Abstract
Background
Colorectal cancer (CRC) often recurs in the peritoneum, although the pattern of peritoneal recurrence (PR) has received less attention. We sought to describe the presentation and risk factors for PR following CRC resection.
Methods
We performed a cohort study of patients undergoing resection of Stage I–III CRC from 2006 to 2007 using merged data from a Commission on Cancer Special Study and the National Cancer Database. We estimated the timing, method of detection, and risk factors for isolated PR.
Results
Here, 8991 patients were included and isolate PR occurred in 77 (0.9%) patients. The median time to PR was 16.2 months (intrquartile range = 9.3–28.0 months) and most patients were identified via new symptoms (36.4%). Pathologic factors associated with increased odds of PR included higher T stage (T3 vs. T2, odds ratio [OR] = 4.8, 95% confidence interval [CI] = 1.5–15.7), N stage (N1 vs. N0, OR = 2.00, CI = 1.1–3.7), and signet ring (OR = 8.2, CI = 3.0–22.3) or mucinous histology (OR = 2.6, CI = 1.5–4.7).
Conclusions
The majority of PR was detected within 18 months and few were identified by surveillance. Advanced T/N stage and signet ring/mucinous histology were associated with increased odds of PR.
Keywords: colorectal cancer, mucinous, peritoneal recurrence, signet ring
1. INTRODUCTION
An estimated 147 950 patients were diagnosed with colorectal cancer (CRC) in 2020, with the majority undergoing potentially curative surgical resection. 1 Recurrence following surgical resection is frequent, ranging from 5% to 33% based on stage, with distant recurrence (DR) being most common. 2 , 3 Although less common than liver or lung, peritoneal recurrence (PR) is of particular interest due to the evolving role of management strategies such as cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Identification of factors associated with PR is critical to understand disease progression, and also to best leverage emerging treatment options.
Risk factors for PR have not been studied in a large national sample in the United States. A regional study of 11 124 CRC patients in Sweden identified advanced T and N stage, emergency surgery, and positive surgical margins as risk factors for isolated PR or concurrent PR and DR. 4 Based on these findings, advanced T stage and perforation are commonly used as enrollment criteria for prospective studies involving second‐look laparoscopy and prophylactic HIPEC. 5 Although less commonly reported, histologic factors such as mucinous or signet‐ring histology have also been identified as risk factors for PR. 6 , 7 However, these other pathologic risk factors are not commonly used as enrollment criteria for studies involving second‐look laparoscopy or prophylactic HIPEC.
As the frequency of PR is low, adequate evaluation requires a large population of patients. We aimed to study isolated PR using data from a large national data set of CRC patients annotated for recurrence. We also aimed to identify risk factors for isolated PR rather than combined PR and DR, as these patients are most likely to benefit from diagnostic and therapeutic strategies targeted to detecting and managing peritoneal disease. The objectives of this study were to describe timing, method of detection, and survival after PR diagnosis following resection for Stage I–III CRC and identify clinical and tumor related factors associated with PR.
2. METHODS
2.1. Patient selection
We analyzed data from a Commission on Cancer (CoC) Special Study on CRC recurrence. The study cohort consisted of adult patients (age ≥ 18 years) identified from the National Cancer Database (NCDB) that underwent definitive resection for Stage I–III CRC between January 1, 2006 and December 31, 2007. Ten patients were randomly selected and stratified by stage at diagnosis and tumor location within the colon or rectum from each CoC accredited facility for further chart abstraction to determine recurrence. Detailed data regarding surveillance, recurrence, and treatment through December 31, 2012 were collected as part of the CoC Special Study on CRC recurrence and merged with data within the NCDB. These methods have been previously described in detail. 3 , 8 We excluded patients with missing information on recurrence, metastatic disease at presentation, and death without documented recurrence (Figure 1). Data for the CoC Special Study on CRC recurrence were collected via a secure web form housed at the NCDB in compliance with the Health Insurance Portability and Accountability Act. Secondary analysis of this de‐identified data was considered exempt by The MD Anderson Cancer Center Institutional Review Board.
Figure 1.
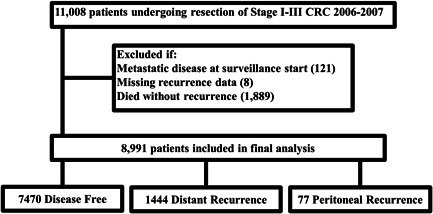
Selection of patients from of National Cancer Database (NCDB)/Commission on Cancer (CoC) cohort. CRC, colorectal cancer.
2.2. Cohorts and variables
Patients that had a recurrence were categorized as having peritoneal, other distant, or locoregional recurrence. The PR cohort consisted of patients with PR alone or concurrent peritoneal and locoregional recurrence. The other DR cohort consisted of patients with distant but not PR. The disease‐free (DF) cohort consisted of patients with no documented recurrence at the date of last follow‐up or the predefined cutoff of 5 years after the surveillance start date. Demographics, tumor characteristics, pathologic reports, treatment information, and facility variables were as defined by the NCDB data standards. 9 Rural versus urban residence was inferred by linking patient zip codes to US census and US Department of Agriculture Economic Research Service data as previously described. 10 Staging was based on the American Joint Committee on Cancer (AJCC) 7th edition staging manual. 11
2.3. Statistical methods
Clinical and demographic characteristics were compared by χ 2, t test, and Kruskal–Wallis tests as appropriate. Median time to recurrence was estimated using the Kaplan–Meier method. Only the first recurrence was analyzed. Patients were censored at the date of last follow‐up or the end of the surveillance period. Differences in demographic, tumor, and facility characteristics were compared for patients with PR versus DR using bivariate and multivariable logistic regression. All variables significant on univariate analysis with a p < 0.2 and a priori decided clinically important variables were included in the final multivariable model. Missing information was included as a separate category. Models were tested for fit by the goodness of fit test. All analyses were performed using Stata MP (version 13.1; StataCorp).
3. RESULTS
Of a total of 11 008 patients, 8991 patients met selection criteria and were included in the analysis. Recurrence of any type occurred in 1521 patients (16.9%). PR alone as the first site of recurrence occurred in 77 (0.9%) of patients, of which 22 (28.5%) also had local recurrence. Demographic data and tumor characteristics for included patients are summarized in Tables 1 and 2, respectively. At baseline there were no significant demographic differences between the groups. Patients with PR most commonly had advanced T stage (96.1% T3 or greater) and higher N stage (70.2% N1 or greater). There were also differences between the PR, DR, and DF cohorts with respect to signet ring histology (9.1% vs. 1.9% vs. 0.7%, respectively), mucinous histology (23.4% vs. 8.7% vs. 9.2%, respectively), and positive margin status (15.6% vs. 8.7% vs. 3.2%, respectively) (p < 0.001).
Table 1.
Patient characteristics by PR of patients undergoing surgery for Stage 1–3 CRC (N = 8991)
| Characteristics | DF (N = 7470) | Nonperitoneal DR (N = 1444) | PR (N = 77) | p | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Age, year | |||||||
| 18–49 | 885 | 11.8 | 206 | 14.3 | 12 | 15.6 | 0.121 |
| 50–64 | 2475 | 33.1 | 479 | 33.2 | 29 | 37.7 | |
| 65–74 | 2038 | 27.3 | 379 | 26.2 | 22 | 28.6 | |
| 75–90 | 2014 | 27 | 365 | 25.3 | 13 | 16.9 | |
| >90 | 58 | 0.8 | 15 | 1 | 1 | 1.3 | |
| Sex | |||||||
| Male | 3537 | 47.3 | 776 | 53.7 | 45 | 58.4 | <0.001 |
| Female | 3930 | 52.6 | 668 | 46.3 | 32 | 41.6 | |
| Unknown | 3 | 0 | 0 | 0 | 0 | 0 | |
| Race | |||||||
| White | 6450 | 86.3 | 1163 | 80.5 | 67 | 87 | <0.001 |
| Black | 679 | 9.1 | 201 | 13.9 | 7 | 9.1 | |
| Others | 341 | 4.6 | 80 | 5.5 | 3 | 3.9 | |
| Comorbidity score* | |||||||
| 0 | 5506 | 73.7 | 1019 | 70.6 | 54 | 70.1 | 0.019 |
| 1 | 1541 | 20.6 | 315 | 21.8 | 20 | 26 | |
| 2 and more | 423 | 5.7 | 110 | 7.6 | 3 | 3.9 | |
| Insurance status | |||||||
| Private | 3109 | 41.6 | 566 | 39.2 | 31 | 40.3 | 0.03 |
| Uninsured | 245 | 3.3 | 52 | 3.6 | 4 | 5.2 | |
| Medicaid | 261 | 3.5 | 70 | 4.8 | 1 | 1.3 | |
| Medicare | 3675 | 49.2 | 710 | 49.2 | 40 | 51.9 | |
| Managed Care | 67 | 0.9 | 9 | 0.6 | 0 | 0 | |
| Unknown | 113 | 1.5 | 37 | 2.6 | 1 | 1.3 | |
| Population density of residence | |||||||
| Metro area | 5831 | 78.1 | 1112 | 77 | 61 | 79.2 | 0.145 |
| Urban area | 1205 | 16.1 | 229 | 15.9 | 10 | 13 | |
| Rural area | 131 | 1.8 | 34 | 2.4 | 4 | 5.2 | |
| Unknown | 303 | 4.1 | 69 | 4.8 | 2 | 2.6 | |
| Facility type | |||||||
| Community | 2023 | 27.1 | 395 | 27.4 | 22 | 28.6 | 0.975 |
| Comprehensive | 4066 | 54.4 | 773 | 53.5 | 41 | 53.2 | |
| Academic/Research | 1353 | 18.1 | 272 | 18.8 | 14 | 18.2 | |
| Others/Unknown | 28 | 0.4 | 4 | 0.3 | 0 | 0 | |
| Facility location | |||||||
| New England | 546 | 7.3 | 93 | 6.4 | 6 | 7.8 | 0.295 |
| Middle Atlantic | 977 | 13.1 | 175 | 12.1 | 12 | 15.6 | |
| South Atlantic | 1517 | 20.3 | 299 | 20.7 | 8 | 10.4 | |
| East North Central | 1549 | 20.7 | 296 | 20.5 | 16 | 20.8 | |
| East South Central | 490 | 6.6 | 106 | 7.3 | 5 | 6.5 | |
| West North Central | 603 | 8.1 | 113 | 7.8 | 10 | 13 | |
| West South Central | 589 | 7.9 | 127 | 8.8 | 3 | 3.9 | |
| Mountain | 334 | 4.5 | 51 | 3.5 | 5 | 6.5 | |
| Pacific | 865 | 11.6 | 184 | 12.7 | 12 | 15.6 | |
Abbreviations: CRC, colorectal cancer; DF, disease‐free; DR, distant recurrence; PR, peritoneal recurrence.
Charlson Comorbidity Index, p calculated via χ 2 test.
Table 2.
Clinicopathologic characteristics by PR of patients undergoing surgery for Stage 1–3 CRC (N = 8991)
| Characteristics | DF (N = 7470) | Nonperitoneal DR (N = 1444) | PR (N = 77) | p | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Cancer site | 0.042 | ||||||
| Right colon | 3613 | 48.4 | 642 | 44.5 | 40 | 51.9 | |
| Left colon | 2860 | 38.3 | 578 | 40 | 29 | 37.7 | |
| Rectum | 997 | 13.3 | 224 | 15.5 | 8 | 10.4 | |
| Tumor stage | <0.001 | ||||||
| I | 2383 | 31.9 | 131 | 9.1 | 0 | 0 | |
| II | 2649 | 35.5 | 401 | 27.8 | 23 | 29.9 | |
| III | 2438 | 32.6 | 912 | 63.2 | 54 | 70.1 | |
| T‐stage | <0.001 | ||||||
| 0/I/1 | 1237 | 16.6 | 49 | 3.4 | 0 | 0 | |
| 2 | 1623 | 21.7 | 152 | 10.5 | 3 | 3.9 | |
| 3 | 4088 | 54.7 | 1007 | 69.7 | 53 | 68.8 | |
| 4 | 452 | 6.1 | 226 | 15.7 | 21 | 27.3 | |
| X, unknown | 70 | 0.9 | 10 | 0.7 | 0 | 0 | |
| N‐stage | <0.001 | ||||||
| 0 | 4983 | 66.7 | 523 | 36.2 | 23 | 29.9 | |
| 1 | 1668 | 22.3 | 451 | 31.2 | 23 | 29.9 | |
| 2 | 729 | 9.8 | 461 | 31.9 | 31 | 40.3 | |
| X, unknown | 90 | 1.2 | 9 | 0.6 | 0 | 0 | |
| Tumor size, mm | <0.001 | ||||||
| <11 | 355 | 4.8 | 15 | 1 | 0 | 0 | |
| 11–20 | 808 | 10.8 | 97 | 6.7 | 3 | 3.9 | |
| >20 | 5711 | 76.5 | 1244 | 86.1 | 73 | 94.8 | |
| Missing | 596 | 8 | 88 | 6.1 | 1 | 1.3 | |
| Tumor histology | <0.001 | ||||||
| Nonmucinous adenocarcinoma | 6734 | 90.1 | 1291 | 89.4 | 52 | 67.5 | |
| Signet‐ring cell | 50 | 0.7 | 27 | 1.9 | 7 | 9.1 | |
| Mucinous | 686 | 9.2 | 126 | 8.7 | 18 | 23.4 | |
| Tumor grade | <0.001 | ||||||
| Well/Moderately differentiated | 6320 | 84.6 | 1141 | 79 | 51 | 66.2 | |
| Poorly | 1074 | 14.4 | 275 | 19 | 23 | 29.9 | |
| Undifferentiated | 74 | 1 | 28 | 1.9 | 3 | 3.9 | |
| Unknown | 2 | 0 | 0 | 0 | 0 | 0 | |
| Total lymph nodes accessed | 0.269 | ||||||
| 0–11 | 2151 | 28.8 | 409 | 28.3 | 23 | 29.9 | |
| 12+ | 5272 | 70.6 | 1023 | 70.8 | 52 | 67.5 | |
| Unknown | 47 | 0.6 | 12 | 0.8 | 2 | 2.6 | |
| Lymphovascular invasion | <0.001 | ||||||
| Yes | 1218 | 16.3 | 505 | 35 | 34 | 44.2 | |
| No | 4691 | 62.8 | 635 | 44 | 31 | 40.3 | |
| Unknown | 1514 | 20.3 | 299 | 20.7 | 11 | 14.3 | |
| Not applicable | 47 | 0.6 | 5 | 0.3 | 1 | 1.3 | |
| Perineural invasion | <0.001 | ||||||
| Yes | 260 | 3.5 | 165 | 11.4 | 9 | 11.7 | |
| No | 3512 | 47 | 557 | 38.6 | 32 | 41.6 | |
| Unknown | 3587 | 48 | 710 | 49.2 | 33 | 42.9 | |
| Not applicable | 111 | 1.5 | 12 | 0.8 | 3 | 3.9 | |
| Surgical margin status | <0.001 | ||||||
| Negative | 7148 | 95.7 | 1300 | 90 | 65 | 84.4 | |
| Positive | 241 | 3.2 | 126 | 8.7 | 12 | 15.6 | |
| Unknown | 81 | 1.1 | 18 | 1.2 | 0 | 0 | |
Note: p calculated via χ 2 test.
Abbreviations: CRC, colorectal cancer; DF, disease‐free; DR, distant recurrence; PR, peritoneal recurrence.
The median time to PR was 16.2 months (interquartile range = 9.3 months to 28.0 months), which was earlier than other DRs (median 21.7 months, p = 0.003). Most PRs (85.3%) occurred within 3 years of surgery. The most common methods of detection included new signs or symptoms (36.4%), evaluation following locoregional recurrence (20.8%), and routine surveillance imaging (14.3%, Table 3). A minority of patients (16.9%) underwent surgery for PR, 49.4% received chemotherapy alone, and 33.8% received no treatment. Survival information is shown in Figure 2. The 5‐year survival rate was 22.8% in patients with PR and 32.6% in patients with DR (p < 0.01).
Table 3.
Method of detection and treatment of PR (N = 77)
| n (%) | |
|---|---|
| Method of detection | |
| Patient/Physician identified sign/symptom | 28 (36.4) |
| Elevated CEA | 12 (13.0) |
| Asymptomatic routine imaging | 11 (14.3) |
| As part of work up for locoregional recurrence | 16 (20.8) |
| Incidental on unrelated imaging | 8 (10.4) |
| Unknown | 4 (5.2) |
| Treatment approach | |
| Surgery alone | 7 (9.1) |
| Chemotherapy alone | 38 (49.4) |
| Combined surgery/chemotherapy | 6 (7.8) |
| None | 26 (33.8) |
Abbreviations: CEA, carcinoembryonic antigen; PR, peritoneal recurrence.
Figure 2.
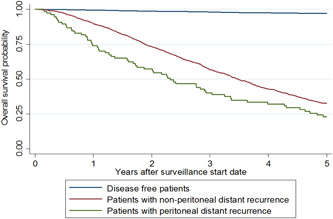
Kaplan‐Meier estimates of overall survival
We used multivariable logistic regression to compare patients with PR to DF patients (Table 4). Tumor factors associated with increased odds of PR included higher T stage (T3 vs. T2 odds ratio [OR] = 4.82, 95% confidence interval [CI] = 1.48–15.72; T4 vs. T2 OR = 12.26, 95% CI = 3.44–43.73), higher N stage (N1 vs. N0 OR = 2.00, 95% CI = 1.08–3.73; N2 vs. N0 OR = 3.72, 95% CI 1.97–6.99), signet ring versus adenocarcinoma histology (OR = 8.22, 95% CI = 3.03–22.32), and mucinous versus adenocarcinoma histology (OR = 2.60, 95% CI = 1.45–4.65). Lymphovascular invasion, perineural invasion, and surgical margins were not significantly associated with increased odds of PR. Demographic factors associated with increased odds of PR were Medicare versus private insurance (OR = 2.55, 95% CI = 1.20–5.44), rural versus metro residence (OR = 3.63, 95% CI = 1.22–10.48), and operative year 2007 versus 2006 (OR = 1.66, 95% CI = 1.02–2.70).
Table 4.
Multivariable logistic regression, factors associated with PR versus no recurrence and PR versus other DR
| Variable | Odds ratio (95% confidence interval) | |
|---|---|---|
| PR compared with DF | PR compared with other DR | |
| Age (years) | ||
| 18–49 | Ref | Ref |
| 50–64 | 1.12 (0.54–2.30) | 1.00 (0.47–2.14) |
| 65–74 | 0.49 (0.19–1.27) | 0.55 (0.20–1.49) |
| 75–90 | 0.28 (0.10–0.80)* | 0.42 (0.14–1.27) |
| >90 | 0.61 (0.07–5.60) | 0.76 (0.07–8.82) |
| Insurance | ||
| Private | Ref | Ref |
| Uninsured | 0.87 (0.29–2.67) | 1.20 (0.38–3.79) |
| Medicaid | 0.36 (0.05–2.70) | 0.32 (0.04–2.45) |
| Medicare | 2.55 (1.20–5.44)* | 2.17 (1.01–4.70) |
| Unknown | 0.98 (0.11–8.17) | 0.83 (0.11–6.52) |
| Population density of residence | ||
| Metro area | Ref | Ref |
| Urban area | 0.69 (0.34–1.40) | 0.77 (0.38–1.58) |
| Rural area | 3.63 (1.22–10.78)* | 2.37 (0.74–7.65) |
| Unknown | 0.49 (0.11–2.16) | 0.53 (0.12–2.29) |
| T‐stage | ||
| 2 | Ref | Ref |
| 3 | 4.82 (1.48–15.72)* | 2.27 (0.67–7.68) |
| 4 | 12.26 (3.44–43.73)* | 2.97 (0.80–11.00) |
| N‐stage | ||
| 0 | Ref | Ref |
| 1 | 2.00 (1.08–3.73)* | 0.92 (0.47–1.81) |
| 2 | 3.72 (1.97–6.99)* | 0.88 (0.44–1.73) |
| Tumor histology | ||
| Nonmucinous adenocarcinoma | Ref | Ref |
| Signet‐ring cell | 8.22 (3.03–22.32)* | 5.31 (1.82–15.49)* |
| Mucinous | 2.60 (1.45–4.65)* | 3.12 (1.70–5.72)* |
| Tumor grade | ||
| Well/Moderately differentiated | Ref | Ref |
| Poorly | 1.05 (0.59–1.88) | 1.17 (0.64–2.12) |
| Undifferentiated | 2.40 (0.65–8.87) | 1.37 (0.34–5.47) |
| Total lymph nodes accessed | ||
| 0–11 | Ref | Ref |
| 12+ | 0.51 (0.30–0.86)* | 0.77 (0.45–1.34) |
| Unknown | 6.98 (1.23–39.45)* | 2.37 (0.39–14.30) |
| Diagnosis year | ||
| 2006 | Ref | Ref |
| 2007 | 1.66 (1.02–2.70)* | 1.68 (1.02–2.75)* |
| Surgery chemotherapy sequence | ||
| No chemotherapy | – | Ref |
| Neo‐adjuvant | – | 1.74 (0.36–8.44) |
| Adjuvant | – | 2.22 (1.07–4.60)* |
| Others | – | 1.87 (0.58–6.01) |
| Lymphovascular invasion | ||
| Yes | Ref | Ref |
| No | 0.60 (0.34–1.09) | 0.94 (0.52–1.69) |
| Unknown | 0.51 (0.39–1.13) | 0.53 (0.24–1.17) |
| Not applicable | 0.99 (0.07–14.89) | 0.43 (0.02–10.31) |
| Perineural invasion | ||
| Yes | Ref | Ref |
| No | 0.76 (0.33–1.75) | 1.55 (0.67–3.58) |
| Unknown | 0.67 (0.29–1.56) | 1.20 (0.53–2.77) |
| Not applicable | 2.23 (0.40–12.36) | 5.29 (0.76–36.72) |
| Surgical margin status | ||
| Negative | Ref | Ref |
| Positive | 2.01 (0.96–4.17) | 1.42 (0.70–2.92) |
Note: Values presented as odds ratio (95% confidence interval).
Abbreviations: DF, disease‐free; DR, distant recurrence; PR, peritoneal recurrence.
p < 0.05.
Compared with patients with other DR, patients with isolated PR were independently more likely to have signet ring versus adenocarcinoma histology (OR 5.31, 95% CI = 1.82–15.49), mucinous versus adenocarcinoma histology (OR = 3.12, 95% CI = 1.70–5.72), operative year 2007 versus 2006 (OR = 1.68, 95% CI = 1.02–2.75), and receipt of adjuvant chemotherapy versus no chemotherapy (OR 2.22, 95% CI = 1.07–4.60). Advanced T and N stage were not associated with PR versus DR.
4. DISCUSSION
In this national sample of patients undergoing surgical resection for Stage I–III CRC, most PRs occurred within 3 years of surgery and were most commonly detected based on the development of concerning symptoms or signs; relatively few were detected by surveillance imaging alone. Notable pathologic factors associated with PR were advanced T and N stage, signet ring histology, and mucinous histology. PR was not associated with tumor grade, lymphovascular or perineural invasion, or positive surgical margins. Surgical treatment for PR was uncommon, but when performed was associated with a 5‐year survival of 22.8%. In contrast, 5‐year OS for patients with other DR undergoing surgery was 32.6%.
We focused on patients with isolated PR, as these patients are most likely to benefit from diagnostic and therapeutic strategies targeted to detect and manage peritoneal disease. Patient or physician identified signs or symptoms was the most common method of detection of PR in our study, consistent with previous studies demonstrating the limited sensitivity of abdominal CT for detecting peritoneal disease, particularly for small lesions. 12 , 13 Detection of subclinical disease is desirable due to the increased likelihood of obtaining complete cytoreduction during subsequent CRS and HIPEC, which is predictive of survival. 14 Our results suggest that current surveillance is suboptimal for detecting most lesions at a subclinical stage. Strategies such as circulating tumor DNA (ctDNA), with or without appropriately timed second‐look laparoscopy, may be considered to identify subclinical peritoneal lesions amenable to complete cytoreduction, but the effectiveness of these strategies need further investigation. 15 , 16 , 17
Although the surveillance strategy for PR has not changed significantly since the study period in our analysis (2006–2007), the management of PR has evolved. We observed that 16.9% of patients underwent surgical management for PR and that the 5‐year survival was 22.8%. CRS/HIPEC is now more frequently performed for PR in experienced centers, though overall evidence remains conflicting. 18 , 19 Whether through CRS/HIPEC or modern chemotherapy regimens, the survival in patients with PR has improved. 20 , 21 The recently completed PRODIGE 7 trial observed a nearly 40% 5‐year survival rate among patients undergoing CRS with or without HIPEC for PR. 22 The management and survival observed in our study may not reflect the advances in modern systemic therapy. It also may reflect the survival differences among patients in routine clinical practice compared with those enrolled in clinical trials. These differences in outcomes, however, highlight the importance of risk stratification and better detection of patients with PR as treatment approaches are improving.
Clinicians have long debated the role of prophylactic CRS/HIPEC in patients at higher risk of developing PR. Several studies have explored the role of prophylactic HIPEC and delayed second‐look laparoscopy in patients at high risk of PR. 15 , 23 , 24 These studies have culminated in two recent randomized clinical trials demonstrating no benefit of adjuvant HIPEC in patients thought to be at high risk for PR. 5 , 25 It is important to consider our findings in the context of these trials. The PROPHYLOCHIP trial enrolled patients with local peritoneal spread, ovarian metastases, or a perforated tumor. Patients underwent prophylactic HIPEC 6 months after resection following adjuvant chemotherapy. 25 The COLOPEC trial enrolled patients with a T4 or perforated tumor and prophylactic HIPEC was performed within 2 months of resection. 5 Histology was not reported in the PROPHYLOCHIP trial and less than 15% of patients in the COLOPEC trial had signet ring or mucinous histology. Our results suggest that patients with signet ring or mucinous tumors are at increased risk for PR. In particular, signet ring and mucinous histology were associated with increased risk of PR versus DR, whereas advanced T and N stage are predictors of any DR.
An analysis of PR following CRC resection was recently conducted using a regional cross‐sectional database of 11 124 CRC patients in Sweden. The authors observed a rate of metachronous PR of 4.2%. 4 This rate was higher than our observed rate, although the study included patients with concurrent PR/DR in their PR cohort, while we assessed isolated PR. Similar small retrospective studies have observed a rate of 4‐5% and have also included concurrent PR/DR patients. 26 , 27 , 28 In these studies, 40% of patients with metachronous PR had concurrent DR, suggesting that this significantly contributed to the difference in PR rate. 27 , 28 A recent analysis on patients with T4 disease demonstrated a 7.9% rate of peritoneal‐only recurrence. 29 In addition, we performed purposeful sampling stratified by stage and, therefore, our cohort includes a population of patients biased towards a lower stage. In our study, 31.9% of patients were of Stage I, whereas in the Swedish study, 15.8% were of Stage I. The rate of PR from our study is not representative of the general population of resected CRC patients.
The analysis has several limitations that must be taken into consideration. First, our cohort may not be reflective of all patients. This was a stage stratified retrospective study of patients that underwent primary resection from 2006 to 2007 and frequency of recurrence cannot be generalized to all Stage I–III CRC patients. Our cohort was also limited to patients treated at CoC‐accredited centers, which may not be representative of all CRC patients treated in the United States. In addition, the random sample of 10 patients from each center, regardless of volume, might over‐represent smaller centers. Second, under‐detection is likely given the low sensitivity of current imaging for detecting PR. Additionally, follow‐up ended at first recurrence, so patients who developed PR after other DR are not recorded. Similarly, patients with initial isolated PR might not have been detected clinically until they developed DR and these patients would not have been identified as having isolated PR in our study. Finally, we could not capture all variables that might impact the probability of PR. For example, our data set did not include information regarding perforation, which has been used as high‐risk enrollment criteria for previous clinical trials of adjuvant HIPEC following CRC resection. 5 , 25 Nonetheless, this study provides “real world” data from a large sample of patients across the United States.
This large retrospective cohort study demonstrates that while relatively uncommon, PR occurs early following CRC resection and is frequently not detected until symptoms develop. PR is associated with poor outcomes and relatively few patients underwent surgical management. We identified advanced T and N stage, signet ring histology, and mucinous histology as risk factors for PR in our cohort. Detection of PR remains a challenge and the effectiveness of emerging methods such as ctDNA, tagged imaging studies, potentially with second‐look laparoscopy in selected high‐risk patients warrants further investigation.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
SYNOPSIS
Peritoneal recurrence (PR) occurs early following CRC resection and is frequently not detected until symptoms develop. Advanced T and N stage, signet ring histology, and mucinous histology were identified as risk factors for PR in our cohort.
ACKNOWLEDGMENTS
This research was supported by a PCORI award – Award Number CE1304‐6855 (George J. Chang). Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number T32 CA090217 (Taylor Aiken). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Aiken T, Hu C‐Y, Uppal A, et al. Peritoneal recurrence after resection for Stage I–III colorectal cancer: a population analysis. J Surg Oncol. 2023;127:678‐687. 10.1002/jso.27175
DATA AVAILABILITY STATEMENT
NA.
REFERENCES
- 1. NCI . 2021. Surveillance, epidemiology, and end results program, cancer stat facts: colorectal cancer. Accessed February 20, 2021. https://seer.cancer.gov/statfacts/html/colorect.html
- 2. Osterman E, Glimelius B. Recurrence risk after up‐to‐date colon cancer staging, surgery, and pathology: analysis of the entire Swedish population. Dis Colon Rectum. 2018;61(9):1016‐1025. 10.1097/dcr.0000000000001158 [DOI] [PubMed] [Google Scholar]
- 3. Zafar SN, Hu CY, Snyder RA, et al. Predicting risk of recurrence after colorectal cancer surgery in the United States: an analysis of a Special Commission on Cancer National Study. Ann Surg Oncol. 2020;27(8):2740‐2749. 10.1245/s10434-020-08238-7 [DOI] [PubMed] [Google Scholar]
- 4. Segelman J, Granath F, Holm T, Machado M, Mahteme H, Martling A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2012;99(5):699‐705. 10.1002/bjs.8679 [DOI] [PubMed] [Google Scholar]
- 5. Klaver CEL, Wisselink DD, Punt CJA, et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): a multicentre, open‐label, randomised trial. Lancet. 2019;4(10):761‐770. 10.1016/s2468-1253(19)30239-0 [DOI] [PubMed] [Google Scholar]
- 6. Nozoe T, Anai H, Nasu S, Sugimachi K. Clinicopathological characteristics of mucinous carcinoma of the colon and rectum. J Surg Oncol. 2000;75(2):103‐107. [DOI] [PubMed] [Google Scholar]
- 7. Pande R, Sunga A, Levea C, et al. Significance of signet‐ring cells in patients with colorectal cancer. Dis Colon Rectum. 2008;51(1):50‐55. 10.1007/s10350-007-9073-7 [DOI] [PubMed] [Google Scholar]
- 8. Snyder RA, Hu CY, Cuddy A, et al. Association between intensity of posttreatment surveillance testing and detection of recurrence in patients with colorectal cancer. JAMA. 2018;319(20):2104‐2115. 10.1001/jama.2018.5816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. NCDB . 2021. Participant user file, data dictionary. Accessed February 20, 2021. https://www.facs.org/quality-programs/cancer/ncdb/puf
- 10. Boffa DJ, Rosen JE, Mallin K, et al. Using The National Cancer Database for outcomes research: a review. JAMA Oncol. 2017;3(12):1722‐1728. 10.1001/jamaoncol.2016.6905 [DOI] [PubMed] [Google Scholar]
- 11. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. Springer; 2010:649. [Google Scholar]
- 12. Koh JL, Yan TD, Glenn D, Morris DL. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann Surg Oncol. 2009;16(2):327‐333. 10.1245/s10434-008-0234-2 [DOI] [PubMed] [Google Scholar]
- 13. Laghi A, Bellini D, Rengo M, et al. Diagnostic performance of computed tomography and magnetic resonance imaging for detecting peritoneal metastases: systematic review and meta‐analysis. Radiol Med. 2017;122(1):1‐15. 10.1007/s11547-016-0682-x [DOI] [PubMed] [Google Scholar]
- 14. Faron M, Macovei R, Goéré D, Honoré C, Benhaim L, Elias D. Linear relationship of peritoneal cancer index and survival in patients with peritoneal metastases from colorectal cancer. Ann Surg Oncol. 2016;23(1):114‐119. 10.1245/s10434-015-4627-8 [DOI] [PubMed] [Google Scholar]
- 15. Elias D, Honoré C, Dumont F, et al. Results of systematic second‐look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Ann Surg. 2011;254(2):289‐293. 10.1097/SLA.0b013e31822638f6 [DOI] [PubMed] [Google Scholar]
- 16. Delhorme JB, Triki E, Romain B, Meyer N, Rohr S, Brigand C. Routine second‐look after surgical treatment of colonic peritoneal carcinomatosis. J Visceral Surg. 2015;152(3):149‐154. 10.1016/j.jviscsurg.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 17. Tie J, Wang Y, Tomasetti C, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med. 2016;8(346):346ra92. 10.1126/scitranslmed.aaf6219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sánchez‐Hidalgo JM, Rodríguez‐Ortiz L, Arjona‐Sánchez Á, et al. Colorectal peritoneal metastases: optimal management review. World J Gastroenterol. 2019;25(27):3484‐3502. 10.3748/wjg.v25.i27.3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klaver CEL, Groenen H, Morton DG, Laurberg S, Bemelman WA, Tanis PJ. Recommendations and consensus on the treatment of peritoneal metastases of colorectal origin: a systematic review of national and international guidelines. Colorectal Dis. 2017;19(3):224‐236. 10.1111/codi.13593 [DOI] [PubMed] [Google Scholar]
- 20. Franko J, Ibrahim Z, Gusani NJ, Holtzman MP, Bartlett DL, Zeh HJ. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer. 2010;116(16):3756‐3762. 10.1002/cncr.25116 [DOI] [PubMed] [Google Scholar]
- 21. Sugarbaker PH, Ryan DP. Cytoreductive surgery plus hyperthermic perioperative chemotherapy to treat peritoneal metastases from colorectal cancer: standard of care or an experimental approach? Lancet Oncol. 2012;13(8):e362‐e369. 10.1016/s1470-2045(12)70210-3 [DOI] [PubMed] [Google Scholar]
- 22. Quénet F, Elias D, Roca L, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open‐label, phase 3 trial. Lancet Oncol. 2021;22(2):256‐266. 10.1016/s1470-2045(20)30599-4 [DOI] [PubMed] [Google Scholar]
- 23. Sammartino P, Sibio S, Biacchi D, et al. Prevention of peritoneal metastases from colon cancer in High‐Risk patients: preliminary results of surgery plus prophylactic HIPEC. Gastroenterol Res Pract. 2012;2012(7):1‐7. 10.1155/2012/141585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morris MC, Dhar VK, Stevenson MA, et al. Adjuvant hyperthermic intraperitoneal chemotherapy (HIPEC) for patients at high‐risk of peritoneal metastases. Surg Oncol. 2019;31:33‐37. 10.1016/j.suronc.2019.09.002 [DOI] [PubMed] [Google Scholar]
- 25. Goéré D, Glehen O, Quenet F, et al. Second‐look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP–PRODIGE 15): a randomised, phase 3 study. Lancet Oncol. 2020;21(9):1147‐1154. 10.1016/s1470-2045(20)30322-3 [DOI] [PubMed] [Google Scholar]
- 26. Choi AH, Farzaneh C, Kejriwal N, et al. Rate of peritoneal carcinomatosis in resected stage II and III colon cancer. Ann Surg Oncol. 2020;27(13):4943‐4948. 10.1245/s10434-020-08689-y [DOI] [PubMed] [Google Scholar]
- 27. Jayne DG, Fook S, Loi C, Seow‐Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89(12):1545‐1550. 10.1046/j.1365-2168.2002.02274.x [DOI] [PubMed] [Google Scholar]
- 28. Nagata H, Ishihara S, Hata K, et al. Survival and prognostic factors for metachronous peritoneal metastasis in patients with colon cancer. Ann Surg Oncol. 2017;24(5):1269‐1280. 10.1245/s10434-016-5732-z [DOI] [PubMed] [Google Scholar]
- 29. Uppal A, Helmink B, Grotz TE, et al. What is the risk for peritoneal metastases and survival afterwards in T4 colon cancers? Ann Surg Oncol. 2022;29(7):4224‐4233. 10.1245/s10434-022-11472-w [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
NA.


