Abstract
During infection of the gastrointestinal tract, salmonellae induce cytokine production and inflammatory responses which are believed to mediate tissue damage in the host. In a previous study, we reported that salmonellae possess the ability to stimulate tumor necrosis factor alpha (TNF-α) accumulation in primary human monocytes, as well as in the human promonocytic cell line U38. In this model system, cytokine upregulation is not due to lipopolysaccharide but is mediated by a released protein. In the present study, TnphoA transposon mutagenesis was used to identify the TNF-α-inducing factor. A mutant Salmonella strain which lacks the ability to induce TNF-α was isolated from a TnphoA library. Genetic analysis of this mutant demonstrated that the hns gene has been interrupted by transposon insertion. The hns gene product is a DNA-binding protein that regulates the expression of a variety of unrelated genes in salmonellae. One of the known targets of histone-like protein H1 is flhDC, the master operon which is absolutely required for flagellar expression. Analysis of other nonflagellated mutant Salmonella strains revealed a correlation between the ability to induce TNF-α and the expression of the phase 1 filament subunit protein FliC. Complementation experiments demonstrated that FliC is sufficient to restore the ability of nonflagellated mutant Salmonella strains to upregulate TNF-α, whereas the phase 2 protein FljB appears to complement to a lesser extent. In addition, Salmonella FliC can confer the TNF-α-inducing phenotype on Escherichia coli, which otherwise lacks the activity. Furthermore, assembly of FliC into complete flagellar structures may not be required for induction of TNF-α.
Salmonellae are gram-negative, facultatively intracellular pathogens of the Enterobacteriaceae family. Depending on the serotype, Salmonella infection can result in clinical syndromes ranging from gastroenteritis to bacteremia and enteric fever. After oral ingestion, salmonellae rapidly reach the bowel and penetrate the intestinal mucosa through M cells (16). By invading the underlying macrophages, salmonellae spread to the mesenteric lymph nodes and, in severe cases, can reach the circulatory system. The ability to invade and survive within macrophages may be an important step in Salmonella pathogenesis (58). Significant tissue damage has been observed during Salmonella infection of murine ligated intestinal loops (3, 4). This process is believed to be mediated by the upregulation of tumor necrosis factor alpha (TNF-α) production by macrophages. The ability of salmonellae to induce TNF-α is well documented (28, 29). In vivo, low levels of TNF-α have been shown to protect mice from Salmonella infection (39, 40), whereas elevated doses cause histopathology and symptoms of septic shock that can be fatal to the host (9, 52).
TNF-α is a proinflammatory cytokine that plays an important role in mechanisms of host defense against intracellular pathogens. Several bacterial components have been described that can affect the levels of TNF-α expression (6, 30, 55). These components are usually secreted or surface exposed but are not necessarily proteins. One of the most potent known inducers of TNF-α is lipopolysaccharide (LPS), which is a major component of the outer membrane of gram-negative bacteria. In the case of salmonellae, outer membrane porins (OMPs) have also been shown to induce TNF-α in human monocytes (17). In contrast, Yersinia strains have evolved mechanisms to downregulate TNF-α production (8), thus increasing their chances of survival in a hostile environment.
Work previously done in our laboratory has discovered a novel mechanism by which salmonellae can activate TNF-α production. Our data showed that Salmonella species can upregulate TNF-α in primary monocytes, as well as in human promonocytic cells, through a released polypeptide(s). This inducer, which is distinct from LPS and the OmpR-regulated OMPs, is not present, or perhaps not active, in several other gram-negative species (12). Partial biochemical characterization revealed that the TNF-α inducer is trypsin sensitive, heat stable, and resistant to urea denaturation and low pH. Two peaks of activity were observed by gel filtration chromatography, corresponding to molecular masses of 110 and 150 kDa.
In the present study, we identified the Salmonella protein responsible for TNF-α induction by using a transposon mutant that lost the ability to cause TNF-α accumulation in human promonocytic cells. Genetic characterization of this mutant provided evidence which points to flagellin as the released TNF-α-inducing polypeptide.
MATERIALS AND METHODS
Cells.
The U38 cell line is a derivative of the human promonocytic line U937 that is stably transfected with a human immunodeficiency virus (HIV) long terminal repeat-chloramphenicol acetyltransferase reporter construct (15). U38 cells were routinely grown in RPMI 1640 medium supplemented with 10% fetal bovine serum and 50-μg/ml gentamicin (complete RPMI) at 37°C in a 5% CO2 atmosphere. U38 cells were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program (Rockville, Md.). Peripheral blood monocytic cells (PBMC) were isolated from healthy human donors. Heparinized whole blood was layered over isolymph at a 3:2 ratio and allowed to stand for 1 h. The buffy coat (top layer) was removed and layered again over isolymph at a 4:3 ratio. The tube was centrifuged at 1,200 × g for 30 min at room temperature, and the top layer containing mononuclear cells was diluted in phosphate-buffered saline (PBS; pH 7.2). The cells were pelleted by centrifugation, and the cell pellet was resuspended in serum-free RPMI. The PBMC were allowed to adhere to tissue culture plates for 2 h at 37°C in 5% CO2. The plates were washed to remove nonadherent cells. The adherent cells were incubated in complete RPMI overnight at 37°C in 5% CO2. To induce LPS tolerance, PBMC were incubated overnight in complete RPMI containing 1-μg/ml Salmonella LPS (Sigma, St. Louis, Mo.).
Bacteria.
The bacterial strains used in this study are described in Table 1. Salmonella typhimurium SJW1103, SJW86, SJW134, SJW1368, SJW2149, and MY605 were generously provided by Robert Macnab. MY605 was always grown at 42°C (nonpermissive temperature). Salmonella enteritidis CDC5str (CD5) was kindly provided by Virginia Miller. S. typhimurium CJD359 and SL1344 were donated by Charles Dorman. The r− m+ Salmonella strain χ3179, which was used as an intermediate strain, was obtained from Roy Curtiss. Escherichia coli CL447 was a gift of Bruce Stocker, and strain SM10λpir was donated by John Mekalanos. Stocks of all strains were stored at −70°C in 25% (vol/vol) glycerol. Bacterial cultures were grown in Luria-Bertani (LB) medium at 37°C on a roller drum. Antibiotics were used at the following concentrations: kanamycin, 45 μg/ml; streptomycin, 100 μg/ml; chloramphenicol, 25 μg/ml. Salmonella strains SJW86 and χ3179 were made competent by the CaCl2 method as previously described (53).
TABLE 1.
Bacterial strains used in this study
| Species and strain | Parent strain | Relevant genotype or phenotype | Reference(s) or source |
|---|---|---|---|
| S. enteritidis | 48 | ||
| CDC5str | CDC5 | Streptomycin resistance | |
| FC32 | CDC5str | hns::TnphoA | This study |
| S. typhimurium | |||
| SL1344 | Wild type | 21 | |
| CJD359 | SL1344 | ompR::Tn10 | 14 |
| SJW1103 | H1-i ΔH2 | 59 | |
| SJW86 | SJW1103 | fliC::Tn10 | R. M. Macnab |
| SJW134 | SJW806 | ΔfliC ΔfljB | 57 |
| SJW1368 | SJW1103 | ΔflhDC | 31, 44 |
| SJW2149 | SJW1103 | ΔfliD | 23 |
| MY605 (MY652) | LT2 | ts flhD | 27; R. M. Macnab |
| χ3179 | LT2 | r−LT m+LT r−SA m+SA r−SB m+SB | 11 |
| E. coli | 60 | ||
| CL447 | C600hag | ΔfliC | |
| SM10λpir | thi thr leu tonA lacY supE recA::RP4− 2Tc::Mu λ pir R6K | 35 |
Preparation of bacterial CM and stimulation of U38 cells.
Overnight bacterial cultures were centrifuged at 5,000 × g for 10 min to pellet bacteria, and the conditioned medium (CM) was sterilized by passage through a 0.2-μm-pore-size Millipore filter. CM were stored at 4°C. When used to stimulate U38 cells, CM was diluted in complete RPMI and added to the cells at the dilutions indicated in each figure legend. All incubations were allowed to proceed for 4 h at 37°C in 5% CO2. At this time, Triton X-100 was added to each sample to a final concentration of 0.5%. Samples were incubated on ice for 5 min and centrifuged for 10 min at 16,000 × g and 4°C to pellet cell debris. In most experiments, the supernatants were frozen at −20°C before being assayed for TNF-α. This protocol served to measure the total levels of TNF-α (soluble and cell associated) in each sample.
ELISA for TNF-α.
The total amount of TNF-α in each sample was determined by using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Cistron, Pine Brook, N.J.). Aliquots (100 μl) of each Triton X-100-treated cell lysate sample were used in the assay in accordance with the manufacturer’s protocol.
Plasmids.
The fliC gene from Salmonella strain CD5 was amplified by PCR using primers that would include the entire gene (5′-CTGGATCCGCATAAAGCGGCTATTTCGCAGGCTAAG-3′ and 5′-CCAAGCTTCAATCGCCGGATTAACGCAGTAAAGAGAG-3′) under the following conditions: 94°C for 1 min, 55°C for 1 min, and 72°C for 3 min for 30 cycles. The 1.6-kb PCR product was gel purified, ligated to generate concatemers, digested with BamHI and HindIII, and cloned into the corresponding sites of pACYC184. The resulting plasmid was called pFW10. The fljB gene was amplified from Salmonella strain SL1344 by using another set of primers (5′-AAGCTAGCGCCTCAAGTGTCGATAACC-3′ and 5′-AAGAGCCCCGAATTCACGGGGCTGAAT-3′). The conditions used were 94°C for 1 min, 51°C for 1 min, and 72°C for 3 min for 30 cycles. The 1.6-kb product was treated as described for fliC, but it was digested with BanII and NheI and cloned into the BanII and XbaI sites of pACYC184. This plasmid was called pFW100. Suicide plasmid pRT733 (a derivative of pJM703.1 [35]) has been used to introduce TnphoA into gram-negative bacteria (51).
TnphoA library and cloning.
TnphoA is a Tn5 derivative containing a truncated form of the alkaline phosphatase gene from E. coli (phoA). When inserted in frame with the signal sequence of a secreted or membrane protein, functional phoA is expressed from the transposon. PhoA activity can then be detected by using the chromogenic substrate 5-bromo-4-chloro-3-indolylphosphate. The TnphoA library of mutants was generated as previously described (48). Exconjugants were selected on LB agar containing kanamycin, streptomycin, and 5-bromo-4-chloro-3-indolylphosphate at 40 μg/ml. Dark blue colonies were isolated, and their CM was tested on U38 cells at a 1:40 dilution for the ability to induce TNF-α. The site of transposition in FC32 was determined by digestion of total chromosomal DNA with SalI and size fractionation on a 0.5% agarose gel. A 100-ng sample of DNA fragments ranging in size from 4 to 8 kb was ligated and diluted 1:100. Primers complementary to the 5′ end of phoA (5′-GTGCAGTAATATCGCCC-3′) and the 3′ end of the kanamycin gene (5′-GACATAGCGTTGGCTACCCG-3′), respectively, were used to amplify the region between the ends of the transposon from 1 ng of DNA. The Expand system (Boehringer Mannheim, Indianapolis, Ind.) was used. The linear product obtained was then sequenced by using the same primers in the DNA sequencing core facility of the Comprehensive Cancer Center of Wake Forest University. The sequences obtained were used to search the GenBank database (Los Alamos, N.Mex.).
Southern blotting.
Southern blotting was performed by using the Genius System (Boehringer Mannheim) in accordance with the manufacturer’s protocol. To isolate genomic DNA from FC32, 30 μl of 10% sodium dodecyl sulfate and 3 μl of proteinase K (20 mg/ml) were added to 567 μl of an overnight culture and the mixture was incubated at 65°C for 1 h. To remove cell debris and denatured proteins, 100 μl of 5 M NaCl and 80 μl of 10% CTAB (hexadecyltrimethylammonium bromide) in 0.7 M NaCl were added and the solution was incubated for an additional 10 min at 65°C. The DNA was then extracted once with chloroform-isoamyl alcohol and twice with phenol-chloroform-isoamyl alcohol. A 1,169-bp BglII fragment of TnphoA was isolated from pRT733 and used as the probe in all of the experiments.
Partial purification of flagella.
Differential centrifugation was used to partially purify flagella from Salmonella strain CD5 as previously described (37). Briefly, bacteria were grown on LB plates for 24 h at 37°C. The culture was then collected into 100 ml of PBS and centrifuged for 15 min at 5,000 × g and 4°C. The bacterial pellet was resuspended in 100 ml of PBS for each 6 g of dry weight and blended in a Sorvall Omnimixer at half speed for 3 min. The sample was then centrifuged at 16,000 × g and 4°C for 15 min to pellet membranes and other cell debris. The supernatant was centrifuged at 40,000 × g and 4°C for 3 h. The pellet was resuspended in 1 ml of LB medium and stored at 4°C. The protein concentration in each sample was determined spectrophotometrically by the method of Bradford (10) with a commercial protein assay solution (Bio-Rad, Hercules, Calif.).
RESULTS
Generation of a TnphoA library in Salmonella strain CD5.
Previous results obtained in our laboratory indicated that salmonellae can induce TNF-α in U38 cells through a released protein (12). To identify this inducer, transposon TnphoA was chosen to generate a library of mutants in S. enteritidis CD5. TnphoA has been used to isolate mutations in exported and membrane proteins. To saturate all of the possible loci for such proteins, 307 PhoA+ clones were isolated from independent matings and the ability of each to induce TNF-α in U38 cells was compared to that of the wild-type CD5 strain. U38 cells were chosen because they do not respond to LPS stimulation (12). Although most of the clones tested induced levels of TNF-α comparable to those of CD5, seven clones showed decreased levels of activity in their CM in the initial screening. Only three of these seven clones failed to reproducibly induce TNF-α in subsequent experiments. The fact that two of these mutants were ampicillin resistant (a marker which is carried on the backbone of pRT733 and is not part of TnphoA) suggested that transposition had resulted in the integration of pRT733 into the chromosome. Because this situation posed a problem in terms of determining the site of transposon insertion, these two mutants were not included in any of the following studies. The remaining clone, named FC32, was chosen for further analysis.
FC32 fails to induce TNF-α in U38 cells.
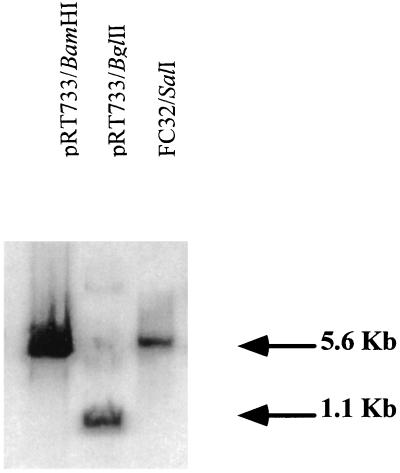
FC32 was repeatedly tested on U38 cells to ensure that the clone had indeed lost TNF-α-inducing activity. When added to U38 cells for 4 h (1:40 dilution), CM from FC32 induced 2% ± 2% of the levels of TNF-α induced by CM from wild-type strain CD5. These results confirmed that FC32 carries a mutation in a gene involved in TNF-α induction. However, the possibility remained that more than one transposition event had occurred in FC32, only one of which was linked to the observed phenotype. To rule out this possibility, Southern blotting was performed with a variety of restriction endonucleases. Regardless of the enzyme used, only a single fragment of FC32 chromosomal DNA hybridized with the TnphoA probe (Fig. 1). In addition, P22 was used to transduce TnphoA into wild-type Salmonella strains CD5 and SL1344. The 100 transductants obtained were screened for kanamycin resistance and PhoA activity. All of them revealed 100% linkage between these two phenotypes. Furthermore, two of these transductants were then chosen at random and their CM was tested on U38 cells for the ability to induce TNF-α. Like FC32, these mutants failed to activate TNF-α (data not shown). Taken together, these results are consistent with the conclusion that only one copy of TnphoA is present in FC32 and that this mutation is responsible for the loss of TNF-α-inducing activity.
FIG. 1.
Southern blot of FC32 chromosomal DNA. A 5-μg sample of DNA was digested with SalI and separated on a 0.5% agarose gel. The DNA was then transferred to a nylon membrane and probed with a 1,169-bp BglII fragment containing transposon TnphoA labeled with the Genius system as described in Materials and Methods. DNA bands were visualized by using the chemiluminescent substrate Lumi-Phos. The sizes of known fragments are shown on the right.
FC32 contains a TnphoA insertion in the hns gene.
To determine the site of TnphoA integration in FC32, chromosomal DNA was digested with SalI and size fractionated. We took advantage of the fact that TnphoA contains a unique SalI site just downstream from the kanamycin resistance gene. The size of the DNA fragment containing the 5′ end of TnphoA generated by SalI restriction was determined by Southern blotting to be approximately 5.5 kb. After digestion with SalI, DNA fragments with sizes ranging between 4 and 8 kb were gel purified and self-ligated to obtain circular products. Primers to the ends of TnphoA were used to amplify the region included between the phoA and kanamycin genes by PCR. The 1-kb linear product was then sequenced with the same two primers, and the nucleotide sequence thus obtained was used to search the GenBank database. The results of this search revealed that TnphoA had transposed 38 bp into the open reading frame of the hns gene, located at 34 min on the Salmonella chromosome (24). TnphoA insertion had generated a premature stop codon in the gene, so that no functional hns gene product could be synthesized in FC32. Sequencing of the PCR product also revealed part of another open reading frame, that of the tdk gene, which is located 600 bp upstream of hns but is transcribed in the opposite direction. This additional piece of information confirmed that TnphoA insertion into FC32 had indeed inactivated the hns gene. The hns gene codes for a transcriptional modulator, histone-like protein H1 (H-NS), which affects the expression of a variety of genes in both E. coli and salmonellae (5, 24). H-NS, however, is itself not an exported protein and does not have a signal sequence. Furthermore, TnphoA insertion into hns occurred out of frame, so that no functional PhoA could be expressed from the hns promoter. It is possible that H-NS, which acts mostly as a repressor, downregulates the endogenous levels of PhoA; this might explain why truncation of H-NS resulted in increased PhoA levels in FC32. Analysis of the linkage map of the Salmonella chromosome revealed that no other gene lies in the same transcriptional unit with hns, so that the phenotype of FC32 was not likely due to a polar effect of TnphoA on a downstream gene.
Nonflagellated Salmonella strains fail to induce TNF-α.
Of the many genes under H-NS control, only a few show decreased expression in H-NS mutants (20). At least two have been studied in E. coli: ompF (which codes for one of the major OMPs) (49) and flhDC (which is the master activator of flagellin expression) (7). We tested the CM from S. typhimurium CJD359, which does not express OmpF because of a mutation in the ompR gene (14), and found that this mutant induced TNF-α to levels comparable to those achieved by the wild-type strain (Table 2). These results suggest that OmpF is not involved in this mechanism of TNF-α activation. We therefore investigated the possible role of flagella in TNF-α induction. Motility assays on soft agar confirmed that, unlike CD5, FC32 is completely nonmotile, but well-defined mutations in flagellar genes were needed to determine whether any of these proteins are responsible for TNF-α induction. To ascertain whether any of the Salmonella flagellar genes are responsible for TNF-α induction in U38 cells, we tested CM from several nonflagellated S. typhimurium strains (Table 1). These four flagellar mutant strains can be divided into two groups based on their flagellar phenotypes: the first two (SJW1368 and MY605) fail to synthesize any of the flagellar proteins, whereas the others (SJW86 and SJW134) have mutations in the gene coding for the main filament subunit, FliC. When tested under standard assay conditions, CM from all these mutants showed a dramatic defect in the ability to induce TNF-α on U38 cells compared to that of wild-type strain SJW1103 (Table 3). Similar results were obtained with human PBMC made LPS tolerant (data not shown). Whereas PBMC responded to CM from wild-type strain SJW1103 with significant TNF-α production, they failed to do so when stimulated with CM from any of the four mutant strains. The fact that loss of fliC in SJW134 and SJW86 is sufficient to cause loss of TNF-α induction suggests that FliC is involved in the process of TNF-α activation in both U38 cells and PBMC.
TABLE 2.
S. typhimurium wild-type and ompR mutant strains induce comparable levels of TNF-α in U38 cells and in PBMC made LPS toleranta
| Salmonella strain | Mean amt (pg) of TNF-α/3 × 106 cells ± SD
|
|
|---|---|---|
| U38 cells | PBMC | |
| SL1344 | 11 ± 2 | 52 ± 8 |
| CJD359 | 14 ± 2 | 39 ± 5 |
U38 cells and PBMC made LPS tolerant were incubated with CM from S. typhimurium wild-type SL1344 or ompR mutant CJD359 at a 1:100 dilution for 4 h. The cells were then lysed, and the amount of TNF-α produced was measured by ELISA. Background TNF-α production in unstimulated cells was below the level of detection. PBMC stimulated with Salmonella LPS (1 μg/ml) failed to express TNF-α to levels above the background.
TABLE 3.
Nonflagellated Salmonella mutants fail to induce TNF-α in U38 cellsa
| Strain whose CM was tested | Mutation(s) | Mean amt (pg) of TNF-α/ 3 × 106 cells ± SD
|
|
|---|---|---|---|
| Expt 1 | Expt 2 | ||
| SJW1103 | 88 ± 16 | 38 ± 3 | |
| SJW86 | fliC, fljB | <1 | <1 |
| SJW134 | fliC, fljB | <1 | <1 |
| MY605 | flhD | <1 | <1 |
| SJW1368 | flhDC | <1 | <1 |
U38 cells (3 × 106 per sample) were incubated with CM from various S. typhimurium strains at a 1:100 dilution. After a 4-h incubation, U38 cells were lysed and the lysates were assayed for TNF-α by ELISA. The results shown are from two independent experiments. Unstimulated U38 cells produced undetectable levels of TNF-α.
The main flagellar subunit protein is responsible for TNF-α induction.
The results obtained with fliC mutants SJW86 and SJW134 provided strong evidence in support of the idea that FliC is the TNF-α inducer. This hypothesis had to be confirmed by introducing fliC into either of these strains to determine if FliC could, by itself, complement the defective phenotype. In studying the effect of FliC, we could not, however, overlook the fact that this is not the only protein that can assemble to form flagellar filaments. Most Salmonella serotypes display phase variation, alternating between the expression of either one of two (and sometimes more) filament subunit proteins (33, 46). Although S. enteritidis is believed to be monophasic (expressing only FliC) (54), S. typhimurium can assemble flagellar filaments composed of either FliC or FljB (50). Deletion of the phase 2 gene fljB in SJW1103 (59) made it impossible to study the effect of endogenous FljB on TNF-α induction in any of its derivative strains. Therefore, a second set of complementation experiments was designed to look at TNF-α activation by FljB. To complement a flagellin mutant with either fliC or fljB, the gene was cloned by PCR from the genomes of wild-type S. enteritidis CD5 (fliC) and S. typhimurium SL1344 (fljB). We chose to clone the two genes from different strains because sequence similarities between the promoter regions of fliC and fljB might have resulted in nonspecific hybridization of the fliC primers to fljB in biphasic strain SL1344. To circumvent this potential problem, fliC was cloned from S. enteritidis CD5, since the phase 2 gene (fljB) is not present in this strain. Each gene was then cloned to include its endogenous promoter, so that the levels of expression from the plasmids would mimic those of the chromosomal genes. fliC and fljB were cloned into pACYC184, a low-copy-number plasmid carrying chloramphenicol resistance, and the resulting constructs were called pFW10 and pFW100. The plasmids were moved into a flagellin mutant (SJW86) by using an r− m+ intermediate Salmonella strain, χ3179. An additional control strain was constructed by introducing pACYC184 (without the insert) into SJW86. The motility of each of these strains was compared to that of wild-type SJW1103 and that of parent strain SJW86. Plasmids pFW10 and pFW100 fully restored the motility of SJW86, whereas the vector alone did not (data not shown). These results confirmed that FliC and FljB expressed from pFW10 and pFW100 are assembled into filaments and fully functional. Next, the CM from each of these strains was used to stimulate U38 cells for 4 h, and the total amount of TNF-α produced by the cells was determined by ELISA. The results of these experiments are shown in Table 4. Consistent with the hypothesis that FliC is directly responsible for TNF-α induction in U38 cells, CM from SJW86(pFW10) (the fliC-reconstituted clone) contained levels of activity comparable to those of wild-type strain SJW1103. Similarly, FljB was able to complement the SJW86 mutant strain, although not to the same degree as FliC. As expected, there was no detectable activity in the CM from SJW86(pACYC184) or in that from SJW86. These results point to FliC and, to a lesser extent, FljB as mediators of TNF-α induction in U38 cells.
TABLE 4.
Effects of FliC and FljB on the ability of flagellin mutants S. typhimurium SJW86 and E. coli CL447 to induce TNF-αa
| Strain whose CM was tested | Complementation | % of wild-type SJW1103 induction of TNF-α | Amt (pg) of TNF-α/3 × 106 cells |
|---|---|---|---|
| S. typhimurium SJW86(pFW10) | fliC | 144 ± 22 | |
| S. typhimurium SJW86(pFW100) | fljB | 49 ± 13 | |
| S. typhimurium SJW86(pACYC184) | 1 ± 1 | ||
| S. typhimurium SJW86 | 1 ± 1 | ||
| E. coli CL447(pFW10) | fliC | 206 ± 16 | |
| E. coli CL447(pFW100) | fljB | 6 ± 2 | |
| E. coli CL447(pACYC184) | <1 | ||
| E. coli CL447 | <1 |
CM from strains SJW86(pFW10), SJW86(pFW100), CL447(pFW10), and CL447(pFW100) was tested for TNF-α-inducing activity on U38 cells. Each CM sample was incubated with 3 × 106 U38 cells at a 1:100 dilution for 4 h. Total TNF-α levels were determined by ELISA. The total amount of TNF-α in each S. typhimurium SJW86 sample is expressed as a percentage of the wild-type SJW1103 level in the corresponding experiment. The results shown are means and standard deviations from three independent experiments. No TNF-α was detected in any of the unstimulated control samples.
E. coli expressing Salmonella FliC induces TNF-α in U38 cells.
The ability of a vector expressing fliC to complement the SJW86 mutant strain confirmed the role of this protein as the main mechanism of TNF-α induction in U38 cells. FliC from E. coli, although closely related to the Salmonella protein, apparently lacks the ability to induce TNF-α in U38 cells (12). We therefore chose E. coli to provide a clean background in which to test the activity of Salmonella FliC in the absence of other Salmonella proteins. FliC− E. coli CL447 was used in these experiments. This strain was transformed with pACYC184, pFW10 (fliC), or pFW100 (fljB), and the motility of the derivative strains was tested on soft agar plates. Only the fliC vector (pFW10) was able to restore the motility of E. coli CL447, whereas the same strain transformed with the fljB vector (pFW100) or pACYC184 was nonmotile. Concurrent with the motility assays, CM from these strains was tested on U38 cells for the presence of TNF-α-inducing activity (Table 4). Consistent with what had been observed with Salmonella strain SJW86(pFW10), E. coli CL447(pFW10) CM was fully capable of activating TNF-α production in U38 cells. Contrary to the data obtained with salmonellae, however, fljB vector pFW100 did not complement fliC mutant E. coli CL447 in terms of motility. We can offer two possible explanations for why pFW100 gave no complementation: either FljB is not expressed in E. coli CL447 (because it requires Salmonella-specific factors that are absent in E. coli) or, perhaps, structural differences between FljB and FliC prevent FljB from being assembled into flagella in CL447 (E. coli is monophasic and normally expresses only FliC). The results obtained with E. coli CL447 confirm the observation made in the complementation experiments in which S. typhimurium SJW86 was used: Salmonella FliC induces TNF-α expression in U38 cells.
Polymerized flagellin and nonpolymerized flagellin induce TNF-α synthesis in U38 cells.
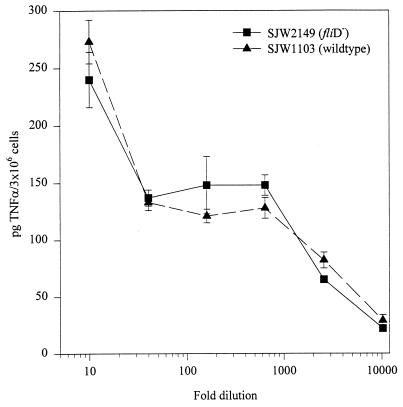
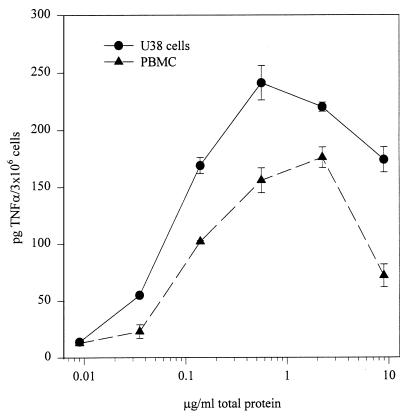
The results of the complementation experiments are consistent with the idea that flagellin is the bacterially released activator of TNF-α in U38 cells. Because the state of aggregation of FliC in active CM is not known, we wanted to determine whether there is a requirement for flagellin monomers to be assembled into filaments to stimulate TNF-α. To answer this question, we first tested CM from S. typhimurium SJW2149, which carries a deletion in the fliD gene. FliD is the filament cap protein, which is located at the distal end of the growing filament and promotes FliC polymerization. In liquid cultures of fliD mutants, FliC is not assembled into filaments and the monomers are released from the bacteria (25). However, when exogenous FliC is added, the local concentrations of FliC may become high enough for a certain degree of productive self-aggregation to be observed (22, 26). Under our test conditions, we expected the former to be the case. As shown in Fig. 2, when CM from this mutant strain was used to stimulate U38 cells, we observed levels of TNF-α induction very similar to those of wild-type strain SJW1103. In a second set of experiments, we partially purified whole flagella from S. enteritidis CD5 and measured their ability to induce TNF-α in U38 cells and LPS-tolerant PBMC. The results of two of these experiments are summarized in Fig. 3. U38 cells and PBMC produced TNF-α in response to the flagellum-enriched preparation in a concentration-dependent manner, with maximum stimulation being observed at a protein concentration of approximately 500 ng/ml. Taken together, these data are consistent with the conclusion that flagellin subunits can activate TNF-α production in U38 cells as part of complete filaments but also when partially unpolymerized.
FIG. 2.
Effect of unpolymerized flagellin subunits on TNF-α activation in U38 cells. Serial dilutions of CM from SJW1103 or SJW2149 (a FliD− strain that fails to polymerize FliC into filaments) were tested on U38 cells as described in Materials and Methods. The amount of TNF-α in the control samples (8 ± 2 pg) has been subtracted from the values shown.
FIG. 3.
Effect of purified flagella on TNF-α expression in U38 cells and LPS-tolerant PBMC. Flagellar structures were isolated from Salmonella strain CD5 and tested on 3 × 106 U38 cells or PBMC at various concentrations. The curves shown are from two representative experiments. Control TNF-α levels were <1 pg/3 × 106 cells in unstimulated U38 cells and 7 ± 1 pg/3 × 106 cells in unstimulated PBMC. TNF-α levels in LPS-stimulated PBMC were 13 ± 4 pg/3 × 106 cells.
DISCUSSION
We describe here a novel mechanism of macrophage activation by salmonellae. The data presented show that Salmonella flagellin, the main subunit of the flagellar filament, can induce high levels of TNF-α production in the human promonocytic cell line U38, as well as in human PBMC. The previous partial characterization of the TNF-α inducer (12) provided data which are consistent with the known biochemical properties of flagellin. In addition, there appears to be no requirement for flagellin to be assembled into filaments to activate TNF-α. Although we cannot completely rule out the possibility that another bacterial component (present in the CM or in the crude flagellar preparations) contributes to TNF-α induction, our genetic data suggest that flagellin is responsible for most, if not all, of this activity. S. typhimurium flagellin expression is subject to phase variation between two different genes, fliC and fljB. The fljB promoter is located within an invertible DNA element (50). In the on orientation, transcription of fljB and of the fliC repressor fljA can proceed. In the off orientation, the fljB operon is not transcribed and FliC is the only subunit protein synthesized. Inversion of the DNA switch is an infrequent event mediated by a site-specific recombinase located within the switch itself. In separate sets of experiments, we have tested the ability of FliC and FljB to complement SJW86, a mutant S. typhimurium strain which does not express either form of the protein. Although SJW86 can be complemented by both genes in terms of motility, fljB does not appear to confer the ability to induce TNF-α to the same levels as fliC. It is possible that this difference in activity is due to inefficient fljB expression in S. typhimurium SJW86. However, the observation that SJW86 complemented with fljB is as motile as SJW86 complemented with fliC suggests that any differences between them in the ability to induce TNF-α expression may, instead, be the result of structural dissimilarities between the two proteins. If this were the case, then the bacteria may have developed this system of phase variation in an effort to optimize colonization while limiting the extent of host cell activation.
Bacterial flagella are very complex in terms of both structure and gene expression (32). The flagellar filament is composed almost entirely of flagellin. The amino and carboxyl termini of flagellin are quite conserved, not only between FliC and FljB, but also in flagellin from different serotypes. In contrast, the central portion of the protein (region IV) is hypervariable and contains most of the antigenic residues (43, 56). Although the possibility that epitopes in this region are the ones involved in TNF-α induction seems attractive, the observation that different Salmonella serotypes possess the activity (12) suggests that there has to be at least a certain degree of conservation in the active epitopes. Additionally, these epitopes would be expected to be exposed in monomeric flagellin and in assembled flagellin, since both forms of the protein show activity on U38 cells.
Another interesting characteristic of Salmonella flagellin is the presence of ɛ-N-methylated lysine residues (1). This modification is not necessary for motility (33), but because it is not a characteristic of eukaryotic proteins, its effect on the immune system may be considerable. The observation that FljB does not restore the TNF-α-inducing phenotype to the same degree as FliC does tends to argue against a role for methylated lysine residues in TNF-α activation. However, the difference in activity between FliC and FljB could be due to the extent to which each protein is methylated. Another way to approach this issue consists of expressing flagellin in a strain that lacks this type of modification. To our knowledge, flagellin lysine residues are not modified in E. coli, but when Salmonella fliC was introduced into the E. coli fliC mutant CL447 (Table 4), the protein was as active as its counterpart expressed in salmonellae. Although not conclusive, these data suggest that modification is not involved and that further studies are needed to identify which residues in FliC are responsible for TNF-α activation.
Flagellar gene expression is highly regulated at the transcriptional level (32). Expression of flhDC, the first operon in the cascade, is influenced by several environmental factors, and its gene products are absolutely required for expression of all of the other flagellar genes. Although the exact mechanism is not known, H-NS has previously been shown to activate flhDC at the transcriptional level in E. coli (7). The FC32 transposon mutant demonstrates a similar scenario in salmonellae. Interestingly, hns mutations in salmonellae result in an attenuated-virulence phenotype (19), an observation which suggests that induction of cytokines such as TNF-α by flagellin contributes to some of the pathology associated with Salmonella infection.
In general, the mechanisms of macrophage activation by facultatively intracellular pathogens such as salmonellae have not been fully elucidated. LPS, which is produced by all gram-negative bacteria, is an extremely potent stimulus for cytokine upregulation. Septic shock is only one example of the effects of cytokine overexpression in response to infection. In some cases, organisms have developed mechanisms to curb the host’s response by secreting proteins that specifically inhibit TNF-α expression in macrophages (8). The Salmonella case illustrated here shows that bacterial components other than LPS may affect cytokine production. Their precise role during infection remains to be determined. However, while naive PBMC are exquisitely sensitive to LPS (its presence in the blood is a rare occurrence), LPS tolerance is a phenomenon that has been described in patients and volunteers exposed to LPS for prolonged periods of time. In these cases, PBMC fail to respond to subsequent LPS stimulation in vitro (18, 38). Tolerance may have developed as a mechanism to prevent the host from mounting a chronic inflammatory response. In the tissues, and particularly in the gastrointestinal tract, resident macrophages are chronically exposed to the LPS released by the normal flora. In addition to tolerance, lack of CD14 expression may also result in LPS nonresponsiveness of lamina propria macrophages in the intestinal mucosa (45). Thus, a different type of stimulus, such as Salmonella flagellin, may activate LPS-nonresponsive or tolerant cells, thus contributing to the processes of macrophage-mediated inflammation and tissue damage that have been observed during Salmonella infection.
Another issue also needs to be addressed in light of the results presented here. Researchers have been able to generate immune responses to particular antigens by inserting them into the flagellin of attenuated Salmonella strains (41, 42). These experiments have yielded promising results in terms of antibody response and protection against subsequent challenge (47). With the discovery of a role for Salmonella flagellin in macrophage activation, the use of chimeric flagellin to present antigenic epitopes to antigen-presenting cells such as macrophages may need more careful examination. In most cases, local TNF-α production in response to flagellin may not have adverse effects; it may, on the contrary, be beneficial to the host. In other cases, however, this approach may prove counterproductive. For example, there is in vitro evidence that TNF-α can, by itself, upregulate HIV production in infected monocytes (34) and that TNF-α mediates most of the effect of salmonellae on HIV replication (2, 36). We may not be able to foresee all of the consequences of the interaction between pathogens coinfecting the same host.
To our knowledge, this is the first time that Salmonella flagellin has been reported to induce TNF-α, but there is evidence that pilin and flagellin from another gram-negative organism, Pseudomonas aeruginosa, can induce interleukin-8 production in respiratory epithelial cells (13). Interestingly, host cells appear to respond to flagellin from two distantly related organisms, salmonellae and pseudomonads, but not to the E. coli protein. Further analysis of the flagellin epitopes responsible for cytokine induction may reveal whether the Salmonella and Pseudomonas proteins use similar mechanisms.
ACKNOWLEDGMENTS
We thank Virginia Miller, Charles Dorman, Robert Macnab, Roy Curtiss, John Mekalanos, and Bruce Stocker for generously donating all of the bacterial strains that made this work possible. We also thank Daniel Wozniak for critically reading the manuscript.
This project was supported by NIH grant AI 38670 (S. B. Mizel), by NIH training grant T32AI07401 (S. B. Mizel), and by an NIH grant to the Comprehensive Cancer Center of Wake Forest University (which supports the DNA sequencing core facility).
REFERENCES
- 1.Ambler R P, Rees M W. ε-N-Methyl-lysine in bacterial flagellar protein. Nature (London) 1959;184:56–57. doi: 10.1038/184056b0. [DOI] [PubMed] [Google Scholar]
- 2.Andreana A, Gollapudi S, Kim C H, Gupta S. Salmonella typhimurium activates human immunodeficiency virus type 1 in chronically infected promonocytic cells by inducing tumor necrosis factor-α production. Biochem Biophys Res Commun. 1994;201:16–23. doi: 10.1006/bbrc.1994.1663. [DOI] [PubMed] [Google Scholar]
- 3.Arnold J W, Klimpel G R, Niesel D W. Tumor necrosis factor alpha (TNFα) regulates intestinal mucus production during salmonellosis. Cell Immunol. 1993;151:336–344. doi: 10.1006/cimm.1993.1243. [DOI] [PubMed] [Google Scholar]
- 4.Arnold J W, Niesel D W, Annable C R, Hess C B, Asuncion M, Cho Y J, Peterson J W, Klimpel G R. Tumor necrosis factor-α mediates the early pathology in Salmonella infection of the gastrointestinal tract. Microb Pathog. 1993;14:217–227. doi: 10.1006/mpat.1993.1021. [DOI] [PubMed] [Google Scholar]
- 5.Atlung T, Ingmer H. H-NS: a modulator of environmentally regulated gene expression. Mol Microbiol. 1997;24:7–17. doi: 10.1046/j.1365-2958.1997.3151679.x. [DOI] [PubMed] [Google Scholar]
- 6.Averill L, Toossi Z, Aung H, Broom W H, Ellner J J. Regulation of production of tumor necrosis factor alpha in monocytes stimulated by the 30-kilodalton antigen of Mycobacterium tuberculosis. Infect Immun. 1995;63:3206–3208. doi: 10.1128/iai.63.8.3206-3208.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertin P, Terao E, Lee E H, Lejeune P, Colson C, Danchin A, Collatz E. The H-NS protein is involved in the biogenesis of flagella in Escherichia coli. J Bacteriol. 1994;176:5537–5540. doi: 10.1128/jb.176.17.5537-5540.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beuscher H U, Rödel F, Forsberg Å, Röllinghoff M. Bacterial evasion of host immune defense: Yersinia enterocolitica encodes a suppressor for tumor necrosis factor alpha expression. Infect Immun. 1995;63:1270–1277. doi: 10.1128/iai.63.4.1270-1277.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beutler B, Grau G E. Tumor necrosis factor in the pathogenesis of infectious diseases. Crit Care Med. 1993;21:S423–S435. [PubMed] [Google Scholar]
- 10.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 11.Bullas L R, Ryu J-I. Salmonella typhimurium LT2 strains which are r− m+ for all three chromosomally located systems of DNA restriction and modification. J Bacteriol. 1983;156:471–474. doi: 10.1128/jb.156.1.471-474.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciacci-Woolwine F, Kucera L S, Richardson S H, Iyer N P, Mizel S B. Salmonellae activate tumor necrosis factor alpha production in a human promonocytic cell line via a released polypeptide. Infect Immun. 1997;65:4624–4633. doi: 10.1128/iai.65.11.4624-4633.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiMango E, Zar H J, Bryan R, Prince A. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J Clin Invest. 1995;96:2204–2210. doi: 10.1172/JCI118275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorman C J, Chatfield S N, Higgins C F, Hayward C, Dougan G. Characterization of porin and ompR mutants of a virulent strain of Salmonella typhimurium: ompR mutants are attenuated in vivo. Infect Immun. 1989;57:2136–2140. doi: 10.1128/iai.57.7.2136-2140.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felber B K, Pavlakis G N. A quantitative bioassay for HIV-1 based on transactivation. Science. 1988;239:184–186. doi: 10.1126/science.3422113. [DOI] [PubMed] [Google Scholar]
- 16.Finlay B B, Siebers A. Mechanisms of mucosal colonization and penetration by bacterial pathogens. In: Roth J A, Bolin C A, Brogden K A, Minior F C, Wannemuehler M J, editors. Virulence mechanisms of bacterial pathogens. 2nd ed. Washington, D.C: ASM Press; 1995. pp. 33–45. [Google Scholar]
- 17.Galdiero F, Cipollaro de L’Ero G, Benedetto N, Galdiero M, Tufano M A. Release of cytokines induced by Salmonella typhimurium porins. Infect Immun. 1993;61:155–161. doi: 10.1128/iai.61.1.155-161.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granowitz E V, Porat R, Mier J W, Orencole S F, Kaplanski G, Lynch E A, Ye K, Vannier E, Wolff S W, Dinarello C A. Intravenous endotoxin suppresses the cytokine response of peripheral blood mononuclear cells of healthy humans. J Immunol. 1993;151:1637–1645. [PubMed] [Google Scholar]
- 19.Harrison J A, Pickard D, Higgins C F, Khan A, Chatfield S N, Ali T, Dorman C J, Hormaeche C E, Dougan G. Role of hns in the virulence phenotype of pathogenic Salmonellae. Mol Microbiol. 1994;13:133–140. doi: 10.1111/j.1365-2958.1994.tb00408.x. [DOI] [PubMed] [Google Scholar]
- 20.Hinton J C D, Santos D S, Seirafi A, Hulton C S J, Pavitt G D, Higgins C F. Expression and mutational analysis of the nucleoid-associated protein H-NS of Salmonella typhimurium. Mol Microbiol. 1992;6:2327–2337. doi: 10.1111/j.1365-2958.1992.tb01408.x. [DOI] [PubMed] [Google Scholar]
- 21.Hoiseth S K, Stocker B A D. Aromatic-dependent S. typhimurium are non-virulent and are effective as live vaccines. Nature (London) 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 22.Homma M, Iino T, Kutsukake K, Yamaguchi S. In vitro reconstitution of flagellar filaments onto hooks of filamentless mutants of Salmonella typhimurium by addition of hook-associated proteins. Proc Natl Acad Sci USA. 1986;83:6169–6173. doi: 10.1073/pnas.83.16.6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Homma M, Kutsukake K, Iino T, Yamaguchi S. Hook-associated proteins essential for flagellar filament formation in Salmonella typhimurium. J Bacteriol. 1984;157:100–108. doi: 10.1128/jb.157.1.100-108.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hulton C S J, Seirafi A, Hinton J C D, Sidebotham J M, Waddell L, Pavitt G D, Owen-Hughes T, Spassky A, Buc H, Higgins C F. Histone-like protein H1 (H-NS), DNA supercoiling, and gene expression in bacteria. Cell. 1990;63:631–642. doi: 10.1016/0092-8674(90)90458-q. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda T, Oosawa K, Hotani H. Self assembly of the filament capping protein, FliD, of bacterial flagella into an annular structure. J Mol Biol. 1996;259:679–686. doi: 10.1006/jmbi.1996.0349. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda T, Yamaguchi S, Hotani H. Flagellar growth in a filament-less Salmonella fliD mutant supplemented with purified hook-associated protein 2. J Biochem. 1993;114:39–44. doi: 10.1093/oxfordjournals.jbchem.a124136. [DOI] [PubMed] [Google Scholar]
- 27.Jones C J, Macnab R M. Flagellar assembly in Salmonella typhimurium: analysis with temperature-sensitive mutants. J Bacteriol. 1990;172:1327–1339. doi: 10.1128/jb.172.3.1327-1339.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung H C, Eckmann L, Yang S-K, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff M F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klimpel G R, Asuncion A, Haithcoat J, Niesel D W. Cholera toxin and Salmonella typhimurium induce different cytokine profiles in the gastrointestinal tract. Infect Immun. 1995;63:1134–1137. doi: 10.1128/iai.63.3.1134-1137.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kostyal D A, Butler G H, Beezhold D H. Mycoplasma hyorhinis molecules that induce tumor necrosis factor alpha secretion by human monocytes. Infect Immun. 1995;63:3858–3863. doi: 10.1128/iai.63.10.3858-3863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kutsukake K, Iino T, Komeda Y, Yamaguchi S. Functional homology of fla genes between Salmonella typhimurium and Escherichia coli. Mol Gen Genet. 1980;178:59–67. doi: 10.1007/BF00267213. [DOI] [PubMed] [Google Scholar]
- 32.Kutsukake K, Ohya Y, Iino T. Transcriptional analysis of the flagellar regulon of Salmonella typhimurium. J Bacteriol. 1990;172:741–747. doi: 10.1128/jb.172.2.741-747.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macnab R M. Flagella and motility. In: Neidhardt F C, Curtiss III R, Ingraham J I, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 123–145. [Google Scholar]
- 34.Mellors J W, Griffith B P, Ortiz M A, Landry M L, Ryan J L. Tumor necrosis factor-α/cachectin enhances human immunodeficiency virus type 1 replication in primary monocytes. J Infect Dis. 1991;163:78–82. doi: 10.1093/infdis/163.1.78. [DOI] [PubMed] [Google Scholar]
- 35.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizel S B, Kucera L S, Richardson S H, Ciacci F, Iyer N P. Regulation of macrophage activation and human immunodeficiency virus production by invasive Salmonella strains. Infect Immun. 1995;63:1820–1826. doi: 10.1128/iai.63.5.1820-1826.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montie T C, Craven R C, Holder I A. Flagellar preparations from Pseudomonas aeruginosa: isolation and characterization. Infect Immun. 1982;35:281–288. doi: 10.1128/iai.35.1.281-288.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munoz C, Carlet J, Fitting C, Misset B, Blériot J-P, Cavaillon J-M. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Invest. 1991;88:1747–1754. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakano Y, Onozuka K, Terada Y, Shinomiya H, Nakano M. Protective effect of recombinant tumor necrosis factor-α in murine salmonellosis. J Immunol. 1990;144:1935–1941. [PubMed] [Google Scholar]
- 40.Nauciel C, Espinasse-Maes F. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect Immun. 1992;60:450–454. doi: 10.1128/iai.60.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newton S M C, Jacob C O, Stocker B A D. Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science. 1989;244:70–72. doi: 10.1126/science.2468182. [DOI] [PubMed] [Google Scholar]
- 42.Newton S M C, Joys T M, Anderson S A, Kennedy R C, Hovi M E, Stocker B A D. Expression and immunogenicity of an 18-residue epitope of HIV gp41 inserted in the flagellar protein of a Salmonella live vaccine. Res Microbiol. 1995;146:203–216. doi: 10.1016/0923-2508(96)80276-2. [DOI] [PubMed] [Google Scholar]
- 43.Newton S M C, Wasley R D, Wilson A, Rosenberg L T, Miller J F, Stocker B A D. Segment IV of a Salmonella flagellin gene specifies flagellar antigen epitopes. Mol Microbiol. 1991;5:419–425. doi: 10.1111/j.1365-2958.1991.tb02124.x. [DOI] [PubMed] [Google Scholar]
- 44.Ohnishi K, Ohto Y, Aizawa S-I, Macnab R M, Iino T. FlgD is a scaffolding protein needed for flagellar hook assembly in Salmonella typhimurium. J Bacteriol. 1994;176:2272–2281. doi: 10.1128/jb.176.8.2272-2281.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith P D, Janoff E N, Mosteller-Barnum M, Merger M, Orenstein J M, Kearney J F, Graham M F. Isolation and purification of CD14-negative mucosal macrophages from normal human small intestine. J Immunol Methods. 1997;202:1–11. doi: 10.1016/s0022-1759(96)00204-9. [DOI] [PubMed] [Google Scholar]
- 46.Smith N H, Selander R K. Molecular genetic basis for complex flagellar antigen expression in a triphasic serovar of Salmonella. Proc Natl Acad Sci USA. 1991;88:956–960. doi: 10.1073/pnas.88.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stocker B A, Newton S M C. Immune responses to epitopes inserted in Salmonella flagellin. Int Rev Immunol. 1994;11:167–178. doi: 10.3109/08830189409061724. [DOI] [PubMed] [Google Scholar]
- 48.Stone B J, Garcia C M, Badger J L, Hassett T, Smith R I F, Miller V L. Identification of novel loci affecting entry of Salmonella enteritidis into eukaryotic cells. J Bacteriol. 1992;174:3945–3952. doi: 10.1128/jb.174.12.3945-3952.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki T, Ueguchi C, Mizuno T. H-NS regulates OmpF expression through micF antisense RNA in Escherichia coli. J Bacteriol. 1996;178:3650–3653. doi: 10.1128/jb.178.12.3650-3653.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szekely E, Simon M. DNA sequences adjacent to flagellar genes and evolution of flagellar-phase variation. J Bacteriol. 1983;155:74–81. doi: 10.1128/jb.155.1.74-81.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor R K, Manoil C, Mekalanos J J. Broad-host-range vectors for delivery of TnphoA: use in genetic analysis of secreted virulence determinants of Vibrio cholerae. J Bacteriol. 1989;171:1870–1878. doi: 10.1128/jb.171.4.1870-1878.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tracey K J, Beutler B, Lowry S F, Merryweather J, Wolpe S, Milsark I W, Hariri R J, Fahey III T J, Zentella A, Albert J D, Shires T, Cerami A. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- 53.Tsai S P, Hartin R J, Ryu J I. Transformation in restriction-deficient Salmonella typhimurium. J Gen Microbiol. 1989;135:2561–2567. doi: 10.1099/00221287-135-9-2561. [DOI] [PubMed] [Google Scholar]
- 54.van Asten A J A M, Zwaagstra K A, Baay M F, Kusters J G, Huis in’t Veld J H J, van der Zeijst B A M. Identification of the domain which determines the g,m serotype of the flagellin of Salmonella enteritidis. J Bacteriol. 1995;177:1610–1613. doi: 10.1128/jb.177.6.1610-1613.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vowels B R, Yang S, Leyden J J. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: implications for chronic inflammatory acne. Infect Immun. 1995;63:3158–3165. doi: 10.1128/iai.63.8.3158-3165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei L-N, Joys T M. Covalent structure of three phase-1 flagellar filament proteins of Salmonella. J Mol Biol. 1985;186:791–803. doi: 10.1016/0022-2836(85)90397-3. [DOI] [PubMed] [Google Scholar]
- 57.Williams A W, Yamaguchi S, Togashi F, Aizawa S-I, Kawagishi I, Macnab R M. Mutations in fliK and flhB affecting flagellar hook and filament assembly in Salmonella typhimurium. J Bacteriol. 1996;178:2960–2970. doi: 10.1128/jb.178.10.2960-2970.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Worton K J, Candy D C A, Wallis T S, Clarke G J, Osborne M P, Haddon S J, Stephen J. Studies on early association of Salmonella typhimurium with intestinal mucosa in vivo and in vitro: relationship to virulence. J Med Microbiol. 1989;29:283–294. doi: 10.1099/00222615-29-4-283. [DOI] [PubMed] [Google Scholar]
- 59.Yamaguchi S, Fujita H, Sugata K, Taira T, Iino T. Genetic analysis of H2, the structural gene for phase-2 flagellin in Salmonella. J Gen Microbiol. 1984;130:255–265. doi: 10.1099/00221287-130-2-255. [DOI] [PubMed] [Google Scholar]
- 60.Zieg J, Silverman M, Hilmen M, Simon M. Recombinational switch for gene expression. Science. 1977;196:170–172. doi: 10.1126/science.322276. [DOI] [PubMed] [Google Scholar]