Abstract
The prevalence and severity of obesity have increased in recent years, likely the result of complex interactions between genes, dietary intake, physical activity, and the environment. The expression of genes favoring the storage of excess calories as fat, which have been selected for over many millennia and are relatively static, has become maladaptive in a rapidly changing environment that minimizes opportunities for energy expenditure and maximizes opportunities for energy intake. The consequences of childhood and adolescent obesity include earlier puberty and menarche in girls, type 2 diabetes and increased incidence of the metabolic syndrome in youth and adults, and obesity in adulthood. These changes are associated with cardiovascular disease as well as with several cancers in adults, likely through insulin resistance and production of inflammatory cytokines. Although concerns have arisen regarding environmental exposures, there have been no formal expert recommendations. Currently, the most important factors underlying the obesity epidemic are the current opportunities for energy intake coupled with limited energy expenditure.
INTRODUCTION
From the perspectives of classification and terminology, prevalence, and recognized consequences, obesity in childhood and youth has been in a state of flux. Currently, “overweight” is defined by a body mass index (BMI)–for-age of ≥85th percentile but <95th percentile in children and adolescents, and “obesity"”is defined as a BMI-for-age of ≥95th percentile (1).
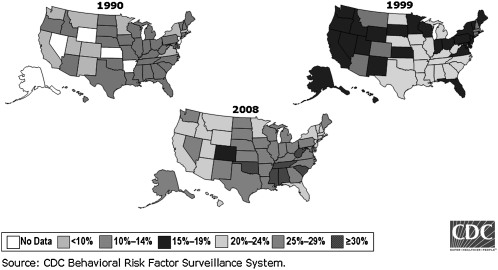
The prevalence and severity of obesity have been increasing in children and adolescents, as well as in adults (2–4), and it is estimated that by 2015 >40% of US adults will be obese (4). The rising prevalence of obesity in this (and other) countries has been identified as the “obesity epidemic.” Data on the rising prevalence of obesity in adults, by state, over the past 2 decades are provided in Figure 1. Adult obesity is linked to comorbidities that have been attributed to estimates as low as 26,000 (6) and as high as 365,000 (7) deaths annually in the United States, with most estimates typically in the range of 100,000–200,000 (8–11). In 2 longitudinal analyses adjusted for smoking status, annual death risk among nonsmokers increased by 12–40% among overweight and by 50–150% among those who were obese (12, 13). In addition, obesity was the second leading cause of preventable, premature death in the United States after smoking (7), and in high-income countries smoking, alcohol consumption, and obesity are the most important causes of cancer (14). Obesity has been estimated to decrease life expectancy by as little as 0.8 (6) to as much as 7 y (11, 15).
FIGURE 1.
Obesity trends among US adults. Obesity is defined as BMI (in kg/m2) ≥30. Reproduced from reference 5.
Multiple studies have shown the association of pediatric and adolescent obesity with obesity in adults. Overweight children are more prone to becoming overweight adults, especially at higher BMIs (16) or if they have an obese parent (17). Almost half of overweight adults were overweight as children, and two-thirds of children in the highest BMI quartile transitioned into the highest BMI quartile as young adults (18). Obesity during the adolescent years is associated with many adverse health consequences (14, 19, 20), and dietary habits, physical inactivity, and rates and degree of obesity become worse with the transition from the teen years into the young adult years (21). Adolescents with a higher BMI experienced 30% higher rates of mortality as young and middle-aged adults, although the persistence of higher BMIs into adulthood accounted for much of the association (22). In addition, Must et al (23) showed that being an obese adolescent was associated with an increased risk of multiple comorbidities in adulthood even if the obesity did not persist.
This article reviews potential factors contributing to the obesity epidemic, the effect of childhood and adolescent obesity on health, and recommendations that health care providers may consider when working with children, their parents, and their communities.
FACTORS CONTRIBUTING TO THE OBESITY EPIDEMIC IN CHILDREN AND ADOLESCENTS
The increasing prevalence of obesity in children is due to complex interactions between genetic and environmental factors. The expression of genes favoring the storage of excess calories as fat—genes that have been selected for over many millennia and are relatively static—becomes maladaptive in a rapidly changing environment that minimizes the opportunities for energy expenditure and maximizes the opportunities for energy intake (24). A recent update of the human obesity gene map reported on hundreds of potential gene loci that affect body weight and adiposity in humans and the mouse model (25), and many believe that the heritability of body fatness is best considered a polygenic, non-Mendelian pattern. However, there are rare syndromes in children that are associated with obesity that are inherited in a Mendelian manner, including leptin deficiency, MC4R polymorphisms, and polymorphisms of the FTO gene (26). A review of the genetics of mouse obesity models is beyond the scope of this article, but the reader may find Leibel's review informative (27). Both early and late feeding practices of infants may have a greater effect on later adiposity than previously thought (28) because premature infants and infants born small-for-gestational-age or large-for-gestational-age are at an increased risk of obesity and its comorbidities (ie, they may develop comorbidities at a lower level of body fatness than appropriate for their gestational age). Being an infant of a mother with diabetes, even if not large for gestational age, conveys an additional risk (29).
Although increasing attention is being paid to the genetic contribution to obesity, the vast majority of studies have examined the effect of environmental and behavioral factors. Recent changes in nutritional and physical activity patterns are considered to have produced changes in the fatness of children. Increased consumption of snacks has contributed disproportionately to energy intake when comparing dietary patterns 12 y apart. For example, snacks contributed 18% of total energy consumed among children aged 6 to 11 y old in 1977, which rose to 24% in 1996; in a similar fashion, snacks contributed to 21% of total energy among youth aged 12 to 18 y old in 1977, which rose to 25% of total energy in 1996 (30). In addition, portion size accounts for 17–19% of the variability in energy intake (31). One study noted that nearly one-third of children and adolescents consumed fast food, and at those encounters they ate a greater number of calories with a greater caloric density than food from other sources; the authors estimated that this pattern would lead to an additional 57 kcal in the daily diet of the average US child and could account for 6 lb weight gain per child per year (32). A recent review of efforts to prevent obesity noted that there was “probable” evidence that consumption of low-energy-dense foods would help decrease the risk of obesity, whereas the consumption of sweetened beverages and the frequency or large portions of energy-dense foods would increase the risk (33). As suggested earlier, fast-food restaurants have a relative abundance of energy-dense foods and sweetened beverages. Increased calcium intake has also been associated with a lower risk of obesity, but this association may represent an overall healthier lifestyle and better diet (34).
There is convincing evidence that increased levels and frequency of physical activity are associated with a decreased risk of obesity (33). Multiple studies have documented physical inactivity by youth (35, 36). Associated with decreased levels of physical activity are increased levels of sedentary activities, such as television viewing, playing video games, and computer activities. A comprehensive review reported that most studies noted a relation between television viewing and adiposity, but that the effect size was very small (37). However, a longitudinal study published later that year reported that the attributable fraction from television viewing >2 h/d was 17% on overweight status [defined in this study population as a BMI (in kg/m2) >25], and 15% on poor fitness, which suggests an important effect on the population (38). About 60% of children's television commercials are food-related, and TV viewing promotes both decreased energy expenditure and increased energy intake (39). Notably, greater changes in dietary habits and physical inactivity occur during the transition between adolescence and adulthood than during other transition periods (21).
Environmental exposures may affect the risk of obesity. A recent editorial reviewed the potential effect of both environmental chemicals and family environment, with potential genetic interaction, on the health outcomes and the timing of puberty in girls (40). For example, phthalate urine concentrations were shown to be associated with central adiposity and insulin resistance in a cross-sectional study of adult males (41), and bisphenol A was shown to inhibit adiponectin release from human adipocytes (42). There are some examples of environmental enablers that favor the gain of body fat, and the reader is encouraged to read the article by Elobeid and Allison (43) for additional information. Abuse, anxiety, depression, and family stress may be associated with obesity, potentially through the adrenal axis and/or stress-related eating patterns (44).
In the United States, as in many other countries, obesity disproportionately affects those with limited economic resources. In a recent systematic review of the literature, minority and lower socioeconomic groups were disproportionately affected by obesity, but the relation was complex (5). Socioeconomic status is linked to other factors, which include parental education, number of parents in the home, parent's age, race-ethnicity, residential and geographical location, and availability of safe recreational areas. Lower socioeconomic status is associated with a reduction of quality-adjusted life years (11), in part mediated through behavioral risk factors such as obesity, smoking, sedentary lifestyle, and alcohol. More specifically, obese 18-y-olds in the US population can expect to live ≈47 quality-adjusted life-years in contrast to 43 quality-adjusted life-years for obese 18-y-olds living under the poverty threshold (11). However, the risk of mortality is greater in the lowest income groups, even after adjustment for those behaviors (45). The disparities in risk of overweight and obese status among those with limited economic resources has persisted and even increased; indigent adolescent males, for example, are more likely to remain overweight, and indigent adolescent females are more likely to become overweight, than their peers with greater economic resources (46). Factors in the built environment may contribute to the relation between lower socioeconomic status and obesity; these factors may include walkability of the neighborhood (47) and a greater number of fast-food restaurants. Individuals who reside in neighborhoods with a greater density of fast-food restaurants are associated with less healthy lifestyles and with increased risk of obesity (48).
EFFECT OF CHILDHOOD AND ADOLESCENT OBESITY
As noted earlier, obesity during the teen years is associated with many adverse health consequences (see discussion below), which include greater rates of mortality as young adults (22). The adolescent years are a time of change in body composition, as well as changes in insulin sensitivity and in concentrations of the adipokines (chemicals produced by the fat cell) such as leptin and adiponectin. Puberty is associated with increases in lean body mass as well as fat mass, with a greater increase in fat mass in girls compared with boys (49). Girls experience a greater increase in fat mass throughout childhood and puberty than boys, and heavier girls experience a greater increase in fat mass during puberty than other girls (50–53). There is a rise in insulin resistance during puberty (54, 55), as well as a worsening of various components of the metabolic syndrome, and concurrently, changes in leptin and adiponectin (56–58). However, pubertal changes in leptin are sex-dependent, and increased concentrations of androgens in males can lead to a decrease in leptin concentrations (59). As previously described, the likelihood of an obese child becoming an obese adult increases with the age of the child independently of the duration of time that the child has been obese (16–18). Childhood obesity is associated with earlier pubertal maturation in girls, and early maturing girls tend to have higher BMIs and body fat at the time of menarche (36, 60–66). However, most girls increase their body fat as they progress through puberty, and therefore a causal relation has not been established. A longitudinal study noted that adult obesity was more strongly associated with childhood obesity than with the timing of puberty (67), which suggests that childhood obesity is the underlying factor for both age of onset of puberty and adult obesity.
Many of the comorbidities associated with obesity are related to several abnormal anthropometric and metabolic changes that tend to cluster and are termed the metabolic syndrome (68). Several definitions have been proposed for the metabolic syndrome in adults; the 2 most commonly used definitions in children and adolescents are modified from the adult criteria and may include the criteria of insulin resistance and type 2 diabetes. The definitions proposed by Cook et al (69) define metabolic syndrome as the presence of ≥3 criteria that include elevated triglycerides (>110 mg/100 mL), low HDL (<40 mg/100 mL), abdominal circumference >90th percentile by sex, elevated fasting glucose (>110 mg/100 mL), and high blood pressure (>90th percentile). The criteria proposed by de Ferranti et al (70) uses ≥3 of 5 criteria: high blood pressure (>90th percentile for age, sex, height), central obesity (waist circumference >75th percentile for age and sex), low HDL (1.3 mmol/L in girls), high fasting glucose (>6.1 mmol/L), and hypertriglyceridemia (>1.1 mmol/L). These definitions, although similar, lead to slightly different outcomes.
Abdominal obesity is the most commonly observed metabolic syndrome phenotype, the one best correlated with other metabolic syndrome phenotypes (71) and the most predictive of the risk of developing the syndrome in children over a 15 y period (72). Thus, it is likely that abdominal obesity that promotes insulin resistance is the most central factor underlying the metabolic syndrome in genetically predisposed individuals due to the increased flux of free fatty acids, increased gluconeogenesis, and decreased insulin clearance by the liver. Alternatively, in a multisite study in adults, insulin resistance as well as fat mass and distribution were independently associated with metabolic risk (73). There has been an increase in the prevalence of metabolic syndrome (74), with increases in hypertension, waist circumference, and hypertriglyceridemia, which account for much of the increased prevalence compared with fasting hyperglycemia and low HDL concentrations (75).
Cook et al (69) noted that the prevalence of metabolic syndrome using the third National Health and Nutrition Examination Survey (NHANES III) data set was 28.7% among adolescents with BMIs >95th percentile. This contrasts to a prevalence of 31.2% using the de Ferranti criteria (70) in the same data set. The prevalence of metabolic syndrome appears to be increasing, with 4.2% prevalence overall in the NHANES III, rising to 6.4% in the NHANES 1999–2000 data (76), and, most recently to 8.6% (77). Earlier studies noted a similar temporal trend in obesity, which suggests that longitudinal changes in the variables associated with metabolic syndrome are due, in part, to the rising prevalence and severity of obesity (78) and that childhood overweight and obesity are most strongly associated with adult clustering of variables associated with the metabolic syndrome (72). The prevalence of metabolic syndrome in adolescents increases with higher BMIs; the odds of metabolic syndrome were 1.55 greater for every half-unit increase in BMI z score (79). The prevalence of metabolic syndrome appears to be greater in male than in female adolescents and in Hispanics and whites when contrasted to blacks (77).
Several studies note the increased risk of several cancers with obesity. An increase of BMI by 5 was associated in men with an increased risk of esophageal adenocarcinoma [relative risk (RR): 1.52], as well as thyroid (RR: 1.33), colon (RR: 1.24), and renal (RR: 1.24) cancers, and, in women, of endometrial (RR: 1.59), gallbladder (RR: 1.59), and esophageal (RR: 1.51) adenocarcinomas, and renal (RR: 1.34) cancer. There was a reduced risk of premenopausal breast cancer with obesity but an increased risk of postmenopausal breast cancer (80). Therefore, the links between childhood and adolescent obesity and adult diseases, specifically cancer and cardiovascular diseases, are noted within this article. A recent review examining the potential links between obesity and cardiovascular disease included fetal origins, epigenetic regulation, metabolic programming, and inflammatory changes whereas other authors have suggested fat patterning, with increased central adiposity, as a link (81).
The “thrifty gene hypothesis,” as proposed by Hales and Barker (82), suggests that malnutrition during fetal and early postnatal life leads to modifications in physiologic functions to improve early survival. The thrifty gene hypothesis should not be confused with the “catch-up growth” hypothesis as described by Cianfarani et al (83) regarding the effects of poor fetal growth on obesity and comorbidity risk. These physiologic and metabolic changes may predispose to later disease, consistent with a longitudinal study that showed that those with coronary artery disease, as well as type 2 diabetes, grew slowly in infancy and fetal life, but increased their BMI rapidly during childhood (84). Epigenetic regulation (changes in gene expression through DNA methylation or histone modification) (85) may affect pre- and postnatal growth patterns and mediate metabolic programming. These links between obesity and cardiovascular disease and type 2 diabetes can be extended to obesity and cancer. For example, several studies describe associations between obesity and breast cancer and the potential contribution of insulin resistance and adipokines (86, 87). There is increasing recognition that fat tissue is a regulator of inflammation (88) and that enlarged adipocytes promote inflammation (89). At a molecular level, leptin and proinflammatory cytokines lead to the induction of aromatase activity, and subsequently, to the production of estrogen (90, 91) as well as insulin resistance. Alternatively, adiponectin resistance in transgenic/knockout mice (genetic models of resistance to insulin's indirect effects on hepatic gluconeogenesis) was shown to exacerbate insulin resistance (92), which implies that adiponectin is a cause rather than a result of insulin resistance. Adiponectin expression is down-regulated by greater insulin concentrations, and several studies have noted an inverse relation between adiponectin concentrations and breast cancer risk (93–96).
Obesity is associated with lower serum vitamin D concentrations; however, it is possible that obese individuals simply store more of this fat-soluble vitamin in a bioinactive form as a result of their adiposity. Low serum vitamin D concentrations were observed in 74% of obese adolescents and 32% of controls. In this study, vitamin D status was influenced by dietary intake of vitamin D, season, race and ethnicity, and degree of adiposity (97). Measures of adiposity, such as BMI and waist circumference, were modestly associated with vitamin D (98). The relation between obesity and vitamin D appears to be related to both dietary factors and decreased subcutaneous conversion (99). The relation between obesity and cancer may also be mediated through vitamin D. Vitamin D deficiency is noted as relating to cancer as well as to other disorders (100, 101). Finally, circulating concentrations of vitamin D have shown an inverse correlation with insulin sensitivity, the incidence of type 2 diabetes, body fatness, and other phenotypes associated with the metabolic syndrome (eg, dyslipidemia) in adults and adolescents (97, 102, 103).
Two factors related to obesity, dietary intake and physical activity, may also affect the risk of cancer. In a review of prospective studies, the only dietary factors strongly associated with cancer were alcohol consumption, obesity, and weight gain (104). In the European Prospective Investigation into Cancer and Nutrition (EPIC) study, there was a decreased incidence of cancers among vegetarians when contrasted to meat eaters (105). Several studies have examined the relation between cancer and physical activity, with many examining the relation between breast cancer and physical activity. Lifetime physical activity was associated with decreased risk of breast cancer; in one study, recreational activity, especially in late adolescence and early adulthood, was protective (106). Other studies also documented the relation between recreational activity and breast cancer (107, 108). One additional study noted that forms of physical activity such as household or occupational activities reduced risk, but recreational activities did not affect the risk of developing breast cancer (109).
RECOMMENDATIONS
Primary prevention through lifestyle and environmental interventions has been suggested as the best approach for addressing cancer worldwide (14), and this approach may serve as the best advice to address the obesity epidemic and the health consequences associated with obesity. However, the health care provider needs to deliver this message in a fashion that does not encourage disordered eating and eating disorders (110). One expert group recommends that health care providers should intervene whenever a child (or adolescent) reaches a BMI above the 85th percentile (111). A comprehensive review noted that promotion of physical activity and reduced television viewing time appears to be helpful to prevention. And there is evidence that school programs while in place may improve physical activity and improve diet behaviors by promoting increased fruit and vegetable consumption (33).
The American Academy of Pediatrics has produced a supplement to address the pediatric and adolescent obesity epidemic, and the following recommendations are adapted from the American Academy of Pediatrics (34, 112). Families should limit consumption of sugar-sweetened beverages as well as limit meals at fast-food restaurants, and families should be encouraged to eat meals together and to eat breakfast. Children and adolescents should limit screen time to 2 h daily, and parents should remove televisions and computers from bedrooms. The American Academy of Pediatrics, as well as other national organizations, recommend 60 min of physical activity daily. In addition to these recommendations, it would be prudent to consider familial growth patterns and history of comorbidities and other risk factors. Pediatricians should become concerned with children at lower levels of body fatness with a strong family history of hypertension, type 2 diabetes, or other metabolic syndrome or cardiovascular comorbidities or with children who were small for gestational age due to their higher probability of developing comorbidities at lower levels of body fatness. In addition, the association of calcium intake with lower weight may be mediated through the association of greater calcium intake with overall better diet (34); there may be a modest effect of fruit and vegetable consumption on preventing obesity (34).
Although there has been concern regarding environmental exposures, the scientific justification is not strong enough at this time, and there have been no formal expert recommendations. If the consumer is interested in reducing exposures to potential endocrine disruptors, products are available through several resources, eg, at http://www.ewg.org/reports/nottoopretty (cited 9 September 2009) and http://www.safecosmetics.org (cited 9 September 2009). An additional approach to minimizing potential endocrine disruptors is the use of organic foods. Organic foods are typically more expensive than nonorganic foods, the purchase of which might be challenging for the budget-minded consumer. In a case in which a decision had to be made about which fruit and vegetables should be organic and which ones need not be, consumer groups such as the Environmental Working Group recommend buying only organic when purchasing foods that contain the highest concentrations of pesticides, otherwise known as “the dirty dozen”: peaches, strawberries, nectarines, apples, spinach, celery, pears, sweet bell peppers, cherries, potatoes, lettuce, and imported grapes (cited 9 September 2009 from http://www.foodnews.org). However, the biggest environmental exposure that one should be concerned about is the ready availability of energy-dense foods at a lower cost per calorie than healthful foods and the ready availability of “competitive foods” in schools. These competitive foods are sold or served in schools in cafeterias and vending machines. In a recent study, students who ate school lunches consumed fewer calories from competitive foods when contrasted with those who consumed competitive foods but did not eat a school lunch (113).
CONCLUSIONS
The prevalence and severity of obesity has increased in recent years and is likely the result of complex interactions among genes, dietary intake, physical activity, and the environment. The consequences of childhood and adolescent obesity include metabolic syndrome and type 2 diabetes in youth and in adulthood and the development of obesity in adulthood. Many of the metabolic, cardiovascular, and oncologic consequences are likely mediated through insulin resistance and production of inflammatory adipokines. The most important factors underlying the obesity epidemic are the current opportunities of energy intake coupled with limited energy expenditure.
Acknowledgments
We acknowledge the cheerful clerical assistance provided by Lynn Hanrahan.
Neither author had a conflict of interest.
REFERENCES
- 1.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007;120:S164–92 [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006;295:1549–55 [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA 2008;299:2401–5 [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev 2007;29:6–28 [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention US obesity trends: trends by state 1985–2008. Updated 20 November 2009. Available from: http://www.cdc.gov/obesity/data/trends.html (cited 16 March 2010)
- 6.Reuser M, Bonneux L, Willekens F. The burden of mortality of obesity at middle and old age is small. A life table analysis of the US Health and Retirement Survey. Eur J Epidemiol 2008;23:601–7 [DOI] [PubMed] [Google Scholar]
- 7.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA 2004;291:1238–45 [DOI] [PubMed] [Google Scholar]
- 8.Danaei G, Ding E, Mozaffarian D, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med 2009;6:e1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flegal KM, Graubard B, Williamson D, Gail M. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA 2007;298:2028–37 [DOI] [PubMed] [Google Scholar]
- 10.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA 2005;293:1861–7 [DOI] [PubMed] [Google Scholar]
- 11.Muennig P, Lubetkin E, Jia H, Franks P. Gender and the burden of disease attributable to obesity. Am J Public Health 2006;96:1662–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med 2006;355:763–78 [DOI] [PubMed] [Google Scholar]
- 13.Lawlor DA, Hart CL, Hole DJ, Davey Smith G. Reverse causality and confounding and the associations of overweight and obesity with mortality. Obesity (Silver Spring) 2006;14:2294–304 [DOI] [PubMed] [Google Scholar]
- 14.Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet 2005;366:1784–93 [DOI] [PubMed] [Google Scholar]
- 15.Peeters A, Barendregt J, Willekens F, Mackenbach JP, Mamun AA, Bonneux L. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med 2003;138:24–32 [DOI] [PubMed] [Google Scholar]
- 16.Guo SS, Chumlea WC. Tracking of body mass index in children in relation to overweight in adulthood. Am J Clin Nutr 1999;70:145S–8S [DOI] [PubMed] [Google Scholar]
- 17.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med 1997;337:869–73 [DOI] [PubMed] [Google Scholar]
- 18.Deshmukh-Taskar P, Nicklas TA, Morales M, Yang SJ, Zakeri I, Berenson GS. Tracking of overweight status from childhood to young adulthood: the Bogalusa Heart Study. Eur J Clin Nutr 2006;60:48–57 [DOI] [PubMed] [Google Scholar]
- 19.Dietz WH. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics 1998;101:518–25 [PubMed] [Google Scholar]
- 20.Dietz WH, Robinson TN. Overweight children and adolescents. N Engl J Med 2005;352:2100–9 [DOI] [PubMed] [Google Scholar]
- 21.Harris KM, Gordon-Larsen P, Chantala K, Udry JR. Longitudinal trends in race/ethnic disparities in leading health indicators from adolescence to young adulthood. Arch Pediatr Adolesc Med 2006;160:74–81 [DOI] [PubMed] [Google Scholar]
- 22.Engeland A, Bjorge T, Tverdal A, Sogaard AJ. Obesity in adolescence and adulthood and the risk of adult mortality. Epidemiology 2004;15:79–85 [DOI] [PubMed] [Google Scholar]
- 23.Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med 1992;327:1350–5 [DOI] [PubMed] [Google Scholar]
- 24.Chung WK, Leibel RL. Considerations regarding the genetics of obesity. Obesity (Silver Spring) 2008;16:S33–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rankinen T, Zuberi A, Chagnon YC, et al. The human obesity gene map: the 2005 update. Obesity (Silver Spring) 2006;14:529–644 [DOI] [PubMed] [Google Scholar]
- 26.Welsh P, Polisecki E, Robertson M, et al. Unraveling the directional link between adiposity and inflammation: a bidirectional Mendelian randomization approach. J Clin Endocrinol Metab 2010;95:93–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leibel RL. Molecular physiology of weight regulation in mice and humans. Int J Obes (Lond) 2008;32(suppl 7):S98–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koletzko B, von Kries R, Monasterolo RC, et al. Can infant feeding choices modulate later obesity risk? Am J Clin Nutr 2009;89:1502S–8S [DOI] [PubMed] [Google Scholar]
- 29.Catalano PM, Farrell K, Thomas A, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr 2009;90:1303–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jahns L, Siega-Riz AM, Popkin BM. The increasing prevalence of snacking among US children from 1977 to 1996. J Pediatr 2001;138:493–8 [DOI] [PubMed] [Google Scholar]
- 31.McConahy KL, Smiciklas-Wright H, Mitchell DC, Picciano MF. Portion size of common foods predicts energy intake among preschool-aged children. J Am Diet Assoc 2004;104:975–9 [DOI] [PubMed] [Google Scholar]
- 32.Bowman SA, Gortmaker SL, Ebbeling CB, Pereira MA, Ludwig DS. Effects of fast-food consumption on energy intake and diet quality among children in a national household survey. Pediatrics 2004;113:112–8 [DOI] [PubMed] [Google Scholar]
- 33.Brown T, Kelly S, Summerbell C. Prevention of obesity: a review of interventions. Obes Rev 2007;8:127–30 [DOI] [PubMed] [Google Scholar]
- 34.Davis MM, Gance-Cleveland B, Hassink S, Johnson R, Paradis G, Resnicow K. Recommendations for prevention of childhood obesity. Pediatrics 2007;120:S229–53 [DOI] [PubMed] [Google Scholar]
- 35.Aaron DJ, Storti KL, Robertson RJ, Kriska AM, LaPorte RE. Longitudinal study of the number and choice of leisure time physical activities from mid to late adolescence: implications for school curricula and community recreation programs. Arch Pediatr Adolesc Med 2002;156:1075–80 [DOI] [PubMed] [Google Scholar]
- 36.Kimm SYS, Glynn NW, Kriska AM, et al. Decline in physical activity in black girls and white girls during adolescence. N Engl J Med 2002;347:709–15 [DOI] [PubMed] [Google Scholar]
- 37.Marshall SJ, Biddle SJH, Gorely T, Cameron N, Murdey I. Relationships between media use, body fatness and physical activity in children and youth: a meta-analysis. Int J Obes Related Metab Disord 2004;28:1238–46 [DOI] [PubMed] [Google Scholar]
- 38.Hancox RJ, Milne BJ, Poulton R. Association between child and adolescent television viewing and adult health: a longitudinal birth cohort study. Lancet 2004;364:257–62 [DOI] [PubMed] [Google Scholar]
- 39.Borzekowski DLG, Robinson TN. The 30-second effect: an experiment revealing the impact of television commercials on food preferences of preschoolers. J Am Diet Assoc 2001;101:42–6 [DOI] [PubMed] [Google Scholar]
- 40.Biro FM, Wolff MS, Kushi LH. Impact of yesterday's genes and today's diet and chemicals on tomorrow's women. J Pediatr Adolesc Gynecol 2009;22:3–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult US males. Environ Health Perspect 2007;115:876–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect 2008;116:1642–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elobeid MA, Allison DB. Putative environmental-endocrine disruptors and obesity: a review. Curr Opin Endocrinol Diabetes Obes 2008;15:403–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vamosi M, Heitmann BL, Kyvik KO. The relation between an adverse psychological and social environment in childhood and the development of adult obesity: a systematic literature review. Obes Rev (Epub ahead of print 30 July. 2009) [DOI] [PubMed] [Google Scholar]
- 45.Lantz PM, House JS, Lepkowski JM, Williams DR, Mero RP, Chen J. Socioeconomic factors, health behaviors, and mortality results from a nationally representative prospective study of US adults. JAMA 1998;279:1703–8 [DOI] [PubMed] [Google Scholar]
- 46.Sherwood NE, Wall M, Neumark-Sztainer D, Story M. Effect of socioeconomic status on weight change patterns in adolescents. Prev Chronic Dis 2009;6:A19. [PMC free article] [PubMed] [Google Scholar]
- 47.Sallis JF, Saelens BE, Frank LD, et al. Neighborhood built environment and income: examining multiple health outcomes. Soc Sci Med 2009;68:1285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li F, Harmer P, Cardinal BJ, Bosworth M, Johnson-Shelton D. Obesity and the built environment: Does the density of neighborhood fast-food outlets matter? Am J Health Promot 2009;23:203–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maynard LM, Wisemandle W, Roche AF, Chumlea WC, Guo SS, Siervogel RM. Childhood body composition in relation to body mass index. Pediatrics 2001;107:344–50 [DOI] [PubMed] [Google Scholar]
- 50.Biro FM, Huang B, Morrison JA, Horn PS, Daniels SR. Body mass index and waist-to-height changes during teen years in girls are influenced by childhood body mass index. J Adolesc Health 2010;46:245–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Demerath EW, Schubert CM, Maynard LM, et al. Do changes in body mass index percentile reflect changes in body composition in children? Data from the Fels Longitudinal Study. Pediatrics 2006;117:e487–95 [DOI] [PubMed] [Google Scholar]
- 52.Freedman DS, Wang J, Maynard LM, et al. Relation of BMI to fat and fat-free mass among children and adolescents. Int J Obes (Lond) 2005;29:1–8 [DOI] [PubMed] [Google Scholar]
- 53.Huang TTK, Johnson MS, Figueroa-Colon R, Dwyer JH, Goran MI. Growth of visceral fat, subcutaneous abdominal fat, and total body fat in children. Obes Res 2001;9:283–9 [DOI] [PubMed] [Google Scholar]
- 54.Ball GDC, Huang TTK, Gower BA, et al. Longitudinal changes in insulin sensitivity, insulin secretion, and beta-cell function during puberty. J Pediatr 2006;148:16–22 [DOI] [PubMed] [Google Scholar]
- 55.Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes 2001;50:2444–50 [DOI] [PubMed] [Google Scholar]
- 56.Retnakaran R, Zinman B, Connelly PW, Harris SB, Hanley AJG. Nontraditional cardiovascular risk factors in pediatric metabolic syndrome. J Pediatr 2006;148:176–82 [DOI] [PubMed] [Google Scholar]
- 57.Weiss R, Taksali SE, Dufour S, et al. The “obese insulin-sensitive” adolescent: importance of adiponectin and lipid partitioning. J Clin Endocrinol Metab 2005;90:3731–7 [DOI] [PubMed] [Google Scholar]
- 58.Winer JC, Zern TL, Taksali SE, et al. Adiponectin in childhood and adolescent obesity and its association with inflammatory markers and components of the metabolic syndrome. J Clin Endocrinol Metab 2006;91:4415–23 [DOI] [PubMed] [Google Scholar]
- 59.Horlick MB, Rosenbaum M, Nicolson M, et al. Effect of puberty on the relationship between circulating leptin and body composition. J Clin Endocrinol Metab 2000;85:2509–18 [DOI] [PubMed] [Google Scholar]
- 60.Adair LS, Gordon-Larsen P. Maturational timing and overweight prevalence in US adolescent girls. Am J Public Health 2001;91:642–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Biro FM, McMahon RP, Striegel-Moore R, et al. Impact of timing of pubertal maturation on growth in black and white female adolescents: the National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr 2001;138:636–43 [DOI] [PubMed] [Google Scholar]
- 62.Demerath EW, Towne B, Chumlea WC, et al. Recent decline in age at menarche: the Fels Longitudinal Study. Am J Hum Biol 2004;16:453–7 [DOI] [PubMed] [Google Scholar]
- 63.Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. Relation of age at menarche to race, time period, and anthropometric dimensions: the Bogalusa Heart Study. Pediatrics 2002;110:e43. [DOI] [PubMed] [Google Scholar]
- 64.Kaplowitz PB, Slora EJ, Wasserman RC, Pedlow SE, Herman-Giddens ME. Earlier onset of puberty in girls: relation to increased body mass index and race. Pediatrics 2001;108:347–53 [DOI] [PubMed] [Google Scholar]
- 65.Power C, Lake JK, Cole TJ. Body mass index and height from childhood to adulthood in the 1958 British born cohort. Am J Clin Nutr 1997;66:1094–101 [DOI] [PubMed] [Google Scholar]
- 66.Wattigney WA, Srinivasan SR, Chen W, Greenlund KJ, Berenson GS. Secular trend of earlier onset of menarche with increasing obesity in black and white girls: the Bogalusa Heart Study. Ethn Dis 1999;9:181–9 [PubMed] [Google Scholar]
- 67.Freedman DS, Khan L, Serdula M, Dietz W, Srinivasan S, Berenson G. The relation of menarcheal age to obesity in childhood and adulthood: the Bogalusa heart study. BMC Pediatr 2003;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haslam DW, James WP. Obesity. Lancet 2005;366:1197–209 [DOI] [PubMed] [Google Scholar]
- 69.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med 2003;157:821–7 [DOI] [PubMed] [Google Scholar]
- 70.de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N. Prevalence of the metabolic syndrome in American adolescents findings from the Third National Health and Nutrition Examination Survey. Circulation 2004;110:2494–7 [DOI] [PubMed] [Google Scholar]
- 71.Anderson PJ, Critchley JA, Chan JC, et al. Factor analysis of the metabolic syndrome: obesity vs insulin resistance as the central abnormality. Int J Obes Relat Metab Disord 2001;25:1782–8 [DOI] [PubMed] [Google Scholar]
- 72.Srinivasan SR, Myers L, Berenson G. Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood. Diabetes 2002;51:204–9 [DOI] [PubMed] [Google Scholar]
- 73.Ferrannini E, Balkau B, Coppack SW, et al. Insulin resistance, insulin response, and obesity as indicators of metabolic risk. J Clin Endocrinol Metab 2007;92:2885–92 [DOI] [PubMed] [Google Scholar]
- 74.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults findings from the third National Health and Nutrition Examination Survey. JAMA 2002;287:356–9 [DOI] [PubMed] [Google Scholar]
- 75.Ford ES, Giles W, Mokdad A. Increasing prevalence of the metabolic syndrome among US adults. Diabetes Care 2004;27:2444–9 [DOI] [PubMed] [Google Scholar]
- 76.Duncan GE, Li SM, Zhou XH. Prevalence and trends of a metabolic syndrome phenotype among US adolescents, 1999–2000. Diabetes Care 2004;27:2438–43 [DOI] [PubMed] [Google Scholar]
- 77.Johnson WD, Kroon JJ, Greenway FL, Bouchard C, Ryan D, Katzmarzyk PT. Prevalence of risk factors for metabolic syndrome in adolescents: National Health and Nutrition Examination Survey (NHANES), 2001–2006. Arch Pediatr Adolesc Med 2009;163:371–7 [DOI] [PubMed] [Google Scholar]
- 78.Srinivasan SR, Myers L, Berenson GS. Rate of change in adiposity and its relationship to concomitant changes in cardiovascular risk variables among biracial (black-white) children and young adults: the Bogalusa Heart Study. Metabolism 2001;50:299–305 [DOI] [PubMed] [Google Scholar]
- 79.Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004;350:2362–74 [DOI] [PubMed] [Google Scholar]
- 80.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–78 [DOI] [PubMed] [Google Scholar]
- 81.Musaad S, Haynes EN. Biomarkers of obesity and subsequent cardiovascular events. Epidemiol Rev 2007;29:98–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hales CN, Barker DJP. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 1992;35:595–601 [DOI] [PubMed] [Google Scholar]
- 83.Cianfarani S, Germani D, Branca F. Low birthweight and adult insulin resistance: the “catch-up growth” hypothesis. Arch Dis Child Fetal Neonatal Ed 1999;81:F71–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barker DJ, Osmond C, Kajantie E, Eriksson JG. Growth and chronic disease: findings in the Helsinki Birth Cohort. Ann Hum Biol 2009;36:445–58 [DOI] [PubMed] [Google Scholar]
- 85.Delcuve GP, Rastegar M, Davie JR. Epigenetic control. J Cell Physiol 2009;219:243–50 [DOI] [PubMed] [Google Scholar]
- 86.Lorincz AM, Sukumar S. Molecular links between obesity and breast cancer. Endocr Relat Cancer 2006;13:279–92 [DOI] [PubMed] [Google Scholar]
- 87.Rose DP, Komninou D, Stephenson GD. Obesity, adipocytokines, and insulin resistance in breast cancer. Obes Rev 2004;5:153–65 [DOI] [PubMed] [Google Scholar]
- 88.Juge-Aubry CE, Henrichot E, Meier CA. Adipose tissue: a regulator of inflammation. Best Pract Res Clin Endocrinol Metab 2005;19:547–66 [DOI] [PubMed] [Google Scholar]
- 89.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr 2006;83:461S–5S [DOI] [PubMed] [Google Scholar]
- 90.Catalano S, Marsico S, Giordano C, et al. Leptin enhances, via AP-1, expression of aromatase in the MCF-7 cell line. J Biol Chem 2003;278:28668–76 [DOI] [PubMed] [Google Scholar]
- 91.Turgeon C, Gingras S, Carrière MC, Blais Y, Labrie F, Simard J. Regulation of sex steroid formation by interleukin-4 and interleukin-6 in breast cancer cells. J Steroid Biochem Mol Biol 1998;65:151–62 [DOI] [PubMed] [Google Scholar]
- 92.Lin HV, Kim JY, Pocai A, et al. Adiponectin resistance exacerbates insulin resistance in insulin receptor transgenic/knockout mice. Diabetes 2007;56:1969–76 [DOI] [PubMed] [Google Scholar]
- 93.Hou WK, Xu YX, Yu T, et al. Adipocytokines and breast cancer risk. Chin Med J 2007;120:1592–6 [PubMed] [Google Scholar]
- 94.Mantzoros C, Petridou E, Dessypris N, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab 2004;89:1102–7 [DOI] [PubMed] [Google Scholar]
- 95.Miyoshi Y, Funahashi T, Kihara S, et al. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res 2003;9:5699–704 [PubMed] [Google Scholar]
- 96.Tworoger SS, Eliassen AH, Kelesidis T, et al. Plasma adiponectin concentrations and risk of incident breast cancer. J Clin Endocrinol Metab 2007;92:1510–6 [DOI] [PubMed] [Google Scholar]
- 97.Alemzadeh R, Kichler J, Babar G, Calhoun M. Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism 2008;57:183–91 [DOI] [PubMed] [Google Scholar]
- 98.McGill AT, Stewart J, Lithander F, Strik C, Poppitt S. Relationships of low serum vitamin D3 with anthropometry and markers of the metabolic syndrome and diabetes in overweight and obesity. Nutr J 2008;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000;72:690–3 [DOI] [PubMed] [Google Scholar]
- 100.Grant WB, Mohr SB. Ecological studies of ultraviolet B, vitamin D and cancer since 2000. Ann Epidemiol 2009;19:446–54 [DOI] [PubMed] [Google Scholar]
- 101.Holick MF.Vitamin D deficiency. N Engl J Med 2007;357:266–81 [DOI] [PubMed] [Google Scholar]
- 102.Liu E, Meigs JB, Pittas AG, et al. Plasma 25-hydroxyvitamin D is associated with markers of the insulin resistant phenotype in nondiabetic adults. J Nutr 2009;139:329–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pittas AG, Lau J, Hu F, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 2007;92:2017–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Michels KB, Mohllajee AP, Roset-Bahmanyar E, Beehler GP, Moysich KB. Diet and breast cancer: a review of the prospective observational studies. Cancer 2007;109:2712–49 [DOI] [PubMed] [Google Scholar]
- 105.Key TJ, Appleby P, Spencer E, Travis R, Roddam A, Allen N. Cancer incidence in vegetarians: results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford). Am J Clin Nutr 2009;89:1620S–6S [DOI] [PubMed] [Google Scholar]
- 106.Kruk J. Lifetime physical activity and the risk of breast cancer: a case-control study. Cancer Detect Prev 2007;31:18–28 [DOI] [PubMed] [Google Scholar]
- 107.Bernstein L, Patel AV, Ursin G, et al. Lifetime recreational exercise activity and breast cancer risk among black women and white women. J Natl Cancer Inst 2005;97:1671–9 [DOI] [PubMed] [Google Scholar]
- 108.Verloop J, Rookus MA, van der Kooy K, van Leeuwen FE. Physical activity and breast cancer risk in women aged 20–54 years. J Natl Cancer Inst 2000;92:128–35 [DOI] [PubMed] [Google Scholar]
- 109.Friedenreich CM, Bryant HE, Courneya KS. Case-control study of lifetime physical activity and breast cancer risk. Am J Epidemiol 2001;154:336–47 [DOI] [PubMed] [Google Scholar]
- 110.Patton GC, Selzer R, Coffey C, Carlin JB, Wolfe R. Onset of adolescent eating disorders: population based cohort study over 3 years. BMJ 1999;318:765–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nader PR, O'Brien M, Houts R, et al. Identifying risk for obesity in early childhood. Pediatrics 2006;118:e594–601 [DOI] [PubMed] [Google Scholar]
- 112.Spear BA, Barlow SE, Ervin C, et al. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics 2007;120:S254–88 [DOI] [PubMed] [Google Scholar]
- 113.Fox MK, Gordon A, Nogales R, Wilson A. Availability and consumption of competitive foods in US public schools. J Am Diet Assoc 2009;109:S57–66 [DOI] [PubMed] [Google Scholar]