Abstract
In clinics, sepsis is a critical disease that often develops into shock and multiple organ dysfunction, leading to a serious threat of death. Patients with sepsis are often accompanied by stress hyperglycemia which is an independent risk factor for poor prognosis in sepsis. Thus, the treatment for stress hyperglycemia has attracted more and more attention, among which intensive insulin therapy is widely concerned. However, the benefits and harms of intensive insulin therapy for sepsis patients remain controversial. What the existing literature discusses mostly are the clinical benefit and hypoglycemia risk of intensive insulin therapy, but there is no conclusion on the target range of blood glucose control, the applicable patients, the timing of treatment initiation, and how to avoid the risk. In this study, we have analyzed and summarized the existing literature, hoping to determine the adverse and clinical benefit of intensive insulin therapy in sepsis. And we attempt to assemble better evidence to propose a better recommendation on hyperglycemia intervention for sepsis patients.
Keywords: Sepsis, Stress hyperglycemia, Intensive insulin therapy, Benefit, Risk
Graphical abstract
1. Introduction
Sepsis is an unusual systemic reaction to infection caused by bacteria, fungi or viruses, which may lead to multisystem organ failure [1]. Multisystem organ failure is frequently associated with significant mortality and substantial medical costs [2]. It is estimated that the incidence of severe sepsis was 0.69% in the USA accompanied by 215000 people dying from the disease annually [2,3]. The epidemiology from Chinese has shown that sepsis affects one fifth of patients in intensive care units (ICUs) with a 90-day mortality rate of 35.5% [4]. Patients with sepsis would occur a series of pathological changes, such as the increase of insulin-antagonistic hormones, excessive release of inflammatory mediators and lipid-derived factors, oxidative stress and endoplasmic reticulum stress, all of which will eventually lead to stress hyperglycemia [5]. According to the findings of Leuven's intensive insulin therapy trial, critically ill patients should be considered as stress hyperglycemia when blood glucose exceeds 6.1 mmol/L [6]. In a prospective analysis of 1000 patients, the incidence of sepsis with stress hyperglycemia was around 40% [7]. Hyperglycemia is an independent risk factor for poor prognosis in sepsis patients [[8], [9], [10]], and it is significantly associated with an increased risk of death [11]. One study found septic patients with stress hyperglycemia had a significantly higher mortality rate than those with normal blood sugar (42.5% vs 13.7%) [8]. Another study reported that patients with blood glucose levels >11.1 mmol/L had a higher mortality rate than those with levels between 5.6 and 11.1 mmol/L and 2.2–5.56 mmol/L (48.6% vs 23.2% vs 9.9%) [12]. Based on those fact that hyperglycemia can result in a poor clinical prognosis and high mortality, it is critical to treat the stress hyperglycemia associated with sepsis.
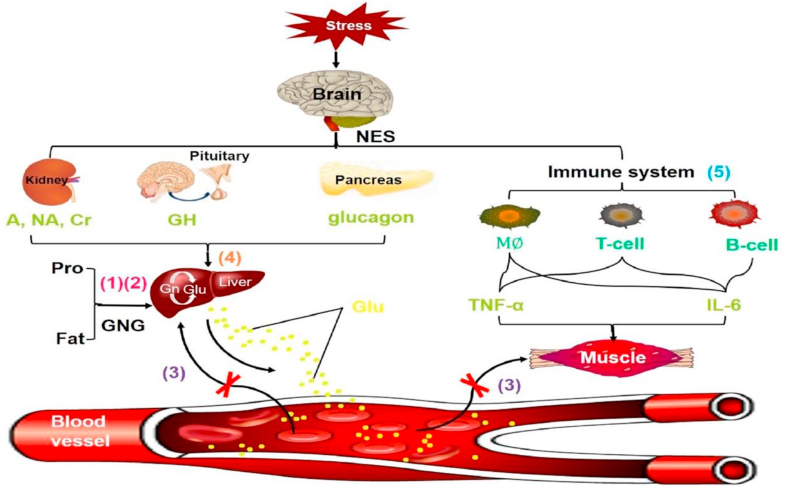
Stress hyperglycemia is primarily induced by the synergistic action of hypothalamus-pituitary-adrenal (HPA) axis, sympathetic adrenal system and cytokines (TNF- α, IL-6, etc.) [13]. The neuroendocrine responses induced by stress are mainly characterized by excessive glycogen decomposition, gluconeogenesis and insulin resistance [14]. Hypoxia, hemorrhage and sepsis are stressors that lead to the highest levels of epinephrine and norepinephrine [15], which will stimulate liver gluconeogenesis and glycogen decomposition. In addition, cytokines (TNF- α, IL-6 etc.) can induce peripheral insulin resistance [14]. For sepsis patients, the changes in the neuroendocrine system and the main causes of hyperglycemia under stress are summarized in Fig. 1 [16]: (1) Accelerated glycogen decomposition, protein metabolism and fat mobilization under high metabolism; (2) The increase of amino acids, fatty acids and lactic acid entering the blood after gluconeogenesis to glucose; (3) Tissues and cells uptake glucose at a reduced rate in a state of sepsis; Furthermore, (4) an increase in counter-regulatory hormones such as cortisol, epinephrine, norepinephrine, growth hormone and glucagon under stress will affect new glucose production, glycogen decomposition and glucose uptake [17]; (5) Tumor necrosis factor-α, IL-6 and other cytokines released by immune cells and other tissues can reduce muscle glucose uptake [17], which also has a very significant effect on blood glucose increase.
Fig. 1.
Mechanism pattern diagram of elevated blood glucose under stress. (1) Metabolic acceleration; (2) Gluconeogenesis; (3) Decreased glucose utilization; (4) Counter-regulatory hormones increase blood glucose; (5) Cytokines decrease glucose uptake. NES: neuroendocrine system; A: adrenaline; NA: noradrenaline; Cr: cortisol; GH: growth hormone; Pro: protein; GNG: gluconeogenesis; Gn: glycogen; Glu: glucose; M : macrophage.
According to different ways of administration, the commonly used hypoglycemic drugs can be classified as oral and injectable drugs. Septic patients are generally in critical condition with the need to reach euglycemia quickly, and the most are unable to take oral medications. In addition, a study has found that dipeptidyl peptidase 4 (DPP4) inhibitors, meglitinides, sodium-dependent glucose transporters 2 (SGLT-2) inhibitors, and alpha-glucosidase inhibitors increase the risk of infection [18]. Therefore, the majority of conventional oral hypoglycemic drugs have some limitations such as slow effect, low drug compliance and even adverse reactions. Despite DPP4-inhibitory drugs have proved to be useful in the treatment of acute respiratory diseases, such as severe acute respiratory syndrome coronavirus 2 (SARS-COV2) [19], the best administration mode for critically ill patients remains injection. Injectable hypoglycemic medications include glucagon-like peptide-1 receptor agonists (GLP1-RAs) and insulin. So far only a few small randomized controlled trials [20,21] have been conducted to assess the efficacy and safety of GLP1-RAs, which reflects the uncertainty of its clinical application. Overall, inpatients who are suffering sepsis complicated by stress hyperglycemia should be given priority to the use of insulin injection. Insulin therapy can be divided into conventional insulin therapy and intensive insulin therapy, and there are some controversial points in some aspects. First of all, the description of the target range of blood glucose control is various across the literature. The latest “Surviving Sepsis Campaign Guidelines” recommended a target blood sugar range of 7.8–10 mmol/L for critically ill patients [22]. However, in several other studies, the target of conventional treatment was 10–11.1 mmol/L [6,23,24]. In this study, intensive insulin therapy is referred to simulate the physiological mode of insulin secretion through continuous subcutaneous or intravenous administration of insulin via an insulin pump, so as to control blood glucose levels. There is literature regarded 6.1–8.3 mmol/L as the goal of tight blood glucose control, and the overall rate of reaching the standard was only 53.9% [5]. Some other studies believe that strictly controlling the level of blood glucose in the range of 4.4–6.1 mmol/L could improve the body's metabolic disorder [6,23]. The second controversial point is whether intensive insulin therapy has a greater clinical benefit and a lower risk of adverse events than conventional insulin therapy. Some researchers have reported that intensive insulin therapy can reduce the mortality, intensive care unit (ICU) stay time and hospitalization time of septic patients [23]. However, there are also some studies claim that intensive glycemic control increases the risk of hypoglycemia [[25], [26], [27], [28]], which may cause serious consequences, such as hypoglycemic coma. As can be seen, intensive insulin therapy is a treatment with both benefit and risk. In 2021 Surviving Sepsis Campaign Guidelines [22], they believed that the balance of this benefit and risk favored initiating insulin therapy at glucose levels >10 mmol/L and thereafter set the target blood glucose range at 7.8–10 mmol/L, but did not recommend a specific protocol. In this review, we analyzed the present literature to determine the safety and clinical benefit of intensive insulin therapy in septic patients. Simultaneously, we attempt to collect better evidence to provide a better reference opinion for clinical decision-making on the target range of blood glucose control, the applicable situation, the timing of starting treatment and how to avoid the risk.
2. Benefit and risk of intensive insulin therapy
2.1. Benefit of intensive insulin therapy
Insulin lowers blood sugar, on the one hand, by promoting more glucose to enter hepatocytes, muscle cells, adipocytes and other tissue cells to synthesize glycogen. On the other hand, it can inhibit fat decomposition and normalize dyslipidemia. What's more, insulin has immunoregulation effects such as inhibiting the overexpression of inflammatory factors and improving the macrophage function [29]. Strictly maintaining glucose levels between 4.4 and 6.1 mmol/L through insulin infusion can significantly reduce mortality and morbidity in critically ill patients [30]. For long-term hospitalized patients who need intensive care, intensive insulin therapy can not only exhibit a lower mortality rate, but also significantly reduce the incidence of sepsis, renal failure and nervous system diseases when compared to conventional treatment [31].
Some clinical trials have shown that intensive insulin therapy is beneficial for patients with the diagnosis of diabetes in terms of preventing complications associated with diabetes mellitus [32]. In a cohort study, the authors observed a significant decrease in the proportion of patients with retinopathy deterioration and increased urinary albumin excretion in the intensive treatment group, as well as an 86% reduction in the risk of new albuminuria in that group [33]. Two other randomized controlled trials have also demonstrated that intensive blood glucose control can reduce the prevalence of neuropathy [34] and the frequency or severity of diabetic microvascular complications (retinopathy and nephropathy) [35]. What is more noteworthy is that the most landmark studies supporting the idea that intensive care patients receiving intensive insulin therapy can reduce morbidity and mortality are two large single-center prospective randomized controlled trials. One of these trials evaluated the effects in surgical patients, which found that the intensive treatment group had a lower hospital mortality rate than the conventional treatment group (37.3% vs 40.0%) [23]. Another study concluded that intensive insulin therapy reduced the mortality of critically ill patients from 8.0% of conventional treatment to 4.6% (P < 0.04) [6]. This reduction is mainly due to a decrease in deaths from sepsis-induced multisystem organ failure. The authors also reported that intensive insulin therapy reduced overall hospital mortality by 34%, decreased bloodstream infections by 46% and acute renal failure requiring dialysis or hemofiltration by 41% [6].
All of the research cited above showed the effectiveness of intensive insulin therapy. Meanwhile, those studies have also mentioned the risk of intensive insulin therapy but have tended to suggest that the clinical benefit of intensive insulin therapy outweighs the risk.
2.2. Risk of intensive insulin therapy
Even though most studies believed that intensive insulin therapy has a significant effect on blood glucose control in patients with sepsis, it does not mean that there is no risk. On the contrary, some clinical research has suggested that intensive insulin therapy has limited clinical benefit in critically ill patients and increases the risk of hypoglycemia.
A meta-analysis of 8432 patients from 29 randomized controlled trials conducted by Wiener et al. [36] reported that intensive insulin therapy was associated with an increased risk of hypoglycemia (13.7% vs 2.5%; RR, 5.13; 95% confidence interval [CI], 4.09–6.43). Additionally, it revealed that there was no difference in overall hospital mortality between intensive insulin therapy and conventional therapy (21.6% vs 23.3%; RR, 0.93; 95% CI, 0.85–1.03), nor did it significantly reduce the risk of new need for dialysis (11.2% vs 12.1%; RR, 0.96; 95% CI, 0.76–1.20). Two other randomized controlled trials described results that intensive treatment group had a higher incidence of severe hypoglycemia (6.8% vs 0.5%, P < 0.001; 17.0% vs 4.1%, P < 0.001) [24,27], which is comparable to the findings of the aforementioned study. However, the difference is that the mortality rate in the intensive treatment group is higher than that in the conventional treatment group (27.5% vs 24.9%, P = 0.02) [27]. The authors also reported that the median number of days in the hospital (P = 0.86) or ICU (P = 0.84) or the median number of days of renal-replacement therapy (P = 0.39) or mechanical ventilation (P = 0.56) between the two treatment groups had no significant difference [27]. Moreover, the study by Brunkhorst et al. showed there was no significant difference in mortality or the mean score for organ failure between the two groups but did note the incidence of serious adverse events in the intensive treatment group was higher than that in the conventional treatment group (10.9% vs 5.2%, P = 0.01) [24].
Since the prevalence of intensive insulin therapy in ICUs is increasing [37], the “Surviving Sepsis Campaign Guidelines” recommended that we should be on guard against a series of problems during intensive insulin therapy. Especially the signs and symptoms of hypoglycemia in a critically ill patient can be misconstrued as another diagnosis, leading to a delay in recognizing the hypoglycemia [[38], [39], [40]]. A large multicenter analysis of evaluating the trend of early BG (that is, within 24 h of ICU admission) and the relationship between blood glucose control and hospital mortality found that both hypoglycemia and hyperglycemia were harmful to critically ill patients [41]. Although both high and low blood sugar levels increase the risk of death [12], hypoglycemia may sometimes be more dangerous. For example, it may be associated with increased severity and higher mortality (<3.89 mmol/L, 38.2% vs 3.89–7.72 mmol/L, 19.5%) in patients with severe sepsis [42]. Therefore, it is necessary to analyze the risk factors for hypoglycemia in sepsis patients receiving intensive insulin therapy, so as to determine a blood glucose range for each ICU that does not cause a significant increase in severe hypoglycemia and is in line with the limitations of nursing and economic resources [43].
To begin with, when eating irregularly or delaying meals, the insulin intensive treatment group is more likely to develop hypoglycemia than the conventional treatment group. Secondly, different patients with different diseases, treatment measures and other factors may result in varying curative effects. Sepsis, hemodialysis, and organ failure have been reported to be risk factors for developing hypoglycemia with intensive insulin therapy [44]. Berghe et al. [45] summed up the data from two previously reported randomized controlled trials [6,23] and discovered that most patients, including those with sepsis at the time of admission, benefited from intensive insulin therapy, but there was no survival benefit for patients with the diagnosis of diabetes [46]. Furthermore, a study comparing the clinical characteristics of patients with and without the diagnosis of diabetes found that up to 84.4% of those with the diagnosis of diabetes died from sepsis [47]. Sathananthan et al. [48] conducted a retrospective study of 1698 patients admitted to ICUs with sepsis secondary to bacterial pneumonia from 2001 to 2012, and also found an increase in mortality in patients with the diagnosis of type 2 diabetes who had lower admission blood glucose.
In addition, rapid normalization of blood sugar levels can be harmful for patients with the diagnosis of diabetes [45]. The mechanism of insulin injury may be related to the anabolic effect that is similar to growth hormone [[49], [50], [51]]. Patients with the diagnosis of diabetes can adapt to chronic hyperglycemia by reducing the expression of glucose transporters (GLUT) in some cell types [52] in order to achieve rapid metabolic control and aggravate complications [53], which may also explain the increased risk of death during insulin therapy in septic patients with the diagnosis of diabetes.
We summarize the literature information involved in analyzing the benefit and risk of intensive insulin therapy, such as type, quality of evidence and number of cases, as shown in Table 1.
Table 1.
Summary of studies included in the analysis of the benefit and risk of intensive insulin therapy.
| References | Type | Quality of Evidence | Number of Cases |
|---|---|---|---|
| [28] | Randomized control trial | Ⅰ | 451 |
| [30] | Cohort study | Ⅱ | 1441 |
| [31] | Randomized control trial | Ⅰ | 1257 |
| [32] | Randomized control trial | Ⅰ | 110 |
| [20] | Randomized control trial | Ⅰ | 1200 |
| [6] | Randomized control trial | Ⅰ | 1548 |
| [33] | Meta analysis | Ⅰ | 8432 |
| [21] | Randomized control trial | Ⅰ | 537 |
| [24] | Randomized control trial | Ⅰ | 6104 |
| [12] | Cohort study | Ⅱ | 502 |
| [39] | Cohort study | Ⅱ | 1158 |
| [41] | Cohort study | Ⅱ | 2272 |
| [45] | Cohort study | Ⅱ | 1698 |
| [46] | Randomized control trial | Ⅰ | 64 |
| [47] | Randomized control trial | Ⅰ | 532 |
Despite the clinical controversy surrounding intensive insulin therapy, we believe that appropriate insulin therapy is crucial for septic patients with elevated blood sugar. Firstly, long-term hyperglycemia will cause pathological changes in various organs of the body, resulting in a series of chronic complications. Regardless of the hypoglycemia risks caused by intensive insulin therapy, the strictest blood sugar control does benefit the most. Secondly, the risk of hypoglycemia is not inevitable. While there is no expert consensus on a standard procedure for risk avoidance of intensive insulin scheme, severe hypoglycemia can be avoided by adjusting the monitoring frequency, administration method, infusion speed and insulin dosage based on the monitoring results of blood glucose level [54].
3. Clinical suggestion of intensive insulin scheme
3.1. Indication and contraindication of intensive insulin scheme
Through the aforementioned analysis of the clinical risk factors of intensive insulin therapy, we can know that not all patients can benefit from intensive insulin therapy. The individual differences may to some extent determine whether patients should receive the treatment, which requires consideration of the indications and contraindications of the treatment.
The most common indication of insulin therapy is the inability to control blood sugar levels through diet, exercise and oral drugs [55]. As all type 1 diabetics require subcutaneous or intravenous insulin therapy whether in the outpatient or inpatient setting, especially in the inpatient setting. Here we mainly discuss indications for intensive insulin therapy in patients with a diagnosis of type 2 diabetes, which include acute illness or surgery, glucose toxicity, contraindications (such as renal or liver failure) to or failure to achieve goals with oral antidiabetic medications, the need for flexible treatment [55], patients with cardiovascular lesions or retinal macular edema, failure to achieve satisfactory blood glucose control on multiple daily injections (MDI) [56], and extreme insulin resistance syndromes such as pregnancy, insulin allergy, and lipodystrophy syndromes [57]. Other indications for intensive insulin therapy include: ketoacidosis, acute infections, severely decompensated patients with clinical symptoms, and dehydration [58]. In addition, we know that a particular concern with septic patients with sepsis in ICUs with regards to subcutaneously administered medications is the risk of malabsorption, particularly for patients receiving vasopressors for hemodynamic support in their shock state. Ideally, patients who are in multi-pressor shock would be receiving an intravenous insulin infusion to avoid the issues associated with malabsorption of subcutaneous medications. And those patients requiring multiple vasopressors or high infusion rates of a single vasopressors would likely still benefit from intravenous insulin infusion rather than the subcutaneous infusion protocol.
Intensive insulin therapy is a common and effective therapeutic method, but there are also situations where the treatment is ineffective and/or dangerous to the patient. Consequently, it is necessary to understand the absolute and relative contraindications of intensive insulin therapy. Absolute contraindications [59] include: severe psychiatric illness; severe, rapidly progressive or proliferative retinopathy; regular exposure to strong magnetic fields. Relative contraindications [59] include: suboptimal adherence to diabetes treatment; suboptimal acceptance of treatment by the patient; poor hygiene and participation in violent sports; sensory (particularly visual) or gestural impairment (physically handicapped); end-stage renal failure and the risk of acidosis; living in extremely cold or heated environments for professional or personal reasons; patients taking underwater diving as a sport or profession; participation in extreme sports.
3.2. Suggestion of intensive insulin regimen in patients with sepsis
In the case of septic patients, while intensive insulin therapy improves clinical outcomes, there is indeed a risk of hypoglycemia. Although hypoglycemia is not an extremely difficult complication to treat, if it is not detected in time and appropriate measures are not taken, it will also bring serious consequences and even lead to the death of patients. Therefore, it is an essential step to monitor the blood glucose levels of septic patients treated with intensive insulin therapy.
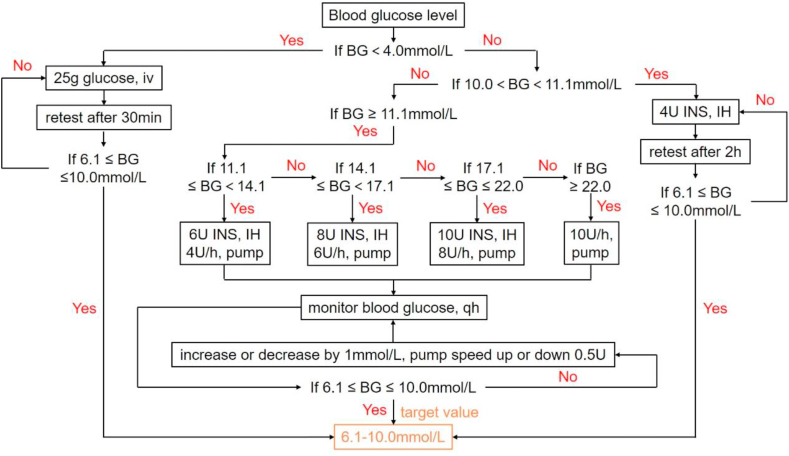
The first “Surviving Sepsis Campaign Guidelines” published in 2004 recommended that blood glucose be monitored frequently (every 30–60 min) after the project start and on a regular basis (every 4 h) once blood sugar levels stabilized [37]. The three subsequent published “Surviving Sepsis Campaign Guidelines” varied slightly in terms of recommendations regarding blood glucose monitoring frequency. They all recommended monitoring blood sugar every 1–2 h until blood glucose levels and the rate of insulin infusion are stable, and then every 4 h [[60], [61], [62]]. However, the mode of administration, the speed of administration and the frequency of monitoring for different blood glucose levels are not fully and specifically reflected in these guidelines. Currently, with reference to these guidelines and clinical practice, our hospital has proposed the following intensive insulin therapy process implemented in ICUs, and it has been applied in clinic. We made the flow chart, as shown in Fig. 2.
Fig. 2.
The intensive insulin therapy process currently implemented in ICUs of our hospital. BG: blood glucose; iv: intravenous injection; INS: insulin; IH: hypodermic injection; qh: quaque hora/every hour.
The target blood glucose level of this process is 6.1–10.0 mmol/L. We divide the patients into three different situations according to their blood glucose levels and take the appropriate measures. (1) Blood glucose (BG) < 4.0 mmol/L: 25 g glucose was injected intravenously and blood glucose levels were retested 30 min later. If the blood glucose level does not reach the target, the above administration method should be continued. (2) 10.0 < BG < 11.1 mmol/L: 4U INS was injected hypodermically and blood glucose was retested after 2 h. If the blood glucose level does not reach the standard, the above administration method should be continued. (3) BG ≥ 11.1 mmol/L: ①11.1 ≤ BG < 14.1 mmol/L: Hypodermic injection of 6U INS or pump insulin at a rate of 4U/h; ②14.1 = BG < 17.1 mmol/L: Hypodermic injection of 8U INS or pump insulin at a rate of 6U/h; ③17.1 = BG ≤ 22.0 mmol/L: Hypodermic injection of 10U INS or pump insulin at a rate of 8U/h; ④BG > 22.0 mmol/L: Pump insulin at a rate of 10U/h. In the third case, checking the blood glucose once every hour after the correction with pump. If the blood glucose level is not up to the standard and increases or decreases by 1 mmol/L, the pump speed is up or down 0.5U. And the blood glucose level was monitored again an hour later for subsequent adjustment of drug administration.
3.3. Matter needing attention and monitoring principle in the implementation of intensive insulin therapy in patients with sepsis
Sepsis is a clinically critical disease that needs special attention. From the above risk analysis, it is known that the risk factors of hypoglycemia in septic patients receiving intensive insulin therapy include: (1) Irregular diet or delayed meals; (2) Organ failure; (3) Patients who need hemodialysis; (4) Patients with the diagnosis of diabetes; (5) Compliance with the prescribed scheme, frequency of blood glucose measurement, use of care point equipment and continuous blood glucose monitoring [63]. The above situation should be closely monitored and evaluated to avoid serious adverse outcomes.
Most critically ill patients have disturbance of consciousness or treatment with mechanical ventilation and sedative drugs, which would make the symptoms be masked and go unnoticed. As a consequence, we should follow the principle of early detection and early treatment to take appropriate monitoring measures for septic patients treated with intensive insulin therapy. Fast blood glucose meter is often used to monitor blood glucose in clinic, and this method is the most common and convenient. Berghe et al. [6] suggested that newly admitted patients be measured once every 1–2 h until their blood glucose levels stabilize at 4.4–6.1 mmol/L, and then once every 4 h. If the blood glucose level is less than 4.4 mmol/L, it should be measured once every hour. In the blood glucose control scheme determined by Kanji et al. [64], it is required to measure blood glucose once every 1–2 h, but when the blood glucose value is ≤ 3.9 mmol/L or ≥ 14.1 mmol/L, the measurement frequency should be increased to once every 30 min. Although there are slight differences in the frequency of blood glucose monitoring proposed in different literatures, the overall trend is consistent with the recommendations given in guidelines [60,61]. That is, the interval time can be extended when the blood glucose is stable within the target range, and the monitoring frequency should be increased when the blood sugar is too high or too low.
Nevertheless, it is not sufficient to use the biochemical criterion of blood glucose value alone, because blood sugar values below the normal range do not always distinguish between normal and pathological hypoglycemia [65]. Pathological hypoglycemia is defined as a triad of hypoglycemia, symptoms of hypoglycemia and disappearance of symptoms after correction of blood sugar [66]. The symptoms caused by a sudden drop in blood sugar are related to the autonomic nervous system and the central nervous system. The former includes anxiety, palpitation, tremulousness, nausea, sweating and hunger [67,68]. The latter includes fatigue, weakness, seizures, confusion, focal neurological dysfunction and coma [69]. By observing whether patients have these clinical manifestations, we can detect the risk of hypoglycemia in time and treat it as soon as possible to prevent more serious consequences.
What's more, we should also pay attention to the replacement time of insulin configuration solution. Generally speaking, changing the insulin solution every 24 h not only ensures clinical efficacy, but also reduces drug waste and infection risk.
4. Discussion
Hyperglycemia is common in sepsis patients, which is positively correlated with the mortality of sepsis [6]. Recently, intensive insulin therapy, as an important method to improve the metabolic state of the body, has attracted increasing attention and become the research hotspot of sepsis-related hyperglycemia. And more trials investigating the effect of intensive insulin therapy on the prognosis of sepsis have appeared successively, but there are also some limitations. First of all, the descriptions of the insulin therapy goal of septic patients with elevated blood glucose in the guidelines and related literature are inconsistent. Secondly, there is no risk prediction model for hypoglycemia in intensive insulin therapy. Irregular or delayed meals, organ failure, the need for hemodialysis and a history of diabetes mellitus are all high-risk factors for hypoglycemia in patients with sepsis. However, it is important to note that septic patients being treated in ICUs have the potential to be intubated and mechanically ventilated, and those patients would not be receiving “meals” but would likely be receiving continuous tube feeds. Therefore, a delay in meals may not be a concern for ICU patients receiving continuous tube feeds. Definitely, if patients are not receiving any nutrition at the time of treatment and therapy initiation, this can be listed as a risk factor for hypoglycemia. Thirdly, the precautions to avoid the risk of hypoglycemia are unclear. Blood glucose monitoring is the simplest and most effective method. In general, the determination of monitoring frequency is in accordance with the following principles: (1) Severe high or low blood glucose levels are monitored once per 30min; (2) Slightly high or low blood glucose levels are monitored every 1–2 h; (3) When blood glucose levels are stable within the target range, the monitoring frequency should be reduced accordingly. We can also observe whether patients have clinical manifestations of hypoglycemia such as anxiety, tremor, palpitations, sweating, nausea, hunger, weakness, fatigue, confusion, seizures, focal neurological dysfunction and coma to detect and prevent hypoglycemia risks in time.
The reason for the rise of blood sugar in patients with sepsis, on the one hand, is that the stress state alters the normal physiological changes of the body, resulting in stress-induced hyperglycemia. On the other hand, it may be related to the fact that the patient is complicated with diabetes mellitus. Diabetes mellitus is one of the most common concomitant diseases in sepsis patients [70,71]. In a study conducted by Vught et al., a total of 1104 septic patients admitted to ICUs were included and found that 241 (21.8%) had a history of diabetes mellitus [72]. Although a study showed that septic patients with the diagnosis of type 2 diabetes did not increase the overall mortality, the authors also reported that septic patients with the diagnosis of type 2 diabetes had an increased incidence of hypoglycemic events compared with those without type 2 diabetes mellitus [73]. Accordingly, managing blood glucose levels in septic patients with the diagnosis of diabetes will aid in improving the prognosis of patients. The target blood glucose range of these patients may be higher than that of patients with simple sepsis. However, for septic patients with the diagnosis of diabetes, there is currently no evidence-based recommendation for the exact level of blood glucose control. This will be a hot issue that will require more research data to support it.
Concerning the problem of elevated blood sugar in patients suffering from sepsis caused by stress, there have been many studies on the use of intensive insulin therapy to control blood sugar. Some studies showed that intensive insulin therapy can reduce the incidence of complications and improve energy metabolism, thereby improve the prognosis of patients. However, some researches indicated that this method has no significant clinical benefit, but actually increases the occurrence of hypoglycemic events. This contradictory result could be attributed to significant differences in some aspects among the subjects included in various studies, such as the severity of the subject's condition, complications, pathophysiological status and genetic inheritance. Based on the fact that strict blood glucose control can improve prognosis but lead to hypoglycemic events as well, we should weigh its risk and benefit. It has been reported that the septic status of patients affected the accuracy of real-time continuous glucose monitoring (CGM) system in ICUs, and that the accuracy of patients with septic shock was significantly improved [74]. What is more worth mentioning is that this CGM technology could detect more episodes of hypoglycemic [12]. Therefore, the development of reliable and accurate CGM equipment in ICUs will help to avoid hypoglycemia-related side effects and optimize the implementation of intensive insulin therapy regimen in patients with sepsis [46].
Besides, it is difficult to apply the results of highly controlled clinical trials to daily practice [75]. The environment, patients, medical equipment and batch number of drugs selected in a clinical trial may differ from the actual clinical treatment, affecting the curative effect and drawing different conclusions. Simultaneously, the importance of minimizing blood glucose variability, the determination of the best target range, the classification of patients who benefit from returning to normal blood sugar levels and how to avoid hypoglycemic events are also important issues that need to be addressed further [76].
For patients with sepsis, insulin therapy should be initiated when the blood glucose level exceeds 10 mmol/L. Compared with conventional insulin therapy, intensive insulin therapy can improve morbidity and mortality in patients, as well as lead to a higher risk of hypoglycemia. Although the risk of hypoglycemia may only be an indicator of the disease severity and is not directly related to an increase in mortality, however, since the increase in hypoglycemic events is always accompanied by an increase in mortality in patients with sepsis, the possibility that hypoglycemia weakens some of the benefit of intensive insulin therapy cannot be ruled out. Based on the above fact that benefit and risk coexist and the insulin therapy practiced in ICUs of our hospital, it is recommended that blood glucose levels be controlled within the range of 6.1–10.0 mmol/L. Granted, the findings of available studies so far suggest that although hypoglycemic events do occur, they may not appear to the extent that would warrant the exclusion of intensive insulin therapy as a treatment modality in patients with severe sepsis. More importantly, intensive insulin therapy does have a greater clinical benefit and the risk of hypoglycemic events can be detected and prevented timely through blood glucose monitoring. Therefore, in the case of the condition that changes in blood glucose levels can be effectively monitored, intensive insulin therapy could be useful and have a minimal risk of hypoglycemia.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by the Key Research Project of Ningxia Hui Autonomous Region in 2021 (Major Project) [2021BEG01001].
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare no competing interests.
Acknowledgements
Special acknowledgement to Shao Liu, chief pharmacist of the Department of Pharmacy, Xiangya Hospital of Central South University, and Lina Zhang, chief physician of the Department of Critical Medicine of Xiangya Hospital of Central South University, for their help and support to this work.
Contributor Information
Jie Du, Email: xiangyadujie@163.com.
Zhi-cheng Gong, Email: gongzhicheng2013@163.com.
Abbreviations
- ICUs
Intensive care units
- ICU
Intensive care unit
- HPA
Hypothalamus-pituitary-adrenal
- NES
Neuroendocrine system
- A
Adrenaline
- NA
Noradrenaline
- Cr
Cortisol
- GH
Growth hormone
- Pro
Protein
- GNG
Gluconeogenesis
- Gn
Glycogen
- Glu
Glucose
- M
Macrophage
- DPP4
Dipeptidyl peptidase 4
- SGLT-2
Sodium-dependent glucose transporters 2
- SARS-COV2
Severe acute respiratory syndrome coronavirus 2
- GLP1-RAs
Glucagon-like peptide-1 receptor agonists
- GLUT
Glucose transporters
- MDI
Multiple daily injections
- BG
Blood glucose
- iv
intravenous injection
- INS
Insulin
- IH
Hypodermic injection
- qh
quaque hora/every hour
References
- 1.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angus D.C., Linde-Zwirble W.T., Lidicker J., Clermont G., Carcillo J., Pinsky M.R. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Wang H.E., Shapiro N.I., Angus D.C., Yealy D.M. National estimates of severe sepsis in United States emergency departments. Crit. Care Med. 2007;35(8):1928–1936. doi: 10.1097/01.CCM.0000277043.85378.C1. [DOI] [PubMed] [Google Scholar]
- 4.Xie J., Wang H., Kang Y., Zhou L., Liu Z., Qin B., et al. The epidemiology of sepsis in Chinese ICUs: a national cross-sectional survey. Crit. Care Med. 2020;48(3):e209–e218. doi: 10.1097/CCM.0000000000004155. [DOI] [PubMed] [Google Scholar]
- 5.Hirasawa H., Oda S., Nakamura M. Blood glucose control in patients with severe sepsis and septic shock. World J. Gastroenterol. 2009;15(33):4132–4136. doi: 10.3748/wjg.15.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van den Berghe G., Wouters P., Weekers F., Verwaest C., Bruyninckx F., Schetz M., et al. Intensive insulin therapy in critically ill patients. N. Engl. J. Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 7.Plummer M.P., Deane A.M. Dysglycemia and glucose control during sepsis. Clin. Chest Med. 2016;37(2):309–319. doi: 10.1016/j.ccm.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Leonidou L., Michalaki M., Leonaardou A., Polyzogopoulou E., Fouka K., Gerolymos M., et al. Stress-induced hyperglycemia in patients with severe sepsis: a compromising factor for survival. Am. J. Med. Sci. 2008;336(6):467–471. doi: 10.1097/MAJ.0b013e318176abb4. [DOI] [PubMed] [Google Scholar]
- 9.Stegenga M.E., Vincent J.L., Vail G.M., Xie J., Haney D.J., Williams M.D., et al. Diabetes does not alter mortality or hemostatic and inflammatory responses in patients with severe sepsis. Crit. Care Med. 2010;38(2):539–545. doi: 10.1097/CCM.0b013e3181c02726. [DOI] [PubMed] [Google Scholar]
- 10.Esper A.M., Moss M., Martin G.S. The effect of diabetes mellitus on organ dysfunction with sepsis: an epidemiological study. Crit. Care. 2009;13(1):1–6. doi: 10.1186/cc7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green J.P., Berger T., Garg N., Horeczko T., Suarez A., Radeos M.S., et al. Hyperlactatemia affects the association of hyperglycemia with mortality in nondiabetic adults with sepsis. Acad. Emerg. Med. 2012;19(11):1268–1275. doi: 10.1111/acem.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmad S., Khalid R. Blood glucose levels in neonatal sepsis and probable sepsis and its association with mortality. J. Coll. Physicians Surg Pak. 2012;22(1):15–18. [PubMed] [Google Scholar]
- 13.Marik P.E., Bellomo R. Stress hyperglycemia: an essential survival response. Crit. Care. 2013;17(2):1–7. doi: 10.1186/cc12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dungan K.M., Braithwaite S.S., Preiser J.C. Stress hyperglycaemia. Lancet. 2009;373(9677):1798–1807. doi: 10.1016/S0140-6736(09)60553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart B.B., Stanford G.G., Ziegler M.G., Lake C.R., Chernow B. Catecholamines: study of interspecies variation. Crit. Care Med. 1989;17(11):1203–1222. [PubMed] [Google Scholar]
- 16.Jeremitsky E., Omert L.A., Dunham C.M., Wilberger J., Rodriguez A. The impact of hyperglycemia on patients with severe brain injury. J. Trauma. 2005;58(1):47–50. doi: 10.1097/01.TA.0000135158.42242.B1. [DOI] [PubMed] [Google Scholar]
- 17.Orban J.C., Deroche D., Ichai C. [Septic shock: blood glucose regulation] Ann. Fr. Anesth. Reanim. 2006;25(3):275–279. doi: 10.1016/j.annfar.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Rim J., Gallini J., Jasien C., Cui X., Phillips L., Trammell A., et al. Use of oral anti-diabetic drugs and risk of hospital and intensive care unit admissions for infections. Am. J. Med. Sci. 2022;364(1):53–58. doi: 10.1016/j.amjms.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solerte S.B., Di Sabatino A., Galli M., Fiorina P. Dipeptidyl peptidase-4 (DPP4) inhibition in COVID-19. Acta Diabetol. 2020;57(7):779–783. doi: 10.1007/s00592-020-01539-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fayfman M., Galindo R.J., Rubin D.J., Mize D.L., Anzola I., Urrutia M.A., et al. A randomized controlled trial on the safety and efficacy of exenatide therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes. Diabetes Care. 2019;42(3):450–456. doi: 10.2337/dc18-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fushimi N., Shibuya T., Yoshida Y., Ito S., Hachiya H. Dulaglutide-combined basal plus correction insulin therapy contributes to ideal glycemic control in non-critical hospitalized patients. J Diabetes Investig. 2020;11(1):125–131. doi: 10.1111/jdi.13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans L., Rhodes A., Alhazzani W., Antonelli M., Coopersmith C.M., French C., et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–1247. doi: 10.1097/CCM.0000000000005337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van den Berghe G., Wilmer A., Hermans G., Meersseman W., Wouters P.J., Milants I., et al. Intensive insulin therapy in the medical ICU. N. Engl. J. Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 24.Brunkhorst F.M., Engel C., Bloos F., Meier-Hellmann A., Ragaller M., Weiler N., et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N. Engl. J. Med. 2008;358(2):125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 25.Yu W.K., Li W.Q., Wang X.D., Yan X.W., Qi X.P., Li N., et al. Influence and mechanism of a tight control of blood glucose by intensive insulin therapy on human sepsis. Zhonghua Wai Ke Za Zhi [Chinese Journal of Surgery] 2005;43(1):29–32. [PubMed] [Google Scholar]
- 26.Savioli M., Cugno M., Polli F., Taccone P., Bellani G., Spanu P., et al. Tight glycemic control may favor fibrinolysis in patients with sepsis. Crit. Care Med. 2009;37(2):424–431. doi: 10.1097/CCM.0b013e31819542da. [DOI] [PubMed] [Google Scholar]
- 27.Finfer S., Chittock D.R., Su S.Y., Blair D., Foster D., Dhingra V., et al. Intensive versus conventional glucose control in critically ill patients. N. Engl. J. Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 28.Annane D., Cariou A., Maxime V., Azoulay E., D’honneu G., Timsit J.F., et al. Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. JAMA. 2010;303(4):341–348. doi: 10.1001/jama.2010.2. [DOI] [PubMed] [Google Scholar]
- 29.Van den Berghe G.H. Role of intravenous insulin therapy in critically ill patients. Endocr. Pract. 2004;10(Suppl 2):17–20. doi: 10.4158/EP.10.S2.17. [DOI] [PubMed] [Google Scholar]
- 30.Trevelin S.C., Carlos D., Beretta M., da Silva J.S., Cunha F.Q. Diabetes mellitus and sepsis: a challenging association. Shock. 2017;47(3):276–287. doi: 10.1097/SHK.0000000000000778. [DOI] [PubMed] [Google Scholar]
- 31.Hansen T.K., Thiel S., Wouters P.J., Christiansen J.S., Van den Berghe G. Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-binding lectin levels. J. Clin. Endocrinol. Metab. 2003;88(3):1082–1088. doi: 10.1210/jc.2002-021478. [DOI] [PubMed] [Google Scholar]
- 32.Association A.D. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(Suppl 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lachin J.M., Gebuth S., Cleary P., Davis M.D., Nathan D.M. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N. Engl. J. Med. 2000;342(6):381–389. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin C.L., Albers J., Herman W.H., Cleary P., Waberski B., Greene D.A., et al. Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care. 2006;29(2):340–344. doi: 10.2337/diacare.29.02.06.dc05-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohkubo T., Kishikawa H., Araki E., Miyata T., Isami S., Motoyoshi S., et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res. Clin. Pract. 1995;28(2):103–117. doi: 10.1016/0168-8227(95)01064-K. [DOI] [PubMed] [Google Scholar]
- 36.Wiener R.S., Wiener D.C., Larson R.J. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300(8):933–944. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- 37.Dellinger R.P., Carlet J.M., Masur H., Gerlach H., Calandra T., Cohen J., et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2004;30:536–555. doi: 10.1007/s00134-004-2210-z. [DOI] [PubMed] [Google Scholar]
- 38.Mackenzie I., Ingle S., Zaidi S., Buczaski S. Hypoglycaemia? So what. Intensive Care Med. 2006;32:620–621. doi: 10.1007/s00134-006-0100-2. [DOI] [Google Scholar]
- 39.Van den Berghe G. First do no harm hypoglycemia or hyperglycemia. Crit. Care Med. 2006;34(11):2843–2844. doi: 10.1097/01.CCM.0000242913.88721.6E. [DOI] [PubMed] [Google Scholar]
- 40.Boord J.B., Graber A.L., Christman J.W., Powers A.C. Practical management of diabetes in critically ill patients. Am. J. Respir. Crit. Care Med. 2001;164:1763–1767. doi: 10.1164/ajrccm.164.10.2103068. [DOI] [PubMed] [Google Scholar]
- 41.Bagshaw S.M., Egi M., George C., Bellomo R., Australia New Zealand Intensive Care Society Database Management Committee Early blood glucose control and mortality in critically ill patients in Australia. Crit. Care Med. 2009;37(2):463–470. doi: 10.1097/CCM.0b013e318194b097. [DOI] [PubMed] [Google Scholar]
- 42.Kushimoto S., Abe T., Ogura H., Shiraishi A., Saitoh D., Fujishima S., et al. Impact of blood glucose abnormalities on outcomes and disease severity in patients with severe sepsis: an analysis from a multicenter, prospective survey of severe sepsis. PLoS One. 2020;15(3) doi: 10.1371/journal.pone.0229919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merz T.M., Finfer S. Pro/con debate: is intensive insulin therapy targeting tight blood glucose control of benefit in critically ill patients. Crit. Care. 2008;12(2):1–6. doi: 10.1186/cc6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vriesendorp T.M., van Santen S., DeVries J.H., de Jonge E., Rosendaal F.R., Schultz M.J., et al. Predisposing factors for hypoglycemia in the intensive care unit. Crit. Care Med. 2006;34(1):96–101. doi: 10.1097/01.CCM.0000194536.89694.06. [DOI] [PubMed] [Google Scholar]
- 45.Van den Berghe G., Wilmer A., Milants I., Wouters P.J., Bouckaert B., Bruyninckx F., et al. Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes. 2006;55(11):3151–3159. doi: 10.2337/db06-0855. [DOI] [PubMed] [Google Scholar]
- 46.Mebis L., Gunst J., Langouche L., Vanhorebeek I., Van den Berghe G. Indication and practical use of intensive insulin therapy in the critically ill. Curr. Opin. Crit. Care. 2007;13(4):392–398. doi: 10.1097/MCC.0b013e3281c1c9c8. [DOI] [PubMed] [Google Scholar]
- 47.Chou W., Chung M.H., Wang H.Y., Chen J.H., Chen W.L., Guo H.R., et al. Clinical characteristics of hyperglycemic crises in patients without a history of diabetes. J. Diabetes Investig. 2014;5(6):657–662. doi: 10.1111/jdi.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sathananthan M., Sathananthan A., Jeganathan N. Characteristics and outcomes of patients with and without type 2 diabetes mellitus and pulmonary sepsis. J. Intensive Care Med. 2020;35(9):836–843. doi: 10.1177/0885066619833910. [DOI] [PubMed] [Google Scholar]
- 49.Valarini R., Sousa M., Kalil R., Abumrad N., Riella M. Anabolic effects of insulin and amino acids in promoting nitrogen accretion in postoperative patients. JPEN - J. Parenter. Enter. Nutr. 1994;18(3):214–218. doi: 10.1177/0148607194018003214. [DOI] [PubMed] [Google Scholar]
- 50.Takala J., Ruokonen E., Webster N.R., Nielsen M.S., Zandstra D.F., Vundelinckx G., et al. Increased mortality associated with growth hormone treatment in critically ill adults. N. Engl. J. Med. 1999;341(11):785–792. doi: 10.1056/NEJM199909093411102. [DOI] [PubMed] [Google Scholar]
- 51.Hirsch I.B., Coviello A. Intensive insulin therapy in critically ill patients. N. Engl. J. Med. 2002;346(20):1586–1588. doi: 10.1056/nejm200205163462016. [DOI] [PubMed] [Google Scholar]
- 52.Klip A., Tsakiridis T., Marette A., Ortiz P.A. Regulation of expression of glucose transporters by glucose: a review of studies in vivo and in cell cultures. Faseb. J. 1994;8(1):43–53. doi: 10.1096/fasebj.8.1.8299889. [DOI] [PubMed] [Google Scholar]
- 53.Ferris F.L., Davis M.D., Aiello L.M. Treatment of diabetic retinopathy. N. Engl. J. Med. 1999;341(9):667–678. doi: 10.1056/NEJM199908263410907. [DOI] [PubMed] [Google Scholar]
- 54.Pisarchik A.N., Pochepen O.N., Pisarchyk L.A. Increasing blood glucose variability is a precursor of sepsis and mortality in burned patients. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0046582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mayfield J.A., White R.D. Insulin therapy for type 2 diabetes: rescue, augmentation, and replacement of beta-cell function. Am. Fam. Physician. 2004;70(3):489–500. [PubMed] [Google Scholar]
- 56.Pickup J.C., Renard E. Long-acting insulin analogs versus insulin pump therapy for the treatment of type 1 and type 2 diabetes. Diabetes Care. 2008;31(Suppl 2):S140–S145. doi: 10.2337/dc08-s235. [DOI] [PubMed] [Google Scholar]
- 57.Moyes V., Driver R., Croom A., Mirakian R., Chowdhury T.A. Insulin allergy in a patient with Type 2 diabetes successfully treated with continuous subcutaneous insulin infusion. Diabet. Med. 2006;23(2):204–206. doi: 10.1111/j.1464-5491.2006.01811.x. [DOI] [PubMed] [Google Scholar]
- 58.Hanefeld M., Fleischmann H., Siegmund T., Seufert J. Rationale for timely insulin therapy in type 2 diabetes within the framework of individualised treatment: 2020 update. Diabetes Ther. 2020;11(8):1645–1666. doi: 10.1007/s13300-020-00855-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lassmann-Vague V., Clavel S., Guerci B., Hanaire H., Leroy R., Loeuille G.A., et al. When to treat a diabetic patient using an external insulin pump. Expert consensus. Société francophone du diabète (ex ALFEDIAM) 2009. Diabetes Metab. 2010;36(1):79–85. doi: 10.1016/j.diabet.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 60.Dellinger P.R., Levy M.M., Rhodes A., Annane D., Gerlach H., Opal S.M., et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dellinger R.P., Levy M.M., Carlet J.M., Bion J., Parker M.M., Jaeschke R., et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2008;34:17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rhodes A., Evans L.E., Alhazzani W., Levy M.M., Antonelli M., Ferrer R., et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock. Intensive Care Med. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 63.Arabi Y.M., Tamim H.M., Rishu A.H. Hypoglycemia with intensive insulin therapy in critically ill patients: predisposing factors and association with mortality. Crit. Care Med. 2009;37(9):2536–2544. doi: 10.1097/CCM.0b013e3181a381ad. [DOI] [PubMed] [Google Scholar]
- 64.Kanji S., Singh A., Tierney M., Meggison H., Mclntyre L., Hebert P.C. Standardization of intravenous insulin therapy improves the efficiency and safety of blood glucose control in critically ill adults. Intensive Care Med. 2004;30(5):804–810. doi: 10.1007/s00134-004-2252-2. [DOI] [PubMed] [Google Scholar]
- 65.Merimee T.J., Tyson J.E. Stabilization of plasma glucose during fasting: normal variations in two separate studies. N. Engl. J. Med. 1974;291(24):1275–1278. doi: 10.1056/NEJM197412122912404. [DOI] [PubMed] [Google Scholar]
- 66.Whipple A.O. Thesurgical therapy of hyperinsu-linism. J. Int. Chir. 1938;3:237–276. [Google Scholar]
- 67.Hepburn D.A., Deary I.J., Frier B.M., Patrick A.W., Quinn J.D., Fisher B.M. Symptoms of acute insulin-induced hypoglycemia in humans with and without IDDM: factor-analysis approach. Diabetes Care. 1991;14(11):949–957. doi: 10.2337/diacare.14.11.949. [DOI] [PubMed] [Google Scholar]
- 68.Towler D.A., Havlin C.E., Craft S., Cryer P. Mechanism of awareness of hypoglycemia: perception of neurogenic (predominantly cholinergic) rather than neuroglycopenic symptoms. Diabetes. 1993;42(12):1791–1798. doi: 10.2337/diab.42.12.1791. [DOI] [PubMed] [Google Scholar]
- 69.Guettier J.M., Gorden P. Hypoglycemia. Endocrinol Metab. Clin. N. Am. 2006;35(4):753–766. doi: 10.1016/j.ecl.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 70.Martin G.S., Mannino D.M., Eaton S., Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 71.Trevelin S.C., Carlos D., Beretta M., da Silva J.S., Cunha F.Q. Diabetes mellitus and sepsis: a challenging association. Shock. 2017;47(3):276–287. doi: 10.1097/SHK.0000000000000778. [DOI] [PubMed] [Google Scholar]
- 72.van Vught L.A., Scicluna B.P., Hoogendijk A.J., Wiewel M.A., Klein Klouwenberg P.M., Cremer O.L., et al. Association of diabetes and diabetes treatment with the host response in critically ill sepsis patients. Crit. Care. 2016;20(1):1–15. doi: 10.1186/s13054-016-1429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sathananthan M., Sathananthan A., Jeganathan N. Characteristics and outcomes of patients with and without type 2 diabetes mellitus and pulmonary Sepsis. J. Intensive Care Med. 2020;35(9):836–843. doi: 10.1177/0885066619833910. [DOI] [PubMed] [Google Scholar]
- 74.Lorencio C., Leal Y., Bonet A., Bondia J., Palerm C.C., Tache A., et al. Real-time continuous glucose monitoring in an intensive care unit: better accuracy in patients with septic shock. Diabetes Technol. Therapeut. 2012;14(7):568–575. doi: 10.1089/dia.2012.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rothwell P.M. External validity of randomised controlled trials:“to whom do the results of this trial apply?”. Lancet. 2005;365(9453):82–93. doi: 10.1016/S0140-6736(04)17670-8. [DOI] [PubMed] [Google Scholar]
- 76.Preiser J.C., Devos P. Clinical experience with tight glucose control by intensive insulin therapy. Crit. Care Med. 2007;35(9 Suppl):S503–S507. doi: 10.1097/01.CCM.0000278046.24345.C7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.