Abstract
Significance:
Coronary artery disease (CAD) is commonly treated using percutaneous coronary interventions (PCI). However, PCI with stent placement damages the endothelium, and failure to restore endothelial function may result in PCI failure with poor patient outcomes.
Recent Advances:
Oxidative signaling is central to maintaining endothelial function. Potentiation of oxidant production, as observed post-PCI, results in endothelial dysfunction (ED). This review delves into our current understanding of the physiological role that endothelial-derived oxidants play within the vasculature and the effects of altered redox signaling during dysfunction. We then examine the impact of PCI and intracoronary stent placement on oxidant production in the endothelium, which can culminate in stent failure. Finally, we explore how recent advances in PCI and stent technologies aim to mitigate PCI-induced oxidative damage and improve clinical outcomes.
Critical Issues:
Current PCI technologies exacerbate cellular oxidant levels, driving ED. If left uncontrolled, oxidative signaling leads to increased intravascular inflammation, restenosis, and neoatherosclerosis.
Future Directions:
Through the development of novel biomaterials and therapeutics, we can limit PCI-induced oxidant production, allowing for the restoration of a healthy endothelium and preventing CAD recurrence.
Keywords: endothelial dysfunction, redox signaling, oxidants, CAD, PCI
Introduction
Coronary artery disease (CAD), characterized by the development of atherosclerotic plaque, is a complex, chronic inflammatory disease of the arterial wall with macrophage infiltration, accumulation of oxidized lipids, medial inflammation, and endothelial dysfunction (ED) (Libby, 2021). As the disease progresses, stable and unstable plaques can result in neointimal hyperplasia, partial/complete stenosis of the artery, plaque rupture, and thrombotic events. Untreated, the loss of lumen and reduction of blood flow in the stenotic artery has a high risk of major adverse cardiac events (MACE) such as myocardial infarction (MI), stroke, and cardiovascular death (Doenst et al, 2019). Percutaneous coronary interventions (PCI) involve physical devices, most commonly balloons and stents, that are expanded and deployed within stenotic arteries to regain lumen size and restore adequate blood flow.
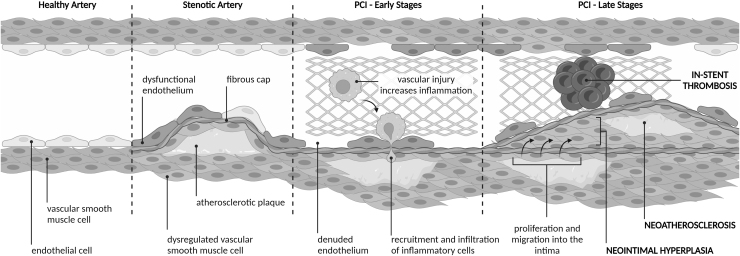
Initially, PCI was by balloon angioplasty alone, expanding stenotic arteries by the inflation of a balloon catheter. Next followed bare-metal stenting (BMS), where a metal mesh stent was permanently placed to expand the stenosed artery. Both angioplasty and stenting cause mechanical injury to the endothelium leading to denudation, ED, vessel injury, and inflammation (Fig. 1). Dysfunction of endothelial cells (ECs) subsequently results in the dysregulation of vascular smooth muscle cells (VSMCs), which proliferate and migrate into the intima causing intimal thickening and neointimal hyperplasia, culminating in arterial restenosis (Fig. 1) (Ebert et al, 2021). In cases of BMS, excessive neointimal hyperplasia, coupled with a high inflammatory response due to stent placement, manifests as in-stent restenosis (Kornowski et al, 1998) where there is a reported restenosis incidence of 9.6% (after 1 year) to 18.1% (after 10 years) and an associated 2% risk of MI (Doyle et al, 2007).
FIG. 1.
The impact of PCI on vessels. In a healthy artery, the endothelium (a single layer of ECs) acts as the barrier between the artery wall and the bloodstream. The endothelium also regulates the proliferation, constriction, and relaxation of VSMCs in the media. In CAD, arteries become stenotic from the development of atherosclerotic plaque, the endothelium becomes dysfunctional, and VSMCs become dysregulated. In the early stages after PCI with stent placement, the lumen area is restored but vascular injury causes endothelial denudation, ED, and increased inflammation. This leads to increased recruitment and infiltration of inflammatory cells into the media. In later stages, neointimal hyperplasia develops from the proliferation and migration of VSMCs into the intima. Neoatherosclerosis then develops within the neointima because of increased inflammatory cell infiltration. In-stent thrombosis can also occur due to delayed re-endothelialization. CAD, coronary artery disease; EC, endothelial cell; ED, endothelial dysfunction; PCI, percutaneous coronary interventions; VSMC, vascular smooth muscle cell.
The occurrence of in-stent restenosis was initially thought to be almost fully abolished with the development of drug-eluting stents (DES), which prevent restenosis with the use of cell-type nonspecific, antiproliferative drugs such as sirolimus or paclitaxel. However, while these drugs reduce neointimal hyperplasia, as they are not cell-type specific they will also stop the regrowth of a healthy endothelium, crucial for artery healing. Impaired/delayed re-endothelialization is also associated with late-stage (>30 days to 1 year) and very late-stage (>1 year) stent thrombosis, with early trials reporting mortality rates of 23.5% from late-stage stent thrombosis and 10.5% from very late-stage stent thrombosis (Kimura et al, 2010).
Reported rates of target lesion revascularization (within 2 years post-DES) only reduced marginally compared with BMS (20% vs. 17.1%, p < 0.001), with no difference in mortality risk with DES use (8.4% vs. 8.4%, p = 0.98) (Malenka et al, 2008). This highlights that endothelial injury and in-stent restenosis after DES implantation remain important clinical issues.
It is important to note that most patients requiring PCI have advanced atherosclerosis with preexisting ED. Additional mechanical injury and endothelial denudation are inevitable consequences of PCI, exacerbating this preexisting ED. Further compounding comorbidities that potentiate ED, including diabetes mellitus, can increase the risk of PCI-mediated complications by up to four-fold (Clare et al, 2022). To develop better PCI treatments that restore healthy vascular function and prevent post-PCI MACE, we must first understand the biochemical and morphological drivers of EC dysfunction, and how current therapies exacerbate these conditions.
We define ED as the inability of ECs to appropriately regulate vascular tone, blood coagulation, vascular inflammation, and VSMC proliferation. The pathological mechanisms behind this loss in EC function are complex but are commonly mediated by the loss of nitric oxide (•NO) bioavailability and dysregulated oxidant production. In this review, we discuss the following: (i) the potential role of endogenously produced •NO and oxidants in regulating healthy vascular function, (ii) the central role of oxidants in ED, (iii) PCI-induced changes to oxidant signaling, and (iv) the novel advances in PCI technologies that tackle changes in oxidant production to restore endothelial function and vascular health.
Reactive Species and Healthy Vascular Function
The vascular endothelium is composed of a single layer of cells that control vascular tone, mediate the immune system, regulate molecule exchange, maintain blood in its fluid state, and coordinate the formation of new blood vessels. In performing these tasks, the production of a range of reactive species by ECs is crucial. Failure to regulate or maintain a balance between the production of these signaling molecules is a hallmark and cause of ED.
The term “reactive species” covers a broad range of free radical and nonradical species with their reactivity centered around oxygen, chlorine, bromine, and nitrogen. The dominant reactive species produced by ECs are the reactive nitrogen species (RNS) derived from •NO, such as peroxynitrite (ONOO−), and the reactive oxygen species (ROS) including superoxide radical anion (O2•−), hydrogen peroxide (H2O2), hydroxyl radicals (•OH), organic peroxides (ROOH), and hypochlorous acid (HOCl).
•NO and RNS: endothelial sources
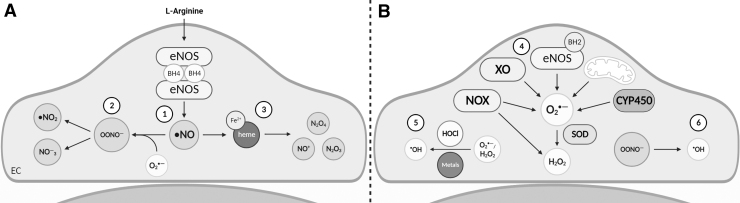
First discovered as the endothelial-derived relaxing factor, •NO is a key mediator of vascular tone and endothelial function (Palmer et al, 1987). In mammals, •NO is synthesized by three key homodimeric nitric oxide synthase (NOS) enzymes: neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS). These enzymes convert the amino acid l-arginine to •NO and citrulline via a two-step oxidation process that is dependent on four redox-dependent cofactors and calcium/calmodulin-mediated transfer of electrons derived from reduced nicotinamide adenine dinucleotide phosphate (NADPH) to NOS heme (Fig. 2A) (Stuehr and Haque, 2019).
FIG. 2.
Endothelial sources of •NO, RNS, and ROS. (A) Endothelial sources of •NO and RNS. l-Arginine is converted to •NO by eNOS ①. To form RNS, •NO reacts with O2•− to form OONO− and peroxynitrous acid (not shown) or is degraded to form •NO2 and NO−3 ②. •NO also reacts with proteins containing Fe2+-heme, forming NO+, N2O4, or N2O3 ③. (B) Endothelial sources of ROS. O2•− production is via activation of enzymes such as uncoupled eNOS, CYP450, and XO, as well as increased mitochondrial respiration or increased NOX activity. Once formed, O2•− is dismutated by SOD to form H2O2. Alternatively, H2O2 is formed directly by various NOX isoforms ④. O2•− and H2O2 react with transition metals (through Fenton reactions) or HOCl to form •OH ⑤. •OH can be formed as a minor product of OONO− degradations ⑥. eNOS, endothelial nitric oxide synthase; H2O2, hydrogen peroxide; HOCl, hypochlorous acid; •NO, nitric oxide; •NO2, nitrogen dioxide; NO−3, nitrate; •OH, hydroxyl radicals; RNS, reactive nitrogen species; ROS, reactive oxygen species; SOD, superoxide dismutase; XO, xanthine oxidase.
•NO has both a short half-life and diffusion distance, owing to its highly reactive nature with heme compounds or other reactive species. One of the fastest •NO reactions is with O2•−, which produces both ONOO− and its acid ONOOH (Fig. 2A). Once formed, ONOO− and ONOOH can oxidize thiolates (S−) to form S-nitrosothiols (RSNO), with a small proportion of ONOO− and ONOOH degraded to form •OH, nitrogen dioxide (•NO2), and nitrate (NO−3) (Ferrer-Sueta et al, 2018; Radi, 2018). RSNO also form through •NO reactions with ferrous heme, forming NO+, or O2/ROS, via N2O4 or N2O3 intermediates (Fig. 2A). As RSNO can transfer their nitroso group via transnitrosation, they are considered by some as a form of RNS.
Cellular RNSO may be present within protein structures as post-translationally modified thiols (also known as S-nitrosation) or small molecules, for example, S-nitrosoglutathione (GSNO). Labile S-nitroso bonds rapidly react with free thiols to generate disulfide bonds impacting protein conformation and/or activity (Wolhuter et al, 2018).
•NO and RNS: vascular tone
Under physiological conditions, ECs produce and release •NO in response to muscarinic receptor stimulation (Palmer et al, 1987), isometric force generation (Wallis and Martin, 2000), shear stress-mediated activation of mechanosensitive channels (Wang et al, 2016), and adrenergic-induced contraction (Fig. 3) (Smith et al, 2020).
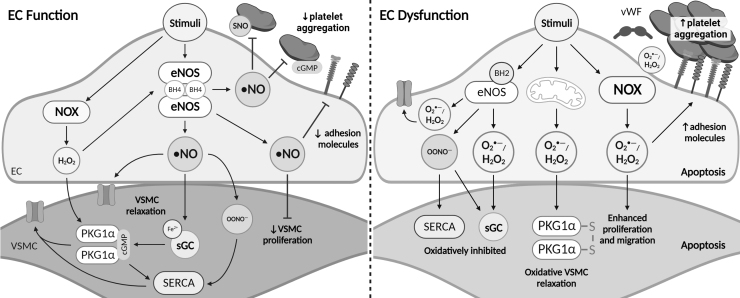
FIG. 3.
•NO, RNS, and ROS in endothelial function and dysfunction. In functional ECs, stimulus-induced eNOS activity elevates •NO production. •NO mediates relaxation of VSMCs via binding to the Fe2+-heme of sGC, which increases cGMP and canonically activates PKG1α. •NO signaling also regulates vascular tone by opening VSMC ion channels (yellow open channel) directly, or via RNS-mediated oxidation of SERCA. H2O2 stabilizes eNOS and regulates arterial tone and blood pressure through cGMP-independent activation of PKG1α. In addition, •NO signaling inhibits platelet aggregation through cGMP signaling, RNS-mediated SNO of platelet proteins, and inhibition of EC adhesion proteins (yellow and purple EC projections). In EC dysfunction, oxidation of the eNOS cofactor BH4 to BH2 uncouples eNOS leading to increased production of O2•− and H2O2. Elevated expression of NOX and oxidant leak from the mitochondria further contribute to O2•−, H2O2, and OONO− levels. Dysregulated oxidant production leads to irreversible oxidations of SERCA, sGC heme, and TRPV4 rendering them inactive and unable to regulate vascular tone. The dominant mechanism of arterial relaxation under these conditions is through oxidative activation of PKG1α, however, prolonged exposure to H2O2 results in EC and VSMC apoptosis. Loss of •NO bioavailability enhances platelet aggregation, adhesion factor expression, and VSMC proliferation. BH2, dihydrobiopterin; BH4, tetrahydrobiopterin; cGMP, 3′,5′-cyclic guanosine monophosphate; PKG1α, protein kinase G subunit 1α; SERCA, sarco/endoplasmic reticulum Ca2+-ATPase; sGC, soluble guanylate cyclase; SNO, S-nitrosation.
•NO initiates arterial relaxation/dilation by decreasing VSMC concentration of, and/or sensitivity to, intracellular calcium. This occurs via activation of •NO smooth muscle receptor soluble guanylate cyclase (sGC). •NO binds to the heme NO and O2 binding domain, and potentially additional unknown sites, resulting in conformational changes of the coiled-coil domain and protein backbone increasing catalytic activity (Horst and Marletta, 2018). Catalytically active sGC increases the conversion of 5′-guanosine triphosphate to 3′,5′-cyclic guanosine monophosphate (cGMP) which canonically activates protein kinase G subunit 1α (PKG1α) (Kim and Sharma, 2021). The •NO/sGC/cGMP/PKG pathway has been shown to regulate arterial tone via inhibition of smooth muscle depolarization by suppressing inositol trisphosphate receptors (IP3Rs), regulating myosin light chain phosphorylation (Kitazawa et al, 2009), and phosphorylation and activation of large conductance calcium-activated potassium channels (BKca) (Alioua et al, 1998).
In addition to the canonical activation of PKG1α, RNS oxidatively activate PKG1α via RSNO-mediated disulfide formation at Cys42 (Burgoyne et al, 2007). Furthermore, ONOO− induces S-glutathiolation of Cys674 of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) and stimulates vasodilation via a thiyl radical or sulfenic acid intermediate (Adachi et al, 2004). Finally, RNS are proposed to directly activate BKca channels through oxidation of redox-active thiols as a mechanism of arterial relaxation (Bolotina et al, 1994).
•NO and RNS: coagulation and inflammation
ECs act as a physical barrier to separate procoagulatory factors in the blood from the reactive tissues in the blood vessel wall, secreting pro- and anti-clotting factors, and regulating platelet activation. •NO inhibits platelet aggregation through the •NO/sGC/PKG pathway with subsequent regulation of the IP3 receptor through inositol 1,4,5-triphosphate IP3 receptor-associated cGMP kinase substrate (Fig. 3) (Antl et al, 2007). However, cGMP-independent inhibition of platelet aggregation has also been reported. •NO-dependent, cGMP-independent mechanisms are associated with decreased exocytosis of platelet granules dependent on the presence of key cysteine residues and can be inhibited using N-acetyl-l-cysteine, which is indicative of protein S-nitrosation (Matsushita et al, 2003). It was concluded that S-nitrosation of platelet granule proteins inhibits exocytosis. However, N-acetyl-l-cysteine is effective at breaking disulfide bonds (Aldini et al, 2018), and therefore, RNS-mediated disulfide formation cannot be mechanistically ruled out.
A recent study has shown that •NO along with RNS, nitrate, and nitrite all have differing platelet inhibition efficacies in a manner dependent on the oxygenation state of the blood (Wajih et al, 2017).
Leukocyte rolling, capture, arrest, and extravasation are regulated by •NO as part of the classic inflammatory response. Acting through cGMP-independent mechanisms, •NO inhibits nuclear factor kappa B (NF-κB), monocyte chemoattractant protein 1, integrin expression, P-selectin, E-selectin, vascular cell adhesion molecule-1 (VCAM-1), and to a lesser extent intercellular adhesion molecule-1 (ICAM-1) (Lefer, 1997). Subsequent studies found that the effects of •NO on NF-κB could be reversed in the presence of dithiothreitol, suggesting the involvement of RNS and thiol oxidation (Marshall and Stamler, 2001). Later works investigating the short-term effects of •NO on leukocyte adhesion (5 min) after tumor necrosis factor α (TNF-α) exposure revealed that increased adhesion was associated with S-nitrosation of protein kinase C (Aguilar et al, 2021). These data suggest that both •NO and RNS mediate the vascular inflammatory response.
•NO and RNS: vascular structure
Endothelial-derived •NO plays a vital role in regulating arterial structure and integrity. In otherwise healthy large- and small-animal studies, the addition of NO donors reduces VSMC proliferation after vascular injury (Pearce et al, 2008). Similarly, endogenous stimulation of •NO formation protects against neointimal formation after carotid ligation injury. This process was sensitive to NOS inhibition and was associated with increased EC survival, decreased inflammatory markers (CD45 antigen, ICAM-1 and VCAM-1), and suppression of VSMC proliferation (Mukai et al, 2006). At the smooth muscle cell level, •NO causes G0/G1 cell arrest inhibiting smooth muscle proliferation through regulation of the ubiquitination system, with recent reports suggesting that this is via RNS-dependent oxidation of redox-active cysteines (Valek et al, 2019).
ROS: EC sources
In ECs, many of the ROS-dependent processes center around O2•− and H2O2. A range of cellular proteins have been shown to produce ROS, with the most notable being the NADPH oxidase family of enzyme complexes (NOX1–NOX5 and DUOX1–DUOX2), which use NADPH to reduce oxygen to O2•− or H2O2 (Fig. 2B). These transmembrane proteins consist of NADPH, FAD, and heme binding domains along with additional activating proteins/factors, with p22phox commonly required for NOX1–NOX4 activation and calcium required for NOX5 and DUOX1–DUOX2 (Bedard and Krause, 2007). In the vasculature, NOX1–NOX5 are expressed in both ECs and VSMCs, with NOX2 predominantly expressed in ECs, NOX1 predominantly expressed in VSMCs, and NOX4 expressed at high levels in both the cell types.
A second major source of O2•− and H2O2 is mitochondrial respiration. O2•− is produced as a by-product of NADH-dependent respiration at a range of sites along the electron transport chain. Under physiological conditions, the majority of O2•− is reduced by superoxide dismutase (SOD) within the mitochondrial matrix, releasing H2O2 (Fig. 2B). While O2•− is predominantly produced in the mitochondrial matrix at two sites, located in complex III and mitochondrial glycerol 3-phosphate dehydrogenase, half of the O2•− is produced directly into the cellular cytosol, thus contributing to cellular O2•− concentrations (Brand, 2020). Mitochondrial O2•− is implicated in a range of diseases and is considered a key contributor to ED (discussed below), however, a role for physiological O2•− signaling is now emerging (Sies et al, 2022).
Although the main role of NOS enzymes is to produce •NO, uncoupled NOS also serve as a significant source of O2•− (Fig. 2B). Uncoupling results in electrons being passed from NADPH to molecular oxygen instead of l-arginine, with O2•− formed instead of •NO (Kietadisorn et al, 2012). NOS uncoupling occurs via (i) oxidation of zinc/thiolate cluster leading to the release of zinc, (ii) decrease in the production, recycling, or oxidation of the NOS cofactor tetrahydrobiopterin (BH4), (iii) increased production of the endogenous NOS inhibitor asymmetric dimethylarginine, and (iv) G-protein-coupled receptor mediated phosphorylation associated with enzyme dysfunction (Wu et al, 2021).
Additional sources of vascular O2•− include xanthine oxidase (XO), an enzyme involved with purine metabolism. Circulating XO binds to the endothelium and produces O2•− and H2O2 via monovalent and divalent electron reduction of molecular oxygen, respectively (Bortolotti et al, 2021). In addition, xenobiotic metabolizing enzyme cytochrome P450 produces O2•− or H2O2 through key reaction stages in its catalytic cycle (Albertolle and Peter Guengerich, 2018).
ROS: vascular tone
Exogenous H2O2 relaxes conduit and resistance arteries with EC50 values commonly in the midmicromolar ranges. The mechanisms involved in relaxation to exogenous H2O2 have previously been reviewed (Stanley et al, 2017). In brief, in ECs, exogenous H2O2 stimulates •NO production via phosphorylation and increased expression/stability of eNOS, increasing EC calcium leading to •NO formation and activation of EC ion channels causing hyperpolarization-mediated relaxation. In VSMCs, exogenous H2O2 causes arterial relaxation through catalase-mediated activation of sGC, oxidative activation of PKG1α, and indirect activation of a range of ion channels (Fig. 3).
Endogenous formation of H2O2 in response to muscarinic (Matoba et al, 2000) or bradykinin receptor stimulation (Matoba et al, 2002) and increased shear stress (Liu et al, 2011) have been shown to mediate arterial relaxation. In support of endogenous H2O2 production directly regulating vascular tone, relaxation can be inhibited by catalase or mutation of Cys42 in PKG1α (Matoba et al, 2000; Prysyazhna et al, 2012). However, with contradictory findings from studies utilizing genetic manipulation of the sources and metabolism of ROS (i.e., altering NOX expression), the field remains divided as to the extent of endogenous H2O2-mediated relaxation under physiological conditions.
ROS: vascular structure
ROS have a role in the embryonic development and maintenance of vascular structure (Sauer and Wartenberg, 2005). Once mature, arterial cells remain under physiological control by ROS. EC proliferation, morphological structure, and survival are regulated by O2•−/H2O2 from NOX2 and NOX4, which mediate extracellular-regulated kinase 1–2 phosphorylation, increased caspase activity, Akt phosphorylation, and phosphorylation of mitogen-activated protein kinase (MAPK) (Peshavariya et al, 2009). NOX-derived ROS also regulate EC migration in response to vascular endothelial growth factor via S-glutathiolation of SERCA2 (Evangelista et al, 2012).
Reducing systems
If left unregulated, endogenously produced ROS/RNS can cause irreversible damage to proteins, lipids, and DNA. Therefore, ECs have multiple reducing systems in place to regulate intracellular oxidant concentrations (Tretter et al, 2021). Direct-acting enzymes, including catalase and glutathione peroxidase 1 (GPx1), work in conjunction with low-molecular-weight antioxidants (lipoic acid, ascorbic acid, and tocopherols) to limit the accumulation of oxidants and maintain reducing conditions within the cell. Elevations in reactive species stimulate the nuclear factor E2-related factor 2 (Nrf2) antioxidant response that transcriptionally regulates the expression of antioxidant systems (Tonelli et al, 2018).
This includes two major thiol-based reducing systems that limit the accumulation of oxidized thiol residues and actively restore oxidized sensor thiols to their reduced state: (i) the glutathione system comprising reduced glutathione (GSH), oxidized glutathione (GSSG), and glutathione reductase (Forman et al, 2009), and (ii) the thioredoxin (Trx) system consisting of peroxiredoxin, Trx, and Trx reductase (TrxR) (Yamawaki et al, 2003). It should be noted that both these families of proteins are more than just an antioxidant defense system. They are recognized as signaling entities in their own right with the capacity to directly regulate cellular signaling. Furthermore, disruption of these reducing systems has been implicated in driving ED (Espinosa-Diez et al, 2018; Kirsch et al, 2016).
•NO, RNS, and ROS in ED
There is growing evidence that the dysregulated production of reactive species in dysfunctional ECs leads to alterations in vascular tone, coagulation, inflammation, and vascular structure contributing to the progression of CAD.
Vascular tone
A key feature of ED is the loss of NO bioavailability resulting in loss of vascular tone. Interestingly, studies show that inhibiting or depleting NOS alone is sufficient to drive ROS production. Decreasing •NO production, by pharmacological inhibition of NOS using L-NAME or siRNA knockdown of eNOS, elevates intracellular H2O2 levels in bovine aortic ECs, in a catalase-sensitive manner (Jin et al, 2009). In ex vivo human coronary arteries, H2O2-mediated dilation in response to flow was demonstrated to be a hallmark of disease (Freed et al, 2014). In patients without CAD, flow-induced arterial dilation is predominantly dependent on the production of •NO. However, in CAD patients, the mechanisms of dilation switch to mitochondrial-derived H2O2, which induces arterial dilation through oxidant activation of PKG1α (Freed et al, 2014; Prysyazhna et al, 2012).
These studies indicate that ED causes a switch from •NO-mediated to H2O2-mediated regulation of vascular tone (Fig. 3). Intriguingly, redox-dead PKG1α mutant mice are hypertensive, suggesting a role for reactive species in the physiological control of blood pressure (Khavandi et al, 2016). Taken together, this suggests that there is a fine balance between oxidant production required to maintain physiology and oxidant production in ED. Recent work has shed light on this area demonstrating that endothelium-dependent dilator responses to sphingosine-1-phosphate, in patients without CAD, are dependent on H2O2-mediated formation of •NO. However, in patients with CAD, the •NO component of dilation is lost with only the H2O2 component remaining. From these data alone, any detrimental issue with H2O2 being the sole mediator of arterial relaxation is not obvious.
However, prolonged exposure of resistance arteries to vasoactive concentrations of H2O2 causes endothelial and smooth muscle cell apoptosis (Shaw et al, 2021). These data highlight that, while H2O2 is a key mediator of vascular tone, reliance on H2O2 during ED is detrimental to vascular health and prolonged H2O2 production may weaken or destroy the endothelium and surrounding arterial tissue.
The relationship between RNS and vascular tone is complex. Work in conduit arteries, including mouse aorta and rabbit carotid arteries, suggests that arterial relaxation in response to EC stimulation is partly through the release of •NO, and is mediated by OONO− formation. OONO− mediates a reversible S-glutathiolation of SERCA leading to increased calcium uptake and smooth muscle relaxation (Fig. 3) (Adachi et al, 2004). However, in disease models such as atherosclerosis, •NO-mediated relaxation is blunted by ROS/RNS-mediated irreversible oxidation of SERCA, rendering it unable to sequester calcium. RNS are further implicated in ED by reducing NO bioavailability through oxidation of BH4 to dihydrobiopterin (BH2) leading to eNOS uncoupling and altering the redox state of smooth muscle sGC, thus decreasing its sensitivity to •NO (Kuzkaya et al, 2003; Tawa et al, 2014). In addition, in an obese mouse model, ED increased iNOS activity and EC NOX1 activity.
The combination of elevated •NO and ROS increased OONO− leading to the oxidation of the transient receptor potential vanilloid receptor 4 regulatory protein, thus inhibiting arterial relaxation and raising blood pressure (Ottolini et al, 2020). Overall, exacerbated RNS production drives ED by inhibiting the key vasodilatory pathways and creating oxidative damage to proteins.
Coagulation and inflammation
With ED, the ability of the circulatory system to maintain blood in its fluid state is compromised. Due to decreased •NO bioavailability, clotting risk is elevated due to loss of its antithrombotic effects (Fig. 3). Furthermore, studies have shown that ROS and RNS generation in ED increases inflammation and clotting risk. In oscillatory shear stress, as associated with atherosclerosis and endothelial disruption, deletion of NOX1 and NOX2 is protective by reducing monocyte and leukocyte adhesion, respectively (Chen et al, 2004; Sorescu et al, 2004). Important factors in this process are the expression of cellular adhesion molecules ICAM-1 and VCAM-1, and the selectins P- and E-selectin.
Additional studies have identified that deletion of the NOX regulatory subunit (p47phox) reduces adhesion and selectin molecule expression (Vendrov et al, 2007). Ramen spectroscopy of human aortic ECs after 3 h of menadione exposure to generate O2•− detected increased expression of the von Willebrand factor, a key mediator of platelet adhesion and blood clotting. ED-induced RNS are similarly deleterious to coagulation. Elevations in ONOO− during CAD significantly accelerate clot formation and factor XIII crosslinking via nitration of fibrinogen (Vadseth et al, 2004).
Vascular structure
Potentiated oxidant production within the endothelium impacts vascular remodeling through the regulation of VSMC proliferation, extracellular matrix composition, and cell-to-cell connections. CAD induces a significant upregulation of O2•− from NOX1 and NOX4 (Szocs et al, 2002). However, the detrimental role of NOX4 has been contested in a recent publication evaluating neointimal cells using scRNAseq in Nox4−/− mice. It was concluded that vascular remodeling occurs irrespective of NOX4 expression, with similar changes detected between WT and Nox4−/− mice (Buchmann et al, 2021). The role of NOX1 appears less contentious. In response to injury, Nox1y/− mice had decreased VSMC proliferation, migration, and apoptosis (Lee et al, 2009). Vascular injury increases NOX2 expression leading to increased RNS formation, as detected by elevated protein nitrotyrosine, and ablation of NOX2 decreased VSMC proliferation and reduced leukocyte adhesion (Chen et al, 2004).
In an atherosclerotic model, deletion of the regulatory subunit for NOX1 and NOX2 (p47phox−/−) resulted in a 50% reduction in lesion size (Barry-Lane et al, 2001). This was associated with a decrease in O2•− and an increase in basal •NO (Judkins et al, 2010). These data highlight the deleterious effects of NOX-derived ROS and their ability to potentiate vascular remodeling.
Altered Endothelial Redox Signaling Following PCI
As CAD progresses, continual changes in redox signaling as part of ED occur and lesion size, along with the extent of vascular remodeling, increases. This results in a decrease in the vessel lumen and the need for intervention heightens. However, interventions utilizing PCI further contribute to ED by impacting redox signaling within the endothelium that increases the risk of stent-related complications. Changes initially arise from the mechanical insult sustained during angioplasty and stent implantation, followed by the effect induced by the physiochemical properties of the stent. Oxidative signaling contributes to the resulting inflammatory response and adverse outcomes, including restenosis and neoatherosclerosis.
Mechanically induced oxidants
The ability of mechanical stresses from PCI to induce damage via induction of ROS is well established. Early canine studies demonstrated that balloon angioplasty results in an acute elevation of oxidant-dependent vasoconstriction (Laurindo et al, 1991). Pretreatment with SOD, but not catalase or •OH scavengers, prevented pathologically enhanced vasoconstriction and the formation of large thrombi. These data indicate that mechanical damage to the endothelium during coronary angioplasty induces pathological levels of O2•−. PCI with stent implantation further mechanically damages the vessel wall resulting in denudation of the endothelium, exposing blood, VSMCs, and extracellular matrix to the stent, thus increasing inflammatory signaling and risk of thrombosis (Fig. 1).
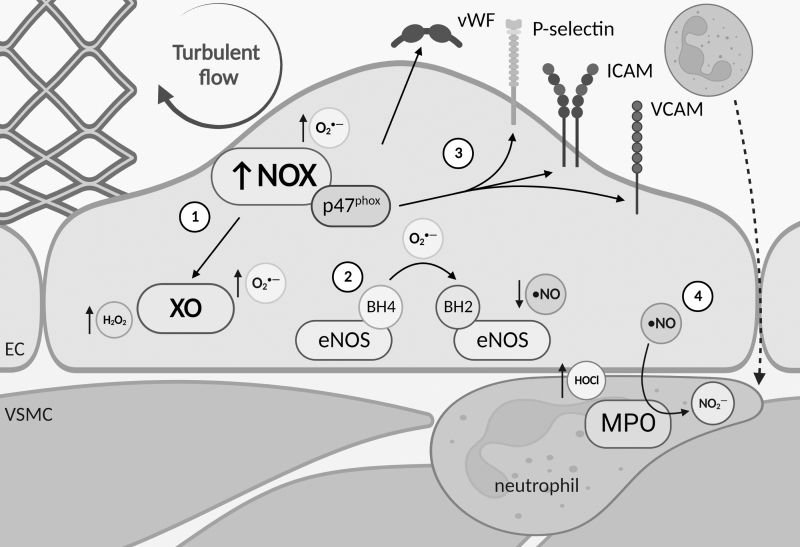
PCI and stent placement also disrupt the flow-generated forces within the vessel, which in turn regulate EC oxidant production (Fig. 4). Oscillatory shear stress generated post-PCI has been shown to inhibit re-endothelialization, thus decreasing the ability of the endothelium to regenerate and increasing the likelihood of thrombosis and neointimal hyperplasia. In ECs exposed to oscillatory shear stress, there is increased O2•− production via NOX directly and NOX-dependent elevations in XO activity (Godbole et al, 2009). Elevations in O2•− decrease •NO bioavailability and push ECs further into a dysfunctional state (Fig. 4). Depletion of •NO and elevated ROS result in increased expression of adhesion molecules and weaker cell–cell junctions, therefore, elevating vascular permeability (Wang et al, 2010).
FIG. 4.
ED is induced by flow disruption. ① Stent implantation typically creates turbulent flow that increases NOX expression in ECs. NOX-derived O2•− increases thrombus risk by elevating the expression of vWF and increasing intracellular ROS production within ECs through activation of XO and ② uncoupling of eNOS via the oxidation of BH4 to BH2, which also decreases NO bioavailability. ③ Downstream signaling from NOX regulatory subunit p47phox leads to elevated expression of EC adhesion factors including P-selectin, ICAM, and VCAM. ④ This increases the recruitment of inflammatory neutrophils to the subendothelial space. MPO within activated neutrophils produces HOCl, which leads to damaging lipid peroxidation, and reduces NO bioavailability through the metabolism of •NO to NO2−. ICAM, intercellular adhesion molecule; MPO, myeloperoxidase; VCAM, vascular cell adhesion molecule; vWF, von Willebrand factor.
Drug-induced oxidants
Second-generation DES have drastically reduced the rates of restenosis; however, they have failed to significantly improve MACE outcomes when compared with BMS. In clinical trials, sirolimus-, paclitaxel-, and, to a lesser extent, zotarolimus-DES have all been associated with prolonged ED (Hofma et al, 2006; Kim et al, 2009; Togni et al, 2007). Clinical trials have shown that 6 months post-paclitaxel-DES implantation, the segment of vessel adjacent to the stented site experienced exercise-induced paradoxical coronary vasoconstriction (Togni et al, 2005). These same vessels had preserved vasodilatory responses to nitroglycerin, indicating they were not insensitive to •NO but that paclitaxel exposure was driving the underlying ED.
The initial elution of drugs from DES has been observed to elevate oxidants, predominantly NOX-mediated O2•− production. A 7-day infusion with sirolimus was found to reduce the endothelium-dependent vasodilatory response of intact rat aorta to acetylcholine (ACh), while VSMC responses to nitroglycerin were unaffected (Jabs et al, 2008). Loss of ACh-mediated vasodilation was coupled with a 40% reduction in •NO production and a significant increase in ROS production from aortic ring segments. Mechanistically, exposure to sirolimus elevated ROS production via increased NOX expression. Similarly, a 7-day infusion of paclitaxel in rats led to reduced flow-mediated dilation and impaired ACh-mediated vasodilation, indicative of ED (Serizawa et al, 2012). These effects are due to paclitaxel-induced increases in NOX expression (subunits p47phox and gp91phox).
Vascular inflammation
The extent of PCI-induced inflammation correlates with short- and long-term prognosis (Gach et al, 2007). The ability of PCI to induce inflammatory signaling cascades has been extensively reviewed (Tucker et al, 2021). In brief, in response to injury, ROS/RNS mediate the upregulation of proinflammatory cytokines, chemokines, and adhesion molecules, resulting in platelet activation and the recruitment of inflammatory cells (Fig. 4). This, combined with PCI-mediated elevations in vascular permeability, results in the recruitment of inflammatory cells within the arterial wall. In addition, the release of microthrombi and the lipid-rich contents from the stented plaque triggers the activation of NOD-like receptor protein 3 inflammasomes, ultimately leading to downstream activation of MAPK and NF-κB signaling pathways resulting in elevated ROS generation (Gordon et al, 2011).
PCI with stent implantation also increases the recruitment of neutrophils containing myeloperoxidase (MPO), resulting in elevated HOCl production in ECs. Interestingly, in the short-term, neutrophil-derived MPO in the subendothelial space activates eNOS. In the presence of H2O2, MPO-derived ROS elevate cytosolic Ca2+, which drives beneficial eNOS phosphorylation and increases •NO production (Thai et al, 2021). However, these beneficial effects are short lived as MPO consumes endothelial-derived •NO, resulting in an overall reduction in NO bioavailability (Fig. 4). In addition, MPO-derived HOCl contributes to lipid peroxidation and endothelial damage further driving ED (Eiserich et al, 2002).
The Colchicine to Prevent Periprocedural Myocardial Injury in Percutaneous Coronary Intervention pilot study found a potential role for the antioxidant colchicine in the reduction of inflammatory markers post-PCI (Cole et al, 2021). Along with its anti-inflammatory properties, colchicine elevates serum GSH levels and thus may be protective by reducing ROS/RNS levels (Das et al, 2000). Similarly, treatment with the antioxidant and lipid-lowering drug nicanartine was found to reduce the inflammatory response to balloon angioplasty in a rabbit model (Wohlfrom et al, 1998). While antioxidant therapies have thus far failed to translate into clinical practice, these data highlight the central role of downstream oxidative signaling during PCI-induced inflammation, which drives ED and contributes to adverse outcomes.
Restenosis: the role of oxidants
Restenosis is typically defined as >75% narrowing of the cross-sectional luminal area by neointimal tissues, made up of VSMCs and proteoglycan-collagenous matrix. A major factor in the development of restenosis is the failure of the endothelium to functionally recover post-PCI, combined with a prolonged inflammatory response. Human studies show that 2 years after sirolimus-DES implantation, stent struts remained uncovered in 20% of patients, corresponding to increased neointimal hyperplasia and up to a 40% risk of subclinical thrombus formation (Takano et al, 2007). A first-in-human clinical trial in 2005 demonstrated that stents coated with anti-CD34 antibodies could capture circulating CD34+ endothelial progenitor cells and increase re-endothelialization post-PCI (Aoki et al, 2005).
However, a follow-up study determined that while early re-endothelialization was improved using this approach, there was no downstream improvement in neointimal thickness at 90 days post-PCI (van Beusekom et al, 2012). Although positive eNOS staining was observed in the regenerated endothelium, there was continuous adhesion of leukocytes to the endothelium, indicating a sustained inflammatory response and decreased NO bioavailability, equating to ED. These data highlight that endothelial coverage is not the only factor to consider when trying to combat restenosis and that endothelial function must be restored to improve clinical outcomes.
Although the initial burst of NOX activation rapidly subsides after stent deployment, low-level O2•− production persists during vascular repair. Combining elevated O2•− with a compensatory upregulation iNOS- and nNOS-derived •NO, to offset impaired eNOS activity, results in the increased formation of RNS that drives inappropriate protein oxidation and hinders endothelial function (Leite et al, 2004).
A recent clinical study has suggested an association between circulating oxidative burden and incidence of restenosis post-PCI when using sirolimus-DES (Ganjali et al, 2022). Elevated SOD is indicative of an increase in antioxidant enzymes in response to elevations in oxidants, thus suggesting that patients with poor prognoses have uncontrolled ROS production. However, upregulation of extracellular SOD post-PCI is counterintuitively beneficial due to its capacity to remove O2•−. Initial studies showed that reduced extracellular SOD expression promoted ROS/RNS production and impaired iNOS-derived NO bioavailability, resulting in loss of vascular tone (Leite et al, 2003). Furthermore, elevation of extracellular SOD by local gene therapy in a hyperlipidemic rabbit model significantly accelerated endothelial recovery and reduced neointimal formation (Brasen et al, 2007).
Attempts have been made to therapeutically reduce the oxidative burden associated with restenosis using third-generation β-blockers to reduce O2•− production via the inhibition of NOX expression and preventing NOS uncoupling (Mollnau et al, 2003). However, a 2-year follow-up from a clinical trial using carvedilol-loaded stents found no significant differences in the neointimal area or the incidence of MACE compared with BMS (10.5% vs. 30.0%, respectively, p = 0.132) (Kim et al, 2011).
Restenosis: the role of the reducing systems
Highlighting the central role that cellular reducing systems play in governing vascular remodeling, GPx1 was recently found to critically regulate neointimal hyperplasia and restenosis following PCI. GPx1 is an important antioxidant enzyme that reduces H2O2 to H2O and, in turn, oxidizes two GSH molecules to GSSG. GPx1 also catalyzes the reduction of lipid peroxides and other organic hydroperoxides. Deficiency of GPx1 in mice was found to drive ED via elevations in O2•− (Dayal et al, 2002; Forgione et al, 2002). PCI-induced damage to the vessel wall has been found to reduce the expression of GPx1 causing knock-on effects on oxido-reductive signaling (Ali et al, 2014). PCI-induced injury in Gpx1−/−Apoe−/− mice resulted in a buildup of H2O2 and a time-dependent increase in GSH resulting in elevated protein S-gluathiolation, likely via sulfenic intermediates (Charles et al, 2007).
S-gluathiolation of the tyrosine phosphatase SHP-2 inactivates the protein and consequently results in the sustained phosphorylation and activation of orphan proto-oncogene receptor tyrosine kinase ROS1. Sustained activation of ROS1 amplifies its ability to activate downstream kinases involved in VSMC proliferation, migration, and apoptosis, resulting in pathological remodeling and restenosis. In addition, GPx1 is a critical regulator of MAPK and NF-κB proinflammatory pathways. Disrupted GPx1-mediated signaling in ECs from Gpx1−/− mice resulted in elevated expression of VCAM-1 on ECs, corresponding to increased adhesion of inflammatory cells to the aortic wall in vivo (Sharma et al, 2016). These data demonstrate that ablation of GPx1 activates the endothelium and facilitates vascular inflammation resulting in ED, which in turn can drive restenosis.
An elevation of GSH-synthesizing enzymes was observed in human restenosis, indicating that elevated reductive signaling post-PCI may be a clinically relevant process (Ali et al, 2014). When combined with evidence that PCI reduces GPx1 expression, these data suggest that elevations in reductive signaling may contribute to the deleterious effects of stents and restenosis. However, an alternative explanation could be that GSH production is simply upregulated in response to exacerbated oxidant production. A failure to upregulate GPx1 may lead to dysregulated oxidation of proteins, via the accumulation of S-glutathiolated thiols, leading to altered redox signaling and progression of neointimal hyperplasia.
In support of this, the presence of a single-nucleotide polymorphism within the GPx1 gene (599C/T) that decreases GPx1 activity correlated to an increased risk of restenosis post-PCI with BMS (OR = 2.9; 95% CI: 1.23 to 6.82) (Shuvalova et al, 2012). The complexities of antioxidant signaling pathways may partially explain the failure of antioxidant therapies in clinical trials and highlight that when targeting redox-mediated processes, there is a need for targeted rather than broad-spectrum therapies.
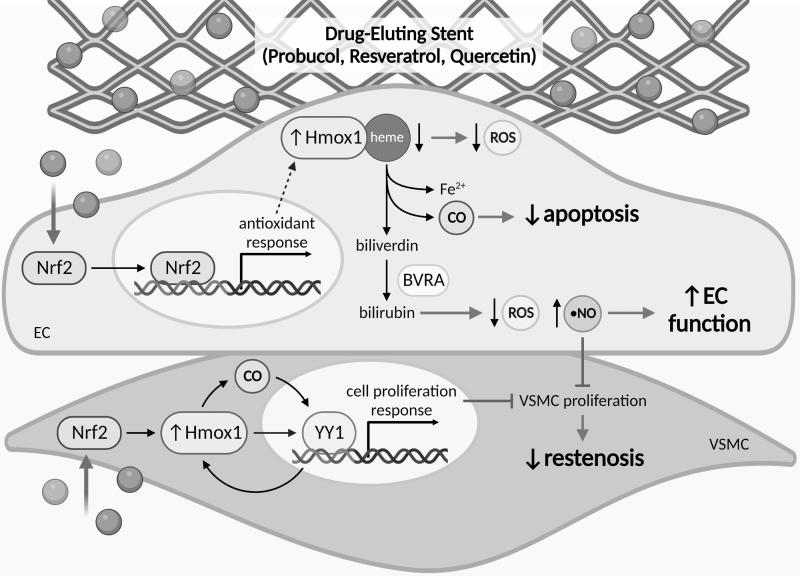
The inducible protein heme oxygenase-1 (Hmox1) is a key player in the vascular reducing system during conditions of inflammation as it plays a protective role against vascular injury by inhibiting neointimal hyperplasia and promoting re-endothelialization (Brouard et al, 2000; Duckers et al, 2001). Hmox1 elicits these opposing effects on VSMC and EC growth through regulation of the yin yang-1 transcription factor (Beck et al, 2010). Hmox1 catalyzes the degradation of heme to Fe2+, CO, and biliverdin, which reduces the pool of free heme and limits ROS production (Fig. 5). Biliverdin is quickly reduced, by biliverdin reductase, to the antioxidant bilirubin, which can improve endothelial function, while the released CO protects ECs from apoptosis (Brouard et al, 2000; Maruhashi et al, 2019). Together, these effects contribute to the protective effects of Hmox1 in the endothelium.
FIG. 5.
Therapeutics targeting Hmox1 to reduce oxidative damage. Antioxidants probucol, resveratrol, and quercetin have been trialed as alternative compounds in association with DES. The uptake of these antioxidants by ECs and VSMCs activates the Nrf2 signaling pathway, through which cytosolic Nrf2 translocates to the nucleus and binds to antioxidant response elements in promoter regions of specific antioxidant response enzymes. The Nrf2 signaling pathway induces expression of Hmox1, which catalyzes the degradation of heme to biliverdin, releasing Fe2+ and CO as by-products, and then, biliverdin is quickly reduced to bilirubin by BVRA. Hmox1 promotes re-endothelialization by decreasing cytosolic heme, which reduces ROS, releasing CO that protects EC from apoptosis, and by increasing antioxidant bilirubin levels, which increase •NO and promote EC function. Increased •NO in EC also facilities inhibition of VSMC proliferation. Induction of Hmox1 expression via Nrf2 signaling, as well as Hmox1-associated CO, can activate the YY1 transcription factor, which in turn activates the cell proliferation response and feeds back to further promote Hmox1 expression. In VSMC, YY1 inhibits VSMC proliferation, which reduces neointimal hyperplasia and restenosis. BVRA, biliverdin reductase; DES, drug-eluting stents; Hmox1, heme oxygenase-1; Nrf2, nuclear factor E2-related factor 2; YY1, Yin Yang 1.
Using the antioxidant probucol to induce Hmox1 expression in rabbits, its systemic administration was found to promote functional re-endothelialization, inhibit VSMC proliferation, and reduce macrophage infiltration resulting in reduced restenosis (Lau et al, 2003; Tanous et al, 2006). A 10-year follow-up of the ISAR-TEST-5 randomized clinical trial, comparing a sirolimus-probucol-DES to a zotarolimus-DES, found that the stents had comparable MACE and very late-stage stent thrombosis, suggesting no added benefit of probucol (Kufner et al, 2020). However, the recent FRIENDLY-OCT randomized trial found an ∼8% increase in stent coverage on a probucol-coated sirolimus-DES compared with a bioresorbable sirolimus-DES (Otaegui Irurueta et al, 2022). This suggests that therapeutically targeting cellular reducing systems may be one mechanism to alleviate PCI-induced oxidative damage and limit restenosis.
Neoatherosclerosis
PCI-induced ED plays a critical role in the formation of neoatherosclerosis, which is characterized by the accumulation of lipid-laden foam cells within the neointima, with/without calcification, and/or a necrotic core. Although the advent of DES reduced the rates of restenosis, the incidence of neoatherosclerosis is significantly greater with first-generation DES than with BMS (30% vs. 16%, p < 0.001) (Nakazawa et al, 2011). Critically, neoatherosclerosis typically develops within a year of DES implantation, whereas in BMS it occurs >1 year post-PCI, indicating that the presence of a DES accelerates neoatherosclerosis formation that may lead to late stent failure (Cui et al, 2016).
Theoretically, re-endothelialization of the vessel wall post-PCI should limit inflammation and prevent neoatherosclerosis. However, in a rabbit model comparing EC function between DES and BMS, while there was comparable EC coverage at 1 month, eNOS expression was significantly lower with DES (sirolimus, zotarolimus, or everolimus) (Torii et al, 2018). Diminished eNOS expression suggests that the regenerated endothelium within these arteries was dysfunctional. As discussed above, DES are known to elevate ROS/RNS within ECs, and therefore, DES may drive neoatherosclerosis via prolonged increases in intracellular oxidant levels.
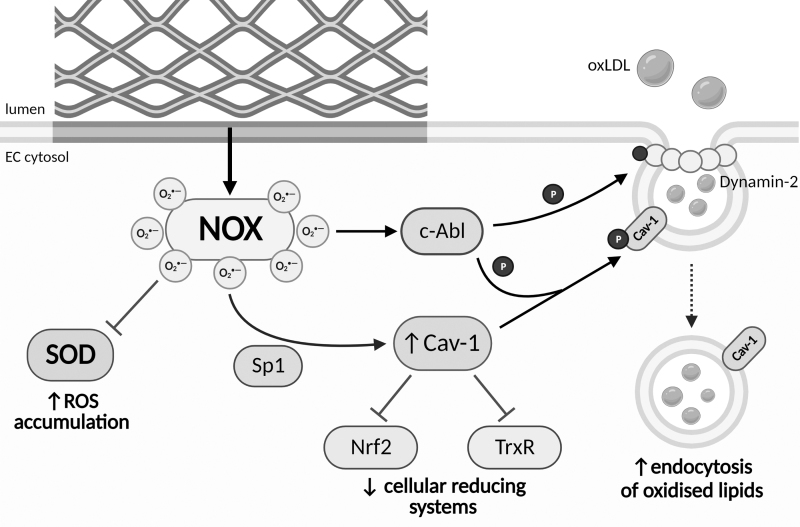
As with atherosclerosis, neoatherosclerosis is an inflammatory process driven by ED that results in the uptake and accumulation of oxidized lipids, mainly oxidized low-density lipoprotein (oxLDL), within the subendothelial space (Fig. 6). Within ECs, lipid transport is largely regulated by caveolin-1 (Cav-1) (Fernandez-Hernando et al, 2009). Cav-1 expression is upregulated in response to ROS via increased binding of Sp1 to the Cav-1 promoter (Fig. 6). Furthermore, ROS induce c-Abl-mediated phosphorylation of Cav-1 and Src-mediated phosphorylation of dynamin-2, promoting Cav-1/dynamin-2 association and driving the formation of caveolae and lipid uptake (Cui et al, 2016). The accumulation of oxidized lipids further drives NOX expression and ROS production, which perpetuates oxidant production within ECs via their effects on eNOS and XO. A buildup of oxLDL potentate inflammatory signaling increases NOX expression, decreases SOD, and limits iNOS expression through inhibition of arginase I/II signaling (Alexander et al, 1991; Barreto et al, 2021).
FIG. 6.
NOX-derived superoxide plays a central role in neoatherosclerosis. As the endothelium repairs, prolonged exposure to stent struts results in the activation of NOX, which drives the formation of neoatherosclerosis. NOX-signaling decreases SOD expression leading to the accumulation of ROS within EC. NOX-derived ROS also increase Sp1-mediated transcription of Cav-1. Cav-1 expression inhibits the Nrf2-mediated antioxidant response and TrxR, resulting in a diminished capacity for ECs to remove oxidants. NOX signaling activates the kinase c-Abl promoting Cav-1 and dynamin-2 phosphorylation, which together elevate the formation and endocytosis of lipid-laden caveolae. High ROS drive the oxidation of intracellular and extracellular lipids within the arterial wall, accelerating the formation of neoatherosclerotic plaques. Cav-1, caveolin-1; TrxR, thioredoxin reductase.
ROS-induced Cav-1 expression also hinders cellular antioxidant responses (Fig. 6). Elevations in Cav-1 inhibit TrxR, consequently limiting Trx-mediated reduction and allowing oxidant accumulation (Volonte and Galbiati, 2009). In addition, Cav-1 inhibits Nrf2, decreasing the transcription of a plethora of antioxidant proteins (Li et al, 2012). Elevated oxidative signaling combined with reduced removal of oxidants ultimately exacerbates ED, VSMC proliferation, and foam cell formation.
Heme-dependent peroxidases also regulate redox signaling and impact neoatherosclerosis. Indoleamine 2,3-dioxygenase (IDO) signaling within atherosclerotic plaques is cardioprotective and has been shown to regulate atherogenesis and inflammation (Cole et al, 2015). CAD patients with higher IDO activity also trend toward a lower 1-year mortality rate (p = 0.081) (Wongpraparut et al, 2021). In the presence of H2O2, IDO1 produces the tryptophan-derived hydroperoxide cis-WOOH, which regulates vasodilation via the oxidation of PKG1α Cys42 (Stanley et al, 2019). This alternative oxidant-mediated vasodilatory response may preserve vascular tone when NO bioavailability is compromised. Conversely, elevated MPO activity correlates with decreased plaque stability due to reduced NO bioavailability and HOCl-mediated accumulation of oxidized lipid species (Rashid et al, 2018).
The Clinical Impact of Novel PCI Technologies
Recent advances in PCI technology and treatment options that target or utilize redox signaling to improve endothelial function have opened the door to a new era of treatments for stenosed arteries. Below we discuss the benefits and limitations of novel therapies developed over the last 2 years with a focus on the effects on redox signaling in the endothelium.
Concomitant medical therapeutics: Hmox1
Hmox1 expression can be induced by the naturally occurring antioxidants resveratrol and quercetin, which have been shown, both alone and in combination, to inhibit neointimal hyperplasia after carotid balloon injury (Fig. 5) (Huang et al, 2009; Khandelwal et al, 2012; Khandelwal et al, 2010). An early rabbit study found that the use of a resveratrol-quercetin-DES accelerated re-endothelialization by 50%, and significantly reduced in-stent stenosis by ∼65% and macrophage infiltration by ∼60%, compared with a BMS control (Kleinedler et al, 2012). In a 2020 study, oral delivery of resveratrol and atorvastatin in combination with a rapamycin (RAPA)-DES improved strut coverage, with areas of high endothelialization in a rabbit model, however, the combined resveratrol/atorvastatin treatment also had increased neointimal thickness, which could potentially develop into restenosis (Chen et al, 2020).
Further in vivo studies found that a 3D-printed resveratrol-loaded stent protected ECs from H2O2-induced apoptosis by promoting •NO release and reducing apoptosis, proinflammatory cytokine (e.g., TNF-α) release, and ROS production (Veerubhotla and Lee, 2022). Resveratrol may also improve vascular tone through oxidative activation of PKG1α (Prysyazhna et al, 2019). Although these studies appear promising, currently no clinical trials are investigating the effect of utilizing resveratrol and/or quercetin in PCI for CAD.
Concomitant medical therapeutics: apolipoprotein A1
Apolipoprotein A1 (apoA1), the major protein constituent of high-density lipoprotein (HDL), has been previously considered a potential treatment target for restenosis. Systemic apoA1 infusions in a murine model of stent deployment resulted in enhanced endothelialization, inhibition of platelet activation, and an overall reduction in neointimal hyperplasia (Vanags et al, 2018). However, apoA1 is susceptible to HOCl-mediated oxidative modifications that result in loss of the atheroprotective properties of HDL. To overcome the oxidant-induced loss of function, oxidation-resistant apoA1 (4WF) was combined with local delivery by an adeno-associated viral-coated stent (Hooshdaran et al, 2022). Preliminary ex vivo studies with 4WF apoA1 were promising, as 4WF maintained its ability to export cholesterol in the presence of HOCl and significantly inhibited VSMC proliferation. However, in subsequent studies using an in vivo porcine model, there was a loss in luminal area with 4WF compared with controls, with increased neointimal thickness and no mitigation of restenosis.
Advances in PCI biomaterials
There has been a shift in recent years to attempt to create stents using materials that are not only robust enough to keep open a blocked artery but that are multifunctional and aim to restore healthy vascular function while reducing stent failure.
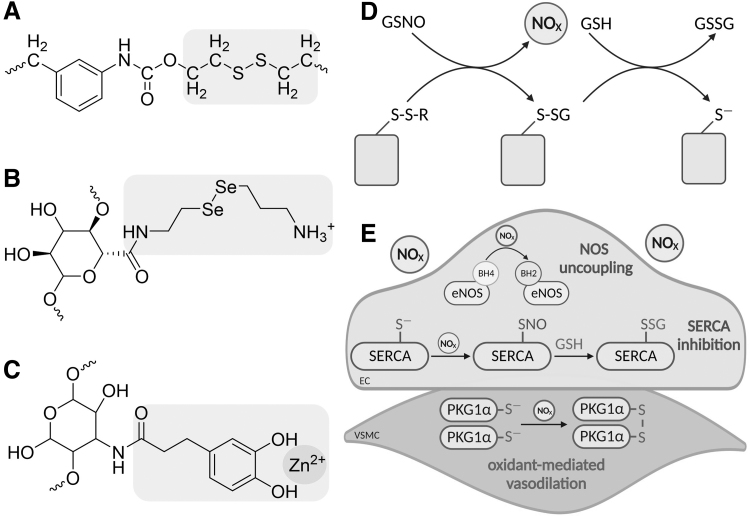
Multiple laboratories have sought to achieve improved endothelial function and increased NO bioavailability by harnessing the properties of redox-active molecules to reduce labile nitroso bonds in circulating RSNO, namely GSNO, to increase local •NO concentrations at the site of stent placement (Fig. 7A–C). Liberated •NO is proposed to improve endothelial function, promote healthy vascular tone via •NO/cGMP signaling, and reduce thrombus formation by decreasing platelet adhesion. One material is a sulfur-mediated polycarbonate polyurethane stent that relies on the reaction of GSNO with disulfide bonds to release NO and in turn S-glutathiolate thiols on the stent surface (Fig. 7A) (Li et al, 2022). The reducing capacity of stent material can be regenerated via reaction with GSH, producing GSSG and a reduced thiol on the stent surface (Fig. 7D).
FIG. 7.
NO-elevating biomaterials. Novel biomaterials including PCU-SS (A), NO hydrogel (B), and Zn2+ bound to catechol-grafted chitosan (C) contain domains (in purple) designed to increase NO bioavailability through the reduction of circulating S-nitrosothiol, predominantly GSNO. (D) Mechanistically, PCU-SS and the NO hydrogel reduce the labile S-nitrosobond in GSNO via nucleophilic attack, resulting in S-glutathiolation of stent surface thiol (or selenium, not shown) and the release of a reactive nitrogen species (NOX). The biomaterial is regenerated using GSH. (E) Elevation of NOX may be detrimental to EC function due to the ability to oxidize BH4 to BH2, resulting in eNOS uncoupling and a decrease in •NO production, and inhibitory S-glutathiolation of SERCA. However, elevated NOX may compensate for decreased •NO by promoting oxidant-mediated vasodilation via disulfide PKG1α in VSMCs. GSH, reduced glutathione; GSNO, S-nitrosoglutathione; PCU-SS, sulfur-mediated polycarbonate polyurethane.
Similarly, an NO-eluting hydrogel has been developed that can be used in conjunction with standard stents (Chen et al, 2021). The hydrogel relies on the reducing potential of selenium in catalyzing GSNO decomposition, which is advantageous as selenium is a better nucleophile with a lower pKa resulting in more efficient reduction of nitroso bonds (Fig. 7B). In a porcine model, the NO-eluting hydrogel improved re-endothelialization, reduced neointimal hyperplasia, and upregulated genes involved in the regulation of vascular tone, including HMOX1. The use of selenium-conjugated stent coatings has also been shown to accelerate endothelial repair and decrease injury-induced inflammation, highlighting the potential for this redox-active molecule to improve endothelial function post-PCI (Zhang et al, 2022b).
However, the liberated “NO” from GSNO will be RNS, likely in the form of HNO, not authentic •NO. As such, this will lack the ability to influence classical •NO/cGMP-mediated vasodilation and may instead add to the oxidative burden in ECs (Fig. 7E). In addition, it is perhaps unlikely that these materials will be efficient at generating NO species in vivo as blood plasma GSH concentrations are typically below 6 μM, thus limiting the capacity of these stent materials to restore their intrinsic reducing capacities (Michelet et al, 1995).
In an effort to promote re-endothelialization and recovery of endothelial function, a functional stent coating that mimics the endothelium has been created (Zhang et al, 2022a). This stent coating utilizes a thiol-ene “click” reaction combining the bioactive Arg-Glu-Asp-Val (REDV) peptide with catechol-grafted chitosan (CS-C) and zinc sulfate. Once in place within the vessel, the REDV peptide promotes re-endothelialization by capturing circulating endothelial progenitor cells (Duan et al, 2019). The CS-C creates a large number of zinc sulfate adsorption sites, which in turn catalyze the release of •NO/RNS, again via the decomposition of circulating RNSO and relying on the redox properties of Zn2+ to coordinate the reduction of the nitroso bond (Fig. 7C).
Transcriptomics comparing aortas from a rabbit model with control stents versus the CS-C/Zn/REDV-coated stents showed an upregulation in cell adhesion markers combined with a decrease in markers for platelet activation and lipid deposition, suggesting that the REDV peptide and •NO/RNS release provide a synergistic effect resulting in the creation a functional endothelium. However, this study had inconsistencies in the impact of the CS-C/Zn/REDV on the expression of matrix metalloproteases, with an apparent upregulation in vivo, which may impact the composition of the vasculature matrix resulting in the weakening of the arterial wall.
It should be noted that previous efforts to create NO-eluting stents have thus far failed to make it into the clinic. Increasing •NO levels in the endothelium has clear benefits in reducing thrombosis and restenosis. However, deleterious by-products of •NO production and metabolism, namely disulfides via RSNO intermediates, must be considered when developing stent technologies (Wolhuter et al, 2018). Moreover, simply increasing •NO may not be enough to improve the long-term function of ECs if intracellular oxidant production is uncontrolled.
To tackle increased ROS production post-PCI, a novel stent was created with an electrospun core-shell nanofiber coloaded with 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPOL) and RAPA that correspondingly serves as an ROS scavenger and VSMC proliferation inhibitor (Wang et al, 2023). Scanning electron microscopy of the luminal side of stented arteries identified significantly higher re-endothelialization with the RAPA/TEMPOL-loaded stent, compared with standard DES or BMS, and ECs were elongated and orientated in the direction of flow. Furthermore, there was a reduction in endothelial adhesion factors (VCAM-1, E-selectin) indicating that RAPA/TEMPOL confers anti-inflammatory and antithrombogenic properties. Notably, neointimal hyperplasia was comparable between the DES and RAPA/TEMPOL-loaded stent at 1 month. Long-term studies are required to determine if this novel stent confers clinical advantages over current DES.
Bioresorbable scaffolds
Bioresorbable stents (BRS), also known as bioabsorbable or biodegradable stents, have the main advantage that they clear from the body within a few years and can theoretically reduce the long-term adverse effects of conventional metallic stents. First-generation BRS came to the market in 2016, however, meta-analysis of randomized trials comparing commercially available BRS to second-generation DES found BRS to be inferior with no clinical advantage (Ke et al, 2020). Nevertheless, while these first-generation BRS failed to positively impact outcomes, the prospect of being able to clear the body of a stent is still an attractive possibility and research into novel BRS is ongoing. The inherent physical and mechanical properties of specific metals, namely Fe, Mg, and Zn, make them attractive candidates for BRS and a great deal of research in the last decade has focused on creating the ideal alloy (Mani et al, 2007).
Fe-based materials are one option for BRS due to their high strength, high elastic modulus, and high ductility. In vivo studies in naïve rabbits found that these stents can be safely implanted and do not exacerbate inflammation, neointimal proliferation, or thrombotic incidents at 18 months postprocedure (Peuster et al, 2001). Pure iron peripheral stents deployed in naïve minipigs were found to be effective and safe, with no signs of iron overload or iron toxicity 1 year postimplantation (Peuster et al, 2006). However, recent studies have shown that corrosion of iron has the strong potential to generate HO•, likely via the Fenton reaction (Scarcello et al, 2020a). HO• is a potent oxidizing agent with significant proinflammatory properties that can affect ECs and the surrounding tissue (Ahsan et al, 2003).
Exposure of isolated rat aortic rings to Fe wire was associated with elevations in Hmox1 expression, uncoupling of eNOS, and decreased NO production within ECs, resulting in impaired endothelium-dependent relaxation (Scarcello et al, 2020b). Concurrent treatment with catalase protected the endothelium, likely by reducing H2O2 and decreasing HO• formed via the Fenton reaction. These data suggest that Fe-based BRS may be deleterious to endothelial function and the effect of HO• production on ECs should be considered when evaluating the biocompatibility of these alloys.
While pure Mg degrades rapidly, biodegradable magnesium alloys are favorable due to their slower degradation rates, biocompatibility, and low thrombogenicity. The release of Mg2+ has been proposed to restore endothelial function by counteracting the pro-oxidative effects of common biodegradable polymers, namely PLLA, by attenuating the expression of inflammatory markers and decreasing mitochondrial ROS production (Ko et al, 2021). Early work showed that an Mg-based BRS had significantly higher re-endothelialization and reduced infiltration of foam cells 35 days post-PCI compared with DES in a rabbit model of neoatherosclerosis (Nicol et al, 2020). In support of this, the use of Mg-BRS was associated with increased vasoconstriction to Ach at 1 year post-PCI (vs. SES, p = 0.003), indicating improved endothelial function (Sabate et al, 2019).
However, a separate 3-year follow-up trial found a higher incidence of target lesion revascularization associated with Mg stents, indicative of increased restenosis and stent failure (CI: −20.7 to −1.2; p = 0.030) (Ortega-Paz et al, 2022).
Perhaps one of the most intriguing studies to come out in 2022 was the development of a hybrid material composed of a biodegradable polymer substrate and double anodic/cathodic metallic layers that comprised Mg and Zn aiming to harness the signaling potential of ROS to improve clinical outcomes (Jeong et al, 2022). This work leads on from the development of an H2O2-generating biomaterial that couples nitinol stents with biodegradable Mg/Zn to reduce VSMC proliferation (Park et al, 2019). While these ROS-producing biomaterials suppressed the proliferation of cultured VSMCs, neither study determined the ability of stents made from these materials to improve in vivo re-endothelialization or endothelial function.
Furthermore, the rationale for the use of these biomaterials to improve PCI outcomes is based on the ability of •NO, not ROS, to suppress VSMC proliferation and improve endothelial function. ROS generated by these composite biomaterials are perhaps even less likely to have beneficial effects on the vasculature, and it is questionable whether increasing ROS production in a state of ED will have beneficial consequences.
Future Directions
Advances in PCI and stent technologies have improved patient outcomes over the last two decades, however, an unmet need remains for novel devices that not only reopen stenotic arteries but also restore healthy vascular function. Currently, drugs used on DES to inhibit VSMC proliferation and reduce restenosis also prevent the restoration of a healthy endothelium. In many cases, these drugs drive oxidant production and reduce •NO bioavailability, therefore enhancing ED.
While we are making strides into developing novel approaches and technologies that can eliminate reactive species or elevate NO bioavailability, as clinical trials have shown, simply removing oxidants or promoting •NO production alone is not sufficient to recover endothelial function post-PCI. If we can better understand the factors that impact oxidative signaling within this complex system, we can create novel stent-based technologies that harness redox signaling to not only improve endothelial function but also restore vascular tone and prevent disease recurrence.
Acknowledgments
All figures, except Figure 7A–C, were created with BioRender.com The chemical structures in Figure 7A–C were drawn using ChemDraw (v19.0.0; Perkin Elmer).
Abbreviations Used
- ACh
acetylcholine
- apoA1
apolipoprotein A1
- BH2
dihydrobiopterin
- BH4
tetrahydrobiopterin
- BKca
large conductance calcium-activated potassium channel
- BMS
bare-metal stenting
- BRS
bioresorbable stents
- BVRA
biliverdin reductase
- CAD
coronary artery disease
- Cav-1
caveolin-1
- cGMP
3′,5′-cyclic guanosine monophosphate
- CI
confidence interval
- CS-C
catechol-grafted chitosan
- DES
drug-eluting stents
- EC
endothelial cell
- ED
endothelial dysfunction
- eNOS
endothelial nitric oxide synthase
- GPx1
glutathione peroxidase 1
- GSH
reduced glutathione
- GSNO
S-nitrosoglutathione
- GSSG
oxidized glutathione
- H2O2
hydrogen peroxide
- HDL
high-density lipoproteins
- Hmox1
heme oxygenase-1
- HOCl
hypochlorous acid
- ICAM-1
intercellular adhesion molecule-1
- IDO
indoleamine 2,3-dioxygenase
- iNOS
inducible nitric oxide synthase
- IP3Rs
inositol trisphosphate receptors
- MACE
major adverse cardiac events
- MAPK
mitogen-activated protein kinase
- MI
myocardial infarction
- MPO
myeloperoxidase
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor kappa B
- nNOS
neuronal nitric oxide synthase
- •NO
nitric oxide
- •NO2
nitrogen dioxide
- NO−3
nitrate
- NOS
nitric oxide synthase
- NOX
NADPH oxidase
- Nrf2
nuclear factor E2-related factor 2
- O2•−
superoxide radical anion
- •OH
hydroxyl radicals
- ONOO−
peroxynitrite
- OR
odds ratio
- oxLDL
oxidized low-density lipoprotein
- PCI
percutaneous coronary interventions
- PCU-SS
sulfur-mediated polycarbonate polyurethane
- PKG1α
protein kinase G subunit 1α
- RAPA
rapamycin
- REDV
Arg-Glu-Asp-Val
- RNS
reactive nitrogen species
- ROOH
organic peroxides
- ROS
reactive oxygen species
- RSNO
S-nitrosothiol
- SERCA
sarco/endoplasmic reticulum Ca2+-ATPase
- sGC
soluble guanylate cyclase
- SNO
S-nitrosation
- SOD
superoxide dismutase
- S−
thiolates
- TEMPOL
4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl
- TNF-α
tumor necrosis factor α
- Trx
thioredoxin
- TrxR
thioredoxin reductase
- VCAM-1
vascular cell adhesion molecule-1
- VSMC
vascular smooth muscle cell
- vWF
von Willebrand factor
- XO
xanthine oxidase
Authors' Contributions
All authors were involved in the conceptualization, writing, review, and editing of the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Jason C. Kovacic acknowledges research support from the National Institutes of Health (R01HL148167), New South Wales health grant RG194194, the Bourne Foundation, and Agilent. Christopher P. Stanley acknowledges research support from a New South Wales Health Early to Mid-Research grant.
References
- Adachi T, Weisbrod RM, Pimentel DR, et al. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med 2004;10(11):1200–1207; doi: 10.1038/nm1119 [DOI] [PubMed] [Google Scholar]
- Aguilar G, Cordova F, Koning T, et al. TNF-alpha-activated eNOS signalling increases leukocyte adhesion through the S-nitrosylation pathway. Am J Physiol Heart Circ Physiol 2021;321(6):H1083–H1095; doi: 10.1152/ajpheart.00065.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahsan H, Ali A, Ali R. Oxygen free radicals and systemic autoimmunity. Clin Exp Immunol 2003;131(3):398–404; doi: 10.1046/j.1365-2249.2003.02104.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertolle ME, Peter Guengerich F. The relationships between cytochromes P450 and H2O2: Production, reaction, and inhibition. J Inorg Biochem 2018;186:228–234; doi: 10.1016/j.jinorgbio.2018.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldini G, Altomare A, Baron G, et al. N-acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radic Res 2018;52(7):751–762; doi: 10.1080/10715762.2018.1468564 [DOI] [PubMed] [Google Scholar]
- Alexander JJ, Graham DJ, Miguel R. Oxygen radicals alter LDL permeability and uptake by an endothelial-smooth muscle cell bilayer. J Surg Res 1991;51(5):361–367; doi: 10.1016/0022-4804(91)90134-8 [DOI] [PubMed] [Google Scholar]
- Ali ZA, de Jesus Perez V, Yuan K, et al. Oxido-reductive regulation of vascular remodeling by receptor tyrosine kinase ROS1. J Clin Invest 2014;124(12):5159–5174; doi: 10.1172/JCI77484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alioua A, Tanaka Y, Wallner M, et al. The large conductance, voltage-dependent, and calcium-sensitive K+ channel, Hslo, is a target of cGMP-dependent protein kinase phosphorylation in vivo. J Biol Chem 1998;273(49):32950–32956; doi: 10.1074/jbc.273.49.32950 [DOI] [PubMed] [Google Scholar]
- Antl M, von Bruhl ML, Eiglsperger C, et al. IRAG mediates NO/cGMP-dependent inhibition of platelet aggregation and thrombus formation. Blood 2007;109(2):552–559; doi: 10.1182/blood-2005-10-026294 [DOI] [PubMed] [Google Scholar]
- Aoki J, Serruys PW, van Beusekom H, et al. Endothelial progenitor cell capture by stents coated with antibody against CD34: The HEALING-FIM (healthy endothelial accelerated lining inhibits neointimal growth-first in man) Registry. J Am Coll Cardiol 2005;45(10):1574–1579; doi: 10.1016/j.jacc.2005.01.048 [DOI] [PubMed] [Google Scholar]
- Barreto J, Karathanasis SK, Remaley A, et al. Role of LOX-1 (lectin-like oxidized low-density lipoprotein receptor 1) as a cardiovascular risk predictor: mechanistic insight and potential clinical use. Arterioscler Thromb Vasc Biol 2021;41(1):153–166; doi: 10.1161/ATVBAHA.120.315421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry-Lane PA, Patterson C, van der Merwe M, et al. p47phox is required for atherosclerotic lesion progression in ApoE(-/-) mice. J Clin Invest 2001;108(10):1513–1522; doi: 10.1172/JCI11927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck K, Wu BJ, Ni J, et al. Interplay between heme oxygenase-1 and the multifunctional transcription factor yin yang 1 in the inhibition of intimal hyperplasia. Circ Res 2010;107(12):1490–1497; doi: 10.1161/CIRCRESAHA.110.231985 [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev 2007;87(1):245–313; doi: 10.1152/physrev.00044.2005 [DOI] [PubMed] [Google Scholar]
- Bolotina VM, Najibi S, Palacino JJ, et al. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature 1994;368(6474):850–853; doi: 10.1038/368850a0 [DOI] [PubMed] [Google Scholar]
- Bortolotti M, Polito L, Battelli MG, et al. Xanthine oxidoreductase: One enzyme for multiple physiological tasks. Redox Biol 2021;41:101882; doi: 10.1016/j.redox.2021.101882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD. Riding the tiger—Physiological and pathological effects of superoxide and hydrogen peroxide generated in the mitochondrial matrix. Crit Rev Biochem Mol Biol 2020;55(6):592–661; doi: 10.1080/10409238.2020.1828258 [DOI] [PubMed] [Google Scholar]
- Brasen JH, Leppanen O, Inkala M, et al. Extracellular superoxide dismutase accelerates endothelial recovery and inhibits in-stent restenosis in stented atherosclerotic Watanabe heritable hyperlipidemic rabbit aorta. J Am Coll Cardiol 2007;50(23):2249–2253; doi: 10.1016/j.jacc.2007.08.038 [DOI] [PubMed] [Google Scholar]
- Brouard S, Otterbein LE, Anrather J, et al. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med 2000;192(7):1015–1026; doi: 10.1084/jem.192.7.1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann GK, Schurmann C, Spaeth M, et al. The hydrogen-peroxide producing NADPH oxidase 4 does not limit neointima development after vascular injury in mice. Redox Biol 2021;45:102050; doi: 10.1016/j.redox.2021.102050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne JR, Madhani M, Cuello F, et al. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science 2007;317(5843):1393–1397; doi: 10.1126/science.1144318 [DOI] [PubMed] [Google Scholar]
- Charles RL, Schroder E, May G, et al. Protein sulfenation as a redox sensor: Proteomics studies using a novel biotinylated dimedone analogue. Mol Cell Proteomics 2007;6(9):1473–1484; doi: 10.1074/mcp.M700065-MCP200 [DOI] [PubMed] [Google Scholar]
- Chen C, Song C, Zhang D, et al. Effect of resveratrol combined with atorvastatin on re-endothelialization after drug-eluting stents implantation and the underlying mechanism. Life Sci 2020;245:117349; doi: 10.1016/j.lfs.2020.117349 [DOI] [PubMed] [Google Scholar]
- Chen Y, Gao P, Huang L, et al. A tough nitric oxide-eluting hydrogel coating suppresses neointimal hyperplasia on vascular stent. Nat Commun 2021;12(1):7079; doi: 10.1038/s41467-021-27368-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Keaney JF Jr., Schulz E, et al. Decreased neointimal formation in Nox2-deficient mice reveals a direct role for NADPH oxidase in the response to arterial injury. Proc Natl Acad Sci U S A 2004;101(35):13014–13019; doi: 10.1073/pnas.0405389101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare J, Ganly J, Bursill CA, et al. The mechanisms of restenosis and relevance to next generation stent design. Biomolecules 2022;12(3):430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J, Htun N, Lew R, et al. Colchicine to prevent periprocedural myocardial injury in percutaneous coronary intervention: The COPE-PCI pilot trial. Circ Cardiovasc Interv 2021;14(5):e009992; doi: 10.1161/CIRCINTERVENTIONS.120.009992 [DOI] [PubMed] [Google Scholar]
- Cole JE, Astola N, Cribbs AP, et al. Indoleamine 2,3-dioxygenase-1 is protective in atherosclerosis and its metabolites provide new opportunities for drug development. Proc Natl Acad Sci U S A 2015;112(42):13033–13038; doi: 10.1073/pnas.1517820112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Liu Y, Zhao F, et al. Neoatherosclerosis after drug-eluting stent implantation: Roles and mechanisms. Oxid Med Cell Longev 2016;2016:5924234; doi: 10.1155/2016/5924234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das D, Pemberton PW, Burrows PC, et al. Antioxidant properties of colchicine in acute carbon tetrachloride induced rat liver injury and its role in the resolution of established cirrhosis. Biochim Biophys Acta 2000;1502(3):351–362; doi: 10.1016/s0925-4439(00)00059-4 [DOI] [PubMed] [Google Scholar]
- Dayal S, Brown KL, Weydert CJ, et al. Deficiency of glutathione peroxidase-1 sensitizes hyperhomocysteinemic mice to endothelial dysfunction. Arterioscler Thromb Vasc Biol 2002;22(12):1996–2002; doi: 10.1161/01.atv.0000041629.92741.dc [DOI] [PubMed] [Google Scholar]
- Doenst T, Haverich A, Serruys P, et al. PCI and CABG for treating stable coronary artery disease: JACC review topic of the week. J Am Coll Cardiol 2019;73(8):964–976; doi: 10.1016/j.jacc.2018.11.053 [DOI] [PubMed] [Google Scholar]
- Doyle B, Rihal CS, O'Sullivan CJ, et al. Outcomes of stent thrombosis and restenosis during extended follow-up of patients treated with bare-metal coronary stents. Circulation 2007;116(21):2391–2398; doi: 10.1161/CIRCULATIONAHA.107.707331 [DOI] [PubMed] [Google Scholar]
- Duan Y, Yu S, Xu P, et al. Co-immobilization of CD133 antibodies, vascular endothelial growth factors, and REDV peptide promotes capture, proliferation, and differentiation of endothelial progenitor cells. Acta Biomater 2019;96:137–148; doi: 10.1016/j.actbio.2019.07.004 [DOI] [PubMed] [Google Scholar]
- Duckers HJ, Boehm M, True AL, et al. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med 2001;7(6):693–698; doi: 10.1038/89068 [DOI] [PubMed] [Google Scholar]
- Ebert MLA, Schmidt VF, Pfaff L, et al. Animal models of neointimal hyperplasia and restenosis: Species-specific differences and implications for translational research. JACC Basic Transl Sci 2021;6(11):900–917; doi: 10.1016/j.jacbts.2021.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiserich JP, Baldus S, Brennan ML, et al. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science 2002;296(5577):2391–2394; doi: 10.1126/science.1106830 [DOI] [PubMed] [Google Scholar]
- Espinosa-Diez C, Miguel V, Vallejo S, et al. Role of glutathione biosynthesis in endothelial dysfunction and fibrosis. Redox Biol 2018;14:88–99; doi: 10.1016/j.redox.2017.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista AM, Thompson MD, Bolotina VM, et al. Nox4- and Nox2-dependent oxidant production is required for VEGF-induced SERCA cysteine-674 S-glutathiolation and endothelial cell migration. Free Radic Biol Med 2012;53(12):2327–2334; doi: 10.1016/j.freeradbiomed.2012.10.546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Hernando C, Yu J, Suarez Y, et al. Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis. Cell Metab 2009;10(1):48–54; doi: 10.1016/j.cmet.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Sueta G, Campolo N, Trujillo M, et al. Biochemistry of peroxynitrite and protein tyrosine nitration. Chem Rev 2018;118(3):1338–1408; doi: 10.1021/acs.chemrev.7b00568 [DOI] [PubMed] [Google Scholar]
- Forgione MA, Weiss N, Heydrick S, et al. Cellular glutathione peroxidase deficiency and endothelial dysfunction. Am J Physiol Heart Circ Physiol 2002;282(4):H1255–H1261; doi: 10.1152/ajpheart.00598.2001 [DOI] [PubMed] [Google Scholar]
- Forman HJ, Zhang H, Rinna A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med 2009;30(1–2):1–12; doi: 10.1016/j.mam.2008.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed JK, Beyer AM, LoGiudice JA, et al. Ceramide changes the mediator of flow-induced vasodilation from nitric oxide to hydrogen peroxide in the human microcirculation. Circ Res 2014;115(5):525–532; doi: 10.1161/CIRCRESAHA.115.303881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gach O, Legrand V, Biessaux Y, et al. Long-term prognostic significance of high-sensitivity C-reactive protein before and after coronary angioplasty in patients with stable angina pectoris. Am J Cardiol 2007;99(1):31–35; doi: 10.1016/j.amjcard.2006.07.059 [DOI] [PubMed] [Google Scholar]
- Ganjali S, Mansouri A, Abbasifard M, et al. Association between oxidative burden and restenosis: A case-control study. Oxid Med Cell Longev 2022;2022:3577761; doi: 10.1155/2022/3577761 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Godbole AS, Lu X, Guo X, et al. NADPH oxidase has a directional response to shear stress. Am J Physiol Heart Circ Physiol 2009;296(1):H152–H158; doi: 10.1152/ajpheart.01251.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JW, Shaw JA, Kirshenbaum LA. Multiple facets of NF-kappaB in the heart: To be or not to NF-kappaB. Circ Res 2011;108(9):1122–1132; doi: 10.1161/CIRCRESAHA.110.226928 [DOI] [PubMed] [Google Scholar]
- Hofma SH, van der Giessen WJ, van Dalen BM, et al. Indication of long-term endothelial dysfunction after sirolimus-eluting stent implantation. Eur Heart J 2006;27(2):166–170; doi: 10.1093/eurheartj/ehi571 [DOI] [PubMed] [Google Scholar]
- Hooshdaran B, Pressly BB, Alferiev IS, et al. Stent-based delivery of AAV2 vectors encoding oxidation-resistant apoA1. Sci Rep 2022;12(1):5464; doi: 10.1038/s41598-022-09524-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst BG, Marletta MA. Physiological activation and deactivation of soluble guanylate cyclase. Nitric Oxide 2018;77:65–74; doi: 10.1016/j.niox.2018.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang BF, Wang W, Fu YC, et al. The effect of quercetin on neointima formation in a rat artery balloon injury model. Pathol Res Pract 2009;205(8):515–523; doi: 10.1016/j.prp.2009.01.007 [DOI] [PubMed] [Google Scholar]
- Jabs A, Gobel S, Wenzel P, et al. Sirolimus-induced vascular dysfunction. Increased mitochondrial and nicotinamide adenosine dinucleotide phosphate oxidase-dependent superoxide production and decreased vascular nitric oxide formation. J Am Coll Cardiol 2008;51(22):2130–2138; doi: 10.1016/j.jacc.2008.01.058 [DOI] [PubMed] [Google Scholar]
- Jeong G, Hwang HW, Chae M, et al. Development of organic/inorganic hybrid materials for fully degradable reactive oxygen species-releasing stents for antirestenosis. Langmuir 2022;38(26):8003–8011; doi: 10.1021/acs.langmuir.2c00791 [DOI] [PubMed] [Google Scholar]
- Jin BY, Sartoretto JL, Gladyshev VN, et al. Endothelial nitric oxide synthase negatively regulates hydrogen peroxide-stimulated AMP-activated protein kinase in endothelial cells. Proc Natl Acad Sci U S A 2009;106(41):17343–17348; doi: 10.1073/pnas.0907409106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judkins CP, Diep H, Broughton BR, et al. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE-/- mice. Am J Physiol Heart Circ Physiol 2010;298(1):H24–H32; doi: 10.1152/ajpheart.00799.2009 [DOI] [PubMed] [Google Scholar]
- Ke J, Zhang H, Huang J, et al. Three-year outcomes of bioresorbable vascular scaffolds versus second-generation drug-eluting stents: Meta-analysis of randomized trials. Medicine (Baltimore) 2020;99(31):e21554; doi: 10.1097/MD.0000000000021554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal AR, Hebert VY, Dugas TR. Essential role of ER-alpha-dependent NO production in resveratrol-mediated inhibition of restenosis. Am J Physiol Heart Circ Physiol 2010;299(5):H1451–H1458; doi: 10.1152/ajpheart.00369.2010 [DOI] [PubMed] [Google Scholar]
- Khandelwal AR, Hebert VY, Kleinedler JJ, et al. Resveratrol and quercetin interact to inhibit neointimal hyperplasia in mice with a carotid injury. J Nutr 2012;142(8):1487–1494; doi: 10.3945/jn.112.162628 [DOI] [PubMed] [Google Scholar]
- Khavandi K, Baylie RA, Sugden SA, et al. Pressure-induced oxidative activation of PKG enables vasoregulation by Ca2+ sparks and BK channels. Sci Signal 2016;9(449):ra100; doi: 10.1126/scisignal.aaf6625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kietadisorn R, Juni RP, Moens AL. Tackling endothelial dysfunction by modulating NOS uncoupling: New insights into its pathogenesis and therapeutic possibilities. Am J Physiol Endocrinol Metab 2012;302(5):E481–E495; doi: 10.1152/ajpendo.00540.2011 [DOI] [PubMed] [Google Scholar]
- Kim C, Sharma R. Cyclic nucleotide selectivity of protein kinase G isozymes. Protein Sci 2021;30(2):316–327; doi: 10.1002/pro.4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Hong YJ, Jeong MH, et al. Two-year clinical outcome after carvedilol-loaded stent implantation in patients with coronary artery disease. Korean J Intern Med 2011;26(1):41–46; doi: 10.3904/kjim.2011.26.1.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Seo HS, Park JH, et al. A prospective, randomized, 6-month comparison of the coronary vasomotor response associated with a zotarolimus- versus a sirolimus-eluting stent: Differential recovery of coronary endothelial dysfunction. J Am Coll Cardiol 2009;53(18):1653–1659; doi: 10.1016/j.jacc.2009.01.051 [DOI] [PubMed] [Google Scholar]
- Kimura T, Morimoto T, Kozuma K, et al. Comparisons of baseline demographics, clinical presentation, and long-term outcome among patients with early, late, and very late stent thrombosis of sirolimus-eluting stents: Observations from the Registry of Stent Thrombosis for Review and Reevaluation (RESTART). Circulation 2010;122(1):52–61; doi: 10.1161/CIRCULATIONAHA.109.903955 [DOI] [PubMed] [Google Scholar]
- Kirsch J, Schneider H, Pagel JI, et al. Endothelial dysfunction, and a prothrombotic, proinflammatory phenotype is caused by loss of mitochondrial thioredoxin reductase in endothelium. Arterioscler Thromb Vasc Biol 2016;36(9):1891–1899; doi: 10.1161/ATVBAHA.116.307843 [DOI] [PubMed] [Google Scholar]
- Kitazawa T, Semba S, Huh YH, et al. Nitric oxide-induced biphasic mechanism of vascular relaxation via dephosphorylation of CPI-17 and MYPT1. J Physiol 2009;587(Pt 14):3587–3603; doi: 10.1113/jphysiol.2009.172189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinedler JJ, Foley JD, Orchard EA, et al. Novel nanocomposite stent coating releasing resveratrol and quercetin reduces neointimal hyperplasia and promotes re-endothelialization. J Control Release 2012;159(1):27–33; doi: 10.1016/j.jconrel.2012.01.008 [DOI] [PubMed] [Google Scholar]
- Ko KW, Choi B, Kang EY, et al. The antagonistic effect of magnesium hydroxide particles on vascular endothelial activation induced by acidic PLGA degradation products. Biomater Sci 2021;9(3):892–907; doi: 10.1039/d0bm01656j [DOI] [PubMed] [Google Scholar]
- Kornowski R, Hong MK, Tio FO, et al. In-stent restenosis: Contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol 1998;31(1):224–230; doi: 10.1016/s0735-1097(97)00450-6 [DOI] [PubMed] [Google Scholar]
- Kufner S, Ernst M, Cassese S, et al. 10-Year outcomes from a randomized trial of polymer-free versus durable polymer drug-eluting coronary stents. J Am Coll Cardiol 2020;76(2):146–158; doi: 10.1016/j.jacc.2020.05.026 [DOI] [PubMed] [Google Scholar]
- Kuzkaya N, Weissmann N, Harrison DG, et al. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: Implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem 2003;278(25):22546–22554; doi: 10.1074/jbc.M302227200 [DOI] [PubMed] [Google Scholar]
- Lau AK, Leichtweis SB, Hume P, et al. Probucol promotes functional reendothelialization in balloon-injured rabbit aortas. Circulation 2003;107(15):2031–2036; doi: 10.1161/01.CIR.0000062682.40051.43 [DOI] [PubMed] [Google Scholar]
- Laurindo FR, da Luz PL, Uint L, et al. Evidence for superoxide radical-dependent coronary vasospasm after angioplasty in intact dogs. Circulation 1991;83(5):1705–1715; doi: 10.1161/01.cir.83.5.1705 [DOI] [PubMed] [Google Scholar]
- Lee MY, San Martin A, Mehta PK, et al. Mechanisms of vascular smooth muscle NADPH oxidase 1 (Nox1) contribution to injury-induced neointimal formation. Arterioscler Thromb Vasc Biol 2009;29(4):480–487; doi: 10.1161/ATVBAHA.108.181925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefer AM. Nitric oxide: Nature's naturally occurring leukocyte inhibitor. Circulation 1997;95(3):553–554; doi: 10.1161/01.cir.95.3.553 [DOI] [PubMed] [Google Scholar]
- Leite PF, Danilovic A, Moriel P, et al. Sustained decrease in superoxide dismutase activity underlies constrictive remodeling after balloon injury in rabbits. Arterioscler Thromb Vasc Biol 2003;23(12):2197–2202; doi: 10.1161/01.ATV.0000093980.46838.41 [DOI] [PubMed] [Google Scholar]
- Leite PF, Liberman M, Sandoli de Brito F, et al. Redox processes underlying the vascular repair reaction. World J Surg 2004;28(3):331–336; doi: 10.1007/s00268-003-7399-4 [DOI] [PubMed] [Google Scholar]
- Li P, Cai W, Li X, et al. Sulfur-mediated polycarbonate polyurethane for potential application of blood-contacting materials. Front Bioeng Biotechnol 2022;10:874419; doi: 10.3389/fbioe.2022.874419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Liu H, Zhou JS, et al. Caveolin-1 inhibits expression of antioxidant enzymes through direct interaction with nuclear erythroid 2 p45-related factor-2 (Nrf2). J Biol Chem 2012;287(25):20922–20930; doi: 10.1074/jbc.M112.352336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. The changing landscape of atherosclerosis. Nature 2021;592(7855):524–533; doi: 10.1038/s41586-021-03392-8 [DOI] [PubMed] [Google Scholar]
- Liu Y, Bubolz AH, Mendoza S, et al. H2O2 is the transferrable factor mediating flow-induced dilation in human coronary arterioles. Circ Res 2011;108(5):566–573; doi: 10.1161/CIRCRESAHA.110.237636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka DJ, Kaplan AV, Lucas FL, et al. Outcomes following coronary stenting in the era of bare-metal vs the era of drug-eluting stents. JAMA 2008;299(24):2868–2876; doi: 10.1001/jama.299.24.2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani G, Feldman MD, Patel D, et al. Coronary stents: A materials perspective. Biomaterials 2007;28(9):1689–1710; doi: 10.1016/j.biomaterials.2006.11.042 [DOI] [PubMed] [Google Scholar]
- Marshall HE, Stamler JS. Inhibition of NF-kappa B by S-nitrosylation. Biochemistry 2001;40(6):1688–1693; doi: 10.1021/bi002239y [DOI] [PubMed] [Google Scholar]
- Maruhashi T, Kihara Y, Higashi Y. Bilirubin and endothelial function. J Atheroscler Thromb 2019;26(8):688–696; doi: 10.5551/jat.RV17035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba T, Shimokawa H, Kubota H, et al. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem Biophys Res Commun 2002;290(3):909–913; doi: 10.1006/bbrc.2001.6278 [DOI] [PubMed] [Google Scholar]
- Matoba T, Shimokawa H, Nakashima M, et al. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest 2000;106(12):1521–1530; doi: 10.1172/JCI10506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, Morrell CN, Cambien B, et al. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell 2003;115(2):139–150; doi: 10.1016/s0092-8674(03)00803-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelet F, Gueguen R, Leroy P, et al. Blood and plasma glutathione measured in healthy subjects by HPLC: Relation to sex, aging, biological variables, and life habits. Clin Chem 1995;41(10):1509–1517. [PubMed] [Google Scholar]
- Mollnau H, Schulz E, Daiber A, et al. Nebivolol prevents vascular NOS III uncoupling in experimental hyperlipidemia and inhibits NADPH oxidase activity in inflammatory cells. Arterioscler Thromb Vasc Biol 2003;23(4):615–621; doi: 10.1161/01.ATV.0000065234.70518.26 [DOI] [PubMed] [Google Scholar]
- Mukai Y, Rikitake Y, Shiojima I, et al. Decreased vascular lesion formation in mice with inducible endothelial-specific expression of protein kinase Akt. J Clin Invest 2006;116(2):334–343; doi: 10.1172/JCI26223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa G, Otsuka F, Nakano M, et al. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol 2011;57(11):1314–1322; doi: 10.1016/j.jacc.2011.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol P, Bulin A, Castellanos MI, et al. Preclinical investigation of neoatherosclerosis in magnesium-based bioresorbable scaffolds versus thick-strut drug-eluting stents. EuroIntervention 2020;16(11):e922–e929; doi: 10.4244/EIJ-D-19-00747 [DOI] [PubMed] [Google Scholar]
- Ortega-Paz L, Brugaletta S, Gomez-Lara J, et al. Magnesium-based resorbable scaffold vs permanent metallic sirolimus-eluting stent in patients with ST-segment elevation myocardial infarction: 3-year results of the MAGSTEMI randomised controlled trial. EuroIntervention 2022;18(5):e389–e396; doi: 10.4244/EIJ-D-21-00651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otaegui Irurueta I, Gonzalez Sucarrats S, Barron Molina JL, et al. Can an ultrathin strut stent design and a polymer free, proendothelializing probucol matrix coating improve early strut healing? The FRIENDLY-OCT trial. An intra-patient randomized study with OCT, evaluating early strut coverage of a novel probucol coated polymer-free and ultra-thin strut sirolimus-eluting stent compared to a biodegradable polymer sirolimus-eluting stent. Int J Cardiol 2022;360:13–20; doi: 10.1016/j.ijcard.2022.04.043 [DOI] [PubMed] [Google Scholar]
- Ottolini M, Hong K, Cope EL, et al. Local peroxynitrite impairs endothelial transient receptor potential vanilloid 4 channels and elevates blood pressure in obesity. Circulation 2020;141(16):1318–1333; doi: 10.1161/CIRCULATIONAHA.119.043385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987;327(6122):524–526; doi: 10.1038/327524a0 [DOI] [PubMed] [Google Scholar]
- Park J, Seo H, Hwang HW, et al. Interface engineering of fully metallic stents enabling controllable H2O2 generation for antirestenosis. Langmuir 2019;35(10):3634–3642; doi: 10.1021/acs.langmuir.8b03753 [DOI] [PubMed] [Google Scholar]
- Pearce CG, Najjar SF, Kapadia MR, et al. Beneficial effect of a short-acting NO donor for the prevention of neointimal hyperplasia. Free Radic Biol Med 2008;44(1):73–81; doi: 10.1016/j.freeradbiomed.2007.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peshavariya H, Dusting GJ, Jiang F, et al. NADPH oxidase isoform selective regulation of endothelial cell proliferation and survival. Naunyn Schmiedebergs Arch Pharmacol 2009;380(2):193–204; doi: 10.1007/s00210-009-0413-0 [DOI] [PubMed] [Google Scholar]
- Peuster M, Hesse C, Schloo T, et al. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials 2006;27(28):4955–4962; doi: 10.1016/j.biomaterials.2006.05.029 [DOI] [PubMed] [Google Scholar]
- Peuster M, Wohlsein P, Brugmann M, et al. A novel approach to temporary stenting: Degradable cardiovascular stents produced from corrodible metal-results 6–18 months after implantation into New Zealand white rabbits. Heart 2001;86(5):563–569; doi: 10.1136/heart.86.5.563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prysyazhna O, Rudyk O, Eaton P. Single atom substitution in mouse protein kinase G eliminates oxidant sensing to cause hypertension. Nat Med 2012;18(2):286–290; doi: 10.1038/nm.2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prysyazhna O, Wolhuter K, Switzer C, et al. Blood pressure-lowering by the antioxidant resveratrol is counterintuitively mediated by oxidation of cGMP-dependent protein kinase. Circulation 2019;140(2):126–137; doi: 10.1161/CIRCULATIONAHA.118.037398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc Natl Acad Sci U S A 2018;115(23):5839–5848; doi: 10.1073/pnas.1804932115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid I, Maghzal GJ, Chen YC, et al. Myeloperoxidase is a potential molecular imaging and therapeutic target for the identification and stabilization of high-risk atherosclerotic plaque. Eur Heart J 2018;39(35):3301–3310; doi: 10.1093/eurheartj/ehy419 [DOI] [PubMed] [Google Scholar]
- Sabate M, Alfonso F, Cequier A, et al. Magnesium-based resorbable scaffold versus permanent metallic sirolimus-eluting stent in patients with ST-segment elevation myocardial infarction: The MAGSTEMI randomized clinical trial. Circulation 2019;140(23):1904–1916; doi: 10.1161/CIRCULATIONAHA.119.043467 [DOI] [PubMed] [Google Scholar]
- Sauer H, Wartenberg M. Reactive oxygen species as signalling molecules in cardiovascular differentiation of embryonic stem cells and tumor-induced angiogenesis. Antioxid Redox Signal 2005;7(11–12):1423–1434; doi: 10.1089/ars.2005.7.1423 [DOI] [PubMed] [Google Scholar]
- Scarcello E, Herpain A, Tomatis M, et al. Hydroxyl radicals and oxidative stress: The dark side of Fe corrosion. Colloids Surf B Biointerfaces 2020a;185:110542; doi: 10.1016/j.colsurfb.2019.110542 [DOI] [PubMed] [Google Scholar]
- Scarcello E, Lobysheva I, Bouzin C, et al. Endothelial dysfunction induced by hydroxyl radicals—The hidden face of biodegradable Fe-based materials for coronary stents. Mater Sci Eng C Mater Biol Appl 2020b;112:110938; doi: 10.1016/j.msec.2020.110938 [DOI] [PubMed] [Google Scholar]
- Serizawa K, Yogo K, Aizawa K, et al. Paclitaxel-induced endothelial dysfunction in living rats is prevented by nicorandil via reduction of oxidative stress. J Pharmacol Sci 2012;119(4):349–358; doi: 10.1254/jphs.12067fp [DOI] [PubMed] [Google Scholar]
- Sharma A, Yuen D, Huet O, et al. Lack of glutathione peroxidase-1 facilitates a pro-inflammatory and activated vascular endothelium. Vasc Pharmacol 2016;79:32–42; doi: 10.1016/j.vph.2015.11.001 [DOI] [PubMed] [Google Scholar]
- Shaw RL, Norton CE, Segal SS. Apoptosis in resistance arteries induced by hydrogen peroxide: Greater resilience of endothelium versus smooth muscle. Am J Physiol Heart Circ Physiol 2021;320(4):H1625–H1633; doi: 10.1152/ajpheart.00956.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuvalova YA, Kaminnyi AI, Meshkov AN, et al. Association between polymorphisms of eNOS and GPx-1 genes, activity of free-radical processes and in-stent restenosis. Mol Cell Biochem 2012;370(1–2):241–249; doi: 10.1007/s11010-012-1419-3 [DOI] [PubMed] [Google Scholar]
- Sies H, Belousov VV, Chandel NS, et al. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat Rev Mol Cell Biol 2022;23(7):499–515; doi: 10.1038/s41580-022-00456-z [DOI] [PubMed] [Google Scholar]
- Smith JF, Lemmey HAL, Borysova L, et al. Endothelial nitric oxide suppresses action-potential-like transient spikes and vasospasm in small resistance arteries. Hypertension 2020;76(3):785–794; doi: 10.1161/HYPERTENSIONAHA.120.15491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorescu GP, Song H, Tressel SL, et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res 2004;95(8):773–779; doi: 10.1161/01.RES.0000145728.22878.45 [DOI] [PubMed] [Google Scholar]
- Stanley CP, Maghzal GJ, Ayer A, et al. Singlet molecular oxygen regulates vascular tone and blood pressure in inflammation. Nature 2019;566(7745):548–552; doi: 10.1038/s41586-019-0947-3 [DOI] [PubMed] [Google Scholar]
- Stanley CP, Maghzal GJ, Stocker R. Roles of Hydrogen Peroxide in the Regulation of Vascular Tone. CRC Press: Boca Raton, FL; 2017. [Google Scholar]
- Stuehr DJ, Haque MM. Nitric oxide synthase enzymology in the 20 years after the Nobel Prize. Br J Pharmacol 2019;176(2):177–188; doi: 10.1111/bph.14533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szocs K, Lassegue B, Sorescu D, et al. Upregulation of Nox-based NAD(P)H oxidases in restenosis after carotid injury. Arterioscler Thromb Vasc Biol 2002;22(1):21–27; doi: 10.1161/hq0102.102189 [DOI] [PubMed] [Google Scholar]
- Takano M, Yamamoto M, Xie Y, et al. Serial long-term evaluation of neointimal stent coverage and thrombus after sirolimus-eluting stent implantation by use of coronary angioscopy. Heart 2007;93(12):1533–1536; doi: 10.1136/hrt.2007.131714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanous D, Brasen JH, Choy K, et al. Probucol inhibits in-stent thrombosis and neointimal hyperplasia by promoting re-endothelialization. Atherosclerosis 2006;189(2):342–349; doi: 10.1016/j.atherosclerosis.2006.01.025 [DOI] [PubMed] [Google Scholar]
- Tawa M, Shimosato T, Iwasaki H, et al. Effects of peroxynitrite on relaxation through the NO/sGC/cGMP pathway in isolated rat iliac arteries. J Vasc Res 2014;51(6):439–446; doi: 10.1159/000371491 [DOI] [PubMed] [Google Scholar]
- Thai T, Zhong F, Dang L, et al. Endothelial-transcytosed myeloperoxidase activates endothelial nitric oxide synthase via a phospholipase C-dependent calcium signalling pathway. Free Radic Biol Med 2021;166:255–264; doi: 10.1016/j.freeradbiomed.2020.12.448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togni M, Raber L, Cocchia R, et al. Local vascular dysfunction after coronary paclitaxel-eluting stent implantation. Int J Cardiol 2007;120(2):212–220; doi: 10.1016/j.ijcard.2006.09.021 [DOI] [PubMed] [Google Scholar]
- Togni M, Windecker S, Cocchia R, et al. Sirolimus-eluting stents associated with paradoxic coronary vasoconstriction. J Am Coll Cardiol 2005;46(2):231–236; doi: 10.1016/j.jacc.2005.01.062 [DOI] [PubMed] [Google Scholar]
- Tonelli C, Chio IIC, Tuveson DA. Transcriptional regulation by Nrf2. Antioxid Redox Signal 2018;29(17):1727–1745; doi: 10.1089/ars.2017.7342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii S, Nakazawa G, Ijichi T, et al. Comparison of the endothelial coverage in everolimus and zotarolimus-eluting stents in normal, atherosclerotic, and bifurcation rabbit iliac arteries. Cardiovasc Interv Ther 2018;33(1):55–61; doi: 10.1007/s12928-016-0437-6 [DOI] [PubMed] [Google Scholar]
- Tretter V, Hochreiter B, Zach ML, et al. Understanding cellular redox homeostasis: A challenge for precision medicine. Int J Mol Sci 2021;23(1):106; doi: 10.3390/ijms23010106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker B, Vaidya K, Cochran BJ, et al. Inflammation during percutaneous coronary intervention-prognostic value, mechanisms and therapeutic targets. Cells 2021;10(6):1391; doi: 10.3390/cells10061391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadseth C, Souza JM, Thomson L, et al. Pro-thrombotic state induced by post-translational modification of fibrinogen by reactive nitrogen species. J Biol Chem 2004;279(10):8820–8826; doi: 10.1074/jbc.M306101200 [DOI] [PubMed] [Google Scholar]
- Valek L, Heidler J, Scheving R, et al. Nitric oxide contributes to protein homeostasis by S-nitrosylations of the chaperone HSPA8 and the ubiquitin ligase UBE2D. Redox Biol 2019;20:217–235; doi: 10.1016/j.redox.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beusekom HM, Ertas G, Sorop O, et al. The Genous endothelial progenitor cell capture stent accelerates stent re-endothelialization but does not affect intimal hyperplasia in porcine coronary arteries. Catheter Cardiovasc Interv 2012;79(2):231–242; doi: 10.1002/ccd.22928 [DOI] [PubMed] [Google Scholar]
- Vanags LZ, Tan JTM, Galougahi KK, et al. Apolipoprotein A-I reduces in-stent restenosis and platelet activation and alters neointimal cellular phenotype. JACC Basic Transl Sci 2018;3(2):200–209; doi: 10.1016/j.jacbts.2017.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerubhotla K, Lee CH. Design of biodegradable 3D-printed cardiovascular stent. Bioprinting 2022;26:e00204; doi: 10.1016/j.bprint.2022.e00204 [DOI] [Google Scholar]
- Vendrov AE, Hakim ZS, Madamanchi NR, et al. Atherosclerosis is attenuated by limiting superoxide generation in both macrophages and vessel wall cells. Arterioscler Thromb Vasc Biol 2007;27(12):2714–2721; doi: 10.1161/ATVBAHA.107.152629 [DOI] [PubMed] [Google Scholar]
- Volonte D, Galbiati F. Inhibition of thioredoxin reductase 1 by caveolin 1 promotes stress-induced premature senescence. EMBO Rep 2009;10(12):1334–1340; doi: 10.1038/embor.2009.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajih N, Basu S, Jailwala A, et al. Potential therapeutic action of nitrite in sickle cell disease. Redox Biol 2017;12:1026–1039; doi: 10.1016/j.redox.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis SJ, Martin W. Conditions permitting suppression of stretch-induced and vasoconstrictor tone by basal nitric oxide activity in porcine cerebral artery. Br J Pharmacol 2000;130(3):567–574; doi: 10.1038/sj.bjp.0703351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Lu J, Yin J, et al. A TEMPOL and rapamycin loaded nanofiber-covered stent favors endothelialization and mitigates neointimal hyperplasia and local inflammation. Bioact Mater 2023;19:666–677; doi: 10.1016/j.bioactmat.2022.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Chennupati R, Kaur H, et al. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J Clin Invest 2016;126(12):4527–4536; doi: 10.1172/JCI87343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Ha CH, Jhun BS, et al. Fluid shear stress stimulates phosphorylation-dependent nuclear export of HDAC5 and mediates expression of KLF2 and eNOS. Blood 2010;115(14):2971–2979; doi: 10.1182/blood-2009-05-224824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfrom M, Hanke S, Kamenz J, et al. Effect of the antioxidant Nicanartine on the proliferative and inflammatory response after experimental balloon angioplasty. Coron Artery Dis 1998;9(12):831–837; doi: 10.1097/00019501-199809120-00008 [DOI] [PubMed] [Google Scholar]
- Wolhuter K, Whitwell HJ, Switzer CH, et al. Evidence against stable protein S-nitrosylation as a widespread mechanism of post-translational regulation. Mol Cell 2018;69(3):438–450 e435; doi: 10.1016/j.molcel.2017.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongpraparut N, Pengchata P, Piyophirapong S, et al. Indoleamine 2,3 dioxygenase (IDO) level as a marker for significant coronary artery disease. BMC Cardiovasc Disord 2021;21(1):353; doi: 10.1186/s12872-021-02140-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Ding Y, Ramprasath T, et al. Oxidative stress, GTPCH1, and endothelial nitric oxide synthase uncoupling in hypertension. Antioxid Redox Signal 2021;34(9):750–764; doi: 10.1089/ars.2020.8112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki H, Haendeler J, Berk BC. Thioredoxin: A key regulator of cardiovascular homeostasis. Circ Res 2003;93(11):1029–1033; doi: 10.1161/01.RES.0000102869.39150.23 [DOI] [PubMed] [Google Scholar]
- Zhang B, Qin Y, Wang Y. A nitric oxide-eluting and REDV peptide-conjugated coating promotes vascular healing. Biomaterials 2022a;284:121478; doi: 10.1016/j.biomaterials.2022.121478 [DOI] [PubMed] [Google Scholar]
- Zhang B, Qin Y, Yang L, et al. An organic selenium and VEGF-conjugated bioinspired coating promotes vascular healing. Biomaterials 2022b;287:121654; doi: 10.1016/j.biomaterials.2022.121654 [DOI] [PubMed] [Google Scholar]