Abstract
IMPORTANCE
Approximately 18.6 million people worldwide are affected by a diabetic foot ulcer each year, including 1.6 million people in the United States. These ulcers precede 80% of lower extremity amputations among people diagnosed with diabetes and are associated with an increased risk of death.
OBSERVATIONS
Neurological, vascular, and biomechanical factors contribute to diabetic foot ulceration. Approximately 50% to 60% of ulcers become infected, and about 20% of moderate to severe infections lead to lower extremity amputations. The 5-year mortality rate for individuals with a diabetic foot ulcer is approximately 30%, exceeding 70% for those with a major amputation. The mortality rate for people with diabetic foot ulcers is 231 deaths per 1000 person-years, compared with 182 deaths per 1000 person-years in people with diabetes without foot ulcers. People who are Black, Hispanic, or Native American and people with low socioeconomic status have higher rates of diabetic foot ulcer and subsequent amputation compared with White people. Classifying ulcers based on the degree of tissue loss, ischemia, and infection can help identify risk of limb-threatening disease. Several interventions reduce risk of ulcers compared with usual care, such as pressure-relieving footwear (13.3% vs 25.4%; relative risk, 0.49; 95% CI, 0.28–0.84), foot skin measurements with off-loading when hot spots (ie, greater than 2 °C difference between the affected foot and the unaffected foot) are found (18.7% vs 30.8%; relative risk, 0.51; 95% CI, 0.31–0.84), and treatment of preulcer signs. Surgical debridement, reducing pressure from weight bearing on the ulcer, and treating lower extremity ischemia and foot infection are first-line therapies for diabetic foot ulcers. Randomized clinical trials support treatments to accelerate wound healing and culture-directed oral antibiotics for localized osteomyelitis. Multidisciplinary care, typically consisting of podiatrists, infectious disease specialists, and vascular surgeons, in close collaboration with primary care clinicians, is associated with lower major amputation rates relative to usual care (3.2% vs 4.4%; odds ratio, 0.40; 95% CI, 0.32–0.51). Approximately 30% to 40% of diabetic foot ulcers heal at 12 weeks, and recurrence after healing is estimated to be 42% at 1 year and 65% at 5 years.
CONCLUSIONS AND RELEVANCE
Diabetic foot ulcers affect approximately 18.6 million people worldwide each year and are associated with increased rates of amputation and death. Surgical debridement, reducing pressure from weight bearing, treating lower extremity ischemia and foot infection, and early referral for multidisciplinary care are first-line therapies for diabetic foot ulcers.
Diabetic foot ulcers affect approximately 18.6 million people worldwide and 1.6 million in the United States each year.1 These ulcers are associated with impaired physical function, reduced quality of life, and increased health care utilization.2,3 If left untreated, foot ulcers can progress to soft tissue infection, gangrene, and limb loss.4 Approximately half of people with a diabetic foot ulcer have lower extremity peripheral artery disease.5 Approximately 50% of ulcers become infected, with up to 20% of these requiring hospitalization; between 15% and 20% of moderate to severe infections eventually lead to a lower-extremity amputation.3,6,7 People with a diabetic foot ulcer have a 5-year mortality rate of 30%, with a mortality rate greater than 70% for people with an above-foot amputation.8 The direct costs of treating diabetic foot ulcers in the United States are estimated to be $9 billion to $13 billion annually.9 This Review summarizes current evidence regarding the epidemiology, pathophysiology, diagnosis, treatment, and prevention of diabetic foot ulcers. A list of common clinical questions and answers related to diabetic foot ulcers is shown in the Box.
Box. Common Questions About Diabetic Foot Ulcers.
How Should Diabetic Foot Ulcers Be Evaluated?
Evaluation of diabetic foot ulcers should include a comprehensive examination of the ulcer (size, depth, signs of infection), assessment for peripheral artery disease with noninvasive vascular laboratory testing, and, if necessary, laboratory measures (erythrocyte sedimentation rate) and imaging studies (plain films followed by magnetic resonance imaging as necessary) for suspected osteomyelitis. Classification of diabetic foot ulcers based on tissue loss, ischemia, and infection can help quantify the risk of amputation.
How Are Diabetic Foot Ulcers Treated?
Treatment for diabetic foot ulcers involves wound care with debridement of necrotic tissue, reduction of weight-bearing pressure on the affected area, management of blood glucose levels ideally to a hemoglobin A1c less than 8%, treatment of infection with appropriate antibiotics, and evaluation for revascularization when peripheral artery disease is present. In some patients, advanced wound treatments may be applied to accelerate healing. A multidisciplinary team approach involving podiatrists, infectious disease specialists, and vascular surgeons, in collaboration with a primary care clinician, can improve outcomes.
How Can the Risk of Diabetic Foot Ulcer Recurrence Be Reduced?
Forty-two percent of patients who have a healed diabetes-related foot ulcer will develop another ulcer within 1 year; therefore, these patients should undergo regular examination of their feet and be treated for callus and other preulcer signs by an appropriately trained clinician. Patients should be educated about proper foot self-care, advised to monitor their foot skin temperatures and off-load when hot spots are found, and provided with and encouraged to wear adequately fitting and pressure-relieving footwear to reduce risk of foot ulcer recurrence.
Methods
We searched PubMed for English-language publications between January 2013 and May 15, 2023, using the search term diabetic foot ulcer, retrieving 2411 articles. Of the 2411 articles identified, 96 were included, consisting of 10 randomized clinical trials, 14 meta-analyses, 10 clinical guidelines, and 62 observational studies.
Epidemiology
More than 550 million people worldwide and 37 million people in the United States have diabetes.10,11 Worldwide, approximately 18.6 million people with diabetes develop a foot ulcer each year.1 Up to approximately 34% of people with type 1 or 2 diabetes develop a foot ulcer during their lifetime.2
About 20% of people with a diabetic foot ulcer will undergo a lower extremity amputation, either minor (ie, part of the foot) or major (ie, above foot).12 Infection and progressive gangrene are the primary causes of lower extremity amputation,4 with approximately 50% of diabetic foot ulcers becoming infected.7,13 Up to approximately 20% of people with a diabetic foot ulcer require hospitalization, and between 15% and 20% of hospitalized patients undergo a lower extremity amputation.6,7,13 In the United States, more than 150 000 nontraumatic lower extremity amputations are performed every year in people with diabetes.11Worldwide, approximately 1.6 million amputations occur each year. Of these, approximately 33% are major amputations.1,14
Inequities in diabetes-related foot complications are common in the United States. Among Medicare beneficiaries (total sample, 92 929) with a newly diagnosed diabetic foot ulcer or diabetic foot infection, the long-term rates of major lower extremity amputation are higher in individuals identifying as non-Hispanic Black (3.8%), Hispanic of any race (2.1%), and Native American (5.1%) than among beneficiaries identifying as non-Hispanic White (1.5%).15 People identifying as non-Hispanic Black and Hispanic of any race have more advanced diabetic foot ulcers and peripheral artery disease at initial presentation and are more likely to undergo lower extremity amputation without a lower extremity revascularization attempt.16–18 Among Medicare beneficiaries (total sample, 643 287), patients identifying as non-Hispanic Black had twice the odds of lower extremity amputation within 1 year of foot ulcer diagnosis after adjusting for multiple covariates (odds ratio [OR], 1.98; 95% CI, 1.93–2.03; P < .001 [absolute rates not provided]) compared with those identifying as non-Hispanic White.19 In addition, diabetic foot ulcers disproportionately affect people with lower socioeconomic status and those living in rural areas.20 For example, in California, the prevalence-adjusted lower extremity amputation rates among individuals with diabetes in low-income neighborhoods was double that among those in higher-income neighborhoods (absolute rates not provided).21 People with diabetes living in rural areas had a higher frequency of major lower extremity amputation (3.4% vs 2.4% in 1 year; adjusted OR, 1.56; 95% CI, 1.48–1.65) compared with those residing in large metropolitan areas, adjusted for sociodemographic and clinical factors.22 The rate of death or major lower extremity amputation was higher in patients with diabetes living in rural areas who identified as non-Hispanic Black (28.0% vs 18.3% of other rural patients with diabetes).23
A meta-analysis of patients with diabetes showed a crude rate of 231 deaths per 1000 person-years in people with a diabetic foot ulcer, compared with 182 deaths per 1000 person-years in those without a diabetic foot ulcer.24 A study of 66 323 US veterans with incident diabetic foot ulcer showed that 3% presented with gangrene, and after adjusting for covariates such as cerebrovascular, cardiovascular, and kidney disease, people with adiabetic foot ulcer and gangrene had an increased risk of mortality (the 1-year survival rate was 62.7% vs 80.8% for the entire cohort of patients with diabetic foot ulcers; HR 1.70 [95% CI, 1.57–1.83]; P < .001).25
Pathophysiology
Diabetic foot ulcers develop as a result of diabetic sensory, motor, and autonomic neuropathy. Sensory neuropathy leads to loss of protective sensation; motor neuropathy causes foot deformity and biomechanical abnormalities, while autonomic neuropathy leads to vis-coelastic changes in the skin, such as skin dryness.2 Callus formation frequently results from these changes (Figure1).2 Minor trauma and inflammation from repetitive impact of the foot while weight-bearing can cause hemorrhage beneath the callus that presents as a full-thickness ulcer (ie, damage extends below the epidermis and dermis into the subcutaneous tissue)on removal of the callus.2 Other mechanisms by which diabetic foot ulcers develop include constant low pressure, eg, from tight shoes causing tissue necrosis, or extremely high pressure, such as a sharp object causing direct mechanical damage.2
Figure 1.
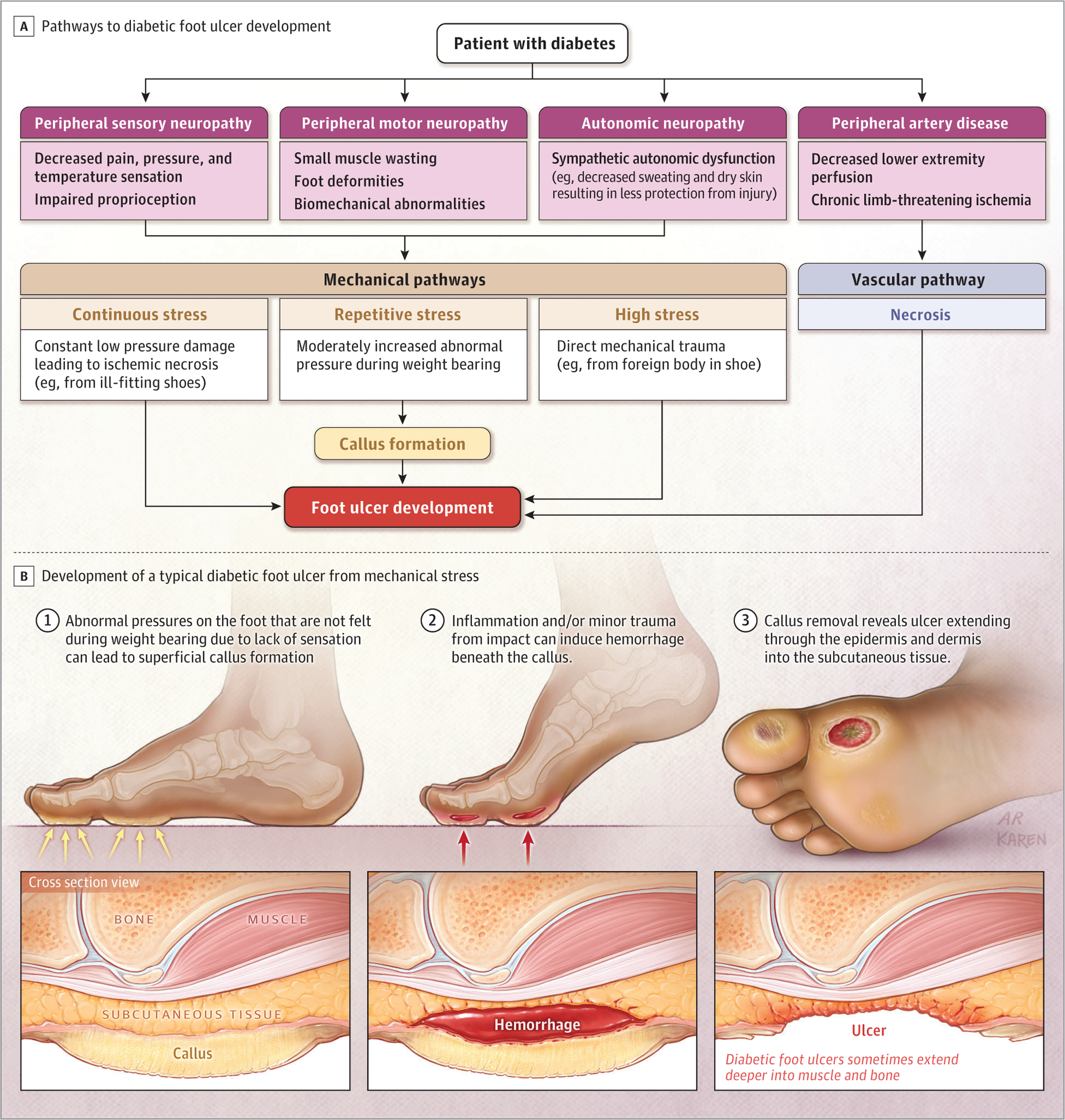
Pathways to Diabetic Foot Ulceration (Mechanical and Ischemic Factors)
Screening for Risk of Diabetic Foot Ulcers
To assess the risk of ulceration in a person with diabetes, the feet are screened annually by a primary care clinician or podiatrist; the screening should include evaluating the feet for neuropathy sufficient to cause loss of protective sensation, peripheral artery disease, and skin breakdown.26 Among patients with a new diabetic foot ulcer, those who had been seen by a podiatrist for preventive care in the year before ulcer development had a lower risk of major lower extremity amputation relative to those who had not seen a podiatrist in the past year(1.20%vs1.84%;OR,0.61[95%CI,0.51–0.72];P < .001).27Acom-prehensive foot examination can be completed by a podiatrist once neuropathy or peripheral artery disease has been diagnosed.28 The history should include questions about any previous diabetic foot complication, which is associated with greater risk of a diabetic foot ulcer (Table1). Pooled data from 8 prospective studies with 1738 participants, 1 retrospective study with 46 participants, and the usual care groups of 9 randomized clinical trials with 636 participants reported that the risk of recurrence of a diabetic foot ulcer was 42% at 1 year, 58% at 3 years, and 65% at 5 years.2
Table 1.
Screening and Follow-Up for Diabetic Foot Ulcer Risk and Active Complications of Diabetic Foot Diseasea
| Category | Ulcer risk | Characteristics | Follow-Up frequency | Prevalence, % |
|---|---|---|---|---|
| Active pathology | Active | Active ulcer, Charcot arthropathy, or infection with or without peripheral artery disease | Rapid referral to specialist/multidisciplinary team | 1.4 |
| 3 | High | In remission: history of diabetic foot ulcer, amputation (minor or major), or end-stage renal disease | 1–3 mo | 1.8 |
| 2 | Moderate | ≥2 Factors among loss of protective sensation, peripheral artery disease, and foot deformity | 3–6 mo | 4.3 |
| 1 | Low | Loss of protective sensation or peripheral artery disease | 6–12 mo | 14.2 |
| 0 | Very low | No loss of protective sensation or peripheral artery disease | Annually | 78.6 |
Based on the International Working Group on the Diabetic Foot (IWGDF) risk classification system for screening and assessment of people with diabetes at risk for foot ulceration and with active disease. Prevalence estimates are adapted from Stang and Leese.29
Individuals with diabetes are assessed for loss of protective sensation as a sign of large fiber neuropathy.28 The Semmes-Weinstein 5.07 monofilament test to assess for absence of pressure sensation at a minimum of 3 sites per foot (likelihood ratio for ulceration range, 11–16) or the 128-Hz tuning fork to assess for absence of vibratory perception (using an on-off technique or timed methods; likelihood ratio range, 16–35) are important components of this assessment.26,30 In the absence of this equipment, the Ipswich Touch Test is an acceptable alternative that can be used to evaluate whether a patient can perceive light touch from an examiner’s index finger applied to 6 or 8 prespecified sites on the feet (likelihood ratio range, 10–15).26
Physical examination should include evaluation for calluses, interdigital maceration, and thickened nails, which may indicate a fun-gal infection and may be associated with increased pressure on the nail bed.28 Digital deformities such as hammer toes or claw toes appear as increased prominence of the interphalangeal joints dorsally and the metatarsal heads on the plantar surface and are common sites of ulceration. The tip of a toe exposed to increased pressure in contact with the ground or shoe is also a common site of ulceration. Assessment of dorsiflexion and plantarflexion ankle range of motion can identify equinus deformity (ie, less than 0 degrees of dorsiflexion at the ankle joint), which increases forefoot plantar pressure. In a prospective study of 1666 people with diabetes, equinus deformity was present in 10.3% .31 Charcot arthropathy, defined as a foot fracture with possible joint dislocation in people with peripheral neuropathy, affects approximately 0.3% of people with diabetes, can lead to significant deformity, and increases the risk of a diabetic foot ulcer, particularly in the midfoot and ankle/hindfoot.32 Although approximately 40% of people with Charcot arthropathy have concomitant tissue loss, a unilateral red, hot, swollen foot without a wound or diagnosis of deep vein thrombosis may indicate Charcot arthropathy.32
Pulse palpation at the ankle and on the foot is a central part of the vascular examination, but palpable pulses have low sensitivity (71.7%) and specificity (72.3%) for detecting peripheral artery disease.33,34 Because peripheral artery disease affects approximately half of people with diabetic foot ulcers,5 clinicians should consider noninvasive testing with the ankle-brachial index or toebrachial index and/or referral to vascular specialists in people with diabetic foot ulcers.35
Assessing Diabetic Foot Ulcers
Classification
Although there are numerous wound classification systems, most focus on the degree of tissue loss, with less emphasis on concomitant infection or ischemia.36,37 Similarly, numerous vascular classifications exist but focus on the degree of ischemia without consideration of tissue loss or infection until the latest stages.38TheWound, Ischemia, Foot Infection (WIfI) classification system was developed and validated as a method to combine all 3 variables (wound, ischemia, and foot infection)and to accurately assess the risk of limb loss for patients with diabetic foot ulcers.38,39 This classification includes assessment of degrees of tissue loss, ischemia, and foot infection as none, mild, moderate, or severe. It assists clinicians in identifying and communicating the severity of diabetic foot ulceration to organize rapid multidisciplinary clinical care (Figure 2).37 A higher score on the WIfI scale is associated with lower extremity amputation and morbidity and can be used to determine the need for revascularization. WIfI scores of 1, 2, 3, and 4 were associated with 1-year amputation rates of 0%, 8%, 11%, and 38%, respectively.39
Figure 2. Wound, Ischemia, and Foot Infection (WIfI) Classification of Limb Threat.
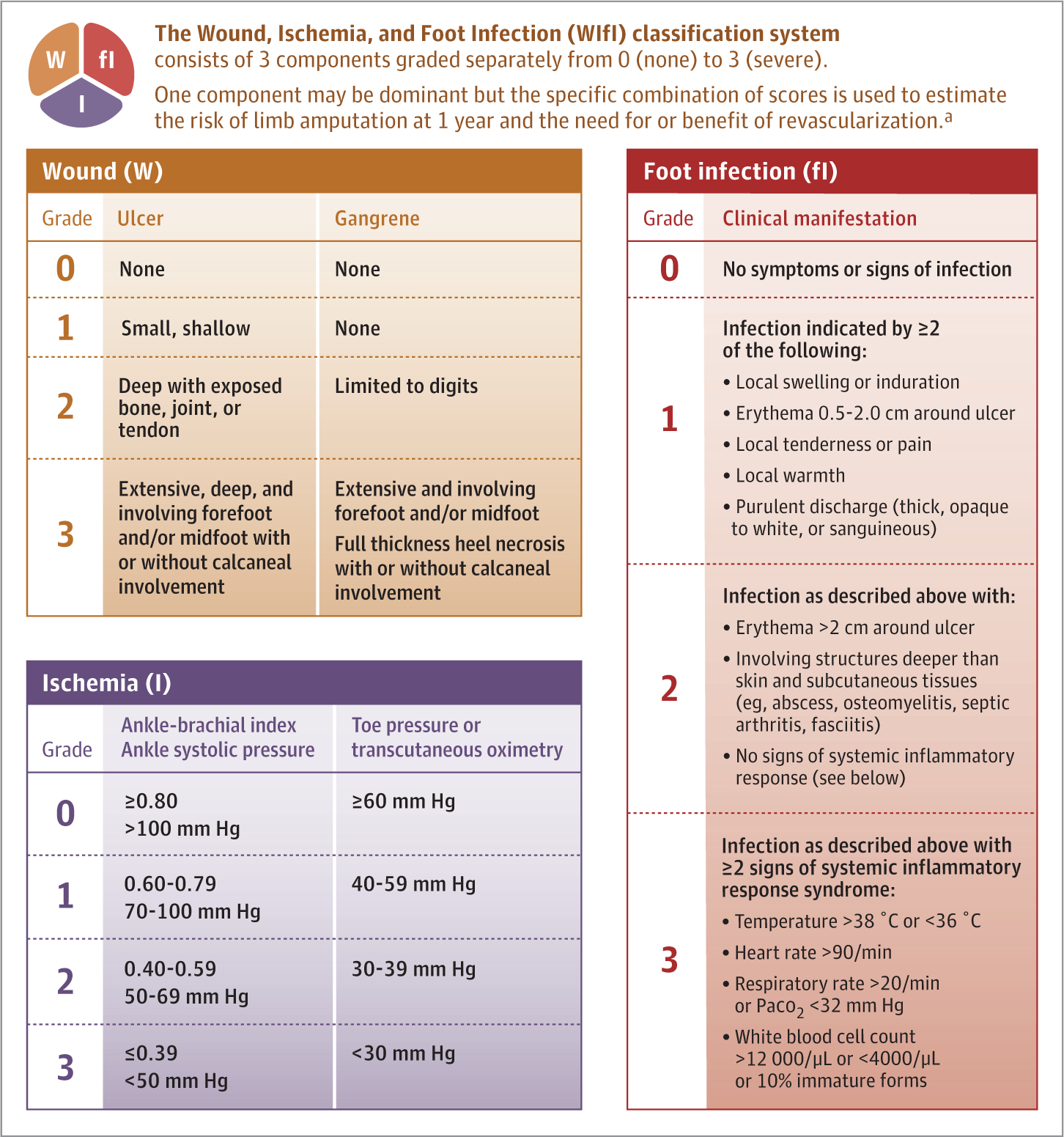
In diabetic foot disease, tissue loss, ischemia, and infection frequently overlap. However, one is frequently more dominant than the other at different times in the life cycle of an acute-on-chronic event. Here, the amount of tissue loss, ischemia, and foot infection can be ordinally graded to help predict outcome and assist in communicating a plan of action.
a A higher score on the WIfI scale38 is associated with lower extremity amputation and morbidity and can be used to determine the need for revascularization. WIfI scores of 1, 2, 3, and 4 were associated with 1-year amputation rates of 0%, 8%, 11%, and 38%, respectively.39 See also Figure 3.
Evaluating Infection
The diagnosis of diabetic foot infection is primarily based on clinical assessment and is suggested by presence of more than 2 signs of inflammation, such as erythema, swelling and possibly purulence, fluctuance, or lymphangiitis.7,13 Randomized clinical trial data are not available to support obtaining wound cultures from all patients with diabetic foot ulcers.7,13 In the absence of other associated diagnoses, an erythrocyte sedimentation rate greater than 70 mm/h can be helpful in improving diagnostic accuracy for osteomyelitis (likelihood ratio, 11).40,41 Particularly among hospitalized patients, for whom the pretest probability of osteomyelitis is high, the combination of a positive probe-to-bone test and plain film radiography can be sufficient to diagnose osteomyelitis (likelihood ratio, 14) without the need for other, more expensive radiological studies.41,42 Magnetic resonance imaging has shown consistent accuracy in identifying osteomyelitis (likelihood ratio, 3.6; sensitivity, 93%; specificity, 75%), particularly when the clinical assessment and plain x-ray findings are equivocal, and may be helpful in identifying occult abscesses or determining the extent of deeper infections.7,43 For diagnosing osteomyelitis, bone biopsy and culture remain the gold standard.7,13
Evaluating Peripheral Artery Disease
Lower extremity peripheral artery disease can be assessed noninvasively with the ankle-brachial index, a ratio of Doppler-recorded arterial pressures of the dorsalis pedis and posterior tibial pressures divided by the brachial artery pressures.35 An ankle-brachial index less than 0.90 is approximately 98% specific and approximately 85% sensitive for peripheral artery disease.35 However, people with diabetes often have medial calcinosis of lower extremity peripheral vessels, resulting in falsely elevated peripheral pressures and a high ankle-brachial index that is insensitive to presence of peripheral artery disease. In these individuals, the toe-brachial index can be measured, since the digital arteries are less commonly affected by medial calcinosis.35 A toe-brachial index less than 0.70 is consistent with peripheral artery disease.33 Toe pressure of 30 mm Hg or greater, transcutaneous oxygen pressure of 25 mm Hg or greater, and skin perfusion pressure of 40 mm Hg or greater have been associated with higher rates of ulcer healing.35 A systematic review and meta-analysis of 25 studies that included 3789 patients reported that transcutaneous oxygen pressure demonstrated high accuracy in predicting ulcer healing and limb amputation.44 The sensitivity and specificity of transcutaneous oxygen pressure for ulcer healing were 0.72 (95% CI, 0.61–0.81) and 0.86 (95% CI, 0.68–0.95), respectively, with a diagnostic OR of 15.81 (95% CI, 3.36–74.45). This is compared with a relatively low prognostic accuracy for ulcer healing using the ankle-brachial index (with cutoffs of <0.9 and ≥1.3), with a sensitivity of 0.48 (95% CI, 0.36–0.61), a specificity of 0.52 (95% CI, 0.42–0.63), and a diagnostic OR of 1.02 (95% CI, 0.40–2.64).44 A skin perfusion pressure greater than 40 mm Hg showed a positive likelihood ratio ranging from 4.86 to 6.40 and corresponding negative likelihood ratios between 0.03 and 0.40 for ulcer healing.45 Another meta-analysis of 4 studies with 104 patients reported that a toe systolic blood pressure less than 30 mm Hg had a 2.09 times higher relative risk (RR) of a nonhealing wound post-amputation compared with values of 30 mm Hg or greater (95% CI, 1.37–3.20; P = .001 [absolute rates not provided]).46 These data are summarized in Table 2.
Table 2.
Assessing Ischemia in the Presence of a Diabetic Foot Ulcer
| Test | Definition | Sensitivity and specificity (wound healing) | Additional notes |
|---|---|---|---|
| Palpation of pulses45 | Palpation of anterior tibial or posterior tibial pulse | 35% sensitive; 100% specific | Pedal pulses that are palpable are associated with high probability of healing (relative risk, 2.26; 95% CI, 2.05–2.49). |
| Ankle-brachial index44 | Ankle pressure compared with arm pressure | 48% sensitive; 52% specific; diagnostic odds ratio, 1.02a | Less useful in patients with diabetes, kidney disease, and diabetic foot ulcers due to falsely elevated ankle pressure from medial calcinosis; low prognostic accuracy for ulcer healing. |
| Toe systolic blood pressure46,47 | Measurement of systolic blood pressure at the toe | 86% sensitive; 58% specific | Toe systolic blood pressure <30 mm Hg is associated with 2.09-fold higher relative risk of nonhealing after partial foot amputation compared with values ≥30 mm Hg (relative risk, 2.09; 95% CI, 1.37–3.20; P =.001). |
| Transcutaneous oximetry44 | Measurement of oxygen tension at the skin surface | 72% sensitive; 86% specific; diagnostic odds ratio, 15.81a | Transcutaneous oxygen pressure ≥25 mm Hg is associated with higher rates of ulcer healing and high accuracy in predicting ulcer healing and limb amputation. |
| Skin perfusion pressure45 | Measurement of blood pressure required to restore microvascular blood flow after occlusion | Skin perfusion pressure ≥40 mm Hg is associated with higher rates of ulcer healing; positive likelihood ratios range from 4.86 to 6.40 and corresponding negative likelihood ratios from 0.03 to 0.40. |
Diagnostic odds ratio is defined as odds of a positive test result in people with disease relative to the odds of a positive test result in those without disease.
Management
People at Risk of Diabetic Foot Ulcer
People in the lowest foot ulcer risk category without loss of protective sensation, peripheral artery disease, or history of foot complications may return for annual follow-up with a primary care clinician or podiatrist (Table 1).26,28 People with diabetes who have an increased risk of foot ulceration should receive education about proper foot self-care and appropriate footwear.28 People with 2 or more risk factors among loss of protective sensation, peripheral artery disease, and foot deformity are considered at moderate risk and should have a shoe specialist consultation (from a podiatrist, pedorthist, or orthotist) for good-quality footwear with appropriate fit that may include therapeutic footwear to reduce pressure.28 People at moderate risk should return for evaluation by a podiatrist every 3 to 6 months (per International Working Group on the Diabetic Foot and American Diabetes Association guidelines, based on expert consensus)28,48; those with peripheral artery disease may require assessment by a vascular specialist.28 Immediate vascular referral is indicated for patients with a diabetic foot ulcer and an ankle pressure less than 50 mm Hg, an ankle-brachial index less than 0.5, a toe pressure less than 30 mm Hg, or a transcutaneous oxygen pressure less than 25 mm Hg.35 Vascular surgery referral should also be considered, regardless of results of vascular study, for patients with peripheral artery disease and a nonhealing diabetic foot ulcer that persists for 4 to 6 weeks despite evidence-based care consisting of pressure off-loading and wound debridement.26,35,49 People with a diabetic foot ulcer that has resolved or who have undergone partial foot amputation for diabetic foot ulcer are in the highest risk category(also called remission).They require pressure-relieving shoes/ orthoses that accommodate foot shape and any deformity present to reduce risk of ulcer recurrence, and this may include custom-made footwear or insoles in extra-depth shoes.28 People with a healed foot ulcer are recommended to return for screening and professional foot care every 1 to 3 months (Table 1).28
People with a healed ulcer may also benefit from dermal thermometry (measuring skin temperature) to identify areas of preulcerative inflammation. A meta-analysis of 772 people in 5 randomized clinical trials showed that at-home skin temperature monitoring, with reduction of steps taken (walking less) in response to hot spots (ie, >2 °C difference between the affected foot and the unaffected foot), decreased risk of developing a diabetic foot ulcer compared with no temperature monitoring (18.7% vs 30.8%; RR, 0.51; 95% CI, 0.31–0.84; P = .008).50
People With a Diabetic Foot Ulcer
A flow chart for management of active diabetic foot complications is shown in Figure 3.
Figure 3. Management of Active Diabetic Foot Complications.
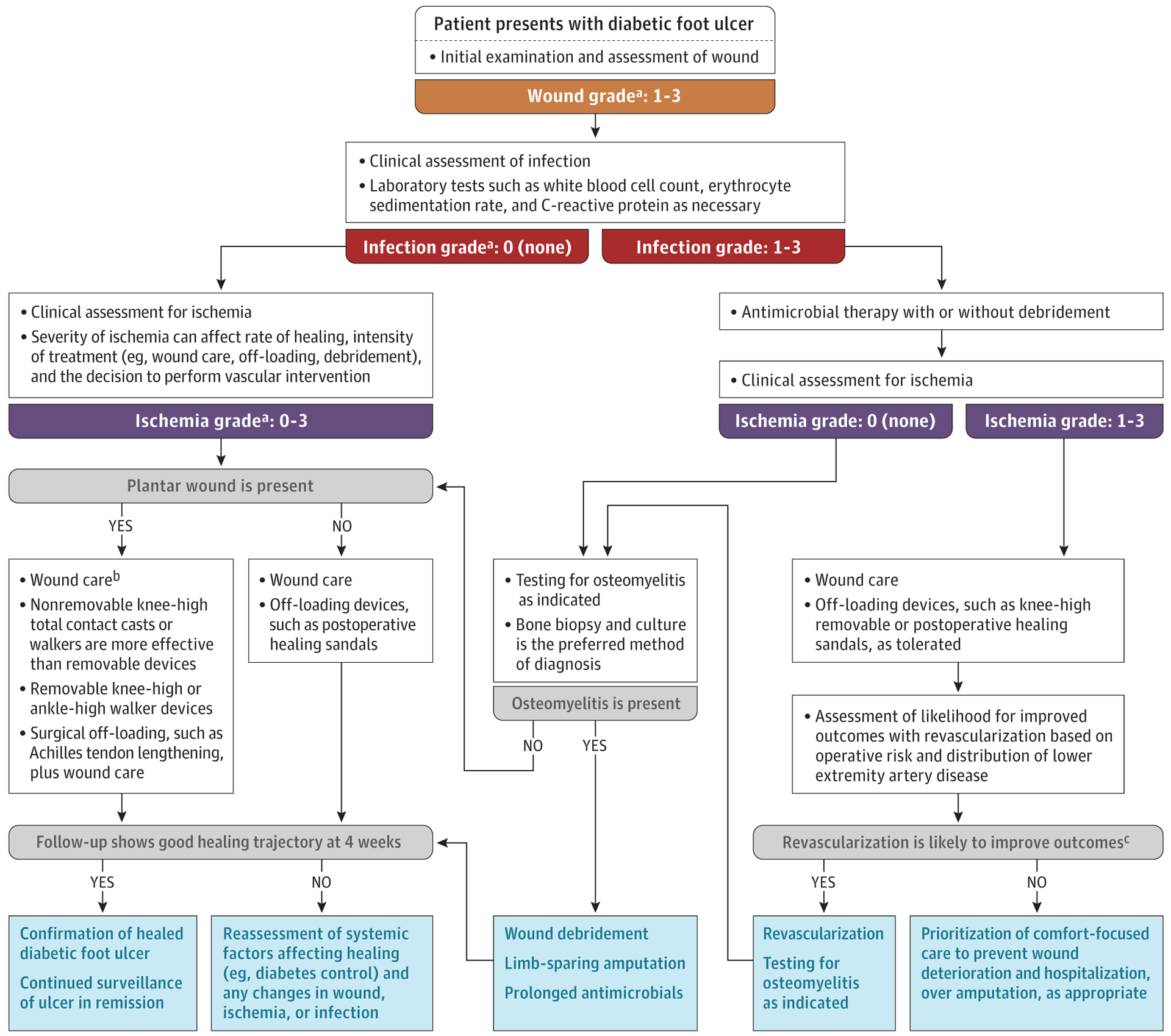
Flowchart for patients with a diabetic foot ulcer based on assessment and treatment of the wound, of ischemia,35,49 and of foot infection.7 Additional detail on off-loading wounds,51 wound management,52 treatment of infection,7 and management of chronic limb threatening ischemia may be found in the International Working Group on the Diabetic Foot guidelines35 and Global Vascular Guidelines.49
a Grading based on the Wound, Ischemia, and Foot Infection (WIfI) classification system. See also Figure 2.
b See also Tables 3, 4, and 5.
c See also Table 6.
Wound Management
Debridement |
Debridement is a standardized approach used to facilitate healing.52 Healing is achieved by eliminating nonviable wound bed and wound edge tissue, including excess callus on the periphery and nonviable dermal tissue, as well as foreign materials and bacterial components. Although guidelines recommend regular debridement, defined as weekly or every other week, randomized clinical trials are lacking.52,53 One study of 154 644 patients with chronic wounds treated at 525 US-based centers (19.0% with diabetic foot ulcers) reported a significant increase in healing (55% vs 28%;P < .001)in people who had weekly debridement vs those who had less frequent debridement.54
Off-Loading |
Off-loading repetitive mechanical stress on the foot, consisting of reducing weight bearing on the ulcer, is an important aspect of treatment and reduces pressure over the wound by spreading force over a larger unit area, thus providing an environment for healing.55 The most effective treatment for off-loading a plantar foot ulcer is a knee-high nonremovable off-loading device, either a total contact cast or a knee-high walker rendered nonremovable.51A total contact cast is a special cast boot applied with minimal padding by a cast technician. The knee-high walker is a prefabricated boot that is generally applied with Velcro or straps. Both the total contact cast and the walker spread force out over a large area, effectively reducing pressure at the ulcer by as much as 80% to 90% compared with a standard shoe.55 Two national surveys of clinicians in the United States and Australia show that the total contact cast was used in only 2% and 15% of patients, respectively, as a primary means of off-loading.56,57 Clinicians frequently cited patient preference as a reason for lack of total contact cast use.56 Alternatively, removable walkers can reduce pressure effectively but do not promote healing as well as nonremovable walkers or total contact casts. Pooled data from 14 randomized clinical trials (1083 patients) showed that nonremovable devices were associated with improved healing compared with removable devices (81.9% vs 66.1%; RR, 1.24; 95% CI, 1.09–1.41; P = .001).58 A study of 20 patients wearing waist-mounted activity monitors and device-mounted monitors reported that patients engaged in only 28% of their total daily activity while wearing the protective boot compared with when it was not worn (345[SD,219] minutes vs 874 [SD,828] minutes;P = .01).59 When patient use of removable devices is suboptimal and the foot ulcer does not heal despite use of nonremovable off-loading devices, surgical reconstruction to help off-load pressure may help.51 For an ulcer on the apex of the lesser toes, flexor tendon tenotomy has become a first-line treatment, based on a recent single-center randomized clinical trial of 16 people with diabetic foot ulcers at the distal plantar digits (100% healing in the tenotomy group vs 37.5% in the group not receiving tenotomy; P = .03).60 Achilles tendon lengthening may reduce risk of ulcer recurrence (RR, 3.4; 95% CI, 1.4–8.2[absolute rates not provided]) for plantar forefootulcers.51,58 These studies are summarized in Table 3.
Table 3.
Reducing Weight-Bearing Pressure on a Diabetic Foot Ulcer
| Off-loading methods | Description | Outcome/benefit |
|---|---|---|
| Knee-high nonremovable off-loading device51 | Total contact cast or knee-high walker rendered nonremovable | Reduces pressure at the ulcer by 80%−90% compared with a standard shoe55; promotes better healing compared with removable devices (relative risk, 1.24; 95% CI, 1.09–1.41 [absolute rates not provided]). First-choice off-loading treatment in international guidelines.51 |
| Removable knee-high and ankle-high walkers51 | Off-loading devices that can be removed by the patient | Reduces pressure effectively but does not promote healing as well as nonremovable walkers or total contact cast. Second-choice off-loading treatment in international guidelines.51 A study of 20 patients wearing waist-mounted activity monitor and device-mounted monitor reported patients engaged in only 28% of their total daily activity while wearing the protective boot compared with when it was not worn (345 [SD, 219] min vs 874 [SD, 828] min; P =.01).59 |
| Felted foam in appropriately fitting shoes | Felted foam applied to at least the ulcer region | Reduces pressure and heals plantar ulcers less effectively than nonremovable and removable devices. Felted foam may be considered in combination with appropriately fitting shoes when off-loading devices are not available or tolerated.51 |
| Flexor tendon tenotomy | Surgical procedure for ulcers on the apex of the lesser toes | 100% vs 37.5% healing ((P = .03) in a single-center randomized clinical trial of 16 patients with diabetic foot ulcers at the distal plantar digits; flexor tendon tenotomy was compared with debridement, moisture-retentive dressings, and nonsurgical off-loading.60 |
| Achilles tendon lengthening | Surgical procedure for plantar forefoot ulcers if nonsurgical treatment fails | One randomized clinical trial of 64 participants showed significantly reduced forefoot plantar pressure compared with presurgical levels (647.2 [SD, 306.7] kPa vs 892.4 [SD, 176.6] kPa; P = .005), a small, nonsignificant effect on healing of foot ulcers when combined with a total contact cast compared with a total contact cast alone (relative risk, 1.10; 95% CI, 0.96–1.27), and reduced risk of recurrence for patients in diabetic foot ulcer remission (relative risk, 3.4; 95% CI, 1.4–8.2).51,61 |
Wound Dressings |
Few data are available regarding the optimal wound dressing for diabetic foot ulcers.52,62 The selection of a wound dressing for a diabetic foot ulcer should be based on wound characteristics, ie, location, presence and/or degree of inflammation, and amount of exudate (Table 4 and Table 5). The dressing should promote a moist environment conducive to tissue growth and epithelial migration without causing excess maceration. It is important to select dressings that remove excess fluid to prevent further tissue inflammation and damage from prolonged contact with the wound or its periphery. In general, hydrogels are preferred for wounds that produce little exudative drainage, while alginates or hydrofibers are recommended for heavily draining wounds.63,64
Table 4.
Wound Healing Dressing Types for Diabetic Foot Ulcersa
| Dressing type | Characteristics and use |
|---|---|
| Alginates | These dressings form a damp gel on absorption, necessitating a secondary dressing. They are conformable, filling dead spaces and managing moderate to heavy exudate effectively. Suitable for wounds with light to moderate serous drainage. |
| Antimicrobial dressings | These dressings contain substances such as silver or iodine that inhibit bacterial growth in the wound, making them suitable for infected wounds or those at high risk of infection. However, it is important to note that, as with each of these categories, there is a lack of strong evidence recommending their use despite their widespread application.52 |
| Collagens | Derived from bovine, equine, porcine, or ovine (sheep) sources, these products help stimulate wound healing. Available in various forms such as gel, pad, paste, powder, and sheets. Some dissolve entirely while others need removal per the manufacturer’s guidelines. A secondary dressing is usually required. Ideal for wounds showing granulation tissue, as they further stimulate its formation. |
| Film dressings | Thin, transparent dressings that foster a moist environment, promoting healing and enabling wound assessment without removal. Ideal for superficial wounds with minimal exudate. |
| Foams | These dressings are capable of absorbing moderate quantities of exudate and can be used under compression. |
| Gauze | Highly permeable dressing material, suitable for wound cleaning, as a cover dressing, and for securing dressings. Gauze is not generally recommended as a primary wound dressing because it can remove healthy granulation tissue during dry dressing changes. |
| Hydrocolloids | These bacteria-proof dressings facilitate autolytic debridement. They are not appropriate for infected wounds as they may damage fragile skin. Ideal for wounds with insignificant serous drainage. |
| Hydrogels | These are glycerin and water-based products available as amorphous gels, sheets, or impregnated dressings. They can be antimicrobial, donate moisture to wounds, assist in autolytic debridement, and possibly reduce pain. They require a secondary dressing and are suitable for low-exudate wounds needing additional moisture. |
Adapted from Sidawy and Perler.63
Table 5.
Wound Healing Therapies for Diabetic Foot Ulcers
| Wound healing therapies | Description | Outcome/benefit |
|---|---|---|
| Dressing selection | Based on wound characteristics, ie, location, inflammation, and amount of exudate | Promote a moist environment conducive to tissue growth and epithelial migration.63,64 |
| Topical fibrin and leucocyte platelet patch | Autologous leucocytes, platelets, and fibrin placed on the wound | Randomized clinical trial of 269 patients reported improved healing at 20 wk with patch compared with standard of care consisting of adequate off-loading, wound debridement, and moisture-balancing dressings (34% vs 22% healed; odds ratio, 1.58; 95% CI, 1.04–2.40).65 |
| Placenta-derived products | Contain growth factors, collagen-rich extracellular matrix, and cells that might accelerate wound healing | Higher likelihood of complete healing at 12–16 wk compared with control dressing (relative risk, 2.0; 95% CI, 1.67–2.39; P < .001).66–68 Data from a meta-analysis of 11 multicenter randomized clinical trials involving 655 people with diabetic foot ulcers. |
| Sucrose octasulfate dressing | Used in treatment of neuroischemic diabetic foot ulcers | Improved healing in a single randomized clinical trial of 240 people with diabetic foot ulcers and mild peripheral artery disease at 20 wk compared with an identical control lipocolloid dressing (48% vs 34%; odds ratio, 2.60; 95% CI, 1.43–4.73).69 |
| Hyperbaric oxygen therapy | Adjunct therapy in neuroischemic or ischemic diabetic foot ulcers when standard of care alone has failed | Improved healing in a single randomized clinical trial of 94 patients receiving hyperbaric oxygen therapy vs sham at 1 year (61% vs 27%; P = .009),70 but more recent trials did not show a significant effect of hyperbaric oxygen therapy.52,71 |
| Topical oxygen therapy | Adjunct therapy in diabetic foot ulcers when standard of care alone has failed | Improved healing over sham controls in a meta-analysis of 492 patients with diabetic foot ulcers: 43.0% in active group vs 28.0% in sham device group (relative risk, 1.59; 95% CI, 1.07–2.37; P = .02).72 |
| Negative pressure wound therapy | Used in treatment of complicated and postoperative wounds in the diabetic foot | Improved healing in a meta-analysis of 943 patients who received negative pressure wound therapy vs standard moisture-balancing dressings (odds ratio, 3.60; 95% CI, 2.38–5.45; P < .001). Effective in resolving wound depth.64,73–75 |
Several new topical therapies have been shown to accelerate wound healing in multicenter randomized clinical trials.52 A topical fibrin and leucocyte platelet patch has reported efficacy in a large randomized clinical trial.65 The patch is made by bedside centrifugation of the patient’s venous blood to generate a disk of autologous leucocytes, platelets, and fibrin that is placed on the wound. The randomized clinical trial reported a significant benefit to those receiving topical treatment at 20 weeks compared with standard of care based on International Working Group on the Diabetic Foot guidelines consisting of off-loading, wound debridement, and moisture-balancing dressings (n = 269; 34% vs 22% healed; OR, 1.58; 95% CI, 1.04–2.40; P = .02).
Placenta-derived products have been studied as potential therapies over the last 2 decades. These products contain growth factors, collagen-rich extracellular matrix, and cells that might accelerate wound healing. Most frequently used placenta-derived treatments are cryopreserved preparations that contain living cells and growth factors, and dehydrated products that contain growth factors but not living cells.66,67 In a meta-analysis of 11 multicenter randomized clinical trials of 655 participants with noninfected, nonischemic diabetic foot ulcers, patients receiving placenta-derived products had a higher incidence of complete resolution of diabetic foot ulcer at 12 to 16 weeks than those with similar care and an alginate or foam control dressing (66.9% vs 34.1%; RR, 2.0; 95% CI, 1.67–2.39; P < .001).68
In the EXPLORER study, a double-blind randomized clinical trial comparing a sucrose octasulfate dressing with an identical control lipocolloid dressing in the treatment of 240 patients with diabetic foot ulcers and concomitant mild peripheral artery disease, healing at 20 weeks was observed among 48% of patients receiving active therapy vs 30% among those receiving standard care (adjusted OR, 2.60; 95% CI, 1.43–4.73; P = .002).69
Hyperbaric oxygen therapy may be an adjunctive therapy in diabetic foot ulcers with concomitant peripheral artery disease when standard of care alone has not attained healing, based on the results of several randomized clinical trials that are not consistent in their outcomes.52 Topical oxygen as an adjunct therapy in diabetic foot ulcers may be considered when standard of care alone has failed (<50% of ulcer healed at 4 weeks), based on recent meta-analyses of randomized clinical trials and systematic reviews.52,72 Pooled results from the most recent meta-analysis showed improved healing with topical oxygen therapy over sham controls (43.0% vs 28.0%; RR, 1.59; 95% CI, 1.07–2.37; P = .02).72
Negative pressure wound therapy has been widely used as an adjunct therapy in the treatment of complicated and postoperative wounds in the diabetic foot for the last 2 decades.52,64 Multiple well-designed randomized clinical trials support its use to improve healing in both partial foot amputations and diabetic foot ulcers.73,74 A meta-analysis of 943 patients with diabetic foot ulcers from 9 randomized clinical trials reported that negative pressure wound therapy was associated with improved healing, compared with treatment with moist wound healing (OR, 3.60; 95% CI, 2.38–5.45; P < .001).74 Negative pressure wound therapy is most effective in resolving wound depth and creating a bed of predominant granulation tissue, at which point it may be discontinued and replaced with skin grafting or wound dressings as described above. Although there are no comparative data, skin grafting may be considered to cover larger defects that might otherwise take longer to heal.76
The American Diabetes Association recently recommended the use of negative pressure wound therapy and topical oxygen therapy as therapies that might be considered in wounds that fail to respond to standard debridement, wound care, and off-loading.64,77 The International Working Group on the Diabetic Foot only recommends considering use of negative pressure wound therapy in post-surgical wounds.52
Treatment of Infected Diabetic Foot Ulcers
Early management of diabetic foot infection reduces the risk of hospitalization and amputation.78 In a study of 668 patients with diabetic foot ulcer infection treated in a single hospital, there was a 0.6% increased risk of major amputation or death for each day that referral to the medical center was delayed (OR, 1.006; 95% CI, 1.003–1.010;P < .001[absolute rates not provided]).78 Although many diabetic foot ulcer infections are superficial, some may require surgical intervention to remove infection in deep soft tissue. In the absence of an acute soft tissue infection in forefoot osteomyelitis, antibiotics may be as effective as surgery.7 A single-center trial randomly assigned 46 patients with forefoot osteomyelitis and diabetic foot ulcers to either surgical resection and a 10-day postoperative antibiotic course or a 90-day course of antimicrobials alone. The median time to healing was 7 weeks in the antibiotic group vs 6 weeks in the surgery group (P = .72). There was no difference in the incidence of minor amputations between groups (16.6% vs 13.6%; P = .34).79
Treatment of Peripheral Artery Disease
Lower extremity revascularization aims to restore pulsatile arterial flow to the foot in chronic limb-threatening ischemia.35,38,80 In patients with chronic limb-threatening ischemia who require revascularization for tissue healing, delayed revascularization is associated with slower healing. A prospective study of 478 patients identified an improved rate of wound healing among patients undergoing revascularization with shorter time to healing among those who received a referral for revascularization within 56 days compared with those who had a longer time to revascularization (hazard ratio [HR], 1.96; 95% CI, 1.52–2.52; P < .001 [absolute rates not provided]).78,81 A retrospective study of 314 patients with diabetes reported that waiting more than 14 days before lower extremity revascularization was associated with a higher rate of major amputation compared with earlier revascularization (OR, 3.1; 95% CI, 1.4–6.9 [absolute rates not provided]).82
A systematic approach may be adopted for revascularization based on overall operative risk assessment and the anatomical distribution of lower extremity artery disease.49 Revascularization should be offered to most patients with chronic limb-threatening ischemia; however, older age, presence and severity of medical comorbidities, impaired functional status, and shorter life expectancy are important preoperative factors to consider when determining whether revascularization is likely to improve outcomes.35,49 Primary lower extremity amputation without revascularization may be appropriate in selected patients, including patients who are non-ambulatory at baseline and patients with frailty.49,83 Both open surgery and endovascular therapy can be appropriate for chronic limb-threatening ischemia.35,49In 1434 patients who were candidates for either surgery (including a single-segment great saphenous vein for bypass) or endovascular treatment (71.8% had diabetes), surgical bypass appeared to be superior to endovascular therapy based on the risk of major adverse limb events, defined as major amputation or a major limb reintervention (repeat bypass graft, graft revision, thrombectomy, or thrombolysis) or death (42.6% vs 57.4%; HR, 0.68; 95% CI, 0.59–0.79).84 Table 6 summarizes the treatment of peripheral artery disease in patients with diabetic foot ulcers.
Table 6.
Treatment of Diabetic Foot Ulcers in Cases of Peripheral Artery Disease
| Treatment of ischemia | Description | Outcome/benefit |
|---|---|---|
| Timely revascularization81,82 | Restores pulsatile arterial flow to the foot in chronic limb-threatening ischemia | In a study of 478 patients with diabetic foot ulcers, faster wound healing for patients undergoing revascularization within 56 d (hazard ratio, 1.96; 95% CI, 1.52–2.52; P < .001).81 In a study of 246 limbs with chronic limb-threatening ischemia, reduced risk of major amputation for patients with revascularization within 14 d (odds ratio, 3.1; 95% CI, 1.4–6.9)82 |
| Primary lower extremity amputation (without salvage attempt)49 | Appropriate in selected patients, including patients nonambulatory at baseline and patients with severe frailty | Offers alternative treatment for patients who are not suitable candidates for revascularization (expert consensus). |
| Surgical bypass vs endovascular therapy84 | Both open surgery and endovascular therapy are used for chronic limb-threatening ischemia | In a randomized clinical trial of 1434 patients who were candidates for either surgery bypass (including single-segment great saphenous vein for bypass) or endovascular treatment (71.8% had diabetes), surgical bypass appeared superior to endovascular therapy in patients with adequate great saphenous vein (hazard ratio, 0.68; 95% CI, 0.59–0.79) for composite outcome of a major adverse limb event (amputation above ankle, major limb intervention, or death) (42.6% vs 57.4%). |
Long-Term Management, Follow-Up, and Outcomes
Multidisciplinary Team Care
Options for long-term management are summarized in Table 7. A multidisciplinary team approach (structured diabetic foot services) has been shown to reduce diabetes-related lower extremity amputation.85 Although the team composition and activities of a multidisciplinary team can vary, it generally includes at least 1 medical specialty clinician (most commonly endocrinology, infectious diseases, or primary care) and 2 or more surgical specialty clinicians (vascular, podiatric, orthopedic, or plastic surgery).85,86 The pooled OR for reduction in major amputation after implementation of a multidisciplinary care team relative to usual care was 0.40 (3.2% vs 4.4%; OR, 0.40; 95% CI, 0.32–0.51; P < .001) across 18 studies and 38 608 participants.86 The team should also include expertise in prescription and management of footwear. A meta-analysis of randomized clinical trials with 1587 participants reported that therapeutic footwear, relative to usual care, significantly reduced incidence of diabetic foot ulcers (13.3% vs 25.4%; RR, 0.49; 95% CI, 0.28–0.84; P < .01).87 The efficacy of therapeutic footwear is supported by the findings of other meta-analyses of randomized clinical trials for therapeutic footwear and for pressure-relieving custom-made footwear.93
Table 7.
Long-Term Management and Prognosis
| Description | Outcome/benefit | |
|---|---|---|
| Multidisciplinary team approach85,86 | Structured diabetic foot services involving various medical and surgical specialties (eg, podiatry, infectious disease, vascular surgery, and primary care) | Significant reduction in major lower extremity amputation relative to usual care (pooled odds ratio, 0.40; 95% CI, 0.32–0.51 [absolute data not available]). |
| Therapeutic footwear87 | Prescription and management of footwear as part of multidisciplinary care | Meta-analysis of 8 randomized clinical trials including 1587 participants showed reduced incidence of diabetic foot ulcer (relative risk, 0.49; 95% CI, 0.28–0.84) with therapeutic footwear relative to those not wearing prescriptive shoes and custom insoles. |
| Rehabilitation, psychological care, and nutrition88–91 | Addressing patients’ mental health, nutritional deficits, and overall quality of life | 47% of people with diabetic foot ulcers have concomitant depression based on a meta-analysis of 11 studies.88 A single-center investigation of 253 patients with diabetic foot ulcers reported that the presence of depressive disorders was associated with a 2-fold increase in mortality risk for any depressive episode (hazard ratio, 2.09; 95% CI, 1.34–3.25) in comparison with an absence of depression.89 A meta-analysis of 1565 patients with diabetic foot ulcers reported lower vitamin D levels (mean difference, −6.48 nmol/L; 95% CI, −10.84to −2.11 nmol/L; P < .004), higher prevalence of vitamin D deficiency (<50 nmol/L) (odds ratio, 1.82; 95% CI, 1.32–2.52; P < .001), and higher prevalence of severe vitamin D deficiency (odds ratio, 2.53; 95% CI, 1.65–3.89; P < .001) compared with 6021 patients with diabetes and no diabetic foot ulcer. |
| Healing rate | Healing rates of diabetic foot ulcers | 30%−40% healing at 12 wk; 23% still unhealed at 12 mo, derived from the US Wound Registry of 71 957 diabetic foot ulcers.92 |
| Recurrence rate in diabetic foot ulcer remission2 | Recurrence rates of diabetic foot ulcers after healing | Pooled data from 8 prospective studies with 1738 participants, 1 retrospective studywith 46 participants, and the usual care groups of 9 randomized clinical trials with 636 participants reported that the risk of recurrence of diabetic foot ulcer was 42% at 1 year, 58% at 3 years, and 65% at 5 years. |
In addition to specialists in wound care, infectious diseases, and vascular care, the team should include clinicians with expertise in rehabilitation, nutrition, and psychological care. Diabetic foot ulcers have a substantial effect on health-related quality of life, and depression in patients with diabetic foot ulcers is common. In a meta-analysis of 11 studies and 2117 people, 47% of patients with diabetic foot ulcers had symptoms of depression.88 Depression has been associated with increased mortality in patients with diabetic foot ulcers. A single-center investigation of 253 patients with diabetic foot ulcers reported that the presence of depressive disorders was associated with a 2-fold increase in mortality for any depressive episode (HR, 2.09; 95% CI, 1.34–3.25 [absolute rates not provided]), minor depressive disorder (HR, 1.93; 95% CI, 1.00–3.74 [absolute rates not provided]), or major depressive disorder (HR, 2.18; 95% CI, 1.31–3.65 [absolute rates not provided]), in comparison with absence of depression.89 Many patients with diabetic foot ulcers have some degree of nutritional deficit.90 A meta-analysis of 1565 patients with diabetic foot ulcers reported lower vitamin D levels (mean difference, −6.48 nmol/L; 95% CI, −10.84 to −2.11 nmol/L; P < .004), higher prevalence of vitamin D deficiency (<50 nmol/L) (73.7% vs 67.3%; OR, 1.82; 95% CI, 1.32–2.52; P = .003), and higher prevalence of severe vitamin D deficiency (36.5% vs 21.6%; OR, 2.53; 95% CI, 1.65–3.89; P < .001) compared with 6021 patients with diabetes and no diabetic foot ulcer.94 Although there are no studies of nutritional interventions, a focus on optimizing glucose control and adequate protein intake may be beneficial.90
Regarding glucose control, a meta-analysis of 47 studies including 12 604 diabetic foot ulcers showed an elevated risk of lower extremity amputation with increased hemoglobin A1c and fasting glucose levels (for hemoglobin A1c ≥8% vs <8%: OR, 4.80 [95% CI, 2.83–8.13]; for fasting glucose ≥126 mg/dL vs <126 mg/dL: OR, 1.46 [95% CI, 1.02–2.09]). However, no relationship was found between hemoglobin A1c category and wound healing.95
High-quality evidence is lacking to support that tight glycemic control reduces the risk of first or recurrent foot ulcers.
Prognosis
Although approximately 30% to 40% of diabetic foot ulcers heal at 12 weeks, 23% of patients have a nonhealed diabetic foot ulcer at 12 months.92 The recurrence rate of diabetic foot ulcers after treatment of any kind is estimated to be 42% at 1 year and 65% at 5 years.2 A recent longitudinal study of 129 patients in diabetic foot ulcer remission found that only 17% had wound recurrence at the identical location, with 48% having a recurrence on the contralateral foot.96 The high rate of recurrence underscores the need for continued surveillance by the patient and medical team.28
Limitations
This Review has limitations. First, non–English-language articles were not included. Second, some relevant publications may have been missed. Third, not all aspects of diabetic foot ulcer treatment or screening were covered.
Conclusions
Diabetic foot ulcers affect approximately 18.6 million people worldwide and are associated with increased rates of amputation and death. Surgical debridement, reducing pressure from weight bearing, treating lower extremity ischemia and foot infection, and early referral for multidisciplinary care are first-line therapies for diabetic foot ulcers.
Funding/Support:
This work is partially supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases awards 1R01124789-01A1 and 1K23DK122126-01 and National Science Foundation Center to Stream Healthcare in Place CNS award 2052578.
Role of the Funder/Sponsor:
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Additional Contributions:
We acknowledge Jacob Wood, MD, University of North Carolina, for assistance with the figures on an earlier version of the manuscript.
Footnotes
Conflict of Interest Disclosures: Dr Armstrong reported receiving consulting fees from Podimetrics, Molnlycke, Cardiovascular Systems Inc, Endo Pharmaceuticals, and Averitas Pharma (GRT US). Dr Boulton reported receiving consulting fees from AOT Inc and Nevro. No other disclosures were reported.
Submissions: We encourage authors to submit papers for consideration as a Review. Please contact Mary McGrae McDermott, MD, at mdm608@northwestern.edu.
REFERENCES
- 1.Zhang Y, Lazzarini PA, McPhail SM, van Netten JJ, Armstrong DG, Pacella RE. Global disability burdens of diabetes-related lower-extremity complications in 1990 and 2016. Diabetes Care. 2020;43(5):964–974. doi: 10.2337/dc19-1614 [DOI] [PubMed] [Google Scholar]
- 2.Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017; 376(24):2367–2375. doi: 10.1056/NEJMra1615439 [DOI] [PubMed] [Google Scholar]
- 3.Petersen BJ, Linde-Zwirble WT, Tan TW, et al. Higher rates of all-cause mortality and resource utilization during episodes-of-care for diabetic foot ulceration. Diabetes Res Clin Pract. 2022;184:109182. doi: 10.1016/j.diabres.2021.109182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ndosi M, Wright-Hughes A, Brown S, et al. Prognosis of the infected diabetic foot ulcer: a 12-month prospective observational study. Diabet Med. 2018;35(1):78–88. doi: 10.1111/dme.13537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prompers L, Huijberts M, Apelqvist J, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe: baseline results from the Eurodiale study. Diabetologia. 2007;50(1):18–25. doi: 10.1007/s00125-006-0491-1 [DOI] [PubMed] [Google Scholar]
- 6.Skrepnek GH, Mills JL Sr, Armstrong DG. A diabetic emergency one million feet long: disparities and burdens of illness among diabetic foot ulcer cases within emergency departments in the United States, 2006–2010. PLoS One. 2015;10 (8):e0134914. doi: 10.1371/journal.pone.0134914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Senneville É, Albalawi Z, van Asten SA, et al. Guidelines on the Diagnosis and Treatment of Foot Infection in Persons With Diabetes: IWGDF/IDSA 2023. Published 2023. Accessed June 12, 2023. https://iwgdfguidelines.org/wp-content/uploads/2023/05/IWGDF-2023-04-Infection-Guideline.pdf [DOI] [PubMed] [Google Scholar]
- 8.Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13(1):16. doi: 10.1186/s13047-020-00383-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rice JB, Desai U, Cummings AKG, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for Medicare and private insurers. Diabetes Care. 2014;37(3):651–658. doi: 10.2337/dc13-2176 [DOI] [PubMed] [Google Scholar]
- 10.International Diabetes Federation. IDF Diabetes Atlas, 10th ed. https://www.diabetesatlas.org [Google Scholar]
- 11.Centers for Disease Control and Prevention. National Diabetes Statistics Report. Updated June 29, 2022. Accessed May 28, 2023. https://www.cdc.gov/diabetes/data/statistics-report/index.html
- 12.McDermott K, Fang M, Boulton AJM, Selvin E, Hicks CW. Etiology, epidemiology, and disparities in the burden of diabetic foot ulcers. Diabetes Care. 2023;46(1):209–221. doi: 10.2337/dci22-0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortes-Penfield NW, Armstrong DG, Brennan MB, et al. Evaluation and management of diabetes-related foot infections. Clin Infect Dis. Published online June 12, 2023. doi: 10.1093/cid/ciad255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behrendt CA, Sigvant B, Szeberin Z, et al. International variations in amputation practice: a VASCUNET report. Eur J Vasc Endovasc Surg. 2018;56(3):391–399. doi: 10.1016/j.ejvs.2018.04.017 [DOI] [PubMed] [Google Scholar]
- 15.Tan TW, Armstrong DG, Concha-Moore KC, et al. Association between race/ethnicity and the risk of amputation of lower extremities among Medicare beneficiaries with diabetic foot ulcers and diabetic foot infections. BMJ Open Diabetes Res Care. 2020;8(1):e001328. doi: 10.1136/bmjdrc-2020-001328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan TW, Shih CD, Concha-Moore KC, et al. Disparities in outcomes of patients admitted with diabetic foot infections. PLoS One. 2019;14(2): e0211481. doi: 10.1371/journal.pone.0211481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blumberg SN, Warren SM. Disparities in initial presentation and treatment outcomes of diabetic foot ulcers in a public, private, and Veterans Administration hospital. J Diabetes. 2014;6(1):68–75. doi: 10.1111/1753-0407.12050 [DOI] [PubMed] [Google Scholar]
- 18.Durazzo TS, Frencher S, Gusberg R. Influence of race on the management of lower extremity ischemia: revascularization vs amputation. JAMA Surg. 2013;148(7):617–623. doi: 10.1001/jamasurg.2013.1436 [DOI] [PubMed] [Google Scholar]
- 19.Miller TA, Campbell JH, Bloom N, Wurdeman SR. Racial disparities in health care with timing to amputation following diabetic foot ulcer. Diabetes Care. 2022;45(10):2336–2341. doi: 10.2337/dc21-2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurst JE, Barn R, Gibson L, et al. Geospatial mapping and data linkage uncovers variability in outcomes of foot disease according to multiple deprivation: a population cohort study of people with diabetes. Diabetologia. 2020;63(3):659–667. doi: 10.1007/s00125-019-05056-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens CD, Schriger DL, Raffetto B, Davis AC, Zingmond D, Roby DH. Geographic clustering of diabetic lower-extremity amputations in low-income regions of California. Health Aff (Millwood). 2014;33(8):1383–1390. doi: 10.1377/hlthaff.2014.0148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akinlotan MA, Primm K, Bolin JN, et al. Racial, rural, and regional disparities in diabetes-related lower-extremity amputation rates, 2009–2017. Diabetes Care. 2021;44(9):2053–2060. doi: 10.2337/dc20-3135 [DOI] [PubMed] [Google Scholar]
- 23.Brennan MB, Powell WR, Kaiksow F, et al. Association of race, ethnicity, and rurality with major leg amputation or death among Medicare beneficiaries hospitalized with diabetic foot ulcers. JAMA Netw Open. 2022;5(4):e228399. doi: 10.1001/jamanetworkopen.2022.8399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saluja S, Anderson SG, Hambleton I, et al. Foot ulceration and its association with mortality in diabetes mellitus: a meta-analysis. Diabet Med. 2020;37(2):211–218. doi: 10.1111/dme.14151 [DOI] [PubMed] [Google Scholar]
- 25.Brennan MB, Hess TM, Bartle B, et al. Diabetic foot ulcer severity predicts mortality among veterans with type 2 diabetes. J Diabetes Complications. 2017;31(3):556–561. doi: 10.1016/j.jdiacomp.2016.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaper NC, van Netten JJ, Apelqvist J, et al. ; IWGDF Editorial Board. Practical guidelines on the prevention and management of diabetes-related foot disease (IWGDF 2023 update). Diabetes Metab Res Rev. Published online May 27, 2023. doi: 10.1002/dmrr.3657 [DOI] [PubMed] [Google Scholar]
- 27.Tolson JP, Aristizabal JC, Urbina DJ, et al. Association of pre-ulcerative foot care and outcomes of diabetic foot ulceration. J Am Podiatr Med Assoc. doi: 10.7547/22-071 [DOI] [PubMed] [Google Scholar]
- 28.Bus SA, Sacco ICN, Monteiro-Soares M, et al. Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2023 update). Diabetes Metab Res Rev. 202311:e3651. doi: 10.1002/dmrr.3651. [DOI] [PubMed] [Google Scholar]
- 29.Stang D, Leese GP. The Scottish Diabetes Foot Action Group 2016 Update of the Diabetic Foot Risk Stratification and Triage System. Published March 1, 2017. Accessed July 1, 2022. https://woundsinternational.com/journal-articles/the-scottish-diabetes-foot-action-group-2016-update-of-the-diabetic-foot-risk-stratification-and-triage-system/
- 30.Kanji JN, Anglin RES, Hunt DL, Panju A. Does this patient with diabetes have large-fiber peripheral neuropathy? JAMA. 2010;303(15):1526–1532. doi: 10.1001/jama.2010.428 [DOI] [PubMed] [Google Scholar]
- 31.Lavery LA, Armstrong DG, Boulton AJM; Diabetex Research Group. Ankle equinus deformity and its relationship to high plantar pressure in a large population with diabetes mellitus. J Am Podiatr Med Assoc. 2002;92(9):479–482. doi: 10.7547/87507315-92-9-479 [DOI] [PubMed] [Google Scholar]
- 32.Wukich DK, Schaper NC, Gooday C, et al. Guidelines on the diagnosis and treatment of active Charcot neuro-osteoarthropathy in persons with diabetes mellitus (IWGDF 2023). Diabetes Metab Res Rev. Published online May 23, 2023. doi: 10.1002/dmrr.3646 [DOI] [PubMed] [Google Scholar]
- 33.Polonsky TS, McDermott MM. Lower extremity peripheral artery disease without chronic limb-threatening ischemia: a review. JAMA. 2021; 325(21):2188–2198. doi: 10.1001/jama.2021.2126 [DOI] [PubMed] [Google Scholar]
- 34.Londero LS, Lindholt JS, Thomsen MD, Hoegh A. Pulse palpation is an effective method for population-based screening to exclude peripheral arterial disease. J Vasc Surg. 2016;63(5):1305–1310. doi: 10.1016/j.jvs.2015.11.044 [DOI] [PubMed] [Google Scholar]
- 35.IWGDF PAD Working Group. Intersocietal PAD Guideline (2023 Update). Accessed June 12, 2023.
- 36.Monteiro-Soares M, Hamilton EJ, Russell DA, et al. Classification of foot ulcers in people with diabetes: a systematic review. Diabetes Metab Res Rev. Published online May 2, 2023. doi: 10.1002/dmrr.3645 [DOI] [Google Scholar]
- 37.Monteiro-Soares M, Hamilton EJ, Russell DA, et al. Guidelines on the classification of foot ulcers in people with diabetes (IWGDF 2023 update). Diabetes Metab Res Rev. Published online May 14, 2023. doi: 10.1002/dmrr.3648 [DOI] [PubMed] [Google Scholar]
- 38.Mills JL Sr, Conte MS, Armstrong DG, et al. ; Society for Vascular Surgery Lower Extremity Guidelines Committee. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014;59(1):220–234. doi: 10.1016/j.jvs.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 39.van Reijen NS, Ponchant K, Ubbink DT, Koelemay MJW. The prognostic value of the WIfI classification in patients with chronic limb threatening ischaemia: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2019;58 (3):362–371. doi: 10.1016/j.ejvs.2019.03.040 [DOI] [PubMed] [Google Scholar]
- 40.Butalia S, Palda VA, Sargeant RJ, Detsky AS, Mourad O. Does this patient with diabetes have osteomyelitis of the lower extremity? JAMA. 2008; 299(7):806–813. doi: 10.1001/jama.299.7.806 [DOI] [PubMed] [Google Scholar]
- 41.Aragón-Sánchez J, Lipsky BA, Lázaro-Martínez JL. Diagnosing diabetic foot osteomyelitis: is the combination of probe-to-bone test and plain radiography sufficient for high-risk inpatients? Diabet Med. 2011;28(2):191–194. doi: 10.1111/j.1464-5491.2010.03150.x [DOI] [PubMed] [Google Scholar]
- 42.Gariani K, Lebowitz D, Kressmann B, Gariani J, Uçkay I. X-ray versus magnetic resonance imaging in diabetic foot osteomyelitis: a clinical comparison. Curr Diabetes Rev. 2021;17(3):373–377. doi: 10.2174/1573399816999200729124134 [DOI] [PubMed] [Google Scholar]
- 43.Lauri C, Tamminga M, Glaudemans AWJM, et al. Detection of osteomyelitis in the diabetic foot by imaging techniques: a systematic review and meta-analysis comparing MRI, white blood cell scintigraphy, and FDG-PET. Diabetes Care. 2017;40 (8):1111–1120. doi: 10.2337/dc17-0532 [DOI] [PubMed] [Google Scholar]
- 44.Wang Z, Hasan R, Firwana B, et al. A systematic review and meta-analysis of tests to predict wound healing in diabetic foot. J Vasc Surg. 2016;63(2) (suppl):29S–36S. doi: 10.1016/j.jvs.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 45.Brownrigg JRW, Hinchliffe RJ, Apelqvist J, et al. ; International Working Group on the Diabetic Foot. Performance of prognostic markers in the prediction of wound healing or amputation among patients with foot ulcers in diabetes: a systematic review. Diabetes Metab Res Rev. 2016;32(suppl 1): 128–135. doi: 10.1002/dmrr.2704 [DOI] [PubMed] [Google Scholar]
- 46.Linton C, Searle A, Hawke F, Tehan PE, Sebastian M, Chuter V. Do toe blood pressures predict healing after minor lower limb amputation in people with diabetes? a systematic review and meta-analysis. Diab Vasc Dis Res. 2020;17(3): 1479164120928868. doi: 10.1177/1479164120928868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tay WL, Lo ZJ, Hong Q, Yong E, Chandrasekar S, Tan GWL. Toe pressure in predicting diabetic foot ulcer healing: a systematic review and meta-analysis. Ann Vasc Surg. 2019;60:371–378. doi: 10.1016/j.avsg.2019.04.011 [DOI] [PubMed] [Google Scholar]
- 48.Boulton AJM, Armstrong DG, Albert SF, et al. ; American Diabetes Association; American Association of Clinical Endocrinologists. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31(8):1679–1685. doi: 10.2337/dc08-9021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Conte MS, Bradbury AW, Kolh P, et al. ; GVG Writing Group. Global Vascular Guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg. 2019;69(6S):3S–125S. doi: 10.1016/j.jvs.2019.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Golledge J, Fernando ME, Alahakoon C, et al. Efficacy of at home monitoring of foot temperature for risk reduction of diabetes-related foot ulcer: a meta-analysis. Diabetes Metab Res Rev. 2022;38 (6):e3549. doi: 10.1002/dmrr.3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bus SA, Armstrong DG, Crews RT, et al. Guidelines on offloading foot ulcers in persons with diabetes—IWGDF 2023 update. Diabetes Metab Res Rev. Published online May 25, 2023. doi: 10.1002/dmrr.3647 [DOI] [PubMed] [Google Scholar]
- 52.Chen P, Vilorio NC, Dhatariya K, et al. Guidelines on interventions to enhance healing of foot ulcers in people with diabetes (IWGDF 2023 update). Diabetes Metab Res Rev. Published online May 25, 2023. doi: 10.1002/dmrr.3644 [DOI] [PubMed] [Google Scholar]
- 53.Eriksson E, Liu PY, Schultz GS, et al. Chronic wounds: treatment consensus. Wound Repair Regen. 2022;30(2):156–171. doi: 10.1111/wrr.12994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilcox JR, Carter MJ, Covington S. Frequency of debridements and time to heal: a retrospective cohort study of 312 744 wounds. JAMA Dermatol. 2013;149(9):1050–1058. doi: 10.1001/jamadermatol.2013.4960 [DOI] [PubMed] [Google Scholar]
- 55.Bus SA. The role of pressure offloading on diabetic foot ulcer healing and prevention of recurrence. Plast Reconstr Surg. 2016;138(3)(suppl): 179S–187S. doi: 10.1097/PRS.0000000000002686 [DOI] [PubMed] [Google Scholar]
- 56.Wu SC, Jensen JL, Weber AK, Robinson DE, Armstrong DG. Use of pressure offloading devices in diabetic foot ulcers: do we practice what we preach? Diabetes Care. 2008;31(11):2118–2119. doi: 10.2337/dc08-0771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raspovic A, Landorf KB. A survey of offloading practices for diabetes-related plantar neuropathic foot ulcers. J Foot Ankle Res. 2014;7:35. doi: 10.1186/s13047-014-0035-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lazzarini PA, Armstrong DG, Crews RT, et al. Effectiveness of offloading interventions for people with diabetes-related foot ulcers: a systematic review and meta analysis. Diabetes Metab Res Rev. Published June 8, 2023. doi: 10.1002/dmrr.3650 [DOI] [PubMed] [Google Scholar]
- 59.Armstrong DG, Lavery LA, Kimbriel HR, Nixon BP, Boulton AJM. Activity patterns of patients with diabetic foot ulceration: patients with active ulceration may not adhere to a standard pressure off-loading regimen. Diabetes Care. 2003;26(9): 2595–2597. doi: 10.2337/diacare.26.9.2595 [DOI] [PubMed] [Google Scholar]
- 60.Andersen JA, Rasmussen A, Engberg S, et al. Flexor tendon tenotomy treatment of the diabetic foot: a multicenter randomized controlled trial. Diabetes Care. 2022;45(11):2492–2500. doi: 10.2337/dc22-0085 [DOI] [PubMed] [Google Scholar]
- 61.Mueller MJ, Sinacore DR, Hastings MK, Strube MJ, Johnson JE. Effect of Achilles tendon lengthening on neuropathic plantar ulcers: a randomized clinical trial. J Bone Joint Surg Am. 2003;85(8):1436–1445. doi: 10.2106/00004623-200308000-00003 [DOI] [PubMed] [Google Scholar]
- 62.Jeffcoate WJ, Vileikyte L, Boyko EJ, Armstrong DG, Boulton AJM. Current challenges and opportunities in the prevention and management of diabetic foot ulcers. Diabetes Care. 2018;41(4): 645–652. doi: 10.2337/dc17-1836 [DOI] [PubMed] [Google Scholar]
- 63.Woelfel S, Shin L, Armstrong DG. Wound care. In: Sidawy MDA, Perler MDB, eds. Rutherford’s Vascular Surgery and Endovascular Therapy. 10th ed. Elsevier; 2022. [Google Scholar]
- 64.Boulton AJM, Armstrong DG, Löndahl M, et al. New Evidence-Based Therapies for Complex Diabetic Foot Wounds. American Diabetes Association; 2022. doi: 10.2337/db2022-02 [DOI] [PubMed] [Google Scholar]
- 65.Game F, Jeffcoate W, Tarnow L, et al. ; LeucoPatch II Trial Team. LeucoPatch system for the management of hard-to-heal diabetic foot ulcers in the UK, Denmark, and Sweden: an observer-masked, randomised controlled trial. Lancet Diabetes Endocrinol. 2018;6(11):870–878. doi: 10.1016/S2213-8587(18)30240-7 [DOI] [PubMed] [Google Scholar]
- 66.Lavery LA, Fulmer J, Shebetka KA, et al. ; Grafix Diabetic Foot Ulcer Study Group. The efficacy and safety of Grafix for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int Wound J. 2014;11(5):554–560. doi: 10.1111/iwj.12329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tettelbach W, Cazzell S, Reyzelman AM, Sigal F, Caporusso JM, Agnew PS. A confirmatory study on the efficacy of dehydrated human amnion/chorion membrane dHACM allograft in the management of diabetic foot ulcers: a prospective, multicentre, randomised, controlled study of 110 patients from 14 wound clinics. Int Wound J. 2019;16(1):19–29. doi: 10.1111/iwj.12976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mohammed YA, Farouk HK, Gbreel MI, et al. Human amniotic membrane products for patients with diabetic foot ulcers. do they help? a systematic review and meta-analysis. J Foot Ankle Res. 2022; 15(1):71. doi: 10.1186/s13047-022-00575-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Edmonds M, Lázaro-Martínez JL, Alfayate-García JM, et al. Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (EXPLORER): an international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol. 2018;6(3):186–196. doi: 10.1016/S2213-8587(17)30438-2 [DOI] [PubMed] [Google Scholar]
- 70.Löndahl M, Katzman P, Nilsson A, Hammarlund C. Hyperbaric oxygen therapy facilitates healing of chronic foot ulcers in patients with diabetes. Diabetes Care. 2010;33(5):998–1003. doi: 10.2337/dc09-1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santema KTB, Stoekenbroek RM, Koelemay MJW, et al. ; DAMO2CLES Study Group. Hyperbaric oxygen therapy in the treatment of ischemic lowerextremity ulcers in patients with diabetes: results of the DAMO2CLES multicenter randomized clinical trial. Diabetes Care. 2018;41(1):112–119. doi: 10.2337/dc17-0654 [DOI] [PubMed] [Google Scholar]
- 72.Carter MJ, Frykberg RG, Oropallo A, et al. Efficacy of topical wound oxygen therapy in healing chronic diabetic foot ulcers: systematic review and meta-analysis. Adv Wound Care (New Rochelle). 2023;12(4):177–186. doi: 10.1089/wound.2022.0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Z, Dumville JC, Hinchliffe RJ, et al. Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus. Cochrane Database Syst Rev. 2018;10(10):CD010318. doi: 10.1002/14651858.CD010318.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen L, Zhang S, Da J, et al. A systematic review and meta-analysis of efficacy and safety of negative pressure wound therapy in the treatment of diabetic foot ulcer. Ann Palliat Med. 2021;10 (10):10830–10839. doi: 10.21037/apm-21-2476 [DOI] [PubMed] [Google Scholar]
- 75.Armstrong DG, Lavery LA; Diabetic Foot Study Consortium. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005;366(9498):1704–1710. doi: 10.1016/S0140-6736(05)67695-7 [DOI] [PubMed] [Google Scholar]
- 76.Rose JF, Giovinco N, Mills JL, Najafi B, Pappalardo J, Armstrong DG. Split-thickness skin grafting the high-risk diabetic foot. J Vasc Surg. 2014;59(6):1657–1663. doi: 10.1016/j.jvs.2013.12.046 [DOI] [PubMed] [Google Scholar]
- 77.ElSayed NA, Aleppo G, Aroda VR, et al. ; American Diabetes Association. tinopathy, neuropathy, and foot care: standards of care in diabetes—2023. Diabetes Care. 2023;46(suppl 1): S203–S215. doi: 10.2337/dc23-S012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin CW, Yang HM, Hung SY, Chen IW, Huang YY. The analysis for time of referral to a medical center among patients with diabetic foot infection. BMC Fam Pract. 2021;22(1):16. doi: 10.1186/s12875-020-01363-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lázaro-Martínez JL, Aragón-Sánchez J, García-Morales E. Antibiotics versus conservative surgery for treating diabetic foot osteomyelitis: a randomized comparative trial. Diabetes Care. 2014;37(3):789–795. doi: 10.2337/dc13-1526 [DOI] [PubMed] [Google Scholar]
- 80.Hicks CW, Canner JK, Sherman RL, Black JH III, Lum YW, Abularrage CJ. Evaluation of revascularization benefit quartiles using the Wound, Ischemia, and Foot Infection classification system for diabetic patients with chronic limb-threatening ischemia. J Vasc Surg. 2021;74(4): 1232–1239. doi: 10.1016/j.jvs.2021.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Elgzyri T, Larsson J, Nyberg P, Thörne J, Eriksson KF, Apelqvist J. Early revascularization after admittance to a diabetic foot center affects the healing probability of ischemic foot ulcer in patients with diabetes. Eur J Vasc Endovasc Surg. 2014;48(4):440–446. doi: 10.1016/j.ejvs.2014.06.041 [DOI] [PubMed] [Google Scholar]
- 82.Noronen K, Saarinen E, Albäck A, Venermo M. Analysis of the elective treatment process for critical limb ischaemia with tissue loss: diabetic patients require rapid revascularisation. Eur J Vasc Endovasc Surg. 2017;53(2):206–213. doi: 10.1016/j.ejvs.2016.10.023 [DOI] [PubMed] [Google Scholar]
- 83.Takeji Y, Yamaji K, Tomoi Y, et al. Impact of frailty on clinical outcomes in patients with critical limb ischemia. Circ Cardiovasc Interv. 2018;11(7): e006778. doi: 10.1161/CIRCINTERVENTIONS.118.006778 [DOI] [PubMed] [Google Scholar]
- 84.Farber A, Menard MT, Conte MS, et al. ; BEST-CLI Investigators. Surgery or endovascular therapy for chronic limb-threatening ischemia. N Engl J Med. 2022;387(25):2305–2316. doi: 10.1056/NEJMoa2207899 [DOI] [PubMed] [Google Scholar]
- 85.Musuuza J, Sutherland BL, Kurter S, Balasubramanian P, Bartels CM, Brennan MB. A systematic review of multidisciplinary teams to reduce major amputations for patients with diabetic foot ulcers. J Vasc Surg. 2020;71(4):1433–1446. doi: 10.1016/j.jvs.2019.08.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Monteiro-Soares M, Vale-Lima J, Martiniano J, Pinheiro-Torres S, Dias V, Boyko EJ. A systematic review with meta-analysis of the impact of access and quality of diabetic foot care delivery in preventing lower extremity amputation. J Diabetes Complications. 2021;35(4):107837. doi: 10.1016/j.jdiacomp.2020.107837 [DOI] [PubMed] [Google Scholar]
- 87.Luo B, Cai Y, Chen D, et al. Effects of special therapeutic footwear on the prevention of diabetic foot ulcers: a systematic review and meta-analysis of randomized controlled trials. J Diabetes Res. 2022;2022:9742665. doi: 10.1155/2022/9742665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jiang FH, Liu XM, Yu HR, Qian Y, Chen HL. The incidence of depression in patients with diabetic foot ulcers: a systematic review and meta-analysis. Int J Low Extrem Wounds. 2022;21(2):161–173. doi: 10.1177/1534734620929892 [DOI] [PubMed] [Google Scholar]
- 89.Winkley K, Sallis H, Kariyawasam D, et al. Five-year follow-up of a cohort of people with their first diabetic foot ulcer: the persistent effect of depression on mortality. Diabetologia. 2012;55(2): 303–310. doi: 10.1007/s00125-011-2359-2 [DOI] [PubMed] [Google Scholar]
- 90.Armstrong DG, Mills JL, Molina M, Molnar JA. Nutrition interventions in adults with diabetic foot ulcers. Guideline Central. Published March 2022. Accessed June 26, 2022. https://www.guidelinecentral.com/guideline/502765/pocket-guide/502768/ [Google Scholar]
- 91.Nabuurs-Franssen MH, Huijberts MS, Nieuwenhuijzen Kruseman AC, Willems J, Schaper NC. Health-related quality of life of diabetic foot ulcer patients and their caregivers. Diabetologia. 2005;48(9):1906–1910. doi: 10.1007/s00125-005-1856-6 [DOI] [PubMed] [Google Scholar]
- 92.Fife CE, Eckert KA, Carter MJ. Publicly reported wound healing rates: the fantasy and the reality. Adv Wound Care (New Rochelle). 2018;7(3):77–94. doi: 10.1089/wound.2017.0743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Van Netten JJ, Raspovic A, Lavery LA, et al. Prevention of foot ulcers in people with diabetes at risk of ulceration: a systematic review and meta-analysis. Diabetes Metab Res Rev. Published online May 27, 2023. doi: 10.1002/dmrr.3652 [DOI] [PubMed] [Google Scholar]
- 94.Lin J, Mo X, Yang Y, Tang C, Chen J. Association between vitamin D deficiency and diabetic foot ulcer wound in diabetic subjects: a meta-analysis. Int Wound J. 2023;20(1):55–62. doi: 10.1111/iwj.13836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lane KL, Abusamaan MS, Voss BF, et al. Glycemic control and diabetic foot ulcer outcomes: a systematic review and meta-analysis of observational studies. J Diabetes Complications. 2020;34(10):107638. doi: 10.1016/j.jdiacomp.2020.107638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Petersen BJ, Rothenberg GM, Lakhani PJ, et al. Ulcer metastasis? anatomical locations of recurrence for patients in diabetic foot remission. J Foot Ankle Res. 2020;13(1):1. doi: 10.1186/s13047-020-0369-3 [DOI] [PMC free article] [PubMed] [Google Scholar]


