Abstract
Mycobacterium tuberculosis establishes infection, progresses towards disease, and is transmitted from the alveolus of the lung. However, the role of the alveolar epithelium in any of these pathogenic processes of tuberculosis is unclear. In this study, lung epithelial cells (A549) were used as a model in which to examine cytotoxicity during infection with either virulent or avirulent mycobacteria in order to further establish the role of the lung epithelium during tuberculosis. Infection of A549 cells with M. tuberculosis strains Erdman and CDC1551 demonstrated significant cell monolayer clearing, whereas infection with either Mycobacterium bovis BCG or Mycobacterium smegmatis LR222 did not. Clearing of M. tuberculosis-infected A549 cells correlated to necrosis, not apoptosis. Treatment of M. tuberculosis-infected A549 cells with streptomycin, but not cycloheximide, demonstrated a significant reduction in the necrosis of A549 cell monolayers. This mycobacterium-induced A549 necrosis did not correlate to higher levels of intracellular or extracellular growth by the mycobacteria during infection. Staining of infected cells with propidium iodide demonstrated that M. tuberculosis induced increased permeation of A549 cell membranes within 24 h postinfection. Quantitation of lactate dehydrogenase (LDH) release from infected cells further demonstrated that cell permeation was specific to M. tuberculosis infection and correlated to A549 cellular necrosis. Inactivated M. tuberculosis or its subcellular fractions did not result in A549 necrosis or LDH release. These studies demonstrate that lung epithelial cell cytotoxicity is specific to infection by virulent mycobacteria and is caused by cellular necrosis. This necrosis is not a direct correlate of mycobacterial growth or of the expression of host cell factors, but is preceded by permeation of the A549 cell membrane and requires infection with live bacilli.
The initiation of infection with Mycobacterium tuberculosis occurs when organisms in small droplet nuclei are inhaled into the alveoli (13, 14, 36). Subsequent passage of these bacilli through the alveolar epithelium is required for the establishment of infection and disease progression (2, 13, 14, 36). Later in disease, destruction of the alveolar epithelium occurs during caseation and liquefaction of the granuloma, leading to transmission of the bacilli (13, 14, 36). The ability of M. tuberculosis to gain passage into the lung tissues and back to the airways through the alveolar epithelium is an important aspect of tuberculosis. Diapedesis of infected alveolar macrophages is most likely the mechanism for passage of the tubercle bacilli into the lungs. However, the precise role of the alveolar epithelial cells during infection is unclear. Human pneumocyte (A549) tissue culture models have been developed to address the role of the alveolar epithelium in the pathogenesis of tuberculosis.
Minimally, the alveolar epithelium represents a physiologic barrier to prevent M. tuberculosis from gaining entry into the bloodstream. Infection of alveolar cells with M. tuberculosis has been shown to alter the bioelectric properties of the infected cells (54). Both virulent M. tuberculosis H37Rv and Erdman and avirulent M. tuberculosis H37Ra and Mycobacterium bovis BCG have been shown to enter and grow within A549 cells (6, 32, 33). However, only infection of A549 cells by virulent M. tuberculosis resulted in clearance of cell monolayers, suggesting that this phenotype is related to the virulence of mycobacteria (32). Cytotoxicity has also been shown in a bilayer system of A549 cells and endothelial cells. Destruction of the epithelial layer occurred upon infection with M. tuberculosis, and the number of virulent M. tuberculosis cells crossing through the bilayer increased after destruction of the epithelial layer (7).
A major question in the pathogenesis of tuberculosis is whether the tissue damage that occurs during disease is mediated solely by the host immune response (47) or whether specific bacterial factors play a role. Determining the mechanisms of cell death caused by infection with M. tuberculosis in the A549 cell model could be important to addressing this question. M. tuberculosis cytotoxicity may be related to invasion efficiency, as virulent M. tuberculosis strains have been shown to be more invasive than avirulent strains or M. bovis BCG (6, 32). Similarly, differences in growth rates may contribute to the cytotoxic phenotype. Cytotoxicity may be due to the release of cytokines by A549 cells upon infection. A549 cells have been shown to release interleukin-8 (IL-8) and MCP-1 upon infection with both H37Rv and H37Ra strains of M. tuberculosis (29, 51), and this has further defined a role for the alveolar epithelium during infection. Interestingly, cytotoxicity is independent of the effects of tumor necrosis factor alpha (TNF-α) secretion or TNF-α-induced apoptosis. A549 cells do not up regulate TNF-α expression (29) or secrete biologically active TNF-α (J. Castro-Garza, F. D. Quinn, and C. H. King, ASM Conf. on Tuberculosis, abstr. no. A-50, 1997) in response to infection with M. tuberculosis. In this report, we examined the cell death caused by virulent and avirulent mycobacteria during infection of human lung epithelial cells and investigated the factors that contribute to this cytotoxic phenotype.
MATERIALS AND METHODS
Bacterial cultures and subcellular fractions.
M. tuberculosis strain Erdman, M. tuberculosis strain CDC1551 (46), and Mycobacterium smegmatis strain LR222 were obtained from the Centers for Disease Control and Prevention culture collection (Atlanta, Ga.). M. bovis BCG strain Pasteur was a kind gift from Barry Bloom (Harvard School of Public Health, Boston, Mass.). Stock 1-ml cultures of the mycobacterial strains were grown by serial 10-fold passage to 100 ml in Middlebrook 7H9 oleic acid, albumin, dextrose, and catalase (OADC) broth until the optical density (600-nm wavelength) was 0.5 to 0.7, corresponding to 1 × 107 to 10 × 107 CFU/ml. This 100-ml stock solution was then dispersed to make a single-cell suspension by applying a 1-min pulse at a setting of 10 to cultures in closed polypropylene tubes at 4°C using a Branson 450 cell sonicator fitted with a cup horn attachment (Branson Ultrasonics, Danbury, Conn.). Aliquots of 0.5 and 1.0 ml were made from this suspension, and these aliquots were immediately quick frozen in an ethanol–dry-ice bath and stored at −70°C until needed.
Before the beginning of each experiment, the aliquots of mycobacteria were thawed, and any bacterial clumps caused by freezing were disrupted by pulse sonication (25, 35). Bacteria were then diluted by serial dilution into tissue culture medium prior to addition to the cell monolayers. All dilutions were verified for uniform dispersal, and accurate multiplicities of infection (MOIs) were confirmed directly for each experiment by performing viable counts of CFU on Middlebrook 7H10 OADC agar.
For experiments using heat-killed or γ-irradiated M. tuberculosis, the aliquots of M. tuberculosis CDC1551 prepared as described above were inactivated either by incubation at 80°C for 60 min or by exposure to 1.2 megarads of γ-irradiation over 12 h at −70°C. CFU counts for these aliquots were determined prior to inactivation, and inactivation of cultures following these treatments was confirmed by plating onto 7H10 plates and observing these plates over a 5-week period. Subcellular fractions from γ-irradiated M. tuberculosis CDC1551 were provided as aseptic lyophilisates by John Belisle (Colorado State University, NIAID contract NOI-AI-75320).
Cell cultures.
Human lung epithelial cells (A549) were obtained from the American Type Culture Collection (Manassas, Va.) at passage number 76. Cells were maintained in modified Eagle's medium (MEM) with Earle's salts and l-glutamine (Gibco/BRL, Grand Island, N.Y.), supplemented with 5% heat-inactivated fetal bovine serum (FBS) (HyClone Laboratories Inc., Logan, Utah), and incubated at 37°C with 5% CO2. Prior to use in experimental assays, the cells were released from the culture flask with 0.25% trypsin (Gibco), washed twice with fresh medium, and seeded onto 6- or 24-well microculture plates as needed. Viable cell counts were confirmed from seeded test wells prior to each experiment using trypan exclusion as previously described (33). All experiments were performed between cell passage numbers 78 and 95.
Necrosis and apoptosis determination in lung epithelial cells.
Analysis of cytotoxicity in lung epithelial cells was performed using an enzyme-linked immunosorbent assay (ELISA) that differentiates between necrosis and apoptosis by measuring the release or sequestering of histone-DNA complexes in affected eukaryotic cells (Roche, Indianapolis, Ind.). Both phenotypes have been measured in A549 and other epithelial cells using this ELISA (8, 28, 34).
Initially, the pore-forming detergent digitonin (1%) and the apoptosis inducer camptothecin (0.005 mM) were used in our model to determine the time of incubation required to detect necrosis and apoptosis with this ELISA. Digitonin or camptothecin was added to uninfected monolayers that were 75% confluent in 24-well plates (∼1 × 105 cells/ml) and incubated at 37°C with 5% CO2. Tissue culture plates were then centrifuged for 5 min at 200 × g, and culture supernatants from the monolayers were collected and filtered through a 0.22-μm-pore-size syringe filter (Millex/GV filters; Millipore Corp., Bedford, Mass.) for the measurement of necrosis. The remaining cells were then treated with a lysis buffer (supplied by Boehringer Mannheim) to lyse the cells and release intracellular histone-DNA complexes. The cell lysates were then collected and filtered through a 0.22-μm-pore-size syringe filter (Millipore). Culture supernatants and cell lysates from each individual well were immediately tested for histone-DNA complexes using the cell death ELISA according to the manufacturer's directions. The levels of necrosis and apoptosis from treated and untreated cells were measured spectrophotometrically at 405 nm (with the reference wavelength of 490 nm subtracted per the manufacturer's directions) and were compared at 4, 24, and 72 h. Untreated monolayers were incubated and measured by ELISA at identical time points to determine the background levels of necrosis and apoptosis in this model. All experiments were tested in triplicate and performed in duplicate.
For the infections, lung epithelial cells were seeded into 24-well tissue culture plates as described above and monolayers were infected with each mycobacterial strain at an equal MOI of 10 bacilli per cell, except for M. smegmatis strain LR222. This strain was used at a viable count equal to an MOI of 50 bacilli per cell to control for the effects of mycobacterial extracellular growth on necrosis or apoptosis in this tissue culture model. All infected cell cultures were incubated at 37°C with 5% CO2. Tissue culture plates were centrifuged, and culture supernatants from infected and uninfected monolayers were collected at 1, 3, and 5 days postinfection as described above. The remaining monolayers were then lysed, and cellular lysates collected as described above. Both the culture supernatants and cell lysates from each individual well were immediately tested as described above, and all data were normalized by subtraction of background levels at each time point. All infections were tested in triplicate, and experiments were repeated three times.
For measurement of necrosis and apoptosis caused by heat-killed or γ-irradiated M. tuberculosis, an inoculum of these inactivated aliquots equal to an MOI of 10 (from CFU per milliliter determined prior to inactivation) was added to A549 cell monolayers. Tissue culture plates were incubated at 37°C with 5% CO2, and mock-infected monolayers were treated for measurement of necrosis and apoptosis as described above.
For measurement of necrosis and apoptosis caused by incubation of subcellular fractions of M. tuberculosis, the protein yields of culture filtrate (CF) and of subcellular fractions of cell wall (CW) and whole-cell lysate (WCL) from 107 CFU of M. tuberculosis/ml were first determined. Briefly, a 10-ml culture of M. tuberculosis CDC1551 was grown in glycerol alanine salts medium (44) for 10 days. A 1-ml aliquot from this preparation was removed and then added to 9 ml of 7H9–OADC, and this preparation was sonicated and plated with serial dilutions onto 7H10 plates as described above. The CF was harvested from the remaining 9 ml of GAS culture, and crude CW and WCL preparations were made from the bacterial pellet of this culture by standard methods (16). These analytical preparations of CW, WCL, and CF were quantitated for their protein content by bicinchoninic acid (BCA) analysis (Pierce Chemical Co., Rockford, Ill.), and the results were compared with the CFU determination. A total of 8 μg of CF protein, 20 μg of WCL protein, and 2.5 μg of CW protein were found to be present in 1 ml of a 107-CFU culture. Tissue culture plates were seeded with A549 cells as above, and viable A549 cells were determined prior to each experiment as above. CF, CW, and WCL fractions were then added at microgram-of-protein-per-CFU equivalents of MOIs of 100 and 500. These 10- and 50-fold excesses were used to address bacterial replication or loss of activity in these γ-irradiated subcellular fractions. Plates were incubated, and cell lysates and supernatants were collected and measured by ELISA as above. All experiments were performed in triplicate.
Effects of inhibitors on the necrosis of A549 cells.
The requirement of bacterial and eukaryotic protein synthesis for the necrosis of A549 cells was determined by incubation with streptomycin sulfate (10) or cycloheximide (43) during the infection of A549 cells with M. tuberculosis strain CDC1551. The MIC of streptomycin sulfate (Sigma, St. Louis, Mo.) for M. tuberculosis strain CDC1551 was determined to be 2 μg/ml (data not shown), and we used a concentration of 10 μg/ml that completely inhibited bacterial growth but was not bactericidal (data not shown). A549 cells were counted and seeded onto 24-well plates as described above. Cells were infected at an MOI of 10 bacilli per cell with M. tuberculosis strain CDC1551 that had been preincubated for 24 h at 37°C either with 10 μg of streptomycin sulfate/ml in MEM–5% FBS or with MEM–5% FBS alone. These preparations of bacilli in either MEM–5% FBS–streptomycin or MEM–5% FBS were directly overlaid onto cell monolayers and were allowed to remain on the cell monolayers throughout the course of infection. Uninfected cells with or without streptomycin sulfate were also included as controls for subtraction of background necrosis, and all data were normalized to these controls. The infected cell cultures were incubated for 5 days, and necrosis was measured as described above at days 1, 3, and 5 postinfection. All infections were conducted in triplicate, and experiments were performed in duplicate.
For experiments to test the effects of cycloheximide on necrosis, we chose a concentration of cycloheximide (50 μg/ml) that inhibited protein synthesis and the exogenous release of chemokines in A549 cells (5, 23, 30, 45), but did not interfere with M. tuberculosis protein synthesis or intracellular growth (27). A549 cells were counted and seeded onto 24-well plates as described above and were treated with cycloheximide for 3 h prior to infection. Cell monolayers with or without cycloheximide were then infected at an MOI of 10 bacilli per cell with M. tuberculosis strain CDC1551. Uninfected cells with or without cycloheximide were included as controls for background necrosis, and all data were normalized to these controls. The infected cell cultures were incubated for 5 days, and necrosis was measured as described above at days 1, 3, and 5 postinfection. All infections were conducted in triplicate wells, and experiments were performed in duplicate.
Measurement of intracellular and extracellular growth of mycobacterial strains.
Six-well plates of A549 cell monolayers (∼1 × 105 cells/ml) were infected with M. tuberculosis strain Erdman or CDC1551 or with M. bovis BCG at an MOI of 10 and then incubated at 37°C with 5% CO2. Entry of M. bovis BCG into A549 cells was verified by electron microscopy of infections at 24 h postinoculation using previously described methods (33). CFU assays were performed at 6, 24, 48, 72, 96, and 120 h postinfection by standard methods (25, 35). Briefly, for determination of extracellular growth, extracellular medium was removed at the above time points from tissue culture wells. Wells were washed twice with phosphate-buffered saline (PBS) to remove any additional extracellular or adherent bacilli. Media and washes were combined, and this suspension was sonicated to achieve single-cell suspensions as described above and was then serially diluted and plated onto 7H10 plates. For determination of intracellular growth, 1 ml of lysis buffer (PBS with 0.25% Tween 80 and 0.016% digitonin) was added to each washed monolayer and tissue culture plates were incubated for 10 min at 37°C according to the standard protocol (25, 35). The lysate was collected, sonicated, serially diluted, and plated as described above. Colonies were counted from extracellular and intracellular growth plates following 14 to 21 days of incubation. All infections were conducted in duplicate and repeated twice.
Measurement of cell permeability and cell membrane distortion in infected lung epithelial cells.
Propidium iodide (PI), a cell membrane-impermeant nuclear stain that stains the nuclei of necrotic porous cells only (53), and annexin V (AV), a dye that binds to negatively charged phospholipids on the outer surface of the plasma membrane of all apoptotic cells and necrotic cells undergoing membrane translocation (48), were used to stain infected A549 cells to determine when M. tuberculosis-infected cells became permeable to PI or susceptible to AV staining.
Lung epithelial cells were seeded into chamber slides (∼1 × 105 cells/ml), and monolayers were infected in duplicate at an MOI of 10 bacilli per cell with mycobacteria as above. At 24 h postinfection, the extracellular bacilli were removed and cell monolayers were washed twice with PBS to remove residual adherent bacilli. Monolayers were overlaid with HEPES buffer supplied by Roche. Individual wells of infected and control cultures were stained with PI and AV (Roche) according to the manufacturer's methods. Slides were washed and fixed with 10% buffered formulin, and cells were viewed at a magnification of ×400 and were counted and photographed using a Zeiss fluorescent microscope (488-nm excitation and 515-nm long pass filter). Uninfected monolayers were also treated with 1% digitonin or 0.005 mM camptothecin and analyzed at 4 h as positive controls for detection of necrotic and apoptotic cells, respectively. Ten fields were counted and recorded for each infection and control assay. All data reported represent the mean and standard deviations of 10 different fields for each infection and control.
Measurement of LDH release from infected A549 cells.
A549 cells were seeded into 24-well plates in a manner similar to that described above, except that the cells were laid down using MEM with 1% FBS, and monolayers were infected with mycobacteria at an MOI of 10 as described above. The release of the cytoplasmic enzyme lactate dehydrogenase (LDH) from infected, permeabilized A549 cells was measured at 6, 24, 48, and 72 h postinfection by using the colorimetric kit from Roche and following the manufacturer's instructions. Background release of LDH was obtained by measurement of untreated cells, and maximum release of LDH was obtained by lysis of uninfected cells per the manufacturer's protocol. The percent LDH release was then determined using the following calculation: [(release of LDH from infected cells background release)/(maximum release of LDH − background release)] × 100. Similarly, heat-killed and γ-irradiated preparations of M. tuberculosis were added to A549 cell monolayers at inoculations equivalent to an MOI of 10 (determined prior to inactivation); subcellular fractions of CF, CW, and WCL were added to monolayers at protein concentrations equivalent to MOIs of 100 and 500; and LDH release was measured from A549 cells following incubation with these preparations as described above. No significant LDH was detected in the MEM–1% FBS tissue culture medium alone, in this medium with each of the above mycobacterial strains, or in the inactivated and subcellular mycobacterial preparations in this medium. All infections, mock infections, and protein overlays were conducted in triplicate and performed in duplicate.
Statistical analysis.
All tests for significance were performed using the Student two-tailed t test, with the statistical program provided with Excel software 97 (Microsoft Corp., Redmond, Wash.). Unless otherwise stated, data were considered significant at a P value of <0.05.
RESULTS
Necrosis of infected lung epithelial cells correlates with cytotoxicity.
In previous studies, M. tuberculosis induced clearing of confluent monolayers upon infection, but M. bovis BCG did not (32). Similar findings were observed in our laboratory when monolayers were infected with M. tuberculosis strain Erdman or CDC1551 versus M. bovis BCG (data not shown). To determine the role of necrosis and apoptosis in the cytotoxicity of lung epithelial cells by infection with M. tuberculosis, a quantitative ELISA was used to measure the release of histone-DNA complexes from the supernatants (necrosis) and lysates (apoptosis) of infected and uninfected lung epithelial cells.
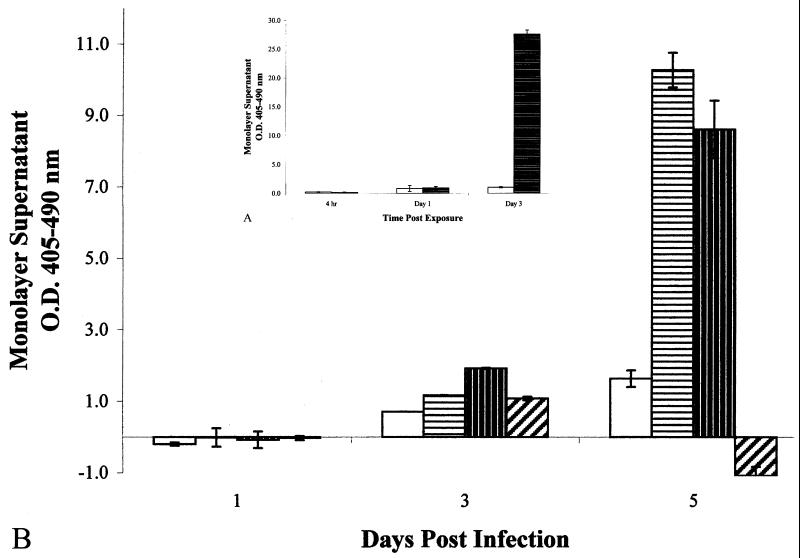
A549 epithelial cells were first tested for susceptibility to necrosis and apoptosis by incubation with either 1% digitonin or 0.005 mM camptothecin. Treatment of A549 cells with digitonin resulted in cellular necrosis within 3 days postoverlay (Fig. 1A), whereas camptothecin treatment resulted in cellular apoptosis within 1 day postoverlay (Fig. 2A), demonstrating the susceptibilities of this cell line and the ability to distinguish between these two cell death phenotypes using this assay.
FIG. 1.
(A) (inset) Optical densities of histone-DNA complexes
released in the culture supernatants of epithelial cell monolayers
exposed to the pore-forming detergent digitonin (at 1%). □,
untreated cells;  , 1%
digitonin-treated cells. Data shown are means from two experiments run
in triplicate. Error bars, standard deviations. (B) Optical densities
of histone-DNA complexes released in the culture supernatants of
infected epithelial cell monolayers. All monolayers were infected at an
MOI of 10:1. □, M. bovis BCG;
, 1%
digitonin-treated cells. Data shown are means from two experiments run
in triplicate. Error bars, standard deviations. (B) Optical densities
of histone-DNA complexes released in the culture supernatants of
infected epithelial cell monolayers. All monolayers were infected at an
MOI of 10:1. □, M. bovis BCG;
 , M. tuberculosis
strain Erdman;
, M. tuberculosis
strain Erdman;  , M.
tuberculosis strain CDC1551;
, M.
tuberculosis strain CDC1551;
 , M. smegmatis. Data
shown are means from three different experiments of triplicate
monolayers. Error bars, standard errors. The values obtained were
normalized by subtraction of background levels of necrosis of
uninfected A549 cell monolayers at each time point.
, M. smegmatis. Data
shown are means from three different experiments of triplicate
monolayers. Error bars, standard errors. The values obtained were
normalized by subtraction of background levels of necrosis of
uninfected A549 cell monolayers at each time point.
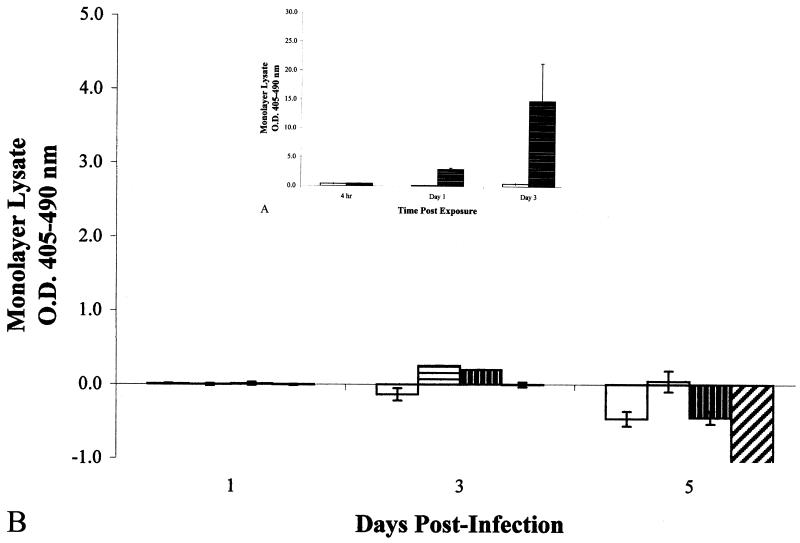
FIG. 2.
(A) (inset) Optical densities of histone-DNA complexes
from the cell lysates of epithelial cell monolayers exposed to 0.005 mM
camptothecin. □, untreated cells;
 , 0.005 mM camptothecin-treated
cells. Data shown are means from two experiments run in triplicate.
Error bars, standard deviations. (B) Optical densities of
intracellular histone-DNA complexes from the cell lysates of
infected epithelial cell monolayers. All monolayers were infected at an
MOI of 10:1. □, M. bovis BCG;
, 0.005 mM camptothecin-treated
cells. Data shown are means from two experiments run in triplicate.
Error bars, standard deviations. (B) Optical densities of
intracellular histone-DNA complexes from the cell lysates of
infected epithelial cell monolayers. All monolayers were infected at an
MOI of 10:1. □, M. bovis BCG;
 , M. tuberculosis
strain Erdman;
, M. tuberculosis
strain Erdman;  , M.
tuberculosis strain CDC1551;
, M.
tuberculosis strain CDC1551;
 , M. smegmatis. Data
shown are means from four different experiments of triplicate
monolayers. Error bars, standard errors. The values obtained were
normalized by subtraction of background levels of apoptosis of
uninfected A549 cell monolayers at each time point.
, M. smegmatis. Data
shown are means from four different experiments of triplicate
monolayers. Error bars, standard errors. The values obtained were
normalized by subtraction of background levels of apoptosis of
uninfected A549 cell monolayers at each time point.
The mode of cytotoxicity after infection of A549 cells by four different strains of mycobacteria was then determined. M. tuberculosis strains Erdman and CDC1551 induced significantly greater necrosis (Fig. 1B) than apoptosis in the infected monolayers (Fig. 2B) by 3 (P = 0.035 and P = 0.021) and 5 (P = 0.021 and P = 0.001) days postinfection, indicating that necrosis was the primary cause of cytotoxicity to the A549 epithelial cell monolayers, and that the time course for induction of necrosis was similar to that shown with digitonin (Fig. 1A). M. tuberculosis strains Erdman and CDC1551 were significantly more necrotic to the monolayers than M. bovis BCG after 5 days postinfection (Fig. 1B) (P = 0.026), correlating to the visual observations of cytotoxicity by these strains (data not shown). Interestingly, M. smegmatis strain LR2222 caused very little cytotoxicity (Fig. 1B), despite the fact that a higher MOI was used (50:1) and a large number of extracellular bacilli had grown in the medium over the 5 days of incubation (data not shown). Although M. tuberculosis strain CDC1551 induced greater necrosis than strain Erdman by 3 days postinfection (Fig. 1B), levels of necrosis by CDC1551 and Erdman were not significantly different (P = 0.776) by day 5 postinfection.
Necrosis of M. tuberculosis-infected lung epithelial cells is reduced by incubation with streptomycin, but not by incubation with cycloheximide.
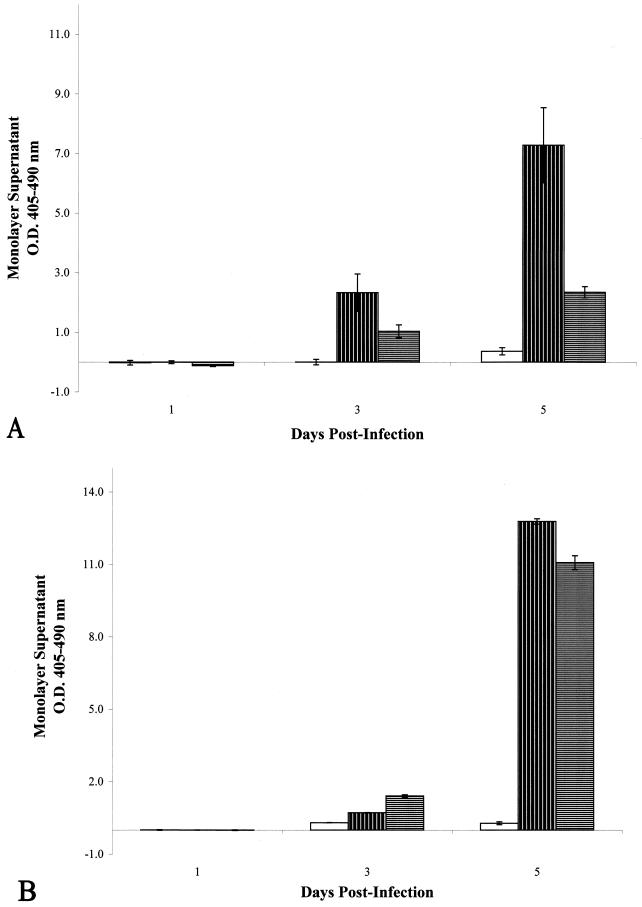
A549 cells infected with M. tuberculosis strain CDC1551 treated with streptomycin demonstrated a significant reduction in necrosis by day 3 postinfection compared to untreated M. tuberculosis CDC1551-infected monolayers (Fig. 3A) (P = 0.027), and this reduction remained significant through day 5 postinfection (P = 0.003), suggesting that bacterial replication or protein synthesis was important for induction of necrosis.
FIG. 3.
(A) Optical densities of histone-DNA complexes released
in the culture supernatants of infected epithelial cell monolayers with
or without treatment using the bacterial protein synthesis inhibitor
streptomycin. □, uninfected, streptomycin-treated cells;
 , M. tuberculosis
CDC1551-infected, untreated cells;
, M. tuberculosis
CDC1551-infected, untreated cells;
 , M. tuberculosis
CDC1551-infected, streptomycin-treated cells. (B) Optical densities of
histone-DNA complexes released in the culture supernatants of infected
epithelial cell monolayers with or without treatment with the
eukaryotic protein synthesis inhibitor cycloheximide. □, uninfected,
cycloheximide-treated cells;
, M. tuberculosis
CDC1551-infected, streptomycin-treated cells. (B) Optical densities of
histone-DNA complexes released in the culture supernatants of infected
epithelial cell monolayers with or without treatment with the
eukaryotic protein synthesis inhibitor cycloheximide. □, uninfected,
cycloheximide-treated cells;  ,
M. tuberculosis CDC1551-infected, untreated cells;
,
M. tuberculosis CDC1551-infected, untreated cells;
 , M.
tuberculosis CDC1551-infected, cycloheximide-treated cells. All
monolayers were infected at an MOI of 10:1. Data shown are means from
two different experiments of triplicate monolayers. Error bars,
standard deviations. The values obtained were normalized by subtraction
of background levels of necrosis of uninfected A549 cell monolayers at
each time point.
, M.
tuberculosis CDC1551-infected, cycloheximide-treated cells. All
monolayers were infected at an MOI of 10:1. Data shown are means from
two different experiments of triplicate monolayers. Error bars,
standard deviations. The values obtained were normalized by subtraction
of background levels of necrosis of uninfected A549 cell monolayers at
each time point.
However, when lung epithelial cells were infected with M. tuberculosis strain CDC1551 after pretreatment of these cells with, and in the presence of, cycloheximide, no reduction in necrosis was observed over the course of the infection (P = 0.372) (Fig. 3B). Eukaryotic protein synthesis, therefore, did not appear to be required for necrosis of A549 cells upon infection with M. tuberculosis.
Growth rates of mycobacteria in A549 cells.
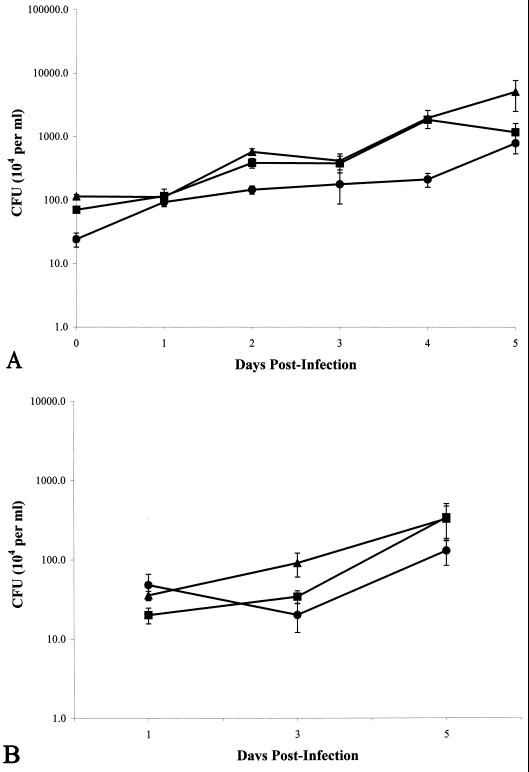
To determine if differences in mycobacterial growth were related to the cytotoxic phenotype, we measured the intracellular and extracellular growth of both strains of M. tuberculosis and that of M. bovis BCG during infection of A549 cells. Verification that M. bovis BCG entered A549 cells was obtained by electron microscopy (data not shown), as has been shown previously for M. tuberculosis (6, 32, 33). Although the numbers of M. tuberculosis CFU obtained from the A549 lysates after 6 h of incubation were greater than those obtained for M. bovis BCG (Fig. 4A), the numbers of intracellular and extracellular CFU obtained for all mycobacterial strains measured at day 1 were similar (Fig. 4). At 3 days postinfection, only M. tuberculosis strain CDC1551 demonstrated more growth than the other two mycobacteria, and this increase was seen only for extracellular growth of this mycobacterium (Fig. 4B). However, this increase was only transient, as all mycobacteria tested demonstrated similar CFU numbers for extracellular growth by day 5 postinfection (Fig. 4B). In addition, while M. tuberculosis CDC1551 continued to grow intracellularly in the A549 cells at day 5 postinfection, the growth of M. tuberculosis Erdman began to wane, and CFU numbers of M. tuberculosis Erdman and M. bovis BCG were similar (Fig. 4A).
FIG. 4.
(A) CFU obtained from intracellular growth of Mycobacterium spp. within A549 cells. Time zero is 6 h postinfection. P < 0.01 for differences between all three mycobacteria at all time points tested, except that no difference was observed between any mycobacteria at 24 h, no difference was observed between M. tuberculosis strain Erdman and M. tuberculosis strain CDC1551 at 24, 48, 72, and 96 h, and no difference was observed between M. bovis BCG and M. tuberculosis strain Erdman at 120 h. (B) CFU obtained from extracellular growth of Mycobacterium spp. cocultured with A549 cells. P < 0.01 at 72 h for M. tuberculosis strain CDC1551 versus M. bovis BCG and at 24 h for M. tuberculosis strain Erdman versus M. tuberculosis strain CDC1551. ▴, M. tuberculosis strain CDC1551; ■, M. tuberculosis strain Erdman; ●, M. bovis BCG. All experiments were performed in duplicate and repeated twice. Error bars, standard deviations.
High or differential growth rates were infrequently observed and did not explain the necrosis induced by infection of A549 cells with mycobacteria. Little extracellular growth occurred for any mycobacteria throughout the course of the experiments (Fig. 4B; also observations by others [7, 29, 35]). Only M. tuberculosis strain CDC1551 demonstrated a greater number of CFU from extracellular growth, and only on day 3 postinfection (Fig. 4B). M. tuberculosis CDC1551 also demonstrated greater CFU numbers for intracellular growth than both M. tuberculosis Erdman and M. bovis BCG at day 5 postinfection (Fig. 4A), though necrosis by both M. tuberculosis strains at day 5 was similar (Fig. 1B). Lastly, M. bovis BCG did not demonstrate any appreciable level of necrosis of A549 cells throughout the course of the experiments (Fig. 1B), despite the fact that similar numbers of intracellular and extracellular CFU were obtained for M. tuberculosis strain Erdman and M. bovis BCG (Fig. 4).
Necrosis of M. tuberculosis-infected lung epithelial cells is preceded by cell permeation.
We next examined cell membrane permeability and distortion to determine if loss of integrity of A549 cellular membranes correlated with necrosis. Initially, A549 cell monolayers were infected with mycobacteria and stained with both PI and AV after 24 h postinfection to identify changes in permeability and/or ionic charge of A549 cell membranes, respectively.
Enumeration of PI-stained cells demonstrated that M. tuberculosis strain Erdman- and M. tuberculosis CDC1551-infected monolayers contained significantly greater numbers of PI-stained cells (7.2 ± 2.5 and 7.8 ± 2.7, respectively) than either BCG-infected (4.2 ± 2.7; P = 0.003) or uninfected (4.7 ± 2.8; P = 0.01) cell monolayers. No difference was observed between monolayers infected with the two strains of M. tuberculosis (P = 0.614) or between BCG-infected and uninfected monolayers (P = 0.689). No significant staining of the cells with AV was evident in any of the monolayers infected with either M. tuberculosis or M. bovis BCG, or in the uninfected monolayers (data not shown). These initial findings suggested that changes in cell permeability, not membrane distortion, correlated with necrosis in this model. In order to better quantitate the permeation of M. tuberculosis-infected A549 cells, we measured the release of the eukaryotic cytoplasmic enzyme LDH from infected A549 cells over time. LDH release was seen only upon infection with M. tuberculosis throughout the course of infection of A549 cells (P = 0.007 for M. tuberculosis strain Erdman versus M. bovis BCG at 48 h postinfection, and P < 0.0001 for M. tuberculosis strain CDC1551 versus M. bovis BCG at 48 h postinfection (Fig. 5). In fact, only a low level of LDH release from M. bovis BCG-infected cells was seen at 48 h, and it was never higher than the background release of uninfected cells at all the other time points (Fig. 5). These data correlated with our results for necrosis (Fig. 1B) and those reported for cell monolayer clearing (32).
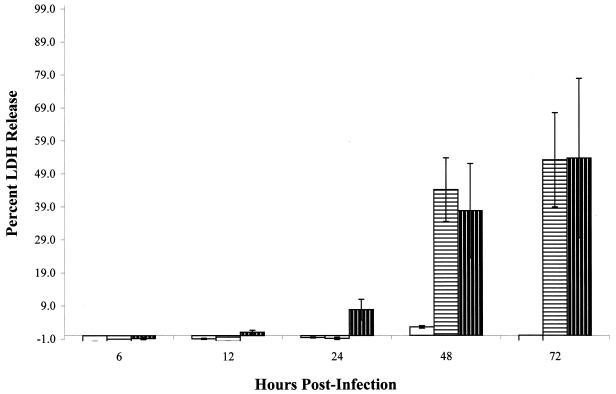
FIG. 5.
LDH release from A549 cell monolayers infected
with Mycobacterium spp. over 3 days. □, M.
bovis BCG;  ,
M. tuberculosis strain Erdman;
,
M. tuberculosis strain Erdman;
 , M.
tuberculosis strain CDC1551. All monolayers were infected at an
MOI of 10. All experiments were performed in triplicate. Error bars,
standard deviations.
, M.
tuberculosis strain CDC1551. All monolayers were infected at an
MOI of 10. All experiments were performed in triplicate. Error bars,
standard deviations.
Interestingly, significant levels of LDH were detected within 24 h postinfection from M. tuberculosis strain CDC1551-infected cells, compared with those from M. tuberculosis strain Erdman-infected cells (P < 0.0001), before any significant growth of the mycobacteria occurred (Fig. 4), suggesting that this rapid release of LDH may be due to inherent properties of this strain. No significant difference in LDH release was seen between M. tuberculosis strains by 48 h postinfection (P = 0.408) (Fig. 5). Consistent with our earlier observations with PI staining and necrosis of A549 cells, the release of LDH from infected cells was specific to infection with M. tuberculosis and could not be explained by differential growth of the mycobacteria.
Necrosis and cell permeation are not induced by incubation of A549 cells with nonviable M. tuberculosis and mycobacterial secreted and subcellular fractions.
We next measured the necrosis of A549 cells following incubation with either nonviable M. tuberculosis or M. tuberculosis subcellular fractions in an attempt to determine what bacterial factors were responsible for this phenotype.
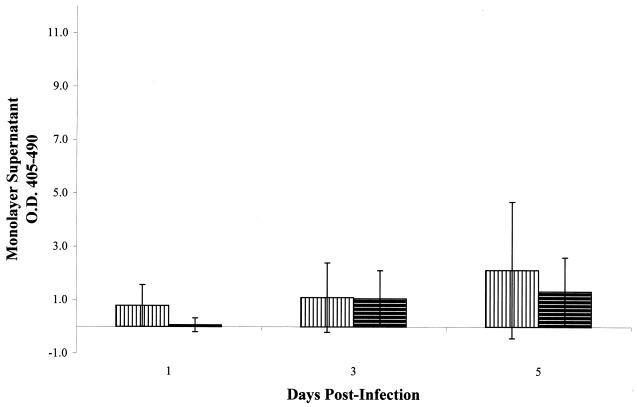
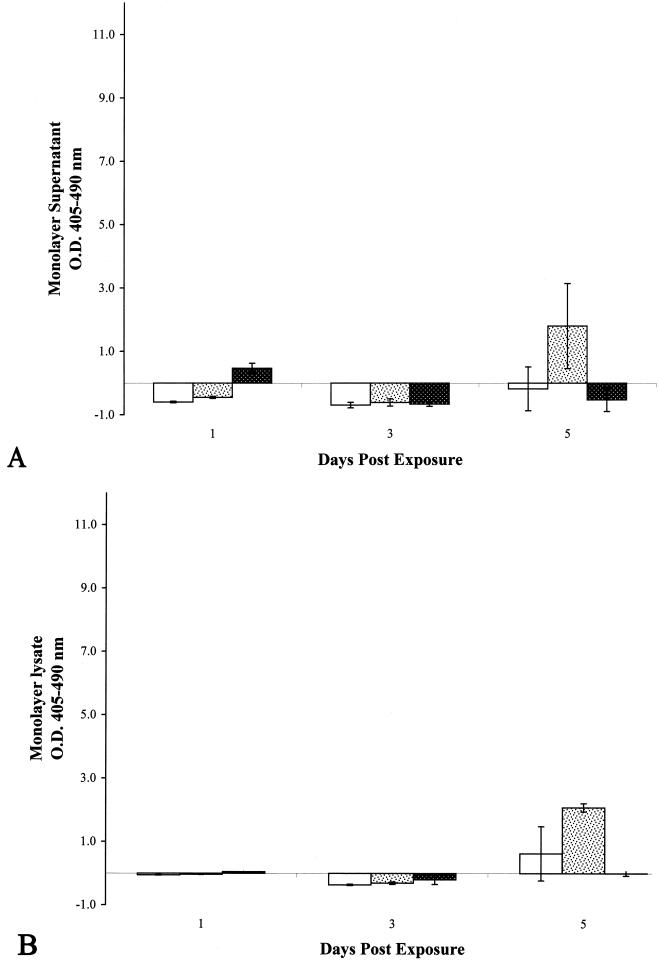
Incubation of A549 cells with either heat-killed or γ-irradiated M. tuberculosis resulted in little to no significant necrosis over the 5-day incubation period (Fig. 6). Further, the addition of a higher inoculum of either preparation did not increase necrosis (data not shown). Necrosis was then measured in A549 cells after addition of high doses of subcellular fractions from γ-irradiated M. tuberculosis to determine whether this phenotype could be induced by these partially purified components. Again, little to no necrosis was observed over the 5-day incubation period with either the mock 100-MOI inoculum (Fig. 7A) or the 500-MOI inoculum (data not shown). M. tuberculosis and its lipoglycan constituents have been shown to induce apoptosis of affected cells (12, 20, 39, 42). Although we did not observe apoptosis of A549 cells infected with viable M. tuberculosis (Fig. 2B), we measured apoptosis induction by these subcellular fractions to determine if similar observations could be made in our laboratory. Induction of A549 cell apoptosis was observed following exposure to these fractions, with the high-lipoglycan-containing CW fraction demonstrating the highest level of apoptosis (Fig. 7B). These results indicate that these preparations contained properties similar to those already reported (39, 42).
FIG. 6.
Optical densities of histone-DNA complexes released in
the culture supernatants of epithelial cell monolayers treated with
inactivated preparations of M. tuberculosis strain
CDC1551.  , heat-killed
M. tuberculosis;
, heat-killed
M. tuberculosis;  ,
γ-irradiated M. tuberculosis. All monolayers were treated
with an inoculum equivalent to an MOI of 10. All experiments were
performed in triplicate. Error bars, standard deviations. The values
obtained were normalized by subtraction of background levels of
necrosis of untreated A549 cell monolayers at each time point.
,
γ-irradiated M. tuberculosis. All monolayers were treated
with an inoculum equivalent to an MOI of 10. All experiments were
performed in triplicate. Error bars, standard deviations. The values
obtained were normalized by subtraction of background levels of
necrosis of untreated A549 cell monolayers at each time point.
FIG. 7.
(A) Optical densities of histone-DNA complexes released in the culture supernatants of epithelial cell monolayers treated with subcellular fractions of M. tuberculosis strain CDC1551. (B) Optical densities of intracellular histone-DNA complexes from the cell lysates of epithelial cell monolayers treated with subcellular fractions of M. tuberculosis strain CDC1551. □, CF; , CW; ▩, WCL. All monolayers were treated with a protein inoculum equivalent to an MOI of 100 bacilli per A549 cell. All experiments were performed in triplicate. Error bars, standard deviations. The values obtained were normalized by subtraction of background levels of necrosis and apoptosis of untreated A549 cell monolayers at each time point.
We next measured LDH release after incubation of A549 cells with either inactivated or subcellular fractions of M. tuberculosis to determine if the early events of cellular necrosis could be attributed to these preparations. Incubation of A549 cells with subcellular mycobacterial fractions and inactivated mycobacteria did not yield any significant release of LDH from A549 cells over the 3-day period (data not shown). In fact, LDH release following exposure to most of these preparations was lower than the background LDH release (data not shown). These results suggest that the cell permeation and subsequent necrosis we observed in A549 cells required infection with viable M. tuberculosis and/or the expression and secretion of factors unique to M. tuberculosis upon exposure to host cells.
DISCUSSION
In this study, we used the A549 epithelial cell line to determine the mode of cytotoxicity observed upon infection with M. tuberculosis. In contrast to the mode of cytotoxicity reported for human macrophages (20, 42), the mode of cytotoxicity induced by M. tuberculosis infection of the A549 epithelial cells was necrosis (Fig. 1B and 2B). Interestingly, we found that none of the mycobacterial strains we tested induced significant levels of apoptosis in these lung epithelial cells, even though A549 cells were sensitive to apoptosis by induction with camptothecin (Fig. 2A) and by infection with other microorganisms (37). Our studies also confirmed previous reports that cytotoxicity is specific to the virulence of the mycobacteria (32).
It was unclear, however, whether host or bacterial cell factors drove M. tuberculosis-specific necrosis. Although it has been shown that A549 cells do not secrete TNF-α during infection with M. tuberculosis (29; Castro-Garza et al., ASM Conf. on Tuberculosis), these cells do secrete chemokines during infection with M. tuberculosis (29, 51) that may contribute to cytotoxicity. In our studies, treatment with streptomycin reduced the degree of necrosis (Fig. 3A), whereas cycloheximide treatment had no significant effect on necrosis (Fig. 3B). These data suggest that the induction of cell death in this model was directly related to infection and growth of M. tuberculosis, and not to the induction of host factors in response to this infection. Interestingly, complete inhibition of necrosis by streptomycin treatment was not observed in this model, suggesting that M. tuberculosis retained the ability to cause necrosis upon cell entry and removal from the medium with streptomycin, or upon denaturation of the antibiotic during the course of the infection.
Since the addition of streptomycin, not cycloheximide, inhibited the necrosis of A549 cells, we hypothesized that either mycobacterial growth or the expression of mycobacterial factors or both were responsible for the cytotoxic phenotype. Our studies demonstrated that the differences in the ability to induce necrosis did not correlate with intracellular and extracellular growth of mycobacteria (Fig. 1B, 4, and 5). Both strains of M. tuberculosis demonstrated equal levels of necrosis at day 5 postinfection, whereas M. bovis BCG did not, even though the numbers of extracellular bacilli for all three mycobacteria and the numbers of intracellular bacilli for M. tuberculosis strain Erdman and M. bovis BCG were similar (Fig. 4). Enumeration of PI-stained infected A549 cells demonstrated significant differences between M. tuberculosis and M. bovis BCG at 24 h postinfection, when there was no difference in the number of CFU between these mycobacteria (Fig. 4). Similarly, LDH release was seen within 24 h postinfection only with M. tuberculosis strain CDC1551 (Fig. 5), though the numbers of intracellular and extracellular bacilli from all three mycobacteria were similar (Fig. 4).
Although the precise mechanisms leading to necrosis are unknown, the induction of necrosis in our model followed a pattern of increasing host cell membrane permeation. As with other bacteria that induce host cell necrosis, such as Legionella pneumophila (22, 55), it is likely that numerous complex events contribute to the M. tuberculosis necrotic phenotype of epithelial cells, including invasion, intracellular growth, differential gene expression, and permeation of the membrane. M. tuberculosis infection of host cells could contribute to this necrosis through secretion of pore-forming molecules, such as previously described hemolysins, or other putative cytotoxic molecules including broad-spectrum esterases or phospholipases (4, 11, 15, 19, 21, 26, 40, 52).
M. tuberculosis strain CDC1551 has been reported to grow faster (31, 38, 46) and possess a different array of lipids than M. tuberculosis strain Erdman (31). The early differences in necrosis observed between these two M. tuberculosis strains were most likely not due to differential intracellular growth, because we did not observe a correlation between growth rate differences and necrosis or LDH release for these two strains (Fig. 1B, 4, and 5). Although this is speculative, the unique lipids present in M. tuberculosis strain CDC1551 could be related to its enhanced ability to cause early cell permeation and cellular necrosis in our model via mechanisms similar to those suggested for other toxigenic mycobacterial lipids, such as the recently described polyketide of Mycobacterium ulcerans (17). Our studies suggest, however, that if such a factor contributes to the cell permeation and necrosis of A549 cells, it is expressed only upon infection or possesses its mode of action from within the host cell. Little to no necrosis, and no LDH release, could be detected in our A549 cell model upon incubation with inactivated preparations or subcellular fractions of M. tuberculosis (Fig. 6 and 7; also data not shown).
Invasion by M. tuberculosis may be a prerequisite for cell permeation and necrosis of A549 cells. M. tuberculosis is invasive to epithelial cells (1) and has two mechanisms for invasion of A549 cells (6). In addition, increases in invasion of, but not adherence to, A549 cells by M. tuberculosis correlates to enhanced cytotoxicity (32). In our studies, only live M. tuberculosis organisms, not inactivated bacilli or subcellular components, were able to permeate the A549 cell membrane (Fig. 5 and data not shown) or induce necrosis of A549 cells (Fig. 1B, 6, and 7A). It is interesting that while both inactivated M. tuberculosis and macromolecules present in its subcellular fractions adhered to A549 cells, only live M. tuberculosis readily entered these cells (our unpublished observations).
Although a role for epithelial cell necrosis during pulmonary tuberculosis remains unclear, epithelial tissues in the lungs are involved at several sites during the disease where necrosis is evident, and in other tissues during the different manifestations of human tuberculosis (3, 14, 24, 41, 50). Early neutrophilic responses which would be expected to arise from the inflammatory effects of early tissue destruction, have not been noted in humans (3, 13, 14, 50) or mice (41) infected with M. tuberculosis. Nonetheless, M. tuberculosis could induce necrosis of infected cells to spread (9), either as a means to escape phagocytosis by alveolar macrophages, or upon release from alveolar macrophages. M. tuberculosis has been shown to be more cytotoxic to A549 cells after passage through macrophages (32). Few bacilli may be present during the establishment of infection; thus the permeation and necrosis observed in our cell model may not be a predominant phenotype at this stage due to the low MOI. However, large numbers of intracellular and extracellular bacilli are present during the latter stages of disease (13, 14, 36). Necrosis of lung epithelial cells may be more likely to occur during these late disease stages and may be an important mechanism enabling the bacilli to escape from the granuloma or the bronchial epithelium during cavitary disease, leading to transmission of the bacilli. Necrotic factors produced by M. tuberculosis, such as those responsible for the cell permeation observed in our model, could also alter phagosomal membranes, thereby affecting the endocytic pathway of M. tuberculosis phagosomes after phagocytosis (18, 49), as suggested for L. pneumophila infection of human macrophages (22, 55).
Therefore, the identification of the mechanisms and requirements, including invasion, that mediate cell permeation and necrosis of epithelial cells after infection with M. tuberculosis will likely provide key insights into the pathogenesis of tuberculosis. We are currently in the process of identifying the perimeters required for, and molecules responsible for, cell permeation and necrosis of A549 cell monolayers through transposon mutagenesis of M. tuberculosis using this A549 cell model.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the American Lung Association (RG029N), by generous donations from Infectious Awareables Inc., and by a grant from the Emory Medical Care Foundation (99001).
We thank E. H. White at the Centers for Disease Control and Prevention in Atlanta, Ga., for electron microscopy and John Belisle at Colorado State University for provision of mycobacterial fractions (NIAID contract NOI-AI-75320). We also thank Ian Orme, Pam Small, John Belisle, and Elizabeth Bonney for helpful discussions and critical review of earlier versions of this report.
REFERENCES
- 1.Arruda S, Bomfim G, Knights R, Huima-Byron T, Riley L W. Cloning of a M. tuberculosisDNA fragment associated with entry and survival inside cells. Science. 1993;261:1454–1457. doi: 10.1126/science.8367727. [DOI] [PubMed] [Google Scholar]
- 2.Balasubrananian B, Wiegeshaus E H, Taylor G T, Smith D W. Pathogenesis of tuberculosis: pathway to apical localization. Tuber Lung Dis. 1994;75:168–178. doi: 10.1016/0962-8479(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 3.Barnes P F, Modlin R L. Human cellular immune responses to Mycobacterium tuberculosis. In: Shinnick T M, editor. Tuberculosis. Berlin, Germany: Springer; 1997. pp. 187–219. [Google Scholar]
- 4.Behr M A, Wilson M A, Gill W P, Salamon H, Schoolnik G K, Rane S, Small P M. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284:1520–1522. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- 5.Bellocq A, Azoulay E, Marullo S, Flahault A, Fouquerary B, Philippe C, Cadranel J, Baud L. Reactive oxygen and nitrogen intermediates increase transforming growth factor-β1 release from human epithelial alveolar cells through two different mechanisms. Am J Respir Cell Mol Biol. 1999;21:128–136. doi: 10.1165/ajrcmb.21.1.3379. [DOI] [PubMed] [Google Scholar]
- 6.Bermudez L E, Goodman J. Mycobacterium tuberculosisinvades and replicates within type II alveolar cells. Infect Immun. 1996;64:1400–1406. doi: 10.1128/iai.64.4.1400-1406.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birkness K A, Deslauriers M, Bartlett J H, White E H, King C H, Quinn F D. An in vitro tissue culture bilayer model to examine early events in Mycobacterium tuberculosisinfection. Infect Immun. 1999;67:653–658. doi: 10.1128/iai.67.2.653-658.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonfoco E, Krainic D, Ankarcrona M, Nicotera P, Lipton S. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-d-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci USA. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrd T F, Green G M, Fowlston S E, Lyons C R. Differential growth characteristics and streptomycin susceptibility of virulent and avirulent Mycobacterium tuberculosisstrains in a novel fibroblast-mycobacterium microcolony assay. Infect Immun. 1998;66:5132–5139. doi: 10.1128/iai.66.11.5132-5139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chopra I, Brennan P. Molecular action of anti-mycobacterial agents. Tuber Lung Dis. 1997;78:89–98. doi: 10.1016/s0962-8479(98)80001-4. [DOI] [PubMed] [Google Scholar]
- 11.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Barrell B G, et al. Deciphering the biology of Mycobacterium tuberculosisfrom the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 12.Cree I A, Nurbhai S, Milne G, Beck J S. Cell death in granulomata: the role of apoptosis. J Clin Pathol. 1987;40:1314–1319. doi: 10.1136/jcp.40.11.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dannenberg A M., Jr Pathogenesis of pulmonary tuberculosis. Am Rev Respir Dis. 1982;125:25–29. doi: 10.1164/arrd.1982.125.3P2.25. [DOI] [PubMed] [Google Scholar]
- 14.Dannenberg A M, Jr, Tomashefski J K., Jr . Pathogenesis of pulmonary tuberculosis. In: Fishman A P, editor. Pulmonary diseases and disorders. 2nd ed. Vol. 3. New York, N.Y: McGraw-Hill; 1988. pp. 1821–1842. [Google Scholar]
- 15.Deshpande R G, Khan M B, Bhat D A, Navalkar R G. Isolation of a contact-dependent haemolysin from Mycobacterium tuberculosis. J Med Microbiol. 1997;46:233–238. doi: 10.1099/00222615-46-3-233. [DOI] [PubMed] [Google Scholar]
- 16.Dobos K M, Swiderek K, Khoo K-H, Brennan P J, Belisle J T. Evidence for glycosylation sites on the 45-kDa glycoprotein of Mycobacterium tuberculosis. Infect Immun. 1995;63:2846–2853. doi: 10.1128/iai.63.8.2846-2853.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George K M, Chatterjee D, Gunawardana G, Welty D, Hayman J, Lee R, Small P L. Mycolactone: a polyketide toxin from Mycobacterium ulceransrequired for virulence. Science. 1999;283:854–857. doi: 10.1126/science.283.5403.854. [DOI] [PubMed] [Google Scholar]
- 18.Hasan Z, Schlax C, Kuhn L, Lefkovits I, Young D, Thole J, Pieters J. Isolation and characterization of the mycobacterial phagosome: segregation from the endosomal/lysosomal pathway. Mol Microbiol. 1997;24:545–553. doi: 10.1046/j.1365-2958.1997.3591731.x. [DOI] [PubMed] [Google Scholar]
- 19.Johansen K A, Gill R E, Vasil M L. Biochemical and molecular analysis of phospholipase C and phospholipase D activity in mycobacteria. Infect Immun. 1996;64:3259–3266. doi: 10.1128/iai.64.8.3259-3266.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keane J, Balcewicz-Sablinska M K, Remold H G, Chupp G L, Meek B B, Fenton M J, Kornfield H. Infection by Mycobacterium tuberculosispromotes human alveolar macrophage apoptosis. Infect Immun. 1997;65:298–304. doi: 10.1128/iai.65.1.298-304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King C H, Mundayoor S, Crawford J T, Shinnick T M. Expression of contact-dependent cytolytic activity by Mycobacterium tuberculosisand isolation of the genomic locus that encodes the activity. Infect Immun. 1993;61:2708–2712. doi: 10.1128/iai.61.6.2708-2712.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirby J E, Vogel J P, Andrews H L, Isberg R R. Evidence for pore-forming ability by Legionella pneumophila. Mol Microbiol. 1998;27:323–336. doi: 10.1046/j.1365-2958.1998.00680.x. [DOI] [PubMed] [Google Scholar]
- 23.Koyama S, Sato E, Nomura H, Kubo K, Miura M, Yamashita T, Nagai S, Izumi T. Monocyte chemotactic factors released from type II pneumocyte-like cells in response to TNF-α and IL-1α. Eur Respir J. 1999;13:820–828. doi: 10.1183/09031936.99.13482099. [DOI] [PubMed] [Google Scholar]
- 24.Lack E E, Connor D H. Tuberculosis. In: Connor D H, Chandler F W, Schwartz D A, Manz H J, Lack E E, editors. Pathology of infectious diseases. Vol. 1. Stamford, Conn: Appleton and Lange; 1997. pp. 857–868. [Google Scholar]
- 25.Laochumroonvorapong P, Paul S, Manca C, Freedman V H, Kaplan G. Mycobacterial growth and sensitivity to H2O2killing in human monocytes in vitro. Infect Immun. 1997;65:4850–4857. doi: 10.1128/iai.65.11.4850-4857.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leao S, Rocha C L, Murillo L A, Parra C A, Patarroyo M E. A species-specific nucleotide sequence of Mycobacterium tuberculosis encodes a protein that exhibits hemolytic activity when expressed in Escherichia coli. Infect Immun. 1995;63:4301–4306. doi: 10.1128/iai.63.11.4301-4306.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee B Y, Horwitz M A. Identification of macrophage and stress-induced proteins of Mycobacterium tuberculosis. J Clin Investig. 1995;96:245–249. doi: 10.1172/JCI118028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leist M, Single B, Castoldi A F, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin Y, Zhang M, Barnes P F. Chemokine production by a human alveolar epithelial cell line in response to Mycobacterium tuberculosis. Infect Immun. 1998;66:1121–1126. doi: 10.1128/iai.66.3.1121-1126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lund L R. Expression of urokinase-type plasminogen activator, its receptor and type-1 plasminogen activator inhibitor is differently regulated by inhibitors of protein synthesis in human cancer cell lines. FEBS Lett. 1996;383:139–144. doi: 10.1016/0014-5793(96)00223-2. [DOI] [PubMed] [Google Scholar]
- 31.Manca C, Tsenova L, Barry III C E, Bergtold A, Freeman S, Haslett P A J, Musser J M, Freedman V H, Kaplan G. Mycobacterium tuberculosisCDC1551 induces a more vigorous host response in vivo and in vitro, but is not more virulent than other clinical isolates. J Immunol. 1999;162:6740–6746. [PubMed] [Google Scholar]
- 32.McDonough K A, Kress Y. Cytotoxicity for lung epithelial cells is a virulence-associated phenotype of Mycobacterium tuberculosis. Infect Immun. 1995;63:4802–4811. doi: 10.1128/iai.63.12.4802-4811.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta P K, King C H, White E H, Murtagh J J, Jr, Quinn F D. Comparison of in vitro models for the study of Mycobacterium tuberculosisinvasion and intracellular replication. Infect Immun. 1996;64:2673–2679. doi: 10.1128/iai.64.7.2673-2679.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miquel K, Pradine A, Sun J. GGTI-298 induces G0-G1 block and apoptosis whereas FT1-277 causes G2-M enrichment in A549 cells. Cancer Res. 1997;57:1846–1850. [PubMed] [Google Scholar]
- 35.Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guérin. J Exp Med. 1994;180:1499–1509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nardell E. Pathogenesis of tuberculosis. In: Reichman L B, Hershfield S E, editors. Tuberculosis. New York, N.Y: Marcel Dekker Inc.; 1993. pp. 103–122. [Google Scholar]
- 37.O'Donnell D R, Milligan L, Stark J M. Induction of CD95 (Fas) and apoptosis in respiratory epithelial cell cultures following respiratory syncytial virus infection. Virology. 1999;257:198–207. doi: 10.1006/viro.1999.9650. [DOI] [PubMed] [Google Scholar]
- 38.Orme I M. Virulence of recent notorious Mycobacterium tuberculosisisolates. Tuber Lung Dis. 1999;79:379–381. doi: 10.1054/tuld.1999.0221. [DOI] [PubMed] [Google Scholar]
- 39.Ozeki Y, Kaneda K, Fujiwara N, Morimoto M, Oka S, Yano I. In vivo induction of apoptosis in the thymus by administration of mycobacterial cord factor (trehalose 6,6′-dimycolate) Infect Immun. 1997;65:1793–1799. doi: 10.1128/iai.65.5.1793-1799.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quinn F D, Newman G, King C H. Virulence determinants of Mycobacterium tuberculosis. In: Shinnick T M, editor. Tuberculosis. New York, N.Y: Springer-Verlag; 1996. pp. 131–156. [DOI] [PubMed] [Google Scholar]
- 41.Rhoades E R, Frank A A, Orme I M. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tuber Lung Dis. 1997;78:57–66. doi: 10.1016/s0962-8479(97)90016-2. [DOI] [PubMed] [Google Scholar]
- 42.Rojas M, Barrera L F, Puzo G, Garcia L F. Differential induction of apoptosis by virulent Mycobacterium tuberculosisin resistant and susceptible murine macrophages: role of nitric oxide and mycobacterial products. J Immunol. 1997;159:1352–1361. [PubMed] [Google Scholar]
- 43.Satav J G, Katyare S S, Fatterparker P, Sreenivasan A. Study of protein synthesis in rat liver mitochondria use of cycloheximide. Eur J Biochem. 1977;73:287–296. doi: 10.1111/j.1432-1033.1977.tb11318.x. [DOI] [PubMed] [Google Scholar]
- 44.Takayama K, Schnoes H K, Armstrong E L, Boyle R W. Site of inhibitory action of isoniazid in the synthesis of mycolic acids in Mycobacterium tuberculosis. J Lipid Res. 1975;16:308–317. [PubMed] [Google Scholar]
- 45.Tewari S, Duerbeck N B, Ross-Duggan J, Noble E P. In vitro protein synthesis by inner membranes of rat brain mitochondria. Res Commun Chem Pathol Pharmacol. 1978;22:385–400. [PubMed] [Google Scholar]
- 46.Valway S E, Sanchez M P C, Shinnick T M, Orme I, Agerton T, Hoy D, Jones J S, Westmoreland H, Onorato I M. An outbreak involving extensive transmission of a virulent strain of Mycobacterium tuberculosis. N Engl J Med. 1998;338:633–639. doi: 10.1056/NEJM199803053381001. [DOI] [PubMed] [Google Scholar]
- 47.Vanham G, Toossi Z, Hirsch C S, Wallis R S, Schwander S K, Rich E A, Ellner J J. Examing a paradox in the pathogenesis of human pulmonary tuberculosis: immune activation and suppression/anergy. Tuber Lung Dis. 1997;78:145–158. doi: 10.1016/s0962-8479(97)90021-6. [DOI] [PubMed] [Google Scholar]
- 48.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labeled annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 49.Via L E, Deretic D, Ulmer R J, Hibler N S, Huber L A, Deretic V. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J Biol Chem. 1997;272:13326–13331. doi: 10.1074/jbc.272.20.13326. [DOI] [PubMed] [Google Scholar]
- 50.Von Lichtenberg F. Mycobacterial diseases. In: Van Lichtenberg F, editor. Pathology of infectious diseases. New York, N.Y: Raven Press; 1991. pp. 173–187. [Google Scholar]
- 51.Wickremasinghe M I, Thomas L H, Friedland J S. Pulmonary epithelial cells are a source of IL-8 in the response to Mycobacterium tuberculosis: essential role of IL-1 from infected monocytes in a NF-κB-dependent network. J Immunol. 1999;163:3936–3947. [PubMed] [Google Scholar]
- 52.Wren B W, Stabler R A, Das S S, Butcher P D, Mangan J A, Clarke J D, Casali N, Parish T, Stoker N G. Characterization of a haemolysin from Mycobacterium tuberculosis with homology to a virulence factor of Serpulina hyodysenteriae. Microbiology. 1998;44:1205–1211. doi: 10.1099/00221287-144-5-1205. [DOI] [PubMed] [Google Scholar]
- 53.Yeh C J, His B L, Faulk W P. Propidium iodide as a nuclear marker in immunofluorescence. II. Use with cellular identification and viability studies. J Immunol Methods. 1981;43:269–275. doi: 10.1016/0022-1759(81)90174-5. [DOI] [PubMed] [Google Scholar]
- 54.Zhang M, Kim K J, Iyer D, Lin Y, Belisle J, McEnery K, Crandall E D, Barnes P F. Effects of Mycobacterium tuberculosison the bioelectric properties of the alveolar epithelium. Infect Immun. 1997;65:692–698. doi: 10.1128/iai.65.2.692-698.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zuckman D M, Hung J B, Roy C R. Pore-forming activity is not sufficient for Legionella pneumophilaphagosome trafficking and intracellular growth. Mol Microbiol. 1999;32:990–1001. doi: 10.1046/j.1365-2958.1999.01410.x. [DOI] [PubMed] [Google Scholar]