Abstract
The SARS-CoV-2 virus has been identified as the infectious agent that led to the COVID-19 pandemic, which the world has seen very recently. Researchers have linked the SARS-CoV-2 outbreak to bats for the zoonotic spread of the virus to humans. Coronaviruses have a crown-like shape and positive-sense RNA nucleic acid. It attaches its spike glycoprotein to the host angiotensin-converting enzyme 2 (ACE2) receptor. Coronavirus genome comprises 14 ORFs and 27 proteins, spike glycoprotein being one of the most critical proteins for viral pathogenesis. Many mammals and reptiles, including bats, pangolins, ferrets, snakes, and turtles, serve as the principal reservoirs for this virus. But many experimental investigations have shown that certain domestic animals, including pigs, chickens, dogs, cats, and others, may also be able to harbor this virus, whether they exhibit any symptoms. These animals act as reservoirs for SARS-CoV, facilitating its zoonotic cross-species transmission to other species, including humans. In this review, we performed a phylogenetic analysis with multiple sequence alignment and pairwise evolutionary distance analysis, which revealed the similarity of ACE2 receptors in humans, chimpanzees, domestic rabbits, house mice, and golden hamsters. Pairwise RMSD analysis of the spike protein from some commonly reported SARS-CoV revealed that bat and pangolin coronavirus shared the highest structural similarity with human coronavirus. In a further experiment, molecular docking confirmed a higher affinity of pig, bat, and pangolin coronavirus spike proteins’ affinity to the human ACE2 receptor. Such comprehensive structural and genomic analysis can help us to forecast the next likely animal source of these coronaviruses that may infect humans. To combat these zoonotic illnesses, we need a one health strategy that considers the well-being of people and animals and the local ecosystem.
Keywords: SARS-CoV-2, Mammals and reptiles, Phylogenetic analysis, RMSD analysis, Molecular docking, One health strategy
1. Introduction
The recent worldwide epidemic, COVID-19, and subsequent global catastrophe have been traced back to a small virus called SARS-CoV-2 [1]. Due to zoonotic transmission, three significant strains of SARS-CoV have been identified to date: the original strain, first reported in 2002, Middle East Respiratory Syndrome CoV (MERS-CoV) in 2013, and the most recent strain, Severe Acute Respiratory Syndrome CoV 2 (SARS-CoV-2), which was primarily attributed to China in 2019 [[2], [3], [4]]. Although SARS-CoV-2 is less pathogenic but spreads extremely quickly; this has led to a considerable increase in the number of cases of SARS-CoV-2 patients throughout the globe [5]. SARS-CoV-2 is classified as a member of the coronaviridae family, which is comprised of the primary coronavirus genus known as Beta coronavirus [6,7]. Its nucleic acid core is composed of a single-stranded RNA of the positive-sense type, ranging in size from 26 to 32 kilobases and 50–200 nm in diameter [8,9]. It codes for four structural proteins and sixteen other proteins that do not have a role in the structure. The viral genome has a leader sequence and an open reading frame (ORF1 a/b) in the 5′ untranslated region (5′ UTR). Also, it includes spike protein (S), envelope (E) protein, membrane/matrix (M) protein, and accessory proteins (ORF 3,6,7a, 7b, 8 and 9b), nucleoprotein (N), and a 3′ untranslated region (3′ UTR) [[10], [11], [12]].
In December 2019, the coronavirus was found in seafood at a wholesale market in Wuhan, Hubei Province, China, as well as in a wet market for live animals. On the 31st of December 2019, a number of instances of pneumonia were reported in China [13,14]. Mild respiratory issues to serious upper respiratory tract infections that caused acute respiratory distress syndrome and deadly interstitial pneumonia were among the symptoms [15]. The etiological agent of COVID-19, which was given the designation SARS-CoV-2 on January 7, 2020, was also identified. Due to its rapid global spread and increased death rates in over 200 nations, the World Health Organization declared this a pandemic [16]. The Wuhan market is thought to have been an ideal location for the direct contact of SARS-CoV-2 from wildlife to humans, which would have resulted in the modification, mutation, and genetic recombination that lead to the transmission of the disease into humans [17,18]. SARS-CoV-2 posed a major threat by achieving some mutations as part of its natural evolution, making it difficult to prevent by designing vaccines. Some of its mutations even enhanced its transmissibility by allowing increased host receptor binding and evasion from the host immune response [19].
SARS-CoV-2 infection resembles most of the typical clinical features of common influenza flu, making it challenging to differentiate from each other [20]. Both exert headache, myalgia, malaise, anorexia, and sore throat as common symptoms at the early stage of infection. Even diagnostic methods of influenza are also applicable to the SARS-CoV-2, including viral culture, serology, rapid antigen testing, reverse transcription polymerase chain reaction (RT-PCR), and immunofluorescence assays. Luckily, some antiviral drugs and monoclonal antibody treatments are available to treat these two diseases. However, they are not fully effective at the later stage of infection when the symptoms intensify. Different research facilities have developed several vaccines against SARS-CoV-2 following different strategies that seem to work on large populations. Large-scale vaccination also decreased the occurrence of the new viral infection.
SARS-CoV-2 is often reported to be sourced from animals, leading to transmission to humans, followed by human-to-human transmission later [21]. However, neither the origin nor the transmission mechanism of SARS-CoV-2 has been conclusively established. Vertebrate transmission is still poorly understood, and researchers are skeptical of their potential roles as reservoirs or amplifying hosts. There are no barriers to entry for SARS-CoV-2; thus, it may infect and exploit a wide variety of animals [22]. There are likely many domestic and wild animals that may serve as reservoirs or intermediate hosts [23,24]. Even though the natural host is well-documented, the animal that most likely served as a vector for spreading the disease, which ultimately affected humans, remains unknown [25]. As reviewed by Sharun et al. (2021), several mammals and birds such as cats, ferrets, raccoon dogs, cynomolgus macaques, white-tailed deer, rabbits, and Egyptian fruit bats are susceptible to SARS-CoV-2 infection. Moreover, intra and inter-species transmission can occur via contact and air [26]. Although most of these incidents are reported in experimental infections, natural infections have also been found in several pets-cats and dogs, zoo animals-tigers, lions, gorillas, and farmed mink and ferrets (Table 1). This terrible virus is thought to have its native reservoir in bats, while the pangolin is thought to serve as an intermediary host for the virus [27]. It has been discovered that horseshoe bat SARS-CoV-2 has a genetic similarity of 96% with bat SARS-CoV-2 (designated RaTG13) [28]. This highly homologous virus has been identified from the bat (Rhinolophus affinis) [29]. The Masked palm civet, the Asian palm civet, Stoliczka's trident bat, and the Chinese rufous horseshoe bat are some of the other animal species that are thought to be closely related to one another [30]. Recently cats, dogs, minks, tigers, and lions have also become susceptible to SARS-CoV-2 [[31], [32], [33], [34]]. It is also reported that betacoronavirus infection can be found in kept, feral or wild animals that have the potential to act as viral reservoirs. That leads us to dive deeper into the multi-omic analyses of closely related SARS-CoV animal reservoirs that might pose a future threat as a pandemic [26]. As a result, identifying the host, should it be available, is essential for taking control of the terrible pandemic scenario.
Table 1.
Some commonly reported animal coronaviruses, their common name, susceptibility and clinical symptoms.
| Animal species | CoV genus | CoV subgenus | CoV common name | Susceptibility | Clinical symptoms | References |
|---|---|---|---|---|---|---|
| Mice | Beta CoV | Embecovirus | Rat coronavirus | Experimental | Gastrointestinal diseases and diarrhea | [[35], [36], [37]] |
| Hamster | Beta CoV | Sarbecovirus | Severe acute respiratory syndrome coronavirus 2 | Experimental | Weight loss | [38,39] |
| Cats | Alpha CoV | Tegacovirus | Feline coronavirus | Natural | Peritonitis, Enteritis | [40,41] |
| Ferrets | Alpha CoV | Minacovirus | Ferret coronavirus | Natural | Bloody stool, dehydration, lethargy, weight loss | [[42], [43], [44]] |
| Tree shrew | Beta CoV | Sarbecovirus | Severe acute respiratory syndrome coronavirus 2 | Experimental | Increased body temperature | [45] |
| Pig | Alpha CoV | Tegacovirus | Porcine respiratory coronavirus (PRCV) | Natural | Respiratory disease | [[46], [47], [48]] |
| Beta CoV | Embecovirus | Porcine hemagglutinating encephalomyelitis virus (pHEV) | Natural | Neurological and/or enteric disease | [49,50] | |
| Delta CoV | Buldecovirus | Porcine deltacoronavirus (PDCoV) | Natural | Gastroenteritis | [[51], [52], [53]] | |
| Alpaca | Alpha CoV | Duvinacovirus | Alpaca alphacoronavirus (ACoV) | Natural | Respiratory disease | [54,55] |
| Beta CoV | Embecovirus | BCoV-like CoV | Natural | Gastroenteritis and/or respiratory disease | [56,57] | |
| Dromedary camel | Alpha CoV | Duvinacovirus | Dromedary camel alphacoronavirus | Natural | Respiratory disease | [58,59] |
| Beta CoV | Embecovirus | BCoV-like dromedary camel CoV UAE-HKU-23 |
Natural | Gastroenteritis | [60] | |
| Beta CoV | Merbecovirus | Dromedary camel Middle East respiratory syndrome coronavirus (MERS-CoV) | Natural | Respiratory disease | [[61], [62], [63]] | |
| Cattle | Beta CoV | Embecovirus | Bovine coronavirus (BCoV) | Natural | Gastroenteritis and/or respiratory disease | [[64], [65], [66]] |
| Sheep | Beta CoV | Embecovirus | BCoV-like CoV | Natural | Gastroenteritis | [67,68] |
| Bat | Alpha CoV | Pedacovirus | Bt-CoV | Natural | No clinical signs | [69] |
| Beta CoV | Merbecovirus | Natural | ||||
| Pangolin | Beta CoV | Sarbecovirus | Pangolin coronavirus | Natural | Cough, shortness of breath and dyspnea | [70] |
Genomic surveillance by performing regular whole-genome sequencing and analysis must be performed to detect any previously unknown or new variant of SARS-CoV [19]. A vast number of genomic and proteomic analyses are available currently that are applicable for multi-omics monitoring of animal genomes and any potential threats [71]. This study provides a summary of what is already known about the evidence of SARS-CoV-2 in various animal species. Additionally, genomic and proteomics analyses of potential animal host receptors are performed in order to determine which animal species might be associated with an increased risk of recurrent SARS-CoV-2 infection in human populations.
2. SARS-CoV-2 biology
Coronaviruses are enveloped positive single-stranded RNA viruses that belong to the largest virus group Nidovirales which includes Coronoviridae, Arteriviridae, Mesoniviridae, and Roniviridae families [72]. The coronaviridae family comprises subfamilies- Coronavirinae and Torovirinae. Based on the genome structure and expression, the Coronavirinae subfamily is further classified into four genera: Alphacoronavirus, Betacoronavirus, Gammacoronavirus, And Deltacoronavirus [73]. The first-two genera usually infect mammals, while the second two infect birds. Subsequently, SARS-CoV-2 has a greater genetic homogeneity with the aforementioned coronavirus genera, establishing its origin in the beta coronavirus genera.
2.1. Structure morphology
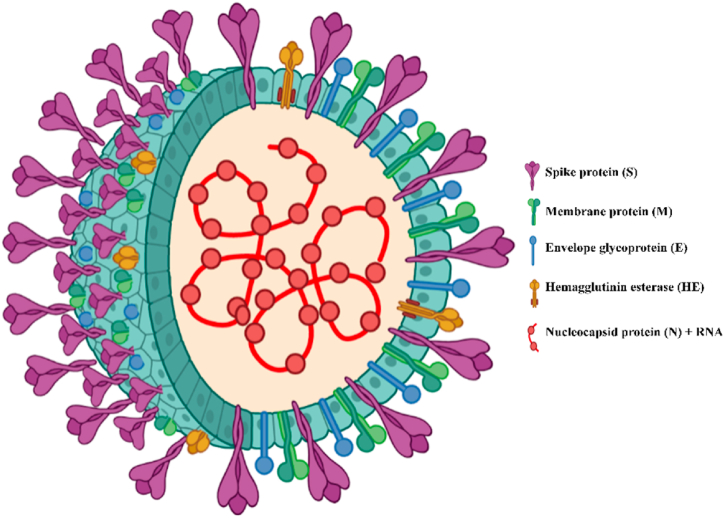
SARS-COV-2 possesses a spherical or elliptic nucleocapsid structure with spikes on the surface and a roughly 60–140 nm diameter, which is often pleomorphic in form [74]. The complete viroid consists mainly of four components-spike (S), nucleocapsid (N), membrane (M), and envelope (E) (Fig. 1) [75,76]. Envelope protein is a transmembrane protein consisting of three domains that act as ion-channeling viroporin and contain a motif involved in protein-protein interaction for pathogenesis [77]. The most abundant membrane-associated M protein supports the viral nucleocapsid and attaches it to the nucleocapsid component [78]. M protein is a membrane glycoprotein comprising three transmembrane domains that support the viral envelope. It can adopt two conformational states and plays a major role in shaping the virion, coronavirus assembly, and interaction with major structural proteins [79,80]. Viral assembly, release, and pathogenesis are associated with a crucial coat protein that is transiently found in the envelope [81]. N proteins are the core viral proteins that directly interact with the RNA genome to form the helically symmetric nucleocapsid. They get activated by the phosphorylation of protein residues by glycogen synthase kinase 3 (GSK3)- which was confirmed by the restriction of viral replication by inhibiting the GSK3 [82,83]. Structural analysis of M, N, and E proteins of SARS-COV-2 showed over 90% similarity among them. However, reduced genetic similarity was observed when the S protein was analyzed against a reference genome. While higher genomic similarity was observed in the betacoronaviruses, genes encoding the spike glycoprotein were found to be least conserved, with a genomic identity of 74–83% [84].
Fig. 1.
Structure of SARS-CoV-2 showing significant components of the virus particle.
2.2. Genomic arrangement
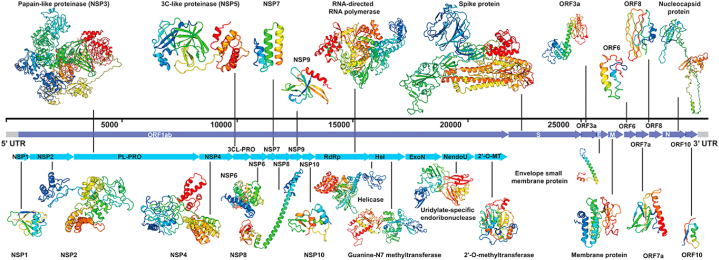
Coronaviruses possess RNA with a length of approximately 27.6–31.6 kb and 38% G + C content that encode approximately 9860 amino acids (Fig. 2). Genome mapping revealed two untranslated regions flanking the whole genome-a 265 nucleotide long 5′ UTR region and 358 nucleotides long 3′ UTR region [85]. It's 5′ end contains a cap region, while the 3′ end includes a poly (A) tail and various open reading frames (ORFs) [86]. This large genome houses replicase enzymes, structural proteins, and some sub-proteins scattered throughout the RNA. Based on the advanced sequencing technology, it was found that the SARS-COV-2 genome contains 14 ORFs that encode 27 proteins. Being located at the 5′ end, coronavirus replicase dominates two-thirds of the genome that comprise two overlapping ORFs (ORF1a and ORF1b) encoding 15 non-structural proteins (NSPs) named nsp1 to nsp10 and from nsp12 to nsp16 respectively [87]. While 3′ region encodes for four structural proteins (S, E, M, and N) and eight secondary proteins [88,89].
Fig. 2.
Genome-wide arrangement of all proteins of SARS-CoV-2 as modeled by Zhang lab [90].
2.3. Major viral proteins
Like other CoVs, SARS-COV-2 houses the largest RNA genome that encodes several structural and non-structural proteins (nsp) [91,92]. These non-structural proteins undergo post-translational modifications and co-operatively regulate of replication process [93]. ORF1a encodes pp1a (440–500 kDa), which further gets spliced into 11 nsps. Whereas, due to the ribosomal frameshift translation of ORF1b continues and encodes a large polypeptide pp1ab (740–810 kDa) that produces 16 nsps [94]. These 16 nsps play a crucial role in pathogenesis inside infected host cells [95].
2.4. Spike glycoprotein
Among the four major components of coronavirus viroid, spike glycoprotein plays the most crucial role by mediating virus particle entry into the host cells [[96], [97], [98]]. It extends outwards from the viral surface and forms homotrimers that bind to the host cell's Angiotensin-converting enzyme 2 (ACE2) receptor [99,100]. The S protein contains two major functional subunits- S1 and S2. The S1 subunit binds the virus particle to the host receptor, S2 subunit fuse viruses to the host cells. The S1 subunit consists of the N-terminal domain (NTD) and receptor binding domain (RBD) that facilitate the virus's binding to the host cell. S2 subunit includes- fusion peptide (FP) required for viral entry and release, heptad repeat 1 and 2 (HR1, HR2), central helix (CH), and connector domain (CD). It also contains a transmembrane domain (TM) and cytoplasmic tail (CT). Host protease enzyme cleaves the S1/S2 protease cleavage site at the fusion site of the S1 and S2 subunit to activate the proteins critical for the fusion of the virus membrane and host cells.
2.5. Main protease
The Viral protein responsible for exerting replication and transcription of the viral gene in the host cell is the central protease. The homodimer main protease consists of protomer A and protomer B and contains three subdomains [101]. Compared to other genera of coronaviruses, S1, S1′, S2 and S4 are the four conserved regions that the active site of the main protease maintains. Inhibitors are anchored to the cysteine thiol residue via a covalent bond in S1′ site, which is crucial to maintain its antiviral activity [102].
2.6. Papain-like protease
As we know, ORF1ab produces large polypeptides comprising several that must be cleaved into their functional form [103]. Papain-like proteases come into action in this step of processing polyproteins. As deposited in PDB, the structural attributes of papain-like protease have demonstrated a homotrimeric fold in its structure. It is involved in cleavage activity and found to be conserved in SARS-CoV and MERS-CoV [104]. Researchers were intrigued at this structural similarity of SARS-COV-2 papain-like protease to explore drug components and several other inhibitors [105].
2.7. RNA-dependent RNA polymerase (RdRp)
RdRp, also known as nsp12, is part of a multisubunit system responsible for the replication and transcription of coronaviruses. It enables viral nucleic acid synthesis and plays an active role in the replication and transcription of coronaviruses with nsp7 and nsp8 as cofactors [106]. The structure of SARS-CoV-2 contains a right-hand RdRp domain with a nidovirus-specific N-terminal extension that together forms a nidovirus RdRp-associated nucleotidyltransferase (NiRAN) structure [107]. Like other viral polymerases, the SARS-CoV polymerase consists of three subdomains-a finger, a palm, and a thumb subdomain [108]. In this structure, a cavity where template-directed RNA synthesis is regulated by the RdRp motifs is formed by the positively charged primer-template entry, NTP entry, and nascent strand exit paths [109].
3. Viral infection and replication
Coronaviruses also use host cell machinery to replicate and cause infection like other viruses. Significant steps in the proliferation of coronaviruses include adhesion and entry into the host cell, translation of RNA-dependent RNA polymerase, transcription of the viral genome, translation of structural and non-structural proteins, replication, assembly, and release from the infected cell.
The binding of the viral particle to the host cell is mediated by the Receptor Binding Domain (RBD) of spike protein to the host ACE2 receptor [110,111]. Host cell protease mediates the cleavage of S1/S2 subunits in the spike protein and activates them. The binding of the S1 subunit to the host receptor alters the conformation of the S2 subunit, leading to the insertion of the virus into the host cell membrane that releases the viral nucleocapsid in the host cytoplasm [112]. Following that, the viral genome starts to translate the ORF1a and ORF1b region, leading to the formation of PP1a and PP1b. After that, the mentioned polypeptides get cleaved with the help of papain-like protease of viral origin into the non-structural proteins required for the upcoming viral transcription and translation (Fig. 3).
Fig. 3.
Mechanism of viral replication inside the host cell.
Like other RNA viruses, SARS-COV-2 starts to exploit the host's replication machinery at this stage. The genomic RNA then serves as a template, while other non-structural proteins produced from PP1a and PP1b at earlier stages participate in the synthesis of negative-sense RNA, genome replication, and subgenomic RNA [[113], [114], [115]]. Some nsps associated with N protein, host proteins, and endoplasmic reticulum (ER) compose coronavirus replication machinery where viral RNA synthesis occurs [116].
New viral particles are formed in the ER-Golgi intermediate compartment (ERGIC). Subsequently, the M protein regulates the formation of mature virus particles from structural proteins and the viral genome [[117], [118], [119]]. In this stage, M and E proteins form the coronavirus envelope [120]. Meanwhile, M proteins regulate the formation of the viral envelope, while the interaction between M and S proteins and M and N proteins mediates the accumulation of other viral proteins at the assembly site [121]. Finally, the complete virion assembled in ERGIC fuse to the host cell vesicles and exits the cell through the secretory pathway [115,122].
3.1. Pathogenesis and interaction with the immune system
Once the virus starts replicating and disseminating among the neighboring cells, the signaling molecules of the immune system start activating the expression of necessary genes and initiate the reactions associated with the immune response. The way SARS-CoV interacts with the host can be discussed in two distinct but interconnected phases comprising the innate and adaptive immune systems.
The innate immune response is vital to inhibit CoV replication of CoVs and help initiate the adaptive immune response against the viral infection. This response is triggered by the interaction between specific molecules present on the cell surface, and cytoplasm is known as pattern recognition receptors (PRRs) and pathogen-associated molecular patterns (PAMPs) that are part of the viral genome and proteins [123,124]. Some prominent PAMP of CoVs is intermediate double-stranded RNA, 5′- triphosphate-bearing RNA, and M and N proteins. Intracellular PRRs like-retinoic acid-inducible receptor-I (RIG-I), melanoma differentiation-associated protein 5 (MAD-5), and toll-like receptors (TLRs): TLR-2,3,4,7,8 sense these PAMPs [[125], [126], [127], [128]]. Interferon (IFN) 3 and 7 and NFκB pathway are expressed due to the sensitization by PAMPs, which initiate the expression of antiviral interferon-stimulated genes (ISGs) [127,128]. Following activation of these ISGs, an abundant amount of IFNs and other pro-inflammatory cytokines like interleukin (IL)-1,6,8 and tumor necrosis factor- β (TNF- β) are synthesized [129]. These cytokines perform a key role in inhibiting and inactivating viral replication machinery. However, CoV's non-structural proteins (nsps), such as NSP-1 of SARS-COV-2, interact antagonistically with the signaling pathway that type 1 IFN initiates.
CD8+, CD4+, T-cytotoxic lymphocytes, and T-helper lymphocytes play a central role in adaptive immunity against SARS-CoV-2 [127,130]. Also, dendritic cells are crucial in activating an adaptive immune response. They internalize crucial viral components and present them to the T-lymphocytes leading to a tailored immune response against viral infection [131]. CD4+ T cells produce pro-inflammatory IL-17, which signals monocytes and neutrophils to secrete other cytokines: IL-1, IL-6, IL-8, IL-21, and TNF- β [127,132]. They also activate the B-lymphocytes to produce antibodies against viral particles. Follicular T helper cells induce B-lymphocytes to produce plasma cells and produce antibodies [133]. Additionally, C5a and C3a subunits of a complementary system are activated during inflammation, suggesting a destructive effect on the virus rather than protection against infection [127]. CD8+ T cells play the most critical role through their cytotoxic mechanism and induction of long-lasting memory cells. Production of IFN-γ and TNF-α with other cytotoxic mediators like perforin and granzyme B is directly induced by the memory CD8+ cells [131]. However, CoVs managed some evasive mechanisms to escape the cellular and humoral immune response against it. For example, SARS-CoV alters T-cell activation by hindering DC's maturation, which is essential for antigen processing and presentation. Conversely, MERS-CoV possesses the remarkable ability to induce apoptosis that leads to severe lymphopenia [127,131].
4. Potential animal origin of SARS-CoV-2
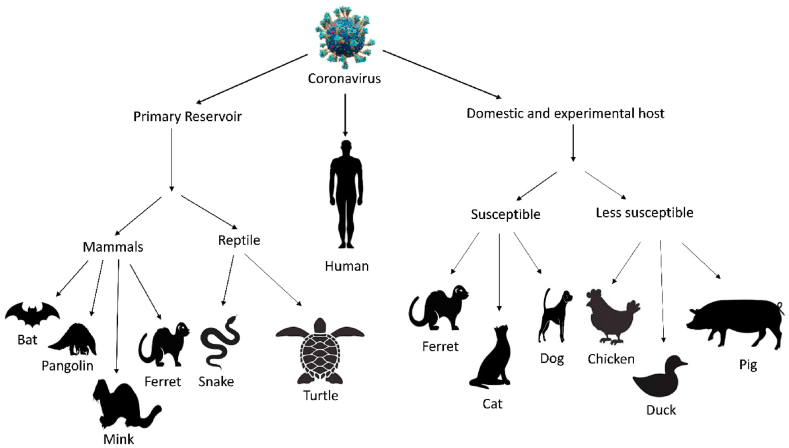
At the primary stage of the outbreak of SARS-CoV-2, the virus was regarded as a human-manipulated or laboratory-made virus [134]. After analyzing the whole genome, the bat is suspected as an intermediate host of SARS-CoV-2. Bat can carry different viruses without infection [135,136]. Currently, it has been found that SARS-CoV-2 and Bat CoV RaTG13 (a virus strain identified from Rhinolophus affinis bat) share 96% genomic homology. Another bat species, Rhinolophus yunnanensis, exhibit 96.2% identical sequence with SARS-CoV-2 [137]. So, it is clear that the origin and natural reservoir of SARS-CoV-2 is a bat. However, direct human-to-bat interaction is infrequent. So, it has been speculated that spillover of SARS-CoV-2 to humans from an intermediate host might occur rather than direct transmission from bat to human (Fig. 4). Hence, no specific animal origin as the intermediate host cannot be identified with rigid information till now [138].
Fig. 4.
Potential animals hosts of SARS-CoV-2.
5. Wildlife as the intermediate host
5.1. Pangolin (Pangolin-CoV)
Pangolin-CoV's whole genome sequence reveals that Malayan pangolin (Manis javanica) shows 85–92% nucleotide sequence homology with SARS-COV-2 and bat CoV [[138], [139],140]. Pangolin is a nocturnal animal species widely used in South Asia, not in China, for its medicinal property in Traditional Chinese medicine. Outbreaks are first reported in China during SARS-CoV-2 [141,142]. In China, this endangered species, their meat, and dried powder are being sold at lucrative prices [143]. They are illegally smuggled into China by wildlife traffickers for the black-market trade and are named Pangolin –CoV [10,144,145].
The pangolin-CoV (MP789) isolates shared the highest genetic resemblance with SARS-CoV-2 than any other pangolin species [146,147]. Even in the case of ACE 2 binding, it shows more affinity than bat CoV [[148], [149], [150]]. Pangolin-CoV possesses five amino acids identical to SARS-CoV-2, but only one amino acid resembles in both bat CoV and SARS-CoV-2 [151,152]. The receptor Binding Domain (RBD) of pangolin CoV 2 and SARS-CoV-2 are quite similar and have the strongest affinity for binding with the ACE2 protein of humans [70]. The RBD of the S protein from pangolin-CoV shows 97.4% amino acid sequence similarity with SARS-CoV-2 [153]. In a study, it has been found that the envelope, membrane, nucleocapsid, and spike protein of pangolin CoV, isolated from 17 to 25 Malayalam pangolins, exhibit 90.7–100% amino acid identity with SARS-CoV-2 [154]. However, from phylogenetic analysis, it has also been found that the source of SARS-CoV-2 is not directly from Pangolin CoV2 [152,155]. The speculation indicates that the natural origin of SARS-CoV-2 is bat-CoV-RaTG13, and later it is transmitted to pangolin as an intermediate host followed by the recombination of bat-CoV (RaTG13) genomic background [156,157] [24].
5.2. Mink
According to a virus-host prediction (VHP) based study by Guo et al. (2020), bat and mink coronaviruses possess almost similar pathogenic cycle to SARS-CoV-2. Mink has been domesticated primarily in Netherlands farms because of its fur. An outbreak started on a farm in the Netherlands on April 23, 2020 and expanded over time to other European countries nearby and the United States and Canada [158]. An investigation reveals that the virus has been transmitted from a worker infected by COVID-19 [159]. Another study reports that at least two employees contracted COVID-19 after consuming mink milk, demonstrating the likelihood of the virus spreading from animal to human and animal to animal [160]. Because of its transmission capability, infection in mink has become a serious and severe problem worldwide [161,162]. Mink is suspected because the mink virus can be mutated and transferred to humans, which might be lethal [163]. However, a recent study showed that mutations in the mink virus reduce the lethality and infectivity of the virus in humans. Some mink viruses that have been found in mink can be a good source for designing effective vaccines [164].
5.3. Reptile
According to the RBD and ACE 2 receptor interaction, turtles such as Chrysemys picta bellii, Chelonia mydas, and Pelodiscus sinensis have been considered potential intermediate hosts of SARS-CoV-2 [165]. In another experiment, the same scientist found that the snake coronavirus could gain the ability to cause disease in humans by homologous recombination [166]. While Zhang C. et al. (2020) proved that not a snake but a frog might be an intermediate host based on RCSU analysis [167]. However, recently it has been found that ACE2 of snakes and turtles lost their affinity to bind to the S protein of SARS-CoV-2, suggesting reptiles cannot be a potential intermediate host of SARS-CoV-2 [168].
5.4. Ferret
A recent investigation revealed the susceptibility of a ferret to SARS-CoV-2 [169]. Ferret has been extensively studied in the laboratory to study the pathogenesis of respiratory influenza A virus (IAV), and its transmission to other ferrets and animals [170]. Type-II alveolar and glandular epithelial cells of the ferret's trachea and bronchi contain ACE2, the preferred host receptor of SARS [171]. SARS-CoV-2 in ferrets replicates very fast in both the upper and lower respiratory tract, resulting in increased body temperature, sneezing, and alveolar damage [172,173]. Infection occurs after four days of virus shade up in nasal washes, saliva, urine, and feces, and infection is disseminated to naive ferrets by direct contact and air transmission [174,175]. Many experiments have taken place to prove the presence of SARS-CoV-2 in ferrets. In one study, SARS-CoV-2 was derived from infected ferrets' body fluids, urine, and fecal specimens [176]. Shi et al., (2020) assessed the virulence of two viruses isolated from the samples collected from the Wuhan seafood market and a possible symptomatic patient [177]. They also inoculated the virus into the upper respiratory tract of the ferret. However, no traces of the virus have been found in the trachea, lungs, heart, liver, spleen, kidneys, pancreas, small intestine, and brain afterward [178]. Kim et al. (2020) reported pulmonary histopathological changes in ferrets contacted with SARS-CoV-2 directly or indirectly [179]. However, a study confirmed ACE2 was not actively involved in attachment by SARS-CoV-2, and the affinity is relatively insignificant compared to humans [180].
6. Domestic animals and feral carnivores as the intermediate host
Being suspected of hosting SARS-CoV-2, several domestic animals were experimentally exposed to the virus through the intranasal route. Cats and ferrets were found to be highly susceptible to SARS-CoV-2, while dogs showed less susceptibility to SARS-CoV-2 [177]. Raccoon dogs, however, are considered an animal reservoir for SARS-CoV-2 transmission [181]. However, chickens, ducks, and pigs showed lower susceptibility [[182], [183], [184]].
SARS-CoV-2 has been detected multiple times in cats. Research at Harbin Veterinary Research Institute concluded that a cat could be infected with SARS-CoV-2 and pose a potential threat of viral spread to other cats [185]. In an experiment on Chinese sub-adult cats, after inoculation of the virus, viral particles were detected in different parts of the respiratory tract (tracheas, lungs, nasal cavity), soft palates of mouth, as well as in the small intestines of euthanized cats, and the virus shedding has also been detected in other cats examining their rectal swabs and fecal samples. Severe lesions in the upper and lower respiratory tracts, including the lungs, have been discovered, and seroconversion and neutralizing antibodies have been monitored in exposed cats [184]. SARS-CoV-2 has been detected in cats' upper respiratory tracts in Belgium and Hong Kong with no signs of severe diseases or death [184,186]. However, in a study conducted in France, nine cats were kept in close contact with COVID-19-positive coworkers, but no viral shedding was found from the cats, while the coworkers were also found positive. So, it is tough to draw a general conclusion. Hence, based on different experiments and the evidence of viral load in inoculated and exposed cats, cats are regarded as animal models that are susceptible to SARS-CoV-2 and may potentially transmit the virus to other mammals.
In an experimental study, no traces of SARS-CoV-2 have been found in chicken and ducks' bodies. All swabs, organ samples, and contact animals isolated from chickens and ducks were negative [187]. Another experiment has been done with poultry animals (chickens, turkeys, ducks, quail, and geese). The result indicated no symptoms of COVID-19, and no disease and virus replication had been detected [188]. An embryonated hen's eggs are contaminated with SARS-CoV-2 and conventional influenza virus in a recent experiment. Later, they did not find any disease transmission through generation; after a few passages, no traces were found [189]. Pigs' conventional piglets are inoculated with SARS-CoV-2 via intranasal, intratracheal, intramuscular, and intravenous routes. No viral replication has been observed, although seroconversion has been detected parentally. Thus, pigs are considered a potential agent of vaccine designing [190].
7. Phylogenetic analysis of host receptors and major viral proteins
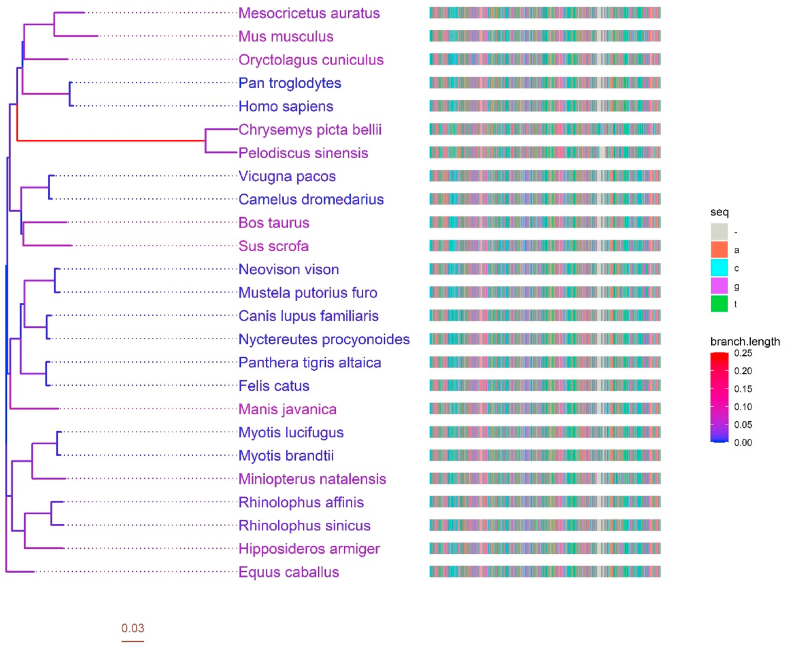
Host receptors coding for angiotensin-converting enzyme 2 (ACE2) have been reported to play a vital role in interacting with viral spike protein in cellular penetration. Thus, animals reported for potential SARS-CoV-2 transmission or susceptibility were analyzed for their similarity in the gene sequence of the ACE2 coding region. While turtles (incorporated as outgroup for phylogenetic analysis), rarely reported for SARS-CoV-2, showed the highest evolutionary distance, other species were close to each other in the context of evolutionary divergence. Such data was also supported by a separate pairwise distance matrix calculation, visualized in a heatmap associated with a dendrogram.
As seen in the phylogenetic analysis and sequence alignment, the human ACE2 receptor shares a fair amount of conserved region with bats, pangolin, cats, European rabbits, pigs, and cattle and also showed similarity with other species (Fig. 5). These data also show that the ACE2 coding region has been conserved in these species over the evolutionary periods, indicating the inter-species transmission of coronavirus infection and susceptibility of these animals to the viral infection.
Fig. 5.
Phylogenetic analysis of the angiotensin-converting enzyme 2 (ACE2) receptor gene sequence in different mammals, including humans. The ACE2 sequences were downloaded from the NCBI nucleotide database (https://www.ncbi.nlm.nih.gov/nuccore). The tree was generated using the ggtree package, and the multiple sequence alignment (MSA) was generated using the MSA package in R, with each color in the MSA representing a nucleotide.
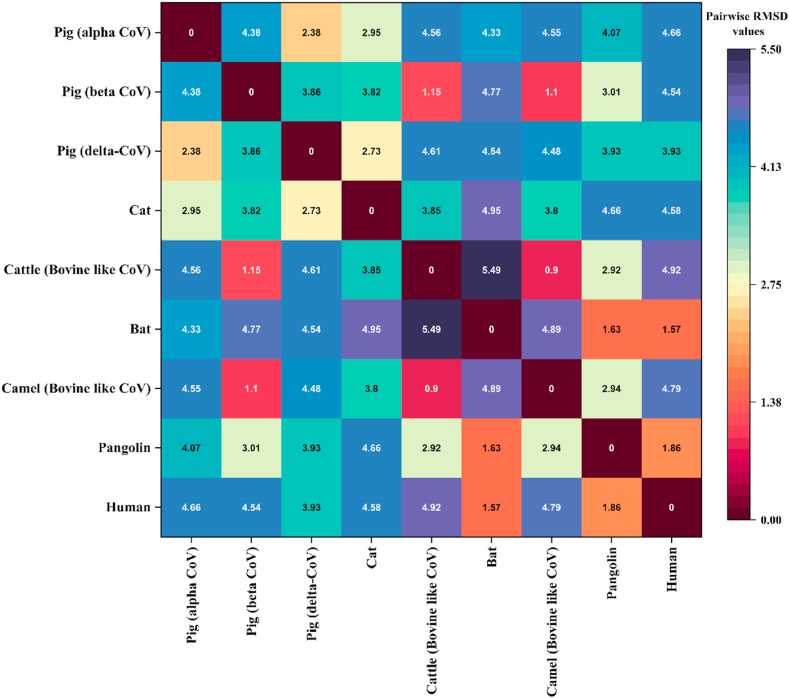
Despite the phylogenetic position, humans have a lower pairwise distance with Brandt's bat (Myotis brandtii), which also shares a more downward pairwise distance with camel (Camelus dromedaries)- one of the prime suspects of MERS-CoV in 2012. Another feature revealed from this evolutionary distance matrix analysis was that pangolin (Manis javanica), suspected as an intermediate host of SARS-CoV-2, has a lower distance from camels, alpaca and raccoon dogs, each of which has been reported as the potential zoonotic origin of SARS-CoV-2 (Fig. 6).
Fig. 6.
Dendrogram and heatmap depicting pairwise distance of the ACE2 gene sequence among animals, including humans. The sequence of ACE2 coding genes was downloaded from the NCBI nucleotide database (https://www.ncbi.nlm.nih.gov/nuccore). The pairwise distance matrix was calculated using the Tamura-Nei model with a gamma parameter (α) of 1.00 for rate variation among sites. Analyses were conducted using MEGA X software, and the heat map visualization with dendrogram analysis was performed using ‘Heatmap with Dendrogram’ in OriginLab 2021 pro.
8. Protein-protein docking simulation of major viral proteins with human ACE2 receptor
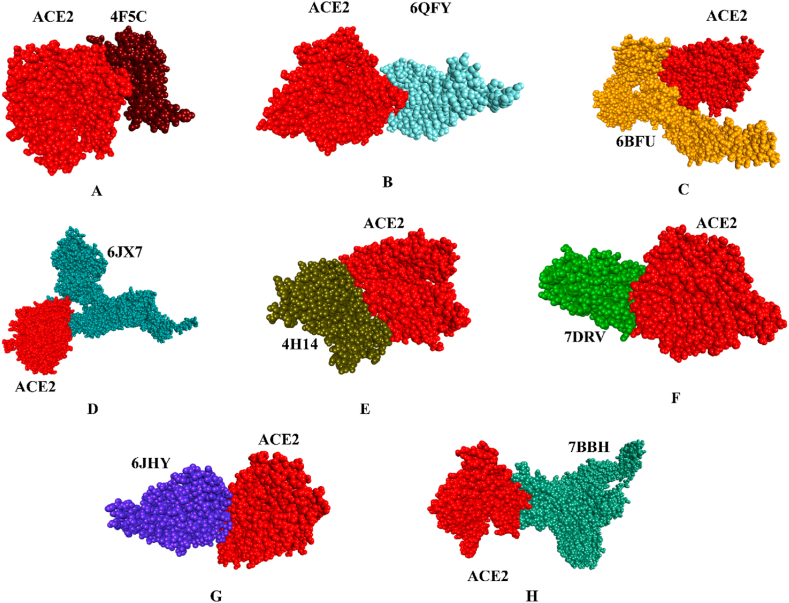
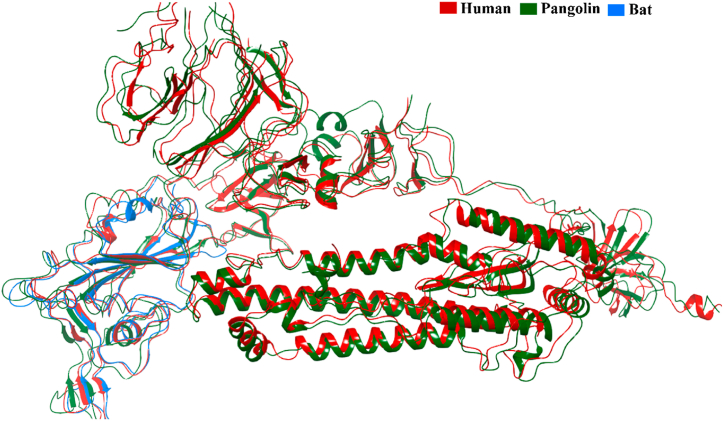
To identify the possible evolutionary notion of animals, as mentioned earlier, as primary or intermediate hosts of the SARS virus to human susceptibility, we had been rigorously docking the spike glycoprotein of the virus that three-dimensional structure synthesized from each of the respective animals with human ACE2 (PDB ID - 1R42: A) protein. Based on the availability of the crystal structure, we selected spike protein of Porcine respiratory coronavirus (PRCV) (4F5C:C), Porcine hemagglutinating encephalomyelitis virus (pHEV) (6QFY:A), and Porcine delta-coronavirus (PDCoV) (6BFU:A) from the pig; Feline coronavirus from cats (6JX7:A); Bovine coronavirus (BCoV) (4H14:A) from cattle; Bat coronavirus (7DRV:C) from mice and BCoV-like dromedary camel CoV UAE-HKU-23 (6JHY:A) from the camel. A web server tool called pyDock (https://life.bsc.es/pid/pydockweb) was used to conduct protein-protein molecular docking experiments to investigate the binding process. Wherein alpha CoV of pig manifested the best docking score of −38.713 kcal/mol, followed by rat coronavirus (−34.729 kcal/mol) and pangolin coronavirus (−35.89 kcal/mol) with human ACE2 receptor. While others articulated a −12.756 to −29.953 kcal/mol score (Table 2). Fig. 7 displayed all of the binding poses of protein-protein interaction. That imminent association brings about the possibility of mostly prone evolutionary invasion of bovine-like CoV (Fig. 7E), porcine respiratory coronavirus (PRCV) (Fig. 7A), and bat coronavirus toward humans (Fig. 7F). Their respective spike glycoproteins superimposed structure analysis unraveled those sequences closed affinities demonstrating lower RMSD values <2 Å than others. These are unfolded in Fig. 8, Fig. 9. Contrarily, other proteins also have an excellent affinity to human ACE2 receptors, which cannot be neglected. There was an unfathomable report of the presence of SARS-CoV in Camelus dromedaries [191]. Thus, our findings lend credence to SARS-CoV-2 may have evolved from a fusion of sequences already present in bats and pangolins consistent with previous scientific findings [192,193].
Table 2.
Protein-protein docking interaction among different SARS CoV with human ACE2 receptor.
| Animals | PDB ID | Score | Electrostatics | Desolvation | Vdw |
|---|---|---|---|---|---|
| Pig (alpha CoV) | 4F5C:C | −38.713 | −23.676 | −14.498 | −5.385 |
| Pig (beta CoV) | 6QFY:A | −23.517 | −21.215 | −3.892 | 15.894 |
| Pig (delta-CoV) | 6BFU:A | −21.781 | −8.343 | −12.538 | −8.994 |
| Cat | 6JX7:A | −12.756 | −7.487 | −8.21 | 29.416 |
| Cattle (Bovine like CoV) | 4H14:A | −29.953 | −25.084 | −5.328 | 4.594 |
| Bat | 7DRV:C | −34.729 | −19.612 | −20.142 | 50.252 |
| Camel (Bovine like CoV) | 6JHY:A | −24.298 | −16.936 | −8.652 | 12.904 |
| Pangolin | 7BBH:A | −35.89 | −14.169 | −22.66 | 9.391 |
| Human | 7CAB:A | −20.639 | −19.305 | −14.284 | 34.499 |
Fig. 7.
Protein-protein docking simulating interaction, (A) 1R42-4F5C:C; (B) 1R42-6QFY:A; (C) 1R42-6BFU:A; (D) 1R42-6JX7:A; (E) 1R42-4H14:A; (F) 1R42-7DRV:C; (G) 1R42-6JHY:A; (H) 1R42-7BBH:A.
Fig. 8.
Pairwise RMSD values among different animals' spike glycoprotein structure of coronavirus.
Fig. 9.
Superimposed spike glycoprotein structure of coronavirus among human, pangolin and bat.
Meanwhile, one filed survey postulated that sporadic infections could not be ruled out among pigs, although the widespread spread of SARS-CoV-2 is quite improbable [194]. Our findings significantly visualized the pig's spike glycoprotein's excellent binding affinities with human ACE2 receptors than others. That may also be a prediction for the next coronavirus animal host.
9. One health approach towards SARS-COV-2 infection
The One Health Approach is a transdisciplinary integrated disease prevention and management strategy that places equal emphasis on the health of animals and humans and the health of the surrounding ecosystem [195]. The “One Health” approach is a theory that connects the well-being of humans to that of closely related species as well as to the environmental niche that we all occupy [196]. Because of the COVID-19 pandemic, we must reevaluate the “One health approach” in order to include the idea of tackling the most recent pandemic we have been dealing with over the course of the previous two years.
One health approach may be split into two distinct categories, each of which is intricately linked to the overarching environmental and ethical framework. The first method is known as the Prudential One Health Approach (POHA). This method takes an all-encompassing approach to disease prevention and treatment, but it never loses sight of the importance of the individual patient, even if just subconsciously. It marks a significant step forward in protecting human health as well as some environmental variables that are beneficial to the well-being of certain other living beings. The second strategy is known as the Radical One Health Approach (ROHA), and it takes a holistic view of both the natural environment and the living ecosystem, rather than focusing just on the perspective of humans. It requires true epistemological and ethical transformation, which does not seem straightforward to execute via rational policy.
Since SARS-CoV-2 is a zoonotic virus transmitted between people and animals, one health approach can be a great tool to surveil and approach disease control [197]. It causes respiratory disease and can easily navigate the interspecies barrier [198,199]. There are significant cases where animals were also infected by this virus worldwide [178]. Various species were infected by this virus, from household pets to wildlife [200]. The rise in the human population, which made it possible for people to settle in previously deserted areas, is only one of the factors that has contributed to a change in how people and other animals interact with one another and their environment [201,202]. Animals become integral to our daily lives, whether for subsistence, transportation, work, recreation, or other reasons. Being in such close quarters with animals raised the risk of contracting zoonotic infections, which are diseases formerly seen exclusively in one or a few species of animals. Second, the planet's climate is shifting drastically because of widespread deforestation and widespread industrial farming. These alterations to the environment and the natural habitats of animals are another factor that puts people and animals closer together, which may lead to the spread of infection from animals to humans [203]. The increased mobility of humans, animals, and animal products is largely attributable to international travel and commerce expansion. Because of this, infectious illnesses may quickly go from one nation to another [204,205]. Hence, personalized immunity demands more emphasis on preventing such zoonotic viral pandemics. Nutritional and dietary supplements can enhance both animal and human immunity, resulting in less severe symptoms during viral infections [206]. Moreover, they can also interfere with viral replication and host cellular mechanisms [207]. Since nutritional interventions require a long time to contribute to the whole population, they will not exert immediate response. However, we can expect a positive boost in personal immunity in the long run. Coronaviruses are known for their genomic diversity and rapid mutation strategy facilitating their inter and intra-species zoonosis. Hence, focusing only on the human coronavirus will not be enough to address this global problem. The prevention and control of animal coronavirus warrants increased attention in this regard. Several natural phytochemicals have been reported for their antiviral activity against different animal coronaviruses [208]. For example-quercetin, griffithsin, terpene derivatives, flavonoids and lectins are all reported for their inhibitory action against viral replication in animals hosts. In vitro and in vivo experiments to confirm these natural products' therapeutic efficiency are highly recommended to find active antiviral compounds.
We have a continual interaction with the biosphere that surrounds us, which includes both the animate (pets or other animals we nurture for their meat, milk, or eggs) and inanimate worlds (plants and herbs we produce for nourishment). In contrast, an ecosystem is the interrelationship between various components of the environment, both living and nonliving, that are visible to the naked eye. On the other hand, recent discoveries have shown that everything in people, animals, and plants is a link in an unbroken chain that extends from the macro to the microenvironment. This has led us to regard a single health strategy as a crucial instrument to combat zoonotic pandemics.
10. Conclusion
Numerous wild animals and domestic animals, which serve as natural reservoirs or intermediate hosts, have been shown to harbor the SARS-CoV-2 virus. The spike glycoproteins of the coronaviruses that are carried by these animals and by human coronaviruses have a number of major structural similarities to one another. In addition, these spike proteins' affinity for human ACE2 receptors is significantly increased. According to our findings of this structure- and genomics-based comprehensive research, the spike proteins of human, bat, and pangolin coronaviruses all share a considerable structural similarity. Phylogenetic analysis and pairwise evolutionary distance analysis showed a significant similarity between the human ACE2 receptor and that of chimpanzees, domestic rabbits, house mice, and golden hamsters. The findings of the molecular docking analysis indicate that the spike protein of pig coronavirus exhibits a greater affinity for human ACE2 receptors compared to those of bat and pangolin. Since the bat was shown to be a possible source of transmission in the most recent COVID epidemic, pigs and pangolins, have the potential to become the next animal reservoir that may be accountable for any forthcoming human coronavirus outbreaks. Hence, one health approach and a particular concern are needed to monitor any unusual mutation in these animal coronaviruses. Genetic surveillance and such metagenomic analysis might help us address the next pandemic and help researchers and healthcare professionals to take proper preventive measures.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article. </p>
Data availability statement
Data will be made available on request.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are obliged to the Ethnobotany and Pharmacognosy Lab, Department of Botany, University of Chittagong, Bangladesh, for providing all the laboratory facilities to complete this study.
References
- 1.Johansen M.D., Irving A., Montagutelli X., Tate M.D., Rudloff I., Nold M.F., Hansbro N.G., Kim R.Y., Donovan C., Liu G., Faiz A., Short K.R., Lyons J.G., McCaughan G.W., Gorrell M.D., Cole A., Moreno C., Couteur D., Hesselson D., Triccas J., Neely G.G., Gamble J.R., Simpson S.J., Saunders B.M., Oliver B.G., Britton W.J., Wark P.A., Nold-Petry C.A., Hansbro P.M. Animal and translational models of SARS-CoV-2 infection and COVID-19. Mucosal Immunol. 2020;13:877–891. doi: 10.1038/s41385-020-00340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez‐Morales A.J., Dhama K., Sharun K., Tiwari R., Bonilla‐Aldana D.K. Susceptibility of felids to coronaviruses. Vet. Rec. 2020;186 doi: 10.1136/vr.m1671. [DOI] [PubMed] [Google Scholar]
- 3.Malik Y.S., Sircar S., Bhat S., Sharun K., Dhama K., Dadar M., Tiwari R., Chaicumpa W. Emerging novel coronavirus (2019-nCoV)-current scenario, evolutionary perspective based on genome analysis and recent developments. Vet. Q. 2020;40:68–76. doi: 10.1080/01652176.2020.1727993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.A.A. Rabaan, S.H. Al-Ahmed, S. Haque, R. Sah, R. Tiwari, Y.S. Malik, K. Dhama, M.I. Yatoo, D.K. Bonilla-Aldana, A.J. Rodriguez-Morales, SARS-CoV-2, SARS-CoV, and MERS-COV: A comparative overview, Infez Med. 28 (n.d.) 174–184.. [PubMed]
- 5.Damas J., Hughes G.M., Keough K.C., Painter C.A., Persky N.S., Corbo M., Hiller M., Koepfli K.P., Pfenning A.R., Zhao H., Genereux D.P., Swofford R., Pollard K.S., Ryder O.A., Nweeia M.T., Lindblad-Toh K., Teeling E.C., Karlsson E.K., Lewin H.A. Broad host range of SARS-CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates. Proc. Natl. Acad. Sci. U. S. A. 2020;117:22311–22322. doi: 10.1073/pnas.2010146117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao J., Cui W., Tian B. The potential intermediate hosts for SARS-CoV-2. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.580137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahdy M.A.A., Younis W., Ewaida Z. An overview of SARS-CoV-2 and Animal infection. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.596391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., Zhong W., Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leroy E.M., Ar Gouilh M., Brugère-Picoux J. The risk of SARS-CoV-2 transmission to pets and other wild and domestic animals strongly mandates a one-health strategy to control the COVID-19 pandemic. One Health. 2020;10 doi: 10.1016/j.onehlt.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhama K., Khan S., Tiwari R., Sircar S., Bhat S., Malik Y.S., Singh K.P., Chaicumpa W., Bonilla-Aldana D.K., Rodriguez-Morales A.J. Coronavirus disease 2019-COVID-19. Clin. Microbiol. Rev. 2020;33 doi: 10.1128/CMR.00028-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han Q., Lin Q., Jin S., You L. Coronavirus 2019-nCoV: A brief perspective from the front line. J. Infect. 2020;80:373–377. doi: 10.1016/j.jinf.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. China novel coronavirus investigating and research team, A novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2019;382(2020):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin. Microbiol. Infect. 2020;26:729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization, Coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed January 20, 2023).
- 17.Ghosh S., Malik Y.S. Drawing comparisons between SARS-CoV-2 and the Animal coronaviruses. Microorganisms. 2020;8:1840. doi: 10.3390/microorganisms8111840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiwari R., Dhama K., Sharun K., Iqbal Yatoo M., Malik Y.S., Singh R., Michalak I., Sah R., Bonilla-Aldana D.K., Rodriguez-Morales A.J. COVID-19: animals, veterinary and zoonotic links. Vet. Q. 2020;40:169–182. doi: 10.1080/01652176.2020.1766725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shehata A.A., Parvin R., Nagy A., Wang Y., Azhar T.M., Attia Y.A., Azhar E.I., Paul A.K., Rahmatullah M. An overview of the ongoing challenges in SARS-CoV-2 global control. Ger. J. Microbiol. 2021;1:1–18. [Google Scholar]
- 20.Parvin R., Hossain I., Hasan A., Afrin S.Z., Shehata A.A. Influenza and coronavirus zoonoses: an overview on pandemic events, viral genome, replication and emergency preparedness. Ger. J. Microbiol. 2022;2:1–11. 10.51585/gjm.2022.3.0016. [Google Scholar]
- 21.Ji W., Wang W., Zhao X., Zai J., Li X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J. Med. Virol. 2020;92:433–440. doi: 10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leroy E.M., Ar Gouilh M., Brugère-Picoux J. The risk of SARS-CoV-2 transmission to pets and other wild and domestic animals strongly mandates a one-health strategy to control the COVID-19 pandemic. One Health. 2020;10 doi: 10.1016/j.onehlt.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tiwari R., Dhama K., Sharun K., Iqbal Yatoo M., Malik Y.S., Singh R., Michalak I., Sah R., Bonilla-Aldana D.K., Rodriguez-Morales A.J. COVID-19: animals, veterinary and zoonotic links. Vet. Q. 2020;40:169–182. doi: 10.1080/01652176.2020.1766725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao J., Cui W., Tian B. The potential intermediate hosts for SARS-CoV-2. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.580137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharun K., Dhama K., Pawde A.M., Gortázar C., Tiwari R., Bonilla-Aldana D.K., Rodriguez-Morales A.J., de la Fuente J., Michalak I., Attia Y.A. SARS-CoV-2 in animals: potential for unknown reservoir hosts and public health implications. Vet. Q. 2021;41:181–201. doi: 10.1080/01652176.2021.1921311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam T.T.-Y., Jia N., Zhang Y.-W., Shum M.H.-H., Jiang J.-F., Zhu H.-C., Tong Y.-G., Shi Y.-X., Ni X.-B., Liao Y.-S., Li W.-J., Jiang B.-G., Wei W., Yuan T.-T., Zheng K., Cui X.-M., Li J., Pei G.-Q., Qiang X., Cheung W.Y.-M., Li L.-F., Sun F.-F., Qin S., Huang J.-C., Leung G.M., Holmes E.C., Hu Y.-L., Guan Y., Cao W.-C. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. 2020;583:282–285. doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- 28.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdel-Moneim A.S., Abdelwhab E.M. Evidence for SARS-COV-2 infection of animal hosts. Pathogens. 2020;9:1–27. doi: 10.3390/pathogens9070529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X., Zai J., Zhao Q., Nie Q., Li Y., Foley B.T., Chaillon A. Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J. Med. Virol. 2020;92:602–611. doi: 10.1002/jmv.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halfmann P.J., Hatta M., Chiba S., Maemura T., Fan S., Takeda M., Kinoshita N., Hattori S., Sakai-Tagawa Y., Iwatsuki-Horimoto K., Imai M., Kawaoka Y. Transmission of SARS-CoV-2 in domestic cats. N. Engl. J. Med. 2020;383:592–594. doi: 10.1056/NEJMc2013400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sit T.H.C., Brackman C.J., Ip S.M., Tam K.W.S., Law P.Y.T., To E.M.W., Yu V.Y.T., Sims L.D., Tsang D.N.C., Chu D.K.W., Perera R.A.P.M., Poon L.L.M., Peiris M. Infection of dogs with SARS-CoV-2. Nature. 2020;586:776–778. doi: 10.1038/s41586-020-2334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fenollar F., Mediannikov O., Maurin M., Devaux C., Colson P., Levasseur A., Fournier P.E., Raoult D. Mink, SARS-CoV-2, and the human-Animal interface. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.663815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McAloose D., Laverack M., Wang L., Killian M.L., Caserta L.C., Yuan F., Mitchell P.K., Queen K., Mauldin M.R., Cronk B.D., Bartlett S.L., Sykes J.M., Zec S., Stokol T., Ingerman K., Delaney M.A., Fredrickson R., Ivančić M., Jenkins-Moore M., Mozingo K., Franzen K., Bergeson N.H., Goodman L., Wang H., Fang Y., Olmstead C., McCann C., Thomas P., Goodrich E., Elvinger F., Smith D.C., Tong S., Slavinski S., Calle P.P., Terio K., Torchetti M.K., Diel D.G. From people to panthera: natural sars-cov-2 infection in tigers and lions at the bronx zoo. mBio. 2020;11:1–13. doi: 10.1128/mBio.02220-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacLachlan N.J., Dubovi E.J. In: Fenner's Veterinary Virology. fifth ed. MacLachlan N.J., F.V.V E.J.B.T., Dubovi Fifth E., editors. Academic Press; Boston: 2017. Coronaviridae; pp. 435–461. [DOI] [Google Scholar]
- 36.Otto G.M., Franklin C.L., Clifford C.B. Laboratory Animal Medicine. Elsevier; 2015. Biology and diseases of rats; pp. 151–207. [DOI] [Google Scholar]
- 37.So R.T.Y., Chu D.K.W., Miguel E., Perera R.A.P.M., Oladipo J.O., Fassi-Fihri O., Aylet G., Ko R.L.W., Zhou Z., Cheng M.-S., Kuranga S.A., Roger F.L., Chevalier V., Webby R.J., Woo P.C.Y., Poon L.L.M., Peiris M. Diversity of dromedary camel coronavirus HKU23 in african camels revealed multiple recombination events among closely related betacoronaviruses of the subgenus embecovirus. J. Virol. 2019;93:151–207. doi: 10.1128/JVI.01236-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sia S.F., Yan L.-M., Chin A.W.H., Fung K., Choy K.-T., Wong A.Y.L., Kaewpreedee P., Perera R.A.P.M., Poon L.L.M., Nicholls J.M., Peiris M., Yen H.-L. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583(7818):834–838. doi: 10.1038/s41586-020-2342-5. 583 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahdy M.A.A., Younis W., Ewaida Z. An overview of SARS-CoV-2 and Animal infection. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.596391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoskins J.D. Coronavirus infection in cats. Vet Clin North Am Small Anim Pract. 1993;23:1. doi: 10.1016/S0195-5616(93)50001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.P. NC, B. JF, F. K, F. A, B. J An enteric coronavirus infection of cats and its relationship to feline infectious peritonitis. Am. J. Vet. Res. 1981;42:368–377. [PubMed] [Google Scholar]
- 42.Provacia L.B.V., Smits S.L., Martina B.E., Raj V.S., Doel P.v.d., Amerongen G.v., Moorman-Roest H., Osterhaus A.D.M.E., Haagmans B.L. Enteric coronavirus in ferrets, The Netherlands. Emerg. Infect. Dis. 2011;17:1570. doi: 10.3201/EID1708.110115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.M. J, K. M, M. RK Ferret coronavirus-associated diseases. Vet Clin North Am Exot Anim Pract. 2010;13:543–560. doi: 10.1016/J.CVEX.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li T.-C., Yoshizaki S., Kataoka M., Doan Y.H., Ami Y., Suzaki Y., Nakamura T., Takeda N., Wakita T. Determination of ferret enteric coronavirus genome in laboratory ferrets. Emerg. Infect. Dis. 2017;23:1568–1570. doi: 10.3201/eid2309.160215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Y., Wang J., Kuang D., Xu J., Yang M., Ma C., Zhao S., Li J., Long H., Ding K., Gao J., Liu J., Wang H., Li H., Yang Y., Yu W., Yang J., Zheng Y., Wu D., Lu S., Liu H., Peng X. Susceptibility of tree shrew to SARS-CoV-2 infection. Sci. Rep. 2020;10:1–10. doi: 10.1038/s41598-020-72563-w. (2020) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim L., Hayes J., Lewis P., Parwani A.V., Chang K.O., Saif L.J. Molecular characterization and pathogenesis of transmissible gastroenteritis coronavirus (TGEV) and porcine respiratory coronavirus (PRCV) field isolates co-circulating in a swine herd. Arch. Virol. 2000;145:1133–1147. doi: 10.1007/S007050070114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brockmeier S.L., Loving C.L., Nicholson T.L., Palmer M.V. Coinfection of pigs with porcine respiratory coronavirus and Bordetella bronchiseptica. Vet. Microbiol. 2008;128:36–47. doi: 10.1016/J.VETMIC.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saif L.J., Wang Q., Vlasova A.N., Jung K., Xiao S. 2019. Coronaviruses, Diseases of Swine; pp. 488–523. [DOI] [Google Scholar]
- 49.Moutelikova R., Prodelalova J. First detection and characterisation of porcine hemagglutinating encephalomyelitis virus in the Czech Republic. Vet Med (Praha) 2019;64:60–66. doi: 10.17221/95/2018-VETMED. [DOI] [Google Scholar]
- 50.Mora-Díaz J.C., Piñeyro P.E., Houston E., Zimmerman J., Giménez-Lirola L.G. Porcine hemagglutinating encephalomyelitis virus: A review. Front. Vet. Sci. 2019;6 doi: 10.3389/fvets.2019.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boley P.A., Alhamo M.A., Lossie G., Yadav K.K., Vasquez-Lee M., Saif L.J., Kenney S.P. Porcine deltacoronavirus infection and transmission in poultry, United States - volume 26, number 2—february 2020 - emerging infectious diseases journal - CDC. Emerg. Infect. Dis. 2020;26:255–264. doi: 10.3201/EID2602.190346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu H., Jung K., Vlasova A.N., Chepngeno J., Lu Z., Wang Q., Saif L.J. Isolation and characterization of porcine deltacoronavirus from pigs with diarrhea in the United States. J. Clin. Microbiol. 2015;53:1537–1548. doi: 10.1128/JCM.00031-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Z. J, C. J, L. Y, D. S, S. H, Z. X, L. J, C. L, Z. X, W. X, J. Z, F. L Pathogenicity of porcine deltacoronavirus (PDCoV) strain NH and immunization of pregnant sows with an inactivated PDCoV vaccine protects 5-day-old neonatal piglets from virulent challenge. Transbound Emerg Dis. 2020;67:572–583. doi: 10.1111/TBED.13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Decaro N., Lorusso A. Novel human coronavirus (SARS-CoV-2): A lesson from animal coronaviruses. Vet. Microbiol. 2020;244 doi: 10.1016/J.VETMIC.2020.108693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crossley B., Mock R., Callison S., Hietala S. Identification and characterization of a novel Alpaca respiratory coronavirus most closely related to the human coronavirus 229E. Viruses. 2012;4:3689–3700. doi: 10.3390/v4123689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castilla D., Escobar V., Ynga S., Llanco L., Manchego A., Lázaro C., Navarro D., Santos N., Rojas M. Enteric viral infections among domesticated South American camelids: first detection of mammalian orthoreovirus in camelids. Animals. 2021;11:1455. doi: 10.3390/ani11051455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luna L., Brandão P.E., Maturrano L., Rosadio R., Silva F.D.F., Soares R.M., Gregori F. Betacoronavirus 1 in alpacas (Vicugna pacos) in the high Peruvian andes. Small Rumin. Res. 2015;133:7. doi: 10.1016/J.SMALLRUMRES.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woo P., Lau S., Fan R., Lau C., Wong E., Joseph S., Tsang A., Wernery R., Yip C., Tsang C.-C., Wernery U., Yuen K.-Y. Isolation and characterization of dromedary camel coronavirus UAE-HKU23 from dromedaries of the Middle East: minimal serological cross-reactivity between MERS coronavirus and dromedary camel coronavirus UAE-HKU23. Int. J. Mol. Sci. 2016;17:691. doi: 10.3390/ijms17050691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Corman V.M., Eckerle I., Memish Z.A., Liljander A.M., Dijkman R., Jonsdottir H., Ngeiywa K.J.Z.J., Kamau E., Younan M., Al Masri M., Assiri A., Gluecks I., Musa B.E., Meyer B., Müller M.A., Hilali M., Bornstein S., Wernery U., Thiel V., Jores J., Drexler J.F., Drosten C. Link of a ubiquitous human coronavirus to dromedary camels. Proc. Natl. Acad. Sci. USA. 2016;113:9864–9869. doi: 10.1073/PNAS.1604472113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woo P., Lau S., Fan R., Lau C., Wong E., Joseph S., Tsang A., Wernery R., Yip C., Tsang C.-C., Wernery U., Yuen K.-Y. Isolation and characterization of dromedary camel coronavirus UAE-HKU23 from dromedaries of the Middle East: minimal serological cross-reactivity between MERS coronavirus and dromedary camel coronavirus UAE-HKU23. Int. J. Mol. Sci. 2016;17:691. doi: 10.3390/ijms17050691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Omrani A.S., Al-Tawfiq J.A., Memish Z.A. Middle east respiratory syndrome coronavirus (Mers-coV): Animal to human interaction. Pathog. Glob. Health. 2015;109:354–362. doi: 10.1080/20477724.2015.1122852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reusken C.B.E.M., Haagmans B.L., Koopmans M.P.G. Dromedary camels and Middle East respiratory syndrome: MERS coronavirus in the “ship of the desert”. Ned. Tijdschr. Geneeskd. 2014;158:A7806. [PubMed] [Google Scholar]
- 63.Eckstein S., Ehmann R., Gritli A., Ben Yahia H., Diehl M., Wölfel R., Ben Rhaiem M., Stoecker K., Handrick S., Ben Moussa M. Prevalence of Middle East respiratory syndrome coronavirus in dromedary camels, Tunisia. Emerg. Infect. Dis. 2021;27:1964–1968. doi: 10.3201/eid2707.204873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hasoksuz M., Kayar A., Dodurka T., Ilgaz A. Detection of respiratory and enteric shedding of bovine coronaviruses in cattle in northwestern Turkey. Acta Vet. Hung. 2005;53:137–146. doi: 10.1556/AVet.53.2005.1.13. [DOI] [PubMed] [Google Scholar]
- 65.Vlasova A.N., Saif L.J. Bovine coronavirus and the Associated diseases. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.643220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saif L.J. Bovine respiratory coronavirus, veterinary clinics of North America. Food Animal Practice. 2010;26:349–364. doi: 10.1016/j.cvfa.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zappulli V., Ferro S., Bonsembiante F., Brocca G., Calore A., Cavicchioli L., Centelleghe C., Corazzola G., De Vreese S., Gelain M.E., Mazzariol S., Moccia V., Rensi N., Sammarco A., Torrigiani F., Verin R., Castagnaro M. Pathology of coronavirus infections: A review of lesions in Animals in the one-health perspective. Animals. 2020;10:2377. doi: 10.3390/ani10122377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pass D., Penhale W., Wilcox G., Batey R. Intestinal coronavirus-like particles in sheep with diarrhoea. Vet. Rec. 1982;111:106–107. doi: 10.1136/vr.111.5.106. [DOI] [PubMed] [Google Scholar]
- 69.Watanabe S., Masangkay J.S., Nagata N., Morikawa S., Mizutani T., Fukushi S., Alviola P., Omatsu T., Ueda N., Iha K., Taniguchi S., Fujii H., Tsuda S., Endoh M., Kato K., Tohya Y., Kyuwa S., Yoshikawa Y., Akashi H. Bat coronaviruses and experimental infection of bats, the Philippines. Emerg. Infect. Dis. 2010;16:1217–1223. doi: 10.3201/eid1608.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lam T.T.-Y., Jia N., Zhang Y.-W., Shum M.H.-H., Jiang J.-F., Zhu H.-C., Tong Y.-G., Shi Y.-X., Ni X.-B., Liao Y.-S., Li W.-J., Jiang B.-G., Wei W., Yuan T.-T., Zheng K., Cui X.-M., Li J., Pei G.-Q., Qiang X., Cheung W.Y.-M., Li L.-F., Sun F.-F., Qin S., Huang J.-C., Leung G.M., Holmes E.C., Hu Y.-L., Guan Y., Cao W.-C. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. 2020;583:282–285. doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- 71.Sikdar S., Das T., Sajib E.H., Rahman K.M.U., Siddiki A.Z., Uddin M.B. Multi-OMICS and molecular biology perspective in Buffalo genome. J. Buffalo Sci. 2021;10:21–31. [Google Scholar]
- 72.Zhou Z., Qiu Y., Ge X. The taxonomy, host range and pathogenicity of coronaviruses and other viruses in the Nidovirales order. Animal Diseases. 2021;1:1–28. doi: 10.1186/s44149-021-00005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Woo P.C.Y., Lau S.K.P., Lam C.S.F., Lau C.C.Y., Tsang A.K.L., Lau J.H.N., Bai R., Teng J.L.L., Tsang C.C.C., Wang M., Zheng B.-J., Chan K.-H., Yuen K.-Y. Discovery of seven novel mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of Alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of Gammacoronavirus and Deltacoronavi. J. Virol. 2012;86:3995–4008. doi: 10.1128/jvi.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu I.T.S., Qiu H., Tse L.A., Wong T.W. Severe acute respiratory syndrome beyond amoy gardens: completing the incomplete legacy. Clin. Infect. Dis. 2014;58:683–686. doi: 10.1093/cid/cit797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. Features, evaluation, and treatment of coronavirus. StatPearls. 2020:19. [PubMed] [Google Scholar]
- 76.Brian D.A., Baric R.S. Coronavirus genome structure and replication. Curr. Top. Microbiol. Immunol. 2005;287:1–30. doi: 10.1007/3-540-26765-4_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16 doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Il Kim Y., Kim S.G., Kim S.M., Kim E.H., Park S.J., Yu K.M., Chang J.H., Kim E.J., Lee S., Casel M.A.B., Um J., Song M.S., Jeong H.W., Lai V.D., Kim Y., Chin B.S., Park J.S., Chung K.H., Foo S.S., Poo H., Mo I.P., Lee O.J., Webby R.J., Jung J.U., Choi Y.K. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe. 2020;27:704–709.e2. doi: 10.1016/j.chom.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Neuman B.W., Kiss G., Kunding A.H., Bhella D., Baksh M.F., Connelly S., Droese B., Klaus J.P., Makino S., Sawicki S.G., Siddell S.G., Stamou D.G., Wilson I.A., Kuhn P., Buchmeier M.J. A structural analysis of M protein in coronavirus assembly and morphology. J. Struct. Biol. 2011;174:11–22. doi: 10.1016/j.jsb.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16 doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fung T.S., Liu D.X. Human coronavirus: host-pathogen interaction. Annu. Rev. Microbiol. 2019;73:529–557. doi: 10.1146/annurev-micro-020518-115759. [DOI] [PubMed] [Google Scholar]
- 83.Luk H.K.H., Li X., Fung J., Lau S.K.P., Woo P.C.Y. Molecular epidemiology, evolution and phylogeny of SARS coronavirus. Infect. Genet. Evol. 2019;71:21–30. doi: 10.1016/j.meegid.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fuk-Woo Chan J., Kok K.-H., Zhu Z., Chu H., Kai-Wang To K., Yuan S., Yuen K.-Y. vol. 9. Taylor & Francis; 2020. pp. 221–236. (Genomic Characterization of the 2019 Novel Human-Pathogenic Coronavirus Isolated from a Patient with Atypical Pneumonia after Visiting Wuhan). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Navas-Martín S., Weiss S.R. Coronavirus replication and pathogenesis: implications for the recent outbreak of severe acute respiratory syndrome (SARS), and the challenge for vaccine development. J. Neurovirol. 2004;10:75–85. doi: 10.1080/13550280490280292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Naqvi A.A.T., Fatima K., Mohammad T., Fatima U., Singh I.K., Singh A., Atif S.M., Hariprasad G., Hasan G.M., Hassan M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim. Biophys. Acta, Mol. Basis Dis. 2020;1866 doi: 10.1016/j.bbadis.2020.165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peng Zhou, Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fung T.S., Liu D.X. Human coronavirus: host-pathogen interaction. Annu. Rev. Microbiol. 2019;73:529–557. doi: 10.1146/annurev-micro-020518-115759. [DOI] [PubMed] [Google Scholar]
- 90.Zhang C., Zheng W., Huang X., Bell E.W., Zhou X., Zhang Y. 2020. Modeling of the SARS-COV-2 Genome Using I-TASSER. [Google Scholar]
- 91.Lai M.M., Stohlman S.A. Comparative analysis of RNA genomes of mouse hepatitis viruses. J. Virol. 1981;38:661–670. doi: 10.1128/jvi.38.2.661-670.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mousavizadeh L., Ghasemi S. Genotype and phenotype of COVID-19: their roles in pathogenesis. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fehr A.R., Perlman S. Coronaviruses: Methods and Protocols. Springer; New York: 2015. Coronaviruses: An overview of their replication and pathogenesis; pp. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim D., Lee J.-Y., Yang J.-S., Kim J.W., Kim V.N., Chang H. The architecture of SARS-CoV-2 transcriptome. Cell. 2020;181:914–921.e10. doi: 10.1016/j.cell.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O'Meara M.J., Rezelj V.V., Guo J.Z., Swaney D.L., Tummino T.A., Hüttenhain R., Kaake R.M., Richards A.L., Tutuncuoglu B., Foussard H., Batra J., Haas K., Modak M., Kim M., Haas P., Polacco B.J., Braberg H., Fabius J.M., Eckhardt M., Soucheray M., Bennett M.J., Cakir M., McGregor M.J., Li Q., Meyer B., Roesch F., Vallet T., Mac Kain A., Miorin L., Moreno E., Naing Z.Z.C., Zhou Y., Peng S., Shi Y., Zhang Z., Shen W., Kirby I.T., Melnyk J.E., Chorba J.S., Lou K., Dai S.A., Barrio-Hernandez I., Memon D., Hernandez-Armenta C., Lyu J., Mathy C.J.P., Perica T., Pilla K.B., Ganesan S.J., Saltzberg D.J., Rakesh R., Liu X., Rosenthal S.B., Calviello L., Venkataramanan S., Liboy-Lugo J., Lin Y., Huang X.P., Liu Y.F., Wankowicz S.A., Bohn M., Safari M., Ugur F.S., Koh C., Savar N.S., Tran Q.D., Shengjuler D., Fletcher S.J., O'Neal M.C., Cai Y., Chang J.C.J., Broadhurst D.J., Klippsten S., Sharp P.P., Wenzell N.A., Kuzuoglu-Ozturk D., Wang H.Y., Trenker R., Young J.M., Cavero D.A., Hiatt J., Roth T.L., Rathore U., Subramanian A., Noack J., Hubert M., Stroud R.M., Frankel A.D., Rosenberg O.S., Verba K.A., Agard D.A., Ott M., Emerman M., Jura N., von Zastrow M., Verdin E., Ashworth A., Schwartz O., d'Enfert C., Mukherjee S., Jacobson M., Malik H.S., Fujimori D.G., Ideker T., Craik C.S., Floor S.N., Fraser J.S., Gross J.D., Sali A., Roth B.L., Ruggero D., Taunton J., Kortemme T., Beltrao P., Vignuzzi M., García-Sastre A., Shokat K.M., Shoichet B.K., Krogan N.J. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen Y., Guo Y., Pan Y., Zhao Z.J. Structure analysis of the receptor binding of 2019-nCoV. Biochem. Biophys. Res. Commun. 2020;525:135–140. doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peng Zhou, Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li W., Moore M., Vasilieva N., Sui J., Nature S.W., undefined . Nature.Com.; 2003. Angiotensin-converting Enzyme 2 Is a Functional Receptor for the SARS Coronavirus. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li F., Li W., Farzan M., Science S.H., undefined Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science.Sciencemag.Org. 2005 doi: 10.1126/science.1113611. n.d. [DOI] [PubMed] [Google Scholar]
- 101.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang X., You T., Liu X., Yang X., Bai F., Liu H., Liu X., Guddat L.W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 102.Yang H., Xie W., Xue X., Yang K., Ma J., Liang W., Zhao Q., Zhou Z., Pei D., Ziebuhr J., Hilgenfeld R., Kwok Y.Y., Wong L., Gao G., Chen S., Chen Z., Ma D., Bartlam M., Rao Z. Design of wide-spectrum inhibitors targeting coronavirus main proteases. PLoS Biol. 2005;3 doi: 10.1371/journal.pbio.0030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thiel V., Ivanov K.A., Kos Putics A., Hertzig T., Schelle B., Bayer S., Weißbrich B., Snijder E.J., Rabenau H., Doerr H.W., Gorbalenya A.E., Ziebuhr J. Mechanisms and enzymes involved in SARS coronavirus genome expression. Microbiologyresearch.Org. 2003;84:2305–2315. doi: 10.1099/vir.0.19424-0. [DOI] [PubMed] [Google Scholar]
- 104.Fu Z., Huang B., Tang J., Liu S., Liu M., Ye Y., Liu Z., Xiong Y., Cao D., Li J., Niu X., Zhou H., Zhao Y.J., Zhang G., Huang H. Structural basis for the inhibition of the papain-like protease of SARS-CoV-2 by small molecules. Biorxiv.Org. 2020 doi: 10.1101/2020.07.17.208959. [DOI] [Google Scholar]
- 105.Rut W., Lv Z., Zmudzinski M., Patchett S., Nayak D., Snipas S.J., El Oualid F., Huang T.T., Bekes M., Drag M., Olsen S.K. Activity profiling and structures of inhibitor-bound SARS-CoV-2-PLpro protease provides a framework for anti-COVID-19 drug design. bioRxiv. 2020 doi: 10.1101/2020.04.29.068890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Subissi L., Posthuma C.C., Collet A., Zevenhoven-Dobbe J.C., Gorbalenya A.E., Decroly E., Snijder E.J., Canard B., Imbert I. One severe acute respiratory syndrome coronavirus protein complex integrates processive RNA polymerase and exonuclease activities. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E3900–E3909. doi: 10.1073/pnas.1323705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lehmann K.C., Gulyaeva A., Zevenhoven-Dobbe J.C., Janssen G.M.C., Ruben M., Overkleeft H.S., Van Veelen P.A., Samborskiy D.V., Kravchenko A.A., Leontovich A.M., Sidorov I.A., Snijder E.J., Posthuma C.C., Gorbalenya A.E. Discovery of an essential nucleotidylating activity associated with a newly delineated conserved domain in the RNA polymerase-containing protein of all nidoviruses. Nucleic Acids Res. 2015;43:8416–8434. doi: 10.1093/nar/gkv838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mcdonald S.M. vol. 4. Wiley Interdiscip Rev RNA; 2013. pp. 351–367. (RNA Synthetic Mechanisms Employed by Diverse Families of RNA Viruses). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gao Y., Yan L., Huang Y., Liu F., Zhao Y., Cao L., Wang T., Sun Q., Ming Z., Zhang L., Ge J., Zheng L., Zhang Y., Wang H., Zhu Y., Zhu C., Hu T., Hua T., Zhang B., Yang X., Li J., Yang H., Liu Z., Xu W., Guddat L.W., Wang Q., Lou Z., Rao Z. 2020. Structure of the RNA-dependent RNA Polymerase from COVID-19 Virus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry Depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wan Y., Shang J., Graham R., Baric R.S., Li F., Wan C.Y. Receptor recognition by the novel coronavirus from Wuhan: an Analysis based on Decade-long structural studies of SARS coronavirus. Am Soc Microbiol. 2021;94 doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lai M.M.C., Cavanagh D. Elsevier; 1997. The Molecular Biology of Coronaviruses; pp. 1–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hagemeijer M.C., De Haan C.A.M. Studying the dynamics of coronavirus replicative structures. Methods Mol. Biol. 2015;1282:261–269. doi: 10.1007/978-1-4939-2438-7_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu X., Liu Y., Weiss S., Arnold E., Sarafianos S.G., Ding J. Molecular model of SARS coronavirus polymerase: implications for biochemical functions and drug design. Nucleic Acids Res. 2003;31:7117–7130. doi: 10.1093/nar/gkg916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Luk H.K.H., Li X., Fung J., Lau S.K.P., Woo P.C.Y. Molecular epidemiology, evolution and phylogeny of SARS coronavirus. Infect. Genet. Evol. 2019;71:21–30. doi: 10.1016/j.meegid.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ziebuhr J., Snijder E.J., Gorbalenya A.E. Virus-encoded proteinases and proteolytic processing in the Nidovirales. J. Gen. Virol. 2000;81:853–879. doi: 10.1099/0022-1317-81-4-853. [DOI] [PubMed] [Google Scholar]
- 117.Bos E.C.W., Luytjes W., Van Der Meulen H., Koerten H.K., Spaan W.J.M. The production of recombinant infectious DI-particles of a murine coronavirus in the absence of helper virus. Virology. 1996;218:52–60. doi: 10.1006/viro.1996.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tooze J., Tooze S., Warren G. Replication of coronavirus MHV-A59 in sac- cells: determination of the first site of budding of progeny virions. Eur. J. Cell Biol. 1984;33:281–293. [PubMed] [Google Scholar]
- 119.Lai M.M.C., Cavanagh D. Elsevier; 1997. The Molecular Biology of Coronaviruses; pp. 1–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bos E.C.W., Luytjes W., Van Der Meulen H., Koerten H.K., Spaan W.J.M. The production of recombinant infectious DI-particles of a murine coronavirus in the absence of helper virus. Virology. 1996;218:52–60. doi: 10.1006/viro.1996.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hogue B.G., Machamer C.E. Nidoviruses. ASM Press; 2014. Coronavirus structural proteins and virus Assembly; pp. 179–200. [DOI] [Google Scholar]
- 122.Naqvi A.A.T., Fatima K., Mohammad T., Fatima U., Singh I.K., Singh A., Atif S.M., Hariprasad G., Hasan G.M., Hassan M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim. Biophys. Acta, Mol. Basis Dis. 2020;1866 doi: 10.1016/j.bbadis.2020.165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., Pan P., Wang W., Hu D., Liu X., Zhang Q., Wu J. Coronavirus infections and immune responses. J. Med. Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.de Wilde A.H., Snijder E.J., Kikkert M., van Hemert M.J. Curr Top Microbiol Immunol. Springer Verlag; 2018. Host factors in coronavirus replication; pp. 1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li J., Liu Y., Zhang X. Murine coronavirus induces type I interferon in oligodendrocytes through recognition by RIG-I and MDA5. J. Virol. 2010;84:6472–6482. doi: 10.1128/JVI.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cervantes-Barragan L., Züst R., Weber F., Spiegel M., Lang K.S., Akira S., Thiel V., Ludewig B. Control of coronavirus infection through plasmacytoid dendritic-cell- derived type I interferon. Blood. 2007;109:1131–1137. doi: 10.1182/blood-2006-05-023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., Pan P., Wang W., Hu D., Liu X., Zhang Q., Wu J. Coronavirus infections and immune responses. J. Med. Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.de Wilde A.H., Snijder E.J., Kikkert M., van Hemert M.J. Curr Top Microbiol Immunol. Springer Verlag; 2018. Host factors in coronavirus replication; pp. 1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Deeks S.G., Odorizzi P.M., Sekaly R.P. The interferon paradox: can inhibiting an antiviral mechanism advance an HIV cure? J. Clin. Invest. 2017;127:103–105. doi: 10.1172/JCI91916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Channappanavar R., Zhao J., Perlman S. T cell-mediated immune response to respiratory coronaviruses. Immunol. Res. 2014;59:118–128. doi: 10.1007/s12026-014-8534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Channappanavar R., Zhao J., Perlman S. T cell-mediated immune response to respiratory coronaviruses. Immunol. Res. 2014;59:118–128. doi: 10.1007/s12026-014-8534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., Zhang X., Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Azkur A.K., Akdis M., Azkur D., Sokolowska M., van de Veen W., Brüggen M.C., O'Mahony L., Gao Y., Nadeau K., Akdis C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19, Allergy. European Journal of Allergy and Clinical Immunology. 2020;75:1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rabi F.A., Al Zoubi M.S., Kasasbeh G.A., Salameh D.M., Al-Nasser A.D. SARS-CoV-2 and coronavirus disease 2019: what We know so far. Pathogens. 2020;9:231. doi: 10.3390/pathogens9030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang G., Cowled C., Shi Z., Huang Z., Bishop-Lilly K.A., Fang X., Wynne J.W., Xiong Z., Baker M.L., Zhao W., Tachedjian M., Zhu Y., Zhou P., Jiang X., Ng J., Yang L., Wu L., Xiao J., Feng Y., Chen Y., Sun X., Zhang Y., Marsh G.A., Crameri G., Broder C.C., Frey K.G., Wang L.-F., Wang J. Comparative Analysis of bat genomes provides insight into the evolution of flight and immunity. Science. 1979;339(2013):456–460. doi: 10.1126/science.1230835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Olival K.J., Hosseini P.R., Zambrana-Torrelio C., Ross N., Bogich T.L., Daszak P. Host and viral traits predict zoonotic spillover from mammals. Nature. 2017;546:646–650. doi: 10.1038/nature22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Adhikari S.P., Meng S., Wu Y.-J., Mao Y.-P., Ye R.-X., Wang Q.-Z., Sun C., Sylvia S., Rozelle S., Raat H., Zhou H. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9:29. doi: 10.1186/s40249-020-00646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xiao K., Zhai J., Feng Y., Zhou N., Zhang X., Zou J.-J., Li N., Guo Y., Li X., Shen X., Zhang Z., Shu F., Huang W., Li Y., Zhang Z., Chen R.-A., Wu Y.-J., Peng S.-M., Huang M., Xie W.-J., Cai Q.-H., Hou F.-H., Chen W., Xiao L., Shen Y. Isolation of SARS-CoV-2-related coronavirus from Malayan pangolins. Nature. 2020;583:286–289. doi: 10.1038/s41586-020-2313-x. [DOI] [PubMed] [Google Scholar]
- 140.do Vale B., Lopes A.P., Fontes M. da C., Silvestre M., Cardoso L., Coelho A.C. Bats, pangolins, minks and other animals - villains or victims of SARS-CoV-2? Vet. Res. Commun. 2021;45:1–19. doi: 10.1007/s11259-021-09787-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wong G., Bi Y.-H., Wang Q.-H., Chen X.-W., Zhang Z.-G., Yao Y.-G. Zoonotic origins of human coronavirus 2019 (HCoV-19/SARS-CoV-2): why is this work important? Zool. Res. 2020;41:213–219. doi: 10.24272/j.issn.2095-8137.2020.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Volpato G., Fontefrancesco M.F., Gruppuso P., Zocchi D.M., Pieroni A. Baby pangolins on my plate: possible lessons to learn from the COVID-19 pandemic. J. Ethnobiol. Ethnomed. 2020;16:19. doi: 10.1186/s13002-020-00366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Volpato G., Fontefrancesco M.F., Gruppuso P., Zocchi D.M., Pieroni A. Baby pangolins on my plate: possible lessons to learn from the COVID-19 pandemic. J. Ethnobiol. Ethnomed. 2020;16:19. doi: 10.1186/s13002-020-00366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu P., Jiang J.-Z., Wan X.-F., Hua Y., Li L., Zhou J., Wang X., Hou F., Chen J., Zou J., Chen J. Are pangolins the intermediate host of the 2019 novel coronavirus (SARS-CoV-2)? PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Touati R., Haddad-Boubaker S., Ferchichi I., Messaoudi I., Ouesleti A.E., Triki H., Lachiri Z., Kharrat M. Comparative genomic signature representations of the emerging COVID-19 coronavirus and other coronaviruses: high identity and possible recombination between Bat and Pangolin coronaviruses. Genomics. 2020;112:4189–4202. doi: 10.1016/j.ygeno.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dimonaco N.J., Salavati M., Shih B.B. Computational Analysis of SARS-CoV-2 and SARS-like coronavirus diversity in human, bat and pangolin populations. Viruses. 2020;13:49. doi: 10.3390/v13010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang Y.-Z., Holmes E.C. A genomic perspective on the origin and emergence of SARS-CoV-2. Cell. 2020;181:223–227. doi: 10.1016/j.cell.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lam T.T.-Y., Jia N., Zhang Y.-W., Shum M.H.-H., Jiang J.-F., Zhu H.-C., Tong Y.-G., Shi Y.-X., Ni X.-B., Liao Y.-S., Li W.-J., Jiang B.-G., Wei W., Yuan T.-T., Zheng K., Cui X.-M., Li J., Pei G.-Q., Qiang X., Cheung W.Y.-M., Li L.-F., Sun F.-F., Qin S., Huang J.-C., Leung G.M., Holmes E.C., Hu Y.-L., Guan Y., Cao W.-C. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. 2020;583:282–285. doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- 150.Luan J., Lu Y., Jin X., Zhang L. Spike protein recognition of mammalian ACE2 predicts the host range and an optimized ACE2 for SARS-CoV-2 infection. Biochem. Biophys. Res. Commun. 2020;526:165–169. doi: 10.1016/j.bbrc.2020.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhao J., Cui W., Tian B.-P. The potential intermediate hosts for SARS-CoV-2. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.580137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li X., Zai J., Zhao Q., Nie Q., Li Y., Foley B.T., Chaillon A. Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J. Med. Virol. 2020;92:602–611. doi: 10.1002/jmv.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.do Vale B., Lopes A.P., Fontes M. da C., Silvestre M., Cardoso L., Coelho A.C. Bats, pangolins, minks and other animals - villains or victims of SARS-CoV-2? Vet. Res. Commun. 2021;45:1–19. doi: 10.1007/s11259-021-09787-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xiao K., Zhai J., Feng Y., Zhou N., Zhang X., Zou J.-J., Li N., Guo Y., Li X., Shen X., Zhang Z., Shu F., Huang W., Li Y., Zhang Z., Chen R.-A., Wu Y.-J., Peng S.-M., Huang M., Xie W.-J., Cai Q.-H., Hou F.-H., Chen W., Xiao L., Shen Y. Isolation of SARS-CoV-2-related coronavirus from Malayan pangolins. Nature. 2020;583:286–289. doi: 10.1038/s41586-020-2313-x. [DOI] [PubMed] [Google Scholar]
- 155.Liu P., Jiang J.-Z., Wan X.-F., Hua Y., Li L., Zhou J., Wang X., Hou F., Chen J., Zou J., Chen J. Are pangolins the intermediate host of the 2019 novel coronavirus (SARS-CoV-2)? PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Flores-Alanis A., Sandner-Miranda L., Delgado G., Cravioto A., Morales-Espinosa R. The receptor binding domain of SARS-CoV-2 spike protein is the result of an ancestral recombination between the bat-CoV RaTG13 and the pangolin-CoV MP789. BMC Res. Notes. 2020;13:398. doi: 10.1186/s13104-020-05242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nadeem M.S., Zamzami M.A., Choudhry H., Murtaza B.N., Kazmi I., Ahmad H., Shakoori A.R. Origin, potential therapeutic targets and treatment for coronavirus disease (COVID-19) Pathogens. 2020;9:307. doi: 10.3390/pathogens9040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhao J., Cui W., Tian B.-P. The potential intermediate hosts for SARS-CoV-2. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.580137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Oreshkova N., Molenaar R.J., Vreman S., Harders F., Oude Munnink B.B., Hakze-van der Honing R.W., Gerhards N., Tolsma P., Bouwstra R., Sikkema R.S., Tacken M.G., de Rooij M.M., Weesendorp E., Engelsma M.Y., Bruschke C.J., Smit L.A., Koopmans M., van der Poel W.H., Stegeman A. SARS-CoV-2 infection in farmed minks, The Netherlands, April and May 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.23.2001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Enserink M. Coronavirus rips through Dutch mink farms, triggering culls. Science. 1979;368(2020) doi: 10.1126/science.368.6496.1169. 1169–1169. [DOI] [PubMed] [Google Scholar]
- 161.Mallapaty S. COVID mink analysis shows mutations are not dangerous - yet. Nature. 2020;587:340–341. doi: 10.1038/d41586-020-03218-z. [DOI] [PubMed] [Google Scholar]
- 162.Munnink B.B.O., Sikkema R.S., Nieuwenhuijse D.F., Molenaar R.J., Munger E., Molenkamp R., Van Der Spek A., Tolsma P., Rietveld A., Brouwer M., Bouwmeester-Vincken N., Harders F., Van Der Honing R.H., Wegdam-Blans M.C.A., Bouwstra R.J., GeurtsvanKessel C., Van Der Eijk A.A., Velkers F.C., Smit L.A.M., Stegeman A., Van Der Poel W.H.M., Koopmans M.P.G. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science. 1979;371(2021):172–177. doi: 10.1126/science.abe5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.WHO/Europe | Mink-strain of COVID-19 virus in Denmark, (n.d.)..
- 164.Konishi T. SARS-CoV-2 mutations among minks show reduced lethality and infectivity to humans. PLoS One. 2021;16 doi: 10.1371/journal.pone.0247626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lu S., Zhao Y., Yu W., Yang Y., Gao J., Wang J., Kuang D., Yang M., Yang J., Ma C., Xu J., Qian X., Li H., Zhao S., Li J., Wang H., Long H., Zhou J., Luo F., Ding K., Wu D., Zhang Y., Dong Y., Liu Y., Zheng Y., Lin X., Jiao L., Zheng H., Dai Q., Sun Q., Hu Y., Ke C., Liu H., Peng X. Comparison of SARS-CoV-2 infections among 3 species of non-human primates. bioRxiv. 2020 doi: 10.1101/2020.04.08.031807. 2020.04.08.031807. [DOI] [Google Scholar]
- 166.Lu S., Zhao Y., Yu W., Yang Y., Gao J., Wang J., Kuang D., Yang M., Yang J., Ma C., Xu J., Qian X., Li H., Zhao S., Li J., Wang H., Long H., Zhou J., Luo F., Ding K., Wu D., Zhang Y., Dong Y., Liu Y., Zheng Y., Lin X., Jiao L., Zheng H., Dai Q., Sun Q., Hu Y., Ke C., Liu H., Peng X. Comparison of SARS-CoV-2 infections among 3 species of non-human primates. bioRxiv. 2020 doi: 10.1101/2020.04.08.031807. 2020.04.08.031807. [DOI] [Google Scholar]
- 167.Zhang C., Zheng W., Huang X., Bell E.W., Zhou X., Zhang Y. Protein structure and sequence reanalysis of 2019-nCoV genome refutes snakes as its intermediate host and the unique similarity between its spike protein insertions and HIV-1. J. Proteome Res. 2020;19:1351–1360. doi: 10.1021/acs.jproteome.0c00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Luan J., Jin X., Lu Y., Zhang L. SARS‐CoV‐2 spike protein favors ACE2 from Bovidae and Cricetidae. J. Med. Virol. 2020;92:1649–1656. doi: 10.1002/jmv.25817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sawatzki K., Hill N.J., Puryear W.B., Foss A.D., Stone J.J., Runstadler J.A. Host barriers to SARS-CoV-2 demonstrated by ferrets in a high-exposure domestic setting. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2025601118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Enkirch T., von Messling V. Ferret models of viral pathogenesis. Virology. 2015;479–480:259–270. doi: 10.1016/j.virol.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.van den Brand J.M.A., Haagmans B.L., Leijten L., van Riel D., Martina B.E.E., Osterhaus A.D.M.E., Kuiken T. Pathology of experimental SARS coronavirus infection in cats and ferrets. Vet. Pathol. 2008;45:551–562. doi: 10.1354/vp.45-4-551. [DOI] [PubMed] [Google Scholar]
- 172.van den Brand J.M.A., Haagmans B.L., Leijten L., van Riel D., Martina B.E.E., Osterhaus A.D.M.E., Kuiken T. Pathology of experimental SARS coronavirus infection in cats and ferrets. Vet. Pathol. 2008;45:551–562. doi: 10.1354/vp.45-4-551. [DOI] [PubMed] [Google Scholar]
- 173.Chu Y.-K., Ali G.D., Jia F., Li Q., Kelvin D., Couch R.C., Harrod K.S., Hutt J.A., Cameron C., Weiss S.R., Jonsson C.B. The SARS-CoV ferret model in an infection-challenge study. Virology. 2008;374:151–163. doi: 10.1016/j.virol.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Martina B.E.E., Haagmans B.L., Kuiken T., Fouchier R.A.M., Rimmelzwaan G.F., van Amerongen G., Peiris J.S.M., Lim W., Osterhaus A.D.M.E. SARS virus infection of cats and ferrets. Nature. 2003;425 doi: 10.1038/425915a. 915–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kim Y.-I., Kim S.-G., Kim S.-M., Kim E.-H., Park S.-J., Yu K.-M., Chang J.-H., Kim E.J., Lee S., Casel M.A.B., Um J., Song M.-S., Jeong H.W., Lai V.D., Kim Y., Chin B.S., Park J.-S., Chung K.-H., Foo S.-S., Poo H., Mo I.-P., Lee O.-J., Webby R.J., Jung J.U., Choi Y.K. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe. 2020;27:704–709.e2. doi: 10.1016/j.chom.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Richard M., Kok A., de Meulder D., Bestebroer T.M., Lamers M.M., Okba N.M.A., Fentener van Vlissingen M., Rockx B., Haagmans B.L., Koopmans M.P.G., Fouchier R.A.M., Herfst S. SARS-CoV-2 is transmitted via contact and via the air between ferrets. Nat. Commun. 2020;11:3496. doi: 10.1038/s41467-020-17367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shi J., Wen Z., Zhong G., Yang H., Wang C., Huang B., Liu R., He X., Shuai L., Sun Z., Zhao Y., Liu P., Liang L., Cui P., Wang J., Zhang X., Guan Y., Tan W., Wu G., Chen H., Bu Z., Bu Z. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 2020;368(6494):1016–1020. doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Abdel-Moneim A.S., Abdelwhab E.M. Evidence for SARS-COV-2 infection of animal hosts. Pathogens. 2020;9:1–27. doi: 10.3390/pathogens9070529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kim Y.-I., Kim S.-G., Kim S.-M., Kim E.-H., Park S.-J., Yu K.-M., Chang J.-H., Kim E.J., Lee S., Casel M.A.B., Um J., Song M.-S., Jeong H.W., Lai V.D., Kim Y., Chin B.S., Park J.-S., Chung K.-H., Foo S.-S., Poo H., Mo I.-P., Lee O.-J., Webby R.J., Jung J.U., Choi Y.K. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe. 2020;27:704–709.e2. doi: 10.1016/j.chom.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Conceicao C., Thakur N., Human S., Kelly J.T., Logan L., Bialy D., Bhat S., Stevenson-Leggett P., Zagrajek A.K., Hollinghurst P., Varga M., Tsirigoti C., Tully M., Chiu C., Moffat K., Silesian A.P., Hammond J.A., Maier H.J., Bickerton E., Shelton H., Dietrich I., Graham S.C., Bailey D. The SARS-CoV-2 Spike protein has a broad tropism for mammalian ACE2 proteins. PLoS Biol. 2020;18 doi: 10.1371/journal.pbio.3001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Freuling C.M., Breithaupt A., Müller T., Sehl J., Balkema-Buschmann A., Rissmann M., Klein A., Wylezich C., Höper D., Wernike K., Aebischer A., Hoffmann D., Friedrichs V., Dorhoi A., Groschup M.H., Beer M., Mettenleiter T.C. Susceptibility of raccoon dogs for experimental SARS-CoV-2 infection. Emerg. Infect. Dis. 2020;26:2982–2985. doi: 10.3201/eid2612.203733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Meekins D.A., Morozov I., Trujillo J.D., Gaudreault N.N., Bold D., Carossino M., Artiaga B.L., Indran S.V., Kwon T., Balaraman V., Madden D.W., Feldmann H., Henningson J., Ma W., Balasuriya U.B.R., Richt J.A. Susceptibility of swine cells and domestic pigs to SARS-CoV-2. Emerg. Microb. Infect. 2020;9:2278–2288. doi: 10.1080/22221751.2020.1831405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schlottau K., Rissmann M., Graaf A., Schön J., Sehl J., Wylezich C., Höper D., Mettenleiter T.C., Balkema-Buschmann A., Harder T., Grund C., Hoffmann D., Breithaupt A., Beer M. SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: an experimental transmission study. Lancet Microbe. 2020;1:e218–e225. doi: 10.1016/S2666-5247(20)30089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Shi J., Wen Z., Zhong G., Yang H., Wang C., Huang B., Liu R., He X., Shuai L., Sun Z., Zhao Y., Liu P., Liang L., Cui P., Wang J., Zhang X., Guan Y., Tan W., Wu G., Chen H., Bu Z., Bu Z. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 2020;368:1016–1020. doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Opriessnig T., Huang Y. Coronavirus disease 2019 (COVID‐19) outbreak: could pigs be vectors for human infections? Xenotransplantation. 2020;27 doi: 10.1111/xen.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Li X. Can cats become infected with Covid-19? Vet. Rec. 2020;186:457–458. doi: 10.1136/vr.m1455. [DOI] [PubMed] [Google Scholar]
- 187.Weingartl H.M., Copps J., Drebot M.A., Marszal P., Smith G., Gren J., Andonova M., Pasick J., Kitching P., Czub M. Susceptibility of pigs and chickens to SARS coronavirus. Emerg. Infect. Dis. 2004;10:179–184. doi: 10.3201/eid1002.030677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Suarez D.L., Pantin-Jackwood M.J., Swayne D.E., Lee S.A., DeBlois S.M., Spackman E. Lack of susceptibility to SARS-CoV-2 and MERS-CoV in poultry. Emerg. Infect. Dis. 2020;26:3074–3076. doi: 10.3201/eid2612.202989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Barr I.G., Rynehart C., Whitney P., Druce J. SARS-CoV-2 does not replicate in embryonated hen's eggs or in MDCK cell lines. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.25.2001122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vergara‐Alert J., Rodon J., Carrillo J., Te N., Izquierdo‐Useros N., Rodríguez de la Concepción M.L., Ávila‐Nieto C., Guallar V., Valencia A., Cantero G., Blanco J., Clotet B., Bensaid A., Segalés J. Transbound Emerg Dis; 2020. Pigs Are Not Susceptible to SARS‐CoV‐2 Infection but Are a Model for Viral Immunogenicity Studies. tbed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Casasnovas J.M., Margolles Y., Noriega M.A., Guzmán M., Arranz R., Melero R., Casanova M., Corbera J.A., Jiménez-de-Oya N., Gastaminza P. Nanobodies protecting from lethal SARS-CoV-2 infection target receptor binding epitopes preserved in virus variants other than omicron. Front. Immunol. 2022:1797. doi: 10.3389/fimmu.2022.863831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Temmam S., Vongphayloth K., Baquero E., Munier S., Bonomi M., Regnault B., Douangboubpha B., Karami Y., Chrétien D., Sanamxay D. Bat coronaviruses related to SARS-CoV-2 and infectious for human cells. Nature. 2022;604:330–336. doi: 10.1038/s41586-022-04532-4. [DOI] [PubMed] [Google Scholar]
- 193.Nga N.T.T., Latinne A., Thuy H.B., Van Long N., Ngoc P.T.B., Anh N.T.L., Van Thai N., Phuong T.Q., Van Thai H., Hai L.K. Evidence of SARS-CoV-2 related coronaviruses circulating in Sunda pangolins (Manis javanica) confiscated from the illegal wildlife trade in Viet Nam. Front. Public Health. 2022;10 doi: 10.3389/fpubh.2022.826116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sikkema R.S., Tobias T., Oreshkova N., de Bruin E., Okba N., Chandler F., Hulst M.M., Rodon J., Houben M., van Maanen K. Experimental and field investigations of exposure, replication and transmission of SARS-CoV-2 in pigs in The Netherlands. Emerg. Microb. Infect. 2022;11:91–94. doi: 10.1080/22221751.2021.2011625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Berger-González M., Pelikan K., Zinsstag J., Ali S.M., Schelling E. Transdisciplinary research and one health. One Health: The Theory and Practice of Integrated Health Approaches. 2020:57–70. doi: 10.1079/9781789242577.0057. [DOI] [Google Scholar]
- 196.Zinsstag J., Schelling E., Crump L., Whittaker M., Tanner M., Stephen C., editors. One Health: the Theory and Practice of Integrated Health Approaches. second ed. CABI; 2021. [DOI] [Google Scholar]
- 197.Kelly T.R., Machalaba C., Karesh W.B., Crook P.Z., Gilardi K., Nziza J., Uhart M.M., Robles E.A., Saylors K., Joly D.O., Monagin C., Mangombo P.M., Kingebeni P.M., Kazwala R., Wolking D., Smith W., Mazet J.A.K. Implementing One Health approaches to confront emerging and re-emerging zoonotic disease threats: lessons from PREDICT. One Health Outlook. 2020;2:1. doi: 10.1186/s42522-019-0007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shehata A.A., Attia Y.A., Rahman MdT., Basiouni S., El-Seedi H.R., Azhar E.I., Khafaga A.F., Hafez H.M. Diversity of coronaviruses with particular Attention to the interspecies transmission of SARS-CoV-2. Animals. 2022;12:378. doi: 10.3390/ani12030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wong A.C.P., Lau S.K.P., Woo P.C.Y. Interspecies jumping of bat coronaviruses. Viruses. 2021;13:2188. doi: 10.3390/v13112188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dróżdż M., Krzyżek P., Dudek B., Makuch S., Janczura A., Paluch E. Current state of knowledge about role of pets in zoonotic transmission of SARS-CoV-2. Viruses. 2021;13:1149. doi: 10.3390/v13061149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jo W.K., Oliveira‐Filho E.F., Rasche A., Greenwood A.D., Osterrieder K., Drexler J.F. Potential zoonotic sources of SARS‐CoV‐2 infections. Transbound Emerg Dis. 2021;68:1824–1834. doi: 10.1111/tbed.13872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Banerjee A., Doxey A.C., Mossman K., Irving A.T. Unraveling the zoonotic origin and transmission of SARS-CoV-2. Trends Ecol. Evol. 2021;36:180–184. doi: 10.1016/j.tree.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tilak R. Emergence of a zoonotic pathogen - novel coronavirus (SARS-CoV-2) in the context of changing environment. J. Comm. Dis. 2020;52:18–24. doi: 10.24321/0019.5138.202021. [DOI] [Google Scholar]
- 204.Khanh N.C., Thai P.Q., Quach H.-L., Thi N.-A.H., Dinh P.C., Duong T.N., Mai L.T.Q., Nghia N.D., Tu T.A., Quang L.N., Quang T.D., Nguyen T.-T., Vogt F., Anh D.D. Transmission of SARS-CoV 2 during long-haul flight. Emerg. Infect. Dis. 2020;26:2617–2624. doi: 10.3201/eid2611.203299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mehta S.H., Clipman S.J., Wesolowski A., Solomon S.S. Holiday gatherings, mobility and SARS-CoV-2 transmission: results from 10 US states following Thanksgiving. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-96779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Islam M., Haque M., Rahman M., Hossen F., Reza M., Barua A., Al Marzan A., Das T., Kumar Baral S., He C. A review on measures to rejuvenate immune system: natural mode of protection against coronavirus infection. Front. Immunol. 2022;13:1013. doi: 10.3389/fimmu.2022.837290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Alagawany M., Attia Y.A., Farag M.R., Elnesr S.S., Nagadi S.A., Shafi M.E., Khafaga A.F., Ohran H., Alaqil A.A., Abd El-Hack M.E. The strategy of boosting the immune system under the COVID-19 pandemic. Front. Vet. Sci. 2021;7 doi: 10.3389/fvets.2020.570748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Attia Y.A., Alagawany M.M., Farag M.R., Alkhatib F.M., Khafaga A.F., Abdel-Moneim A.-M.E., Asiry K.A., Mesalam N.M., Shafi M.E., Al-Harthi M.A. Phytogenic products and phytochemicals as a candidate strategy to improve tolerance to coronavirus. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.573159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.