Preamble
The American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) initiated the hepatitis C guidance project (hereafter HCV guidance) in 2013. The AASLD-IDSA HCV guidance website (www.HCVGuidelines.org) disseminates up-to-date, peer-reviewed, unbiased, evidence-based recommendations to aid clinicians making decisions regarding the testing, management, and treatment of hepatitis C virus (HCV) infection. Utilizing a web-based system enables timely and nimble distribution of the HCV guidance, which is periodically updated in near real time as necessitated by emerging research data, recommendations from public health agencies, the availability of new therapeutic agents, or other significant developments affecting the rapidly evolving hepatitis C arena. The value and utility of the online HCV guidance to the community of hepatitis C care providers throughout the world is evidence by the nearly 10 million pageviews by 1.5 million users originating from 228 countries and territories since the January 2014 launch of the website. A major update of the HCV guidance was released electronically in November 2019. This HCV guidance update summarizes and highlights key new or amended recommendations since the previous October 2018 print publication.(1)
The advent of safe, well tolerated, and highly efficacious (>95% cure rate)(2) direct-acting antiviral (DAA) therapy for HCV infection has ushered in a new era in which elimination of hepatitis C is conceivable. In 2016, the World Health Organization (WHO) proposed a global health sector strategy to eliminate hepatitis C as a public health threat by 2030 and developed an action plan to facilitate this goal.(3) In response to the WHO action plan, the National Academies of Science, Engineering, and Medicine (NASEM) developed a U.S. strategy for the elimination of hepatitis C.(4) Key elements of the elimination plan include improved detection of undiagnosed cases, increased linkage and access to care for newly diagnosed persons, and expanded treatment access. Many of the recommendations included in the latest update to the HCV guidance and highlighted herein align with and support the goals of the NASEM and WHO strategies to move from control to eventual elimination of hepatitis C. Topics addressed include universal and risk-based hepatitis C screening; simplified treatment algorithms for treatment-naive adults without cirrhosis or with compensated cirrhosis; hepatitis C management in the pediatric population; acute hepatitis C testing and management; and transplantation of organs from HCV-viremic donors into HCV-negative recipients. For detailed evidence reviews related to these topics and information addressing other aspects of HCV testing and management, see the online HCV guidance (www.HCVGuidelines.org).
Process
The HCV guidance was developed and is updated by a volunteer panel (representing AASLD and IDSA) of hepatology and infectious diseases clinicians with hepatitis C expertise using an evidence-based review of available data, including information presented at scientific conferences and published in peer-reviewed journals. Based on scientific evidence and expert opinion, recommendations are rated by the level of evidence (I, II, or III) and the strength of the recommendation (A, B or C) using a system adapted from the American College of Cardiology and American Heart Association.(5, 6) See the original AASLD-IDSA hepatitis C guidance publication(7) or the HCV guidance website for additional details about the processes and methods employed. All recommendations are reviewed and approved by the governing boards of AASLD and IDSA.
The HCV guidance panel classifies therapeutic regimens as recommended, alternative, or not recommended based on patient factors (i.e., treatment naive versus experienced, cirrhosis status, and comorbidities) and viral characteristics (i.e., genotype, subtype, resistance-associated substitutions). Recommended regimens are considered equivalent; alternative regimens are effective but, compared to recommended regimens, have potential disadvantages, limitations for use in certain patient populations, or less supporting data.
Universal and Risk-Based Hepatitis C Screening and Follow-Up
The identification of risk factors associated with contracting HCV infection served as the basis for the risk-based hepatitis C screening recommendations issued by the U.S. Centers for Disease Control and Prevention (CDC) in 1998.(8) Although sensitive for the identification of persons with chronic HCV infection, risk-based screening failed to identify the majority of individuals with HCV infection due to both clinician and patient barriers.(9–12) Analysis of the 2003–2010 National Health and Nutrition Examination Survey (NHANES) prevalence data demonstrated that approximately three fourths of individuals with chronic hepatitis C in the United States belonged to the 1945 through 1965 birth cohort.(13) Based on these data, both CDC and the U.S. Preventive Services Task Force (USPSTF) recommended one-time hepatitis C screening of all individuals in this birth cohort (1945 through 1965) regardless of risk factors.(14, 15) Since these recommendations were established in 2012, HCV epidemiology in the United States has changed. Hepatitis C infection incidence nearly quadrupled from 2010 to 2017, primarily driven by increased injection drug use related to the opioid epidemic.(16–19) CDC viral hepatitis surveillance data indicate progressively increasing acute HCV infection incidence each year from 2009 through 2017. Most of these new HCV infections occurred in persons born after 1965 with those aged 20–39 years accounting for the majority of cases. This ongoing trend has spurred interest in expanding HCV screening among the general U.S. population. Several modeling studies suggest the cost-effectiveness of such an approach.(20–23) Accordingly, the AASLD-IDSA guidance HCV screening and follow-up recommendations have been updated and include newly recommended universal HCV screening for all adults aged 18 years or older followed by periodic testing for persons with ongoing risk behaviors and/or exposures.
One-Time, Universal Hepatitis C Screening for Adults
Recommendations
-
1
One-time, routine, opt out HCV screening is recommended for all individuals aged 18 years or older. (I, B)
In light of the inadequacy of targeted HCV case finding using risk-based and birth cohort HCV screening,(24, 25) investigators have modeled the cost-effectiveness of one-time universal HCV screening for adults aged ≥18 years. Independent studies using different modeling techniques demonstrate that one-time universal screening for adults aged ≥18 years is cost-effective (<$30,000/quality-adjusted life years) compared with birth-cohort screening.(20, 26) Additionally, the cost-effectiveness of nontargeted HCV screening has proven robust in a variety of venues including correctional,(27) prenatal,(28, 29) and primary care(30) settings, and substance use treatment centers.(31, 32) Given the current epidemiology of HCV disease in the United States, the cost-effectiveness of universal HCV screening, the high efficacy of DAA therapy, and the myriad liver-related and other health benefits of virologic cure,(33–38) the HCV guidance panel recommends universal, one-time, opt out HCV screening of adults aged ≥18 years. Although neither CDC nor the USPSTF currently recommend universal HCV screening in adults, CDC initiated a peer review process to consider such a recommendation in July 2019. Similarly, in August 2019, the USPSTF published a draft recommendation for universal HCV screening among adults aged 18–79 years. The USPSTF draft universal hepatitis C screening recommendation differs from that of the AASLD-IDSA HCV guidance by setting an upper age limit of 79 years. The HCV guidance panel does not recommend an age limit for universal adult HCV screening due to the excellent quality of life of many octogenarians and the association between advanced age and more rapid HCV disease progression.
The HCV guidance panel’s new universal screening recommendation is intended to enhance HCV case finding among adults not included in the 1945 through 1965 birth cohort and aligns with the WHO and NASEM goals of eliminating HCV as a public health threat by 2030. This is particularly important for men and women aged 20–39 years due to the disproportionate overlapping impact of the opioid epidemic and associated injection drug use and the rising rate of incident HCV infections in this age group. Universal HCV screening also bypasses the inherent barriers in ascertaining an accurate risk factor assessment.
Risk-Based HCV Testing
Recommendations
-
2
One-time HCV testing should be performed for all persons younger than 18 years old with behaviors, exposures, or conditions or circumstances associated with an increased risk of HCV infection. (I, B)
-
3
Periodic repeat HCV testing should be offered to all persons with behaviors, exposures, or conditions or circumstances associated with an increased risk of HCV exposure. (IIa, C)
-
4
Annual HCV testing is recommended for all persons who inject drugs and for men with human immunodeficiency virus (HIV) infection who have unprotected sex with men. (IIa, C)
One-time, risk-based HCV screening is recommended for persons younger than 18 years old with current or past behaviors, exposures, or conditions or circumstances associated with an increased risk of HCV infection (see Table 1). There is currently insufficient evidence to support universal HCV screening in the pediatric population.
People with ongoing risk factor(s) for HCV infection remain vulnerable for as long as the behavior, exposure, condition, or circumstance persists, thereby warranting periodic repeat HCV testing. There is a paucity of data addressing the optimal frequency of repeat testing thereby leaving the periodicity to the clinician’s discretion on a case-by-case basis with consideration of an individual’s risk for HCV infection or reinfection. People who inject drugs (PWID) and men with HIV infection who have unprotected sex with men are exceptions to this guidance. Because of the high incidence and prevalence of HCV infection in these populations,(39–47) at least annual HCV testing is recommended. Given that many PWID lack access to or eschew traditional healthcare delivery systems, integration of HCV testing services into substance use treatment programs, needle/syringe service programs, and acute detoxification programs expands the opportunities to accomplish periodic HCV testing in this key population.(48–50)
Table 1.
Behaviors, Exposures, or Conditions or Circumstances Associated With an Increased Risk of HCV Infection
| Risk Behaviors | • Injection drug use (current or ever, including those who injected only once) • Intranasal illicit drug use • Men who have sex with men |
| Risk Exposures | • Persons on long-term hemodialysis (ever) • Persons with percutaneous/parenteral exposures in an unregulated setting • Healthcare, emergency medical, and public safety workers after needle stick, sharps, or mucosal exposures to HCV-infected blood • Children born to HCV-infected women • Persons who were ever incarcerated • Prior recipients of blood transfusion(s) or organ transplant, including persons who: ○ Were notified that they received blood from a donor who later tested positive for HCV ○ Received a transfusion of blood or blood components, or underwent an organ transplant prior to July 1992 ○ Received clotting factor concentrates produced prior to 1987 |
| Risk Conditions and Circumstances | • HIV infection • Sexually active persons about to start pre-exposure prophylaxis (PrEP) for HIV • Unexplained chronic liver disease and/or chronic hepatitis, including elevated alanine aminotransferase (ALT) levels • Solid organ donors (deceased and living) and solid organ transplant recipients |
Initial HCV Testing and Follow-Up
Recommendations
-
5
HCV-antibody testing with reflex HCV RNA polymerase chain reaction testing is recommended for initial HCV screening. (I, A)
-
6
Among persons with a negative HCV-antibody test who were exposed to HCV within the prior 6 months, HCV-RNA or follow-up HCV-antibody testing 6 months or longer after exposure is recommended. HCV-RNA testing can also be considered for immunocompromised persons. (I, C)
-
7
Among persons at risk for reinfection after previous spontaneous or treatment-related viral clearance, HCV-RNA testing is recommended because a positive HCV-antibody test is expected. (I, C)
-
8
Persons found to have a positive HCV-antibody test and negative results for HCV RNA by polymerase chain reaction should be informed that they do not have evidence of current (active) HCV infection but are not protected from reinfection. (I, A)
-
9
Quantitative HCV-RNA testing is recommended prior to initiation of antiviral therapy to document the baseline level of viremia (i.e., baseline viral load). (I, A)
-
10
HCV genotype testing may be considered for those in whom it may alter treatment recommendations. (I, A)
HCV-antibody testing using a U.S. Food and Drug Administration (FDA) approved assay (laboratory-based or point-of-care) is recommended for initial HCV screening.(51, 52) The sensitivity and specificity of the lone FDA-approved point-of-care test (OraQuick HCV Rapid Antibody Test; OraSure Technologies Inc., Bethlehem, Pennsylvania) are similar to laboratory-based assays.(53, 54) A positive HCV-antibody test indicates current (active) HCV infection (acute or chronic), a past resolved infection, or rarely a false positive result.(55) A test to detect HCV viremia is necessary to confirm active HCV infection (see Figure 1). Ideally, a positive HCV-antibody test automatically reflexes to HCV-RNA testing. This approach requires a single blood collection and avoids a return visit for confirmatory testing, a major barrier in the continuum of care.(56) Collection of dried blood spots is an option for sequential HCV-antibody and reflex HCV-RNA testing. Dried blood spot collection can be accomplished with a fingerstick rather than venipuncture and transport does not require an intact cold chain, making this an advantageous testing option in rural areas and among people for whom phlebotomy is a testing barrier.(57) An FDA-approved quantitative or qualitative HCV-RNA assay with a detection level of ≤25 IU/mL should be used.
HCV-RNA testing is required to detect reinfection after previous spontaneous or treatment-related viral clearance because HCV-antibody positivity is expected (see Figure 1). Immunocompromised persons and those with possible HCV exposure in the prior 6 months may be HCV antibody negative due to delayed or failed seroconversion(58) or being in the seroconversion window period,(52) respectively. HCV-RNA testing is a consideration for these individuals, particularly for those with known risk factor(s).
Persons who have a reactive HCV-antibody test and a negative (not detected) HCV-RNA test should be informed that they do not have evidence of current HCV infection. Although additional testing is typically unnecessary, HCV-RNA testing can be repeated for persons with ongoing HCV infection risk or if there is a high index of suspicion for recent infection. If either the clinician or patient wish to determine whether a positive HCV-antibody test in the absence of HCV viremia represents a resolved HCV infection or a biologic false positive, repeat testing with a different HCV-antibody assay can be undertaken. A false positive typically does not occur with two different assays.(51, 59)
Quantitative HCV-RNA testing is recommended prior to initiating antiviral therapy to determine baseline viremia (viral load), which may affect treatment duration with ledipasvir/sofosbuvir therapy. With the advent of pangenotypic DAA regimens, HCV genotyping is no longer universally required prior to treatment initiation. Pretreatment genotyping is recommended for persons with a prior HCV treatment failure because DAA regimen selection and duration may differ by genotype. Pretreatment genotyping is not required for treatment-naive patients without cirrhosis if a pangenotypic regimen is used.
Figure 1. Recommended Testing for Diagnosis of Current HCV Infection or Reinfection.
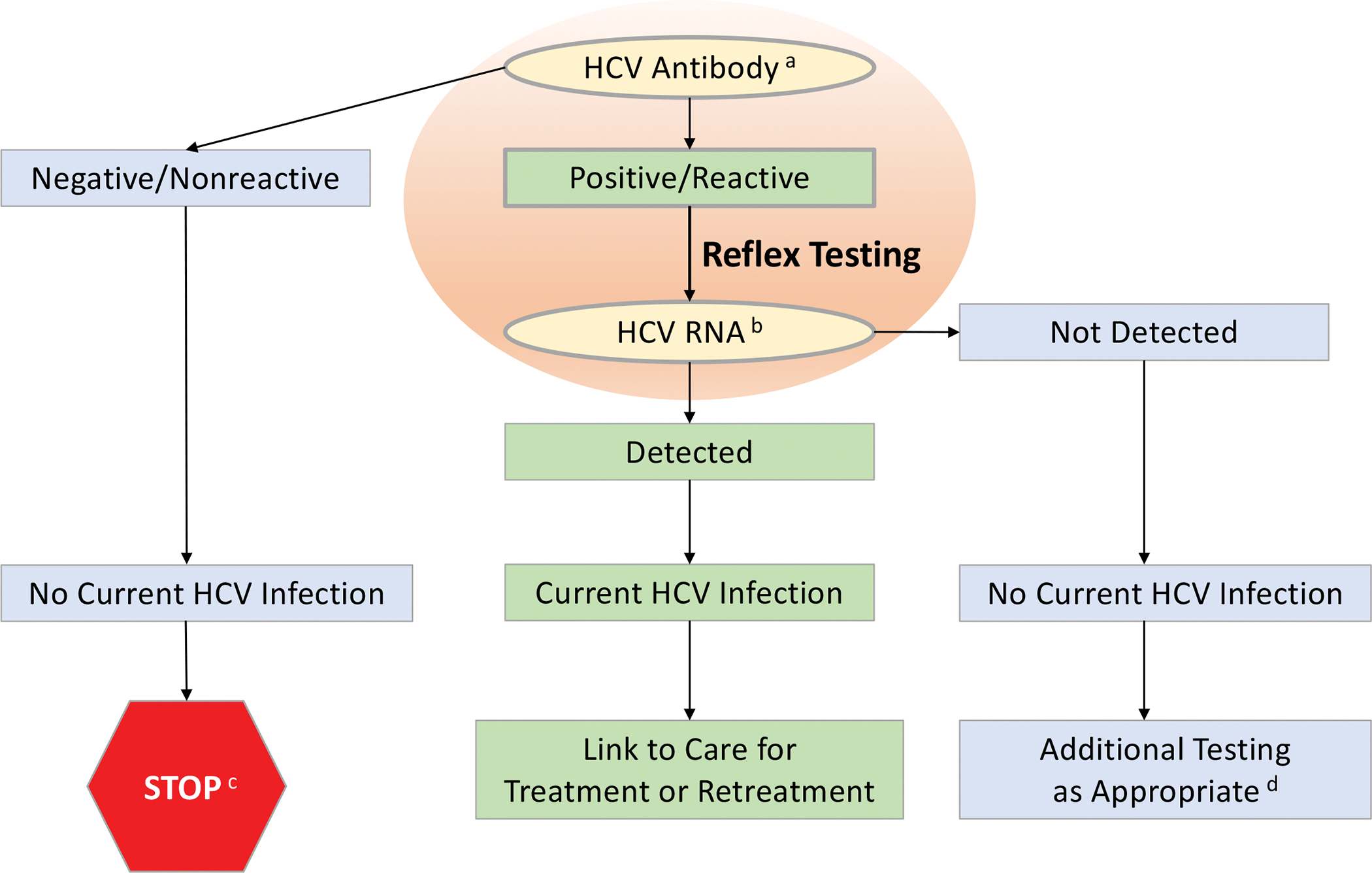
a For diagnosis of current initial HCV infection, begin with HCV-antibody testing.
b For recurrent HCV infection, begin with HCV-RNA testing.
c For persons who might have been exposed to HCV within the past 6 months, testing for HCV RNA or follow-up testing for HCV antibody should be performed. For persons who are immunocompromised, testing for HCV RNA should be performed.
d To differentiate past, resolved HCV infection from biologic false positivity for HCV antibody, testing with another HCV-antibody assay can be considered. Repeat HCV-RNA testing if the person tested is suspected to have had HCV exposure within the past 6 months or has clinical evidence of HCV disease, or if there is concern regarding the handling or storage of the test specimen.
Adapted from Centers for Disease Control and Prevention.(51)
Counseling and Clinical Care for Persons With Active HCV Infection
Recommendations
-
11
Persons with current HCV infection should receive education and interventions aimed at reducing liver disease progression and preventing HCV transmission. (IIa, B)
-
12
Abstinence from alcohol and, when appropriate, interventions to facilitate cessation of alcohol consumption should be advised for all persons with HCV infection. (IIa, B)
-
13
All persons with HCV infection should be provided education about how to prevent HCV transmission to others. (I, C)
-
14
Evaluation for advanced fibrosis using noninvasive markers (or liver biopsy, if required) is recommended for all persons with HCV infection to facilitate an appropriate decision regarding HCV treatment strategy, and to determine the need for initiating additional measures for cirrhosis management (e.g., hepatocellular carcinoma screening). (I, A)
-
15
Evaluation for other conditions that may accelerate liver fibrosis, including hepatitis B and HIV infections, is recommended for all persons with active HCV infection. (IIb, B)
-
16
Vaccination against hepatitis A and hepatitis B is recommended for all susceptible persons with HCV infection. (IIa, C)
-
17
Vaccination against pneumococcal infection is recommended for all persons with cirrhosis. (IIa, C)
Upon diagnosis of active HCV infection, patients require counseling and certain clinical interventions prior to initiation of antiviral therapy. Prevention of further liver damage is crucial. To that end, counseling patients to abstain from alcohol takes priority because of associations between excess alcohol use and incident or progressive fibrosis, and the development of hepatocellular carcinoma (HCC).(60–69) There is no known safe level of alcohol use for patients with chronic hepatitis C. All patients with chronic hepatitis C, especially those with advanced fibrosis or cirrhosis, should be advised to abstain from alcohol use.(70–72) Persons suffering from alcohol use disorder require treatment for this condition; consider referring these individuals to an addiction specialist. Ongoing alcohol use, however, is not a contraindication to antiviral therapy. Data indicate that ongoing alcohol use does not affect therapeutic outcomes with DAA regimens among treatment-adherent patients.(73)
From a public health perspective, educating persons with HCV infection about how to avoid transmitting the virus to others (Table 2) serves as an essential primary prevention measure to curb and eventually eliminate the hepatitis C epidemic. Exposure to infected blood is the primary mode of HCV transmission. Epidemics of acute HCV due to sexual transmission in men with HIV infection who have sex with men have also been described.(74–77)
Assessing liver disease severity is an essential component of the workup for all persons with newly diagnosed chronic hepatitis C as this factor influences initial and follow-up evaluation. This assessment (i.e., presence or absence of cirrhosis) can usually be accomplished with noninvasive tests (Table 3). Liver biopsy is rarely required but is a consideration if other causes of liver disease are suspected.
Persons with known or suspected cirrhosis are at increased risk for complications of advanced liver disease and require frequent follow-up. They should also avoid hepatotoxic drugs, such as excessive acetaminophen (>2 g/d) and certain herbal supplements. Nephrotoxic drugs (e.g., nonsteroidal anti-inflammatory drugs) should also be avoided. Ongoing imaging surveillance for HCC and gastroesophageal varices is recommended for patients with cirrhosis.(78–80) Cirrhosis with portal hypertension portends a greater likelihood of developing future hepatic complications in untreated patients.(81, 82) Transient elastography provides point-of-care information regarding liver stiffness and can reliably distinguish patients with a high versus low likelihood of cirrhosis.(83–85)
Screening for hepatitis B virus (HBV) with an FDA-approved hepatitis B surface antigen (HBsAg) assay and HIV with an FDA-approved HIV-antigen/antibody test is recommended because these coinfections are associated with a poorer HCV prognosis.(86–90) Persons who test positive for HBsAg require additional monitoring during HCV treatment due to HBV reactivation risk.(91) Anti-HBV therapy is another consideration for these patients. For persons who test negative for HBsAg but positive for hepatitis B core antibodies (with or without hepatitis B surface antibodies) have resolved HBV infection and no further workup or additional monitoring is needed.(92)
Primary prevention measures for persons without coinfection include counseling about how to avoid contracting HIV and HBV, and immunization against HBV and hepatitis A virus (HAV) as needed. CDC also recommends pneumococcal vaccination for all persons with chronic liver disease.(93)
Table 2.
Measures to Prevent HCV Transmission
| HCV-infected persons should be counseled to avoid sharing toothbrushes and dental or shaving equipment, and be cautioned to cover any bleeding wound to prevent the possibility of others coming into contact with their blood. |
| Persons should be counseled to stop using illicit drugs and enter substance abuse treatment. Those who continue to inject drugs should be counseled to: • Avoid reusing or sharing syringes, needles, water, cotton, and other drug preparation equipment. • Use new sterile syringes and filters, and disinfected cookers. • Clean the injection site with a new alcohol swab. • Dispose of syringes and needles after 1 use in a safe, puncture-proof container. |
| Persons with HCV infection should be advised not to donate blood and to discuss HCV serostatus prior to donation of body organs, other tissue, or semen. |
| Persons with HIV/HCV coinfection and those with multiple sexual partners or sexually transmitted infections should be encouraged to use barrier precautions to prevent sexual transmission. Other persons with HCV infection should be counseled that the risk of sexual transmission is low and may not warrant barrier protection. |
| Household surfaces and implements contaminated with visible blood from an HCV-infected person should be cleaned using a dilution of one part household bleach to nine parts water. Gloves should be worn when cleaning up blood spills. |
Table 3.
Noninvasive Tests to Assess Liver Disease Severity
| Liver-directed physical exam (normal in most patients) |
| Routine blood tests (eg, ALT, AST, albumin, bilirubin, international normalized ratio [INR], and CBC with platelet count) |
| Serum fibrosis marker panels |
| Transient elastography |
| Liver imaging (eg, ultrasound or CT scan) |
| AST-to-platelet ratio index (APRI) |
| FIB-4 score |
Universal Treatment of Adults With Chronic Hepatitis C and Simplified Treatment Algorithms
Chronic HCV infection is an important infectious cause of death in the United States and a major contributor to morbidity and mortality from viral hepatitis globally. The availability of safe, effective, well tolerated therapy substantially facilitates the goal of expanding HCV treatment as recommended in the HCV elimination strategies of WHO(3) and NASEM.(4) Overall, DAA regimens successfully cure HCV infection in >95% of treated persons.(2) Moreover, the development of coformulated, pangenotypic regimens that require relatively short treatment durations has greatly simplified HCV antiviral therapy administration. Despite these remarkable therapeutic improvements, in 2015, only 7.4% of persons with diagnosed HCV had begun antiviral treatment.(94) Although more recent limited data indicate increased DAA access and uptake, this has been uneven geographically and across different patient populations.(95–97) Thus, only a minority of persons with HCV infection obtain the many health benefits of successful treatment. From a public health perspective, successful HCV treatment also supports primary prevention by decreasing the population of persons capable of transmitting the virus, thereby reducing the incidence of HCV infection.
Universal Treatment of Adults With HCV Infection
Recommendation
-
18
Antiviral treatment is recommended for all adults with acute or chronic HCV infection, except those with a short life expectancy that cannot be remediated by HCV therapy, liver transplantation, or another directed therapy. (I, A)
Eradicating hepatitis C infection results in numerous health benefits, including reduced rates of all-cause mortality, cirrhosis, hepatic decompensation, and HCC.(33, 37, 98–110) Successful treatment also confers improvement in extrahepatic manifestations of HCV disease, including cryoglobulinemic vasculitis(111–116) and HCV-related non-Hodgkin lymphoma and other lymphoproliferative disorders,(117–125) as well as improved productivity and quality of life.(34, 35, 126–131) Given these and other benefits associated with virologic cure, the HCV guidance panel strongly recommends antiviral treatment for all adults with acute or chronic HCV infection (except those with a short life expectancy that cannot be remediated). Importantly, this recommendation includes persons with ongoing substance use (alcohol or drugs). Several studies demonstrate that treatment-committed individuals in this disproportionately affected population achieve sustained virologic response (SVR) rates with DAA therapy comparable to those without known, current substance use.(73, 132–139) The universal treatment recommendation represents a principal tenet of the HCV guidance along with newly recommended universal hepatitis C screening of adults. The HCV guidance panel urges healthcare providers caring for adults to encourage hepatitis C screening and treatment (if positive) because DAA therapy is safe and cures HCV infection in most people.(2)
Simplified HCV Treatment Algorithms for Treatment-Naive Adults Without Cirrhosis or With Compensated Cirrhosis
One approach to improving access to curative HCV treatment is expanding the number of healthcare providers administering antiviral therapy. Data demonstrate that HCV treatment can be effectively provided by a broad range of healthcare professionals with differing expertise—including specialists, primary care physicians, nurse practitioners, clinical pharmacy specialists, physician assistants, and registered nurses—without compromising treatment efficacy or safety.(95, 140) Consequently, the HCV guidance panel developed simplified HCV treatment algorithms for treatment-naive adults (without cirrhosis or with compensated cirrhosis), which align with the NASEM plan to eliminate HCV as a U.S. public health burden by 2030. These simplified treatment algorithms are designed to be used by any healthcare provider knowledgeable about HCV disease and treatment, including those without extensive experience who have timely access to a specialist. The simplified treatment algorithms provide concise, clear guidance on pretreatment assessment, on-treatment monitoring, assessment of response, and post-treatment management (see Figures 2 and 3).
Figure 2.
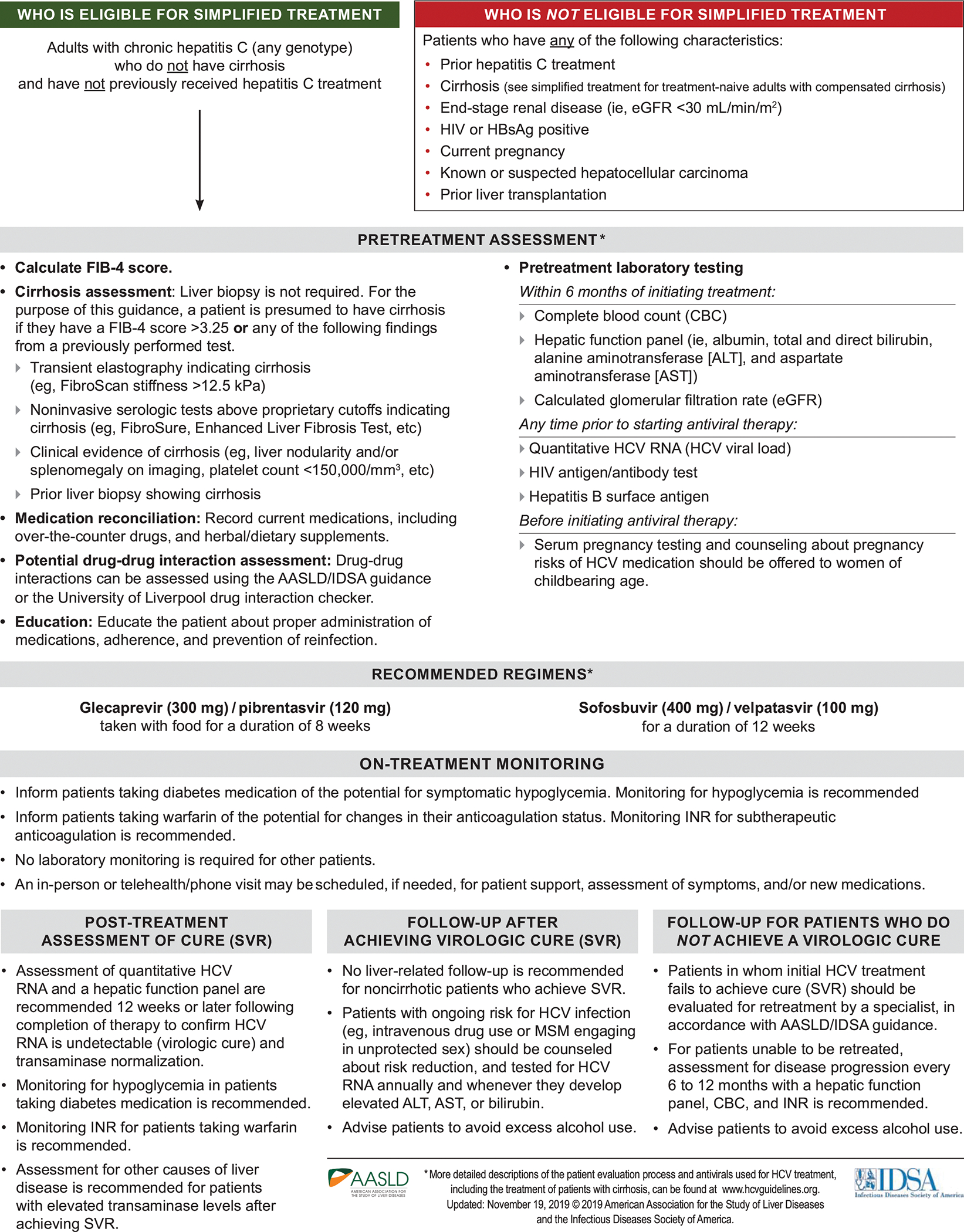
Recommended Simplified HCV Treatment Algorithm for Treatment-Naive Adults Without Cirrhosis
Figure 3.
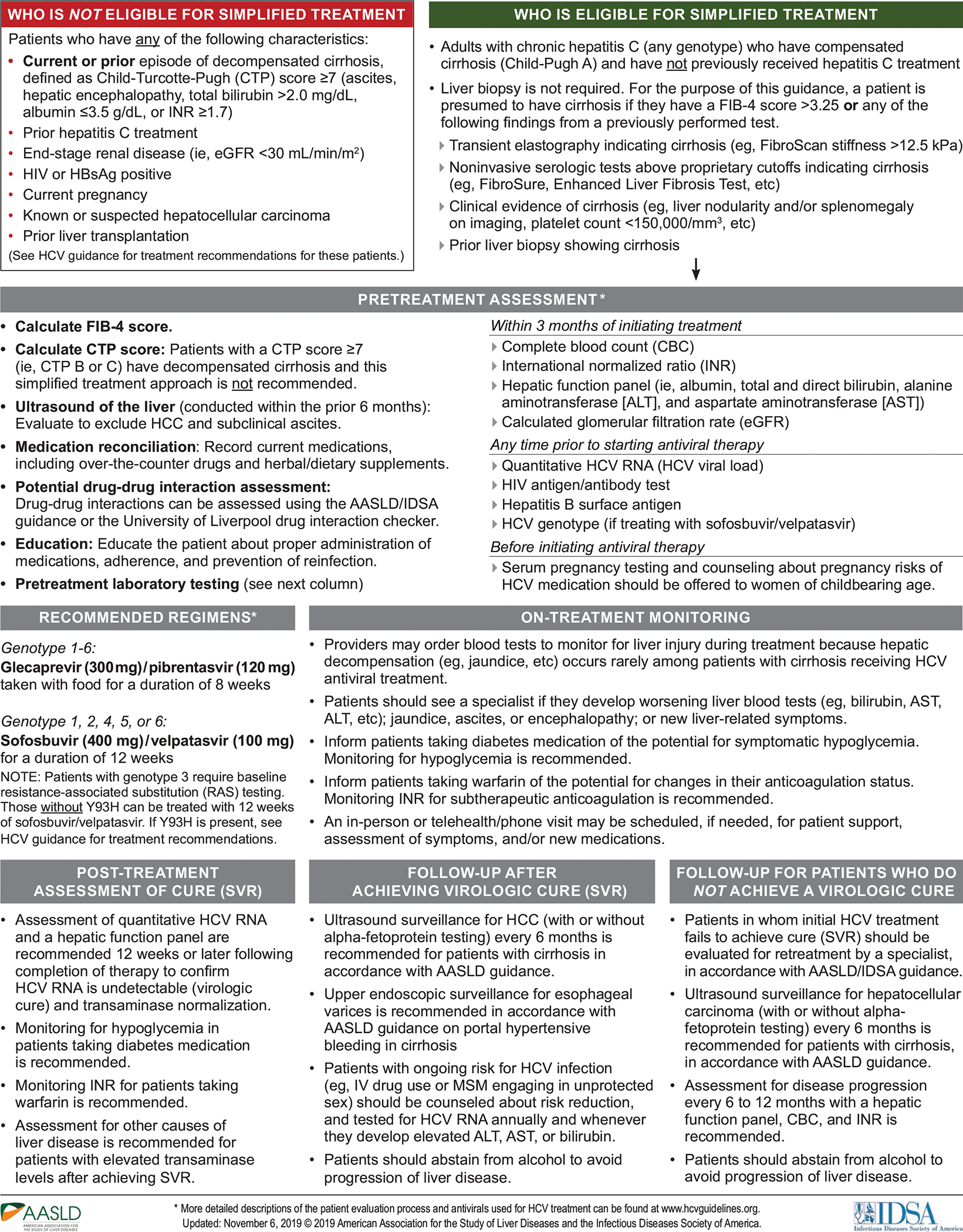
Recommended Simplified HCV Treatment Algorithm for Treatment-Naive Adults With Compensated Cirrhosis
Simplified HCV Treatment Algorithm for Treatment-Naive Adults Without Cirrhosis
The simplified HCV treatment algorithm for adults without cirrhosis (see Figure 2) applies to persons aged ≥18 years who have not been previously treated for their infection and do not have evidence of cirrhosis as defined by the noninvasive parameters specified in the HCV guidance. Evidence of cirrhosis includes a FIB-4 score >3.25 or any of the following findings from a previously performed test: transient elastography indicating cirrhosis (e.g., FibroScan [Echosens, Paris, France] stiffness >12.5 kPa); noninvasive serologic tests that exceed proprietary cutoffs (e.g., FibroSure [BioPredictive, Paris, France], Enhanced Liver Fibrosis Test [Siemens Healthcare, Erlangen, Germany], etc.); clinical evidence of cirrhosis (e.g., liver nodularity and/or splenomegaly on imaging, platelet count <150,000/mm3, etc.); and/or prior liver biopsy showing cirrhosis. This simplified treatment algorithm is not recommended for persons with HIV and/or HBV infection, prior liver transplantation, HCC, end-stage renal disease (i.e., eGFR <30 mL/min/m2), and/or current pregnancy because they require more nuanced care. See the online HCV guidance for management and treatment recommendations for these patients.
The pretreatment evaluation should include an assessment for cirrhosis, medication reconciliation, drug-drug interactions, and patient education regarding treatment administration and the importance of adherence, and transmission prevention. Recommended pretreatment laboratory testing is conducted to confirm chronic HCV infection and exclude decompensated liver disease, HBV and/or HIV coinfection, end-stage renal disease, and pregnancy prior to treatment initiation.
Clearance of HCV infection with DAA therapy can improve hepatic function and thereby affect the safety and efficacy of some concomitantly administered medications. Real-world data indicate an association between DAA therapy and reduced glycemia, particularly among people with diabetes.(141–144) Patients taking diabetes medication(s) should be informed of the potential for symptomatic hypoglycemia during and after DAA therapy. Glucose monitoring during and after DAA treatment is recommended; dosage adjustments of diabetes medication(s) may be needed. Real-world data also indicate an association between DAA therapy and a clinically significant reduction in warfarin dose-response.(145, 146) Patients taking warfarin should be informed of the potential for a change in their anticoagulation status. INR monitoring for subtherapeutic anticoagulation is recommended during and after DAA treatment; warfarin dosage adjustments may be needed. For others, on-treatment laboratory monitoring is not required unless a patient experiences treatment-related side effects or there are adherence concerns.
Several well-designed, robust clinical trials have demonstrated the safety(147) and high curative efficacy of glecaprevir/pibrentasvir(148–158) and sofosbuvir/velpatasvir(159–164) among treatment-naive persons without cirrhosis regardless of HCV genotype. These findings have been confirmed in real-world cohort studies for both glecaprevir/pibrentasvir(165–167) and sofosbuvir/velpatasvir.(167–171) Based on these data, 8 weeks of glecaprevir/pibrentasvir or 12 weeks of sofosbuvir/velpatasvir is recommended for adults eligible for the simplified treatment algorithm.
To assess treatment response, HCV-RNA and hepatic aminotransferase testing is recommended 12 or more weeks after completing DAA treatment. Undetectable HCV RNA represents SVR and virologic cure. In the absence of cirrhosis, persons who attain SVR require no liver-specific follow-up. For those with ongoing HCV risk factors, risk-reduction counseling is recommended as well as HCV-RNA testing annually or anytime an increase in hepatic aminotransferase levels occurs. Recurrent HCV viremia after attainment of SVR represents either reinfection or a relapse (i.e., reemergence of the originally infecting HCV strain).(172, 173) With reinfection, treatment approaches are identical to those for initial treatment. If relapse is suspected or cannot be ruled out, such patients should be managed by clinicians with expertise in managing HCV treatment failure.
Persons who attain SVR but have persistently elevated hepatic aminotransferase levels require evaluation for other causes of liver disease. Individuals for whom treatment fails can often be successfully retreated; see the online HCV guidance for management and antiviral regimen recommendations for treatment-experienced persons. If retreatment is delayed or not feasible, assessment for liver disease progression every 6–12 months is recommended as specified in Figure 2. Advise all patients, regardless of SVR, to avoid excess alcohol intake to prevent liver damage.
Simplified HCV Treatment Algorithm for Treatment-Naive Adults With Compensated Cirrhosis
The simplified HCV treatment algorithm for adults with compensated cirrhosis (see Figure 3) applies to persons aged ≥18 years who have not been previously treated for their infection and have evidence of compensated cirrhosis (i.e., Child-Turcotte-Pugh [CTP] class A) but not decompensated cirrhosis (i.e., CTP class B or C). Noninvasive evidence of cirrhosis mirrors the parameters specified in the previous section and are shown in Figure 3. Calculation of the CTP score is recommended to differentiate compensated versus decompensated cirrhosis. A CTP score ≥7 or any history of decompensation disqualifies these patients for the simplified treatment algorithm. Recommended pretreatment assessment also includes clinical evaluation for ascites and hepatic encephalopathy, and ultrasound imaging of the liver within the prior 6 months to evaluate for HCC and subclinical ascites; any of these clinical or imaging findings are contraindications to use of the simplified treatment algorithm. See the online HCV guidance for treatment and management of persons with decompensated cirrhosis. Other circumstances and comorbid conditions that disqualify a patient for use of the simplified treatment algorithm mirror those described in the previous section and are shown in Figure 3. Similarly, medication reconciliation, assessment for potential drug-drug interactions, and pretreatment education and counseling are the same as for treatment-naive patients without cirrhosis (see Figure 3).
Pretreatment laboratory assessment of patients with compensated cirrhosis eligible for use of the simplified treatment algorithm includes CBC, INR, a hepatic function panel, and eGFR within 3 months of initiating antiviral therapy. Quantitative HCV RNA, HIV antigen/antibody, and HBsAg tests are recommended any time prior to initiating DAA therapy. Notably, pretreatment genotype testing is recommended if sofosbuvir/velpatasvir therapy is planned because of the necessity for baseline RAS testing in persons with cirrhosis and genotype 3 infection.
Because new-onset hepatic decompensation develops rarely during HCV DAA treatment, clinicians may opt for on-treatment blood tests to detect liver injury. Patients who experience deteriorating hepatic laboratory parameters and/or new-onset jaundice, ascites, encephalopathy, or other new liver-related signs or symptoms should promptly see a liver specialist. On-treatment monitoring of blood glucose levels and INR are recommended for persons on diabetes medications or warfarin, respectively, with dosage adjustments as warranted (see previous section for a more detailed discussion).
Multiple rigorous clinical trials have demonstrated the safety(139) and high curative efficacy of glecaprevir/pibrentasvir(148, 174–177) and sofosbuvir/velpatasvir(160, 162–164, 171, 178–180) among treatment-naive adults with compensated cirrhosis, regardless of HCV genotype. These findings have been confirmed in real-world cohort studies for both glecaprevir/pibrentasvir(153, 165–167, 181–183) and sofosbuvir/velpatasvir.(169, 170, 184–189) Based on these data, recommended regimens for adults eligible for the simplified treatment algorithm are 8 weeks of glecaprevir/pibrentasvir for patients with genotype 1–6, or 12 weeks of sofosbuvir/velpatasvir for those with genotype 1, 2, 4, 5, or 6. Pretreatment RAS testing is recommended for persons with genotype 3 because only those without a baseline NS5A Y93H RAS are eligible for a 12-week course of sofosbuvir/velpatasvir. Patients with genotype 3 and a baseline Y93H RAS should be treated with glecaprevir/pibrentasvir or an alternative regimen (see the online HCV guidance).
HCV-RNA and aminotransferase testing are recommended 12 or more weeks after completion of DAA therapy to assess treatment response. Undetectable HCV RNA represents SVR and virologic cure. Ultrasound surveillance for HCC (with or without alpha-fetoprotein testing) every 6 months after treatment completion is recommended for patients with cirrhosis, regardless of achieving SVR.(78) Upper endoscopic surveillance for esophageal varices is recommended, consistent with AASLD guidance on portal hypertensive bleeding in cirrhosis.(190) Advise all patients to abstain from alcohol use to reduce the risk of liver disease progression. Risk-reduction counseling is recommended for persons with ongoing HCV risk factors. HCV-RNA testing annually or anytime an increase in hepatic aminotransferase levels occurs is also recommended for these persons. Recurrent HCV viremia after attainment of SVR represents either reinfection or a relapse.(172, 173) With reinfection, the treatment approaches are identical to those for initial treatment. If relapse is suspected or cannot be ruled out, such patients should be managed by clinicians with expertise in managing HCV treatment failure. Persons who attain SVR but experience persistently elevated hepatic aminotransferase levels require evaluation for other causes of liver disease.
Individuals in whom initial treatment fails should be evaluated by a specialist for retreatment, which often proves successful. See the online HCV guidance for management and antiviral regimen recommendations for treatment-experienced persons. If retreatment is delayed or not feasible, assessment for liver disease progression every 6–12 months is recommended as specified in Figure 3.
HCV in the Pediatric Population
An estimated 3.5 million to 5.0 million children and adolescents worldwide have chronic HCV infection,(191, 192) including an estimated 23,000 to 46,000 pediatric patients in the United States.(193) Vertical transmission accounts for most HCV infections in the pediatric population.(194) The rate of mother-to-child transmission of HCV infection is approximately 5%, although rates are higher among women with inadequately controlled HIV coinfection, and in women with HCV RNA >6 log10 IU/mL.(195–201) Universal prenatal hepatitis C screening, as recommended by the HCV guidance panel, is expected to facilitate improved identification of at-risk infants who require HCV testing.(202–204) This will likely result in better HCV disease case finding in the pediatric population.
Antiviral treatment of children and adolescents with HCV infection has been previously limited to adolescents aged ≥12 years due to the absence of FDA-approved regimens for younger children. Recent and anticipated FDA approval of additional regimens for children aged 3 through 11 years present an opportunity to expand HCV treatment in the pediatric population. Modeling data indicate that HCV DAA therapy is cost-effective in children as young as 12 years(205) and is anticipated to be so for the 3- through 11-year-old age group.
Testing of Perinatally Exposed Children and Siblings of Children With HCV Infection
Recommendations
-
19
All children born to women with acute or chronic hepatitis C should be tested for HCV infection. Antibody-based testing is recommended at or after 18 months of age. (I, A)
-
20
Testing with an HCV-RNA assay can be considered in the first year of life, but the optimal timing of such testing is unknown. (IIa, C)
-
21
Testing with an HCV-RNA assay can be considered as early as 2 months of age. (IIa, B)
-
22
Repetitive HCV-RNA testing prior to 18 months of age is not recommended. (III, A)
-
23
Children who are anti-HCV positive after 18 months of age should be tested with an HCV-RNA assay after age 3 to confirm chronic hepatitis C infection. (I, A)
-
24
The siblings of children with vertically acquired chronic hepatitis C should be tested for HCV infection, if born from the same mother. (I, C)
The HCV guidance panel recommends HCV-antibody testing at age ≥18 months for children born to women with HCV infection. Earlier antibody testing is not recommended due to maternal anti-HCV, which can persist in the infant’s serum for up to 18 months.(206, 207) For infants with a positive HCV-antibody test at or after 18 months of age, the HCV guidance panel recommends HCV-RNA testing at age ≥3 years to determine chronic infection versus spontaneous viral clearance. Approximately 25%−50% of vertically infected infants spontaneously resolve HCV infection by 4 years of age,(191, 200, 208–211) although spontaneous viral clearance can occur later in childhood.(212–214)
HCV-RNA testing can be considered in the first year beginning at 2 months of age, particularly in the setting of concern about loss to follow-up. Detectable HCV RNA during the first year of life reliably correlates to anti-HCV positivity at 18 months.(215) Repetitive HCV-RNA testing prior to 18 months of age is not recommended. Hepatitis C screening is indicated for siblings of children with vertically acquired, chronic HCV infection if born to the same mother and not previously tested for HCV infection.
Counseling Parents and Children Regarding HCV Transmission and Prevention
Recommendations
-
25
Parents should be informed that hepatitis C is not transmitted by casual contact and, as such, children with HCV infection do not pose a risk to other children and can participate in school, sports, and athletic activities, and engage in all other regular childhood activities without restrictions. (I, B)
-
26
Parents should be informed that universal precautions should be followed at school and in the home of children with HCV infection. Educate families and children about the risk and routes of HCV transmission, and the techniques for avoiding blood exposure, such as avoiding the sharing of toothbrushes, razors, and nail clippers, and the use of gloves and dilute bleach to clean up blood. (I, B)
Children with HCV infection often face discrimination and stigmatization in school and child-care settings, usually driven by public misconceptions about contracting hepatitis C. Further, well-intentioned parents and caregivers may limit the activities of a child with HCV infection due to concerns about their health.(216, 217) Clinicians caring for these children should counsel and assure parents that their child poses no threat to others because HCV is not transmitted by casual contact in the absence of blood exposure. Children with HCV infection can and should fully participate in school and extracurricular activities of their choosing without restrictions. Educate parents and appropriately aged children and adolescents about how to prevent HCV transmission in the home and at school. This includes implementing universal precautions for preventing transmission of bloodborne pathogens, covering open wounds, cleaning blood-contaminated surfaces with dilute bleach, and avoiding sharing personal hygiene items that might be contaminated with blood, such as toothbrushes, razors, nail clippers, and so on (see Table 2).
Sexual transmission of HCV occurs but inefficiently except among men with HIV infection who have unprotected sex with men.(218–220) Encourage adolescents with HIV/HCV coinfection and those with multiple sexual partners and/or sexually transmitted infections (STIs) to use barrier precautions to prevent transmission of HCV and other STIs. Counsel other adolescents with HCV infection that the risk of sexual transmission is low, but barrier precautions are recommended to prevent HIV and other STIs.
Monitoring and Medical Management
Recommendations
-
27
Routine liver biochemistries at initial diagnosis and at least annually thereafter are recommended to assess for HCV disease progression. (I, C)
-
28
Appropriate vaccinations are recommended for children with HCV infection who are not immune to HBV and/or HAV to prevent these infections. (I, C)
-
29
Disease severity assessment via routine laboratory testing and physical examination, as well as use of evolving noninvasive modalities (i.e., transient elastography, imaging, or serum fibrosis markers) is recommended for all children with chronic hepatitis C. (I, B)
-
30
Children with cirrhosis should undergo HCC surveillance and endoscopic surveillance for varices per standard recommendations. (I, B)
-
31
Hepatotoxic drugs should be used with caution in children with chronic hepatitis C after assessment of potential risks versus benefits of treatment. Use of corticosteroids, cytotoxic chemotherapy, or therapeutic doses of acetaminophen are not contraindicated in children with chronic hepatitis C. (II, C)
-
32
Solid organ transplantation and bone marrow transplantation are not contraindicated in children with chronic hepatitis C. (II, C)
-
33
Anticipatory guidance about the potential risks of alcohol for progression of liver disease is recommended for adolescents with chronic HCV infection and their families. Abstinence from alcohol and interventions to facilitate cessation of alcohol consumption, when appropriate, are advised for all persons with chronic HCV infection. (I, C)
The initial assessment of children with chronic HCV infection includes exclusion of other causes of liver disease, assessment of HCV disease severity, and detection of extrahepatic manifestations of HCV (uncommon in children). Children with chronic HCV infection usually appear clinically well on physical examination; hepatomegaly occurs in ≤10% of patients.(221, 222) Assessment of hepatic laboratory parameters, including albumin, aminotransferase levels, total bilirubin, INR, and platelet count, is recommended every 6–12 months. Persistently or intermittently elevated hepatic aminotransferase levels occur in approximately 50% of children with chronic HCV infection.(221) Serum aminotransferase levels, however, do not consistently correlate with HCV liver disease severity.(223) Laboratory testing for concomitant infections with HBV (i.e., HBsAg, anti-HBc, and anti-HBs testing) and HIV (i.e., anti-HIV), and immunity to HAV (i.e., anti-HAV IgG) are also recommended due to shared risk factors and the need to vaccinate nonimmune children against HAV and HBV. Serum fibrosis markers hold promise to assess hepatic disease severity but require further validation in the pediatric population.(224–226)
For pediatric patients with suspected advanced liver disease, initial assessment using liver ultrasound imaging to evaluate for splenomegaly and/or venous collaterals is recommended to avoid ionizing radiation exposure. Although liver biopsy remains the gold standard to assess inflammation grade and fibrosis stage, sampling variability and potential adverse events (e.g., bleeding) are problematic.(227–231) Additionally, most clinicians and patients (or their parents) prefer noninvasive alternatives to determine the presence or absence of cirrhosis, particularly in the pediatric population. Ultrasound-based, liver elastography appears increasingly promising for monitoring children and adolescents with chronic HCV infection.(232–236)
HCV-related liver disease generally progresses more slowly in children and adolescents compared to adults, although disease progression is unpredictable.(191, 221, 237–239) Despite a paucity of data evaluating risk factors for HCV disease progression in the pediatric population, children with comorbid conditions (e.g., obesity with nonalcoholic fatty liver disease, congenital heart disease with elevated right heart pressures, and HIV and/or HBV coinfection) and those receiving hepatotoxic drugs require careful monitoring.(87–90, 191, 226, 240)
Advanced HCV-related liver disease develops infrequently in children and teens, usually occurring more than 30 years after initial infection.(241–243) Cirrhosis is uncommon and HCC even more rare, occurring almost exclusively in those with cirrhosis.(213, 214, 242, 244–253) Limited evidence suggests that children with chronic hepatitis C and a history of childhood leukemia may be at increased risk of developing HCC.(254, 255) The HCV guidance panel recommends HCC surveillance using liver ultrasound imaging (with or without alpha-fetoprotein testing) every 6 months for pediatric HCV patients with cirrhosis, consistent with AASLD guidance for HCC surveillance in adults.(78) A baseline endoscopy to detect esophageal varices and every 3 years thereafter (in the absence viral clearance) is advisable for these patients. Successful HCV DAA therapy substantially reduces the risk for cirrhosis complications.(256, 257)
In children with HCV-related advanced fibrosis or cirrhosis, medications known to accelerate hepatic fibrosis (e.g., methotrexate) should be avoided, if possible. Although corticosteroids and other immunosuppressants may enhance HCV replication, they are not contraindicated in children with HCV infection and should be prescribed for appropriate indications based on the overall risks versus benefits. Notably, icteric flares of HCV—as reported in children and adults with chronic HBV infection—have not been reported in children receiving an organ transplant or cytotoxic chemotherapy. Although underlying liver disease is a risk factor for the development of hepatic veno-occlusive disease following bone marrow transplantation,(258, 259) chronic HCV infection should not delay this therapy.
No dosage adjustments are necessary for commonly prescribed medications such as antimicrobial, antiepileptic, and cardiovascular agents. Nonsteroidal anti-inflammatory drugs and aspirin should be avoided, if possible, for patients with cirrhosis and esophageal varices due gastrointestinal bleeding and nephrotoxicity risks. Acetaminophen is a safe and effective analgesic for children and adolescents with chronic HCV infection when dosed per packaging recommendations.
Alcohol abstinence is strongly advised to reduce the risk for liver disease progression.(61, 63, 64, 66–72) Similarly, counsel appropriately aged, untreated pediatric patients and their parents about the importance of maintaining a healthy body weight due to the deleterious effects of insulin resistance on HCV-related fibrosis progression.(260–271)
Whom and When to Treat Among Children and Adolescents With HCV Infection
Recommendations
-
34
DAA treatment with an approved regimen is recommended for all children and adolescents with HCV infection aged ≥3 years as they will benefit from antiviral therapy, regardless of disease severity. (I, B)
-
35
The presence of extrahepatic manifestations—such as cryoglobulinemia, rashes, and glomerulonephritis—as well as advanced fibrosis should lead to early antiviral therapy to minimize future morbidity and mortality. (I, C)
Although advanced HCV-related liver disease occurs uncommonly in children and adolescents, hepatic fibrosis progresses over time and complications may develop during early adulthood. The rationale for treating persons with HCV infection in the pediatric population mirrors that for adults, i.e., to reduce disease-related morbidity and mortality. Additionally, curative DAA therapy during childhood or adolescence supports the HCV treatment as transmission prevention paradigm, a pillar of the 2017 NASEM hepatitis C elimination strategy.(4) The extension of pediatric HCV antiviral treatment to 3- through 11-year-olds comes at a critical inflexion point in the hepatitis C epidemic, given the recent increase in HCV infection among women of childbearing age(272–275) and the fact that an estimated 29,000 women with HCV infection gave birth each year from 2011 to 2014.(18)
HCV Antiviral Therapy for Children and Adolescents Aged ≥3 Years, Without Cirrhosis or With Compensated Cirrhosis (Child-Pugh A)
Recommendations for Treatment-Naive and Interferon-Experienced Patients
-
36
An 8-week course of the daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) is recommended for treatment-naive adolescents aged ≥12 years or weighing ≥45 kg with any HCV genotype, without cirrhosis or with compensated cirrhosis. (I, B)
-
37
A 12-week course of the combination of ledipasvir/sofosbuvir (weight-based dosing, see Table 4) is recommended for treatment-naive or interferon-experienced children aged ≥3 years with HCV genotype 1, 4, 5, or 6 infection, without cirrhosis or with compensated cirrhosis. (I, B)
The high rate of HCV clearance with DAA regimens previously demonstrated in adults are increasingly being replicated in the pediatric population. Eight weeks of the daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) gained FDA approval for use in treatment-naive or interferon-experienced adolescents aged ≥12 years or weighing ≥45 kg with any HCV genotype infection, without cirrhosis or with compensated cirrhosis (Child-Pugh A). Although the registration trial included only adolescents with genotype 1 through 4,(276) glecaprevir/pibrentasvir garnered FDA approval for all genotypes based on the safety and efficacy of the regimen demonstrated in adults.(148–150, 152–157, 165–167, 277) The recommendations for use of glecaprevir/pibrentasvir in treatment-experienced adolescents is also based on clinical trial data from adults.(154, 156, 176, 278–280) Given its pangenotypic activity, and safety and efficacy records in adults, the HCV guidance panel recommends glecaprevir/pibrentasvir as the first choice for adolescent HCV treatment. Coadministration of carbamazepine, efavirenz-containing regimens, and St. John’s wort should be avoided because these compounds may decrease circulating concentrations of glecaprevir and pibrentasvir.
In August 2019, the FDA approved an expansion of pediatric indications for ledipasvir/sofosbuvir to include the 3- through 11-year-old age group in addition to the ≥12 years adolescent group for specific clinical scenarios. Dosing is weight based (see Table 4). Twelve weeks of ledipasvir/sofosbuvir is recommended for treatment-naive children and adolescents aged ≥3 years with genotype 1, 4, 5, or 6, without cirrhosis or with compensated cirrhosis (Child-Pugh A). This regimen is also recommended for interferon-experienced (± ribavirin, with or without an HCV protease inhibitor) children and adolescents aged ≥3 years with genotype 1 or 4. A 12-week course is recommended for patients without cirrhosis; 24 weeks is recommended for those with compensated cirrhosis. Three clinical trials supporting the approval of ledipasvir/sofosbuvir in the pediatric population aged ≥3 years demonstrated high SVR12 rates comparable to those observed in adults.(281–283) Limited real-world data further corroborate these findings.(284, 285)
In September 2019, the FDA newly approved weight-based sofosbuvir plus ribavirin for treatment-naive and interferon-experienced (± ribavirin) children aged ≥3 years with genotype 2 or 3, without cirrhosis or with compensated cirrhosis (Child-Pugh A). A 12-week course is recommended for pediatric patients without cirrhosis and 24 weeks is recommended for those with compensated cirrhosis (see the online HCV guidance for dosing recommendations). The registration trial conducted in children aged 3 to <12 years demonstrated an SVR12 of 98%.(286) The use of sofosbuvir plus ribavirin is further supported by clinical trial data involving adolescents(287) and adults with genotype 2 or 3 infection.(288–291) At the time of manuscript preparation, sofosbuvir (plus ribavirin) remained the only FDA-approved DAA for children 3 through 11 years with genotype 2 or 3 infection. However, recent clinical trials evaluating weight-based dosing of sofosbuvir/velpatasvir(292) and glecaprevir/pibrentasvir(293) are expected to lead to FDA approval for children aged 3 through 11 years. The HCV guidance panel recommends awaiting approval of a pangenotypic regimen unless there is a compelling need for immediate antiviral treatment of children aged 3 through 11 years with genotype 2 or 3 infection.
DAA-experienced pediatric HCV patients are rarely encountered in clinical practice. Due to a paucity of data in the pediatric population, DAA-experienced children and adolescents with HCV infection should be treated using the adult HCV guidance while under the supervision of a pediatric HCV specialist. Similarly, decompensated cirrhosis and recurrent HCV after liver transplantation are rare clinical scenarios; children and adolescents with these conditions require specialty care. See the online HCV guidance for additional information about treatment of these children and adolescents.
As with adults, testing for active HBV infection (i.e., HBsAg, anti-HBc, and anti-HBs) is recommended prior to initiating HCV DAA therapy in pediatric patients due to the risk for HBV reactivation during or after treatment.(92, 294, 295) Additionally, on-treatment and post-treatment glucose monitoring for hypoglycemia in children and adolescents with diabetes and INR monitoring in those taking warfarin is recommended due to potential alterations in dose-response relationships associated with DAA-related HCV viral clearance.(141–146)
Table 4.
Weight-Based Dosing of Ledipasvir/Sofosbuvir for Children Aged ≥3 Years
| Body Weight | Once Daily Dose of Ledipasvir/Sofosbuvir |
|---|---|
| <17 kg | 33.75 mg/150 mg |
| 17 to <35 kg | 45 mg/200 mg |
| ≥35 kg | 90 mg/400 mg per day |
Acute HCV Infection
Acute HCV infection is arbitrarily defined as the first 6 months of infection. Patients are often minimally symptomatic or experience nonspecific symptoms (e.g., fatigue, anorexia, mild or moderate abdominal pain, low-grade fever, nausea, and/or vomiting) with mild to moderate elevations in hepatic aminotransferases.(296) Jaundice occurs in a minority of patients (< 25%) and fulminant hepatic failure is extremely rare.(297, 298) Despite difficulties in diagnosis and reporting, CDC estimates that 44,300 acute HCV infections occurred in the United States in 2017 with the upper limit of the confidence interval being 151,000 cases.(299) As previously noted, increased injection drug use associated with the opioid epidemic accounts for much of the recent increase in acute HCV infection incidence. An estimated 75% of persons acutely infected with HCV progress to chronic infection, although this number varies depending on host factors (e.g., age at exposure, sex, HIV coinfection, and IL28B genotype).
Substantial fluctuations in viremia during acute HCV infection may lead to higher HCV RNA levels in this phase of the infection compared with those seen during chronic infection.(300, 301) Drawing on the analogy from HIV infection and limited evidence with HCV, acute HCV infection may be associated with an increased risk for transmission.(302) Conversely, antiviral treatment during acute infection has the potential to reduce transmission to other susceptible individuals (i.e., treatment as prevention). Modeling studies support treatment of acute HCV infection, demonstrating cost-effectiveness assuming high treatment efficacy with a relatively short treatment duration—requisite conditions that currently exist.(303, 304) Successful HCV treatment as prevention of transmission has been demonstrated in several cohorts of HIV-positive men who have sex with men (MSM) where unrestricted access to DAA therapy resulted in an approximately 50% decrease in acute HCV infections.(305)
Data addressing optimal treatment approaches for acute HCV infection continue to evolve. In the interim, current guidance is to treat with regimens and durations as recommended for chronic HCV infection until additional data on abbreviated treatment regimens become available.
Diagnosis of Acute HCV Infection
Recommendation
-
38
HCV-antibody and HCV-RNA testing are recommended when acute HCV infection is suspected due to known exposure, clinical presentation, or elevated aminotransferase levels (see Figure 4). (I, C)
HCV RNA typically becomes reliably detectable within 2–3 weeks after viral exposure(306–309); HCV antibody seroconversion among immunocompetent persons typically occurs 2–3 months after exposure, on average.(308–311) Therefore, the best laboratory evidence to support a diagnosis of acute HCV infection is a positive HCV-RNA test in the setting of a negative HCV-antibody test (identification during the seronegative window period) or a positive HCV-antibody test after a prior negative anti-HCV test (seroconversion).(51, 52, 312) Rarely, these approaches may be misleading such as in immunosuppressed individuals with impaired antibody production.(58, 313, 314)
Laboratory-based diagnosis of acute HCV infection is most straightforward when there has been a discrete, known or suspected exposure (e.g., after new onset or a change in drug injection practice, a percutaneous needlestick exposure to HCV-infected blood, a potentially nonsterile tattoo, or sexual contact). Baseline HCV-antibody and HCV-RNA testing should be performed within 48 hours of the exposure to document baseline HCV infection status (see Figure 4).
If baseline testing is negative, repeat testing for HCV antibody and HCV RNA is recommended. The frequency can be tailored based on management objectives (e.g., monthly testing to identify and treat acute infection). If the baseline HCV-antibody test is positive but HCV RNA is undetectable, repeat HCV-RNA and ALT testing is recommended to identify acute reinfection. When baseline HCV-antibody and HCV-RNA testing are both positive, the person most likely already has chronic infection from a prior exposure.
Individuals suspected of having acute HCV infection often do not have a discrete exposure and/or have no prior baseline testing, making a definitive diagnosis of acute infection more challenging (see Table 5). Acute infection should be suspected if there is a new rise in the ALT level without an alternative cause.(315, 316) Acute infection should also be suspected when there are low (especially <104 IU/mL) or fluctuating (>1 log10 IU/mL) HCV-RNA levels, or spontaneous clearance during follow-up. These patterns do not commonly occur outside of the acute phase of HCV infection.(300, 301, 317) Patients suspected of having acute HCV infection also require laboratory evaluation to exclude other or coexisting causes of acute hepatitis (e.g., HAV, HBV, hepatitis delta virus superinfection if chronically infected with HBV, hepatitis E virus [in the correct clinical scenario], and autoimmune hepatitis).(318) HIV testing is also recommended.
Figure 4. Testing Algorithm for Discrete, Recognized HCV Exposurea.

a Often there is no discrete exposure and/or the entry to healthcare occurs with jaundice or elevated liver enzymes. In those instances, baseline testing cannot be performed and the diagnosis of acute HCV infection is based on clinical criteria (see text).
b Repeat HCV Ab is not needed if the test is positive at baseline. Frequency of testing can be tailored based on risk of exposure.
c If there were additional exposures in the preceding 6 months, a newly diagnosed patient who is HCV RNA and HCV Ab positive may still be in the acute phase. Symptoms, elevated ALT, and/or fluctuations in virus levels may distinguish acute from chronic HCV infection.
d Baseline testing should be performed within 48 hours of exposure to determine existing infection status, including HCV RNA, HCV Ab, and ALT.
Table 5.
Interpretation of Blood Tests for Diagnosis of Acute HCV Infection
| Test | Interpretation for Diagnosis of Acute HCV |
|---|---|
| HCV antibody | • Test may be negative during the first 6 weeks after exposure. • Seroconversion may be delayed or absent in immunosuppressed individuals. • Presence of HCV antibody alone does not distinguish between acute vs chronic infection. |
| HCV RNA | • Viral fluctuations >1 log10 IU/mL may indicate acute HCV infection. • HCV RNA may be transiently negative during acute HCV infection. • Presence of HCV RNA alone does not distinguish between acute vs chronic infection. |
| ALT | • Fluctuating ALT peaks suggest acute infection. • ALT may be normal during acute HCV infection. • ALT may be elevated due to other liver insults, such as alcohol consumption. |
Medical Management and Transmission Prevention
Recommendations
-
39
After the initial diagnosis of acute HCV with viremia (defined as quantifiable RNA), HCV treatment should be initiated without awaiting spontaneous resolution. (I, B)
-
40
Counseling is recommended for patients with acute HCV infection to avoid hepatotoxic insults, including hepatotoxic drugs (e.g., acetaminophen) and alcohol consumption, and to reduce the risk of HCV transmission to others. (I, C)
-
41
Referral to an addiction medicine specialist is recommended for patients with acute HCV infection related to substance use. (I, B)
The HCV guidance panel newly recommends initiating DAA therapy upon initial diagnosis of acute HCV infection without awaiting possible spontaneous clearance (i.e., a test and treat strategy). Real-world data demonstrate a reduction in HCV viremia incidence and prevalence with unrestricted access to HCV therapy.(305, 319) Mathematical modeling studies also suggest that scaling up DAA treatment can reduce HCV incidence and prevalence, especially among populations at highest risk of onward transmission (e.g., MSM and PWID).(303, 320–322) Additionally, delay introduced by waiting for spontaneous clearance may increase the number of patients lost to follow-up.
Counseling persons with acute HCV infection to reduce behaviors that could result in virus transmission (e.g., sharing injection equipment and engaging in high-risk sexual practices) is recommended. Because the risk of transmission of other bloodborne, sexually transmitted infections (e.g., HIV and HBV) is higher in the acute phase of infection, some experts counsel persons with acute HCV to consider using barrier precautions, even in a stable monogamous relationship. For persons with acute HCV infection who have a history of recent injection drug use, referral to harm reduction services and an addiction medicine specialist is recommended as needed.(323–326)
Monitoring with hepatic panels (ALT, AST, bilirubin, and INR in the setting of an increasing bilirubin level) is recommended at 2-week to 4-week intervals until resolution of acute hepatitis C.(316) Hepatic laboratory parameters typically improve rapidly with antiviral treatment. Alteration of dosages of concomitant medications that are metabolized by hepatic enzymes is unnecessary unless there is concern for developing acute liver failure (e.g., increasing bilirubin level and prolongation of INR). Acetaminophen and alcohol consumption should be avoided during acute HCV infection.(327–329)
Patients with acute HCV infection rarely require hospitalization unless nausea and vomiting are severe. Although acute liver failure is very rare (<1%), it represents a serious and life-threatening complication of acute HCV infection. Patients with an INR >1.5 and those who exhibit any signs of acute liver failure (e.g., hepatic encephalopathy) should be immediately referred to a liver transplant center. Use of HCV antiviral regimens in acute liver failure should be managed by a clinician experienced in HCV treatment, ideally in consultation with a liver transplant specialist.
HCV infection spontaneously clears in 20%−50% of untreated patients.(330) Clearance of acute HCV infection usually occurs within 6 months of the estimated time of infection. Only 11%–14% of those who remain viremic at 6 months spontaneously clear the infection at a later time.(331, 332) Predictors of spontaneous clearance include presentation with jaundice, elevated ALT, HBsAg positivity, female sex, younger age, genotype 1 infection, and host genetic polymorphisms, most notably those near the IL28B gene.(330, 333)
Patients who experience spontaneous clearance do not require antiviral therapy. They do, however, require counseling about the risk of reinfection and testing at least annually for this development in the setting of ongoing risk behaviors. Notably, transient suppression of viremia sometimes occurs in persons with acute HCV infection, even among those who progress to chronic infection. Thus, a single undetectable HCV-RNA test result is insufficient to declare spontaneous clearance.(300, 333, 334)
Acute HCV Infection Treatment
Recommendation
-
42
Due to high efficacy and safety, the same regimens that are recommended for chronic HCV infection are recommended for acute infection. (IIa, C)
Data are emerging regarding treatment of acute HCV infection with abbreviated courses of DAA regimens in both HCV monoinfection and HIV/HCV coinfection.(335–338) There are presently insufficient data, however, to recommend abbreviated courses of any approved DAA regimens. Until more definitive data are available, recommended treatment is as described for chronic hepatitis C infection in the online HCV guidance. Pangenotypic regimens, as recommended in the simplified HCV treatment section, represent the preferred choice for eligible patients. For patients who are ineligible for simplified HCV treatment, genotyping may be considered to guide DAA regimen selection.
Organ Transplantation From HCV-Viremic Donors to HCV-Negative Recipients
In 2018, 8,250 liver transplantations were performed in the United States, the largest number ever performed in a single year.(339) Despite annual increases in the number of liver transplantations performed in the United States in the 10-year period from 2009 through 2018, more than 14,000 liver transplantation candidates died awaiting the procedure.(339) Given the sizable chasm between the number of waitlisted liver transplantation candidates and the pool of available organs, some transplant programs are turning to a previously untapped pool of organs from deceased HCV-viremic donors; historically, these organs were discarded with rare exception.(340) Coincident with the marked increase in drug overdose deaths among PWID in the United States,(341) this pool of donor organs has sadly increased substantially.(342) In stark contrast to this tragic loss of life, the development of safe and highly effective(2) DAA therapy provides an opportunity to consider use of allografts from HCV-viremic donors in HCV-negative recipients because iatrogenic HCV infection can be cured with retention of allograft function in the majority of cases. Recent data indicate increasing acceptance of these organs among HCV-negative recipients.(340, 343, 344) Although early outcome data are encouraging, the overall experience is limited and many ethical issues and scientific questions remain, such as avoidance of selection bias, the optimal timing of DAA therapy, detailed evaluation of drug-drug interactions between DAAs and immunosuppressants, and long-term graft and patient outcomes. Additional research is needed to clarify short-term and long-term risks and benefits, and to determine and refine optimal clinical management practices.
Considerations for Use of HCV-Viremic Donor Organs in HCV-Negative Recipients
Recommendations
-
43Informed consent should include the following elements (I, C):
- Risk of transmission from an HCV-viremic donor (and with a U.S. Public Health Service defined increased risk donor, the potential risks for other viral infections)
- Risk of liver disease if HCV treatment is not available or treatment is unsuccessful
- Benefits, specifically reduced waiting time and possibly lower waiting list mortality
- Unknown long-term consequences (hepatic and extrahepatic) of HCV exposure (even if cure is attained)
- Risk of allograft failure
- Risk of HCV transmission to partner
-
44Transplant centers should have a programmatic strategy to (I, C):
- Document informed consent
- Assure access to HCV treatment and retreatment(s), as necessary
- Ensure long-term follow-up of recipients (beyond SVR12)
The informed consent process for HCV-negative patients contemplating accepting an organ from an HCV-viremic donor must address not only the potential benefits and risks of the procedure itself but also the known HCV-specific risks as well as long-term uncertainties. Given the mismatch between the number of organ transplantation candidates and the pool of available organs, willingness to accept an allograft from an HCV-viremic donor can reduce waitlist time(345, 346) and improve access to transplantation,(347) thereby reducing the risk of dying while awaiting an available organ.(348–350)
To make an informed decision to consent to accepting an organ from an HCV-viremic donor, the recipient needs to understand the high risk of HCV infection. All donors undergo HCV-antibody testing and nucleic acid testing for HCV RNA. Donors who are HCV antibody positive and HCV RNA negative pose a very low risk of HCV transmission to the recipient.(351–353) Rarely, high risk donors with very recent HCV exposure who are anti-HCV positive and HCV RNA negative may pose a transmission risk.(354) These increased risk donors are identified according to guidelines issued by the U.S. Public Health Service.(355) Increased risk donors may also pose a risk for HIV and HBV transmission depending on their specific risk factors. Patients who receive an allograft from an increased risk donor require monitoring after transplantation to detect HCV transmission(353, 355) as well as possible HBV and/or HIV infection if the donor was at increased risk for these infections. HCV-viremic donors pose the highest risk for HCV transmission to allograft recipients.
Transplant recipients need to understand the risks conferred on them in the event of iatrogenic HCV infection from the allograft. This includes the necessity of HCV antiviral therapy and the risk of liver disease if such treatment is unavailable or unsuccessful. Additionally, there is a risk of DAA-associated allograft rejection and possible loss,(356–362) and/or reduced allograft function.(361, 363–366) Because use of allografts from HCV-viremic donors in HCV-negative recipients is a recent development in transplant medicine, there are no data on possible long-term hepatic and extrahepatic adverse effects associated with HCV exposure, even among those cured of the infection. Allograft recipients who are HCV viremic can potentially sexually transmit the virus to their partner(s), particularly among MSM.(74, 367–372)
The HCV guidance panel recommends that all programs performing HCV-viremia discordant solid organ transplantations have a strategy to execute and document a rigorous informed consent process; assure access to HCV treatment and retreatment, as needed; and ensure long-term follow-up of organ recipients to monitor for potential late consequences of HCV exposure and allograft function.
Treatment of HCV-Negative Recipients of Allografts from HCV-Viremic Donors
Recommendation Regarding Timing of DAA Therapy
-
45
Prophylactic/preemptive DAA therapy with a pangenotypic regimen is recommended. (II, B)
Recommendations for DAA Therapy
-
46
An 8-week course of the pangenotypic daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) is recommended. (I, C)
-
47
A 12-week course of the pangenotypic daily fixed-dose combination of sofosbuvir (400 mg)/velpatasvir (100 mg) is recommended. (I, C)
-
48
A 12-week course of the daily fixed-dose combination of ledipasvir (90 mg)/sofosbuvir (400 mg) is recommended for patients with genotype 1, 4, 5, or 6 only. (I, C)
Initiation of DAA therapy for HCV-negative recipients of an allograft from an HCV-viremic donor can occur prophylactically/preemptively (i.e., perioperatively without confirmation of HCV viremia in the recipient) or reactively after documentation of HCV viremia. The goal is to undertake DAA therapy as early as clinically possible to avoid the development of acute hepatitis and other complications of HCV infection. Emerging data suggest that initiating prophylactic/preemptive DAA therapy before viremia occurs reduces the likelihood of complications, such as fibrosing cholestatic hepatitis.(359, 373–376) The prophylactic/preemptive approach may also allow for a shorter duration of DAA treatment,(359, 377) although this is not currently recommended outside of a clinical trial setting.
Because genotyping of HCV-viremic donors is not routinely performed, only a pangenotypic DAA regimen (i.e., glecaprevir/pibrentasvir or sofosbuvir/velpatasvir) should be used if opting for a prophylactic/preemptive treatment approach. With the reactive treatment approach, genotyping can be used to guide DAA regimen selection if a pangenotypic regimen is not utilized. Although clinical trial data demonstrate the safety and efficacy of elbasvir/grazoprevir among HCV-negative kidney transplant recipients of allografts from HCV-viremic donors,(378–381) it is recommended as an alternative regimen due to the necessity for baseline RAS testing and the need for addition of ribavirin to the regimen if RASs are present.
Several other important considerations should be taken account when selecting a DAA regimen for these patients. Protease inhibitors should be avoided in the presence of moderate to severe liver dysfunction (i.e., Child-Pugh B or C).(382) Assessment of drug-drug interactions is crucial as some medications are contraindicated or not recommended during DAA therapy (e.g., high-dose proton pump inhibitors [twice daily dosing], amiodarone [contraindicated with sofosbuvir-inclusive regimens], and certain statins [eg, atorvastatin], among others). Importantly, complex interactions occur between DAAs and calcineurin inhibitors.(383–388) Coadministration of elbasvir/grazoprevir and cyclosporine leads to a 15-fold increase in grazoprevir and a 2-fold increase in elbasvir area under the concentration-time curve;(389) this DAA regimen and immunosuppressant combination should be avoided. A 40%−50% increase in tacrolimus level is predicted with coadministration of elbasvir/grazoprevir(389); no dosing adjustments are anticipated but tacrolimus levels should be monitored. Please see the online HCV guidance for additional information about drug-drug interactions between DAAs and immunosuppressants as well as other medications.
Limited short-term data from liver,(340, 357, 390, 391) kidney,(358, 378–380, 390–394) heart,(359, 373, 390, 395) and lung(359, 374) transplant programs performing solid organ transplantations involving HCV-viremic donors and HCV-negative recipients are encouraging. However, the overall number of published cases is limited and treatment approaches varied. Known risks include DAA treatment failure with emergence of complex RASs and possible severe or rapidly progressive liver disease.(358, 374, 396, 397) Due to the limited, heterogeneous experience to date and lack of long-term safety data, strong consideration should be given to performing these transplantations under institutional review board approved protocols as recommended by the American Society of Transplantation consensus panel.(353)
Acknowledgment:
We thank the able staffs of AASLD and IDSA, particularly Sheila Tynes, Audrey Davis-Owino, Vita Washington, and Vincent Keane for project management, and administrative and technical support of the HCV guidance project.
Financial Support:
Funding for the hepatitis C guidance project is provided exclusively by AASLD and IDSA. The hepatitis C guidance panel members serve as uncompensated volunteers.
Abbreviations:
- AASLD
American Association for the Study of Liver Diseases
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CBC
complete blood count
- CDC
U.S. Centers for Disease Control and Prevention
- CTP
Child-Turcotte-Pugh
- DAA
direct-acting antiviral
- FDA
U.S. Food and Drug Administration
- HAV
hepatitis A virus
- HBsAg
hepatitis B virus surface antigen
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- IDSA
Infectious Diseases Society of America
- INR
international normalized ratio
- MSM
men who have sex with men
- NASEM
National Academies of Science, Engineering, and Medicine
- PWID
people who inject drugs
- STI
sexually transmitted infection
- SVR
sustained virologic response
- USPSTF
U.S. Preventive Services Task Force
- WHO
World Health Organization
Footnotes
Hepatitis C guidance panel members and authors and their affiliations are listed at the end of the article.
AASLD-IDSA HCV Guidance Panel Members and Authors
Current and Recent Panel Members Involved in Development of Guidance
Chairs
Marc G. Ghany, M.D., M.H.Sc.
Investigator, Liver Diseases Branch
National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health
Bethesda, MD
Kristen M. Marks, M.D.
Assistant Professor of Medicine, Weill Cornell Medicine
New York, NY
Timothy R. Morgan, M.D.
Chief of Hepatology, Veterans Affairs Long Beach Healthcare System
Long Beach, CA
David L. Wyles, M.D.
Chief Division of Infectious Diseases, Denver Health
Denver, CO
Members
Andrew I. Aronsohn, M.D.
Assistant Professor of Medicine, University of Chicago Medical Center
Chicago, IL
Debika Bhattacharya, M.D.
Assistant Clinical Professor of Medicine, University of California Los Angeles
Santa Monica, CA
Tina Broder, M.S.W., M.P.H.
Administrative Manager, Building Equity and Alignment for Impact
Calabasas, CA
Oluwaseun O. Falade-Nwulia, M.B.B.S., M.P.H.
Assistant Professor of Medicine, Johns Hopkins University School of Medicine
Baltimore, MD
Jordan J. Feld, M.D., M.P.H.
Assistant Professor of Medicine and Research Director of Gastroenterology, Medicine
Toronto Western Hospital Liver Center
Toronto, ON Canada
Stuart C. Gordon, M.D.
Wayne State University School of Medicine, Director of Hepatology, Henry Ford Health System
Detroit, MI
Theo Heller, M.D.
Chief of Translational Hepatology Unit, Liver Diseases Branch
National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health
Bethesda, MD
Ravi R. Jhaveri M.D., F.I.D.S.A., F.P.I.D.S., F.A.A.P.
Associate Division Head, Pediatric Infectious Diseases
Ann & Robert H. Lurie Children’s Hospital of Chicago
Professor of Pediatrics, Feinberg Northwestern School of Medicine
Chicago, IL
Maureen M. Jonas, M.D.
Clinical Director, Hepatology
Director, Center for Childhood Liver Disease/Professor of Pediatrics
Harvard Medical School, Boston Children’s Hospital
Boston, MA
Jennifer J. Kiser, Pharm.D.
Assistant Professor and Associate Director of the Center for Translational Pharmacokinetics and Pharmacogenomics, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences
Aurora, CO
Benjamin P. Linas, M.D., M.P.H.
Assistant Professor of Medicine, Boston University School of Medicine
Boston, MA
Vincent Lo Re, M.D., M.S.C.E.
Associate Professor of Medicine and Epidemiology, University of Pennsylvania Perelman School of Medicine, Division of Infectious Diseases and Center for Clinical Epidemiology and Biostatistics
Philadelphia, PA
Marion G. Peters, M.D.
Chief of Hepatology Research, University of California San Francisco
San Francisco, CA
K. Rajender Reddy, M.D.
Director of Hepatology, Hospital of the University of Pennsylvania
Philadelphia, PA
Andrew Reynolds
Hepatitis C Wellness Manager, San Francisco AIDS Foundation
San Francisco, CA
John D. Scott, M.D., M.Sc., F.I.D.S.A.
Associate Professor of Medicine
Hepatitis and Liver Clinic, University of Washington
Seattle, WA
Gloria Searson, A.C.S.W.
Executive Director, Coalition on Positive Health Empowerment
New York, NY
Philip Spradling, M.D.
U.S. Centers for Disease Control and Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention
Atlanta, GA
Norah A. Terrault, M.D.
Professor of Medicine, Chief of the Division of Gastrointestinal and Liver Diseases, University of Southern California Keck School of Medicine
Los Angeles, CA
Stacey B. Trooskin, M.D., Ph.D.
Jonathan Lax Treatment Center, Philadelphia FIGHT, Viral Hepatitis Program
Philadelphia, PA
Elizabeth C. Verna, M.D., M.S.
Columbia University Irving Medical Center
New York, NY
John B. Wong, M.D.
Professor of Medicine and Chief of the Division of Clinical Decision Making, Tufts University School of Medicine
Boston, MA
Ann E. Woolley, M.D., M.P.H.
Instructor in Medicine, Department of Infectious Diseases, Brigham and Women’s Hospital, Harvard Medical School
Boston, MA
Kimberly A. Workowski, M.D.
Professor of Medicine, Emory University School of Medicine
Atlanta, GA
AASLD-IDSA HCV Guidance Project Scientific Writer, Editor, and Author
Tina M. St. John, M.D.
Owner and Principal, St. John Health Communications & Consulting
Vancouver, WA
AASLD-IDSA HCV Guidance Panel Financial Relationships With Commercial Entities
Chairs
Dr. Ghany has no relevant financial affiliations to disclose (updated November 12, 2019).
Dr. Marks was awarded research grants, paid to her institution, from Gilead Sciences and Merck & Co. (updated November 12, 2019).
Dr. Morgan was awarded research grants, paid to his institution, from AbbVie, GENFIT, Gilead Sciences, and Merck & Co. (updated November 12, 2019).
Dr. Wyles was awarded research grants, paid to his institution, from Gilead Sciences (updated November 18, 2019).
Members
Dr. Aronsohn has no relevant financial affiliations to disclose (updated November 12, 2019).
Dr. Bhattacharya has no relevant financial affiliations to disclose (updated November 13, 2018).
Ms. Broder has no relevant financial affiliations to disclose (updated November 13, 2018).
Dr. Falade-Nwulia (recent but not a current member of the HCV guidance panel) has no relevant financial affiliations to disclose (updated November 29, 2018).
Dr. Feld serves as a scientific consultant to AbbVie, Arbutus Biopharma, Enanta Pharmaceuticals, Gilead Sciences, F. Hoffmann-La Roche, and Merck & Co. He was awarded research grants, paid to his institution, from AbbVie, FUJIFILM Wako Chemicals U.S.A., Gilead Sciences, and Janssen Pharmaceuticals (updated November 12, 2019).
Dr. Gordon was awarded research grants, paid to his institution, from AbbVie, Gilead Sciences, and Merck & Co. (updated November 12, 2019).
Dr. Heller has no relevant financial affiliations to disclose (updated November 12, 2019).
Dr. Jhaveri serves as a scientific consultant for AstraZeneca. He received research grants, paid to his institution, from AbbVie and Gilead Sciences (updated October 31, 2019).
Dr. Jonas serves as a scientific consultant to Gilead Sciences Pediatric HCV Advisory Board. She was awarded research grants, paid to her institution, from AbbVie, F. Hoffmann-La Roche, Gilead Sciences, and Merck & Co. (updated November 12, 2019).
Dr. Kiser was awarded research grants, paid to her institution, from Gilead Sciences and ViiV Healthcare (updated November 12, 2019).
Dr. Linas has no relevant financial affiliations to disclose (updated November 12, 2019).
Dr. Lo Re has no relevant financial affiliations to disclose (updated November 18, 2019).
Dr. Peters’ (recent but not a current member of the HCV guidance panel) husband is an employee/officer/director and has stock options at F. Hoffmann-La Roche (updated November 12, 2019).
Dr. Reddy serves on advisory boards for AbbVie, Gilead Sciences, Merck & Co., and Vertex Pharmaceuticals. He was awarded research grants, paid to his institution, from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Pharmaceutica, and Merck & Co. (updated November 18, 2019).
Mr. Reynolds’ organization was awarded educational grants from AbbVie and Gilead Sciences (updated November 12, 2019).
Dr. Scott has no relevant financial affiliations to disclose (updated November 12, 2019).
Ms. Searson’s organization was awarded grants from AbbVie, Gilead Sciences, and Merck & Co. (updated November 12, 2019).
Dr. Spradling has no relevant financial affiliations to disclose (updated November 12, 2019).
Dr. Terrault serves on the medical affairs board of Gilead Sciences (uncompensated) and the advisory board of Intercept Pharmaceuticals. She was awarded research grants, paid to her institution, from Gilead Sciences (updated November 12, 2019).
Dr. Trooskin (recent but not a current member of the HCV guidance panel) is a consultant for Gilead Sciences. She was awarded research grants, paid to her institution, from Gilead Sciences and the Gilead Foundation (updated November 12, 2019).
Dr. Verna serves on an advisory board of Gilead Sciences. She was awarded research grants, paid to her institution, from Salix Pharmaceuticals (updated November 13, 2018).
Dr. Wong has no relevant financial affiliations to disclose. Dr. Wong is a member of the USPSTF; this article does not necessarily represent the views and policies of the USPSTF Force (updated November 1, 2019).
Dr. Woolley has no relevant financial affiliations to disclose (updated November 18, 2019).
Dr. Workowski was awarded research grants, paid to her institution, from Gilead Sciences (updated November 18, 2019).
DR. MARC GHANY (Orcid ID : 0000-0003-2837-0188)
DR. TIMOTHY MORGAN (Orcid ID : 0000-0003-1328-0307)
DR. DEBIKA BHATTACHARYA (Orcid ID : 0000-0002-2136-7763)
DR. NORAH TERRAULT (Orcid ID : 0000-0003-4143-1950)
References
- 1.Hepatitis C guidance 2018 update: AASLD-IDSA recommendations for testing, managing, and treating hepatitis C virus infection. Clin Infect Dis 2018;67:1477–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med 2017;166:637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016–2021: Towards Ending Viral Hepatitis. Geneva: WHO Document and Production Services, 2016:1–56. [Google Scholar]
- 4.National Academies of Sciences, Engineering, and Medicine. 2017. A National Strategy for the Elimination of Hepatitis B and C: Phase Two Report. Washington, DC: The National Academies Press, 2017:1–202. [PubMed] [Google Scholar]
- 5.Methodology manual and policies from the ACCF/AHA task force on practice guidelines. Dallas, TX: American College of Cardiology Foundation and American Heart Association, Inc.; 2010. [Google Scholar]
- 6.Shiffman RN, Shekelle P, Overhage JM, Slutsky J, Grimshaw J, Deshpande AM. Standardized reporting of clinical practice guidelines: a proposal from the Conference on Guideline Standardization. Ann Intern Med 2003;139:493–498. [DOI] [PubMed] [Google Scholar]
- 7.Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 2015;62:932–954. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep 1998;47:1–39. [PubMed] [Google Scholar]
- 9.Kuncio DE, Newbern EC, Fernandez-Vina MH, Herdman B, Johnson CC, Viner KM. Comparison of risk-based hepatitis C screening and the true seroprevalence in an urban prison system. J Urban Health 2015;92:379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waruingi W, Mhanna MJ, Kumar D, Abughali N. Hepatitis C Virus universal screening versus risk based selective screening during pregnancy. J Neonatal Perinatal Med 2015;8:371–378. [DOI] [PubMed] [Google Scholar]
- 11.Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001–2008. Hepatology 2012;55:1652–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology 2010;51:729–733. [DOI] [PubMed] [Google Scholar]
- 13.Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, Holmberg SD. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med 2014;160:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moyer VA. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2013;159:349–357. [DOI] [PubMed] [Google Scholar]
- 15.Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Teo CG, Jewett A, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep 2012;61:1–32. [PubMed] [Google Scholar]
- 16.Rosenberg ES, Rosenthal EM, Hall EW, Barker L, Hofmeister MG, Sullivan PS, Dietz P, et al. Prevalence of hepatitis C virus infection in US states and the District of Columbia, 2013 to 2016. JAMA Netw Open 2018;1:e186371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zibbell JE, Asher AK, Patel RC, Kupronis B, Iqbal K, Ward JW, Holtzman D. Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. Am J Public Health 2018;108:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ly KN, Jiles RB, Teshale EH, Foster MA, Pesano RL, Holmberg SD. Hepatitis C virus infection among reproductive-aged women and children in the United States, 2006 to 2014. Ann Intern Med 2017;166:775–782. [DOI] [PubMed] [Google Scholar]
- 19.Suryaprasad AG, White JZ, Xu F, Eichler BA, Hamilton J, Patel A, Hamdounia SB, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006–2012. Clin Infect Dis 2014;59:1411–1419. [DOI] [PubMed] [Google Scholar]
- 20.Eckman MH, Ward JW, Sherman KE. Cost effectiveness of universal screening for hepatitis C virus infection in the era of direct-acting, pangenotypic treatment regimens. Clin Gastroenterol Hepatol 2019;17:930–939.e939. [DOI] [PubMed] [Google Scholar]
- 21.Buti M, Dominguez-Hernandez R, Casado MA, Sabater E, Esteban R. Healthcare value of implementing hepatitis C screening in the adult general population in Spain. PLoS One 2018;13:e0208036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong WWL, Tu HA, Feld JJ, Wong T, Krahn M. Cost-effectiveness of screening for hepatitis C in Canada. Cmaj 2015;187:E110–e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coffin PO, Scott JD, Golden MR, Sullivan SD. Cost-effectiveness and population outcomes of general population screening for hepatitis C. Clin Infect Dis 2012;54:1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh YH, Rothman RE, Laeyendecker OB, Kelen GD, Avornu A, Patel EU, Kim J, et al. Evaluation of the Centers for Disease Control and Prevention recommendations for hepatitis C virus testing in an urban emergency department. Clin Infect Dis 2016;62:1059–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyons MS, Kunnathur VA, Rouster SD, Hart KW, Sperling MI, Fichtenbaum CJ, Sherman KE. Prevalence of diagnosed and undiagnosed hepatitis C in a midwestern urban emergency department. Clin Infect Dis 2016;62:1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barocas JA, Tasillo A, Eftekhari Yazdi G, Wang J, Vellozzi C, Hariri S, Isenhour C, et al. Population-level outcomes and cost-effectiveness of expanding the recommendation for age-based hepatitis C testing in the United States. Clin Infect Dis 2018;67:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He T, Li K, Roberts MS, Spaulding AC, Ayer T, Grefenstette JJ, Chhatwal J. Prevention of hepatitis C by screening and treatment in U.S. prisons. Ann Intern Med 2016;164:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaillon A, Rand EB, Reau N, Martin NK. Cost-effectiveness of universal hepatitis C virus screening of pregnant women in the United States. Clin Infect Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tasillo A, Eftekhari Yazdi G, Nolen S, Schillie S, Vellozzi C, Epstein R, Randall L, et al. Short-term effects and long-term cost-effectiveness of universal hepatitis C testing in prenatal care. Obstet Gynecol 2019;133:289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Assoumou SA, Tasillo A, Leff JA, Schackman BR, Drainoni ML, Horsburgh CR, Barry MA, et al. Cost-effectiveness of one-time hepatitis C screening strategies among adolescents and young adults in primary care settings. Clin Infect Dis 2018;66:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schackman BR, Gutkind S, Morgan JR, Leff JA, Behrends CN, Delucchi KL, McKnight C, et al. Cost-effectiveness of hepatitis C screening and treatment linkage intervention in US methadone maintenance treatment programs. Drug Alcohol Depend 2018;185:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schackman BR, Leff JA, Barter DM, DiLorenzo MA, Feaster DJ, Metsch LR, Freedberg KA, et al. Cost-effectiveness of rapid hepatitis C virus (HCV) testing and simultaneous rapid HCV and HIV testing in substance abuse treatment programs. Addiction 2015;110:129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carrat F, Fontaine H, Dorival C, Simony M, Diallo A, Hezode C, De Ledinghen V, et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet 2019;393:1453–1464. [DOI] [PubMed] [Google Scholar]
- 34.Gerber L, Estep M, Stepanova M, Escheik C, Weinstein A, Younossi ZM. Effects of viral eradication with ledipasvir and sofosbuvir, with or without ribavirin, on measures of fatigue in patients with chronic hepatitis C virus infection. Clin Gastroenterol Hepatol 2016;14:156–164.e153. [DOI] [PubMed] [Google Scholar]
- 35.Younossi ZM, Stepanova M, Henry L, Gane E, Jacobson IM, Lawitz E, Nelson D, et al. Effects of sofosbuvir-based treatment, with and without interferon, on outcome and productivity of patients with chronic hepatitis C. Clin Gastroenterol Hepatol 2014;12:1349–1359.e1313. [DOI] [PubMed] [Google Scholar]
- 36.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med 2013;158:329–337. [DOI] [PubMed] [Google Scholar]
- 37.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, Duarte-Rojo A, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012;308:2584–2593. [DOI] [PubMed] [Google Scholar]
- 38.Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol 2011;9:509–516. [DOI] [PubMed] [Google Scholar]
- 39.Valencia La Rosa J, Ryan P, Alvaro-Meca A, Troya J, Cuevas G, Gutierrez J, Moreno S. HCV seroconversion in a cohort of people who use drugs followed in a mobile harm reduction unit in Madrid: Breaking barriers for HCV elimination. PLoS One 2018;13:e0204795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newsum AM, Stolte IG, van der Meer JT, Schinkel J, van der Valk M, Vanhommerig JW, Buve A, et al. Development and validation of the HCV-MOSAIC risk score to assist testing for acute hepatitis C virus (HCV) infection in HIV-infected men who have sex with men (MSM). Euro Surveill 2017;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vallejo F, Barrio G, Brugal MT, Pulido J, Toro C, Sordo L, Espelt A, et al. High hepatitis C virus prevalence and incidence in a community cohort of young heroin injectors in a context of extensive harm reduction programmes. J Epidemiol Community Health 2015;69:599–603. [DOI] [PubMed] [Google Scholar]
- 42.Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014;58:1–10. [DOI] [PubMed] [Google Scholar]
- 43.Witt MD, Seaberg EC, Darilay A, Young S, Badri S, Rinaldo CR, Jacobson LP, et al. Incident hepatitis C virus infection in men who have sex with men: a prospective cohort analysis, 1984–2011. Clin Infect Dis 2013;57:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bravo MJ, Vallejo F, Barrio G, Brugal MT, Molist G, Pulido J, Sordo L, et al. HCV seroconversion among never-injecting heroin users at baseline: no predictors identified other than starting injection. Int J Drug Policy 2012;23:415–419. [DOI] [PubMed] [Google Scholar]
- 45.Linas BP, Wong AY, Schackman BR, Kim AY, Freedberg KA. Cost-effective screening for acute hepatitis C virus infection in HIV-infected men who have sex with men. Clin Infect Dis 2012;55:279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wandeler G, Gsponer T, Bregenzer A, Gunthard HF, Clerc O, Calmy A, Stockle M, et al. Hepatitis C virus infections in the Swiss HIV Cohort Study: a rapidly evolving epidemic. Clin Infect Dis 2012;55:1408–1416. [DOI] [PubMed] [Google Scholar]
- 47.Williams IT, Bell BP, Kuhnert W, Alter MJ. Incidence and transmission patterns of acute hepatitis C in the United States, 1982–2006. Arch Intern Med 2011;171:242–248. [DOI] [PubMed] [Google Scholar]
- 48.Aronson ID, Bennett A, Marsch LA, Bania TC. Mobile technology to increase HIV/HCV testing and overdose prevention/response among people who inject drugs. Front Public Health 2017;5:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barocas JA, Brennan MB, Hull SJ, Stokes S, Fangman JJ, Westergaard RP. Barriers and facilitators of hepatitis C screening among people who inject drugs: a multi-city, mixed-methods study. Harm Reduct J 2014;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butler K, Day C, Dietze P, Bruno R, Alati R, Burns L. The potential reach of opioid substitution settings to deliver HCV care to people who inject drugs in Australia. J Subst Abuse Treat 2015;58:90–94. [DOI] [PubMed] [Google Scholar]
- 51.Centers for Disease Control and Prevention. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep 2013;62:362–365. [PMC free article] [PubMed] [Google Scholar]
- 52.Alter MJ, Kuhnert WL, Finelli L. Guidelines for laboratory testing and result reporting of antibody to hepatitis C virus. Centers for Disease Control and Prevention. MMWR Recomm Rep 2003;52:1–13, 15. [PubMed] [Google Scholar]
- 53.Chevaliez S, Poiteau L, Rosa I, Soulier A, Roudot-Thoraval F, Laperche S, Hezode C, et al. Prospective assessment of rapid diagnostic tests for the detection of antibodies to hepatitis C virus, a tool for improving access to care. Clin Microbiol Infect 2016;22:459.e451–456. [DOI] [PubMed] [Google Scholar]
- 54.Lee SR, Kardos KW, Schiff E, Berne CA, Mounzer K, Banks AT, Tatum HA, et al. Evaluation of a new, rapid test for detecting HCV infection, suitable for use with blood or oral fluid. J Virol Methods 2011;172:27–31. [DOI] [PubMed] [Google Scholar]
- 55.Pawlotsky JM. Use and interpretation of virological tests for hepatitis C. Hepatology 2002;36:S65–S73. [DOI] [PubMed] [Google Scholar]
- 56.Mera J, Vellozzi C, Hariri S, Carabin H, Drevets DA, Miller A, Reilley B, et al. Identification and clinical management of persons with chronic hepatitis C virus infection - Cherokee Nation, 2012–2015. MMWR Morb Mortal Wkly Rep 2016;65:461–466. [DOI] [PubMed] [Google Scholar]
- 57.Lange B, Roberts T, Cohn J, Greenman J, Camp J, Ishizaki A, Messac L, et al. Diagnostic accuracy of detection and quantification of HBV-DNA and HCV-RNA using dried blood spot (DBS) samples - a systematic review and meta-analysis. BMC Infect Dis 2017;17:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomson EC, Nastouli E, Main J, Karayiannis P, Eliahoo J, Muir D, McClure MO. Delayed anti-HCV antibody response in HIV-positive men acutely infected with HCV. Aids 2009;23:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vermeersch P, Van RM, Lagrou K. Validation of a strategy for HCV antibody testing with two enzyme immunoassays in a routine clinical laboratory. J Clin Virol 2008;42:394–398. [DOI] [PubMed] [Google Scholar]
- 60.Marcellin P, Pequignot F, Delarocque-Astagneau E, Zarski JP, Ganne N, Hillon P, Antona D, et al. Mortality related to chronic hepatitis B and chronic hepatitis C in France: evidence for the role of HIV coinfection and alcohol consumption. J Hepatol 2008;48:200–207. [DOI] [PubMed] [Google Scholar]
- 61.Safdar K, Schiff ER. Alcohol and hepatitis C. Semin Liver Dis 2004;24:305–315. [DOI] [PubMed] [Google Scholar]
- 62.Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, Beasley P, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology 2002;36:1206–1213. [DOI] [PubMed] [Google Scholar]
- 63.Harris DR, Gonin R, Alter HJ, Wright EC, Buskell ZJ, Hollinger FB, Seeff LB. The relationship of acute transfusion-associated hepatitis to the development of cirrhosis in the presence of alcohol abuse. Ann Intern Med 2001;134:120–124. [DOI] [PubMed] [Google Scholar]
- 64.Bellentani S, Pozzato G, Saccoccio G, Crovatto M, Croce LS, Mazzoran L, Masutti F, et al. Clinical course and risk factors of hepatitis C virus related liver disease in the general population: report from the Dionysos study. Gut 1999;44:874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, Vidaud M, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology 1999;30:1054–1058. [DOI] [PubMed] [Google Scholar]
- 66.Corrao G, Arico S. Independent and combined action of hepatitis C virus infection and alcohol consumption on the risk of symptomatic liver cirrhosis. Hepatology 1998;27:914–919. [DOI] [PubMed] [Google Scholar]
- 67.Wiley TE, McCarthy M, Breidi L, McCarthy M, Layden TJ. Impact of alcohol on the histological and clinical progression of hepatitis C infection. Hepatology 1998;28:805–809. [DOI] [PubMed] [Google Scholar]
- 68.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997;349:825–832. [DOI] [PubMed] [Google Scholar]
- 69.Noda K, Yoshihara H, Suzuki K, Yamada Y, Kasahara A, Hayashi N, Fusamoto H, et al. Progression of type C chronic hepatitis to liver cirrhosis and hepatocellular carcinoma--its relationship to alcohol drinking and the age of transfusion. Alcohol Clin Exp Res 1996;20:95A–100A. [DOI] [PubMed] [Google Scholar]
- 70.Hagstrom H Alcohol consumption in concomitant liver disease: how much is too much? Curr Hepatol Rep 2017;16:152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Younossi ZM, Zheng L, Stepanova M, Venkatesan C, Mir HM. Moderate, excessive or heavy alcohol consumption: each is significantly associated with increased mortality in patients with chronic hepatitis C. Aliment Pharmacol Ther 2013;37:703–709. [DOI] [PubMed] [Google Scholar]
- 72.Westin J, Lagging LM, Spak F, Aires N, Svensson E, Lindh M, Dhillon AP, et al. Moderate alcohol intake increases fibrosis progression in untreated patients with hepatitis C virus infection. J Viral Hepat 2002;9:235–241. [DOI] [PubMed] [Google Scholar]
- 73.Tsui JI, Williams EC, Green PK, Berry K, Su F, Ioannou GN. Alcohol use and hepatitis C virus treatment outcomes among patients receiving direct antiviral agents. Drug Alcohol Depend 2016;169:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jin F, Matthews GV, Grulich AE. Sexual transmission of hepatitis C virus among gay and bisexual men: a systematic review. Sex Health 2017;14:28–41. [DOI] [PubMed] [Google Scholar]
- 75.Urbanus AT, van de Laar TJ, Stolte IG, Schinkel J, Heijman T, Coutinho RA, Prins M. Hepatitis C virus infections among HIV-infected men who have sex with men: an expanding epidemic. AIDS 2009;23:F1–F7. [DOI] [PubMed] [Google Scholar]
- 76.van de Laar T, Pybus O, Bruisten S, Brown D, Nelson M, Bhagani S, Vogel M, et al. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology 2009;136:1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fierer DS, Uriel AJ, Carriero DC, Klepper A, Dieterich D, Mullen MP, Thung SN, et al. Liver fibrosis during an outbreak of acute hepatitis C virus infection in HIV-infected men: a prospective cohort study. J Infect Dis 2008;198:683–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- 79.Fontana RJ, Sanyal AJ, Ghany MG, Lee WM, Reid AE, Naishadham D, Everson GT, et al. Factors that determine the development and progression of gastroesophageal varices in patients with chronic hepatitis C. Gastroenterology 2010;138:2321–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, Del Ninno E, et al. The natural history of compensated cirrhosis due to hepatitis C virus: a 17-year cohort study of 214 patients. Hepatology 2006;43:1303–1310. [DOI] [PubMed] [Google Scholar]
- 81.Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med 2013;158:807–820. [DOI] [PubMed] [Google Scholar]
- 82.Rockey DC, Bissell DM. Noninvasive measures of liver fibrosis. Hepatology 2006;43:S113–S120. [DOI] [PubMed] [Google Scholar]
- 83.Stefanescu H, Rusu C, Lupsor-Platon M, Nicoara Farcau O, Fischer P, Grigoras C, Horhat A, et al. Liver stiffness assessed by ultrasound shear wave elastography from General Electric accurately predicts clinically significant portal hypertension in patients with advanced chronic liver disease. Ultraschall Med 2019. [DOI] [PubMed] [Google Scholar]
- 84.Bonder A, Afdhal N. Utilization of FibroScan in clinical practice. Curr Gastroenterol Rep 2014;16:372. [DOI] [PubMed] [Google Scholar]
- 85.Castera L Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology 2012;142:1293–1302. [DOI] [PubMed] [Google Scholar]
- 86.Pol S, Haour G, Fontaine H, Dorival C, Petrov-Sanchez V, Bourliere M, Capeau J, et al. The negative impact of HBV/HCV coinfection on cirrhosis and its consequences. Aliment Pharmacol Ther 2017;46:1054–1060. [DOI] [PubMed] [Google Scholar]
- 87.Puoti M, Lorenzini P, Cozzi-Lepri A, Gori A, Mastroianni C, Rizzardini G, Mazzarello G, et al. Incidence and progression to cirrhosis of new hepatitis C virus infections in persons living with human immunodeficiency virus. Clin Microbiol Infect 2017;23:267.e261–267.e264. [DOI] [PubMed] [Google Scholar]
- 88.Kruse RL, Kramer JR, Tyson GL, Duan Z, Chen L, El-Serag HB, Kanwal F. Clinical outcomes of hepatitis B virus coinfection in a United States cohort of hepatitis C virus-infected patients. Hepatology 2014;60:1871–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS 2008;22:1979–1991. [DOI] [PubMed] [Google Scholar]
- 90.Zarski JP, Bohn B, Bastie A, Pawlotsky JM, Baud M, Bost-Bezeaux F, Tran van Nhieu J, et al. Characteristics of patients with dual infection by hepatitis B and C viruses. J Hepatol 1998;28:27–33. [DOI] [PubMed] [Google Scholar]
- 91.Lee SW, Lee TY, Yang SS, Peng YC, Yeh HZ, Chang CS. Prevalence of hepatitis B reactivation among Chinese individuals with chronic hepatitis C treated with pan-oral direct-acting antivirals. Gastroenterology Res 2018;11:124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mucke MM, Backus LI, Mucke VT, Coppola N, Preda CM, Yeh ML, Tang LSY, et al. Hepatitis B virus reactivation during direct-acting antiviral therapy for hepatitis C: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2018;3:172–180. [DOI] [PubMed] [Google Scholar]
- 93.Prevention CfDCa. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012;61:816–819. [PubMed] [Google Scholar]
- 94.World Health Organization. Global Hepatitis Report 2017. Geneva: World Health Organization, 2017:viii. [Google Scholar]
- 95.Butt AA, Yan P, Lo Re Iii V, Shaikh OS, Ross DB. Trends in treatment uptake and provider specialty for hepatitis C virus (HCV) infection in the Veterans Affairs Healthcare System: results from the Eeectronically retrieved cohort of HCV-infected veterans (ERCHIVES). Clin Infect Dis 2019;68:857–859. [DOI] [PubMed] [Google Scholar]
- 96.World Health Organization. Progress Report on Access to Hepatitis C Treatment: Focus on Overcoming Barriers in Low- and Middle-Income Countries, March 2018. Geneva: World Health Organization, 2018:2–30. [Google Scholar]
- 97.Lin M, Kramer J, White D, Cao Y, Tavakoli-Tabasi S, Madu S, Smith D, et al. Barriers to hepatitis C treatment in the era of direct-acting anti-viral agents. Aliment Pharmacol Ther 2017;46:992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee YB, Nam JY, Lee JH, Chang Y, Cho H, Cho YY, Cho EJ, et al. Differential effect of HCV eradication and fibrosis grade on hepatocellular carcinoma and all-cause mortality. Sci Rep 2018;8:13651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bashir MH, Fazili J, Madhoun MF, Kanagala R, Chen S, Nusrat S. Impact of sustained virologic response on short-term clinical outcomes in hepatitis C-related cirrhosis. Eur J Gastroenterol Hepatol 2018;30:296–301. [DOI] [PubMed] [Google Scholar]
- 100.Nahon P, Layese R, Bourcier V, Cagnot C, Marcellin P, Guyader D, Pol S, et al. Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in surveillance programs. Gastroenterology 2018;155:1436–1450.e1436. [DOI] [PubMed] [Google Scholar]
- 101.Janjua NZ, Chong M, Kuo M, Woods R, Wong J, Yoshida EM, Sherman M, et al. Long-term effect of sustained virological response on hepatocellular carcinoma in patients with hepatitis C in Canada. J Hepatol 2017;66:504–513. [DOI] [PubMed] [Google Scholar]
- 102.Nahon P, Bourcier V, Layese R, Audureau E, Cagnot C, Marcellin P, Guyader D, et al. Eradication of hepatitis C virus infection in patients with cirrhosis reduces risk of liver and non-liver complications. Gastroenterology 2017;152:142–156.e142. [DOI] [PubMed] [Google Scholar]
- 103.Prenner SB, VanWagner LB, Flamm SL, Salem R, Lewandowski RJ, Kulik L. Hepatocellular carcinoma decreases the chance of successful hepatitis C virus therapy with direct-acting antivirals. J Hepatol 2017;66:1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Simmons B, Saleem J, Heath K, Cooke GS, Hill A. Long-term treatment outcomes of patients infected with hepatitis C virus: a systematic review and meta-analysis of the survival benefit of achieving a sustained virological response. Clin Infect Dis 2015;61:730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Poynard T, Moussalli J, Munteanu M, Thabut D, Lebray P, Rudler M, Ngo Y, et al. Slow regression of liver fibrosis presumed by repeated biomarkers after virological cure in patients with chronic hepatitis C. J Hepatol 2013;59:675–683. [DOI] [PubMed] [Google Scholar]
- 106.Morgan TR, Ghany MG, Kim HY, Snow KK, Shiffman ML, De Santo JL, Lee WM, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology 2010;52:833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mallet V, Gilgenkrantz H, Serpaggi J, Verkarre V, Vallet-Pichard A, Fontaine H, Pol S. Brief communication: the relationship of regression of cirrhosis to outcome in chronic hepatitis C. Ann Intern Med 2008;149:399–403. [DOI] [PubMed] [Google Scholar]
- 108.Veldt BJ, Heathcote EJ, Wedemeyer H, Reichen J, Hofmann WP, Stefan Z, Manns MP, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med 2007;147:677–684. [DOI] [PubMed] [Google Scholar]
- 109.Metwally MA, Zein CO, Zein NN. Regression of hepatic fibrosis and cirrhosis in patients with chronic hepatitis C treated with interferon-based therapy. Gastroenterology 2003;124:1561. [DOI] [PubMed] [Google Scholar]
- 110.Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, Ling MH, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology 2002;122:1303–1313. [DOI] [PubMed] [Google Scholar]
- 111.Dammacco F, Lauletta G, Russi S, Leone P, Tucci M, Manno C, Monaco S, et al. Clinical practice: hepatitis C virus infection, cryoglobulinemia and cryoglobulinemic vasculitis. Clin Exp Med 2019;19:1–21. [DOI] [PubMed] [Google Scholar]
- 112.Lauletta G, Russi S, Pavone F, Vacca A, Dammacco F. Direct-acting antiviral agents in the therapy of hepatitis C virus-related mixed cryoglobulinaemia: a single-centre experience. Arthritis Res Ther 2017;19:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gragnani L, Visentini M, Fognani E, Urraro T, De Santis A, Petraccia L, Perez M, et al. Prospective study of guideline-tailored therapy with direct-acting antivirals for hepatitis C virus-associated mixed cryoglobulinemia. Hepatology 2016;64:1473–1482. [DOI] [PubMed] [Google Scholar]
- 114.Sise ME, Bloom AK, Wisocky J, Lin MV, Gustafson JL, Lundquist AL, Steele D, et al. Treatment of hepatitis C virus-associated mixed cryoglobulinemia with direct-acting antiviral agents. Hepatology 2016;63:408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fabrizi F, Dixit V, Messa P. Antiviral therapy of symptomatic HCV-associated mixed cryoglobulinemia: meta-analysis of clinical studies. J Med Virol 2013;85:1019–1027. [DOI] [PubMed] [Google Scholar]
- 116.Landau DA, Scerra S, Sene D, Resche-Rigon M, Saadoun D, Cacoub P. Causes and predictive factors of mortality in a cohort of patients with hepatitis C virus-related cryoglobulinemic vasculitis treated with antiviral therapy. J Rheumatol 2010;37:615–621. [DOI] [PubMed] [Google Scholar]
- 117.Hirose S, Yamaji Y, Tsuruya K, Ogawa Y, Miyaoka M, Kagawa T. Rapid regression of B-cell non-Hodgkin’s lymphoma after eradication of hepatitis C virus by direct antiviral agents. Case Rep Gastroenterol 2019;13:336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Peveling-Oberhag J, Bankov K, Dultz G, Ballo O, Lohmeyer J, Brunnberg U, Marcu V, et al. miRNA-26b downregulation in peripheral blood mononuclear cells of patients with hepatitis C associated lymphomas is restored by successful interferon-free antiviral therapy. Antivir Ther 2019. [DOI] [PubMed] [Google Scholar]
- 119.Rattotti S, Ferretti VV, Rusconi C, Rossi A, Fogazzi S, Baldini L, Pioltelli P, et al. Lymphomas associated with chronic hepatitis C virus infection: A prospective multicenter cohort study from the Rete Ematologica Lombarda (REL) clinical network. Hematol Oncol 2019;37:160–167. [DOI] [PubMed] [Google Scholar]
- 120.Arcaini L, Besson C, Frigeni M, Fontaine H, Goldaniga M, Casato M, Visentini M, et al. Interferon-free antiviral treatment in B-cell lymphoproliferative disorders associated with hepatitis C virus infection. Blood 2016;128:2527–2532. [DOI] [PubMed] [Google Scholar]
- 121.Takahashi K, Nishida N, Kawabata H, Haga H, Chiba T. Regression of Hodgkin lymphoma in response to antiviral therapy for hepatitis C virus infection. Intern Med 2012;51:2745–2747. [DOI] [PubMed] [Google Scholar]
- 122.Gisbert JP, Garcia-Buey L, Pajares JM, Moreno-Otero R. Systematic review: regression of lymphoproliferative disorders after treatment for hepatitis C infection. Aliment Pharmacol Ther 2005;21:653–662. [DOI] [PubMed] [Google Scholar]
- 123.Svoboda J, Andreadis C, Downs LH, Miller WT, Tsai DE, Schuster SJ. Regression of advanced non-splenic marginal zone lymphoma after treatment of hepatitis C virus infection. Leuk Lymphoma 2005;46:1365–1368. [DOI] [PubMed] [Google Scholar]
- 124.Hermine O, Lefrere F, Bronowicki JP, Mariette X, Jondeau K, Eclache-Saudreau V, Delmas B, et al. Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N Engl J Med 2002;347:89–94. [DOI] [PubMed] [Google Scholar]
- 125.Mazzaro C, Little D, Pozzato G. Regression of splenic lymphoma after treatment of hepatitis C virus infection. N Engl J Med 2002;347:2168–2170. [DOI] [PubMed] [Google Scholar]
- 126.Boscarino JA, Lu M, Moorman AC, Gordon SC, Rupp LB, Spradling PR, Teshale EH, et al. Predictors of poor mental and physical health status among patients with chronic hepatitis C infection: the Chronic Hepatitis Cohort Study (CHeCS). Hepatology 2015;61:802–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kesen O, Kani HT, Yanartas O, Aykut UE, Gok B, Gunduz F, Yilmaz Y, et al. Evaluation of depression, anxiety and quality of life in hepatitis C patients who treated with direct acting antiviral agents. Turk J Gastroenterol 2019;30:801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Younossi ZM, Stepanova M, Feld J, Zeuzem S, Jacobson I, Agarwal K, Hezode C, et al. Sofosbuvir/velpatasvir improves patient-reported outcomes in HCV patients: results from ASTRAL-1 placebo-controlled trial. J Hepatol 2016;65:33–39. [DOI] [PubMed] [Google Scholar]
- 129.Younossi Z, Henry L. Systematic review: patient-reported outcomes in chronic hepatitis C--the impact of liver disease and new treatment regimens. Aliment Pharmacol Ther 2015;41:497–520. [DOI] [PubMed] [Google Scholar]
- 130.Younossi ZM, Stepanova M, Afdhal N, Kowdley KV, Zeuzem S, Henry L, Hunt SL, et al. Improvement of health-related quality of life and work productivity in chronic hepatitis C patients with early and advanced fibrosis treated with ledipasvir and sofosbuvir. J Hepatol 2015;63:337–345. [DOI] [PubMed] [Google Scholar]
- 131.Mathew A, Peiffer LP, Rhoades K, McGarrity TJ. Improvement in quality of life measures in patients with refractory hepatitis C, responding to re-treatment with pegylated interferon alpha −2b and ribavirin. Health Qual Life Outcomes 2006;4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Coffin PO, Santos GM, Behar E, Hern J, Walker J, Matheson T, Kinnard EN, et al. Randomized feasibility trial of directly observed versus unobserved hepatitis C treatment with ledipasvir-sofosbuvir among people who inject drugs. PLoS One 2019;14:e0217471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Macias J, Morano LE, Tellez F, Granados R, Rivero-Juarez A, Palacios R, Rios M, et al. Response to direct-acting antiviral therapy among ongoing drug users and people receiving opioid substitution therapy. J Hepatol 2019;71:45–51. [DOI] [PubMed] [Google Scholar]
- 134.Radley A, Robinson E, Aspinall EJ, Angus K, Tan L, Dillon JF. A systematic review and meta-analysis of community and primary-care-based hepatitis C testing and treatment services that employ direct acting antiviral drug treatments. BMC Health Serv Res 2019;19:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Grebely J, Dalgard O, Conway B, Cunningham EB, Bruggmann P, Hajarizadeh B, Amin J, et al. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol 2018;3:153–161. [DOI] [PubMed] [Google Scholar]
- 136.Scherz N, Bruggmann P, Brunner N. Direct-acting antiviral therapy for hepatitis C infection among people receiving opioid agonist treatment or heroin assisted treatment. Int J Drug Policy 2018;62:74–77. [DOI] [PubMed] [Google Scholar]
- 137.Norton BL, Fleming J, Bachhuber MA, Steinman M, DeLuca J, Cunningham CO, Johnson N, et al. High HCV cure rates for people who use drugs treated with direct acting antiviral therapy at an urban primary care clinic. Int J Drug Policy 2017;47:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Read P, Lothian R, Chronister K, Gilliver R, Kearley J, Dore GJ, van Beek I. Delivering direct acting antiviral therapy for hepatitis C to highly marginalised and current drug injecting populations in a targeted primary health care setting. Int J Drug Policy 2017;47:209–215. [DOI] [PubMed] [Google Scholar]
- 139.Dore GJ, Altice F, Litwin AH, Dalgard O, Gane EJ, Shibolet O, Luetkemeyer A, et al. Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med 2016;165:625–634. [DOI] [PubMed] [Google Scholar]
- 140.Kattakuzhy S, Gross C, Emmanuel B, Teferi G, Jenkins V, Silk R, Akoth E, et al. Expansion of treatment for hepatitis C virus infection by task shifting to community-based nonspecialist providers: a nonrandomized clinical trial. Ann Intern Med 2017;167:311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Drazilova S, Janicko M, Skladany L, Kristian P, Oltman M, Szantova M, Krkoska D, et al. Glucose metabolism changes in patients with chronic hepatitis C treated with direct acting antivirals. Can J Gastroenterol Hepatol 2018;2018:6095097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Abdel Alem S, Elsharkawy A, Fouad R, Adel E, Abdellatif Z, Musa S, Nagy A, et al. Improvement of glycemic state among responders to sofosbuvir-based treatment regimens: single center experience. J Med Virol 2017;89:2181–2187. [DOI] [PubMed] [Google Scholar]
- 143.Pavone P, Tieghi T, d’Ettorre G, Lichtner M, Marocco R, Mezzaroma I, Passavanti G, et al. Rapid decline of fasting glucose in HCV diabetic patients treated with direct-acting antiviral agents. Clin Microbiol Infect 2016;22:462.e461–463. [DOI] [PubMed] [Google Scholar]
- 144.Soriano V, Barreiro P, de Mendoza C. Hypoglycemia in a diabetic patient during hepatitis C therapy. Hepatology 2016;63:2065–2066. [DOI] [PubMed] [Google Scholar]
- 145.Rindone JP, Mellen CK. Reduction in warfarin effect associated with sofosbuvir-velpatasvir. Am J Health Syst Pharm 2017;74:1308–1311. [DOI] [PubMed] [Google Scholar]
- 146.DeCarolis DD, Westanmo AD, Chen YC, Boese AL, Walquist MA, Rector TS. Evaluation of a potential interaction between new regimens to treat hepatitis C and warfarin. Ann Pharmacother 2016;50:909–917. [DOI] [PubMed] [Google Scholar]
- 147.McGlynn EA, Adams JL, Kramer J, Sahota AK, Silverberg MJ, Shenkman E, Nelson DR. Assessing the safety of direct-acting antiviral agents for hepatitis C. JAMA Netw Open 2019;2:e194765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Asselah T, Lee SS, Yao BB, Nguyen T, Wong F, Mahomed A, Lim SG, et al. Efficacy and safety of glecaprevir/pibrentasvir in patients with chronic hepatitis C virus genotype 5 or 6 infection (ENDURANCE-5,6): an open-label, multicentre, phase 3b trial. Lancet Gastroenterol Hepatol 2019;4:45–51. [DOI] [PubMed] [Google Scholar]
- 149.Flamm S, Mutimer D, Asatryan A, Wang S, Rockstroh J, Horsmans Y, Kwo PY, et al. Glecaprevir/pibrentasvir in patients with chronic HCV genotype 3 infection: an integrated phase 2/3 analysis. J Viral Hepat 2019;26:337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Foster GR, Dore GJ, Wang S, Grebely J, Sherman KE, Baumgarten A, Conway B, et al. Glecaprevir/pibrentasvir in patients with chronic HCV and recent drug use: an integrated analysis of 7 phase III studies. Drug Alcohol Depend 2019;194:487–494. [DOI] [PubMed] [Google Scholar]
- 151.Foster GR, Asselah T, Kopecky-Bromberg S, Lei Y, Asatryan A, Trinh R, Zadeikis N, et al. Safety and efficacy of glecaprevir/pibrentasvir for the treatment of chronic hepatitis C in patients aged 65 years or older. PLoS One 2019;14:e0208506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Naganuma A, Chayama K, Notsumata K, Gane E, Foster GR, Wyles D, Kwo P, et al. Integrated analysis of 8-week glecaprevir/pibrentasvir in Japanese and overseas patients without cirrhosis and with hepatitis C virus genotype 1 or 2 infection. J Gastroenterol 2019;54:752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sterling RK, Zeuzem S, Welzel TM, Manns M, Reddy KR, Terrault N, Ben Ari Z, et al. Safety and efficacy of glecaprevir/pibrentasvir for the treatment of HCV genotype 1–6: results of the HCV-Target Study [Abstract 956]. Gastroenterology 2019;156:S1220. [Google Scholar]
- 154.Asselah T, Kowdley KV, Zadeikis N, Wang S, Hassanein T, Horsmans Y, Colombo M, et al. Efficacy of glecaprevir/pibrentasvir for 8 or 12 weeks in patients with hepatitis C virus genotype 2, 4, 5, or 6 infection without cirrhosis. Clin Gastroenterol Hepatol 2018;16:417–426. [DOI] [PubMed] [Google Scholar]
- 155.Chayama K, Suzuki F, Karino Y, Kawakami Y, Sato K, Atarashi T, Naganuma A, et al. Efficacy and safety of glecaprevir/pibrentasvir in Japanese patients with chronic genotype 1 hepatitis C virus infection with and without cirrhosis. J Gastroenterol 2018;53:557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Puoti M, Foster GR, Wang S, Mutimer D, Gane E, Moreno C, Chang TT, et al. High SVR12 with 8-week and 12-week glecaprevir/pibrentasvir therapy: An integrated analysis of HCV genotype 1–6 patients without cirrhosis. J Hepatol 2018;69:293–300. [DOI] [PubMed] [Google Scholar]
- 157.Zeuzem S, Foster GR, Wang S, Asatryan A, Gane E, Feld JJ, Asselah T, et al. Glecaprevir-pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection. N Engl J Med 2018;378:354–369. [DOI] [PubMed] [Google Scholar]
- 158.Kwo PY, Poordad F, Asatryan A, Wang S, Wyles DL, Hassanein T, Felizarta F, et al. Glecaprevir and pibrentasvir yield high response rates in patients with HCV genotype 1–6 without cirrhosis. J Hepatol 2017;67:263–271. [DOI] [PubMed] [Google Scholar]
- 159.Isakov V, Chulanov V, Abdurakhmanov D, Burnevich E, Nurmukhametova E, Kozhevnikova G, Gankina N, et al. Sofosbuvir/velpatasvir for the treatment of HCV: excellent results from a phase-3, open-label study in Russia and Sweden. Infect Dis (Lond) 2019;51:131–139. [DOI] [PubMed] [Google Scholar]
- 160.Wei L, Lim SG, Xie Q, Van KN, Piratvisuth T, Huang Y, Wu S, et al. Sofosbuvir-velpatasvir for treatment of chronic hepatitis C virus infection in Asia: a single-arm, open-label, phase 3 trial. Lancet Gastroenterol Hepatol 2019;4:127–134. [DOI] [PubMed] [Google Scholar]
- 161.Hezode C, Reau N, Svarovskaia ES, Doehle BP, Shanmugam R, Dvory-Sobol H, Hedskog C, et al. Resistance analysis in patients with genotype 1–6 HCV infection treated with sofosbuvir/velpatasvir in the phase III studies. J Hepatol 2018;68:895–903. [DOI] [PubMed] [Google Scholar]
- 162.Jacobson IM, Lawitz E, Gane EJ, Willems BE, Ruane PJ, Nahass RG, Borgia SM, et al. Efficacy of 8 weeks of sofosbuvir, velpatasvir, and voxilaprevir in patients with chronic HCV infection: 2 phase 3 randomized trials. Gastroenterology 2017;153:113–122. [DOI] [PubMed] [Google Scholar]
- 163.Feld JJ, Jacobson IM, Hezode C, Asselah T, Ruane PJ, Gruener N, Abergel A, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med 2015;373:2599–2607. [DOI] [PubMed] [Google Scholar]
- 164.Foster GR, Afdhal N, Roberts SK, Brau N, Gane EJ, Pianko S, Lawitz E, et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med 2015;373:2608–2617. [DOI] [PubMed] [Google Scholar]
- 165.Berg T, Naumann U, Stoehr A, Sick C, John C, Teuber G, Schiffelholz W, et al. Real-world effectiveness and safety of glecaprevir/pibrentasvir for the treatment of chronic hepatitis C infection: data from the German hepatitis C-registry. Aliment Pharmacol Ther 2019;49:1052–1059. [DOI] [PubMed] [Google Scholar]
- 166.D’Ambrosio R, Pasulo L, Puoti M, Vinci M, Schiavini M, Lazzaroni S, Soria A, et al. Real-world effectiveness and safety of glecaprevir/pibrentasvir in 723 patients with chronic hepatitis C. J Hepatol 2019;70:379–387. [DOI] [PubMed] [Google Scholar]
- 167.Drysdale K, Townley C, Mahomed F, Foster GR. Effectiveness of therapy in 16,567 directly-acting antiviral treated people in England: high response rates in genotype 3 hepatitis C infection regardless of degree of fibrosis, but ribavirin improves response in cirrhosis [Abstract LBO-07]. J Hepatol 2019;70:e131. [Google Scholar]
- 168.Mangia A, Milligan S, Khalili M, Fagiuoli S, Shafran S, Carrat F, Quzan D, et al. Global real world evidence of sofosbuvir/velpatasvir as a simple, effective regimen for the treatment of chronic hepatitis C patients: integrated analysis of 12 clinical practice cohorts [Abstract GS-03]. J Hepatol 2019;70:e2–e3. [Google Scholar]
- 169.Nguyen E, Trinh S, Trinh H, Nguyen H, Nguyen K, Do A, Levitt B, et al. Sustained virologic response rates in patients with chronic hepatitis C genotype 6 treated with ledipasvir+sofosbuvir or sofosbuvir+velpatasvir. Aliment Pharmacol Ther 2019;49:99–106. [DOI] [PubMed] [Google Scholar]
- 170.Wu DB, Jiang W, Wang YH, Chen B, Wang ML, Tao YC, Chen EQ, et al. Safety and efficacy of sofosbuvir-based direct-acting antiviral regimens for hepatitis C virus genotype 6 in Southwest China: real-world experience of a retrospective study. J Viral Hepat 2019;26:316–322. [DOI] [PubMed] [Google Scholar]
- 171.von Felden J, Vermehren J, Ingiliz P, Mauss S, Lutz T, Simon KG, Busch HW, et al. High efficacy of sofosbuvir/velpatasvir and impact of baseline resistance-associated substitutions in hepatitis C genotype 3 infection. Aliment Pharmacol Ther 2018;47:1288–1295. [DOI] [PubMed] [Google Scholar]
- 172.Sarrazin C, Isakov V, Svarovskaia ES, Hedskog C, Martin R, Chodavarapu K, Brainard DM, et al. Late relapse versus hepatitis C virus reinfection in patients with sustained virologic response after sofosbuvir-based therapies. Clin Infect Dis 2017;64:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Simmons B, Saleem J, Hill A, Riley RD, Cooke GS. Risk of late relapse or reinfection with hepatitis C virus after achieving a sustained virological response: a systematic review and meta-analysis. Clin Infect Dis 2016;62:683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Brown RS Jr., Buti M, Rodrigues L, Chulanov V, Chuang WL, Aguilar H, Horvath G, et al. Glecaprevir/pibrentasvir for 8 weeks in treatment-naive patients with chronic HCV genotypes 1–6 and compensated cirrhosis: the EXPEDITION-8 trial. J Hepatol 2019. [DOI] [PubMed] [Google Scholar]
- 175.Rockstroh JK, Lacombe K, Viani RM, Orkin C, Wyles D, Luetkemeyer AF, Soto-Malave R, et al. Efficacy and safety of glecaprevir/pibrentasvir in patients coinfected with hepatitis C virus and human immunodeficiency virus type 1: the EXPEDITION-2 study. Clin Infect Dis 2018;67:1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Forns X, Lee SS, Valdes J, Lens S, Ghalib R, Aguilar H, Felizarta F, et al. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis 2017;17:1062–1068. [DOI] [PubMed] [Google Scholar]
- 177.Kwo PY, Wyles DL, Wang S, Poordad F, Gane E, Maliakkal B, Shiffman ML, et al. 100% SVR4 with ABT-493 and ABT-530 with or without ribavirin in treatment-naive HCV genotype 3-infected patients with cirrhosis. J Hepatol 2016;64:S208. [Google Scholar]
- 178.Esteban R, Pineda JA, Calleja JL, Casado M, Rodriguez M, Turnes J, Morano Amado LE, et al. Efficacy of sofosbuvir and velpatasvir, with and without ribavirin, in patients with hepatitis C virus genotype 3 infection and cirrhosis. Gastroenterology 2018;155:1120–1127.e1124. [DOI] [PubMed] [Google Scholar]
- 179.Asselah T, Bourgeois S, Pianko S, Zeuzem S, Sulkowski M, Foster GR, Han L, et al. Sofosbuvir/velpatasvir in patients with hepatitis C virus genotypes 1–6 and compensated cirrhosis or advanced fibrosis. Liver Int 2018;38:443–450. [DOI] [PubMed] [Google Scholar]
- 180.Younossi ZM, Stepanova M, Jacobson IM, Asselah T, Gane EJ, Lawitz E, Foster GR, et al. Sofosbuvir and velpatasvir with or without voxilaprevir in direct-acting antiviral-naive chronic hepatitis C: patient-reported outcomes from POLARIS 2 and 3. Aliment Pharmacol Ther 2018;47:259–267. [DOI] [PubMed] [Google Scholar]
- 181.Persico M, Aglitti A, Milella M, Coppola C, Messina V, Claar E, Gentile I, et al. Real-life glecaprevir/pibrentasvir in a large cohort of patients with hepatitis C virus infection: the MISTRAL study. Liver Int 2019;39:1852–1859. [DOI] [PubMed] [Google Scholar]
- 182.Ogawa E, Furusyo N, Nakamuta M, Nomura H, Satoh T, Takahashi K, Koyanagi T, et al. Glecaprevir and pibrentasvir for Japanese patients with chronic hepatitis C genotype 1 or 2 infection: Results from a multicenter, real-world cohort study. Hepatol Res 2019;49:617–626. [DOI] [PubMed] [Google Scholar]
- 183.Toyoda H, Atsukawa M, Watanabe T, Nakamuta M, Uojima H, Nozaki A, Takaguchi K, et al. Real-world experience of 12-week DAA regimen of glecaprevir and pibrentasvir in patients with chronic HCV infection. J Gastroenterol Hepatol 2019. [DOI] [PubMed] [Google Scholar]
- 184.Belperio PS, Shahoumian TA, Loomis TP, Mole LA, Backus LI. Real-world effectiveness of daclatasvir plus sofosbuvir and velpatasvir/sofosbuvir in hepatitis C genotype 2 and 3. J Hepatol 2019;70:15–23. [DOI] [PubMed] [Google Scholar]
- 185.Janjua NZ, Darvishian M, Wong S, Yu A, Rossi C, Ramji A, Yoshida EM, et al. Effectiveness of ledipasvir/sofosbuvir and sofosbuvir/velpatasvir in people who inject drugs and/or those in opioid agonist therapy. Hepatol Commun 2019;3:478–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lv DD, Wang ML, Chen EQ, Wu DB, Tao YC, Zhang DM, Tang H. A retrospective study of the efficacy of sofosbuvir plus NS5A inhibitors for patients with hepatitis C virus genotype-2 chronic infection. Eur J Gastroenterol Hepatol 2019;31:382–388. [DOI] [PubMed] [Google Scholar]
- 187.Gayam V, Tiongson B, Khalid M, Mandal AK, Mukhtar O, Gill A, Garlapati P, et al. Sofosbuvir based regimens in the treatment of chronic hepatitis C genotype 1 infection in African-American patients: a community-based retrospective cohort study. Eur J Gastroenterol Hepatol 2018;30:1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liu CH, Huang YJ, Yang SS, Chang CH, Yang SS, Sun HY, Liu CJ, et al. Generic sofosbuvir-based interferon-free direct acting antiviral agents for patients with chronic hepatitis C virus infection: a real-world multicenter observational study. Sci Rep 2018;8:13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tao YC, Deng R, Wang ML, Lv DD, Yuan M, Wang YH, Chen EQ, et al. Satisfactory virological response and fibrosis improvement of sofosbuvir-based regimens for Chinese patients with hepatitis C virus genotype 3 infection: results of a real-world cohort study. Virol J 2018;15:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2017;65:310–335. [DOI] [PubMed] [Google Scholar]
- 191.Indolfi G, Easterbrook P, Dusheiko G, El-Sayed MH, Jonas MM, Thorne C, Bulterys M, et al. Hepatitis C virus infection in children and adolescents. Lancet Gastroenterol Hepatol 2019;4:477–487. [DOI] [PubMed] [Google Scholar]
- 192.Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 2014;61:S45–S57. [DOI] [PubMed] [Google Scholar]
- 193.Jhaveri R, Grant W, Kauf TL, McHutchison J. The burden of hepatitis C virus infection in children: estimated direct medical costs over a 10-year period. J Pediatr 2006;148:353–358. [DOI] [PubMed] [Google Scholar]
- 194.Indolfi G, Azzari C, Resti M. Perinatal transmission of hepatitis C virus. J Pediatr 2013;163:1549–1552.e1541. [DOI] [PubMed] [Google Scholar]
- 195.Jhaveri R, Hashem M, El-Kamary SS, Saleh DA, Sharaf SA, El-Mougy F, Abdelsalam L, et al. Hepatitis C virus (HCV) vertical transmission in 12-month-old infants born to HCV-infected women and assessment of maternal risk factors. Open Forum Infect Dis 2015;2:ofv089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Benova L, Mohamoud YA, Calvert C, Abu-Raddad LJ. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis 2014;59:765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Delotte J, Barjoan EM, Berrebi A, Laffont C, Benos P, Pradier C, Bongain A. Obstetric management does not influence vertical transmission of HCV infection: results of the ALHICE group study. J Matern Fetal Neonatal Med 2014;27:664–670. [DOI] [PubMed] [Google Scholar]
- 198.Cottrell EB, Chou R, Wasson N, Rahman B, Guise JM. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2013;158:109–113. [DOI] [PubMed] [Google Scholar]
- 199.Shebl FM, El-Kamary SS, Saleh DA, Abdel-Hamid M, Mikhail N, Allam A, El-Arabi H, et al. Prospective cohort study of mother-to-infant infection and clearance of hepatitis C in rural Egyptian villages. J Med Virol 2009;81:1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mast EE, Hwang LY, Seto DS, Nolte FS, Nainan OV, Wurtzel H, Alter MJ. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis 2005;192:1880–1889. [DOI] [PubMed] [Google Scholar]
- 201.Ceci O, Margiotta M, Marello F, Francavilla R, Loizzi P, Francavilla A, Mautone A, et al. Vertical transmission of hepatitis C virus in a cohort of 2,447 HIV-seronegative pregnant women: a 24-month prospective study. J Pediatr Gastroenterol Nutr 2001;33:570–575. [DOI] [PubMed] [Google Scholar]
- 202.Epstein RL, Sabharwal V, Wachman EM, Saia KA, Vellozzi C, Hariri S, Linas BP. Perinatal transmission of hepatitis C virus: defining the cascade of care. J Pediatr 2018;203:34–40.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Page K, Leeman L, Bishop S, Cano S, Bakhireva LN. Hepatitis C cascade of care among pregnant women on opioid agonist pharmacotherapy attending a comprehensive prenatal program. Matern Child Health J 2017;21:1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ohmer S, Honegger J. New prospects for the treatment and prevention of hepatitis C in children. Curr Opin Pediatr 2016;28:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nguyen J, Barritt ASt, Jhaveri R. Cost effectiveness of early treatment with direct-acting antiviral therapy in adolescent patients with hepatitis C virus infection. J Pediatr 2019;207:90–96. [DOI] [PubMed] [Google Scholar]
- 206.Aniszewska M, Kowalik-Mikolajewska B, Pokorska-Spiewak M, Marczynska M. [Anti-HCV testing as a basic standard of monitoring HCV mother-to-child infection: advantages and disadvantages of the method]. Przegl Epidemiol 2012;66:341–345. [PubMed] [Google Scholar]
- 207.England K, Pembrey L, Tovo PA, Newell ML. Excluding hepatitis C virus (HCV) infection by serology in young infants of HCV-infected mothers. Acta Paediatr 2005;94:444–450. [DOI] [PubMed] [Google Scholar]
- 208.Garazzino S, Calitri C, Versace A, Alfarano A, Scolfaro C, Bertaina C, Vatrano S, et al. Natural history of vertically acquired HCV infection and associated autoimmune phenomena. Eur J Pediatr 2014;173:1025–1031. [DOI] [PubMed] [Google Scholar]
- 209.Farmand S, Wirth S, Loffler H, Woltering T, Kenzel S, Lainka E, Henneke P. Spontaneous clearance of hepatitis C virus in vertically infected children. Eur J Pediatr 2012;171:253–258. [DOI] [PubMed] [Google Scholar]
- 210.Yeung LT, To T, King SM, Roberts EA. Spontaneous clearance of childhood hepatitis C virus infection. J Viral Hepat 2007;14:797–805. [DOI] [PubMed] [Google Scholar]
- 211.European Paediatric Hepatitis C Virus Network. A significant sex--but not elective cesarean section--effect on mother-to-child transmission of hepatitis C virus infection. J Infect Dis 2005;192:1872–1879. [DOI] [PubMed] [Google Scholar]
- 212.Indolfi G, Mangone G, Bartolini E, Moriondo M, Azzari C, Resti M. Hepatitis C viraemia after apparent spontaneous clearance in a vertically infected child. Lancet 2016;387:1967–1968. [DOI] [PubMed] [Google Scholar]
- 213.Iorio R, Giannattasio A, Sepe A, Terracciano LM, Vecchione R, Vegnente A. Chronic hepatitis C in childhood: an 18-year experience. Clin Infect Dis 2005;41:1431–1437. [DOI] [PubMed] [Google Scholar]
- 214.Vogt M, Lang T, Frosner G, Klingler C, Sendl AF, Zeller A, Wiebecke B, et al. Prevalence and clinical outcome of hepatitis C infection in children who underwent cardiac surgery before the implementation of blood-donor screening. N Engl J Med 1999;341:866–870. [DOI] [PubMed] [Google Scholar]
- 215.Honegger JR, Crim L, Gowda C, Sanchez PJ. Polymerase chain reaction (PCR) for detection of vertically acquired hepatitis C virus (HCV) infection in early infancy [Abstract 2215]. Open Forum Infect Dis 2018;5:S654. [Google Scholar]
- 216.Rodrigue JR, Balistreri W, Haber B, Jonas MM, Mohan P, Molleston JP, Murray KF, et al. Impact of hepatitis C virus infection on children and their caregivers: quality of life, cognitive, and emotional outcomes. J Pediatr Gastroenterol Nutr 2009;48:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nydegger A, Srivastava A, Wake M, Smith AL, Hardikar W. Health-related quality of life in children with hepatitis C acquired in the first year of life. J Gastroenterol Hepatol 2008;23:226–230. [DOI] [PubMed] [Google Scholar]
- 218.Vaux S, Chevaliez S, Saboni L, Sauvage C, Sommen C, Barin F, Alexandre A, et al. Prevalence of hepatitis C infection, screening and associated factors among men who have sex with men attending gay venues: a cross-sectional survey (PREVAGAY), France, 2015. BMC Infect Dis 2019;19:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tieu HV, Laeyendecker O, Nandi V, Rose R, Fernandez R, Lynch B, Hoover DR, et al. Prevalence and mapping of hepatitis C infections among men who have sex with men in New York City. PLoS One 2018;13:e0200269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Schmidt AJ, Falcato L, Zahno B, Burri A, Regenass S, Mullhaupt B, Bruggmann P. Prevalence of hepatitis C in a Swiss sample of men who have sex with men: whom to screen for HCV infection? BMC Public Health 2014;14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.European Paediatric Hepatitis C Virus Network. Three broad modalities in the natural history of vertically acquired hepatitis C virus infection. Clin Infect Dis 2005;41:45–51. [DOI] [PubMed] [Google Scholar]
- 222.Rerksuppaphol S, Hardikar W, Dore GJ. Long-term outcome of vertically acquired and post-transfusion hepatitis C infection in children. J Gastroenterol Hepatol 2004;19:1357–1362. [DOI] [PubMed] [Google Scholar]
- 223.Casiraghi MA, De Paschale M, Romano L, Biffi R, Assi A, Binelli G, Zanetti AR. Long-term outcome (35 years) of hepatitis C after acquisition of infection through mini transfusions of blood given at birth. Hepatology 2004;39:90–96. [DOI] [PubMed] [Google Scholar]
- 224.Nielsen J, Christensen VB, Borgwardt L, Rasmussen A, Ostrup O, Kjaer MS. Prognostic molecular markers in pediatric liver disease - Are there any? Biochim Biophys Acta Mol Basis Dis 2019;1865:577–586. [DOI] [PubMed] [Google Scholar]
- 225.Pokorska-Spiewak M, Kowalik-Mikolajewska B, Aniszewska M, Pluta M, Marczynska M. Clinical usefulness of new noninvasive serum biomarkers for the assessment of liver fibrosis and steatosis in children with chronic hepatitis C. Clin Exp Hepatol 2017;3:198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mack CL, Gonzalez-Peralta RP, Gupta N, Leung D, Narkewicz MR, Roberts EA, Rosenthal P, et al. NASPGHAN practice guidelines: Diagnosis and management of hepatitis C infection in infants, children, and adolescents. J Pediatr Gastroenterol Nutr 2012;54:838–855. [DOI] [PubMed] [Google Scholar]
- 227.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology 2009;49:1017–1044. [DOI] [PubMed] [Google Scholar]
- 228.Schiano TD, Azeem S, Bodian CA, Bodenheimer HC Jr., Merati S, Thung SN, Hytiroglou P. Importance of specimen size in accurate needle liver biopsy evaluation of patients with chronic hepatitis C. Clin Gastroenterol Hepatol 2005;3:930–935. [DOI] [PubMed] [Google Scholar]
- 229.Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003;38:1449–1457. [DOI] [PubMed] [Google Scholar]
- 230.Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002;97:2614–2618. [DOI] [PubMed] [Google Scholar]
- 231.Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology 1994;20:15–20. [PubMed] [Google Scholar]
- 232.Hwang JY, Yoon HM, Kim JR, Lee JS, Jung AY, Kim KM, Cho YA. Diagnostic performance of transient elastography for liver fibrosis in children: a systematic review and meta-analysis. AJR Am J Roentgenol 2018;211:W257–w266. [DOI] [PubMed] [Google Scholar]
- 233.Behairy Bel S, Sira MM, Zalata KR, Salama el SE, Abd-Allah MA. Transient elastography compared to liver biopsy and morphometry for predicting fibrosis in pediatric chronic liver disease: Does etiology matter? World J Gastroenterol 2016;22:4238–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Geng XX, Huang RG, Lin JM, Jiang N, Yang XX. Transient elastography in clinical detection of liver cirrhosis: A systematic review and meta-analysis. Saudi J Gastroenterol 2016;22:294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lewindon PJ, Balouch F, Pereira TN, Puertolas-Lopez MV, Noble C, Wixey JA, Ramm GA. Transient liver elastography in unsedated control children: Impact of age and intercurrent illness. J Paediatr Child Health 2016;52:637–642. [DOI] [PubMed] [Google Scholar]
- 236.Lee CK, Perez-Atayde AR, Mitchell PD, Raza R, Afdhal NH, Jonas MM. Serum biomarkers and transient elastography as predictors of advanced liver fibrosis in a United States cohort: the Boston children’s hospital experience. J Pediatr 2013;163:1058–1064.e1052. [DOI] [PubMed] [Google Scholar]
- 237.Mizuochi T, Takano T, Yanagi T, Ushijima K, Suzuki M, Miyoshi Y, Ito Y, et al. Epidemiologic features of 348 children with hepatitis C virus infection over a 30-year period: a nationwide survey in Japan. J Gastroenterol 2018;53:419–426. [DOI] [PubMed] [Google Scholar]
- 238.Bortolotti F, Verucchi G, Camma C, Cabibbo G, Zancan L, Indolfi G, Giacchino R, et al. Long-term course of chronic hepatitis C in children: from viral clearance to end-stage liver disease. Gastroenterology 2008;134:1900–1907. [DOI] [PubMed] [Google Scholar]
- 239.Resti M, Jara P, Hierro L, Azzari C, Giacchino R, Zuin G, Zancan L, et al. Clinical features and progression of perinatally acquired hepatitis C virus infection. J Med Virol 2003;70:373–377. [DOI] [PubMed] [Google Scholar]
- 240.Delgado-Borrego A, Healey D, Negre B, Christofi M, Sabharwal S, Ludwig DA, Chung RT, et al. Influence of body mass index on outcome of pediatric chronic hepatitis C virus infection. J Pediatr Gastroenterol Nutr 2010;51:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Diagnosis Jhaveri R. and management of hepatitis C virus-infected children. Pediatr Infect Dis J 2011;30:983–985. [DOI] [PubMed] [Google Scholar]
- 242.Goodman ZD, Makhlouf HR, Liu L, Balistreri W, Gonzalez-Peralta RP, Haber B, Jonas MM, et al. Pathology of chronic hepatitis C in children: liver biopsy findings in the Peds-C Trial. Hepatology 2008;47:836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Minola E, Prati D, Suter F, Maggiolo F, Caprioli F, Sonzogni A, Fraquelli M, et al. Age at infection affects the long-term outcome of transfusion-associated chronic hepatitis C. Blood 2002;99:4588–4591. [DOI] [PubMed] [Google Scholar]
- 244.Rumbo C, Fawaz RL, Emre SH, Suchy FJ, Kerkar N, Morotti RA, Shneider BL. Hepatitis C in children: a quaternary referral center perspective. J Pediatr Gastroenterol Nutr 2006;43:209–216. [DOI] [PubMed] [Google Scholar]
- 245.Indolfi G, Guido M, Azzari C, Resti M. Histopathology of hepatitis C in children, a systematic review: implications for treatment. Expert Rev Anti Infect Ther 2015;13:1225–1235. [DOI] [PubMed] [Google Scholar]
- 246.Mohan P, Barton BA, Narkewicz MR, Molleston JP, Gonzalez-Peralta RP, Rosenthal P, Murray KF, et al. Evaluating progression of liver disease from repeat liver biopsies in children with chronic hepatitis C: a retrospective study. Hepatology 2013;58:1580–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Harris HE, Mieli-Vergani G, Kelly D, Davison S, Gibb DM, Ramsay ME. A national sample of individuals who acquired hepatitis C virus infections in childhood or adolescence: risk factors for advanced disease. J Pediatr Gastroenterol Nutr 2007;45:335–341. [DOI] [PubMed] [Google Scholar]
- 248.Castellino S, Lensing S, Riely C, Rai SN, Davila R, Hayden RT, Fleckenstein J, et al. The epidemiology of chronic hepatitis C infection in survivors of childhood cancer: an update of the St Jude Children’s Research Hospital hepatitis C seropositive cohort. Blood 2004;103:2460–2466. [DOI] [PubMed] [Google Scholar]
- 249.Jara P, Resti M, Hierro L, Giacchino R, Barbera C, Zancan L, Crivellaro C, et al. Chronic hepatitis C virus infection in childhood: clinical patterns and evolution in 224 white children. Clin Infect Dis 2003;36:275–280. [DOI] [PubMed] [Google Scholar]
- 250.Badizadegan K, Jonas MM, Ott MJ, Nelson SP, Perez-Atayde AR. Histopathology of the liver in children with chronic hepatitis C viral infection. Hepatology 1998;28:1416–1423. [DOI] [PubMed] [Google Scholar]
- 251.Garcia-Monzon C, Jara P, Fernandez-Bermejo M, Hierro L, Frauca E, Camarena C, Diaz C, et al. Chronic hepatitis C in children: a clinical and immunohistochemical comparative study with adult patients. Hepatology 1998;28:1696–1701. [DOI] [PubMed] [Google Scholar]
- 252.Guido M, Rugge M, Jara P, Hierro L, Giacchino R, Larrauri J, Zancan L, et al. Chronic hepatitis C in children: the pathological and clinical spectrum. Gastroenterology 1998;115:1525–1529. [DOI] [PubMed] [Google Scholar]
- 253.Kage M, Fujisawa T, Shiraki K, Tanaka T, Fujisawa T, Kimura A, Shimamatsu K, et al. Pathology of chronic hepatitis C in children. Child Liver Study Group of Japan. Hepatology 1997;26:771–775. [DOI] [PubMed] [Google Scholar]
- 254.Gonzalez-Peralta RP, Langham MR Jr., Andres JM, Mohan P, Colombani PM, Alford MK, Schwarz KB. Hepatocellular carcinoma in 2 young adolescents with chronic hepatitis C. J Pediatr Gastroenterol Nutr 2009;48:630–635. [DOI] [PubMed] [Google Scholar]
- 255.Strickland DK, Jenkins JJ, Hudson MM. Hepatitis C infection and hepatocellular carcinoma after treatment of childhood cancer. J Pediatr Hematol Oncol 2001;23:527–529. [DOI] [PubMed] [Google Scholar]
- 256.Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Mandorfer M, Kozbial K, Schwabl P, Freissmuth C, Schwarzer R, Stern R, Chromy D, et al. Sustained virologic response to interferon-free therapies ameliorates HCV-induced portal hypertension. J Hepatol 2016;65:692–699. [DOI] [PubMed] [Google Scholar]
- 258.Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, Arat M, et al. Sinusoidal obstruction syndrome/veno-occlusive disease: current situation and perspectives-a position statement from the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant 2015;50:781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kumar S, DeLeve LD, Kamath PS, Tefferi A. Hepatic veno-occlusive disease (sinusoidal obstruction syndrome) after hematopoietic stem cell transplantation. Mayo Clin Proc 2003;78:589–598. [DOI] [PubMed] [Google Scholar]
- 260.Petta S, Camma C, Di Marco V, Macaluso FS, Maida M, Pizzolanti G, Belmonte B, et al. Hepatic steatosis and insulin resistance are associated with severe fibrosis in patients with chronic hepatitis caused by HBV or HCV infection. Liver Int 2011;31:507–515. [DOI] [PubMed] [Google Scholar]
- 261.Cua IH, Hui JM, Kench JG, George J. Genotype-specific interactions of insulin resistance, steatosis, and fibrosis in chronic hepatitis C. Hepatology 2008;48:723–731. [DOI] [PubMed] [Google Scholar]
- 262.Moucari R, Asselah T, Cazals-Hatem D, Voitot H, Boyer N, Ripault MP, Sobesky R, et al. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology 2008;134:416–423. [DOI] [PubMed] [Google Scholar]
- 263.Petta S, Camma C, Di Marco V, Alessi N, Cabibi D, Caldarella R, Licata A, et al. Insulin resistance and diabetes increase fibrosis in the liver of patients with genotype 1 HCV infection. Am J Gastroenterol 2008;103:1136–1144. [DOI] [PubMed] [Google Scholar]
- 264.Lo Iacono O, Venezia G, Petta S, Mineo C, De Lisi S, Di Marco V, Rodolico V, et al. The impact of insulin resistance, serum adipocytokines and visceral obesity on steatosis and fibrosis in patients with chronic hepatitis C. Aliment Pharmacol Ther 2007;25:1181–1191. [DOI] [PubMed] [Google Scholar]
- 265.Svegliati-Baroni G, Bugianesi E, Bouserhal T, Marini F, Ridolfi F, Tarsetti F, Ancarani F, et al. Post-load insulin resistance is an independent predictor of hepatic fibrosis in virus C chronic hepatitis and in non-alcoholic fatty liver disease. Gut 2007;56:1296–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Bugianesi E, Marchesini G, Gentilcore E, Cua IH, Vanni E, Rizzetto M, George J. Fibrosis in genotype 3 chronic hepatitis C and nonalcoholic fatty liver disease: Role of insulin resistance and hepatic steatosis. Hepatology 2006;44:1648–1655. [DOI] [PubMed] [Google Scholar]
- 267.Taura N, Ichikawa T, Hamasaki K, Nakao K, Nishimura D, Goto T, Fukuta M, et al. Association between liver fibrosis and insulin sensitivity in chronic hepatitis C patients. Am J Gastroenterol 2006;101:2752–2759. [DOI] [PubMed] [Google Scholar]
- 268.D’Souza R, Sabin CA, Foster GR. Insulin resistance plays a significant role in liver fibrosis in chronic hepatitis C and in the response to antiviral therapy. Am J Gastroenterol 2005;100:1509–1515. [DOI] [PubMed] [Google Scholar]
- 269.Fartoux L, Poujol-Robert A, Guechot J, Wendum D, Poupon R, Serfaty L. Insulin resistance is a cause of steatosis and fibrosis progression in chronic hepatitis C. Gut 2005;54:1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Muzzi A, Leandro G, Rubbia-Brandt L, James R, Keiser O, Malinverni R, Dufour JF, et al. Insulin resistance is associated with liver fibrosis in non-diabetic chronic hepatitis C patients. J Hepatol 2005;42:41–46. [DOI] [PubMed] [Google Scholar]
- 271.Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, McCaughan GW, et al. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression [corrected]. Gastroenterology 2003;125:1695–1704. [DOI] [PubMed] [Google Scholar]
- 272.Nolen LD, O’Malley JC, Seeman SS, Bruden DJT, Apostolou A, McMahon BJ, Bruce MG. Hepatitis C in pregnant American Indian and Alaska native women; 2003–2015. Int J Circumpolar Health 2019;78:1608139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Watts T, Stockman L, Martin J, Guilfoyle S, Vergeront JM. Increased risk for mother-to-infant transmission of hepatitis C virus among Medicaid recipients - Wisconsin, 2011–2015. MMWR Morb Mortal Wkly Rep 2017;66:1136–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Koneru A, Nelson N, Hariri S, Canary L, Sanders KJ, Maxwell JF, Huang X, et al. Increased hepatitis C virus (HCV) detection in women of childbearing age and potential risk for vertical transmission - United States and Kentucky, 2011–2014. MMWR Morb Mortal Wkly Rep 2016;65:705–710. [DOI] [PubMed] [Google Scholar]
- 275.Kuncio DE, Newbern EC, Johnson CC, Viner KM. Failure to test and identify perinatally infected children born to hepatitis C virus-infected women. Clin Infect Dis 2016;62:980–985. [DOI] [PubMed] [Google Scholar]
- 276.Jonas MM, Squires RH, Rhee SM, Lin CW, Bessho K, Feiterna-Sperling C, Hierro L, et al. Pharmacokinetics, safety, and efficacy of glecaprevir/pibrentasvir in adolescents with chronic hepatitis C virus: part 1 of the DORA study. Hepatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Kwo P, Gane EJ, Peng CY, Pearlman B, Vierling JM, Serfaty L, Buti M, et al. Effectiveness of elbasvir and grazoprevir combination, with or without ribavirin, for treatment-experienced patients with chronic hepatitis C infection. Gastroenterology 2017;152:164–175.e164. [DOI] [PubMed] [Google Scholar]
- 278.Zeuzem S, Feld J, Wang S, Bourliere M, Wedemeyer H, Gane E, Flisiak R, et al. ENDURANCE-1: efficacy and safety of 8- versus 12-week treatment with ABT-493/ABT-530 in patients with chronic HCV genotype 1 infection. In: 67th Annual Meeting of the American Association for the Study of Liver Diseases; 2016 November 11–15, 2016; Boston, MA; 2016. [Google Scholar]
- 279.Asselah T, Reesink H, Gerstoft J, de Ledinghen V, Pockros PJ, Robertson M, Hwang P, et al. Efficacy of elbasvir and grazoprevir in participants with hepatitis C virus genotype 4 infection: A pooled analysis. Liver Int 2018;38:1583–1591. [DOI] [PubMed] [Google Scholar]
- 280.Wyles D, Poordad F, Wang S, Alric L, Felizarta F, Kwo PY, Maliakkal B, et al. Glecaprevir/pibrentasvir for hepatitis C virus genotype 3 patients with cirrhosis and/or prior treatment experience: a partially randomized phase 3 clinical trial. Hepatology 2018;67:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Schwarz KB, Rosenthal P, Murray KF, Honegger JR, Hardikar W, Hague R, Mittal N, et al. Ledipasvir-sofosbuvir for 12 weeks in children 3 to <6 years old with chronic hepatitis C. Hepatology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Murray KF, Balistreri WF, Bansal S, Whitworth S, Evans HM, Gonzalez-Peralta RP, Wen J, et al. Safety and efficacy of ledipasvir-sofosbuvir with or without ribavirin for chronic hepatitis C in children ages 6–11. Hepatology 2018;68:2158–2166. [DOI] [PubMed] [Google Scholar]
- 283.Balistreri WF, Murray KF, Rosenthal P, Bansal S, Lin CH, Kersey K, Massetto B, et al. The safety and effectiveness of ledipasvir-sofosbuvir in adolescents 12–17 years old with hepatitis C virus genotype 1 infection. Hepatology 2017;66:371–378. [DOI] [PubMed] [Google Scholar]
- 284.El-Araby HA, Behairy BE, El-Guindi MA, Adawy NM, Allam AA, Sira AM, Khedr MA, et al. Generic sofosbuvir/ledipasvir for the treatment of genotype 4 chronic hepatitis C in Egyptian children (9–12 years) and adolescents. Hepatol Int 2019. [DOI] [PubMed] [Google Scholar]
- 285.El-Karaksy H, Mogahed EA, Abdullatif H, Ghobrial C, El-Raziky MS, El-Koofy N, El-Shabrawi M, et al. Sustained viral response in genotype 4 chronic hepatitis C virus-infected children and adolescents treated with sofosbuvir/ledipasvir. J Pediatr Gastroenterol Nutr 2018;67:626–630. [DOI] [PubMed] [Google Scholar]
- 286.Rosenthal P, Schwarz KB, Gonzalez-Peralta RP, Lin CH, Kelly DA, Nightingale S, Balistreri WF, et al. Sofosbuvir and ribavirin therapy for children aged 3 to <12 years with hepatitis C virus genotype 2 or 3 infection. Hepatology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Wirth S, Rosenthal P, Gonzalez-Peralta RP, Jonas MM, Balistreri WF, Lin CH, Hardikar W, et al. Sofosbuvir and ribavirin in adolescents 12–17 years old with hepatitis C virus genotype 2 or 3 infection. Hepatology 2017;66:1102–1110. [DOI] [PubMed] [Google Scholar]
- 288.Sulkowski MS, Naggie S, Lalezari J, Fessel WJ, Mounzer K, Shuhart M, Luetkemeyer AF, et al. Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection. JAMA 2014;312:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, Illeperuma A, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med 2014;370:1993–2001. [DOI] [PubMed] [Google Scholar]
- 290.Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, Shiffman ML, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med 2013;368:1867–1877. [DOI] [PubMed] [Google Scholar]
- 291.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013;368:1878–1887. [DOI] [PubMed] [Google Scholar]
- 292.Jonas MM, Romero R, Sokal EM, Rosenthal P, Verucchi G, Lin C, Wen J, et al. Safety and efficacy of sofosbuvir/velpatasvir in pediatric patients 6 to <18 years old with chronic hepatitis C infection [abstract 748]. The Liver Meeting. Boston, Massachusetts; 2019. [Google Scholar]
- 293.Jonas MM, Lon HK, Rhee S, Gilmour SM, Gonzalez-Peralta RP, Leung D, Ling SC, et al. Pharmacokinetics of glecaprevir/pibrentasvir in children with chronic HCV infection: interim analysis of part 2 of the DORA study [abstract 1551]. The Liver Meeting. Boston, Massachusetts; 2019. [Google Scholar]
- 294.Chen G, Wang C, Chen J, Ji D, Wang Y, Wu V, Karlberg J, et al. Hepatitis B reactivation in hepatitis B and C coinfected patients treated with antiviral agents: a systematic review and meta-analysis. Hepatology 2017;66:13–26. [DOI] [PubMed] [Google Scholar]
- 295.Bersoff-Matcha SJ, Cao K, Jason M, Ajao A, Jones SC, Meyer T, Brinker A. Hepatitis B Virus reactivation associated with direct-acting antiviral therapy for chronic hepatitis C virus: a review of cases reported to the US Food and Drug Administration adverse event reporting system. Ann Intern Med 2017;166:792–798. [DOI] [PubMed] [Google Scholar]
- 296.Loomba R, Rivera MM, McBurney R, Park Y, Haynes-Williams V, Rehermann B, Alter HJ, et al. The natural history of acute hepatitis C: clinical presentation, laboratory findings and treatment outcomes. Aliment Pharmacol Ther 2011;33:559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Tracy B, Shrestha R, Stein L, Bhasin D, Pollinger H, Rubin RA. Liver transplantation for fulminant genotype 2a/c hepatitis C virus marked by a rapid recurrence followed by cure. Transpl Infect Dis 2017;19. [DOI] [PubMed] [Google Scholar]
- 298.Farci P, Alter HJ, Shimoda A, Govindarajan S, Cheung LC, Melpolder JC, Sacher RA, et al. Hepatitis C virus-associated fulminant hepatic failure. N Engl J Med 1996;335:631–634. [DOI] [PubMed] [Google Scholar]
- 299.Centers for Disease Control and Prevention. Viral Hepatitis Surveillance United States, 2017. Atlanta: Centers for Disease Control and Prevention, 2019:3. [Google Scholar]
- 300.Hajarizadeh B, Grady B, Page K, Kim AY, McGovern BH, Cox AL, Rice TM, et al. Patterns of hepatitis C virus RNA levels during acute infection: the InC3 study. PLoS One 2015;10:e0122232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Hajarizadeh B, Grebely J, Applegate T, Matthews GV, Amin J, Petoumenos K, Hellard M, et al. Dynamics of HCV RNA levels during acute hepatitis C virus infection. J Med Virol 2014;86:1722–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Hisada M, O’Brien TR, Rosenberg PS, Goedert JJ. Virus load and risk of heterosexual transmission of human immunodeficiency virus and hepatitis C virus by men with hemophilia. The Multicenter Hemophilia Cohort Study. J Infect Dis 2000;181:1475–1478. [DOI] [PubMed] [Google Scholar]
- 303.Popping S, Hullegie SJ, Boerekamps A, Rijnders BJA, de Knegt RJ, Rockstroh JK, Verbon A, et al. Early treatment of acute hepatitis C infection is cost-effective in HIV-infected men-who-have-sex-with-men. PLoS One 2019;14:e0210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Bethea ED, Chen Q, Hur C, Chung RT, Chhatwal J. Should we treat acute hepatitis C? A decision and cost-effectiveness analysis. Hepatology 2018;67:837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Boerekamps A, van den Berk GE, Lauw FN, Leyten EM, van Kasteren ME, van Eeden A, Posthouwer D, et al. Declining hepatitis C virus (HCV) incidence in Dutch human immunodeficiency virus-positive men who have sex with men after unrestricted access to HCV therapy. Clin Infect Dis 2018;66:1360–1365. [DOI] [PubMed] [Google Scholar]
- 306.Busch MP, Murthy KK, Kleinman SH, Hirschkorn DF, Herring BL, Delwart EL, Racanelli V, et al. Infectivity in chimpanzees (Pan troglodytes) of plasma collected before HCV RNA detectability by FDA-licensed assays: implications for transfusion safety and HCV infection outcomes. Blood 2012;119:6326–6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Glynn SA, Wright DJ, Kleinman SH, Hirschkorn D, Tu Y, Heldebrant C, Smith R, et al. Dynamics of viremia in early hepatitis C virus infection. Transfusion 2005;45:994–1002. [DOI] [PubMed] [Google Scholar]
- 308.Hoofnagle JH. Hepatitis C: the clinical spectrum of disease. Hepatology 1997;26:15s–20s. [DOI] [PubMed] [Google Scholar]
- 309.Barrera JM, Bruguera M, Ercilla MG, Gil C, Celis R, Gil MP, del Valle Onorato M, et al. Persistent hepatitis C viremia after acute self-limiting posttransfusion hepatitis C. Hepatology 1995;21:639–644. [PubMed] [Google Scholar]
- 310.Public Health Service US. Updated U.S. Public Health Service guidelines for the management of cccupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep 2001;50:1–52. [PubMed] [Google Scholar]
- 311.Alter MJ, Margolis HS, Krawczynski K, Judson FN, Mares A, Alexander WJ, Hu PY, et al. The natural history of community-acquired hepatitis C in the United States. The Sentinel counties chronic non-A, non-B hepatitis study team. N Engl J Med 1992;327:1899–1905. [DOI] [PubMed] [Google Scholar]
- 312.Cox AL, Netski DM, Mosbruger T, Sherman SG, Strathdee S, Ompad D, Vlahov D, et al. Prospective evaluation of community-acquired acute-phase hepatitis C virus infection. Clin Infect Dis 2005;40:951–958. [DOI] [PubMed] [Google Scholar]
- 313.Vanhommerig JW, Thomas XV, van der Meer JT, Geskus RB, Bruisten SM, Molenkamp R, Prins M, et al. Hepatitis C virus (HCV) antibody dynamics following acute HCV infection and reinfection among HIV-infected men who have sex with men. Clin Infect Dis 2014;59:1678–1685. [DOI] [PubMed] [Google Scholar]
- 314.Chamot E, Hirschel B, Wintsch J, Robert CF, Gabriel V, Deglon JJ, Yerly S, et al. Loss of antibodies against hepatitis C virus in HIV-seropositive intravenous drug users. AIDS 1990;4:1275–1277. [DOI] [PubMed] [Google Scholar]
- 315.Kim AY, Nagami EH, Birch CE, Bowen MJ, Lauer GM, McGovern BH. A simple strategy to identify acute hepatitis C virus infection among newly incarcerated injection drug users. Hepatology 2013;57:944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Blackard JT, Shata MT, Shire NJ, Sherman KE. Acute hepatitis C virus infection: a chronic problem. Hepatology 2008;47:321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.McGovern BH, Birch CE, Bowen MJ, Reyor LL, Nagami EH, Chung RT, Kim AY. Improving the diagnosis of acute hepatitis C virus infection with expanded viral load criteria. Clin Infect Dis 2009;49:1051–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Kushner T, Serper M, Kaplan DE. Delta hepatitis within the Veterans Affairs medical system in the United States: prevalence, risk factors, and outcomes. J Hepatol 2015;63:586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Salazar-Vizcaya L, Wandeler G, Fehr J, Braun D, Cavassini M, Stoeckle M, Bernasconi E, et al. Impact of direct-acting antivirals on the burden of HCV infection among persons who inject drugs and men who have sex with men in the Swiss HIV cohort study. Open Forum Infect Dis 2018;5:ofy154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Martin NK, Thornton A, Hickman M, Sabin C, Nelson M, Cooke GS, Martin TCS, et al. Can hepatitis C virus (HCV) direct-acting antiviral treatment as prevention reverse the HCV epidemic among men who have sex with men in the United Kingdom? Epidemiological and modeling insights. Clin Infect Dis 2016;62:1072–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Martin NK, Hickman M, Hutchinson SJ, Goldberg DJ, Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis 2013;57:S39–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Martin NK, Vickerman P, Grebely J, Hellard M, Hutchinson SJ, Lima VD, Foster GR, et al. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology 2013;58:1598–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Artenie AA, Zang G, Daniel M, Fortier E, Jutras-Aswad D, Puzhko S, Bruneau J. Short-term injection drug use changes following hepatitis C virus (HCV) assessment and treatment among persons who inject drugs with acute HCV infection. Int J Drug Policy 2017;47:239–243. [DOI] [PubMed] [Google Scholar]
- 324.Bruneau J, Zang G, Abrahamowicz M, Jutras-Aswad D, Daniel M, Roy E. Sustained drug use changes after hepatitis C screening and counseling among recently infected persons who inject drugs: a longitudinal study. Clin Infect Dis 2014;58:755–761. [DOI] [PubMed] [Google Scholar]
- 325.Litwin AH, Harris KA Jr., Nahvi S, Zamor PJ, Soloway IJ, Tenore PL, Kaswan D, et al. Successful treatment of chronic hepatitis C with pegylated interferon in combination with ribavirin in a methadone maintenance treatment program. J Subst Abuse Treat 2009;37:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Strathdee SA, Latka M, Campbell J, O’Driscoll PT, Golub ET, Kapadia F, Pollini RA, et al. Factors associated with interest in initiating treatment for hepatitis C Virus (HCV) infection among young HCV-infected injection drug users. Clin Infect Dis 2005;40:S304–S312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Proeschold-Bell RJ, Patkar AA, Naggie S, Coward L, Mannelli P, Yao J, Bixby P, et al. An integrated alcohol abuse and medical treatment model for patients with hepatitis C. Dig Dis Sci 2012;57:1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Dieperink E, Ho SB, Heit S, Durfee JM, Thuras P, Willenbring ML. Significant reductions in drinking following brief alcohol treatment provided in a hepatitis C clinic. Psychosomatics 2010;51:149–156. [DOI] [PubMed] [Google Scholar]
- 329.Whitlock EP, Polen MR, Green CA, Orleans T, Klein J. Behavioral counseling interventions in primary care to reduce risky/harmful alcohol use by adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2004;140:557–568. [DOI] [PubMed] [Google Scholar]
- 330.Kamal SM. Acute hepatitis C: a systematic review. Am J Gastroenterol 2008;103:1283–1297. [DOI] [PubMed] [Google Scholar]
- 331.Grebely J, Page K, Sacks-Davis R, van der Loeff MS, Rice TM, Bruneau J, Morris MD, et al. The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology 2014;59:109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Page K, Hahn JA, Evans J, Shiboski S, Lum P, Delwart E, Tobler L, et al. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J Infect Dis 2009;200:1216–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Mosley JW, Operskalski EA, Tobler LH, Buskell ZJ, Andrews WW, Phelps B, Dockter J, et al. The course of hepatitis C viraemia in transfusion recipients prior to availability of antiviral therapy. J Viral Hepat 2008;15:120–128. [DOI] [PubMed] [Google Scholar]
- 334.Villano SA, Vlahov D, Nelson KE, Cohn S, Thomas DL. Persistence of viremia and the importance of long-term follow-up after acute hepatitis C infection. Hepatology 1999;29:908–914. [DOI] [PubMed] [Google Scholar]
- 335.Naggie S, Fierer DS, Hughes MD, Kim AY, Luetkemeyer A, Vu V, Roa J, et al. Ledipasvir/sofosbuvir for 8 weeks to treat acute hepatitis C virus infections in men with human immunodeficiency virus infections: sofosbuvir-containing regimens without iInterferon for treatment of acute HCV in HIV-1 infected individuals. Clin Infect Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Deterding K, Spinner CD, Schott E, Welzel TM, Gerken G, Klinker H, Spengler U, et al. Ledipasvir plus sofosbuvir fixed-dose combination for 6 weeks in patients with acute hepatitis C virus genotype 1 monoinfection (HepNet Acute HCV IV): an open-label, single-arm, phase 2 study. Lancet Infect Dis 2017;17:215–222. [DOI] [PubMed] [Google Scholar]
- 337.Rockstroh JK, Bhagani S, Hyland RH, Yun C, Dvory-Sobol H, Zheng W, Brainard DM, et al. Ledipasvir-sofosbuvir for 6 weeks to treat acute hepatitis C virus genotype 1 or 4 infection in patients with HIV coinfection: an open-label, single-arm trial. Lancet Gastroenterol Hepatol 2017;2:347–353. [DOI] [PubMed] [Google Scholar]
- 338.Matthews GV, Bhagani S, Van Der Valk M, Rockstroh J, Kim AY, Thurnheer C, Feld JJ, et al. Short course duration sofosburiv/velpatasvir is inferior to standard duration therapy in the treatment of recently acquired HCV infection: results from REACT study [abstract LP2]. The Liver Meeting. Boston, Massachusetts; 2019. [Google Scholar]
- 339.Organ Procurement and Transplantation Network, National Data. US Department of Health and Human Services website. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/. Accessed October 27, 2019.
- 340.Cotter TG, Paul S, Sandikci B, Couri T, Bodzin AS, Little EC, Sundaram V, et al. Increasing utilization and excellent initial outcomes following liver transplant of hepatitis C virus (HCV)-viremic donors into HCV-negative recipients: outcomes following liver transplant of HCV-viremic donors. Hepatology 2019;69:2381–2395. [DOI] [PubMed] [Google Scholar]
- 341.Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants - United States, 2015–2016. MMWR Morb Mortal Wkly Rep 2018;67:349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Goldberg DS, Blumberg E, McCauley M, Abt P, Levine M. Improving organ utilization to help overcome the tragedies of the opioid epidemic. Am J Transplant 2016;16:2836–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Potluri VS, Goldberg DS, Mohan S, Bloom RD, Sawinski D, Abt PL, Blumberg EA, et al. National trends in utilization and 1-year outcomes with transplantation of HCV-viremic kidneys. J Am Soc Nephrol 2019;30:1939–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Bowring MG, Kucirka LM, Massie AB, Ishaque T, Bae S, Shaffer AA, Garonzik Wang J, et al. Changes in utilization and discard of HCV antibody-positive deceased donor kidneys in the era of direct-acting antiviral therapy. Transplantation 2018;102:2088–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Bhamidimarri KR, Ladino M, Pedraza F, Guerra G, Mattiazzi A, Chen L, Ciancio G, et al. Transplantation of kidneys from hepatitis C-positive donors into hepatitis C virus-infected recipients followed by early initiation of direct acting antiviral therapy: a single-center retrospective study. Transpl Int 2017;30:865–873. [DOI] [PubMed] [Google Scholar]
- 346.Scalea JR, Barth RN, Munivenkatappa R, Philosophe B, Cooper M, Whitlow V, LaMattina JC. Shorter waitlist times and improved graft survivals are observed in patients who accept hepatitis C virus+ renal allografts. Transplantation 2015;99:1192–1196. [DOI] [PubMed] [Google Scholar]
- 347.Sageshima J, Troppmann C, McVicar JP, Santhanakrishnan C, de Mattos AM, Perez RV. Impact of willingness to accept hepatitis C seropositive kidneys among hepatitis C RNA-positive waitlisted patients. Transplantation 2018;102:1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Sawinski D, Forde KA, Lo Re V 3rd, Goldberg DS, Cohen JB, Locke JE, Bloom RD, et al. Mortality and kidney transplantation outcomes among hepatitis C virus-seropositive maintenance dialysis patients: a retrospective cohort study. Am J Kidney Dis 2019;73:815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Shelton BA, Sawinski D, Mehta S, Reed RD, MacLennan PA, Locke JE. Kidney transplantation and waitlist mortality rates among candidates registered as willing to accept a hepatitis C infected kidney. Transpl Infect Dis 2018;20:e12829. [DOI] [PubMed] [Google Scholar]
- 350.Kucirka LM, Peters TG, Segev DL. Impact of donor hepatitis C virus infection status on death and need for liver transplant in hepatitis C virus-positive kidney transplant recipients. Am J Kidney Dis 2012;60:112–120. [DOI] [PubMed] [Google Scholar]
- 351.Bari K, Luckett K, Kaiser T, Diwan T, Cuffy M, Schoech MR, Safdar K, et al. Hepatitis C transmission from seropositive, nonviremic donors to non-hepatitis C liver transplant recipients. Hepatology 2018;67:1673–1682. [DOI] [PubMed] [Google Scholar]
- 352.Selzner N, Berenguer M. Should organs from hepatitis C-positive donors be used in hepatitis C-negative recipients for liver transplantation? Liver Transpl 2018;24:831–840. [DOI] [PubMed] [Google Scholar]
- 353.Levitsky J, Formica RN, Bloom RD, Charlton M, Curry M, Friedewald J, Friedman J, et al. The American Society of Transplantation consensus conference on the use of hepatitis C viremic donors in solid organ transplantation. Am J Transplant 2017;17:2790–2802. [DOI] [PubMed] [Google Scholar]
- 354.Suryaprasad A, Basavaraju SV, Hocevar SN, Theodoropoulos N, Zuckerman RA, Hayden T, Forbi JC, et al. Transmission of hepatitis C virus from organ donors despite nucleic acid test screening. Am J Transplant 2015;15:1827–1835. [DOI] [PubMed] [Google Scholar]
- 355.Seem DL, Lee I, Umscheid CA, Kuehnert MJ. PHS guideline for reducing human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission through organ transplantation. Public Health Rep 2013;128:247–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Karkout KA, Al Sherif S, Hussein Q, Albawardi A, Boobes Y. Possible acute rejection associated with the use of the new anti-hepatitis C virus medications. Avicenna J Med 2019;9:32–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Kwong AJ, Wall A, Melcher M, Wang U, Ahmed A, Subramanian A, Kwo PY. Liver transplantation for hepatitis C virus (HCV) non-viremic recipients with HCV viremic donors. Am J Transplant 2019;19:1380–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Molnar MZ, Nair S, Cseprekal O, Yazawa M, Talwar M, Balaraman V, Podila PSB, et al. Transplantation of kidneys from hepatitis C-infected donors to hepatitis C-negative recipients: single center experience. Am J Transplant 2019. [DOI] [PubMed] [Google Scholar]
- 359.Woolley AE, Singh SK, Goldberg HJ, Mallidi HR, Givertz MM, Mehra MR, Coppolino A, et al. Heart and lung transplants from HCV-infected donors to uninfected recipients. N Engl J Med 2019;380:1606–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Zaky Z, Herlitz L, Augustine J. The impact of direct antiviral therapy for hepatitis C (DAA) on acute rejection and donor specific antibody formation in kidney transplant recipients, evidence from surveillance biopsies. Transplantation 2018;102:S325. [Google Scholar]
- 361.Fernandez I, Munoz-Gomez R, Pascasio JM, Baliellas C, Polanco N, Esforzado N, Arias A, et al. Efficacy and tolerability of interferon-free antiviral therapy in kidney transplant recipients with chronic hepatitis C. J Hepatol 2017;66:718–723. [DOI] [PubMed] [Google Scholar]
- 362.Saxena V, Khungar V, Verna EC, Levitsky J, Brown RS, Jr., Hassan MA, Sulkowski MS, et al. Safety and efficacy of current direct-acting antiviral regimens in kidney and liver transplant recipients with hepatitis C: results from the HCV-TARGET study. Hepatology 2017;66:1090–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Faisal N, Bilodeau M, Aljudaibi B, Hirch G, Yoshida EM, Hussaini T, Ghali MP, et al. Impact of sofosbuvir-based regimens for the treatment of hepatitis C after liver transplant on renal function: results of a Canadian national retrospective study. Exp Clin Transplant 2019;17:59–63. [DOI] [PubMed] [Google Scholar]
- 364.Fernandez-Ruiz M, Polanco N, Garcia-Santiago A, Munoz R, Hernandez AM, Gonzalez E, Mercado VR, et al. Impact of anti-HCV direct antiviral agents on graft function and immunosuppressive drug levels in kidney transplant recipients: a call to attention in the mid-term follow-up in a single-center cohort study. Transpl Int 2018;31:887–899. [DOI] [PubMed] [Google Scholar]
- 365.Dharancy S, Coilly A, Fougerou-Leurent C, Duvoux C, Kamar N, Leroy V, Tran A, et al. Direct-acting antiviral agent-based regimen for HCV recurrence after combined liver-kidney transplantation: results from the ANRS CO23 CUPILT study. Am J Transplant 2017;17:2869–2878. [DOI] [PubMed] [Google Scholar]
- 366.Lubetzky M, Chun S, Joelson A, Coco M, Kamal L, Ajaimy M, Gaglio P, et al. Safety and efficacy of treatment of hepatitis C in kidney transplant recipients with directly acting antiviral agents. Transplantation 2017;101:1704–1710. [DOI] [PubMed] [Google Scholar]
- 367.Nijmeijer BM, Koopsen J, Schinkel J, Prins M, Geijtenbeek TB. Sexually transmitted hepatitis C virus infections: current trends, and recent advances in understanding the spread in men who have sex with men. J Int AIDS Soc 2019;22 Suppl 6:e25348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Medland NA, Chow EP, Bradshaw CS, Read TH, Sasadeusz JJ, Fairley CK. Predictors and incidence of sexually transmitted Hepatitis C virus infection in HIV positive men who have sex with men. BMC Infect Dis 2017;17:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.van Santen DK, van der Helm JJ, Del Amo J, Meyer L, D’Arminio Monforte A, Price M, Beguelin CA, et al. Lack of decline in hepatitis C virus incidence among HIV-positive men who have sex with men during 1990–2014. J Hepatol 2017;67:255–262. [DOI] [PubMed] [Google Scholar]
- 370.Chan DP, Sun HY, Wong HT, Lee SS, Hung CC. Sexually acquired hepatitis C virus infection: a review. Int J Infect Dis 2016;49:47–58. [DOI] [PubMed] [Google Scholar]
- 371.Page EE, Nelson M. Hepatitis C and sex. Clin Med (Lond) 2016;16:189–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Hagan H, Jordan AE, Neurer J, Cleland CM. Incidence of sexually transmitted hepatitis C virus infection in HIV-positive men who have sex with men. Aids 2015;29:2335–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Bethea ED, Gaj K, Gustafson JL, Axtell A, Lebeis T, Schoenike M, Turvey K, et al. Pre-emptive pangenotypic direct acting antiviral therapy in donor HCV-positive to recipient HCV-negative heart transplantation: an open-label study. Lancet Gastroenterol Hepatol 2019;4:771–780. [DOI] [PubMed] [Google Scholar]
- 374.Cypel M, Feld JJ, Galasso M, Pinto Ribeiro RV, Marks N, Kuczynski M, Kumar D, et al. Prevention of viral transmission during lung transplantation with hepatitis C-viraemic donors: an open-label, single-centre, pilot trial. Lancet Respir Med 2019. [DOI] [PubMed] [Google Scholar]
- 375.Kapila N, Al-Khalloufi K, Bejarano PA, Vanatta JM, Zervos XB. Fibrosing cholestatic hepatitis after kidney transplantation from HCV-viremic donors to HCV-negative recipients: A unique complication in the DAA era. Am J Transplant 2019. [DOI] [PubMed] [Google Scholar]
- 376.Martini S, Salizzoni M, David E, Tandoi F, Fonio P, Delsedime L, Strona S, et al. Favorable short-term outcome of hepatitis C virus-positive liver graft with bridging fibrosis: A plea for very early viral eradication. Hepatology 2017;65:2116–2118. [DOI] [PubMed] [Google Scholar]
- 377.Gupta G, Yakubu I, Bhati CS, Zhang Y, Kang L, Patterson JA, Andrews-Joseph A, et al. Ultra-short duration direct acting antiviral prophylaxis to prevent virus transmission from hepatitis C viremic donors to hepatitis C negative kidney transplant recipients. Am J Transplant 2019. [DOI] [PubMed] [Google Scholar]
- 378.Durand CM, Bowring MG, Brown DM, Chattergoon MA, Massaccesi G, Bair N, Wesson R, et al. Direct-acting antiviral prophylaxis in kidney transplantation from hepatitis C virus-infected donors to noninfected recipients: an open-label nonrandomized Trial. Ann Intern Med 2018;168:533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Reese PP, Abt PL, Blumberg EA, Van Deerlin VM, Bloom RD, Potluri VS, Levine M, et al. Twelve-month outcomes after transplant of hepatitis C-infected kidneys into uninfected recipients: a single-group trial. Ann Intern Med 2018;169:273–281. [DOI] [PubMed] [Google Scholar]
- 380.Goldberg DS, Abt PL, Reese PP. Transplanting HCV-infected kidneys into uninfected recipients. N Engl J Med 2017;377:1105. [DOI] [PubMed] [Google Scholar]
- 381.Goldberg DS, Abt PL, Blumberg EA, Van Deerlin VM, Levine M, Reddy KR, Bloom RD, et al. Trial of transplantation of HCV-infected kidneys into uninfected recipients. N Engl J Med 2017;376:2394–2395. [DOI] [PubMed] [Google Scholar]
- 382.Caro L, Wenning L, Guo Z, Fraser IP, Fandozzi C, Talaty J, Panebianco D, et al. Effect of hepatic impairment on the pharmacokinetics of grazoprevir, a hepatitis C virus protease inhibitor. Antimicrob Agents Chemother 2017;61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Frey A, Piras-Straub K, Walker A, Timm J, Gerken G, Herzer K. The influence of immunosuppressants on direct-acting antiviral therapy is dependent on the hepatitis C virus genotype. Transpl Infect Dis 2018;20. [DOI] [PubMed] [Google Scholar]
- 384.Ahmed A, Lutchman GA, Kwo PY. Drug-drug interactions in hepatitis C virus treatment: Do they really matter? Clin Liver Dis (Hoboken) 2017;10:111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Smolders EJ, Pape S, de Kanter CT, van den Berg AP, Drenth JP, Burger DM. Decreased tacrolimus plasma concentrations during HCV therapy: a drug-drug interaction or is there an alternative explanation? Int J Antimicrob Agents 2017;49:379–382. [DOI] [PubMed] [Google Scholar]
- 386.Badri PS, Parikh A, Coakley EP, Ding B, Awni WM, Dutta S, Menon RM. Pharmacokinetics of tacrolimus and cyclosporine in liver transplant recipients receiving 3 direct-acting antivirals as treatment for hepatitis C infection. Ther Drug Monit 2016;38:640–645. [DOI] [PubMed] [Google Scholar]
- 387.Dick TB, Lindberg LS, Ramirez DD, Charlton MR. A clinician’s guide to drug-drug interactions with direct-acting antiviral agents for the treatment of hepatitis C viral infection. Hepatology 2016;63:634–643. [DOI] [PubMed] [Google Scholar]
- 388.Burgess S, Partovi N, Yoshida EM, Erb SR, Azalgara VM, Hussaini T. Drug interactions with direct-acting antivirals for hepatitis C: implications for HIV and transplant patients. Ann Pharmacother 2015;49:674–687. [DOI] [PubMed] [Google Scholar]
- 389.Feng HP, Caro L, Fandozzi CM, Guo Z, Talaty J, Wolford D, Panebianco D, et al. Pharmacokinetic interactions between elbasvir/grazoprevir and immunosuppressant drugs in healthy volunteers. J Clin Pharmacol 2018;58:666–673. [DOI] [PubMed] [Google Scholar]
- 390.Kapila N, Narayanan Menon KV, Al-Khalloufi K, Vanatta JM, Murgas C, Reino D, Ebaid S, et al. HCV NAT positive solid organ allografts transplanted into HCV negative recipients: A real-world experience. Hepatology 2019. [DOI] [PubMed] [Google Scholar]
- 391.Shah AP, Cameron A, Singh P, Frank AM, Fenkel JM. Successful treatment of donor-derived hepatitis C viral infection in three transplant recipients from a donor at increased risk for bloodborne pathogens. Transpl Infect Dis 2017;19. [DOI] [PubMed] [Google Scholar]
- 392.Friebus-Kardash J, Gackler A, Kribben A, Witzke O, Wedemeyer H, Treckmann J, Herzer K, et al. Successful early sofosbuvir-based antiviral treatment after transplantation of kidneys from HCV-viremic donors into HCV-negative recipients. Transpl Infect Dis 2019;21:e13146. [DOI] [PubMed] [Google Scholar]
- 393.La Hoz RM, Sandikci B, Ariyamuthu VK, Tanriover B. Short-term outcomes of deceased donor renal transplants of HCV uninfected recipients from HCV seropositive nonviremic donors and viremic donors in the era of direct-acting antivirals. Am J Transplant 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Franco A, Moreso F, Merino E, Sancho A, Kanter J, Gimeno A, Balibrea N, et al. Renal transplantation from seropositive hepatitis C virus donors to seronegative recipients in Spain: a prospective study. Transpl Int 2019;32:710–716. [DOI] [PubMed] [Google Scholar]
- 395.Madan S, Patel SR, Rahgozar K, Saeed O, Murthy S, Vukelic S, Sims DB, et al. Utilization rates and clinical outcomes of hepatitis C positive donor hearts in the contemporary era. J Heart Lung Transplant 2019;38:907–917. [DOI] [PubMed] [Google Scholar]
- 396.Graziadei IW, Zoller HM, Schloegl A, Nachbaur K, Pfeiffer KP, Mark W, Mikuz G, et al. Early viral load and recipient interleukin-28B rs12979860 genotype are predictors of the progression of hepatitis C after liver transplantation. Liver Transpl 2012;18:671–679. [DOI] [PubMed] [Google Scholar]
- 397.Wiesner RH, Sorrell M, Villamil F. Report of the first International Liver Transplantation Society expert panel consensus conference on liver transplantation and hepatitis C. Liver Transpl 2003;9:S1–9. [DOI] [PubMed] [Google Scholar]


