Abstract
Chk1 is a multifunctional protein kinase that plays essential roles in cell survival and cell cycle checkpoints. Chk1 is phosphorylated at multiple sites by several protein kinases, but the precise effects of these phosphorylations are largely unknown. Using a knockout-knockin system, we examined the abilities of Chk1 mutants to reverse the defects of Chk1-null cells. Wild-type Chk1 could rescue all the defects of Chk1-null cells. Like endogenous Chk1, wild-type Chk1 localized in both the cytoplasm and the nucleus, and its centrosomal association was enhanced by DNA damage. The mutation at S345 resulted in mitotic catastrophe, impaired checkpoints, and loss of the ability to localize in the cytoplasm, but the mutant retained the ability to be released from chromatin upon encountering genotoxic stressors. In contrast, the mutation at S317 resulted in impaired checkpoints and loss of chromatin release upon encountering genotoxic stressors, but its mutant retained the abilities to prevent mitotic catastrophes and to localize in the cytoplasm, suggesting the distinct effects of these phosphorylations. The forced immobilization of S317A/S345A in centrosomes resulted in the prevention of apoptosis in the presence or absence of DNA damage. Thus, two-step phosphorylation of Chk1 at S317 and S345 appeared to be required for proper localization of Chk1 to centrosomes.
Maintaining the genomic stability of a cell requires a complex network of cell cycle checkpoint mechanisms that prevent cell cycle progression when damage to DNA is encountered or key processes are not completed (24, 28). After detection of specific DNA or DNA-protein structures, a signal is transduced to effector molecules that implement checkpoint-dependent responses, such as cell cycle arrest or apoptosis.
In mammals, signals initiated by DNA damage sensors very rapidly transduce to ATM and ATR kinases, which are both extremely large proteins with homology to phosphoinositide 3-kinases (PIKKs) that phosphorylate a great number of substrates, including Chk1 (1, 32). ATM is mainly activated in response to DNA double-strand breaks. ATR forms a heterodimer with ATRIP that binds to UV-damaged DNA or to RPA-coated single-stranded DNA (45). ATR is also activated during a checkpoint response in the Xenopus system (12, 19). Very recently, Kumagai et al. reported that TopBP1 directly activates the ATR-ATRIP complex in vitro (20). Because both protein complexes localize to sites of DNA damage, it is possible that TopBP1 activates ATR-ATRIP in the locality of checkpoint-inducing DNA structure. In addition, ATM regulates the recruitment of ATR to sites of DNA damage, leading to double-strand break-induced Chk1 phosphorylation (2, 13).
The checkpoint kinases Chk1 and Chk2 were first identified in fission yeast as essential for cell cycle arrest before mitosis in response to DNA damage or DNA replication blockage, respectively (26, 37). Unlike Chk2, examination of mouse cells deficient in Chk1 revealed multiple essential roles for this kinase in cell cycle regulation and checkpoint responses (21, 36). Although the activation mechanism of Chk2 has recently been investigated in some detail (42), the manner in which Chk1 function is regulated remains largely elusive. The C-terminal region of Chk1 possesses several conserved SQ/TQ motifs, in which serine or threonine residues are the phosphorylation sites preferred by PIKK. Indeed, S317 and S345 of Chk1 were phosphorylated in vivo in response to DNA damage or a DNA replication block (11, 12, 43), and phosphorylation at these sites was blocked in cells lacking the kinase ATR (21) and was markedly inhibited in cells with a reduced amount of Rad17 (44) or an absence of Hus1 (40). Notably, these phosphorylations were also observed under unperturbed cell cycle conditions, suggesting that phosphorylation of Chk1 might also regulate an unperturbed cell cycle (14). Checkpoint-dependent phosphorylation has been suggested to regulate Chk1 function, since phosphorylated Chk1 has higher kinase activity than has unphosphorylated Chk1 and since each Chk1 mutant, S317A or S345A, showed poor activation in response to DNA damage (43). The crystal structure of the Chk1 protein revealed an open kinase conformation, implying that it is catalytically active in the unmodified form (6); however, the C-terminal domain of Chk1 negatively regulates Chk1 kinase activity, and checkpoint-dependent phosphorylation of this region has been suggested to reverse this autoinhibition (16). In addition to phosphorylation by PIKK, S280 and S301 of Chk1 were phosphorylated in vivo by protein kinase B (17, 34) and by Cdk1 (33), respectively, although the physiological significance of their phosphorylation is largely unknown.
Very recently, another regulatory mechanism of Chk1 function has been described (35). Checkpoint-dependent release of Chk1 from chromatin was found to be important for the proper functioning of Chk1 activity, and this release was dependent on PIKK. Thus, phosphorylation of Chk1 could also affect its cellular localization, thereby regulating checkpoint responses. For example, S345, but not T323 (homologous to mammalian S317), appeared to be required for checkpoint function in fission yeast (4), and this phosphorylation enhanced the accumulation of Chk1 in the nucleus through interaction with 14-3-3 protein (8). However, in mammals, the centrosomal association of Chk1 plays an important role in preventing premature activation of cyclin B-Cdk1 during an unperturbed cell cycle (18). Thus, there remains some controversy as to where Chk1 really functions. To evaluate the physiological significance of Chk1 phosphorylations and to elucidate the mechanism by which they regulate Chk1 activity, knockout-knockin experiments are obviously required.
With the use of conditional Chk1 knockout cells exogenously expressing its mutants, we report here that both S317 and S345 were required for proper checkpoint responses, but S317 was dispensable for cell survival in the absence of DNA damage or replication stress. We also demonstrated that centrosomal association could complement the phenotypes of the Chk1 phosphorylation site mutants, suggesting that the centrosomal association of Chk1 may be regulated by its phosphorylation. Finally, endogenous Chk1-deficient cells expressing the Chk1 mutants showed that a loss of checkpoint function per se caused chromosomal instability.
MATERIALS AND METHODS
Establishment of cells expressing mutants of Chk1.
Twenty micrograms of pCAGGS-Chk1 (wild type) and its mutants (S317A, S345A, S317AS345A, S280A, S280E, S301A, S357A/S366A, S317E, and S345E) and 5 μg of PGK-Hyg vector were linearized with SalI and KpnI, respectively. The linearized DNA was electroporated into Chk1lox/− embryonic stem (ES) cells with a Gene Pulser II (27). Cells were selected with 0.15 mg/ml hygromycin B for 7 days. Single colonies were screened by reverse transcription-PCR with a set of primers: Chk1sc-05 (5′-CAGCCCCTCATACATTGATAAATT-3′) and Myc-04 (5′-TGCATATTCAGATCCTCTTCTGAG-3′).
Antibodies.
The antibodies used were as follows: anti-Chk1 (sc-8408 [Santa Cruz] for Western blotting and C9358 [Sigma] for immunohistochemistry), anti-phospho-Ser345-Chk1 (no. 2341; Cell Signaling), anti-phospho-Ser317-Chk1 (ab2834-50; abcam), anti-phospho-Ser10 histone H3 (06-570; Upstate), anti-Chk2 (sc-9064; Santa Cruz), anti-p53 (NCL-p53-505; Novocasta Laboratory), anti-IKKα (sc-7182; Santa Cruz), anti-Orc2 (sc-13238; Santa Cruz), anti-Nek2 (sc-33167; Santa Cruz), anti-lamin B (33-2000; Zymed laboratories), anti-pericentrin (PRB-432C [Covance] and 611815 [BD Transduction Laboratory]), anti-phospho-Tyr15-Cdk1 (219440; Calbiochem), and anti-β-actin (ab2676-100; abcam).
G2 checkpoint assay.
The G2 checkpoint assay was assessed using phospho-histone H3 staining as described previously (41). In brief, cells were harvested, washed with phosphate-buffered saline (PBS), and fixed in 70% cold ethanol overnight. The fixed cells were washed twice with PBS, permeabilized in 0.25% Triton-PBS on ice for 10 min, and then washed with PBS and suspended in 100 μl of α-phospho-histone H3 antibody in 1% bovine serum albumin-PBS for 3 h at room temperature. The resultant cells were washed with PBS and incubated in α-rabbit immunoglobulin G fluorescein isothiocyanate-conjugated antibody diluted 1:100 with 1% bovine serum albumin-PBS for 30 min at room temperature. The immunostained cells were then washed twice with PBS and stained in 50 μg/ml propidium iodide-100 μg/ml RNase A solution for 1 h and subjected to fluorescence-activated cell sorter analysis.
Radio-resistant DNA synthesis.
Chk1-disrupted ES cells (1 × 106) were cultured with ES medium containing 10 nCi/ml [14C]thymidine (Amersham) for 24 h, washed with PBS, and cultured again for 30 min in ES medium. The cells were then X-irradiated (10 Gy), cultured for 1 h, and then pulse-labeled with ES medium containing 2.5 μCi/ml [3H]thymidine for 30 min. The labeled cells were harvested and fixed in ice-cold 70% ethanol overnight. The fixed cells were applied to membranes on the filtration plate, and the membranes were washed with 70% ethanol and then 95% ethanol (Nalgen Nunc; no. 255984). Radioactivity on the membranes was determined with a liquid scintillation counter. Radio-resistant DNA synthesis (RDS) was determined by the radioactivity of 3H divided by that of 14C.
For experiments using cells expressing Chk1 transiently, 3 × 106 Chk1Δ/− ES cells were transfected with 9 μg of pCAGGS-Chk1-PACT or pCAGGS-Chk1, cultured for an additional 12 h, and then analyzed for RDS assay.
Subcellular fractionation.
Subcellular fractionation of mammalian cells was performed according to a previously reported method (25, 35). In brief, 6 × 106 ES cells were suspended in 400 μl of solution A (10 mM HEPES 7.9, 10 mM KCl, 1.5 mM MgCl2, 0.34 M sucrose, 10% glycerol, 1 mM dithiothreitol, protease and phosphatase inhibitors), and Triton X-100 was added to a final concentration of 0.1%. The cells were incubated on ice for 5 min, and cytoplasmic (S1) and nuclear fractions were harvested by centrifugation at 1,300 × g for 4 min. The isolated nuclei were then washed in solution A, lysed in 400 μl solution B (3 mM EDTA, 0.2 mM EGTA, 1 mM DTT, protease and phosphatase inhibitors), and incubated on ice for 10 min. The soluble nuclear (S2) and chromatin fractions were harvested by centrifugation at 1,700 × g for 4 min. The isolated chromatin (P2) was then washed in solution B, centrifuged at 10,000 × g for 1 min, and resuspended in 400 μl Laemmle sample buffer. The P2 fraction was sonicated four times for 30 s each time using a Biorupter.
Immunostaining in situ.
Cells on glass slides were fixed in −20°C methanol-acetone (1:1) for 7 min. Immunofluorescence microscopy was performed with the combinations of antibodies specified in the figure legends as described previously (18).
Transfection and TUNEL staining.
Chk1lox/− ES cells were infected with adenoviruses expressing Cre recombinase to disrupt the endogenous Chk1 allele. Cells (1 × 106) deficient in endogenous Chk1 were cultured for 24 h. The cells were then cotransfected with 1 μg of pME18S-GFP and 3 μg of pCAGGS-Chk1-PACT or pCAGGS-Chk1 using Lipofectamine 2000 Reagent (Invitrogen). Twenty-four hours after transfection, cells were harvested and fixed in 2% paraformaldehyde for 30 min at room temperature. Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining was performed with an In Situ Cell Death Detection Kit, TMR red (Roche Diagnostics). TUNEL-positive, GFP-positive cells were counted under a microscope.
RESULTS
Establishment of Chk1lox/− ES cells that exogenously express Chk1 phosphorylation site mutants.
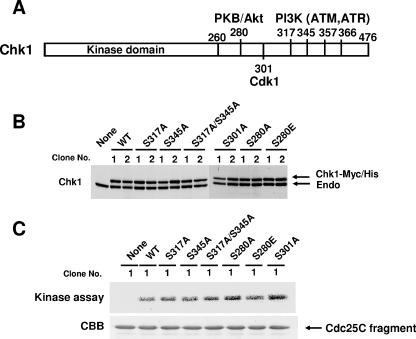
In mammalian cells, Chk1 is known to be phosphorylated at multiple sites by PIKK and other kinases in response to DNA damage or a DNA replication block (Fig. 1A). However, it is not known whether these phosphorylations are essential for Chk1 functions or what effect they have on cell cycle arrest or cell survival. To address these questions, we performed knockout-knockin experiments using Chk1lox/− ES cells. To avoid any effect arising from a difference in the amounts of mutant proteins expressed and clonal effects, we selectively used cell lines that express the mutant proteins at a level similar to that of endogenous Chk1 protein (Fig. 1B). To confirm whether wild-type or mutant Chk1 proteins exogenously expressed in ES cells really have kinase activity, we examined this activity in vitro using immunoprecipitates (lysates from clone no. 1) obtained with anti-Myc antibody and a Cdc25C fragment as a substrate and found that these mutants exhibited kinase activity similar to that of wild-type Chk1 (Fig. 1C).
FIG. 1.
Establishment of Chk1lox/− ES cells expressing wild-type Chk1 and its phosphorylation site mutants. (A) Schematic presentation of Chk1 sites targeted for phosphorylation by several kinases, including PIKK, Akt/protein kinase B, and Cdk1. (B) Expression of wild-type (WT) Chk1 and its mutants. Two groups of independent Chk1lox/− ES cells expressing wild-type or mutant Chk1-Myc-His were harvested, and the lysates were subjected to immunoblotting using anti-Chk1 antibodies. Endo, endogenous Chk1. (C) The lysates from cells (clone no.1) were also immunoprecipitated using anti-Myc antibodies, and the precipitates were subjected to an in vitro kinase assay using the GST-Cdc25C fragment as a substrate (CBB).
Cells expressing S317A and S345A mutants were defective in cell cycle checkpoints.
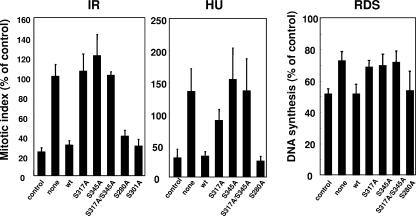
In the previous study, we found that Chk1 protein was not detected and cell cycle checkpoints were completely defective at 30 h after infection with adenoviruses expressing Cre, as described previously (27). Therefore, we examined the abilities of wild-type or mutant Chk1 proteins using cell lines of clone no. 1 to reverse the loss of cell cycle checkpoint functions in response to several genotoxic stresses at this time point. With respect to the G2/M checkpoint, prevention of mitotic entry in response to infrared radiation was almost completely reversed by wild-type Chk1, S280A, and S301A, but not by the mutants S317A, S345A, and S317A/S345A (Fig. 2, left panel). Similar results were observed when cells were treated with hydroxyurea (HU) (Fig. 2, middle panel). With respect to the intra-S phase checkpoint, as with the G2/M checkpoint, Chk1-dependent RDS was not restored by S317A, S345A, or S317A/S345A mutants (Fig. 2, right panel). Similar results were also observed when clone no. 2 cell lines were used (data not shown). Taken together, these results suggested that phosphorylation of Chk1 at S317 and S345 may be indispensable for cell cycle checkpoint functions. Although S357 and S366 are also conserved phosphorylation sites targeted by PIKK, a previous report demonstrated that these sites were not likely to be phosphorylated in vivo in the event of a DNA replication block (43). Consistent with this, the S357A/S366A double mutant reversed the cell death observed in Chk1-depleted cells (data not shown). S317E and S345E did not restore checkpoint functions (data not shown), suggesting that replacement of the Ser residue with Glu did not mimic the phosphorylation status in this case, as suggested previously (4, 11).
FIG. 2.
Mutations of Chk1 in S317 and S345 abrogated Chk1-dependent checkpoint functions. Chk1lox/− ES cells expressing wild-type (wt) Chk1 or its mutants were infected with adenoviruses expressing Cre recombinase. Cells were treated with infrared radiation (5 Gy) or HU (2.5 mM) 30 h after infection, the mitotic indices were determined by counting phospho-histone H3-positive cells, and the results are presented as percentages of the control (no treatment; mitotic index, 3.7 ± 0.6). RDS was determined as described in Materials and Methods. The data are means plus standard deviations of at least three independent experiments. None, no exogenous Chk1.
Mutation at S317, but not at S345, did not cause mitotic catastrophe.
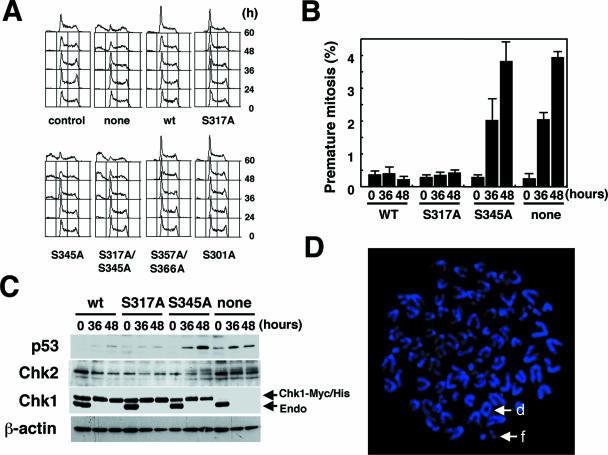
Chk1 is a multifunctional kinase that regulates cell cycle progression and cell cycle checkpoints. Accordingly, we next asked whether mutants of Chk1 could prevent the cell death observed in Chk1-depleted ES cells. We found a significant increase in the population of the sub-G1 fraction in Chk1-depleted cells (Fig. 3A, none), and a similar increase was observed in cells expressing S345A and S317A/S345A mutants. In contrast, cells expressing S317A, as well as the wild type, S357A/S366A, and S301A, did not undergo cell death (Fig. 3A). Consistent with this, premature mitosis measured by the counting of phospho-histone H3-positive cells with less than a 4 N DNA content was significantly increased in cells expressing S345A mutants, whereas this increase was not detected at all in cells expressing the wild type or the S317A mutant (Fig. 3B). These results suggested that phosphorylation of S317 was dispensable for the prevention of premature mitosis.
FIG. 3.
Chk1 mutated at S317, but not at S345, retained its function in cell survival. (A) Cell cycle distribution patterns of cells expressing wild-type (wt) Chk1 or its mutants. Chk1lox/− ES cells expressing wild-type Chk1 or its mutants were infected with adenoviruses expressing Cre recombinase. The cell cycle distribution patterns were analyzed by FACScan at the indicated times. (B) Mutation in S317, but not S345, did not cause premature mitosis. Chk1lox/− ES cells expressing wild-type Chk1 or its mutants were infected with adenoviruses as for panel A. Premature mitosis was determined at the indicated times by counting phospho-histone H3-positive cells with less than a 4 N DNA content using FACScan. The data are means plus standard deviations of at least three independent experiments. (C) Mutation at S345 resulted in the activation of the Chk2-p53-dependent DNA damage checkpoint. Chk1lox/− ES cells expressing wild-type Chk1 or its mutants were infected with adenoviruses as for panel A. Cells were harvested at the indicated times, and the lysates were subjected to immunoblotting using the indicated antibodies. (D) Increased chromosomal aberrations in Chk1Δ/− ES cells expressing S317A. Chromosomes were stained with DAPI (4′,6′-diamidino-2-phenylindole) and observed under a Zeiss axioplan imaging 2 microscope. Metaphase chromosome spread of Chk1Δ/− ES cells expressing S317A exhibited tetraploidy. d and f indicate a dicentric and a chromosome fragment.
Previously, we demonstrated that apoptosis induced by Chk1 depletion was a mitotic catastrophe that occurred via the ATM/ATR-p53-dependent DNA damage checkpoint (27). Therefore, we determined whether apoptosis in cells expressing S345A was really due to mitotic catastrophe through the activation of the DNA damage checkpoint. A significant increase in the amount of p53 protein and modification of Chk2 were readily detected in cells expressing S345A (Fig. 3C), suggesting activation of the Chk2-dependent DNA damage checkpoint pathway. These changes were not detected in cells expressing the wild type or the S317A mutant. The knockout of the endogenous Chk1 was equally efficient in wild-type, S317A, and S345A cell lines. Thus, phosphorylation of S317 appeared to be dispensable for prevention of mitotic catastrophe, but phosphorylation of S345 may be required. Because the status of p53 activity in ES cells is contentious, we examined whether Chk1 depletion caused the induction of Noxa and Puma expression, which was known to be p53 dependent. A significant induction of Noxa and Puma expression levels was detected in Chk1-deficient ES cells (see Fig. S1 in the supplemental material), suggesting that p53 was active in our ES cells.
Increased chromosomal abnormalities in cells expressing S317A.
Given that cells expressing S317A were defective in cell cycle checkpoint function but capable of reversing a nonviable phenotype, we then asked whether Chk1-dependent checkpoint signaling is of crucial importance for the maintenance of chromosomal integrity, which protects cells from tumorigenesis. To address this question, we performed cytogenetic studies with S345A cells or S317A cells. The metaphase spreads 36 h after Chk1 depletion from different genotypes were analyzed for chromosome alterations, such as dicentric chromosomes, fragments (acentric and centric), and breaks (chromosome and chromatid). Chromosome numbers were also calculated for each sample. As many as 150 metaphases per genotype were analyzed in this study. In cells expressing wild-type Chk1, 3.7% of the cells showed chromosomal aberrations. Surprisingly, although S345A cells showed defects in phenotypes at cell cycle checkpoints, only 7.6% of metaphase chromosomes displayed structural abnormalities, presumably due to elimination of abnormal cells by mitotic catastrophe. S317A cells (15.1%) were more prone to chromosomal abnormalities than were S345A cells. In addition, 25% of S317A cells with abnormal chromosomes exhibited tetraploidy, which was not detected at all in control cells or S345A cells (Fig. 3D). These chromosomal abnormalities were not detected in cells expressing endogenous Chk1 (data not shown).
Phosphorylation of Chk1 during normal S phase and subcellular localization of its phosphorylated forms.
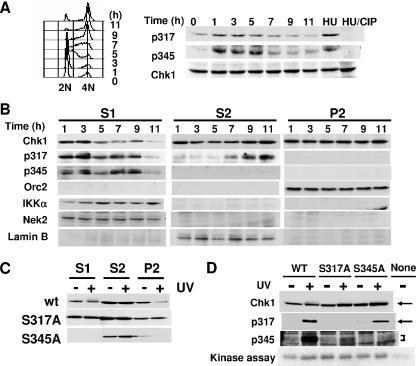
Our results suggested that phosphorylation of Chk1 at S345 is a prerequisite for prevention of premature mitosis during normal S phase. We next examined whether this site is really phosphorylated in vivo under unperturbed conditions. ES cells were synchronized at the G1/S boundary by a nocodazole/mimosine treatment and then released into S phase. Fluorescence-activated cell sorter analysis revealed that S phase started at 1 h and was completed 9 h after release (Fig. 4A, left). Immunoblotting using antibodies specific to phospho-Chk1 that is phosphorylated at S317 and S345 revealed that phosphorylation at S317 was detected at late G1 to late S phase. In contrast, Chk1 phosphorylated at S345 was not detected at late G1 phase but peaked at early to middle S phase and then decreased at late S phase. Similar patterns of Chk1 phosphorylation were also observed in cells synchronized at M phase by nocodazole treatment alone, although synchronization appeared less effective, eliminating the possibility that Chk1 phosphorylations were due to DNA damage induced by mimosine treatment (see Fig. S2 in the supplemental material). The bands of Chk1 phosphorylated at S317 and at S345 were enhanced by HU treatment and almost completely disappeared after treatment with phosphatase, confirming the specificities of these phosphospecific antibodies (Fig. 4A, right).
FIG. 4.
Subcellular localization of phosphorylated forms of Chk1 during unperturbed S phase. (A) Chk1 is phosphorylated at S317 and S345 during unperturbed S phase. ES cells were synchronized at late G1 phase by a nocodazole/mimosine treatment and then released into S phase. Cell cycle distribution was analyzed by FACScan (left). Cells were harvested at the indicated times after release into S phase, and the lysates were then subjected to immunoblotting using antibodies specific to phospho-Chk1 phosphorylated at S317 and S345 as indicated. (B) Phospho-Chk1 that was phosphorylated at S345 predominantly localized in the cytoplasmic fraction. ES cells were synchronized and released into S phase and harvested as for panel A. The cell lysates were then fractionated as described in Materials and Methods. Each fraction was subjected to immunoblotting using the indicated antibodies. (C) Chk1Δ/− ES cells expressing the indicated Chk1 were treated with or without UV light (500 J/m2). The treated cells were harvested, and the lysates were fractionated as for panel B. Each fraction was then subjected to immunoblotting using anti-Chk1 antibodies. wt, wild type. (D) Chk1Δ/− ES cells expressing the indicated Chk1 were treated with or without UV light as for panel C. The treated cells were harvested, and the soluble fractions (S1 plus S2) were then subjected to immunoblotting using the indicated antibodies. The soluble lysates were also immunoprecipitated using anti-Myc antibodies, and the precipitates were subjected to an in vitro kinase assay using a GST-Cdc25C fragment as a substrate.
To investigate the subcellular localization of phosphorylated Chk1, we fractionated extracts of synchronized ES cells by a centrifugation-based method and obtained fractions of cytoplasmic proteins (S1) and soluble nuclear proteins (S2) and a chromatin-enriched fraction (P2). Immunoblotting using anti-Orc2, anti-lamin B, anti-Nek2, and anti-IKKα antibodies revealed predominant localization of Orc2 in P2, lamin B in S2, and Nek2 and IKKα proteins in the S1 fraction, indicating that fractionation of the extracts was successful (5, 15). Chk1 was detected in all fractions at almost the same level, although it was decreased in the S1 fraction at late S phase. The Chk1 phosphorylated at S317 predominantly appeared in the S1 fraction at early S phase, but it decreased during late S phase. Inversely, it was detected in the S2 fraction at late S phase. Interestingly, Chk1 phosphorylated at S345 was detected only in the S1 fraction. Taken together, these results suggested that phosphorylation of Chk1 at S345, but not at S317, might be important for cytoplasmic localization of Chk1 during S phase.
Very recently, Smits et al. reported that PIKK-dependent phosphorylation of Chk1 leads to its release from chromatin, which is important for transmitting the DNA damage signal (35). Therefore, we next determined which Chk1 phosphorylation site was necessary for chromatin dissociation upon DNA damage. Exogenously expressed wild-type Chk1 effectively dissociated from the chromatin fraction in response to DNA damage induced by UV (Fig. 4C). This dissociation was not observed in S317A cells but was obvious in S345A cells. S345A proteins were not present in the S1 fraction in the presence or absence of DNA damage, further supporting the idea that phosphorylation of Chk1 at S345 is important for its cytoplasmic localization. Consistent with this, a mobility shift in Chk1 upon DNA damage was detected only in the cytoplasmic fraction. Taken together with the fact that Chk1 that was phosphorylated at S317 was not detected in the chromatin fraction (Fig. 4B), phosphorylation at this site appeared to be important for chromatin dissociation in response to DNA damage.
We then asked whether one phosphorylation of Chk1 affects the other phosphorylation upon DNA damage. In the soluble fraction (S1 plus S2), the protein amounts of wild-type Chk1 and its mutants were at the same level. Antibodies specific to phospho-S317 and phospho-S345 did not detect S317A and S345A proteins at all in the absence or presence of DNA damage, respectively, confirming the specificity of these antibodies. Interestingly, phosphorylation of Chk1 at S317 was strongly enhanced in wild-type Chk1 and S345A upon DNA damage induced by UV, suggesting that the phosphorylation of Chk1 at S345 did not affect the phosphorylation at S317 (Fig. 4D). In contrast, phosphorylation of Chk1 at S345 was strongly enhanced in wild-type Chk1 but was very weak in S317A upon DNA damage, suggesting that phosphorylation of S317 is required for the proper phosphorylation of S345 in response to activation of DNA damage checkpoints.
Centrosomal targeting of Chk1 complements its phosphorylation site mutations.
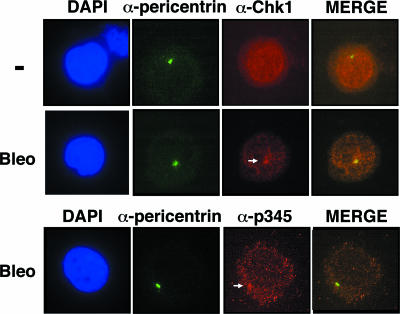
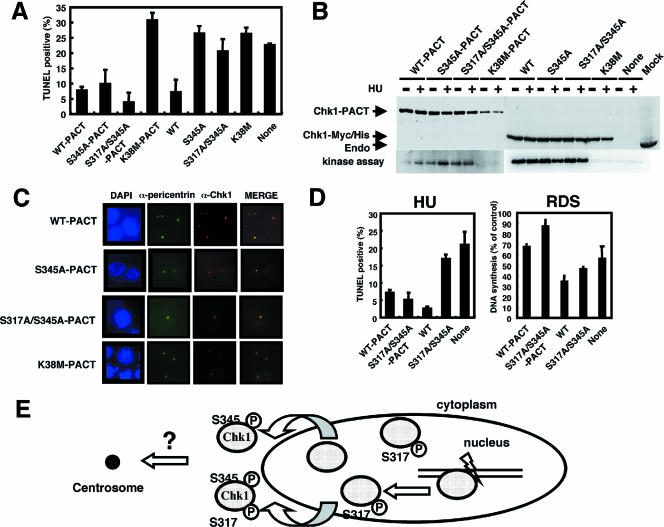
Given that the mutation at S345 abolished all functions tested, the possibility was raised that cytoplasmic localization of Chk1 might be important for its function. In this regard, centrosome-associated Chk1 has been reported to shield centrosomal Cdk1 from premature activation (18). Therefore, we first confirmed centrosomal localization of the endogenous Chk1 and examined the effect of DNA damage on its localization. We used anti-pericentrin antibodies to detect centrosomes. Although centrosomal signals of Chk1 appeared in trace amounts in the absence of DNA damage, they were significantly enhanced by bleomycin treatment. Interestingly, anti-phospho-S345 antibodies revealed the predominant localization of the phosphorylated form of Chk1 at S345 in the cytoplasm and its colocalization with centrosomes (Fig. 5), confirming the results of cell fractionation (Fig. 4B). In addition, similar results were also observed in Chk1-depleted cells expressing wild-type Chk1 or the S317A or S345A mutant, showing that trace centrosomal signals of wild-type Chk1 were enhanced by DNA damage. S345A did not appear to localize at centrosomes (see Fig. S3 in the supplemental material). Thus, we speculated that phosphorylation of Chk1 at S345 was required only for its centrosomal targeting and that targeting centrosomal Chk1 with mutations in its phosphorylation sites could prevent apoptosis in the presence or absence of genotoxic stressors. Interestingly, a recent study revealed that immobilizing a kinase-dead mutant of Chk1 within the centrosome by fusing it to the PACT domain of AKAP450 suppressed endogenous Chk1 activity and thus induced premature activation of Cdk1 at centrosomes (18). Using a similar approach, we transfected wild-type or mutant pCAGGS-Chk1-PACT. With the use of a green fluorescent protein (GFP) expression vector, we confirmed that transfection efficiency was more than 85% under these experimental conditions (data not shown). The results showed that immobile S345A-PACT and S317A/S345A-PACT were capable of inhibiting apoptosis in Chk1-deficient cells to an extent similar to that of wild-type Chk1-PACT, but the kinase-dead mutant (K38M)-PACT was not (Fig. 6A). S345A and S317A/S345A mutants without a PACT domain failed to inhibit apoptosis. Immunoblotting revealed that the expression levels of these proteins were almost the same (Fig. 6B). Immunostaining in situ revealed that wild-type Chk1-PACT and its mutants strictly localized at centrosomes (Fig. 6C). Interestingly, immobile S317A/S345A-PACT was also capable of inhibiting apoptosis in the presence of HU (Fig. 6D). Consistent with this, wild-type Chk1-PACT, but not K38M-PACT, colocalized with centrosome-associated phosphorylation of Cdk1 at Y15 (see Fig. S4 in the supplemental material), suggesting the inhibition of Cdk1 at centrosomes. In contrast, wild-type PACT and S317A/S345A-PACT failed to rescue the defect in intra-S phase checkpoint function, presumably due to the fact that the target of the intra-S phase checkpoint may be nuclear Cdk2 but not centrosomal Cdk1 (Fig. 6D). Taken together, these results suggested that centrosomal targeting of Chk1 suppressed both checkpoint defects and nonviable phenotypes arising from mutations in its phosphorylation sites.
FIG. 5.
Centrosomal association of Chk1 was enhanced by DNA damage. ES cells were stained in situ using anti-pericentrin (centrosome marker), anti-Chk1, and anti-phospho-Chk1 at Ser345 antibodies. Immunofluorescence microscopy was performed as described in Materials and Methods. Bleo, bleomycin treatment (6 h); −, no treatment; DAPI, DAPI (4′,6′-diamidino-2-phenylindole) staining. The arrows indicate centrosomes.
FIG. 6.
Centrosomal targeting of Chk1 was capable of complementing Chk1 mutations at S317 and S345. (A) pCAGGS Chk1-PACT, pCAGGS Chk1, and pME18S-GFP were cotransfected into Chk1Δ/− ES cells as described in Materials and Methods. The transfected cells were harvested 24 h after transfection, and TUNEL staining of GFP-positive cells was analyzed with an immunofluorescence microscope. Apoptosis was determined by measuring TUNEL-positive cells. The data are means plus standard deviations of at least three independent experiments. WT, wild type. (B) Protein expression of the indicated forms of Chk1-PACT, Chk1-Myc/His, and endogenous Chk1 was detected by immunoblotting using anti-Chk1 antibodies. The lysates were immunoprecipitated using anti-Myc antibodies, and the precipitates were subjected to an in vitro kinase assay using a GST-Cdc25C fragment as a substrate. (C) Chk1Δ/− ES cells expressing Chk1-PACT were stained in situ using the indicated antibodies. Immunofluorescence microscopy was performed as described in Materials and Methods. DAPI, DAPI (4′,6′-diamidino-2-phenylindole) staining. (D) pCAGGS Chk1-PACT or pCAGGS Chk1 and pME18S-GFP were cotransfected into Chk1Δ/− ES cells as for panel A. The transfected cells were treated with HU (2.5 mM), and TUNEL-positive cells were then determined as for panel A. The data are means plus standard deviations of at least three independent experiments. For RDS, pCAGGS Chk1-PACT or pCAGGS Chk1 was transfected into Chk1Δ/− ES cells. RDS was analyzed as described in Materials and Methods. (E) Model for regulation of Chk1 translocation into centrosomes by two-step phosphorylation in response to DNA damage or during unperturbed S phase. The requirement for phosphorylation of Chk1 at Ser345 for centrosomal association is not known.
DISCUSSION
On the basis of phenotypes from Chk1−/− mice (21, 36), Chk1 is suggested to be a multifunctional kinase that serves as a checkpoint mediator and an essential factor for cell survival. Chk1 is phosphorylated in response to several types of DNA damage or stalled DNA replication in a manner dependent on PIKK. However, it can be phosphorylated even in the absence of DNA damage or stalled replication (14). In addition, Chk1 is also phosphorylated by Akt (17, 34) and Cdk1 (33) in vivo. Thus, these phosphorylations might be essential for transducing signals to downstream targets. To address this question directly and to elucidate the mechanisms related to how these phosphorylations regulate Chk1 function, we performed knockout-knockin experiments using Chk1lox/− cells and several phosphorylation site mutants.
The most striking difference between the phosphorylation mutants S317A and S345A was in their abilities to restore the viability of Chk1-deficient cells. Mutation at S317, but not at S345, resulted in a complete loss of checkpoint function but allowed the cell to survive. Consistent with this, S345 was specifically phosphorylated at early to middle S phase under unperturbed conditions. Studies of the subcellular localization of phosphorylated forms of Chk1 revealed that Chk1 phosphorylated at S345 was predominantly detected in the cytoplasmic fraction (Fig. 4B and 5), suggesting that phosphorylation at this site promoted cytoplasmic localization of Chk1. Thus, the S345A, but not the S317A, mutant was hardly detected in the cytoplasmic fraction. Chk1 phosphorylated at S317 was detected in increased amounts in the soluble nuclear fraction toward late S phase, by which time the total phosphorylation of Chk1 at S345 had gradually decreased (Fig. 4A). Thus, it might be possible that dephosphorylation of Chk1 at S345 triggered the movement of Chk1 from the cytoplasm and its accumulation in the nucleus. Kramer et al. have reported that the centrosomal association of Chk1 protein appeared to be important for preventing premature mitosis (18). Our results indicated that the centrosomal localization of Chk1 could complement Chk1 mutations in the phosphorylation sites (S345A or S317A/S345A). Taken together, the phosphorylation of Chk1 at S345 appeared to be important for its cytoplasmic localization and the subsequent association of Chk1 with centrosomes. Consistent with this notion, the mobility shift in Chk1 upon DNA damage was detected only in the cytoplasmic fraction (Fig. 4C), further supporting the idea that phosphorylation of Chk1 triggered its cytoplasmic accumulation. While it was reported that mutations at S317 and S345 abrogated the nuclear accumulation of the Chk1 protein, revealing the presence of mutant Chk1 in both the nucleus and cytoplasm (14), we found that wild-type Chk1 was present in both these locations but that Chk1 phosphorylated at S345 predominantly localized in the cytoplasm. Although we cannot explain these differences, it might be that the presence of endogenous Chk1 affects the localization of mutant Chk1. Alternatively, a higher level of expression of a mutant Chk1 might also affect its subcellular localization.
A very recent report has shown that a rapid release of Chk1 from chromatin promotes the cell cycle checkpoint response and that this release is correlated with the phosphorylation of Chk1 in a manner dependent on PIKK (35). Our results also indicated that the S345A mutant, as well as wild-type Chk1, was effectively released from chromatin but the S317A mutant was not. Thus, phosphorylation of Chk1 at S317 but not at S345 might trigger Chk1 release from chromatin. These findings suggest a model for how Chk1 phosphorylation contributes to S-phase progression and to the response to DNA damage (Fig. 6E). After assembling at sites of damaged DNA, active PIKKs trigger the phosphorylation of chromatin-bound Chk1 at S317 and S345, leading to the rapid dissociation of Chk1 from chromatin and its accumulation in the cytoplasm. This accumulation likely enhances the centrosomal association of the Chk1 protein, preventing the activation of cyclin B-Cdk1. During unperturbed S phase, phosphorylation of soluble nuclear Chk1 at S345 may be sufficient for preventing premature mitosis, presumably due to the activation of PIKK in the entire nuclear region. In addition, S345 phosphorylation of only a small portion of the Chk1 pool might be sufficient for preventing mitosis until completion of DNA replication under unperturbed conditions. However, upon DNA damage, phosphorylation at S317 of chromatin-bound Chk1 at the damaged sites and its subsequent release from chromatin appeared to be necessary, since PIKKs are predominantly activated at sites of DNA damage (9, 22, 45). In such cases, phosphorylation at S317 and S345 of a large portion of the Chk1 pool could be necessary, because a prolonged inhibition of Cdk1 activity appeared to be critical until DNA damage was completely repaired. Interestingly, in response to DNA damage, preceding phosphorylation of S317 appeared to be required for effective phosphorylation of S345 (Fig. 4D), suggesting the possibility that phosphorylation of S345 might be coupled with the dissociation of Chk1 from the chromatin fraction upon DNA damage. However, we cannot exclude the possibility that phosphorylation of S317 led to the conformational changes in the Chk1 molecule, and thus, PIKK could easily access an S345 residue.
The requirement for phosphorylation of Chk1 for cell cycle checkpoint function has been studied in yeast. S317 and S345 (T323 and S345 in fission yeast) are the conserved consensus phosphorylation sites targeted by PIKK in humans, mice, Xenopus, and fission yeast. However, in fission yeast, S345, but not T323, was necessary for checkpoint function, and no phenotypic consequence of a mutation at T323 was observed, although both sites appeared to be phosphorylated in vivo (4). In addition, phosphorylation of S345 created the 14-3-3 protein (Rad24) binding site and subsequently enhanced the nuclear accumulation of the Chk1 protein (8). Thus, although Chk1 pathways, including those used by upstream effectors and downstream targets, are well known to be conserved among eukaryotes, the molecular basis of their interplay is somewhat varied.
With the use of mutants (S317A) that lack checkpoint function but retain their roles in cell survival, we could now examine whether a loss of checkpoint function per se really caused chromosomal instability. Because upstream effectors of Chk1, including Rad9 (7, 29), Rad17 (38), Hus1 (39), and ATR (3), are essential for sustaining cell survival, the chromosomal instability observed in their respective null cells might not arise solely from a loss of checkpoint functions, but also from the loss of a role in cell survival. Our present results clearly indicate that the loss of checkpoint function caused chromosomal instability, including breaks and dicentrics. Interestingly, we found a significant increase in the number of cells with tetraploidy among cells with S317A, but not in those with S345A or wild-type Chk1. Recent works have emphasized the significance of tetraploidy in tumor development (10, 30) and proposed the existence of a checkpoint that eliminates tetraploid cells in a p53-dependent manner (10, 23). Thus, Chk1 might be involved in this tetraploidy checkpoint,because it could function upstream of p53 (31). Further study is obviously required to address this issue.
In conclusion, our studies suggest that phosphorylation of Chk1 plays multiple roles in Chk1 regulation. Forcing Chk1 to localize in the centrosomes was sufficient to restore the Chk1 function of the phosphorylation site mutants. Therefore, centrosomal localization of Chk1 appears to be involved in both checkpoint signaling and cell survival, and two-step phosphorylation of Chk1 promotes this process.
Supplementary Material
Acknowledgments
We thank M. Shimada for critical reading of our manuscript and H. Kojima for technical assistance. We thank K. Nakayama and N. Ishida for subcellular fractionation.
This work was supported in part by the Ministry of Education, Science, Sports, and Culture of Japan through Grants-in-Aid for Scientific Research on Priority Area (A) and for Scientific Research (B) (to M.N.).
Footnotes
Published ahead of print on 22 January 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Abraham, R. T. 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15:2177-2196. [DOI] [PubMed] [Google Scholar]
- 2.Adams, K. E., A. L. Medhurst, D. A. Dart, and N. D. Lakin. 2006. Recruitment of ATR to sites of ionising radiation-induced DNA damage requires ATM and components of the MRN protein complex. Oncogene 25:3894-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, E. J., and D. Baltimore. 2000. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 14:397-402. [PMC free article] [PubMed] [Google Scholar]
- 4.Capasso, H., C. Palermo, S. Wan, H. Rao, U. P. John, M. J. O'Connell, and N. C. Walworth. 2002. Phosphorylation activates Chk1 and is required for checkpoint-mediated cell cycle arrest. J. Cell Sci. 115:4555-4564. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter, P. B., P. R. Mueller, and W. G. Dunphy. 1996. Role for a Xenopus Orc2-related protein in controlling DNA replication. Nature 379:357-360. [DOI] [PubMed] [Google Scholar]
- 6.Chen, P., C. Luo, Y. Deng, K. Ryan, J. Register, S. Margosiak, A. Tempczyk-Russell, B. Nguyen, P. Myers, K. Lundgren, C. C. Kan, and P. M. O'Connor. 2000. The 1.7 Å crystal structure of human cell cycle checkpoint kinase Chk1: implications for Chk1 regulation. Cell 100:681-692. [DOI] [PubMed] [Google Scholar]
- 7.Dang, T., S. Bao, and X. F. Wang. 2005. Human Rad9 is required for the activation of S-phase checkpoint and the maintenance of chromosomal stability. Genes Cells 10:287-295. [DOI] [PubMed] [Google Scholar]
- 8.Dunaway, S., H. Y. Liu, and N. C. Walworth. 2005. Interaction of 14-3-3 protein with Chk1 affects localization and checkpoint function. J. Cell Sci. 118:39-50. [DOI] [PubMed] [Google Scholar]
- 9.Falck, J., J. Coates, and S. P. Jackson. 2005. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature 434:605-611. [DOI] [PubMed] [Google Scholar]
- 10.Fujiwara, T., M. Bandi, M. Nitta, E. V. Ivanova, R. T. Bronson, and D. Pellman. 2005. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature 437:1043-1047. [DOI] [PubMed] [Google Scholar]
- 11.Gatei, M., K. Sloper, C. Sorensen, R. Syljuasen, J. Falck, K. Hobson, K. Savage, J. Lukas, B. B. Zhou, J. Bartek, and K. K. Khanna. 2003. Ataxia-telangiectasia-mutated (ATM) and NBS1-dependent phosphorylation of Chk1 on Ser-317 in response to ionizing radiation. J. Biol. Chem. 278:14806-14811. [DOI] [PubMed] [Google Scholar]
- 12.Guo, Z., A. Kumagai, S. X. Wang, and W. G. Dunphy. 2000. Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts. Genes Dev. 14:2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jazayeri, A., J. Falck, C. Lukas, J. Bartek, G. C. Smith, J. Lukas, and S. P. Jackson. 2006. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat. Cell Biol. 8:37-45. [DOI] [PubMed] [Google Scholar]
- 14.Jiang, K., E. Pereira, M. Maxfield, B. Russell, D. M. Goudelock, and Y. Sanchez. 2003. Regulation of Chk1 includes chromatin association and 14-3-3 binding following phosphorylation on Ser-345. J. Biol. Chem. 278:25207-25217. [DOI] [PubMed] [Google Scholar]
- 15.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621-663. [DOI] [PubMed] [Google Scholar]
- 16.Katsuragi, Y., and N. Sagata. 2004. Regulation of Chk1 kinase by autoinhibition and ATR-mediated phosphorylation. Mol. Biol. Cell 15:1680-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King, F. W., J. Skeen, N. Hay, and E. Shtivelman. 2004. Inhibition of Chk1 by activated PKB/Akt. Cell Cycle 3:634-637. [PubMed] [Google Scholar]
- 18.Kramer, A., N. Mailand, C. Lukas, R. G. Syljuasen, C. J. Wilkinson, E. A. Nigg, J. Bartek, and J. Lukas. 2004. Centrosome-associated Chk1 prevents premature activation of cyclin-B-Cdk1 kinase. Nat. Cell Biol. 6:884-891. [DOI] [PubMed] [Google Scholar]
- 19.Kumagai, A., Z. Guo, K. H. Emami, S. X. Wang, and W. G. Dunphy. 1998. The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J. Cell Biol. 142:1559-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumagai, A., J. Lee, H. Y. Yoo, and W. G. Dunphy. 2006. TopBP1 activates the ATR-ATRIP complex. Cell 124:943-955. [DOI] [PubMed] [Google Scholar]
- 21.Liu, Q., S. Guntuku, X. S. Cui, S. Matsuoka, D. Cortez, K. Tamai, G. Luo, S. Carattini-Rivera, F. DeMayo, A. Bradley, L. A. Donehower, and S. J. Elledge. 2000. Chk1 is an essential kinase that is regulated by Atr and required for the G2/M DNA damage checkpoint. Genes Dev. 14:1448-1459. [PMC free article] [PubMed] [Google Scholar]
- 22.Lukas, C., J. Falck, J. Bartkova, J. Bartek, and J. Lukas. 2003. Distinct spatiotemporal dynamics of mammalian checkpoint regulators induced by DNA damage. Nat. Cell Biol. 5:255-260. [DOI] [PubMed] [Google Scholar]
- 23.Margolis, R. L. 2005. Tetraploidy and tumor development. Cancer Cell 8:353-354. [DOI] [PubMed] [Google Scholar]
- 24.Melo, J., and D. Toczyski. 2002. A unified view of the DNA-damage checkpoint. Curr. Opin. Cell Biol. 14:237-245. [DOI] [PubMed] [Google Scholar]
- 25.Mendez, J., and B. Stillman. 2000. Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol. Cell. Biol. 20:8602-8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murakami, H., and H. Okayama. 1995. A kinase from fission yeast responsible for blocking mitosis in S phase. Nature 374:817-819. [DOI] [PubMed] [Google Scholar]
- 27.Niida, H., S. Tsuge, Y. Katsuno, A. Konishi, N. Takeda, and M. Nakanishi. 2005. Depletion of Chk1 leads to premature activation of Cdc2-cyclin B and mitotic catastrophe. J. Biol. Chem. 280:39246-39252. [DOI] [PubMed] [Google Scholar]
- 28.O'Connell, M. J., N. C. Walworth, and A. M. Carr. 2000. The G2-phase DNA-damage checkpoint. Trends Cell Biol. 10:296-303. [DOI] [PubMed] [Google Scholar]
- 29.Roos-Mattjus, P., B. T. Vroman, M. A. Burtelow, M. Rauen, A. K. Eapen, and L. M. Karnitz. 2002. Genotoxin-induced Rad9-Hus1-Rad1 (9-1-1) chromatin association is an early checkpoint signaling event. J. Biol. Chem. 277:43809-43812. [DOI] [PubMed] [Google Scholar]
- 30.Shi, Q., and R. W. King. 2005. Chromosome nondisjunction yields tetraploid rather than aneuploid cells in human cell lines. Nature 437:1038-1042. [DOI] [PubMed] [Google Scholar]
- 31.Shieh, S. Y., J. Ahn, K. Tamai, Y. Taya, and C. Prives. 2000. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 14:289-300. [PMC free article] [PubMed] [Google Scholar]
- 32.Shiloh, Y. 2003. ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer 3:155-168. [DOI] [PubMed] [Google Scholar]
- 33.Shiromizu, T., H. Goto, Y. Tomono, J. Bartek, G. Totsukawa, A. Inoko, M. Nakanishi, F. Matsumura, and M. Inagaki. 2006. Regulation of mitotic function of Chk1 through phosphorylation at novel sites by cyclin-dependent kinase 1 (Cdk1). Genes Cells 11:477-485. [DOI] [PubMed] [Google Scholar]
- 34.Shtivelman, E., J. Sussman, and D. Stokoe. 2002. A role for PI 3-kinase and PKB activity in the G2/M phase of the cell cycle. Curr. Biol. 12:919-924. [DOI] [PubMed] [Google Scholar]
- 35.Smits, V. A., P. M. Reaper, and S. P. Jackson. 2006. Rapid PIKK-dependent release of Chk1 from chromatin promotes the DNA-damage checkpoint response. Curr. Biol. 16:150-159. [DOI] [PubMed] [Google Scholar]
- 36.Takai, H., K. Tominaga, N. Motoyama, Y. A. Minamishima, H. Nagahama, T. Tsukiyama, K. Ikeda, K. Nakayama, and M. Nakanishi. 2000. Aberrant cell cycle checkpoint function and early embryonic death in Chk1−/− mice. Genes Dev. 14:1439-1447. [PMC free article] [PubMed] [Google Scholar]
- 37.Walworth, N., S. Davey, and D. Beach. 1993. Fission yeast chk1 protein kinase links the rad checkpoint pathway to cdc2. Nature 363:368-371. [DOI] [PubMed] [Google Scholar]
- 38.Wang, X., L. Zou, H. Zheng, Q. Wei, S. J. Elledge, and L. Li. 2003. Genomic instability and endoreduplication triggered by RAD17 deletion. Genes Dev. 17:965-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss, R. S., T. Enoch, and P. Leder. 2000. Inactivation of mouse Hus1 results in genomic instability and impaired responses to genotoxic stress. Genes Dev. 14:1886-1898. [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss, R. S., S. Matsuoka, S. J. Elledge, and P. Leder. 2002. Hus1 acts upstream of chk1 in a mammalian DNA damage response pathway. Curr. Biol. 12:73-77. [DOI] [PubMed] [Google Scholar]
- 41.Xu, B., and M. B. Kastan. 2004. Analyzing cell cycle checkpoints after ionizing radiation. Methods Mol. Biol. 281:283-292. [DOI] [PubMed] [Google Scholar]
- 42.Xu, X., L. M. Tsvetkov, and D. F. Stern. 2002. Chk2 activation and phosphorylation-dependent oligomerization. Mol. Cell. Biol. 22:4419-4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao, H., and H. Piwnica-Worms. 2001. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol. Cell. Biol. 21:4129-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou, L., D. Cortez, and S. J. Elledge. 2002. Regulation of ATR substrate selection by Rad17-dependent loading of Rad9 complexes onto chromatin. Genes Dev. 16:198-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zou, L., and S. J. Elledge. 2003. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300:1542-1548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.