Abstract
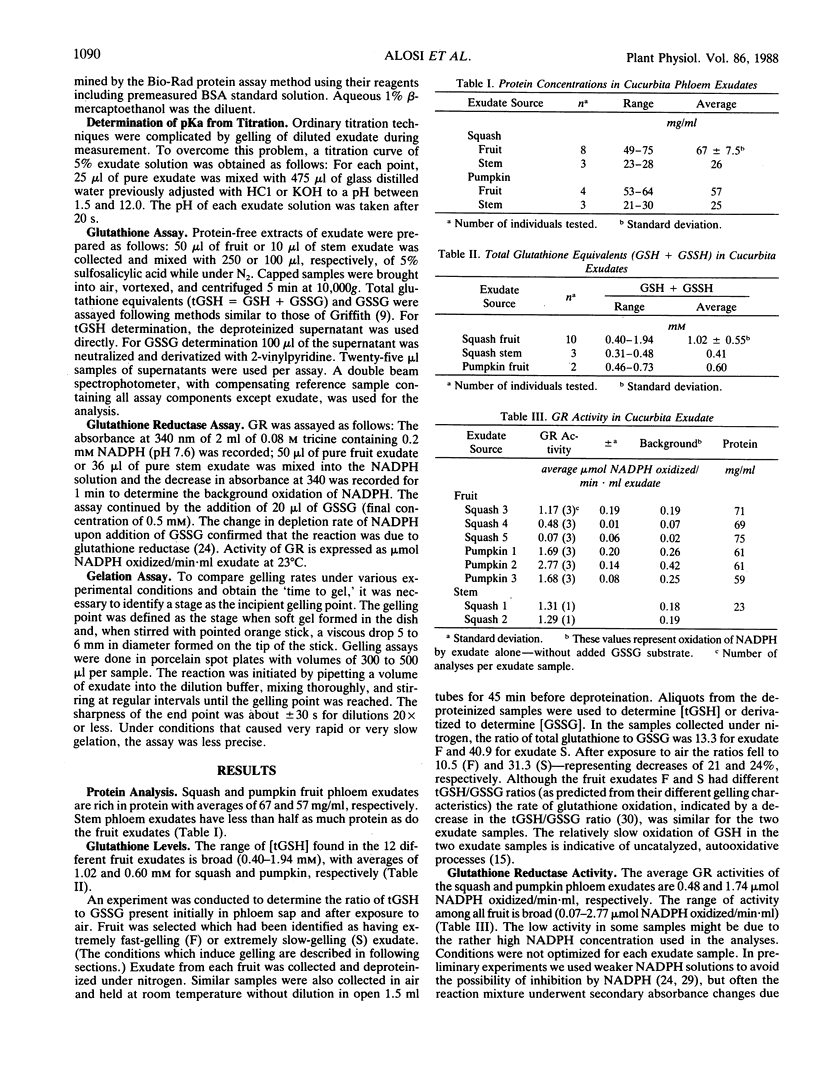
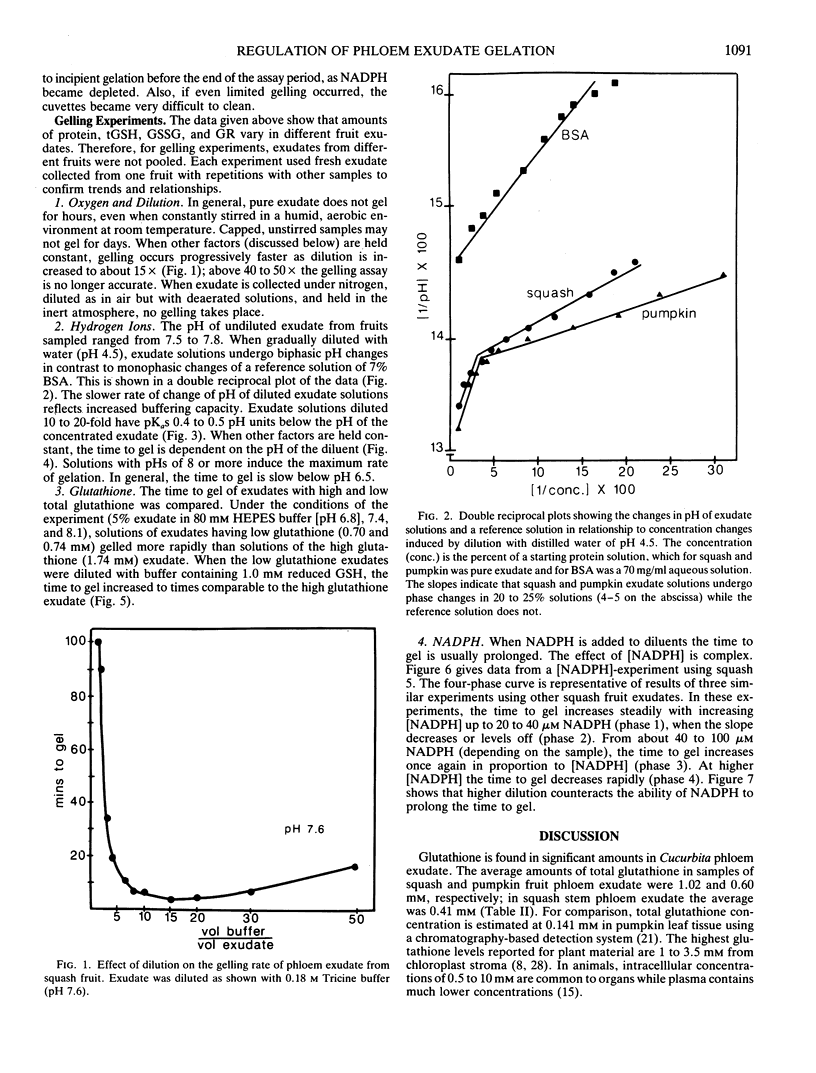
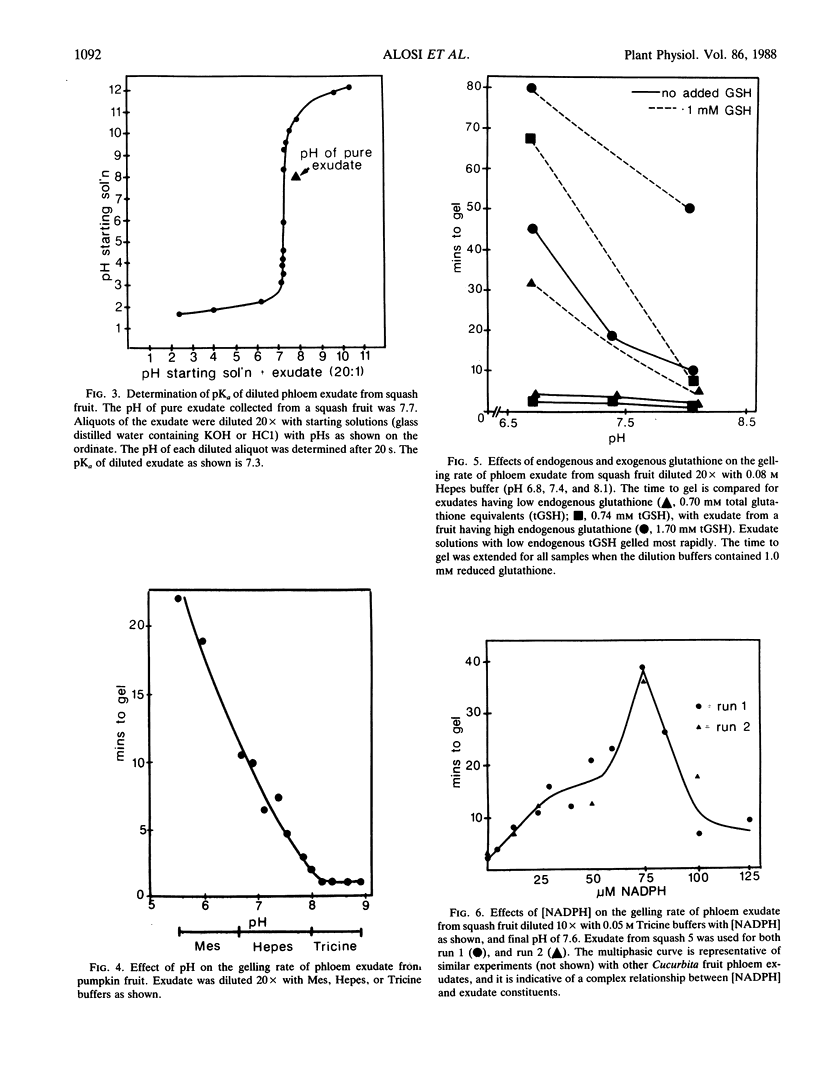
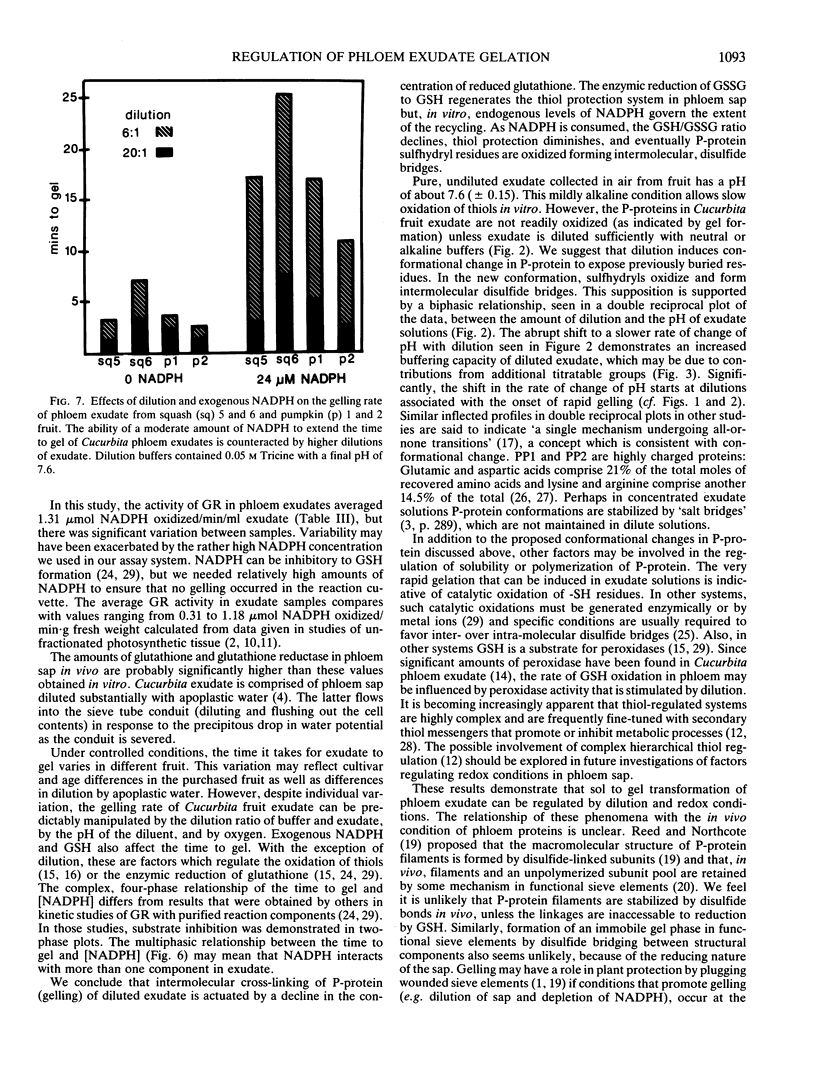
The average glutathione equivalent concentration in phloem exudate collected from squash fruit (Cucurbita moschata [Duchesne] Poir. var Butternut) and pumpkin fruit (Cucurbita pepo [L.] var Jack-o-lattern) was 1.02 and 0.60 millimolar, respectively. Glutathione reductase (EC 1.6.4.2) activity in phloem exudate from squash and pumpkin fruit averaged 0.48 and 1.74 micromole NADPH oxidized per minute per milliliter, respectively. Protein concentrations in fruit phloem exudates averaged 67 milligrams per milliliter for squash and 57 milligrams per milliliter for pumpkin. The phloem-specific P-proteins account for most of the protein content of exudate. Pure exudate from fruit does not gel for hours or days, but when diluted with neutral or alkaline aqueous solutions, exudate gels rapidly. Exudate solutions undergo biphasic pH changes with dilution. We suggest that P-protein undergoes conformational change upon dilution, exposing titratable groups and sulfhydryl residues. Oxidation of the latter forms the intermolecular disulfide bridges of the gel. The gelation of diluted exudate is regulated by factors (oxygen, pH, glutathione, NADPH) which affect the maintenance of reduced sulfhydryl residues and the activity of glutathione reductase. While these factors may also act in vivo to regulate redox conditions in phloem, their relationship to hypothetical sol/gel transitions or motile and nonmotile phases in the transport conduit is unknown.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. K. A lectin from the exudate of the fruit of the vegetable marrow (Cucurbita pepo) that has a specificity for beta-1,4-linked N-acetylglucosamine oligosaccharides. Biochem J. 1979 Oct 1;183(1):133–137. doi: 10.1042/bj1830133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J. J., Gamble P. E., Hatfield J. L., Quisenberry J. E. Plant morphological and biochemical responses to field water deficits: I. Responses of glutathione reductase activity and paraquat sensitivity. Plant Physiol. 1985 Oct;79(2):415–419. doi: 10.1104/pp.79.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski P. P., Anderson J. W. Light-dependent Reduction of Oxidized Glutathione by Ruptured Chloroplasts. Plant Physiol. 1978 Feb;61(2):221–225. doi: 10.1104/pp.61.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T. C., Wada K., Buchanan B. B., Holmgren A. Reduction of purothionin by the wheat seed thioredoxin system. Plant Physiol. 1987 Oct;85(2):446–451. doi: 10.1104/pp.85.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Misra H. P. Generation of superoxide free radical during the autoxidation of thiols. J Biol Chem. 1974 Apr 10;249(7):2151–2155. [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Subunit structure and interactions of the phloem proteins of Cucurbita maxima (pumpkin). Eur J Biochem. 1983 Aug 15;134(3):561–569. doi: 10.1111/j.1432-1033.1983.tb07603.x. [DOI] [PubMed] [Google Scholar]
- Rennenberg H., Filner P. Stimulation of h(2)s emission from pumpkin leaves by inhibition of glutathione synthesis. Plant Physiol. 1982 Apr;69(4):766–770. doi: 10.1104/pp.69.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartio T. Disulfide-bonded polymerization of plasma fibronectin in the presence of metal ions. J Biol Chem. 1986 Jul 15;261(20):9433–9437. [PubMed] [Google Scholar]
- Walker T. S. The purification and some properties of a protein causing gelling in phloem sieve tube exudate from Cucurbita pepo. Biochim Biophys Acta. 1972 Feb 29;257(2):433–444. doi: 10.1016/0005-2795(72)90296-6. [DOI] [PubMed] [Google Scholar]
- Worthington D. J., Rosemeyer M. A. Glutathione reductase from human erythrocytes. Catalytic properties and aggregation. Eur J Biochem. 1976 Aug 1;67(1):231–238. doi: 10.1111/j.1432-1033.1976.tb10654.x. [DOI] [PubMed] [Google Scholar]
- Ziegler D. M. Role of reversible oxidation-reduction of enzyme thiols-disulfides in metabolic regulation. Annu Rev Biochem. 1985;54:305–329. doi: 10.1146/annurev.bi.54.070185.001513. [DOI] [PubMed] [Google Scholar]


