Abstract
Soil salinity and saline irrigation water are major constraints in sugarcane affecting the production of cane and sugar yield. To understand the salinity induced responses and to identify novel genomic resources, integrated de novo transcriptome and small RNA sequencing in sugarcane wild relative, Erianthus arundinaceus salt tolerant accession IND 99-907 and salt-sensitive sugarcane genotype Co 97010 were performed. A total of 362 known miRNAs belonging to 62 families and 353 miRNAs belonging to 63 families were abundant in IND 99-907 and Co 97010 respectively. The miRNA families such as miR156, miR160, miR166, miR167, miR169, miR171, miR395, miR399, miR437 and miR5568 were the most abundant with more than ten members in both genotypes. The differential expression analysis of miRNA reveals that 221 known miRNAs belonging to 48 families and 130 known miRNAs belonging to 42 families were differentially expressed in IND 99-907 and Co 97010 respectively. A total of 12,693 and 7982 miRNA targets against the monoploid mosaic genome and a total of 15,031 and 12,152 miRNA targets against the de novo transcriptome were identified for differentially expressed known miRNAs of IND 99-907 and Co 97010 respectively. The gene ontology (GO) enrichment analysis of the miRNA targets revealed that 24, 12 and 14 enriched GO terms (FDR < 0.05) for biological process, molecular function and cellular component respectively. These miRNAs have many targets that associated in regulation of biotic and abiotic stresses. Thus, the genomic resources generated through this study are useful for sugarcane crop improvement through biotechnological and advanced breeding approaches.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-023-03867-7.
Keywords: Sugarcane, miRNA, Transcriptome, Small RNA, Salinity, miRNA targets
Introduction
MicroRNAs (miRNAs) are endogenous, single-stranded non-coding regulatory RNA molecules with a length of 18–24 bp involved in post-transcriptional regulation of gene expression in eukaryotes (Zhang et al. 2006; Yang et al. 2021a). The plant miRNA-guided post-transcriptional regulation involves the recognition of targets based on their complementarity or near complementarity between miRNA and their target mRNA (Brodersen et al. 2008). Plant miRNA interacts with 3′ UTR, coding regions and 5′ UTR regions of target genes (Hausser et al. 2013) to regulate the gene expression either through mRNA cleavage or translational repression (Gu and Kay 2010; Orang et al. 2014; Li et al. 2018). The biogenesis of miRNA in plants involves precise, complex and highly regulated signalling networks (Wang et al. 2019a; Gao et al. 2021) and, there are several evidences to elucidate the role of miRNA in regulating the biotic and abiotic stresses responses in many plant species (Sun et al. 2015; Aravind et al. 2017; Fu et al. 2017; Xie et al. 2017; Zhang et al. 2017; Parmar et al. 2020; Yang et al. 2020).
Salinity and saline water are major constraints in crop production in arid and semi-arid climatic regions (Manoj et al. 2019; Hassani et al. 2020; Hussain et al. 2020). Each day, around 2000 hectares of saline soil was generated globally in turn responsible for 10–25% yield reduction in many crops (Shahid et al. 2018). Salt stress causes two kinds of stresses to plants, namely the osmatic and ionic stresses (Munns and Tester 2008). The salt-induced osmatic stress causes physiological drought stress to plants by reducing the availability of soil moisture. The ionic stress causes the excessive accumulation of salts in tissues such as sodium and chlorine resulted in plasmolysis and cell death (Katsuhara 1997; Huh et al. 2002). The sessile plants exhibits three types of tolerance mechanisms such as tolerance to osmotic stress, toxic ion exclusion and tissue tolerance (Munns and Tester 2008). These tolerant mechanisms are governed by complex, precise and genotypic-specific networks of genes, moderating the responses to salinity stresses at signalling and metabolic pathways (Gupta and Huang 2014; Parihar et al. 2015; Bandehagh and Taylor 2020; Shahid et al. 2020). Several reports has shown that miRNA regulating the salinity stress responses in plant species (Xie et al. 2017; Parmar et al. 2020; Yang et al. 2020).
The significance of miRNA in temporal and spatial regulation of their target gene expression in many metabolic pathways are well studied in many plant species. The selective biogenesis of miRNA, their transportation, miRNA mediated gene regulation and miRNA degradation are highly specific and governed by network of several genes in responses to environmental stimuli (Jung et al. 2015; Von Born et al. 2018; Tolstyko et al. 2019). The miRNAs are regulating the gene expression by interacting with 5′ UTR and coding regions resulting in down regulation of the target genes (Fang and Rajewsky 2011) and modulation of protein turnover through transcriptional repression by interacting with 5′ UTR regions (Lytle et al. 2007). The interaction of miRNA with 3′ UTR regions resulted in translational upregulation of target genes (Vasudevan and Steitz 2007) and miRNA mediated upregulation of target genes (Stevens 2008; Vasudevan 2012; Orang et al. 2014). The miRNA and their target interactions in NGS based genome-wide miRNA-mRNA expression studies were categorized into four types of interactions viz., both miRNA and their targets were upregulated as Type-1 interaction, down regulation of both miRNA and their target genes as Type-2, neutral expression as Type-3 and opposite expression as Type-4 interactions (Aravind et al. 2017). The miRNA regulating their target genes related to abiotic stresses were reported in crop species such as miR164b and OsmiR535 in rice (Jiang et al. 2019; Yue et al. 2020), miRNVL5 in cotton (Gao et al. 2016), miR172a in soybean (Pan et al. 2016), miR172a and miR172b in rice and wheat (Cheng et al. 2021) and miR528 in Bentgrass (Yuan et al. 2015). With advent of NGS, now it easier to dissect out the genome-wide miRNA and their targets associated with regulation of biotic and abiotic stresses and it has been demonstrated in many crop species (Parmar et al. 2020; Yuan et al. 2020; Zhou et al. 2020; Bohra et al. 2021; Li et al. 2021a; Yang et al. 2021a, b).
Erianthus arundinaceus [Retzius] Jeswiet) is a cane forming wild relative of sugarcane (Welker et al. 2015, 2019) and harbours many genes for agronomic traits and tolerances to biotic and abiotic stresses (Uwatoko et al. 2011; Augustine et al. 2015a,b; Narayan et al. 2019, 2021; Clarancia et al. 2020; Swathik Clarancia et al. 2021; 2023). E. arundinaceus is a cross-compatible with sugarcane and has been utilized in our sugarcane breeding programmes, and resulted in identification many genetic stocks with high biomass production potential and tolerant to biotic and abiotic stresses (Kumar et al. 2015; Premachandran et al. 2017; Wang et al. 2019b; Meena et al. 2020). Sugarcane is a glycophyte and salinity stress directly affect the accumulation of biomass and sucrose at all stages of crop growth (Saxena et al. 2010; Yunita et al. 2020; Zhao et al. 2020). The transcriptome and miRNA genomic resources for distant wild relatives such as salinity tolerant E. arundinaceus and salt-sensitive sugarcane cultivars help to decipher the role of miRNA in regulating gene expression and salt responsive metabolic pathways. Hence, we adopted the integrated comparative small RNA and de novo transcriptome analysis to understand the miRNA mediated post-transcriptional regulation of salt responsive genes and pathways in E. arundinaceus genotype IND 99-907 and salt-sensitive sugarcane genotype Co 97010. The accession IND 99-907 collected from the saline soil of Ernakulum district, Kerala (Nair and Somarajan 2003) and characterized for salt tolerance through morphological, anatomical, physiological parameters. We have identified the differentially expressed salt responsive genes in IND 99-907 and Co 97010 through de novo comparative transcriptome analysis (Vignesh et al. 2021) and the present study aimed at identification of salinity responsive miRNA and their targets through integrated small RNA-transcriptome sequencing approaches. The genomic resources developed through this study is first of kind, between sugarcane and its distant relative E. arundinaceus, and useful for crop improvement through marker-assisted breeding and biotechnological approaches.
Material and methods
Plant material, total RNA isolation, library preparation and small RNA sequencing
The details of the experimental material and methodologies followed for sampling of roots from the stressed and control treatments of the salt tolerant E. arundinaceus accession IND 99-907 and salt-sensitive sugarcane variety Co 97010 were previously described in de novo salt transcriptome studies (Vignesh et al. 2021). The total RNA was isolated by using TRIzol reagent followed by quality check using Agilent 2200 Tape Station System (Liu et al. 2014). The RNA integrity (RIN) values ≥ 7.0 were used for library preparation using NEBNext Multiplex Small RNA Library Preparation Kit (New England Biolabs) as per the manufacturer’s instructions and libraries were sequenced using Illumina HiSeq 2500 platform in 1 × 50 bp single end format.
Pre-processing of raw reads and filtering of raw reads
The quality of raw reads of Small RNA were checked using FastQC (Andrews 2015) and the adapter sequences (5′ RNA Adapter = > 5′ GTTCAGAGTTCTACAGTCCGACGATC; 3′ RNA Adapter = > 3′ AGATCGGAAGAGCACACGTCTGAACTC) were removed using the Cutadapt tool (Martin 2011). Reads with 18 to 30 nucleotides with Phred score above 30 (Q30) were filtered using Trimmomatic (Bolger et al. 2014). Non-regulatory RNAs such as rRNA, tRNA, snRNA and snoRNA were filtered out by aligning clean reads with SILVA (https://www.arb-silva.de/), GtRNAdb (http://gtrnadb.ucsc.edu/) and Rfam database (http://rfam.sanger.ac.uk/) with a maximum mismatch of 0 using bowtie2 (Langmead and Salzberg 2013) and resultant reads were used for miRNA predictions.
Prediction of miRNAs and annotation
The clean reads filtered after removal of non-regulatory RNAs were aligned against the monocot miRNA database of the miRBase (http://www.mirbase.org/) using bowtie program (Langmead et al. 2009) with a maximum mismatch (−n 2) for the prediction of the known miRNA. The unaligned reads were used for prediction of novel miRNA using Sugarcane monoploid genome (Garsmeur et al. 2018) as the reference genome. The abundance of miRNAs in each samples were estimated using SAMtool (Li et al. 2009) and novel miRNAs were identified by using miRDeep2 tool (Friedländer et al. 2012).
Differential expression analysis of miRNA and their target predictions
The differential expression analysis of miRNAs were performed using DESeq2 (Love et al. 2014) and their targets were predicted using psRNA target server (Dai et al. 2018) (http://plantgrn.noble.org/psRNATarget/) against the databases of sugarcane monoploid genome (Garsmeur et al. 2018) and assembled salinity transcriptome (Vignesh et al. 2021).
Validation of Small RNA-Seq and their targets
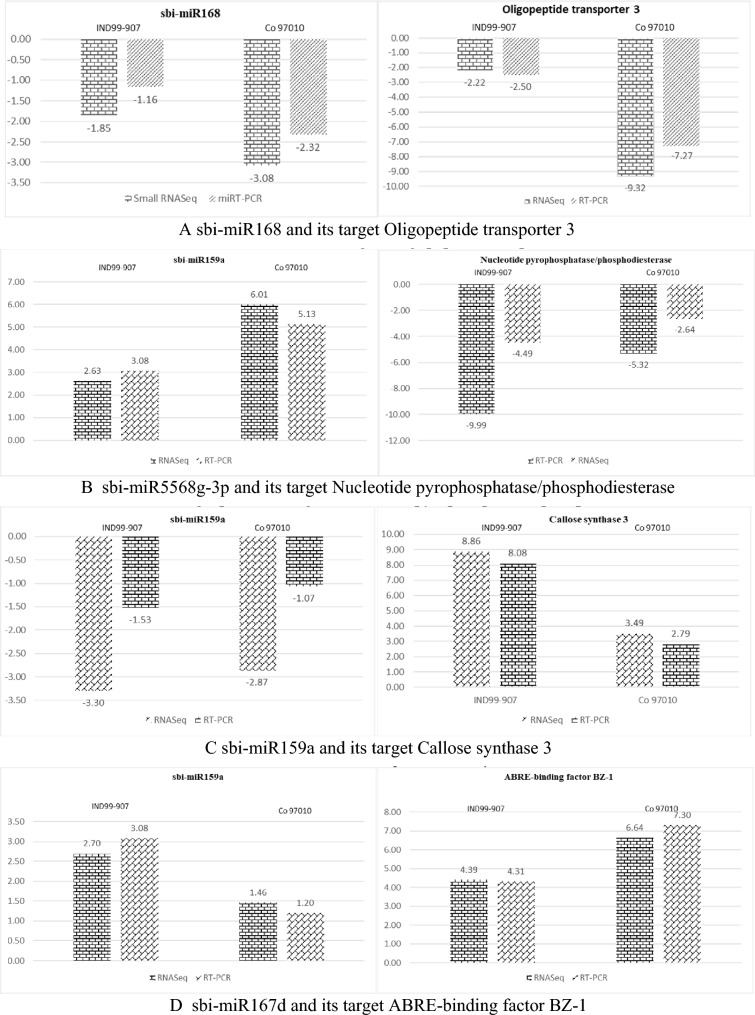
Four miRNAs such as sbi-miR159a, sbi-miR168, sbi-miR5568g-3p, sbi-miR167d and their targets such as Callose synthase 3, Oligopeptide transporter 3, Nucleotide pyrophosphatase/phosphodiesterase and ABRE-binding factor BZ-1 were chosen for validation of Small RNA-Seq (Supplementary Table 1). The total RNA isolation from the stressed and control root samples of IND 99-907 and Co 97010 was described in our previous studies (Vignesh et al. 2021) and normalized RNA samples were used for validation. The cDNA of mature miRNAs for stressed and control root samples of IND 99-907 and Co 97010 were synthesized using miScript II RT Kit (Qiagen) as per the manufacturer’s instructions. Briefly, a total of 2.0 µg total RNA was added with 4.0 µL of 5 × miScript HiSpec Buffer, 2 μL of 10 × miScript Nucleics Mix, 2 μL of miScript Reverse Transcriptase Mix and volume made up to 20.0 μL using RNase-free water. 5S RNA was used as an internal control for expressions analysis of miRNAs (Su et al. 2017) using miScript SYBR® Green PCR Kit (Qiagen) as per the manufacturer’s instructions. Briefly, 10 μL of 2 × QuantiTect SYBR Green PCR Master Mix, 2 μL of 10 × miScript Universal Primer, 2 Μl of 10 × miScript Primer Assay, and template 10–20 ng of cDNA of miRNA and volume made up to 20 μL using RNase free water. Quantitative real time PCR conditions followed were explained elsewhere (Dharshini et al. 2016; Narayan et al. 2021). The relative expression of miRNA was estimated using 2−ΔΔCT method (Su et al. 2017). The miRNA targets were validated as per the protocol described in our previous studies (Vignesh et al. 2021).
Results
Small RNA sequencing data and pre-processing
The small RNA sequencing of control and stressed root samples of IND 99-907 and Co 97010 was performed using Illumina HiSeq 2500 platform in 1 × 50 bp single-end format and labelled as 907C, 907S, 97010C and 97010S respectively. A total of 865,221,680 raw reads were generated, which includes 210,986,172, 214,385,900, 197,546,113 and 242,303,495 raw reads of 907C, 907S, 97010C and 97010S respectively. The high-quality reads with minimum Phred score ≥ 30 and length of 18–30 nucleotides after the removal of adopters, rRNA, tRNA, snRNA, snoRNA resulted in 58,716,698, 86,602,825, 57,530,483 and 66,998,728 clean reads from libraries of 907C, 907S, 97010C and 97010S respectively (Table 1). The length distribution for clean reads showed that 21 nucleotide reads were the most abundant (Fig. 1) and more than 8 million clean reads were length of 21 nucleotides. Among the four libraries, the maximum 21 nucleotide reads were observed in 907C samples and the lowest in 907S libraries.
Table 1.
Pre-processing of small RNA libraries of control and stresses samples of IND 99-907 and Co 97010
| Type | 907C | 907S | 97010C | 97010S |
|---|---|---|---|---|
| Raw reads | 210,986,172 | 214,385,900 | 197,546,113 | 242,303,495 |
| Length < 18 | 65,870,723 | 117,605,365 | 67,905,970 | 80,108,269 |
| Length > 30 | 159,385,521 | 179,557,250 | 135,127,012 | 179,496,276 |
| tRNAs | 133,147 | 8,738,922 | 239,337 | 687,129 |
| rRNAs | 2,154,031 | 770,157 | 2,227,623 | 2,241,794 |
| snRNAs | 130,069 | 148,874 | 191,261 | 263,420 |
| snoRNAs | 11,389 | 52,380 | 22,003 | 30,293 |
| Cleaned reads (Length = 18–30) | 58,716,698 | 86,602,825 | 57,530,483 | 66,998,728 |
| Q30% | 74.82% | 92.35% | 69.50% | 75.25% |
Whereas, 907C and 907S denotes control and stress samples of E. arundinaceus respectively; 97010C and 97010S denotes control and stress samples of sugarcane cultivar Co 97010 respectively
Fig. 1.
Small RNA length distribution of four sequenced libraries
Identification and differential expression of salt responsive known miRNAs
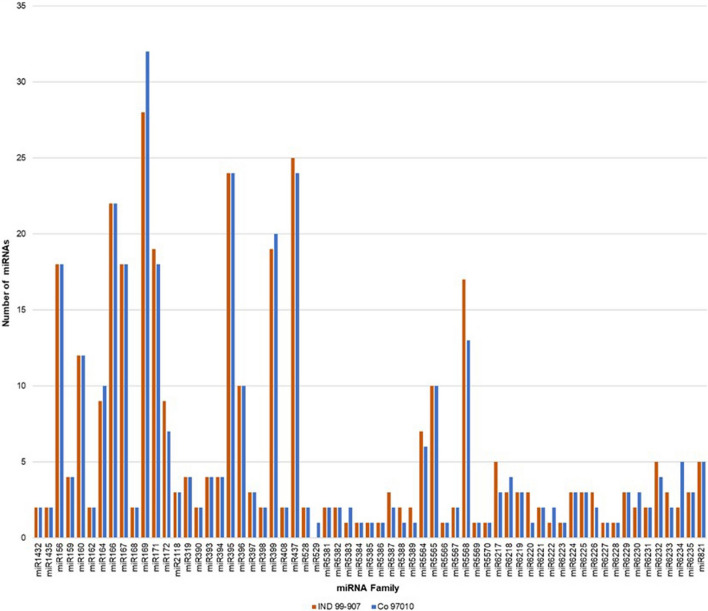
Non-coding non-regulatory RNAs such as rRNAs tRNAs, and other RNA families were filtered out by aligning with SILVA (https://www.arb-silva.de/), GtRNAdb (http://gtrnadb.ucsc.edu/) and Rfam database (http://rfam.sanger.ac.uk/) respectively. The resultant clean reads were used for prediction of miRNA by aligning against the monocot miRNA data of miRBase database. A total of 362 known miRNAs belonging to 62 families in IND 99–907 and 353 miRNAs belonging to 63 families in Co 97010 were identified (Fig. 2). The miRNA families such as miR156, miR160, miR166, miR167, miR169, miR171, miR395, miR399, miR437 and miR5568 were the most abundant with more than ten members in both IND 99-907 and Co 97010 libraries. The miR169 family was the most abundant with 28 members in IND 99-907 and 32 members in Co 97010. Other miRNA families such as miR437 class were enriched with 25 members in IND 99-907 and 24 members in Co 97010, miR395 class with 24 members and miR166 class with 22 members in both genotypes. Only 1 miRNA was abundant in 11 and 9 miRNA families in IND 99-907 and Co 97010 respectively.
Fig. 2.
Distribution of miRNAs families in IND 99-907 and Co 97010
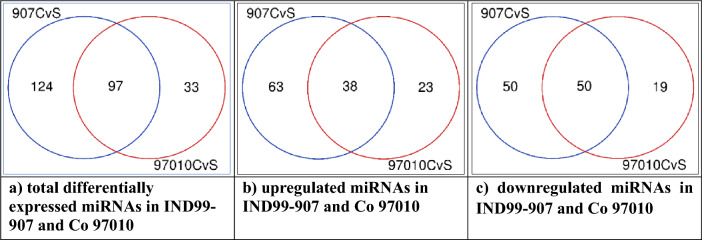
The differential expression analysis between control and stressed libraries resulted in identification of 221 miRNAs belonging to 48 families in IND 99-907 and 130 miRNAs belonging to 42 families in Co 97010 (p < 0.05). Among the 221 differentially expressed (DE) miRNA in IND 99-907, 101 miRNAs were upregulated and 100 miRNAs were downregulated (logFC > 1; logFC < −1). Similarly in Co 97010, 61 miRNAs were upregulated and 69 of them were downregulated. Among the differentially expressed miRNAs, 97 miRNAs were expressed in both genotypes, whereas, 124 and 33 miRNAs were uniquely expressed in IND 99-907 and Co 97010 respectively. Among unique DE miRNAs, 38 miRNAs were upregulated in both genotypes whereas 63 and 23 miRNAs were uniquely upregulated in IND 99-907 and Co 97010 respectively. Similarly, 50 and 19 miRNAs were uniquely downregulated in IND 99-907 and Co 97010 respectively, whereas 50 miRNAs were downregulated in both genotypes (Fig. 3). Among the 101 upregulated miRNAs in IND 99-907, 10 miRNAs were most abundant with expression fold change higher than 5.0 (logFC) and miR5568b-5p was abundant with log2FC = 6.597. Among 61 upregulated miRNAs in Co 97010, 37 miRNAs were abundant with expression fold-change greater than 5.0 and miR437r was abundant with log2FC = 7.771.
Fig. 3.
Venn diagram showing the unique and shared miRNAs between IND 99-907 and Co 97010; a total differentially expressed miRNAs, b upregulated miRNAs and c) downregulated miRNAs
Identification of salt responsive novel miRNAs in four libraries
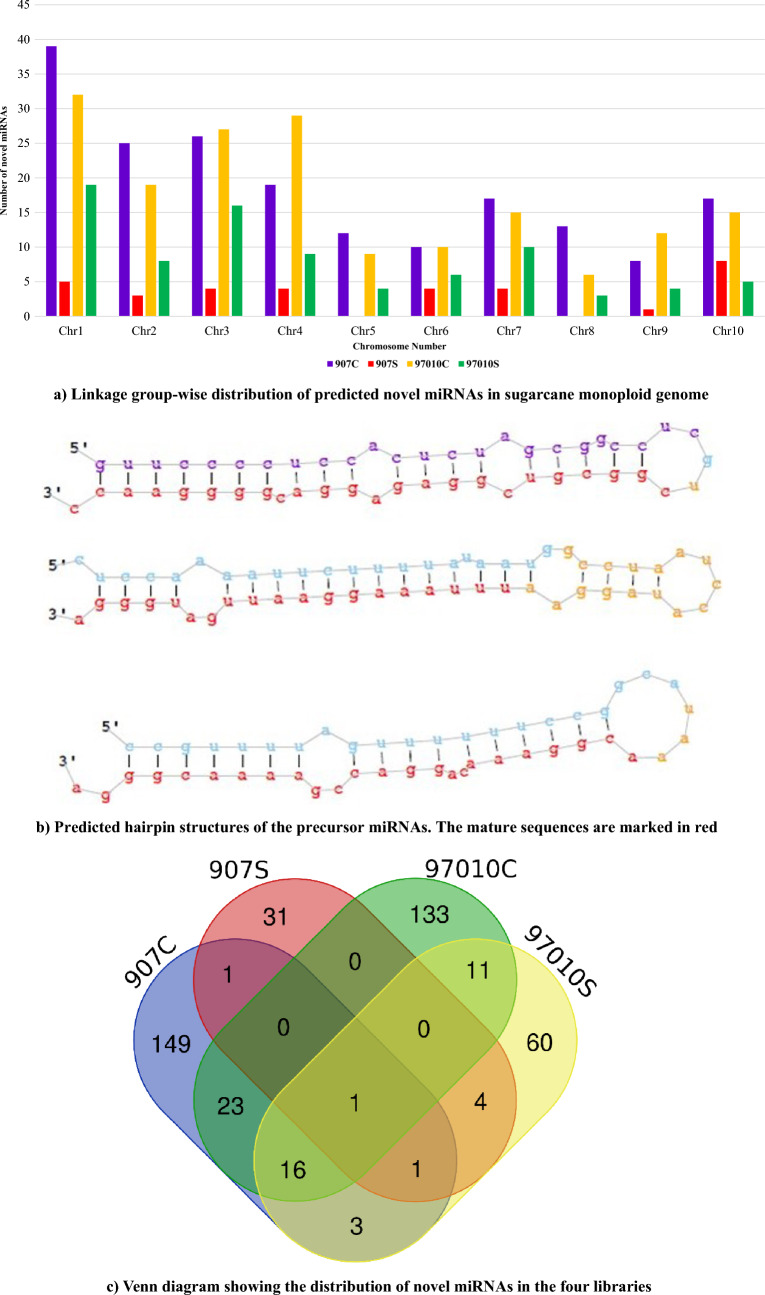
The unaligned reads after the identification of known miRNA from the miRBase were mapped against sugarcane monoploid reference genome (Garsmeur et al. 2018) using miRDeep2 tool for identification of novel miRNAs. A total 6,032,125 (10.78%), 27,406,017 (35.60%), 7,999,544 (14.65%) and 15,178,810 (23.77%) reads were mapped against sugarcane monoploid genome. As per the standard miRNA annotation protocol (Meyers et al. 2008), miRNA with a copy number less than 10 were discarded (Yang et al. 2020) which resulted in identification of a total 194, 38, 184 and 96 novel miRNA from 907C, 907S, 97010C and 97010S libraries respectively. The novel miRNA identified were having 18–25 nucleotide length and distributed among all ten linkage groups (Fig. 4A).
Fig. 4.
A Linkage group-wise distribution of predicted novel miRNAs in sugarcane monoploid genome, B predicted hairpin structures of the precursor miRNAs and the mature sequences are marked in red, and C Venn diagram showing the distribution of novel miRNAs in the four libraries
A total of 194 novel miRNAs were identified in 907C library and among them, 87 were having ( +) strand and 98 were having (−) strand. Similarly, 16, 90 and 45 novel miRNAs were present in the ( +) strand and 22, 97 and 53 novel miRNAs were present in the (−) strands of 907S, 97010C and 97010S libraries respectively. With regards to linkage group-wise distribution, the highest number of novel miRNA were located on LG-1, a total of 39 and 19 novel miRNA in control libraries whereas 8 and 32 novel miRNA in stressed libraries were located on LG1 respectively. In the library 907S, the chromosomal distribution was < 10 with no novel miRNAs predicted in chromosomes 5 and 8, whereas, only 1 novel miRNA was observed in chromosome 9. A total of 10, 6, 7 and 4 novel miRNAs had mature read counts greater than 1000 in the four libraries of 907C, 907S, 97010C and 97010S respectively. With regards to read counts, novel miRNAs viz., Sh09_38450, Sh03_9706, Sh09_41462 and Sh01_6609 were recorded the highest number of read counts of 13,066, 10,129, 15,460 and 13,108 reads in the libraries 907C, 907S, 97010C and 97010S respectively. In IND 99-907, only three novel miRNAs were abundant in both control and stressed libraries. A total of 28 novel miRNAs were abundant in both libraries of Co 97010. With regards to cross comparison of novel miRNA between IND 99-907 and Co 97010, novel miRNA (149) were more abundant in the control libraries as compared to 139 novel miRNAs in stressed libraries (Fig. 4C). All of the predicted novel miRNAs had significant randfold p values with no similarity domains in Rfam database. Both precursor (pre-miRNA) and star (miRNA*) sequences were present for predicted novel miRNAs supporting the miRNA biogenesis. The structures of the novel miRNAs were predicted and few of the representative structures of novel miRNAs showing mature miRNAs located on the stem arms of the hairpin secondary structures are presented in Fig. 4B. Moreover, the minimum free energies (MFE) of the precursor sequences were calculated and it ranged from − 68.70 to − 7.00 kcal/mol, − 44.70 to − 8.70 kcal/mol, − 56.40 to − 8.10 kcal/mol and − 68.90 to − 2.60 kcal/mol with an average length of 63, 59, 63 and 62 nucleotide for 907C, 907S, 97010C and 97010S libraries respectively.
Target gene prediction of miRNA and their annotation
To identify the target genes of miRNA, differentially expressed miRNA from IND 99-907 and Co 97010 (p < 0.05) were targeted against the monoploid mosaic sugarcane genome, our de novo salt transcriptome assemblies and differentially expressed genes (DEGs) (p < 0.05) under stressed and control libraries of IND 99-907 and Co 97010. The gene annotation for the DEGs was obtained by performing a blastn search against the annotated cDNA sequences of the sugarcane monoploid genome. The miRNA target prediction against the monoploid mosaic sugarcane genome resulted in identification of 12,693 gene targets for 221 DE miRNA in IND 99-907 and 7982 gene targets for 130 DE miRNA in Co 97010. In the case of our salt transcriptome assembly (Vignesh et al. 2021), a total of 15,031 and 12,152 miRNA targets were predicted for DE miRNA of IND 99-907 and Co 97010 respectively. Further, to study the miRNA-mRNA based on their expression profiles, DE miRNAs were specifically targeted against 7731 and 6159 DEGs of IND 99-907 and Co 97010 respectively and a total of 4378 and 2744 target genes or DEGs were identified in IND 99-907 and Co 97010 respectively. The predicted interactions between miRNAs and their targets showed that same miRNAs targeting multiple genes as well as the same gene targeted by multiple miRNAs.
Expression patterns of miRNA and their interaction with targets
To analyse the salinity stress response of two contrasting genotypes IND 99-907 and Co 97010, both miRNA and their target expression analysis was studied decipher their interactions. The expression patterns between miRNA and their targets were classified into three categories viz., type I interaction: both miRNA and their targets were upregulated, type II Interaction: both miRNA and their targets were down-regulated, type III interaction: miRNA was upregulated and their targets were down regulated, and type IV interactions: miRNA was down regulated and its targets were upregulated (Aravind et al. 2017). A total of 594, 222, 225 and 402 known miRNA in IND 99-907, and 190, 280, 188 and 245 known miRNA in Co 97010 were interacted with their targets in type I, II, III and type IV pattern respectively.
Gene ontology (GO) enrichment analysis for miRNA targets
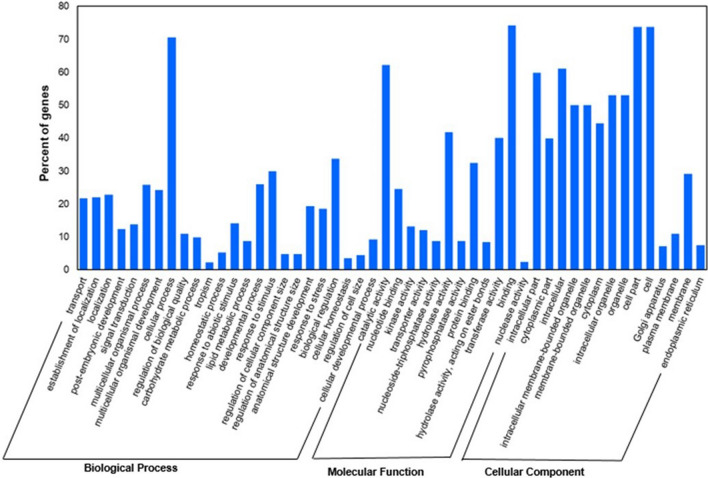
The GO enrichment analysis of the miRNA targets were carried out to elucidate the functional roles of miRNA and their targets for salt responsive biological, molecular and cellular function in IND 99-907 and Co 97010. We identified 24, 12 and 14 enriched GO terms (FDR < 0.05) in three categories biological process, molecular function and cellular component respectively (Fig. 5). The salt responsive biological processes such as transport (GO:0006810), signal transduction (GO:0007165), response to abiotic stimulus (GO:0009628), response to stress (GO:0006950) and cellular developmental processes (GO:0048869) GO terms were significantly enriched under salinity stress. In the salt responsive molecular function category, binding (GO:0005488), transporter activity (GO:0005215) and hydrolase activity (GO:0016787) GO terms were significantly enriched and intracellular part (GO:0044424) and cytoplasmic part (GO:0044444) of cellular components GO terms were significantly enriched under salinity stress.
Fig. 5.
Overview of GO annotations of potential target genes of miRNAs derived from the transcriptome studies of E. arundinaceus accession IND 99-907 and salinity sensitive sugarcane cultivar Co 97010. The data represents three GO categories namely biological process, molecular function and cellular component
Validation of small RNA-Seq and their targets
We have randomly selected four miRNA (sbi-miR159a, sbi-miR168, sbi-miR5568g-3p, sbi-miR167d) and their targets (Callose synthase 3, Oligopeptide transporter 3, Nucleotide pyrophosphatase/phosphodiesterase and ABRE-binding factor BZ-1) for validation of Small RNA-Seq (Supplementary Table 1) in E. arundinaceus accession IND 99-907 and salinity sensitive sugarcane cultivar Co 97010. The differential expression pattern of miRNA in Small RNA-Seq and their targets in Transcriptome studies were comparable with expression pattern in RT-PCR (Fig. 6).
Fig. 6.
Validation of randomly selected four miRNA and their targets in salt tolerant E. arundinaceus accession IND 99-907 and salinity sensitive sugarcane cultivar Co 97010
Discussion
The modern sugarcane varieties are salt sensitive and salinity stress causes the significant reduction in crop growth and biomass accumulation in sugarcane (Saxena et al. 2010; Uwatoko et al. 2011; Zhao et al. 2020; Mohanan et al. 2021). E. arundinaceus is a wild relative of sugarcane with high biomass production potential and harbours many genes for agronomic, biotic and abiotic stresses (Uwatoko et al. 2011; Augustine et al. 2015a,b). Though the miRNA studies were reported for various physiological, biotic and abiotic stresses in sugarcane such as aerenchyma cell formation (Queiroz de Pinho Tavares et al. 2020), cold stress (Yuan et al. 2020), aluminium stress (da Silva et al. 2019), smut disease (Su et al. 2017) and bacilliform virus (Ashraf et al. 2020), the role of miRNA in regulating of salt responsive mechanism in sugarcane is poorly understood. The abundance of miRNA and their role in regulation for growth, developmental, physiological, biotic and abiotic stress tolerances were well studied and reviewed in many crops (Gao et al. 2016; Bai et al. 2018; Cui et al. 2020; Waheed and Zeng 2020). The genome-wide comparative profiling of salt responsive miRNA helps to uncover the possible role of miRNA in conferring the salt stress tolerance in salt tolerant E. arundinaceus genotype IND 99-907 and salt-sensitive sugarcane genotype Co 97010.
Next generation sequencing approaches are frequently used for identification of genome-wide identification of miRNA and their targets in many crops like rice (Chopperla et al. 2020), wheat (Zhou et al. 2019; Hou et al. 2020), maize (Li et al. 2016), sorghum (Katiyar et al. 2015), foxtail millet (Yi et al. 2013) and many other crops. The reduction in cost of sequencing and recent advances in bioinformatic analysis of NGS data (Muir et al. 2016; Li et al. 2019) paved the way for genome-wide discovery of miRNA in non-model crops like citrus (Xie et al. 2017), Dimocarpus longan (Xu et al. 2020), Camellia sinensis (Jeyaraj et al. 2017), Osmanthus fragrans (Shi et al. 2021), Achyranthes bidentata (Yang et al. 2021a) and many other species. In our studies, we investigated the comparative miRNA profiling to identify the salt responsive conserved and novel miRNA associated with salt tolerance in IND 99-907 and Co 97010. We have generated 58.72, 86.60, 57.53 and 66.99 million of clean reads from the control and stressed samples of IND 99-907 and Co 97010 respectively and clean reads generated in our studies were higher than previous reports in sugarcane (da Silva et al. 2017; Su et al. 2017; Yang et al. 2017) and recent reports in other plant species (Chopperla et al. 2020; Hou et al. 2020; Yang et al. 2020, 2021a, b; Li et al. 2021a; Shi et al. 2021). The sRNA distribution was ranged from 18 to 25 nucleotide length and 21 nucleotide sRNAs were more abundant in both stressed and control libraries of IND 99-907 and Co 97010. This is in concurrence with abundance of 21 nucleotide sRNA under salinity stress in other crops (Sun et al. 2015; Xie et al. 2017; Yang et al. 2020).
The reference genome sequences are lacking in E. arundinaceus and only the monoploid mosaic genome of sugarcane is available in public databases (Garsmeur et al. 2018) made us to use of our salinity root transcriptome data for prediction of comparative salt responsive miRNA targets in IND 99-907 and Co 97010. Besides, the transcriptome-based miRNA predictions are more informative in uncovering the regulatory network of miRNA in non-model crops which are lacking of the complete genome sequences (Xie et al. 2017; Chen et al. 2019; Zhou et al. 2020; Yang et al. 2021b) justifying the use integrated miRNA-transcriptome profiling to decipher the regulatory network of miRNA and their targets for salinity tolerance. Small RNAs were mapped on to the mature miRNA data on to the sorghum miRNA in miRbase, we identified 362 known miRNAs belonging to 62 families in IND 99-907 and 353 miRNAs belonging to 63 families in Co 97010. The most of the known miRNA identified in IND 99-907 and Co 97010 were reported in other species (Chavez Montes et al. 2014) reaffirms to confidence in sRNA data generated in our studies. We have adopted statistically stringent steps in identification of novel miRNA by aligning against sugarcane monoploid genome (Garsmeur et al. 2018) and minimum threshold of MFEI was − 0.85 (− 68.70 to − 7.00 kcal/mol, − 44.70 to − 8.70 kcal/mol, − 56.40 to − 8.10 kcal/mol and − 68.90 to − 2.60 kcal/mol with an average length of 63, 59, 63 and 62 nt for the four libraries 907C, 907S, 97010C and 97010S respectively) precisely resolve the mature novel miRNA identifications. The stringent bioinformatic and reliable methodologies adopted in our studies in identification of known and novel miRNA helps to uncover the regulatory role of miRNA for salinity stress tolerance in IND 99-907 and Co 97010.
Several studies have demonstrated the role of miRNA in regulating the physiological and agronomic traits and tolerances to biotic and abiotic stresses in sugarcane such as abundance of 100 known miRNAs families for aluminium stress (da Silva et al. 2019), 8 known and 31 novel miRNAs for aerenchyma formation in roots, about 260 known miRNAs for smut disease (Su et al. 2017) and 200 known miRNAs for cold acclimation (Yang et al. 2017). In our studies, we observed 117 known miRNA belongs to 38 families in Co 97010 and 146 known miRNAs belong to 44 families in IND 99-907. Overall, total of 156 known miRNAs belongs to 48 miRNA families were abundant in both genotypes under salinity stress. Most of the known miRNAs were abundant in both genotypes in our studies indicating the complex interactions between known miRNA and their targets. Notably, sbi-miR5568b-5p showed the highest differential upregulation in IND 99-907 and that of sbi-miR5568g-5p upregulated differentially expressed in Co 97010. The families of miR5568 are highly conserved in both monocots and dicots (Chavez Montes et al. 2014), their abundance was reported for salinity stress in wild emmer wheat (Feng et al. 2017) and known to regulate the several abiotic stress tolerance through modulating the target genes such as HSP20 (Nagaraju et al. 2020) and NF-Y genes in sorghum (Maheshwari et al. 2019). In our studies, five miRNAs belonging to miR5568 family were upregulated and positively regulating the 11 unigenes and negatively regulating the two unigenes in IND 99-907. In IND 99-907, sbi-miR5568d-3p upregulated (4.12 logFC) has targets in upregulating the unigenes such as Protein HUA2-LIKE 3 (20.21 log2FC), callose synthase 3-like (8.86 log2FC) and tetratricopeptide repeat protein 38 isoform X2 (7.93 log2FC) and these targets are associated with biosynthesis of nuclear factor, biosynthesis of callose and abscisic acid signalling (Rosado et al. 2006; Vatén et al. 2011; Jali et al. 2014), whereas in Co 97010, seven miRNA belongs to seven different families have targets in 57 differentially expressed salt responsive unigenes.
The differential expression of miRNA and regulation of their target genes under salinity stress is reported in many crops (Ding et al. 2009; Wang et al. 2019c; Kouhi et al. 2020; Parmar et al. 2020) including sugarcane (Ferreira et al. 2012; Carnavale Bottino et al. 2013; Yang et al. 2017; Silva et al. 2021). In our studies, a total of 221 miRNA belonging to 48 families in IND 99-907 and 130 miRNA belonging to 42 families in Co 97010 were identified. Among them, miRNA families such as sbi-miR156, sbi-miR319, sbi-miR397, sbi-miR1432, sbi-miR2118, sbi-miR5564, sbi-miR6220 in IND 99-907 and sbi-miR390, sbi-miR5381, sbi-miR5567, sbi-miR6226, sbi-miR6235 in Co 97010 were uniquely and differentially expressed in our studies. The uniquely differentially expressed miRNA families of IND 99-907 characterized for their role in regulating the various biotic and abiotic stresses such as miR156 and sbi-miR319 for salt tolerance (Zhou et al. 2013; Kouhi et al. 2020), miR1432, miR2118 and miR6220 for drought tolerance (Wu et al. 2015; Nagaraju et al. 2019; Li et al. 2021b); miR397 and miR5564 for herbicide tolerance, nutrient deficiency and biomass accumulation in many crops (Pan et al. 2017; Patel et al. 2019; Zhu et al. 2021).
Many known miRNA regulating various biotic and abiotic stresses are also found in our study. The miRNA miR5568 was reported to upregulated under salinity stress (Feng et al. 2017) and its family members viz., sbi-miR5568b-5p, sbi-miR5568e-3p and sbi-miR5568g-3p were upregulated only in IND 99-907, whereas sbi-miR5568g-5p, sbi-miR5568e-5p and sbi-miR5568f-3p were expressed only in Co 97010. The monocot specific miR6220 (Chavez Montes et al. 2014) family member sbi-miR6220-5p was upregulated in IND 99-907 and did not differentially expressed in Co 97010. Besides, miR160 regulates the leaf development and conferring tolerance to drought (Yang et al. 2019), In this study, sbi-miR160b was upregulated in salinity tolerant clone IND 99-907 and sbi-miR160f was down regulated in salinity sensitive genotype Co 97010. MiR396 regulating the vernalization and triggering the immune response to biotic stresses, its family member, miR396a was upregulated only in IND 99–907, miR396b was upregulated in IND 99-907 and down regulated in Co 97010. Similarly, 221 and 130 known miRNA were differentially expressed in IND 99-907 and Co 97010 provides insight into the role of miRNA and their targets conferring salt tolerance in E. arundinaceus.
The sequencing of vascular plants revealed that miR6220 family was monocot specific, less conserved across plant systems, 21 and 24 nucleotide types were most abundant and their abundance ranged 1–10 per million reads (Chavez Montes et al. 2014). The abundance of miR6220 was reported for water stress in S. narenga (Liu et al. 2018), drought and salt tolerance in sorghum (Katiyar et al. 2015; Nagaraju et al. 2019), salt stress in wheat (Feng et al. 2017) and phosphate deficiency in maize (Gupta et al. 2017). In our study, sbi-miR6220-3p were abundant in both IND 99-907 and Co 97010, whereas, sbi-miR6220-5p expressed only in IND 99-907. The predicted targets for sbi-miR6220-5p in our salt transcriptome data (Vignesh et al. 2021) were phosphoglucan phosphatase DSP4 (ERI-C_DN2351_c0_g1_i4.p1), callose synthase 3-like (ERI-S_DN0_c1_g3_i3.p1) and chromatin modification-related protein EAF1 B isoform X1 (ERI-S_DN1267_c0_g1_i27.p1). The abundance of miR6220 under salt tolerance was reported in Aegilops tauschii (Feng et al. 2017) and it is a part of histone acetyltransferase complex of EF1B associated with DNA repair mechanism (Auger et al. 2008). Callose synthase 3-like associated biosynthesis of callose and its accumulation in plasmodesmata regulate the movement of transcriptional factors and miRNA (Vatén et al. 2011) and phosphoglucan phosphatase DSP4 associated with starch degradation during the cold acclimation (Berrocal-Lobo et al. 2011).
The miR6232 abundant in plant system (Nithin et al. 2017) and are regulating the tolerances to biotic and abiotic stresses in plants. It has target genes for abiotic stress tolerance such as HSP70 (Buyuk et al. 2016) and sorghum nuclear factor Y family (Maheshwari et al. 2019). In our study, sbi-miR6232b-5p was abundant under stressed condition and sbi-miR6232a-5p under controlled condition IND 99-907 and its expression was not found in Co 97010. The target identification from our transcriptome data has showed that miR6232b-5p has targets in 13 differentially expressed unigenes in stressed sample of IND 99-907 and eight unigenes in control sample of IND 99-907. The differentially expressed targets and miRNA analysis showed that miR6232b-5p binds to 3′ UTR regions of pre-mRNA splicing factor CWC22 (ERI-S_DN5828_c0_g1_i99.p2, upregulated by 19.47 logFC) and regulates the mRNA processing and confer tolerance to abiotic stress tolerance (Steckelberg et al. 2012; Han et al. 2017; Zhu et al. 2020). The miR6232b-5p interacts with 3′ UTR and coding regions of the chromatin modification protein EAF1-B (ERI-S_DN1267_c0_g1_i27.p1; upregulated by 20.42 logFC), thereby regulates the DNA repair mechanism during abiotic stress tolerance (Auger et al. 2008; Feng et al. 2017). The FAB1D phosphatidyl inositol kinase has targets for miR6232b-5p in the coding regions and FAB1D (ERI-S_DN5272_c0_g1_i5.p1, upregulated by 9.38 logFC) associated with synthesis of phospholipids from phosphatidylinositol and regulates various physiological responses in development, biotic and abiotic stress responses (Serrazina et al. 2014; Hirano and Sato 2019; Mueller-Roeber and Pical 2002). The TRRAP (ERI-S_DN4770_c0_g1_i40.p1, upregulated by 9.19 logFC) regulates the replication, transcription and DNA repair (McMahon et al. 1998; Murr et al. 2007), has target for miR6232-5p at 3′ UTR region. Besides, other upregulated targets for miR6232b-5p are MAPKKK (ERI-S_DN2233_c0_g1_i38.p1, upregulated by 7.66 logFC) associated with ABA signal transduction has targets at 3′ UTR, aldehyde oxidase isoforms (ERI-S_DN16627_c1_g1_i1.p1, upregulated by 3.11 logFC) regulated the ABA biosynthesis and secondary metabolites (Omarov et al. 2003; Colasuonno et al. 2017; Srivastava et al. 2017) has targets at coding regions, plasma membrane type calcium transporting ATPase 5 (ERI-S_DN2288_c0_g1_i14.p1, upregulated by 1.92 logFC) regulates calcium mediated signalling during abiotic stresses (Huda et al. 2013) has target at coding regions. Sucrose Transport Protein SUT4 and protein RDM16 regulates the DNA methylation and cytokinin signalling (Sharma et al. 2017; Lv et al. 2021) has targets at coding regions for miR6232b-5p.
Conclusion
We performed the comparative transcriptome and miRNA profiling analysis between salinity stressed and control root samples of salt tolerant E. arundinacesus clone IND 99-907 and salt-sensitive sugarcane genotype Co 97010. Differentially expressed miRNA used to predict their target mRNAs using transcriptome data. We identified differentially expressed miRNA in both genotypes and their uniquely expression in control and stressed samples in salt tolerant and salt-sensitive sugarcane genotypes. The gene ontology analysis of miRNA targets predicted from transcriptome data revealed that many salt responsive processes such as transport, signal transduction, response to abiotic stimulus, response to stress and cellular developmental processes GO terms were significantly enriched under salinity stress. Many target genes related to biotic and abiotic stresses were differentially expressed in our studies. Further studies are required to overexpress miRNA and their targets for utilization in the crop improvement programmes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are respectfully thanking and acknowledging the financial support from ‘Science and Engineering Research Board, Department of Science and Technology (DST-SERB)’ under Early Career Research Award (File No.: DST/SERB/ECR/2017/000349). We are also thankful to AgriGenome Labs Pvt Ltd, Cochin, India for transcriptome and small RNA sequencing work. We thankfully acknowledge the ICAR-Sugarcane Breeding Institute, Coimbatore for kind support for the study.
Author contributions
PV & CM: equal contributions to research work or shared first co-authors and bioinformatics data analysis. CM, CA: project formulation and execution of field work. PV, CM, CA: project formulation, bioinformatics flow of data analysis, designed the experiment for performing genome assembly and differential gene expression. KS, CM, CA: execution of field work. CM: uploaded the raw data of small RNA Seq to NCBI website. CM, VS, HKM, KS: RNA isolation and performed qRT-PCR validation. PS, CM: prepared all tables, figures and wrote the manuscript. CM, CA: overall supervised the experiment. All authors read the manuscript and approved.
Funding
This research was supported by the financial grant received from ‘Science and Engineering Research Board, Department of Science and Technology (DST-SERB)’ under the “Early Career Research Award (File No.: DST/SERB/ECR/2017/000349)”.
Data availability
The Illumina raw reads of samples of transcriptome were deposited in NCBI BIO PROJECT with accession number PRJNA716503 and raw data of Small RNA-Seq of stressed and control samples of IND 99-907 and Co 97010 were deposited in NCBI BIO PROJECT with accession number PRJNA888452, PRJNA888470, PRJNA889536 and PRJNA889539 respectively.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Experimental material involves the research between sugarcane wild relative Erianthus arundinaceus and modern sugarcane genotype (Saccharum spp) is in compliance with institutional, national, and international guidelines and legislation.
Footnotes
$Palanisamy Vignesh and Channappa Mahadevaiah shared first authors and authors with equal contributions.
Contributor Information
Channappa Mahadevaiah, Email: C.Mahadevaiah@icar.gov.in.
Chinnaswamy Appunu, Email: cappunu@gmail.com.
References
- Andrews S (2015) FASTQC A quality control tool for high throughput sequence data. In: Babraham Inst. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/. Accessed Dec 2021
- Aravind J, Rinku S, Pooja B, Shikha M, Kaliyugam S, Mallikarjuna MG, Kumar A, Rao AR, Nepolean T. Identification, characterization, and functional validation of drought-responsive microRNAs in subtropical maize inbreds. Front Plant Sci. 2017;8:941. doi: 10.3389/fpls.2017.00941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf F, Ashraf MA, Hu X, Zhang S. A novel computational approach to the silencing of sugarcane Bacilliform Guadeloupe a virus determines potential host-derived MicroRNAs in sugarcane (Saccharum officinarum L.) Peer J. 2020;8:e8359. doi: 10.7717/peerj.8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger A, Galarneau L, Altaf M, Nourani A, Doyon Y, Utley RT, Cronier D, Allard S, Cote J. Eaf1 is the platform for NuA4 molecular assembly that evolutionarily links chromatin acetylation to ATP-dependent exchange of histone H2A variants. Mol Cell Biol. 2008;28:2257–2270. doi: 10.1128/mcb.01755-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine SM, Narayan JA, Syamaladevi DP, Appunu C, Chakravarthi M, Ravichandran V, Subramonian N. Erianthus arundinaceus HSP70 (EaHSP70) overexpression increases drought and salinity tolerance in sugarcane (Saccharum spp. hybrid) Plant Sci. 2015;232:23–34. doi: 10.1016/j.plantsci.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Augustine SM, Ashwin Narayan J, Syamaladevi DP, Appunu C, Chakravarthi M, Ravichandran V, Tuteja N, Subramonian N. Overexpression of EaDREB2 and pyramiding of EaDREB2 with the pea DNA helicase gene (PDH45) enhance drought and salinity tolerance in sugarcane (Saccharum spp. hybrid) Plant Cell Rep. 2015;34:247–263. doi: 10.1007/s00299-014-1704-6. [DOI] [PubMed] [Google Scholar]
- Bai Q, Wang X, Chen X, Shi G, Liu Z, Guo C, Xiao K. Wheat miRNA TaemiR408 acts as an essential mediator in plant tolerance to Pi deprivation and salt stress via modulating stress-associated physiological processes. Front Plant Sci. 2018;9:1–17. doi: 10.3389/fpls.2018.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandehagh A, Taylor NL. Can alternative metabolic pathways and shunts overcome salinity induced inhibition of central carbon metabolism in crops? Front Plant Sci. 2020;11:1–18. doi: 10.3389/fpls.2020.01072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrocal-Lobo M, Ibanez C, Acebo P, Ramos A, Perez-Solis ES, Collada C, Casado R, Aragoncillo C, Allona I. Identification of a homolog of Arabidopsis DSP4 (SEX4) in chestnut: its induction and accumulation in stem amyloplasts during winter or in response to the cold. Plant, Cell Environ. 2011;34:1693–1704. doi: 10.1111/j.1365-3040.2011.02365.x. [DOI] [PubMed] [Google Scholar]
- Bohra A, Gandham P, Rathore A, Thakur V, Saxena RK, Naik SS, Varshney RK, Singh NP. Identification of microRNAs and their gene targets in cytoplasmic male sterile and fertile maintainer lines of pigeonpea. Planta. 2021;253:1–15. doi: 10.1007/s00425-021-03568-6. [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen P, Sakvarelidze-Achard L, Bruun-Rasmussen M, Dunoyer P, Yamamoto YY, Sieburth L, Voinnet O. Widespread translational inhibition by plant miRNAs and siRNAs. Sci. 2008;320(5880):1185–1190. doi: 10.1126/science.1159151. [DOI] [PubMed] [Google Scholar]
- Buyuk I, Inal B, Ilhan E, Tanriseven M, Aras S, Erayman M. Genome-wide identification of salinity responsive HSP70 s in common bean. Mol Biol Rep. 2016;43:1251–1266. doi: 10.1007/s11033-016-4057-0. [DOI] [PubMed] [Google Scholar]
- Carnavale Bottino M, Rosario S, Grativol C, Thiebaut F, Rojas CA, Farrineli L, Hemerly AS, Ferreira PC. High-throughput sequencing of small RNA transcriptome reveals salt stress regulated microRNAs in sugarcane. PLoS ONE. 2013;8:1–12. doi: 10.1371/journal.pone.0059423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez Montes RA, Rosas-Cárdenas DF, De Paoli E, Accerbi M, Rymarquis LA, Mahalingam G, Marsch-Martínez N, Meyers BC, Green PJ, De Folter S. Sample sequencing of vascular plants demonstrates widespread conservation and divergence of microRNAs. Nat Commun. 2014;5:1–15. doi: 10.1038/ncomms4722. [DOI] [PubMed] [Google Scholar]
- Chen H, Yang Q, Chen K, Zhao S, Zhang C, Pan R, Cai T, Deng Y, Wang X, Chen Y, Chu W. Integrated microRNA and transcriptome profiling reveals a miRNA-mediated regulatory network of embryo abortion under calcium deficiency in peanut (Arachis hypogaea L.) BMC Genom. 2019;20:1–17. doi: 10.1186/s12864-019-5770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, He Q, Tang S, Wang H, Zhang X, Lv M, Liu H, Gao Q, Zhou Y, Wang Q, Man X. The miR172/IDS1 signaling module confers salt tolerance through maintaining ROS homeostasis in cereal crops. New Phytol. 2021;230:1017–1033. doi: 10.1111/nph.17211. [DOI] [PubMed] [Google Scholar]
- Chopperla R, Mangrauthia SK, Bhaskar Rao T, Balakrishnan M, Balachandran SM, Prakasam V, Channappa G. A comprehensive analysis of MicroRNAs expressed in susceptible and resistant rice cultivars during Rhizoctonia solani AG1-IA infection causing sheath blight disease. Int J Mol Sci. 2020;21:1–25. doi: 10.3390/ijms21217974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarancia PS, Naveenarani M, Valarmathi R, Suresha GS, Hemaprabha G, Ram B, Appunu C. Isolation, characterization and expression analysis of novel water deficit stress-responsive DEEPER ROOTING 1 (DRO1) gene from drought-tolerant Erianthus arundinaceus. J Sugarcane Res. 2020;10(1):1–11. doi: 10.37580/JSR.2020.1.10.1-11. [DOI] [Google Scholar]
- Colasuonno P, Marcotuli I, Lozito ML, Simeone R, Blanco A, Gadaleta A. Characterization of aldehyde oxidase (AO) genes involved in the accumulation of carotenoid pigments in wheat grain. Front Plant Sci. 2017;8:1–11. doi: 10.3389/fpls.2017.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Wang JJ, Zhao JH, Fang YY, He XF, Guo HS, Duan CG. A Brassica miRNA regulates plant growth and immunity through distinct modes of action. Mol Plant. 2020;13:231–245. doi: 10.1016/j.molp.2019.11.010. [DOI] [PubMed] [Google Scholar]
- da Silva MD, de Oliveira Silva RL, Neto JR, Benko-Iseppon AM, Kido EA. Genotype-dependent regulation of drought-responsive genes in tolerant and sensitive sugarcane cultivars. Gene. 2017;633:17–27. doi: 10.1016/j.gene.2017.08.022. [DOI] [PubMed] [Google Scholar]
- da Silva RG, Rosa-Santos TM, Franca SD, Kottapalli P, Kottapalli KR, Zingaretti SM. Microtranscriptome analysis of sugarcane cultivars in response to aluminum stress. PLoS ONE. 2019;14:1–12. doi: 10.1371/journal.pone.0217806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Zhuang Z, Zhao PX. PsRNATarget: a plant small RNA target analysis server (2017 release) Nucleic Acids Res. 2018;46(W1):W49–54. doi: 10.1093/nar/gky316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharshini S, Chakravarthi M, Manoj VM, Naveenarani M, Kumar R, Meena M, Ram B, Appunu C. De novo sequencing and transcriptome analysis of a low temperature tolerant Saccharum spontaneum clone IND 00–1037. J Biotechnol. 2016;231:280–294. doi: 10.1016/j.jbiotec.2016.05.036. [DOI] [PubMed] [Google Scholar]
- Ding D, Zhang L, Wang H, Liu Z, Zhang Z, Zheng Y. Differential expression of miRNAs in response to salt stress in maize roots. Ann Bot. 2009;103:29–38. doi: 10.1093/aob/mcn205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z, Rajewsky N. The impact of miRNA target sites in coding sequences and in 3′UTRs. PLoS ONE. 2011;6(3):e18067. doi: 10.1371/journal.pone.0018067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (2008) Harmonized world soil database (version 1.0)
- Feng K, Nie X, Cui L, Deng P, Wang M, Song W. Genome-wide identification and characterization of salinity stress-responsive miRNAS in wild emmer wheat (Triticum turgidum ssp. dicoccoides) Genes (basel) 2017;8:1–28. doi: 10.3390/genes8060156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira TH, Gentile A, Vilela RD, Costa GG, Dias LI, Endres L, Menossi M. microRNAs associated with drought response in the bioenergy crop sugarcane (Saccharum spp.) PLoS ONE. 2012;7:e46703. doi: 10.1371/journal.pone.0046703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander MR, Mackowiak SD, Li N, Chen W, Rajewsky N. MiRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012;40:37–52. doi: 10.1093/nar/gkr688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu R, Zhang M, Zhao Y, He X, Ding C, Wang S, Feng Y, Song X, Li P, Wang B. Identification of salt tolerance-related microRNAs and their targets in maize (Zea mays L.) using high-throughput sequencing and degradome analysis. Front Plant Sci. 2017;8:1–13. doi: 10.3389/fpls.2017.00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Yang L, Zeng HQ, Zhou ZS, Yang ZM, Li H, Sun D, Xie F, Zhang B. A cotton miRNA is involved in regulation of plant response to salt stress. Sci Rep. 2016;6:1–14. doi: 10.1038/srep19736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Nie J, Wang H. MicroRNA biogenesis in plant. Plant Growth Regul. 2021;93:1–12. doi: 10.1007/s10725-020-00654-9. [DOI] [Google Scholar]
- Garsmeur O, Droc G, Antonise R, Grimwood J, Potier B, Aitken K, Jenkins J, Martin G, Charron C, Hervouet C, Costet L. A mosaic monoploid reference sequence for the highly complex genome of sugarcane. Nat Commun. 2018;9(1):2638. doi: 10.1038/s41467-018-05051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S, Kay MA. How do miRNAs mediate translational repression? Silence. 2010;1:1–5. doi: 10.1186/1758-907X-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta B, Huang B. Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genom. 2014;701596:1–18. doi: 10.1155/2014/701596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Kumari M, Kumar H, Varadwaj PK. Genome-wide analysis of miRNAs and Tasi-RNAs in Zea mays in response to phosphate deficiency. Funct Integr Genom. 2017;17:335–351. doi: 10.1007/s10142-016-0538-4. [DOI] [PubMed] [Google Scholar]
- Han N, Ji XL, Du YP, He X, Zhao XJ, Zhai H. Identification of a novel alternative splicing variant of VvPMA1 in grape root under salinity. Front Plant Sci. 2017;8:1–10. doi: 10.3389/fpls.2017.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani A, Azapagic A, Shokri N. Predicting long-term dynamics of soil salinity and sodicity on a global scale. Proc Natl Acad Sci USA. 2020;117:33017–33027. doi: 10.1073/PNAS.2013771117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausser J, Syed AP, Bilen B, Zavolan M. Analysis of CDS-located miRNA target sites suggests that they can effectively inhibit translation. Genome Res. 2013;23:604–615. doi: 10.1101/gr.139758.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Sato MH. Diverse physiological functions of FAB1 and phosphatidylinositol 3, 5-bisphosphate in plants. Front Plant Sci. 2019;10:1–8. doi: 10.3389/fpls.2019.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou G, Du C, Gao H, Liu S, Sun W, Lu H, Kang J, Xie Y, Ma D, Wang C. Identification of microRNAs in developing wheat grain that are potentially involved in regulating grain characteristics and the response to nitrogen levels. BMC Plant Biol. 2020;20:1–21. doi: 10.1186/s12870-020-2296-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh GH, Damsz B, Matsumoto TK, Reddy MP, Rus AM, Ibeas JI, Narasimhan ML, Bressan RA, Hasegawa PM. Salt causes ion disequilibrium-induced programmed cell death in yeast and plants. Plant J. 2002;29:649–659. doi: 10.1046/j.0960-7412.2001.01247.x. [DOI] [PubMed] [Google Scholar]
- Hussain MI, Farooq M, Muscolo A, Rehman A. Crop diversification and saline water irrigation as potential strategies to save freshwater resources and reclamation of marginal soils—a review. Environ Sci Pollut Res. 2020;27:28695–28729. doi: 10.1007/s11356-020-09111-6. [DOI] [PubMed] [Google Scholar]
- Jali SS, Rosloski SM, Janakirama P, Steffen JG, Zhurov V, Berleth T, Clark RM, Grbic V. A plant-specific HUA2-LIKE (HULK) gene family in Arabidopsis thaliana is essential for development. Plant J. 2014;80:242–254. doi: 10.1111/tpj.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaraj A, Zhang X, Hou Y, Shangguan M, Gajjeraman P, Li Y, Wei C. Genome-wide identification of conserved and novel microRNAs in one bud and two tender leaves of tea plant (Camellia sinensis) by small RNA sequencing, microarray-based hybridization and genome survey scaffold sequences. BMC Plant Biol. 2017;17:1–16. doi: 10.1186/s12870-017-1169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Zhou L, Chen W, Ye N, Xia J, Zhuang C. Overexpression of a microRNA-targeted NAC transcription factor improves drought and salt tolerance in Rice via ABA-mediated pathways. Rice. 2019;12:1–1. doi: 10.1186/s12284-019-0334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KW, Jeong TU, Hwang MJ, Kim K, Ahn KH. Phosphate adsorption ability of biochar/Mg-Al assembled nanocomposites prepared by aluminum-electrode based electro-assisted modification method with MgCl2 as electrolyte. Bioresour Technol. 2015;198:603–610. doi: 10.1016/j.biortech.2015.09.068. [DOI] [PubMed] [Google Scholar]
- Katiyar A, Smita S, Muthusamy SK, Chinnusamy V, Pandey DM, Bansal KC. Identification of novel drought-responsive microRNAs and trans-acting siRNAs from Sorghum bicolor (L.) Moench by high-throughput sequencing analysis. Front Plant Sci. 2015;6:1–21. doi: 10.3389/fpls.2015.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuhara M. Apoptosis-like cell death in barley roots under salt stress. Plant Cell Physiol. 1997;38:1091–1093. doi: 10.1093/oxfordjournals.pcp.a029277. [DOI] [Google Scholar]
- Kouhi F, Sorkheh K, Ercisli S. MicroRNA expression patterns unveil differential expression of conserved miRNAs and target genes against abiotic stress in safflower. PLoS ONE. 2020;15:1–18. doi: 10.1371/journal.pone.0228850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Appunu C, Anna Durai A, Premachandran MN, Raffee Viola V, Ram B, Mahadevaiah C, Meena MR, Manjunatha T. Genetic confirmation and field performance comparison for yield and quality among advanced generations of Erianthus arundinaceus, E. bengalense and Saccharum spontaneum cyto-nuclear genome introgressed sugarcane intergeneric hybrids. Sugar Tech. 2015;17:379–385. doi: 10.1007/s12355-014-0333-2. [DOI] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2013;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10(3):1–10. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. 1000 Genome project data processing subgroup. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Liu Z, Gao L, Wang L, Gao M, Jiao Z, Qiao H, Yang J, Chen M, Yao L, Liu R. Genome-wide identification and characterization of micrornas in developing grains of Zea mays L. PLoS ONE. 2016;11:1–18. doi: 10.1371/journal.pone.0153168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Xu R, Li N. MicroRNAs from plants to animals, do they define a new messenger for communication? Nutr Metab. 2018;15:1–21. doi: 10.1186/s12986-018-0311-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wu K, Ruan C, Pan J, Wang Y, Long H. Cost-reduction strategies in massive genomics experiments. Mar Life Sci Technol. 2019;1:15–21. doi: 10.1007/s42995-019-00013-2. [DOI] [Google Scholar]
- Li H, Meng H, Sun X, Deng J, Shi T, Zhu L, Lv Q, Chen Q. Integrated microRNA and transcriptome profiling reveal key miRNA-mRNA interaction pairs associated with seed development in Tartary buckwheat (Fagopyrum tataricum) BMC Plant Biol. 2021;21:1–20. doi: 10.1186/s12870-021-02914-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zheng YP, Zhou XH, Yang XM, He XR, Feng Q, Zhu Y, Li GB, Wang H, Zhao JH, Hu XH. Rice miR1432 fine-tunes the balance of yield and blast disease resistance via different modules. Rice. 2021;14:1–27. doi: 10.1186/s12284-021-00529-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MH, Borg S, Padmanaban A, Saligrama D, Walker DM, Inche A, Elliott J, Schmidt E. Automated assessment of next generation sequencing library preparation workflow for quality and quantity using the Agilent 2200 TapeStation System. J Biomol Tech. 2014;25:S18. [Google Scholar]
- Liu X, Zhang R, Ou H, Gui Y, Wei J, Zhou H, Tan H, Li Y. Comprehensive transcriptome analysis reveals genes in response to water deficit in the leaves of Saccharum narenga (Nees ex Steud) hack. BMC Plant Biol. 2018;18(1):1–16. doi: 10.1186/s12870-018-1428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014 doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv B, Hu K, Tian T, Wei K, Zhang F, Jia Y, Tian H, Ding Z. The pre-mRNA splicing factor RDM16 regulates root stem cell maintenance in Arabidopsis. J Integr Plant Biol. 2021;63:662–678. doi: 10.1111/jipb.13006. [DOI] [PubMed] [Google Scholar]
- Lytle JR, Yario TA, Steitz JA. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5′ UTR as in the 3′ UTR. Proc Natl Acad Sci USA. 2007;104:9667–9672. doi: 10.1073/pnas.0703820104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari P, Kummari D, Palakolanu SR, Nagasai Tejaswi U, Nagaraju M, Rajasheker G, Jawahar G, Jalaja N, Rathnagiri P, Kavi Kishor PB. Genome-wide identification and expression profile analysis of nuclear factor Y family genes in Sorghum bicolor L. (Moench) PLoS ONE. 2019;14:1–27. doi: 10.1371/journal.pone.0222203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoj VM, Anunanthini P, Swathik PC, Dharshini S, Ashwin Narayan J, Manickavasagam M, Sathishkumar R, Suresha GS, Hemaprabha G, Ram B, Appunu C. Comparative analysis of glyoxalase pathway genes in Erianthus arundinaceus and commercial sugarcane hybrid under salinity and drought conditions. BMC Genom. 2019;19:1–6. doi: 10.1186/s12864-018-5349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet J. 2011;17(1):10–12. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- McMahon SB, Van Buskirk HA, Dugan KA, Copeland TD, Cole MD. The novel ATM-related protein TRRAP is an essential cofactor for the c- Myc and E2F oncoproteins. Cell. 1998;94:363–374. doi: 10.1016/S0092-8674(00)81479-8. [DOI] [PubMed] [Google Scholar]
- Meena MR, Kumar R, Ramaiyan K, Chhabra ML, Raja AK, Krishnasamy M, Kulshreshtha N, Pandey SK, Ram B. Biomass potential of novel interspecific and intergeneric hybrids of Saccharum grown in sub-tropical climates. Sci Rep. 2020;10:1–12. doi: 10.1038/s41598-020-78329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers BC, Axtell MJ, Bartel B, Bartel DP, Baulcombe D, Bowman JL, Cao X, Carrington JC, Chen X, Green PJ, Griffiths-Jones S. Criteria for annotation of plant microRNAs. Plant Cell. 2008;20:3186–3190. doi: 10.1105/tpc.108.064311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanan MV, Pushpanathan A, Padmanabhan S, Sasikumar T, Jayanarayanan AN, Selvarajan D, Ramalingam S, Ram B, Chinnaswamy A. Overexpression of glyoxalase III gene in transgenic sugarcane confers enhanced performance under salinity stress. J Plant Res. 2021;134:1083–1094. doi: 10.1007/s10265-021-01300-9. [DOI] [PubMed] [Google Scholar]
- Mueller-Roeber B, Pical C. Inositol phospholipid metabolism in Arabidopsis. Characterized and putative isoforms of inositol phospholipid kinase and phosphoinositide-specific phospholipase C. Plant Physiol. 2002;130(1):22–46. doi: 10.1104/pp.004770.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir P, Li S, Lou S, Wang D, Spakowicz DJ, Salichos L, Zhang J, Weinstock GM, Isaacs F, Rozowsky J, Gerstein M. The real cost of sequencing: scaling computation to keep pace with data generation. Genome Biol. 2016;17:1–9. doi: 10.1186/s13059-016-0917-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Murr R, Vaissiere T, Sawan C, Shukla V, Herceg Z. Orchestration of chromatin-based processes: mind the TRRAP. Oncogene. 2007;26:5358–5372. doi: 10.1038/sj.onc.1210605. [DOI] [PubMed] [Google Scholar]
- Nagaraju M, Kumar SA, Reddy PS, Kumar A, Rao DM, Kavi Kishor PB. Genome-scale identification, classification, and tissue specific expression analysis of late embryogenesis abundant (LEA) genes under abiotic stress conditions in Sorghum bicolor L. PLoS ONE. 2019;14:1–27. doi: 10.1371/JOURNAL.PONE.0209980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraju M, Reddy PS, Kumar SA, Kumar A, Rajasheker G, Rao DM, Kishor PK. Genome-wide identification and transcriptional profiling of small heat shock protein gene family under diverse abiotic stress conditions in Sorghum bicolor (L.) Int J Biol Macromol. 2020;142:822–834. doi: 10.1016/j.ijbiomac.2019.10.023. [DOI] [PubMed] [Google Scholar]
- Nair NV, Somarajan K (2003) Diversity for Saccharum germplasm in Kerala. IPGRI Plant Genet Resour Newsl 135: 40–43. ISSN 1020–3362
- Narayan JA, Dharshini S, Manoj VM, Padmanabhan TS, Kadirvelu K, Suresha GS, Subramonian N, Ram B, Premachandran MN, Appunu C. Isolation and characterization of water-deficit stress-responsive α-expansin 1 (EXPA1) gene from Saccharum complex. Biotech. 2019;9:1–3. doi: 10.1007/2Fs13205-019-1719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan JA, Chakravarthi M, Nerkar G, Manoj VM, Dharshini S, Subramonian N, Premachandran MN, Kumar RA, Surendar KK, Hemaprabha G, Ram B. Overexpression of expansin EaEXPA1, a cell wall loosening protein enhances drought tolerance in sugarcane. Ind Crops Prod. 2021;159:113035. doi: 10.1016/j.indcrop.2020.113035. [DOI] [Google Scholar]
- Nithin C, Thomas A, Basak J, Bahadur RP. Genome-wide identification of miRNAs and lncRNAs in Cajanus cajan. BMC Genom. 2017;18:1–14. doi: 10.1186/s12864-017-4232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omarov R, Dräger D, Tischner R, Lips H. Aldehyde oxidase isoforms and subunit composition in roots of barley as affected by ammonium and nitrate. Physiol Plant. 2003;117:337–342. doi: 10.1034/j.1399-3054.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- Orang AV, Safaralizadeh R, Kazemzadeh-Bavili M. Mechanisms of miRNA-mediated gene regulation from common downregulation to mRNA-specific upregulation. Int J Genom. 2014;2014:1–15. doi: 10.1155/2014/970607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WJ, Tao JJ, Cheng T, Bian XH, Wei W, Zhang WK, Ma B, Chen SY, Zhang JS. Soybean miR172a improves salt tolerance and can function as a long-distance signal. Mol Plant. 2016;9:1337–1340. doi: 10.1016/j.molp.2016.05.010. [DOI] [PubMed] [Google Scholar]
- Pan L, Zhao H, Yu Q, Bai L, Dong L. miR397/laccase gene mediated network improves tolerance to fenoxaprop-P-ethyl in Beckmannia syzigachne and Oryza sativa. Front Plant Sci. 2017;8:1–14. doi: 10.3389/fpls.2017.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar P, Singh S, Singh R, Singh VP, Prasad SM. Effect of salinity stress on plants and its tolerance strategies: a review. Environ Sci Pollut Res. 2015;22:4056–4075. doi: 10.1007/s11356-014-3739-1. [DOI] [PubMed] [Google Scholar]
- Parmar S, Gharat SA, Tagirasa R, Chandra T, Behera L, Dash SK, Shaw BP. Identification and expression analysis of miRNAs and elucidation of their role in salt tolerance in rice varieties susceptible and tolerant to salinity. PLoS ONE. 2020;15:1–26. doi: 10.1371/journal.pone.0230958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P, Yadav K, Srivastava AK, Suprasanna P, Ganapathi TR. Overexpression of native Musa-miR397 enhances plant biomass without compromising abiotic stress tolerance in banana. Sci Rep. 2019;9:1–15. doi: 10.1038/s41598-019-52858-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premachandran MN, Sobhakumari VP, Lekshmi M, Raffee Viola V. Genome characterization of in vitro induced amphiploids of an intergeneric hybrid Erianthus arundinaceus × Saccharum spontaneum. Sugar Tech. 2017;19:386–393. doi: 10.1007/s12355-016-0482-6. [DOI] [Google Scholar]
- Queiroz de Pinho Tavares E, Camara Mattos Martins M, Grandis A, Romim GH, Rusiska Piovezani A, Weissmann Gaiarsa J, Silveira Buckeridge M. Newly identified miRNAs may contribute to aerenchyma formation in sugarcane roots. Plant Direct. 2020;4(3):e00204. doi: 10.1002/pld3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado A, Schapire AL, Bressan RA, Harfouche AL, Hasegawa PM, Valpuesta V, Botella MA. The Arabidopsis tetratricopeptide repeat-containing protein TTL1 is required for osmotic stress responses and abscisic acid sensitivity. Plant Physiol. 2006;142:1113–1126. doi: 10.1104/pp.106.085191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena P, Srivastava RP, Sharma ML. Studies on salinity stress tolerance in sugarcane varieties. Sugar Tech. 2010;12:59–63. doi: 10.1007/s12355-010-0011-y. [DOI] [Google Scholar]
- Serrazina S, Dias FV, Malhó R. Characterization of FAB1 phosphatidylinositol kinases in Arabidopsis pollen tube growth and fertilization. New Phytol. 2014;203(3):784–793. doi: 10.1111/nph.12836. [DOI] [PubMed] [Google Scholar]
- Shahid SA, Zaman M, Heng L. Chapter 1. Introduction to soil salinity, sodicity and diagnostics techniques. In: Zaman M, Shahid SA, Heng L, editors. Guideline for salinity assessment, mitigation and adaptation using nuclear and related techniques. Cham, Switzerland: Springer Nature Switzerland AG; 2018. pp. 1–42. [Google Scholar]
- Shahid MA, Sarkhosh A, Khan N, Balal RM, Ali S, Rossi L, Gómez C, Mattson N, Nasim W, Garcia-Sanchez F. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy. 2020;10:1–34. doi: 10.3390/agronomy10070938. [DOI] [Google Scholar]
- Sharma RD, Bogaerts B, Goyal N. RDM16 and STA1 regulate differential usage of exon/intron in RNA directed DNA methylation pathway. Gene. 2017;609:62–67. doi: 10.1016/j.gene.2017.01.027. [DOI] [PubMed] [Google Scholar]
- Shi Y, Xia H, Cheng X, Zhang L. Genome-wide miRNA analysis and integrated network for flavonoid biosynthesis in Osmanthus fragrans. BMC Genom. 2021;22:1–11. doi: 10.1186/s12864-021-07439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JDOL, da Silva RG, Nogueira LDF, Zingaretti SM. MicroRNAs regulate tolerance mechanisms in sugarcane (Saccharum spp.) under aluminum stress. Crop Breed Appl Biotechnol. 2021;21:1–8. doi: 10.1590/1984-70332021v21n1a5. [DOI] [Google Scholar]
- Srivastava S, Brychkova G, Yarmolinsky D, Soltabayeva A, Samani T, Sagi M. Aldehyde oxidase 4 plays a critical role in delaying silique senescence by catalyzing aldehyde detoxification. Plant Physiol. 2017;173:1977–1997. doi: 10.1104/pp.16.01939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckelberg AL, Boehm V, Gromadzka AM, Gehring NH. CWC22 connects Pre-mRNA splicing and exon junction complex assembly. Cell Rep. 2012;2:454–461. doi: 10.1016/j.celrep.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Stevens K. When microRNAs activate translation. Nat Methods. 2008;5:122–123. doi: 10.1038/nmeth0208-122a. [DOI] [Google Scholar]
- Su Y, Zhang Y, Huang N, Liu F, Su W, Xu L, Ahmad W, Wu Q, Guo J, Que Y. Small RNA sequencing reveals a role for sugarcane miRNAs and their targets in response to Sporisorium scitamineum infection. BMC Genom. 2017;18:1–19. doi: 10.1186/s12864-017-3716-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Xu L, Wang Y, Yu R, Zhu X, Luo X, Gong Y, Wang R, Limera C, Zhang K, Liu L. Identification of novel and salt-responsive miRNAs to explore miRNA-mediated regulatory network of salt stress response in radish (Raphanus sativus L.) BMC Genomics. 2015;16:1–16. doi: 10.1186/s12864-015-1416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swathik Clarancia P, Valarmathi R, Suresha GS, Hemaprabha G, Appunu C. Isolation and characterization of drought responsive aldehyde dehydrogenase (ALDH) gene from drought tolerant wild relative of sugarcane Erianthus arundinaceus. J Sugarcane Res. 2021;11(2):180–190. doi: 10.37580/JSR.2021.1.11.56-65. [DOI] [Google Scholar]
- Swathik Clarancia P, Naveenarani M, Ashwin Narayan J, Krishna SS, Thirugnanasambandam PP, Valarmathi R, Suresha GS, Gomathi R, Kumar RA, Manickavasagam M, Jegadeesan R. Genome-wide identification, characterization and expression analysis of plant nuclear factor (NF-Y) gene family transcription factors in Saccharum spp. Genes. 2023;14(6):1147. doi: 10.3390/genes14061147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolstyko E, Lezzhov A, Solovyev A. Identification of miRNA precursors in the phloem of Cucurbita maxima. PeerJ. 2019;2019:1–13. doi: 10.7717/peerj.8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwatoko N, Tanaka M, Saito A, Gau M. Establishment of plant regeneration system in Erianthus arundinaceus (Retz.) Jeswiet, a potential biomass crop. Grassl Sci. 2011;57:231–237. doi: 10.1111/j.1744-697X.2011.00234.x. [DOI] [Google Scholar]
- Vasudevan S. Posttranscriptional upregulation by microRNAs. Wiley Interdiscip Rev RNA. 2012;3:311–330. doi: 10.1002/wrna.121. [DOI] [PubMed] [Google Scholar]
- Vasudevan S, Steitz JA. AU-rich-rlement-mediated upregulation of translation by FXR1 and Argonaute 2. Cell. 2007;128:1105–1118. doi: 10.1016/j.cell.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatén A, Dettmer J, Wu S, Stierhof YD, Miyashima S, Yadav SR, Roberts CJ, Campilho A, Bulone V, Lichtenberger R, Lehesranta S. Callose biosynthesis regulates symplastic trafficking during root development. Dev Cell. 2011;21:1144–1155. doi: 10.1016/j.devcel.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Vignesh P, Mahadevaiah C, Parimalan R, Valarmathi R, Dharshini S, Nisha S, Suresha GS, Swathi S, Mahadeva Swamy HK, Sreenivasa V, Mohanraj K, Hemaprabha G, Ram B, Appunu C. Comparative de novo transcriptome analysis identifies salinity stress responsive genes and metabolic pathways in sugarcane and its wild relative Erianthus arundinaceus [Retzius] Jeswiet. Sci Rep. 2021;11(1):24514. doi: 10.1038/s41598-021-03735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Born P, Bernardo-Faura M, Rubio-Somoza I. An artificial miRNA system reveals that relative contribution of translational inhibition to miRNA-mediated regulation depends on environmental and developmental factors in Arabidopsis thaliana. PLoS ONE. 2018;13:1–14. doi: 10.1371/journal.pone.0192984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed S, Zeng L. The critical role of miRNAs in regulation of flowering time and flower development. Genes (basel) 2020;11:1–24. doi: 10.3390/genes11030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Mei J, Ren G. Plant microRNAs: biogenesis, homeostasis, and degradation. Front Plant Sci. 2019;10:1–12. doi: 10.3389/fpls.2019.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Li R, Wang H, Qi B, Jiang X, Zhu Q, Cai D, Tang X, Zhao Q. Sweetcane (Erianthus arundinaceus) as a native bioenergy crop with environmental remediation potential in southern China: a review. GCB Bioenergy. 2019;11:1012–1025. doi: 10.1111/gcbb.12600. [DOI] [Google Scholar]
- Wang W, Liu D, Chen D, Cheng Y, Zhang X, Song L, Hu M, Dong J, Shen F. MicroRNA414c affects salt tolerance of cotton by regulating reactive oxygen species metabolism under salinity stress. RNA Biol. 2019;16:362–375. doi: 10.1080/15476286.2019.1574163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker CA, Souza-Chies TT, Longhi-Wagner HM, Peichoto MC, McKain MR, Kellogg EA. Phylogenetic analysis of Saccharum S.L. (Poaceae; Andropogoneae), with emphasis on the circumscription of the south American species. Am J Bot. 2015;102:248–263. doi: 10.3732/ajb.1400397. [DOI] [PubMed] [Google Scholar]
- Welker CA, McKain MR, Vorontsova MS, Peichoto MC, Kellogg EA. Plastome phylogenomics of sugarcane and relatives confirms the segregation of the genus Tripidium (Poaceae: Andropogoneae) Taxon. 2019;68:246–267. doi: 10.1002/tax.12030. [DOI] [Google Scholar]
- Wu BF, Li WF, Xu HY, Qi LW, Han SY. Role of cin-miR2118 in drought stress responses in Caragana intermedia and Tobacco. Gene. 2015;574:34–40. doi: 10.1016/j.gene.2015.07.072. [DOI] [PubMed] [Google Scholar]
- Xie R, Zhang J, Ma Y, Pan X, Dong C, Pang S, He S, Deng L, Yi S, Zheng Y, Lv Q. Combined analysis of mRNA and miRNA identifies dehydration and salinity responsive key molecular players in citrus roots. Sci Rep. 2017;7(1):42094. doi: 10.1038/srep42094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Chen X, Chen Y, Zhang Q, Su L, Chen X, Chen Y, Zhang Z, Lin Y, Lai Z. Genome-wide identification of miRNAs and their targets during early somatic embryogenesis in Dimocarpus longan Lour. Sci Rep. 2020;10(1):4626. doi: 10.1038/s41598-020-60946-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zhang X, Su Y, Zou J, Wang Z, Xu L, Que Y. miRNA alteration is an important mechanism in sugarcane response to low-temperature environment. BMC Genomics. 2017;18:1–18. doi: 10.1186/s12864-017-4231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Wang Y, Teotia S, Wang Z, Shi C, Sun H, Gu Y, Zhang Z, Tang G. The interaction between miR160 and miR165/166 in the control of leaf development and drought tolerance in Arabidopsis. Sci Rep. 2019;9(1):2832. doi: 10.1038/s41598-019-39397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Zhu P, Kang H, Liu L, Cao Q, Sun J, Dong T, Zhu M, Li Z, Xu T. High-throughput deep sequencing reveals the important role that microRNAs play in the salt response in sweet potato (Ipomoea batatas L.) BMC Genomics. 2020;21:1–6. doi: 10.1186/s12864-020-6567-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Zhang L, Yang Y, Schmid M, Wang Y. Mirna mediated regulation and interaction between plants and pathogens. Int J Mol Sci. 2021;22(6):2913. doi: 10.3390/ijms22062913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YH, Li MJ, Yi YJ, Li RF, Li CX, Yang H, Wang J, Zhou JX, Shang S, Zhang ZY. Integrated miRNA-mRNA analysis reveals the roles of miRNAs in the replanting benefit of Achyranthes bidentata roots. Sci Rep. 2021;11(1):1628. doi: 10.1038/s41598-021-81277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Xie S, Liu Y, Qi X, Yu J. Genome-wide characterization of microRNA in foxtail millet (Setaria italica) BMC Plant Biol. 2013;13:1–15. doi: 10.1186/1471-2229-13-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Li Z, Li D, Yuan N, Hu Q, Luo H. Constitutive expression of rice microRNA528 alters plant development and enhances tolerance to salinity stress and nitrogen starvation in creeping bentgrass. Plant Physiol. 2015;169:576–593. doi: 10.1104/pp.15.00899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Li Z, Yuan N, Hu Q, Zhou M, Zhao J, Li D, Luo H. MiR396 is involved in plant response to vernalization and flower development in Agrostis stolonifera. Hortic Res. 2020;7:173. doi: 10.1038/s41438-020-00394-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue E, Cao H, Liu B. Osmir535, a potential genetic editing target for drought and salinity stress tolerance in Oryza sativa. Plants. 2020;9:1–10. doi: 10.3390/plants9101337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunita R, Hartati RS, Suhesti S, Syafaruddin Response of bululawang sugarcane variety to salt stress. IOP Conf Ser Earth Environ Sci. 2020;418(1):012060. doi: 10.1088/1755-1315/418/1/012060. [DOI] [Google Scholar]
- Zhang B, Pan X, Cobb GP, Anderson TA. Plant microRNA: a small regulatory molecule with big impact. Dev Biol. 2006;289:3–16. doi: 10.1016/j.ydbio.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Zhang JW, Long Y, Xue MD, Xiao XG, Pei XW. Identification of microRNAs in response to drought in common wild rice (Oryza rufipogon Griff.) shoots and roots. PLoS ONE. 2017;12:1–18. doi: 10.1371/journal.pone.0170330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Zhu K, Momotaz A, Gao X. Sugarcane plant growth and physiological responses to soil salinity during tillering and stalk elongation. Agric. 2020;10:1–13. doi: 10.3390/agriculture10120608. [DOI] [Google Scholar]
- Zhou M, Li D, Li Z, Hu Q, Yang C, Zhu L, Luo H. Constitutive expression of a miR319 gene alters plant development and enhances salt and drought tolerance in transgenic creeping bentgrass. Plant Physiol. 2013;161:1375–1391. doi: 10.1104/pp.112.208702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Zheng S, Liu R, Lu L, Zhang C, Zhang L, Yant L, Wu Y. The genome-wide impact of cadmium on microRNA and mRNA expression in contrasting Cd responsive wheat genotypes. BMC Genom. 2019;20:1–19. doi: 10.1186/s12864-019-5939-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Luo S, Hameed S, Xiao D, Zhan J, Wang A, He L. Integrated mRNA and miRNA transcriptome analysis reveals a regulatory network for tuber expansion in Chinese yam (Dioscorea opposita) BMC Genomics. 2020;21:1–18. doi: 10.1186/s12864-020-6492-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Mao F, Tian Y, Lin X, Gu L, Gu H, Qu LJ, Wu Y, Wu Z. The features and regulation of co-transcriptional splicing in Arabidopsis. Mol Plant. 2020;13:278–294. doi: 10.1016/j.molp.2019.11.004. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Li D, Cong L, Lu X. Identification of microRNAs involved in crosstalk between nitrogen, phosphorus and potassium under multiple nutrient deficiency in sorghum. Crop J. 2021;9:465–475. doi: 10.1016/j.cj.2020.07.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Illumina raw reads of samples of transcriptome were deposited in NCBI BIO PROJECT with accession number PRJNA716503 and raw data of Small RNA-Seq of stressed and control samples of IND 99-907 and Co 97010 were deposited in NCBI BIO PROJECT with accession number PRJNA888452, PRJNA888470, PRJNA889536 and PRJNA889539 respectively.