Abstract
Background
The long-term health consequences of COVID-19 remain largely unclear. The aim of this study was to describe the long-term health consequences of patients with COVID-19 who have been discharged from hospital and investigate the associated risk factors, in particular disease severity.
Methods
We did an ambidirectional cohort study of patients with confirmed COVID-19 who had been discharged from Jin Yin-tan Hospital (Wuhan, China) between Jan 7 and May 29, 2020. Patients who died before follow-up; patients for whom follow-up would be difficult because of psychotic disorders, dementia, or readmission to hospital; those who were unable to move freely due to concomitant osteoarthropathy or immobile before or after discharge due to diseases such as stroke or pulmonary embolism; those who declined to participate; those who could not be contacted; and those living outside of Wuhan or in nursing or welfare homes were all excluded. All patients were interviewed with a series of questionnaires for evaluation of symptoms and health-related quality of life, underwent physical examinations and a 6-min walking test, and received blood tests. A stratified sampling procedure was used to sample patients according to their highest seven-category scale during their hospital stay as 3, 4, and 5–6, to receive pulmonary function test, high resolution CT of the chest, and ultrasonography. Enrolled patients who had participated in the Lopinavir Trial for Suppression of SARS-CoV-2 in China received SARS-CoV-2 antibody tests. Multivariable adjusted linear or logistic regression models were used to evaluate the association between disease severity and long-term health consequences.
Findings
In total, 1733 of 2469 discharged patients with COVID-19 were enrolled after 736 were excluded. Patients had a median age of 57·0 years (IQR 47·0–65·0) and 897 (52%) were male and 836 (48%) were female. The follow-up study was done from June 16 to Sept 3, 2020, and the median follow-up time after symptom onset was 186·0 days (175·0–199·0). Fatigue or muscle weakness (52%, 855 of 1654) and sleep difficulties (26%, 437 of 1655) were the most common symptoms. Anxiety or depression was reported among 23% (367 of 1616) of patients. The proportions of 6-min walking distance less than the lower limit of the normal range were 17% for those at severity scale 3, 13% for severity scale 4, and 28% for severity scale 5–6. The corresponding proportions of patients with diffusion impairment were 22% for severity scale 3, 29% for scale 4, and 56% for scale 5–6, and median CT scores were 3·0 (IQR 2·0–5·0) for severity scale 3, 4·0 (3·0–5·0) for scale 4, and 5·0 (4·0–6·0) for scale 5–6. After multivariable adjustment, patients showed an odds ratio (OR) of 1·61 (95% CI 0·80–3·25) for scale 4 versus scale 3 and 4·60 (1·85–11·48) for scale 5–6 versus scale 3 for diffusion impairment; OR 0·88 (0·66–1·17) for scale 4 versus scale 3 and OR 1·76 (1·05–2·96) for scale 5–6 versus scale 3 for anxiety or depression, and OR 0·87 (0·68–1·11) for scale 4 versus scale 3 and 2·75 (1·61–4·69) for scale 5–6 versus scale 3 for fatigue or muscle weakness. Of 94 patients with blood antibodies tested at follow-up, the seropositivity (96·2% vs 58·5%) and median titres (19·0 vs 10·0) of the neutralising antibodies were significantly lower compared with at the acute phase. 107 of 822 participants without acute kidney injury and with an estimated glomerular filtration rate (eGFR) of 90 mL/min per 1·73 m2 or more at acute phase had eGFR less than 90 mL/min per 1·73 m2 at follow-up.
Interpretation
At 6 months after acute infection, COVID-19 survivors were mainly troubled with fatigue or muscle weakness, sleep difficulties, and anxiety or depression. Patients who were more severely ill during their hospital stay had more severe impaired pulmonary diffusion capacities and abnormal chest imaging manifestations, and are the main target population for intervention of long-term recovery.
Funding
National Natural Science Foundation of China, Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, National Key Research and Development Program of China, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, and Peking Union Medical College Foundation.
Research in context.
Evidence before this study
We searched PubMed for follow-up studies regarding long-term consequences of COVID-19 from database inception up to Nov 5, 2020, without any language restrictions. The search terms were (COVID-19 OR SARS-CoV-2 OR Coronavirus disease 2019 OR 2019-nCoV) AND (survivor* OR recover* OR persistent OR follow up OR discharge* OR long term OR sequelae). The studies reported that patients with COVID-19 discharged from hospitals might have persistent symptoms, abnormal patterns in chest imaging manifestations, impaired lung functions, and poor quality of life. However, the representativeness of the studies and the explicitness of provided information were insufficient due to small numbers of cases and the short duration of follow-up (up to about 3 months after discharge). The long-term health consequences of discharged patients with COVID-19 and the associated risk factors were still unknown.
Added value of this study
To our knowledge, this study is the largest cohort study (n=1733) with the longest follow-up duration for the consequences of adult patients discharged from hospital recovering from COVID-19. Our findings showed that 68% of patients reported at least one symptom at 6 months after symptom onset, and the proportion was higher in females. The most common symptoms were fatigue or muscle weakness and sleep difficulties. Additionally, 23% of patients reported anxiety or depression at follow-up. The percentage of patients with pulmonary diffusion abnormality during follow-up was higher in patients with more severe disease at acute phase. These patients also had a higher CT score at follow-up. Ground glass opacity and irregular lines were the most common patterns at follow-up. In multivariable analysis, females and participants with severity scale 5–6 had a higher prevalence of lung diffusion impairment, and anxiety or depression. The seropositivity of the neutralising antibodies, N-IgM, RBD-IgM, and S-IgM, N-IgA, RBD-IgA, and S-IgA antibodies, and RBD-IgG, and neutralising antibody titres at follow-up were significantly lower than at acute phase.
Implications of all the available evidence
At 6 months after symptom onset, patients with COVID-19 had symptoms of fatigue or muscle weakness, sleep difficulties, and anxiety or depression. Patients with a more severe illness during their hospital stay had increasingly impaired pulmonary diffusion capacities and abnormal chest imaging manifestations, and these are the patients who are the main target population for intervention of long-term recovery. The decline of neutralising antibodies raises concern for SARS-CoV-2 re-infection. The risk of re-infection should be monitored for patients who present with new symptoms of COVID-19.
Introduction
As of Jan 4, 2021, the global pandemic of COVID-19—an emerging infectious disease caused by SARS-CoV-2—has resulted in more than 83 million confirmed cases with more than 1·8 million deaths. The epidemiological and clinical characteristics, pathogenesis, and complications of patients with COVID-19 at acute phase have been explicitly described,1, 2 but the long-term consequences of the illness remain largely unclear.
Long-term follow-up studies on persistent symptoms, and lung function, physical, and psychological problems of discharged patients are urgently required.3 Only a few studies with small sample sizes have been published, with the longest follow-up duration being 3 months following discharge from hospital.4, 5, 6, 7, 8 Some persisting symptoms such as fatigue and dyspnoea,4, 8 impaired pulmonary function,5, 7 and chest image abnormalities6 were reported in patients following hospital discharge, but the full spectrum of post-discharge characteristics is still unknown. Furthermore, no studies have yet reported the extra-pulmonary organ manifestations that could persist after damage in acute stage or are newly onset after discharge.
We aimed to describe the long-term consequences of COVID-19 in patients after hospital discharge and identify the potential risk factors, including disease severity, associated with these consequences.
Methods
Study design and participants
This ambidirectional cohort study was done at Jin Yin-tan Hospital, the first designated hospital for patients with COVID-19 in Wuhan, Hubei, China. We included all patients with laboratory confirmed COVID-19 who were discharged from Jin Yin-tan Hospital between Jan 7 and May 29, 2020. We excluded the following patients: (1) those who died before the follow-up visit; (2) those for whom follow-up would be difficult owing to psychotic disorder, dementia, or readmission to hospital attributed to underlying diseases; (3) those who were unable to move freely due to concomitant osteoarthropathy or immobile before or after discharge due to diseases such as stroke or pulmonary embolism; (4) those who declined to participate; (5) those unable to be contacted; and (6) those living outside of Wuhan or in nursing or welfare homes. All discharged patients met uniform discharge criteria according to the Chinese clinical guidance for COVID-19 pneumonia diagnosis and treatment issued by the National Health Commission (ie, no fever for three consecutive days, improvement in respiratory symptoms, obvious resolution and recovery of acute lesion in lung imaging, and two negative test results for SARS-CoV-2 24 h apart).9
The study was approved by the Research Ethics Commission of Jin Yin-tan Hospital (KY-2020–78.01). Written informed consent was obtained from all study participants.
Procedures
We defined the acute phase as the time between symptom onset and hospital discharge. Clinical data for acute phase were retrieved from electronic medical records, including demographic characteristics (age, sex, education, and cigarette smoking), clinical characteristics (self-reported comorbidities, symptom onset time, and chest images), laboratory test results, and treatment (corticosteroids, intravenous immunoglobulin, antibiotics, thymosin, and antivirals including lopinavir–ritonavir, arbidol, chloroquine phosphate, and hydroxychloroquine). The disease severity was characterised by the highest seven-category scale during the hospital stay (termed the severity scale),10 which consisted of the following categories: 1, not admitted to hospital with resumption of normal activities; 2, not admitted to hospital, but unable to resume normal activities; 3, admitted to hospital but not requiring supplemental oxygen; 4, admitted to hospital but requiring supplemental oxygen; 5, admitted to hospital requiring high-flow nasal cannula (HFNC), non-invasive mechanical ventilation (NIV), or both; 6, admitted to hospital requiring extracorporeal membrane oxygenation, invasive mechanical ventilation (IMV), or both; and 7, death. Data were managed using REDCap electronic data capture tools to minimise missing inputs and allow for real-time data validation and quality control.
The appointment for the follow-up visit was set by trained medical staff via telephone. All participants were contacted in the order of their symptom onset date documented in their medical record. If the follow-up appointment was missed, the patient was given two opportunities to reschedule the visit.
Follow-up consultations were done in the outpatient clinic of Jin Yin-tan Hospital. All participants were interviewed face-to-face by trained physicians and asked to complete a series of questionnaires, including a self- reported symptom questionnaire (appendix pp 5–6), the modified British Medical Research Council (mMRC) dyspnoea scale, the EuroQol five-dimension five-level (EQ-5D-5L) questionnaire, the EuroQol Visual Analogue Scale (EQ-VAS), and an ischaemic stroke and cardiovascular event registration form.11 For the symptom questionnaire, participants were asked to report newly occurring and persistent symptoms, or any symptoms worse than before COVID-19 development. The mMRC scale is a five-category scale to characterise the level of dyspnoea with physical activity in which higher scores correspond with increased dyspnoea.12 The EQ-5D-5L is a validated questionnaire to evaluate patient quality of life by assessment of the following five factors: mobility, self-care, usual activities, pain or discomfort, and anxiety or depression. Categorisation within each factor is divided into five levels that range from no problems to extreme problems.13 The EQ-VAS is a patient's subjective assessment of generic health ranging from 0 to 100, with higher scores representing better subjective health experience.14 They also underwent a physical examination and a 6-min walking test.
Venous blood samples were collected from all participants who attended follow-up appointments for complete blood count, serum creatinine, haemoglobin, and glycated haemoglobin A1c (HbA1c). Furthermore, SARS-CoV-2 antibody concentrations were measured for participants that had previously been enrolled in the Lopinavir Trial for Suppression of SARS-CoV-2 in China (LOTUS).10 Plasma samples at acute phase, collected with a median duration of 23 days (IQR 20–26) after illness onset, and follow-up were analysed simultaneously. The immunoglobulin (Ig) M, IgA, and IgG antibodies against the nucleoprotein, spike protein, and the receptor binding domain of the spike protein were evaluated by use of enzyme-linked immunosorbent assay. The neutralising antibodies were titred on Vero cells by use of a microneutralisation assay. The detailed test method was described in our previous antibody studies.15, 16
Additionally, a stratified disproportional random sampling procedure according to severity scale was used to select patients to undergo pulmonary function test, ultrasonography of lower limb veins and abdomen, and chest high-resolution CT (HRCT). Patients requiring HFNC, NIV, or IMV (severity scale ≥5) were all invited to receive the pulmonary function test, ultrasound, and HRCT of chest. The ratio used to select patients not requiring supplemental oxygen (severity scale 3) and those requiring supplemental oxygen (severity scale 4) was 1:2.
The pulmonary function test was done in the Lung Function Laboratory of Jin Yin-tan Hospital using the Master Screen PFT (Vyaire Medical GmbH, Hoechberg, Germany) according to American Thoracic Society guidelines.17 Chest HRCT was in the supine position during end-inspiration (SIEMENS SOMATOM PERSPECTIVE 64 CT scanner). Images were reconstructed at 1 mm slice thickness, with 1 mm increment, 512 mm × 512 mm. The final chest CT images during the hospital stay and the follow-up image were cross-compared. The CT features were evaluated by one experienced radiologist and one pulmonologist. We used a validated artificial intelligence software to calculate the extent of anatomic involvement of each of the five lobes, which was defined as the volume ratio of pneumonia lesions to each lung lobe,18 and then calculated a semi-quantitative CT score to assess the pulmonary involvement.19, 20 Briefly, the score was calculated for each of the five lobes considering the extent of anatomic involvement, as follows: 0, no involvement; 1, less than 5% involvement; 2, 5–25% involvement; 3, 26–50% involvement; 4, 51–75% involvement; and 5, more than 75% involvement. The total CT score was the sum of the five lobe scores (0–25). Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease-Epidemiology Collaboration equation.21 Detailed diagnostic criteria for acute kidney injury, diabetes, and deep venous thrombosis of lower limb veins are presented in the appendix (p 3). The primary outcomes included symptoms (fatigue or muscle weakness, sleep difficulties, hair loss, and smell disorder), exercise capacity (distance walked in 6 min), health-related quality of life (pain or discomfort, anxiety or depression, mobility, personal care, and usual activity), lung function, and chest CT pattern at follow-up. The secondary outcomes included extrapulmonary organ function (including eGFR, HbA1c, deep venous thrombosis of lower limbs, and ultrasonographic features of kidney, liver, spleen, and pancreas) and antibody titres and seropositivity.
Statistical analysis
Demographic characteristics and long-term health consequences of COVID-19 in patients were presented as median (IQR) for continuous variables and expressed as absolute values along with percentages for categorical variables. Participants were categorised into three groups according to their severity scale during their hospital stay: scale 3, not requiring supplemental oxygen; scale 4, requiring supplemental oxygen; or scale 5–6, requiring HFNC, NIV, or IMV. Demographic characteristics and long-term consequences across participants with different categories of severity scale were shown. Long-term health consequences for males and females were also shown. For the comparison of symptoms, exercise capacity, and health-related quality of life between males and females, we used the Mann-Whitney U test, χ2 test, or Fisher's exact test where appropriate. Multivariable adjusted logistic regression models were used to estimate the odds ratios (ORs) and 95% CIs for association between disease severity and categorical outcomes. For association between disease severity and continuous outcomes, multivariable adjusted linear regression models were used to estimate the β estimates and 95% CIs. Confounders including age, sex, cigarette smoking (never-smoker, current smoker, and former smoker), education (college or higher, middle school or lower), comorbidity (hypertension, diabetes, cardiovascular diseases, cerebrovascular diseases, malignant tumour, chronic obstructive pulmonary disease, and chronic kidney disease), corticosteroids, antivirals (lopinavir–ritonavir, arbidol, chloroquine phosphate, and hydroxychloroquine), and intravenous immunoglobulin were adjusted for. The comparison of antibody test results at acute phase and follow-up was done with paired t tests for antibody titres and a McNemar test for antibody seropositivity.
Multivariable adjusted logistic regression analysis was also used for exploring risk factors associated with diffusion impairment, anxiety or depression, and fatigue or muscle weakness, and linear regression analysis was used to assess the percentage change in CT score from acute phase to follow-up. The percentage change was calculated by use of the following formula: (CT score at acute phase – CT score at follow-up)/CT score at acute phase × 100. For associations of age, cigarette smoking, and education with outcome measure, the variables adjusted for the association of disease severity with consequences (age, sex, cigarette smoking, education, comorbidity, corticosteroids, antivirals, and intravenous immunoglobulin) were all included in the models, except for comorbidity. For association of comorbidity with outcome, the aforementioned variables were all included. For association of other factors including sex, corticosteroids, antiviral, and intravenous immunoglobulin with outcome, disease severity and the aforementioned variables were included in the model. All tests were two-sided, and a p value less than 0·05 was considered statistically significant. We included all participants for whom the variables of interest were available in the final analysis, without imputing missing data. All statistical analyses were done with SAS, version 9.4.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
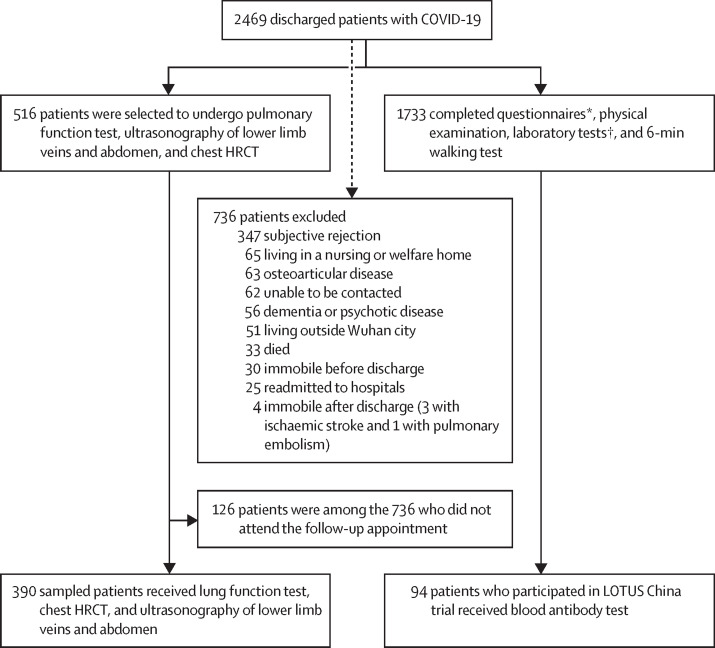
A total of 2469 patients with COVID-19 were discharged from Jin Yin-tan Hospital between Jan 7 and May 29, 2020, and the follow-up study was done from June 16, 2020, to Sept 3, 2020. 736 patients were excluded because they did not attend follow-up appointments for several reasons, which are outlined in figure 1 . Notably, 33 (1·3%) of the 2469 patients died after discharge mainly due to exacerbation of underlying pulmonary, heart, or kidney disease, and the detailed characteristics are shown in the appendix (pp 7–9). 25 patients were readmitted to hospital for underlying disease complications when contacted by telephone for follow-up, with one of them admitted for respiratory failure caused by underlying pulmonary fibrosis. Three patients developed ischaemic strokes, and one patient had an acute pulmonary embolism due to deep venous thrombosis of lower limbs after discharge. Finally, 1733 adult participants were enrolled for questionnaire interview, physical examination, laboratory tests, and a 6-min walking test. 94 of 1733 patients received a blood antibody test. 390 of 516 sampled patients ascertained as eligible received a lung function test, chest HRCT, and ultrasonography of lower limb veins and abdomen (figure 1). The 126 remaining sampled patients did not undergo these tests because they were among the 736 patients who did not attend the follow-up appointment.
Figure 1.
Flow chart of patients with COVID-19 discharged from Jin Yin-tan Hospital between Jan 7, and May 29, 2020
HRCT=high resolution CT. LOTUS=Lopinavir Trial for Suppression of SARS-CoV-2 in China. *A series of questionnaires included a self-reported symptom questionnaire, the modified British Medical Research Council dyspnoea scale, the EuroQol five-dimension five-level questionnaire, the EuroQol Visual Analogue Scale, and an ischaemic stroke and cardiovascular event registration form. †Laboratory tests included a white cell count, lymphocyte count, serum creatinine, haemoglobin, and glycosylated haemoglobin.
The demographic and clinical characteristics of participants retrieved from electronic medical records during acute phase are shown in table 1 . The median age of the enrolled participants was 57·0 years (IQR 47·0–65·0), with 897 (52%) males and 836 (48%) females. The most common comorbidity was hypertension (505 patients, 29%), followed by diabetes (207 patients, 12%), and cardiovascular disease (128 patients, 7%). 1172 (68%) of 1733 participants required oxygen therapy during their hospital stay, and 122 (7%) required HFNC, NIV, or IMV. 76 participants (4%) were admitted to the intensive care unit (ICU). The median duration of hospital stay was 14·0 days (10·0–19·0) and time exclusively in the ICU was 14·0 (6·5–25·5) days. The proportion of males was higher among participants with a higher severity scale: 49% (214 of 439) for severity scale 3, 52% (605 of 1172) for scale 4, and 64% (78 of 122) for scale 5–6. The median duration from symptom onset to follow-up visit is 186·0 (175·0–199·0) days and the median time from discharge to follow-up visit is 153·0 (146·0–160·0) days (table 1).
Table 1.
Characteristics of enrolled patients
| Total (n=1733) | Scale 3: not requiring supplemental oxygen (n=439) | Scale 4: requiring supplemental oxygen (n=1172) | Scale 5–6: requiring HFNC, NIV, or IMV (n=122) | |||
|---|---|---|---|---|---|---|
| Age, years | 57·0 (47·0–65·0) | 57·0 (46·0–65·0) | 57·0 (48·0–65·0) | 56·0 (48·0–65·0) | ||
| Sex | ||||||
| Male | 897 (52%) | 214 (49%) | 605 (52%) | 78 (64%) | ||
| Female | 836 (48%) | 225 (51%) | 567 (48%) | 44 (36%) | ||
| Education | ||||||
| College or higher | 499/1558 (32%) | 132/405 (33%) | 322/1045 (31%) | 45/108 (42%) | ||
| Middle school or lower | 1059/1558 (68%) | 273/405 (67%) | 723/1045 (69%) | 63/108 (58%) | ||
| Cigarette smoking | ||||||
| Never-smoker | 1585/1731 (92%) | 408 (93%) | 1071/1170 (92%) | 106 (87%) | ||
| Current smoker | 102/1731 (6%) | 19 (4%) | 69/1170 (6%) | 14 (11%) | ||
| Former smoker | 44/1731 (3%) | 12 (3%) | 30/1170 (3%) | 2 (2%) | ||
| Comorbidities | ||||||
| Hypertension | 505 (29%) | 129 (29%) | 331 (28%) | 45 (37%) | ||
| Diabetes | 207 (12%) | 60 (14%) | 132 (11%) | 15 (12%) | ||
| Cardiovascular diseases | 128/1732 (7%) | 41/438 (9%) | 72 (6%) | 15 (12%) | ||
| Cerebrovascular diseases | 47/1732 (3%) | 11 (3%) | 35/1171 (3%) | 1 (1%) | ||
| Malignant tumour | 44 (3%) | 9 (2%) | 33 (3%) | 2 (2%) | ||
| Chronic obstructive pulmonary disorder | 31 (2%) | 6 (1%) | 24 (2%) | 1 (1%) | ||
| Chronic kidney disease | 27 (2%) | 4 (1%) | 21 (2%) | 2 (2%) | ||
| Systolic blood pressure ≥140 mm Hg | 398/1724 (23%) | 121 (28%) | 251/1166 (22%) | 26/119 (22%) | ||
| Diastolic blood pressure ≥90 mm Hg | 386/1724 (22%) | 115 (26%) | 253/1166 (22%) | 18/119 (15%) | ||
| Highest seven-category scale during hospital stay | ||||||
| 3: admitted to hospital, not requiring supplemental oxygen | 439 (25%) | 439 (100%) | NA | NA | ||
| 4: admitted to hospital, requiring supplemental oxygen | 1172 (68%) | NA | 1172 (100%) | NA | ||
| 5: admitted to hospital, requiring HFNC or NIV or both | 112 (6%) | NA | NA | 112 (92%) | ||
| 6: admitted to hospital, requiring ECMO or IMV or both | 10 (1%) | NA | NA | 10 (8%) | ||
| Treatment received during hospital stay | ||||||
| Corticosteroids | 398 (23%) | 38 (9%) | 275 (23%) | 85 (70%) | ||
| Antivirals | 943 (54%) | 222 (51%) | 648 (55%) | 73 (60%) | ||
| Lopinavir–ritonavir | 236 (14%) | 40 (9%) | 164 (14%) | 32 (26%) | ||
| Arbidol | 831 (48%) | 202 (46%) | 568 (48%) | 61 (50%) | ||
| Chloroquine phosphate | 4 (<1%) | 0 | 3 (<1%) | 1 (1%) | ||
| Hydroxychloroquine | 2 (<1%) | 1 (<1%) | 1 (<1%) | 0 | ||
| Antibiotics | 1339 (77%) | 254 (58%) | 965 (82%) | 120 (98%) | ||
| Thymosin | 289 (17%) | 68 (15%) | 202 (17%) | 19 (16%) | ||
| Intravenous immunoglobulin | 345 (20%) | 37 (8%) | 238 (20%) | 70 (57%) | ||
| Length of hospital stay, days | 14·0 (10·0–19·0) | 11·0 (8·0–16·0) | 14·0 (10·0–18·0) | 35·0 (22·0–51·0) | ||
| ICU admission | 76 (4%) | 0 | 32 (3%) | 44 (36%) | ||
| Length of ICU stay, days | 14·0 (6·5–25·5) | NA | 7·0 (2·5–18·0) | 20·0 (10·0–41·5) | ||
| Time from symptom onset to admission, days | 15·0 (11·0–25·0) | 20·5 (12·0–43·0) | 14·0 (10·0–22·0) | 13·0 (11·0–17·0) | ||
| Time from discharge to follow-up, days | 153·0 (146·0–160·0) | 151·0 (140·0–156·0) | 154·0 (150·0–160·0) | 157·0 (135·0–169·0) | ||
| Time from symptom onset to follow-up, days | 186·0 (175·0–199·0) | 187·0 (175·0–198·0) | 184·0 (175·0–196·0) | 205·0 (189·5–217·0) | ||
Data are n (%), n/N (%), or median (IQR). The differing denominators used indicate missing data. HFNC=high-flow nasal cannula for oxygen therapy. NIV=non-invasive ventilation. IMV=invasive mechanical ventilation. NA=not applicable. ECMO=extracorporeal membrane oxygenation. ICU=intensive care unit.
68% of patients (1119 of 1650) reported at least one symptom at follow-up (table 2 ) and a higher percentage was observed in females (appendix pp 10–11). The prevalence of presenting at least one symptom among participants with scale 5–6 was higher than those with scale 3 (OR 2·74 [95% CI 1·42–5·30]). The most common symptoms after discharge were fatigue or muscle weakness (855 [52%] of 1654) and sleep difficulties (437 [26%] of 1655; table 2). The prevalence of an mMRC score greater than or equal to 1 was significantly higher in participants with scale 5–6 than those with scale 3 (OR 2·18 [95% CI 1·30–3·65]; table 2). Full details of the EQ-5D-5L questionnaire are presented in the appendix (pp 12–13). Participants with scale 5–6 had more problems in mobility, pain or discomfort, and anxiety or depression than did those with scale 3 (all p<0·05; table 2). 23% (367 of 1616) of participants reported anxiety or depression at follow-up, which was more common in females (appendix pp 10–11). Compared with participants with scale 3, participants with scale 5–6 presented with shorter walking distance in meters in 6 min (487·5 [IQR 412·0–525·0] vs 490·0 [448·5–535·0]) and a higher proportion of less than the lower limit of the normal range (LLN); however, no significant difference was observed for participants with scale 4. The proportion of patients with a 6-min walking distance less than LLN was 17% (74 of 428) for scale 3, 13% (144 of 1147) for scale 4, and 28% (32 of 114) for scale 5–6 (table 2).
Table 2.
Symptoms, exercise capacity, and health-related quality of life at follow-up according to severity scale
| Total (n=1733) |
Seven-category scale |
OR or β (95% CI) |
|||||
|---|---|---|---|---|---|---|---|
| Scale 3: not requiring supplemental oxygen (n=439) | Scale 4: requiring supplemental oxygen (n=1172) | Scale 5–6: requiring HFNC, NIV or IMV (n=122) | Scale 4 vs 3 | Scale 5 −6 vs 3 | |||
| Symptoms | |||||||
| Any one of the following symptoms | 1119/1650 (68%) | 295/419 (70%) | 722/1111 (65%) | 102/120 (85%) | OR 0·77 (0·59 to 1·00) | OR 2·74 (1·42 to 5·30)* | |
| Fatigue or muscle weakness | 855/1654 (52%) | 218/419 (52%) | 547/1115 (49%) | 90/120 (75%) | OR 0·87 (0·68 to 1·11) | OR 2·75 (1·61 to 4·69)† | |
| Sleep difficulties | 437/1655 (26%) | 115/419 (27%) | 280/1116 (25%) | 42/120 (35%) | OR 0·89 (0·68 to 1·17) | OR 1·70 (1·03 to 2·81)* | |
| Hair loss | 359/1655 (22%) | 93/419 (22%) | 236/1116 (21%) | 30/120 (25%) | OR 1·01 (0·76 to 1·36) | OR 1·46 (0·84 to 2·55) | |
| Smell disorder | 176/1655 (11%) | 45/419 (11%) | 112/1116 (10%) | 19/120 (16%) | OR 0·95 (0·64 to 1·41) | OR 1·33 (0·66 to 2·69) | |
| Palpitations | 154/1655 (9%) | 43/419 (10%) | 96/1116 (9%) | 15/120 (13%) | OR 0·86 (0·57 to 1·31) | OR 1·50 (0·72 to 3·13) | |
| Joint pain | 170/1649 (10%) | 57/420 (14%) | 94/1110 (8%) | 19/119 (16%) | OR 0·53 (0·37 to 0·77)† | OR 0·77 (0·40 to 1·50) | |
| Decreased appetite | 138/1655 (8%) | 42/419 (10%) | 82/1116 (7%) | 14/120 (12%) | OR 0·67 (0·44 to 1·01) | OR 1·15 (0·55 to 2·43) | |
| Taste disorder | 120/1655 (7%) | 33/419 (8%) | 77/1116 (7%) | 10/120 (8%) | OR 0·89 (0·57 to 1·41) | OR 0·98 (0·42 to 2·30) | |
| Dizziness | 101/1655 (6%) | 33/419 (8%) | 61/1116 (5%) | 7/120 (6%) | OR 0·76 (0·48 to 1·21) | OR 1·00 (0·40 to 2·54) | |
| Diarrhoea or vomiting | 80/1652 (5%) | 30/418 (7%) | 45/1114 (4%) | 5/120 (4%) | OR 0·51 (0·30 to 0·85)* | OR 0·27 (0·08 to 0·86)* | |
| Chest pain | 77/1649 (5%) | 20/420 (5%) | 53/1110 (5%) | 4/119 (3%) | OR 0·96 (0·55 to 1·68) | OR 0·67 (0·21 to 2·22) | |
| Sore throat or difficult to swallow | 69/1655 (4%) | 28/419 (7%) | 36/1116 (3%) | 5/120 (4%) | OR 0·50 (0·29 to 0·86)* | OR 0·59 (0·18 to 1·89) | |
| Skin rash | 47/1655 (3%) | 15/419 (4%) | 28/1116 (3%) | 4/120 (3%) | OR 0·58 (0·29 to 1·14) | OR 0·54 (0·14 to 2·13) | |
| Myalgia | 42/1649 (3%) | 13/420 (3%) | 24/1110 (2%) | 5/119 (4%) | OR 0·75 (0·36 to 1·55) | OR 1·95 (0·59 to 6·46) | |
| Headache | 34/1649 (2%) | 9/420 (2%) | 22/1110 (2%) | 3/119 (3%) | OR 0·95 (0·42 to 2·12) | OR 1·65 (0·38 to 7·07) | |
| Low-grade fever | 2/1655 (<1%) | 0 | 2/1116 (<1%) | 0 | NA | NA | |
| mMRC score | |||||||
| 0 | 1195/1614 (74%) | 323/425 (76%) | 801/1078 (74%) | 71/111 (64%) | 1 (ref) | 1 (ref) | |
| ≥1 | 419/1614 (26%) | 102/425 (24%) | 277/1078 (26%) | 40/111 (36%) | OR 1·11 (0·84 to 1·47) | OR 2·18 (1·30 to 3·65)* | |
| EQ-5D-5L questionnaire‡ | |||||||
| Mobility: problems with walking around | 113/1621 (7%) | 25/426 (6%) | 72/1083 (7%) | 16/112 (14%) | OR 1·06 (0·63 to 1·78) | OR 2·46 (1·11 to 5·44)* | |
| Personal care: problems with washing or dishing | 11/1621 (1%) | 0 | 10/1083 (1%) | 1/112 (1%) | NA | NA | |
| Usual activity: problems with usual activity | 25/1610 (2%) | 5/425 (1%) | 15/1075 (1%) | 5/110 (5%) | OR 1·10 (0·35 to 3·50) | OR 3·39 (0·73 to 15·68) | |
| Pain or discomfort | 431/1615 (27%) | 111/422 (26%) | 274/1081 (25%) | 46/112 (41%) | OR 0·87 (0·66 to 1·13) | OR 1·96 (1·20 to 3·20)* | |
| Anxiety or depression | 367/1616 (23%) | 98/425 (23%) | 233/1080 (22%) | 36/111 (32%) | OR 0·88 (0·66 to 1·17) | OR 1·76 (1·05 to 2·96)* | |
| Quality of life§ | 80·0 (70·0 to 90·0) | 80·0 (70·0 to 90·0) | 80·0 (75·0 to 90·0) | 80·0 (70·0 to 87·5) | β 2·68 (−1·55 to 6·90) | β −2·35 (−10·60 to 5·94) | |
| Distance walked in 6 min, m | 495·0 (440·0 to 538·0) | 490·0 (448·5 to 535·0) | 495·0 (443·0 to 540·0) | 487·5 (412·0 to 525·0) | β 1·07 (−6·77 to 8·91) | β −35·30 (−51·00 to −19·60)† | |
| Percentage of predicted value¶ | 87·7 (79·1 to 95·8) | 86·3 (78·3 to 94·6) | 88·4 (80·1 to 96·6) | 81·5 (73·7 to 92·3) | β 0·48 (−1·13 to 2·10) | β −6·74 (−9·98 to −3·51)† | |
| Less than lower limit of the normal range‖ | 250/1689 (15%) | 74/428 (17%) | 144/1147 (13%) | 32/114 (28%) | OR 0·92 (0·65 to 1·31) | OR 4·33 (2·29 to 8·18)† | |
| eGFR<90 mL/min per 1·73 m2 | 487/1393 (35%) | 121/338 (36%) | 326/967 (34%) | 40/88 (45%) | OR 0·86 (0·63 to 1·19) | OR 1·44 (0·76 to 2·70) | |
Data are n/N (%) or median (IQR), unless otherwise specified. The differing denominators used indicate missing data. OR=odds ratio. HFNC=high-flow nasal cannula for oxygen therapy. NIV=non-invasive ventilation. IMV=invasive mechanical ventilation. NA=not applicable. mMRC=modified British Medical Research Council. EQ-5D-5L=EuroQol five-dimension five-level questionnaire. eGFR=estimated glomerular filtration rate.
p<0·05.
p<0·001.
Detailed results of EQ-5D-5L questionnaire are presented in the appendix (pp 12–13).
Quality of life was assessed using the EuroQol Visual Analogue Scale, ranging from 0 (worst imaginable health) to 100 (best imaginable health).
Predicted values were calculated according to the method of Enright and Sherrill.22
The lower limit of the normal range was calculated by subtracting 153 m from the predicted value for males or by subtracting 139 m for females.
390 of the 516 patients ascertained as eligible received lung function tests, chest HRCT, and ultrasonography of lower limb veins and abdomen. A total of 349 participants completed the lung function test, and 41 were unable to complete it due to poor compliance. The proportion of participants with lung diffusion impairment was 22% (18 of 83) for scale 3, 29% (48 of 165) for scale 4, and 56% (48 of 86) for scale 5–6 (table 3 ). A significant difference was observed between scale 3 and scale 5–6, but not between scale 3 and scale 4. In the subgroup analysis by sex, both males and females with scale 5–6, and males with scale 4 had higher prevalence for decreased lung diffusion capacity than did those with scale 3 (all p<0·05) (appendix p 14). Decreased total lung capacity (<80% of predicted values) did not show a significant difference in participants with scale 4 or scale 5–6 compared with those with scale 3 (table 3).
Table 3.
Lung function and chest CT at follow-up according to severity scale
|
Seven-category scale |
OR or β (95% CI) |
||||
|---|---|---|---|---|---|
| Scale 3: not requiring supplemental oxygen | Scale 4: requiring supplemental oxygen | Scale 5–6: requiring HFNC, NIV, or IMV | Scale 4 vs 3 | Scale 5–6 vs 3 | |
| Lung function | |||||
| Number of patients | 89 | 172 | 88 | ||
| FEV1 <80%, % of predicted | 7 (8%) | 4 (2%) | 11 (13%) | OR 0·14 (0·03 to 0·68)* | OR 0·50 (0·09 to 2·93) |
| FVC <80%, % of predicted | 3 (3%) | 1 (1%) | 10 (11%) | OR 0·11 (0·01 to 1·59) | OR 2·09 (0·19 to 23·02) |
| FEV1/FVC <70% | 7 (8%) | 13 (8%) | 2 (2%) | OR 0·91 (0·29 to 2·80) | OR 0·26 (0·03 to 1·93) |
| TLC <80%, % of predicted | 9/83 (11%) | 17/165 (10%) | 30/86 (35%) | OR 0·89 (0·33 to 2·42) | OR 3·00 (0·93 to 9·67) |
| FRC <80%, % of predicted | 5/83 (6%) | 6/165 (4%) | 16/84 (19%) | OR 0·61 (0·17 to 2·16) | OR 3·93 (0·97 to 15·82) |
| RV <80%, % of predicted | 16/83 (19%) | 28/164 (17%) | 43/86 (50%) | OR 0·76 (0·33 to 1·75) | OR 2·75 (1·03 to 7·37)* |
| DLCO <80%, % of predicted† | 18/83 (22%) | 48/165 (29%) | 48/86 (56%) | OR 1·61 (0·80 to 3·25) | OR 4·60 (1·85 to 11·48)* |
| Chest CT | |||||
| Number of patients | 95 | 163 | 95 | ||
| At least one abnormal CT pattern | 49 (52%) | 87/161 (54%) | 50/92 (54%) | OR 0·93 (0·53 to 1·64) | OR 0·81 (0·38 to 1·72) |
| GGO | 39 (41%) | 78/161 (48%) | 41/92 (45%) | OR 1·19 (0·68 to 2·09) | OR 0·93 (0·44 to 1·98) |
| Irregular lines | 10 (11%) | 24/161 (15%) | 22/92 (24%) | OR 1·46 (0·60 to 3·52) | OR 1·89 (0·64 to 5·61) |
| Consolidation | 0 | 4/161 (2%) | 0 | NA | NA |
| Interlobular septal thickening | 1 (1%) | 2/161 (1%) | 0 | NA | NA |
| Subpleural line | 6 (6%) | 5/161 (3%) | 4/92 (4%) | NA | NA |
| Reticular pattern | 0 | 1/161 (1%) | 1/92 (1%) | NA | NA |
| Volume of lung lesions, cm3 | 1·6 (0·6 to 5·6) | 3·3 (0·8 to 12·4) | 29·1 (4·6 to 77·3) | β 7·45 (−12·40 to 27·28) | β 34·37 (7·74 to 61·00)* |
| Volume of consolidation, cm3 | 0·2 (0·1 to 0·4) | 0·3 (0·1 to 1·0) | 1·6 (0·2 to 4·4) | β 0·19 (−1·97 to 2·35) | β 3·05 (0·14 to 5·95)* |
| Volume of GGO, cm3 | 1·4 (0·6 to 4·7) | 2·9 (0·7 to 10·0) | 26·3 (4·3 to 73·3) | β 7·26 (−10·70 to 25·25) | β 31·32 (7·16 to 55·48)* |
| Volume ratio of lung lesion to total lung, % | 0·0 (0·0 to 0·1) | 0·1 (0·0 to 0·3) | 0·7 (0·1 to 2·2) | β −0·06 (−1·36 to 1·24) | β 1·44 (−0·30 to 3·18) |
| Volume ratio of consolidation to total lung, % | 0·0 (0·0 to 0·0) | 0·0 (0·0 to 0·0) | 0·0 (0·0 to 0·1) | NA | NA |
| Volume ratio of GGO to total lung, % | 0·0 (0·0 to 0·1) | 0·1 (0·0 to 0·2) | 0·6 (0·1 to 1·9) | β −0·07 (−1·20 to 1·07) | β 1·23 (−0·29 to 2·76) |
| CT score | 3·0 (2·0 to 5·0) | 4·0 (3·0 to 5·0) | 5·0 (4·0 to 6·0) | β 0·33 (−0·19 to 0·84) | β 1·25 (0·56 to 1·95)‡ |
Data are absolute values, n (%), n/N (%), or median (IQR), unless otherwise specified. OR=odds ratio. HFNC=high-flow nasal cannula for oxygen therapy. NIV=non-invasive ventilation. IMV=invasive mechanical ventilation. FEV1=forced expiratory volume in one second. FVC=forced vital capacity. TLC=total lung capacity. FRC=functional residual capacity. RV=residual volume. DLCO=diffusion capacity for carbon monoxide. GGO=ground glass opacity. NA=not applicable.
p<0·05.
Carbon monoxide diffusion capacity was not corrected for haemoglobin.
p<0·001.
A total of 353 participants completed chest HRCT at follow-up. The median CT scores are 3·0 (IQR 2·0–5·0) for participants at scale 3, 4·0 (3·0–5·0) for participants at scale 4, and 5·0 (4·0–6·0) among participants at scale 5–6, with a significant difference between scale 3 and scale 5–6 (p=0·0005; table 3), which is also observed in the subgroup analysis by sex (appendix pp 16–17). Additionally, males at scale 4 had a significantly higher CT score than did those at scale 3 (p=0·028). Ground glass opacity (GGO) is the most common HRCT pattern at follow-up, followed by irregular lines (table 3). The consolidation in acute phase is nearly full resolution at follow-up (appendix p 18). The detailed comparison of chest CT images during hospital stay and follow-up is shown in the appendix (p 18). Dynamic changes of chest images of a 41-year-old man with SARS-CoV-2 infection who received NIV during his hospital stay are shown in the appendix (pp 21–22). Bilateral consolidation, subpleural line, and GGO before discharge were almost completely absorbed approximately 5 months after discharge.
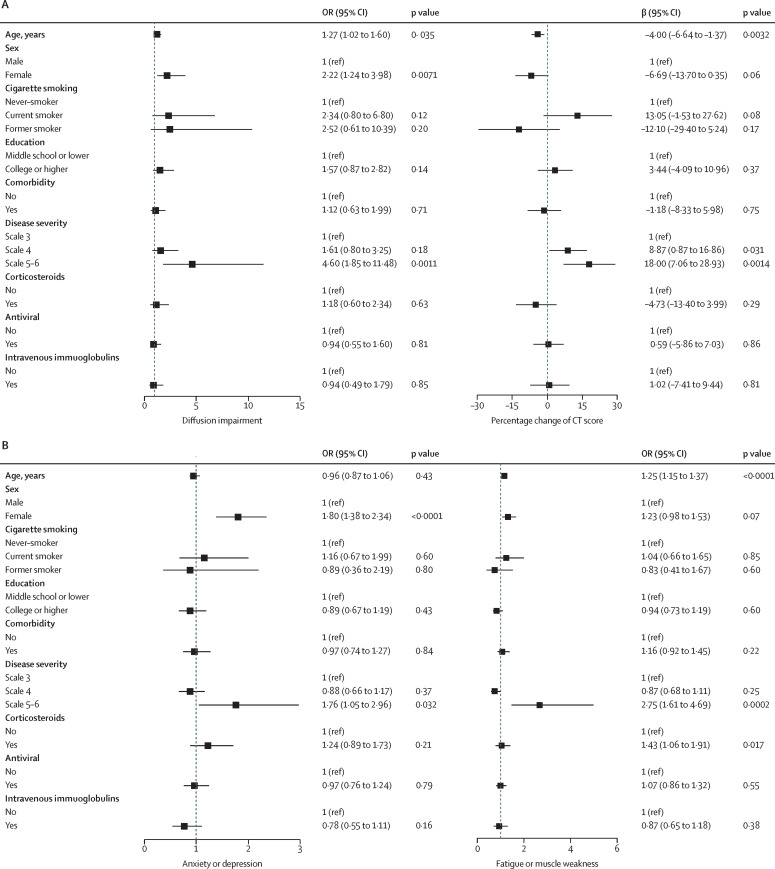
After multivariable adjustment, participants at scale 5–6 showed an OR of 4·60 (95% CI 1·85–11·48) for diffusion impairment, OR 1·76 (1·05–2·96) for anxiety or depression, and OR 2·75 (1·61–4·69) for fatigue or muscle weakness, compared with participants at scale 3 (figure 2 ). Prevalence of diffusion impairment, anxiety or depression, and fatigue or muscle weakness for participants with scale 4 was not significant compared with scale 3. The percentage change of CT score from acute phase to follow-up was higher among participants with scale 4 and 5–6 than in those with scale 3. Females had an OR of 2·22 (95% CI 1·24–3·98) for diffusion impairment and OR 1·80 (1·38–2·34) for anxiety or depression compared with males. Age was positively associated with diffusion impairment and fatigue and muscle weakness, and negatively associated with percentage of CT score changed, with the prevalence of diffusion impairment being 27% higher (OR 1·27, 95% CI 1·02–1·60) and fatigue or muscle weakness being 25% higher (OR 1·25 [1·15–1·37]) per 10-year increase of age, and percentage of CT score changed from acute phase to follow-up 4% lower (1·37–6·64) per 10-year increase of age (figure 2). No significant association of age with anxiety or depression was observed.
Figure 2.
Risk factors associated with diffusion impairment and CT score (A), and anxiety or depression and fatigue or muscle weakness (B)
For associations of age, cigarette smoking, and education with outcome measure, the variables including age, sex, cigarette smoking, education, corticosteroids, antivirals, and intravenous immunoglobulin were all included in the models. For association of comorbidity with outcome, the aforementioned variables were all included together with comorbidity. For association of other factors including sex, corticosteroid, antiviral, and intravenous immunoglobulin with outcome, disease severity and the aforementioned variables were included in the model. OR (95% CI) or β (95% CI) for age indicates the risk of diffusion impairment, CT score, anxiety or depression, and fatigue or muscle weakness per 10-year age increase. OR=odds ratio.
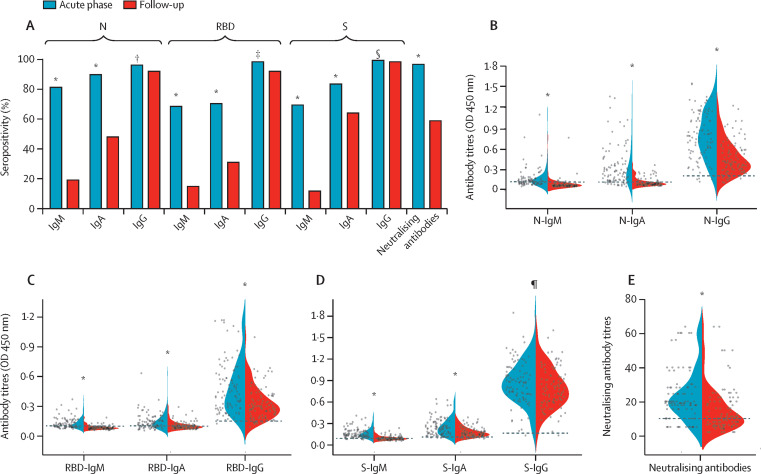
Plasma samples of 94 patients who participated in the LOTUS China trial10 were collected. The seropositivity (96·2% vs 58·5%) and median titres (19·0 vs 10·0) of the neutralising antibodies were significantly lower than at acute phase (figure 3 ). The seropositivity of N-IgM, RBD-IgM, S-IgM, N-IgA, RBD-IgA, S-IgA, and RBD-IgG at follow-up significantly decreased compared with that at acute phase (figure 3A). However, the seropositivity of N-IgG and S-IgG antibodies did not show significant change. More than 90% of participants tested positive for all three IgG antibodies at follow-up. The longitudinal changes in antibody concentrations were further evaluated, with the N-IgM, RBD-IgM, S-IgM, N-IgA, RBD-IgA, S-IgA, N-IgG, RBD-IgG, and S-IgG concentrations, and neutralising antibody titres waning over time (figure 3B–E). However, heterogeneous responses were observed in IgG against N, RBD, and S proteins. Compared with the concentrations at acute phase, antibody concentrations increased by more than 20% in seven (7%) participants for N-IgG, in ten (11%) for RBD-IgG, and in 20 (21%) for S-IgG at follow-up. The concentrations decreased by more than 20% in 76 (81%) participants for N-IgG, in 64 (68%) for RBD-IgG, and in 28 (30%) for S-IgG (appendix p 23).
Figure 3.
Temporal changes of seropositivity and antibody titres against SARS-CoV-2
(A) Seropositivity of each antibody indicated by the y-axis. Violin plots show the distribution of each antibody feature N (B), RBD (C), S (D), and neutralising antibodies (E) split across baseline and follow-up plasma samples of 94 individuals. The horizontal lines are used to indicate the value used to diagnose positivity from the antibody test. The comparison of antibody test results at acute phase and follow-up was done with paired t tests for antibody titres and a McNemar test for antibody positive rates. Plasma samples at acute phase were collected during hospital stay with a median duration of 23 (IQR 20–26) days from illness onset. OD=optical density. p values indicate a comparison between acute phase and follow-up. *p<0·0001. †p=0·29. ‡p=0·039. §p=1·00. ¶p=0·021.
The dynamic changes of white blood cell count, lymphocyte count, and haemoglobin concentrations from symptom onset to follow-up, classified by severity scale, are presented in the appendix (pp 24–25). 488 patients had lymphocytopenia (lymphocyte count <0·8 × 109 per L) during the acute phase. Among those whose lymphocyte counts were available at follow-up, 97% had lymphocyte counts 0·8 × 109 per L or more. 58 patients without self-reported history of diabetes were newly diagnosed with the condition at follow-up. Among 13 of these patients with HbA1c tested during their hospital stay, one patient showed normal HbA1c concentrations at acute phase, but abnormal concentrations at follow-up. Of the 390 participants who received ultrasonography, no deep venous thrombosis of lower limbs was observed. The abdominal ultrasound results in these patients were also normal (appendix p 19).
The distribution of kidney function at acute phase and follow-up is presented in the appendix (p 26). Among participants with eGFR available at follow-up, 35% (487 of 1393) had decreased eGFR (<90 mL/min per 1·73 m2; table 2). 101 (6%) of 1706 patients had acute kidney injury at acute phase. Among participants with eGFR available both at acute phase and follow-up, 479 of 1378 had decreased eGFR at follow-up. Of 1016 participants with non-acute kidney injury and normal eGFR value at acute phase, 822 had eGFR available at follow-up, with 107 (13%) presenting with decreased eGFR (appendix p 26).
Discussion
To our knowledge, this is the largest cohort study with the longest follow-up duration assessing the health consequences of adult patients discharged from hospital recovering from COVID-19. We found that at 6 months after symptom onset, most patients endorsed at least one symptom, particularly fatigue or muscle weakness, sleep difficulties, and anxiety or depression. More severely ill patients had increased prevalence of pulmonary diffusion abnormality, fatigue or muscle weakness, and anxiety or depression. The seropositivity and titres of the neutralising antibodies were significantly lower than at acute phase.
We found that fatigue or muscle weakness, sleep difficulties, and anxiety or depression were common, even at 6 months after symptom onset. This is consistent with data from previous SARS long-term follow-up studies. Canadian researchers found that most SARS survivors had good physical recovery from their illness, but 33% reported a significant decrement in mental health 1 year later.23 A follow-up study of SARS survivors showed that 40% of patients still had a chronic fatigue problem for a mean period of 41·3 months after SARS.24 We found that female sex and severity of illness were risk factors for persistent psychological symptoms. Female SARS survivors had higher stress levels and higher levels of depression and anxiety.25 In a 3-month follow-up survey of 538 patients with COVID-19, Xiong and colleagues8 found that physical decline or fatigue, post-activity polypnoea, and alopecia were more common in females than in males. The underlying mechanism of the psychiatric consequences of COVID-19 is likely to be multifactorial and might include the direct effects of viral infection, the immunological response, corticosteroid therapy, ICU stay, social isolation, and stigma.26
The results of lung function assessment in this study showed that a considerable proportion (22–56% across different severity scales) of participants had a pulmonary diffusion abnormality 6 months after symptom onset. This was consistent with findings that the most common abnormal CT pattern was pulmonary interstitial change (GGOs and irregular lines), which were similar to the long-term lung manifestations of SARS27 or influenza.28 Respiratory viral infection might potentially induce distinct fibroblast activation in the convalescence phase.29 The disease severity in the acute phase was found to be associated with pulmonary diffusion abnormality and percentage change of CT score in the multivariable analysis. Our results did not suggest that corticosteroids could accelerate the recovery of lung injury on pulmonary function assessment and chest imaging, although evidence has shown the benefits of corticosteroid treatment for patients with COVID-19.30, 31 Whether the remaining radiological or pulmonary diffusion abnormalities completely resolve needs to be investigated in further follow-up studies.
In this study, we found that the seropositivity and median titres of the neutralising antibodies were significantly lower than at acute phase. In a report assessing 30 082 patients with mild-to-moderate COVID-19, although antibody titres were stable over a period of 3 months, a modest decline was observed at the 5-month timepoint.32 Among asymptomatic individuals, 81% had a reduction of neutralising antibody concentrations during the early convalescent phase.33 The decline of neutralising antibodies observed in the present study and other studies raises concern for SARS-CoV-2 re-infection. The risk of re-infection should be monitored for patients who present with compatible symptoms of COVID-19.
Our study also investigated long-term extrapulmonary organ manifestations and death during follow-up. For example, persistent renal dysfunctions were observed, some participants were newly diagnosed with diabetes, and venous thromboembolic diseases (including cardiovascular and cerebrovascular events) occurred. Angiotensin-converting enzyme 2—enriched in the renal proximal tubule34, 35—could mediate the entry of SARS-CoV-2 into epithelial cells to accumulate and cause cytotoxicity and inflammatory cell infiltration. A previous study reported that persistent impairment in renal function can occur following an episode of acute kidney injury, with the potential to progress to end-stage kidney disease with dialysis.36 The limitation of serum creatinine to diagnose acute kidney injury has been underscored, which might result in underestimation of patients with acute kidney injury at acute phase.37 For the first time, we showed that 13% of patients without acute kidney injury and with normal eGFR at the acute phase had decreased eGFR at follow-up. The persistent follow-up of discharged patients with COVID-19 is necessary and essential, not only to understand the association between extrapulmonary diseases and SARS-CoV-2 infection, but also to find ways to reduce morbidity and mortality by efficient prevention.
This study has several limitations. Firstly, the baseline data of pulmonary function and 6-min walking distance are unavailable. However, the proportion of patients with chronic pulmonary and heart disease in this cohort is fairly low, although self-reported by patients, which might have resulted in underestimation. The observed impaired pulmonary function and exercise capacity cannot be directly attributed to COVID-19. Furthermore, the proportion of current or former smokers collected at admission might have been underestimated considering the emergency state of hospitals at the early stage of the COVID-19 pandemic. With updated baseline characteristics of study participants according to data collected from subsequent follow-up (appendix p 20), the independent risk factors for diffusion impairment, CT score, anxiety or depression, and fatigue or muscle weakness were not substantially changed (appendix pp 27–28). Secondly, for new symptom onset after COVID-19, the data were not stratified further to determine whether the symptoms were persistent following COVID-19, worsened after COVID-19 recovery, or occurred post-discharge. Thirdly, patients with mild COVID-19 symptoms who had stayed in Fangcang shelter hospitals38 were not enrolled. Further efforts are needed to compare the long-term outcomes between inpatients and outpatients. Finally, the number of participants with SARS-CoV-2 antibody test results both at acute phase and follow-up was limited. In the future, a larger sample is needed to clarify the dynamic changes of antibodies against SARS-CoV-2.
At 6 months after symptom onset, fatigue or muscle weakness and sleep difficulties were the main symptoms of patients who had recovered from COVID-19. Prevalence of anxiety or depression as an important psychological complication and impaired pulmonary diffusion capacities were higher in patients with more severe illness. These results support that those with severe disease need post-discharge care. Longer follow-up studies in a larger population are necessary to understand the full spectrum of health consequences from COVID-19.
Data sharing
As subsequent follow-up investigations for COVID-19 are in progress, data collected for the study, including individual participant data, and a data dictionary defining each field in the set, will not be made available to others. When all follow-up investigations are finished, data might be made available.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This work was supported by the National Natural Science Foundation of China (82041011/H0104); Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS 2018-I2M-1–003 and 2020-I2M-CoV19–005); the National Key Research and Development Program of China (2018YFC1200102); and Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis (2020ZX09201001). This work was also supported by Peking Union Medical College Foundation (the China Evergrande Group, Jack Ma Foundation, Sino Biopharmaceutical, Ping An Insurance [Group], and New Sunshine Charity Foundation). We thank all patients who participated in this study and their families. We also thank all the staff of this follow-up study team (Qiongya Wang, Ying Liu, Wen Liu, and colleagues). We also appreciate the input from Geyi Wen from the China-Japan Friendship Hospital (Beijing, China) to the study design.
Contributors
CW, BC, DZ, JW, YeW, and LR conceived and designed the study and took responsibility for the integrity of the data and the accuracy of the data analysis. BC, YeW, LH, XG, GF, LS, JX, LG, and GW drafted the manuscript. BC, XG, GF, YeW, LH, LS, LR, LG, and GW did the analysis and all authors critically revised the manuscript for important intellectual content and gave final approval for the version to be published. DZ, CH, LK, XL, XZ, YeW, LH, DC, JL, ZH, ST, YZ, LC, DX, YaL, CL, LP, and YoL completed the follow-up work. DZ, CH, LK, XL, XZ, YeW, LH, DC, JL, ZH, ST, YZ, LC, DX, YaL, CL, LP, ML, WX, YiW, and JZ collected the data. BC, JW, DZ, and XG accessed and verified the underlying data. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 2.Cevik M, Kuppalli K, Kindrachuk J, Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ. 2020;371 doi: 10.1136/bmj.m3862. [DOI] [PubMed] [Google Scholar]
- 3.Yelin D, Wirtheim E, Vetter P, et al. Long-term consequences of COVID-19: research needs. Lancet Infect Dis. 2020;20:1115–1117. doi: 10.1016/S1473-3099(20)30701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carfi A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y, Tan C, Wu J, et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21:163. doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu C, Ye L, Xia R, et al. Chest CT and clinical follow-up of discharged patients with COVID-19 in Wenzhou City, Zhejiang, China. Ann Am Thorac Soc. 2020;17:1231–1237. doi: 10.1513/AnnalsATS.202004-324OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao YM, Shang YM, Song WB, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiong Q, Xu M, Li J, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infection. 2021;27:89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.China National Health Commission Chinese clinical guidance for COVID-19 pneumonia diagnosis and treatment. http://kjfy.meetingchina.org/msite/news/show/cn/3337.html
- 10.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie W, Wu Y, Wang W, et al. A longitudinal study of carotid plaque and risk of ischemic cardiovascular disease in the Chinese population. J Am Soc Echocardiogr. 2011;24:729–737. doi: 10.1016/j.echo.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 13.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 15.Ren L, Fan G, Wu W, et al. Antibody responses and clinical outcomes in adults hospitalized with severe COVID-19: a post hoc analysis of LOTUS China trial. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1247. published online Aug 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) Clin Infect Dis. 2020;71:778–785. doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Thoracic Society Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 18.Liu F, Zhang Q, Huang C, et al. CT quantification of pneumonia lesions in early days predicts progression to severe illness in a cohort of COVID-19 patients. Theranostics. 2020;10:5613–5622. doi: 10.7150/thno.45985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francone M, Iafrate F, Masci GM, et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol. 2020;30:6808–6817. doi: 10.1007/s00330-020-07033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 23.Tansey CM, Louie M, Loeb M, et al. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch Intern Med. 2007;167:1312–1320. doi: 10.1001/archinte.167.12.1312. [DOI] [PubMed] [Google Scholar]
- 24.Lam MH, Wing YK, Yu MW, et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 2009;169:2142–2147. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- 25.Lee AM, Wong JG, McAlonan GM, et al. Stress and psychological distress among SARS survivors 1 year after the outbreak. Can J Psychiatry. 2007;52:233–240. doi: 10.1177/070674370705200405. [DOI] [PubMed] [Google Scholar]
- 26.Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie L, Liu Y, Xiao Y, et al. Follow-up study on pulmonary function and lung radiographic changes in rehabilitating severe acute respiratory syndrome patients after discharge. Chest. 2005;127:2119–2124. doi: 10.1378/chest.127.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai L, Gu L, Cao B, et al. Clinical features of pneumonia caused by 2009 influenza A(H1N1) virus in Beijing, China. Chest. 2011;139:1156–1164. doi: 10.1378/chest.10-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyd DF, Allen EK, Randolph AG, et al. Exuberant fibroblast activity compromises lung function via ADAMTS4. Nature. 2020;587:466–471. doi: 10.1038/s41586-020-2877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 34.Du M, Cai G, Chen F, Christiani DC, Zhang Z, Wang M. Multiomics evaluation of gastrointestinal and other clinical characteristics of COVID-19. Gastroenterology. 2020;158:2298. doi: 10.1053/j.gastro.2020.03.045. 301.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forni LG, Darmon M, Ostermann M, et al. Renal recovery after acute kidney injury. Intensive Care Med. 2017;43:855–866. doi: 10.1007/s00134-017-4809-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatraju PK, Wurfel MM, Himmelfarb J. Trajectory of kidney function: the canary in sepsis. Am J Respir Crit Care Med. 2020;202:1211–1212. doi: 10.1164/rccm.202007-2627ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen S, Zhang Z, Yang J, et al. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet. 2020;395:1305–1314. doi: 10.1016/S0140-6736(20)30744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
As subsequent follow-up investigations for COVID-19 are in progress, data collected for the study, including individual participant data, and a data dictionary defining each field in the set, will not be made available to others. When all follow-up investigations are finished, data might be made available.