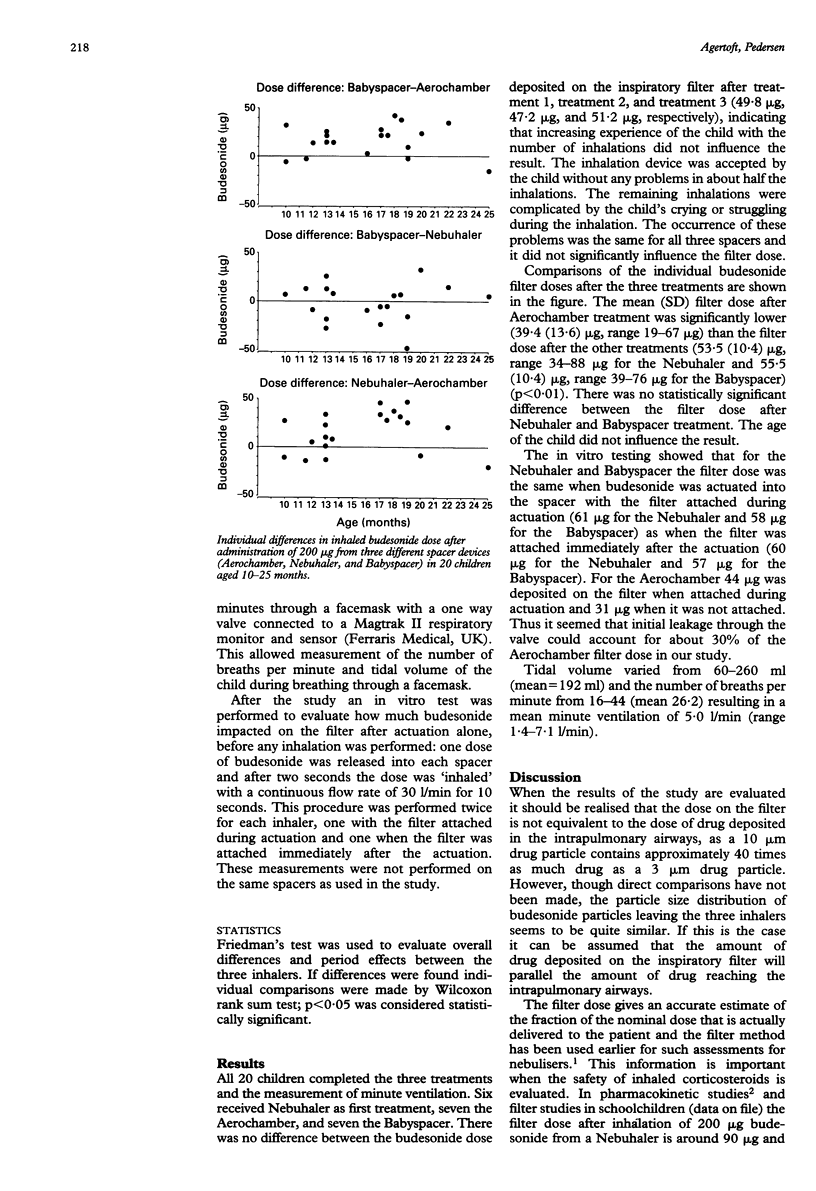
Abstract
The budesonide dose delivered to the patient from three different spacer devices (Nebuhaler = 750 ml, Aerochamber = 140 ml, and Babyspacer = 260 ml) was assessed by measuring the budesonide dose deposited on a filter inserted between the spacer outlet and the mouth of the patient. Twenty children aged 10-25 months were given a single dose of 200 micrograms budesonide from each spacer device in a randomised crossover study. All spacers had a facemask attached and a one way valve system. The children breathed through the inhalation system for 30 seconds. Furthermore, the minute ventilation of the children through a tightly fitting facemask was measured. The filter dose of budesonide was significantly lower after Aerochamber treatment (39.4 micrograms, range 19-67 micrograms) than after Nebuhaler (53.5 micrograms, range 34-88 micrograms) and Babyspacer (55.5 micrograms, range 39-76 micrograms) treatment. The minute ventilation of the children varied from 1.4 l/min to 7.0 l/min (mean 5.0 l/min). This was sufficient to empty all spacers within the 30 seconds of inhalation. It is concluded that spacer volume does not seem to be so important for children aged 10-25 months as long as spacers with a volume lower than 750 ml are used.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crimi N., Palermo F., Cacopardo B., Vancheri C., Oliveri R., Palermo B., Mistretta A. Bronchodilator effect of Aerochamber and Inspirease in comparison with metered dose inhaler. Eur J Respir Dis. 1987 Sep;71(3):153–157. [PubMed] [Google Scholar]
- Everard M. L., Clark A. R., Milner A. D. Drug delivery from holding chambers with attached facemask. Arch Dis Child. 1992 May;67(5):580–585. doi: 10.1136/adc.67.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Evans H. E. Evaluation of inhalation aids of metered dose inhalers in asthmatic children. Chest. 1987 Mar;91(3):366–369. doi: 10.1378/chest.91.3.366. [DOI] [PubMed] [Google Scholar]
- Lødrup Carlsen K. C., Nikander K., Carlsen K. H. How much nebulised budesonide reaches infants and toddlers? Arch Dis Child. 1992 Sep;67(9):1077–1079. doi: 10.1136/adc.67.9.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S., Ostergaard P. A. Nasal inhalation as a cause of inefficient pulmonal aerosol inhalation technique in children. Allergy. 1983 Apr;38(3):191–194. doi: 10.1111/j.1398-9995.1983.tb01605.x. [DOI] [PubMed] [Google Scholar]
- Pedersen S., Steffensen G., Ekman I., Tönnesson M., Borgå O. Pharmacokinetics of budesonide in children with asthma. Eur J Clin Pharmacol. 1987;31(5):579–582. doi: 10.1007/BF00606634. [DOI] [PubMed] [Google Scholar]


