Abstract
Background
The neutrophil to high-density lipoprotein cholesterol ratio (NHR) is independently associated with the severity of various diseases. However, its association with acute biliary pancreatitis (ABP) remains unknown.
Methods
This study included 1335 eligible patients diagnosed with ABP from April 2016 to December 2022. Patients were divided into low- and high-NHR level groups using an optimal cut-off value determined utilizing Youden’s index. Multivariate logistic regression analysis was used to investigate the correlation between NHR and ABP severity. Multivariate analysis-based limited restricted cubic spline (RCS) method was used to evaluate the nonlinear relationship between NHR and the risk of developing moderate or severe ABP.
Results
In this study, multivariate logistic regression analysis indicated an independent association between NHR and ABP severity (p < .001). The RCS analysis showed a linear correlation between NHR and the risk of developing moderate or severe ABP (P for non-linearity > 0.05), and increased NHR was found to be independently associated with a more severe form of the disease.
Conclusions
Our study suggests that NHR is a simple and practical independent indicator of disease severity, serving as a potential novel predictor for patients with ABP.
Keywords: Acute biliary pancreatitis, neutrophil to high-density lipoprotein cholesterol ratio, disease severity
KEY MESSAGES
This study is the first to report on the independent association between the neutrophil to high-density lipoprotein cholesterol ratio (NHR) and acute biliary pancreatitis (ABP) severity.
The restricted cubic spline analysis showed a linear correlation between NHR and the risk of developing moderate or severe ABP.
Increased NHR levels are independently associated with a more severe degree of the disease.
Introduction
Acute pancreatitis (AP) is a dynamic inflammatory disease with a severity that can change over its course [1,2]. The Revised Atlanta Classification categorizes it into mild (MAP), moderate–severe (MSAP), and severe pancreatitis (SAP) [2]. While AP has a 2% mortality rate, SAP may reach a mortality rate of 15%–20% [3,4]. Accurate and early identification of individuals prone to developing critical illness is crucial, as it enables close monitoring and timely treatment upon admission, ultimately reducing complications and mortality [5].
Acute biliary pancreatitis (ABP) is the most common type of AP, accounting for approximately 40% of AP cases [6] and has a mortality rate ranging from 5% to 20% [7]. Risk factors for ABP include female gender, age over 70 years, and the presence of gallstones smaller than 5 mm [8]. Male gender is associated with severe forms of ABP and high mortality [9]. Pseudocysts, necrosis, mortality, and the need for treating complications are more common in ABP than in other types of AP [10]. Inflammation plays a significant role in the occurrence and development of AP [11]. Haematologic inflammation indicators, such as white blood cell (WBC) count, C-reactive protein, neutrophil–lymphocyte ratio, platelet–lymphocyte ratio, and systemic inflammatory response index, are independently associated with the severity of ABP [12–15]. Additionally, the neutrophil to high-density lipoprotein cholesterol ratio (NHR) is associated with the severity of various diseases, including acute ischaemic stroke, cardiovascular disease, and Parkinson’s disease [16–18]. However, no studies have investigated the association between NHR and ABP severity. This study aimed to address this gap.
Materials and methods
Patients
This study included patients with ABP referred to Yijishan Hospital of Wannan Medical College from April 2016 to December 2022. Exclusion criteria comprised: (1) previous history of pancreatitis, recurrent pancreatitis, or chronic pancreatitis; (2) age under 18; (3) more than 48 h from symptom onset to admission; (4) pregnancy; (5) chronic liver or kidney disorders; (6) autoimmune disease; (7) malignant tumour or blood proliferative disease; (8) use of lipid-lowering drugs within the past 6 months; and (9) incomplete data. Due to the retrospective nature of the study, ethics approval and informed consent from patients were waived by the Scientific Research and New Technology Institutional Review Board of Yijishan Hospital of Wannan Medical College in accordance with national legislation and institutional requirements.
Data collection and outcomes
We collected patient data, including haematological parameters upon admission, comorbidities, and clinical variables. The NHR was calculated as neutrophil count divided by the high-density lipoprotein cholesterol (HDL-C) value [19]. The outcome measure was the severity of ABP based on the Revised Atlanta Classification [2]. In this study, MSAP and SAP were merged into the non-MAP group.
Statistical analysis
Continuous variables were presented as median (25th–75th percentile) or mean ± standard deviation and compared using the Mann–Whitney U test or independent sample t-test. Categorical variables were presented as frequency and percentage and compared using the chi-square or Fisher’s exact tests. The area under the receiver operating characteristic (ROC) curve was used to analyse the predictive accuracy of NHR. The optimal NHR cut-off value was determined using Youden’s index [20]. Based on the optimal cut-off value, patients were categorized into low- and high-NHR level groups. The Delong test was utilized to compare the value of the area under the ROC curve [21]. Univariate logistic analysis was used to identify the associations between variables and ABP severity. Non-significant variables (p > .05) were not included in the multivariate analysis. Multivariate logistic regression was used to determine the independent association between NHR and ABP severity. Odds ratio (OR) with the corresponding 95% confidence intervals (95% CIs) were calculated. Variance inflation factor (VIF) values were calculated to detect the degree of multicollinearity among the variables, with VIF < 10 indicating no multicollinearity [22]. The nonlinear relationship between NHR (as a continuous variable) and the risk of developing non-MAP was measured using restricted cubic spline (RCS) based on multivariate analysis [23]. Furthermore, a prediction model incorporating NHR and other independent indicators for severity evaluation was established. Internal validation was performed by calculating the adjusted Harrell’s concordance index (C-index) utilizing 1000 resamples through the bootstrapping method [24]. A p-value <.05 was considered statistically significant. Statistical analyses were performed using SPSS (version 25.0), R (version 4.0.2), and MedCalc (Version 15.2). Two-sided p-values <.05 were considered statistically significant.
Results
Baseline characteristics
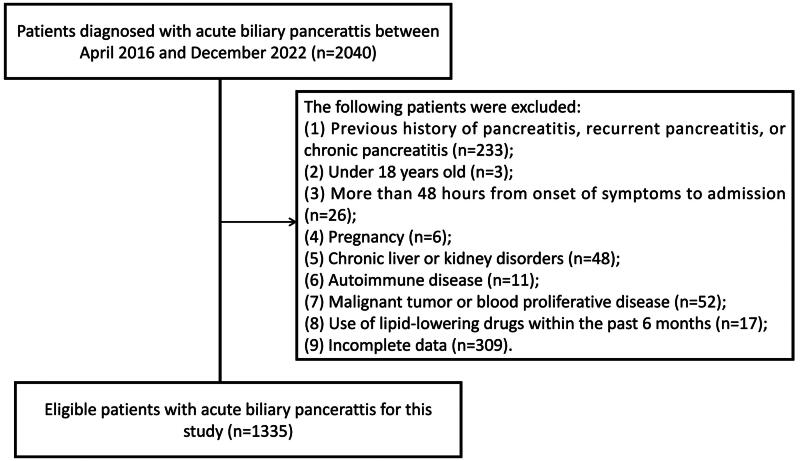
A total of 1335 eligible patients with ABP (1087 with MAP and 248 with non-MAP) were enrolled in this study (Figure 1). Their mean age was 60.8 ± 16.3 years, with males comprising 48.9% (n = 644). The non-MAP group exhibited significantly higher values for WBC, neutrophils, blood glucose (GLU), blood urea nitrogen (BUN), red cell distribution width (RDW), and NHR, while the HDL-C, albumin (ALB), total cholesterol (TC), lymphocytes (LYM), and calcium values were lower compared to the MAP group. No significant differences were observed between the two groups in terms of gender, age, diabetes status, creatinine (Cr), and platelet (PLT) count. The baseline characteristics of patients are summarized in Table 1.
Figure 1.
Flow chart of the patient selection process.
Table 1.
Baseline characteristics of patients with acute biliary pancreatitis.
| Variables | All patients (n = 1335) | MAP (n = 1087) | Non-MAP (n = 248) | p-Value |
|---|---|---|---|---|
| Gender (n, %) | .282 | |||
| Male | 644 (48.2%) | 532 (48.9%) | 112 (45.2%) | |
| Female | 691 (51.8%) | 555 (51.1%) | 136 (54.8%) | |
| Age (years) | 60.8 ± 16.3 | 60.6 ± 16.2 | 61.4 ± 16.7 | .484 |
| Hypertension (n, %) | .030 | |||
| Yes | 475 (35.6%) | 372 (34.2%) | 103 (41.5%) | |
| No | 860 (64.4%) | 715 (65.8%) | 145 (58.5%) | |
| Diabetes (n, %) | .295 | |||
| Yes | 218 (16.3%) | 172 (15.8%) | 46 (18.5%) | |
| No | 1117 (83.7%) | 915 (84.2%) | 202 (81.5%) | |
| ALB (g/L) | 35.6 ± 6.0 | 36.1 ± 5.8 | 33.0 ± 6.2 | <.001 |
| ALT (U/L) | 78.0 (25.0–195.0) | 85.0 (26.0–210.0) | 46.5 (22.0–123.5) | <.001 |
| AST (U/L) | 48.0 (22.0–129.0) | 50.0 (22.0–137.0) | 38.5 (22.0–88.5) | .015 |
| BUN (mmol/L) | 5.3 (3.9–7.2) | 5.2 (3.8–6.8) | 6.3 (4.1–10.1) | <.001 |
| Cr (µmol/L) | 67.6 (54.7–83.2) | 67.8 (54.9–81.2) | 67.4 (51.6–99.1) | .218 |
| GLU (mmol/L) | 6.6 (5.2–8.7) | 6.3 (5.0–8.1) | 7.8 (5.9–10.6) | <.001 |
| TC (mmol/L) | 3.8 (3.1–4.6) | 3.8 (3.3–4.6) | 3.4 (2.6–4.3) | <.001 |
| TG (mmol/L) | 1.0 (0.6–1.6) | 1.0 (0.6–1.6) | 1.1 (0.7–1.6) | .026 |
| HDL-C (mmol/L) | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.1 ± 0.4 | <.001 |
| LDL-C (mmol/L) | 2.0 (1.5–2.6) | 2.1 (1.6–2.6) | 1.7 (1.3–2.4) | <.001 |
| Ca (mmol/L) | 2.0 ± 0.2 | 2.1 ± 0.2 | 1.9 ± 0.3 | <.001 |
| WBC (109/L) | 10.9 (7.3–14.6) | 10.4 (7.0–14.1) | 13.2 (9.8–17.1) | <.001 |
| NEUT (109/L) | 9.0 (5.4–12.8) | 8.5 (4.9–12.3) | 11.8 (8.1–15.3) | <.001 |
| LYM (109/L) | 0.9 (0.7–1.3) | 1.0 (0.7–1.4) | 0.8 (0.6–1.2) | <.001 |
| RDW (%) | 13.3 (12.8–14.0) | 13.2 (12.7–13.9) | 13.6 (12.9–14.3) | <.001 |
| PLT (109/L) | 171.8 ± 67.1 | 171.2 ± 66.4 | 174.6 ± 70.0 | .459 |
| AMY (U/L) | 468.0 (145.0–1122.0) | 456.0 (145.0–1106.0) | 563.5 (140.5–1168.8) | .480 |
| LIP (U/L) | 424.4 (105.0–954.4) | 422.4 (110.5–961.7) | 438.5 (90.9–894.2) | .569 |
| NHR | 7.5 (4.4–11.4) | 6.8 (4.0–10.2) | 11.0 (6.8–15.4) | <.001 |
Data are presented as numbers, mean ± standard deviation, median (25th–75th percentiles), or frequency [percentage (%)].
Abbreviations: MAP: mild acute biliary pancreatitis; non-MAP: moderate and severe acute biliary pancreatitis; ALB: albumin; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BUN: blood urea nitrogen; Cr: creatinine; GLU: glucose; TC: total cholesterol; TG: triglyceride; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; Ca: calcium; WBC: white blood count; NEUT: neutrophil; LYM: lymphocyte; RDW: red cell distribution width; PLT: platelet; AMY: amylase; LIP: lipase; NHR: neutrophil high-density lipoprotein cholesterol ratio.
Baseline characteristics and laboratory data between the low and high groups
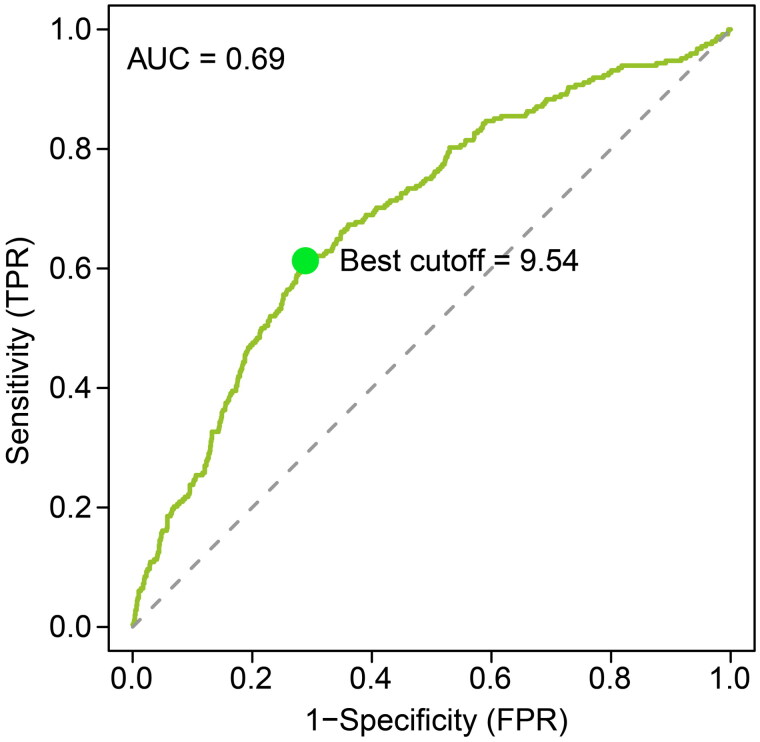
As previously mentioned, based on the optimal cut-off value of NHR (9.54), patients were divided into two groups (Figure 2). The BUN, Cr, GLU, WBC, neutrophil, and RDW values of the high-NHR level group were significantly higher than those of the low-NHR group, while the ALB, TC, HDL-C, and calcium values were significantly lower than those of the low-NHR level group. The proportion of males was significantly higher in the high-NHR level group than in the low-NHR level group. No significant differences were observed in age and diabetes status between the two groups (Table 2). Additionally, ROC analysis revealed that NHR exhibited superior predictive capability compared to WBC, neutrophil–lymphocyte ratio, platelet–lymphocyte ratio, and systemic inflammatory response index (all Delong test p-values <.01, Supplementary Table 1).
Figure 2.
Receiver operating curve of the NHR for prediction of disease severity in patients with acute biliary pancreatitis.
Table 2.
Comparison of characteristics between high- and low-NHR groups.
| Variables | Total (n = 1335) |
||
|---|---|---|---|
| NHR < 9.54 (n = 869) | NHR > 9.54 (n = 466) | p-Value | |
| Gender (n, %) | <.001 | ||
| Male | 384 (44.2%) | 260 (55.8%) | |
| Female | 485 (55.8%) | 206 (44.2%) | |
| Age (years) | 60.6 ± 16.1 | 61.1 ± 16.5 | .540 |
| Hypertension (n, %) | .004 | ||
| Yes | 285 (32.8%) | 190 (40.8%) | |
| No | 584 (67.2%) | 276 (59.2%) | |
| Diabetes (n, %) | .090 | ||
| Yes | 131 (15.1%) | 87 (18.7%) | |
| No | 738 (84.9%) | 379 (81.3%) | |
| ALB (g/L) | 36.7 ± 5.5 | 33.5 ± 6.3 | <.001 |
| ALT (U/L) | 82.0 (24.0–204.0) | 68.5 (26.0–168.5) | .534 |
| AST (U/L) | 50.0 (22.0–133.0) | 42.0 (22.8–116.3) | .265 |
| BUN (mmol/L) | 5.1 (3.8–6.6) | 5.9 (4.2–8.8) | <.001 |
| Cr (µmol/L) | 66.8 (54.9–79.9) | 69.1 (54.1–89.6) | .009 |
| GLU (mmol/L) | 6.2 (4.9–7.8) | 7.4 (5.7–9.9) | <.001 |
| TC (mmol/L) | 3.9 (3.3–4.7) | 3.5 (2.8–4.3) | <.001 |
| TG (mmol/L) | 0.9 (0.5–1.5) | 1.1 (0.8–1.8) | <.001 |
| HDL-C (mmol/L) | 1.3 ± 0.4 | 1.0 ± 0.3 | <.001 |
| LDL-C (mmol/L) | 2.1 (1.6–2.6) | 1.9 (1.4–2.4) | <.001 |
| Ca (mmol/L) | 2.1 ± 0.2 | 2.0 ± 0.2 | <.001 |
| WBC (109/L) | 8.4 (6.1–11.3) | 15.4 (12.7–18.7) | <.001 |
| NEUT (109/L) | 6.6 (4.1–9.5) | 13.8 (11.0–16.7) | <.001 |
| LYM (109/L) | 1.0 (0.7–1.4) | 0.8 (0.6–1.2) | <.001 |
| RDW (%) | 13.2 (12.7–13.8) | 13.5 (13.0–14.2) | <.001 |
| PLT (109/L) | 168.8 ± 62.4 | 177.4 ± 74.7 | .025 |
| AMY (U/L) | 462.0 (156.0–1142.0) | 484.0 (121.8–1025.5) | .262 |
| LIP (U/L) | 428.0 (110.8–997.8) | 411.6 (93.6–852.5) | .161 |
| NHR | 5.3 (3.3–7.3) | 13.5 (11.2–18.3) | <.001 |
Data are presented as numbers, mean ± standard deviation, median (25th–75th percentiles), or frequency [percentage (%)].
Abbreviations: NHR: neutrophil high-density lipoprotein cholesterol ratio; ALB: albumin; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BUN: blood urea nitrogen; Cr: creatinine; GLU: glucose; TC: total cholesterol; TG: triglyceride; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; Ca: calcium; WBC: white blood count; NEUT: neutrophil; LYM: lymphocyte; RDW: red cell distribution width; PLT: platelet; AMY: amylase; LIP: lipase.
High NHR is independently associated with ABP severity
After covariate adjustment, the multivariate logistic analysis indicated that high NHR was independently associated with increased disease severity (OR: 2.026; 95% CI: 1.347–3.049; p < .001) (Table 3). To enhance prediction accuracy, we integrated NHR with other independent indicators, developing a prediction model: −0.340 + 0.090 × BUN + 0.108 × GLU + 0.163 × RDW − 2.577 × calcium + 0.038 × NHR (C-index = 0.761, Hosmer–Lemeshow test: p > .05). Internal validation yielded an adjusted C-index of 0.756, confirming the reliability of the prediction model. ROC curve analysis revealed that the predictive ability of the model surpassed that of NHR alone (Supplementary Table 1).
Table 3.
NHR is independently associated with the severity of patients with acute biliary pancreatitis in multivariate analyses.
| Variables | OR | 95% CI | p-Value |
|---|---|---|---|
| Hypertension | .31 | ||
| No | Ref | ||
| Yes | 0.842 | 0.601–1.173 | |
| ALB (g/L) | 0.974 | 0.946–1.003 | .08 |
| ALT (U/L) | 0.999 | 0.998–1.000 | .14 |
| BUN (mmol/L) | 1.089 | 1.034–1.149 | <.01 |
| Cr (µmol/L) | 1.001 | 0.996–1.004 | .792 |
| GLU (mmol/L) | 1.129 | 1.077–1.185 | <.001 |
| TC (mmol/L) | 0.936 | 0.784–1.101 | .439 |
| LDL-C (mmol/L) | 0.885 | 0.674–1.157 | .374 |
| Ca (mmol/L) | 0.140 | 0.062–0.308 | <.001 |
| WBC (109/L) | 1.003 | 0.969–1.038 | .862 |
| RDW (%) | 1.146 | 1.009–1.296 | .033 |
| NHR | <.001 | ||
| Low | Ref | ||
| High | 2.026 | 1.347–3.049 |
Sex, age, hypertension (no/yes), diabetes mellitus (no/yes), NHR, ALB, ALT, aspartate aminotransferase, BUN, Cr, GLU, TC, triglyceride, LDL-C, Ca, WBC, lymphocyte, RDW, and platelet were included in the univariate logistic analysis. Variables that did not have a significant effect on outcome (p > 0.05) in the univariate analysis were not included in the multivariate logistic analysis.
Abbreviations: ALB: albumin; ALT: alanine aminotransferase; BUN: blood urea nitrogen; Cr: creatinine; GLU: glucose; TC: total cholesterol; LDL-C: low-density lipoprotein cholesterol; Ca: calcium; WBC: white blood cell; RDW: red cell distribution width; NHR: neutrophil high-density lipoprotein cholesterol ratio; OR: odds ratio; CI: confidence interval.
Linear relationship between the NHR and disease severity
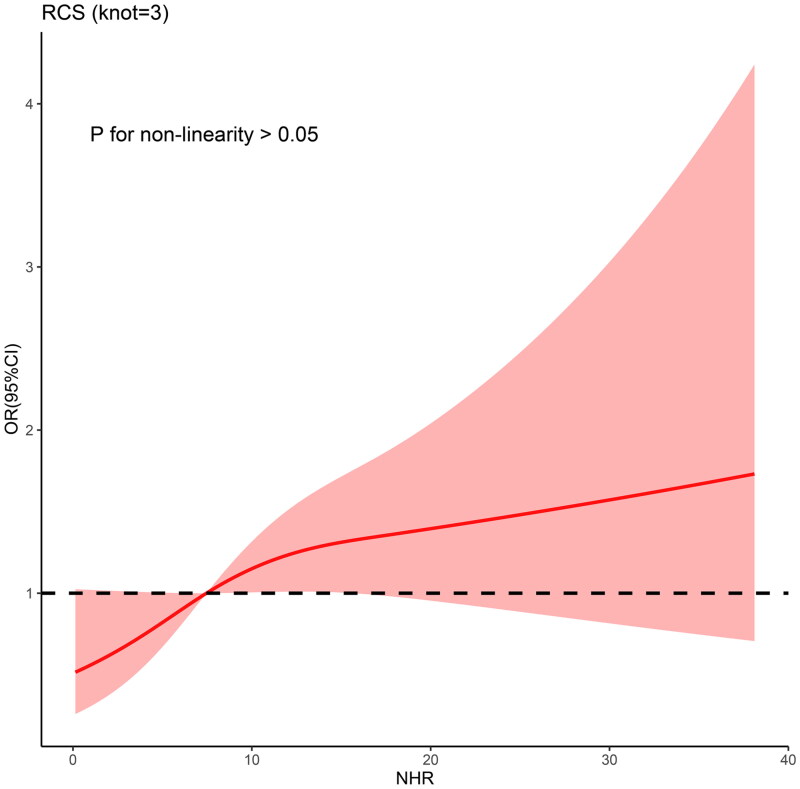
As illustrated in Figure 3, the curve displayed a consistent upward trend with increasing NHR. A linear association was observed between NHR and the risk of developing non-MAP (P for non-linearity > 0.05). NHR was shown to be positively correlated with disease severity, suggesting that the risk of developing non-MAP increased with an increase in NHR.
Figure 3.
Associations of the NHR and the risk of developing moderate or severe acute biliary pancreatitis using restricted cubic spline.
Discussion
In this study, we investigated the association between NHR and ABP severity. Our results revealed that lower NHR levels were independently associated with lower disease severity. Furthermore, we found a linear positive correlation between NHR and the risk of developing non-MAP. NHR is a conventional, inexpensive, and readily available blood indicator and serves as an effective, independent indicator of the severity of various diseases [16–18,25]. Higher NHR values were independently associated with a higher risk of haemorrhagic transformation in patients with acute ischaemic stroke [16] and with cardiovascular risk in healthy individuals [17]. Higher NHR values were also significantly associated with a higher risk of developing metabolic syndrome in the general population [25]. Conversely, lower NHR levels were negatively associated with the severity of Parkinson’s disease [18]. Similarly, our study indicates that a lower NHR is independently associated with a lower severity of ABP. While NHR is recognized for its effectiveness in predicting AP severity in clinical practice, this is the first study to explore its association with ABP severity, to the best of our knowledge.
Neutrophils play a central role in AP development [26], generating pro-inflammatory chemotactic signals and recruiting a large number of inflammatory cells to the site of inflammation. Infiltrated neutrophils act synergistically, producing oxygen-free radicals that contribute to persistent inflammation and local tissue damage [27–31]. Inflammatory signals secreted by pancreatic acinar cells, recruit and stimulate circulating inflammatory cells, including neutrophils [32]. Overstimulation of these inflammatory cells triggers local and systemic inflammatory reactions, such as acute respiratory distress syndrome and renal failure, ultimately resulting in high rates of mortality and morbidity [32]. Gukovskaya et al. demonstrated that neutrophil depletion or inhibition of neutrophil infiltration reduces trypsin activity and tissue damage in AP [33]. Furthermore, neutrophil count can predict the early risk of persistent organ failure in patients with ABP [14]. Regarding HDL-C, some researchers have pointed out that reduced HDL leads to a more severe systemic inflammatory response [34]. During inflammation, HDL-C binds to endotoxins, facilitating their removal from the body [35,36]. Zhang et al. reported that decreased HDL-C in patients with AP represents an impairment of anti-inflammatory function, potentially causing damage to pancreatic acinar cells [37]. As systemic inflammatory response syndrome is a major contributor to organ failure in patients with AP [38,39], HDL-C may prevent disease progression by inhibiting the inflammatory response. Additionally, decreased HDL-C serves as an indicator of organ failure within 48 h of hospitalization in patients with AP and is independently associated with a high risk of persistent organ failure in this pathology [40,41]. Hence, the combination of neutrophils and HDL-C primarily reflects the inflammatory status, potentially predicting the ABP severity.
In this study, we found no correlation between gender and severity. However, the high-NHR level group had a significantly higher proportion of male patients than the low-NHR level group, indicating that male patients with ABP may exhibit higher levels of inflammation compared to females. This aligns with the findings of Shen et al. who reported that gender is not independently associated with organ failure risk in patients with ABP, but males are more likely to experience local complications; these differences between males and females could be attributed to the impact of sex hormones on the inflammatory response [9]. Nevertheless, the specific mechanism underlying this phenomenon warrants further exploration.
This study has certain limitations. First, it is a retrospective, single-centered study, leading to unavoidable selection bias. Validation through prospective multicentre research including a larger number of patients is needed. Second, since ABP is the most common type of AP in China, the usefulness of NHR in predicting the severity of other types of AP remains uncertain. Third, the specific mechanisms underlying the relationship between NHR and ABP severity are still unclear.
Conclusions
In conclusion, our study reveals that NHR is independently associated with ABP severity, suggesting its potential use in identifying patients at high risk of developing non-MAP. These findings hold promise for improving the assessment of disease severity in patients with ABP.
Supplementary Material
Funding Statement
This research received no external funding.
Author contributions
Conceptualization: W.W. Methodology: L.Y. and W.W. Software: L.Y. and W.W. Validation: C.Y.H. and W.W. Formal analysis: L.Y. and W.W. Investigation: L.Y. and W.W. Resources: C.Y.H. Data curation: L.Y. and W.W. Writing – original draft preparation: L.Y. Writing – review and editing: C.Y.H. and W.W. Visualization: W.W. Supervision: W.W. and C.Y.H. Project administration: W.W. and L.Y. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
The authors report no conflict of interest.
Data availability statement
The data presented in this study are available on request from the corresponding author, upon reasonable request.
References
- 1.Sui Y, Zhao Z, Zhang Y, et al. Fibrinogen-like protein 1 as a predictive marker for the incidence of severe acute pancreatitis and infectious pancreatic necrosis. Medicina. 2022;58(12):1. doi: 10.3390/medicina58121753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis – 2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–8. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 3.Johnson CD, Besselink MG, Carter R.. Acute pancreatitis. BMJ. 2014;349:g4859. doi: 10.1136/bmj.g4859. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Zheng R, Gao F, et al. Association between high-density lipoprotein cholesterol and apolipoprotein A-I and severe acute pancreatitis: a case-control study. Eur J Gastroenterol Hepatol. 2021;33(12):1517–1523. doi: 10.1097/MEG.0000000000002095. [DOI] [PubMed] [Google Scholar]
- 5.Hong W-D, Chen X-R, Jin S-Q, et al. Use of an artificial neural network to predict persistent organ failure in patients with acute pancreatitis. Clinics. 2013;68(1):27–31. doi: 10.6061/clinics/2013(01)rc01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frossard J-L, Steer ML, Pastor CM.. Acute pancreatitis. Lancet. 2008;371(9607):143–152. doi: 10.1016/S0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- 7.Guo X, Li Y, Lin H, et al. A nomogram for clinical estimation of acute biliary pancreatitis risk among patients with symptomatic gallstones: a retrospective case-control study. Front Cell Infect Microbiol. 2022;12:935927. doi: 10.3389/fcimb.2022.935927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bougard M, Barbier L, Godart B, et al. Management of biliary acute pancreatitis. J Visc Surg. 2019;156(2):113–125. doi: 10.1016/j.jviscsurg.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Shen H-N, Wang W-C, Lu C-L, et al. Effects of gender on severity, management and outcome in acute biliary pancreatitis. PLoS One. 2013;8(2):e57504. doi: 10.1371/journal.pone.0057504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamal A, Akshintala VS, Kamal MM, et al. Does etiology of pancreatitis matter? Differences in outcomes among patients with post-endoscopic retrograde cholangiopancreatography, acute biliary, and alcoholic pancreatitis. Pancreas. 2019;48(4):574–578. doi: 10.1097/MPA.0000000000001283. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz-Moormann P, Schwerk W, Sinn P.. Histological alterations of the preampullary common bile and pancreatic duct in acute biliary and nonbiliary pancreatitis. Digestion. 1986;34(2):93–100. doi: 10.1159/000199316. [DOI] [PubMed] [Google Scholar]
- 12.Tang D, Gu J, Ao Y, et al. Clinical efficacy of endoscopic retrograde cholangiopancreatography in the treatment of acute biliary pancreatitis: a meta-analysis. Wideochir Inne Tech Maloinwazyjne. 2022;17(4):561–578. doi: 10.5114/wiitm.2022.119902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu Q, Pan X, Cao Y, et al. Clinical evaluation of continuous renal replacement therapy in combination with ultrasound-guided percutaneous transhepatic gallbladder drainage for acute severe biliary pancreatitis: a retrospective study. Kidney Blood Press Res. 2017;42(6):1023–1032. doi: 10.1159/000485437. [DOI] [PubMed] [Google Scholar]
- 14.Tang J, Chen T, Ni W, et al. Dynamic nomogram for persistent organ failure in acute biliary pancreatitis: development and validation in a retrospective study. Dig Liver Dis. 2022;54(6):805–811. doi: 10.1016/j.dld.2021.06.033. [DOI] [PubMed] [Google Scholar]
- 15.Silva-Vaz P, Abrantes AM, Morgado-Nunes S, et al. Evaluation of prognostic factors of severity in acute biliary pancreatitis. Int J Mol Sci. 2020;21(12):4300. doi: 10.3390/ijms21124300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang R, Jin F, Zheng L, et al. Neutrophil to high-density lipoprotein ratio is associated with hemorrhagic transformation in patients with acute ischemic stroke. J Inflamm Res. 2022;15:6073–6085. doi: 10.2147/JIR.S381036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan X, Zhang X, Ban J, et al. Association of neutrophil to high-density lipoprotein cholesterol ratio with cardiac ultrasound parameters and cardiovascular risk: a cross-sectional study based on healthy populations. J Inflamm Res. 2023;16:1853–1865. doi: 10.2147/JIR.S406102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Z, Fan Q, Wu S, et al. Compared with the monocyte to high-density lipoprotein ratio (MHR) and the neutrophil to lymphocyte ratio (NLR), the neutrophil to high-density lipoprotein ratio (NHR) is more valuable for assessing the inflammatory process in Parkinson’s disease. Lipids Health Dis. 2021;20(1):35. doi: 10.1186/s12944-021-01462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi K, Hou J, Zhang Q, et al. Neutrophil-to-high-density-lipoprotein-cholesterol ratio and mortality among patients with hepatocellular carcinoma. Front Nutr. 2023;10:1127913. doi: 10.3389/fnut.2023.1127913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walter SD. The partial area under the summary ROC curve. Stat Med. 2005;24(13):2025–2040. doi: 10.1002/sim.2103. [DOI] [PubMed] [Google Scholar]
- 21.DeLong ER, DeLong DM, Clarke-Pearso DL.. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 22.Liao Y, Yin G, Fan X.. The positive lymph node ratio predicts survival in T1–4N1–3M0 non-small cell lung cancer: a nomogram using the SEER database. Front Oncol. 2020;10:1356. doi: 10.3389/fonc.2020.01356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrell FE, Lee KL, Pollock BG.. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988;80(15):1198–1202. doi: 10.1093/jnci/80.15.1198. [DOI] [PubMed] [Google Scholar]
- 24.Tong C, Wang W, He C.. m1A methylation modification patterns and metabolic characteristics in hepatocellular carcinoma. BMC Gastroenterol. 2022;22(1):93. doi: 10.1186/s12876-022-02160-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen T, Chen H, Xiao H, et al. Comparison of the value of neutrophil to high-density lipoprotein cholesterol ratio and lymphocyte to high-density lipoprotein cholesterol ratio for predicting metabolic syndrome among a population in the Southern Coast of China. Diabetes Metab Syndr Obes. 2020;13:597–605. doi: 10.2147/DMSO.S238990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue J, Sharma V, Habtezion A.. Immune cells and immune-based therapy in pancreatitis. Immunol Res. 2014;58(2–3):378–386. doi: 10.1007/s12026-014-8504-5. [DOI] [PubMed] [Google Scholar]
- 27.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6(3):173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Wu W, Dong L, et al. Neutrophil to lymphocyte ratio predicts persistent organ failure and in-hospital mortality in an Asian Chinese population of acute pancreatitis. Medicine. 2016;95(37):e4746. doi: 10.1097/MD.0000000000004746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamed M, Reza G, Mona P, et al. Investigating the relationship between the severity of coronary artery disease and inflammatory factors of MHR, PHR, NHR, and IL-25. Med J Islam Repub Iran. 2021;35:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong W, He Y, Bao H, et al. Diagnostic value of neutrophil-lymphocyte ratio for predicting the severity of acute pancreatitis: a meta-analysis. Dis Markers. 2020;2020:9731854. doi: 10.1155/2020/9731854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soehnlein O, Lindbom L.. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10(6):427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 32.Yang Z-W, Meng X-X, Xu P.. Central role of neutrophil in the pathogenesis of severe acute pancreatitis. J Cell Mol Med. 2015;19(11):2513–2520. doi: 10.1111/jcmm.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gukovskaya AS, Vaquero E, Zaninovic V, et al. Neutrophils and NADPH oxidase mediate intrapancreatic trypsin activation in murine experimental acute pancreatitis. Gastroenterology. 2002;122(4):974–984. doi: 10.1053/gast.2002.32409. [DOI] [PubMed] [Google Scholar]
- 34.Murphy AJ, Woollard KJ.. High-density lipoprotein: a potent inhibitor of inflammation. Clin Exp Pharmacol Physiol. 2009;37(7):710–718. doi: 10.1111/j.1440-1681.2009.05338.x. [DOI] [PubMed] [Google Scholar]
- 35.Ni Q, Yu Z, Zhang P, et al. High-density lipoprotein cholesterol level as an independent protective factor against aggravation of acute pancreatitis: a case–control study. Front Endocrinol. 2023;14:1077267. doi: 10.3389/fendo.2023.1077267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Catapano AL, Pirillo A, Bonacina F, et al. HDL in innate and adaptive immunity. Cardiovasc Res. 2014;103(3):372–383. doi: 10.1093/cvr/cvu150. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Guo F, Li S, et al. Decreased high density lipoprotein cholesterol is an independent predictor for persistent organ failure, pancreatic necrosis and mortality in acute pancreatitis. Sci Rep. 2017;7(1):8064. doi: 10.1038/s41598-017-06618-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh VK, Wu BU, Bollen TL, et al. Early systemic inflammatory response syndrome is associated with severe acute pancreatitis. Clin Gastroenterol Hepatol. 2009;7(11):1247–1251. doi: 10.1016/j.cgh.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 39.Sharma D, Jakkampudi A, Reddy R, et al. Association of systemic inflammatory and anti-inflammatory responses with adverse outcomes in acute pancreatitis: preliminary results of an ongoing study. Dig Dis Sci. 2017;62(12):3468–3478. doi: 10.1007/s10620-017-4813-6. [DOI] [PubMed] [Google Scholar]
- 40.Venegas-Tamayo AR, Peña-Veites OM, Hernández-González MA, et al. Decreased HDL-C levels as a predictor of organ failure in acute pancreatitis in the emergency department. Life. 2023;13(7):1602. doi: 10.3390/life13071602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong W, Lin S, Zippi M, et al. High-density lipoprotein cholesterol, blood urea nitrogen, and serum creatinine can predict severe acute pancreatitis. Biomed Res Int. 2017;2017:1648385–1648387. doi: 10.1155/2017/1648385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author, upon reasonable request.