Abstract
Neu1 is a sialidase enzyme that plays a crucial role in the regulation of glycosylation in a variety of cellular processes, including cellular signaling and inflammation. In recent years, numerous evidence has suggested that human NEU1 is also involved in the pathogenesis of various respiratory diseases, including lung infection, chronic obstructive pulmonary disease (COPD), asthma, and pulmonary fibrosis. This review paper aims to provide an overview of the current research on human NEU1 and respiratory diseases.
Introduction
Respiratory disease refers to a group of disorders that affect the respiratory system, including the lungs, airways, and other structures involved in breathing. These diseases can range from mild conditions such as the common cold to more severe illnesses like pneumonia and chronic obstructive pulmonary disease (COPD). Previously, much attention has been paid to the role of Neu1from viral and bacterial in human respiratory diseases. In recent years, there has been growing interest in the role of human Neuraminidases 1 (Neu1), a sialidase enzyme, in the development and progression of respiratory diseases [1–10].
Neu1, as the most abundant sialidase located in lysosomes and on the cell membrane of mammal, is an enzyme that plays a crucial role in the removal of sialic acid residues from glycoproteins and glycolipids. Sialic acids are important components of cell surface molecules and are involved in various cellular processes, including cell adhesion, signaling, and immune response. The production, secretion, and activity of sialidase can also be regulated by proteins that bind to sialidases, especially protective protein/cathepsin A (PPCA) binding to NEU1. The binding of glycosidase β-Gal, PPCA and NEU1 formed lysosomal multiprotein complex, which regulate the intralysosomal catabolism of sialylated macromolecules [11]. Neu1, PPCA and elastic binding protein (EBP) are together assembled the elastin receptor complex (ERC), which modulated the assembly of elastic fibers [12]. Dysregulation of Neu1 activity has been implicated in several diseases, including cancer, metabolism disorder, neurodegenerative disorders, and respiratory diseases. It has been proved that NEU1 is expressed in human airway smooth muscle cells, epithelial and microvascular endothelial cells, fibroblasts, and in the lungs of patients with idiopathic pulmonary fibrosis (IPF) [1–6]. Neu1 was upregulated in lung tissues of patients with COPD and asthma, suggesting that it may play a role in the development of these diseases [1, 2]. It has been reported that Neu1 was upregulated in airway smooth muscle cells from patients with asthma, and inhibition of Neu1 reduced airway hyperresponsiveness in mice with asthma [3, 4]. Consistent with this, Neu1 expression was increased in lung tissue samples from patients with interstitial lung disease (ILD), correlated with increased inflammation and mucus production in the airways [5, 6]. Above results indicated that the abnormally high expression of Neu1 was correlated with the high immune response in human respiratory diseases.
Neu1 has also been implicated in the pathogenesis of pulmonary fibrosis, a progressive and often fatal lung disease. Previous study demonstrated that Neu1 was upregulated in lung tissues of patients with pulmonary fibrosis, and that inhibition of Neu1 reduced lung fibrosis in a mouse model of the disease [5–7]. While the exact mechanisms by which Neu1 contributes to lung disease are not yet fully understood, it is believed that Neu1 plays a role in the regulation of cell signaling pathways, inflammation, and extracellular matrix remodeling. Thus, targeting Neu1 may represent a promising therapeutic approach for the treatment of diverse respiratory diseases.
In this review, we used keywords such as “Neu1”, “sialidase 1”, “respiratory disease”, “lung infection”, “COPD”, “asthma”, and “pulmonary fibrosis” to identify relevant articles on PubMed. Here, we will summary the recent understanding of Neu1 and its relevance for pulmonary health and disease (Fig. 1), illustrating its potential clinical application.
Fig. 1.
Schematic view of the role of the Neu1 in respiratory disease
NEU1 and lung injury
Airway epithelial cells (EC) express sialylated receptors that recognize bacterial pathogens, and mediate its interaction with host cells. P. aeruginosa (Pa) as a Gram-negative, flagellated, opportunistic human pathogen, adhered to ECs by its flagellin interacted with cell surface sialoprotein transmembrane mucin 1 (MUC1). Simeon and colleague found that flagellin from Pa could promote bacterial adhesion to and invasion of ECs by Neuraminidase 1 (NEU1)-dependent MUC1 ectodomain desialylation in vitro [8]. Subsequently, they also verified that Neu1 provoked the shedding of MUC1-ED from airway, which suppressed Pa lung infection in BALB/c mice [9]. Recently, Simeon’ team reported that the concentration of desialylated MUC1-ED and flagellin expression in Pa were dramatically increased in bronchoalveolar lavage fluid (BALF) harvested from Pa-infected patients [10], this indicated that measurement of MUC1-ED in BALF levels might serve as a guide for antibiotic therapy in patients with Pa-infections. In summary, different in vivo experiments confirmed that NEU1 might modulate the desialylation of human ECs receptors to resist the pulmonary bacterial infections.
NEU1 highly expressed in vascular endothelia of different human tissues. It has been reported that NEU1 overexpressed in human lung microvascular EC (HMVEC-Ls) inhibited their migration into wound [1]. Subsequent research found that NEU1 mediated inhibition of angiogenesis in human pulmonary microvascular ECs (HPMECs) through desialylation of CD31 [13]. The results of further research manifested that NEU1-CD31 interaction might drive by src family kinases during angiogenesis process for postconfluent HPMECs. According to a in vivo study, Neuraminidase promoted desialylation in polymorphonuclear leukocytes, potentiated inflammatory response and vascular collapse in LPS-induced acute lung injury [14]. These studies indicated that NEU1 has negative effect on the pulmonary reprogram after acute injury.
NEU1 and pulmonary fibrosis
As typical feature of pulmonary fibrosis, epithelial abnormalities, vascular remodeling, abnormal wound healing and angiogenesis caused histopathological changes and functional decline of the lungs. Numerous evidence implicated various genes, including cell surface receptors, their ligands, extracellular matrix molecules and downstream signaling pathways, were involved in pathogenesis of pulmonary fibrosis [15, 16]. The imbalance between pro-inflammatory and regulatory immune cell subsets is a cardinal cause of pulmonary fibrosis. The preponderance of evidence demonstrated that elevated NEU1 expression regulated the desialylation and activity of receptors (such as platelet-derived growth factor receptor and Toll like receptors) in epithelial and endothelial cells involved in the development of pulmonary fibrosis [17, 18]. It has been verified that elevated expression of NEU1 sialidase provokes pulmonary fibrosis by aggravated lymphocytic infiltration and collagen accumulation in human [3]. Neu1-dependent CD31 desialylation modulated the capillary-like tube formation, which was correlated with pulmonary angiogenesis in lungs of patient with IPF [12, 19].
Selective inhibition of Neu1 attenuated bleomycin-induced lung inflammation and fibrosis by mediated desialylation of Muc1 ectodomain [8]. Recent study showed that sialidases was higher in male compared to female mice induced by bleomycin, high NEU1 expression closely correlated with CD11b+ macrophages in BALF from lung tissue of bleomycin induced female mice. Both NEU2 and NEU3 levels were associated with some inflammation and fibrosis markers independent on gender [20]. Meanwhile another study found that abnormally serum sialidase NEU3 level promoted fibrosis through serum amyloid P (SAP) desialylation acceleration and IL-10 accumulation by PBMC in idiopathic pulmonary fibrosis (IPF) patients [21]. Consistent with this, pulmonary fibrosis was strongly attenuated in bleomycin-induced Neu3 knockout mice [22]. Recent research confirmed that inhibition of NEU3-mediated TGF-β1 activation might rescued the lung injury caused by bleomycin [9]. These studies suggested that both Neu1 and Neu3 might have pronounced effects on pulmonary fibrosis process.
NEU1 and asthma
Asthma is an airway disease, characterized with increased serum levels of IgE and inflammation being caused by elevated levels of T(H)2-type cytokines. T helper 2 (Th2) cells are abundant in type 2 (T2) asthma. CD4+ T cells accumulated in the airway are associated the development of asthma [23]. The cell surface adhesion receptor cluster of differentiation 44 (CD44) as a highly glycosylated molecule, interacted with hyaluronic acid (HA), to participate in the airway accumulation of CD4+ T cells in murine model of asthma [3, 4]. Th2-mediated airway inflammation was caused by CD44–HA interactions through Neu1-meidated desialylation in the airway of asthma mouse model. Another study has demonstrated Neu1 sialidase activity in respiratory airway epithelia regulated EGFR- and MUC1-dependent signaling and bacterial adhesion in vitro [2]. It has been reported that NEU1 interacted with Toll like receptors (TLR2, 3, 4) and activated TLR signaling [24]. TLR3 could recognize human rhinovirus (HRV) RNA and highly expressed in lung epithelial cells post-HRV infection [25]. Martin and colleague demonstrated that higher cytokine expression in asthmatics is correlated with decrease of NEU1-mediated TLR3 activation in asthmatic airway cells after HRV infection in children [26]. These reports supported that Neu1 might have the potential for further applications in asthma prevention or treatment.
NEU1 and tumors of lung
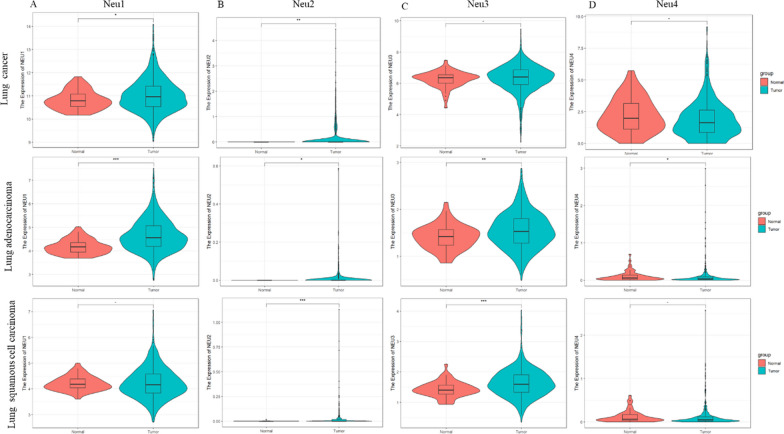
NEU-1 exhibited pronounced effects on the development of several cancers by regulated the cancer cells' proliferation and migration, including hepatocellular cancer, pancreatic carcinoma and breast cancer. This sialidase [27, 28]. RNA-seq data of 1093/556/538 patients with lung cancer (LC), lung adenocarcinoma (LA) or lung squamous cell carcinoma (LSCC) were extracted from the TCGA database, and the expression of NEU1 in tumors of lung and paracancer tissue samples was analyzed using R language, as shown in Fig. 2. Higher transcriptional level of NEU1 was found to be remarkably correlated with LC and LA in human based on bioinformatical analysis (P = 0.05 for LC, P = 2.61*10–16 for LA), but has no significant change in LSCC (Fig. 2A). NEU2 level all increased in different LC (Fig. 2B). The expression of NEU3 is both remarkably raised in LA or LSCC, except in LC (Fig. 2C), this indicated that NEU3 expression can be used to distinguish LC from other two LA. Although NEU4 expression was only upregulated in LA (Fig. 2D), but its constitutive expression in lung was low, NEU4 was not detected in paracancer tissue of most patient with LA. It suggested that Neu4 was not proper to be an accurate marker of LA. Those results indicated that the different sialidase had specific effects on tumors of lung.
Fig. 2.
Expression of NEU1 in lung carcinoma in TCGA database. A Expression of NEU1 in lung cancer (LC), lung adenocarcinoma (LA) or lung squamous cell carcinoma(LSCC); B expression of NEU2 in LC/LA/LSCC; C expression of NEU3 in LC/LA/LSCC; D expression of NEU4 in LC/LA/LSCC; (lung cancer (n = 1093) lung adenocarcinoma (n = 556), lung squamous cell carcinoma (n = 538) ***p < 0.001, **p < 0.01, *p < 0.05, − p < 1)
Lung cancer is the most common cancer and the leading cause of cancer-related deaths, including small-cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) [29, 30]. Several evidence indicates that Neu1 participated in multistage tumorigenesis through binding with matrix metalloproteinase-9 and G protein-coupled receptor tethered to receptor tyrosine kinases (RTKs) and TOLL-like receptor (TLRs) [31, 32]. The p53 tumor suppressor gene mutation highly occurred in lung cancer, involved in promoting cell migration and tumor metastasis [30]. Recent study found that mutant p53 (p53-R273H) promoted NSCLC cell mobility by accelerated NEU1 transcription via activation of AKT signaling [33]. Previous reports demonstrated that Neu1-meidated HA-CD44 binding play an important role in asthma [3], while HA-CD44 binding is also correlated with tumorigenesis or metastasis in lung cancer [34, 35]. It has been proved deglycosylation by neuraminidase induced CD44-HA binding in human lung cancer cell lines [36], but whether NEU1 is the key neuraminidase for HA deglycosylation in lung cancer remains unclear. NEU1 was also identified to be correlated with levels of drug resistance in lung cancer [34, 35]. The association between abnormally high expression of Neu1 and poor prognosis of lung cancer needs further investigation.
NEU1 and COVID-19
Neu1 is correlated with immune cell activation, including T cells, B cells, and monocytes [36–41]. Upregulation of Neu1 activity in activated murine lymphocytes only increased production of IL-4 and have no effect on other Th1 cytokine such as IFN-γ, while raised the production of IFN-γ in activated human T lymphocytes [42]. Neu1 regulate B cell activation via altered the sialic-acid-binding immunoglobulin-like lectins (Siglecs) CD22 organization in plasma membrane [43, 44]. Endogenous Neu1 sialidase activity is significantly increased during the process of monocytes differentiate into macrophages, which trigger MHC II-enriched compartments [45, 46].
Abnormally high expression of NEU1 interacted with MMP-9, contributed to neutrophil overactivation from COVID-19 patients with severe infections [47]. NEU1 inhibitors (oseltamivir and zanamivir) dampened neutrophil dysfunction and improved infection control as well as host survival in pulmonary infection. SARS-CoV-2 infected human cells through angiotensin-converting enzyme 2 (ACE2) on host plasma. ACE2, as a sialylated glycoprotein, its sialic acids are vital for SARS-CoV-2 infection [48, 49]. Reduced NEU1 activity might aggravate SARS-CoV-2 infectious through promoting excessive lysosomal exocytosis in host cells [50], but this hypothesis needs more clinical and basic evidences to support. In addition, some patients recovered from COVID-19 didn’t produce detectable neutralizing antibodies [51], whether this phenotype is associated the immune dysfunction caused by NEU1 deficiency in cellular response, we should learn it more accurately and deeply. Recent studied revealed that host NEU1 interplay with MMP-9 caused neutrophil overactivation by shedding Sia during severe infections including in sepsis and COVID-19 [47]. The newest report shows that highly glycosylated N protein of SARS-CoV-2 and HCoV-OC43 was regulated by host NEU1. Neu5Ac2en-OAcOMe, a newly selectively Neu1 inhibitor targeted intracellular sialidase, remark reduced HCoV-OC43 and SARS-CoV-2 replication in vitro and rescued mice from HCoV-OC43 infection-induced death [52]. However, it is important to note that the role of NEU1 in COVID-19 is still not fully understood and further research is needed to elucidate its precise mechanisms and implications in the disease.
NEU1 inhibitor
Sialidases are involved in several human disorders such as metabolic diseases, infectious, lung diseases, kidney diseases, cardiovascular diseases, and cancers. Neuraminidase inhibitors (NAIs) designed for bacterial or viral have different activity against human neuraminidase in treatment of patients with influenza and chronic respiratory disease ([53–58], Table 1). Consistent with this, sialidase inhibitors DANA and oseltamivir (Tamiflu) strongly attenuated pulmonary fibrosis induced by bleomycin in the mouse model [8, 59] C9-pentylamide analogues of DANA show the inhibition potency toward NEU1 [57], C9-butyl-amide-2-deoxy-2,3-dehydro-N-acetylneuraminic acid (C9-BA-DANA), turned out to be the most selective inhibitor for NEU1, inhibited endogenous and ectopically expressed sialidase activity [58, 60]. C5, C9-modified DANA analogues increased potency of DANA analogues against NEU2 [61, 62].C9-triazolyl DANA derivatives exhibited remarkably increased activity for NEU3 [63].C9-4-hydroxymethyltriazolyl DANA (C9-4HMT-DANA) is active against NEU4 [64]. Although these inhibitors might provide potential tools for investigation of the specific role of human NEU isoenzymes in biological systems, but the activity of these compounds in vivo needed further study.
Table 1.
Summary of Neuraminidase inhibitors (NAIs)
| Compound | IC50 of human neuraminidase | References | |||
|---|---|---|---|---|---|
| Neu1 | Neu2 | Neu3 | Neu4 | ||
| Zanamivir | > 500 μM | (7.8 ± 2.0) μM | (4.0 ± 0.6) μM | (47 ± 6) μM | [48, 49] |
| Oseltamivir | > 500 μM | > 500 μM | > 500 μM | > 500 μM | |
| Peramivir | > 500 μM | (70 ± 7) mM | > 500 μM | > 500 μM | |
| 2-deoxy-2,3-didehydro-N-acetylneuraminic acid (DANA) | (49 ± 8) μM | (37 ± 6) μM | (7.7 ± 0.8) μM | (8.3 ± 1.0) μM | [50] |
| Neu5AcN32en | (24 ± 2) μM | (22 ± 3) μM | (4.4 ± 0.9) μM | (5.8 ± 0.8) μM | |
| C9-butyl-amide-2-deoxy-2,3-dehydro-N-acetylneuraminic acid (C9-BA-DANA) | (3.4 ± 0.2) μM | > 500 μM | (110 ± 40) μM | (220 ± 50) μM | |
| C9-biphenyltriazolyl DANA(C9-4BPT-DANA) | > 500 μM | (32 ± 5) μM | (0.7 ± 0.1) μM | (0.52 ± 0.1) μM | [51] |
| C9-4-hydroxymethyltriazolyl DANA(C9-4HMT-DANA) | (620 ± 10) μM | (240 ± 20) μM | (19.7 ± 2.3) μM | (60 ± 20) μM | [52] |
Traditional Chinese medicine (TCM) has its unique advantages in the treatment of human diseases. It is an important method to screen the effective drugs for the target of human diseases from TCM. Plenty of Chinese herbs and their extracts also exhibited strong sialidases inhibitory activity, including Lonicerae Japonicae Flos, Scutellariae Radix, Olyra latifolia L. leaves, Huanglian Jiedu Decoction and others [65–68]. Our study found that dipsacoside B ameliorated APAP-induced hepatotoxicity by prohibiting Neu1 [69]. The newest evidence supported that salvianolic acid B show strong ability to inhibit Neu1 activity during renal fibrosis development [70]. Interfering peptides (IntPep) targeting the transmembrane (TM) domains 2 of human membrane NEU1 has been proved to disrupt NEU1 dimerization and efficiently block the sialidase activity at the plasma membrane [71]. In a word, Neu1 inhibitors have shown promise as potential therapeutic agents in various diseases and conditions, but further research is needed to fully understand their mechanisms of action and evaluate their efficacy and safety.
Concluding remarks and future challenges
Numerous studies have provided evidence for the involvement of NEU1 in the pathogenesis of respiratory diseases. NEU1 plays a crucial role in the regulation of glycosylation and is involved in the pathogenesis of various respiratory diseases. NEU-mediated mucin1 extracellular ectodomain (MUC1-ED) desialylation regulates pulmonary collagen deposition, fibrosis, bacterial adhesion, and viral infection. While the molecular mechanism of MUC1-ED desialylation mediated recruitment of NEU1 under Pa infection is still unclear. At the same time, with the advances of medical and measure methods, could MUC1-ED and/or flagellin levels in BALF be a rapid diagnostic assay to identify patients with Pa lung infections independent of bacterial culture or genotyping techniques?
NEU1 and NEU3 are abundant sialidases in the lung. Abnormal expression of these sialidases may be a potent marker to distinguish different tumors of lung, but the specific role of these sialidases and the correlated mechanism involved in development of tumors of lung, is needed more and more strong evidence to support.
Although accumulating evidence demonstrated that neuraminidase inhibitors designed for viral or bacterial shown the inhibitory activity of human NEU1 at cellular or animal levels, the clinical studies of the pharmacology effect of NAIs are rare. The direct interaction between NAIs and NEU1 should be explored by a more reliable method, such as the surface plasmon resonance (SPR) assay, affinity chromatographic methods and other methods. Comprehensive studies on efficacy, safety and toxicity of NAIs in humans are urgently to proceed. Targeting Neu1 may represent a promising therapeutic approach for the treatment of these diseases.
Overall, human NEU1 mediated MUC1 desialylation, human NEU1 modulated immune cell differentiate and activation, contribute to the development and progression of various inflammatory, fibrotic, and fibro-inflammatory human pathologies. Further research focusing on the details of human NEU isoenzymes in biological systems is needed to fully understand the mechanisms underlying the involvement of Neu1 in these conditions and to explore its potential as a therapeutic target for the treatment of respiratory diseases. It is promising that NAIs will be effective treatment of various human respiratory diseases.
Author contributions
SRM and DDL wrote the main manuscript text, and AYW provided significant input. NL drew graphics, YQ and GXZ made the table, ZBW carry out bioinformatical analysis. JSJ and YLZ reviewed and edited the manuscript and approved the final version. All authors have read and approved the manuscript.
Funding
This work is financially supported by the National Natural Science Foundation of China (project reference no. 81900780) and The Nanjing Research Center for Infectious Diseases of Integrated Traditional Chinese and Western Medicine (YBZX2022).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interest to declare with regards to the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shiran Mei, Dingding Li and Aoyi Wang have contributed equally to this work.
Contributor Information
Yanliang Zhang, Email: fsyy00404@njucm.edu.cn.
Shujun Jiang, Email: jiangshujun@njucm.edu.cn.
References
- 1.Cross AS, Hyun SW, Miranda-Ribera A, Feng C, Liu A, Nguyen C, et al. NEU1 and NEU3 sialidase activity expressed in human lung microvascular endothelia: NEU1 restrains endothelial cell migration, whereas NEU3 does not. J Biol Chem. 2012;287(19):15966–15980. doi: 10.1074/jbc.M112.346817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lillehoj EP, Hyun SW, Feng C, Zhang L, Liu A, Guang W, et al. NEU1 sialidase expressed in human airway epithelia regulates epidermal growth factor receptor (EGFR) and MUC1 protein signaling. J Biol Chem. 2012;287(11):8214–8231. doi: 10.1074/jbc.M111.292888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katoh S, Maeda S, Fukuoka H, Wada T, Moriya S, Mori A, et al. A crucial role of sialidase Neu1 in hyaluronan receptor function of CD44 in T helper type 2-mediated airway inflammation of murine acute asthmatic model. Clin Exp Immunol. 2010;161(2):233–234. doi: 10.1111/j.1365-2249.2010.04165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katoh S. Critical involvement of CD44 in T helper type 2 cell-mediated eosinophilic airway inflammation in a mouse model of acute asthma. Front Immunol. 2022;12:811600. doi: 10.3389/fimmu.2021.811600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luzina IG, Lockatell V, Hyun SW, Kopach P, Kang PH, Noor Z, et al. Elevated expression of NEU1 sialidase in idiopathic pulmonary fibrosis provokes pulmonary collagen deposition, lymphocytosis, and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2016;310(10):L940–L954. doi: 10.1152/ajplung.00346.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luzina IG, Lillehoj EP, Lockatell V, Hyun SW, Lugkey KN, Imamura A, et al. Therapeutic effect of neuraminidase-1-selective inhibition in mouse models of bleomycin-induced pulmonary inflammation and fibrosis. J Pharmacol Exp Ther. 2021;376(1):136–146. doi: 10.1124/jpet.120.000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karhadkar TR, Meek TD, Gomer RH. Inhibiting sialidase-induced TGF-β1 activation attenuates pulmonary fibrosis in mice. J Pharmacol Exp Ther. 2021;376(1):106–117. doi: 10.1124/jpet.120.000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lillehoj EP, Hyun SW, Liu A, Guang W, Verceles AC, Luzina IG, et al. NEU1 sialidase regulates membrane-tethered mucin (MUC1) ectodomain adhesiveness for Pseudomonas aeruginosa and decoy receptor release. J Biol Chem. 2015;290(30):18316–18331. doi: 10.1074/jbc.M115.657114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lillehoj EP, Guang W, Hyun SW, Liu A, Hegerle N, Simon R, et al. Neuraminidase 1-mediated desialylation of the mucin 1 ectodomain releases a decoy receptor that protects against Pseudomonas aeruginosa lung infection. J Biol Chem. 2019;294(2):662–678. doi: 10.1074/jbc.RA118.006022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verceles AC, Bhat P, Nagaria Z, Martin D, Patel H, Ntem-Mensah A, et al. MUC1 ectodomain is a flagellin-targeting decoy receptor and biomarker operative during Pseudomonas aeruginosa lung infection. Sci Rep. 2021;11(1):22725. doi: 10.1038/s41598-021-02242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonten EJ, Annunziata I, d'Azzo A. Lysosomal multienzyme complex: pros and cons of working together. Cell Mol Life Sci. 2014;71(11):2017–2032. doi: 10.1007/s00018-013-1538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennasroune A, Romier-Crouzet B, Blaise S, Laffargue M, Efremov RG, et al. Elastic fibers and elastin receptor complex: neuraminidase-1 takes the center stage. Matrix Biol. 2019;84:57–67. doi: 10.1016/j.matbio.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Lee C, Liu A, Miranda-Ribera A, Hyun SW, Lillehoj EP, Cross AS, et al. NEU1 sialidase regulates the sialylation state of CD31 and disrupts CD31-driven capillary-like tube formation in human lung microvascular endothelia. J Biol Chem. 2014;289(13):9121–9135. doi: 10.1074/jbc.M114.555888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng C, Zhang L, Nguyen C, Vogel SN, Goldblum SE, Blackwelder WC, et al. Neuraminidase reprograms lung tissue and potentiates lipopolysaccharide-induced acute lung injury in mice. J Immunol. 2013;191(9):4828–4837. doi: 10.4049/jimmunol.1202673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh V, Ulasov I, Gupta S, Singh A, Roy VK, Kharwar RK. Idiopathic pulmonary fibrosis: where do we stand and how far to go? Discov Med. 2024;36(180):22. doi: 10.24976/Discov.Med.202436180.3. [DOI] [PubMed] [Google Scholar]
- 16.Mutsaers SE, Miles T, Prêle CM, Hoyne GF. Emerging role of immune cells as drivers of pulmonary fibrosis. Pharmacol Ther. 2023;252:108562. doi: 10.1016/j.pharmthera.2023.108562. [DOI] [PubMed] [Google Scholar]
- 17.Jayanth P, Amith SR, Gee K, Szewczuk MR. Neu1 sialidase and matrix metalloproteinase-9 cross-talk is essential for neurotrophin activation of Trk receptors and cellular signaling. Cell Signal. 2010;22(8):1193–1205. doi: 10.1016/j.cellsig.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Hinek A, Bodnaruk TD, Bunda S, Wang Y, Liu K. Neuraminidase-1, a subunit of the cell surface elastin receptor, desialylates and functionally inactivates adjacent receptors interacting with the mitogenic growth factors PDGF-BB and IGF-2. Am J Pathol. 2008;173(4):1042–1056. doi: 10.2353/ajpath.2008.071081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Y, Zhang J, Zheng JX. Angiogenesis and pulmonary fibrosis. Zhonghua Jie He He Hu Xi Za Zhi. 2023;46(2):197–202. doi: 10.3760/cma.j.cn112147-20220511-00397. [DOI] [PubMed] [Google Scholar]
- 20.Pilling D, Sahlberg K, Chen W, Gomer RH. Changes in lung sialidases in male and female mice after bleomycin aspiration. Exp Lung Res. 2022;48(9–10):291–304. doi: 10.1080/01902148.2022.2144548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W, Karhadkar TR, Ryu C, Herzog EL, Gomer RH. Reduced sialylation and bioactivity of the antifibrotic protein serum amyloid P in the sera of patients with idiopathic pulmonary fibrosis. Immunohorizons. 2020;4(6):352–362. doi: 10.4049/immunohorizons.2000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karhadkar TR, Chen W, Gomer RH. Attenuated pulmonary fibrosis in sialidase-3 knockout (Neu3−/−) mice. Am J Physiol Lung Cell Mol Physiol. 2020;318(1):L165–L179. doi: 10.1152/ajplung.00275.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lloyd CM, Saglani S. T cells in asthma: influences of genetics, environment, and T-cell plasticity. J Allergy Clin Immunol. 2013;131(5):1267–1274. doi: 10.1016/j.jaci.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Chen GY, Brown NK, Wu W, Khedri Z, Yu H, Chen X, et al. Broad and direct interaction between TLR and Siglec families of pattern recognition receptors and its regulation by Neu1. Elife. 2014;3:e04066. doi: 10.7554/eLife.04066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hewson CA, Jardine A, Edwards MR, Laza-Stanca V, Johnston SL. Toll-like receptor 3 is induced by and mediates antiviral activity against rhinovirus infection of human bronchial epithelial cells. J Virol. 2005;79(19):12273–12279. doi: 10.1128/JVI.79.19.12273-12279.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pech M, Weckmann M, König IR, Franke A, Heinsen FA, Oliver B, et al. Rhinovirus infections change DNA methylation and mRNA expression in children with asthma. PLoS ONE. 2018;13(11):e0205275. doi: 10.1371/journal.pone.0205275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toussaint K, Appert-Collin A, Morjani H, Albrecht C, Sartelet H, Romier-Crouzet B, Maurice P, Duca L, Blaise S, Bennasroune A. Neuraminidase-1: a sialidase involved in the development of cancers and metabolic diseases. Cancers. 2022;14(19):4868. doi: 10.3390/cancers14194868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haxho F, Neufeld RJ, Szewczuk MR. Neuraminidase-1: a novel therapeutic target in multistage tumorigenesis. Oncotarget. 2016;7(26):40860–40881. doi: 10.18632/oncotarget.8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 30.Yin X, Li Y, Wang H, Jia T, Wang E, Luo Y, et al. Small cell lung cancer transformation: from pathogenesis to treatment. Semin Cancer Biol. 2022;86(Pt 2):595–606. doi: 10.1016/j.semcancer.2022.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Liu YC, Yen HY, Chen CY, Chen CH, Cheng PF, Juan YH, et al. Sialylation and fucosylation of epidermal growth factor receptor suppress its dimerization and activation in lung cancer cells. Proc Natl Acad Sci USA. 2011;108(28):11332–11337. doi: 10.1073/pnas.1107385108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uemura T, Shiozaki K, Yamaguchi K, Miyazaki S, Satomi S, Kato K, et al. Contribution of sialidase NEU1 to suppression of metastasis of human colon cancer cells through desialylation of integrin beta4. Oncogene. 2009;28(9):1218–1229. doi: 10.1038/onc.2008.471. [DOI] [PubMed] [Google Scholar]
- 33.Lv T, Lv H, Fei J, Xie Y, Lian D, Hu J, et al. p53–R273H promotes cancer cell migration via upregulation of neuraminidase-1. J Cancer. 2020;11(23):6874–6882. doi: 10.7150/jca.44718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Efeoglu E, Henry M, Clynes M, Meleady P. Label-free quantitative proteomics analysis of adriamycin selected multidrug resistant human lung cancer cells. Biomolecules. 2022;12(10):1401. doi: 10.3390/biom12101401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song JM, Im J, Nho RS, Han YH, Upadhyaya P, Kassie F. Hyaluronan-CD44/RHAMM interaction-dependent cell proliferation and survival in lung cancer cells. Mol Carcinog. 2019;58(3):321–333. doi: 10.1002/mc.22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsubara Y, Katoh S, Taniguchii H, Oka M, Kadota J, Kohno S. Expression of CD44 variants in lung cancer and its relationship to hyaluronan binding. J Int Med Res. 2000;28(2):78–90. doi: 10.1177/147323000002800203. [DOI] [PubMed] [Google Scholar]
- 37.Chen XP, Enioutina EY, Daynes RA. The control of IL-4 gene expression in activated murine T lymphocytes: a novel role for neu-1 sialidase. J Immunol. 1997;158(7):3070–3080. doi: 10.4049/jimmunol.158.7.3070. [DOI] [PubMed] [Google Scholar]
- 38.Landolfi NF, Leone J, Womack JE, Cook RG. Activation of T lymphocytes results in an increase in H-2-encoded neuraminidase. Immunogenetics. 1985;22(2):159–167. doi: 10.1007/BF00563513. [DOI] [PubMed] [Google Scholar]
- 39.Landolfi NF, Cook RG. Activated T-lymphocytes express class I molecules which are hyposialylated compared to other lymphocyte populations. Mol Immunol. 1986;23(3):297–309. doi: 10.1016/0161-5890(86)90057-X. [DOI] [PubMed] [Google Scholar]
- 40.Katoh S, Miyagi T, Taniguchi H, Matsubara Y, Kadota J, Tominaga A, et al. Cutting edge: an inducible sialidase regulates the hyaluronic acid binding ability of CD44-bearing human monocytes. J Immunol. 1999;162(9):5058–5061. doi: 10.4049/jimmunol.162.9.5058. [DOI] [PubMed] [Google Scholar]
- 41.Gee K, Kozlowski M, Kumar A. Tumor necrosis factor-alpha induces functionally active hyaluronan-adhesive CD44 by activating sialidase through p38 mitogen-activated protein kinase in lipopolysaccharide-stimulated human monocytic cells. J Biol Chem. 2003;278(39):37275–37287. doi: 10.1074/jbc.M302309200. [DOI] [PubMed] [Google Scholar]
- 42.Nan X, Carubelli I, Stamatos NM. Sialidase expression in activated human T lymphocytes influences production of IFN-gamma. J Leukoc Biol. 2007;81(1):284–296. doi: 10.1189/jlb.1105692. [DOI] [PubMed] [Google Scholar]
- 43.Meyer SJ, Linder AT, Brandl C, Nitschke L. B cell siglecs–news on signaling and its interplay with ligand binding. Front Immunol. 2018;9:2820. doi: 10.3389/fimmu.2018.02820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tran HT, Li C, Chakraberty R, Cairo CW. NEU1 and NEU3 enzymes alter CD22 organization on B cells. Biophys Rep. 2022;2(3):100064. doi: 10.1016/j.bpr.2022.100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stamatos NM, Liang F, Nan X, Landry K, Cross AS, Wang LX, et al. Differential expression of endogenous sialidases of human monocytes during cellular differentiation into macrophages. FEBS J. 2005;272(10):2545–2556. doi: 10.1111/j.1742-4658.2005.04679.x. [DOI] [PubMed] [Google Scholar]
- 46.Liang F, Seyrantepe V, Landry K, Ahmad R, Ahmad A, Stamatos NM, et al. Monocyte differentiation up-regulates the expression of the lysosomal sialidase, Neu1, and triggers its targeting to the plasma membrane via major histocompatibility complex class II-positive compartments. J Biol Chem. 2006;281(37):27526–27538. doi: 10.1074/jbc.M605633200. [DOI] [PubMed] [Google Scholar]
- 47.de Oliveira FR, Amaral FC, Souza CF, Mendes DAGB, Wanderley CWS, Lorenzini CB, et al. Neuraminidase is a host-directed approach to regulate neutrophil responses in sepsis and COVID-19. Br J Pharmacol. 2023;180(11):1460–1481. doi: 10.1111/bph.16013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shajahan A, Supekar NT, Gleinich AS, Azadi P. Deducing the N and o-glycosylation profile of the spike protein of novel coronavirusSARS-CoV-2. Glycobiology. 2020;30:981–988. doi: 10.1093/glycob/cwaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwegmann-Wessels C, Herrler G. Sialic acids as receptor determinants for coronaviruses. Glycoconj J. 2006;23:51–58. doi: 10.1007/s10719-006-5437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bongiovanni A, Cusimano A, Annunziata I, d'Azzo A. Sialylation of host proteins as targetable risk factor for COVID-19 susceptibility and spreading: a hypothesis. FASEB Bioadv. 2021;3(3):192–197. doi: 10.1096/fba.2020-00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu F, Wang A, Liu M, Wang Q, Chen J, Xia S, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv. 2020 doi: 10.1101/2020.03.30.20047365. [DOI] [Google Scholar]
- 52.Yang D, Wu Y, Turan I, Keil J, Li K, Chen MH, et al. Targeting intracellular Neu1 for coronavirus infection treatment. iScience. 2023;26(2):106037. doi: 10.1016/j.isci.2023.106037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williamson JC, Pegram PS. Neuraminidase inhibitors in patients with underlying airways disease. Am J Respir Med. 2002;1(2):85–90. doi: 10.1007/BF03256597. [DOI] [PubMed] [Google Scholar]
- 54.Richards MR, Guo T, Hunter CD, Cairo CW. Molecular dynamics simulations of viral neuraminidase inhibitors with the human neuraminidase enzymes: insights into isoenzyme selectivity. Bioorg Med Chem. 2018;26(19):5349–5358. doi: 10.1016/j.bmc.2018.05.035. [DOI] [PubMed] [Google Scholar]
- 55.Hata K, Koseki K, Yamaguchi K, Moriya S, Suzuki Y, Yingsakmongkon S, et al. Limited inhibitory effects of oseltamivir and zanamivir on human sialidases. Antimicrob Agents Chemother. 2008;52(10):3484–3491. doi: 10.1128/AAC.00344-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo T, Héon-Roberts R, Zou C, Zheng R, Pshezhetsky AV, Cairo CW. Selective inhibitors of human neuraminidase 1 (NEU1) J Med Chem. 2018;61(24):11261–11279. doi: 10.1021/acs.jmedchem.8b01411. [DOI] [PubMed] [Google Scholar]
- 57.Magesh S, Moriya S, Suzuki T, Miyagi T, Ishida H, Kiso M. Design, synthesis, and biological evaluation of human sialidase inhibitors. Part 1: selective inhibitors of lysosomal sialidase (NEU1) Bioorg Med Chem Lett. 2008;18(2):532–537. doi: 10.1016/j.bmcl.2007.11.084. [DOI] [PubMed] [Google Scholar]
- 58.Guo T, Dätwyler P, Demina E, Richards MR, Ge P, Zou C, et al. Selective inhibitors of human neuraminidase 3. J Med Chem. 2018;61(5):1990–2200. doi: 10.1021/acs.jmedchem.7b01574. [DOI] [PubMed] [Google Scholar]
- 59.Karhadkar TR, Pilling D, Cox N, Gomer RH. Sialidase inhibitors attenuate pulmonary fibrosis in a mouse model. Sci Rep. 2017;7(1):15069. doi: 10.1038/s41598-017-15198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hyun SW, Liu A, Liu Z, Cross AS, Verceles AC, Magesh S, et al. The NEU1-selective sialidase inhibitor, C9-butyl-amide-DANA, blocks sialidase activity and NEU1-mediated bioactivities in human lung in vitro and murine lung in vivo. Glycobiology. 2016;26(8):834–849. doi: 10.1093/glycob/cww060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y, Cao H, Yu H, Chen Y, Lau K, Qu J, et al. Identifying selective inhibitors against the human cytosolic sialidase NEU2 by substrate specificity studies. Mol BioSyst. 2011;7:1060–1072. doi: 10.1039/c0mb00244e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khedri Z, Li Y, Cao H, Qu J, Yu H, Muthana MM, et al. Synthesis of selective inhibitors against V. cholerae sialidase and human cytosolic sialidase NEU2. Org Biomol Chem. 2012;10:6112–6120. doi: 10.1039/c2ob25335f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zou Y, Albohy A, Sandbhor M, Cairo CW. Inhibition of human neuraminidase 3 (NEU3) by C9-triazole derivatives of 2,3- didehydro-n-acetyl-neuraminic acid. Bioorg Med Chem Lett. 2010;20:7529–7533. doi: 10.1016/j.bmcl.2010.09.111. [DOI] [PubMed] [Google Scholar]
- 64.Albohy A, Zhang Y, Smutova V, Pshezhetsky AV, Cairo CW. Identification of selective nanomolar inhibitors of the human neuraminidase, NEU4. ACS Med Chem Lett. 2013;4:532–537. doi: 10.1021/ml400080t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou X, Li H, Shi Z, Gao S, Wei S, Li K, et al. Inhibition activity of a traditional Chinese herbal formula Huang-Lian-Jie-Du-Tang and its major components found in its plasma profile on neuraminidase-1. Sci Rep. 2017;7(1):15549. doi: 10.1038/s41598-017-15733-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang T, Xiao M, Wong CK, Mok KC, Zhao X, Ti H, et al. A traditional multi-herb formulation, exerts anti-influenza effects in vitro and in vivo via neuraminidase inhibition and immune regulation. BMC Complement Altern Med. 2018;18(1):150. doi: 10.1186/s12906-018-2216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Albrecht C, Akissi ZLE, Yao-Kouassi PA, Alabdul Magid A, Maurice P, Duca L, et al. Identification and evaluation of new potential inhibitors of human neuraminidase 1 extracted from Olyra latifolia L.: a preliminary study. Biomedicines. 2021;9(4):411. doi: 10.3390/biomedicines9040411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang JY, Chen QQ, Li J, Zhang L, Qi LW. Neuraminidase 1 and its inhibitors from Chinese herbal medicines: an emerging role for cardiovascular diseases. Am J Chin Med. 2021;49(4):843–862. doi: 10.1142/S0192415X21500403. [DOI] [PubMed] [Google Scholar]
- 69.Chen S, Li M, Jiang W, Zheng H, Qi LW, Jiang S. The role of Neu1 in the protective effect of dipsacoside B on acetaminophen-induced liver injury. Ann Transl Med. 2020;8(13):823. doi: 10.21037/atm-19-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen QQ, Liu K, Shi N, Ma G, Wang P, Xie HM, et al. Neuraminidase 1 promotes renal fibrosis development in male mice. Nat Commun. 2023;14(1):1713. doi: 10.1038/s41467-023-37450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Albrecht C, Kuznetsov AS, Appert-Collin A, Dhaideh Z, Callewaert M, Bershatsky YV, et al. Transmembrane peptides as a new strategy to inhibit neuraminidase-1 activation. Front Cell Dev Biol. 2020;8:611121. doi: 10.3389/fcell.2020.611121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.