Abstract
Objectives:
To assess antiretroviral therapy (ART) coverage among pregnant women living with HIV and compare the characteristics of women who received and did not receive ART during pregnancy in Zambia.
Methods:
A cross-sectional study was conducted at urban and rural health facilities in Southern Province, Zambia, from 2016-2019. Pregnant women living with HIV delivering at study sites were enrolled and administered a questionnaire, and the results of infant diagnostic testing for HIV at birth was documented.
Results:
1184 mother/infant pairs were enrolled. ART coverage was 93.7%. Most women who did not receive ART during pregnancy reported HIV diagnosis at delivery (18.0%) or during pregnancy (57.7%). The primary reported reason for not receiving ART was not wanting to take the drugs. Women who did not receive ART during pregnancy were significantly younger, less likely to have disclosed their HIV-infection status to others, and less likely to have received antenatal care than women who received ART. ART use correlated with higher levels of education in urban but not rural sites. Overall, 1.0% of infants were infected with HIV at birth, including 0.8% of infants born to women who received ART and 4.1% of infants born to women who did not.
Conclusions:
Most women received ART according to guidelines, resulting in low perinatal transmission rates of HIV to infants. Efforts to increase ART coverage and prevent vertical transmission should focus on identifying incident HIV infections during pregnancy and strengthening counseling for newly diagnosed pregnant women.
Keywords: HIV, pediatrics, sub-Saharan Africa, prevention of mother-to-child HIV transmission
Introduction
In 2020, an estimated 19.3 million women were living with HIV globally and 150,000 children were newly infected, with 84% of new infections among children reported in sub-Saharan Africa (1). Great strides have been made in reducing the number of children acquiring HIV infection, down from an estimated 490,000 in 2000 (2), primarily through expansion of services to prevent mother-to-child transmission (PMTCT) and provision of antiretroviral therapy (ART) to pregnant women living with HIV. Globally, 85% of pregnant women living with HIV had access to ART in 2020 (3).
The scale-up of PMTCT programs was driven by the 2010 adoption of a global plan to reduce the number of incident infections in infants and young children by 90% and decrease the risk of mother-to-child transmission of HIV to <5% (4). To achieve these goals, targets set included that 90% of women receive perinatal ART. The plan focused on 22 priority countries with the most pregnant women living with HIV, including 21 in sub-Saharan Africa. However, progress towards eliminating vertical HIV transmission has slowed in recent years as expansion of services in focus countries has stalled (5).
Multiple barriers exist that prevent access to, receipt of, and adherence to ART for pregnant women living with HIV in sub-Saharan Africa. A study in Uganda following the implementation of Option B+ (i.e. pregnant women start ART regardless of disease status and continue for life) highlighted the individual barriers of poor knowledge of the benefits of ART for PMTCT, drug side effects, stigma and discrimination, and participant denial; the interpersonal barrier of non-disclosure; the community barrier of religious beliefs; and the health system barriers of inaccessibility, lack of confidentiality, and poor documentation (6). Many of these concerns were also noted in a 2018 meta-analysis of perinatal HIV transmission in sub-Saharan Africa, with the addition of the following challenges: late initiation of ART, non-adherence, non-involvement of men in antenatal care, and lack of community-led PMTCT programs (7).
For programs to expand coverage and effectively curtail mother-to-child HIV transmission in the era of universal ART, a better understanding of the differences between pregnant women who receive and do not receive ART is needed. To address these gaps, a cross-sectional study was conducted among pregnant women living with HIV in Southern Province, Zambia. Zambia is one of the priority countries in the global plan (4), with an estimated population of 17.9 million people (8), including 850,000 women living with HIV and 8300 newly infected children in 2020 (1). ART coverage among pregnant women living with HIV increased from 58% in 2010 to 80% in 2020 (1).
Methods
Study setting
This analysis was nested within the Novel Screening for Exposed Babies (NSEBA) Study which was conducted from 2016 to 2019 in urban (Livingstone City) and rural (Macha) areas of Southern Province, Zambia (Supplemental Figure 1). Livingstone is a large city, approximately 480 km south of the capital of Lusaka, with an estimated population of 134,349 in 2010 (9). Macha is a rural area located approximately 350 km south of Lusaka, with an estimated population density of 25–45 residents per km2 and primarily populated by subsistence farmers. The estimated HIV prevalence in Southern Province was 12.4% among adults 15–49 years of age in 2018 (10).
Healthcare in Zambia is provided at three levels corresponding to the services provided: primary (urban/rural health centers [UHCs/RHCs]/health posts), secondary (district hospitals), and tertiary (specialized services). Maternity and ART services are provided at primary (UHCs/RHCs) and secondary facilities, and complicated cases are referred to tertiary facilities. Women living with HIV receive ART at HIV clinics at the facilities. However, pregnant women are seen and receive ART through Maternal and Child Health clinics until 3 months after the baby is weaned.
During the study period, universal treatment of pregnant women living with HIV was implemented throughout Zambia. HIV testing was recommended for children exposed to HIV starting at birth (or first contact) through at least 6 weeks post-weaning (11).
NSEBA study overview
The objective of the NSEBA Study was to evaluate strategies for implementing point-of-care technologies for early infant diagnosis of HIV in urban and rural Zambia. This included a cross-sectional validation study of a point-of-care platform for diagnosing HIV infection at birth in Livingstone City from February 2016 to August 2018 and a cohort study to validate a point-of-care testing platform for diagnosing HIV from birth through the post-weaning period in the Macha area from February 2016 to December 2019 (12). Hospitals and UHCs/RHCs providing maternity services were selected based on the numbers of women living with HIV delivering at the facility and infants receiving HIV diagnostic testing in prior years. This cross-sectional analysis was performed using data collected at birth from the Livingstone and Macha sites.
NSEBA study procedures at birth
At Macha sites (1 hospital and 4 RHCs) and for the first year at Livingstone sites (1 hospital and 1 UHC), all clinically stable infants born to women living with HIV in the maternity wards were eligible for participation (Supplemental Table 1). In the second year, additional sites in Livingstone (2 UHCs) were added and eligibility at all sites in Livingstone was restricted to clinically stable infants born to high-risk women living with HIV to achieve the primary aim of the NSEBA study. High-risk was defined as not receiving ART throughout pregnancy or starting ART during pregnancy based on national treatment guidelines in effect at that time (11).
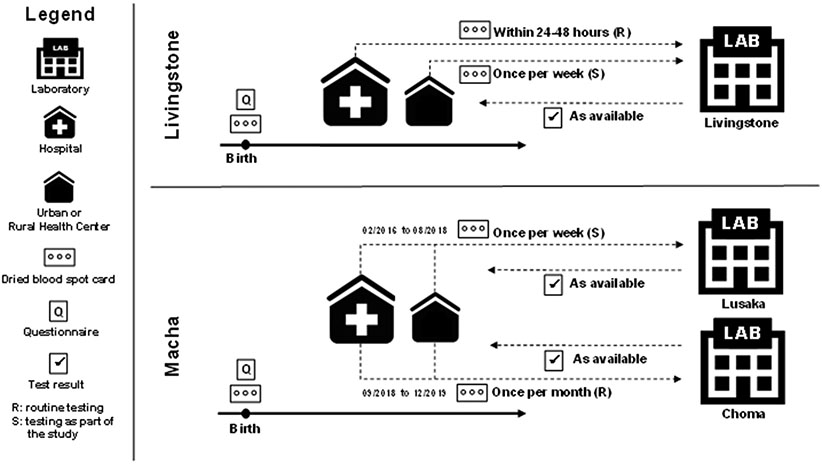
Potentially eligible infants were identified by study staff through daily surveillance of admission and delivery registers, and their mothers were approached for participation in the study. Mothers willing to participate provided written informed consent and were administered a questionnaire to collect information on demographic characteristics, antenatal care, HIV testing history, and receipt of ART. Dried blood spots (DBS) were collected on filter paper from the infant for nucleic acid-based testing at a central laboratory (Figure 1). Although national guidelines recommended testing at birth, only the hospital in Livingstone routinely did this. At the other sites, testing at birth was offered to participants as a study activity (in Macha it became routinely available in 2018). Study staff received test results for study participants and provided them to clinic staff who conveyed results to the mother through routine clinic procedures.
Figure 1: Study overview and procedures.
Analytic sample
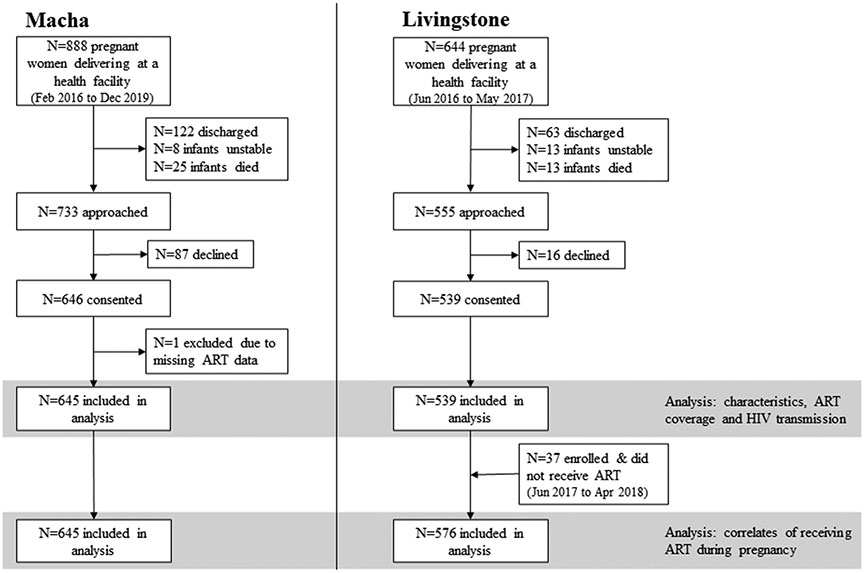
The analytic sample to estimate ART coverage and rates of mother-to-child HIV transmission included: 1) all infants enrolled at the birth visit at the Macha sites from February 2016 to December 2019 (n=645; one child excluded due to missing maternal ART data); and 2) all infants enrolled at the Livingstone sites from June 2016 to May 2017 (n=539) (Figure 2).
Figure 2: Enrollment flowchart by site.
ART: antiretroviral therapy
The analytic sample to describe women who did not receive ART, assess reasons for not receiving ART, and evaluate correlates of not receiving ART included the two groups from the first analysis, as well as infants born to women living with HIV who did not receive ART enrolled at the Livingstone sites from June 2017 to April 2018 (n=37). These infants were included to better capture the range of reasons provided and increase power for detecting correlates of not receiving ART.
Definitions
Maternal ART was primarily defined by self-report; where available, HIV clinic records were referenced for confirmation in approximately one-third of cases. ART start date (or whether known to be receiving ART prior to pregnancy without a start date) was collected, allowing for differentiation between women initiating ART prior to or during pregnancy. For this analysis, maternal ART use was dichotomized as any use (initiation of ART prior to or during pregnancy) versus none. Timing of maternal HIV diagnosis was defined based on self-reported testing history and results.
Statistical analysis
ART coverage was estimated as the proportion of women receiving any ART during pregnancy. As ART coverage varies by population and HIV program, estimates were calculated overall and by location and year. Rates of mother-to-child HIV transmission at birth were estimated as the proportion of infants testing positive and were calculated overall and by location and maternal ART. The characteristics of women were analyzed separately by site and compared using chi-squared tests for categorical variables and Wilcoxon rank-sum test for continuous variables.
The characteristics of women who did not receive ART, including timing of maternal HIV diagnosis and testing history, were summarized by location. Self-reported reasons for not receiving ART were summarized by location among women diagnosed with HIV prior to or during pregnancy. Correlates of not receiving ART during pregnancy were evaluated by comparing the characteristics of women who did and did not receive ART in each location, using chi-squared tests for categorical variables and Wilcoxon rank-sum tests for continuous variables.
For all analyses, p-values less than 0.05 were considered statistically significant. All analyses were conducted using Stata Statistical Software, Release 17 (StataCorp LLC, College Station, TX).
Ethics statement
This study was approved by the Institutional Review Boards at Macha Research Trust and the Johns Hopkins Bloomberg School of Public Health, and by the National Health Research Authority in Zambia. Written informed consent was obtained for participation in the study, including publication of results, by trained study staff from a parent or guardian of HIV-exposed infants.
Results
Characteristics of the study population
Overall, 1532 pregnant women living with HIV were identified and 1184 (77.3%) HIV-exposed infants were enrolled, including 645 from sites in Macha and 539 from sites in Livingstone (Figure 2).
Maternal characteristics are presented in Table 1. Women enrolled from the rural Macha area were significantly different from those enrolled in urban Livingstone. They had a lower level of education, were more likely to be farmers, were less likely to own a cell phone, and had longer travel times to the clinic using more varied modes of transportation. Women from the two areas also differed on several pregnancy and HIV-related characteristics (Table 1). Although receipt of antenatal care was nearly universal across both sites, women in Macha were more likely to attend ≥4 antenatal care visits, to receive antenatal care for more than 4 months of their pregnancy, and to have been diagnosed with HIV infection prior to pregnancy, compared with women in Livingstone.
Table 1:
Characteristics of pregnant women living with HIV in southern Zambia, overall and by site
| Livingstone (N=539) | Macha (N=645) | Total (N=1184) | |||||
|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | p-valuec | |
| Demographic characteristics | |||||||
| Age in years, median (IQR) | 30 | (25, 35) | 30 | (24, 36) | 30 | (24, 35) | 0.16 |
| Missing | 0 | 1 | 1 | ||||
| Maternal education | <0.001 | ||||||
| None | 8 | 1.5 | 19 | 3.0 | 27 | 2.3 | |
| Primary | 104 | 19.4 | 344 | 53.9 | 448 | 38.1 | |
| Secondary | 357 | 66.5 | 258 | 40.4 | 615 | 52.3 | |
| Some college/technical | 68 | 12.7 | 17 | 2.7 | 85 | 7.2 | |
| Missing | 2 | 7 | 9 | ||||
| Owns cell phone | 437 | 81.1 | 444 | 69.1 | 881 | 74.5 | <0.001 |
| Missing | 0 | 2 | 2 | ||||
| Transportation time (minutes), median (IQR) | 15 | (10, 20) | 60 | (30, 120) | 30 | (15, 60) | <0.001 |
| ≤30min | 475 | 91.5 | 158 | 26.8 | 633 | 57.1 | |
| >30min - 1hr | 30 | 5.8 | 213 | 36.2 | 243 | 21.9 | |
| >1hr - 2hr | 10 | 1.9 | 123 | 20.9 | 133 | 12.0 | |
| >2hr - 3hr | 3 | 0.6 | 46 | 7.8 | 49 | 4.4 | |
| >3hr - 5hr | 1 | 0.2 | 30 | 5.1 | 31 | 2.8 | |
| >5hr | 0 | 0 | 19 | 3.2 | 19 | 1.7 | |
| Missing | 20 | 56 | 76 | ||||
| Transportation methods | |||||||
| Walk | 41 | 7.6 | 177 | 27.5 | 218 | 18.4 | <0.001 |
| Bicycle | 0 | 0 | 87 | 13.5 | 87 | 7.4 | <0.001 |
| Public/Group transport | 3 | 0.6 | 128 | 19.9 | 131 | 11.1 | <0.001 |
| Motor vehicle (Car/Vehicle/Motorcycle/Scooter) | 379 | 70.3 | 159 | 24.7 | 538 | 45.5 | <0.001 |
| Ambulance | 115 | 21.3 | 46 | 7.1 | 161 | 13.6 | <0.001 |
| Other | 1 | 0.2 | 48 | 7.5 | 49 | 4.1 | <0.001 |
| Missing | 0 | 1 | 1 | ||||
| Employment | <0.001 | ||||||
| Farmer | 17 | 3.2 | 522 | 81.1 | 539 | 45.6 | |
| Non-farmer | 300 | 55.7 | 65 | 10.1 | 365 | 30.9 | |
| Student | 1 | 0.2 | 6 | 0.9 | 7 | 0.6 | |
| Unemployed | 221 | 41.0 | 51 | 7.9 | 272 | 23.0 | |
| Missing | 0 | 1 | 1 | ||||
| Pregnancy and HIV-related characteristics | |||||||
| Received antenatal care | 533 | 98.9 | 639 | 99.5 | 1172 | 99.2 | 0.20 |
| Missing | 0 | 3 | 3 | ||||
| # of antenatal care visits | 0.002 | ||||||
| 1 to 3 visits | 249 | 47.3 | 243 | 38.3 | 492 | 42.3 | |
| 4 to 8 visits | 278 | 52.8 | 392 | 61.7 | 670 | 57.7 | |
| Missing | 12 | 10 | 22 | ||||
| Duration of antenatal care | 0.001 | ||||||
| 0 to 4 months | 351 | 69.9 | 270 | 59.7 | 621 | 65.1 | |
| 5 to 9 months | 151 | 30.1 | 182 | 40.3 | 333 | 34.9 | |
| Missing | 37 | 193 | 230 | ||||
| Place of deliverya | |||||||
| Clinic | 193 | 35.8 | 291 | 45.3 | 484 | 41.0 | <0.001 |
| Hospital | 338 | 62.7 | 313 | 48.7 | 651 | 55.1 | |
| Home | 8 | 1.5 | 39 | 6.1 | 47 | 4.0 | |
| Missing | 0 | 2 | 2 | ||||
| Timing of HIV diagnosis | |||||||
| Before pregnancy | 319 | 59.2 | 494 | 76.6 | 813 | 68.7 | <0.001 |
| During pregnancy | 216 | 40.1 | 141 | 21.9 | 357 | 30.2 | |
| During delivery/labor | 4 | 0.7 | 10 | 1.6 | 14 | 1.2 | |
| Missing | 0 | 0 | 0 | ||||
| HIV status disclosedb | - | 371 | 98.7 | 371 | 98.7 | ||
| Husband/boyfriend | - | 333 | 88.6 | 333 | 88.6 | ||
| Parents | - | 271 | 72.1 | 271 | 72.1 | ||
| Children | - | 57 | 15.2 | 57 | 15.2 | ||
| Other relatives | - | 157 | 41.8 | 157 | 41.8 | ||
| Friends | - | 55 | 14.6 | 55 | 14.6 | ||
| Neighbors | - | 36 | 9.6 | 36 | 9.6 | ||
| Other | - | 1 | 0.3 | 1 | 0.3 | ||
| Missing | 539 | 269 | 808 | ||||
Seven women in the Macha area gave birth on way to facility. Their places of delivery were marked as the place of their enrollment (N=4 for hospital, N=3 for clinic).
Question added after study start on August 14, 2017
Wilcoxon rank-sum test for continuous variables and chi-squared test for categorical variables
ART coverage, regimens, and mother-to-child transmission of HIV
ART coverage was estimated to be 93.7% (1109/1184) and was significantly higher in Macha (95.0%; 613/645) than Livingstone (92.0%; 496/539; p=0.03), although the difference was small. No significant differences were observed by clinic in either area (Supplemental Figure 2). Among women diagnosed prior to pregnancy, 97.7% (794/813) received ART during pregnancy, including 86.4% (648/750) who had initiated ART prior to the pregnancy. Among women diagnosed during pregnancy, 88.2% (315/357) received ART during pregnancy.
Among women who received ART (n=1109), information on regimen type was available for 1088 (98.1%). All women reported receiving combination ART, primarily (96.9%; 1051/1085) tenofovir disoproxil fumarate plus lamivudine or emtricitabine plus efavirenz (TDF/XTC/EFV). Most (71.6%; 794/1109) women receiving ART reported being diagnosed with HIV prior to pregnancy.
All infants were tested for HIV, and valid results were available for 97.7% (1157/1184). Overall, 1.0% (12/1157) of infants were infected with HIV at birth, including 1.5% (8/539) in Livingstone and 0.7% (4/618) in Macha. Infants born to women who did not receive ART were significantly more likely to be infected with HIV (4.1% [3/74], compared to 0.8% [9/1083]; p=0.008; Figure 3).
Figure 3: Mother-to-child transmission of HIV by maternal receipt of antiretroviral therapy, overall and by site.
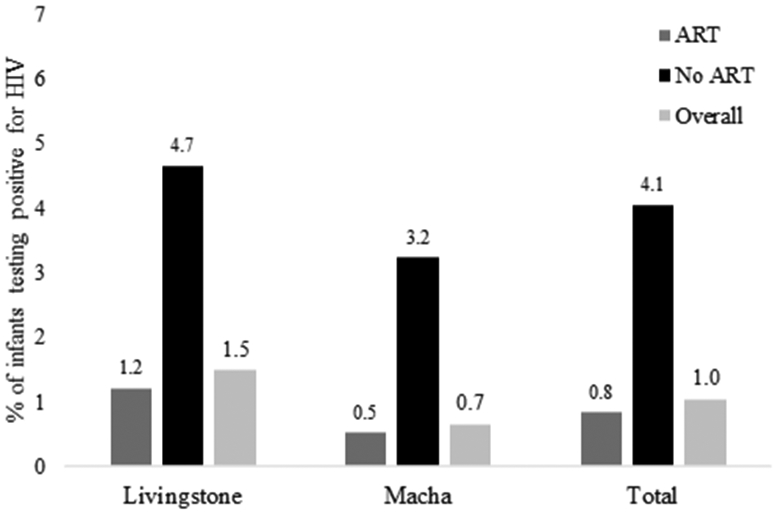
ART: antiretroviral therapy
HIV testing history and reasons for not receiving ART
A total of 112 women enrolled from the Macha (n=32) and Livingstone (n=43 enrolled in the first year and n=37 added from the second year of enrollment) sites did not receive ART (Table 2). Most women reported being diagnosed with HIV either at labor and delivery (18.0%; 20/111) or during pregnancy (57.7%; 64/111), but 24.3% (27/111) were diagnosed prior to pregnancy (see Supplemental Table 2 for participant characteristics by timing of diagnosis). Maternal HIV testing history was reviewed to evaluate missed opportunities for testing and earlier diagnosis (Supplemental Table 3). More than half of women diagnosed with HIV during pregnancy tested negative for HIV prior to pregnancy (59.4%; 38/64), and approximately half diagnosed during pregnancy were diagnosed in the second trimester (49.2%; 31/64). Two-thirds (65.0%; 13/20) of women diagnosed with HIV at labor and delivery had previously tested negative for HIV (50.0% during pregnancy and 60.0% prior to pregnancy). Of those diagnosed at labor and delivery who tested negative during pregnancy, 40.0% (4/10) had last tested negative during the third trimester. Most women diagnosed at labor and delivery who did not report testing during pregnancy received antenatal care (60.0%; 6/10).
Table 2:
Correlates of not receiving antiretroviral therapy during pregnancy among women living with HIV, overall and by site
| Livingstone (N=576) | Macha (N=645) | Total (N=1221) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No ART (N=80) | ART (N=496) |
p-valuec | No ART (N=32) |
ART (N=613) |
p-valuec | No ART (N=112) |
ART (N=1109) |
p-valuec | |||||||
| N | % | N | % | N | % | N | % | N | % | N | % | ||||
| Demographic characteristics | |||||||||||||||
| Age in years, median (IQR) | 26 | (23, 32) | 30 | (25, 35) | 0.005 | 23 | (19, 28) | 31 | (24, 36) | <0.001 | 25 | (21, 31.5) | 30 | (25, 36) | <0.001 |
| Missing | 0 | 0 | 0 | 1 | 0 | 1 | |||||||||
| Maternal education | 0.08 | 0.019 | 0.34 | ||||||||||||
| None | 2 | 2.5 | 6 | 1.2 | 0 | 0.0 | 19 | 3.1 | 2 | 1.8 | 25 | 2.3 | |||
| Primary | 24 | 30.0 | 92 | 18.6 | 10 | 31.3 | 334 | 55.1 | 34 | 30.4 | 426 | 38.7 | |||
| Secondary | 46 | 57.5 | 332 | 67.2 | 20 | 62.5 | 238 | 39.3 | 66 | 58.9 | 570 | 51.8 | |||
| Some college/Technical | 8 | 10.0 | 64 | 13.0 | 2 | 6.3 | 15 | 2.5 | 10 | 8.9 | 79 | 7.2 | |||
| Missing | 0 | 2 | 0 | 7 | 0 | 9 | |||||||||
| Paternal education | <0.001 | 0.022 | 0.05 | ||||||||||||
| None | 2 | 2.8 | 0 | 0 | 0 | 0.0 | 17 | 3.4 | 2 | 2.1 | 17 | 1.8 | |||
| Primary | 8 | 11.3 | 16 | 3.5 | 2 | 8.0 | 178 | 35.3 | 10 | 10.4 | 194 | 20.2 | |||
| Secondary | 52 | 73.2 | 321 | 70.6 | 21 | 84.0 | 279 | 55.4 | 73 | 76.0 | 600 | 62.6 | |||
| Some college/Technical | 9 | 12.7 | 118 | 25.9 | 2 | 8.0 | 30 | 6.0 | 11 | 11.5 | 148 | 15.4 | |||
| Missing | 9 | 41 | 7 | 109 | 16 | 150 | |||||||||
| Maternal employment | 0.034 | <0.001 | <0.001 | ||||||||||||
| Farmer | 5 | 6.3 | 15 | 3.0 | 18 | 56.3 | 504 | 82.4 | 23 | 20.5 | 519 | 46.8 | |||
| Non-farmer | 34 | 42.5 | 283 | 57.1 | 6 | 18.8 | 59 | 9.6 | 40 | 35.7 | 342 | 30.9 | |||
| Student | 1 | 1.3 | 1 | 0.2 | 0 | 0.0 | 6 | 1.0 | 1 | 0.9 | 7 | 0.6 | |||
| Unemployed | 40 | 50.0 | 197 | 39.7 | 8 | 25.0 | 43 | 7.0 | 48 | 42.9 | 240 | 21.7 | |||
| Missing | 0 | 0 | 0 | 1 | 0 | 1 | |||||||||
| Owns cell phone | 55 | 68.8 | 403 | 81.3 | 0.010 | 22 | 71.0 | 422 | 69.0 | 0.81 | 77 | 69.4 | 825 | 74.5 | 0.24 |
| Missing | 0 | 0 | 1 | 1 | 1 | 1 | |||||||||
| Transportation time | 0.30 | 0.40 | 0.007 | ||||||||||||
| ≤30min | 68 | 87.2 | 438 | 91.8 | 12 | 42.9 | 146 | 26.0 | 80 | 75.5 | 584 | 56.3 | |||
| >30min - 1hr | 9 | 11.5 | 26 | 5.5 | 7 | 25.0 | 206 | 36.7 | 16 | 15.1 | 232 | 22.4 | |||
| >1hr - 2hr | 1 | 1.3 | 9 | 1.9 | 5 | 17.9 | 118 | 21.0 | 6 | 5.7 | 127 | 12.2 | |||
| >2hr - 3hr | 0 | 0.0 | 3 | 0.6 | 2 | 7.1 | 44 | 7.8 | 2 | 1.9 | 47 | 4.5 | |||
| >3hr - 5hr | 0 | 0.0 | 1 | 0.2 | 2 | 7.1 | 28 | 5.0 | 2 | 1.9 | 29 | 2.8 | |||
| >5hr | 0 | 0.0 | 0 | 0 | 0 | 0.0 | 19 | 3.4 | 0 | 0 | 19 | 1.8 | |||
| Missing | 2 | 19 | 4 | 52 | 6 | 71 | |||||||||
| Pregnancy and HIV-related characteristics | |||||||||||||||
| Received antenatal care | 74 | 92.5 | 491 | 99.0 | <0.001 | 31 | 96.9 | 608 | 99.7 | 0.024 | 105 | 93.8 | 1099 | 99.4 | <0.001 |
| Missing | 0 | 0 | 0 | 3 | 0 | 3 | |||||||||
| # of antenatal care visits | <0.001 | 0.67 | <0.001 | ||||||||||||
| 1 to 3 visits | 50 | 67.6 | 215 | 44.3 | 13 | 41.9 | 230 | 38.1 | 63 | 60.0 | 445 | 40.9 | |||
| 4 to 8 visits | 24 | 32.4 | 270 | 55.7 | 18 | 58.1 | 374 | 61.9 | 42 | 40.0 | 644 | 59.1 | |||
| Missing | 6 | 11 | 1 | 9 | 7 | 20 | |||||||||
| Duration of antenatal care | 0.041 | 0.71 | 0.034 | ||||||||||||
| 0 to 4 months | 58 | 80.6 | 316 | 68.7 | 10 | 55.6 | 260 | 59.9 | 68 | 75.6 | 576 | 64.4 | |||
| 5 to 9 months | 14 | 19.4 | 144 | 31.3 | 8 | 44.4 | 174 | 40.1 | 22 | 24.4 | 318 | 35.6 | |||
| Missing | 8 | 36 | 14 | 179 | 22 | 215 | |||||||||
| Place of deliverya | 0.005 | 0.42 | 0.10 | ||||||||||||
| Facility (hospital or clinic) | 75 | 93.8 | 489 | 98.6 | 29 | 90.6 | 575 | 94.1 | 104 | 92.9 | 1064 | 96.1 | |||
| Home | 5 | 6.3 | 7 | 1.4 | 3 | 9.4 | 36 | 5.9 | 8 | 7.1 | 43 | 3.9 | |||
| Missing | 0 | 0 | 0 | 2 | 0 | 2 | |||||||||
| Timing of HIV diagnosis | <0.001 | <0.001 | <0.001 | ||||||||||||
| Before pregnancy | 20 | 25.3 | 307 | 61.9 | 7 | 21.9 | 487 | 79.5 | 27 | 24.3 | 794 | 71.6 | |||
| During pregnancy | 49 | 62.0 | 189 | 38.1 | 15 | 46.9 | 126 | 20.6 | 64 | 57.7 | 315 | 28.4 | |||
| During delivery/labor | 10 | 12.7 | 0 | 0 | 10 | 31.3 | 0 | 0.0 | 20 | 18.0 | 0 | 0 | |||
| Missing | 1 | 0 | 0 | 0 | 1 | 0 | |||||||||
| Status disclosedb | 17 | 70.8 | - | - | 11 | 68.8 | 360 | 100.0 | <0.001 | 28 | 70.0 | 360 | 100.0 | <0.001 | |
| Missing | 56 | 496 | 16 | 253 | 72 | 749 | |||||||||
| Disclosed to husband/boyfriend | 12 | 50.0 | - | - | 5 | 31.3 | 328 | 91.1 | <0.001 | 17 | 42.5 | 328 | 91.1 | <0.001 | |
| Status disclosed among those with diagnosis before delivery/laborb | 17 | 73.9 | - | - | 7 | 70.0 | 360 | 100.0 | <0.001 | 24 | 72.7 | 360 | 100 | <0.001 | |
| Disclosed to husband/boyfriend | 12 | 52.2 | - | - | 4 | 40.0 | 328 | 91.1 | <0.001 | 16 | 48.5 | 328 | 91.1 | <0.001 | |
| Missing | 46 | 496 | 12 | 253 | 58 | 749 | |||||||||
Seven women gave birth on way to facility. Their places of delivery were marked as the place of their enrollment (N=4 for hospital, N=3 for clinic).
Question added after study start on August 14, 2017
Wilcoxon rank-sum test for continuous variables chi-squared test for categorical variables
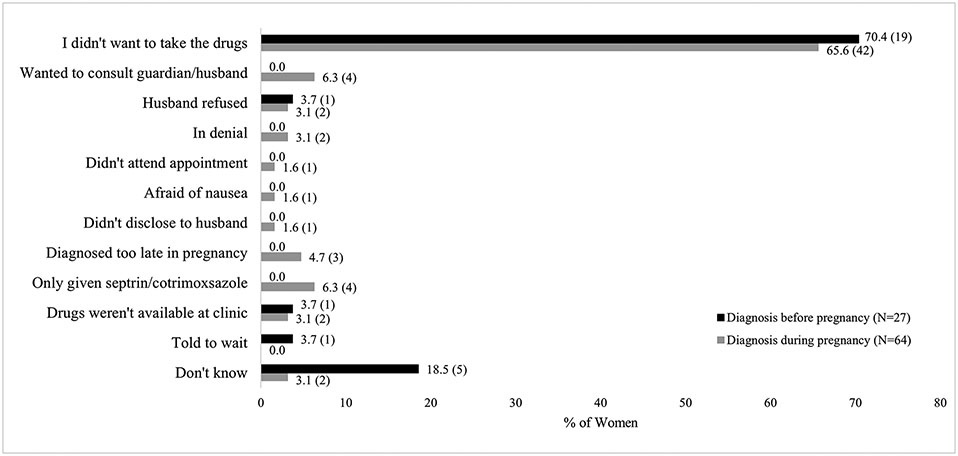
Among women diagnosed prior to or during pregnancy, the primary reason for not receiving ART during pregnancy was that they did not want to take the medication (70.4% [19/27] and 65.6% [42/64] among women diagnosed prior to or during pregnancy, respectively; Figure 4). Other individual and clinic-level reasons were also reported (Figure 4).
Figure 4: Self-reported reasons for not receiving antiretroviral therapy during pregnancy among women diagnosed with HIV before or during pregnancy.
Note: 27 women diagnosed with HIV before pregnancy and 64 diagnosed during pregnancy provided reasons for not receiving antiretroviral therapy during pregnancy. Data labels represent % (N).
Correlates of not receiving ART during pregnancy
Correlates of not receiving ART are presented in Table 2. At all sites, women who did not receive ART were younger, less likely to have disclosed their HIV status to another person, including their partner, and less likely to have received antenatal care, compared to women who received ART.
Other demographic correlates varied by site. In Livingstone, women who did not receive ART were less likely to have completed secondary school or higher, less likely to have a partner who completed secondary school or higher, less likely to own a cell phone, and more likely to be unemployed, although these differences were not all statistically significant. In contrast, in the Macha area, women who did not receive ART were significantly more likely to have completed secondary school or higher, more likely to have a partner who completed secondary school or higher, and more likely to be unemployed or employed in an area other than farming.
Discussion
This study, conducted in one rural and one urban area in southern Zambia from 2016-2019, found that 93.7% of pregnant women living with HIV received ART, with little variation across setting and time. Women who did not receive ART were more likely to be younger and to have been diagnosed with HIV during pregnancy or at labor and delivery, and less likely to have disclosed their status to their partner or relatives. As expected, infants born to women who received ART were significantly less likely to be diagnosed with HIV infection at birth.
The ART coverage estimate of 93.7% among pregnant women residing in Livingstone and Macha during 2016 to 2019 indicates successful implementation of HIV and PMTCT programs in both areas. This level of ART coverage is higher than national estimates (range of 78-86% during 2016-2019) but similar to regional estimates for southern and eastern Africa (90-96% during 2016-2019) (13). Option B+ was adopted in Zambia in 2013 (14) and a test and treat policy was adopted in 2017 (15), with both policies implemented quickly in the study areas. Prior studies conducted in the Macha area document the dramatic progress that has been made. In 2010-2012, only 80% of women seeking early infant diagnostic testing had received ART during pregnancy and only 64% had received a combination regimen (16). In a similar study conducted in 2013-2015, 83% of women had received ART during pregnancy and 80% had received a combination regimen (17). In comparison, 95% of women in the Macha area in this study received ART and all received combination regimens as recommended. ART coverage in Macha remained stable across all four years of the study, suggesting that reaching the remaining 5% of women in need will be challenging. Given the near universal coverage of antenatal care and strong implementation of the HIV and PMTCT programs in this area, additional efforts will need to focus on addressing the reasons these women fail to receive ART.
While some pregnant women in this study failed to receive ART due to clinic-level challenges, most women reported not wanting to take the drugs. The profile of pregnant women not receiving ART included younger age, diagnosis during pregnancy or labor and delivery, and non-disclosure of their HIV status to others. Some missed opportunities for testing occurred, but most women had a history of prior negative HIV tests and these likely represent incident infections rather than new diagnoses of long-term infections. This is consistent with recent HIV incidence data from Zambia showing a peak annual HIV incidence of 1.16% among women aged 25-35 years (18), and regional data indicating that adolescent girls and young women bear a disproportionate share of new infections (19). Learning of a new HIV diagnosis can be difficult to accept, leading to delays in ART initiation (20). Even after accepting the diagnosis, fears around HIV-associated stigma from partners, family, and friends can prevent disclosure of their HIV status, thus depriving women of both the emotional and logistical support necessary to successfully initiate and adhere to ART (20-23). These findings support current recommendations (24) to test all pregnant women early in pregnancy with retesting in the third trimester and suggest that strengthening both testing and counseling services in antenatal care could help to reach some of the women who were missed and increase ART coverage.
Differences between sites were found in the relationship between education and ART during pregnancy. In Livingstone, ART receipt was correlated with higher levels of maternal and paternal education, consistent with studies that found higher education to be associated with increased knowledge about HIV and ART and increased uptake of HIV and PMTCT services (25-28). In contrast, in the Macha area, ART receipt was correlated with lower levels of maternal and paternal education. In this small rural community, fears around disclosure of HIV status among those with higher educational attainment, including professionals working in and around the clinics, may have decreased engagement with HIV and PMTCT services. Other studies have identified stigma as an obstacle to ART adherence during pregnancy among women with higher levels of education or in higher socioeconomic classes (6). These findings highlight the importance of understanding underlying reasons for not receiving ART across diverse geographic regions so that interventions can be appropriately targeted to reach all women in need.
As expected, receipt of ART reduced the risk of HIV transmission to the infant (29-31). In this study, 0.8% and 4.1% of infants born to women who did and did not receive ART during pregnancy were found to be infected. These results are similar to prior studies and trials that have found rates of transmission in the perinatal period of 0.8-2.9% (32, 33) and 4.3-10.4% (34-37) among women who did and did not receive ART during pregnancy. The overall low rate of transmission of 1.0% in this study reflects the high ART coverage observed in this population.
This study had several limitations. First, the study was conducted at health facilities and therefore did not include women delivering at home who did not bring the child to the maternity ward for assessment. In 2018, an estimated 82% of women in Southern Province reported delivering in a health facility, with a higher proportion in urban than rural areas (10). Consequently, the exclusion of women delivering at home is unlikely to have had a major impact on the results, although ART coverage may have been overestimated, especially in the Macha area. Second, the study only enrolled infants who were stable enough for early infant diagnostic testing, and therefore did not enroll mothers of infants who died or were clinically unstable. Given the potential negative impact of untreated HIV infection on birth outcomes (38), this exclusion may also have led to overestimation of ART coverage and underestimation of HIV transmission in this study. Third, while the study asked women why they had not received ART during pregnancy, specific reasons for not wanting to take the drugs were not ascertained. Lastly, this study was conducted in health facilities in two areas of Southern Province. While the estimates of ART coverage provide important information for both urban and rural settings in Southern Province, they may not be representative of ART coverage throughout Zambia. However, many of the correlates of not receiving ART during pregnancy were consistent across sites and are likely to be generalizable to other areas with high coverage in Zambia and sub-Saharan Africa.
In conclusion, this study found high uptake of ANC and PMTCT services in both urban and rural areas of southern Zambia, resulting in low rates of HIV transmission to infants in the perinatal period. This is a remarkable achievement and attests to the strength of the HIV program and the commitment of the Government of Zambia and its partners to eliminating mother-to-child transmission of HIV. While most women received care according to guidelines, some missed opportunities for testing, diagnosis and treatment were identified. Increasing ART coverage further and reaching the remaining women in need will require sustaining current program activities and additional efforts to focus on identifying incident infections during pregnancy through repeated testing, strengthening counseling and support for women newly diagnosed during pregnancy, and addressing context-specific concerns about testing and care so that all women living HIV and their infants can receive the full health benefits of available treatment.
Supplementary Material
Sources of Funding:
This work was supported by grants from the National Institute of Allergy and Infectious Diseases (5R01AI116324) and the Thrasher Research Fund (12647). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Thrasher Research Fund.
References
- 1.The Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS Data 2021. Geneva; 2021. Report No.: JC3032E. [Google Scholar]
- 2.The Joint United Nations Programme on HIV/AIDS (UNAIDS). AIDS by the numbers. Geneva; 2016. [PubMed] [Google Scholar]
- 3.The Joint United Nations Programme on HIV/AIDS (UNAIDS). Fact Sheet 2021. Geneva; 2021. [Google Scholar]
- 4.The Joint United Nations Programme on HIV/AIDS (UNAIDS). Global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2011. Report No.: JC2137E. [Google Scholar]
- 5.The Joint United Nations Programme on HIV/AIDS (UNAIDS). Start Free, Stay Free, AIDS Free: Final report on 2020 targets. Geneva; 2021. Report No.: JC3031E. [Google Scholar]
- 6.King R, Matovu JN, Rujumba J, Wavamunno P, Amone A, Gabagaya G, et al. PMTCT Option B+ 2012 to 2018 - Taking stock: barriers and strategies to improve adherence to Option B+ in urban and rural Uganda. Afr J AIDS Res. 2020;19(2):135–46. [DOI] [PubMed] [Google Scholar]
- 7.Yah CS, Tambo E. Why is mother to child transmission (MTCT) of HIV a continual threat to new-borns in sub-Saharan Africa (SSA). J Infect Public Health. 2019;12(2):213–23. [DOI] [PubMed] [Google Scholar]
- 8.World Bank Group. Population, total - Zambia: World Bank Group; 2022. [Internet]. [cited 2022 Aug 05]. Available from: https://data.worldbank.org/indicator/SP.POP.TOTL?locations=ZM. [Google Scholar]
- 9.City Population. Country statistics - Zambia (2010 census) [Internet]. [cited 2021 Dec 13]. Available from: https://www.citypopulation.de/en/zambia/cities/.
- 10.Zambia Statistics Agency, Ministry of Health (MOH) Zambia, ICF. Zambia Demographic and Health Survey 2018. Lusaka, Zambia, and Rockville, Maryland, USA; 2019. Report No.: FR361. [Google Scholar]
- 11.Ministry of Health (MOH). Zambia consolidated guidelines for treatment & prevention of HIV infection. Lusaka: Republic of Zambia; 2016. [Google Scholar]
- 12.Sutcliffe CG, Mutanga J, Moyo N, Agarwal AK, Schue JL, Hamahuwa M, et al. Point-of-care p24 antigen detection for early infant diagnosis of HIV infection: cross-sectional and longitudinal studies in Zambia. BMC Infect Dis. 2021;21(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AIDSinfo. Coverage of pregnant women who receive ARV for PMTCT [Internet]. UNAIDS; 2020. [cited 2021 Dec 13]. Available from: https://aidsinfo.unaids.org. [Google Scholar]
- 14.Ministry of Health (MOH), Ministry of Community Development Mother and Child Health (MCDMCH). Zambia consolidated guidelines for treatment and prevention of HIV infection. Lusaka: Republic of Zambia; 2013. [Google Scholar]
- 15.Ministry of Health (MOH). Zambia consolidated guidelines for prevention and treatment of HIV infection. Lusaka: Republic of Zambia; 2018. [Google Scholar]
- 16.Sutcliffe CG, van Dijk JH, Hamangaba F, Mayani F, Moss WJ. Turnaround time for early infant HIV diagnosis in rural Zambia: A chart review. PLoS One. 2014;9(1):e87028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sitler L, Hamahuwa M, Deutsch-Feldman M, Sinywimaanzi K, Thuma PE, Moss WJ, et al. Countdown to zero: Correlates of PMTCT among HIV-infected women in rural Zambia [conference presentation]: INTEREST Conference, Lilongwe, Malawi; 2017. [Google Scholar]
- 18.Ministry of Health (MOH). Zambia Population-based HIV Impact Assessment (ZAMPHIA) 2016: Final Report. Lusaka: Republic of Zambia; 2019. [Google Scholar]
- 19.The Joint United Nations Programme on HIV/AIDS (UNAIDS). Global AIDS update - Seizing the moment: Tackling entrenched inequalities to end epidemics. Geneva; 2020. Report No.: JC2991. [Google Scholar]
- 20.Mukose AD, Bastiaens H, Makumbi F, Buregyeya E, Naigino R, Musinguzi J, et al. What influences uptake and early adherence to Option B+ (lifelong antiretroviral therapy among HIV positive pregnant and breastfeeding women) in Central Uganda? A mixed methods study. PLoS One. 2021;16(5):e0251181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laher F, Cescon A, Lazarus E, Kaida A, Makongoza M, Hogg RS, et al. Conversations with mothers: Exploring reasons for prevention of mother-to-child transmission (PMTCT) failures in the era of programmatic scale-up in Soweto, South Africa. AIDS Behav. 2012;16(1):91–8. [DOI] [PubMed] [Google Scholar]
- 22.Watt MH, Knippler ET, Knettel BA, Sikkema KJ, Ciya N, Myer L, et al. HIV disclosure among pregnant women initiating ART in Cape Town, South Africa: Qualitative perspectives during the pregnancy and postpartum periods. AIDS Behav. 2019;22(12):3945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinuthia J, Singa B, McGrath CJ, Odeny B, Langat A, Katana A, et al. Prevalence and correlates of non-disclosure of maternal HIV status to male partners: a national survey in Kenya. BMC Public Health. 2018;18:671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. Geneva: World Health Organization; 2021. [PubMed] [Google Scholar]
- 25.Haile ZT, Teweldeberhan AK, Chertok IRA. Correlates of women’s knowledge of mother-to-child transmission of HIV and its prevention in Tanzania: a population-based study. AIDS Care. 2016;28(1):70–8. [DOI] [PubMed] [Google Scholar]
- 26.Saka AO, Onyeneho CA, Ndikom CM. Perception and utilization of prevention of mother-to-child transmission of human immunodeficiency virus (HIV) services among women living with HIV. Eur J Midwifery. 2021;5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azuogu BN, Ossai EN, Aniwada EC, Madubueze UC, Azuogu VC, Agu AP, et al. Knowledge and utilization of PMTCT services among women accessing antenatal care in private health facilites in Abakaliki, Nigeria. West Afr J Med. 2021;38(8):713–8. [PubMed] [Google Scholar]
- 28.Akal CG, Afework DT. Status of prevention of mother-to-child transmission (PMTCT) services utilization and factors affecting PMTCT service uptake by pregnant women attending antenatal care clinic in selected health facilities of Afar Regional State, Ethiopia. J Environ Public Health. 2018;2018:5127090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muyunda B, Musonda P, Mee P, Todd J, Michelo C. Effectiveness of lifelong ART (Option B+) in the prevention of mother-to-child transmission of HIV programme in Zambia: Observations based on routinely collected health data. Front Public Health. 2020;7:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mutanga JN, Mutembo S, Ezeamama AE, Fubisha RC, Sialondwe D, Simuchembu B, et al. Tracking progress toward elimination of mother to child transmission of HIV in Zambia: Findings from the Early Infant Diagnosis of HIV program (2009–2017). J Trop Pediatr. 2020;66(1):56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gumede-Moyo S, Filteau S, Munthali T, Todd J, Musonda P. Implementation effectiveness of revised (post-2010) World Health Organization guidelines on prevention of mother-to-child transmission of HIV using routinely collected data in sub-Saharan Africa: A systematic literature review. Medicine (Baltimore). 2017;96(40):e8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shapiro RL, Hughes MD, Ogwu A, Kitch D, Lockman S, Moffat C, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362:2282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kilewo C, Karlsson K, Ngarina M, Massawe A, Lyamuya E, Swai A, et al. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. J Acquir Immune Defic Syndr. 2009;52(3):406–16. [DOI] [PubMed] [Google Scholar]
- 34.Wiktor SZ, Ekpini E, Karon JM, Nkengasong J, Maurice C, Severin ST, et al. Short-course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Côte d'Ivoire: a randomised trial. Lancet. 1999;353(9155):781–5. [DOI] [PubMed] [Google Scholar]
- 35.Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354(9181):795–802. [DOI] [PubMed] [Google Scholar]
- 36.Shaffer N, Chuachoowong R, Mock PA, Bhadrakom C, Siriwasin W, Young NL, et al. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomised controlled trial. Bangkok Collaborative Perinatal HIV Transmission Study Group. Lancet. 1999;353(9155):773–80. [DOI] [PubMed] [Google Scholar]
- 37.Bertolli J, St Louis ME, Simonds RJ, Nieburg P, Kamenga M, Brown C, et al. Estimating the timing of mother-to-child transmission of human immunodeficiency virus in a breast-feeding population in Kinshasa, Zaire. J Infect Dis. 1996;174(4):722–6. [DOI] [PubMed] [Google Scholar]
- 38.Wedi COO, Kirtley S, Hopewell S, Corrigan R, Kennedy SH, Hemelaar J. Perinatal outcomes associated with maternal HIV infection: a systematic review and meta-analysis. Lancet HIV. 2016;3(1):e33–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.