-Aldrich, St. Louis, MO, USA) were added to the culture solution. Moreover, 50 ng/mL of RhBMP-2 (Sigma-Aldrich) and 0~200 ng/mL of OPG-Fc (Amgen, Thousand Oaks, CA, USA) were added to the culture solution.
Cell viability assay
After seeding on 96 well plates at 5×103 cells/well density, the MC3T3-E1 cells were grown in growth media for 24 hours. Next, the growth media was removed and the MC3T3-E1 cells were differentiated for 1, 3, and 7 days in differentiation media, where either rhBMP-2 or OPG-Fc was added. Cell viability after the differentiation was evaluated using Cell Counting Kit-8 (Dojindo Molecular Technologies, Inc., Rockville, MD, USA). The absorbance of each well was measured using a microplate reader (Tecan Group Ltd., Zurich, Switzerland).
Alkaline phosphatase assay
The MC3T3-E1 cells were grown in growth media for 24 hours after seeding them in 24 well plates at 2×104 cells/well. After removing the growth media, the MC3T3-E1 cells were differentiated for 1, 3, 7, and 8 days in differentiation media with an addition of either rhBMP-2 or OPG-Fc. The differentiation level of osteoblasts was assessed using an alkaline phosphatase assay (ALP) colorimetric assay kit (BioVision, Milpitas, CA, USA). The kit was used according to the manufacturer’s instructions and the absorbance of each well was measured using a microplate reader (Tecan Group Ltd.). A bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific Inc., Rockford, IL, USA) was used for the correction of the measured values in protein concentration.
RNA extraction and real-time polymerase chain reaction
RNA was extracted using TRI Reagent (Molecular Research Center, Inc., Cincinnati, OH, USA) and cDNA was compounded from the separated RNA using the SuperScript Vilo cDNA synthesis kit (Invitrogen, Burlington, ON, Canada). A real-time polymerase chain reaction (PCR) was conducted on the synthesized cDNA with the CFX96 Real-time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using FastStart Essential DNA Green Master (Roche, Mannheim, Germany). The analysis condition for real-time PCR was 95°C for 10 seconds, 60°C for 10 seconds, 72°C for 10 seconds, with 40 cycles. The sequence of the used primer was as follows: β-actin: 5’- tgttaccaactgggacgaca-3’, 5’-ggggtgttgaaggtctcaaa-3’; Runx2: 5’-cccagccacctttacctaca-3’, 5’-agagatatggagtgctgctg-3’; OPN: 5’-tgagaccgtcactgctagta-3’, 5’-aggtcctcatctgtggcatc-3’.
Western blotting
The MC3T3-E1 cells were grown in growth media for 24 hours after seeding them in 6 well plates at 1×105 cells/well. Next, the growth media was removed and the MC3T3-E1 cells were differentiated for 1, 3, 7, and 8 days in differentiation media, where either rhBMP-2 or OPG-Fc was added.
After cleansing the cells with phosphate-buffered saline, a mixture of lysis buffer (Thermo Fisher Scientific Inc., Waltham, MA, USA), protease inhibitor, and phosphatase inhibitor (both from Roche) was added to the reaction above ice for 15 minutes. To obtain the protein supernatant, centrifugation was conducted for 15 minutes at 4°C, 12,000 rpm. The BCA analysis was implemented on the obtained supernatant to measure the protein concentration. A 30 μg protein sample was obtained to go under electro-phoresis using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and the protein was transferred from gel to a nitrocellulose membrane (Amersham, Chicago, IL, USA). The membrane was blocked for an hour using TBS-T solution (20 mM Tris, 137 mM NaCl, 0.05% Tween-20) with an addition of 5% skim milk, and then was reacted with a primary and secondary antibody. Anti-GAPDH (1:10000, ab8245; Abcam plc, Cambridge, UK), anti-Runx2 (1:1000, ab23981; Abcam plc), and anti-osteopontin (OPN) (1:1000, ab63856; Abcam plc) were used as the primary antibodies. Peroxidase AffiniPure Donkey Anti-Mouse IgG (H+L) and Peroxidase-conjugated AffiniPure Donkey Anti-Rabbit IgG (H+L) (Jackson Immunoresearch, Baltimore, MD, USA) were used as the secondary antibodies. After revealing the blotted membrane in Immobilon Western blotting (Merck Millipore, Burlington, VT, USA), manifestation of protein was confirmed with a chemiluminescence imaging analysis device (Fusion Solo 6S, Vilber Lourmat, Marne-la-Vallée, France).
Statistical analysis
Cell viability and ALP activity experiments were performed in triplicates and data are shown as mean±standard deviation from 3 independent experiments. SPSS 25.0 (IBM Co., Armonk, NY, USA) was used for the statistical analysis. Statistical significance was tested using Student’s t-test, where p-values less than 0.05 were regarded as significant.
RESULTS
Effect of the combination of rhBMP-2 and OPG-Fc on the viability and differentiation level of MC3T3-E1 cells
While differentiating the MC3T3-E1 cells for 1, 3, and 7 days, cell viability was assessed using a treatment of 10~200 ng/mL OPG-Fc (data not shown). Treatment with 10 ng/mL of OPG-Fc for 7 days produced an approximate 9% increase, compared to the control group, while 7-day treatment with 150 ng/mL OPG-Fc showed an approximate 15% decrease, compared to that of the control group. However, the treatment concentration and time of OPG-Fc did not indicate a statistically significant impact on the viability of preosteoblasts.
MC3T3-E1 cells were differentiated for 1, 3, and 7 days with 10~200 ng/mL OPG-Fc treatment and the level of differentiation of osteoblasts was evaluated (data not shown). Differentiation of osteoblasts increased by approximately 31% compared to the control group in the case of 1-day treatment with 100 ng/mL OPG-Fc. The increment was largest under this condition, but the result was not statistically significant (p=0.246). The differentiation level of osteoblasts tended to decrease with higher OPG-Fc treatment concentrations and longer treatment periods.
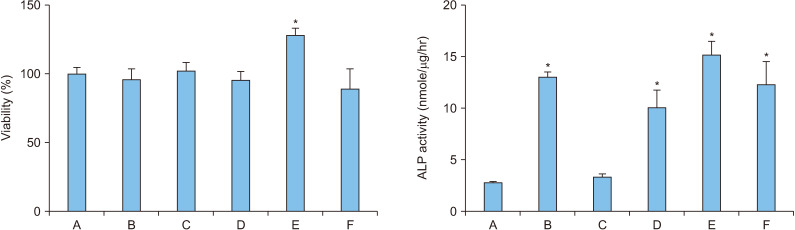
Our previous study proved that 7-day treatment with 50 ng/mL rhBMP-2 was effective for the differentiation of MC3T3-E1 cells [8]. Hence, the present study confirmed the level of differentiation of MC3T3-E1 cells by combining the condition of 7-day treatment with 50 ng/mL rhBMP-2 and 1-day treatment with 100 ng/mL OPG-Fc (Figure 1).
Figure 1.
Viability and alkaline phosphatase (ALP) activity in osteoblasts (MC3T3-E1) following treatment with recombinant human bone morphogenetic protein-2 (rhBMP-2)/osteoprotegerin (OPG-Fc). A: control, B: 50 ng/mL rhBMP-2 for 8 days, C: 100 ng/mL OPG-Fc for 8 days, D: 50 ng/mL rhBMP-2 and 100 ng/mL OPG-Fc for 8 days, E: 50 ng/mL rhBMP-2 for 7 days and 100 ng/mL OPG-Fc for 1 day, F: 100 ng/mL OPG-Fc for 1 day and 50 ng/mL rhBMP-2 for 7 days. Error bars represent mean±standard deviation. *p<0.05 compared with the control group.
The cell viability significantly increased by approximately 28% compared to that of the control group when MC3T3-E1 cells were treated with 50 ng/mL rhBMP-2 for 7 days, followed by 1-day treatment with 100 ng/mL OPG-Fc (p=0.027). The differentiation level of osteoblasts significantly increased approximately 4.5 times that of the control group when MC3T3-E1 cells were treated with 50 ng/mL rhBMP-2 only for 8 days (p=0.019). Seven-day treatment with 50 ng/mL rhBMP-2 followed by 1-day treatment with 100 ng/mL OPG-Fc produced the highest differentiation level, where the osteoblasts differentiation significantly increased by approximately 5.3 times that of the control group (p=0.015) and increased by approximately 17% from 8-day treatment with rhBMP-2 only.
Effect of combined rhBMP-2 and OPG-Fc on the expression of mRNA in Runx2 and OPN in MC3T3-E1 cells
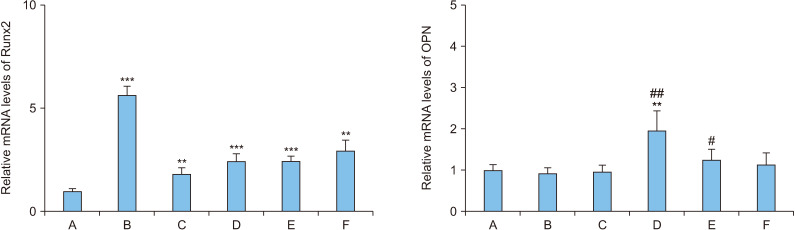
The expression of mRNA in Runx2 and OPN, which are involved in the differentiation of osteoblasts, was examined under the 7-day treatment with 50 ng/mL rhBMP-2 and 1-day treatment with 100 ng/mL OPG-Fc (Figure 2).
Figure 2.
The levels of mRNA in osteoblasts (MC3T3-E1) following treatment with recombinant human bone morphogenetic protein-2 (rhBMP-2)/osteoprotegerin (OPG-Fc). A: control, B: 50 ng/mL rhBMP-2 for 8 days, C: 100 ng/mL OPG-Fc for 8 days, D: 50 ng/mL rhBMP-2 and 100 ng/mL OPG-Fc for 8 days, E: 50 ng/mL rhBMP-2 for 7 days and 100 ng/mL OPG-Fc for 1 day, F: 100 ng/mL OPG-Fc for 1 day and 50 ng/mL rhBMP-2 for 7 days. Error bars represent mean±standard deviation. Runx2: runt domain-containing transcription factor 2, OPN: osteopontin. **p<0.01 and ***p<0.001, compared with the control group. #p<0.05 and ##p<0.01, compared with the B group.
The expression of Runx2 mRNA significantly increased by approximately 5.6 times that of the control group when MC3T3-E1 cells were treated with rhBMP-2 only for 8 days (p<0.001). The differentiation level of the osteoblasts was the second highest under the 1-day treatment condition with OPG-Fc, followed by the 7-day treatment with rhBMP-2, which significantly increased by approximately 2.9 times compared to the control group (p=0.008). The expression of Runx2 mRNA under 7-day treatment condition with rhBMP-2 followed by 1-day treatment with OPG-Fc, which produced the highest differentiation level (Figure 1), significantly increased by approximately 2.5 times that of the control group (p<0.001).
The expression of OPN mRNA significantly increased by approximately 2.0 times that of the control group when MC3T3-E1 cells were treated simultaneously with rhBMP-2 and OPG-Fc for 8 days (p=0.004) and the increase was approximately 2.1 times with the 8-day treatment condition with rhBMP-2 only (p=0.003). The expression of OPN mRNA with 7-day treatment with rhBMP-2 followed by 1-day treatment with OPG-Fc, which produced the highest differentiation level (Figure 1), increased by approximately 24% from that of the control group and by approximately 34% from that under 8-day treatment condition with rhBMP-2 only, demonstrating statistical significance (p=0.030).
Effect of combined rhBMP-2 and OPG-Fc on the expression of proteins in Runx2 and OPN in MC3T3-E1 cells
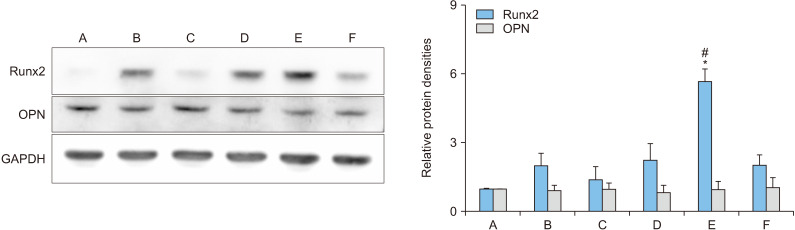
The expression of proteins in Runx2 and OPN that are involved in the differentiation of osteoblasts, was explored with 7-day treatment with 50 ng/mL rhBMP-2 and 1-day treatment with 100 ng/mL OPG-Fc (Figure 3).
Figure 3.
Protein levels in osteoblasts (MC3T3-E1) following treatment with recombinant human bone morphogenetic protein-2 (rhBMP-2)/osteoprotegerin (OPG-Fc). A: control, B: 50 ng/mL rhBMP-2 for 8 days, C: 100 ng/mL OPG-Fc for 8 days, D: 50 ng/mL rhBMP-2 and 100 ng/mL OPG-Fc for 8 days, E: 50 ng/mL rhBMP-2 for 7 days and 100 ng/mL OPG-Fc for 1 day, F: 100 ng/mL OPG-Fc for 1 day and 50 ng/mL rhBMP-2 for 7 days. Error bars represent mean±standard deviation. Runx2: runt domain-containing transcription factor 2, OPN: osteopontin. *p<0.05 compared with the control group. #p<0.05 compared with the B group.
In the 7-day treatment condition with rhBMP-2 followed by 1-day treatment with OPG-Fc, the expression of Runx2 protein significantly increased, reaching approximately 5.7 times that of the control group (p=0.043). The result was approximately 2.8 times the result obtained in the 8-day treatment condition with rhBMP-2 only, which was almost double the level observed in the control group (p=0.040).
The expression of OPN protein increased by approximately 7% compared to the control group when MC3T3-E1 cells were treated with OPG-Fc for 1 day, followed by 7-day treatment with rhBMP-2. However, no significant change from the control group was observed with other rhBMP-2 and OPG-Fc conditions.
DISCUSSION
This study explored the optimal concentration and time for the factors that are involved in osteogenesis to identify the condition that increases bone formation and regeneration. A previous study compared the impact of rhBMP-2, which is involved in the differentiation of preosteoblasts, and ALN, a bone resorption inhibitor, on preosteoblast differentiation [8]. The study found that treating with 50 ng/mL rhBMP-2 for 7 days and then treating with 5 µmol/L ALN for the next 3 days increased osteoblastogenesis. Furthermore, Yu et al. [10] reported that ALP activity, which indicates the level of differentiation from preosteoblasts to osteoblasts, was approximately 67% higher in the OPG overexpression group than the control group and the expression of mRNA of Smad1 and Akt1, which are transcription factors involved in differentiation, also increased by approximately twofold.
In the current study, cell viability and ALP activity were compared by altering OPG-Fc concentration and time while differentiating from preosteoblasts to osteoblasts. Treatment with OPG-Fc with 10~200 ng/mL concentrations for 1, 3, or 7 days did not produce a statistically significant effect for cell viability. ALP activity increased most prominently in 1-day treatment with 100 ng/mL OPG-Fc. In our previous research, ALP activity increased the most in the case of 3-day treatment with ALN at 5 µmol/L concentration [8]. OPG-Fc also increased ALP activity in the differentiating preosteoblasts, where ALP activity increased with shorter treatment time compared to that of ALN.
Hence, this study examined the effect on preosteoblasts by combining conditions proven to be effective in enhancing osteoblastogenesis, including 7-day treatment with 50 ng/mL rhBMP-2 and 1-day treatment with 100 ng/mL OPG-Fc. Cell viability and ALP activity of differentiating preosteoblasts showed highest and significant increases in of 7-day treatment condition with 50 ng/mL rhBMP-2 followed by 1-day treatment with 100 ng/mL OPG-Fc (p=0.015). Under this condition, the ALP activity was higher than in the case of 8-day treatment with rhBMP-2 only. BMP-2 is currently a drug used as a single treatment, so it can be considered a positive control that increases ALP activity. As a result of comparing combinations that increased ALP activity compared to condition treated only with 50 ng/mL rhBMP-2, 7-day treatment with 50 ng/mL rhBMP-2 followed by 1-day treatment with 100 ng/mL OPG-Fc showed the greatest increase and was consistent with the cell viability results. This finding was consistent with the previous study result where osteoblastogenesis increased with the 7-day treatment with rhBMP-2 first and 3-day treatment with ALN afterward [8]. An in vivo experiment which stimulated osteoblast activity in the early phase and then suppressed osteoclasts, reported that bone remodeling increased most prominently in a group where rhBMP-2 was first released, followed by ALN emission [11].
Although a previous study that conducted an experiment on critical-sized bone defects using mice reported that locally transferred BMP-2 and systematically administered OPG-Fc were more effective at inhibiting osteoclasts and improving bone healing than the case of a single BMP-2 treatment, the reaction was quantified histologically and through imaging [12]. The present study compared the expression of osteoblast differentiation factors, Runx2 and OPN, by combining 50 ng/mL rhBMP-2 for 7 days and 100 ng/mL OPG-Fc for 1 day treatment conditions. The expression of Runx2 mRNA was significantly higher than that of the control group in 7-day treatment with rhBMP-2 followed by 1-day treatment with OPG-Fc (p<0.001). The expression of OPN mRNA increased by approximately 24% compared to the control group but no statistical significance was observed. Because the action of Runx2, an early major transcription factor, is essential for preosteoblast differentiation by BMP-2, we confirmed that Runx2 mRNA expression increased in all conditions except the control group. A recent study reported that RANKL reverse signaling in osteoblasts may prepare osteoblasts for further maturation and that vesicular RANK stimulates osteoblast differentiation [13]. RANKL reverse signaling by OPG-Fc is thought to influence OPN mRNA expression in BMP-2-driven preosteoblast differentiation. Therefore, among the combination of rhBMP-2 and OPG-Fc groups, OPN mRNA expression is thought to have increased in 8-day treatment with 50 ng/mL rhBMP-2 and 100 ng/mL OPG-Fc, and 7-day treatment with rhBMP-2 followed by 1-day treatment with OPG-Fc. The expression of Runx2 protein was significantly higher than that of the control group in the case of 7-day treatment with rhBMP-2 followed by 1-day treatment with OPG-Fc (p=0.043). The expression of OPN protein with other rhBMP-2 and OPG-Fc conditions demonstrated no difference from the control group. Under serial treatment with 50 ng/mL rhBMP-2 for 7 days followed by 5 µmol/L ALN for 3 days, the expression of Runx2 mRNA and protein increased, while the mRNA expression of OPN decreased 28% compared to that of the control group [8]. Among the combination of rhBMP-2 and OPG-Fc groups, the increase in Runx2 protein in 7-day treatment with rhBMP-2 followed by 1-day treatment with OPG-Fc implies that OPG-Fc treated in preosteoblasts first stimulated by BMP-2 increased the expression of Runx2 protein in the BMP-2/Runx2 pathway. In this experimental condition, Runx2, which is involved in the early stages of preosteoblast differentiation, acted as a major factor in preosteoblast differentiation, so ALP activity is thought to have increased the most in 7-day treatment with rhBMP-2 followed by 1-day treatment with OPG-Fc. A previous study observed the effects of concentration and time on clinical immune inhibitors of dexamethasone and BMP-2 using rat bone marrow stromal cells and reported a similar tendency [14]. The study showed that ALP activity was higher when the BMP-2/Dex ratio was 1/6 during 4, 7, and 14 days than any other concentration combinations and that Runx2, OPN and OCN mRNA expression also demonstrated greater increase during 4, 7, and 14 days under that condition.
CONCLUSION
Treating preosteoblasts with rhBMP-2 first and then with OPG-Fc improved differentiation ability to osteoblasts and upwardly adjusted the mRNA expression of Runx2 and OPN, which are differentiation-related factors, and protein expression of Runx2.
This outcome implies that the combination of rhBMP-2 and OPG-Fc enhances the efficacy of the differentiation of osteoblasts. This study proposed a combination of concentration and time for a new drug that is applicable to serial administration aimed at boosting osteogenic effects.
ACKNOWLEDGMENTS
None.
Footnotes
FUNDING
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korean Government (MSIP) (grant No. NRF-2018M3C1B7020722 and NFR-2021R1F1A1060970).
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: S.H.K., D.S.Y., and C.N.S. Data curation: S.H.K., H.J.C., and S.M.L. Investigation: S.H.K., H.J.C., and C.N.S. Funding acquisition: C.N.S. Methodology: H.J.C., S.M.L., and C.N.S. Project administration: C.N.S. Validation: all authors. Visualization: H.J.C., S.M.L., and C.N.S. Writing-review and editing: all authors. Approval of the final manuscript: all authors.
REFERENCES
- 1.Amarasekara DS, Kim S, Rho J. Regulation of osteoblast differentiation by cytokine networks. Int J Mol Sci. 2021;22:2851. doi: 10.3390/ijms22062851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quinlan E, Thompson EM, Matsiko A, O'Brien FJ, López-Noriega A. Long-term controlled delivery of rhBMP-2 from collagen-hydroxyapatite scaffolds for superior bone tissue regeneration. J Control Release. 2015;207:112–9. doi: 10.1016/j.jconrel.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 3.Lackington WA, Gehweiler D, Zhao E, Zderic I, Nehrbass D, Zeiter S, et al. Interleukin-1 receptor antagonist enhances the therapeutic efficacy of a low dose of rhBMP-2 in a weight-bearing rat femoral defect model. Acta Biomater. 2022;149:189–97. doi: 10.1016/j.actbio.2022.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Tateiwa D, Kaito T, Hashimoto K, Okada R, Kodama J, Kushioka J, et al. Selective retinoic acid receptor γ antagonist 7C is a potent enhancer of BMP-induced ectopic endochondral bone formation. Front Cell Dev Biol. 2022;10:802699. doi: 10.3389/fcell.2022.802699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aspenberg P, Agholme F, Magnusson P, Fahlgren A. Targeting RANKL for reduction of bone loss around unstable implants: OPG-Fc compared to alendronate in a model for mechanically induced loosening. Bone. 2011;48:225–30. doi: 10.1016/j.bone.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 6.Huang P, Wang Y, Chi ZY, Yang ZY, Ni J, Yang WJ, et al. [In vitro study of combination rhOPG-Fc and alendronate on inhibition osteoclast] Zhonghua Wai Ke Za Zhi. 2005;43:812–6. Chinese. [PubMed] [Google Scholar]
- 7.Wang Y, Huang P, Tang PF, Chan KM, Li G. Alendronate (ALN) combined with osteoprotegerin (OPG) significantly improves mechanical properties of long bone than the single use of ALN or OPG in the ovariectomized rats. J Orthop Surg Res. 2011;6:34. doi: 10.1186/1749-799X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SH, Choi HJ, Yoon DS, Son CN. Serial administration of rhBMP-2 and alendronate enhances the differentiation of osteoblasts. Int J Rheum Dis. 2021;24:1266–72. doi: 10.1111/1756-185X.14189. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Q, Chen S, Shi J, Li F, Shi X, Hu X, et al. Coupled OPG-Fc on decellularized aortic valves by EDC/NHS attenuates rat MSCs calcification in vitro. ASAIO J. 2019;65:197–204. doi: 10.1097/MAT.0000000000000796. [DOI] [PubMed] [Google Scholar]
- 10.Yu H, de Vos P, Ren Y. Overexpression of osteoprotegerin promotes preosteoblast differentiation to mature osteoblasts. Angle Orthod. 2011;81:100–6. doi: 10.2319/050210-238.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee D, Wufuer M, Kim I, Choi TH, Kim BJ, Jung HG, et al. Sequential dual-drug delivery of BMP-2 and alendronate from hydroxyapatite-collagen scaffolds for enhanced bone regeneration. Sci Rep. 2021;11:746. doi: 10.1038/s41598-020-80608-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bougioukli S, Jain A, Sugiyama O, Tinsley BA, Tang AH, Tan MH, et al. Combination therapy with BMP-2 and a systemic RANKL inhibitor enhances bone healing in a mouse critical-sized femoral defect. Bone. 2016;84:93–103. doi: 10.1016/j.bone.2015.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikebuchi Y, Aoki S, Honma M, Hayashi M, Sugamori Y, Khan M, et al. Coupling of bone resorption and formation by RANKL reverse signalling. Nature. 2018;561:195–200. doi: 10.1038/s41586-018-0482-7. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Lin D, Li B, Hong H, Jiang C, Yuan Y, et al. BMP-2/CPC scaffold with dexamethasone-loaded blood clot embedment accelerates clinical bone regeneration. Am J Transl Res. 2022;14:2874–93. [PMC free article] [PubMed] [Google Scholar]