Abstract
Adenoviruses bearing lesions in the E1B 55-kDa protein (E1B 55-kDa) gene are restricted by the cell cycle such that mutant virus growth is most impaired in cells infected during G1 and least restricted in cells infected during S phase (F. D. Goodrum and D. A. Ornelles, J. Virol. 71:548–561, 1997). A similar defect is reported here for E4 orf6-mutant viruses. An E4 orf3-mutant virus was not restricted for growth by the cell cycle. However, orf3 was required for enhanced growth of an E4 orf6-mutant virus in cells infected during S phase. The cell cycle restriction may be linked to virus-mediated mRNA transport because both E1B 55-kDa- and E4 orf6-mutant viruses are defective at regulating mRNA transport at late times of infection. Accordingly, the cytoplasmic-to-nuclear ratio of late viral mRNA was reduced in G1 cells infected with the mutant viruses compared to that in G1 cells infected with the wild-type virus. By contrast, this ratio was equivalent among cells infected during S phase with the wild-type or mutant viruses. Furthermore, cells infected during S phase with the E1B 55-kDa- or E4 orf6-mutant viruses synthesized more late viral protein than did cells infected during G1. However, the total amount of cytoplasmic late viral mRNA was greater in cells infected during G1 than in cells infected during S phase with either the wild-type or mutant viruses, indicating that enhanced transport of viral mRNA in cells infected during S phase cannot account for the difference in yields in cells infected during S phase and in cells infected during G1. Thus, additional factors affect the cell cycle restriction. These results indicate that the E4 orf6 and orf3 proteins, in addition to the E1B 55-kDa protein, may cooperate to promote cell cycle-independent adenovirus growth.
The adenovirus type 5 (Ad5) early region 1B 55-kDa oncoprotein (E1B 55-kDa) functions to overcome restrictions imposed on Ad growth by the cell cycle. In a randomly cycling population of cells, the E1B 55-kDa-deletion virus dl338 is restricted for growth such that it produces progeny virus in only a fraction of infected cells. By contrast, the wild-type virus produces progeny in nearly every infected cell. By analyzing virus growth in synchronized populations of cells, we demonstrated that the growth of the mutant virus was severely restricted in cells infected during G1 whereas this restriction was partially relieved in cells infected at the onset of S phase (26). Furthermore, we have recently demonstrated that the cell cycle restriction of the E1B 55-kDa-mutant virus can be overcome by infecting cells at 39°C (27). Because the E1B 55-kDa-mutant virus is not defective for viral DNA synthesis, we concluded that the role of the E1B 55-kDa protein in promoting cell cycle-independent virus growth resides in the late phase of viral replication (26).
At late times in the lytic infection, the E1B 55-kDa protein facilitates the transport of late viral transcripts and a subset of cellular transcripts while inhibiting the transport of most cellular transcripts (4, 6, 42, 54, 76). The E1B 55-kDa protein mediates viral and cellular mRNA transport in a complex with the early region 4 (E4) orf6 protein and possibly primate cell-specific cellular factors (10, 28, 30, 52). Consequently, viral mutants that fail to express either the E1B 55-kDa or the E4 orf6 gene products are defective for late viral gene expression and viral replication (10, 14, 30, 54). The transport of several cellular messages also requires the E1B 55-kDa protein late in Ad infection. These include the heat shock protein 70, β-tubulin, and the interferon-inducible Mx-A and 6-16 mRNAs. Transport of this subset of cellular mRNAs correlates with activation of these genes late in infection (47, 76). The ability of the E1B 55-kDa-mutant virus to grow selectively in S-phase cells suggests the possibility that a host cell factor compensates for the defect in virus-mediated mRNA transport in the absence of the E1B 55-kDa protein (26). Furthermore, other viral proteins that function in the control of mRNA transport may also contribute to cell cycle-independent Ad growth.
The E4 orf6 and orf3 proteins have redundant and overlapping functions, some of which are related to functions of the E1B 55-kDa protein (10, 11, 14). Each of these E4 gene products independently augments viral DNA synthesis, late viral gene expression, shutoff of host protein synthesis, and production of progeny virus (10, 11, 30, 34). Both the orf3 and orf6 proteins stabilize late mRNAs in the nucleus and thus enhance cytoplasmic accumulation of late Ad mRNAs (10, 11, 65, 66). Furthermore, the orf3 and orf6 proteins have been shown to affect mRNA splice site selection (50, 51).
The E1B 55-kDa and E4 orf6 proteins contribute to cellular transformation in cooperation with E1A. Both the E1B 55-kDa and E4 orf6 proteins independently bind and inhibit transcriptional activation mediated by the cellular growth suppressor, p53 (16, 37, 48, 77, 79). Inhibition of p53-mediated transactivation by the E1B 55-kDa protein is required for transformation by both the weakly oncogenic group C and the highly oncogenic group A Ads (37, 77, 80, 81). In cooperation with the E1A and E1B proteins, the E4 orf6 protein transforms baby rat kidney cells (48), converts the nontumorigenic 293 cell line (29) into a tumorigenic cell line in nude mice, and blocks p53-dependent apoptosis (46). Furthermore, coexpression of the E1B 55-kDa and E4 orf6 proteins decreases the stability of p53 in both transformed and productively infected cells (46, 48, 60, 70). Despite the relationship between these viral proteins and p53, the failure of the E1B 55-kDa-mutant virus to replicate independently of the cell cycle is not due to a failure to abrogate p53 function (27, 64). Recently, the E4 orf3 gene has been shown to cooperate with E1A, E1B, and E4 orf6 in transforming nonpermissive primary rat cells (49).
In this work, a series of mutant Ads were analyzed to determine if other Ad gene products related to the E1B 55-kDa protein contributed to cell cycle-independent virus growth. Mutant viruses that fail to express the E4 orf6 protein were restricted by the cell cycle for growth in a manner similar to that of the E1B 55-kDa-mutant virus. Therefore, the cell cycle restriction to virus growth may be linked to the defect in virus-mediated mRNA transport shared by the E1B 55-kDa- and E4 orf6-mutant viruses. Consistent with this suggestion, the cytoplasmic-to-nuclear ratio of late viral mRNA was reduced in G1 cells infected with the E1B 55-kDa- or E4 orf6-mutant viruses compared to that in G1 cells infected with the wild-type virus. By contrast, this ratio, which serves as an indirect measure of mRNA transport, was equivalent among cells infected during S phase with the wild-type or mutant viruses. Additionally, cells infected during S phase with the mutant viruses synthesized more late viral protein than did cells infected during G1. Nevertheless, comparison of total cytoplasmic levels of late transcripts suggested that the cell cycle restriction may not result entirely from the inability to accumulate late viral transcripts in the cytoplasm. Finally, the growth restriction of mutant viruses that failed to express both the E4 orf3 and orf6 proteins could not be overcome by infecting cells during S phase. These results demonstrate a requirement for E4 orf3 and orf6, as well as the E1B 55-kDa protein, in promoting cell cycle-independent virus growth.
MATERIALS AND METHODS
Cell culture.
Cell culture media, cell culture supplements, and serum were obtained from Life Technologies (Gaithersburg, Md.) through the Tissue Culture Core Laboratory of the Comprehensive Cancer Center of Wake Forest University. HeLa (ATCC CCL 2; American Type Culture Collection, Manassas, Va.), 293 (29), and W162 (72) cells were maintained as monolayers in Dulbecco modified Eagle’s minimal essential medium (DMEM) supplemented with 10% newborn calf serum, 100 U of penicillin, and 100 μg of streptomycin per ml. Cells were maintained in subconfluent adherent cultures in a 5% CO2 atmosphere at 37°C by passaging them twice weekly at a 1:10 dilution.
Synchronization of the HeLa cell cycle was achieved by a combination of mitotic detachment and hydroxyurea block as previously described (26). The degree of synchrony in each experiment was monitored by the DNA content of individual cells in parallel cell cultures by fluorescence-activated cell sorting as described previously (26). All flow cytometric analyses were conducted by the Steroid Receptor Laboratory in cooperation with the Hematology Flow Cytometry Laboratory of North Carolina Baptist Hospital.
Viruses.
The viruses used in these studies are described in Table 1. The phenotypically wild-type Ad5 parent virus dl309 used in these studies lacks a portion of the region encoding the E3 region that has been shown to be dispensable for growth in tissue culture (35). The E1B-mutant virus dl338 contains a 524-bp deletion in the 55-kDa protein coding region (54). Mutant virus dl1520D contains an 827-bp deletion in the region encoding the 55-kDa protein in combination with a stop codon to ensure that a truncated 55-kDa product cannot be expressed (5). The E1B 55-kDa-mutant virus dl110 contains a 472-bp deletion between nucleotide sequence positions 2333 and 2804 of the E1B coding region (4). E1B 55-kDa mutants S380, R443, and A143 were constructed by making in-frame 12-bp insertions in the DNA sequence of the E1B gene (78). The E1B 19-kDa-mutant virus dl337 contains a 146-bp deletion in the E1B 19-kDa coding region between nucleotide sequence positions 1770 and 1960 (53).
TABLE 1.
Description of Adsa
| Genotype | Virus | Modification | Reference |
|---|---|---|---|
| Wild type | dl309 | Phenotypically wild-type virus lacking a portion of E3 region | 35 |
| E1B 55-kDa-mutant | dl338 | 521-bp deletion in E1B 55-kDa region | 43 |
| dl1520 | 827-bp deletion combined with a stop codon | 5 | |
| S380 | 12-bp insertion in E1B 55-kDa region at codon 380 | 78 | |
| R443 | 12-bp insertion in E1B 55-kDa region at codon 443 | 78 | |
| A143 | 12-bp insertion in E1B 55-kDa region at codon 143 | 78 | |
| E1B 19-kDa-mutant | dl337 | 146-bp deletion in E1B 19-kDa region | 43 |
| E4 orf3-mutant | inorf3 | 8-bp insertion in E4 orf3 region | 34 |
| E4 orf6-mutant | dl355 | 14-bp deletion in E4 orf6 region | 14 |
| dl355* | 14-bg deletion in E4 orf6 region | 34 | |
| inorf6/inorf6/7 | 2-bp insertion disrupting both orf6 and orf6/7 | 34 | |
| E4 orf3-orf6-mutant | inorf3/inorf6 | 8-bp insertion in E4 orf3 and 8-bp insertion in E4 orf6 | 34 |
| dl355*/inorf3 | dl355* mutant and inorf3 mutant combined | 34 | |
| E4-deletion | dl366* | 2,269-bp deletion of entire E4 region | 34 |
| dl366*+orf1-2 | dl366* mutant with orf1-2 added back | 34 | |
| dl366*+orf3 | dl366* mutant with orf3 added back | 34 | |
| dl366*+orf4 | dl366* mutant with orf4 added back | 34 |
Mutant viruses dl338, dl1520, S380, R443, A143, dl337, and dl355 were constructed in the dl309 background. All other mutant viruses contain an intact E3 region.
E4-mutant virus dl355 contains a 14-bp deletion to disrupt orf6 in the wild-type dl309 background (30). The E4 mutation in dl355* is the same as that in dl355; however, the E3 region has been restored in dl355* (34). The E4-mutant virus dl366* contains a 2,269-bp deletion to disrupt the entire E4 coding region in a wild-type E3 background (34). The E4-mutant viruses, dl366*+orf1-2, dl366*+orf3, and dl366*+orf4, have orf1-2, orf3, and orf4 of E4 restored, respectively. Mutant virus E4-inorf3 contains an 8-bp insertion at the SspI restriction site (96.4 map units; 1 map unit is approximately 360 bp) disrupting the orf3 coding region. The mutant virus dl355*/inorf3 contains the E4 orf6 14-bp deletion with the 8-bp insertion in E4 orf3. The E4 inorf3/inorf6 contains 8-bp insertions at the SspI restriction sites at 93.0 map units (orf6) and 96.4 map units (orf3). The E4 inorf6/inorf6/7 mutant contains a 2-bp insertion at an AccI restriction site (94.6 map units) that disrupts both the E4 orf6 and orf6/7 coding regions (34).
The propagation of these viruses has been described elsewhere (10, 35). In brief, virus stocks were prepared by infecting 293 cells for E1B-mutant viruses or W162 cells for E4 region-mutant viruses at a low multiplicity of infection. Virus was harvested 4 to 5 days postinfection from a concentrated freeze-thaw lysate by sequential centrifugation in discontinuous and equilibrium cesium chloride gradients (36). The gradient-purified virus was supplemented with 5 volumes of 12 mM HEPES (pH 7.4)–120 mM NaCl–0.1 mg of bovine serum albumin (Fraction V; Life Technologies, Inc.) per ml–50% glycerol (Fisher Scientific, Pittsburgh, Pa.) and stored at −20°C. The titer of each virus stock was determined by plaque assays using 293 cells (36) and W162 cells (72) for the E1B- and E4-mutant viruses, respectively.
For infection with Ad, cells were passaged 16 to 24 h prior to infection to a density of 3 × 104 cells per cm2. Cells were washed once with phosphate-buffered saline (PBS), and the final wash was replaced with virus (3 to 20 PFU per cell) in Ad infection medium (PBS supplemented with 0.2 mM CaCl2, 0.2 mM MgCl2, 2% calf serum, 100 U of penicillin, and 100 μg of streptomycin per ml). The virus was added at one-fourth the normal culture volume, and the cells were gently rocked for 60 min at 37°C. The virus suspension was then replaced with normal growth medium, and the infected cells were returned to 37°C.
Electron microscopy.
Infected HeLa cells were processed for transmission electron microscopy at a time when virus production had reached a maximum and the integrity of the cell was well preserved. For most virus infections, cells were processed 24 h postinfection; for viruses that suffer delays in viral DNA synthesis, cells were processed 36 h postinfection. The infected HeLa cells were mechanically removed from the culture dish and pooled with the supernatant medium before harvesting to ensure quantitative recovery of the infected cells. The cells were washed with 0.2 M sodium cacodylate, pH 7.2, and fixed with 2.5% gluteraldehyde in the same buffer. The fixed cells were prepared for transmission electron microscopy as previously described (26).
Plaque assays for viral yields.
Detailed methods for Ad plaque assays have been described elsewhere (36). In brief, virus was harvested from cells in culture medium 48 to 72 h postinfection by multiple cycles of freezing and thawing. The cell lysates were clarified by centrifugation and serially diluted in infection medium for infection of 293 and W162 cells for plaque assay of E1B 55-kDa- and E4-mutant viruses, respectively. After incubation with the diluted virus for 1 h, the infected cells were overlaid with 0.7% SeaKem ME agarose (FMC BioProducts, Rockland, Maine) in DMEM supplemented with 0.75% sodium bicarbonate and 4% newborn calf serum. The cells were overlaid with additional agarose in growth medium on the third day after infection. Plaques were visualized by staining with neutral red in an agarose overlay on the seventh day after infection. Data were typically collected from three dilutions in each series of dilutions. The virus yield (PFU per milliliter) was determined by linear regression and expressed as the number of PFU per infected cell. Replicate samples were compared with the two-tailed Student t test.
Late viral protein synthesis.
Late viral protein synthesis was analyzed by pulse-labeling infected HeLa cells for 1 h with 0.1 mCi of 35S-labeled amino acids (Tran35S-label; ICN Biochemicals) per ml in cysteine- and methionine-free DMEM supplemented with 2% fetal bovine serum at 32 h postinfection. Cells were then scraped into PBS, pelleted, and lysed in 2% sodium dodecyl sulfate (SDS)–125 mM Tris (pH 6.8)–20% glycerol–5% 2-mercaptoethanol–100 mM dithiothreitol–0.01% bromophenol blue. Protein from 1 × 105 to 2 × 105 cell equivalents was separated by SDS-polyacrylamide gel electrophoresis (PAGE) on an 8% polyacrylamide gel with a 36:1 ratio of acrylamide to N,N′-methylenebisacrylamide (Polysciences, Warrington, Pa.). PAGE gels were fixed in 15% glacial acetic acid–7.5% methanol. Proteins were quantified with the use of a Molecular Dynamics PhosphorImager and ImageQuant analysis software (Molecular Dynamics, Sunnyvale, Calif.). Ad late proteins were identified by using virion standards that were synthesized in the presence of 14C-labeled mixed amino acids (Amersham, Arlington Heights, Ill.) and gradient purified from 293 cells infected with the wild-type Ad, dl309, as described previously (36).
Cell fractionation and RNA isolation.
All solutions used for RNA purification and in vitro transcription were prepared from diethyl pyrocarbonate-treated water as described in Ausubel et al. (3). RNA was isolated from the nucleus and cytoplasm of Ad-infected HeLa cells at 16 to 18 h postinfection. Approximately 4 × 106 infected cells were scraped in ice-cold PBS, pelleted at 1,000 × g at 4°C, and then suspended in 0.15 ml of isotonic buffer (140 mM NaCl, 10 mM Tris-Cl [pH 7.4], 1.5 mM MgCl2) containing 10 mM vanadyl-adenosine complex. An equal volume of isotonic buffer with 1% (vol/vol) Nonidet P-40 (Calbiochem) was added to the cells on ice with gentle mixing. After 5 min, the nuclei were pelleted at 1,000 × g and briefly washed with isotonic buffer containing 0.5% Nonidet P-40. After again the nuclei were again pelleted, the supernatant liquid was pooled as the cytoplasmic fraction.
Cytoplasmic RNA was isolated with the use of Trizol LS (Life Technologies) as recommended by the manufacturer, and the purified RNA was stored in 0.01 mM sodium acetate, pH 5.2. Nuclear RNA was isolated as described in Ausubel et al. (3). In brief, the nuclear pellet was resuspended in isotonic buffer containing 10 mM vanadyl-adenosine complex to which 10 volumes of a solution containing 4 M guanidine thiocyanate (Life Technologies), 20 mM sodium acetate (pH 5.2), 0.1 mM dithiothreitol, and 0.5% N-lauroyl sarcosine was added. The volume was adjusted to 2.5 ml with the same solution, and the mixture was incubated for 60 to 90 min at room temperature with gentle mixing. The entire mixture was layered on top of a 5.7 M cesium chloride cushion in an SW-51 (Beckman) centrifuge tube and centrifuged at 35,000 rpm at 16°C for 16 to 20 h. The RNA pellet was recovered and dissolved in 0.01 mM sodium acetate, pH 5.2. The concentration of nuclear and cytoplasmic RNA was normalized on the basis of initial cell number at 1.33 × 107 cells per ml. Polyadenylated RNA was purified from cytoplasmic RNA by three sequential rounds of purification with oligo(dT) cellulose (New England Biolabs).
RNase protection assays.
DNA fragments obtained by thermal cycle amplification corresponding to Ad5 DNA spanning the polyadenylation site of the L3 and L5 gene were subcloned into pGEM7z(+) (Promega) for transcription in vitro. The primers used for the L3 gene (5′-TTCCTAACTTTGACGCGGTA-3′ and 5′-TTCGACAGGAAACCGTGTG-3′) generated a 442-bp thermal cycle product corresponding to bases 27811 through 28252 of the Ad5 genome. The primers used for the L5 gene (5′-TTCAGCTTATCCAAAATCTCACG-3′ and 5′-TTCGCCTTGGTTTGCTT-3′) generated a 513-bp product corresponding to bases 32545 through 33058 of the Ad5 genome. Radiolabeled RNA complementary to the sequences surrounding the L3 and L5 polyadenylation site was synthesized in vitro with SP6 RNA polymerase as recommended by the manufacturer (Promega) with [α-32P]CTP (ICN; 10 mCi/ml, >400 Ci/mmol). The DNA template was removed by digestion with RNase-free DNase (RQ1 DNase; Promega), and the radioactive RNA was purified by sequential ethanol precipitation from 0.125% SDS, 0.5 M ammonium acetate, 25 μg of tRNA carrier per ml, and then 2.5 M ammonium acetate. The RNA was resuspended in 0.04 ml of 0.01 mM sodium acetate, and the amount of radioactive transcript was quantified by liquid scintillation counting.
The levels of L3 and L5 RNA were determined by RNase protection assays (9, 45). Hybridization reaction mixtures were prepared in a final volume of 20 μl containing 5 μl of NaCl-EDTA solution (3.6 M NaCl, 30 mM EDTA [pH 7.4]) and 15 μl containing an excess (2 × 106 cpm) of 32P-labeled RNA probe synthesized in vitro and total cytoplasmic or nuclear RNA isolated from 5 × 105 cells. After hybridization overnight at 65°C under a drop of mineral oil, the hybrids were digested with 150 μl of RNase cocktail (5 mM EDTA [pH 7.4], 30 μg of RNase P1 [500 U/mg; Sigma] per ml, 10 μg of RNase T1 [300,000 U/mg; Sigma] per ml) for 1 h at room temperature. Digestions were stopped with 20 μl of N-LS cocktail (1% N-lauroyl sarcosine [Sigma] and 1 μg of proteinase K [Calbiochem] per ml), and the protected products were precipitated at −20°C after the addition of 150 μl of GTC solution (4 M guanidine thiocyanate, 0.5% N-lauroyl sarcosine, 25 mM sodium citrate, 0.7% 2-mercaptoethanol), 0.05 mg of tRNA per ml as a carrier, and 0.3 ml of isopropanol (8). The protected RNA hybrid was precipitated with tRNA carrier and 2.5 volumes of ethanol. The RNA pellet was resuspended in RNA loading buffer (90% deionized formamide, 10 mM EDTA [pH 7.4], 0.2% bromophenol blue, 0.2% xylene cyanol) and denatured at ≥90°C for 3 min. Protected fragments were separated on a denaturing 6% polyacrylamide minigel containing urea. Protected fragments were quantified with the use of a Molecular Dynamics PhosphorImager and ImageQuant analysis software.
Computer-assisted graphics.
Radioactive samples were visualized with the use of a Molecular Dynamics PhosphorImager and ImageQuant analysis software. Radioactive images were obtained as 16-bit gray-scale images and then converted to 8-bit gray-scale images. The density ranges were adjusted for printing without further contrast or image enhancement. The digitized images were imported at 300 dpi into the graphic software, Canvas (Deneba Software, Miami, Fla.), operating on a Macintosh microcomputer to create the final figures.
RESULTS
Ad mutants that exhibit a potential cell cycle restriction for growth produce virus in only a fraction of infected cells.
The cell cycle restriction for growth of the E1B 55-kDa mutant virus dl338 was initially identified by analyzing Ad-infected HeLa cells by electron microscopy. This approach permits analysis of the Ad infection at the level of the individual cell. Only 20% of the HeLa cells infected with dl338 produced progeny virions, although all cells were infected and synthesized viral DNA. By contrast, the wild-type virus dl309 produced virus in nearly every infected cell (26). In the work presented here, we evaluated other Ad mutants with defects in viral genes related to the E1B 55-kDa gene for a potential cell cycle restriction. These include mutant viruses containing lesions in the E4 orf3 and orf6 genes. Asynchronous HeLa cells were infected with the wild-type virus dl309, an E1B 55-kDa-mutant virus, dl1520, an E4 orf3-mutant virus, inorf3, or an E4 orf6-mutant virus, dl355, at multiplicities of 20 PFU per cell. The cells were then processed for transmission electron microscopy to determine the fraction of infected cells producing virus (Fig. 1 and Table 2).
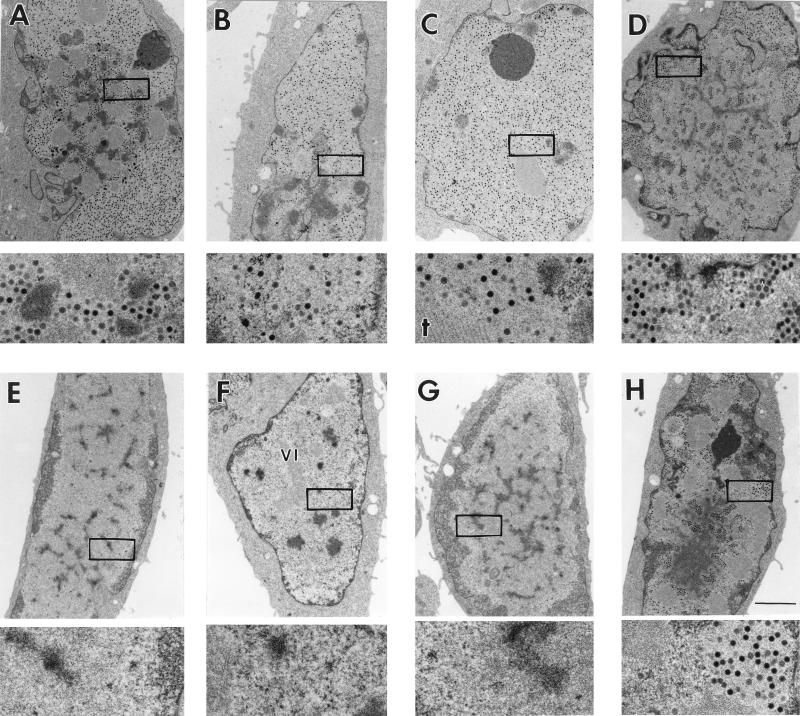
FIG. 1.
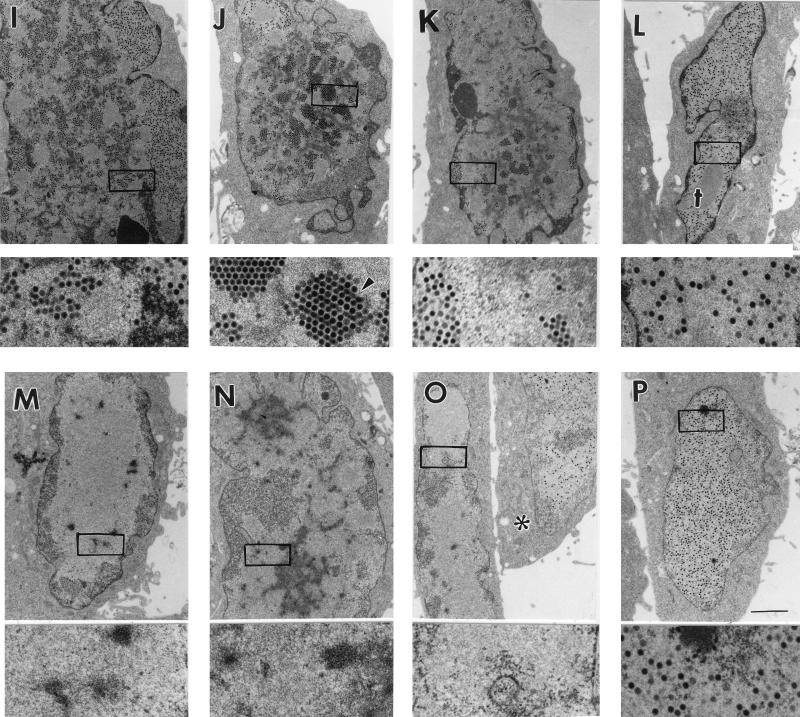
E1B 55-kDa- and E4 orf6-mutant viruses, but not an E4 orf3-mutant virus, produce virus in only a fraction of infected cells. Monolayers of HeLa cells were infected with the wild-type virus dl309 (A to D), the E1B 55-kDa mutant virus dl1520 (E to H), the E4 Orf3-mutant inorf3 (I to L), or the E4 orf6-mutant virus dl355 (M to P), at a multiplicity of 20 PFU/cell. At 24 h postinfection, cells were fixed in 2.5% glutaraldehyde, embedded, and sectioned for transmission electron microscopy. Nearly all (>95%) of the cells infected with the wild-type virus contained electron-dense viral particles in the nucleus. Four representative wild-type virus-infected cells are shown in panels A through D. Virions can be easily seen in the high-magnification images, representing a fivefold magnification of the boxed region of the nucleus from the low-magnification image. Of the four representative cells infected with the E1B-mutant virus (E to H), only the cell shown in panel H contains virus particles in the nucleus. Nearly all (>95%) cells infected with the E4 orf3-mutant virus produced virus, as can be seen in the four representative cells shown in panels I through L. Of the five E4 orf6-mutant virus-infected cells (M to P), only the cell in panel P and one of the cells in panel O (marked by an asterisk) contained virus particles in their nuclei, although all cells were infected. Representative viral inclusions are marked VI. A crystalline array of virus particles in panel J is marked by an arrowhead, and crystalline aggregates of viral proteins in panels C and L are marked by dagger (†). Bars, 2 μm.
TABLE 2.
Fraction of cells producing mutant virus
| Relevant genotype | Virus | Virus-producing cells (%)a
|
|
|---|---|---|---|
| Asyncb (n) | S phase (n) | ||
| Wild type | dl309 | 95 (8) | 95 (4) |
| E1B 55-kDa-mutant | dl1520 | 21 (8) | 58 (4) |
| orf6-mutant | dl355 | 35 (3) | 60 (2) |
| orf3-mutant | inorf3 | 95 (3) | 96 (1) |
| orf3-orf6-mutant | inorf3/inorf6 | 27 (3) | 22 (2) |
| dl355*/inorf3 | 10 (3) | 14 (2) | |
| E4-deletion | dl366* | 3 (2) | ND |
| dl366*+orf1-2 | 24 (1) | 24 (1) | |
| dl366*+orf3 | 28 (2) | 40 (1) | |
| dl366*+orf4 | 19 (1) | 14 (1) | |
HeLa cells that were clearly infected were scored for the presence or absence of virus particles. At least 200 cells per experiment were counted, and each value represents an average of cells producing virus. All measurements from multiple experiments (n > 1) were within ±8%. n, number of independent experiments. ND, not determined.
G1 cells were used for dl520, inorf3, and dl355*/inorf3 infections instead of asynchronous (Async) cells.
Cells infected with each of the viruses as shown in Fig. 1 exhibit morphological evidence of virus infection. The nuclear membrane has become crenulated, chromatin is marginated and displaced to the outer periphery of the nuclear membrane, and the nucleolus has changed in appearance. In addition, nuclei of all infected cells contained viral inclusions (example marked VI in Fig. 1F) which are the intranuclear sites of viral DNA synthesis and accumulation (12, 57, 71) and RNA biogenesis (55, 58, 75). The wild-type virus produced progeny in nearly every infected HeLa cell (Fig. 1A to D and Table 2). Virus particles are small, densely stained, and uniform in size and shape as seen in the higher-magnification image below each cell. As previously reported (26), mock-infected cell nuclei were devoid of any structures resembling viral inclusions or particles (data not shown).
More than 1,000 cells infected with the E1B 55-kDa-mutant virus dl1520 were evaluated. Only 21% of these infected cells contained progeny virions (Table 2). This is similar to results obtained with the E1B 55-kDa-mutant virus dl338 (26). Of the four representative cells shown in Fig. 1E to H, only the cell in panel H contains virus, as can be seen in the fivefold-higher-magnification image.
In contrast to cells infected with the E1B 55-kDa-mutant virus, nearly all cells infected with the E4 orf3-mutant virus inorf3 produced progeny virions (Fig. 1I to L and Table 2). These results suggest that, like the wild-type virus, the E4 orf3-mutant virus is not restricted by the cell cycle for growth. Crystalline arrays of virus particles (arrowhead, Fig. 1J) and crystalline aggregates of viral proteins (marked by daggers in Fig. 1C and L) were more commonly observed in E4 orf3-mutant-infected cells than in cells infected with other viruses.
In cells infected with the E4 orf6-mutant virus dl355, virus was produced in 35% of the infected cells (Fig. 1M to P and Table 2). Of the representative cells infected with dl355 shown in Fig. 1, only one of the cells in panel O (marked by an asterisk) and the cell in panel P contained progeny virions, indicating that the growth of this E4 orf6-mutant virus may be restricted by the cell cycle.
Cells were synchronized and infected at the onset of S phase with each of the mutant viruses to determine if mutant viruses restricted to growth in a fraction of infected cells were restricted by the cell cycle (Table 2). We previously demonstrated that cells infected during S phase with the E1B 55-kDa-mutant virus dl338 produced virus in a greater percentage of infected cells (up to 75%) than did cells infected during G1 or asynchronous growth (26) (Table 3). The fraction of dl355-infected cells producing virus increased from an average of 35% in asynchronously infected cells to 60% in cells infected during S phase. Therefore, this mutant is restricted by the cell cycle for growth similarly to the E1B 55-kDa-mutant viruses. The E4 orf3-mutant virus inorf3 was not restricted by the cell cycle for growth as indicated by the experiments whose results are shown in Fig. 1 and Table 2. In three independent experiments, inorf3 produced progeny virus in an average of 95% of the cells infected during asynchronous growth. Not surprisingly, this fraction was not increased by infecting synchronized HeLa cells during S phase.
TABLE 3.
Fraction of cells producing E1B-mutant virus
| Relevant genotype | Virus | Virus-producing cells (%)a
|
|
|---|---|---|---|
| Async (n) | S phase (n) | ||
| Wild type | dl309 | 95 (8) | 95 (4) |
| 55-kDa-mutant | dl338 | 22 (8) | 67 (3) |
| A143 | 25 (3) | 68 (3) | |
| S380 | 90 (3) | ND | |
| R443 | 56 (3) | 56 (3) | |
| 19-kDa-mutant | dl337 | 95 (2) | ND |
HeLa cells that were clearly infected were scored for the presence or absence of virus particles. At least 200 cells per experiment were counted, and each value represents an average of cells producing virus. All measurements were within ±8%. n, number of independent experiments. ND, not determined. Async, asynchronous.
A virus bearing mutations in both E4 orf3 and orf6 genes (inorf3/inorf6) produced progeny virus in 27% of asynchronous cells (Table 2). This result is similar to the growth restriction observed for the E4 orf6-mutant virus dl355. Surprisingly, when cells were infected at the onset of S phase with the double mutant virus, the fraction of cells producing virus did not increase. A similar result was obtained with a second E4 orf3-orf6 double-mutant virus, dl355*/inorf3. This mutant virus was restricted for growth in both cells infected during G1 and cells infected during S phase, producing virus in 10 and 14% of the infected cells, respectively. Thus, the growth restriction of the E4 orf3-orf6-mutant viruses cannot be ameliorated by infecting cells during S phase. These results indicate that the E4 orf3 protein is required for enhanced growth of orf6-mutant viruses in cells infected during S phase.
The virus lacking all E4 genes, dl366*, produced virus in only 3% of the infected cells. This result is expected, since viruses that lack the entire E4 coding region fail to synthesize viral DNA (11, 30, 34). Virus growth is substantially restored for viruses that contain one or two E4 open reading frames in the dl366* background. The E4-deletion virus containing only orf1 and orf2 of E4, dl366*+orf1-2, produced progeny virus in 24% of the cells infected during asynchronous growth. The E4-deletion virus with orf3 restored, dl366*+orf3, produced virus in 28% of the cells infected during asynchronous growth. Finally, the E4-deletion virus with orf4 restored, dl366*+orf4, produced progeny in 19% of cells infected during asynchronous growth. These results indicate that each of these deletion viruses may be restricted for growth by the cell cycle. However, like the E4 orf3-orf6-double-mutant viruses, the fraction of cells infected with dl366*+orf1-2 or dl366*+orf4 did not increase when cells were infected during S phase. By contrast, S-phase cells infected with dl366*+orf3 produced progeny in 40% of the infected cells. Apparently, inclusion of orf3 in the large dl366* deletion background conferred a limited ability to overcome the growth restriction in a cell infected during S phase. These morphological results suggest a role for the E4 orf3 protein in abating cell cycle restrictions imposed on virus growth in the absence of the E4 orf6 protein.
E4-mutant viruses that are restricted by the cell cycle for growth produce greater yields of virus in cells infected during S phase.
Virus yields were measured from cells infected during G1 or S phase by plaque assay to confirm the growth restriction of the E4-mutant viruses deduced by electron microscopy. If growth of these mutant viruses is restricted by the cell cycle, then cells infected during S phase should produce greater yields of virus. Note that it is the stage of the cell cycle at the time of infection that determines the outcome of the E1B 55-kDa-mutant virus infection, since cells no longer continue to progress through the cell cycle shortly after infection (25). For these experiments, synchronized HeLa cells were infected during S phase or G1 with each E4-mutant virus at a multiplicity of infection of 3 to 10. At 48 to 72 h postinfection, viral yields were determined by titer on W162 cells. The two-tailed Student t test was used to determine if the average yield of virus obtained from S-phase-infected cells and from G1-infected cells differed. The results shown in Fig. 2 demonstrate a cell cycle restriction for growth of mutant viruses lacking the E4 orf6 gene.
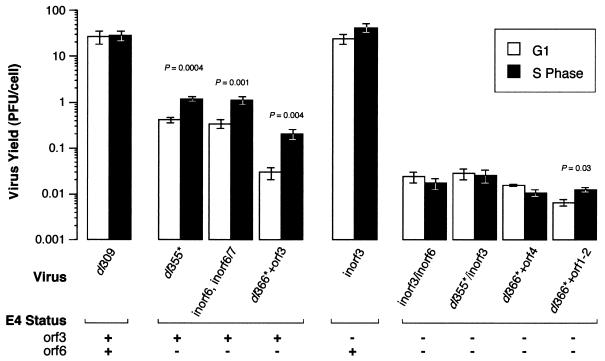
FIG. 2.
HeLa cells infected during S phase produce greater yields of E4 orf6-mutant virus, but not E4 orf3-mutant virus, than do cells infected during G1. HeLa cells were synchronized to S phase or G1. Cells were infected with the wild-type virus (dl309), the E4-mutant viruses lacking orf6 (dl355*, inorf6/inorf6/7, and dl366*+orf3), the E4 orf3-mutant virus (inorf3), or the E4-mutant viruses lacking orf3 and orf6 (inorf3/inorf6, dl355*/inorf3, dl366*+or4, and dl366*±orf1-2) at a multiplicity of 3 to 10 PFU per cell. Cells were lysed 48 to 72 h postinfection, and virus yields were measured by plaque assays with W162 cells. For the wild-type virus, 1 PFU measured with W162 cells corresponds to approximately 100 infectious units in HeLa cells. The results shown are averages of three to nine independent infections performed in four independent experiments. Yields are expressed as PFU per cell. Viruses that produced significantly different yields between cells infected during S phase and cells infected during G1 are identified by the associated P values derived from the two-tailed Student t test. All other comparisons of S with G1 were not statistically different, with P values of >0.5. The status of the E4 orf3 and orf6 genes in each mutant virus is indicated, where a plus sign indicates a wild-type gene and a minus sign indicates a functionally null gene.
The wild-type virus dl309 produced nearly equivalent yields of virus in cells infected during G1 and in cells infected during S phase (Fig. 2). By the convention that a P value of ≤0.05 is significant, growth of the wild-type virus was not significantly affected by the stage of the cell cycle at the time of infection. Three mutant viruses that expressed the E4 orf3 protein, but not the orf6 protein, produced greater amounts of virus in cells infected during S phase than in cells infected during G1. The differences in yields from cells infected during S phase and from cells infected during G1 with these E4 orf6-mutant viruses were significant (P ≤ 0.004). The E4 orf6-mutant virus dl355* produced 2.9-fold-greater yields of virus in cells infected during S phase than in cells infected during G1. This increase is similar to that measured for S-phase cells infected with the E1B 55-kDa-mutant viruses dl338 and dl1520 compared to cells infected during asynchronous growth (Fig. 3). The inorf6/inorf6/7 virus is unable to express both the orf6 protein and the related orf6/7 protein and produced 3.3-fold-greater yields of virus in cells infected during S phase than in cells infected during G1. The contribution of the mutation in orf6/7 to the growth restriction is not known. However, since an E4 orf6/7-mutant virus grows to wild-type virus yields (30) and the inorf6/inorf6/7-mutant virus is not more restricted for growth than is the inorf6-mutant virus (34), the predominant defect in this virus is expected to be due to the mutation in orf6. Finally, the dl366*+orf3 virus exhibited a similar S-phase enhancement, producing sevenfold more virus in S-phase cells than in G1 cells. Note that dl366*+orf3 expresses only the orf3 protein of the E4 region. These results, which indicate that orf6-mutant viruses grow more effectively in cells infected during S phase, are consistent with the morphological data of Fig. 1 and Table 2.
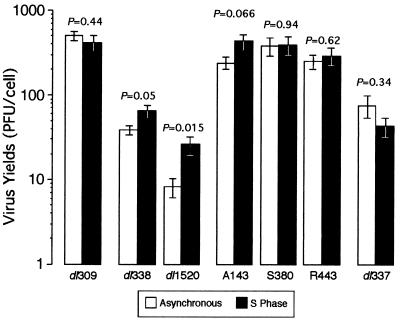
FIG. 3.
E1B 55-kDa-mutant viruses defective for late gene expression produce greater yields of virus in S-phase cells than in asynchronous cells. Asynchronous or S-phase HeLa cells were infected with either the wild-type virus (dl309), and E1B 55-kDa-mutant virus (dl338, A143, R443, or S380), or an E1B 19-kDa-mutant virus (dl337) at a multiplicity of 3 PFU per cell. Cells were lysed 48 h postinfection, and virus yields were measured by plaque assays with 293 cells. Yields are averages from multiple experiments and are expressed as PFU per cell. Asynchronous values are derived from five experiments, and S-phase values were derived from three experiments, except for dl337. Both asynchronous and S-phase values for dl337 were derived from three experiments. The standard errors of the means are shown. The probabilities indicated above each set of bars derived from a two-tailed Student t test and represent the probabilities that the two averages are from the same population.
The inorf3 virus grew to near-wild-type yields in S-phase or G1 cells, indicating that inorf3 is not restricted by the cell cycle for growth (Fig. 2). Although the average yield of inorf3 virus increased 1.6-fold in cells infected during S phase over that measured for cells infected during G1, this value is not likely significant (P = 0.23).
Also in agreement with the results in Table 2, three mutant viruses (inorf3/inorf6, dl355*/inorf3, and dl366*+orf4) that fail to express both the E4 orf3 and orf6 proteins failed to produce greater yields of virus in cells infected during S phase than in cells infected during G1. Indeed, these double-mutant viruses produced slightly reduced yields of virus in cells infected during G1. By contrast, the mutant virus dl366*+orf1-2 produced a greater yield of virus in cells infected during S phase than in cells infected during G1. Although the associated P value of 0.03 indicates that this difference is significant, the 1.9-fold increase in yield from S-phase-infected cells is not associated with an increase in the fraction of S-phase-infected cells producing dl366*+orf1-2 (24% ± 8%) over that of G1-infected cells (24% ± 8%) (Table 2). Nonetheless, it remains possible that the orf1 or orf2 products affect growth of the virus in a cell cycle-dependent manner.
We cannot exclude the possibility that the delay in viral DNA synthesis in cells infected with the E4 orf3-orf6 double-mutant viruses contributes to the failure of these viruses to replicate to greater yields in cells infected during S phase. However, a defect in viral DNA synthesis seems unlikely to be the cause of reduced growth, since viral yields are measured after 48 h postinfection and, by this time of infection, the amount of viral DNA in asynchronous cells infected with the E4 orf3-orf6 double-mutant viruses has nearly reached wild-type levels (34). Nevertheless, it is possible that mutant viruses defective for viral DNA synthesis suffer greater competition for DNA synthetic machinery in the cell infected during S phase, since cellular DNA synthesis is inhibited less efficiently in cells infected during S phase than in cells infected during G1 (33).
We could not detect virus production from HeLa cells infected during S phase with the virus lacking the entire E4 region, dl366*. However, in four independent infections, the production of dl366* in cells infected during G1 could be measured by plaque assay. The yield of virus from cells synchronized to G1 was comparable to the yield of virus from an asynchronous population of cells (data not shown). Although not conclusive, these observations suggest that the dl366* virus grows best in cells infected during G1 and may not grow appreciably in cells infected during S phase. In this respect, growth of the large E4-deletion virus dl366* resembles that of other E4 orf3-orf6 double-mutant viruses.
These experiments establish a cell cycle restriction for growth of E4 orf6-mutant viruses. As for the E1B 55-kDa-mutant viruses, this cell cycle restriction can be partially overcome by infecting cells during S phase. In addition, the growth benefit of the E4 orf6-mutant virus derived from infecting cells during S phase is dependent on the presence of the E4 orf3 gene.
Cell cycle restriction for growth of the E1B 55-kDa-mutant viruses correlates with the defect in virus-mediated mRNA transport.
The E1B 55-kDa and the E4 orf6 proteins function as a complex to mediate viral and cellular mRNA transport in the infected cell. Since both the E1B 55-kDa- and E4 orf6-mutant viruses are defective for mRNA transport and cell cycle-independent growth, we sought to determine if the cell cycle restriction may be linked to the defect in virus-mediated mRNA transport. This notion was further supported by the analysis of three viruses bearing in-frame insertions in the E1B 55-kDa gene. These mutant viruses direct the synthesis of a stable E1B 55-kDa protein that is defective predominantly in one of three properties attributed to the E1B 55-kDa protein (37, 78). A143 is primarily defective for late viral gene expression, which may stem from the defect in virus-mediated mRNA transport. S380 is primarily defective for binding p53 and transformation, and R443 is defective for transcriptional silencing. The insertion mutants were evaluated for their ability to replicate independently of the cell cycle to determine if the loss of a specific property of the E1B 55-kDa protein correlates with cell cycle-restricted virus growth. The fraction of infected HeLa cells producing these mutant viruses was determined by electron microscopy (Table 3), and yield of mutant virus from HeLa cells infected during S phase or asynchronous HeLa cells were infected at a multiplicity of 20 PFU per cell. Cells were processed for transmission electron microscopy at 24 h postinfection. For the measurements of virus yields, S-phase or asynchronous HeLa cells were infected at a multiplicity of 3 PFU per cell. Cells were lysed at 48 h postinfection, and viral yields were determined by titer on 293 cells.
As reported previously (26), the E1B 55-kDa-mutant virus dl338 produces virus in approximately 22% of the cells infected during asynchronous growth (Table 3). However, when cells were infected during S phase, virus was produced in an average of 67% of the infected cells. By contrast, the wild-type virus dl309 produces virus in nearly every infected cell irrespective of the stage of the cell cycle at the time of infection.
The insertion mutant virus A143 produced virus in 25% of the cells infected during asynchronous growth (Table 3). When cells were synchronized and infected with A143 at the onset of S phase, virus was produced in 68% of the infected cells, indicating a cell cycle restriction resembling that of the deletion mutant viruses. The E1B 55-kDa-insertion mutant S380 produced progeny in nearly all infected cells, similar to the wild-type virus. Additionally, S380-infected cells typically contained more virus particles than did cells infected with the wild-type virus (data not shown). This result is consistent with the results of Berk and associates (78), who found that cells infected with S380 have a greater burst size and produce greater yields of virus than do cells infected with the wild-type virus. R443 produced virus in 56% of cells infected during asynchronous growth. It is not clear how this intermediate phenotype should be interpreted. Since the fraction of cells infected with R443 containing virus did not change when cells were infected during S phase, the ability to silence transcription may not bear on cell cycle-independent virus growth. Replication of R443 may be restricted to a fraction of infected cells by mechanisms other than a cell cycle restriction. Of the representative insertion mutant viruses analyzed, only A143 exhibited a restriction to virus growth that was alleviated in cells infected during S phase. If the defect in late gene expression of A143 stems from the defect in mRNA transport, this would suggest that the failure of the virus to regulate viral and cellular mRNA transport may underlie the cell cycle restriction.
Additionally, asynchronous cells infected with the E1B 19-kDa-mutant virus dl337 were analyzed by electron microscopy. This mutant virus produced progeny virus in nearly every infected cell. Therefore, this virus was not restricted by the cell cycle for growth. The E1B 19-kDa protein is a functional homologue of the cellular protein Bcl-2 and effectively inhibits p53-mediated apoptosis (15, 61). This result further supports the finding that the cell cycle restriction is not due to the failure to inhibit p53 (27).
The yield of virus obtained from cells infected during S phase compared to that for cells infected during asynchronous growth is consistent with the fraction of virus-producing cells measured by electron microscopy (Fig. 3). The wild-type virus was not significantly affected by the stage of the cell cycle at the time of infection. However, within any given experiment, the yield of wild-type virus from asynchronous or G1-infected cells exceeded that from S-phase-infected cells. By contrast, both E1B 55-kDa-deletion mutant viruses dl338 and dl1520 produced two- to threefold-greater yields in S-phase-infected cells than in asynchronously infected cells. Consistent with these results, we previously reported that cells infected during S phase with dl338 produced sevenfold-greater yields of virus than did cells infected during G1 (26). Although S-phase-infected cells exhibit the greatest permissivity for E1B 55-kDa-mutant virus growth, infection of an S-phase cell does not fully restore growth of the E1B 55-kDa-mutant virus to wild-type levels.
Each of the E1B-insertion mutant viruses analyzed here was not as defective for growth as were the deletion mutants. Nonetheless, the mutant virus A143 produced 1.6-fold more virus in cells infected during S phase than in cells infected during asynchronous growth. Although this difference was marginally significant (P = 0.067), the growth restriction of A143 was reduced by infecting cells during S phase. By contrast, S380 and R443 produced nearly equivalent yields of virus in both S-phase and asynchronously growing cells. In this respect, the growth of the S380 and R443 mutant viruses resembled the growth of the wild-type virus in S-phase and asynchronous cells. The growth of these insertion mutants is consistent with the suggestion that the cell cycle restriction is linked to a defect in the control of mRNA transport. It remains unclear why the difference in yield of A143 failed to reflect the more dramatic changes in virus production observed by electron microscopy. Compared to asynchronously infected cells, cells infected during S phase with A143 contained as much as threefold more cells producing progeny virus (Table 3), although these cells produced only 1.6-fold more virus (Fig. 3).
Consistent with the findings summarized in Table 3, growth of the E1B 19-kDa-mutant virus dl337 was not enhanced in cells infected during S phase (Fig. 3). Rather, the yield of this mutant virus was greater in cells infected during asynchronous growth than in cells infected during S phase, although this difference is not likely to be significant (P = 0.34). The growth of dl337 demonstrates that not all viruses that are defective for growth are restricted by the cell cycle.
Late viral gene expression is partially restored in HeLa cells infected during S phase with the E1B 55-kDa- or E4 orf6-mutant viruses.
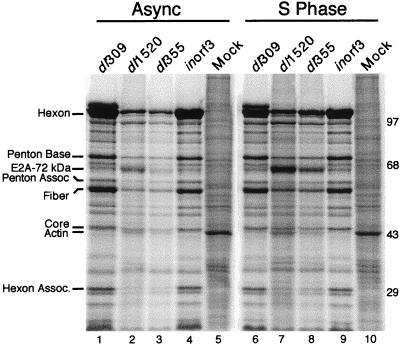
The E1B 55-kDa- and the E4 orf6-mutant viruses are defective for late gene expression due, in part, to reduced transport of late viral messages (14, 30, 43, 54). If the defects in mRNA transport and late gene expression contribute to the cell cycle restriction for growth of the E1B 55-kDa- and E4 orf6-mutant viruses, then late viral gene expression should be at least partially restored in cells infected during S phase with either of these mutant viruses. The results shown in Fig. 4 and Table 4 are consistent with this idea. The gel shown in Fig. 4 is representative of four independent experiments that are summarized in Table 4. For these experiments, cells were infected as an asynchronous population or synchronized and infected at the onset of S phase with dl309, dl1520, dl355, or inorf3 or mock infected. At 32 h postinfection, protein synthesis was evaluated by metabolically labeling the cells with 35S-amino acids for 1 h. Protein from equivalent numbers of cells was separated by SDS-PAGE and analyzed by phosphorescence imaging.
FIG. 4.
The E1B 55-kDa-mutant and E4 orf6-mutant viruses synthesize greater levels of viral late proteins in HeLa cells infected during S phase than in asynchronous (Async) cells. Asynchronous or S-phase HeLa cells were mock infected or infected with the wild-type virus dl309, the E1B 55-kDa-mutant virus dl1520, the E4 orf6-mutant virus dl355, or the E4 orf3-mutant virus inorf3 at a multiplicity of 20 PFU per cell. At 32 h postinfection, cells were labeled with 35S-labeled amino acids for 1 h. Proteins from 105 cells (per lane) were separated by SDS-PAGE. Proteins were visualized and quantified by phosphorescence imaging. The migrations and masses (in kilodaltons) or molecular weight standards are indicated to the right of the gel. The positions of six Ad late proteins were determined with Ad virion standards labeled with 14C-amino acids and are indicated to the left of the gel. The positions of the E2A 72-kDA protein and the cellular actin protein are also indicated. The gel shown is representative of four independent experiments.
TABLE 4.
Relative levels of late viral protein synthesized in asynchronous- or S-phase-infected HeLa cells
| Late protein | % of wild-type protein synthesized in asynchronous cellsa
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Async
|
S phase
|
|||||||
| dl309 | dl1520 | dl355 | inorf3 | dl309 | dl1520 | dl355 | inorf3 | |
| Hexon | 100 | 18 | 16 | 93 | 77 | 38 | 27 | 95 |
| Penton base | 100 | 20 | 16 | 76 | 92 | 45 | 29 | 98 |
| Penton associated | 100 | 36 | 25 | 76 | 84 | 98 | 53 | 87 |
| Fiber | 100 | 13 | 11 | 55 | 72 | 38 | 21 | 63 |
| Core | 100 | 54 | 31 | 70 | 107 | 124 | 59 | 93 |
| Hexon associated | 100 | 13 | 8 | 77 | 64 | 26 | 19 | 71 |
| Avg | 100 | 25 | 18 | 74 | 83 | 61 | 35 | 84 |
HeLa cells were synchronized and infected at the onset of S phase or during asynchronous (Async) growth with dl309, dl1520, dl355, or inorf3 or were mock infected. At 32 h postinfection, cells were pulse-labeled with 35S-amino acids for 1 h and then lysed. Proteins were separated on an 8% polyacrylamide gel and quantified by phosphorescence imaging. Late proteins synthesized in asynchronous cells infected with dl309 were normalized to 100 to calculate the relative percentages of protein synthesized in cells infected during asynchronous growth with the mutant viruses or cells infected during S phase with the wild-type or mutant viruses. Each value represents an average from four independent experiments. All measurements were within ±10%.
In cells infected during asynchronous growth, the E1B 55-kDa- and E4 orf6-mutant viruses synthesized reduced levels of late viral proteins (Fig. 4). These results are consistent with the defect in virus-mediated mRNA transport and with the results of others (30, 74). By contrast, cells infected with the E4 orf3-mutant virus inorf3 appeared to synthesize levels of late viral proteins equivalent to those of wild-type virus-infected cells. Cells infected during S phase synthesized slightly reduced levels of viral late proteins when infected with the wild-type virus but greater levels when cells were infected with the dl1520 or dl355 mutant virus compared to cells infected during asynchronous growth with these viruses.
For reasons that are not well understood, the E1B 55-kDa- and the E4 orf6-mutant viruses overproduce the early region 2A (E2A) 72-kDa DNA-binding protein (14, 43, 74). The E2A protein is readily identified in Fig. 4. Curiously, expression of this protein at late times in infection mimicked that of late viral proteins in HeLa cells infected with the E1B 55-kDa- and E4 orf6-mutant viruses in that its synthesis was enhanced in cells infected during S phase (Fig. 4; compare lanes 2 and 3 with 7 and 8).
The synthesis of six late viral proteins was quantified. These results are summarized in Table 4, where they are expressed as a relative percentage of the corresponding value for the wild-type virus measured in cells infected during asynchronous growth. S-phase cells infected with the wild-type virus synthesize late viral proteins to an average level of 83% of those synthesized in cells infected during asynchronous growth. These results are consistent with the finding that asynchronous or G1 cells are slightly better suited for replication of the wild-type Ad than are S-phase cells (26). Likewise, inorf3 synthesized similar amounts of late viral proteins in cells infected during S phase and in cells infected during asynchronous growth, consistent with the lack of a cell cycle restriction for inorf3 virus growth. By contrast, late viral gene expression in cells infected during S phase with either dl1520 or dl355 was increased relative to that of cells infected during asynchronous growth. The average level of late viral protein synthesis in cells infected during S phase increased from 25 to 61% for dl1520 and from 18 to 35% for dl355 compared to that for cells infected during asynchronous growth. Similar results were obtained for cells labeled at 24 h postinfection (data not shown). These results are consistent with the suggestion that S-phase cells provide a host factor that may enhance late gene expression of the E1B 55-kDa- and E4 orf6-mutant viruses (26).
The effect of Ad infection on cellular gene expression can be seen in the intensity of the band corresponding to the 42-kDa actin protein (prominent in mock-infected cells) and by the overall density of the lane, which is most evident in the portion of the gel above the resolved protein bands (Fig. 4). The shutoff of host protein synthesis by the wild-type virus dl309 is especially evident by the near loss of the actin band in either asynchronously or S-phase-infected cells. By contrast, the E1B 55-kDa-mutant dl1520 and the E4 orf6-mutant dl355 viruses were defective for host cell shutoff. For example, actin was more intensely labeled in cells infected with dl1520 or dl355 than in cells infected with dl309 (Fig. 4; compare lanes 2 and 3 to 1). Cells infected with inorf3 exhibit levels of cellular gene expression similar to that of cells infected with dl309 (Fig. 3; compare lanes 4 and 9 to 1 and 6).
Cells infected during S phase were more resistant to the shutoff of host gene expression than were cells infected during asynchronous growth. The density of cellular background and the actin band increased two- to threefold in cells infected during S phase with dl309 and inorf3 over those in cells infected during asynchronous growth (Fig. 4; compare lanes 6 and 9 to 1 and 4). Furthermore, the cellular protein background was up to fourfold greater in cells infected during S phase with either the dl1520 or dl355-mutant virus than in asynchronous cells infected with the same viruses (Fig. 4; compare lanes 7 and 8 to 2 and 3). The reason for the increase in cellular gene expression in cells infected during S phase is not known, but the increase could reflect a diminished ability of the virus to inhibit host protein synthesis or an overall increase in the export of mRNA to the cytoplasm.
Cytoplasmic levels of L3 and L5 late viral mRNAs are greater in cells infected during G1 than in cells infected during S phase.
Late viral protein synthesis was partially restored in cells infected during S phase with the E1B 55-kDa- or E4 orf6-mutant virus. To determine if this reflected greater levels of late viral mRNA in the cytoplasm of cells infected during S phase, cells were synchronized and infected and RNA was isolated at 16 h postinfection. Levels of processed late viral transcripts in the cytoplasmic and nuclear fractions were measured by RNase protection assays with probes that spanned the polyadenylation sites of the L3 and L5 families of late viral transcripts.
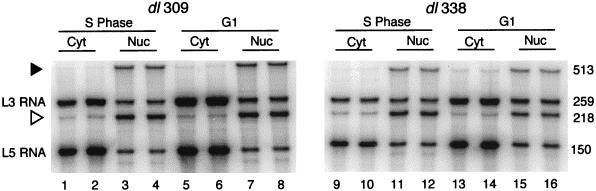
Representative results from analyzing L3 and L5 mRNA obtained from cells infected during S phase or G1 with the wild-type virus dl309 or E1B 55-kDa-mutant virus dl338 are shown in Fig. 5. For both the dl309 and the dl338 infections, the 259 (L3)- and 150 (L5)-nucleotide products expected for RNA that was polyadenylated at the L3 and L5 sites, respectively, were present in greater amounts in the cytoplasmic fraction than in the nuclear fraction of both S-phase and G1 cells. The 513- and 218-nucleotide products (arrowheads in Fig. 5) were derived from readthrough or precursor transcripts that extended beyond the L3 and L5 polyadenylation sites, respectively. The readthrough products were more abundant in the nuclear fraction, consistent with these protected products representing incompletely processed forms of the transcripts.
FIG. 5.
Relative levels of cytoplasmic (Cyt) and nuclear (Nuc) L3 and L5 late viral transcripts determined by RNase protection assays. S-phase or G1 HeLa cells were mock infected or infected with the wild-type virus dl309 or the E1B 55-kDa-mutant virus dl338 at a multiplicity of 10 PFU per cell. At 16 h postinfection, cells were fractionated, and total RNA was isolated from both cytoplasmic and nuclear fractions. L3 and L5 transcript levels were determined by RNase protection assays with RNA probes that span the polyadenylation site of the L3 and L5 families of transcripts, respectively. Protected hybrids were resolved on polyacrylamide-urea minigels. Results of a representative protection assay for S-phase and G1 cells infected with dl309 or dl338 are shown. The positions of readthrough transcripts that extended beyond the L3 (filled arrowhead) and L5 (open arrowhead) polyadenylation sites and the mature transcripts (L3 or L5) are indicated to the left of the gel. The nucleotide length (bases) of each product is indicated to the right of the gel. Duplicate RNase protection results are in adjacent lanes.
Several control experiments established that the RNase-protected products were specific for viral RNA. No signal was detected in assays of mock-infected cells, indicating that cellular sequences present did not protect the L3 or L5 probe (data not shown). In addition, the protected fragments were due to virus-specific RNA and not to viral DNA, because the protected products were not affected by digestion with RNase-free DNase but were abolished by treatment with RNase A. Furthermore, no protected products were detected in RNase protection assays containing only yeast tRNA and probe RNA (data not shown).
Cells infected with the wild-type virus dl309 during G1 contained more cytoplasmic L3 and L5 than did cells infected during S phase. This result seemed reasonable because the wild-type virus appears to grow best in cells infected during G1. Therefore, we expected that cells infected during S phase with the E1B 55-kDa-mutant virus dl338 would contain greater levels of cytoplasmic viral mRNA than would cells infected during G1. However, the levels of L3 and L5 transcripts were actually greater in cells infected during G1 than in cells infected during S phase. These results are similar to those for the wild-type virus infection (Fig. 5; compare lanes 1 and 2 to 5 and 6 and lanes 9 and 10 to 13 and 14).
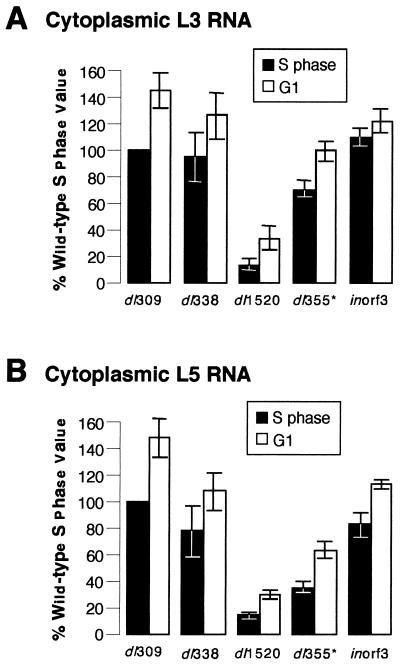
The levels of cytoplasmic viral L3 and L5 mRNA in cells infected with the wild-type virus dl309, the E1B 55-kDa-mutant viruses dl338 and dl1520, the E4 orf6-mutant virus dl355*, or the E4 orf3-mutant virus inorf3 were quantified by phosphorescence imaging. The graphs shown in Fig. 6 are averages derived from three independent experiments for which up to 10 RNase protection experiments were performed. The cytoplasmic levels of L3 and L5 transcripts from cells infected during S phase with the wild-type virus were normalized to 100%. The cytoplasmic levels of the L3 and L5 transcripts from cells infected with the wild-type virus during G1 or from cells infected with the mutant viruses during S phase or G1 are therefore represented as percentages of the value measured in S-phase cells infected with the wild-type virus. For all viruses analyzed, cells infected during G1 contained greater cytoplasmic levels of late viral mRNA than did cells infected during S phase. Cells infected during G1 with the wild-type or mutant viruses had on average 70% more cytoplasmic L3 (Fig. 6A) and L5 (Fig. 6B) mRNA than did cells infected during S phase. Although the amount of cytoplasmic viral mRNA varied significantly between experiments, within each experiment, cells infected during G1 always had more cytoplasmic L3 and L5 transcripts than did cells infected during S phase. These results imply that the levels of cytoplasmic viral mRNA cannot account for the difference in late viral protein synthesis in cells infected during S phase and G1 (Fig. 4 and Table 4).
FIG. 6.
Cytoplasmic fractions from cells infected during G1 contain greater levels of L3 and L5 viral transcripts than do those from cells infected during S phase. S-phase or G1 HeLa cells were infected with the wild-type virus dl309, the E1B 55-kDa-mutant virus dl338 or dl1520, the E4 orf6-mutant virus dl355, or the E4 orf3-mutant virus inorf3. Total RNA was isolated from cytoplasmic fractions at 16 h postinfection. L3 and L5 transcript levels were determined by RNase protection assay as described in Fig. 5. The cytoplasmic levels of L3 and L5 transcripts from S-phase cells infected with dl309 were normalized to 100 to determine the relative percentages of L3 (A) and L5 (B) transcripts in cytoplasmic fractions from cells infected during S phase with the mutant viruses or infected during G1 with the wild-type or mutant viruses. The results summarized in panels A and B are averages obtained from 10 experiments for dl309, 5 experiments for dl338, 8 experiments for dl1520, 6 experiments for dl355, and 2 experiments for inorf3. The standard errors of the means for each experiment are shown.
The differences in cytoplasmic levels of late viral mRNAs between cells infected during S phase and cells infected during G1 cannot be accounted for by differences in the amount of total RNA present between cells infected during S phase and cells infected during G1. Total RNA and poly(A)+ RNA were isolated from the cytoplasm of infected cells and quantitated by absorption spectroscopy. Cells infected during S phase with the wild-type or mutant viruses contained greater amounts of total and poly(A)+ RNA in the cytoplasm than did cells infected during G1 (data not shown). These results are consistent with the observation that total RNA content increases as noninfected cells progress from G1 through S phase (22, 68). Furthermore, these findings support the notion that Ad-infected cells retain characteristics possessed by the cell at the time of infection (25).
Cells infected during S phase or G1 with the wild-type or mutant viruses were also analyzed to determine if steady-state cytoplasmic levels of cellular transcripts were affected by the stage of the cell cycle at the time of infection. Probes for β-actin and glyceraldehyde-3-phosphate dehydrogenase were used for RNase protection assays as described above. In three independent experiments, equivalent levels of β-actin and glyceraldehyde-3-phosphate dehydrogenase transcript were measured in the cytoplasm of cells infected during S phase and cells infected during G1 (data not shown).
The cytoplasmic levels of L3 and L5 mRNA from G1 cells infected with the mutant viruses dl338, dl1520, and dl355* were reduced compared to those in cells infected with the wild-type virus (Fig. 6). This difference most likely stems from the defect in viral mRNA transport (4, 42, 54). Interestingly, the amount of late viral mRNA in the cytoplasm of dl1520-infected cells was considerably less than that measured for dl338-infected cells, although both viruses are expected to be functionally null for the E1B 55-kDa protein. This observation is consistent with dl1520 exhibiting a greater defect for growth than dl338, as seen in Fig. 3 and as discussed previously (5, 27).
Late viral mRNA transport is partially restored in cells infected during S phase with the E1B 55-kDa- and E4 orf6-mutant viruses.
Although cells infected during G1 contained more late viral mRNA in the cytoplasm than did cells infected during S phase, cells infected during S phase with the E1B 55-kDa- or E4 orf6-mutant virus appeared to exhibit enhanced mRNA transport compared to cells infected during G1 with these mutant viruses. To serve as an indirect measure of mRNA transport, we determined the cytoplasmic-to-nuclear ratio of L3 and L5 mRNA. The amount of viral mRNA in the cytoplasm and nucleus was determined by RNase protection experiments such as that presented in Fig. 5. Because this RNase protection assay identifies mRNA only by the polyadenylation event, it is possible that some of the nuclear mRNA included incompletely processed forms, whereas all of the specific product in the cytoplasm was most likely to be mature mRNA. Consequently, the measure of the cytoplasmic-to-nuclear RNA ratio may underestimate the ratio of mature cytoplasmic mRNA to mature nuclear mRNA.
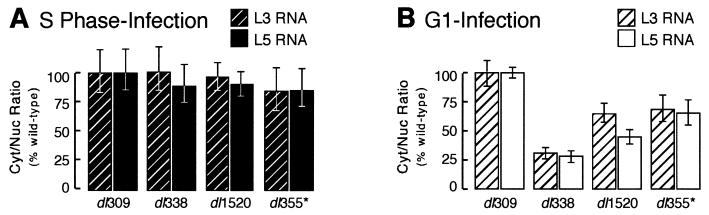
Greater cytoplasmic-to-nuclear ratios were measured for the wild-type virus when cells were infected during G1 than when cells were infected during S phase. For cells infected during S phase with the wild-type virus, characteristic cytoplasmic-to-nuclear ratios were 2.4 ± 0.5 and 1.9 ± 0.3 for L3 and L5 transcripts, respectively. By contrast, cells infected during G1 with the wild-type virus typically had cytoplasmic-to-nuclear ratios for L3 and L5 mRNAs of 3.3 ± 0.5 and 2.4 ± 0.2, respectively.
The cytoplasmic-to-nuclear ratios for L3 and L5 mRNAs from the mutant virus infections are represented as a fraction of the ratio determined for the wild-type infection in Fig. 7. The cytoplasmic-to-nuclear ratios for both L3 and L5 mRNAs from cells infected with the mutant viruses during S phase were statistically indistinguishable from that of the wild-type virus. This similarity in the efficiency of mRNA transport is due not only to increased cytoplasmic-to-nuclear ratios for late viral mRNA in cells infected with the E1B 55-kDa and E4 orf6-mutant viruses during S phase but also to a corresponding decrease in the cytoplasmic-to-nuclear ratios for late viral mRNA in S-phase cells infected with the wild-type virus. By contrast, the cytoplasmic-to-nuclear ratios of viral L3 and L5 transcripts from cells infected during G1 with the mutant viruses was as much as fivefold reduced compared to the wild-type virus infection. These results indicate that the transport of late viral mRNAs is greater in cells infected during S phase with the E1B 55-kDa- and E4 orf6-mutant viruses than in cells infected during G1. From these results, we conclude that Ad-mediated mRNA transport is differentially regulated depending on the stage of the cell cycle at the time of infection. Nonetheless, this apparent increase in transport did not result in greater levels of cytoplasmic viral mRNAs (Fig. 6).
FIG. 7.
S-phase cells infected with the E1B 55-kDa-mutant or the E4 orf6-mutant virus yield greater cytoplasmic (Cyt)-to-nuclear (Nuc) ratios for L3 and L5 transcripts than do G1 cells. S-phase or G1 HeLa cells were mock infected or infected with the wild-type virus dl309, the E1B 55-kDa-mutant virus dl338 or dl1520, or the E4 orf6-mutant virus dl355 at a multiplicity of 10 PFU per cell. At 16 h postinfection, cells were fractionated and total RNA was isolated from both cytoplasmic and nuclear fractions. L3 and L5 transcript levels were determined by RNase protection assays with RNA probes that span the polyadenylation sites of the L3 and L5 families of transcripts, respectively. Relative levels of cytoplasmic and nuclear L3 and L5 mRNAs were determined by phosphorescence imaging and were normalized to total RNA to account for inconsistencies in RNA recovery. The cytoplasmic-to-nuclear ratios from representative S-phase (A) and G1 (B) infections are expressed as percentages of the ratios for the wild-type infections. The standard errors of the means are shown.
DISCUSSION
We have demonstrated a role for the E4 orf6 protein similar to that of the E1B 55-kDa protein in overcoming cell cycle restrictions imposed on virus growth. E1B 55-kDa- and E4 orf6-mutant viruses that are defective for virus-mediated mRNA transport were restricted by the cell cycle for growth (Fig. 1 and 2; Tables 2 and 3). Cells infected during S phase with the E1B 55-kDa- or E4 orf6-mutant viruses had greater cytoplasmic-to-nuclear ratios of L3 and L5 late transcripts (Fig. 7) and synthesized greater levels of late proteins (Fig. 4 and Table 4) than did cells infected with these mutant viruses during G1 or asynchronous growth. Furthermore, these mutant viruses produced greater yields of virus from cells that were infected during S phase (Fig. 2 and 3). Nevertheless, factors other than late viral gene expression are involved in permitting cell cycle-independent replication, since accumulation of late viral messages in the cytoplasm of mutant-infected cells did not correlate with enhanced growth in cells infected during S phase (Fig. 6). An E4 orf3-mutant virus was not restricted by the cell cycle for replication (Fig. 1 and 2 and Table 2). However, enhanced growth of the E4 orf6-mutant viruses in cells infected during S phase required expression of the E4 orf3 protein (Fig. 2 and Table 2). These studies have demonstrated novel roles for the E4 orf6 and orf3 proteins in promoting cell cycle-independent Ad growth in cooperation with the E1B 55-kDa protein.
In addition to promoting cell cycle-independent growth, the E1B 55-kDa and E4 orf6 proteins function as a complex to mediate both viral and cellular mRNA transport (4, 6, 30, 42, 54, 76). The function of the E1B 55-kDa and E4 orf6 proteins in mediating viral and cellular mRNA transport, and thus gene expression, late in infection may underlie cell cycle-independent Ad growth. When cells were infected during S phase with the E1B 55-kDa- or E4 orf6-mutant virus, the defect in late viral gene expression was partially restored such that cells infected during S phase with the mutant virus synthesized greater levels of late viral proteins than did cells infected during asynchronous growth (Fig. 4 and Table 4). Interestingly, the defect in late gene expression and growth can also be ovecome by infecting and maintaining cells at elevated temperatures during the infection (27, 32, 42, 74). Furthermore, the cell cycle restriction was alleviated in cells infected at elevated temperatures in a manner resembling that observed in cells infected during S phase such that mutant virus was produced in approximately 70% of the infected cells (27). These findings suggest that a common mechanism may underlie cell cycle restriction and virus-mediated mRNA transport, since both defects exhibit a cold-sensitive phenotype.
We measured cytoplasmic and nuclear levels of the L3 and L5 late viral transcripts in cells infected during S phase or G1 to more directly analyze the link between virus-mediated mRNA transport and the cell cycle restriction (Fig. 5). The cytoplasmic-to-nuclear ratio of viral transcripts reflects the combined contributions of transport from the nucleus to the cytoplasm and stability of these transcripts in the respective cellular compartment (54). Because the stability of late viral mRNA did not vary with the infecting virus or stage of the cell cycle (data not shown), we conclude that the cytoplasmic-to-nuclear ratio provides a reasonable measure of viral mRNA transport. Cells infected during G1 with the wild-type virus had greater cytoplasmic-to-nuclear ratios of L3 and L5 mRNA than did cells infected during S phase with either wild-type or mutant virus (Fig. 7). By contrast, cells infected during S phase with the E1B 55-kDa- or E4 orf6-mutant virus had greater cytoplasmic-to-nuclear ratios of L3 and L5 mRNA than did cells infected during G1 with these mutant viruses. In addition, this ratio was equivalent among S-phase cells infected with either wild-type or mutant virus. Therefore, the apparent ability of the mutant viruses to transport viral mRNA in cells infected during S phase with wild-type-like efficiency results from an increase in transport of late viral mRNA in cells infected with the mutant viruses and a decrease in transport of late viral mRNA in cells infected with the wild-type virus during S phase. These results indicate that the transport of late viral mRNA is differentially regulated in response to the stage of the cell cycle at the time of infection.
Although the transport of late viral mRNA was restored to wild-type efficiency in S-phase cells infected with the E1B 55-kDa- or E4 orf6-mutant viruses (Fig. 7), greater cytoplasmic levels of late viral transcripts were measured in cells infected during G1 with either the wild-type or mutant viruses than in cells infected during S phase (Fig. 6). The basis for enhanced accumulation of late viral mRNA in the G1-infected cell is not known, but the accumulation may, for example, reflect differential rates of viral transcription as a function of the cell cycle. Nonetheless, the enhanced synthesis of late viral proteins and enhanced virus growth seen for the S-phase-infected cell was unique to the cell cycle-restricted mutant viruses analyzed here. Perhaps other factors contribute to an increased translational efficiency of the late viral messages in S-phase cells infected with the RNA transport-defective viruses (Fig. 4 and Table 4). Also, since the differential transport of mRNA that occurs as a function of the cell cycle did not impact steady-state levels of cytoplasmic late viral mRNA, we suggest that this control may have a greater significance in regulating expression of cellular genes that impact Ad growth.
Perhaps cells infected during S phase are more promiscuous for the transport of all mRNA. As a result, late viral transcript levels are reduced by the increase in cellular message transport and the resulting competition for limited transport machinery. This suggestion is consistent with the finding that cells infected during S phase with the wild-type or mutant Ads synthesize more cellular proteins than do cells infected during G1 (Fig. 4). If growth of the wild-type virus is facilitated by host cell shutoff, then the reduced shutoff seen in S-phase-infected cells may hinder replication of the wild-type virus (Fig. 3 and 4 and Table 4). The RNA transport-defective viruses, on the other hand, may derive greater benefit from the promiscuous RNA transport apparent in the S-phase-infected cell. This benefit may lie not so much in the transport of late viral genes as in the enhanced transport and expression of a subset of cellular genes. Conceivably, expression of these cellular genes requires the E1B 55-kDa–E4 orf6 protein complex in cells infected during stages of the cell cycle other than S phase. Transcripts of several cellular genes, such as the hsp-70 and β-tubulin genes and the interferon-inducible genes Mx-A and 6-16, are selectively transported late in Ad infection. The selective transport of host transcripts depends on the E1B 55-kDa protein and stimulation of their transcription in the late phase of infection (47, 76). Perhaps the transport of host transcripts such as these is facilitated for the purpose of viral replication.
It has been previously suggested that a positively acting factor may be recruited to the viral inclusions that are nuclear compartments of viral RNA processing by the E1B 55-kDa–E4 orf6 complex to facilitate mRNA transport and cell cycle-independent virus growth (52). Perhaps this factor is abundant in S-phase cells and can promote the transport of RNA in the absence of the E1B 55-kDa–E4 orf6 complex. As suggested by our previous work, such a factor may be unique to primate cells (28). Several cellular proteins function in both mRNA transport and cell cycle regulation such that these processes may be linked to some extent (21, 23, 41, 62, 63, 67, 69). These proteins may be targets of the E1B 55-kDa or E4 orf6 proteins. For example, a novel cellular heterogeneous nuclear ribonucleoprotein, E1B AP5, associates with the E1B 55-kDa protein and overcomes the E1B 55-kDa-dependent inhibition of cellular mRNA export when overexpressed (24). It would be of interest to know if the expression or function of E1B AP5 is differentially regulated through the cell cycle.
In contrast to the E1B 55-kDa- and E4 orf6-mutant viruses, the E4 orf3-mutant virus inorf3 produced virus in nearly every infected cell and thus was not restricted by the cell cycle for growth (Fig. 1). Nevertheless, in the absence of the E4 orf6 gene, the orf3 gene was essential for enhanced replication of the E4 orf6-mutant viruses in S-phase-infected cells (Table 2 and Fig. 2). Growth of the E4 orf3-orf6 double-mutant viruses in cells infected during G1 was restricted to a fraction of infected cells, similar to that of the E4 orf6-mutant viruses (Table 2). However, when cells were infected during S phase with the E4 orf3-orf6-double-mutant viruses the fraction of infected cells producing virus did not increase (Table 2) and these cells did not produce greater yields of virus than did cells infected during G1 (Fig. 2). These results indicate that E4 orf3-orf6 double-mutant viruses may be restricted for growth by mechanisms other than the cell cycle.
It is not clear what mechanism other than the cell cycle restriction may limit virus production to a fraction of infected cells. Perhaps there is a limited probability (20 to 30%) that a cell infected with the E1B 55-kDa- or E4 orf6-mutant virus will produce progeny virus. This probability can be modulated by a number of factors such as the stage of the cell cycle at the time of infection, the temperature at which the infection is maintained, and expression of the E4 orf3 gene. Although the specific function of the E4 orf3 gene product in promoting cell cycle-independent growth is not understood, these results demonstrate that, in the absence of other E4 proteins, the E4 orf3 protein acts in the S-phase-infected cell to overcome restrictions on virus growth. In this regard, it is interesting to note that expression of an unidentified E4 gene product(s) in recombinant, replication-defective Ad vectors other than the orf6 protein resulted in dysregulation of the cell cycle and inappropriate cyclin expression (73). Perhaps the E4 orf3 protein contributes to such an effect.
Since the restriction for the E1B 55-kDa-mutant viruses is similar to that for the E4 orf6-mutant viruses, we speculate that the E1B 55-kDa-mutant viruses may also rely on the presence of the E4 orf3 gene for enhanced growth in cells infected during S phase. The interaction of these two viral proteins is suggested by the findings that the E1B 55-kDa protein is physically associated with the E4 orf3 protein (49) and colocalizes with the E4 orf3 protein in the absence of other E4 proteins (39). Preliminary results suggest that enhanced growth of the E1B 55-kDa-mutant virus requires an intact E4 orf3 gene (25). Such a finding would imply that the E4 orf3 protein is not necessary for cell cycle-independent replication in the presence of the E1B 55-kDa and E4 orf6 proteins but is necessary to establish the permissive state in S-phase cells infected with the E1B 55-kDa or E4 orf6-mutant virus. If substantiated, these results have implications for the development of oncolytic Ad vectors for the treatment of cancer. These results would predict that the E4 orf3 gene may be essential for the selective replication of oncolytic Ad vectors that lack the E1B 55-kDa gene in tumor cells (7, 31).
The contribution of the E4 orf3 protein to enhanced growth of E4 orf6-mutant viruses in S-phase cells may be linked to its role in disrupting nuclear domains called ND10s or promyelocytic leukemia oncogenic domains. Redistribution of proteins sequestered within ND10s, such as PML and Sp100, may trigger cell cycle dysregulation and oncogenesis, as in acute promyelocytic leukemia (reviewed in reference 17). The functions of ND10-associated proteins are not well understood, but the proteins have been hypothesized to promote cellular events analogous to the activation of a viral program of replication and may function in cell cycle regulation (2, 17–19). The morphology of ND10s changes with cell cycle progression, becoming more amorphous and diffuse, perhaps reflecting an active state, when cells enter S phase (19). Furthermore, localization of PML and Sp100 to interchromatin granules suggests that ND10-associated proteins may function in mRNA metabolism (56). The E1A and E1B 55-kDa proteins localize to ND10s early in infection (13, 18). Late in infection, some ND10-associated proteins are translocated to the viral inclusions (18, 56). Perhaps the E4 orf3 protein disrupts these domains to make ND10-associated proteins accessible to E1B 55-kDa and E4 orf6 proteins for the promotion of cell cycle-independent replication or virus-mediated mRNA transport. In the absence of the E1B 55-kDa or E4 orf6 proteins during Ad infection, ND10-associated proteins may contribute to the virus infection when cells are infected during S phase with a virus that expresses the E4 orf3 protein. Intriguingly, the ICP0 protein of herpes simplex virus type 1 (20, 40, 44, 59) and the IE1 and IE2 proteins of human cytomegalovirus (1, 38) also induce redistribution of ND10-associated proteins to viral inclusions, strongly suggesting a role for these proteins in viral replication.
In summary, we have demonstrated a role for the E4 orf6 protein in overcoming cell cycle restrictions imposed on virus growth. This role is similar to that described for the E1B 55-kDa protein (26). Our results indicate that the lytic infection of Ad is differentially affected by the stage of the cell cycle at the time of infection with respect to virus-mediated mRNA transport, late gene expression, and virus growth. Interestingly, the E4 orf3 protein was required for enhanced replication of E4 orf6-mutant viruses in cells infected during S phase. The mechanisms employed by Ad to promote cell cycle-independent virus growth and their relationship to mRNA biogenesis warrant continued investigation.
ACKNOWLEDGMENTS
This work was supported in part by Public Health Service grants AI35589 from the National Institute of Allergy and Infectious Disease and CA77342 from the National Cancer Institute and a grant from the North Carolina Biotechnology Center. Tissue culture reagents and services were provided by the Tissue Culture Core Laboratory of the Comprehensive Cancer Center of Wake Forest University, supported in part by NIH grant CA12197.
We gratefully acknowledge Tom Shenk (Princeton University) for the dl309, dl337, dl338, and dl355 viruses; Arnie Berk (UCLA) for the dl1520D, A143, S380, and R443 viruses; Pat Hearing (SUNY at Stony Brook) for the inorf3, inorf6, inorf6/7, inorf3/inorf6, dl355*, dl355*/inorf3, dl366*, dl366*+orf1-2, dl366*+orf3, and dl366*+orf4 viruses; and Gary Ketner (Johns Hopkins) for the W162 cells. We thank Doug Lyles and Griff Parks (Wake Forest University) for critically reading the manuscript and for scientific discussions. We also thank Ken Grant and Nora Zbieranski of Wake Forest University for invaluable assistance with electron microscopy and fluorescence-activated cell sorting analysis, respectively.
REFERENCES
- 1.Ahn J H, Hayward G S. The major immediate-early proteins IE1 and IE2 of human cytomegalovirus colocalize with and disrupt PML-associated nuclear bodies at very early times in infected permissive cells. J Virol. 1997;71:4599–4613. doi: 10.1128/jvi.71.6.4599-4613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ascoli C A, Maul G G. Identification of a novel nuclear domain. J Cell Biol. 1991;112:785–795. doi: 10.1083/jcb.112.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K S, editors. Current protocols in molecular biology. Vol. 2. New York, N.Y: Greene Publishing Associates and John Wiley and Sons, Inc.; 1993. [Google Scholar]
- 4.Babiss L E, Ginsberg H S, Darnell J E. Adenovirus E1B proteins are required for accumulation of late viral mRNA and for effects on cellular mRNA translation and transport. Mol Cell Biol. 1985;5:2552–2558. doi: 10.1128/mcb.5.10.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker D D, Berk A J. Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology. 1987;156:107–121. doi: 10.1016/0042-6822(87)90441-7. [DOI] [PubMed] [Google Scholar]
- 6.Beltz G A, Flint S J. Inhibition of HeLa cell protein synthesis during adenovirus infection. Restriction of cellular messenger RNA sequences to the nucleus. J Mol Biol. 1979;131:353–373. doi: 10.1016/0022-2836(79)90081-0. [DOI] [PubMed] [Google Scholar]
- 7.Bischoff J R, Kim D H, Williams A, Heise C, Horn S, Muna M, Ng L, Nye J A, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicate selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 8.Bordonaro M, Saccomanno C F, Nordstrom J L. An improved T1/A ribonuclease protection assay. BioTechniques. 1994;16:428–430. [PubMed] [Google Scholar]
- 9.Brewer G, Ross J. Messenger RNA turnover in cell-free extracts. Methods Enzymol. 1990;181:202–209. doi: 10.1016/0076-6879(90)81122-b. [DOI] [PubMed] [Google Scholar]
- 10.Bridge E, Ketner G. Interaction of adenoviral E4 and E1B products in late gene expression. Virology. 1990;174:345–353. doi: 10.1016/0042-6822(90)90088-9. [DOI] [PubMed] [Google Scholar]
- 11.Bridge E, Ketner G. Redundant control of adenovirus late gene expression by early region 4. J Virol. 1989;63:631–638. doi: 10.1128/jvi.63.2.631-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brigati D J, Myerson D, Leary J J, Spalholz B, Travis S Z, Fong C K Y, Hsiung G D, Ward D C. Detection of viral genomes in cultured cells and paraffin-embedded tissue sections using biotin-labeled hybridization probes. Virology. 1983;126:32–50. doi: 10.1016/0042-6822(83)90460-9. [DOI] [PubMed] [Google Scholar]
- 13.Carvalho T, Seeler J S, Ohman K, Jordan P, Pettersson U, Akusjarvi G, Carmo-Fonseca M, Dejean A. Targeting of adenovirus E1A and E4-ORF3 proteins to nuclear matrix-associated PML bodies. J Cell Biol. 1995;131:45–56. doi: 10.1083/jcb.131.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutt J R, Shenk T, Hearing P. Analysis of adenovirus early region 4-encoded polypeptides synthesized in productively infected cells. J Virol. 1987;61:543–552. doi: 10.1128/jvi.61.2.543-552.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debbas M, White E. Wild-type p53 mediates apoptosis by E1A, which is inhibited by E1B. Genes Dev. 1993;7:546–554. doi: 10.1101/gad.7.4.546. [DOI] [PubMed] [Google Scholar]
- 16.Dobner T, Horikoshi N, Rubenwolf S, Shenk T. Blockage by adenovirus E4orf6 of transcriptional activation by p53 tumor suppressor. Science. 1996;262:1470–1473. doi: 10.1126/science.272.5267.1470. [DOI] [PubMed] [Google Scholar]
- 17.Doucas V, Evans R M. The PML nuclear compartment and cancer. Biochim Biophys Acta. 1996;1288:M25–M29. doi: 10.1016/s0304-419x(96)00028-5. [DOI] [PubMed] [Google Scholar]
- 18.Doucas V, Ishov A M, Romo A, Juguilon H, Weitzman M D, Evans R M, Maul G G. Adenovirus replication is couple with the dynamic properties of the PML nuclear structure. Genes Dev. 1996;10:196–207. doi: 10.1101/gad.10.2.196. [DOI] [PubMed] [Google Scholar]
- 19.Dyck J A, Maul G G, Miller W, Chen J D, Kakizuka A, Evans R M. A novel macromolecular structure is a target of the promyelocyte-retinoic acid receptor oncoprotein. Cell. 1994;76:333–343. doi: 10.1016/0092-8674(94)90340-9. [DOI] [PubMed] [Google Scholar]
- 20.Everett R D, Maul G G. HSV-1 IE protein Vmw110 causes redistribution of PML. EMBO J. 1994;13:5062–5069. doi: 10.1002/j.1460-2075.1994.tb06835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fornerod M, Ohno M, Yoshida M, Mattaj I W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 22.Fujikawa-Yamamoto K. The relationship between length of the cell cycle duration and RNA content in HeLa S3 cells. Cell Struct Funct. 1983;8:303–308. doi: 10.1247/csf.8.303. [DOI] [PubMed] [Google Scholar]
- 23.Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- 24.Gabler S, Schutt H, Groitl P, Wolf H, Shenk T, Dobner T. E1B 55-kilodalton-associated protein: a cellular protein with RNA-binding activity implicated in nucleocytoplasmic transport of adenovirus and cellular mRNAs. J Virol. 1998;72:7960–7971. doi: 10.1128/jvi.72.10.7960-7971.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodrum, F. D., and D. A. Ornelles. Unpublished results.
- 26.Goodrum F D, Ornelles D A. The early region 1B 55-kilodalton oncoprotein of adenovirus relieves growth restrictions imposed on viral replication by the cell cycle. J Virol. 1997;71:548–561. doi: 10.1128/jvi.71.1.548-561.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodrum F D, Ornelles D A. p53 status does not determine outcome of E1B 55-kilodalton mutant adenovirus lytic infection. J Virol. 1998;72:9479–9490. doi: 10.1128/jvi.72.12.9479-9490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodrum F D, Shenk T, Ornelles D A. Adenovirus early region 4 34-kilodalton protein directs the nuclear localization of the early region 1B 55-kilodalton protein in primate cells. J Virol. 1996;70:6323–6335. doi: 10.1128/jvi.70.9.6323-6335.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham F L, Smiley J, Russell W C, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–72. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 30.Halbert D N, Cutt J R, Shenk T. Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host cell shutoff. J Virol. 1985;56:250–257. doi: 10.1128/jvi.56.1.250-257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heise C, Sampson-Johannes A, Williams A, McCormick F, von Hoff D D, Kirn D H. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639–644. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 32.Ho Y-S, Galos R, Williams J. Isolation of type 5 adenovirus mutants with a cold-sensitive host range phenotype: genetic evidence of an adenovirus transformation maintenance function. Virology. 1982;122:109–124. doi: 10.1016/0042-6822(82)90381-6. [DOI] [PubMed] [Google Scholar]
- 33.Hodge L D, Scharff M D. Effect of adenovirus on host cell DNA synthesis in synchronized cells. Virology. 1969;37:554–564. doi: 10.1016/0042-6822(69)90273-6. [DOI] [PubMed] [Google Scholar]
- 34.Huang M M, Hearing P. Adenovirus early region 4 encodes two gene products with redundant effects in lytic infection. J Virol. 1989;63:2605–2615. doi: 10.1128/jvi.63.6.2605-2615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones N, Shenk T. Isolation of Ad5 host range deletion mutants defective in transformation of rat embryo cells. Cell. 1979;17:683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- 36.Jones N, Shenk T. Isolation of deletion and substitution mutants of adenovirus type 5. Cell. 1978;13:181–186. doi: 10.1016/0092-8674(78)90148-4. [DOI] [PubMed] [Google Scholar]
- 37.Kao C C, Yew P R, Berk A J. Domains required for in vitro association between the cellular p53 and the adenovirus 2 E1B 55kD proteins. Virology. 1990;179:806–814. doi: 10.1016/0042-6822(90)90148-k. [DOI] [PubMed] [Google Scholar]
- 38.Kelly C, van Driel R, Wilkinson G W G. Disruption of PML-associated nuclear bodies during human cytomegalovirus infection. J Gen Virol. 1995;76:2887–2893. doi: 10.1099/0022-1317-76-11-2887. [DOI] [PubMed] [Google Scholar]
- 39.Konig C, Roth J, Dobbelstein M. Adenovirus type 5 E4orf3 protein relieves p53 inhibition by E1B-55-kilodalton protein. J Virol. 1999;73:2253–2262. doi: 10.1128/jvi.73.3.2253-2262.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korioth F, Gieffers C, Maul G G, Frey J. Molecular characterization of NDP52, a novel protein of the nuclear domain 10, which is redistributed upon virus infection and interferon treatment. J Cell Biol. 1995;130:1–13. doi: 10.1083/jcb.130.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kornbluth S, Dasso M, Newport J. Evidence for a dual role for TC4 protein in regulating nuclear structure and cell cycle progression. J Cell Biol. 1994;125:705–719. doi: 10.1083/jcb.125.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leppard K N, Shenk T. The adenovirus E1B-55 kD protein influences mRNA transport via an intranuclear effect on RNA metabolism. EMBO J. 1989;8:2329–2336. doi: 10.1002/j.1460-2075.1989.tb08360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Logan J, Pilder S, Shenk T. Functional analysis of adenovirus type-5 early region 1B. Cancer Cells. 1984;2:527–532. [Google Scholar]
- 44.Maul G G, Guldner H H, Spivack J G. Modification of discrete nuclear domains induced by herpes simplex virus type 1 immediate early gene product ICPO. J Gen Virol. 1993;74:2679–2690. doi: 10.1099/0022-1317-74-12-2679. [DOI] [PubMed] [Google Scholar]
- 45.Melton D A, Krieg P A, Rebagliati M R, Maniatis T, Zinn K, Greene M R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing the bacteriophage SP6 promoter. Nucleic Acids Res. 1984;12:7035–7055. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore M, Horikoshi N, Shenk T. Oncogenic potential of the E4orf6 protein. Proc Natl Acad Sci USA. 1996;93:1195–11301. doi: 10.1073/pnas.93.21.11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore M, Schaack J, Baim S B, Morimoto R I, Shenk T. Induced heat shock mRNAs escape the nucleocytoplasmic transport block in adenovirus-infected HeLa cells. Mol Cell Biol. 1987;7:4505–4512. doi: 10.1128/mcb.7.12.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nevels M, Rubenwolf S, Spruss T, Wolf H, Dobner T. The adenovirus E4orf6 protein can promoter E1A/E1B-induced focus formation by interfering with p53 tumor suppressor function. Proc Natl Acad Sci USA. 1997;94:1206–1211. doi: 10.1073/pnas.94.4.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nevels M, Tauber B, Kremmer E, Spruss T, Wolf H, Dobner T. Transforming potential of the adenovirus type 5 E4orf3 protein. J Virol. 1999;73:1591–1600. doi: 10.1128/jvi.73.2.1591-1600.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nordqvist K, Ohman K, Akusjarvi G. Human adenovirus encodes two proteins which have opposite effects on accumulation of alternatively spliced mRNAs. Mol Cell Biol. 1994;14:437–445. doi: 10.1128/mcb.14.1.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohman K, Nordqvist K, Akusjarvi G. Two adenovirus proteins with redundant activities in virus growth facilitate tripartite leader mRNA accumulation. Virology. 1993;194:50–58. doi: 10.1006/viro.1993.1234. [DOI] [PubMed] [Google Scholar]
- 52.Ornelles D A, Shenk T. Localization of the adenovirus early region 1B 55-kilodalton protein during lytic infection: association with nuclear viral inclusions requires the early region 4 34-kilodalton protein. J Virol. 1991;65:424–439. doi: 10.1128/jvi.65.1.424-429.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pilder S, Logan J, Shenk T. Deletion of the gene encoding the adenovirus 5 early region 1B 21,000-molecular-weight polypeptide leads to degradation of viral and host cell DNA. J Virol. 1984;52:664–671. doi: 10.1128/jvi.52.2.664-671.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pilder S, Moore M, Logan J, Shenk T. The adenovirus E1B-55K transforming polypeptide modulates transport or cytoplasmic stabilization of viral and host cell mRNAs. Mol Cell Biol. 1986;6:470–476. doi: 10.1128/mcb.6.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Puvion-Dutilleul F, Bachellerie J-P, Visa N, Puvion E. Rearrangements of intranuclear structures involved in RNA processing in response to adenovirus infection. J Cell Biol. 1994;107:1457–1468. doi: 10.1242/jcs.107.6.1457. [DOI] [PubMed] [Google Scholar]
- 56.Puvion-Dutilleul F, Chelbi-Alix M K, Koken M, Quignon F, Puvion E, deThe H. Adenovirus infection induces rearrangements in the intranuclear distribution of the nuclear-body associated PML protein. Exp Cell Res. 1995;218:9–16. doi: 10.1006/excr.1995.1125. [DOI] [PubMed] [Google Scholar]
- 57.Puvion-Dutilleul F, Puvion E. Analysis by in situ hybridization and autoradiography of sites of replication and storage of single- and double-stranded adenovirus type 5 DNA in lytically infected HeLa cells. J Struct Biol. 1990;103:280–289. doi: 10.1016/1047-8477(90)90046-f. [DOI] [PubMed] [Google Scholar]
- 58.Puvion-Dutilleul F, Roussev R, Puvion E. Distribution of viral RNA molecules during the adenovirus type 5 infectious cycle in HeLa cells. J Struct Biol. 1992;108:209–220. doi: 10.1016/1047-8477(92)90021-2. [DOI] [PubMed] [Google Scholar]
- 59.Puvion-Dutilleul F, Venturini L, Guillemin M C, deThe H, Puvion E. Sequestration of PML and Sp100 proteins in an intranuclear viral structure during herpes simplex virus type 1 infection. Exp Cell Res. 1995;221:448–461. doi: 10.1006/excr.1995.1396. [DOI] [PubMed] [Google Scholar]
- 60.Querido E, Marcellus R C, Lai A, Charbonneau R, Teodoro J G, Ketner G, Branton P E. Regulation of p53 levels by the E1B 55-kilodalton protein and E4orf6 in adenovirus-infected cells. J Virol. 1997;71:3788–3798. doi: 10.1128/jvi.71.5.3788-3798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rao L, Debbas M, Sabbatini D, Hockenberry D, Korsmeyer S, White E. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc Natl Acad Sci USA. 1992;89:7742–7746. doi: 10.1073/pnas.89.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ren M, Coutavas E, D’Eustachio P, Rush M G. Effects of mutant Ran/TC4 proteins on cell cycle progression. Mol Cell Biol. 1994;14:4216–4224. doi: 10.1128/mcb.14.6.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ren M, Drivas G, D’Eustachio P, Rush M G. Ran/Tc4: a small nuclear GTP-binding protein that regulates DNA synthesis. J Cell Biol. 1993;120:313–323. doi: 10.1083/jcb.120.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rothmann T, Hengstermann A, Whitaker N J, Scheffner M, zur Hausen H. Replication of ONYX-015, a potential anticancer adenovirus, is independent of p53 status in tumor cells. J Virol. 1998;72:9470–9478. doi: 10.1128/jvi.72.12.9470-9478.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sandler A B, Ketner G. Adenovirus early region 4 is essential for normal stability of late nuclear RNAs. J Virol. 1989;63:624–630. doi: 10.1128/jvi.63.2.624-630.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sandler A B, Ketner G. The metabolism of host mRNAs in cells infected by an adenovirus E4 mutant. Virology. 1991;181:319–326. doi: 10.1016/0042-6822(91)90498-z. [DOI] [PubMed] [Google Scholar]
- 67.Schlenstedt G, Saaverda C, Loeb J D J, Cole C N, Silver P A. The GTP-bound form of the yeast Ran/TC4 homologue blocks nuclear protein import and the appearance of poly(A)+ RNA in the cytoplasm. Proc Natl Acad Sci USA. 1995;92:225–229. doi: 10.1073/pnas.92.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Skog S, Tribukait B. Discontinuous RNA and protein synthesis and accumulation during cell cycle of Ehrlich ascites tumour cells. Exp Cell Res. 1985;159:510–518. doi: 10.1016/s0014-4827(85)80024-0. [DOI] [PubMed] [Google Scholar]
- 69.Stade K, Ford C S, Guthrie C, Weis K. Exportin 1 (CRM1p) is an essential nuclear export factor. Cell. 1997;90:1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- 70.Steegenga W T, van Laar T, Roteco N, Mandarino A, Shavarts A, van der Eb A J, Jochemsen A G. Adenovirus E1A proteins inhibit activation of transcription by p53. Mol Cell Biol. 1996;16:2101–2109. doi: 10.1128/mcb.16.5.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Voelkerding K, Klessig D F. Identification of two nuclear subclasses of the adenovirus type 5-encoded DNA binding protein. J Virol. 1986;60:353–362. doi: 10.1128/jvi.60.2.353-362.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weinberg D H, Ketner G. A cell line that supports the growth of a defective early region 4 deletion mutant of human adenovirus type 2. Proc Natl Acad Sci USA. 1983;80:5383–5386. doi: 10.1073/pnas.80.17.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wersto R P, Rosenthal E R, Seth P K, Eissa N T, Donahue R E. Recombinant, replication-defective adenovirus gene transfer vectors induce cell cycle dysregulation and inappropriate expression of cyclin proteins. J Virol. 1998;72:9491–9502. doi: 10.1128/jvi.72.12.9491-9502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Williams J, Karger B D, Ho Y S, Castiglia C L, Mann T, Flint S J. The adenovirus E1B 495R protein plays a role in regulating the transport and stability of the viral late messages. Cancer Cells. 1986;4:275–284. [Google Scholar]
- 75.Wolgemuth D J, Hsu M-T. Visualization of nascent RNA transcripts and simultaneous transcription and replication in viral nucleoprotein complexes from adenovirus 2-infected HeLa cells. J Mol Biol. 1981;147:247–268. doi: 10.1016/0022-2836(81)90440-x. [DOI] [PubMed] [Google Scholar]
- 76.Yang U-C, Huang W, Flint S J. mRNA export correlates with activation of transcription in human subgroup C adenovirus-infected cells. J Virol. 1996;70:4071–4080. doi: 10.1128/jvi.70.6.4071-4080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yew P R, Berk A J. Inhibition of p53 transactivation required for transformation by adenovirus early 1B protein. Nature. 1992;357:82–85. doi: 10.1038/357082a0. [DOI] [PubMed] [Google Scholar]
- 78.Yew P R, Kao C C, Berk A J. Dissection of functional domains in the adenovirus 2 early 1B 55kD polypeptide by suppressor-linked insertional mutagenesis. Virology. 1990;179:795–805. doi: 10.1016/0042-6822(90)90147-j. [DOI] [PubMed] [Google Scholar]
- 79.Yew R P, Lui X, Berk A J. Adenovirus E1B oncoprotein tethers a transcriptional repression domain to p53. Genes Dev. 1994;8:190–202. doi: 10.1101/gad.8.2.190. [DOI] [PubMed] [Google Scholar]
- 80.Zantema A, Fransen J A M, Davis-Oliver A, Ramaekers F C S, Vooijs G P, DeLeys B, van der Eb A J. Localization of the E1B proteins of adenovirus type 5 in transformed cells as revealed by interaction with monoclonal antibodies. Virology. 1985;142:44–58. doi: 10.1016/0042-6822(85)90421-0. [DOI] [PubMed] [Google Scholar]
- 81.Zantema A, Schrier P I, Davis-Oliver A, van Laar T, Vaessen R T M J, van der Eb A J. Adenovirus serotype determines association and localization of the large E1B tumor antigen and cellular tumor antigen p53 in transformed cells. Mol Cell Biol. 1985;5:3084–3091. doi: 10.1128/mcb.5.11.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]