Abstract
The cell-mediated immune response has been documented to be the major protective immune mechanism in mice infected genitally with the agent of mouse pneumonitis (MoPn), a biovar of Chlamydia trachomatis. Moreover, there is strong evidence to indicate that gamma interferon (IFN-γ) is a major effector mechanism of the cell-mediated immune response. Previous studies from this laboratory have also reported that the dominant cell population in the genital tract is the CD4 Th1 population. When experiments were performed by the enzyme-linked immunospot assay, high numbers of cells producing IFN-γ were found in the genital tract, concomitant with resolution of the infection; however, in addition, an increase in IFN-γ-producing cells which were CD4− was seen early in the infection. Since natural killer (NK) cells produce IFN-γ and have been found to participate in the early responses in other infections, we hypothesized that NK cells are responsible for early IFN-γ production in the murine chlamydial model. NK cells were quantified by the standard YAC-1 cytotoxicity assay and were found to appear in the genital tract as early as 12 h after intravaginal infection with MoPn. The cells were confirmed to be NK cells by abrogation of YAC-1 cell cytotoxicity by treatment in vitro and in vivo with anti-asialo-GM1. The early IFN-γ response could also be depleted by treatment with anti-asialo-GM1, indicating that NK cells were responsible for the production of this cytokine. Of interest was our observation that depletion of NK cells also exacerbated the course of infection in the mice and elicited a Th2 response, as indicated by a marked increase in immunoglobulin G1 antibody. Thus, these data demonstrate that NK cells are not only responsible for the production of IFN-γ early in the course of chlamydial genital tract infection but are also, via IFN-γ, a significant factor in the development of the Th1 CD4 response and in the control of the infection.
Chlamydia trachomatis is the leading cause of sexually transmitted diseases in developed countries and may lead to serious sequelae, including infertility and ectopic pregnancy, in women. While a variety of strategies for prevention of this disease are being evaluated, the development of a vaccine to either prevent infection or prevent pathologic changes remains a viable option. In order to produce a successful vaccine, an understanding of the protective immunological mechanisms is required. Nevertheless, information on the protective immune response in humans is limited; thus, continued studies in animal models are essential to acquire this information.
It has become clear in recent years that the cell-mediated immune response plays an important role in the protective immune response to chlamydial genital infections (19). Whether cell-mediated immunity (CMI) functions alone or in concert with the humoral immune response is not completely clear at this point and appears to depend upon the animal model being used. While the guinea pig infected with the agent of guinea pig inclusion conjunctivitis requires both antibody and CMI for resolution of and resistance to chlamydial genital infection (18), the murine model infected with the C. trachomatis agent of mouse pneumonitis (MoPn) requires only CMI for elimination of genital infection and for protection against reinfection (17). These data have been confirmed recently by experiments using B-cell knockout mice (26). Studies in our laboratory as well as others have also demonstrated that this protective response is dependent primarily upon the CD4 T-cell response (16, 25). Ramsey and Rank (16) first demonstrated that MoPn-specific T-cell lines enriched for CD4 cells were more effective in the elimination of genital infection than were CD8-enriched lines. Su and Caldwell (25) confirmed that CD4 spleen cells were more effective in resolving infection than were CD8 cells, and Morrison et al. (14) observed that mice deficient in either class II major histocompatibility complex or CD4 cells had much longer infections than immunologically intact animals. While CD8 cell lines and clones were able to resolve MoPn genital infections, they were less efficient in doing so than CD4 lines and clones (8, 9, 16). Moreover, mice deficient in β2-microglobulin (class I deficient) were able to resolve genital infection quite readily (14).
Data from our laboratory have also shown that the primary CD4 subclass responsible for the resolution of the infection is the Th1 subclass, as demonstrated by the ability of a Th1 clone to resolve genital infection in nude mice (9) and by the preponderance of Th1 cells in the genital tract and draining lymph nodes following MoPn genital infection (2). Of significance also was the observation that mice immunized by the subcutaneous route produced a predominant Th2 response in the genital tract in contrast to immunization by the mucosal route, which elicited a predominant Th1 response (13). When given a challenge infection in the genital tract, mice with the predominant Th2 response demonstrated little immunity to the challenge in contrast to a high level of immunity in animals with a Th1 response. While the mechanism employed by the Th1 cells is not known for certain, there has been a large amount of data to demonstrate that gamma interferon (IFN-γ) has antichlamydial activity (1) and is required for resolution of MoPn genital and respiratory infections (20, 29).
Thus, while it would appear that the Th1 response plays an important role in chlamydial genital infection, there is little known with regard to how this response is regulated in chlamydial infections. Certainly, it has been well documented in other intracellular infections, such as those with leishmania (24) and listeria (5), that NK cells are important in the production of IFN-γ which can up-regulate the Th1 response. Interestingly, when Cain and Rank (2) assessed the Th1 response by the enzyme-linked immunospot (ELISPOT) assay, they observed a marked increase in the number of IFN-γ-producing cells in the genital tract 7 days after intravaginal infection. These cells were not eliminated by treatment in vitro with anti-CD4 antibody, suggesting that the IFN-γ was produced by a cell type other than CD4 cells (2a). A logical suspect for the production of IFN-γ at this stage in the infection is the NK cell. Therefore, it was the purpose of this study to examine the role of NK cells in the production of the early IFN-γ response and to determine if NK cells participate in the development of the Th1 cell response resulting from intravaginal infection with MoPn. Moreover, it has become clear that the early events in the host response to an infection can profoundly affect the outcome of the infection; thus, it was also a goal of this study to determine the effect of the early cytokine response in the control of a primary chlamydial genital infection.
MATERIALS AND METHODS
Experimental animals.
Female BALB/c mice at 4 to 5 weeks of age were purchased from Harlan Sprague-Dawley, Indianapolis, Ind. Animals were housed in an environmentally controlled room with a cycle of 12 h of light and 12 h of darkness. Animals were routinely used in experiments when they were 6 weeks old.
Chlamydia culture and infection of mice.
The C. trachomatis biovar MoPn, which was originally obtained from the American Type Culture Collection and has been continually passaged in our laboratory for approximately 20 years, was used throughout this study. MoPn organisms for infection purposes were grown on McCoy cell monolayers, and elementary bodies were purified, titrated, and stored in sucrose-phosphate buffer (2-SP) (23) by established procedures. MoPn for use as an antigen was cultured on HeLa cells in order to avoid cross-reactivity to the host cells.
Seven days prior to infection, mice were subcutaneously injected with a single dose of 2.5 mg of progesterone in the form of Depo-Provera (Upjohn, Kalamazoo, Mich.) in order to induce a state of anestrus in the mice and thus eliminate any potential effect of the stage of the estrous cycle. This was particularly important in this study because we were studying various immunological parameters over a short 5- to 7-day period at the very onset of infection. Lack of estrous cycle synchrony in this study might have introduced major variables which would have made interpretation of the data difficult. For genital infection, mice were anesthetized by the intraperitoneal injection of pentobarbital sodium (50 mg/kg of body weight) and infected intravaginally with 107 inclusion-forming units (IFU) in 30 μl of 2-SP buffer.
Preparation of MNCs.
Mononuclear cells (MNCs) were extracted from the genital tracts and various secondary lymphoid tissue samples from mice at various times after infection. To prepare genital tract-associated MNCs, the genital tracts from infected mice were pooled, minced thoroughly, treated with 10 ml of sterile 0.5% type I collagenase (Sigma Chemicals, St. Louis, Mo.) at 37°C for 1 h with brief agitation every 15 min, and filtered through 70-μm-pore-size nylon cell strainers (Falcon; Becton Dickinson, Paramus, N.J.) with an additional 40 ml of RPMI 1640 medium to remove cell debris. The MNCs were then enriched over a Ficoll-Hypaque gradient (Lympholyte M; Cedarlane Laboratories, Hornby, Ontario, Canada). The contaminating erythrocytes were lysed with a 0.85% NH4Cl solution (pH 7.2). MNCs of secondary lymphoid tissues were prepared by gentle teasing of the pooled tissues in RPMI 1640 medium and enriched over a Ficoll-Hypaque gradient. MNCs derived from lymph nodes are usually free of erythrocyte contamination and did not require NH4Cl treatment. The viability of the resulting MNC preparation was routinely greater than 95%, as assessed by trypan blue exclusion.
Phenotyping of genital tract-associated MNCs.
Phenotyping of MNCs was performed by standard flow cytometric methodology. Cells were stained with predetermined dilutions of fluorescein isothiocyanate (FITC)-conjugated anti-CD3 (immunoglobulin G2b [IgG2b]) monoclonal antibodies (MAbs), FITC-conjugated polyclonal anti-mouse Ig (IgA, IgM, and IgG) (Sigma), FITC-conjugated rat anti-mouse Mac-3 MAb (IgG1) (Pharmingen, San Diego, Calif.), and appropriate isotype-matched control antibodies for 30 min at 4°C. The stained cells were washed twice with phosphate-buffered saline (PBS) containing 0.2% bovine serum albumin and 0.02% NaN3. Flow cytometric analysis was performed on a FACScan, and mean channel fluorescence was calculated by using the Lysis II and WinMDI software packages (Becton Dickinson, Mountain View, Calif.).
Analysis of NK cell activity.
The NK cell activity of cells derived from various tissues was determined by measuring the specific cytolytic activity against 51Cr-labeled YAC-1 target cells in a standard 4-h chromium release assay at several effector/target (E/T) ratios, as described previously (7) with slight modifications. Briefly, 2 × 106 YAC-1 cells in 10 ml of RPMI 1640 medium (Cell-Gro) containing 10% fetal calf serum, 100 U of penicillin per ml, and 100 μg of streptomycin per ml were labeled with 100 μCi of sodium chromate (ICN) for 12 h at 37°C in a CO2 incubator. A total of 104 thoroughly washed YAC-1 target cells in 100 μl of complete medium were then distributed into wells of round-bottomed microtiter plates containing effector cells at various concentrations. Each test sample was plated in triplicate. The microtiter plates were incubated at 37°C for 4 h. The percentage of specific 51Cr release was calculated as follows: 100 × (cpm of test sample − cpm of medium)/(cpm of maximum control − cpm of medium control), where cpm is counts per minute. The spontaneous release of target cells was usually 6 to 7% and never exceeded 10%.
Titration of anti-asialo-GM1 rabbit antibody.
Although asialo-GM1 (Waco Bioproducts, Richmond, Va.) has been widely used as a surface marker to identify NK cells, other cell types also express this surface antigen (12). Therefore, it was necessary to determine the most appropriate concentration of anti-asialo-GM1 antibody to attain maximal depletion of NK-like activity with a minimal effect on other cell types. In this titration experiment, the criteria for selecting the best titer of this antibody was a maximal depletion of in vitro cytotoxicity against YAC-1 cells, concomitant with a minimal effect on the CD3+ T-cell population, as determined by a fluorescence-activated cell sorter. A serial dilution of anti-asialo-GM1 antibody and a fixed concentration of freshly prepared rabbit complement (1:10 dilution) were used to selectively deplete asialo-GM1-positive cells in MNCs derived from the spleens of BALB/c mice. Medium alone and complement alone were also included in this titration study as controls. For in vivo use of anti-asialo-GM1 antibody, various amounts of the antibody were injected intraperitoneally into mice at indicated times, and the efficacy of such treatment was determined by analyzing NK-like activity and CD3+ T-cell population as above. Control mice received equivalent amounts of an irrelevant rabbit antibody (inactivated rabbit anti-herpes simplex virus [HSV] serum). The experiments indicated that a 1:200 dilution and 50 μl of anti-asialo-GM1 rabbit antibody per injection were the optimal concentrations for in vitro and in vivo use, respectively.
Selective depletion of T cells or NK cells.
Depletion of selective T cells or NK cells was performed both in vitro and in vivo with the appropriate antibodies and dilutions discussed above. In vitro, complement-mediated cytotoxicity was used to selectively deplete T cells or NK cells. Briefly, a minimum of 107 MNCs was incubated with 100 μg of anti-CD3 MAb (Gibco Laboratories, Grand Island, N.Y.) or a 1:200 dilution of rabbit anti-asialo-GM1 antibody for 1 h at 4°C with constant agitation. The treated MNCs were washed and then incubated with appropriately diluted low Tox-M rabbit complement (Accurate Chemical & Scientific Corp., Westbury, N.Y.) for 1 h at 37°C. After the cells were thoroughly washed, the resulting cell suspension was used as a CD3+ T-cell-depleted or NK cell-depleted suspension, as described above.
In vivo, polyclonal anti-asialo-GM1 antibody was used to deplete NK cells. Mice were injected intraperitoneally with 50 μl of anti-asialo-GM1 or an equivalent amount of an irrelevant rabbit antibody (inactivated rabbit anti-HSV serum) on days −3, −1, 1, and 3 after MoPn infection. The efficacy of such treatment was evaluated by the standard YAC-1 cell cytotoxicity assay.
Measurement of MoPn-specific antibodies.
IgG1 and IgG2a antibodies to MoPn were measured by a standard enzyme-linked immunosorbent assay as previously described (13). Peroxidase-labeled antibodies to murine IgG1 and IgG2a were obtained from Southern Biotechnology Associates, Inc., Birmingham, Ala.
Analysis of IL-4 and IFN-γ production.
A modification of the ELISPOT assay, originally described by Taguchi et al. (27), was used to determine the profile of cytokine production of genital tract-associated MNCs. Briefly, nitrocellulose-based 96-well plates (Millititer HA; Millipore Corporation, Marlborough, Mass.) were coated with the primary antibody (2 μg/ml) directed against either murine IL-4 or murine IFN-γ (Pharmingen). After the plates were coated overnight at 4°C, they were washed with PBS containing 0.05% Tween 20 (PBS-Tween; pH 7.4), and blocked with PBS containing 5% fetal calf serum for 1 h at 37°C in the CO2 incubator. To determine the cytokine production, MNCs were extracted from iliac lymph nodes (ILN) at indicated times after infection and were stimulated in vitro with a Renografin-purified preparation of elementary bodies (3) (5 μg/ml), derived from infected HeLa cells, at 37°C overnight. The stimulated MNCs were distributed into each well in triplicate at various concentrations and incubated for an additional 20 h in the incubator. The plates were then washed extensively with PBS-Tween to remove unbound cells, followed by an overnight incubation at 4°C with a secondary biotinylated antibody (4 μg/ml), directed against either IL-4 or IFN-γ. After a thorough washing, the plates were incubated with 2.5 μg of avidin-peroxidase (Vector, Burlingame, Calif.) per ml for 1 h, followed by color development with 3-amino-9-ethylcarbazole. Spots or cytokine-producing cells were enumerated with the aid of a dissecting microscope. The mean number of spots derived from the triplicate samples was used to calculate the spot-forming cells per million cells.
RT-PCR.
Reverse transcription-PCR (RT-PCR) was used to verify the expression of transcripts of specific cytokines in the genital tracts of mice early in the infection. This technique basically involves three major steps, including isolation of total RNA from infected genital tracts, reverse transcription of the first-strand cDNA from total RNA, and amplification of sequences of interest from cDNA by PCR.
(i) RNA extraction.
Total RNA was extracted from the genital tracts of mice at the indicated times after MoPn infection, using commercially available TRI REAGENT (Molecular Research Center, Inc., Cincinnati, Ohio) according to the manufacturer’s recommendation. Briefly, the genital tracts of five mice were pooled, minced, and digested with collagenase, as described earlier for preparation of MNCs. The resulting cell suspensions after digestion were directly lysed with 1 ml of TRI REAGENT. The homogenates were left for 5 min at room temperature to permit complete dissociation of nucleoprotein complex before the addition of 0.1 ml of 1-bromo-3-chloropropane. The resulting mixtures were vigorously shaken, incubated at 25°C for 10 min, and centrifuged at 12,000 × g for 14 min for phase separation. The aqueous phase containing RNA was transferred to a fresh tube. The RNA was precipitated by adding 0.5 ml of isopropanol, and the solution was centrifuged. The resulting precipitates were washed once with 1 ml of 75% ethanol and centrifuged at 7,500 × g for 5 min. After a brief drying period, RNA pellets were dissolved with diethylpyrocarbonate-treated water and quantified at 260 nm in a spectrophotometer (Hitachi Instrument, Inc., Houston, Tex.). The RNA samples were stored at −80°C or immediately used for the first-strand cDNA synthesis.
(ii) First-strand cDNA synthesis.
The cDNA was synthesized directly from total RNA by using a commercially available kit purchased from CLONTECH Laboratories, Inc. (Palo Alto, Calif.). Briefly, a 20-μl mixture contained 1 μg of total RNA, 20 pmol of oligo(dT)18 primer, 0.5 mM each of the four deoxynucleoside triphosphates, 20 U of RNase inhibitor, Tris buffer (50 mM Tris-HCl [pH 8.3], 75 mM KCl, 3 mM MgCl2), and 200 U of Moloney murine leukemia virus reverse transcriptase were incubated at 42°C for 1 h, followed by 5 min at 95°C to stop the reaction. The final reaction mixture was diluted to a final volume of 100 μl by the addition of 80 μl of diethylpyrocarbonate-treated H2O and stored either at 4°C for use in 2 weeks or at −20°C for a much later usage.
(iii) Amplification of target signals in cDNA by PCR.
The standard PCR was used to amplify the active transcripts of targeted cytokines indirectly from cDNA. The cytokine-specific oligonucleotides, designed to amplify only cDNA, but not genomic DNA, were either custom-made or purchased from Clontech Laboratories, Inc. The sequences of these amplimers and the expected sizes after amplification are summarized in Table 1. For amplification by PCR, 5 μl of the diluted cDNA sample was typically used as the template in a total volume of a 50-μl PCR mixture, containing 0.2 mM each of the four deoxynucleoside triphosphates, 0.4 μM each of 5′- and 3′-end primers, Tris buffer (10 mM Tris-HCl, [pH 8.4], 50 mM KCl, 1.5 mM MgCl2) and 2.0 U of Ampli Taq polymerase (CLONTECH Laboratories, Inc.). A DNA thermocycler (model 480; Perkin-Elmer, Norwalk, Conn.) was set to the following conditions for all the amplimers, (except IL-12 p40): (i) an initial denaturation step for 3 min at 94°C, (ii) 30 cycles of PCR, with 1 cycle consisting of denaturation at 94°C for 45 s, primer annealing at 60°C for 45 s, and elongation at 72°C for 2 min, and (iii) a single final extension step of 7 min at 72°C. To amplify IL-12 p40 sequences, the following PCR conditions were used: (i) 3 cycles with each cycle consisting of denaturation at 94°C for 45 s, annealing at 58°C for 1 min and 15 s, extension at 72°C for 1 min 45 s and (ii) 27 cycles, with each cycle consisting of 94°C for 35 s, 58°C for 45 s, and 72°C for 1 min and 15 s. To visualize the amplified signals, an aliquot of 10 μl of the resulting PCR product was electrophoresed onto 2% agarose gels in Tris-acetate-EDTA buffer at pH 8.0. The HaeIII fragments of φX174 replicative-form DNA (Gibco-BRL, Life Technologies) were included as molecular size markers. After electrophoresis, the gels were stained with ethidium bromide and documented with ImageStore 7500 transilluminator (Ultra Violet Products Inc., Upland, Calif.). The assessment of active glyceraldehyde-3-phosphate dehydrogenase (G3PDH) transcripts was used as a positive control and to calibrate the amount of RNA.
TABLE 1.
Sequences of G3PDH- and cytokine-specific amplimers and expected sizes after amplification
| Specific cytokine | Strain specificitya | Sequence of oligonucleotide | Expected size (bp) after amplification |
|---|---|---|---|
| IFN-γ | + | TGC ATC TTG GCT TTG CAG CTC TTC CTC ATG GC | 365 |
| − | TGG ACC TGT GGG TTG TTG ACC TCA AAC TTG GC | ||
| TNF-α | + | TTC TGT CTA CTG AAC TTC GGG GTG ATC GGT CC | 354 |
| − | GTA TGA GAT AGC AAA TCG GCT GAC GGT GTG GG | ||
| IL-4 | + | CCA GCT AGT TGT CAT CCT GCT CTT CTT TCT CG | 357 |
| − | CAG TGA TGT GGA CTT GGA CTC ATT CAT GGT GC | ||
| IL-10 | + | ATG CAG GAC TTT AAG GGT TAC TTG GGT T | 455 |
| − | ATT TCG GAG AGA GGT ACA AAC GAG GTT T | ||
| IL-12 (p40) | + | CCA CTC ACA TCT GCT GCT CCA CAA G | 266 |
| − | ACT TCT CAT AGT CCC TTT GGT CCA G | ||
| MIP | + | ATG AAG GTC TCC ACC ACT GCC | 279 |
| − | TCA GGC AAT CAG TTC CAG TTC CAG GTC AGT GAT GTA TTC | ||
| G3PDH | + | ACC ACA GTC CAT GCC ATC AC | 450 |
| − | TCC ACC ACC CTG TTG CTG TA |
+, positive-sense strand; −, negative-sense strand.
Statistics.
Unless otherwise indicated, all data were analyzed by using a one-tailed t test with P of <0.05 as the maximal value for statistical significance. All experiments were repeated at least once.
RESULTS
Characterization of the early cytokine profile in the genital tracts of BALB/c mice intravaginally infected with MoPn.
Previously, we demonstrated that a prominent IFN-γ response could be detected in the genital tract as early as 7 days after intravaginal infection with MoPn (2) and that this response did not appear to be related to a CD4 response (2a). However, since these data represented a single time point and since it has become increasingly clear that the early events in the host response to an infection are significant with regard to the nature of the acquired immune response, we wanted to determine when specific cytokines were expressed following intravaginal infection with chlamydiae. A total of 30 mice were intravaginally infected with MoPn, and groups of five mice each were killed at 6, 12, 18, 24, 36, and 48 h after infection. Five age-matched, uninfected mice were also included as the control, which was designated 0 h. Spleen cells derived from healthy uninfected mice were stimulated with concanavalin A and were used as a source of RNA for a positive control for the expression of tumor necrosis factor alpha (TNF-α), macrophage inflammatory protein (MIP), IFN-γ, IL-10, and IL-4. The genital tracts were pooled and digested with collagenase, and the resulting cell pellets were directly used for the total RNA extraction without further enrichment for MNCs. The RNA was used to assess cytokine expression by RT-PCR.
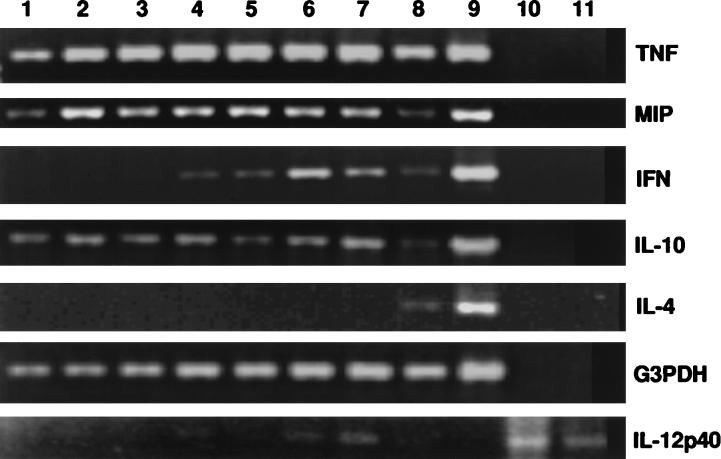
When the kinetics of expression of each individual cytokine were analyzed, it became clear that the expression of various cytokines occurred quite early in the infection process. TNF-α, MIP, and IL-10 were constitutively expressed in uninfected animals (Fig. 1); however, TNF-α and MIP genes appeared to be up-regulated as early as 6 h after infection. IFN-γ expression was first detected 12 h after infection, and the expression of the inducible p40 subunit of IL-12 was detected at 18 h. Since IL-12 has been shown to be an upstream cytokine, which is required for promoting IFN-γ production by NK cells, the 6-h delay in IL-12 expression, compared to IFN-γ expression, was somewhat unexpected, although it is possible that the RT-PCR conditions used in our study to detect IL-12 were not optimal, thereby reducing the sensitivity in detecting specific transcripts. Similar results were obtained when the experiment was repeated. IL-4 was not detected at any time during the 48-h period after infection. Of interest in this experiment was the observation that IFN-γ was expressed as early as 12 h after infection, suggesting the rapid recruitment of cells capable of producing IFN-γ to the local site. The fact that IFN-γ expression occurs so quickly would also suggest that the responsible cell is normally present either in the local site or in the circulation and does not require induction.
FIG. 1.
Kinetics of early cytokine expression in the genital tracts of mice intravaginally infected with MoPn. Total RNAs were extracted from genital tracts of five BALB/c mice at various times after MoPn genital infection. The RT-PCR was used to amplify specific signals of various cytokines and G3PDH (housekeeping gene) with specific primers. The resulting PCR products were resolved after electrophoresis onto a 2% agarose gel. For all the samples except IL-12 p40, lanes 1 to 7, specific signals at 0, 6, 12, 18, 24, 36, and 48 h after infection, respectively; lane 8, concanavalin A-stimulated splenocyte control; lane 9, positive plasmid control; lane 10, negative control; lane 11, empty. For IL-12 p40, lanes 1 to 7, signals at 0, 6, 12, 18, 24, 36, and 48 h after infection, respectively; lane 8, concanavalin A-stimulated control; lane 9, negative control; lanes 10 and 11, positive sample controls.
Determination of NK cell activity in tissues of MoPn-infected mice.
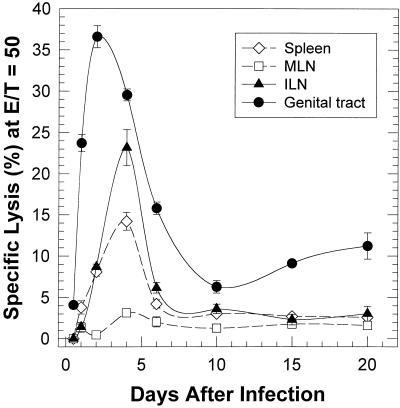
Since NK cells are primary effector cells in the innate immune response and have been implicated as a source of IFN-γ in other infectious disease models, we undertook to determine whether they could be detected in the genital tract and ILN early in chlamydial genital infection as well. Thus, in order to determine the kinetics of the NK response, several groups of mice (10 mice/group; 7 weeks old) were intravaginally infected with 107 IFU of MoPn. MNCs were collected from pooled genital tracts, ILN, mesenteric lymph nodes (MLN), and spleens at 2, 4, 6, 10, 15, and 20 days after infection. When the total number of MNCs derived from genital tracts was determined, it was observed that there was a dramatic increase in MNC number from a baseline count of 105 cells to 2.8 × 106 as early as 2 days after infection, with the peak number 6 days after infection (2.8 × 107).
NK cytolytic activity in each of the MNC preparations was determined by the standard NK cytotoxicity assay by using YAC-1 cells as targets. As shown in Fig. 2, NK activity in the genital tract, as judged by YAC-1 cell cytotoxicity, increased over the baseline as early as 12 h after infection. Because of the low cell recovery, we were able to evaluate the NK activity in the genital tracts and ILNs only at 12 h after infection at an E/T ratio of 12:1. NK activity continued to increase rapidly in the genital tract with the peak response occurring 2 days after infection. However, by 10 days after the infection, NK activity had returned to lower, albeit still positive levels. The NK responses in both the ILN and the spleen followed similar kinetics but reached peak levels about 1 day later than cells from the genital track. While the peak response in each tissue was significantly higher than the baseline levels (P < 0.0001 by two-way analysis of variance), the maximum response in the ILN and the spleen was lower than that of the genital tract. Although NK activity was readily detectable in the genital tract, ILN, and spleen, only a minimal NK response was observed in the MLN, indicating that events necessary to activate the NK response did not occur in the MLN. In particular, chlamydiae or chlamydial antigen may not have reached the MLN at this early stage of infection.
FIG. 2.
Kinetics of NK cell activity in mice intravaginally infected with MoPn. The NK cell activity in the MNCs derived from spleens, MLNs, ILNs, and genital tracts of infected mice were evaluated in vitro by a standard 4-h chromium assay, using 51Cr-labeled NK-sensitive YAC-1 cells as target cells, at an E/T ratio of 50 to 1. The spontaneous release of the labeled YAC-1 cells never exceeded 10% and was usually 6 to 7%. Each point indicates the mean of triplicate samples ± standard deviation.
Confirmation of NK cell identity.
While NK cells are routinely characterized with regard to their ability to lyse YAC-1 cells, it was important to confirm that this activity in the genital tract cell suspension was indeed caused by NK cells. Rabbit anti-asialo-GM1 antibody has been successfully used to deplete NK cells, both in vitro and in vivo (6, 12). Thus, to verify the identity of NK cells in BALB/c mice, asialo-GM1-positive cells were selectively depleted from MNC derived from either genital tracts or ILN in vitro by complement-mediated cytotoxicity. Since the expression of asialo-GM1 antigen is not restricted solely to NK cells and may be expressed by other cells, such as T cells, monocytes, and liver cells (12), we therefore determined an appropriate dilution of anti-asialo-GM1 antiserum to deplete NK cells and minimally affect other cells. In addition, because T cells are also present at this time, the extracted cell population was also treated with anti-CD3.
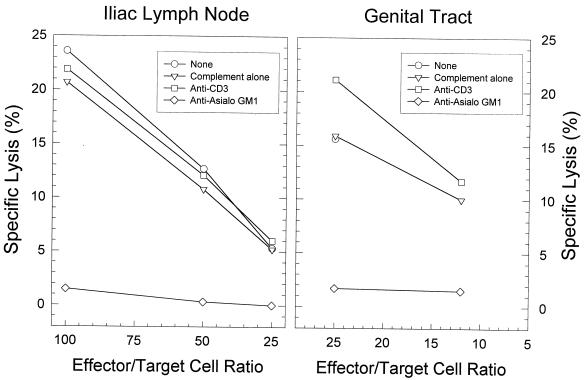
When the MNCs obtained from the genital tract and ILN 4 days after infection were treated with anti-asialo-GM1 and complement, the level of NK cytotoxic activity was significantly reduced compared with that in control cells treated with complement alone or in untreated controls (P < 0.01 according to a one-tailed t test) (Fig. 3). The percentage of MNCs from infected ILN which were CD3 positive remained unaltered (P > 0.05 when assessed by a chi-square test) by in vitro treatment with anti-asialo-GM1 (39.1 ± 0.8) compared to controls treated with complement alone (42.7 ± 1.2). There were insufficient cells from the genital tract to determine the percentage of CD3-positive cells. In contrast, genital tract and ILN MNCs depleted of CD3-positive cells (percentage of CD3+ MNCs, 17.5 ± 1.6; P < 0.001) retained toxicity for YAC-1 cells. These data suggested that the lysis of YAC-1 cells seen with cells derived early after MoPn infection was indeed mediated by NK cells and not by T cells.
FIG. 3.
Elimination of NK cell cytotoxicity for YAC-1 cells in the ILN and genital tract by in vitro treatment with anti-asialo-GM1 but not anti-CD3. Cell suspensions containing NK cells were collected 4 days after genital infection with MoPn.
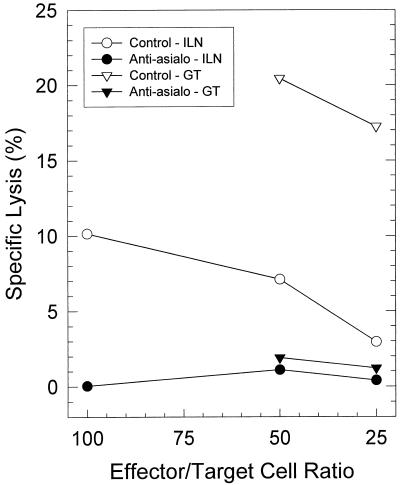
To confirm that NK activity could be removed from mice in vivo by anti-asialo-GM1 treatment, we intraperitoneally injected mice with either a predetermined amount of anti-asialo-GM1 antibody (50 μl) or an equivalent amount of irrelevant rabbit serum at −3, −1, +1, and +3 days after MoPn infection. MNCs were extracted from genital tracts and ILN on day 4 postinfection and assayed for in vitro toxicity against YAC-1 cells. Similar to the in vitro experiments, the percentage of CD3+ cells from infected ILN and genital tracts remained unaffected by in vivo treatment with anti-asialo-GM1 compared to that from untreated controls and in contrast to Mac-1-expressing cells which were significantly reduced (Table 2). Mac-1 has been demonstrated to be present on NK cells. However, as shown in Fig. 4, this treatment resulted in a nearly complete depletion of cytotoxic activity against YAC-1 cells from MNCs derived from the genital tracts and ILNs compared to those of control mice (P < 0.01 by one-tailed t-test) in two independent experiments. Therefore, it is clear that multiple injections of mice with anti-asialo-GM1 antibody can efficiently remove NK cells. These data also indicated that the cytolytic activity against YAC-1 cells was NK cell dependent.
TABLE 2.
Intraperitoneal injection of mice with anti-asialo-GM1 antibody resulted in a significant reduction of Mac-1-positive cells in the cellular infiltrates derived from the genital tracts of MoPn-infected mice
| Cellular source and surface marker | Expt no. | % of positively stained cells in mice:
|
|
|---|---|---|---|
| Control | Anti-asialo-GM1 treated | ||
| ILN | |||
| CD3 | 1 | 51.6 | 57.0 |
| 2 | 42.2 | 50.4 | |
| Mac-1 | 1 | 6.1 | 7.1 |
| 2 | 1.3 | 1.1 | |
| B220 | 1 | 7.0 | 6.8 |
| 2 | 20.0 | 20.4 | |
| Genital tract | |||
| CD3 | 1 | 33.8 | 38.5 |
| 2 | 27.2 | 28.5 | |
| Mac-1 | 1 | 50.7 | 32.9a |
| 2 | 51.6 | 24.7b | |
| B220 | 1 | 11.0 | 8.0 |
| 2 | 3.0 | 7.8 | |
Significantly different from the value obtained for the control (P < 0.025 by chi-square test.
Significantly different from the value obtained for the control mice (P < 0.001 by chi-square test).
FIG. 4.
Elimination of NK cell cytotoxicity for YAC-1 cells in the ILN and genital tract (GT) by in vivo treatment of mice with anti-asialo-GM1. Cell suspensions containing NK cells were collected 4 days after genital infection with MoPn.
NK cells are responsible for the early local IFN-γ response in MoPn-infected mice.
We have shown that a strong IFN-γ response was induced in genital tracts and ILNs of mice within a few days of chlamydial genital infection. Because both T cells and NK cells are potent IFN-γ producers, the identity of the effector cells mediating the early IFN-γ response in MoPn-infected mice was not certain. However, since in a preliminary study, selective depletion of CD4+ T cells did not alter the frequency of IFN-γ-producing cells in genital tracts and ILNs (2a), the role of NK cells in the production of the early local IFN-γ response was evaluated.
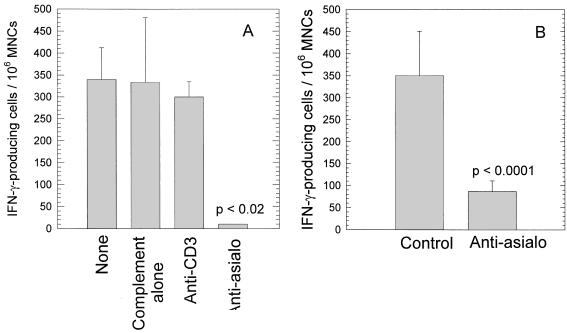
MNCs were extracted from the ILNs of 20 mice at day 4 after MoPn infection, and T cells or NK cells were selectively depleted in vitro by complement-mediated cytotoxicity with specific anti-CD3 antibody or anti-asialo-GM1 rabbit antibody, respectively. The ELISPOT assay for IFN-γ-producing cells was performed on the resulting cell populations. The ILN were chosen rather than the genital tracts because of difficulties in performing the ELISPOT assay on genital tract lymphocytes. In addition, previous data from our laboratory have shown that cellular and molecular events in the genital tract are accurately reflected by the ILN as well. Controls included cells treated with complement alone and cells that were not treated. As shown in Fig. 5, only the population of cells treated with anti-asialo-GM1 had a significantly lower number of IFN-γ-producing cells. Treatment with anti-CD3, while depleting T cells, did not alter the number of cells secreting IFN-γ. Thus, these data strongly suggest that IFN-γ in the ILN early after chlamydial infection is produced by NK cells.
FIG. 5.
Reduction in the number of IFN-γ-producing cells by two treatments as determined by the ELISPOT assay. (A) In vitro treatment of cells from the ILN with anti-asialo-GM1 and complement; (B) in vivo treatment of mice with anti-asialo-GM1. Cells were harvested from the ILN 4 days after infection with MoPn and assessed for IFN-γ-producing cells by the ELISPOT assay.
To further confirm that NK cells are indeed the primary effector cells responsible for the early IFN-γ response in ILN, we also depleted NK cells in vivo before and during the course of chlamydial infection by multiple injections with anti-asialo-GM1 antibody. Age-matched mice receiving an equivalent amount of irrelevant rabbit serum were included as controls. At day 4 after infection, MNCs were extracted from ILN and the total number of IFN-γ-producing cells was determined by the ELISPOT assay. Similar to the in vitro results, animals treated with anti-asialo-GM1 had a marked reduction in the number of IFN-γ-producing cells detected in the ILN (Fig. 5). These results confirmed the results derived from the in vitro depletion study and indicated that NK cells appear to be the major source of IFN-γ early in the course of MoPn genital infection.
Although the cellular response in the ILN reflects the events in the genital tract, it was still important to demonstrate that depletion of NK cells from the genital tract also reduced the level of IFN-γ in this site as well. It has proven difficult to obtain positive results with the ELISPOT assay early in the infection course; therefore, we examined genital tract tissue for the presence of IFN-γ RNA transcripts in animals infected with MoPn and in infected animals treated with anti-asialo-GM1. Because good expression of IFN-γ-specific transcripts was detected in the genital tracts of mice 36 h after infection, we selected this time point to determine the transcription signals of IFN-γ gene in genital tracts. Thus, two groups of five mice each were treated either with anti-asialo-GM1 antibody to deplete NK cells or with irrelevant rabbit serum as a control. At 36 h after infection, genital tracts from five mice of each group were harvested and pooled for preparation of MNCs. Total RNA extracted from the resulting MNCs was processed by RT-PCR. In vivo depletion of NK cells resulted in a lack of IFN-γ expression in genital tracts compared to that in control mice (Fig. 6), thereby supporting the observations in the ILN that NK cells are responsible for the production of IFN-γ early in the course of chlamydial genital infection.
FIG. 6.
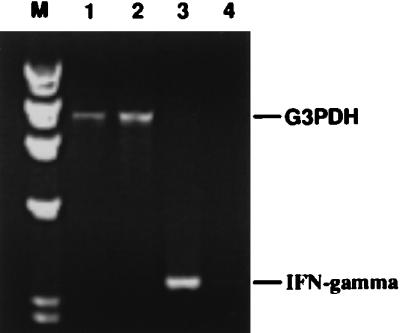
In vivo treatment of mice with anti-asialo-GM1 antibody resulted in a significant reduction in IFN-γ-specific transcripts in the genital tracts of mice intravaginally infected with MoPn. Total RNA was extracted from the genital tracts of mice treated with anti-asialo-GM1 or of untreated mice 36 h after chlamydial infection. RT-PCR was used to amplify signals for IFN-γ and G3PDH (housekeeping gene) by using specific primers. The resulting PCR products were electrophoresed on an agarose gel. Lanes 1 and 2, G3PDH-specific bands of mice with NK cells and NK-depleted mice, respectively; lanes 3 and 4 IFN-γ-specific PCR product for mice with NK cells and NK-depleted mice, respectively; lane M, molecular size markers.
Effect of NK depletion on the course of MoPn genital infection.
IFN-γ produced by Th1 cells has been documented to be important in the resolution of murine chlamydial genital infections; however, as indicated by the above data, IFN-γ is also elaborated by NK cells early in the course of the infection. Since NK cells are present in the tissue so early in the course of infection, it is of interest to determine whether NK cells have an impact on the development of the immune response to chlamydiae and whether they play a role in the control of the early portion of the infection. In order to evaluate the possible contribution to the early host response by NK cells, two groups of animals were infected with 107 IFU of MoPn in the genital tract and were treated either with anti-asialo-GM1 or an irrelevant antibody. The course of the infection was monitored by assessing IFU on cervical swabs at various times after infection. At the end of the experiment, serum was obtained from all mice and the levels of IgG1 and IgG2a were measured to determine the relative Th1 and Th2 responses.
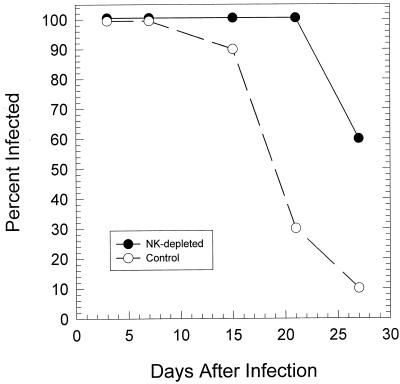
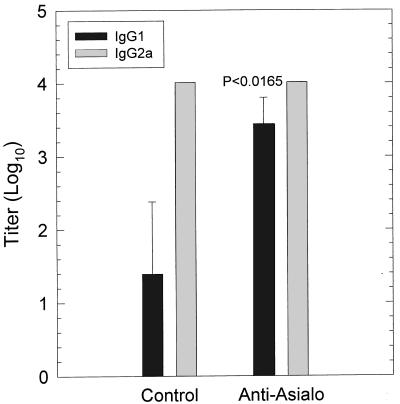
In two separate experiments, there was no significant difference in the number of chlamydial IFU early in the infection (days 3 to 6) (data not shown); however, the infection in anti-asialo-GM1-treated mice was significantly prolonged than in control animals (P < 0.005) (Fig. 7). Interestingly, when IgG1 and IgG2a levels were determined 30 days after infection, there was a significant increase in IgG1 antibody to MoPn in mice treated with anti-asialo-GM1 (P < 0.017) (Fig. 8). These data suggested that depletion of NK cells resulted in a shift from a Th1 dominant response to more of a Th2 response. Thus, it is likely that depletion of NK cells deprived the animal of the early IFN-γ response which is necessary to inhibit the Th2 response. Consequently, the increase in the Th2 response at the expense of the Th1 response resulted in less-efficient clearance of the infection.
FIG. 7.
Course of infection in mice depleted of NK cells by treatment with anti-asialo-GM1. Each point represents the percentage of 10 mice (two separate experiments of 5 mice each) positive for chlamydiae in the genital tract as determined by isolation in tissue culture. The infection was significantly prolonged in NK-depleted mice.
FIG. 8.
Titers of serum IgG1 and IgG2a antibodies to MoPn in mice depleted of NK cells by treatment with anti-asialo-GM1 30 days after intravaginal infection with MoPn. A significant increase in IgG1 was observed in NK-depleted mice.
DISCUSSION
It has become increasingly clear that the early cellular and molecular events after infection not only are important in the regulation of the early infection but also strongly influence the nature of the acquired immune response and the ultimate outcome of the disease. While the critical role of the early events in infection has been documented for some intracellular organisms, there is little information available on the nature of the early events in the genital tract following infection with chlamydiae. Interestingly, during our experiments in which we observed the appearance of IFN-γ-producing cells concomitantly with resolution of infection, we also noted a large number of IFN-γ-producing cells appearing as early as 7 days after infection. Since preliminary experiments suggested that this early IFN-γ response was not mediated by CD4 cells, we hypothesized that the cellular source of the IFN-γ was NK cells.
Initially, we confirmed that IFN-γ, as measured by RT-PCR, was up-regulated beginning as early as 12 to 18 h after intravaginal infection with chlamydiae and that high levels were attained by 36 h after infection. When the NK response was assessed in the genital tract by the YAC-1 cytotoxicity assay, an increase in NK cells in the genital tract was also observed as early as 12 to 24 h after infection, with the peak number being seen 48 to 96 h after infection. Thus, these data demonstrated a strong temporal association between the appearance of NK cells in the genital tract and the up-regulation of the local IFN-γ response. An increase in NK activity was also observed in the ILN and spleen, although it was delayed by several days in comparison to the genital tract. The fact that the killing of the YAC-1 cells was indeed caused by NK cells was confirmed by the abrogation of the cytotoxic response by genital tract and ILN mononuclear cells by the in vitro treatment with rabbit anti-asialo-GM1 and complement but not with anti-CD3 and complement. A similar reduction in YAC-1 cytotoxicity was seen when mice were treated in vivo with anti-asialo-GM1.
In order to confirm that the IFN-γ response was dependent upon the presence of NK cells, MoPn-infected mice were depleted of NK cells by in vitro and in vivo treatment with anti-asialo-GM1. In both experiments, when the number of cells producing IFN-γ was quantified from the ILN by the ELISPOT assay, a significant reduction in number was seen in the groups treated with anti-asialo-GM1. In vitro treatment of ILN cells with anti-CD3 did not alter the number of cells producing IFN-γ. Finally, when the transcription of IFN-γ was determined in genital tract lymphocytes from mice treated with anti-asialo-GM1, a marked decrease in transcripts was also seen. Thus, these data strongly indicate that NK cells trafficking to or within the genital tract as a result of chlamydial genital infection are responsible for the production of IFN-γ early in the infection course.
The appearance of NK cells following chlamydial respiratory infection has been previously documented by Williams et al. (30, 31) when they observed an increase in cytotoxicity for YAC-1 cells by spleen cells 5 days after intranasal inoculation with MoPn. The finding that IFN-γ production by NK cells occurs early in the infection is not surprising in that similar results have been observed in mice infected with MoPn in the respiratory tract (30) and in other murine models of infection (5, 24). Williams et al. (30) also reported that IFN-γ could be detected in lung homogenates of immunologically intact mice as well as the lung homogenates of nude and SCID mice. Treatment of SCID mice with anti-asialo-GM1 was able to reduce the amount of IFN-γ recovered. In murine listeriosis, subcutaneous inoculation of a sublethal dose of Listeria monocytogenes induced the early appearance of IFN-γ-producing NK cells in the draining lymph nodes (5). The peak level of NK response occurred at 24 h after infection. Scharton and Scott (24) also found that NK cells were the source of IFN-γ early in infection of mice with Leishmania major.
Of importance in the current study is the observation that NK cells appear locally very quickly in response to a relatively localized infection in the genital tract and may thus act as a first line of defense with respect to the production of IFN-γ. However, recent studies of MoPn infection in mice deficient in IFN-γ receptors or IFN-γ did not show any increase in the number of organisms early in the infection course but rather demonstrated longer duration of infections in comparison to immunologically intact controls (4, 11). These data coupled with the data in our study would suggest that the primary role of the early IFN-γ production by NK cells is to down-regulate the Th2 response, thereby allowing expression of a strong Th1 response which has been shown to be essential for resolution of the infection in the murine model. However, unfortunately for the host, a number of studies have also suggested that pathologic changes may be associated with the development of a CMI response (15, 21, 28). Thus, the role of NK cells may have both positive and negative consequences.
Finally, since the data presented here strongly indicate that NK cells are responsible for the production of IFN-γ, one would expect that depletion of NK cells would modify the course of MoPn genital infection. Indeed, when mice were depleted of NK cells by treatment with anti-asialo-GM1, the infection was prolonged compared with that in the controls. In addition, assessment of the IgG subclass response to chlamydial antigen indicated a significant increase in IgG1. In contrast, IgG2a was the dominant antibody in untreated mice. Therefore, these data support those of previous studies in the murine model that demonstrated an important role for CD4 Th1 cells in the resolution of chlamydial genital infection (9, 16, 25). It was apparent that depletion of NK cells effected an up-regulation of the Th2 response. With an increase in Th2 cells, and thus, an increase in IL-4 and IL-10 production, one would anticipate a down-regulation of the Th1 response. Although the Th2 response was increased, the Th1 response was probably not totally abrogated, based on the presence of IgG2a. Nevertheless, the data indicate that functional NK cells are necessary for optimal clearance of the infection.
The data presented in this study provide new information on the regulation of the Th1 response to chlamydial genital infection and demonstrate an important role of NK cells similar to what has been described for other infectious systems. They further support the significance of key events occurring in the first 96 h of the infection; i.e., the nature of the acquired immune response which develops is dependent upon these early events. Clearly, a critical event for the initiation of the immune response and the eventual outcome of the infection and disease is the initial cytokine and chemokine profile which is elicited upon infection of the host cell by chlamydiae or the exposure of certain cells to bacterial products. In this regard, Rasmussen et al. (22) have recently demonstrated that infection of endocervical cells by C. trachomatis can evoke the production of both IL-1 and IL-8 which play important roles in the initiation of the inflammatory response. Moreover, Ingalls et al. (10) have shown that chlamydial lipopolysaccharide can elicit the production of TNF-α, which is also intimately involved in a variety of roles in the inflammatory response. Regardless of the stimulus for NK targeting of the genital tract infection, NK cells do indeed play a significant role in the reduction of the level of infection early in the disease and contribute to the development of the protective immune response.
ACKNOWLEDGMENT
This study was supported by grant number AI26328 from the National Institute of Allergy and Infectious Diseases from the National Institutes of Health.
REFERENCES
- 1.Byrne G I, Krueger D A. Lymphokine-mediated inhibition of Chlamydia replication in mouse fibroblasts is neutralized by anti-gamma interferon immunoglobulin. Infect Immun. 1983;42:1152–1158. doi: 10.1128/iai.42.3.1152-1158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cain T K, Rank R G. Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1995;63:1784–1789. doi: 10.1128/iai.63.5.1784-1789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Cain, T. K., and R. G. Rank. Unpublished data.
- 3.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotter T W, Ramsey K H, Miranpuri G S, Poulsen C E, Byrne G I. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun. 1997;65:2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunn P L, North R J. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect Immun. 1991;59:2892–2900. doi: 10.1128/iai.59.9.2892-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godeny E K, Gauntt C J. Murine natural killer cells limit coxsackievirus B3 replication. J Immunol. 1987;139:913–918. [PubMed] [Google Scholar]
- 7.Hauser W E, Sharma S D, Remington J S. Natural killer cells induced by acute and chronic Toxoplasma infection. Cell Immunol. 1982;69:330–346. doi: 10.1016/0008-8749(82)90076-4. [DOI] [PubMed] [Google Scholar]
- 8.Igietseme J U, Magee D M, Williams D M, Rank R G. Role for CD8+ T cells in antichlamydial immunity defined by chlamydia-specific T-lymphocyte clones. Infect Immun. 1994;62:5195–5197. doi: 10.1128/iai.62.11.5195-5197.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Igietseme J U, Ramsey K H, Magee D M, Williams D M, Kincy T J, Rank R G. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific Th1 lymphocyte clone. Reg Immunol. 1993;5:317–324. [PubMed] [Google Scholar]
- 10.Ingalls R R, Rice P A, Qureshi N, Takayama K, Lin J S, Golenbock D T. The inflammatory cytokine response to Chlamydia trachomatis infection is endotoxin mediated. Infect Immun. 1995;63:3125–3130. doi: 10.1128/iai.63.8.3125-3130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson M, Schön K, Ward M, Lycke N. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect Immun. 1997;65:1032–1044. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasai M, Iwamori M, Nagai Y, Okumura K, Tada T. A glycolipid on the surface of mouse natural killer cells. Eur J Immunol. 1980;10:175–180. doi: 10.1002/eji.1830100304. [DOI] [PubMed] [Google Scholar]
- 13.Kelly K A, Robinson E A, Rank R G. Initial route of antigen administration alters the T-cell cytokine profile produced in response to the mouse pneumonitis biovar of Chlamydia trachomatis following genital infection. Infect Immun. 1996;64:4976–4983. doi: 10.1128/iai.64.12.4976-4983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrison R P, Feilzer K, Tumas D B. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4661–4668. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patton D L, Kuo C, Wang S, Halbert S A. Distal tubal obstruction induced by repeated Chlamydia trachomatis salpingeal infection in pig-tailed macaques. J Infect Dis. 1987;155:1292–1299. doi: 10.1093/infdis/155.6.1292. [DOI] [PubMed] [Google Scholar]
- 16.Ramsey K H, Rank R G. Resolution of chlamydial genital infection with antigen-specific T-lymphocyte lines. Infect Immun. 1991;59:925–931. doi: 10.1128/iai.59.3.925-931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramsey K H, Soderberg L S F, Rank R G. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect Immun. 1988;56:1320–1325. doi: 10.1128/iai.56.5.1320-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rank R G. Role of the immune response. In: Barron A L, editor. Microbiology of Chlamydia. Boca Raton, Fla: CRC Press; 1988. pp. 217–234. [Google Scholar]
- 19.Rank R G, Bavoil P M. Prospects for a vaccine against Chlamydia genital disease. 2. Immunity and vaccine development. Bull Inst Pasteur. 1996;94:55–82. [Google Scholar]
- 20.Rank R G, Ramsey K H, Pack E A, Williams D M. Effect of gamma interferon on resolution of murine chlamydial genital infection. Infect Immun. 1992;60:4427–4429. doi: 10.1128/iai.60.10.4427-4429.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rank R G, Sanders M M, Patton D L. Increased incidence of oviduct pathology in the guinea pig after repeat vaginal inoculation with the chlamydial agent of guinea pig inclusion conjunctivitis. J Sex Transm Dis. 1995;22:48–54. doi: 10.1097/00007435-199501000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen S J, Eckmann L, Quayle A J, Shen L, Zhang Y X, Anderson D J, Fierer J, Stephens R S, Kagnoff M F. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Investig. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schachter J. Chlamydiae (psittacosis-lymphogranuloma venereum-trachoma group) In: Lennette E H, Balows A, Hausler W J Jr, Truant J P, editors. Manual of clinical microbiology. 3rd ed. Washington, D.C: American Society for Microbiology; 1980. pp. 357–364. [Google Scholar]
- 24.Scharton T M, Scott P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su H, Caldwell H D. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect Immun. 1995;63:3302–3308. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su H, Feilzer K, Caldwell H D, Morrison R P. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect Immun. 1997;65:1993–1999. doi: 10.1128/iai.65.6.1993-1999.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taguchi T, McGhee J R, Coffman R L, Beagley K W, Eldridge J H, Takatsu K, Kiyono H. Detection of individual mouse splenic T cells producing IFN-gamma and IL-5 using the enzyme-linked immunospot (ELISPOT) assay. J Immunol Methods. 1990;128:65–73. doi: 10.1016/0022-1759(90)90464-7. [DOI] [PubMed] [Google Scholar]
- 28.Tuffrey M, Alexander F, Taylor-Robinson D. Severity of salpingitis in mice after primary and repeated inoculation with a human strain of Chlamydia trachomatis. J Exp Pathol. 1990;71:403–410. [PMC free article] [PubMed] [Google Scholar]
- 29.Williams D M, Byrne G I, Grubbs B, Marshal T J, Schachter J. Role in vivo for gamma interferon in control of pneumonia caused by Chlamydia trachomatis in mice. Infect Immun. 1988;56:3004–3006. doi: 10.1128/iai.56.11.3004-3006.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams D M, Grubbs B G, Schachter J, Magee D M. Gamma interferon levels during Chlamydia trachomatis pneumonia in mice. Infect Immun. 1993;61:3556–3558. doi: 10.1128/iai.61.8.3556-3558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams D M, Schachter J, Grubbs B. Role of natural killer cells in infection with the mouse pneumonitis agent (murine Chlamydia trachomatis) Infect Immun. 1987;55:223–226. doi: 10.1128/iai.55.1.223-226.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]