Abstract
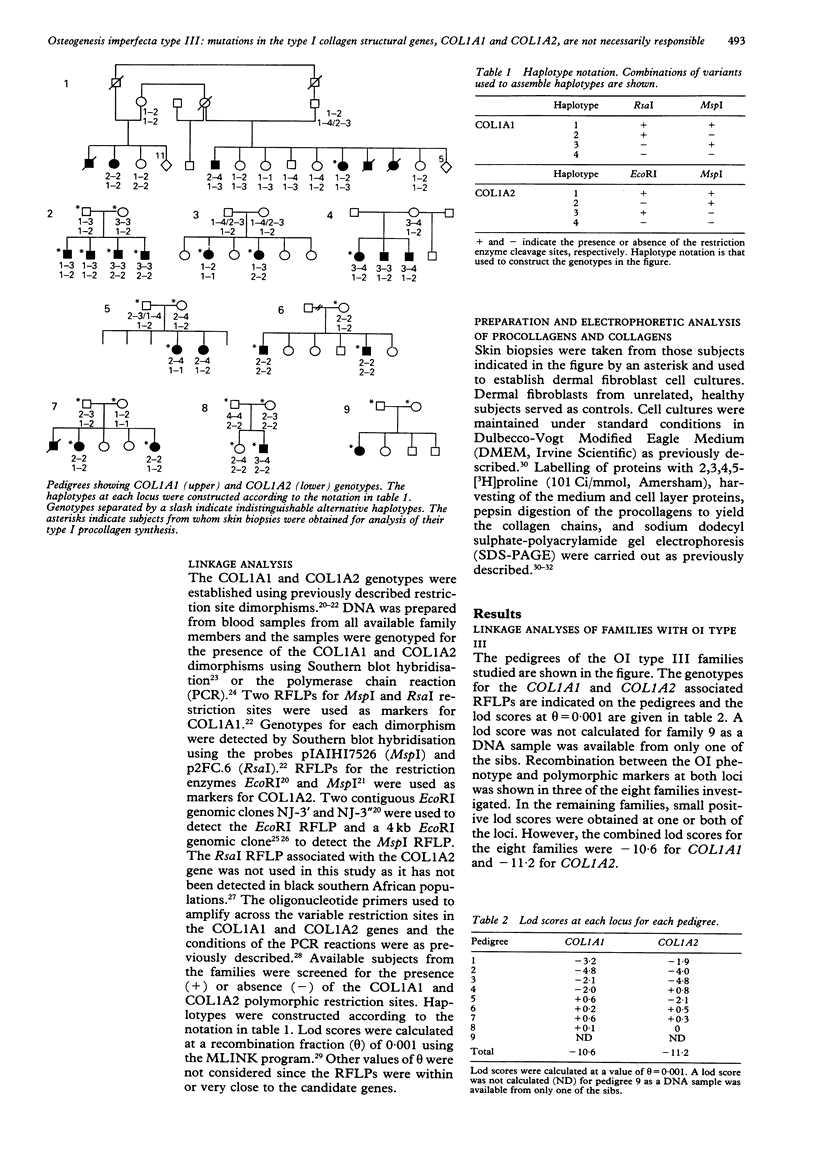
Most forms of osteogenesis imperfecta are caused by dominant mutations in either of the two genes, COL1A1 and COL1A2, that encode the pro alpha 1(I) and pro alpha 2(I) chains of type I collagen, respectively. However, a severe, autosomal recessive form of OI type III with a comparatively high frequency has been recognised in the black populations of southern Africa. We preformed linkage analyses in eight OI type III families using RFLPs associated with the COL1A1 and COL1A2 loci to determine whether mutations in the genes for type I collagen were responsible for this form of OI. Recombination between the OI phenotype and polymorphic markers at both loci was shown in three of the eight families investigated. The combined lod scores for the eight families were -10.6 for COL1A1 and -11.2 for COL1A2. Further, we examined the type I procollagen produced by skin fibroblast cultures derived from 15 affected and 12 unaffected subjects from the above eight families plus one further family. We found no evidence for defects in the synthesis, structure, secretion, or post-translational modification of the chains of type I procollagen produced by any of the family members. These results suggest that mutations within or near the type I collagen structural genes are not responsible for this form of OI.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitchison K., Ogilvie D., Honeyman M., Thompson E., Sykes B. Homozygous osteogenesis imperfecta unlinked to collagen I genes. Hum Genet. 1988 Mar;78(3):233–236. doi: 10.1007/BF00291667. [DOI] [PubMed] [Google Scholar]
- Baker R., Lynch J., Ferguson L., Priestley L., Sykes B. PCR detection of five restriction site dimorphisms at the type I collagen loci COL1A1 and COL1A2. Nucleic Acids Res. 1991 Aug 11;19(15):4315–4315. doi: 10.1093/nar/19.15.4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsh G. S., Byers P. H. Reduced secretion of structurally abnormal type I procollagen in a form of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5142–5146. doi: 10.1073/pnas.78.8.5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J. F., Chan D., Walker I. D., Rogers J. G., Cole W. G. Lethal perinatal osteogenesis imperfecta due to the substitution of arginine for glycine at residue 391 of the alpha 1(I) chain of type I collagen. J Biol Chem. 1987 May 25;262(15):7021–7027. [PubMed] [Google Scholar]
- Beighton P., Versfeld G. A. On the paradoxically high relative prevalence of osteogenesis imperfecta type III in the black population of South Africa. Clin Genet. 1985 Apr;27(4):398–401. doi: 10.1111/j.1399-0004.1985.tb02282.x. [DOI] [PubMed] [Google Scholar]
- Bonadio J., Holbrook K. A., Gelinas R. E., Jacob J., Byers P. H. Altered triple helical structure of type I procollagen in lethal perinatal osteogenesis imperfecta. J Biol Chem. 1985 Feb 10;260(3):1734–1742. [PubMed] [Google Scholar]
- Byers P. H., Tsipouras P., Bonadio J. F., Starman B. J., Schwartz R. C. Perinatal lethal osteogenesis imperfecta (OI type II): a biochemically heterogeneous disorder usually due to new mutations in the genes for type I collagen. Am J Hum Genet. 1988 Feb;42(2):237–248. [PMC free article] [PubMed] [Google Scholar]
- Byers P. H., Wallis G. A., Willing M. C. Osteogenesis imperfecta: translation of mutation to phenotype. J Med Genet. 1991 Jul;28(7):433–442. doi: 10.1136/jmg.28.7.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Solal L., Bonaventure J., Maroteaux P. Dominant mutations in familial lethal and severe osteogenesis imperfecta. Hum Genet. 1991 Jul;87(3):297–301. doi: 10.1007/BF00200907. [DOI] [PubMed] [Google Scholar]
- Cohn D. H., Byers P. H. Clinical screening for collagen defects in connective tissue diseases. Clin Perinatol. 1990 Dec;17(4):793–809. [PubMed] [Google Scholar]
- Cohn D. H., Starman B. J., Blumberg B., Byers P. H. Recurrence of lethal osteogenesis imperfecta due to parental mosaicism for a dominant mutation in a human type I collagen gene (COL1A1). Am J Hum Genet. 1990 Mar;46(3):591–601. [PMC free article] [PubMed] [Google Scholar]
- Dalgleish R., Trapnell B. C., Crystal R. G., Tolstoshev P. Copy number of a human type I alpha 2 collagen gene. J Biol Chem. 1982 Nov 25;257(22):13816–13822. [PubMed] [Google Scholar]
- Deak S. B., Nicholls A., Pope F. M., Prockop D. J. The molecular defect in a nonlethal variant of osteogenesis imperfecta. Synthesis of pro-alpha 2(I) chains which are not incorporated into trimers of type I procollagen. J Biol Chem. 1983 Dec 25;258(24):15192–15197. [PubMed] [Google Scholar]
- Grobler-Rabie A. F., Brebner D. K., Vandenplas S., Wallis G., Dalgleish R., Kaufman R. E., Bester A. J., Mathew C. G., Boyd C. D. Polymorphism of DNA sequence in the human pro alpha 2(I) collagen gene. J Med Genet. 1985 Jun;22(3):182–186. doi: 10.1136/jmg.22.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobler-Rabie A. F., Wallis G., Brebner D. K., Beighton P., Bester A. J., Mathew C. G. Detection of a high frequency RsaI polymorphism in the human pro alpha 2(I) collagen gene which is linked to an autosomal dominant form of osteogenesis imperfecta. EMBO J. 1985 Jul;4(7):1745–1748. doi: 10.1002/j.1460-2075.1985.tb03845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan F., Beighton P. Autosomal recessive inheritance of osteogenesis imperfecta. Clin Genet. 1975 Aug;8(2):107–111. doi: 10.1111/j.1399-0004.1975.tb04398.x. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M. Easy calculations of lod scores and genetic risks on small computers. Am J Hum Genet. 1984 Mar;36(2):460–465. [PMC free article] [PubMed] [Google Scholar]
- Nicholls A. C., Osse G., Schloon H. G., Lenard H. G., Deak S., Myers J. C., Prockop D. J., Weigel W. R., Fryer P., Pope F. M. The clinical features of homozygous alpha 2(I) collagen deficient osteogenesis imperfecta. J Med Genet. 1984 Aug;21(4):257–262. doi: 10.1136/jmg.21.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack M., Constantinou C. D., Kalia K., Nielsen K. B., Prockop D. J. Substitution of serine for alpha 1(I)-glycine 844 in a severe variant of osteogenesis imperfecta minimally destabilizes the triple helix of type I procollagen. The effects of glycine substitutions on thermal stability are either position of amino acid specific. J Biol Chem. 1989 Nov 25;264(33):19694–19699. [PubMed] [Google Scholar]
- Pruchno C. J., Cohn D. H., Wallis G. A., Willing M. C., Starman B. J., Zhang X. M., Byers P. H. Osteogenesis imperfecta due to recurrent point mutations at CpG dinucleotides in the COL1A1 gene of type I collagen. Hum Genet. 1991 May;87(1):33–40. doi: 10.1007/BF01213088. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sandberg M. M. Matrix in cartilage and bone development: current views on the function and regulation of major organic components. Ann Med. 1991 Aug;23(3):207–217. doi: 10.3109/07853899109148050. [DOI] [PubMed] [Google Scholar]
- Sillence D. O., Barlow K. K., Cole W. G., Dietrich S., Garber A. P., Rimoin D. L. Osteogenesis imperfecta type III. Delineation of the phenotype with reference to genetic heterogeneity. Am J Med Genet. 1986 Mar;23(3):821–832. doi: 10.1002/ajmg.1320230309. [DOI] [PubMed] [Google Scholar]
- Sillence D. O., Senn A., Danks D. M. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979 Apr;16(2):101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starman B. J., Eyre D., Charbonneau H., Harrylock M., Weis M. A., Weiss L., Graham J. M., Jr, Byers P. H. Osteogenesis imperfecta. The position of substitution for glycine by cysteine in the triple helical domain of the pro alpha 1(I) chains of type I collagen determines the clinical phenotype. J Clin Invest. 1989 Oct;84(4):1206–1214. doi: 10.1172/JCI114286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes B., Ogilvie D., Wordsworth P., Anderson, Jones N. Osteogenesis imperfecta is linked to both type I collagen structural genes. Lancet. 1986 Jul 12;2(8498):69–72. doi: 10.1016/s0140-6736(86)91609-0. [DOI] [PubMed] [Google Scholar]
- Sykes B., Ogilvie D., Wordsworth P., Wallis G., Mathew C., Beighton P., Nicholls A., Pope F. M., Thompson E., Tsipouras P. Consistent linkage of dominantly inherited osteogenesis imperfecta to the type I collagen loci: COL1A1 and COL1A2. Am J Hum Genet. 1990 Feb;46(2):293–307. [PMC free article] [PubMed] [Google Scholar]
- Tajima S., Ting J. P., Pinnell S. R., Kaufman R. E. Isolation and characterization of a human pro alpha 2(I) collagen gene segment. J Invest Dermatol. 1984 Mar;82(3):265–269. doi: 10.1111/1523-1747.ep12260213. [DOI] [PubMed] [Google Scholar]
- Tsipouras P., Myers J. C., Ramirez F., Prockop D. J. Restriction fragment length polymorphism associated with the pro alpha 2(I) gene of human type I procollagen. Application to a family with an autosomal dominant form of osteogenesis imperfecta. J Clin Invest. 1983 Oct;72(4):1262–1267. doi: 10.1172/JCI111082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenplas S., Wiid I., Grobler-Rabie A., Brebner K., Ricketts M., Wållis G., Bester A., Boyd C., Måthew C. Blot hybridisation analysis of genomic DNA. J Med Genet. 1984 Jun;21(3):164–172. doi: 10.1136/jmg.21.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viljoen D., Beighton P. Osteogenesis imperfecta type III: an ancient mutation in Africa? Am J Med Genet. 1987 Aug;27(4):907–912. doi: 10.1002/ajmg.1320270417. [DOI] [PubMed] [Google Scholar]
- Wallis G. A., Starman B. J., Zinn A. B., Byers P. H. Variable expression of osteogenesis imperfecta in a nuclear family is explained by somatic mosaicism for a lethal point mutation in the alpha 1(I) gene (COL1A1) of type I collagen in a parent. Am J Hum Genet. 1990 Jun;46(6):1034–1040. [PMC free article] [PubMed] [Google Scholar]
- Wenstrup R. J., Shrago-Howe A. W., Lever L. W., Phillips C. L., Byers P. H., Cohn D. H. The effects of different cysteine for glycine substitutions within alpha 2(I) chains. Evidence of distinct structural domains within the type I collagen triple helix. J Biol Chem. 1991 Feb 5;266(4):2590–2594. [PubMed] [Google Scholar]
- Wenstrup R. J., Willing M. C., Starman B. J., Byers P. H. Distinct biochemical phenotypes predict clinical severity in nonlethal variants of osteogenesis imperfecta. Am J Hum Genet. 1990 May;46(5):975–982. [PMC free article] [PubMed] [Google Scholar]


