Abstract
Introduction
Mpox, formerly known as monkeypox, is a public health emergency most commonly presenting with a painful rash and several systemic findings. However, there are several conditions that may mimic its presentation.
Objective
This narrative review provides a focused overview of mpox mimics for emergency clinicians.
Discussion
Mpox is a global health emergency. The disease is primarily spread through contact, followed by the development of a centrifugally-spread rash that evolves from macules to papules to vesicles to pustules. This is often associated with lymphadenopathy and fever. As the rash is one of the most common presenting signs of the infection, patients mpox may present to the emergency department (ED) for further evaluation. There are a variety of mimics of mpox, including smallpox, varicella, primary and secondary syphilis, acute retroviral syndrome, and genital herpes simplex virus.
Conclusion
Knowledge of mpox and its mimics is vital for emergency clinicians to differentiate these conditions and ensure appropriate diagnosis and management.
Keywords: Mpox, Monkeypox, Rash, Lesions, Infectious diseases, Dermatology
1. Introduction
Since an initial cluster of mpox (formerly known as monkeypox) was identified in the United Kingdom in May 2022, confirmed cases in the largest multi-country mpox outbreak to date have continued to increase worldwide, prompting declarations of a national health emergency in the U.S. and a global health emergency by the World Health Organization (WHO) [1]. As of December 21, 2022, there have been 83,424 confirmed cases and 72 deaths in 110 countries, with 29,740 cases and 20 deaths in the U.S [2]. There are several characteristic features of the mpox exanthem, including the progression of a painful centrifugally-spread rash that evolves from macules to papules to vesicles to pustules, which when combined with other distinct characteristics, such as regional lymphadenopathy, should increase suspicion for this diagnosis [[3], [4], [5]]. While in the 2022 outbreak the disease appears to be self-limited with an extremely low mortality rate (primarily associated with complications including encephalitis), morbidity can be significant due to painful skin and oral lesions, limiting oral intake and requiring hospitalization [1,2,6,7]. Given the potential morbidity and transmission through droplets, direct skin-to-skin contact, or contact with contaminated surfaces, prompt recognition and management are imperative to limit spread and reduce potential morbidity in vulnerable populations [5]. While definitive diagnosis of mpox is made with polymerase chain reaction (PCR), this assay is not universally available, and test results are unlikely to return in the span of an emergency department (ED) visit. Emergency clinicians may see increased volumes of patients seeking assessment for vague complaints of “rash” or “lesions” and must therefore be able to distinguish the clinical findings in order to prioritize testing and begin proper treatment.
There are a variety of potentially deadly skin conditions, including acute generalized exanthematous pustulosis (AGEP), Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), pemphigus vulgaris, meningococcemia, toxic-shock syndrome, and others. Many other infectious diseases can also result in rash, including smallpox; varicella; herpes simplex virus (HSV); measles; hand, foot, mouth disease; molluscum contagiosum; human immunodeficiency virus (HIV); syphilis; disseminated gonococcal infection (DGI); lymphogranuloma venereum (LGV); chancroid; staphylococcal scalded skin syndrome (SSSS); Rocky Mountain spotted fever (RMSF); and fungal infection. Table 1 provides an overview of these conditions. However, many of these conditions do not typically present with a progressive rash with papules, vesicles, or pustules. This review will provide a focused differential of dermatologic conditions that may resemble mpox for the emergency clinician, including smallpox, varicella, syphilis, acute retroviral syndrome, and genital herpes.
Table 1.
Key findings and features of potential mimics of mpox.
| Condition | Cutaneous manifestations |
|---|---|
| Smallpox | Initial macules become papules then vesicles and pustules; deep-seated, firm, umbilicated lesions Oropharyngeal lesions Prominent lymphadenopathy |
| Varicella | Simultaneous fever with pruritic rash; superficial papules and vesicles with irregular borders and centripetal distribution in various stages |
| Genital herpes (herpes simplex virus; HSV) | Grouped 2–4 mm vesicles with underlying erythema, progressing to vesiculopustules with erosions and ulcerations Can have umbilicated appearance and be pruritic |
| Measles | Initial development of Koplik spots prior to rash Erythematous maculopapular lesions spreading cephalocaudally and centrifugally |
| Hand, foot, and mouth disease | Uniform evolution of macules to papules then vesicles and pustules with predilection for palms, soles, and oropharynx |
| Molluscum contagiosum | 2–5 mm pruritic, firm, dome-shaped shiny, centrally umbilicated lesions Can have peripheral ‘crown’ of punctiform or radiating vessels |
| Acute retroviral syndrome (human immunodeficiency virus; HIV) | Diffuse, 5–10 mm, well-circumscribed pink-red macules and maculopapules May be mildly pruritic |
| Syphilis | Primary: Single genital papule evolving to a 1–2 cm painless ulceration with non-exudative base Secondary, early: diffuse, macular, flat, pale pink-to-brown elliptical lesions Secondary, late: large, flattened papules or annular lesions |
| Disseminated gonococcal infection (DGI) | Painless macules, papules, or petechiae evolving into pustules with central “gun-metal gray” necrotic areas and surrounding erythema |
| Lymphogranuloma venereum (LGV) | Isolated genital ulcer that heals with 2–6 week interval to development of large painful superficial or deep inguinal lymph nodes; may have rectal mass |
| Chancroid | Erythematous genital papule evolving to pustule, followed by painful ulceration approximately 1–2 cm in diameter with yellow-gray exudate |
| Meningococcemia | May initially present with nonspecific maculopapular rash for 1–2 days Develop focal petechiae on trunk and legs; may involve oral mucosa; may coalesce into purpura and/or cutaneous hemorrhage and necrosis |
| Staphylococcal scalded skin syndrome (SSSS) | Erythema spreading to generalized body progressing to flaccid bullae with shallow erosions Positive Nikolsky sign No mucous membrane involvement |
| Rocky Mountain spotted fever (RMSF) | Blanching erythematous 1–4 mm macules evolving into petechiae beginning on distal extremities and spreading to trunk May later involve palms and soles |
| Toxic shock syndrome (TSS) | Diffuse macular erythroderma Mucous membranes may be involved with hyperemia Desquamation 1–2 weeks after illness onset (often palms and soles) |
| Fungal infection | Cryptococcus: local cellulitis, ulceration, or whitlow at inoculation; umbilicated lesions with AIDS Histoplasmosis: varying papules, plaques, nodules, pustules, ulcers; lesions may resemble molluscum lesions |
| Acute generalized exanthematous pustulosis (AGEP) | Non-follicular pustules on edematous erythema Starts on face and spread to trunk and limbs |
| Erythema multiforme | Initial erythematous round papules with centripetal spread evolving to target lesions <3 cm in diameter with dusky central area, surrounded by ring of pale edema, and erythematous peripheral halo |
| Pemphigus vulgaris | Primary lesions: tense, clear vesicles/bullae on head, trunk, mucosa which then become flaccid and turbid within several days, followed by rupture and areas of sensitive, denuded skin Positive Nikolsky sign |
| Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) | Coalescing erythematous macules with purpuric centers progressing to vesicles and bullae, followed by skin sloughing Hemorrhagic mucosal erosions with white or gray membrane Positive Nikolsky sign |
| Sweet syndrome | Painful, edematous, red or violaceous papules, plaques, and nodules ranging in size up to several cm Oral lesions may be present |
2. Methods
To construct this review, the authors searched Pubmed and Google Scholar for articles using the keywords “monkeypox”, “mpox”, “smallpox”, “varicella”, chickenpox”, “syphilis”, “HIV”, “retroviral syndrome”, “genital herpes” through December 22, 2022. Authors evaluated case reports and series, retrospective and prospective studies, randomized controlled trials, systematic reviews and meta-analyses, and other narrative reviews. Authors also reviewed guidelines and supporting citations of included articles. The literature search was restricted to studies and resources published in English. Three authors with experience in critical appraisal of the literature reviewed all of the resources and decided which resources to include for the review by consensus, with a focus on emergency medicine-relevant articles. When available, systematic reviews and meta-analyses were preferentially selected. These were followed sequentially by randomized controlled trials, prospective studies, retrospective studies, case reports, and other narrative reviews when alternate data were not available. A total of 67 resources were selected for inclusion in this review.
3. Discussion
3.1. Key characteristics of Mpox and related dermatitis
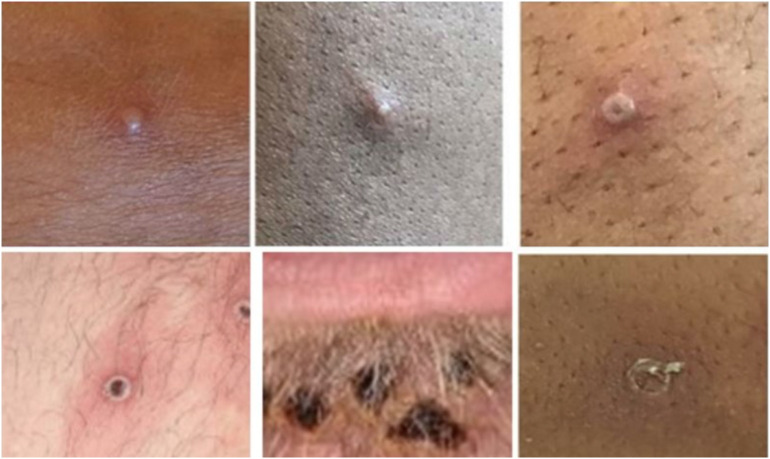
Rash is one of the characteristic features of mpox, occurring in over 97% of cases (Fig. 1 ) [8]. It typically begins on days 1–3 of illness, though it can appear as late as the 11th day of infection [3,9,10]. Initially lesions can be pruritic, painful, indurated, and umbilicated, and they frequently present as macules [11]. Lesions are generally 2–5 mm in diameter, though they may reach 1 cm [[12], [13], [14]]. The macules uniformly progress over 1–2 days to papules (day 3 of illness), then to vesicles containing clear liquid (days 4–5), and finally to pustules filled with yellow fluid (days 6–7), which then crust over and form scabs (days 7–14) (Fig. 2 ) [11]. The patient is considered contagious until all scabs have fallen off [11]. The sequential nature of the rash helps distinguish mpox from one of its dermatologic mimics: varicella (chickenpox). In varicella, all four stages of the rash appear concomitantly. However, another dermatologic mimic, smallpox, can present with the majority of similar-stage lesions simultaneously [15].
Fig. 1.
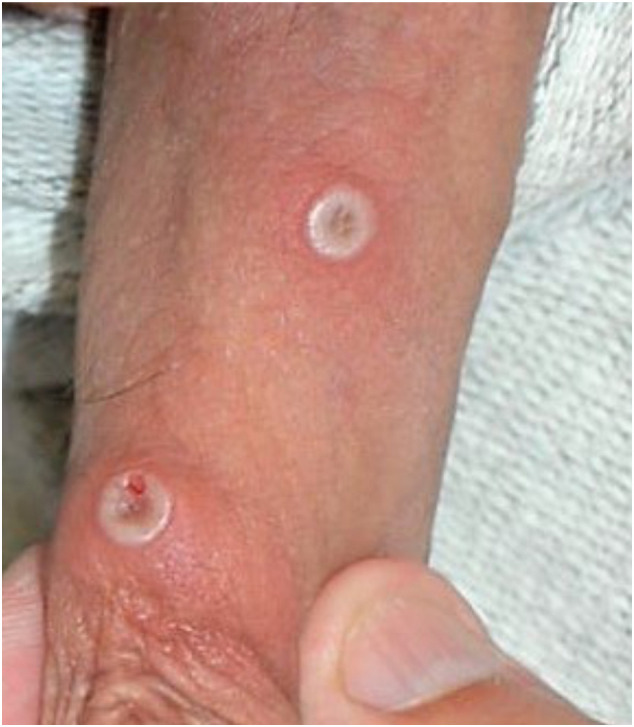
Mpox lesions on penis. Available from https://commons.m.wikimedia.org/wiki/File:Monkeypox_lesions.jpg.
Fig. 2.
Mpox lesion stages. Available from https://commons.m.wikimedia.org/wiki/File:Stages_of_monkeypox_lesion_development.jpg.
When considering past outbreaks of mpox as well as sporadic cases prior to the current outbreak, the lesions of mpox most commonly spread in a centrifugal manner to involve the chest, arms, and legs, followed by the hands and feet [3,9,10,16]. In prior outbreaks, involvement of the whole body was common within 24 h of the first lesion, and mpox could also involve the oral mucosa, genitalia, and conjunctivae [1]. The distribution of the rash involved the face in 95% of cases, upper extremities in 81.3%, palms and soles in 75%, lower extremities in 65.6%, oral mucosa in 70%, genitalia in 30%, and conjunctivae in 20% [3,11].
The 2022 outbreak differs, however, in several regards. The current global outbreak has disproportionately affected men-who-have-sex-with-men (MSM), as well as those with HIV, with 36–42% of those affected HIV positive [7,17,18]. Transmission through close skin-to-skin contact within sexual networks has led to new patterns in clinical presentations and dermatologic findings. Recent reports note the current rash, involving both skin and mucous membranes, can be the initial manifestation of illness in more than half of infected patients [[17], [18], [19]]. In a small subset of these patients, the mucocutaneous rash is the only manifestation of mpox infection [17,18]. The number of mpox lesions on the skin or mucous membranes is also lower in the current outbreak; more than half of patients had 10 or fewer lesions, frequently noted in the perineal region (genital and ano-rectal areas) – importantly, a very large percentage of patients with fewer genital lesions presented with 1 single mpox sore [17]. In the current outbreak, penile, ano-rectal, and oral-pharyngeal lesions are seen with significant frequency [7,17,20]. Aside from the rash, a number of other symptoms are typically present. The most recent data as of December 21, 2022, demonstrate fever in 64%, malaise in 63%, chills in 59%, pruritis in 59%, headache in 54%, lymphadenopathy in 54%, myalgia in 53%, and gastrointestinal symptoms in up to 18% [8]. Patients may also present with severe sore throat and dysphagia, as well as ulcerative lesions in the posterior oropharynx. Proctitis may present with anorectal pain (38%), purulent discharge/bleeding (21%), or tenesmus (20%) [8]. This may be accompanied by lesions in the perianal area.
3.2. Mimics
While multiple different infectious illnesses can present with similar clinical and dermatologic manifestations, smallpox and varicella likely represent the most similar appearing entities.
3.2.1. Smallpox
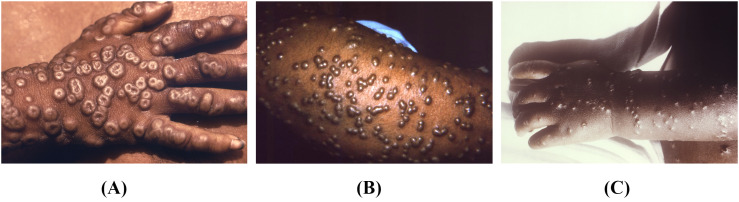
Smallpox results from a primary infection with variola virus (VARV), a member of the Poxviridae family, orthopoxvirus genus alongside mpox. Responsible for approximately 500 million deaths over several hundred years, this highly infectious and lethal virus was eradicated in 1980 after a focused vaccination campaign; overlap between smallpox immunization and mpox infection has rates of cross-protection as high as 85% [[21], [22], [23], [24], [25], [26], [27], [28], [29]]. Humans are the only known host and reservoir for smallpox. The virus can persist in aerosolized form in cool, dry environments, but it is effectively killed by ultraviolet light and most common hospital disinfectants [22,24,26].
Following an incubation period of approximately 7–14 days, patients with smallpox typically have a febrile prodrome lasting 1–4 days, followed by the onset of a rash [23,24,26]. Lesions typically first occur in the oropharynx, and this early period is when respiratory concentrations have the highest potential for transmission [[22], [23], [24],26]. It is difficult to differentiate between smallpox and mpox lesions on physical examination, as both evolve from macules to papules to vesicles and then pustules, and are deep-seated, firm, and round, with central umbilication (Fig. 3 ) [22,24]. In milder cases of the variant variola minor, papules may fail to evolve to vesicles or pustules [23]. Clinically, the regional lymphadenopathy classically associated with mpox is the only distinguishing feature [24]. However, there is no significant direct comparison of the frequencies and findings in populations under surveillance for smallpox and mpox, as the latter was discovered largely after the eradication of the former [21,24]. Given its implications with regard to bioterrorism and the associated illness severity, cases of suspected smallpox mandate immediate isolation, public health notification, and confirmatory testing [24,26].
Fig. 3.
A) Multiple umbilicated maculopapular lesions seen on the hand of a patient with smallpox. Available from https://phil.cdc.gov/Details.aspx?pid=16064. B) Late-stage smallpox rash on the arm with numerous pustules. Available from https://phil.cdc.gov/Details.aspx?pid=10467. C) Maculopapular rash with scattered umbilicated lesions in a patient with smallpox. Available from https://phil.cdc.gov/Details.aspx?pid=16055.
3.2.2. Varicella
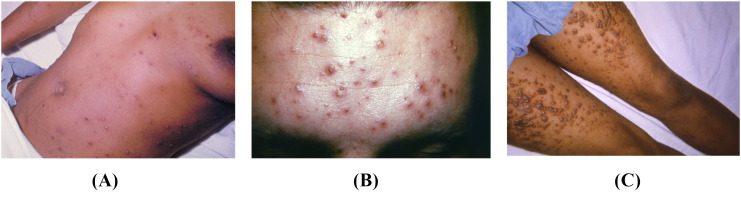
Chickenpox results from a primary infection with varicella zoster virus (VZV), a member of the herpesviridae family. Unlike mpox, VZV has no known animal reservoir and is transmitted only through human-to-human contact [[30], [31], [32]]. Primary VZV infection classically presents with abrupt onset of fever coinciding with a new pruritic rash [30,32,33]. The rash of VZV infection differs from that of mpox in that it is superficial and non-umbilicated, has irregular borders, is distributed centripetally, is found in various stages throughout the body, and spares the palms and soles (Fig. 4 ) [4,30,[32], [33], [34]]. Lesions are considered contagious until crusts form, and lesions fully heal within approximately 1–2 weeks [35,36]. The rash associated with chickenpox is classically described as resembling a “dew-drop on a rose petal.” Most complications stem from a bacterial superinfection of the lesions, which occurs in approximately 5% of cases [[35], [36], [37]]. Secondary VZV infections, better known as shingles, occur due to viral reactivation within dorsal root ganglia. They can occur at any age, likely due to temporary or ongoing immunocompromise, and present with painful vesicles with surrounding erythema following a dermatomal distribution [38].
Fig. 4.
A) Multiple lesions in different stages of healing, present on different locations on the body in this patient with chickenpox. Available from https://phil.cdc.gov/Details.aspx?pid=2901. B) Centripetal distribution of rash with lesions in multiple stages in a patient with chickenpox. Available from https://phil.cdc.gov/Details.aspx?pid=12559. C) In this patient with chickenpox, some of the papules filled with fluid have ruptured, causing a crusty appearance. Available from https://phil.cdc.gov/Details.aspx?pid=21510.
While many factors appear to clinically distinguish VZV from mpox, several studies document overlapping signs and symptoms. In fact, up to 50% of patients in Africa who were misdiagnosed as mpox were found to have VZV instead [39,40]. Even more challenging, previous epidemiologic studies document lesion polymerase chain reaction (PCR) testing being simultaneously positive for VZV and mpox in up to 12% of patients [[30], [31], [32], [33]]. Several theories exist regarding potential coinfection, including simultaneous onset of VZV and mpox, mpox infection with secondary shingles, and immunocompromise from one virus leading to secondary infection with either mpox or primary VZV [[30], [31], [32], [33]]. Many documented cases describe an “atypical Varicella” manifestation, which includes up to 86% with lesions on the palms or soles, 85% with uniform size and stage of lesions, and 70% with the predominant lymphadenopathy usually considered pathognomonic for mpox [30,33,34]. These discoveries further emphasize the importance of lesion testing to correlate with clinical findings.
3.3. Other illnesses
3.3.1. Syphilis
Syphilis is a bacterial infection caused by Treponema pallidum [[41], [42], [43]]. The organisms gain access to subcutaneous tissues through a small abrasion, resulting in infection at the location of inoculation. The mean inoculation phase is 21 days but can last up to 90 days [43]. Organisms evade the local host immune response and cause a primary chancre at the initial inoculation site, which is known as primary syphilis. The lesion starts as a papule that is usually painless, which then ulcerates to form the classic primary chancre [42,43]. This chancre is 1–2 cm with a nonexudative base and is typically isolated, indurated, and sharply demarcated [44,45]. Regional lymphadenopathy is often present. Patients who are immunocompromised may have several lesions.
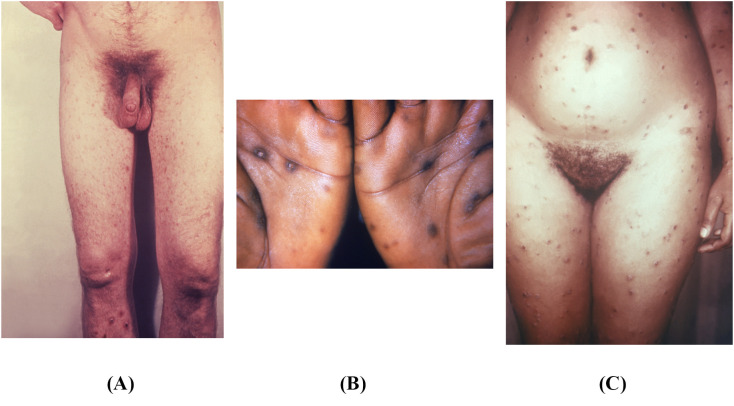
The primary lesion most commonly resolves in 6 weeks. Following the primary chancre, syphilis becomes systemic in 20–25% of cases, resulting in secondary syphilis 4–8 weeks after the primary chancre [46]. Signs and symptoms of secondary syphilis include a rash involving the palms and soles, which is the most characteristic finding (Fig. 5 ). However, over 20% of patients do not notice this rash [44,45]. The rash can take any form but is classically diffuse and symmetric, with macular or papular eruptions on the trunk and extremities [42,43,47]. Early secondary lesions are macular, flat, pale, and pink to brown in color; 4–30 mm in diameter; and elliptical in shape [42,43,47]. The central area is typically discolored, while the periphery blends into normal skin. Small papular or pustular lesions may be present, as are follicular, vesicular, and lichenoid lesions. Late secondary lesions are large, papular, and flattened. Annular lesions may also be present [42,43,47].
Fig. 5.
A) This patient was diagnosed with secondary syphilis. Note the diffuse pustulopapular rash. Available from https://phil.cdc.gov/Details.aspx?pid=17885. B) This photograph portrays a papulosquamous rash on the palms, seen in secondary syphilis. Available from https://phil.cdc.gov/Details.aspx?pid=17043. C) In this image, this patient with secondary syphilis has a diffuse maculopapular rash. Available from https://phil.cdc.gov/Details.aspx?pid=17929.
3.3.2. Acute retroviral syndrome
HIV was first described in 1985 [48]. The disease has been reported in a variety of patient populations, including injection drug users, MSM, recipients of blood products, and healthcare workers with needle-stick or body fluid exposure [49]. The time to development of symptoms after exposure is usually 2–4 weeks. The constellation of signs and symptoms is known as acute retroviral syndrome, which typically includes fever, sore throat, lymphadenopathy, rash, myalgias, headache, diarrhea, and weight loss [[50], [51], [52], [53], [54], [55]]. Painful mucocutaneous ulcers may also be present. These are usually shallow and sharply demarcated ulcers, characterized by an area of erythema surrounding a white base. They are often found on the anus, oral mucosa, penis, and esophagus [56]. A generalized rash may occur 2–3 days after fever onset and lasts 5–8 days [56]. The rash is characterized by 5–10 mm well-circumscribed, pink or red macules or maculopapules on the trunk, face, and extremities. The palms and soles may also be involved [56]. Lesions can be mildly pruritic, and pustules, vesicles, and urticaria can also be present [[57], [58], [59], [60]]. While no sign or symptom is specific for the diagnosis, a prolonged symptom duration and mucocutaneous ulcers are suggestive of HIV. Considering the disease is integral to diagnosis.
3.3.3. Genital herpes
Herpes simplex virus is a significant public health issue and includes both herpes simplex virus type 1 (HSV-1) and herpes simplex virus type 2 (HSV-2) [61,62]. Both strains may cause genital herpes, though HSV-2 is the predominant cause. Transmission occurs during sexual intercourse, and most transmissions occur when the patient is unaware they are infected [63,64]. Primary infection occurs when the patient does not have antibodies to HSV-1 or HSV-2. The incubation period ranges from 2 to 12 days, and the presentation varies significantly [62,65,66]. Skin lesions start as 2–4 mm vesicles with underlying erythema appearing in groups. These progress to vesicles, pustules, erosions, and then areas of ulceration. The vesicles and pustules can have an umbilicated appearance [65,66]. These lesions resolve on average within 19 days [65,66]. Women tend to have more severe symptoms. The lesions are typically painful but may be pruritic. Up to 98% have pain and pruritus along the lesions, 80% of patients have tender lymphadenopathy, 67% have systemic systems (e.g., headache, malaise, fever, myalgias), and 63% have dysuria [62,65,66]. Recurrent infection occurs with reactivation of genital HSV, which is typically less severe with shorter duration of symptoms [62,[65], [66], [67]].
4. Conclusion
Mpox most commonly presents with a painful rash and several systemic findings, including lymphadenopathy and fever. Focusing on the differential diagnosis of febrile illness associated with vesiculobullous lesions and lymphadenopathy, a variety of conditions can mimic its presentation, including smallpox, varicella, syphilis, acute retroviral syndrome, and genital herpes. An understanding of mpox and its mimics can assist emergency clinicians.
CRediT authorship contribution statement
Brit Long: Writing – review & editing, Writing – original draft, Visualization, Validation, Conceptualization. Stephen Y. Liang: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Conceptualization. Brandon M. Carius: Writing – review & editing, Writing – original draft, Visualization, Validation. Summer Chavez: Writing – review & editing, Writing – original draft, Visualization, Conceptualization. Michael Gottlieb: Writing – review & editing, Visualization, Validation, Supervision, Conceptualization. Alex Koyfman: Writing – review & editing, Visualization, Supervision, Conceptualization. William J. Brady: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Conceptualization.
Declaration of Competing Interest
None.
Acknowledgements
All authors conceived the idea for this manuscript and contributed substantially to the writing and editing of the review. This manuscript did not utilize any grants, and it has not been presented in abstract form. This clinical review has not been published, it is not under consideration for publication elsewhere, its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder. This review does not reflect the views or opinions of the U.S. Government, Department of Defense, Defense Health Agency, U.S. Army, U.S. Air Force, or SAUSHEC EM Residency Program.
References
- 1.World Health Organization Monkeypox. 2022. https://www.who.int/news-room/fact-sheets/detail/monkeypox Accessed 11/18/2022.
- 2.U.S. Centers for Disease Control and Prevention Monkeypox. 2022 Outbreak Cases and Data. 2022. https://www.cdc.gov/poxvirus/monkeypox/response/2022/index.html Accessed 12/22/2022.
- 3.Huhn G.D., Bauer A.M., Yorita K., et al. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin Infect Dis. 2005;41(12):1742–1751. doi: 10.1086/498115. [DOI] [PubMed] [Google Scholar]
- 4.MacNeil A., Reynolds M.G., Carroll D.S., et al. Monkeypox or varicella? Lessons from a rash outbreak investigation in the Republic of the Congo. Am J Trop Med Hyg. 2009;80(4):503–507. [PubMed] [Google Scholar]
- 5.Long B., Koyfman A., Gottlieb M., et al. Monkeypox: a focused narrative review for emergency medicine clinicians. Am J Emerg Med. 2022;61:34–43. doi: 10.1016/j.ajem.2022.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basgoz N., Brown C.M., Smole S.C., et al. Case 24-2022: a 31-year-old man with perianal and penile ulcers, rectal pain, and rash. N Engl J Med. 2022;387(6):547–556. doi: 10.1056/NEJMcpc2201244. [DOI] [PubMed] [Google Scholar]
- 7.Thornhill J.P., Barkati S., Walmsley S., et al. Monkeypox virus infection in humans across 16 countries - April-June 2022. N Engl J Med. 2022;387(8):679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Centers for Disease Control and Prevention Monkeypox cases by age and gender, race/ethnicity, and symptoms. 2022. https://www.cdc.gov/poxvirus/monkeypox/response/2022/demographics.html Accessed 12/22/2022.
- 9.Osadebe L., Hughes C.M., Shongo Lushima R., et al. Enhancing case definitions for surveillance of human monkeypox in the Democratic Republic of Congo. PLoS Negl Trop Dis. 2017;11(9) doi: 10.1371/journal.pntd.0005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yinka-Ogunleye A., Aruna O., Dalhat M., et al. Outbreak of human monkeypox in Nigeria in 2017-18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19(8):872–879. doi: 10.1016/S1473-3099(19)30294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Centers for Disease Control and Prevention Monkeypox. Clinical recognition. 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html Accessed 10/6/2022.
- 12.Breman J.G., Kalisa R., Steniowski M.V., Zanotto E., Gromyko A.I., Arita I. Human monkeypox, 1970-79. Bull World Health Organ. 1980;58(2):165–182. [PMC free article] [PubMed] [Google Scholar]
- 13.Di Giulio D.B., Eckburg P.B. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4(1):15–25. doi: 10.1016/S1473-3099(03)00856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nalca A., Rimoin A.W., Bavari S., Whitehouse C.A. Reemergence of monkeypox: prevalence, diagnostics, and countermeasures. Clin Infect Dis. 2005;41(12):1765–1771. doi: 10.1086/498155. [DOI] [PubMed] [Google Scholar]
- 15.Jezek Z., Szczeniowski M., Paluku K.M., Mutombo M. Human monkeypox: clinical features of 282 patients. J Infect Dis. 1987;156(2):293–298. doi: 10.1093/infdis/156.2.293. [DOI] [PubMed] [Google Scholar]
- 16.Petersen E., Kantele A., Koopmans M., et al. Human Monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect Dis Clin North Am. 2019;33(4):1027–1043. doi: 10.1016/j.idc.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel A., Bilinska J., Tam J.C.H., et al. Clinical features and novel presentations of human monkeypox in a Central London Centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378 doi: 10.1136/bmj-2022-072410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarin-Vicente E.J., Alemany A., Agud-Dios M., et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet. 2022;400(10353):661–669. doi: 10.1016/S0140-6736(22)01436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inigo Martinez J., Gil Montalban E., Jimenez Bueno S., et al. Monkeypox outbreak predominantly affecting men who have sex with men, Madrid, Spain, 26 April to 16 June 2022. Euro Surveill. 2022;27(27) doi: 10.2807/1560-7917.ES.2022.27.27.2200471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orviz E., Negredo A., Ayerdi O., et al. Monkeypox outbreak in Madrid (Spain): clinical and virological aspects. J Infect. 2022;85(4):412–417. doi: 10.1016/j.jinf.2022.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shchelkunov S.N., Totmenin A.V., Babkin I.V., et al. Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett. 2001;509(1):66–70. doi: 10.1016/S0014-5793(01)03144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alibek K. Smallpox: a disease and a weapon. Int J Infect Dis. 2004;8(Suppl. 2):S3–S8. doi: 10.1016/j.ijid.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Slifka M.K., Hanifin J.M. Smallpox: the basics. Dermatol Clin. 2004;22(3):263–274. doi: 10.1016/j.det.2004.03.002. [vi] [DOI] [PubMed] [Google Scholar]
- 24.Moore Z.S., Seward J.F., Lane J.M. Smallpox. Lancet. 2006;367(9508):425–435. doi: 10.1016/S0140-6736(06)68143-9. [DOI] [PubMed] [Google Scholar]
- 25.Rimoin A.W., Mulembakani P.M., Johnston S.C., et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci U S A. 2010;107(37):16262–16267. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theves C., Biagini P., Crubezy E. The rediscovery of smallpox. Clin Microbiol Infect. 2014;20(3):210–218. doi: 10.1111/1469-0691.12536. [DOI] [PubMed] [Google Scholar]
- 27.Doshi R.H., Guagliardo S.A.J., Doty J.B., et al. Epidemiologic and ecologic investigations of Monkeypox, Likouala department, Republic of the Congo, 2017. Emerg Infect Dis. 2019;25(2):281–289. doi: 10.3201/eid2502.181222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silva N.I.O., de Oliveira J.S., Kroon E.G., Trindade G.S., Drumond B.P. Here, there, and everywhere: the wide host range and geographic distribution of zoonotic Orthopoxviruses. Viruses. 2020;13(1) doi: 10.3390/v13010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simpson K., Heymann D., Brown C.S., et al. Human monkeypox - after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38(33):5077–5081. doi: 10.1016/j.vaccine.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macneil A., Reynolds M.G., Braden Z., et al. Transmission of atypical varicella-zoster virus infections involving palm and sole manifestations in an area with monkeypox endemicity. Clin Infect Dis. 2009;48(1):e6–e8. doi: 10.1086/595552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoff N.A., Morier D.S., Kisalu N.K., et al. Varicella coinfection in patients with active Monkeypox in the Democratic Republic of the Congo. Ecohealth. 2017;14(3):564–574. doi: 10.1007/s10393-017-1266-5. [DOI] [PubMed] [Google Scholar]
- 32.Hughes C.M., Liu L., Davidson W.B., et al. A tale of two viruses: coinfections of Monkeypox and varicella zoster virus in the Democratic Republic of Congo. Am J Trop Med Hyg. 2020;104(2):604–611. doi: 10.4269/ajtmh.20-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung J., McCollum A.M., Radford K., et al. Varicella in Tshuapa Province, Democratic Republic of Congo, 2009-2014. Trop Med Int Health. 2019;24(7):839–848. doi: 10.1111/tmi.13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tumewu J., Wardiana M., Ervianty E., et al. An adult patient with suspected of monkeypox infection differential diagnosed to chickenpox. Infect Dis Rep. 2020;12(Suppl. 1):8724. doi: 10.4081/idr.2020.8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veraldi S., Schianchi R. Chickenpox, impetigo, and anetoderma. Pediatr Dermatol. 2006;23(3):305–306. doi: 10.1111/j.1525-1470.2006.00246.x. [DOI] [PubMed] [Google Scholar]
- 36.Freer G., Pistello M. Varicella-zoster virus infection: natural history, clinical manifestations, immunity and current and future vaccination strategies. New Microbiol. 2018;41(2):95–105. [PubMed] [Google Scholar]
- 37.Pollard A.J., Isaacs A., Hermione Lyall E.G., et al. Potentially lethal bacterial infection associated with varicella zoster virus. BMJ. 1996;313(7052):283–285. doi: 10.1136/bmj.313.7052.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen J.I. Clinical practice: Herpes zoster. N Engl J Med. 2013;369(3):255–263. doi: 10.1056/NEJMcp1302674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer H., Perrichot M., Stemmler M., et al. Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. J Clin Microbiol. 2002;40(8):2919–2921. doi: 10.1128/JCM.40.8.2919-2921.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rimoin A.W., Kisalu N., Kebela-Ilunga B., et al. Endemic human monkeypox, Democratic Republic of Congo, 2001-2004. Emerg Infect Dis. 2007;13(6):934–937. doi: 10.3201/eid1306.061540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larsen S.A. Syphilis. Clin Lab Med. 1989;9(3):545–557. [PubMed] [Google Scholar]
- 42.Golden M.R., Marra C.M., Holmes K.K. Update on syphilis: resurgence of an old problem. JAMA. 2003;290(11):1510–1514. doi: 10.1001/jama.290.11.1510. [DOI] [PubMed] [Google Scholar]
- 43.French P. Syphilis BMJ. 2007;334(7585):143–147. doi: 10.1136/bmj.39085.518148.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chapel T.A. The variability of syphilitic chancres. Sex Transm Dis. 1978;5(2):68–70. doi: 10.1097/00007435-197804000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Chapel T.A. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980;7(4):161–164. doi: 10.1097/00007435-198010000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Clark E.G.D. N. The Oslo study of the natural course of untreated syphilis: an epidemiologic investigation based on a re-study of the Boeck-Bruusgaard material. Med Clin North Am. 1964;48(3):613–623. [Google Scholar]
- 47.Baughn R.E., Musher D.M. Secondary syphilitic lesions. Clin Microbiol Rev. 2005;18(1):205–216. doi: 10.1128/CMR.18.1.205-216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cooper D.A., Gold J., Maclean P., et al. Acute AIDS retrovirus infection. Definition of a clinical illness associated with seroconversion. Lancet. 1985;1(8428):537–540. doi: 10.1016/s0140-6736(85)91205-x. [DOI] [PubMed] [Google Scholar]
- 49.Tindall B., Cooper D.A., Donovan B., Penny R. Primary human immunodeficiency virus infection. Clinical and serologic aspects. Infect Dis Clin North Am. 1988;2(2):329–341. [PubMed] [Google Scholar]
- 50.Niu M.T., Stein D.S., Schnittman S.M. Primary human immunodeficiency virus type 1 infection: review of pathogenesis and early treatment intervention in humans and animal retrovirus infections. J Infect Dis. 1993;168(6):1490–1501. doi: 10.1093/infdis/168.6.1490. [DOI] [PubMed] [Google Scholar]
- 51.Daar E.S., Little S., Pitt J., et al. Diagnosis of primary HIV-1 infection. Los Angeles County primary HIV infection recruitment network. Ann Intern Med. 2001;134(1):25–29. doi: 10.7326/0003-4819-134-1-200101020-00010. [DOI] [PubMed] [Google Scholar]
- 52.Kared H., Lelievre J.D., Donkova-Petrini V., et al. HIV-specific regulatory T cells are associated with higher CD4 cell counts in primary infection. AIDS. 2008;22(18):2451–2460. doi: 10.1097/QAD.0b013e328319edc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Braun D.L., Kouyos R.D., Balmer B., Grube C., Weber R., Gunthard H.F. Frequency and spectrum of unexpected clinical manifestations of primary HIV-1 infection. Clin Infect Dis. 2015;61(6):1013–1021. doi: 10.1093/cid/civ398. [DOI] [PubMed] [Google Scholar]
- 54.Robb M.L., Eller L.A., Kibuuka H., et al. Prospective study of acute HIV-1 infection in adults in East Africa and Thailand. N Engl J Med. 2016;374(22):2120–2130. doi: 10.1056/NEJMoa1508952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crowell T.A., Colby D.J., Pinyakorn S., et al. Acute retroviral syndrome is associated with high viral burden, CD4 depletion, and immune activation in systemic and tissue compartments. Clin Infect Dis. 2018;66(10):1540–1549. doi: 10.1093/cid/cix1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lapins J., Gaines H., Lindback S., Lidbrink P., Emtestam L. Skin and mucosal characteristics of symptomatic primary HIV-1 infection. AIDS Patient Care STDS. 1997;11(2):67–70. doi: 10.1089/apc.1997.11.67. [DOI] [PubMed] [Google Scholar]
- 57.Calabrese L.H., Proffitt M.R., Levin K.H., Yen-Lieberman B., Starkey C. Acute infection with the human immunodeficiency virus (HIV) associated with acute brachial neuritis and exanthematous rash. Ann Intern Med. 1987;107(6):849–851. doi: 10.7326/0003-4819-107-6-849. [DOI] [PubMed] [Google Scholar]
- 58.de Jong M.D., Hulsebosch H.J., Lange J.M. Clinical, virological and immunological features of primary HIV-1 infection. Genitourin Med. 1991;67(5):367–373. doi: 10.1136/sti.67.5.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Molina J.M., Welker Y., Ferchal F., Decazes J.M., Shenmetzler C., Modai J. Hepatitis associated with primary HIV infection. Gastroenterology. 1992;102(2):739. doi: 10.1016/0016-5085(92)90138-o. [DOI] [PubMed] [Google Scholar]
- 60.Rizzardi G.P., Tambussi G., Lazzarin A. Acute pancreatitis during primary HIV-1 infection. N Engl J Med. 1997;336(25):1836–1837. doi: 10.1056/NEJM199706193362516. [DOI] [PubMed] [Google Scholar]
- 61.Sucato G., Wald A., Wakabayashi E., Vieira J., Corey L. Evidence of latency and reactivation of both herpes simplex virus (HSV)-1 and HSV-2 in the genital region. J Infect Dis. 1998;177(4):1069–1072. doi: 10.1086/515261. [DOI] [PubMed] [Google Scholar]
- 62.Gupta R., Warren T., Wald A. Genital herpes. Lancet. 2007;370(9605):2127–2137. doi: 10.1016/S0140-6736(07)61908-4. [DOI] [PubMed] [Google Scholar]
- 63.Schillinger J.A., McKinney C.M., Garg R., et al. Seroprevalence of herpes simplex virus type 2 and characteristics associated with undiagnosed infection: New York City, 2004. Sex Transm Dis. 2008;35(6):599–606. doi: 10.1097/OLQ.0b013e3181666fb1. [DOI] [PubMed] [Google Scholar]
- 64.Workowski K.A., Bachmann L.H., Chan P.A., et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1–187. doi: 10.15585/mmwr.rr7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corey L., Adams H.G., Brown Z.A., Holmes K.K. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983;98(6):958–972. doi: 10.7326/0003-4819-98-6-958. [DOI] [PubMed] [Google Scholar]
- 66.Kimberlin D.W., Rouse D.J. Clinical practice. Genital Herpes N Engl J Med. 2004;350(19):1970–1977. doi: 10.1056/NEJMcp023065. [DOI] [PubMed] [Google Scholar]
- 67.Whitley R.J., Kimberlin D.W., Roizman B. Herpes simplex viruses. Clin Infect Dis. 1998;26(3):541–553. doi: 10.1086/514600. quiz 554–545. [DOI] [PubMed] [Google Scholar]