ABSTRACT
The retroviral Gag protein of human immunodeficiency virus type 1 (HIV-1) plays a central role in the selection of unspliced viral genomic RNA (gRNA) for packaging into new virions. Previously, we demonstrated that full-length HIV-1 Gag undergoes nuclear trafficking, where it associates with unspliced viral RNA (USvRNA) at transcription sites. To further examine the kinetics of HIV-1 Gag nuclear localization, we used biochemical and imaging techniques to determine the timing of HIV-1 entry into the nucleus. We also aimed to determine more precisely Gag’s subnuclear distribution to test the hypothesis that Gag associates with euchromatin, the transcriptionally active region of the nucleus. We observed that HIV-1 Gag localized to the nucleus at low expression levels shortly after its synthesis in the cytoplasm, suggesting that nuclear trafficking was not strictly concentration dependent. Furthermore, we found that HIV-1 Gag preferentially localized to the transcriptionally active euchromatin fraction compared to the heterochromatin-rich region in a latently infected T-cell line (J-Lat 10.6) treated with latency-reversal agents. Interestingly, HIV-1 Gag was more closely co-localized with euchromatin-associated histone marks near the nuclear periphery, the preferred location of HIV-1 proviral integration. Although the precise function of Gag’s association with histones in transcriptionally active chromatin regions remains uncertain, together with previous reports, this finding is consistent with a potential role for euchromatin-associated Gag molecules to initiate the selection of newly transcribed USvRNA in the nucleus for incorporation into virions.
IMPORTANCE
The traditional view of retrovirus assembly posits that packaging of gRNA by HIV-1 Gag occurs in the cytoplasm or at the plasma membrane. However, our previous studies showing that HIV-1 Gag enters the nucleus and binds to USvRNA at transcription sites suggest that gRNA selection may occur in the nucleus. In the present study, we observed that HIV-1 Gag trafficked to the nucleus and co-localized with USvRNA within 8 hours of expression. In infected T cells (J-Lat 10.6) reactivated from latency and in a HeLa cell line stably expressing an inducible Rev-dependent HIV-1 construct, we found that Gag preferentially localized with euchromatin histone marks associated with enhancer and promoter regions near the nuclear periphery, which is the favored site HIV-1 integration. These observations support the innovative hypothesis that HIV-1 Gag associates with euchromatin-associated histones to localize to active transcription sites, promoting capture of newly synthesized gRNA for packaging.
KEYWORDS: HIV-1 Gag, retrovirus assembly, nuclear trafficking, euchromatin localization, subcellular fractionation, HIV-1 latency reversal
INTRODUCTION
Retroviruses reverse transcribe their genomic RNA (gRNA) into double-stranded proviral DNA that stably integrates into the host chromosome. Following integration, which typically occurs near the periphery of the nucleus (1–5), host transcription machinery is used to generate nascent unspliced viral RNA (USvRNA) that is selected by Gag and packaged as genomes into new virions [reviewed in references (6, 7)]. The retroviral Gag polyprotein is the major structural polyprotein and contains several conserved domains, including matrix, capsid (CA), nucleocapsid (NC), and p6, which are individually cleaved during virus maturation [reviewed in reference (8)]. To select gRNA for incorporation into assembling virions, the NC domain of Gag recognizes and selectively binds to the psi packaging signal positioned at the 5′ end of the gRNA transcript, forming a ribonucleoprotein (RNP) complex [reviewed in references (7, 9)]. Deletion of the NC domain diminishes Gag’s ability to package gRNA into new virus particles and decreases virus-like particle production (10). Although the binding of Gag to gRNA has been well characterized using in vitro and cell-based studies, the mechanisms that govern the gRNA selection process are being reexamined after the discovery of Gag nuclear trafficking (11–13).
Gag proteins of various retroviruses, including human immunodeficiency virus type-1 (HIV-1) (11–13), Rous sarcoma virus (RSV) (12, 14–19), and mouse leukemia virus (20), have been found to undergo nuclear localization [reviewed in reference (21)]. For RSV, reduction of Gag nuclear trafficking results in a decrease in packaging of gRNA into virions (17), indicating that efficient genome packaging depends not only on NC binding but also on nuclear trafficking of Gag. We have shown that both RSV and HIV Gag proteins co-localize with their cognate USvRNAs at distinct foci in the perichromatin space and at transcriptional burst sites, suggesting that a subset of Gag traffics to proviral integration sites where new RNA is synthesized (13, 16). However, the timing of HIV-1 viral ribonucleoprotein complex (vRNP) formation has remained undefined, and it is unclear whether Gag engages with host factors in specific subdomains of the nucleus. In this work, we examined the temporospatial properties of HIV-1 Gag nuclear entry, its association with HIV-1 USvRNA, and its three-dimensional (3D) co-localization with specific chromatin marks.
The nucleus is the control center of the cell where genome replication, transcription, and splicing occur. These processes are highly compartmentalized into specific subnuclear domains, including chromosome territories and nuclear splicing speckles, enabling the cell to efficiently coordinate the expression of thousands of genes [reviewed in reference (22)]. Aside from subnuclear compartments, chromatin organization and genome architecture also play a major role in gene regulation and cellular differentiation [reviewed in references (23, 24)]. During HIV-1 infection, the viral core containing the preintegration complex enters the nucleus, where the reverse-transcribed DNA is loaded with histones and then preferentially integrates into active gene locations near the nuclear periphery (1–5, 25). The transition between active and latent HIV-1 infection is mediated by the chromatin state and regulated by CCCTC-binding factor (CTCF) (26–30). Condensation of the chromatin surrounding the HIV-1 integrated provirus promotes transcriptional repression, allowing the establishment of latency and creating a long-lived reservoir of infected T cells [reviewed in reference (31)]. These series of events permit the virus to remain undetected and evade host immune responses. Accordingly, recent efforts to combat HIV-1 infection, such as the “shock and kill” method, aim to reverse HIV-1 latency through the use of latency-reversal agents to trigger cell death in infected T cells (32–35).
One factor that influences HIV-1 transcriptional control is epigenetic modifications that alter the chromatin structure. For example, H3 and H4 acetylations are associated with increased HIV-1 transcription through the recruitment of histone acetyltransferases to the proviral long terminal repeats (LTRs) (36–38). Conversely, H3 methylation leads to HIV-1 transcriptional repression by restricting LTR access and promoting heterochromatin formation with sequestration of HIV-1 Tat (39–45). Additionally, HIV-1 integration sites and latency are driven by the presence of CTCF insulation of nearby topological associating domains (29, 30). Together, these studies demonstrate that the spatial properties of nuclear proteins and chromatin architecture can influence HIV-1 gene expression.
Learning more about the spatial biology and temporal distributions of the nuclear population of HIV-1 Gag is important to uncover novel functions of Gag that have yet to be explored. Many fundamental questions about the mechanism and role of HIV-1 Gag nuclear trafficking remain unanswered. An example of a novel role for HIV-1 Gag in the nucleus is the report that HIV-1 Gag interacts with an RNA demethylation enzyme in the nucleus, which influences the efficiency of gRNA packaging (46). In our recent work, we showed that co-localization of HIV-1 Gag and USvRNA increases in cells treated with the transcription inhibitors actinomycin D and DRB, suggesting that Gag binds USvRNA co-transcriptionally (13). As a follow up to those studies, in this work, we examined whether HIV-1 Gag traffics to areas of the nucleus enriched in euchromatin based on its localization to sites of active vRNA transcription.
MATERIALS AND METHODS
Plasmid, cells, and inductions
HIV-1 Gag-mCerulean rtTA HeLa cells contain a doxycycline-inducible HIV-1 viral DNA stably integrated into a HeLa cell line containing rtTA, as previously described (13). Briefly, the original HIV-1 plasmid was constructed from the HIV-1 NL4.3 strain containing 24 copies of the MS2-MBL stem loops (47) (a kind gift from Dr. Nathan Sherer, University of Wisconsin-Madison). The plasmid was modified by deletion of 21 MS2-MBL loops, substitution of the HIV-1 LTR with a dox-inducible promoter at both the 5′ and 3′ ends of the provirus (48), excision of a 1.9-kb region of the pol gene using MscI sites, and replacement of the nef gene with rtTA. The resulting DNA was cloned into the Piggybac vector (System Biosciences, Palo Alto, CA, USA) containing a selective marker for puromycin drug resistance. A stable cell line was created using selection with puromycin (Cytiva HyClone, Malborough, MA, USA) and the correct sequence of the insertion confirmed by DNA sequencing of the cellular genomic DNA (data not shown). The HeLa cell line was maintained in Dulbecco’s modified Eagle medium containing 10% fetal bovine serum. Expression of the HIV-1 construct was induced by treating cells with doxycycline (GoldBio, St. Louis, MO, USA) at various concentrations, as explained in the Results. J-Lat T cells (clone 10.6, obtained through the NIH AIDS Reagent Program) (49), derived from Jurkat T cells, which express low levels of the CD4 receptor (50), were maintained in RPMI medium supplemented with 10% fetal bovine serum. Both cell lines were grown in the presence of penicillin, streptomycin, and amphotericin B at 38°C and 5% CO2. Reversal of latent HIV-1 gene expression in J-Lat 10.6 cells was performed by treatment with prostratin (Sigma, St. Louis, MO, USA) or tumor necrosis factor alpha (TNF-α) (Abcam, Cambridge, MA, USA), as noted in the Results.
Subcellular fractionation
Fractionation of HIV-1 Gag-mCerulean rtTA HeLa cells was performed as described with minor modifications (13). Briefly, cells were grown in 10-cm2 cell culture dishes (Genesee Scientific GenClone, San Diego, CA, USA), doxycycline induced, trypsinized, washed with cold 1× phosphate-buffered saline (PBS), and centrifuged at 16,000 × g at 4°C for 30 s to remove residual culture medium. Cells were resuspended in 400-µL cold cytoplasmic lysis buffer [10-mM HEPES, pH 7.9, 10-mM KCl, 0.1-mM EDTA, 0.4% (vol/vol) Nonidet P40, 1-mM dithiothreitol (DTT), Roche Protease Inhibitor cOmplete tablets EDTA free (MilliporeSigma, St. Louis, MO, USA) to 1×] and incubated on ice for 5 min, followed by centrifugation at 16,000 × g at 4°C for 5 min. Supernatants were collected as the cytoplasmic fraction, and pellets were resuspended in 200-µL cold nuclear lysis buffer (20-mM HEPES, pH 7.9, 100-mM NaCl, 1-mM DTT, Roche Protease Inhibitor cOmplete tablets EDTA free) in the presence of OmniCleave (Biosearch Technologies, Novato, CA, USA). Pellets were incubated in a 37°C water bath for 10 min, and cell suspensions were brought up to 400-mM NaCl and 1-mM EDTA. Pellets were vortexed for 15 s and rotated end-over-end at 4°C for 20 min. Samples were spun at 16,000 × g at 4°C for 10 min, and the supernatant was collected as the nuclear fraction.
Fractionation of J-Lat 10.6 cells was performed similarly as described above with minor modifications for the cytoplasmic lysis. Prior to lysis, J-Lat 10.6 cells were grown in T75 flasks (Corning Inc., Corning, NY, USA) and induced by prostratin or TNF-α treatment. Cells were resuspended in 1,000-µL cold cytoplasmic lysis buffer (10-mM HEPES, pH 7.9, 10-mM KCl, 2-mM Mg acetate, 3-mM CaCl2, 340-mM sucrose, 1-mM DTT, Roche Protease Inhibitor cOmplete tablets EDTA to 1× and incubated on ice for 5 min. Nonidet P40 (0.05%) was added to samples and immediately vortexed for 15 s followed by centrifugation at 3,500 × g at 4°C for 10 min. Supernatants were collected as the cytoplasmic fraction. Nuclear fractions were extracted as described above.
Chromatin fractionation of HIV-1 Gag-mCerulean rtTA HeLa and J-Lat 10.6 cells were performed as described by Chase et al. (51). Following induction and harvest, cells were resuspended in 1,000-µL cold sucrose buffer containing 10-mM HEPES, pH 7.9, 10-mM KCl, 2-mM Mg acetate, 3-mM CaCl2, 340-mM sucrose, 1-mM DTT, and Roche Protease Inhibitor cOmplete tablets EDTA to 1× and incubated on ice for 5 min, followed by centrifugation at 3,500 × g at 4°C for 10 min. The supernatant was collected as the cytoplasmic fraction. Cell pellets were then resuspended in 1,000-µL cold nucleoplasm extraction buffer [50-mM HEPES, pH 7.9, 150-mM K acetate, 1.5-mM MgCl2, 0.1% (vol/vol) Nonidet P40, 1-mM DTT, Roche Protease Inhibitor cOmplete tablets EDTA free] and homogenized using Dounce homogenizers on ice. Samples were transferred into fresh tubes and rotated end-over-end at 4°C for 20 min. The supernatant was collected as the nucleoplasm fraction after centrifuging samples at 16,000 × g at 4°C for 10 min. Remaining cell pellets were resuspended in 500-µL cold nuclease incubation buffer [50-mM HEPES, pH 7.9, 10-mM NaCl, 1.5-mM MgCl2, 1-mM DTT, 100-U/mL Omnicleave (Biosearch Technologies), Roche Protease Inhibitor cOmplete tablets EDTA free] and incubated in a 37°C water bath for 10 min. The buffer in the cell suspension was adjusted to 150-mM NaCl and incubated on ice for 20 min. Samples were centrifuged at 16,000 × g at 4°C for 10 min, and the supernatant was collected as the euchromatin fraction. Cell pellets were then resuspended in 500-µL cold chromatin extraction buffer (50-mM HEPES 7.9, 500-mM NaCl, 1.5-mM MgCl2, 0.1% Triton X-100, 1-mM DTT, Roche Protease Inhibitor cOmplete tablets EDTA free) and incubated on ice for 20 min. Samples were centrifuged at 16,000 × g at 4°C for 10 min, and the supernatant was collected as the heterochromatin fraction. Chromatin fractionations in experiments involving treatment with 18.5-nM romidepsin (Sigma) were performed as described above with drug treatment prior to fractionations.
Western blot analysis
After cell fractionation, the protein concentration of each sample was measured using the Bradford Protein Assay protein with Coomassie protein assay reagent (Thermo Fisher Scientific-Invitrogen, Waltham, MA, USA). Next, 5 µg of each protein fraction was added to 1× loading buffer (250-mM Tris, pH 6.8, 40% glycerol, 8% β-mercapto-ethanol, 0.4% bromophenol blue, 8% SDS) and subjected to immunoblot analysis using the Stain-Free Imaging Technology (BioRad, Hercules, CA, USA). After SDS-PAGE, gels were transferred to polyvinylidene fluoride membrane and blocked in 5% milk 1× Tris-buffered saline with 0.1% Tween-20 for 30 min. Gag was detected using purified mouse anti-p24 supernatant from anti-HIV-1 Gag hybridoma 183 [National Institutes of Health (NIH) AIDS Reagent Program, Division of AIDS, NIAID, NIH, Germantown, MD, USA] at 1:2,000 dilution. Antibodies targeting calnexin (1:3,000; Enzo, cat #ADI-SPA-865-F), mediator complex subunit 4 (Med4) (1:5,000; Abcam, cat #Ab129170), H3K4me3 (1:5,000; Abcam, cat #Ab8580), H3K27ac (1:2,500; Abcam, cat #Ab4729), H3K9me3 (1:1,000; Active Motif, cat #39065), and total H3 (1:10,000; ProteinTech, cat #68345-1-Ig) were used to assess fraction purities. Mouse (1:5,000) and rabbit (1:10,000) secondary horseradish peroxidase-conjugated antibodies (Jackson ImmunoResearch, West Grove, PA, USA; mouse cat #711-036-152, rabbit cat #715-036-150) were used with Pierce ECL Western blotting substrate (Thermo Scientific, cat #32106) and visualized by a ChemiDox MP Imager (BioRad). Densitometry analysis was performed using ImageLab (BioRad) with adjusted total band volume data for each lane extracted for analysis. To account for the differences in the volume of nuclear versus cytoplasmic compartments, the adjusted total band volume derived from the signal representing 5 µg of protein loaded in each lane of the Western blot was normalized to represent 1% of the total protein of each fraction. The adjusted cytoplasmic and nuclear band volumes were used to calculate the percentage of cytoplasmic or nuclear signal by dividing each fraction by the sum of the total band volume from both fractions. Three independent biological replicates were performed for each experiment. The mean percentage ± standard error of the mean (SEM) of nuclear Gag and the mean percentage of chromatin marks ± SEM in the nuclear fractions in the three replicates were statistically analyzed using two- and one-way analyses of variance (ANOVAs), respectively, with statistical significance defined as P < 0.5.
Single-molecule fluorescence in situ hybridization
HIV-1 Gag-mCerulean rtTA HeLa cells were induced with doxycycline (2 µg/mL) for 24 hours, washed with PBS and fixed with 3.7% formaldehyde in 1× PBS for 10 min. Single-molecule fluorescence in situ hybridization (smFISH) was performed using Stellaris RNA FISH protocol for adherent cells (Biosearch Technologies). The 48 RNA FISH probes targeting the gag gene were labeled with Quasar570 to specifically visualize USvRNA at the level of single-molecule detection, as described previously (13, 52). After fixation, cells were washed twice with 1× PBS and permeabilized with 70% EtOH overnight at 4°C, then rehydrated in wash buffer (10% formamide, 2× saline-sodium phosphate-EDTA [SSPE], diethyl pyrocarbonate [DEPC] water) for 20 min at room temperature. Coverslips with cells were inverted onto RNA smFISH probes diluted 1:50 in hybridization buffer (10% dextran, 10% formamide, 2× subsp., DEPC water) in a humid chamber overnight at 37°C. Coverslips were then washed with wash buffer for 30 min at 37°C, followed by 4′,6-diamidino-2-phenylindole (DAPI) staining (1:1,000 in wash buffer) for 30 min at 37°C. Coverslips were mounted onto microscope slides using ProLong Diamond Antifade Mountant (Thermo Fisher Scientific-Invitrogen) and cured for 72 hours.
Immunofluorescence of histone marks
HIV-1 Gag-mCerulean rtTA HeLa cells were briefly washed with PBS and fixed using 3.7% formaldehyde for 10 min at room temperature. Following fixation, cells were washed three times for 5 min with PBS and permeabilized with permeabilization buffer (0.12-M KCl, 0.02-M NaCl, 0.01-M Tris-HCl, pH 7.7, 0.1% Triton X-100) for 10 min. Cells were washed again three times with the same solution and blocked with 3% bovine serum albumin in PBS for 10 min. Primary antibodies against H3K4me3 (1:600; Abcam, cat #Ab8580), H3K27ac (1:600; Abcam, cat #Ab4729), and H3K9me3 (1:600; Active Motif, cat #39766) were diluted in blocking buffer and applied to an inverted coverslip containing cells for 45 min at room temperature. Cells were then washed three times using blocking buffer for 5 min each, incubated with anti-rabbit AlexaFluor 647 secondary antibody (1:1,000; Life Technologies, cat #A31573) for 45 min at room temperature, washed three times with PBS, and mounted onto microscope slides using ProLong Diamond Antifade Mountant (Thermo Fisher Scientific-Invitrogen).
Confocal microscopy
Cells on coverslips were imaged using a Leica SP8 laser scanning confocal microscope (Leica Microsystems, Buffalo Grove, IL, USA). Imaging was performed using a white light laser and a ×63/NA1.4 oil immersion objective lens with sequential scanning of each channel with a frame average of 4 at 400-Hz imaging speed using hybrid detectors. Gag-mCerulean was excited at 458-nm laser line, and fluorescent emission was detected from 463 to 520 nm. USvRNA detected by smFISH probes labeled by Quasar570 was excited using 548-nm laser, and emission was captured from 553 to 635 nm. All histone marks were excited by 647-nm laser and captured from 652 to 751 nm. Single fluorophore controls were imaged separately to ensure no cross talk occurred. Images for the HIV-1 Gag time course experiments were processed by Gaussian filters, and histograms were adjusted to best visualize nuclear foci. Images of Gag and histone mark co-localization were processed by deconvolution using Huygens Image Processing Software (Scientific Volume Imaging). The 3D surface renderings of Z-stacks, cross-sections, and co-localization channels were generated in Imaris v.10.0 image analysis software (Bitplane).
For co-localization experiments presented in Movies S1 to S3, surface renderings were rotated 360° along the Y-plane followed by a scan through of the Z-stack using the orthogonal clipping plane function to allow visualization of co-localized complexes within the nucleus. Movies created for experiments involving the visualization of histone marks (Movies S3 to S5) were made by first rotating 3D surface renderings 360° along the Y-plane and then rotating 90° along the X-plane. Cells were zoomed in to visualize the co-localized Gag:histone spots (white).
Quantitative image analysis
To measure the intensity of Gag localization in cytoplasmic and nuclear compartments for the time course experiments, fluorescent intensity analysis was performed using ImageJ (53). Images were selected in Leica LAS X by isolating a single Z plane through the center of the cell nucleus. Nuclei were traced in ImageJ and the fluorescent intensity was measured. Background subtraction was performed by sampling a small region outside the cell and averaged with all cell replicates to obtain the corrected total cell fluorescence.
To determine the percent of Gag and USvRNA co-localization, Imaris image analysis software (Bitplane) was used to calculate Mander’s coefficient based on the HIV-1 Gag and USvRNA signal from images. The nuclear signal for each channel was isolated by creating a mask of the nucleus generated from surface rendering of the DAPI stain. Co-localization analysis between Gag and USvRNA was performed using the co-localization function to generate a co-localization channel and to obtain Mander’s coefficient. The same methods were employed to determine the co-localization of HIV-1 Gag with various histone marks. To measure the distances of co-localized spots from the chromatin periphery, a surface rendering of the histone mark fluorescence was made, and the spot function was used to generate spots from the Gag:histone co-localization channel. The distance from Gag:histone co-localized spots to the periphery of the surface was measured. Statistical tests were used to determine significance, defined as P < 0.05, as indicated in the figure legends.
RESULTS
HIV-1 Gag nuclear trafficking and co-localization with USvRNA occurred at early time points after induction of HIV-1 gene expression
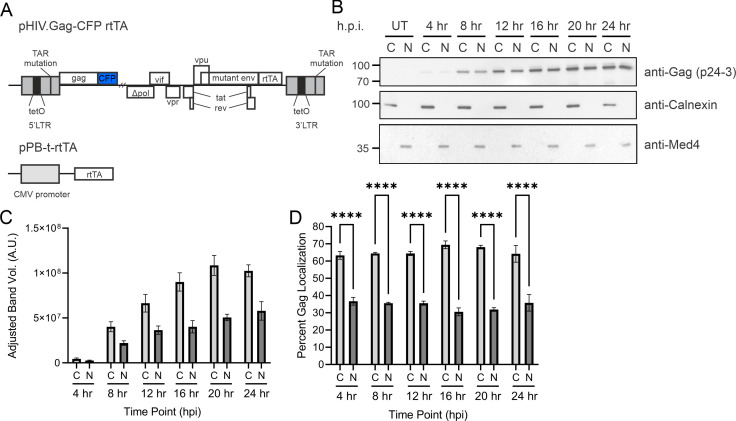
To examine the temporospatial dynamics of HIV-1 Gag nuclear localization, we constructed a HeLa cell line containing an integrated, HIV-1 genome expressing HIV-1 Gag-mCerulean controlled by a doxycycline promoter, which allowed synchronization of gene expression (Fig. 1A). In this construct, the insertion of the mCerulean gene in-frame at the 3′ end of the Gag sequence resulted in the elimination of viral protease activity, allowing the study of full-length Gag trafficking without the presence of free CA, which would have been a confounding factor in these experiments. We performed a time course using the HIV-1 Gag-mCerulean rtTA HeLa cell line by inducing HIV-1 gene expression with doxycycline (2 µg/mL). Subcellular fractionation was performed every 4 hours for 24 hours to measure the amount of Gag protein present in cytoplasmic and nuclear fractions (Fig. 1B, n = 3). Gag expression was detected by Western blot using an anti-p24 antibody targeting the CA domain of Gag, and quantification of band intensities was measured by densitometry analysis (Fig. 1C). There was no detectable Gag expression in untreated cells (Fig. 1B), so no further analysis of these samples was performed. Purity of the nuclear and cytoplasmic fractions was assessed using antibodies against calnexin (a cytoplasmic protein) and Med4 (a nuclear-localized protein), and the immunoblots consistently did not detect contamination of the nuclear or cytoplasmic fractions (Fig. 1B).
Fig 1.
Gag nuclear localization occurred within 4 hours in a stable HeLa cell line expressing HIV-1 using a doxycycline-inducible promoter. (A) Schematic of the doxycycline-inducible HIV-1 viral integrant expressing Gag-mCerulean and rtTA plasmids integrated in HeLa cells (HIV-1 Gag-mCerulean rtTA HeLa). The viral expression construct contains a deletion in pol, frameshift mutation in env, mutation of the trans-activation responsive region (TAR), substitution of nef with rtTA, and insertion of TetO sites at the promoter. (B) HIV-1 Gag-mCerulean rtTA HeLa cells were induced with dox (2 µg/mL) in a time-dependent fashion every 4 hours, and subcellular fractionation was performed to isolate cytoplasmic and nuclear lysates. Western blotting was performed to observe full-length HIV-1 Gag. Fraction purity was assessed by calnexin (cytoplasmic) and Med4 (nuclear). (C) Densitometry of immunoblots of cytoplasmic and nuclear fractions at each time point shown as adjusted band volumes. Background subtraction was performed to obtain adjusted band volume. (D) Percent Gag localization was determined by (fraction band volume) / (total band volume of cytoplasmic and nuclear within each time point). Analysis was compiled over three independent replicates. Error bars indicate standard errors of the mean. Statistical significance: ****P < 0.0001.
Our results indicated that HIV-1 Gag was present in cytoplasmic and nuclear fractions as early as 4 hours post-induction (h.p.i.; Fig. 1B and C), and expression in each fraction increased through 20 hours, reaching a plateau at 24 hours. At each time point, Gag levels were statistically significantly higher in the cytoplasmic fractions compared to the nuclear fractions (Fig. 1D), with a range of approximately 30%–37% of Gag in the nuclear fraction. The percentage of Gag in the nucleus compared to that in the cytoplasm remained relatively constant throughout the 24-hour period (Fig. 1D), indicating that the nuclear population of Gag was either stable or replenished after it was depleted. The presence of full-length Gag in the nucleus at early time points (4–8 hours) and low levels of protein expression indicated that Gag nuclear trafficking occurred shortly after Gag production began and suggested that nuclear entry was not driven primarily by a concentration gradient.
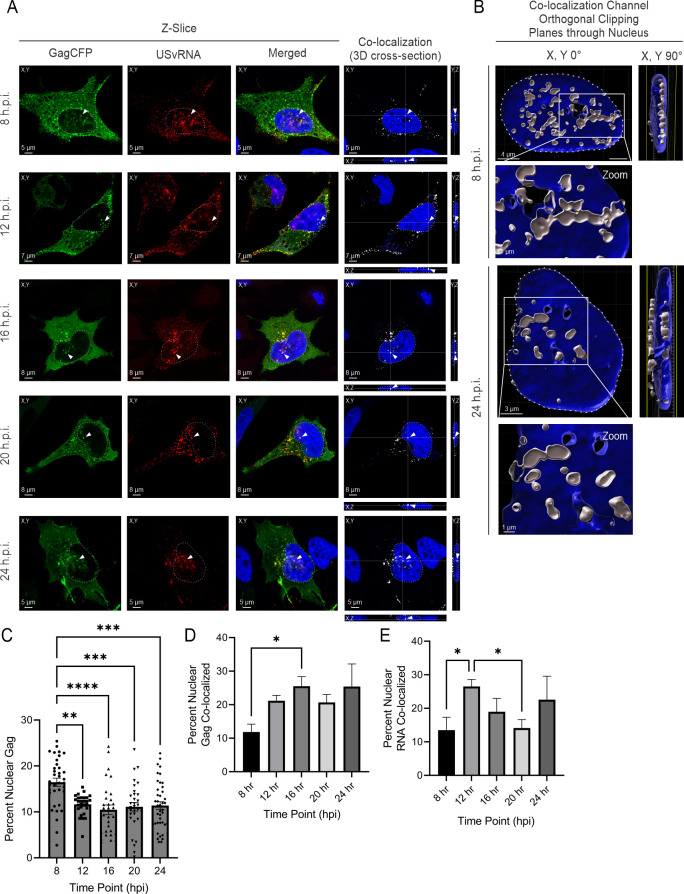
To examine HIV-1 Gag-mCerulean subcellular localization using an imaging approach, cells at several time points (8, 12, 16, 20, and 24 h.p.i.) were imaged through the center of the nuclear plane using confocal microscopy. Beginning at 8 h.p.i, both focal and diffuse Gag-mCerulean fluorescence (green) were observed within the nucleus (nuclear DNA stained blue by DAPI; Fig. 2A), in the cytoplasm, and at the plasma membrane. To detect HIV-1 USvRNA, FISH was performed using a set of oligonucleotide probes targeting the gag gene, as we previously described (13). At each time point, Gag and USvRNA co-localization was assessed using Imaris, with distinct co-localized foci appearing as yellow in the nucleus, cytoplasm, and at the plasma membrane (Merged image panels in Fig. 2A). The 3D cross-sections of Z-stacks confirmed Gag:USvRNA co-localization in the nucleus in the X, Y, and Z planes, indicated by arrows (white, co-localization channel). Additionally, a surface rendering of the nucleus and Gag-USvRNA spots based on the white co-localization channel was created to confirm co-localization was occurring inside the nucleus as defined by the DAPI signal (8 and 24 h.p.i., shown in Fig. 2B). Analysis of 3D renderings of Z-stacks from individual cells imaged at the 8- and 24-h.p.i. time points demonstrated Gag-USvRNA co-localization in the nucleus at numerous individual foci (see also Video S1 and Video S2, respectively).
Fig 2.
HIV-1 Gag co-localized with unspliced vRNA (USvRNA) shortly after Gag expression in integrated HeLa cells. (A) Confocal images of HIV-1 Gag-mCerulean rtTA HeLa cells at 8, 12, 16, 20, and 24 h.p.i. Visualization of Gag-mCerulean (false-colored green) and USvRNA (false-colored red) in the XY plane. Nuclei of cells were visualized by DAPI stain (blue). Cross-sections of Z-stacks subjected to co-localization analysis identified Gag and USvRNA co-localized signal (white) across XY, YX, and YZ planes. (B) 3D surface renderings of the nucleus (DAPI, blue) and the Gag-USvRNA co-localization channel (white) depict 8 and 24 h.p.i. time points. Bisecting the nucleus with an orthogonal clipping plane and rotating it at 90° demonstrate that Gag and USvRNA co-localization occurs within the nucleus. (C) The percentage of nuclear Gag was calculated from corrected cell fluorescence of confocal images. Quantitative analysis of HIV-1 Gag-mCerulean rtTA HeLa cells induced with doxycycline measuring nuclear HIV-1 Gag co-localization with USvRNA (D) and USvRNA co-localization with Gag (E) by Mander’s coefficient. Error bars indicate standard errors of the mean. Statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
To quantitate nuclear Gag levels obtained by confocal imaging, nuclear Gag fluorescence was measured from a single Z-plane through the center of the nucleus (n = 30–41 cells per data point across three independent biological replicates). Comparison of Gag levels in each subcellular location yielded similar trends as observed in Fig. 1, with more Gag in the cytoplasm compared to the nucleus. In contrast to the Western blot analysis, the percentage of nuclear Gag was observed highest at 8 h.p.i. (16.5% ± 0.9%) and decreased to 11.6% ± 0.4% by 12 h.p.i., where it remained relatively constant throughout the remaining time points (Fig. 2C). It should be noted that there was a lower nuclear-to-cytoplasmic ratio of Gag detected using fluorescence imaging compared to Western blotting. This discrepancy may be attributed to several factors. First, the number of cells analyzed was different for each method; the fractionation and Western blot analysis were performed using a large population of cells (n = 9 × 106), whereas imaging was performed on a single-cell basis and represented a smaller number (n = 30–41 cells). Second, because the HIV-1 Gag-mCerulean rtTA HeLa is a polyclonal cell line with varying numbers of integrated proviral constructs, viral transcription and Gag expression levels varied from cell to cell even though all cells were treated with equal concentrations of doxycycline. Another factor is the difference in sensitivity of immunoblotting, with the ability to detect Gag by chemiluminescence being more efficient than visualization of individual molecules of Gag-mCerulean in single cells, where single fluorescence molecules or faint Gag foci below the threshold of detection would not be measured. Finally, it is important to note that the imaging analysis was performed by analyzing a single Z-slice through the center of the nucleus, which did not include Gag molecules above or below the plane that were nonetheless located within the 3D volume of the nucleus. Bearing in mind the numerous explanations for discrepancies in the absolute amounts, the overall trend of nuclear Gag localization was similar for both methods.
Additionally, single-cell images obtained at each time point containing HIV-1 Gag and USvRNA (n = 9–26 cells) were analyzed in 3D using the Imaris signal-based co-localization function to measure the overall fluorescence intensity of the Gag and USvRNA signals and to obtain Mander’s coefficient for each channel. At 8 h.p.i., we observed that nuclear Gag co-localized with USvRNA (11.9% ± 2.3%, Fig. 2D), and nuclear USvRNA co-localized with HIV-1 Gag (13.5% ± 3.9%, Fig. 2E) to similar degrees. At 12 h.p.i., the percentage of nuclear USvRNA that was co-localized with Gag increased (26.5% ± 2.1%) and then plateaued, followed by a slight decrease in co-localization at the 24-hour time point (22.6% ± 7.0%, Fig. 2E). The percentage of Gag co-localized with USvRNA increased after 8 h.p.i. and remained relatively constant through 16 h.p.i. (25.5% ± 2.8%, Fig. 2D). The overall percentage of HIV-1 Gag co-localized with USvRNA was similar to that previously reported by Tuffy et al. (13), although a different analytical method (object-based co-localization) was used in that study.
HIV-1 Gag preferentially localized to the euchromatin-associated protein fraction
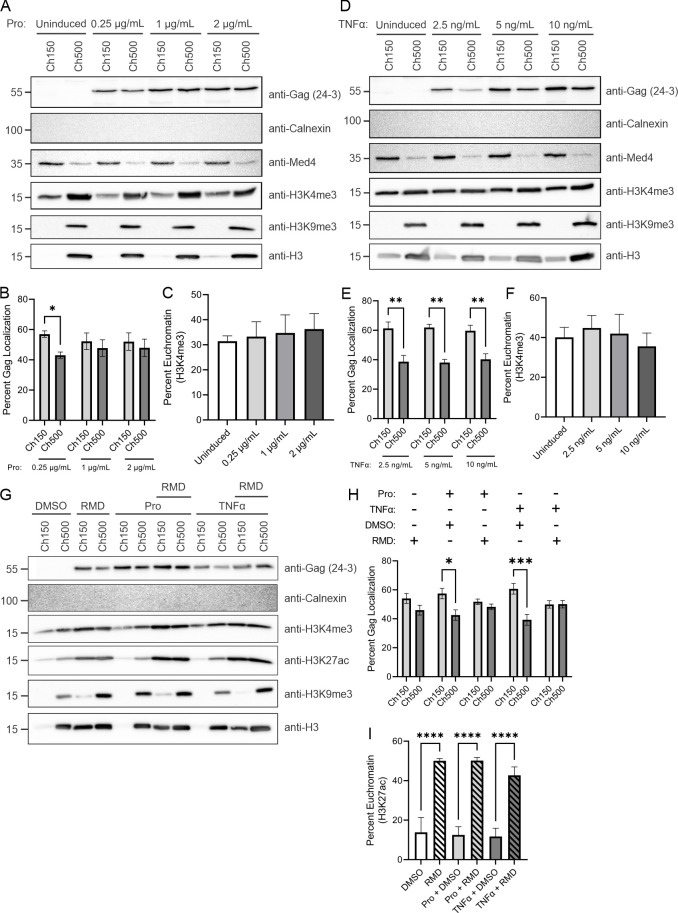
Our previous results suggest that a population of full-length Gag localizes to the proviral transcription site in HeLa and T cells (13), indicating that Gag may be associated with transcriptionally active euchromatin. To test this idea, we examined whether Gag localized to specific chromatin-associated regions using a differential salt fractionation method (51). To begin, we examined whether the level of HIV-1 Gag expression affected its localization to specific subsets of chromatin. HIV-1 Gag-mCerulean rtTA HeLa cells were treated with varying concentrations of doxycycline (2–2,000 ng/mL) to regulate the level of Gag expression. After isolation of nuclei, extraction of euchromatin and heterochromatin-associated proteins was achieved using buffers containing either 150-nM NaCl or 500-nM NaCl, respectively, and Western blotting was performed to measure the level of Gag present in the euchromatin (Ch150) and heterochromatin (Ch500) protein fractions (Fig. 3A; n = 3). Each fraction was also analyzed using antibodies against calnexin (cytoplasm), Med4 (nuclear), tri-methylated H3K4 (H3K4me3, euchromatin), H3K9 (H3K9me3, heterochromatin), and total H3 histone (chromatin). H3K4me3 marks are associated with active promoters, and H3K9me3 marks are located at constitutive heterochromatin regions (54–57). As reported by Chase et al., chromatin fractions extracted using low salt buffer contained proteins involved in active transcription that were distinct from the high salt chromatin fraction, despite the presence of H3K4me3 in both chromatin fractions (51). Our results, consistent with Chase et al., indicated that the H3 histone signal was primarily in the Ch500 fraction (51). Interestingly, we found that HIV-1 Gag signal was barely detectable at 2 ng/mL and then increased in a dose-dependent fashion starting at 20 ng/mL with maximum reached at 200 mg/mL with no further increase at 2,000 ng/mL, suggesting saturation of the doxycycline-induction system (Fig. 3A and S1A), consistent with previous reports that this promoter reached maximal HIV-1 p24 release into cell supernatants at a dose of 500-ng/mL doxycycline (48). Densitometry analysis of Western blots revealed that HIV-1 Gag preferentially localized with euchromatin (69.1% ± 6.3%) compared to heterochromatin (30.9% ± 6.3%, P = 0.032) at lower levels of Gag expression (20-ng/mL doxycycline;) (Fig. 3B). Similar results were obtained using 200-ng/mL doxycycline induction, with 58.1% ± 0.9% of Gag in the euchromatin and 41.9% ± 0.9% in heterochromatin fraction (P = 0.001). At 2,000 ng/mL, the highest doxycycline induction level used, the ratio of Gag in euchromatin versus heterochromatin fractions remained significantly higher but moved closer to being equivalent in each fraction (53.4% ± 0.03% and 46.6% ± 0.03%, respectively). Three different concentrations of the chromatin lysates were loaded onto the gel, and the Gag signal was detected using immunoblotting, indicating that the Gag proteins were within the linear range of detection (Fig. S1E). To determine whether treatment with doxycycline altered the distribution of chromatin proteins, H3K4me3, H3K9me3, and total H3 histone ratios for euchromatin and heterochromatin were assessed and found to be unchanged across all drug concentrations (Fig. 3C; Fig. S1).
Fig 3.
HIV-1 Gag preferentially localized to the euchromatin protein fractions in HeLa cells containing integrated HIV-1 DNA. (A) HIV-1 Gag-mCerulean rtTA HeLa cells were induced using 2-, 20-, 200, and 2,000-ng/mL doxycycline (dox). Gag was detected in euchromatin (Ch150) and heterochromatin (Ch500) fractions by immunoblot using anti-p24 antibody. Calnexin (cytoplasmic), Med4 (nuclear), H3K4me3 (euchromatin), H3K9me3 (heterochromatin), and total H3 (chromatin) were used to assess fraction purity. (B) Analysis of detected bands from Western blot measuring percent gag localization. Due to the lack of signal, 2-ng/mL dox induction was excluded from the analysis. (C) Percent euchromatin was analyzed from H3K4me3 Western blot signals, showing there were no changes in euchromatin levels resulting from dox treatment. (D) HIV-1 Gag-mCerulean rtTA HeLa cells were treated with 20-ng/mL dox in the presence of romidepsin (RMD) or vehicle control (dimethyl sulfoxide [DMSO]), fractionated, and analyzed by Western blot. Anti-p24 antibody was used to detect the Gag protein, and the same fractions were analyzed using anti-calnexin (cytoplasm), H3K4me3 (euchromatin), H3K27ac (euchromatin), H3K9me3 (heterochromatin), and H3 (total chromatin). (E) The percentage of Gag localization was determined via analysis of Gag band volumes. (F) Percent euchromatin was analyzed by H3K4me3 band volume, demonstrating that the addition of RMD increased euchromatin formation, as expected. Error bars indicate standard errors of the mean. Statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
We next asked whether opening the chromatin structure to adopt a more transcriptionally-active state would affect HIV-1 Gag localization. To promote euchromatin formation, cells were treated with 18.5 nM (10 ng/mL) of histone deacetylase inhibitor (HDACi) romidepsin (RMD), which effectively reactivates latently infected CD4+ T cells, resulting in a 10-fold increase in HIV-1 virion production (58). Based on the prior experiment, a dose of 20-ng/mL doxycycline was initially used to induce Gag expression for 24 hours (Fig. 3D). Treatment with doxycycline alone resulted in higher levels of Gag in euchromatin (65.1% ± 4.8%) compared to heterochromatin (34.9% ± 4.8%, P = 0.013), similar to that shown in Fig. 3B (Fig. 3E). Of note, cells treated with RMD alone (without doxycycline) expressed slightly higher levels of Gag than the dimethyl sulfoxide control, with more Gag in the euchromatin (68.8% ± 6.2%) compared to the heterochromatin fractions (31.2% ± 6.2%, P = 0.015; Fig. 3E). This result suggests that changing the global chromatin environment with RMD treatment promoted HIV-1 gene expression and enhanced Gag localization to the transcriptionally active chromatin fraction, independently of the doxycycline-sensitive promoter. Cells treated with the combination of doxycycline and RMD displayed increased Gag expression by 4.2-fold compared to doxycycline treatment alone, with equivalent levels of Gag in the chromatin fractions (Fig. 3D and E), suggesting that the movement of Gag into the euchromatin fraction was saturable. To assess the effectiveness of the HDACi drug treatment on facilitating euchromatin formation, chromatin fractions were assessed by Western blotting using antibodies against H3K4me3 (associated with active promoters), acetylated H3K27 (H3K27ac, associated with active enhancer regions) (59), H3K9me3 (heterochromatin), and total H3 histone (Fig. S2). Cells treated with RMD exhibited increased H3K4me3 and H3K27ac histone marks, signifying a shift toward euchromatin formation, as expected (Fig. 3F; Fig. S2B). H3K9me3 histone marks were found primarily in the heterochromatin fractions (Fig. S2C). The total H3 histone antibody detected a higher proportion of H3-associated marks in the euchromatin in the presence of RMD, indicating that a subset of H3 was modified and moved into the transcriptionally active chromatin region, consistent with the efficacy of the HDACi to alter the global chromatin landscape (Fig. S2D). Calnexin antibody was used to demonstrate that chromatin fractions did not contain cytoplasmic contamination. Taken together, these results indicate that Gag preferentially localized to euchromatin, most prominently at low to moderate levels of Gag expression, suggesting that the factor that allows Gag to accumulate in euchromatin may be limiting in the cell.
HIV-1 Gag nuclear trafficking in infected T cells reactivated from latency
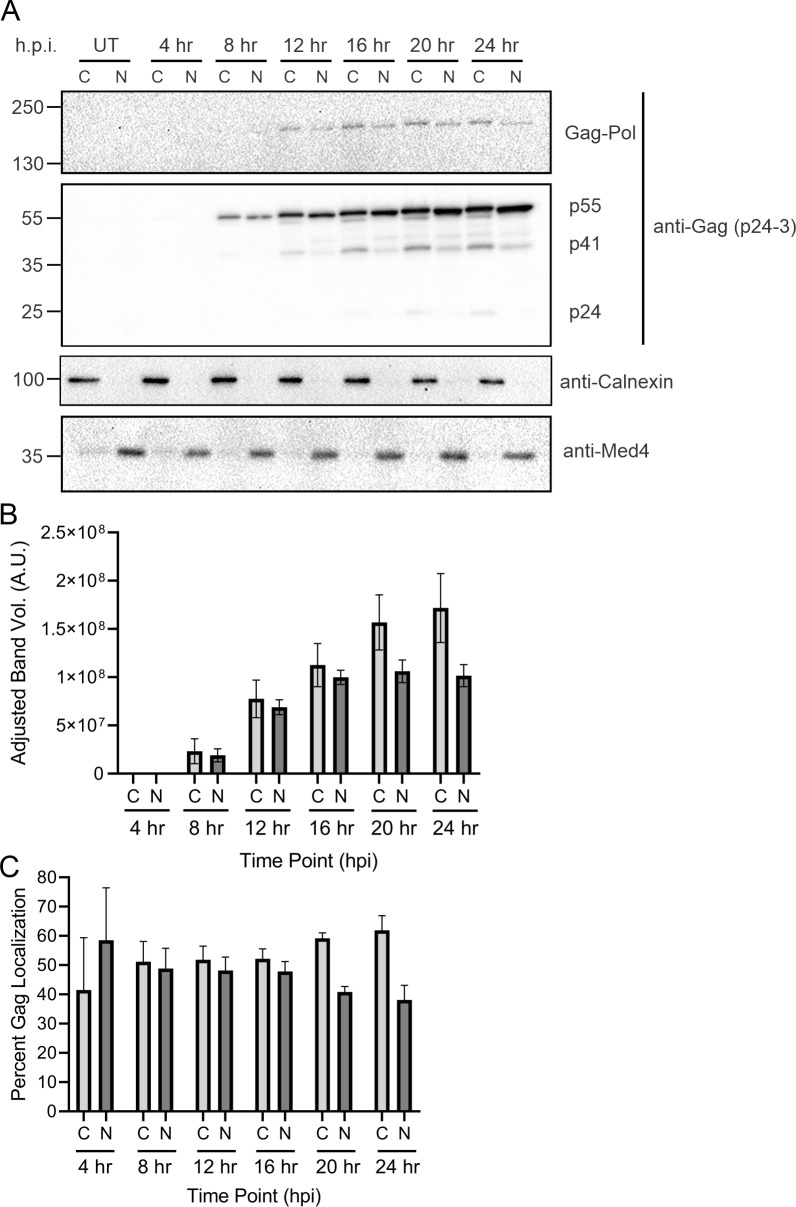
Based on the results observed in HeLa cells, we examined the timing and subcellular distribution of Gag in infected T cells reactivated from latency using a model system more representative of natural infection. We previously showed that HIV-1 Gag traffics to the nucleus in J-Lat 10.6 cells treated with the latency-reversal agent prostratin (13), a protein kinase C (PKC) agonist that acts by stimulating PKC to activate the NF-κB signaling pathway (60). To determine the timing of Gag expression and nuclear trafficking in T cells, we performed a 24-hour time course following latency reversal. Cells were treated with prostratin (1 µg/mL), and every 4 hours, subcellular fractionation and Western blotting (n = 3) were performed. Gag was detected in both cytoplasmic and nuclear fractions in J-Lat 10.6 cells starting at 4 h.p.i. (Fig. 4A and B). Although not statistically significant, the amount of Gag in the nucleus was higher (58.5% ± 17.9%) compared to that in the cytoplasm (41.5% ± 17.9%), whereas all other time points exhibited slightly higher cytoplasmic levels of Gag (Fig. 4C). The nuclear Gag concentrations decreased over the 24-hour period, similar to the HeLa cell model, with the exception of the last time point. These subtle differences in the timing of Gag localization between the HeLa and J-Lat 10.6 cell line may be attributed to the cell type or the presence of Gag-Pol, which is absent in the HIV-1 Gag-mCerulean rtTA HeLa cells. Interestingly, Gag-Pol was noted to be present in the nuclear fraction beginning at 12 h.p.i. (Fig. 4A), which has not been previously reported and warrants additional study.
Fig 4.
Nuclear Gag localization occurred within 8 hours in latently infected T cells treated with latency-reversal agents. (A) J-Lat 10.6 cells were treated with prostratin (2 µg/mL) every 4 hours for a total of 24 hours. Cells were subjected to subcellular fractionation to isolate cytoplasmic and nuclear protein fractions and probed via Western blot using anti-p24 antibody. Calnexin (cytoplasmic) and Med4 (nuclear) were used to assess fraction purity. (B) Immunoblot analysis of cytoplasmic and nuclear fractions at each time point was performed by measuring band volumes with background subtraction. (C) Percent of Gag localization was determined as shown in Fig. 1D.
HIV-1 Gag preferentially localized to euchromatin in latently infected T cells treated with latency-reversal agents
Previously, we reported that Gag and USvRNA co-localize at proviral transcriptional burst sites in reactivated J-Lat 10.6 cells, suggesting that Gag traffics to sites of USvRNA transcription upon reactivation of viral RNA expression after latency reversal (13). To determine whether Gag was associated with the euchromatin-associated protein fraction under these conditions, we assessed the chromatin localization of Gag after reactivation of HIV-1 gene expression in J-Lat 10.6 cells using varying concentrations of prostratin (Fig. 5A, n = 3) and TNF-α (Fig. 5D, n = 3). In chronically infected T cells, HIV-1 relies on activation of the tumor necrosis factor receptor by TNF-α to promote NK-κB recruitment to the HIV-1 LTR promoter (61–63). Thus, the use of TNF-α mimics natural reactivation of latently infected T cells. Densitometry analysis of Western blots revealed that Gag expression increases with increasing prostratin and TNF-α concentrations (Fig. S3A and F, respectively) and preferentially localized with euchromatin, particularly at lower expression levels (Fig. 5B and E, respectively). In chromatin fractions of J-Lat 10.6 cells reactivated by 0.25 µg/mL of prostratin, we found that 57.0% ± 1.3% of Gag localized to euchromatin and only 43.0% ± 1.3% localized to heterochromatin (Fig. 5B, P = 0.011). The euchromatin:heterochromatin ratio of Gag was equivalent at higher levels of Gag expression. Interestingly, J-Lat 10.6 cells reactivated with 2.50-ng/mL TNF-α exhibited much higher Gag localization to the euchromatin (61.3% ± 4.3%) compared to heterochromatin (38.7% ± 4.3%, P = 0.002; Fig. 5E). The preferential localization of Gag to euchromatin over heterochromatin was observed in cells treated at all concentrations of TNF-α, ranging from 2.5 to 10 ng/mL. Treatment of cells with prostratin or TNF-α did not alter euchromatin mark expression, as shown by the stable levels of H3K4me3 in the Ch150 fractions (Fig. 5C and F, respectively), indicating that the change in Gag localization was specific. Furthermore, measurement of H3K4me3, H3K9me3, and total H3 histone levels in chromatin fractions did not exhibit significant changes from prostratin or TNF-α treatment (Fig. S3). Quantitative assessment of the chromatin lysates immunoblots was performed across three concentrations, affirming that the Gag bands measured were in the linear range (Fig. S3E and J).
Fig 5.
HIV-1 Gag preferentially localized to euchromatin in reactivated latently infected T cells. Western blot of J-Lat 10.6 euchromatin (Ch150) and heterochromatin (Ch500) protein fractions following induction with various concentrations of prostratin (A) or TNF-α (D). Percent Gag localization was determined from the adjusted band volume from Western blots of chromatin fractions following induction by prostratin (B) and TNF-α (E). HIV-1 Gag preferentially localized to the euchromatin fraction at lower levels of drug treatment. Percent euchromatin was analyzed by H3K4me3 band volume for both prostratin (C) and TNF-α (F) inductions. Neither of these drugs used altered euchromatin formation. (G) J-Lat 10.6 cells were separated into euchromatin (Ch150) and heterochromatin (Ch500) following prostratin or TNF-α induction in the presence of RMD. A Western blot probed using anti-p24 antibody was used to determine HIV-1 Gag localization. Fraction purity was assessed by antibodies targeting calnexin (cytoplasm), Med4 (nucleus), H3K4me3, H3K27ac (euchromatin), H3K9me3 (heterochromatin), and total H3 (chromatin). (H) Quantitation of Gag localization was determined by measuring the adjusted band volume of the Western blot. (I) Assessment of percent euchromatin was analyzed through H3K27ac adjusted band volumes, demonstrating that the addition of RMD increases euchromatin formation.
The upregulation of HIV-1 gene expression by HDACis in latently infected T cells has been extensively characterized (27, 35, 58, 64, 65); however, we are not aware of any studies that have examined whether HDACi affects Gag subnuclear localization upon latency reversal. To determine how HDACi impacts Gag localization in reactivated T cells, we treated J-Lat 10.6 cells with 18.5-nM RMD (10 ng/mL) combined with either prostratin or TNF-α at concentrations of 0.25 or 2.5 ng/mL, respectively. Chromatin fractions were prepared as previously described, and immunoblotting using anti-p24 antibody was performed to measure Gag levels in each fraction (Fig. 5G, n = 4). Purity of each fraction was assessed using anti-calnexin (cytoplasm), anti-Med4 (nucleus), anti-H3K4me3, and anti-H3K27ac (euchromatin), anti-H3K9me3 (heterochromatin), and anti-H3 (chromatin) antibodies. Similar to the findings shown in Fig. 5A and D, Lat cells treated with prostratin or TNF-α exhibited higher levels of Gag in euchromatin (Fig. 5H; Fig. S4A; P = 0.01 and 0.0002, respectively). Cells treated with RMD also showed a preference for Gag euchromatin localization, although the increase was not statistically significant (54.1% ± 3.4% euchromatin and 46.0% ± 3.4% heterochromatin, P = 0.33). H3K27ac levels were slightly increased in the presence of RMD, verifying the increased formation of euchromatin with drug treatment (Fig. 5I and S4B). The H3K9me3 heterochromatin mark and total H3 expression also increased in the presence of RMD (Fig. S4C and D), suggesting that RMD treatment changes the global chromatin landscape to promote euchromatin formation. However, latency reversal using prostratin or TNF-α plus RMD treatment resulted in an equivalent ratio of Gag in the euchromatin and heterochromatin fractions, consistent with results seen at high levels of Gag induction in HeLa cells (Fig. 3). Altogether, these results indicate that HIV-1 Gag preferentially localizes to the euchromatin under lower expression levels following reactivation, suggesting that localization to transcriptionally active chromatin is a property of HIV-1 Gag in T cells as well as HeLa cells, and in both cases, it is a saturable process.
HIV-1 Gag co-localization with transcriptionally active versus repressive histone marks
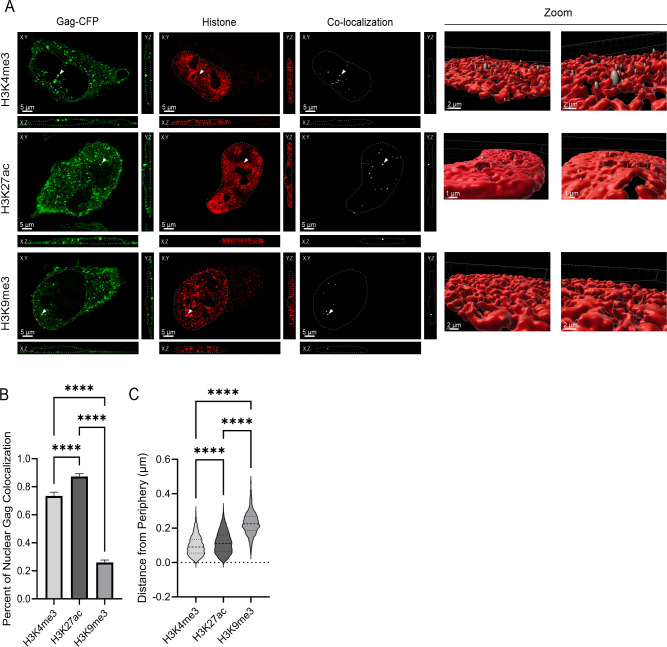
To determine whether we could visualize HIV-1 Gag co-localization with specific chromatin modifications, we performed immunofluorescence to detect H3K4me3, H3K27ac, and H3K9me3 histone marks (red, Fig. 6). Following 24 hours of doxycycline induction of the HIV-1 Gag-mCerulean rtTA HeLa cells, we observed Gag co-localization with each of the three histone marks independently, as indicated by the co-localization channel shown in white (Fig. 6A; n = 32–34 cells over three independent replicates). Imaris co-localization analysis revealed that a higher percentage of nuclear Gag co-localized with the euchromatin marks H3K4me3 and H3K27ac (73.5% ± 0.03% and 87.3% ± 0.02%, respectively) compared to the heterochromatin mark H3K9me3 (25.9% ± 0.02% with P < 0.0001; Fig. 6B), supporting our previous observation from the biochemical chromatin fractionation assays (Fig. 3). Interestingly, Gag was found to co-localize significantly more with H3K27ac histone marks compared to H3K4me3 histone marks (Fig. 6), suggesting Gag may have a specific preference for active enhancer regions.
Fig 6.
HIV-1 Gag co-localized with euchromatin histone marks at the nuclear periphery. (A) Immunofluorescence of euchromatin (H3K4me3 and H3K27ac) and heterochromatin (H3K9me3) histone mark (red) in HIV-1 Gag-mCerulean rtTA HeLa cells were imaged via confocal microscopy. Cross-sections of Z-stacks revealed that HIV-1 Gag (green) co-localizes (white channel) with each histone mark (white arrows) in the XY, XZ, and YZ planes. The chromatin space is outlined in white dashed lines. To examine the co-localized spots in 3D, surface renderings of Z-stacks of cells expressing Gag and stained for each chromatin mark were generated in Imaris, and the Gag:histone co-localization channel was highlighted by the spot function. (B) Mander’s coefficients of Gag co-localization with H3K4me3, H3K27ac, and H3K9me3 were calculated using the Imaris co-localization function. (C) The distance of Gag:histone co-localized spots to the periphery of the chromatin surface rendering was measured using Imaris. Error bars denote standard errors of the mean. Statistical significance: ****P < 0.0001.
Next, we examined the localization of HIV-1 Gag in 3D chromatin space. To address this question, we created a surface rendering of the chromatin space (red) and used the Imaris spot function to determine the position of co-localization of Gag with the chromatin marks. Over 800 co-localized spots consisting of Gag and chromatin marks spots were generated and used for analysis. As shown in zoomed-in panels in Fig. 6A, Gag co-localization with H3K4me3 (n = 807) and H3K27ac (n = 1463) preferentially occurred near the periphery of the nucleus, in some cases extending beyond the outer limits of the chromatin space (Video S3 and Video S4, respectively). Conversely, Gag and H3K9me3 (n = 882) co-localized foci were more centrally located and appeared largely buried in the 3D rendering, with far fewer spots protruding from the chromatin mask (Video S5).
Additionally, we measured the distance between Gag-histone co-localized spots and the periphery of the chromatin space, defined by the fluorescence signal of the histone channel in 3D space. Interestingly, we found that Gag co-localized with the euchromatin marks H3K4me3 and H3K27ac more toward with the nuclear periphery when compared to the heterochromatin mark. Gag co-localized with H3K27ac marks at a mean distance of 0.12 µm ± 0.002 µm from the nuclear periphery and co-localized with H3K4me3 at an average of 0.10 µm ± 0.002 µm from the outer edge of the chromatin border (P < 0.0001, Fig. 6C). Strikingly, co-localization of Gag with the heterochromatin histone mark H3K9me3 occurred significantly farther away from the outer border of the chromatin (at a mean distance of 0.23 µm ± 0.002 µm, P < 0.0001; Fig. 6C) compared to the euchromatin marks, indicating that HIV-1 Gag more closely associated with the transcriptionally active marks associated with promoters and enhancers near the periphery of the chromatin space compared to the heterochromatin region.
DISCUSSION
A major role of HIV-1 Gag is to orchestrate virion assembly by binding and selecting USvRNA to serve as the gRNA for packaging into nascent virus particles. Historically, it was widely accepted that HIV-1 Gag resided only in the cytoplasm, and that the initial Gag-gRNA interaction occurred at the plasma membrane. However, with more sensitive light microscopy methods and advances in biochemical techniques, the HIV-1 Gag polyprotein has been detected in the nucleus by independent laboratories (12, 13, 46). In our published work, we showed that HIV-1 Gag interacts with USvRNA in the nucleus at distinct foci in the perichromatin space and at HIV-1 transcriptional burst sites (13), suggesting that selection of USvRNA by Gag may occur co-transcriptionally. Consistent with our observation that a population of HIV-1 Gag is present in the nucleus, Pereira-Montecinos et al. demonstrated that the Gag polyprotein in the nucleus interacts with an RNA demethylase (46). These investigators found that the association of Gag with this nuclear demethylase influences m6A modification of HIV-1 full-length RNA, promoting gRNA packaging (46). Furthermore, proteomic analyses from several groups, along with our unpublished data, identified potential Gag-interacting factors that have roles in transcription, splicing, chromatin remodeling, and nucleocytoplasmic trafficking (66–70) and (B. Rice and L. Parent, unpublished data). Together, these results suggest that HIV-1 Gag may serve a novel function in the nucleus that is linked to gRNA packaging, although other roles in chromatin remodeling, splicing, or regulation of gene expression are also possible and merit further investigation.
We previously reported that full-length HIV-1 Gag was present in nuclear extracts at 24 hours after induction of HIV-1 gene expression (13). In this work, we found that Gag expression was detected in both nuclear and cytoplasmic fractions at much earlier time points (4 h.p.i) in HeLa cells containing an integrated, doxycycline-inducible, Rev-dependent construct expressing Gag-mCerulean (Fig. 1B and C). Similar temporospatial properties of Gag were observed in J-Lat 10.6 cells treated with latency-reversal agents, with Gag nuclear localization beginning at 8 h.p.i. (Fig. 4A and B). A previous study by Mohammadi et al. demonstrated that HIV-1 USvRNA transcripts arise 8 hours after integration and correlate with the onset of Gag protein translation (71). Our experiments in J-Lat 10.6 cells followed a similar time course, with the reactivation of viral gene expression induced by prostratin or TNF-α in alignment with that report. Given that translation of the viral mRNA Gag occurs shortly after transcription, these data suggest that Gag nuclear translocation occurs shortly after Gag synthesis.
We previously showed that HIV-1 Gag co-localizes with USvRNA in the nucleus at 24 h.p.i (13). In the present study, we observed Gag nuclear localization as early as 4 h.p.i.; therefore, we sought to determine how early Gag was co-localized with USvRNA. Using single-molecule RNA FISH, we observed Gag and USvRNA co-localization in the nucleus beginning at 8 h.p.i. in the HIV-1 Gag-mCerulean HeLa cell line (Fig. 2A). The proportion of HIV-1 Gag co-localized with USvRNA appeared to remain constant for 16 hours, whereas the percentage of USvRNA co-localized with Gag fluctuated (Fig. 2D and E). Our results mimicked those previously reported by Tuffy et al., where roughly a third of the nuclear Gag co-localized with HIV-1 USvRNA by 24 h.p.i. (13). Several studies have described the stochastic nature of HIV-1 transcription with transcriptional bursting, which could explain the variability we observed in the timing of formation of Gag:USvRNA vRNPs (72–75).
It is interesting that only a subset of Gag associates with USvRNA in the nucleus. One possibility is that the binding of Gag to the USvRNA may act to “mark” the RNA for packaging, and since only two copies of RNA are packaged, only a small amount of Gag would be needed in the nucleus. The 5′ leader sequence of the vRNA adopts a different structure to promote dimerization and packaging compared to the mRNA structure used for translation, and the number of guanosines at the 5′ end has also been shown to be different for packaged versus translated transcripts (76–78). It would be interesting to investigate whether the binding of Gag to the USvRNA in the nucleus depends on or promotes an RNA conformation that facilitates packaging, or whether Gag selectively associates with transcripts containing a single guanine at the 5′ start site. Other alternative functions of Gag recruitment to active promoter and enhancer sites include altering the transcriptional profile of cellular genes for the benefit of the virus replication, suppressing co-transcriptional splicing to favor full-length USvRNA and tip the balance toward packaging over subgenomic mRNA export, or recruiting nuclear RNA export machinery to mediate nucleocytoplasmic transport of the genomic vRNP complex.
Together, the studies presented suggest there may be different populations of Gag, one that remains cytoplasmic and another that undergoes nuclear trafficking and associates with nascent USvRNA. A study that examined Gag-containing complexes indicated that several species exist within the cell and bind to various cellular factors with differing contributions to particle formation (79). Moreover, as mentioned above, several mass spectrometry studies have found an association of Gag with a variety of host nuclear and cytoplasmic factors (66–70) (B. Rice and L. Parent, unpublished data), supporting the notion that Gag may form transient complexes with different cellular factors during the journey from the nucleus, through the cytoplasm, and to the plasma membrane. Of note, the apparent molecular weight of nuclear Gag expressed in J-Lat 10.6 cells appears to be slightly smaller compared to cytoplasmic Gag (Fig. 4A). This slight difference in mobility may be due to a post-translational modification such as phosphorylation that could alter Gag trafficking, localization, or function. Further investigation is needed to examine whether nuclear Gag is differentially modified and, if so, to probe the relevance for Gag trafficking and function.
HIV-1 integration, latency, and reactivation are influenced by the chromatin state of the infected host cell [reviewed in reference (80)]. Potential Gag-interacting factors identified by proteomic analyses include chromatin-associated proteins (66–70) (B. Rice and L. Parent, unpublished data). Moreover, histones were found to be packaged in HIV-1 particles released from infected cells (81). These data prompted us to investigate whether nuclear Gag preferentially localized to heterochromatin or transcriptionally active euchromatin. We found that Gag expressed by either doxycycline induction of a HeLa cell line or treatment of J-Lat 10.6T cells with latency-reversal agents, such as prostratin or RMD (28, 58, 64, 82), preferentially localized with euchromatin (Fig. 3 and 5, respectively). Conversely, higher Gag expression resulted in a near equal ratio of Gag in euchromatin and heterochromatin fractions, suggesting that the amount of Gag associated with euchromatin is saturable.
The regulation of chromatin conformation is achieved through the methylation and acetylation of histones. Several marks, including H3K4me3 and H3K27ac, associate with actively transcribing regions, whereas H3K9me3 associates with transcriptionally silent regions [as reviewed in reference (83)]. HIV-1 proviral DNA undergoes histone loading, particularly with H3 and H4 histones after nuclear import prior to integration (84). In the present study, our results indicate the HIV-1 Gag preferentially associates with euchromatin marks H3K4me3 and H3K27ac compared to a heterochromatin mark, supporting the chromatin fractionation results (Fig. 6B). As previously discussed, H3K4me3 histone specifically marks active promoter regions, whereas H3K27ac marks active enhancer regions (54, 55, 59). Distal enhancers, such as those marked by H3K27ac, physically interact with promoter regions through forced chromatin looping to promote transcription [reviewed in reference (85)]. Interestingly, Gag exhibited slightly higher associations with H3K27ac marks compared to H3K4me3 (Fig. 6B). It was shown that both 5′ and 3′ LTR regions of integrated provirus are enriched with H3K4me3, and HIV-1 expression is dependent on H3K4 methylation by P-TEFb recruited by Tat (86–88). Little is known about the relationship between H3K27ac and HIV-1 transcription, but the activation of H3K4me3-enriched genes is interdependent on the presence of H3K27ac (88, 89). Therefore, it is plausible that Gag promotes chromatin loop interactions or utilizes enhancer histone marks to locate the active viral transcription site.
HIV-1 Gag primarily co-localized with euchromatin marks located near the nuclear periphery (Fig. 6C). It is possible that the preferential localization of HIV-1 Gag to histone marks in open chromatin is important for the co-transcriptional selection of gRNA for packaging into virions. This hypothesis is further supported by the finding that the site of HIV-1 integration occurs with higher frequency in active gene bodies near the outer region of the nucleus, perhaps to facilitate vRNA export through the nuclear pores (1, 2). A previous report demonstrates that proviral activation of HIV-1 alters chromatin accessibility of neighboring genes (90). Further experiments, such as ChIP-Seq or ATAC-Seq, would be helpful to pinpoint the specific motifs or DNA elements that play a role in directing Gag trafficking within the nucleus and whether Gag itself alters nearby chromatin structure.
In summary, in these studies investigating the temporospatial properties of nuclear Gag, we demonstrated that a subset of nascent HIV-1 Gag undergoes nuclear localization and traffics to actively transcribing regions of the chromosome, where HIV-1 proviral integration preferentially occurs (Fig. 7). These events could facilitate the production of new virus particles following latency reversal by selecting USvRNA for packaging prior to nuclear export. However, the precise mechanism by which this phenomenon occurs remains uncertain and requires further investigation to decipher the exact role(s) of HIV-1 Gag nuclear trafficking following reactivation of proviral gene expression in latently infected cells.
Fig 7.
Proposed model of HIV-1 Gag localization to the euchromatin at the nuclear periphery to facilitate gRNA packaging. Our results demonstrate that HIV-1 Gag is imported into the nucleus shortly after induction of viral gene expression or reactivation from latency. Once within the nucleus, HIV-1 Gag preferentially localizes with euchromatin marks associated with active promoters and enhancers near the outer edge of the nucleus. The model proposes that a subset of HIV-1 Gag is imported to the nucleus and associates with euchromatin marks at the nuclear periphery, where the provirus tends to be integrated. HIV-1 Gag may select and bind USvRNA co-transcriptionally for packaging into new virions. This figure was created with BioRender.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute On Drug Abuse of the National Institutes of Health (NIH) under award number R21DA053689 to L.J.P. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The work was also partially funded by a training grant (NCI T32 CA060395-22 to J.C.; PI, Craig Meyers). All confocal imaging was performed at the Advanced Light Microscopy Core (RRID:SCR_022526), which is funded, in part, by the Pennsylvania State University College of Medicine via the Office of the Vice Dean of Research and Graduate Students and the Pennsylvania Department of Health using Tobacco Settlement Funds (Commonwealth Universal Research Enhancement).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the University. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions.
We thank Alan Cochrane and Nicholas Buchkovich for constructive comments on the manuscript and Parent laboratory members for their important contributions to this work: Rebecca Maldonado and Greg Lambert for technical advice, guidance on data interpretation, and constructive comments on the manuscript, and Malgorzata Sudol for technical assistance. The following reagent was obtained through the NIH HIV Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH: J-Lat full-length cells (10.6), ARP-9849, contributed by Dr. Eric Verdin.
Contributor Information
Leslie J. Parent, Email: lparent@pennstatehealth.psu.edu.
Viviana Simon, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/jvi.01179-23.
Legends for Fig. S1 to S4.
Quantitative assessment of full-length Gag expression and euchromatin mark in HIV-1 Gag-mCerulean rtTA HeLa cells.
Quantitative assessment of full-length Gag expression and euchromatin mark in HIV-1 Gag-mCerulean rtTA HeLa cells in the presence of RMD.
Quantitative assessment of full-length Gag expression and euchromatin mark in latently-infected J-Lat 10.6 cells induced with either prostratin or TNFα.
Quantitative assessment of full-length Gag expression and euchromatin mark in latently infected J-Lat 10.6 cells induced treated with RMD.
Legends for Movies S1 to S5.
HIV-1 Gag co-localization with USvRNA at 8 hpi.
HIV-1 Gag co-localization with USvRNA at 24 hpi.
HIV-1 Gag co-localization with euchromatin marker H3K4me3.
HIV-1 Gag co-localization with euchromatin marker H3K27ac.
HIV-1 Gag co-localization with heterochromatin marker H3K9me3.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Dieudonné M, Maiuri P, Biancotto C, Knezevich A, Kula A, Lusic M, Marcello A. 2009. Transcriptional competence of the integrated HIV-1 provirus at the nuclear periphery. EMBO J 28:2231–2243. doi: 10.1038/emboj.2009.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Albanese A, Arosio D, Terreni M, Cereseto A. 2008. HIV-1 pre-integration complexes selectively target decondensed chromatin in the nuclear periphery. PLoS One 3:e2413. doi: 10.1371/journal.pone.0002413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burdick RC, Deleage C, Duchon A, Estes JD, Hu W-S, Pathak VK. 2022. Intranuclear positions of HIV-1 proviruses are dynamic and do not correlate with transcriptional activity. mBio 13:e0325621. doi: 10.1128/mbio.03256-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marini B, Kertesz-Farkas A, Ali H, Lucic B, Lisek K, Manganaro L, Pongor S, Luzzati R, Recchia A, Mavilio F, Giacca M, Lusic M. 2015. Nuclear architecture dictates HIV-1 integration site selection. Nature 521:227–231. doi: 10.1038/nature14226 [DOI] [PubMed] [Google Scholar]
- 5. Lelek M, Casartelli N, Pellin D, Rizzi E, Souque P, Severgnini M, Di Serio C, Fricke T, Diaz-Griffero F, Zimmer C, Charneau P, Di Nunzio F. 2015. Chromatin organization at the nuclear pore favours HIV replication. Nat Commun 6:6483. doi: 10.1038/ncomms7483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rabson AB, Graves BJ. 1997. Synthesis and processing of viral RNA. In Coffin JM, Hughes SH, Varmus HE (ed), Retroviruses. Cold Spring Harbor Laboratory Press, New York. [PubMed] [Google Scholar]
- 7. D’Souza V, Summers MF. 2005. How retroviruses select their genomes. Nat Rev Microbiol 3:643–655. doi: 10.1038/nrmicro1210 [DOI] [PubMed] [Google Scholar]
- 8. Mattei S, Schur FKM, Briggs JAG. 2016. Retrovirus maturation - an extraordinary structural transformation. Curr Opin Virol 18:27–35. doi: 10.1016/j.coviro.2016.02.008 [DOI] [PubMed] [Google Scholar]
- 9. Jouvenet N, Lainé S, Pessel-Vivares L, Mougel M. 2011. Cell biology of retroviral RNA packaging. RNA Biol 8:572–580. doi: 10.4161/rna.8.4.16030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Muriaux D, Darlix JL. 2010. Properties and functions of the nucleocapsid protein in virus assembly. RNA Biol 7:744–753. doi: 10.4161/rna.7.6.14065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grewe B, Hoffmann B, Ohs I, Blissenbach M, Brandt S, Tippler B, Grunwald T, Uberla K. 2012. Cytoplasmic utilization of human immunodeficiency virus type 1 genomic RNA is not dependent on a nuclear interaction with gag. J Virol 86:2990–3002. doi: 10.1128/JVI.06874-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lochmann TL, Bann DV, Ryan EP, Beyer AR, Mao A, Cochrane A, Parent LJ. 2013. NC-mediated nucleolar localization of retroviral gag proteins. Virus Res 171:304–318. doi: 10.1016/j.virusres.2012.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tuffy KM, Maldonado RJK, Chang J, Rosenfeld P, Cochrane A, Parent LJ. 2020. HIV-1 gag forms ribonucleoprotein complexes with unspliced viral RNA at transcription sites. Viruses 12:1281. doi: 10.3390/v12111281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scheifele LZ, Garbitt RA, Rhoads JD, Parent LJ. 2002. Nuclear entry and CRM1-dependent nuclear export of the rous sarcoma virus gag polyprotein. Proc Natl Acad Sci U S A 99:3944–3949. doi: 10.1073/pnas.062652199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scheifele LZ, Kenney SP, Cairns TM, Craven RC, Parent LJ. 2007. Overlapping roles of the rous sarcoma virus gag P10 domain in nuclear export and Virion core morphology. J Virol 81:10718–10728. doi: 10.1128/JVI.01061-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maldonado RJK, Rice B, Chen EC, Tuffy KM, Chiari EF, Fahrbach KM, Hope TJ, Parent LJ, Goff SP. 2020. Visualizing association of the retroviral gag protein with unspliced viral RNA in the nucleus. mBio 11:e00524-20. doi: 10.1128/mBio.00524-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garbitt-Hirst R, Kenney SP, Parent LJ. 2009. Genetic evidence for a connection between rous sarcoma virus gag nuclear trafficking and genomic RNA packaging. J Virol 83:6790–6797. doi: 10.1128/JVI.00101-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scheifele LZ, Ryan EP, Parent LJ. 2005. Detailed mapping of the nuclear export signal in the rous sarcoma virus gag protein. J Virol 79:8732–8741. doi: 10.1128/JVI.79.14.8732-8741.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Butterfield-Gerson KL, Scheifele LZ, Ryan EP, Hopper AK, Parent LJ. 2006. Importin-B family members mediate alpharetrovirus gag nuclear entry via interactions with matrix and nucleocapsid. J Virol 80:1798–1806. doi: 10.1128/JVI.80.4.1798-1806.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nash MA, Meyer MK, Decker GL, Arlinghaus RB. 1993. A subset of Pr65Gag is nucleus associated in murine leukemia virus-infected cells. J Virol 67:1350–1356. doi: 10.1128/jvi.67.3.1350-1356.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stake MS, Bann DV, Kaddis RJ, Parent LJ. 2013. Nuclear trafficking of retroviral RNAs and gag proteins during late steps of replication. Viruses 5:2767–2795. doi: 10.3390/v5112767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spector DL. 2001. Nuclear domains. J Cell Sci 114:2891–2893. doi: 10.1242/jcs.114.16.2891 [DOI] [PubMed] [Google Scholar]
- 23. Venkatesh S, Workman JL. 2015. Histone exchange, chromatin structure and the regulation of transcription. Nat Rev Mol Cell Biol 16:178–189. doi: 10.1038/nrm3941 [DOI] [PubMed] [Google Scholar]
- 24. Ghosh RP, Meyer BJ. 2021. Spatial organization of chromatin: emergence of chromatin structure during development. Annu Rev Cell Dev Biol 37:199–232. doi: 10.1146/annurev-cellbio-032321-035734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Selyutina A, Persaud M, Lee K, KewalRamani V, Diaz-Griffero F. 2020. Nuclear import of the HIV-1 core precedes reverse transcription and uncoating. Cell Rep 32:108201. doi: 10.1016/j.celrep.2020.108201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Verdin E, Paras P, Lint C. 1993. Chromatin disruption in the promoter of HIV-1 during transcriptional activation. EMBO J 12:3249–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van Lint C, Emiliani S, Ott M, Verdin E. 1996. Transcriptional activation and chromatin remodeling of the HIV-I promoter in response to histone acetylation. EMBO J 15:1112–1120. doi: 10.1002/j.1460-2075.1996.tb00449.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van Lint C, Emiliani S, Verdin E. 1996. The expression of a small fraction of cellular genes is changed in response to histone hyperacetylation. Gene Expr 5:245–253. [PMC free article] [PubMed] [Google Scholar]
- 29. Jefferys SR, Burgos SD, Peterson JJ, Selitsky SR, Turner A-M, James LI, Tsai Y-H, Coffey AR, Margolis DM, Parker J, Browne EP, Silvestri G. 2021. Epigenomic characterization of latent HIV infection identifies latency regulating transcription factors. PLoS Pathog 17:e1009346. doi: 10.1371/journal.ppat.1009346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rheinberger M, Costa AL, Kampmann M, Glavas D, Shytaj IL, Sreeram S, Penzo C, Tibroni N, Garcia-Mesa Y, Leskov K, Fackler OT, Vlahovicek K, Karn J, Lucic B, Herrmann C, Lusic M. 2023. Genomic profiling of HIV-1 integration in microglia cells links viral integration to the topologically associated domains. Cell Rep 42:112110. doi: 10.1016/j.celrep.2023.112110 [DOI] [PubMed] [Google Scholar]
- 31. Taube R, Peterlin BM. 2013. Lost in transcription: molecular mechanisms that control HIV latency. Viruses 5:902–927. doi: 10.3390/v5030902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Elliott JH, Wightman F, Solomon A, Ghneim K, Ahlers J, Cameron MJ, Smith MZ, Spelman T, McMahon J, Velayudham P, et al. 2014. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog 10:e1004473. doi: 10.1371/journal.ppat.1004473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, Richman DD, Hudgens MG, Bosch RJ, Coffin JM, Eron JJ, Hazuda DJ, Margolis DM. 2012. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 487:482–485. doi: 10.1038/nature11286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Archin NM, Kirchherr JL, Sung JAM, Clutton G, Sholtis K, Xu Y, Allard B, Stuelke E, Kashuba AD, Kuruc JD, Eron J, Gay CL, Goonetilleke N, Margolis DM. 2017. Interval dosing with the HDAC inhibitor vorinostat effectively reverses HIV latency. J Clin Invest 127:3126–3135. doi: 10.1172/JCI92684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Søgaard OS, Graversen ME, Leth S, Olesen R, Brinkmann CR, Nissen SK, Kjaer AS, Schleimann MH, Denton PW, Hey-Cunningham WJ, Koelsch KK, Pantaleo G, Krogsgaard K, Sommerfelt M, Fromentin R, Chomont N, Rasmussen TA, Østergaard L, Tolstrup M, Siliciano RF. 2015. The depsipeptide romidepsin reverses HIV-1 latency in vivo. PLoS Pathog 11:e1005142. doi: 10.1371/journal.ppat.1005142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Col E, Caron C, Seigneurin-Berny D, Gracia J, Favier A, Khochbin S. 2001. The histone acetyltransferase, hGCN5, interacts with and acetylates the HIV transactivator, tat. J Biol Chem 276:28179–28184. doi: 10.1074/jbc.M101385200 [DOI] [PubMed] [Google Scholar]
- 37. Hottiger MO, Nabel GJ. 1998. Interaction of human immunodeficiency virus type 1 tat with the transcriptional coactivators p300 and CREB binding protein. J Virol 72:8252–8256. doi: 10.1128/JVI.72.10.8252-8256.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Benkirane M, Chun RF, Xiao H, Ogryzko VV, Howard BH, Nakatani Y, Jeang KT. 1998. Activation of integrated provirus requires histone acetyltransferase: p300 and P/CAF are coactivators for HIV-1 tat. J Biol Chem 273:24898–24905. doi: 10.1074/jbc.273.38.24898 [DOI] [PubMed] [Google Scholar]
- 39. Lachner M, O’Carroll D, Rea S, Mechtler K, Jenuwein T. 2001. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410:116–120. doi: 10.1038/35065132 [DOI] [PubMed] [Google Scholar]
- 40. Marban C, Redel L, Suzanne S, Van Lint C, Lecestre D, Chasserot-Golaz S, Leid M, Aunis D, Schaeffer E, Rohr O. 2005. COUP-TF interacting protein 2 represses the initial phase of HIV-1 gene transcription in human microglial cells. Nucleic Acids Res 33:2318–2331. doi: 10.1093/nar/gki529 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41. Azzaz AM, Vitalini MW, Thomas AS, Price JP, Blacketer MJ, Cryderman DE, Zirbel LN, Woodcock CL, Elcock AH, Wallrath LL, Shogren-Knaak MA. 2014. Human heterochromatin protein 1Α promotes nucleosome associations that drive chromatin condensation. J Biol Chem 289:6850–6861. doi: 10.1074/jbc.M113.512137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rohr O, Lecestre D, Chasserot-Golaz S, Marban C, Avram D, Aunis D, Leid M, Schaeffer E. 2003. Recruitment of tat to heterochromatin protein HP1 via interaction with CTIP2 inhibits human immunodeficiency virus type 1 replication in microglial cells. J Virol 77:5415–5427. doi: 10.1128/jvi.77.9.5415-5427.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Friedman J, Cho W-K, Chu CK, Keedy KS, Archin NM, Margolis DM, Karn J. 2011. Epigenetic silencing of HIV-1 by the histone H3 lysine 27 methyltransferase enhancer of zeste 2. J Virol 85:9078–9089. doi: 10.1128/JVI.00836-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pearson R, Kim YK, Hokello J, Lassen K, Friedman J, Tyagi M, Karn J. 2008. Epigenetic silencing of human immunodeficiency virus (HIV) transcription by formation of restrictive chromatin structures at the viral long terminal repeat drives the progressive entry of HIV into latency. J Virol 82:12291–12303. doi: 10.1128/JVI.01383-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tripathy MK, McManamy MEM, Burch BD, Archin NM, Margolis DM. 2015. H3K27 demethylation at the proviral promoter sensitizes latent HIV to the effects of vorinostat in ex vivo cultures of resting CD4 + T cells. J Virol 89:8392–8405. doi: 10.1128/JVI.00572-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pereira-Montecinos C, Toro-Ascuy D, Ananías-Sáez C, Gaete-Argel A, Rojas-Fuentes C, Riquelme-Barrios S, Rojas-Araya B, García-de-Gracia F, Aguilera-Cortés P, Chnaiderman J, Acevedo ML, Valiente-Echeverría F, Soto-Rifo R. 2022. Epitranscriptomic regulation of HIV-1 full-length RNA packaging. Nucleic Acids Res 50:2302–2318. doi: 10.1093/nar/gkac062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pocock GM, Becker JT, Swanson CM, Ahlquist P, Sherer NM. 2016. HIV-1 and M-PMV RNA nuclear export elements program viral genomes for distinct cytoplasmic trafficking behaviors. PLoS Pathog 12:e1005565. doi: 10.1371/journal.ppat.1005565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Das AT, Tenenbaum L, Berkhout B. 2016. Tet-on systems for doxycycline-inducible gene expression. Curr Gene Ther 16:156–167. doi: 10.2174/1566523216666160524144041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jordan A, Bisgrove D, Verdin E. 2003. HIV reproducibly establises a latent infection after acute infection of T cells in vitro. EMBO J 22:1868–1877. doi: 10.1093/emboj/cdg188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW. 1999. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci U S A 96:5215–5220. doi: 10.1073/pnas.96.9.5215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chase GP, Rameix-Welti M-A, Zvirbliene A, Zvirblis G, Götz V, Wolff T, Naffakh N, Schwemmle M. 2011. Influenza virus ribonucleoprotein complexes gain preferential access to cellular export machinery through chromatin targeting. PLoS Pathog 7:e1002187. doi: 10.1371/journal.ppat.1002187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Orjalo A, Johansson HE, Ruth JL. 2011. Stellaris fluorescence in situ hybridization (FISH) probes: a powerful tool for mRNA detection. Nat Methods 8:i–ii. doi: 10.1038/nmeth.f.349 [DOI] [Google Scholar]
- 53. Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH image to imagej: 25 years of image analysis. Nat Methods 9:671–675. doi: 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, Barrera LO, Van Calcar S, Qu C, Ching KA, Wang W, Weng Z, Green RD, Crawford GE, Ren B. 2007. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet 39:311–318. doi: 10.1038/ng1966 [DOI] [PubMed] [Google Scholar]
- 55. Heintzman ND, Hon GC, Hawkins RD, Kheradpour P, Stark A, Harp LF, Ye Z, Lee LK, Stuart RK, Ching CW, Ching KA, Antosiewicz-Bourget JE, Liu H, Zhang X, Green RD, Lobanenkov VV, Stewart R, Thomson JA, Crawford GE, Kellis M, Ren B. 2009. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature 459:108–112. doi: 10.1038/nature07829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nakayama J, Rice JC, Strahl BD, Allis CD, Grewal SI. 2001. Role of histone H3 Lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292:110–113. doi: 10.1126/science.1060118 [DOI] [PubMed] [Google Scholar]
- 57. Martens JHA, O’Sullivan RJ, Braunschweig U, Opravil S, Radolf M, Steinlein P, Jenuwein T. 2005. The profile of repeat-associated histone lysine methylation states in the mouse epigenome. EMBO J. 24:800–812. doi: 10.1038/sj.emboj.7600545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wei DG, Chiang V, Fyne E, Balakrishnan M, Barnes T, Graupe M, Hesselgesser J, Irrinki A, Murry JP, Stepan G, Stray KM, Tsai A, Yu H, Spindler J, Kearney M, Spina CA, McMahon D, Lalezari J, Sloan D, Mellors J, Geleziunas R, Cihlar T. 2014. Histone deacetylase inhibitor romidepsin induces HIV expression in CD4 T cells from patients on suppressive antiretroviral therapy at concentrations achieved by clinical dosing. PLoS Pathog 10:e1004071. doi: 10.1371/journal.ppat.1004071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, Steine EJ, Hanna J, Lodato MA, Frampton GM, Sharp PA, Boyer LA, Young RA, Jaenisch R. 2010. Histone H3K27Ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci U S A 107:21931–21936. doi: 10.1073/pnas.1016071107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Williams SA, Chen LF, Kwon H, Fenard D, Bisgrove D, Verdin E, Greene WC. 2004. Prostratin antagonizes HIV latency by activating NF-κB. J Biol Chem 279:42008–42017. doi: 10.1074/jbc.M402124200 [DOI] [PubMed] [Google Scholar]
- 61. Osborn L, Kunkel S, Nabel GJ. 1989. Tumor necrosis factor α and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kB. Proc Natl Acad Sci U S A 86:2336–2340. doi: 10.1073/pnas.86.7.2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Duh EJ, Maury WJ, Folks TM, Fauci AS, Rabson AB. 1989. Tumor necrosis factor α activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-κB sites in the long terminal repeat. Proc Natl Acad Sci U S A 86:5974–5978. doi: 10.1073/pnas.86.15.5974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Griffin GE, Leung K, Folks TM, Kunkel S, Nabel GJ. 1989. Activation of HIV gene expression during monocyte differentiation by induction of NF-kB. Nature 339:70–73. doi: 10.1038/339070a0 [DOI] [PubMed] [Google Scholar]
- 64. Jønsson KL, Tolstrup M, Vad-Nielsen J, Kjær K, Laustsen A, Andersen MN, Rasmussen TA, Søgaard OS, Østergaard L, Denton PW, Jakobsen MR. 2015. Histone deacetylase inhibitor romidepsin inhibits de novo HIV-1 infections. Antimicrob Agents Chemother 59:3984–3994. doi: 10.1128/AAC.00574-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kiernan RE, Vanhulle C, Schiltz L, Adam E, Xiao H, Maudoux F, Calomme C, Burny A, Nakatani Y, Jeang KT, Benkirane M, Van Lint C. 1999. HIV-1 tat transcriptional activity is regulated by acetylation. EMBO J. 18:6106–6118. doi: 10.1093/emboj/18.21.6106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Engeland CE, Brown NP, Börner K, Schümann M, Krause E, Kaderali L, Müller GA, Kräusslich H-G. 2014. Proteome analysis of the HIV-1 gag interactome. Virology 460–461:194–206. doi: 10.1016/j.virol.2014.04.038 [DOI] [PubMed] [Google Scholar]
- 67. Jäger S, Cimermancic P, Gulbahce N, Johnson JR, McGovern KE, Clarke SC, Shales M, Mercenne G, Pache L, Li K, et al. 2012. Global landscape of HIV-human protein complexes. Nature 481:365–370. doi: 10.1038/nature10719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ritchie C, Cylinder I, Platt EJ, Barklis E. 2015. Analysis of HIV-1 gag protein interactions via biotin ligase tagging. J Virol 89:3988–4001. doi: 10.1128/JVI.03584-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li Y, Frederick KM, Haverland NA, Ciborowski P, Belshan M. 2016. Investigation of the HIV-1 matrix interactome during virus replication. Proteomics Clinical Apps 10:156–163. doi: 10.1002/prca.201400189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Le Sage V, Cinti A, Valiente-Echeverría F, Mouland AJ. 2015. Proteomic analysis of HIV-1 gag interacting partners using proximity-dependent biotinylation. Virol J 12:138. doi: 10.1186/s12985-015-0365-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mohammadi P, Desfarges S, Bartha I, Joos B, Zangger N, Muñoz M, Günthard HF, Beerenwinkel N, Telenti A, Ciuffi A, Emerman M. 2013. 24 hours in the life of HIV-1 in a T cell line. PLoS Pathog 9:e1003161. doi: 10.1371/journal.ppat.1003161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tantale K, Garcia-Oliver E, Robert M-C, L’Hostis A, Yang Y, Tsanov N, Topno R, Gostan T, Kozulic-Pirher A, Basu-Shrivastava M, Mukherjee K, Slaninova V, Andrau J-C, Mueller F, Basyuk E, Radulescu O, Bertrand E. 2021. Stochastic pausing at latent HIV-1 promoters generates transcriptional bursting. Nat Commun 12:4503. doi: 10.1038/s41467-021-24462-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Skupsky R, Burnett JC, Foley JE, Schaffer DV, Arkin AP, Friedman N. 2010. HIV promoter integration site primarily modulates transcriptional burst size rather than frequency. PLoS Comput Biol 6:e1000952. doi: 10.1371/journal.pcbi.1000952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Burnett JC, Miller-Jensen K, Shah PS, Arkin AP, Schaffer DV, Bieniasz PD. 2009. Control of stochastic gene expression by host factors at the HIV promoter. PLoS Pathog 5:e1000260. doi: 10.1371/journal.ppat.1000260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Singh A, Razooky B, Cox CD, Simpson ML, Weinberger LS. 2010. Transcriptional bursting from the HIV-1 promoter is a significant source of stochastic noise in HIV-1 gene expression. Biophys J 98:L32–L34. doi: 10.1016/j.bpj.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nikolaitchik OA, Liu S, Kitzrow JP, Liu Y, Rawson JMO, Shakya S, Cheng Z, Pathak VK, Hu W-S, Musier-Forsyth K. 2021. Selective packaging of HIV-1 RNA genome is guided by the stability of 5' untranslated region polyA stem. Proc Natl Acad Sci U S A 118:e2114494118. doi: 10.1073/pnas.2114494118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nikolaitchik OA, Somoulay X, Rawson JMO, Yoo JA, Pathak VK, Hu W-S. 2020. Unpaired guanosines in the 5' untranslated region of HIV-1 RNA act synergistically to mediate genome packaging. J Virol 94:e00439-20. doi: 10.1128/JVI.00439-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Brown JD, Kharytonchyk S, Chaudry I, Iyer AS, Carter H, Becker G, Desai Y, Glang L, Choi SH, Singh K, Lopresti MW, Orellana M, Rodriguez T, Oboh U, Hijji J, Ghinger FG, Stewart K, Francis D, Edwards B, Chen P, Case DA, Telesnitsky A, Summers MF. 2020. Structural basis for transcriptional start site control of HIV-1 RNA fate. Science 368:413–417. doi: 10.1126/science.aaz7959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Deng Y, Hammond JA, Pauszek R, Ozog S, Chai I, Rabuck-Gibbons J, Lamichhane R, Henderson SC, Millar DP, Torbett BE, Williamson JR. 2021. Discrimination between functional and non-functional cellular gag complexes involved in HIV-1 assembly: characterizing intracellular HIV-1 gag complexes. J Mol Biol 433:166842. doi: 10.1016/j.jmb.2021.166842 [DOI] [PubMed] [Google Scholar]
- 80. Agosto LM, Gagne M, Henderson AJ. 2015. Impact of chromatin on HIV replication. Genes (Basel) 6:957–976. doi: 10.3390/genes6040957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chertova E, Chertov O, Coren LV, Roser JD, Trubey CM, Bess JW, Sowder RC, Barsov E, Hood BL, Fisher RJ, Nagashima K, Conrads TP, Veenstra TD, Lifson JD, Ott DE. 2006. Proteomic and biochemical analysis of purified human immunodeficiency virus type 1 produced from infected monocyte-derived macrophages. J Virol 80:9039–9052. doi: 10.1128/JVI.01013-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Reuse S, Calao M, Kabeya K, Guiguen A, Gatot J-S, Quivy V, Vanhulle C, Lamine A, Vaira D, Demonte D, et al. 2009. Synergistic activation of HIV-1 expression by deacetylase inhibitors and prostratin: implications for treatment of latent infection. PLoS One 4:e6093. doi: 10.1371/journal.pone.0006093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bannister AJ, Kouzarides T. 2011. Regulation of chromatin by histone modifications. Cell Res 21:381–395. doi: 10.1038/cr.2011.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Machida S, Depierre D, Chen HC, Thenin-Houssier S, Petitjean G, Doyen CM, Takaku M, Cuvier O, Benkirane M. 2020. Exploring histone loading on HIV DNA reveals a dynamic nucleosome positioning between unintegrated and integrated viral genome. Proc Natl Acad Sci U S A 117:6822–6830. doi: 10.1073/pnas.1913754117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Schoenfelder S, Fraser P. 2019. Long-range enhancer–promoter contacts in gene expression control. Nat Rev Genet 20:437–455. doi: 10.1038/s41576-019-0128-0 [DOI] [PubMed] [Google Scholar]
- 86. Park J, Lim CH, Ham S, Kim SS, Choi BS, Roh TY. 2014. Genome-wide analysis of histone modifications in latently HIV-1 infected T cells. AIDS 28:1719–1728. doi: 10.1097/QAD.0000000000000309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhou M, Deng L, Lacoste V, Park HU, Pumfery A, Kashanchi F, Brady JN, Kumar A. 2004. Coordination of transcription factor phosphorylation and histone methylation by the P-TEFb kinase during human immunodeficiency virus type 1 transcription. J Virol 78:13522–13533. doi: 10.1128/JVI.78.24.13522-13533.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhao W, Xu Y, Wang Y, Gao D, King J, Xu Y, Liang F-S. 2021. Investigating crosstalk between H3K27 acetylation and H3K4 trimethylation in CRISPR/dCas-based epigenome editing and gene activation. Sci Rep 11:15912. doi: 10.1038/s41598-021-95398-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tie F, Banerjee R, Stratton CA, Prasad-Sinha J, Stepanik V, Zlobin A, Diaz MO, Scacheri PC, Harte PJ. 2009. CBP-mediated acetylation of histone H3 lysine 27 antagonizes Drosophila polycomb silencing. Development 136:3131–3141. doi: 10.1242/dev.037127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Shah R, Gallardo CM, Jung YH, Clock B, Dixon JR, McFadden WM, Majumder K, Pintel DJ, Corces VG, Torbett BE, Tedbury PR, Sarafianos SG. 2022. Activation of HIV-1 proviruses increases downstream chromatin accessibility. iScience 25:105490. doi: 10.1016/j.isci.2022.105490 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Legends for Fig. S1 to S4.
Quantitative assessment of full-length Gag expression and euchromatin mark in HIV-1 Gag-mCerulean rtTA HeLa cells.
Quantitative assessment of full-length Gag expression and euchromatin mark in HIV-1 Gag-mCerulean rtTA HeLa cells in the presence of RMD.
Quantitative assessment of full-length Gag expression and euchromatin mark in latently-infected J-Lat 10.6 cells induced with either prostratin or TNFα.
Quantitative assessment of full-length Gag expression and euchromatin mark in latently infected J-Lat 10.6 cells induced treated with RMD.
Legends for Movies S1 to S5.
HIV-1 Gag co-localization with USvRNA at 8 hpi.
HIV-1 Gag co-localization with USvRNA at 24 hpi.
HIV-1 Gag co-localization with euchromatin marker H3K4me3.
HIV-1 Gag co-localization with euchromatin marker H3K27ac.
HIV-1 Gag co-localization with heterochromatin marker H3K9me3.