Abstract
Immune checkpoint inhibitors (ICIs) have revolutionized cancer management and have been widely applied; however, they still have some limitations in terms of efficacy and toxicity. There are multiple treatment regimens in Traditional Chinese Medicine (TCM) that play active roles in combination with Western medicine in the field of oncology treatment. TCM with ICIs works by regulating the tumor microenvironment and modulating gut microbiota. Through multiple targets and multiple means, TCM enhances the efficacy of ICIs, reverses resistance, and effectively prevents and treats ICI-related adverse events based on basic and clinical studies. However, there have been few conclusions on this topic. This review summarizes the development of TCM in cancer treatment, the mechanisms underlying the combination of TCM and ICIs, existing studies, ongoing trials, and prospects for future development.
Keywords: Traditional Chinese Medicine, Immune checkpoint inhibitor, Immunotherapy, Tumor microenvironment
Introduction
Cancer is one of the leading causes of death globally, with almost 10 million cancer-related deaths reported in 2020 [1]. Recently, immunotherapies, represented by immune checkpoint inhibitors (ICIs) have revolutionized cancer management. Immune checkpoints can be expressed by tumor cells to escape immunosurveillance [2, 3]. The most common immune checkpoints are cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and programmed cell death 1 (PD-1) [4]. ICIs are monoclonal antibodies targeting immune checkpoint molecules that can restore the body’s antitumor immune response and promote T cell-mediated clearance of tumor cells by blocking the inhibitory signaling pathways of T cells. ICIs have become the first-line treatment for a variety of solid and liquid tumors. Despite the development of ICIs treatment, numerous problems remain, including uncertain efficacy, immune checkpoint inhibitor-related adverse events (irAEs), and drug resistance.
China is a large country with high cancer burden. The integration of Traditional Chinese Medicine (TCM) and Western medicine is a special therapy that is implemented in more than 70% of cancer patients in China [5]. TCM treatments include the use of Chinese medicine monomers, extracts, traditional compound prescriptions (a prescription consisting of two or more TCM herbs), Chinese medicine patents (a TCM product composed of TCM herbs and processed according to the prescribed prescription and preparation process [6]), acupuncture, exercise (slow, gentle, and symmetrical movements represented by Tai Chi, Baduanjin, Yijinjing, and Wuqinxi [7]), and moxibustion (burning of the herb moxa over acupuncture points [8]) [9]. Previous studies have suggested that TCM can suppress angiogenesis, growth, and metastasis of tumor cells, and prompt their apoptosis [10, 11], which has achieved excellent curative outcomes in the treatment of cancer.
Yin and Yang, a concept derived from ancient Chinese philosophy, describe how opposite or contrary forces may be complementary, interconnected, and interdependent. Yin refers to things, nature, or body functions that are cold, downward, inert, dim, internal, material, declining, and inhibiting [12, 13]. Yang is the opposite of Yin. Hence, the concepts of Yin (negative regulation) and Yang (positive regulation) are often associated with immune function [14] and maintenance of the immune balance [15]. Studies have increasingly demonstrated the use of TCM for combining ICIs through immunomodulation, enhancing the efficacy of ICIs, reducing the incidence of irAEs, and treating irAEs [16, 17]. Nevertheless, the combination of TCM and ICIs has received limited attention, and its mechanism and efficacy remain unclear. Herein, we aimed to provide an overview of the combination of TCM and ICIs including potential mechanisms, current studies, and our perspective on the future.
Development of TCM in oncology treatment
The connotation of the tumor was first discussed in The Yellow Emperor’s Inner Canon, which was a classical work of TCM more than 2000 years ago [18]. This masterpiece has proposed various classifications and the corresponding names of tumors including “Shijia”, “Changqin”, “Jinliu”, “Jiju”, “Yege”, etc. Through long-term clinical practice, TCM has summarized the etiology of tumors as external causes of environmental factors, for instance, environmental pollution, and six pathogenic factors from the environment, namely, wind, cold, summer-heat, dampness, dryness, and fire; internal injuries caused by emotional factors (seven emotions consisting of joy, anger, worry, thought, grief, fear, and surprise), overstraining, and improper diet are also discussed.
TCM has a unique understanding of the pathogenesis and development of oncology, including dysregulation of Yin and Yang, disturbances in circulation of the two basic substances of Qi and Xue, and disorders of Zang and Fu representing the internal organs and their function. Hence, TCM has proposed that the overall treatment principles are strengthening body resistance and eliminating evil, which is regulating Yin and Yang, Qi and Xue, and Zang and Fu.
In the past 70 years, integrated TCM and Western medicine have been the most distinctive methods for the treatment of cancers in China. The main treatment methods include TCM combined with radiotherapy [19], chemotherapy, targeted therapy [20], immunotherapy, and maintenance therapy for end-stage patients. During the different phases of cancer, TCM has shown a significant antitumor effect, reversing drug resistance, alleviating clinical symptoms, decreasing treatment-related adverse events, improving quality of life, and extending overall survival (including precancerous lesions [21], neoadjuvant treatment [22], adjuvant treatment [23], supportive care [24], and prevention of recurrence [25]).
Various TCM methods have been used to develop generalizable treatment regimens. As early as 1997, China’s State Food and Drug Administration had already approved the use of Kanglaite (extracted from the Chinese herb, Coicis semen yokuinin) for hepatic cell carcinoma (HCC) (odds ratio [OR] = 2.57, 95% confidence interval [CI] 2.10–3.16, P < 0.00001) [26] and non-small cell lung cancer (NSCLC) (relative risk [RR] = 1.45, 95% CI 1.31–1.60, P < 0.00001) [27], which would increase the objective response rate (ORR) combined with chemotherapy [28]. Aside from the development of TCM injections and oral Chinese patent medicine [29], new drugs were discovered from TCM, such as Haier granules [30] and Icaritin [31], which were recommended by the Chinese Society of Clinical Oncology clinical guidelines as standard drugs.
Furthermore, basic research has been conducted on the complex and systematic anticancer mechanisms of TCM at the molecular and cellular levels, particularly in immunomodulation [16]. Ginseng [32, 33] and Astragalus [34] have been shown to affect both innate and adaptive immunity. The mechanisms of TCM on DNA methylation, histone modification, and regulation of noncoding RNAs are being explored [35, 36]. Artificial intelligence-assisted TCM promises to become a new growth point [37]. Using digital data, more solid evidence for TCM will be provided.
In recent years, TCM guidelines and international cooperation have been developed in the field of cancer [19]. Increasing international communication and standardizing TCM has contributed to its development. Consequently, TCM deserves more attention in the immuno-oncology era.
Mechanisms of TCM impacts ICI therapy and irAEs
Regulation of tumor microenvironment: stromal cells, immune cells, and hypoxia
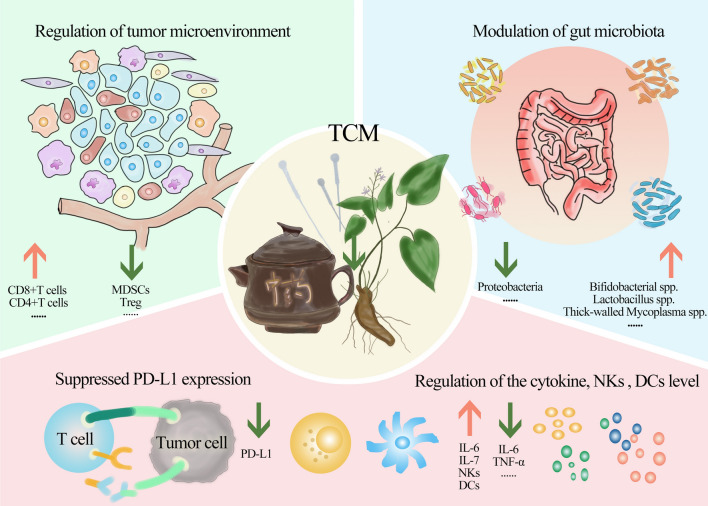
The tumor microenvironment (TME) is the environment where tumors originate and develop. Growing evidence suggests that TCM can influence stromal components, immune cells, and metabolic status to reverse the resistance of ICIs, enhance clinical efficacy, and prevent and reduce the severity of irAEs [38, 39].
TCM can inhibit angiogenesis by regulating vascular endothelial growth factor (VEGF) expression, reducing the activity of angiogenic factor receptors, or inhibiting endothelial cell proliferation [40]. Various herbs, including Astragalus Membranaceus and Curcuma Wenyujin [41] promote vascular normalization in tumor-derived endothelial cells of HCC. These effects prevent abnormal vasculature and high interstitial pressure within the tumor, resulting in a high level of immune cell infiltration and ICIs penetration [42].
TCM also plays a crucial role in regulating immune cell infiltration and activity in the TME, including T cells, natural killer cells (NKs), regulatory cells (Tregs), tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs). The sensitization effect of TCM is enhanced by higher infiltration of CD8 + and CD4 + T cells, while immunity suppressors and TAMs are decreased [43]. TCM balances immune responses and tolerances in terms of treating and preventing irAEs. Despite the lack of a complete understanding of how irAEs function, increasing research suggests that TCM can be used to treat irAEs by enhancing immunosuppressive cells [44, 45]. TCM appears to have a double-edged sword for sensitizing efficacy and for treating irAEs. Consistent with this, TCM exerts its effects both in the treatment of infection [46] and autoimmune diseases [47, 48].
Hypoxia is another characteristic of TME that hinders ICI efficacy [49]. TCM alleviates tumor hypoxia in the TME by suppressing hypoxia-inducible factor 1α (HIF-1α) [50] and lactate [51]. For example, Rhodiola crenulate, a well-known Chinese herb, can resist hypoxia through increased VEGF, poor perfusion, and inhibition of hypoxia-inducible transcription factor signaling [52–54].
Modulation of gut microbiota
First, the gut microbiota influences systemic immune function and antitumor immunity. Several studies have demonstrated that the diversity of gut microbiota affects the response of ICIs [55]. A variety of species, including Bifidobacterium [56], Akkermansia, and Alistipes [57] can boost ICI responses. The gut microbiota also affects the incidence and severity of irAEs [58, 59]. Patients with melanoma who are treated with ICIs and rich in Bacteroides dorei are more likely to develop irAEs [60].
The interaction between the gut microbiota and TCM is a key mechanism for the combination of TCM and ICIs [61]. On the one hand, gut microbiota transforms TCM compounds into active chemicals. On the other hand, TCM regulates the gut microbiota to increase immunity, promote the effects induced by ICIs, balance the immune response, and treat irAEs [62]. Basic studies, such as those on the TCM Shaoyao Ruangan mixture and berberine suggest that TCM leads to an increase in the diversity of gut microbiota, wherein a higher abundance of Bifidobacterial [63], Lactobacillus [64], Firmicutes, and a lower abundance of Proteobacteria [65] is noted, which are associated with good ICI efficacy, fewer irAEs, and milder symptoms.
Other possible mechanisms
PD-L1 is expressed in tumor cells, and the combination of PD-L1 and PD-1 leads to tumor immune escape. As a natural immune checkpoint inhibitor, TCM can suppress PD-L1, which inhibits PD-1/PD-L1 interaction. As a result of the deubiquitinating activity of berberine’s, PD-L1 is ubiquitinated and degraded, inhibiting the PD-1/PD-L1 axis [66]. Bu-Fei Decoction was found to suppress the expression of PD-L1 by Pang et al. [67]. Zhang et al. [68] demonstrated that the TCM compound prescription CFF-1 suppressed PD-L1 expression in prostate cancer cells in a time- and dose-dependent manner. However, high expression of PD-L1 is considered a prerequisite for PD-1/PD-L1 immunotherapy [69], and whether the TCM potentiates ICIs through this mechanism is unclear.
The bidirectional function of cytokines has also been demonstrated during ICI treatment. Cytokines such as interleukin-2 (IL-2) and IL-12 have previously been introduced to increase intertumoral lymphocyte infiltration and anti-tumor immunity [70, 71], but their production in response to ICIs can increase irAEs. Cytokine inhibitors targeting tumor necrosis factor-α (TNF-α), IL-6, and IL-17A have been increasingly used to treat irAEs [72]. Some TCM treatments can enhance cytokine production, whereas others can prevent these cytokines from increasing to treat irAEs [73, 74]. Diammonium glycyrrhizinate [75], extracted and purified from liquorices, improves the production of IL-6 and IL-7, protecting the liver from injury. However, TCM can inhibit cytokine storm including IL-6, and TNF-α [76].
There is also promising evidence that TCM can increase NKs infiltration in lymph nodes and immune organs, enhance dendritic cell (DC) activity, and improve the efficacy of ICIs [77, 78] Fig. 1.
Fig. 1.
Mechanisms of TCM impacts the ICIs therapy and irAEs
TCM enhances the efficacy of ICIs and reverses drug resistance
Clinical and experimental studies have shown that TCM and ICIs are more effective in combination. The synergistic effect of TCM has been demonstrated in mouse models of colon carcinoma [79–87], lung cancer [88, 89], breast cancer [84, 87], melanoma [91–94], ovarian cancer [95], HCC [96], and acute T lymphoblastic leukemia [97] (Table 1).
Table 1.
The basic research on TCM enhances the efficacy of ICIs
| Cancers (cells) | Type | TCM | Formulation | ICIs | Upregulate | Downregulate | References |
|---|---|---|---|---|---|---|---|
| Mechanism: regulation of tumor microenvironment | |||||||
| Colon carcinoma (CT26) | In vivo and in vitro | Andrographis paniculata | Monomer | Anti-PD-1 | CD4 + and CD8 + T cells, IFN-γ, and granzyme B + | COX2 and PGE2 | Liu W et al. [79] |
| Colon carcinoma (MC38) | In vivo and in vitro | Sanguisorbae Radix | Extract | Anti-PD-1 | CD8 + T cells | NA | Lee EJ et al. [86] |
| Colon carcinoma (CMT93 and HCT116) | In vivo and in vitro | Pien-Tze-Huang | Compound prescription | Anti-PD-1 and anti-PD-L1 | CD3 + and CD8 + T cells, IFN-γ, and granzyme B + | p-STAT3, IRF1, and PD-L1 expression | Chen Q et al. [88] |
| Colon carcinoma (CT26) and breast cancer (4T1) | In vivo | Ginseng-derived nanoparticles | Monomer | Anti-PD-1 | CD4 + and CD8 + T cells, CCL5, CXCL9, granzyme B + , IFN-γ, TNF-α, and IL-2 | M2/M1 ratio | Han X et al. [84] |
| Colon carcinoma (MC38) | In vivo | Dahuang Fuzi Baijiang decoction | Compound prescription | Anti-PD-1 | CD8 + T and PD-1int T cells | PD-1hiTim3 + Tex | Xu Y et al. [83] |
| Colon carcinoma (MC38) | In vivo | Atractylenolide I | Monomer | Anti-PD-1 | CD8 + T cells | NA | Xu H et al. [85] |
| Breast cancer (4T1 or MDA-MB-231) | In vivo | Salvianolic acid B | Monomer | Anti-PD-L1 | CD8 + T cells, granzyme B + , IFN-γ | PD-1 expression | Qian C et al. [91] |
| Melanoma (B16F10) and colon carcinoma (MC38) and | In vivo and in vitro | Icaritin | Monomer | Anti-PD-1 and anti-CTLA-4 | CD8 + T cells | PD-L1 expression on MDSCs and neutrophils | Hao H et al. [93] |
| Melanoma (B16-F10) | In vivo and in vitro | Ailanthone | Monomer | Anti-PD-L1 | CD4 + and CD8 + T cells, granzyme B + , IFN-γ, M1 | Tregs, M2, MDSCs, and PD-L1 expression | Yu P et al. [94] |
| Lung cancer (A549 or lewis lung carcinoma) | In vivo and in vitro | Cryptotanshinone | Monomer | Anti-PD-L1 | CD4 + and CD8 + T cells, CXCL9, CXCL11, granzyme B + , IFN-γ, and perforin | DCs (CD11c + /CD45 +) | Liu S et al. [90] |
| Ovarian cancer (JHOC-5), colon cancer (MC38 or CT26) | In vivo and in vitro | Curcumin | Monomer | Anti-PD-1 and anti-PD-L1 | CD8 + T cells, IFN-γ, DCs in the dLNs and spleens | STAT3 and IL-6 | Hayakawa T et al. [95] |
| Hepatocellular carcinoma (Hepa1-6) | In vivo | Bufalin | Monomer | Anti-PD-1 | CD4 + and CD8 + T cells, macrophage, IFN-γ, TNF-α, and IL-10 | M2 phenotype, p50 NF-κB, and TGF-β | Yu Z et al. [96] |
| Mechanism: modulation of gut microbiota | |||||||
| Colon carcinoma (CT26) | In vivo | Gegen Qinlian decoction | Compound prescription | Anti-PD-1 | s__Bacteroides_acidifaciens, s__uncultured_organism_g__norank_f__Bacteroidales_S24-7_group, CD8 + T cells, IL-1, and IFN-γ | CD4 + T cells | Lv J et al. [81] |
| Colon carcinoma (MC38) | In vivo | Jujube powder | TCM | Anti-PD-L1 | Diversity index of gut microbiota, and Lachnospiraceae | Prevotellaceae | Wang L et al. [80] |
| Lung cancer (lewis lung cancer) and melanoma (B16-F10) | In vivo | Ginseng polysaccharides | Monomer | Anti-PD-1 | Diversity index of gut microbiota, and Bacteroides (B. vulgatus and P. distasonis), CD8 + T cells, granzyme B + , IFN-γ, TNF-α, and SCFAs abundance | Treg cells, IDO activity, kynurenine/tryptophan ratio | Huang J et al. [89] |
| Mechanism: others | |||||||
| Melanoma (B16) | In vivo and in vitro | Juzentaihoto | Compound prescription | Anti-PD-1 | IL-12, IFN-γ, and NKs activity | NA | Ishikawa S et al. [93] |
| Colon carcinoma (CT26) | In vivo and in vitro | Jiedu Sangen decoction | Compound prescription | Anti-PD-L1 | E-cadherin | N-cadherin, β-actin, Slug, Snail, Vimentin, PI3K, P-AKT, and AKT | Shan F et al. [82] |
| Melanoma (B16F10) | In vivo | Astragalus membranaceus polysaccharides | Monomer | Anti-PD-L1 | NKs, IFN-γ, CD4 + and CD8 + T cells in mesenteric lymph nodes and lung | B16 cells | Hwang J et al. [92] |
| Acute T lymphoblastic leukemia (Jurkat cells) | In vitro | YIV-906 (inspired by Huang Qin Tang) | Compound prescription | Anti-PD-1 | Nuclear factor of activated T cells activity | NA | Lam W et al. [97] |
TCM traditional Chinese medicine, ICIs immune checkpoint inhibitors, PD-1 programmed death-1, IFN-γ interferon-γ, COX2 cyclooxygenase-2, PGE2 prostaglandin E2, NA not available, PD-L1 programmed cell death-ligand 1, p-STAT3 phosphorylated signal transducer and activator of transcription 3, IRF1 interferon regulatory factor 1,CCL5 chemokine (C-C motif) ligand 5, CXCL9 chemokine (C-X-C motif) ligand 9, CXCL11 chemokine (C-X-C motif) ligand 11, TNF-α tumor necrosis factor-α, IL-2 interleukin-2, M2 macrophages 2, M1 macrophages 1, PD-1int T cells intermediate PD-1 expression, PD-1hiTim3 + Tex T-cell immunoglobulin domain and mucin domain 3 + subset with intermediate expression of PD-1, MDSCs myeloid-derived suppressor cells, Tregs regulatory cells, DCs dendritic cells, dLNs draining lymph nodes, IL-6 interleukin-6, IL-10 interleukin-10, p50 NF-κB nuclear factor kappa-B1, TGF-β tumor necrosis factor-β, IL-1 interleukin-1, SCFAs short chain fatty acids, IDO indole-3-pyruvate, IL-12 interleukin-12, NKs natural killer cells, PI3K phosphoinositide-3 kinase, P-AKT phosphorylated protein kinase B
By modulating the TME, Andrographolide, the main bioactive component of TCM Andrographis paniculate, inhibits tumor growth (tumor weight of 1.57 g from 2.53 g) and induces apoptosis with an increase in infiltration and function of CD4 + and CD8 + T lymphocytes with increased tumor suppression cytokines, including interferon-γ (IFN-γ) (combination group vs. anti-PD-1 group:16.5 ± 1.7% vs. 10 ± 1.6%, P < 0.05), perforin, granzyme B, recombinant factor related apoptosis ligand (FasL), and TNF-α in colon cancer mouse models combined with anti-PD-1 [79]. By enhancing the infiltration of CD8 + T and CD4 + T cells in the TME, Cryptotanshinone accelerates anti-PD-L1 activity in a lung cancer model with high expression of chemokine (C-X-C motif) ligand 9 (CXCL9), CXCL11, and granzyme B + [89]. One Ginseng-derived nanoparticle reprograms TAMs to increase chemokine (C-C motif) ligand 5 (CCL5) and CXCL9 secretion to recruit CD8 + T cells, which synergizes with anti-PD-1 [84]. Sanguisorbae Radix efficiently enhances tumor-infiltrating CD8 + T cell activation by blocking the PD-1/PD-L1 interaction in colorectal cancer (CRC), promoting the efficacy of anti-PD-1 [86]. Salviaric acid B, another active ingredient in salvia, potentiates CD8 + T cell infiltration in the TME along with endothelial protection resulting in the normalization of vascular function and inducing a positive efficacy of anti-PD-L1 in breast carcinoma models [87]. Of note, Ailanthone plays a synergistic effect by reducing the infiltration of immunosuppressive Tregs (combination group vs. vehicle + anti-PD-L1:5.13 vs. 18) in melanoma model [94]. In the presentation of tumor antigens, curcumin administration restored the T cell stimulatory activity of murine DCs in murine tumor models, leading to synergistic antitumor activity with anti-PD-1/PD-L1 [95].
TCM also enhances the effects of ICIs in regulating the gut microbiota. In a lung cancer model, the combination therapy of oral ginseng polysaccharides and anti-PD-1 sensitizes the antitumor effect by increasing Muribaculum abundance in the combination group compared to the anti-PD-1 alone group [88]. Oral jujube powder [80] elevates the alpha diversity index of gut microbiota and the abundance of Lachnospiraceae, leading to promising efficacy of anti-PD-L1 against the colon tumor model. The classic TCM formulation Gegen Qinlian Decoction [81] has been shown to significantly improve antitumor efficacy by continuously enriching Bacteroides acidifaciens and, Peptococcaceae over time.
Based on other possible mechanisms, Hwang et al. [91] found that intranasal treatment with a membranaceus polysaccharide activated DCs in the mesenteric lymph nodes (mLNs), and stimulated NKs and T cells in the mLNs, and enhance anti-PD-L1 activity in B16 melanoma cells. Ishikawa et al. [93] also indicated that the TCM compound prescription Juzentaihoto increases IL-2, IFN-γ, and NKs activity, leading to a promising effect of anti-PD-1.
Studies of patients treated with TCM and ICIs, particularly in English, have reported limited results. In a retrospective study conducted by Tsao et al. [98], neutrophil-to-lymphocyte ratios were decreased by astragalus polysaccharide injections, ICIs, and chemotherapy (treatment group vs. control group: 0.11 vs. 0.52, P = 0.003). The overall survival (OS) was prolonged, but not statistically significant (treatment group vs. control group: 26.1 months vs. 25.4 months, P = 0.76). The ORR for Xiaoyan decoction was higher than that for nivolumab alone in a randomized clinical trial [99] for advanced NSCLC (treatment group vs. control group: 57.14% vs. 28.00%, P < 0.05).
Currently, 15 clinical trials are registered with ClinicalTrials.gov and the Chinese Clinical Trial Registry (ChiCTR) (Table 2). In those clinical trials, TCM compounds (11, 73.3%) were favored over monomers and other treatments. These compounds include Xianglian Wan (ChiCTR1900026300), Yiqijiedu compound (ChiCTR2000036977, ChiCTR2100041920), Bushen Tiaoyuan Recipe (ChiCTR2000032287), Shenlingbaizhu Powder (ChiCTR2200061279), HuGuXiaoJiTang (NCT05378334), Fuzheng Kangai Granule (ChiCTR2200055453), Gegen Qinlian Tablets (ChiCTR2100051747), Fuzi Lizhong Pill (ChiCTR2200058126), and Wenyang Tongluo Recipe (ChiCTR2200055330), which include ancient classic prescriptions as well as self-prescribed medications. Combination regimens typically used anti-PD-1 rather than anti-PD-L1 or anti-CTLA-4 (12, 80.0%). The cancer type with the most ongoing trials was lung cancer (10, 66.7%). Randomized and parallel clinical trials have been widely conducted to test whether TCM can enhance the efficacy of ICIs, which is associated with robust evidence.
Table 2.
Ongoing clinical trials on enhancing the effect of ICIs by TCM
| Year-author | Registry number | Cancer type | TCM | Formulation | Intervention | Controlled | Sample size | Primary outcomes |
|---|---|---|---|---|---|---|---|---|
| RCT | ||||||||
| 2019-Zhong Y | ChiCTR1900026300 | Malignant tumors | Xianglian Wan | Compound prescription | ICIs + Xianglian Wan | ICIs + Xianglian Wan placebo | 44/44 | PFS and intestinal flora |
| 2020-Li X | ChiCTR2000040911 | Lung cancer | Astragalus Polysaccharide | Monomer | Anti-PD-1(Carrelizumab) + apatinib mesylate + Astragalus polysaccharide injection | Anti-PD-1(carrelizumab) + apatinib mesylate | 30/30 | PFS |
| 2020-Yang J | ChiCTR2000036977 | NSCLC | Yiqijiedu compound | Compound prescription | Anti-PD-1(Pembrolizumab) + Yijiedufang Compound | Anti-PD-1(pembrolizumab) | 30/30 | PFS and MST |
| 2021-Yang J | ChiCTR2100041920 | NSCLC | Yiqijiedu compound | Compound prescription | Anti-PD-1 + Yijiedufang Compound | Anti-PD-1 + placebo | 40/40 | PFS and OS |
| 2021-Tian W | ChiCTR2100045870 | HCC | Huaier Granules | Chinese patent medicine | Anti-PD-L1(atelizumab) + bevacizumab + Huaier Granules | Anti-PD-L1(atelizumab) + bevacizumab | 90/90 | Tumor size, AFP, and safety |
| 2021-Yan D | ChiCTR2100051276 | NSCLC | Baicalin combination | Monomer | Anti-PD-1 + baicalin combination | Anti-PD-1 | 76/76 | Tumor size |
| 2022-Wu W | ChiCTR2200055453 | NSCLC | Fuzheng Kangai Granule | Compound prescription | Anti-PD-1 + Fuzheng Kangai Granule | Anti-PD-1 + placebo | 30/30 | PFS |
| 2022-Feng C | ChiCTR2200061279 | NSCLC | Shenlingbaizhu Powder | Compound prescription | Anti-PD-1 + Shenlingbaizhu Powder | Anti-PD-1 | 88/88 | PFS |
| 2022-Zhang H | NCT05378334 | NSCLC | HuGuXiaoJiTang | Compound prescription | Anti-PD-1 + chemotherapy + HGXJT | Anti-PD-1 + chemotherapy | 41/41 | DCR |
| Single-arm study | ||||||||
| 2021-Li P | ChiCTR2100049159 | HCC | TCM | Compound prescription | Anti-PD-1(carrelizumab) + TCM | NA | 50 | PFS |
| 2021-Cui H | ChiCTR2100051747 | LUSC | GegenQinlian Tablets | Compound prescription | Anti-PD-1 + GegenQinlian Tablets | NA | 27 | ORR and DCR |
| 2021-Cao Y | ChiCTR2100046431 | Lung cancer | Gensing Polysaccharides | Compound prescription | ICIs + Gensing Polysaccharides | NA | 45 | ORR and DCR |
| 2022-Wang G | ChiCTR2200058126 | Pancreatic cancer | Fuzi Lizhong Pill | Compound prescription | Anti-PD-1(carrelizumab) + albumin paclitaxel + apatinib ± Fuzi Lizhong Pill | NA | 30 | ORR |
| Observational study | ||||||||
| 2020-Wang L | ChiCTR2000032287 | NSCLC | Bushen Tiaoyuan Recipe | Compound prescription | Anti-PD-1 + chemotherapy + Bushen Tiaoyuan Recipe | Anti-PD-1 + chemotherapy + placebo | 55/55 | ORR, AEs, and cancer fatigue score |
| 2022-Zhang F | ChiCTR2200055330 | Stomach Cancer | Wenyang Tongluo Recipe | Compound prescription | Anti-PD- 1(toripalimab) + Wenyang Tongluo Recipe + apatinib | Anti- PD-1(toripalimab) + apatinib | 30/30 | Tumor size and tumor marker |
TCM traditional Chinese medicine, RCT randomized clinical trial, ICIs immune checkpoint inhibitors, PFS progression-free survival, PD-1 programmed death-1, NSCLC none-small-cell lung cancer, MST median survival time, OS overall survival, HCC hepatocellular carcinoma, PD-L1 programmed cell death-ligand 1, AFP alpha-fetoprotein, DCR disease control rate, NA not available, LUSC lung squamous cell carcinoma, ORR objective response rate, AEs adverse events
TCM in the treatment and prevention of irAEs
The relatively high incidence of irAEs limits the use of ICIs. Effective precautions and treatments may help patients benefit more from ICI therapies. TCM for irAEs has been studied in a few experiments [100]. A practical reason is the lack of a robust preclinical mouse model; even though some models such as the ICI-associated myocarditis mouse model [101], are being developed.
Evidence from clinical studies and case reports suggests that TCM can exert therapeutic effects on irAEs (Table 3). A 56-year-old man with gastric carcinoma developed immune-related cystitis after five cycles of anti-PD-1 plus paclitaxel and tegafur treatment. The patients recovered without recurrence of their lower urinary tract symptoms after taking the TCM formulation Chai-Ling-Tang orally for 2 months [102]. Beyond compound formulation, Li et al. [103] reported that acupuncture treatment significantly relieved the symptoms of limb numbness and fatigue in a 63-year-old patient with immune-related Guillain-Barré syndrome (GBS). As demonstrated in this case, acupuncture effectively treated ICI-induced GBS in the absence of a significant response to intravenous gamma globulin.
Table 3.
Clinical results of TCM in treatment and precaution of irAEs
| Year-Author | Cancer type | TCM | Formulation | Immunotherapy | IrAE | Outcomes |
|---|---|---|---|---|---|---|
| RCT | ||||||
| 2022-Wu L [104] | NSCLC | Qigui Yishen Decoction | Compound prescription | Anti-PD-1 or anti-PD-L1 | Immune checkpoint inhibitor-related AKI | Lower BUN, Scr, and higher eGFR |
| 2022-Min M [105] | Malignant tumour | Heat-sensitive moxibustion | Moxibustion | Anti-PD-1 | Prevention | Lower incidence of immune checkpoint inhibitor-related gastrointestinal toxicity |
| 2021-Zhou Y [106] | Malignant tumour | Yifei Decoction | Yifei Decoction | ICIs | Immune checkpoint inhibitor-related pneumonitis | All grades reduced |
| 2022-Xu Q [107] | Malignant tumour | Topical TCM | Compound prescription | ICIs | Immune checkpoint inhibitor-related skin toxicity | Lower incidence of immune checkpoint inhibitor-related skin toxicity |
| Case report | ||||||
| 2022-Wang Z [102] | gastric carcinoma | Chai-Ling -Tang | Compound prescription | Anti-PD-1(sintilimab) + paclitaxel + tegafur | Immune checkpoint inhibitor-related cystitis | Recovered |
| 2022-Li J [103] | lung cancer | Acupuncture | Acupuncture | Anti-PD-1(tislelizumab) + docetaxel | Guillain–Barre Syndrome | Relieved |
TCM traditional Chinese medicine, irAE immune checkpoint inhibitor-related adverse event, NSCLC none-small-cell lung cancer, PD-1 programmed death-1, PD-L1 programmed cell death-ligand 1, AKI acute kidney injury, BUN blood urea nitrogen, Scr serum creatinine, eGFR estimated glomerular filtration Rate, ICIs immune checkpoint inhibitors
The kidney function of 48 patients with acute kidney injury caused by ICIs was evaluated in a prospective randomized study [104]. Qigui Yishen Decoction significantly reduced blood urea nitrogen (BUN) (treatment group vs. control treatment:4.03 ± 0.82 mmol/L vs. 8.59 ± 1.08 mmol/L, P < 0.01), and serum creatinine (Scr) levels (treatment group vs. control treatment:60.03 ± 7.32 μmol/L vs. 150.59 ± 26.78 μmol/L, P < 0.01), and estimated glomerular filtration rate (eGFR) (treatment group vs. control treatment:60.03 ± 8.32 min/L vs. 47.59 ± 6.78 min/L, P < 0.05) when compared to corticosteroids alone. Meanwhile, the severity of acute kidney injury caused by ICIs has decreased. Similarly, another TCM compound formulation Yifei Decoction [106] in combination with corticosteroids had good efficiency in decreasing the grades of ICI-related pneumonia (combination group vs. corticosteroids group: 1.63 ± 0.74 vs. 1.82 ± 0.78, P < 0.05) and acceptable tolerability, but without statistical differences.
The external application of Chinese medicine seems to have attracted more attention in the field of irAEs (Table 4). Researchers have compared Pi-Yan-Ning, a Chinese patent medicine, to corticosteroids in the treatment of maculopapular rash caused by ICI grade 2–3 (ChiCTR2200059263). Additionally, electroacupuncture is being investigated as an effective treatment for irAE symptoms without affecting OS or progression-free survival (PFS) (ChiCTR2200059759).
Table 4.
Ongoing clinical trials of TCM in treatment and precaution of irAEs
| Year-author | Registry number | Study type | IrAE | TCM | Formulation | Intervention | Controlled | Sample size | Primary outcomes |
|---|---|---|---|---|---|---|---|---|---|
| 2022-Ma X | ChiCTR2200059759 | Cohort study | NA | Electroacupuncture | Acupuncture | Electroacupuncture stimulation of Zusanli acupoint | Without any acupuncture/Sham acupuncture stimulation of Zusanli acupoint | 100/100/100 | OS |
| 2022-Shu Q | ChiCTR2200059263 | RCT | Skin | Pi-Yan-Ning | Compound prescription | Pi-Yan-Ning | Corticosteroide | 25/25 | Median time of disease remission and drug efficacy |
IrAE immune checkpoint inhibitors-related adverse event, TCM traditional Chinses medicine, NA not available, OS overall survival, RCT randomized controlled trial
TCM has also been regarded as having a preventive effect on irAEs in addition to its therapeutic benefit. According to Xu Q [107], Chinese medicine external treatment reduced the incidence of ICI-related cutaneous adverse events (treatment group vs. control group: 29.17% vs. 58.33%, P = 0.042). Heat-sensitization moxibustion as a traditional therapeutic technique [105] was tested in 40 patients who received anti-PD-1 therapy with a lower incidence of ICI-related gastrointestinal toxicity (treatment group vs. control group: 30.0% vs. 65.0%, P = 0.027) and grades (P = 0.007).
Challenges and future perspectives
With the widespread use of ICIs, cancer patients with limited treatment have been living longer. However, the treatment is limited by primary and acquired resistance, unclear efficacy, and unpredictable toxicity. In China, oncology treatment has benefited from the integration of TCM and Western medicine for a long time. Multiple means of treatment can be applied to all stages of the tumor, focusing on different groups of the population based on syndrome differentiation [18]. TCM also helps improve the efficacy of ICIs and the prevention and treatment of irAEs, which is a completely new perspective. Therefore, research on the combination of TCM and ICIs is of practical significance.
The present studies, however, have many shortcomings. First, the bidirectional regulation of TCM combined with ICIs requires further research. Based on the literature review, TCM may have a synergistic effect and prevent irAEs owing to its multiple components, targets, and pathways. Astragalus, for example, can both enhance the efficacy of ICIs [92] and treat ICI-related acute kidney injury (AKI) [104]. Moxibustion improves immune function and lowers the incidence of ICI-related gastrointestinal side effects [105]. Despite this, research on the most obvious and crucial advantages of TCM combined with ICIs is underappreciated.
Second, our findings indicate some degree of incongruence between basic research and clinical trials. Basic research in this field is prone to utilizing TCM monomers rather than traditional compound prescriptions and Chinese medicine patents commonly utilized in clinical practice and clinical trials. In the case of Ginseng, which has been shown to have distinct synergic effects, has not been tested in clinical trials, let alone transformed into a mature medicine. Thus, in our review, we found that basic experiments and clinical studies focused on TCM in different lines, with little overlap. In the future, a stronger research team consisting of TCM physicians, Western medicine physicians, basic researchers, pharmacologists, and clinical trialists could collaborate more closely to improve translational research on TCM combined with ICIs. In addition, series studies are more important than single studies.
Third, it remains to be determined whether the existing standards for evaluating the clinical efficacy of modern medicine are applicable to TCM in combination with ICIs. In addition, we should pursue more diverse types of studies in this field, not just randomized clinical trials (RCTs) and case reports. The existing evaluation, RCT, only focuses on one medicine, and low-certainty case reports of the combination of TCM and ICIs cannot reflect its real-world effectiveness, even though the integration of TCM and Western medicine covers a large number of patients, particularly in China. The existing study modes and evaluation efficacy standards cannot adjust for TCM’s primary features of syndrome differentiation and treatment, individualized treatment, and holistic concepts. TCM combined with ICIs should be evaluated using new standards in future studies. Further clinical evidence, such as real-world studies, may reflect the real clinical condition of TCM in the era of immunotherapy.
Furthermore, there are new issues in immunotherapy that warrant TCM involvement. Examples include using TCM to overcome primary and acquired resistance to ICIs, examining the association between TCM syndromes, treatment regimens, the incidence of irAEs, combining TCM and ICIs for special populations, and artificial intelligence-assisted TCM combined with ICIs.
Acknowledgements
We thank all the frontline doctors, nurses, disease control workers, and researchers who demonstrated selflessness in the face of COVID-19 in China.
Abbreviations
- AKI
Acute kidney injury
- AKT
Protein kinase B
- BUN
Blood urea nitrogen
- CCL5
Chemokine (C-C motif) ligand 5
- CI
Confidence interval
- CTLA-4
Cytotoxic T lymphocyte-associated protein 4
- CRC
Colorectal cancer
- COX2
Cyclooxygenase-2
- CXCL9
Chemokine (C-X-C motif) ligand 9
- DC
Dendritic cell
- dLNs
Draining lymph nodes
- eGFR
Estimated glomerular filtration rate
- FasL
Factor related apoptosis ligand
- GBS
Guillain-Barré syndrome
- HCC
Hepatic cell carcinoma
- HIF-1α
Hypoxia-inducible factor 1α
- IDO
Indole-3-pyruvate
- IL-2
Interleukin-2
- ICIs
Immune checkpoint inhibitors
- IFNγ
Interferon-γ
- IrAEs
Immune checkpoint inhibitor-related adverse events
- IRF1
Interferon regulatory factor 1
- MDSCs
Myeloid-derived suppressor cells
- mLNs
Mesenteric lymph nodes
- M1
Macrophages 1
- M2
Macrophages 2
- NA
Not available
- NKs
Natural killer cells
- NSCLC
None-small-cell lung cancer
- OR
Odds ratio
- ORR
Objective response rate
- OS
Overall survival
- PD-1
Programmed death-1
- PD-1int T cells
Intermediate PD-1 expression
- PD-L1
Programmed cell death-ligand 1
- PFS
Progression-free survival
- PGE2
Prostaglandin E2
- RCT
Randomized controlled trial
- RR
Relative risk
- SCFAs
Short chain fatty acids
- Scr
Serum creatinine
- TAMs
Tumor-associated macrophages
- TCM
Traditional Chinese medicine
- TGF-β
Tumor necrosis factor-β
- TNF-α
Tumor necrosis factor-α
- TME
Tumor microenvironment
- Tregs
Regulatory cells
- VEGF
Vascular endothelial growth factor
Author contributions
YY and JZ designed this manuscript; XZ, ZH, HD, and XL collected the relevant works of previous studies; YY and ZL completed the original draft; SW, JZ, and HC modified the draft critically. All authors read and approved the final manuscript
Funding
This study was supported by National Natural Science Foundation of China (No. 82004151), which was led by SW.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author declares that there are no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jia-bin Zheng, Email: dr_zhengjiabin@foxmail.com.
Hui-juan Cui, Email: cuihj1963@sina.com.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Johnson DB, Nebhan CA, Moslehi JJ, et al. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19(4):254–267. doi: 10.1038/s41571-022-00600-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hegde PS, Karanikas V, Evers S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin Cancer Res. 2016;22(8):1865–1874. doi: 10.1158/1078-0432.CCR-15-1507. [DOI] [PubMed] [Google Scholar]
- 4.Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–249. doi: 10.1146/annurev-pathol-042020-042741. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Lou Y, Wang J, et al. Research status and molecular mechanism of the traditional Chinese medicine and antitumor therapy combined strategy based on tumor microenvironment. Front Immunol. 2021;11:609705. doi: 10.3389/fimmu.2020.609705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia Y, Sun J, Zhao Y, et al. Chinese patent medicine for osteoporosis: a systematic review and meta-analysis. Bioengineered. 2022;13(3):5581–5597. doi: 10.1080/21655979.2022.2038941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li R, Chen H, Feng J, et al. Effectiveness of traditional Chinese exercise for symptoms of knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Int J Environ Res Public Health. 2020;17(21):7873. doi: 10.3390/ijerph17217873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlaeger JM, Stoffel CL, Bussell JL, et al. Moxibustion for cephalic version of breech presentation. J Midwifery Womens Health. 2018;63(3):309–322. doi: 10.1111/jmwh.12752. [DOI] [PubMed] [Google Scholar]
- 9.Hsiao WL, Liu L. The role of traditional Chinese herbal medicines in cancer therapy–from TCM theory to mechanistic insights. Planta Med. 2010;76(11):1118–1131. doi: 10.1055/s-0030-1250186. [DOI] [PubMed] [Google Scholar]
- 10.Jia L, Ma S, Hou X, et al. The synergistic effects of traditional Chinese herbs and radiotherapy for cancer treatment. Oncol Lett. 2013;5(5):1439–1447. doi: 10.3892/ol.2013.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng W, Cheng Z, Xing D, Zhang M. Asparagus polysaccharide suppresses the migration, invasion, and angiogenesis of hepatocellular carcinoma cells partly by targeting the HIF-1α/VEGF signalling pathway in vitro. Evid Based Complement Alternat Med. 2019;2019:3769879. doi: 10.1155/2019/3769879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Yu Y, Cai J, et al. Chinese medicine dictionary. Beijing: People’s Medical Publishing House; 2005. [Google Scholar]
- 13.Wang B, Dong X. Chinese-english bilingual glossary of traditional Chinese medicine. Beijing: Science Press; 1993. [Google Scholar]
- 14.Chen H, Li S, Cho W, et al. The role of acupoint stimulation as an adjunct therapy for lung cancer: a systematic review and meta-analysis. BMC Complement Altern Med. 2013;13:362. doi: 10.1186/1472-6882-13-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao X. Immunology in China: the past, present and future. Nat Immunol. 2008;9(4):339–342. doi: 10.1038/ni0408-339. [DOI] [PubMed] [Google Scholar]
- 16.Zhang N, Xiao X. Integrative medicine in the era of cancer immunotherapy: challenges and opportunities. J Integr Med. 2021;19(4):291–294. doi: 10.1016/j.joim.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Zhang X, Wang Y, et al. Application of immune checkpoint targets in the anti-tumor novel drugs and traditional Chinese medicine development. Acta Pharm Sin B. 2021;11(10):2957–2972. doi: 10.1016/j.apsb.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Wang S, Zhang Y, et al. Traditional Chinese medicine and cancer: history, present situation, and development. Thorac Cancer. 2015;6(5):561–569. doi: 10.1111/1759-7714.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, Kan J, Fan T, et al. Efficacy of the nourishing yin and clearing heat therapy based on traditional Chinese medicine in the prevention and treatment of radiotherapy-induced oral mucositis in nasopharyngeal carcinomas: a systematic review and meta-analysis of thirty randomized controlled trials. Evid Based Complement Alternat Med. 2022;2022:4436361. doi: 10.1155/2022/4436361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Qiu H, Li C, et al. The positive role of traditional Chinese medicine as an adjunctive therapy for cancer. Biosci Trends. 2021;15(5):283–298. doi: 10.5582/bst.2021.01318. [DOI] [PubMed] [Google Scholar]
- 21.Xu W, Li B, Xu M, et al. Traditional Chinese medicine for precancerous lesions of gastric cancer: a review. Biomed Pharmacother. 2022;146:112542. doi: 10.1016/j.biopha.2021.112542. [DOI] [PubMed] [Google Scholar]
- 22.Chan Y, Cheung F, Zhang C, et al. Ancient Chinese Medicine herbal formula Huanglian Jiedu decoction as a neoadjuvant treatment of chemotherapy by improving diarrhea and tumor response. Front Pharmacol. 2020;11:252. doi: 10.3389/fphar.2020.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang H, Li M, Du K, et al. Traditional Chinese medicine for adjuvant treatment of breast cancer: Taohong Siwu decoction. Chin Med. 2021;16(1):129. doi: 10.1186/s13020-021-00539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao X, Bu Y, Jia Q. Traditional Chinese medicine as supportive care for the management of liver cancer: past, present, and future. Genes Dis. 2019;7(3):370–379. doi: 10.1016/j.gendis.2019.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Li JW, Qin YN, et al. Clinical observation on the effect of Chinese medicine-“TCM formula” intervention on recurrence and metastasis of triple negative breast cancer. Complement Ther Med. 2020;52:102456. doi: 10.1016/j.ctim.2020.102456. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Liu X, Ma J, 2019. The clinical efficacy and safety of kanglaite adjuvant therapy in the treatment of advanced hepatocellular carcinoma: a PRISMA-compliant meta-analysis. Biosci Rep. [DOI] [PMC free article] [PubMed]
- 27.Huang X, Wang J, Lin W, et al. Kanglaite injection plus platinum-based chemotherapy for stage III/IV non-small cell lung cancer: a meta-analysis of 27 RCTs. Phytomedicine. 2020;67:153154. doi: 10.1016/j.phymed.2019.153154. [DOI] [PubMed] [Google Scholar]
- 28.Tsao SY. Perspectives of traditional Chinese medicine to patch up immune checkpoint blockers. Explor Target Antitumor Ther. 2022;3(5):676–693. doi: 10.1016/j.phymed.2019.153154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nourmohammadi S, Aung TN, Cui J, et al. Effect of compound kushen injection, a natural compound mixture, and its identified chemical components on migration and invasion of colon, brain, and breast cancer cell lines. Front Oncol. 2019;9:314. doi: 10.3389/fonc.2019.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Q, Shu C, Laurence AD, et al. Effect of Huaier granule on recurrence after curative resection of HCC: a multicentre, randomised clinical trial. Gut. 2018;67(11):2006–2016. doi: 10.1136/gutjnl-2018-315983. [DOI] [PubMed] [Google Scholar]
- 31.Yu Z, Guo J, Hu M, et al. Icaritin exacerbates mitophagy and synergizes with doxorubicin to induce immunogenic cell death in hepatocellular carcinoma. ACS Nano. 2020;14(4):4816–4828. doi: 10.1021/acsnano.0c00708. [DOI] [PubMed] [Google Scholar]
- 32.Wong A, Che C, Leung K. Recent advances in ginseng as cancer therapeutics: a functional and mechanistic overview. Nat Prod Rep. 2015;32(2):256–272. doi: 10.1039/c4np00080c. [DOI] [PubMed] [Google Scholar]
- 33.Yim NH, Kim YS, Chung HS. Inhibition of programmed death receptor-1/programmed death ligand-1 interactions by ginsenoside metabolites. Molecules. 2020;25(9):2068. doi: 10.3390/molecules25092068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taixiang W, Munro AJ, Guanjian L. Chinese medical herbs for chemotherapy side effects in colorectal cancer patients. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD004540.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiang Y, Guo Z, Zhu P, et al. Traditional Chinese medicine as a cancer treatment: modern perspectives of ancient but advanced science. Cancer Med. 2019;8(5):1958–1975. doi: 10.1002/cam4.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao H, Ren S, Yang H, et al. Peppermint essential oil: its phytochemistry, biological activity, pharmacological effect and application. Biomed Pharmacother. 2022;154:113559. doi: 10.1016/j.biopha.2022.113559. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Shi X, Li L, et al. The impact of artificial intelligence on traditional Chinese medicine. Am J Chin Med. 2021;49(6):1297–1314. doi: 10.1016/j.biopha.2022.113559. [DOI] [PubMed] [Google Scholar]
- 38.McKean WB, Moser JC, Rimm D, et al. Biomarkers in precision cancer immunotherapy: promise and challenges. Am Soc Clin Oncol Educ Book. 2020;40:e275–e291. doi: 10.1200/EDBK_280571. [DOI] [PubMed] [Google Scholar]
- 39.Gide TN, Wilmott JS, Scolyer RA, et al. Primary and acquired resistance to immune checkpoint inhibitors in metastatic melanoma. Clin Cancer Res. 2018;24(6):1260–1270. doi: 10.1158/1078-0432.CCR-17-2267. [DOI] [PubMed] [Google Scholar]
- 40.Han S, Li P. Progress of research in antitumor mechanisms with Chinese medicine. Chin J Integr Med. 2009;15(4):316–320. doi: 10.1007/s11655-009-0316-4. [DOI] [PubMed] [Google Scholar]
- 41.Zang W, Bian H, Huang X, et al. Traditional Chinese Medicine (TCM) astragalus membranaceus and curcuma wenyujin promote vascular normalization in tumor-derived endothelial cells of human hepatocellular carcinoma. Anticancer Res. 2019;39(6):2739–2747. doi: 10.2187/anticanres.13400. [DOI] [PubMed] [Google Scholar]
- 42.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 43.Wei J, Liu Z, He J, et al. Traditional Chinese medicine reverses cancer multidrug resistance and its mechanism. Clin Transl Oncol. 2022;24(3):471–482. doi: 10.1007/s12094-021-02716-4. [DOI] [PubMed] [Google Scholar]
- 44.da Rocha ML, Oliveira LE, Patrício Santos CC, et al. Antinociceptive and anti-inflammatory effects of the monoterpene α, β-epoxy-carvone in mice. J Nat Med. 2013;67:743–749. doi: 10.1007/s11418-012-0738-8. [DOI] [PubMed] [Google Scholar]
- 45.Qiao J, Xu LH, He J, et al. Cucurbitacin E exhibits anti-inflammatory effect in RAW 264.7 cells via suppression of NF-κB nuclear translocation. Inflamm Res. 2013;62:461–469. doi: 10.1007/s00011-013-0598-z. [DOI] [PubMed] [Google Scholar]
- 46.Huang K, Zhang P, Zhang Z, et al. Traditional Chinese medicine (TCM) in the treatment of COVID-19 and other viral infections: efficacies and mechanisms. Pharmacol Ther. 2021;225:107843. doi: 10.1016/j.pharmthera.2021.107843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J, Zhu F, Huang W, et al. Therapeutic effect and mechanism of acupuncture in autoimmune diseases. Am J Chin Med. 2022;50(3):639–652. doi: 10.1142/S0192415X22500252. [DOI] [PubMed] [Google Scholar]
- 48.Wu N, Yuan T, Yin Z, et al. Network pharmacology and molecular docking study of the chinese miao medicine Sidaxue in the treatment of rheumatoid arthritis. Drug Des Devel Ther. 2022;16:435–466. doi: 10.2147/DDDT.S330947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scharping NE, Menk AV, Whetstone RD, et al. Efficacy of PD-1 blockade is potentiated by metformin-induced reduction of tumor hypoxia. Cancer Immunol Res. 2017;5(1):9–16. doi: 10.1158/2326-6066.CIR-16-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin J, Qiu S, Wang P, et al. Cardamonin inhibits breast cancer growth by repressing HIF-1α-dependent metabolic reprogramming. J Exp Clin Cancer Res. 2019;38(1):377. doi: 10.1186/s13046-019-1351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li J, Cao GY, Zhang XF, et al. Chinese medicine she-xiang-xin-tong-ning, containing moschus, corydalis and ginseng, protects from myocardial ischemia injury via angiogenesis. Am J Chin Med. 2020;48(1):107–126. doi: 10.1142/S0192415X20500068. [DOI] [PubMed] [Google Scholar]
- 52.Lee SY, Li MH, Shi LS, et al. Rhodiola crenulata extract alleviates hypoxic pulmonary edema in rats. Evid Based Complement Alternat Med. 2013;2013:718739. doi: 10.1155/2013/718739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang YN, Liu ZZ, Feng ZM, et al. Lignans from the root of rhodiola crenulata. J Agric Food Chem. 2012;60(4):964–972. doi: 10.1021/jf204660c. [DOI] [PubMed] [Google Scholar]
- 54.Fan F, Xu N, Sun Y, et al. Uncovering the metabolic mechanism of salidroside alleviating microglial hypoxia inflammation based on microfluidic chip-mass spectrometry. J Proteome Res. 2022;21(4):921–929. doi: 10.1021/acs.jproteome.1c00647. [DOI] [PubMed] [Google Scholar]
- 55.Li W, Deng Y, Chu Q, Zhang P. Gut microbiome and cancer immunotherapy. Cancer Lett. 2019;447:41–47. doi: 10.1016/j.canlet.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 56.Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 58.Gori S, Inno A, Belluomini L, et al. Gut microbiota and cancer: How gut microbiota modulates activity, efficacy and toxicity of antitumoral therapy. Crit Rev Oncol Hematol. 2019;143:139–147. doi: 10.1016/j.critrevonc.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 59.Tan B, Chen MJ, Guo Q, et al. Clinical-radiological characteristics and intestinal microbiota in patients with pancreatic immune-related adverse events. Thorac Cancer. 2021;12(12):1814–1823. doi: 10.1111/1759-7714.13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Usyk M, Pandey A, Hayes RB, et al. Bacteroides vulgatus and Bacteroides dorei predict immune-related adverse events in immune checkpoint blockade treatment of metastatic melanoma. Genome Med. 2021;13(1):160. doi: 10.1186/s13073-021-00974-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen Y, Yuan M, Chen Y, et al. The gut microbiota and traditional chinese medicine: a new clinical frontier on cancer. Curr Drug Targets. 2021;22(11):1222–1231. doi: 10.2174/1389450122666210412141304. [DOI] [PubMed] [Google Scholar]
- 62.Li X, Wu D, Niu J, et al. Intestinal flora: a pivotal role in investigation of traditional Chinese medicine. Am J Chin Med. 2021;49(2):237–268. doi: 10.1142/S0192415X21500130. [DOI] [PubMed] [Google Scholar]
- 63.Zhen H, Qian X, Fu X, et al. Regulation of Shaoyao Ruangan mixture on intestinal flora in mice with primary liver cancer. Integr Cancer Ther. 2019;18:1534735419843178. doi: 10.1177/1534735419843178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lyu M, Wang YF, Fan GW, et al. Balancing herbal medicine and functional food for prevention and treatment of cardiometabolic diseases through modulating gut microbiota. Front Microbiol. 2017;8:2146. doi: 10.3389/fmicb.2017.02146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen H, Zhang F, Li R, et al. Berberine regulates fecal metabolites to ameliorate 5-fluorouracil induced intestinal mucositis through modulating gut microbiota. Biomed Pharmacother. 2020;124:109829. doi: 10.1016/j.biopha.2020.109829. [DOI] [PubMed] [Google Scholar]
- 66.Liu Y, Liu X, Zhang N, et al. Berberine diminishes cancer cell PD-L1 expression and facilitates antitumor immunity via inhibiting the deubiquitination activity of CSN5. Acta Pharm Sin B. 2020;10(12):2299–2312. doi: 10.1016/j.apsb.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pang L, Han S, Jiao Y, et al. Bu Fei decoction attenuates the tumor associated macrophage stimulated proliferation, migration, invasion and immunosuppression of non-small cell lung cancer, partially via IL-10 and PD-L1 regulation. Int J Oncol. 2017;51:25–38. doi: 10.3892/ijo.2017.4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y, Wei Y, Jiang S, et al. Traditional Chinese medicine CFF-1 exerts a potent anti-tumor immunity to hinder tumor growth and metastasis in prostate cancer through EGFR/JAK1/STAT3 pathway to inhibit PD-1/PD-L1 checkpoint signaling. Phytomedicine. 2022;99:153939. doi: 10.1016/j.phymed.2022.153939. [DOI] [PubMed] [Google Scholar]
- 69.Liang Y, Yu M, Zhou C, et al. Variation of PD-L1 expression in locally advanced cervical cancer following neoadjuvant chemotherapy. Diagn Pathol. 2020;15(1):67. doi: 10.1186/s13000-020-00977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Panelli MC. White R, Foster M, et al. Forecasting the cytokine storm following systemic interleukin (IL)-2 administration. J Transl Med. 2004;2(1):17. doi: 10.1186/1479-5876-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sangro B, Mazzolini G, Ruiz J, et al. Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors. J Clin Oncol. 2004;22(8):1389–1397. doi: 10.1200/JCO.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 72.Kang JH, Bluestone JA, Young A. Predicting and preventing immune checkpoint inhibitor toxicity: targeting cytokines. Trends Immunol. 2021;42(4):293–311. doi: 10.1016/j.it.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y, Wang X, Li Y, et al. Xuanfei baidu decoction reduces acute lung injury by regulating infiltration of neutrophils and macrophages via PD-1/IL17A pathway. Pharmacol Res. 2022;176:106083. doi: 10.1016/j.phrs.2022.106083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Piao S, Chen C, Wang X, et al. Lycium barbarum polysaccharides enhance T-Cell function by inhibiting programmed death-ligand 1 via phosphoinositide-3-kinase/protein kinase B suppression in bladder cancer cells. Curr Top Nutraceutical Res. 2020;19(3):295–302. doi: 10.3729/ctnr2641-452X.19,295-302. [DOI] [Google Scholar]
- 75.Feng C, Wang H, Yao C, et al. Diammonium glycyrrhizinate, a component of traditional Chinese medicine Gan-Cao, prevents murine T-cell-mediated fulminant hepatitis in IL-10- and IL-6-dependent manners. Int Immunopharmacol. 2007;7(10):1292–1298. doi: 10.1016/j.intimp.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 76.Dai Y, Qiang W, Gui Y, et al. A large-scale transcriptional study reveals inhibition of COVID-19 related cytokine storm by traditional Chinese medicines. Sci Bull. 2021;66(9):884–888. doi: 10.1016/j.scib.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang H, Fang J, Fan X, et al. Advances in molecular mechanisms for traditional Chinese medicine actions in regulating tumor immune responses. Front Pharmacol. 2020;11:1009. doi: 10.3389/fphar.2020.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu X, Yang H, Chen X, et al. Nano-herb medicine and PDT induced synergistic immunotherapy for colon cancer treatment. Biomaterials. 2021;269:120654. doi: 10.1016/j.biomaterials.2021.120654. [DOI] [PubMed] [Google Scholar]
- 79.Liu W, Fan T, Li M, et al. Andrographolide potentiates PD-1 blockade immunotherapy by inhibiting COX2-mediated PGE2 release. Int Immunopharmacol. 2020;81:106206. doi: 10.1016/j.intimp.2020.106206. [DOI] [PubMed] [Google Scholar]
- 80.Wang L, Jing N, Liu X, et al. Nurturing and modulating gut microbiota with jujube powder to enhance anti-PD-L1 efficiency against murine colon cancer. J Funct Foods. 2019;64:103647. doi: 10.1016/j.jff.2019.103647. [DOI] [Google Scholar]
- 81.Lv J, Jia Y, Li J, et al. Gegen Qinlian decoction enhances the effect of PD-1 blockade in colorectal cancer with microsatellite stability by remodelling the gut microbiota and the tumour microenvironment. Cell Death Dis. 2019;10(6):415. doi: 10.1038/s41419-019-1638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shan F, Sun L, Zhang L, et al. Inhibition to epithelial-mesenchymal transition and metastatic potential in colorectal cancer cell by combination of traditional Chinese medicine formulation Jiedu Sangen decoction and PD-L1 inhibitor. Integr Cancer Ther. 2020;19:1534735420972486. doi: 10.1177/1534735420972486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu Y, Wang H, Wang T, et al. Dahuang Fuzi Baijiang decoction restricts progenitor to terminally exhausted T cell differentiation in colorectal cancer. Cancer Sci. 2022;113(5):1739–1751. doi: 10.1111/cas.15311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Han X, Wei Q, Lv Y, et al. Ginseng-derived nanoparticles potentiate immune checkpoint antibody efficacy by reprogramming the cold tumor microenvironment. Mol Ther. 2022;30(1):327–340. doi: 10.1016/j.ymthe.2021.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu H, Van der Jeught K, Zhou Z, et al. Atractylenolide I enhances responsiveness to immune checkpoint blockade therapy by activating tumor antigen presentation. J Clin Invest. 2021;131(10):e146832. doi: 10.1016/j.ymthe.2021.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee EJ, Kim JH, Kim TI, et al. Sanguisorbae radix suppresses colorectal tumor growth through PD-1/PD-L1 blockade and synergistic effect with pembrolizumab in a humanized PD-L1-expressing colorectal cancer mouse model. Front Immunol. 2021;12:737076. doi: 10.3389/fimmu.2021.737076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen Q, Hong Y, Weng S, et al. Traditional Chinese medicine Pien-Tze-Huang inhibits colorectal cancer growth and immune evasion by reducing β-catenin transcriptional activity and PD-L1 expression. Front Pharmacol. 2022;13:828440. doi: 10.3389/fphar.2022.828440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang J, Liu D, Wang Y, et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut. 2022;71(4):734–745. doi: 10.1136/gutjnl-2020-321031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu S, Han Z, Trivett AL, et al. Cryptotanshinone has curative dual anti-proliferative and immunotherapeutic effects on mouse Lewis lung carcinoma. Cancer Immunol Immunother. 2019;68(7):1059–1071. doi: 10.1007/s00262-019-02326-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qian C, Yang C, Tang Y, et al. Pharmacological manipulation of Ezh2 with salvianolic acid B results in tumor vascular normalization and synergizes with cisplatin and T cell-mediated immunotherapy. Pharmacol Res. 2022;182:106333. doi: 10.1016/j.phrs.2022.106333. [DOI] [PubMed] [Google Scholar]
- 91.Hwang J, Zhang W, Dhananjay Y, et al. Astragalus membranaceus polysaccharides potentiate the growth-inhibitory activity of immune checkpoint inhibitors against pulmonary metastatic melanoma in mice. Int J Biol Macromol. 2021;182:1292–1300. doi: 10.1016/j.ijbiomac.2021.05.073. [DOI] [PubMed] [Google Scholar]
- 92.Hao H, Zhang Q, Zhu H, et al. Icaritin promotes tumor T-cell infiltration and induces antitumor immunity in mice. Eur J Immunol. 2019;49(12):2235–2244. doi: 10.1002/eji.201948225. [DOI] [PubMed] [Google Scholar]
- 93.Ishikawa S, Ishikawa T, Tezuka C, et al. Efficacy of Juzentaihoto for tumor immunotherapy in B16 melanoma metastasis model. Evid Based Complement Alternat Med. 2017;2017:6054706. doi: 10.1155/2017/6054706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu P, Wei H, Li K, et al. The traditional chinese medicine monomer Ailanthone improves the therapeutic efficacy of anti-PD-L1 in melanoma cells by targeting c-Jun. J Exp Clin Cancer Res. 2022;41(1):346. doi: 10.1186/s13046-022-02559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hayakawa T, Yaguchi T, Kawakami Y. Enhanced anti-tumor effects of the PD-1 blockade combined with a highly absorptive form of curcumin targeting STAT3. Cancer Sci. 2020;111(12):4326–4335. doi: 10.1111/cas.14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu Z, Li Y, Li Y, et al. Bufalin stimulates antitumor immune response by driving tumor-infiltrating macrophage toward M1 phenotype in hepatocellular carcinoma. J Immunother Cancer. 2022;10(5):e004297. doi: 10.1136/jitc-2021-004297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lam W, Hu R, Liu SH, et al. YIV-906 enhances nuclear factor of activated T-cells (NFAT) activity of T cells and promotes immune checkpoint blockade antibody action and CAR T-cell activity. Front Pharmacol. 2023;13:1095186. doi: 10.3389/fphar.2022.1095186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsao SM, Wu TC, Chen J, et al. Astragalus polysaccharide injection (PG2) normalizes the neutrophil-to-lymphocyte ratio in patients with advanced lung cancer receiving immunotherapy. Integr Cancer Ther. 2021;20:1534735421995256. doi: 10.1177/1534735421995256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu F, Ma L, Song R, et al. Effect of Xiaoyan Decoction combined with PD-1 mab in neoadjuvant therapy of advanced NSCLC. Chin J Lung Dis. 2022;15(01):79–81. doi: 10.3877/cma.j.issn.1674-6902.2022.01.022. [DOI] [Google Scholar]
- 100.Morad G, Helmink BA, Sharma P, et al. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021;184(21):5309–5337. doi: 10.1016/j.cell.2021.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wei SC, Meijers WC, Axelrod ML, et al. A Genetic mouse model recapitulates immune checkpoint inhibitor-associated myocarditis and supports a mechanism-based therapeutic intervention. Cancer Discov. 2021;11(3):614–625. doi: 10.1158/2159-8290.CD-20-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Z, Zhu L, Huang Y, et al. Successful treatment of immune-related cystitis by Chai-Ling-Tang (Sairei-To) in a gastric carcinoma patient: case report and literature review. Explore. 2022;S1550–8307(22):00042–48. doi: 10.1016/j.explore.2022.04.002. [DOI] [PubMed] [Google Scholar]
- 103.Li J, Xu D, Liu Y, et al. Acupuncture treatment of Guillain-Barré syndrome after using immune checkpoint inhibitors: a case report. Front Neurol. 2022;13:908282. doi: 10.3389/fneur.2022.908282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu L, Mi C, Xu Z, et al. Clinical study of Qigui Yishen decoction in treating acute kidney injury of tumor patients after application of PD-1/PD-L1 inhibitor. China Mod Dr. 2021;59(23):124–128. [Google Scholar]
- 105.Min M, Wu K, Zhu W. Application effect of heat-sensitive moxibustion of PD-1 inhibitor-related gastrointestinal toxicity on malignancy tumor patients. Nurs Pract Res. 2022;19(11):1704–1708. [Google Scholar]
- 106.Zhou Y, Wei Y, Chen J, et al. Clinical observation on Yifei decoction in treating immune checkpoint inhibitor associated pneumonia. Zhejiang J Tradit Chin Med. 2021;56(08):561–563. doi: 10.3969/j.issn.0411-8421.2021.08.007. [DOI] [Google Scholar]
- 107.Xu Q. Observation on the efficacy pf traditional Chinese medicine in preventing and treating immune-related adverse reactions. China Pract Med. 2022;17(05):177–179. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Liu J, Liu X, Ma J, 2019. The clinical efficacy and safety of kanglaite adjuvant therapy in the treatment of advanced hepatocellular carcinoma: a PRISMA-compliant meta-analysis. Biosci Rep. [DOI] [PMC free article] [PubMed]
Data Availability Statement
Not applicable.