Abstract
In mouse fetal gonads, germ cell development is accompanied by changes in cell cycle mode in response to external signals and intrinsic mechanisms of cells. During fetal development, male germ cells undergo G0/G1 arrest, while female germ cells exit the mitotic cell cycle and enter meiosis. In fetal testes, NANOS2 and CYP26B1 force germ cells to stay in G0/G1 arrest phase, preventing them from entering the meiotic cell cycle. In the fetal ovary, external signals, such as RA, BMP, and WNT, promote the competency of female germ cells to enter the meiotic cell cycle. MEIOSIN and STRA8 ensure the establishment of the meiotic cell cycle by activating meiotic genes, such that meiotic entry coincides with the S phase. This review discusses germ cell development from the viewpoint of cell cycle regulation and highlights the mechanism of the entry of germ cells into meiosis.
Keywords: Cell cycle, Germ cell, Meiosis, MEIOSIN, STRA8
Different Modes of Cell Cycle Regulation in Early Germ Cell Development
In mammalian germ cell development, differences in cell cycle regulation play a fundamental role in the reproductive strategy of each sex. During mouse embryogenesis, sexually bipotent germ cells are specified as primordial germ cells (PGCs) by the actions of BMP and WNT signals, which comprise approximately 50–80 cells [1]. These sexually potent PGCs migrate through the hindgut and dorsal mesentery and eventually colonize the genital ridge by E10.5 (Fig. 1). During the migratory phase, PGCs proliferate to increase the number to ~25,000 cells by E13.5 [2, 3]. Since the proliferation of germ cells during this period principally determines the number of gamete sources in gonads, an active mitotic cell cycle plays a pivotal role in ensuring reproductive capacity in life.
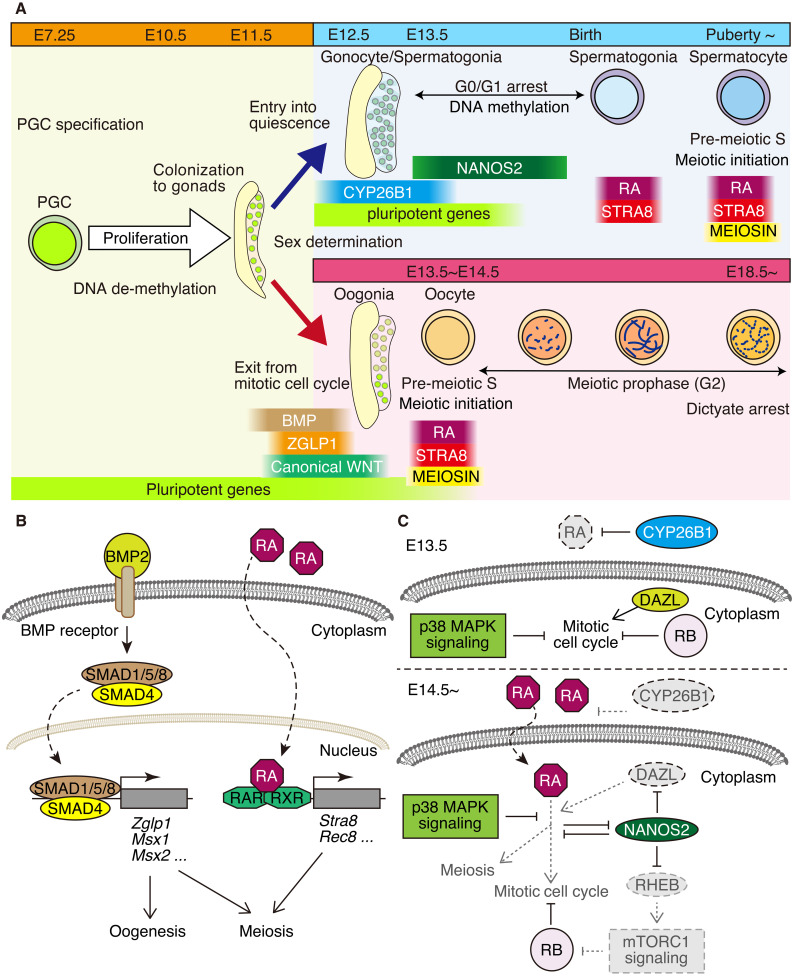
Fig. 1.
Cell cycle regulation associated with germ cell development. (A) Schematic illustration of the cell cycle events associated with germ cell development in the testis (upper right) and fetal ovary (lower right). The periods of key gene expressions (STRA8, MEIOSIN. CYP26B1, NANOS2) and extrinsic signal executions (RA, BMP, WNT) are shown along the developmental stages. In females, the meiotic cell cycle starts around E13.5-E14.5 and is followed by meiotic prophase and subsequently by dictyate/diplotene arrest. In fetal testis, meiotic entry is suppressed by CYP26B1 and NANOS2, leading to G0/G1 arrest of the germ cells. After birth, the meiotic cell cycle is accompanied by the differentiation of spermatocytes. (B) BMP signaling confers oogenic gene activation and the competency for meiosis to female germ cells. RA signaling is mediated by RARs (RARα, RARβ, RARγ) and retinoid X receptors (RXRs), which are bound by RA. RA signaling activates the meiotic program via the activation of the key genes for meiosis, such Stra8 and Rec8. (C) At E13.5 (upper), CYP26B1 degrades RA, preventing male germ cells from responding to RA. p38 MAPK signaling and NANOS2 suppress RA signaling in male germ cells. At E14.5 onward (lower), CYP26B1 expression decreases. Alternatively, NANOS2 inhibits mTORC1 activity via the suppression of Rheb. RB suppresses cell cycle progression. The attenuated pathways are shown in gray.
Initially, PGCs were sexually neutral, and their sexual development depended on the surrounding environment. Approximately E11.5, gonadal somatic cells undergo sex-specific differentiation mediated by genetic cues, and germ cells adopt the sex of the surrounding somatic cells. With respect to sex-specific differentiation of germ cells, the most noteworthy event at E13.5 onward is that while male germ cells undergo cell cycle arrest at the G0/G1 phase, those of females quit the mitotic cell cycle and enter the meiotic cell cycle (Fig. 1). Therefore, the change in the cell cycle mode leads to fundamental differences between male and female germ cell development.
External Signals for Oogenic Development and Meiotic Entry in Female Germ Cells
Meiosis occurs between males and females at different periods during germ cell development. In mouse fetal ovaries, meiotic entry is restricted within a narrow window at approximately E13.5-E14.5 (Fig. 1A). In mice, female germ cells/oogonia quit the mitotic cell cycle, enter meiosis after sex determination [4,5,6], and downregulate pluripotency genes [3]. Meiotic entry propagates in a wave from the anterior to posterior pole with radial geometry in mouse fetal ovaries [7,8,9,10]. In the fetal ovary of cynomolgus monkeys, meiotic entry is observed approximately 10–12 weeks post-fertilization (wpf), which occurs earlier in the germ cells located in the inner cortex than in the outer cortex [11]. In human females, oogonia differentiate into oocytes at approximately 10–11 weeks of gestation (gw), and the majority of oocytes show meiotic gene expression at approximately 14–17 weeks of gestation [12, 13]. Another subpopulation of oogonia continues to express pluripotency markers until approximately gw of 19–20. After gw 20, the majority of oocytes initiate meiosis and get arrested at the diplotene stage [14].
Germ cells in the fetal ovary require several external signals to become competent for meiotic entry. Upon feminization of somatic cells, pre-granulosa cells express BMP2 in response to WNT signaling. WNT signaling and the downstream transcription factor β-catenin play a role in osteogenic differentiation to allow meiotic entry in the fetal ovary in a timely manner [15, 16]. BMP signaling leads to oogenic gene activation and meiosis competency in female germ cells (Fig. 1B) [17]. SMAD4 and SMAD1/5/8 act as transducers of BMP signaling, which is assumed to mediate the activation of the oogenic program, and their absence leads to male-type differentiation even in the ovary [18]. Furthermore, under BMP signaling, ZGLP1 activates key genes involved in oogenesis, including meiosis [19]. MSX1 and MSX2 have been hypothesized to be required for meiosis in female germ cells in response to BMP signaling [20]. Thus, BMP signaling directs multiple regulatory pathways to coordinate oogonial development before meiotic entry.
Retinoic acid (RA) is one of the key signals that instruct germ cells to express meiotic genes, such as stimulated by retinoic acid gene 8 (Stra8) and ensure timely entry into meiosis in the fetal ovary [4, 5, 21, 22]. RA signaling is mediated by retinoic acid receptors (RARs; RARα, RARβ, and RARγ) and retinoid X receptors (RXRs) that are bound by RA (Fig. 1B). RARs and RXRs form heterodimers and bind to retinoic acid responsive elements (RAREs) of target genes that are required for meiosis and other oogonial development. Two sources of RA are known [21, 23]. One is the mesonephros, from which the RA diffuses towards the fetal gonad, and the other is the somatic cells in the fetal ovary (Fig. 1B). Retinaldehyde dehydrogenase ALDH1A (ALDH1A1, ALDH1A2, and ALDH1A3) synthesizes RA from the retinol [21, 23]. Since RA or RAR-agonists upregulate the expression of meiotic genes, and pan-RAR antagonists suppress their expression in embryonic ovaries [4, 5], it has been assumed that female germ cells respond to RA prior to meiotic entry. However, genetic studies of mice lacking ALDH1A families or RARs provided contrasting evidence, demonstrating that RA signaling was dispensable for meiosis [23,24,25,26]. These studies imply that RA signaling is not the sole determinant of meiotic initiation and/or that other signaling substances that are metabolized by CYP26B1 may trigger meiotic entry. Altogether, external signals by RA and BMP synergistically coordinate oogonial development and meiotic entry into the fetal ovary.
G0/G1 Cell Cycle Arrest in Fetal Male Germ Cells
In fetal testes, male germ cells exit the mitotic cell cycle and enter G0/G1 arrest at approximately E13.5, which contrasts with female germ cells entering meiosis at the corresponding time (Fig. 1A) [27,28,29,30]. During G0/G1 arrest, fetal male germ cells (gonocytes/pro-spermatogonia) undergo de novo CpG methylation, and a subset of them subsequently establish a stem cell population called spermatogonial stem cells (SSCs) shortly after birth. During puberty, male germ cells enter meiosis when differentiation into spermatocytes is initiated. Thereafter, meiosis continuously occurs in the testes throughout life.
There are cell-intrinsic factors that direct male germ cells to G0/G1 arrest rather than meiotic entry. The retinoblastoma protein family (RB, p107, and p130) is known to be a negative regulator of S phase entry in the cell cycle and plays a pivotal role in cell cycle arrest. RB binds to E2F and represses S-phase-related gene expression [31]. Phosphorylation of RB by CDK4/6 leads to its dissociation from E2F, reducing the expression of S-phase-related genes. After germ cell sex determination, the phosphorylation level of RB is high in male germ cells from E12.5-E13.5 but decreases at E14.5-P1.5, which coincides with G0/G1 arrest [30]. Disruption of RB results in the active proliferation of male germ cells at E14.5, indicating that RB is required for G0/G1 arrest. Furthermore, STRA8 and meiosis-associated genes are ectopically upregulated in RB-deficient gonocytes, suggesting that the suppression of meiotic entry is compromised in the absence of RB [30]. However, even in the absence of RB, male germ cells eventually stop proliferating at later embryonic stages and are consequently removed by apoptosis, suggesting the existence of both RB-dependent and RB-independent mechanisms that arrest the cell cycle in fetal male germ cells [28, 30]. Similar phenomena have been reported for transcription factor BCN2-deficient gonocytes, which undergo premature re-entry into the cell cycle and exhibit ectopic expression of meiotic genes [32]. These findings imply that cell cycle arrest is indispensable for male germ cell development because it blocks meiotic cell cycle entry.
Similar to embryonic females, RA is synthesized at and secreted from the mesonephros in embryonic males [5, 26]. Although female germ cells receive RA signals, male germ cells are refractory to RA. In fetal testes, cytochrome P450 enzyme CYP26B1 degrades RA, preventing male germ cells from responding to RA (Fig. 1C) [4, 5, 33, 34]. When CYP26B1 expression decreases after E14.5, male germ cells alternatively exhibit cell-intrinsic mechanisms to prevent RA signaling and ectopic entry into meiosis.
NANOS families are evolutionarily conserved RNA-binding proteins with three orthologous genes in the mammalian genome [35]. Nanos2 is specifically expressed in male germ cells and precedes G0/G1 arrest. NANOS2 forms a complex with the RNA-binding proteins DND1 and deadenylation enzyme [36] and represses numerous genes that promote cell cycle and female-type germ cell differentiation, thereby maintaining gonocytes in G0/G1 arrest. One of the critical NANOS2 targets is Dazl, which encodes an RNA-binding protein and is required for acquiring competence to enter the meiotic prophase [37]. If the degradation-resistant form of Dazl that lacks the 3′-UTR is expressed, gonocytes exit G0/G1 arrest and resume the cell cycle [38]. Furthermore, NANOS2 suppresses the ectopic expression of RA-responsive genes, such as Stra8 in fetal testes; otherwise, male germ cells would exhibit signs of activation of meiosis-related genes [39]. Altogether, abrogation of cell cycle arrest leads to ectopic signs of meiotic entry in gonocytes, due to, at least in part, the susceptibility to ectopic RA signaling.
Reciprocally, ectopic RA signaling suppressed NANOS2 expression in male germ cells (Fig. 1C). This effect is counteracted by p38 mitogen-activated protein kinase (MAPK) signaling, which is required for cell cycle arrest at the G1 phase in a variety of differentiated cells. The active phosphorylated form of p38 is present in both male and female germ cells at E11.5 but is restricted to male germ cells at later stages. Abrogation of MAPK signaling by the inhibitor SB203580 leads to the downregulation of Nanos2 and concomitant ectopic signs of meiotic entry [40]. Thus, MAPK signaling promotes the entry of male germ cells in the G0/G1 arrest phase, antagonizing the meiotic entry [40, 41].
The mTORC1 pathway promotes cell cycle progression in response to stimuli, such as growth factors, hormones, and cellular stress. NANOS2 suppresses mTORC1 activity by directly binding to Rheb mRNA, which encodes an activator of mTORC1 [42]. Although mTORC1 is typically inactive in quiescent male germ cells, it is activated in the absence of NANOS2. As the majority of mTORC1-active cells in Nanos2-KO mice express the mitotic cell cycle marker Ki67, mTORC1 suppression is one of the key mechanisms that induce G1/G0 arrest in embryonic male germ cells. In addition, NANOS2 is known to suppress mTORC1 by trapping mTOR protein onto cytoplasmic mRNP in SSCs [43]. Therefore, NANOS2 represses mTORC1 activity through both post-transcriptional and post-translational mechanisms. Thus, NANOS2 acts as a node for different cascades to initiate and maintain cell cycle arrest.
Meiotic Cell Cycle in the Germ Cell Development
In mammals, the decision to enter meiosis is one of the most critical events for sexual reproduction because meiosis leads to germ cell differentiation into sperm or oocytes. Meiosis is a specialized cell cycle that modifies the mechanism of the canonical mitotic cell cycle such that it undergoes one round of DNA replication, followed by two rounds of chromosome segregation. Meiotic entry always coincides with the “pre-meiotic S” phase, which has a longer duration than the mitotic S phase [44] (Fig. 2A). Meiotic prophase I is a specialized stage that follows the pre-meiotic S phase and is equivalent to G2 phase of the canonical mitotic cell cycle. Meiotic prophase I is substantially prolonged compared to the canonical G2 phase to ensure meiosis-specific chromosome events, such as homolog synapsis, meiotic recombination, and reductional segregation of homologous chromosomes [45,46,47,48,49]. In male mice, approximately ten days are required to complete meiotic prophase I.
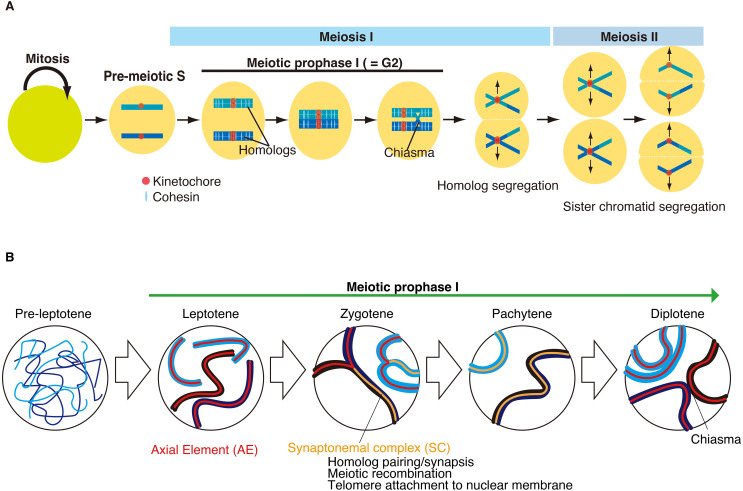
Fig. 2.
Schematic of meiotic cell cycle. (A) Meiosis comprises one round of DNA replication followed by two rounds of chromosome segregation. Meiosis starts from the pre-meiotic S phase followed by meiotic prophase I, which is equivalent to the G2 phase of the cell cycle. Meiosis-specific chromosome events occur during meiotic prophase I. Cohesin plays crucial roles in meiosis-specific chromosomal events during meiotic prophase I. In meiosis I, homologous chromosomes, rather than sister chromatids, are segregated in opposite directions. In meiosis II, sister chromatids are segregated. (B) Schematic illustrations of sequential chromosomal events during meiotic prophase. Pre-leptotene is the transition stage just before meiotic prophase I. Meiotic prophase I is divided into four substages according to chromosome morphology. During meiotic prophase I, sister chromatids are organized into the axial element (AE). Meiosis-specific cohesion is loaded onto the chromatin during leptotene. Homologous chromosomes undergo pairing and synapsis from leptotene to zygotene. The synaptonemal complex (SC) is fully assembled between homologous chromosomes at pachytene. Meiotic recombination generates crossovers between homologous chromosomes, yielding physical linkages called chiasmata. At diplotene, the SC is disassembled.
Meiotic prophase I demonstrates a feature of chromosome dynamics distinct from those of mitosis. Meiotic prophase I is divided into four substages ( leptotene, zygotene, pachytene, and diplotene) according to chromosome morphology and the extent of homolog synapsis (Fig. 2B). During meiotic prophase I, sister chromatids are organized into an axis structure called an axial element (AE). Mitotic-type cohesins (RAD21) are replaced by meiotic-type cohesins (RAD21L and REC8) [49]. Accordingly, the overall chromatin organization drastically changes with the disappearance of topologically associated domains (TAD) [50,51,52]. The pre-leptotene stage is defined with the mitosis-to-meiosis transition just before the start of meiotic prophase I, where the axis structure is yet to be developed, and AE components (e.g., SYCP3) are often seen as aggregates. The cytologically defined pre-leptotene stage partly overlaps with the pre-meiotic G1/S transition of the cell cycle when pre-meiotic DNA replication begins. Leptotene is defined by a chromosome morphology with a developing axis structure. Zygotene is defined as the time when the axis structure is developed and homologous chromosomes are about to undergo synapsis. Pachytene is defined as the time at which homologous chromosomes are fully synchronized by the synaptonemal complex (SC). During pachytene, meiotic recombination generates crossovers between homologous chromosomes, yielding physical linkages known as chiasmata. Diplotene is defined as the time at which homolog synapsis is disassembled. Following meiotic prophase I, homologous chromosomes, rather than sister chromatids, are segregated toward the opposite poles of the spindle by the dissolution of chiasmata in meiotic division I. Finally, in meiotic division II, sister chromatids are segregated to produce haploid gametes.
Sexually Different Regulation of the Meiotic Cell Cycle
The meiotic cell cycle is regulated by sexually different mechanisms that coordinate with the developmental program of germ cells. In mouse fetal ovaries, germ cells enter meiosis within a narrow window around E13.5-E14.5, and subsequently undergo dictyate/diplotene arrest in the middle of the meiotic prophase around birth (Fig. 1A). Dictyate/diplotene arrest is a long-term dormant state in oocytes that persists until the resumption of meiosis [53]. In human females, the dormant state of oocytes persists for more than 40 years. During dictyate/diplotene arrest, oocytes show a large nucleus called the germinal vesicle (GV) and reside in the primordial follicles. Thus, the female reproductive lifespan is determined by the oocyte pool that has undergone meiotic initiation during this limited period in fetal ovaries. From the viewpoint of the cell cycle, dictyate/diplotene arrest occurs in the “G2 phase” and halts undergoing meiotic division I, which is remarkably different from G0/G1 arrest observed in quiescent cells of somatic tissues. The mechanism underlying how oocytes sustain long-term arrest remains elusive. In males, meiosis occurs continuously during puberty in the testis. In contrast to female meiosis, spermatocytes undergo two successive rounds of chromosome segregation.
Remarkable differences are observed in the sensitivity of checkpoint responses to chromosomal errors during the meiotic prophase between spermatocytes and oocytes. Aberrations in meiotic recombination and homolog synapsis during the meiotic prophase are more strictly monitored in spermatocytes than in oocytes. ATR kinase plays a crucial role in meiotic prophase checkpoints that monitor proper homologous synapsis. ATR phosphorylates H2AX along unsynapsed chromosomes, which induces the meiotic silencing of unsynapsed chromosomes (MSUC) /meiotic sex chromosome inactivation (MSCI) [54, 55]. In spermatocytes, most X- and Y-chromosome regions are unsynapsed owing to limited homology and are transcriptionally silenced in the heterochromatic XY body. MSCI is essential for passing the pachytene checkpoints in spermatocytes [56]. Similar sexual differences in meiotic prophase have also been observed in response to heat stress. While the ovaries reside in the abdominal cavity, the testes are always maintained at a lower temperature than that of the body core. Progression of meiotic prophase in testes is sensitive to body core temperature, and spermatocytes with aberrations in meiotic recombination and homolog synapsis under heat stress are eliminated before progressing beyond pachytene [57].
Sexual differences in the mode of the cell cycle were observed during the transition from meiosis I to meiosis II [58]. In male meiosis, there is a short period of the interphase-like stage called “interkinesis” between meiosis I and meiosis II, during which chromosomes are decondensed, and the nuclear membrane is reassembled. In females, chromosomes are persistently condensed after completion of the first meiotic division until meiosis II, without such an interphase-like stage. This is partly due to different CDK1-Cyclin B activities accompanied by the regulation of the anaphase-promoting complex (APC/c) during the meiosis I-meiosis II transition between spermatocytes and oocytes [59].
MEIOSIN and STRA8 Activate Meiotic Cell Cycle Program
Although the mechanism of meiotic initiation is fundamental, how germ cells regulate the mitosis-to-meiosis transition remains unclear. Stimulated by retinoic acid gene 8 (Stra8) was originally identified as a gene that is induced by RA in mouse P19 embryonic carcinoma cells [60]. STRA8 is transiently expressed in the pre-meiotic germ cells of embryonic ovaries and postnatal testes, and its temporal expression corresponds to the pre-leptotene stage, the earliest time point of meiotic prophase I. In females, STRA8 is expressed around E13.5-E14.5, which coincides with the meiotic entry in the embryonic ovary [8,9,10, 22]. In males, STRA8 is transiently expressed twice during germ cell development, the first of which occurs in differentiating spermatogonia, and the second of which occurs at the time of differentiation into spermatocytes [61,62,63,64] (Fig. 1A). While meiosis does not occur at the first expression of STRA8 in spermatogonia, it occurs at the second stage of STRA8 expression in spermatocytes [61,62,63,64] (Fig. 1A). It is unclear why meiosis is induced only at the second expression of STRA8 but not at the first expression of STRA8 in the testis. Since Stra8 KO germ cells fail to undergo normal meiosis in males and females [61, 65,66,67], STRA8 has been assumed to play a key role in entry into meiosis. Although the significance of STRA8 in meiotic initiation is well known, the molecular function of STRA8 remains elusive.
Our proteomic approach identified a previously uncharacterized protein, Gm4969 as an STRA8-interacting factor, which was named meiosis initiator (MEIOSIN) [68]. MEIOSIN was transiently co-expressed with STRA8 in pre-leptotene spermatocytes and oocytes. Since expression of STRA8 is still observed in Meiosin KO and vice versa, expression of Meiosin and Stra8 is regulated independently of each other. MEIOSIN/STRA8 co-expressing pre-leptotene germ cells overlap with EdU incorporation, representing de novo DNA replication. Thus, the co-expression of MEIOSIN and STRA8 coincides with the pre-meiotic S phase, the time at which meiosis is initiated (Fig. 3A). While STRA8 is observed in the cytoplasm and nuclei, MEIOSIN is exclusively localized in the nuclei of spermatocytes and oocytes. In the absence of MEIOSIN, STRA8 fails to localize to the nucleus and remains largely in the cytosol. This indicates that MEIOSIN plays a role in the nuclear translocation of STRA8.
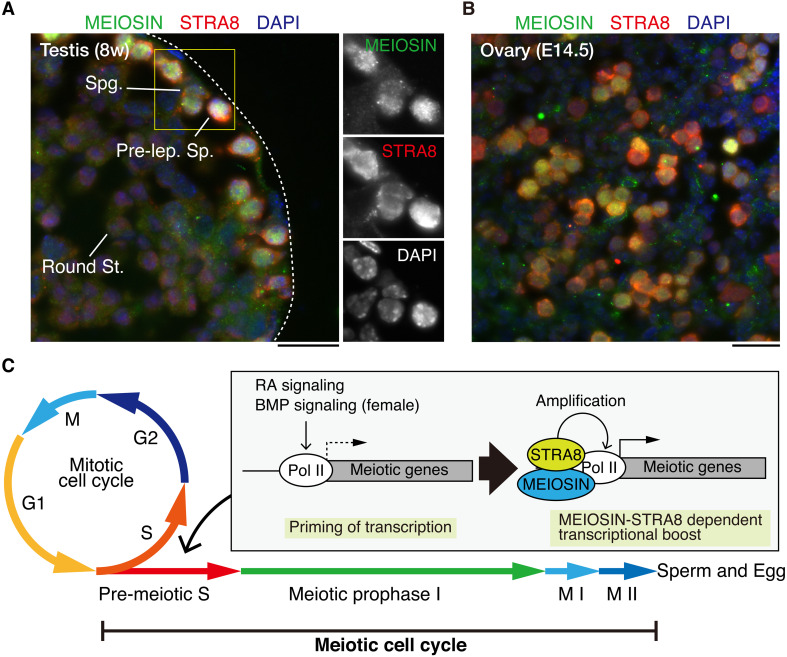
Fig. 3.
MEIOSIN and STRA8 direct meiotic initiation in germ cells. (A) STRA8 and MEIOSIN proteins are co-expressed in the pre-leptotene spermatocyte in the seminiferous stages VII-VIII. STRA8 is also expressed in the spermatogonium that is negative for MEIOSIN. Enlarged images are shown on the right. Pre-lep. Sp.: Pre-leptotene spermatocyte. Round Sp.: Round spermatid. Spg.: Spermatogonium. Scale bar: 25 μm. (B) Embryonic (E14.5) ovary section was stained as in (A). STRA8 and MEIOSIN proteins are co-expressed in the pre-leptotene germ cells. Scale bar: 25 μm. The samples were prepared and the images were acquired as described previously [68]. (C) Schematic illustration of sequential activation of meiotic prophase genes. Meiotic genes were primed before meiotic entry. BMP signaling also contributes to transcriptional priming, at least in females. MEIOSIN and STRA8 amplify meiotic gene transcription.
MEIOSIN protein possesses DNA-binding domains, basic helix–loop–helix (HLH), and high mobility group (HMG) box domains. Similarly, STRA8 possesses an HLH domain and an HMG-like domain [65, 69]. MEIOSIN and STRA8 form a complex that binds to the transcriptional start sites of numerous genes in the preleptotene germ cells [68, 69] (Fig. 3B). Crucially, the target genes bound by MEIOSIN and STRA8 are associated with meiotic prophase processes, such as meiotic chromosome dynamics and meiotic recombination. These include genes encoding meiosis-specific chromosome components (Sycp2, Sycp3, Hormad1, and Hormad2), synaptonemal complex (Sycp1, Syce1, Syce2, Syce3, and Tex12), meiotic cohesin subunits (Rad21L, Smc1β, and Stag3), meiotic recombination components (Prdm9, Dmc1, Spo11, Top6BL, Meiob, Ankrd31, Hsf2BP, Brme1, and Msh5), and meiosis-specific telomere proteins (Kash5, Majin, Terb1, and Sun1). Indeed, MEIOSIN/STRA8 target meiotic genes were downregulated in Meiosin KO and Stra8 KO spermatocytes. This suggested that the MEIOSIN-STRA8 complex acts as a transcriptional activator of meiosis-related genes to initiate meiosis in germ cells. Expression of MEIOSIN and STRA8, which coincides with the pre-meiotic S phase, suggests that the MEIOSIN-STRA8 complex coordinates meiotic entry with the cell cycle by robust transcriptional activation of the target genes for the involvement of a meiotic program in the S phase.
Meiotic gene expression has been hypothesized to be activated via STRA8-dependent and STRA8-independent pathways [70, 71]. Indeed, some meiotic genes, such as Sycp3 and Rec8 are already expressed at low levels prior to meiosis in an RA-dependent manner [70,71,72,73,74]. Meiotic gene promoters are poised by the enrichment of H3K4me2 and RNA polymerase II prior to meiosis [75] and primed with basal transcription. This hypothesis is exemplified by the fact that the expression of the MEIOSIN/STRA8 target genes is still detected at residual levels in Meiosin KO and Stra8 KO [68, 69]. The MEIOSIN-STRA8 complex probably boosts the transcription of genes primed for meiotic entry, which is in line with the proposed action of STRA8, which amplifies the transcription of a broad range of meiotic genes [69].
MEIOSIN and STRA8 are Required for Switching the Cell Cycle from Mitosis to Meiosis
In the testes and embryonic ovaries of Meiosin KO and Stra8 KO mice, although germ cells fail to enter meiotic prophase and do not show any signs of homolog synapsis or meiotic recombination, mitotic metaphase cells marked by histone H3Ser10 phosphorylation accumulate [76]. Furthermore, in Meiosin KO, aberrant mitotic-like cells emerge with ectopic misexpression of mitotic Cyclin A2, which should be suppressed at meiotic prophase in the wild-type [77,78,79]. These aberrant mitotic-like cells in Meiosin KO and Stra8 KO were eliminated by apoptosis. This phenotype could be attributed in part to the downregulation of MEIOC and YTHDC2, which bind to RNA and destabilize mitotic cell cycle-associated transcripts at the post-transcriptional level [77,78,79,80,81,82]. In Meioc KO and Ythdc2 KO mice, germ cells failed to maintain meiotic prophase, showing ectopic expression of mitotic cyclin and metaphase chromosome condensation [77,78,79], which is also observed in Meiosin KO and Stra8 KO mice [61, 68]. This evidence suggests that if meiotic gene expression programs are not installed in germ cells at a proper time, the exit of the mitotic cell cycle and entry into meiosis will fail.
It should be noted that a small population of Meiosin KO ovarian germ cells differentiate into mature oocyte-like cells exhibiting germinal vesicles (GV) and zona pellucida even though they fail to undergo meiotic prophase [68]. This phenomenon has also been observed in Stra8 KO female [67]. GV oocyte-like cells isolated from Meiosin KO ovaries can progress to a metaphase-like status in the culture, similar to WT oocytes. As WT oocytes undergo meiotic recombination during meiotic prophase, 20 pairs of bivalents with chiasma are observed during metaphase I. However, Meiosin KO oocyte-like cells possess 40 sister chromatid-like mitotic cells, suggesting that they have skipped meiotic prophase. This suggests that some oocyte-like cells undergo folliculogenesis without passing through the mitosis-to-meiosis transition in Meiosin KO and Stra8 KO females. This hypothesis is further supported by evidence that the development of oocyte-like cells can be directly induced from pluripotent stem cells by overexpressing key transcription factors in vitro [83]. Therefore, meiotic gene activation is genetically independent of oocyte development. Taken together, MEIOSIN and STRA8 play a role in switching the cell cycle from mitosis to meiosis in germ cells.
Conclusion
The fate of sexually neutral germ cells is determined by the cell cycle during development. Whether fetal germ cells undergo the meiotic cell cycle or G0/G1 arrest is induced by external signals and intrinsic mechanisms after mitotic proliferation. NANOS2 and CYP26B1 are key regulators that direct male germ cells to G0/G1 arrest and prevent them from entering the meiotic cell cycle. In the fetal ovary, RA, BMP, and WNT signaling force female germ cells to become competent in the meiotic cell cycle. MEIOSIN and STRA8 ensure the establishment of the meiotic cell cycle by activating meiotic genes, such that meiotic entry coincides with the S phase. Thus, the decision of germ cell fate is determined around E13.5 -14.5 by the change in cell cycle mode from the active mitotic cell cycle.
There are several issues yet to be understood regarding the regulation of the decision to enter the meiotic cell cycle. How the expression of MEIOSIN and STRA8 is regulated by upstream pathways [84, 85], and how the activation of the meiotic program synchronizes with the S phase of the cell cycle, are critical questions that are required to understand the precise mechanism of the meiotic cell cycle. Given that non-canonical subunits of basal transcriptional initiation complex TF IID are expressed in germ cells [86,87,88], how MEIOSIN and STRA8 transactivate meiotic genes together with germ cell-type TF IID should be examined. Furthermore, how meiotic initiation and subsequent prophase are regulated in sexually distinct ways is not well understood. The sexually dimorphic mechanisms of meiotic initiation should be considered in the context of the cell cycle, which requires further investigation.
Conflict of Interests
The authors declare no conflicts of interest.
Acknowledgments
This work was supported in part by a JSPS KAKENHI grant (#20K22638) to R.S; JSPS KAKENHI grants (#19H05743, #20H03265, #22K19315, #22H04922, and #23H00379) to K.I.; a grant from AMED PRIME [23gm6310021h0001) to K.I.; grants from Astellas Foundation for Research on Metabolic Disorders; Takeda Science Foundation to K.I. This publication was supported by JSPS KAKENHI (22HP2009).
References
- 1.Ginsburg M, Snow MH, McLaren A. Primordial germ cells in the mouse embryo during gastrulation. Development 1990; 110: 521–528. [DOI] [PubMed] [Google Scholar]
- 2.Tam PP, Snow MH. Proliferation and migration of primordial germ cells during compensatory growth in mouse embryos. J Embryol Exp Morphol 1981; 64: 133–147. [PubMed] [Google Scholar]
- 3.Saitou M, Yamaji M. Primordial germ cells in mice. Cold Spring Harb Perspect Biol 2012; 4: a008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci USA 2006; 103: 2474–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P. Retinoid signaling determines germ cell fate in mice. Science 2006; 312: 596–600. [DOI] [PubMed] [Google Scholar]
- 6.Spiller C, Koopman P, Bowles J. Sex determination in the mammalian germline. Annu Rev Genet 2017; 51: 265–285. [DOI] [PubMed] [Google Scholar]
- 7.Soygur B, Jaszczak RG, Fries A, Nguyen DH, Malki S, Hu G, Demir N, Arora R, Laird DJ. Intercellular bridges coordinate the transition from pluripotency to meiosis in mouse fetal oocytes. Sci Adv 2021; 7: eabc6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menke DB, Koubova J, Page DC. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol 2003; 262: 303–312. [DOI] [PubMed] [Google Scholar]
- 9.Yao HH, DiNapoli L, Capel B. Meiotic germ cells antagonize mesonephric cell migration and testis cord formation in mouse gonads. Development 2003; 130: 5895–5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bullejos M, Koopman P. Germ cells enter meiosis in a rostro-caudal wave during development of the mouse ovary. Mol Reprod Dev 2004; 68: 422–428. [DOI] [PubMed] [Google Scholar]
- 11.Mizuta K, Katou Y, Nakakita B, Kishine A, Nosaka Y, Saito S, Iwatani C, Tsuchiya H, Kawamoto I, Nakaya M, Tsukiyama T, Nagano M, Kojima Y, Nakamura T, Yabuta Y, Horie A, Mandai M, Ohta H, Saitou M. Ex vivo reconstitution of fetal oocyte development in humans and cynomolgus monkeys. EMBO J 2022; 41: e110815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo F, Yan L, Guo H, Li L, Hu B, Zhao Y, Yong J, Hu Y, Wang X, Wei Y, Wang W, Li R, Yan J, Zhi X, Zhang Y, Jin H, Zhang W, Hou Y, Zhu P, Li J, Zhang L, Liu S, Ren Y, Zhu X, Wen L, Gao YQ, Tang F, Qiao J. The Transcriptome and DNA Methylome Landscapes of Human Primordial Germ Cells. Cell 2015; 161: 1437–1452. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Dong J, Yan L, Yong J, Liu X, Hu Y, Fan X, Wu X, Guo H, Wang X, Zhu X, Li R, Yan J, Wei Y, Zhao Y, Wang W, Ren Y, Yuan P, Yan Z, Hu B, Guo F, Wen L, Tang F, Qiao J. Single-cell RNA-seq analysis maps development of human germline cells and gonadal niche interactions. Cell Stem Cell 2017; 20: 858–873.e4. [DOI] [PubMed] [Google Scholar]
- 14.Jørgensen A, Rajpert-De Meyts E. Regulation of meiotic entry and gonadal sex differentiation in the human: normal and disrupted signaling. Biomol Concepts 2014; 5: 331–341. [DOI] [PubMed] [Google Scholar]
- 15.Le Rolle M, Massa F, Siggers P, Turchi L, Loubat A, Koo BK, Clevers H, Greenfield A, Schedl A, Chaboissier MC, Chassot AA. Arrest of WNT/β-catenin signaling enables the transition from pluripotent to differentiated germ cells in mouse ovaries. Proc Natl Acad Sci USA 2021; 118: e2023376118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chassot AA, Gregoire EP, Lavery R, Taketo MM, de Rooij DG, Adams IR, Chaboissier MC. RSPO1/β-catenin signaling pathway regulates oogonia differentiation and entry into meiosis in the mouse fetal ovary. PLoS One 2011; 6: e25641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyauchi H, Ohta H, Nagaoka S, Nakaki F, Sasaki K, Hayashi K, Yabuta Y, Nakamura T, Yamamoto T, Saitou M. Bone morphogenetic protein and retinoic acid synergistically specify female germ-cell fate in mice. EMBO J 2017; 36: 3100–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Q, Fukuda K, Kato Y, Zhou Z, Deng CX, Saga Y. Sexual fate change of XX germ cells caused by the deletion of SMAD4 and STRA8 independent of somatic sex Reprogramming. PLoS Biol 2016; 14: e1002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagaoka SI, Nakaki F, Miyauchi H, Nosaka Y, Ohta H, Yabuta Y, Kurimoto K, Hayashi K, Nakamura T, Yamamoto T, Saitou M. ZGLP1 is a determinant for the oogenic fate in mice. Science 2020; 367: eaaw4115. [DOI] [PubMed] [Google Scholar]
- 20.Le Bouffant R, Souquet B, Duval N, Duquenne C, Hervé R, Frydman N, Robert B, Habert R, Livera G. Msx1 and Msx2 promote meiosis initiation. Development 2011; 138: 5393–5402. [DOI] [PubMed] [Google Scholar]
- 21.Bowles J, Feng CW, Miles K, Ineson J, Spiller C, Koopman P. ALDH1A1 provides a source of meiosis-inducing retinoic acid in mouse fetal ovaries. Nat Commun 2016; 7: 10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng CW, Burnet G, Spiller CM, Cheung FKM, Chawengsaksophak K, Koopman P, Bowles J. Identification of regulatory elements required for Stra8 expression in fetal ovarian germ cells of the mouse. Development 2021; 148: dev194977. [DOI] [PubMed] [Google Scholar]
- 23.Chassot AA, Le Rolle M, Jolivet G, Stevant I, Guigonis JM, Da Silva F, Nef S, Pailhoux E, Schedl A, Ghyselinck NB, Chaboissier MC. Retinoic acid synthesis by ALDH1A proteins is dispensable for meiosis initiation in the mouse fetal ovary. Sci Adv 2020; 6: eaaz1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar S, Chatzi C, Brade T, Cunningham TJ, Zhao X, Duester G. Sex-specific timing of meiotic initiation is regulated by Cyp26b1 independent of retinoic acid signalling. Nat Commun 2011; 2: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vernet N, Condrea D, Mayere C, Féret B, Klopfenstein M, Magnant W, Alunni V, Teletin M, Souali-Crespo S, Nef S, Mark M, Ghyselinck NB. Meiosis occurs normally in the fetal ovary of mice lacking all retinoic acid receptors. Sci Adv 2020; 6: eaaz1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellutti L, Abby E, Tourpin S, Messiaen S, Moison D, Trautmann E, Guerquin MJ, Rouiller-Fabre V, Habert R, Livera G. Divergent roles of CYP26B1 and endogenous retinoic acid in mouse fetal gonads. Biomolecules 2019; 9: 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Western PS, Miles DC, van den Bergen JA, Burton M, Sinclair AH. Dynamic regulation of mitotic arrest in fetal male germ cells. Stem Cells 2008; 26: 339–347. [DOI] [PubMed] [Google Scholar]
- 28.Spiller CM, Wilhelm D, Koopman P. Retinoblastoma 1 protein modulates XY germ cell entry into G1/G0 arrest during fetal development in mice. Biol Reprod 2010; 82: 433–443. [DOI] [PubMed] [Google Scholar]
- 29.Miles DC, van den Bergen JA, Sinclair AH, Western PS. Regulation of the female mouse germ cell cycle during entry into meiosis. Cell Cycle 2010; 9: 408–418. [DOI] [PubMed] [Google Scholar]
- 30.Du G, Oatley MJ, Law NC, Robbins C, Wu X, Oatley JM. Proper timing of a quiescence period in precursor prospermatogonia is required for stem cell pool establishment in the male germline. Development 2021; 148: dev194571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harbour JW, Dean DC. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev 2000; 14: 2393–2409. [DOI] [PubMed] [Google Scholar]
- 32.Vanhoutteghem A, Messiaen S, Hervé F, Delhomme B, Moison D, Petit JM, Rouiller-Fabre V, Livera G, Djian P. The zinc-finger protein basonuclin 2 is required for proper mitotic arrest, prevention of premature meiotic initiation and meiotic progression in mouse male germ cells. Development 2014; 141: 4298–4310. [DOI] [PubMed] [Google Scholar]
- 33.MacLean G, Li H, Metzger D, Chambon P, Petkovich M. Apoptotic extinction of germ cells in testes of Cyp26b1 knockout mice. Endocrinology 2007; 148: 4560–4567. [DOI] [PubMed] [Google Scholar]
- 34.Saba R, Wu Q, Saga Y. CYP26B1 promotes male germ cell differentiation by suppressing STRA8-dependent meiotic and STRA8-independent mitotic pathways. Dev Biol 2014; 389: 173–181. [DOI] [PubMed] [Google Scholar]
- 35.Tsuda M, Sasaoka Y, Kiso M, Abe K, Haraguchi S, Kobayashi S, Saga Y. Conserved role of nanos proteins in germ cell development. Science 2003; 301: 1239–1241. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki A, Niimi Y, Shinmyozu K, Zhou Z, Kiso M, Saga Y. Dead end1 is an essential partner of NANOS2 for selective binding of target RNAs in male germ cell development. EMBO Rep 2016; 17: 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gill ME, Hu YC, Lin Y, Page DC. Licensing of gametogenesis, dependent on RNA binding protein DAZL, as a gateway to sexual differentiation of fetal germ cells. Proc Natl Acad Sci USA 2011; 108: 7443–7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kato Y, Katsuki T, Kokubo H, Masuda A, Saga Y. Dazl is a target RNA suppressed by mammalian NANOS2 in sexually differentiating male germ cells. Nat Commun 2016; 7: 11272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki A, Saga Y. Nanos2 suppresses meiosis and promotes male germ cell differentiation. Genes Dev 2008; 22: 430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Q, Fukuda K, Weinstein M, Graff JM, Saga Y. SMAD2 and p38 signaling pathways act in concert to determine XY primordial germ cell fate in mice. Development 2015; 142: 575–586. [DOI] [PubMed] [Google Scholar]
- 41.Ewen K, Jackson A, Wilhelm D, Koopman P. A male-specific role for p38 mitogen-activated protein kinase in germ cell sex differentiation in mice. Biol Reprod 2010; 83: 1005–1014. [DOI] [PubMed] [Google Scholar]
- 42.Shimada R, Koike H, Hirano T, Kato Y, Saga Y. NANOS2 suppresses the cell cycle by repressing mTORC1 activators in embryonic male germ cells. iScience 2021; 24: 102890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Z, Shirakawa T, Ohbo K, Sada A, Wu Q, Hasegawa K, Saba R, Saga Y. RNA binding protein Nanos2 organizes post-transcriptional buffering system to retain primitive state of mouse spermatogonial stem cells. Dev Cell 2015; 34: 96–107. [DOI] [PubMed] [Google Scholar]
- 44.Pratto F, Brick K, Cheng G, Lam KG, Cloutier JM, Dahiya D, Wellard SR, Jordan PW, Camerini-Otero RD. Meiotic recombination mirrors patterns of germline replication in mice and humans. Cell 2021; 184: 4251–4267.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zickler D, Kleckner N. Recombination, pairing, and synapsis of homologs during meiosis. Cold Spring Harb Perspect Biol 2015; 7: a016626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cahoon CK, Hawley RS. Regulating the construction and demolition of the synaptonemal complex. Nat Struct Mol Biol 2016; 23: 369–377. [DOI] [PubMed] [Google Scholar]
- 47.Keeney S, Lange J, Mohibullah N. Self-organization of meiotic recombination initiation: general principles and molecular pathways. Annu Rev Genet 2014; 48: 187–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baudat F, Imai Y, de Massy B. Meiotic recombination in mammals: localization and regulation. Nat Rev Genet 2013; 14: 794–806. [DOI] [PubMed] [Google Scholar]
- 49.Ishiguro KI. The cohesin complex in mammalian meiosis. Genes Cells 2019; 24: 6–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alavattam KG, Maezawa S, Sakashita A, Khoury H, Barski A, Kaplan N, Namekawa SH. Attenuated chromatin compartmentalization in meiosis and its maturation in sperm development. Nat Struct Mol Biol 2019; 26: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vara C, Paytuví-Gallart A, Cuartero Y, Le Dily F, Garcia F, Salvà-Castro J, Gómez-H L, Julià E, Moutinho C, Aiese Cigliano R, Sanseverino W, Fornas O, Pendás AM, Heyn H, Waters PD, Marti-Renom MA, Ruiz-Herrera A. Three-dimensional genomic structure and cohesin occupancy correlate with transcriptional activity during spermatogenesis. Cell Rep 2019; 28: 352–367.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Wang H, Zhang Y, Du Z, Si W, Fan S, Qin D, Wang M, Duan Y, Li L, Jiao Y, Li Y, Wang Q, Shi Q, Wu X, Xie W. Reprogramming of meiotic chromatin architecture during spermatogenesis. Mol Cell 2019; 73: 547–561.e6. [DOI] [PubMed] [Google Scholar]
- 53.Slizynski BM. Meiotic prophase in female mice. Nature 1957; 179: 638. [DOI] [PubMed] [Google Scholar]
- 54.Burgoyne PS, Mahadevaiah SK, Turner JM. The consequences of asynapsis for mammalian meiosis. Nat Rev Genet 2009; 10: 207–216. [DOI] [PubMed] [Google Scholar]
- 55.Turner JM. Meiotic silencing in mammals. Annu Rev Genet 2015; 49: 395–412. [DOI] [PubMed] [Google Scholar]
- 56.Royo H, Polikiewicz G, Mahadevaiah SK, Prosser H, Mitchell M, Bradley A, de Rooij DG, Burgoyne PS, Turner JM. Evidence that meiotic sex chromosome inactivation is essential for male fertility. Curr Biol 2010; 20: 2117–2123. [DOI] [PubMed] [Google Scholar]
- 57.Hirano K, Nonami Y, Nakamura Y, Sato T, Sato T, Ishiguro KI, Ogawa T, Yoshida S. Temperature sensitivity of DNA double-strand break repair underpins heat-induced meiotic failure in mouse spermatogenesis. Commun Biol 2022; 5: 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim J, Ishiguro K, Nambu A, Akiyoshi B, Yokobayashi S, Kagami A, Ishiguro T, Pendas AM, Takeda N, Sakakibara Y, Kitajima TS, Tanno Y, Sakuno T, Watanabe Y. Meikin is a conserved regulator of meiosis-I-specific kinetochore function. Nature 2015; 517: 466–471. [DOI] [PubMed] [Google Scholar]
- 59.Tanno N, Kuninaka S, Fujimura S, Takemoto K, Okamura K, Takeda N, Araki K, Araki M, Saya H, Ishiguro KI. Phosphorylation of the anaphase promoting complex activator FZR1/CDH1 is required for meiosis II entry in mouse male germ cell. Sci Rep 2020; 10: 10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oulad-Abdelghani M, Bouillet P, Décimo D, Gansmuller A, Heyberger S, Dollé P, Bronner S, Lutz Y, Chambon P. Characterization of a premeiotic germ cell-specific cytoplasmic protein encoded by Stra8, a novel retinoic acid-responsive gene. J Cell Biol 1996; 135: 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mark M, Jacobs H, Oulad-Abdelghani M, Dennefeld C, Féret B, Vernet N, Codreanu CA, Chambon P, Ghyselinck NB. STRA8-deficient spermatocytes initiate, but fail to complete, meiosis and undergo premature chromosome condensation. J Cell Sci 2008; 121: 3233–3242. [DOI] [PubMed] [Google Scholar]
- 62.Zhou Q, Li Y, Nie R, Friel P, Mitchell D, Evanoff RM, Pouchnik D, Banasik B, McCarrey JR, Small C, Griswold MD. Expression of stimulated by retinoic acid gene 8 (Stra8) and maturation of murine gonocytes and spermatogonia induced by retinoic acid in vitro. Biol Reprod 2008; 78: 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou Q, Nie R, Li Y, Friel P, Mitchell D, Hess RA, Small C, Griswold MD. Expression of stimulated by retinoic acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: an in vivo study in vitamin A-sufficient postnatal murine testes. Biol Reprod 2008; 79: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Endo T, Romer KA, Anderson EL, Baltus AE, de Rooij DG, Page DC. Periodic retinoic acid-STRA8 signaling intersects with periodic germ-cell competencies to regulate spermatogenesis. Proc Natl Acad Sci USA 2015; 112: E2347–E2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet 2006; 38: 1430–1434. [DOI] [PubMed] [Google Scholar]
- 66.Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AM, Page DC. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci USA 2008; 105: 14976–14980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dokshin GA, Baltus AE, Eppig JJ, Page DC. Oocyte differentiation is genetically dissociable from meiosis in mice. Nat Genet 2013; 45: 877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ishiguro KI, Matsuura K, Tani N, Takeda N, Usuki S, Yamane M, Sugimoto M, Fujimura S, Hosokawa M, Chuma S, Ko MSH, Araki K, Niwa H. MEIOSIN Directs the switch from mitosis to meiosis in mammalian germ cells. Dev Cell 2020; 52: 429–445.e10. [DOI] [PubMed] [Google Scholar]
- 69.Kojima ML, de Rooij DG, Page DC. Amplification of a broad transcriptional program by a common factor triggers the meiotic cell cycle in mice. eLife 2019; 8: e43738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soh YQ, Junker JP, Gill ME, Mueller JL, van Oudenaarden A, Page DC. A gene regulatory program for meiotic prophase in the fetal ovary. PLoS Genet 2015; 11: e1005531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koubova J, Hu YC, Bhattacharyya T, Soh YQ, Gill ME, Goodheart ML, Hogarth CA, Griswold MD, Page DC. Retinoic acid activates two pathways required for meiosis in mice. PLoS Genet 2014; 10: e1004541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chuma S, Nakatsuji N. Autonomous transition into meiosis of mouse fetal germ cells in vitro and its inhibition by gp130-mediated signaling. Dev Biol 2001; 229: 468–479. [DOI] [PubMed] [Google Scholar]
- 73.Evans E, Hogarth C, Mitchell D, Griswold M. Riding the spermatogenic wave: profiling gene expression within neonatal germ and sertoli cells during a synchronized initial wave of spermatogenesis in mice. Biol Reprod 2014; 90: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jan SZ, Vormer TL, Jongejan A, Röling MD, Silber SJ, de Rooij DG, Hamer G, Repping S, van Pelt AMM. Unraveling transcriptome dynamics in human spermatogenesis. Development 2017; 144: 3659–3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sin HS, Kartashov AV, Hasegawa K, Barski A, Namekawa SH. Poised chromatin and bivalent domains facilitate the mitosis-to-meiosis transition in the male germline. BMC Biol 2015; 13: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ishiguro KI, Matsuura K, Tani N, Takeda N, Usuki S, Yamane M, Sugimoto M, Fujimura S, Hosokawa M, Chuma S, Ko MSH, Araki K, Niwa H. MEIOSIN directs the switch from mitosis to meiosis in mammalian germ cells. Dev Cell 2020; 52: 429–445.e10. [DOI] [PubMed] [Google Scholar]
- 77.Abby E, Tourpin S, Ribeiro J, Daniel K, Messiaen S, Moison D, Guerquin J, Gaillard JC, Armengaud J, Langa F, Toth A, Martini E, Livera G. Implementation of meiosis prophase I programme requires a conserved retinoid-independent stabilizer of meiotic transcripts. Nat Commun 2016; 7: 10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soh YQS, Mikedis MM, Kojima M, Godfrey AK, de Rooij DG, Page DC. Meioc maintains an extended meiotic prophase I in mice. PLoS Genet 2017; 13: e1006704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jain D, Puno MR, Meydan C, Lailler N, Mason CE, Lima CD, Anderson KV, Keeney S. ketu mutant mice uncover an essential meiotic function for the ancient RNA helicase YTHDC2. eLife 2018; 7: e30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wojtas MN, Pandey RR, Mendel M, Homolka D, Sachidanandam R, Pillai RS. Regulation of m6A Transcripts by the 3′→5′ RNA Helicase YTHDC2 Is Essential for a Successful Meiotic Program in the Mammalian Germline. Mol Cell 2017; 68: 374–387.e12. [DOI] [PubMed] [Google Scholar]
- 81.Li L, Krasnykov K, Homolka D, Gos P, Mendel M, Fish RJ, Pandey RR, Pillai RS. The XRN1-regulated RNA helicase activity of YTHDC2 ensures mouse fertility independently of m6A recognition. Mol Cell 2022; 82: 1678–1690.e12. [DOI] [PubMed] [Google Scholar]
- 82.Liu R, Kasowitz SD, Homolka D, Leu NA, Shaked JT, Ruthel G, Jain D, Lin H, Keeney S, Luo M, Pillai RS, Wang PJ. YTHDC2 is essential for pachytene progression and prevents aberrant microtubule-driven telomere clustering in male meiosis. Cell Reports 2021; 37: 110110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hamazaki N, Kyogoku H, Araki H, Miura F, Horikawa C, Hamada N, Shimamoto S, Hikabe O, Nakashima K, Kitajima TS, Ito T, Leitch HG, Hayashi K. Reconstitution of the oocyte transcriptional network with transcription factors. Nature 2021; 589: 264–269. [DOI] [PubMed] [Google Scholar]
- 84.Sun S, Jiang Y, Zhang Q, Pan H, Li X, Yang L, Huang M, Wei W, Wang X, Qiu M, Cao L, He H, Yu M, Liu H, Zhao B, Jiang N, Li R, Lin X. Znhit1 controls meiotic initiation in male germ cells by coordinating with Stra8 to activate meiotic gene expression. Dev Cell 2022; 57: 901–913.e4. [DOI] [PubMed] [Google Scholar]
- 85.Ito T, Ohta M, Osada A, Nishiyama A, Ishiguro KI, Tamura T, Sekita Y, Kimura T. Switching defective/sucrose non-fermenting chromatin remodeling complex coordinates meiotic gene activation via promoter remodeling and Meiosin activation in female germline. Genes Cells 2023; 28: 15–28. [DOI] [PubMed] [Google Scholar]
- 86.Zhou H, Grubisic I, Zheng K, He Y, Wang PJ, Kaplan T, Tjian R. Taf7l cooperates with Trf2 to regulate spermiogenesis. Proc Natl Acad Sci USA 2013; 110: 16886–16891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grive KJ, Gustafson EA, Seymour KA, Baddoo M, Schorl C, Golnoski K, Rajkovic A, Brodsky AS, Freiman RN. TAF4b regulates oocyte-specific genes essential for meiosis. PLoS Genet 2016; 12: e1006128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gura MA, Mikedis MM, Seymour KA, de Rooij DG, Page DC, Freiman RN. Dynamic and regulated TAF gene expression during mouse embryonic germ cell development. PLoS Genet 2020; 16: e1008515. [DOI] [PMC free article] [PubMed] [Google Scholar]