Corresponding Author

Key Words: neurocardiology, myocardial infarction, sudden cardiac death, sympathetic nervous system, vagal nerve stimulation
Lost time is never found again.
—Benjamin Franklin1
As perfectly depicted by Franklin’s famous quote, time is of the essence when dealing with acute myocardial ischemia, and the concept that “time is muscle” is the foundation for early reperfusion strategies aimed at restoring coronary blood flow as soon as possible and preventing myocardial cell death. Unfortunately, coronary flow is rarely restored within the “golden hour”, and even when it does, the detrimental cascade of events triggered by ischemia is already in movement. Furthermore, coronary blood flow restoration carries the potential to exacerbate the ischemic injury, a phenomenon better known as reperfusion damage. From a pathophysiological point of view, myocardial ischemia, myocardial infarction (MI), and reperfusion damage are distinct conditions, but they do elicit a common response, represented by a profoundly disrupted cardiac neural afferent signaling, leading, in turn, to an abnormal peripheral and central processing of cardiovascular inputs, and to the detrimental combination of increased, but dysfunctional, sympathetic output and decreased parasympathetic output. All these processes start at the time of coronary occlusion and may be further amplified during reperfusion. This complex, mechanistic view is now very well-established, but it took a long time and many preclinical studies to unravel the pivotal role of parasympathetic withdrawal in the progression of ischemia/reperfusion damage. This was due to the historical misconception that cardiac parasympathetic efferent fibers do not reach the ventricles and, subsequently, to the focus on the effects of vagus nerve stimulation (VNS) as an antiarrhythmic intervention in the setting of acute MI, mostly overlooking its great potential to improve the ischemic substrate. In 1974, Myers2 suggested a direct ventricular protective effect of VNS on ischemic ventricular fibrillation (VF). In the early ’90s,3 we demonstrated in a conscious post-MI canine model with recurrent ischemia that cholinergic antagonism favors VF, whereas right VNS protects against VF. VNS also markedly reduced reperfusion arrhythmias in cats. In both cases, approximately 50% of this protective effect was related to heart rate (HR) reduction while 50% was independent. Finally, by showing that left stellectomy increases cardiac vagal efferent activity, registered at a single-fiber level, Cerati et al4 provided evidence supporting the existence of a strong sympathetic–parasympathetic interaction, that was subsequently confirmed to take place at multiple levels of cardiac neuraxis.
An important advanced was the conclusive demonstration by Tracey et al,5 in the early 2000s, of the so-called cholinergic anti-inflammatory pathway, a neural mechanism that inhibits proinflammatory cytokine release via signals that require α7 cholinergic receptor expression on macrophages and other immunocompetent cells. The discovery that cholinergic neurons reflexively regulate inflammatory responses in real time (within seconds) with an inhibitory effect had a major impact. Calvillo et al6 showed that VNS markedly reduced infarct size following ischemia/reperfusion in rats, demonstrating a pivotal role for the nicotinic receptors expressed in inflammatory. The preconditioning-like beneficial effect of VNS applied before ischemia was found to be mostly associated with the activation, through Gi-coupled muscarinic receptors, of phosphatidylinositol 3-kinase and Akt in cardiomyocytes, leading to the up-regulation of the antiapoptotic protein BCL-2 and the suppression of caspase-3, thus protecting from cell death. Finally, VNS was also shown to have Akt- independent antioxidant effects, to improve mitochondrial function and energy metabolism and to promote angiogenesis of the infarcted heart.
The first conscious animal model of chronic VNS (cVNS) reported by Li et al7 in 2004 assessed cervical cVNS applied for 40 consecutive days starting 14 days after MI, to mimic a heart failure (HF) condition. cVNS improved left ventricular (LV) function, lowered ventricular mass, norepinephrine, and B-type natriuretic peptide (BNP) levels and dramatically improved survival at 140 days. Similar findings were confirmed in 2 different canine models of chronic HF (induced by coronary microembolizations and by high-rate ventricular pacing) and proved to be additional to those achieved with metoprolol alone. Based on these promising data, cervical cVNS (mostly right-sided) has been tested in chronic HF in 4 different clinical trials, with mixed results, possibly due to the lack of an adequate knowledge of the dose–response relationship of electrical VNS and of the subgroups of patients most likely to benefit from VNS.8
In the attempt to improve the therapeutic potential of cVNS for ischemic cardiomyopathy, and taking advantage of new technologies, other preclinical studies were than performed. Machhada et al9 used the elegant technology of optogenetic stimulation to assess the effects of a 4-week program of intermittent activation of cholinergic neurons in the vagal dorsal motor nucleus on LV contractility and exercise capacity in normal and in post-MI rats (starting 48 hours after MI). This purely efferent cVNS with almost no impact on HR was associated with significantly better cardiac function (compared with the corresponding sham-control group) both in normal and in post-MI rats. Specifically, LV function almost normalized in post-MI rats, despite a similar infarct size (at least 30% of LV mass), an effect much more pronounced than the one observed by Li et al.7 The improvement in LV function caused by optogenetic cVNS in normal rats was related to a reduced myocardial expression of GRK2 (G protein–coupled receptor kinase 2) and β-arrestin 2, both involved in β1-adrenergic receptor desensitization and internalization; these data underline the complexity of sympathetic-parasympathetic interaction10 and the apparently paradoxical potential for cVNS to improve sympathetic responses of cardiomyocytes despite its well established anti-adrenergic effects.
In this issue of JACC: Basic to Translational Science, Hadaya et al11 further expand our knowledge on the mechanisms and the effects of cVNS in a post-MI large animal model (minipigs) with a high translational potential. Three groups were studied: control subjects, untreated MI, and MI + right cVNS, with cVNS started 48 hours after. Chronic VNS was delivered according to the principle of the neural fulcrum, defined as a balance point characterized by an equal engagement of afferent and efferent vagal fibers, leading to a null HR response. The effects of cVNS were extensively characterized at multiple levels and by several techniques including: LV function (at baseline and in response to bilateral stellate ganglion stimulation); LV electrical activity (including both activation and repolarization times); ventricular arrhythmia susceptibility assessed by programmed electrical stimulation and cardiac and neuronal structural remodeling, assessed from myocardial samples and neuronal samples from the stellate ganglia and the dorsal root ganglia. Notably, to guarantee therapeutic levels of cVNS, a 4-week titration period was used, during which the stimulation amplitude was systematically titrated to that producing a null HR change at 5 Hz frequency and 250-μs pulse width during the on phase of cVNS. In the MI + cVNS group, cVNS was stopped after titration at the time of acute MI and then resumed 48 hours later at the previously determined intensity. VNS in this model induced a pronounced improvement in cardiac function, a marked reduction in induced ventricular arrhythmias, and the preservation of normal cardiac sympathetic responses compared with untreated MI. These changes were associated with a marked mitigation of the unfavorable electrical and structural consequences of acute MI in the ischemic border zone and at the neuronal level (including both neurons and glial cells). The investigators also showed for the first time in a chronic model, that acute, supra-threshold bilateral VNS yields no additional antiarrhythmic benefits when superimposed over cVNS.
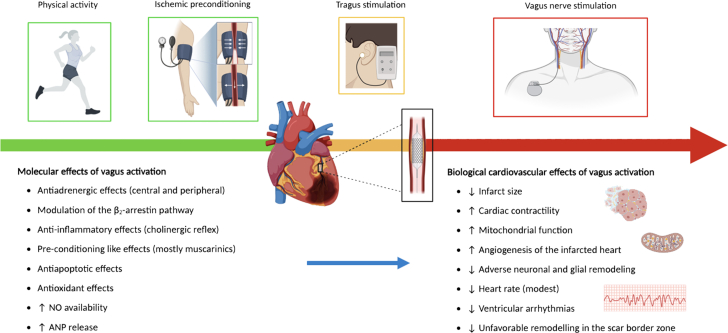
Overall, the study strongly reinforces the concept that the sooner vagal output is increased in the setting of acute MI, the larger the benefit. It must be acknowledged that invasive VNS through device implantation cannot be acutely achieved during MI/reperfusion in humans, but alternative investigational strategies such as tragus nerve stimulation can be hypothesized. Indeed, a pilot clinical study assessed the effects of low-level (50% of the amplitude that lowers HR) right tragus nerve stimulation applied at the time of primary percutaneous coronary intervention and lasting for only 2 hours in patients with ST-segment elevation MI. The study showed a significant reduction in reperfusion-related arrhythmia, infarct size and inflammatory biomarkers, that was accompanied by an improved cardiac function at 7 days.12 Of note, tragus stimulation, although indirectly leading to an increased vagal output, is by itself a purely afferent type of VNS. Notably, parasympathetic activity can be increased noninvasively in all subjects with exercise training (Figure 1), and this may lead to a reduced risk of malignant arrhythmias in case of myocardial infarction. Indeed, we have shown that the physical inactivity significantly increases the risk of VF occurring during a first myocardial infarction in humans.13
Figure 1.
Potential Approaches to Increase Cardiac Vagal Output
Potential approaches to increase cardiac vagal output before (green), during (yellow), and after (red) myocardial ischemia end reperfusion. ANP = atrial natriuretic peptide; NO = nitric oxide. Created with BioRender.com.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Franklin B. Quotations. BrainyQuote. https://www.brainyquote.com/quotes/benjamin_franklin_104457 Accessed July 4, 2023.
- 2.Myers R.W., Pearlman A.S., Hyman R.M., et al. Beneficial effects of vagal stimulation and bradycardia during experimental acute myocardial ischemia. Circulation. 1974;49:943-7. doi: 10.1161/01.cir.49.5.943. [DOI] [PubMed] [Google Scholar]
- 3.Vanoli E., De Ferrari G.M., Stramba-Badiale M., et al. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res. 1991;68:1471-81. doi: 10.1161/01.res.68.5.1471. [DOI] [PubMed] [Google Scholar]
- 4.Cerati D., Schwartz P.J. Single cardiac vagal fiber activity, acute myocardial ischemia, and risk for sudden death. Circ Res. 1991;69:1389-401. doi: 10.1161/01.res.69.5.1389. [DOI] [PubMed] [Google Scholar]
- 5.Tracey K.J. The inflammatory reflex. Nature. 2002;420:853-9. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 6.Calvillo L., Vanoli E., Andreoli E., et al. Vagal stimulation, through its nicotinic action, limits infarct size and the inflammatory response to myocardial ischemia and reperfusion. J Cardiovasc Pharmacol. 2011;58:500-7. doi: 10.1097/FJC.0b013e31822b7204. [DOI] [PubMed] [Google Scholar]
- 7.Li M., Zheng C., Sato T., et al. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004;109:120-4. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- 8.Dusi V., De Ferrari G.M. Vagal stimulation in heart failure. Herz. 2021;46:541–549. doi: 10.1007/s00059-021-05076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machhada A., Hosford P.S., Dyson A., Ackland G.L., Mastitskaya S., Gourine A.V. Optogenetic stimulation of vagal efferent activity preserves left ventricular function in experimental heart failure. J Am Coll Cardiol Basic Trans Science. 2020;5:799–810. doi: 10.1016/j.jacbts.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dusi V., De Ferrari G.M., Mann D.L. Cardiac Sympathetic-Parasympathetic Interaction: The Endless Story of Yin and Yang. J Am Coll Cardiol Basic Trans Science. 2020;5:811–814. doi: 10.1016/j.jacbts.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadaya J., Dajani A.H., Cha S., et al. Vagal nerve stimulation reduces ventricular arrhythmias and mitigates adverse neural cardiac remodeling post-myocardial infarction. J Am Coll Cardiol Basic Trans Science. 2023;8:1100–1118. doi: 10.1016/j.jacbts.2023.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu L., Huang B., Po S.S., et al. Low-level tragus stimulation for the treatment of ischemia and reperfusion injury in patients with ST-segment elevation myocardial infarction: a proof-of-concept study. J Am Coll Cardiol Intv. 2017;10:1511–1520. doi: 10.1016/j.jcin.2017.04.036. [DOI] [PubMed] [Google Scholar]
- 13.De Ferrari G.M., Dusi V., Ruffinazzi M., et al. Physical inactivity is a risk factor for primary ventricular fibrillation. J Am Coll Cardiol. 2019;73:2117–2118. doi: 10.1016/j.jacc.2019.01.063. [DOI] [PubMed] [Google Scholar]