Abstract
Ether glycerolipids extracted from various archaeobacteria were formulated into liposomes (archaeosomes) possessing strong adjuvant properties. Mice of varying genetic backgrounds, immunized by different parenteral routes with bovine serum albumin (BSA) entrapped in archaeosomes (∼200-nm vesicles), demonstrated markedly enhanced serum anti-BSA antibody titers. These titers were often comparable to those achieved with Freund's adjuvant and considerably more than those with alum or conventional liposomes (phosphatidylcholine-phosphatidylglycerol-cholesterol, 1.8:0.2:1.5 molar ratio). Furthermore, antigen-specific immunoglobulin G1 (IgG1), IgG2a, and IgG2b isotype antibodies were all induced. Association of BSA with the lipid vesicles was required for induction of a strong response, and >80% of the protein was internalized within most archaeosome types, suggesting efficient release of antigen in vivo. Encapsulation of ovalbumin and hen egg lysozyme within archaeosomes showed similar immune responses. Antigen-archaeosome immunizations also induced a strong cell-mediated immune response: antigen-dependent proliferation and substantial production of cytokines gamma interferon (Th1) and interleukin-4 (IL-4) (Th2) by spleen cells in vitro. In contrast, conventional liposomes induced little cell-mediated immunity, whereas alum stimulated only an IL-4 response. In contrast to alum and Freund's adjuvant, archaeosomes composed of Thermoplasma acidophilum lipids evoked a dramatic memory antibody response to the encapsulated protein (at ∼300 days) after only two initial immunizations (days 0 and 14). This correlated with increased antigen-specific cell cycling of CD4+ T cells: increase in synthetic (S) and mitotic (G2/M) and decrease in resting (G1) phases. Thus, archaeosomes may be potent vaccine carriers capable of facilitating strong primary and memory humoral, and cell-mediated immune responses to the entrapped antigen.
Immune mechanisms that control diseases include mainly the induction of neutralizing antibodies (humoral immunity) and generation of T cells (cell-mediated immunity), including CD4+ helper (Th) and CD8+ cytotoxic (cytotoxic T-lymphocyte) responses. T helper cells often segregate into dichotomous cytokine-secreting phenotypes: Th1 cells secreting gamma interferon (IFN-γ), interleukin-2 (IL-2), and lymphotoxin aid cell-mediated immunity, whereas Th2 cells producing IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13 facilitate B-cell antibody production (22, 30). CD8+ T cells are principally involved in killing infected targets and tumors (18).
The success of vaccines depends on two key aspects: identification of specific antigenic targets and the ability to evoke a strong and appropriate immune response. Over the past decade, considerable progress has been made toward identification, purification, and/or synthesis of key antigenic determinants of pathogens and tumors (7, 24). However, relatively poor immunogenicity may be expected from such highly purified proteins and/or peptides, limiting their ability to induce a strong protective immune response. While coadministering antigens (Ags) with immunostimulating adjuvants often facilitates a strong immune response, many adjuvants have undesirable side effects such as severe inflammatory responses that preclude their use in humans. Indeed, the only adjuvant currently approved universally for use in humans is alum (aluminum hydroxide), which is a relatively weak potentiator of cell-mediated immune responses (16).
Liposomes composed of synthetic esters have been explored as possible Ag carrier vehicles, and a liposome-based vaccine against hepatitis A has been licensed for humans (2). However, while liposomes provide an antigenic depot, often codelivery of additional adjuvants such as lipid A or cholera toxin (CT) is required for effective immunity (17, 34).
The domain Archaea (archaeobacteria) consists of organisms distinct from eubacterial and eukaryotic cells in part characterized by their unique, polar lipid structures. Archaeal lipids are composed of branched phytanyl chains, which are fully saturated in many species and are attached via ether bonds to the glycerol backbone carbons at the sn-2,3 positions (19, 38). In contrast, conventional phospholipids found in other bacteria and eukarya have fatty acyl chains, which may be unsaturated and attached via ester bonds to the sn-1,2 carbons of the glycerol. The unique structures of archaeal lipids confer considerable stability on liposomal vesicles (archaeosomes) formulated from the total polar lipids (TPL) of the different archaea (40) or from purified lipid subfractions (35). This led us to explore the adjuvant activity of archaeosomes. In earlier studies, we reported that archaeosomes facilitated a strong antibody response to entrapped protein Ags, namely, bovine serum albumin (BSA) and CT B subunit (42).
Adjuvants suited for vaccine formulations should display universal properties of facilitating strong and sustained immune responses against varied immunogens as well as driving the response in appropriate directions to provide protective immunity. With this aspect in mind, the current study was aimed at evaluating the ability of archaeosomes to facilitate the different facets of immune responses against entrapped protein Ags and characterizing the mechanism(s) of adjuvant action. Besides expanding our earlier studies and assessing the ability of various archaeosome types to evoke responses to several protein Ags in mice of varying genetic backgrounds, we provide here evidence for induction of strong Ag-specific Th2 and Th1 (cell-mediated) immunity, memory response, and Ag-dependent cycling of memory T cells.
MATERIALS AND METHODS
Growth of archaea and extraction of lipids.
Halobacterium salinarum (“Halobacterium cutirubrum”) (ATCC 33170), Methanobacterium espanolae GP9 (DSM 5982), Methanobrevibacter smithii ALI (DSM 2375), Methanosphaera stadtmanae MCB-3 (DSM 3091), Thermoplasma acidophilum 122-1B3 (ATCC 27658), Methanosarcina mazei S-6 (DSM 2053), and Natronobacterium magadii MS3 (ATCC 43099) were cultivated in 75- to 250-liter fermentors as described earlier (10). Total lipids were extracted from frozen cell pastes, and the TPL were collected as the acetone-insoluble fraction (10).
Preparation and characterization of archaeosomes and conventional liposomes.
Archaeosomes were composed of the TPL from the different archaea mentioned above except for PGP-0-CH3 archaeosomes. These were prepared from PGP-0-CH3 (phosphatidylglyceromethylphosphate diether analog [20]) isolated from H. salinarum with a purity of at least 79%, determined by negative-ion fast atom bombardment-mass spectrometry. l-α-Dimyristoylphosphatidylcholine (DMPC), l-α-dimyristoylphosphatidylglycerol (DMPG), and cholesterol (CHOL) were purchased from Sigma Chemical Co., St. Louis, Mo., for the preparation of conventional liposomes, defined herein as DMPC-DMPG-CHOL (1.8:0.2:1.5 molar ratio) unless otherwise stated. Vesicles were prepared by pressure extrusion at 23°C with 400-nm-pore-size filters (9). Briefly, 20 mg of dried lipid was hydrated in 1 ml of phosphate-buffered saline (PBS) containing the protein Ag (10 mg/ml). Ag that was not associated with the vesicles was removed by ultracentrifugation (200,000 × gmax for 30 min) three times from 7-ml volumes of PBS. Mean vesicle diameters were determined by number-weighted Gaussian size distributions with a Nicomp Particle sizer (model 370; Nicomp, Santa Barbara, Calif.). The amount of protein incorporated into the vesicles was estimated, after lipid removal (45), by the sodium dodecyl sulfate (SDS)-Lowry method and comparison with standard curves constructed for the relevant protein. The ratio of protein to lipid (micrograms per milligram) is based on the salt-free dry weights of the vesicles.
Encapsulation versus surface localization of Ag in lipid vesicles.
Archaeosomes and conventional liposomes containing BSA were incubated with protease type IV from Streptomyces griseus (Sigma Chemical Co.). The assay consisted of incubating 50 μl of BSA-vesicles with, or without, 0.028 U of protease for 2 h at 35°C. Protease inhibitors, phenylmethylsulfonyl fluoride and leupeptin (Sigma), were then added from ethanolic solutions to achieve 50 μM each. After 0.5 h at ambient temperature, 5 μg of hen egg lysozyme (HEL) (Sigma) was added just prior to vesicle lysis with SDS sample buffer, and samples of 50 μl were placed immediately in a boiling water bath for 3 min. SDS-polyacrylamide gel electrophoresis and quantitative densitometry on bands stained with Coomassie brilliant blue R-250 (Bio-Rad, Richmond, Calif.) were done as described elsewhere (42). Controls were included within each experiment to verify that the protease inhibitors were effective and to confirm digestion of surface-bound Ag. For the latter, BSA was surface bound by incubating empty H. salinarum archaeosomes overnight at 4°C with 10 mg of BSA/ml of PBS, followed by one wash. Interfering bands were absent from all vesicle types lacking Ag.
Mice, immunization, and Ags.
Inbred, 6- to 8-week-old female BALB/c and C57BL/6 mice were purchased from Charles River Laboratories (St. Constant, Canada), and C3H/HeJ mice were from The Jackson Laboratory (Bar Harbor, Maine). Mice were maintained in the animal facility of the Institute for Biological Sciences, National Research Council, in accordance with guidelines of the Canadian Council on Animal Care.
Groups of four to six mice received two immunizations of the Ag in PBS (no adjuvant), in alum or Freund's adjuvant (FA), or entrapped in archaeosomes or conventional liposomes, as specified in the figure legends. Immunization volume was 100 to 200 μl, Ag dose was 8 to 15 μg/injection, and lipid concentration was 0.2 to 1.7 mg/injection. For alum immunizations, the Ag was adsorbed onto Imject alum (Pierce, Rockford, Ill.) according to the manufacturer's protocol. When FA (Sigma Chemical Co.) was used, Ag in PBS was emulsified with 62.5% complete FA in the first injection and incomplete FA for the second. Immunization routes used included intraperitoneal (i.p.), intramuscular (i.m.), and subcutaneous (s.c.) injection at the base of the tail. Ags tested included fatty-acid-free BSA, HEL, and ovalbumin (OVA), all purchased from Sigma Chemical Co.
Evaluation of antibody titers.
Mice were bled either from the tail veins or by cardiac puncture, and blood was collected in Microtainer serum separator tubes (Becton Dickinson, Franklin Lakes, N.J.). After the blood was allowed to clot (1 h at 4°C), the serum was separated by centrifugation and frozen at −20°C until assayed. The antibody levels were determined by indirect Ag-specific enzyme-linked immunosorbent assay (ELISA) (42). Briefly, ELISA plates (enzyme immunoassay microtitration plates, 96 wells and flat bottom; ICN Biomedicals, Inc., Aurora, Ohio) were coated with Ag in PBS (10 μg/ml), and serial twofold dilutions of serum (from individual mice) were assayed in duplicate. Horseradish peroxidase-conjugated goat anti-mouse immunoglobulin (Ig; IgG plus IgM) revealing antibody (Caltag, San Francisco, Calif.) was used to determine total antibody titers of sera. For isotyping of the sera, horseradish peroxidase-conjugated sheep anti-mouse isotype-specific (IgM-, IgG1-, IgG2a-, IgG2b-, or IgG3-specific) revealing antibodies (Serotech Inc.; distributed by Cedarlane Laboratories, Hornby, Canada) were used. The reactions were developed with the ABTS Microwell peroxidase system (Kirkegaard and Perry Laboratories, Gaithersburg, Md.), and absorbance was determined at 415 nm after 15 min. Antibody titers are represented as endpoint dilutions exhibiting an optical density of 0.3 U above background. Samples pertaining to each experiment were evaluated in the same assay.
Ag-specific proliferation of splenocytes.
Splenocytes were obtained on day 28 from individual mice immunized on days 0 and 21. Selective lysis of erythrocytes was performed with Tris-buffered ammonium chloride, pH 7.2 (Sigma Chemical Co.). Cells (5 × 106/ml) were washed twice, and triplicate cultures were set up (72 h, 7% CO2) in RPMI 1640 medium (Gibco-BRL, Life Technologies Inc., Grand Island, N.Y.) in 96-well round-bottom microtiter plates (Falcon; Becton Dickinson) in the presence or absence of Ag. The serum supplement used for culture included either defined equine serum (2%, for BSA-stimulated cultures) or fetal bovine serum (FBS; 8%, for HEL- and OVA-stimulated cultures), obtained from HyClone Laboratories Inc., Logan, Utah. Wells were pulsed with 1 μCi of [3H]thymidine (ICN Pharmaceuticals Canada, Montreal, Quebec, Canada) for 18 h, cultures were harvested onto glass-fiber filters, and the radioactivity incorporated was determined by liquid scintillation counting.
Cytokine assays.
Cytokines produced in supernatants of Ag-stimulated cultures were measured by a sandwich ELISA (29). Antibody pairs used included RA-6A2 (ATCC HB170) and XMG1.2-biotin (8) for IFN-γ, and 11B11 (31) and BVD6-24G2-biotin (Pharmingen Canada Inc., Mississauga, Canada) for IL-4. IFN-γ and IL-4 standards were purchased from ID Labs, London, Canada. Duplicate standard curves were included on each plate. The sensitivities of the ELISAs were as follows: IFN-γ, >100 pg/ml, and IL-4, >15 pg/ml. Regardless of thresholds, samples from each experiment were tested in the same assay.
Cell cycle analysis.
Cell cycle analysis was performed by quantitating the incorporation of DNA-binding dye (propidium iodide) by flow cytometry. Briefly, splenocytes (106) were stained with fluorescein isothiocyanate-conjugated anti-CD4 antibodies (Pharmingen Canada Inc.) for 30 min on ice in 50 μl of RPMI medium containing 8% FBS. Cells were then washed and fixed overnight at 4°C in 1 ml of 70% ice-cold ethanol. Fixed cells were stained with propidium iodide (Calbiochem, La Jolla, Calif.) in the presence of RNase A (100 U/ml; Boehringer Mannheim, Laval, Canada) and analyzed by flow cytometry (EPICS XL; Coulter Corp., Hialeah, Fla.). DNA content histograms gated on CD4+ T cells were obtained with EXPO software (Coulter Corp.). As the ploidy of the DNA increases as cells enter synthetic and mitotic phases, each cell incorporates greater amounts of propidium iodide. Thus, the percentages of cells in various phases of the cell cycle (apoptotic, G1 [resting], S [synthetic], and G2/M [mitotic]) were calculated based on DNA content.
Statistical analyses.
Student's t test was done to determine statistical significance between different immunization groups for the various experiments, whereas analysis of variance (ANOVA) was used for long-term memory data to determine differences within groups (over time). P < 0.05 was considered statistically significant.
RESULTS
Choice of archaeosome types.
The archaeosome types tested were chosen to represent divergent sources of TPL, based on their lipid structures. TPL with high caldarchaeol (tetraether lipids) content (T. acidophilum, >90%; M. espanolae, 65%) are known to form the most stable archaeosomes (40). M. smithii was chosen because it represents the major archaeon resident in the human gut (28) and has a caldarchaeol content of 40 mol%. On the other hand, M. stadtmanae was included occasionally as an example of a human isolate with relatively low humoral, adjuvant properties. The phospholipids and glycolipids of H. salinarum and their sulfated forms are unique and have been characterized extensively (20). M. mazei was of interest as a representative TPL composed almost exclusively of phosphoarchaeols with simple phospho-head groups of inositol, glycerol, serine, and ethanolamine (41). Overall, we attempted to include at least one of the three most promising candidate archaeosomes (M. smithii, T. acidophilum, or H. salinarum) in each experiment.
BSA-archaeosomes induce strong humoral responses in mice of varying genetic backgrounds.
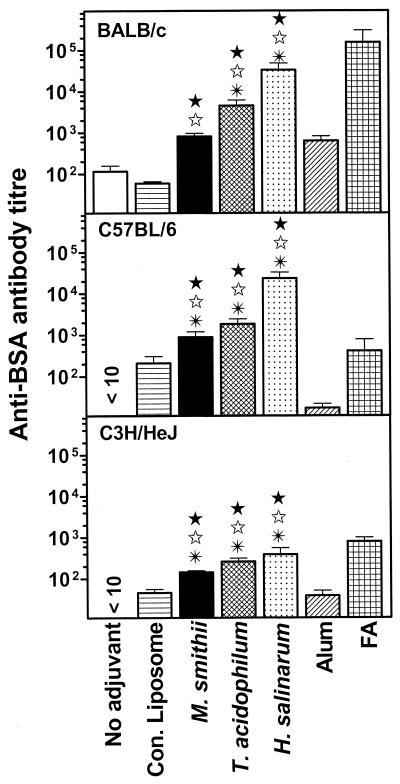
In earlier studies, we have demonstrated the ability of several archaeosome types to induce a strong antibody response to the entrapped protein Ag (42). The three most potent archaeosome adjuvants were tested in the current study for their ability to induce antibody responses in mice of different genetic backgrounds. Inbred mouse strains BALB/c, C57BL/6, and C3H/HeJ were immunized (i.p.) with BSA entrapped in archaeosomes comprised of TPL of different archaea or conventional liposomes or in conjunction with traditional adjuvants such as alum and FA, and anti-BSA antibodies were evaluated in the sera. Entrapment of BSA in archaeosomes resulted in elevated anti-BSA antibody titers (total IgG plus IgM) in all three mouse strains (Fig. 1). The antibody titers achieved by entrapping BSA in archaeosomes were often comparable to that found with the potent FA and superior to those with alum and conventional liposomes. A low Ag dose (10 μg/injection) was used for these experiments, demonstrating the potency of archaeosomes in facilitating a strong humoral response. Immunization with bare Ag (BSA in PBS) failed to evoke an anti-BSA antibody response, reiterating the poor immunogenicity of BSA. Thus, adjuvant activity of archaeosomes is not restricted by major histocompatibility complex haplotype of the mouse strains.
FIG. 1.
Induction of humoral response to entrapped BSA by archaeosomes in mice of varying genetic backgrounds. Mice were immunized i.p. on days 0 and 21 with 10 μg of BSA either in PBS (no adjuvant), encapsulated in conventional liposomes (0.79 mg of lipid/injection) or archaeosomes (composed of TPL of M. smithii [0.49 mg of lipid/injection], T. acidophilum [0.37 mg of lipid/injection], or H. salinarum [0.88 mg of lipid/injection]), adsorbed on alum, or emulsified in FA. Blood was collected on day 28, and sera were analyzed for anti-BSA antibodies (total IgG plus IgM). Values represent means ± standard errors of the means of antibody titers for mice in groups of five. Values statistically significant by Student's t test (P < 0.05) for archaeosomes compared to no-adjuvant controls (★), conventional liposomes (⋆), and/or alum (✠) are indicated.
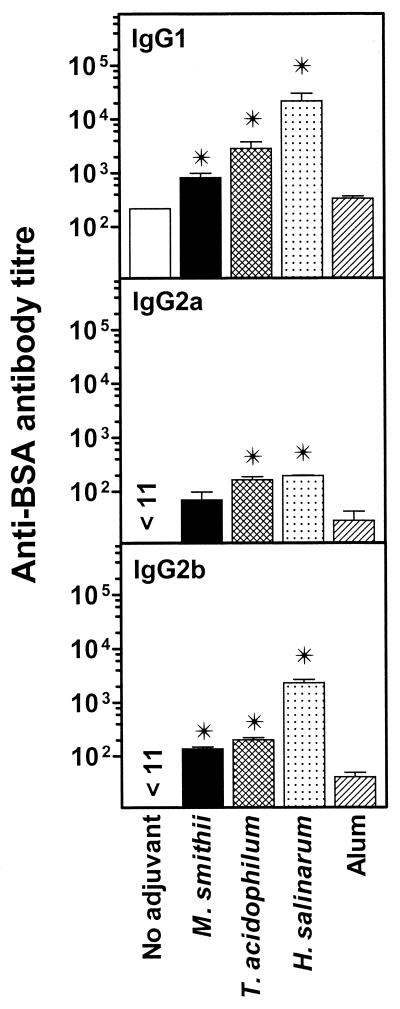
Isotype distribution.
Sera collected from immunized BALB/c mice were analyzed for isotype distribution. Figure 2 indicates the serum distribution of Ag-specific IgG1, IgG2a, and IgG2b antibodies. The overall magnitude of anti-BSA-specific IgG1 antibody titers was higher than that of IgG2a and IgG2b. Nevertheless, titers of all three antibodies were significantly enhanced by BSA-archaeosomes in comparison to those induced after BSA-alum immunization (P < 0.05). The induction of both IgG1 and IgG2a antibodies by archaeosomes suggests efficient major histocompatibility complex class II presentation of the Ag leading to both a humoral (IgG1) and a cell-mediated (IgG2a; complement-fixing antibodies) response. In all cases, very little anti-BSA IgM antibodies were detected, indicating strong class switching, whereas IgG3 antibodies were undetectable (data not shown).
FIG. 2.
Anti-BSA antibody isotypes induced after archaeosome immunization. Sera from BALB/c mice analyzed for total antibody titer (IgG plus IgM) for Fig. 1 were further characterized for antibody isotypes (IgG1, IgG2a, and IgG2b). Values represent means ± standard errors of the means of isotype-specific anti-BSA antibody titers for mice in groups of five. ✠, value statistically significant by Student's t test (P < 0.05) for archaeosomes compared to alum immunization.
Internalization of BSA within archaeosomes.
Because of the lack of information on the stability-degradability of archaeosomes in vivo, it was especially relevant to determine whether the protein Ag had first to be released from the internal space of the archaeosome or whether only surface-bound Ag could be presented from these relatively stable vesicles. Data showed that any BSA which was surface bound was susceptible to protease IV treatment and that surface localization versus internalization of the protein varied with the composition of the vesicle (Table 1). However, the equivalent ability to evoke an antibody response by archaeosome types with >80% internalized Ag (M. smithii and T. acidophilum), compared to those where substantial amounts of the antigen were surface located (H. salinarum), suggested that efficient vesicle degradation had occurred in vivo.
TABLE 1.
Internal versus surface location of BSA encapsulated in various types of lipid vesiclesa
| Vesicle type | Internal BSA (%)b |
|---|---|
| Conventional (DMPC-DMPG-CHOL) | 89 ± 1 |
| M. smithii | 82 ± 7 |
| T. acidophilum | 85 ± 3 |
| H. salinarum | 61 ± 7 |
| M. mazei | 92 ± 1 |
| M. espanolae | 98 ± 0 |
| M. stadtmanae | 76 ± 2 |
| Surface BSA-H. salinarum | 3 ± 1 |
Vesicle types were digested with protease prior to separation by SDS-polyacrylamide gel electrophoresis of BSA from lipids and peptide cleavage products. The intact BSA band stained with Coomassie blue was quantitated by densitometry. BSA adsorbed to the surface of preformed H. salinarum archaeosomes served as a control for enzyme activity.
Values represent means ± standard deviations of internalized BSA, calculated from two preparations for each sample.
BSA must be archaeosome associated to induce strong anti-BSA antibody titers.
To test the effect of Ag encapsulation on the adjuvant action of archaeosomes, mice were immunized with BSA entrapped in archaeosomes or given separate injections of the respective weights-concentrations of empty archaeosomes and BSA. Archaeosome types were chosen to represent a high caldarchaeol (M. espanolae and T. acidophilum), a moderate caldarchaeol (M. smithii), or a simple phosphoarchaeol (M. mazei) composition. Data in Table 2 indicate that association of archaeosomes and Ag, either internalized or surface bound, is required to achieve high antibody titers, by both i.p. and i.m. routes. A significant drop in the titers was observed (for all archaeosome types irrespective of lipid composition) when empty archaeosomes and BSA were administered separately. The TPL of M. espanolae are largely uncharacterized structurally and were excluded from further studies. Similarly, M. mazei archaeosomes were excluded upon discovering that the permeability of these vesicles is dependent upon the salt form of the TPL used in their construction (32).
TABLE 2.
Requirement for encapsulation of BSA within various archaeosomes to achieve an elevated humoral responsea
| Injection | BSA encapsulated | Archaeosome diam (nm)b | Anti-BSA titerc
|
|
|---|---|---|---|---|
| i.p. | i.m. | |||
| M. smithii-BSA | Yes | 195 ± 77 | 3,443 ± 912d | 909.8 ± 152d |
| M. smithii + BSA | No | 207 ± 74 | 482.8 ± 127 | 323.9 ± 66.8 |
| T. acidophilum-BSA | Yes | 63 ± 33 | 1,007 ± 254d | 1,925 ± 502d |
| T. acidophilum + BSA | No | 99 ± 48 | 183.9 ± 65.7 | 271.1 ± 195 |
| M. mazei-BSA | Yes | 136 ± 59 | 919.3 ± 341d | 1,631 ± 163d |
| M. mazei + BSA | No | 132 ± 59 | 99.86 ± 8.59 | 12.46 ± 4.14 |
| M. espanolae-BSA | Yes | 196 ± 75 | 2,729 ± 581d | 1,340 ± 122d |
| M. espanolae + BSA | No | 200 ± 68 | 439.3 ± 68.0 | 202.6 ± 32.9 |
| BSA + FA | 7,588 ± 1,671 | 4,539 ± 349 | ||
| BSA | 151.7 ± 37.6 | 107.7 ± 48.9 | ||
BALB/c mice (four per group) were immunized on days 0 and 14 with archaeosomes containing encapsulated BSA (archaeosome-BSA) or with separate injections of the respective weights of empty archaeosome and BSA (archaeosome + BSA). For injections of nonencapsulated Ag, empty archaeosomes were given i.p. 7 h prior to a separate i.p. injection of BSA in PBS; for i.m. injections, BSA and empty archaeosomes were injected in separate haunches at about the same time. In all cases, 12.5 μg of BSA was administered per injection. Dry weights of archaeosomes in each immunization were 1.67 mg of M. smithii, 0.81 mg of T. acidophilum, 1.53 mg of M. mazei, and 1.35 mg of M. espanolae. T. acidophilum archaeosomes were prepared by hydration in PBS followed by French pressure cell treatment and centrifugation at 8,000 × g to discard the large archaeosomes.
Diameter ± standard deviation.
Humoral response (day 26) determined as titer (endpoint dilution at 0.3 optical density units) of IgG plus IgM antibodies in sera ± standard error of the mean.
Significantly different (P < 0.05) from value for the corresponding nonencapsulated group as calculated by Student's t test.
Archaeosomal lipids induce superior humoral responses compared to conventional liposomal lipids.
Liposomes composed of synthetic ester-lipids (conventional liposomes) have been used in the past to facilitate humoral responses to entrapped Ags (1, 14). In earlier studies (26, 42), as well as the current one (Fig. 1), we have observed that archaeosome-facilitated humoral responses are significantly superior to that of conventional liposomes. The adjuvant action of conventional liposomes was directly compared to that of archaeosomes by immunizing mice with BSA entrapped in M. smithii archaeosomes, conventional DMPC-DMPG liposomes, or vesicles composed of mixtures of archaeal and ester lipid types (Table 3). A potent anti-BSA antibody response was observed when Ag was delivered in vesicles made with 100% M. smithii TPL. This response dropped significantly (P < 0.05) with increasing proportions of DMPC-DMPG in the vesicle composition, although entrapment of Ag in 100% DMPC-DMPG vesicles yielded a moderately higher antibody titer than did bare antigen. Day 35 titers were evaluated after three (injections on days 0, 14, and 31) instead of the usual two immunizations. Even under these conditions, antibody titers induced by conventional liposomes failed to equal that of archaeosomes.
TABLE 3.
Anti-BSA antibody titers in sera of BALB/c mice immunized with M. smithii TPL archaeosomes containing increasing amounts of DMPC-DMPGa
| DMPC-DMPG (wt %) | Vesicle diam (nm) | Vesicle/ injection (mg [dry wt]) | Anti-BSA titerb
|
|
|---|---|---|---|---|
| Day 20 | Day 35 | |||
| 0 | 180 ± 81 | 1.19 | 409.6 ± 29.1 | 638.1 ± 90.2 |
| 50 | 186 ± 57 | 0.86 | 198.8 ± 37.4c | 411.1 ± 62.5 |
| 90 | 152 ± 54 | 1.14 | 125.9 ± 11.4c | 184.8 ± 15.3c |
| 100 | 126 ± 56 | 1.01 | 57.9 ± 21.3c | 139.48 ± 39.5c |
Mice (three per group) were immunized by i.p. injections (days 0, 14, and 31) of various liposome vesicles containing 12.5 μg of encapsulated BSA. Vesicles were prepared from M. smithii TPL, DMPC-DMPG (1.8:0.2 mol%), or mixtures shown as weight percent made from these two compositions.
The humoral response determined as titer (endpoint dilution at 0.3 optical density units) of IgG plus IgM antibodies in sera ± standard error of the mean. Sera from mice in the Ag-alone control groups (12.5 μg of BSA in PBS/injection) had anti-BSA antibody titers of <25 and 64.9 ± 53.9 on days 20 and 35, respectively.
Significantly different (P < 0.01) from titer for 100% M. smithii vesicles (0% DMPC-DMPG) as calculated by Student's t test.
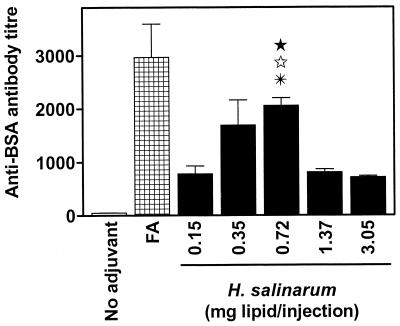
Ag/lipid ratio influences the magnitude of humoral response.
One factor that may influence the adjuvant action of archaeosomes is the ratio of Ag to TPL. To specifically address this issue, mice were immunized with archaeosomes containing varying Ag loading to achieve a constant entrapped-Ag dose of 8 μg/injection within a range of 0.15 to 3.1 mg (dry weight) of BSA-archaeosomes. Data in Fig. 3 demonstrate the dose-dependent effect of H. salinarum lipids on the antibody titers to the entrapped Ag. Interestingly, both very low and high doses of lipids resulted in lowering of the antibody titer, whereas intermediate doses (0.4 to 0.7 mg of lipid/injection) yielded optimum titers. In most experiments herein, archaeosome preparations contained lipid doses of <1 mg/injection.
FIG. 3.
Influence of Ag loading on anti-BSA antibody titers. H. salinarum TPL archaeosomes were loaded with BSA so that 8 μg of Ag was encapsulated in a range from 0.15 to 3.1 mg (dry weight) of archaeosomes. BALB/c mice were immunized i.p. on days 0 and 14 with these formulations. Negative controls included mice immunized with bare Ag (no adjuvant), whereas BSA-FA-immunized mice represent positive controls. Mice were bled on day 21, and sera were analyzed for anti-BSA antibodies. Data represent mean titers ± standard errors of the means for mice in groups of three. Values for medium lipid dose (0.72 mg) are significantly different (P < 0.01) from those for low dose (0.15 mg) (★) and high dose (1.37 mg [⋆] and 3.05 mg [✠]), as calculated by Student's t test.
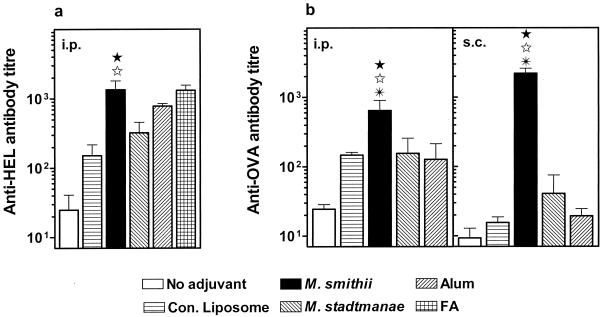
Archaeosomes facilitate humoral responses to other entrapped protein Ags.
In the studies described above, BSA was used as the model Ag for entrapment in archaeosomes and assessment of humoral responses. Potential vaccine adjuvants must possess the capacity to facilitate immune responses to a wide array of proteins irrespective of antigenic properties. Therefore, the immune response to other Ags entrapped within archaeosomes was studied. Figure 4 illustrates the antibody response in mice, obtained after immunization with two other model Ags (HEL and OVA) entrapped within M. smithii and M. stadtmanae archaeosomes. As with BSA, M. smithii archaeosomes induced markedly enhanced antibody responses in comparison to bare Ag (no adjuvant) and conventional liposomes. Interestingly, s.c. immunizations with M. smithii archaeosome-Ag resulted in very strong titers, with a dramatic enhancement (∼2 log) over that of alum (Fig. 4b). Antibody titers induced by M. stadtmanae were relatively lower than that of M. smithii, as has been observed by us in other studies as well (39).
FIG. 4.
Induction of antibodies to entrapped HEL and OVA by archaeosomes. BALB/c mice were immunized i.p. or s.c. on days 0 and 21 with 10 μg of HEL (a) or 15 μg of OVA (b) in PBS (no adjuvant), alum, or FA or encapsulated in either conventional liposomes (1.6 mg of lipid/injection for HEL-liposome and 0.8 mg of lipid/injection for OVA-liposome) or archaeosomes. Archaeosomes were composed of TPL of M. smithii (0.31 mg of lipid/injection for HEL-M. smithii and 0.8 mg of lipid/injection for OVA-M. smithii) or M. stadtmanae (0.25 mg of lipid/injection for HEL-M. stadtmanae and 0.48 mg of lipid/injection for OVA-M. stadtmanae). Blood was collected by cardiac puncture on day 28, and sera were analyzed for HEL (a)- or OVA (b)-specific antibodies. Data represent mean titers ± standard errors of the means for mice in groups of three to six. Antibody titers obtained with M. smithii-Ag immunization are statistically significant (P < 0.04) compared to the no-adjuvant control group (★), conventional liposomes (⋆), and/or alum (✠) as calculated by Student's t test.
Induction of cell-mediated immunity: archaeosomes facilitate Ag-specific proliferation of spleen cells.
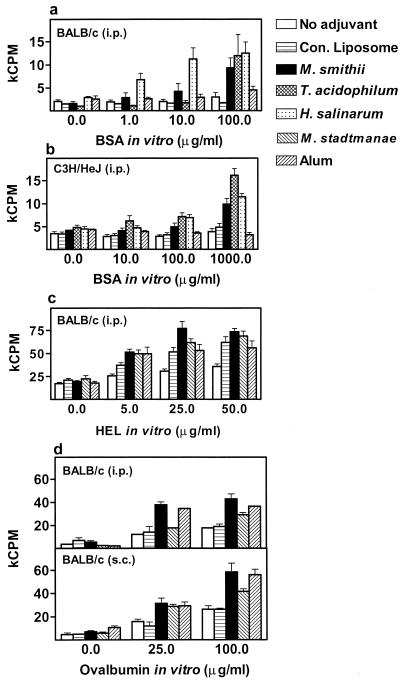
The induction of a cell-mediated response against Ags is often critical for sustained and effective immunity, particularly against intracellular pathogens. One of the first indicators of an effective cell-mediated immune response is the ability of lymphocyte populations of immunized mice to respond to Ag in vitro. Spleen cells from immunized mice were cultured in vitro with varying doses of Ag, and at 72 h the proliferative response was assessed by [3H]thymidine incorporation. Figures 5a and b indicate the BSA-specific proliferation of spleen cells after i.p. immunization. Immunization with BSA precluded the use of FBS-supplemented medium for in vitro Ag stimulation. Therefore, the overall low levels of proliferation noted may be attributed to the inability of horse serum supplement to support strong growth. Interestingly, even under such suboptimal stimulation conditions, a dose-dependent proliferation to BSA was seen with spleen cells of mice immunized with BSA entrapped in archaeosomes. Furthermore, the proliferative response of spleen cells following archaeosome immunization was seen in both BALB/c and C3H/HeJ (lipopolysaccharide [LPS]-hyporesponsive) mice. On the other hand, conventional liposomes as well as alum failed to induce BSA-specific proliferation. Ag-specific proliferation was more pronounced with other Ags (HEL and OVA). Both HEL-archaeosomes and OVA-archaeosomes induced strong Ag-specific proliferation, as did alum (Fig. 5c and d). Immunization by the s.c. route also resulted in similar responses. However, even with robust Ag stimulation, conventional liposomes failed to equal M. smithii archaeosomes in their ability to induce proliferation.
FIG. 5.
Induction of Ag-specific spleen cell proliferation after immunization with Ag-archaeosomes. Mice (BALB/c and/or C3H/HeJ) were immunized i.p. and/or s.c. with BSA-archaeosomes (a and b), HEL-archaeosomes (c), and OVA-archaeosomes (d) as per the same regimen described for Fig. 1 and 4. Archaeosomes were composed of TPL of M. smithii, T. acidophilum, H. salinarum, or M. stadtmanae, as indicated in the figure. Appropriate Ag-alone (no adjuvant), Ag-conventional liposomes, and alum controls were included as indicated. Spleens were harvested on day 28, cells from each mouse were stimulated in triplicate with varying doses of the appropriate Ag in vitro for 72 h and pulsed with [3H]thymidine, and proliferation was assessed. Ag-dependent proliferative response for cells from each mouse was determined. Data represent the mean counts per minute × 1,000 (kCPM) ± standard errors of the means for mice in each group (n = 5 [a and b], n = 6 [c], and n = 3 [d]).
Archaeosomes induce Th1 and Th2 cytokine production by Ag-stimulated spleen cells.
Ag-specific lymphocyte proliferation results in activation of specific T helper responses and cytokine production. While Th2 (IL-4) responses are important for aiding antibody production by B cells, Th1 (IFN-γ) responses are important for induction of effective cell-mediated immunity. Th1 and Th2 responses may be dichotomous, and Ags may themselves skew immunity toward one or the other (22). A superior adjuvant, on the other hand, may possess the desirable capacity of inducing both Th1 and Th2 responses irrespective of antigenic nature.
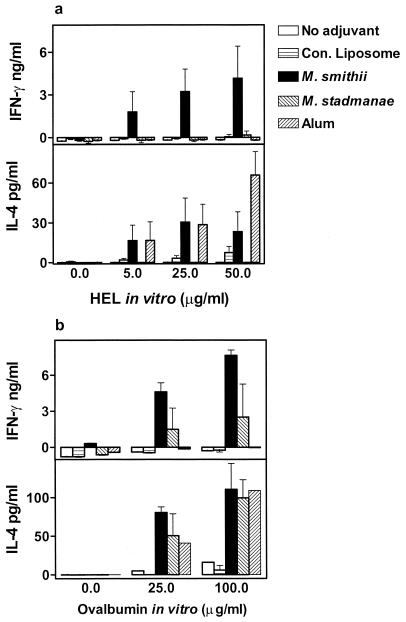
Data in Fig. 6a indicate Ag-dependent cytokine production by cultured spleen cells following i.p. immunization with HEL-archaeosomes. Substantial IFN-γ production was seen only in cultures of cells from M. smithii HEL-archaeosome-immunized mice. The IFN-γ levels showed a clear dose-dependent production in response to stimulation by Ag. Alum failed to evoke any IFN-γ production. On the other hand, both archaeosomes and alum induced IL-4 production. M. stadtmanae archaeosomes and conventional liposomes induced neither IFN-γ nor IL-4, as might have been expected from their poor adjuvanticity. The ability of M. smithii archaeosomes to induce Th1 (IFN-γ) as well as Th2 (IL-4) responses to entrapped Ags was further corroborated with another Ag system. Immunization of mice with OVA-archaeosomes by the s.c. route also resulted in production of both IFN-γ and IL-4 by spleen cells (Fig. 6b). After s.c. immunization with archaeosomes, cytokine production was detectable even in popliteal lymph node cell cultures (data not shown). Interestingly, even the potency of s.c. immunization failed to evoke IFN-γ production by alum. Also, conventional liposomes induced neither IL-4 nor IFN-γ.
FIG. 6.
Induction of both Th1 and Th2 cytokines by M. smithii archaeosomes. BALB/c mice were immunized i.p. with 10 μg of HEL in archaeosomes (a) or s.c. with 15 μg of OVA in archaeosomes (b) on days 0 and 21. Archaeosomes were composed of the TPL of M. smithii or M. stadtmanae. Appropriate Ag alone (no adjuvant), Ag-conventional liposomes (Con. Liposome), and Ag-alum controls were included as shown in the figure. Spleens were harvested on day 28, and Ag-specific stimulation was set up for cells from individual mice as described for Fig. 5. Cytokine levels in 72-h supernatants were determined by ELISA. Values represent means ± standard errors of the means of cytokine production by spleen cells of mice in each group (n = 6 [a] and n = 3 [b]).
T. acidophilum archaeosomes induce a long-term memory response.
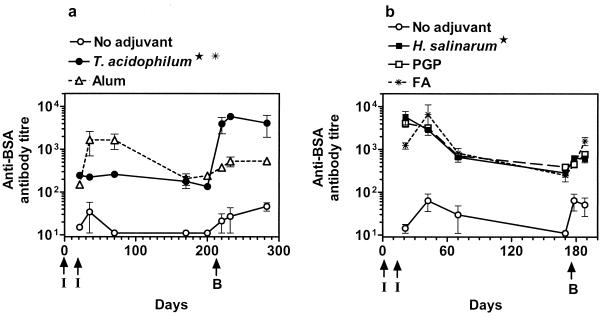
The success of vaccines often depends on the induction of a strong persistent memory response. It was reasoned that archaeosomes, because of their stability properties (40), may sustain Ag in vivo for longer periods than other adjuvant formulations, aiding memory responses.
Mice were immunized on days 0 and 14 with BSA-archaeosomes or other adjuvant-BSA preparations. The antibody titers were monitored in groups of mice at regular intervals, and memory responses were assessed after boosting with bare Ag in the absence of adjuvant (Fig. 7). Figure 7a indicates that T. acidophilum archaeosomes induced a moderately enhanced primary antibody response that was sustained for nearly 200 days (P < 0.02 by ANOVA, for all time points compared to no-adjuvant immunization). Following an Ag-alone boost on day 210, a remarkable memory antibody response was seen (∼2-log enhancement, P = 0.0004) that was sustained to at least 300 days. On the other hand, alum, despite inducing an enhanced primary response (days 35 and 70, P < 0.01 compared to no-adjuvant immunization), showed a subsequent drop in antibody titers and failed to evoke significant memory response (following Ag boost). BSA-archaeosomes prepared from the TPL of M. stadtmanae (1.1 mg/injection) resulted in low initial titers of antibody and little indication of a memory response (data not shown).
FIG. 7.
Ability of archaeosomes to induce a memory response to the encapsulated Ag. BALB/c mice were immunized (indicated by I) i.p. on days 0 and 14 with 15 μg of BSA either in PBS (no adjuvant), in alum, with FA, or encapsulated in archaeosomes. Archaeosomes were composed of TPL of either T. acidophilum (0.53 mg/injection) or H. salinarum (0.9 mg/injection) or were from purified lipid PGP-0-CH3 (0.96 mg/injection). A memory boost (indicated by B) of 25 μg of BSA (without adjuvant) was carried out on either day 210 (a) or day 171 (b). Mice were bled at regular intervals, and anti-BSA antibody titers were determined. Data represent mean titers ± standard errors of the means of mice in groups of four to six. ★, values for archaeosome immunization are significantly different from those for the no-adjuvant control group at all time points (P < 0.01). ✠, values for T. acidophilum group are significantly different over a period of time following memory boost (P < 0.001) as calculated by ANOVA.
In contrast to the T. acidophilum BSA-archaeosome response noted above, BSA-archaeosomes composed of the TPL of M. mazei (0.53 mg/injection), N. magadii (0.34 mg/injection) (data not shown), or H. salinarum (Fig. 7b) failed to evoke a strong memory response despite a substantially enhanced primary response. Vesicles composed of purified PGP-0-CH3 (the major lipid in H. salinarum TPL) showed a near-identical response to TPL of H. salinarum. FA was similar to H. salinarum vesicles by inducing a strong and sustained primary response but failing to evoke an enhanced memory response.
Induction of T-cell memory by T. acidophilum archaeosomes: increased cell cycling of CD4+ cells.
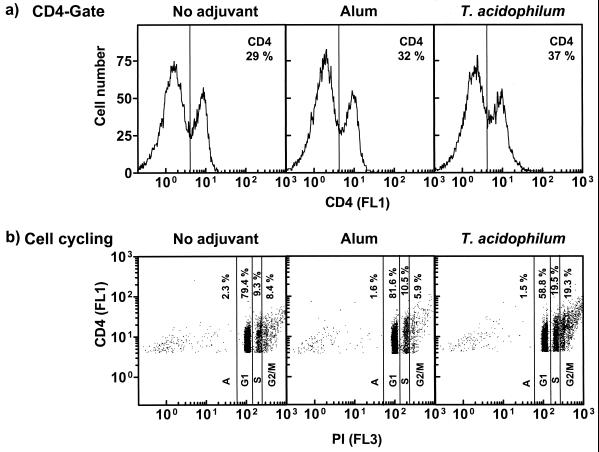
As antibody response to protein Ags is often T cell dependent, we tested if the strong memory antibody response induced by T. acidophilum archaeosomes was accompanied by stimulation of T helper (CD4+) subsets. Thus, the groups of mice used for experimentation in Fig. 7a were challenged again with Ag (25 μg of BSA in PBS, without adjuvant) on day 334. Seven days later, the propensity of cell cycling (based on propidium iodide staining) by splenic CD4+ T cells was analyzed. The frequency of CD4+ staining of spleen cell populations obtained from control (no-adjuvant) or alum- or T. acidophilum archaeosome-immunized mice is shown in Fig. 8a. The total CD4+ cell number was increased from 29% in the controls to 37% in the archaeosome-immunized group. Analysis of cell cycling profiles of the CD4+ cells revealed that antigenic challenge of T. acidophilum archaeosome-immunized mice resulted in a dramatic increase in the numbers of cells in the S (synthetic) and G2/M (mitotic) phase and a concomitant decrease of cells in the G1 (resting) phase (Fig. 8b). In contrast, alum immunization evoked no change in the percentages of cells in the various phases compared to the no-adjuvant control. Thus, archaeosomes induced the development and persistence of memory CD4+ T cells against the entrapped protein Ag.
FIG. 8.
Induction of CD4+ cell cycling following memory responses to entrapped Ag. Mice were immunized with BSA in PBS (no adjuvant), in alum, or entrapped in T. acidophilum archaeosomes as described for Fig. 7. Mice in all groups received an additional booster injection of BSA in PBS (25 μg, i.p., without adjuvant) on day 334. Seven days later, spleens were harvested, and cells within each group were pooled for analysis of cell cycling. Cells were stained with anti-CD4+ fluorescein isothiocyanate-conjugated antibodies and propidium iodide (PI), and PI uptake by CD4+ cells was assessed by flow cytometry. Twenty thousand events per sample were acquired. Cell cycle analysis on gated CD4+ cells was obtained by EXPO software. Histograms in panel a indicate the CD4+ (FL1) gate. Plots in panel b indicate the percentage of CD4+ cells in various phases of the cell cycle (based on PI [FL3] content) in each group. As the amount of DNA per cell increases, the greater is the incorporation of PI and the greater is the number of cells in synthetic and/or mitotic phase. A, apoptotic phase; G1, resting phase; S, synthetic phase; and G2/M, mitotic phase.
DISCUSSION
Of the various archaeosome types tested, those composed of the TPL of T. acidophilum, H. salinarum, and M. smithii possessed the strongest adjuvant properties, often equalling that of FA and generally surpassing that of alum. The potency of archaeosomes was evident even with low Ag doses of 8 to 15 μg associated with <1 mg of lipid per injection. We have related the structural properties of the TPL from the two human archaea M. smithii and M. stadtmanae to their contrasting adjuvant properties (39) and found that, although the lipids present in each TPL were remarkably similar, quantitatively there were large differences. Both archaetidyl inositol and β-Glcp-(1,6)-β-Glcp-(1,1)-archaeol were associated with low adjuvant activity, whereas major lipids having phosphoserine head groups were found in M. smithii TPL. However, the divergent lipid compositions of other potent archaeosomes suggest that archaeal lipid structures in general can confer adjuvant activity.
Interestingly, all three archaeosome preparations that induce the strongest responses had differentially localized (surface versus internalized) Ags (Table 1). It is currently unknown whether the localization of the Ag on the archaeosome carrier is of significance to phagocytosis and presentation in vivo. However, the ability of archaeosomes with most of the Ag internalized to induce a strong primary immune response suggests vesicle degradation in vivo, leading to Ag release and presentation. This was particularly striking for T. acidophilum archaeosomes (with >80% internalized Ag), because these are composed primarily of caldarchaeol (tetraether) lipids known to form a monolayer arrangement by spanning the archaeosome membrane (5) and thus represent the most stable archaeosomes in vitro (35, 40). However, conventional liposomes failed to induce strong antibody responses, even though >85% of the Ag was internalized within the vesicle, suggesting that immunomodulatory properties, along with in vivo stability and the ability to deliver Ag to Ag-presenting cells (APCs), are important for adjuvant activity.
Association of the Ag with the archaeosomes, as opposed to independent injections of the vesicle and Ag, yielded the strongest antibody response (Table 2). Liposomes are phagocytosed by APCs and have been used to deliver drugs and/or toxins to phagocytes (15, 44). Thus, association of Ag with archaeosomal vesicles probably ensured direct delivery of the Ag to the APCs for effective processing and presentation. Indeed, we have noted that archaeosomes are phagocytosed to a greater extent than are conventional liposomes (43). However, as some archaeosomes (M. espanolae and M. smithii) exhibited some adjuvant activity even when empty archaeosomes and Ag were injected separately, these appear to serve as both immunomodulators and Ag carriers.
As the genetic background of the host may differentially influence antigenic responses, the adjuvant activity of archaeosomes was assessed in different mouse strains. Three different mouse strains were chosen to reflect either a Th2-biased (BALB/c), a Th1-biased (C57BL/6), or an LPS-hyporesponsive (C3H/HeJ) host environment (6, 21, 33). Although the overall magnitude of antibody response varied between the different mouse strains, archaeosomes generally induced humoral responses equalling or surpassing those of alum and FA in all strains (Fig. 1).
Serum Ig isotype patterns of immunized mice revealed that both Ag-specific IgG1 and Ag-specific IgG2a antibodies were induced by the archaeosomes (Fig. 2). As a strong humoral response and induction of IL-4 induce switching toward IgG1 and a cell-mediated response and induction of IFN-γ induce switching to IgG2a (37), it appeared that both effector arms were influenced by archaeosomes. This was corroborated by the induction of Ag-specific proliferation (Fig. 5), as well as the induction of cytokines IFN-γ (Th1) and IL-4 (Th2) by spleen cells of archaeosome-immunized mice (Fig. 6). In contrast, adjuvants such as alum (Fig. 6) (16) and CT (46) induce exclusively a Th2 response, while others such as immunostimulating complexes evoke primarily Th1 immunity (36). Furthermore, the ability of archaeosomes to induce cell-mediated responses to various Ags (BSA, HEL, and OVA), as well as by different immunization routes, suggests a potential “universal” adjuvant function.
One of the important aspects of vaccine delivery is the ability to generate long-term memory. The failure of several initially protective vaccine formulations is due to the lack of this long-term effect. Although several archaeosomes induced a strong primary antibody response that was sustained for prolonged lengths of time (>100 days), this did not always correlate with a strong memory response. T. acidophilum archaeosomes induced the most dramatic memory response to antigenic challenge (Fig. 7). The parallel induction of strong cell cycling in CD4+ cells suggested efficient maintenance of T-cell memory as well (Fig. 8). Memory T cells have been characterized as CD44hi (11). Interestingly, there was also a moderate increase (10 to 15%) in the expression of CD44hi CD4+ cells in the spleens of mice immunized with T. acidophilum archaeosomes (data not shown), reiterating the potent memory response. It has been suggested that Ag persistence may enhance the maintenance of long-term memory (4, 27). The internalization of >80% of the Ag within T. acidophilum archaeosomes (Table 1), coupled with an anticipated in vivo stability of these unilayer caldarchaeol vesicles, may indeed have allowed maintenance of Ag for longer durations. Indeed, the less stable H. salinarum vesicles that had substantial amounts of the Ag localized on their surfaces (<60% internalized) induced a strong primary response but failed to induce a significant memory response. Overall, the availability of various archaeosome types that associate Ags differently may provide a convenient tool for modulation of primary and/or memory responses suited to a particular vaccine application.
Bacterial structures have provided a major source of immunoadjuvants. Peptidoglycans and the monophosphoryl lipid A or lipid A components of LPS are examples that can modulate the immune system without themselves being immunogenic (3). Similarly, the advantage of archaeosomes appears to be in their ability to modulate both immune effector arms. Furthermore, we have not observed significant induction of antilipid antibodies after several repeated archaeosome immunizations (G. D. Sprott et al., unpublished observations). The exact mechanism(s) underlying the effectiveness of archaeosomes and other bacterial adjuvants remains unclear. Bacterial products may be immunomodulatory because they mimic the microbial structures that provide the danger signal of infection to the host (3). Moreover, the recognition of defined microbial components by phylogenetically ancient receptors present on APCs may result in immune activation. The presence of CD14, the LPS receptor on macrophages and neutrophils, is one such example (25). Also, phosphatidylserine which is expressed on apoptotic cells and recognized by CD36 on macrophages (12) is found as archaetidylserine and caldarchaetidylserine analogs in the TPL of some archaeal strains, notably M. smithii (39). Archaeosomes appear to be phagocytosed by APCs to a greater extent than conventional liposomes (43). Whether this interaction is mediated by specific receptors and/or results in differential activation of the APCs, and thereby enhances Ag presentation, still needs to be determined.
In our earlier studies, we reported archaeosomes to be superior in inducing antibody responses to the entrapped Ag in comparison to several conventional liposome formulations (42). In this study, we observed archaeosomes to be distinctly superior to conventional liposomes (DMPC-DMPG-CHOL) in their ability to induce cell-mediated immunity as well. Conventional liposomes have often been used to render protective protein and peptide Ags immunogenic, as well as to detoxify and/or deliver other potential adjuvants such as CT, lipid A, and cytokines (17, 23, 34). Liposomes are versatile systems for effective delivery of Ags. However, the main impediments to the use of conventional liposomes as vaccine adjuvants have been the need to formulate complex mixtures with other immunoadjuvants, the consequent uncertainty of safety in humans, and the lack of cost-effectiveness in obtaining purified lipids. Archaeosomes may surpass such impediments, as they are independently effective and clearly superior to alum in their adjuvant action. Furthermore, M. smithii is a natural inhabitant of the human colon (28), suggesting that lipids from such archaeobacteria may be most readily accepted for vaccine formulations. Our studies with mice so far have failed to indicate any archaeosome-related toxicity even in the long term. A lack of toxicity may stem from the eukaryote-like lipid structures, which bear phytanyl alkyl chains, as do fat-soluble vitamins. Indeed, a detailed study of mice with archaeosomes composed of the main polar lipid of T. acidophilum has indicated no toxicity (13).
A number of immunoadjuvants including CT, lipid A, MF-59, and chitosan as well as particulate delivery systems such as ISCOMS, proteosomes, nonionic surfactant vesicles, and microspheres are being studied for their efficacy and potential use in humans (3, 24, 36). The exponential rise in the number of adjuvants being tested in recent years emphasizes the critical need for novel universal immunostimulants for use with next-generation vaccines. The advantages of archaeosomes appear to be twofold: firstly in their ability to provide an antigenic depot like microspheres and conventional liposomes and secondly in their immunostimulation potential like that of CT, lipid A, and ISCOMS. As a carrier system, archaeosomes are highly stable (40) and capable of sustaining Ag for prolonged periods. As immunostimulants, archaeosomes induce both Th1 and Th2 responses. The efficacy of archaeosomes for protective immunization, their mechanism(s) of action, and safety require further study. Nevertheless, the available data indicate that archaeosomes may hold great promise as vaccine delivery vehicles.
ACKNOWLEDGMENTS
We are grateful to Lise Deschatelets and Perry Fleming for archaeal lipid production.
Footnotes
National Research Council of Canada publication no. 40403.
REFERENCES
- 1.Alving C R, Koulchin V, Glenn G M, Rao M. Liposomes as carriers of peptide antigens: induction of antibodies and cytotoxic T lymphocytes to conjugated and unconjugated peptides. Immunol Rev. 1995;145:5–31. doi: 10.1111/j.1600-065x.1995.tb00075.x. [DOI] [PubMed] [Google Scholar]
- 2.Ambrosch F, Wiedermann G, Jonas S, Althaus B, Finkel B, Gluck R, Herzog C. Immunogenicity and protectivity of a new liposomal hepatitis A vaccine. Vaccine. 1997;15:1209–1213. doi: 10.1016/s0264-410x(97)00015-7. [DOI] [PubMed] [Google Scholar]
- 3.Audibert F M, Lise L D. Adjuvants: current status, clinical perspectives and future prospects. Immunol Today. 1993;14:281–284. doi: 10.1016/0167-5699(93)90046-N. [DOI] [PubMed] [Google Scholar]
- 4.Bell E B, Sparshott S M, Bunce C. CD4+ T-cell memory, CD45R subsets and the persistence of antigen: a unifying concept. Immunol Today. 1998;19:60–64. doi: 10.1016/s0167-5699(97)01211-5. [DOI] [PubMed] [Google Scholar]
- 5.Beveridge T J, Choquet C G, Patel G B, Sprott G D. Freeze-fracture planes of methanogen membranes correlate with the content of tetraether lipids. J Bacteriol. 1993;175:1191–1197. doi: 10.1128/jb.175.4.1191-1197.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bix M, Wang Z E, Thiel B, Schork N J, Locksley R M. Genetic regulation of commitment to interleukin 4 production by a CD4+ T cell-intrinsic mechanism. J Exp Med. 1998;188:2289–2299. doi: 10.1084/jem.188.12.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boon T, Coulie P G, Van den Eynde B. Tumor antigens recognized by T cells. Immunol Today. 1997;18:267–268. doi: 10.1016/s0167-5699(97)80020-5. [DOI] [PubMed] [Google Scholar]
- 8.Cherwinski H M, Schumacher J H, Brown K D, Mosmann T R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987;166:1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choquet C G, Patel G B, Beveridge T J, Sprott G D. Formation of unilamellar liposomes from total polar lipid extracts of methanogens. Appl Environ Microbiol. 1992;58:2894–2900. doi: 10.1128/aem.58.9.2894-2900.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choquet C G, Patel G B, Beveridge T J, Sprott G D. Stability of pressure-extruded liposomes made from archaeobacterial ether lipids. Appl Microbiol Biotechnol. 1994;42:375–384. doi: 10.1007/BF00902745. [DOI] [PubMed] [Google Scholar]
- 11.Dutton R W, Bradley L M, Swain S L. T cell memory. Annu Rev Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 12.Fadok V A, Warner M L, Bratton D L, Henson P M. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (alpha v beta 3) J Immunol. 1998;161:6250–6257. [PubMed] [Google Scholar]
- 13.Freisleben H J, Bormann J, Litzinger D C, Lehr F, Rudolph P, Schatton W, Huang L. Toxicity and biodistribution of liposomes of the main phospholipid from the archaeobacterium Thermoplasma acidophilum. J Liposome Res. 1995;5:215–223. [Google Scholar]
- 14.Gregoriadis G. The immunological adjuvant and vaccine carrier properties of liposomes. J Drug Target. 1994;2:351–356. doi: 10.3109/10611869408996809. [DOI] [PubMed] [Google Scholar]
- 15.Gregoriadis G. Engineering liposomes for drug delivery: progress and problems. Trends Biotechnol. 1995;13:527–537. doi: 10.1016/S0167-7799(00)89017-4. [DOI] [PubMed] [Google Scholar]
- 16.Gupta R K, Rost B E, Reyveld E, Siber G S. Adjuvant properties of aluminum and calcium compounds. In: Powell M F, Newman M J, editors. Vaccine design: the subunit and adjuvant approach. New York, N.Y: Plenum Press; 1995. pp. 229–248. [DOI] [PubMed] [Google Scholar]
- 17.Harokopakis E, Hajishengallis G, Michalek S M. Effectiveness of liposomes possessing surface-linked recombinant B subunit of cholera toxin as an oral antigen delivery system. Infect Immun. 1998;66:4299–4304. doi: 10.1128/iai.66.9.4299-4304.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henkart P A. CTL effector functions. Semin Immunol. 1997;9:85–86. doi: 10.1006/smim.1997.0064. [DOI] [PubMed] [Google Scholar]
- 19.Kates M. Archaebacterial lipids: structure, biosynthesis and function. Biochem Soc Symp. 1992;58:51–72. [PubMed] [Google Scholar]
- 20.Kates M, Moldoveanu N, Stewart L C. On the revised structure of the major phospholipid of Halobacterium salinarum. Biochim Biophys Acta. 1993;1169:46–53. doi: 10.1016/0005-2760(93)90080-s. [DOI] [PubMed] [Google Scholar]
- 21.Krishnan L, Guilbert L J, Wegmann T G, Belosevic M, Mosmann T R. T helper 1 response against Leishmania major in pregnant C57BL/6 mice increases implantation failure and fetal resorptions: correlation with increased IFN-γ and TNF and reduced IL-10 production by placental cells. J Immunol. 1996;156:653–662. [PubMed] [Google Scholar]
- 22.Krishnan L, Mosmann T R. Functional subpopulation of CD4+ T lymphocytes. In: Kimber I, Selgrade M K, editors. T lymphocyte subpopulations in immunotoxicology. Chichester, United Kingdom: John Wiley & Sons; 1998. pp. 7–32. [Google Scholar]
- 23.Lachman L B, Ozpolat B, Rao X M. Cytokine-containing liposomes as vaccine adjuvants. Eur Cytokine Netw. 1996;7:693–698. [PubMed] [Google Scholar]
- 24.Leclerc C, Ronco J. New approaches in vaccine development. Immunol Today. 1998;19:300–302. doi: 10.1016/s0167-5699(98)01282-1. [DOI] [PubMed] [Google Scholar]
- 25.Lei M G, Chen T Y, Morrison D C. Lipopolysaccharide/lipid A receptors on lymphocytes and macrophages. Int Rev Immunol. 1990;6:223–235. doi: 10.3109/08830189009056633. [DOI] [PubMed] [Google Scholar]
- 26.Makabi Panzu B, Sprott G D, Patel G B. Coenzyme Q10 in vesicles composed of archaeal ether lipids or conventional lipids enhances the immuno-adjuvanticity to encapsulated protein. Vaccine. 1998;16:1504–1510. doi: 10.1016/s0264-410x(98)00018-8. [DOI] [PubMed] [Google Scholar]
- 27.Markiewicz M A, Girao C, Opferman J T, Sun J, Hu Q, Agulnik A A, Bishop C E, Thompson C B, Ashton Rickardt P G. Long-term T cell memory requires the surface expression of self-peptide/major histocompatibility complex molecules. Proc Natl Acad Sci USA. 1998;95:3065–3070. doi: 10.1073/pnas.95.6.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller T L, Wolin M J. Enumeration of Methanobrevibacter smithii in human feces. Arch Microbiol. 1982;131:14–18. doi: 10.1007/BF00451492. [DOI] [PubMed] [Google Scholar]
- 29.Mosmann T R, Fong T A. Specific assays for cytokine production by T cells. J Immunol Methods. 1989;116:151–158. doi: 10.1016/0022-1759(89)90198-1. [DOI] [PubMed] [Google Scholar]
- 30.Mosmann T R, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 31.Ohara J, Paul W E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985;315:333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- 32.Patel G B, Agnew B J, Jarrell H C, Sprott G D. Stability of liposomes prepared from the total polar lipids of Methanosarchina mazei is affected by the specific salt form of the lipids. J Liposome Res. 1999;9:229–245. [Google Scholar]
- 33.Poltorak A, He X, Smirnova I, Liu M Y, Huffel C V, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 34.Richards R L, Rao M, Wassef N M, Glenn G M, Rothwell S W, Alving C R. Liposomes containing lipid A serve as an adjuvant for induction of antibody and cytotoxic T-cell responses against RTS, S malaria antigen. Infect Immun. 1998;66:2859–2865. doi: 10.2307/1366431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ring K, Henkel B, Valenteijn A, Gutermann R. Studies on the permeability and stability of liposomes derived from a membrane-spanning bipolar archaeobacterial tetraetherlipid. In: Schmidt K H, editor. Liposomes as drug carriers. Stuttgart, Germany: Thieme; 1986. pp. 101–123. [Google Scholar]
- 36.Sjolander A, Cox J C, Barr I G. ISCOMs: an adjuvant with multiple functions. J Leukoc Biol. 1998;64:713–723. doi: 10.1002/jlb.64.6.713. [DOI] [PubMed] [Google Scholar]
- 37.Snapper C M, Paul W E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 38.Sprott G D. Structures of archaeobacterial membrane lipids. J Bioenerg Biomembr. 1992;24:555–566. doi: 10.1007/BF00762348. [DOI] [PubMed] [Google Scholar]
- 39.Sprott G D, Brisson J, Dicaire C J, Pelletier A K, Deschatelets L A, Krishnan L, Patel G B. A structural comparison of the total polar lipids from the human archaea Methanobrevibacter smithii and Methanosphaera stadtmanae and its relevance to the adjuvant activities of their liposomes. Biochim Biophys Acta. 1999;1440:275–288. doi: 10.1016/s1388-1981(99)00130-4. [DOI] [PubMed] [Google Scholar]
- 40.Sprott G D, Dicaire C J, Fleming L P, Patel G B. Stability of liposomes prepared from archaeobacterial lipids and phosphatidylcholine mixtures. Cells Mater. 1996;6:143–155. [Google Scholar]
- 41.Sprott G D, Dicaire C J, Patel G B. The ether lipids of Methanosarcina mazei and other Methanosarcina species, compared by fast atom bombardment mass spectrometry. Can J Microbiol. 1994;40:837–843. [Google Scholar]
- 42.Sprott G D, Tolson D L, Patel G B. Archaeosomes as novel antigen delivery systems. FEMS Microbiol Lett. 1997;154:17–22. doi: 10.1111/j.1574-6968.1997.tb12618.x. [DOI] [PubMed] [Google Scholar]
- 43.Tolson D L, Latta R K, Patel G B, Sprott G D. Uptake of archaeobacterial and conventional liposomes by phagocytic cells. J Liposome Res. 1996;6:755–776. [Google Scholar]
- 44.Verma J N, Wassef N M, Wirtz R A, Atkinson C T, Aikawa M, Loomis L D, Alving C R. Phagocytosis of liposomes by macrophages: intracellular fate of liposomal malaria antigen. Biochim Biophys Acta. 1991;1066:229–238. doi: 10.1016/0005-2736(91)90191-a. [DOI] [PubMed] [Google Scholar]
- 45.Wessel D, Flugge U I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- 46.Williams N A, Hirst T R, Nashar T O. Immune modulation by the cholera-like enterotoxins: from adjuvant to therapeutic. Immunol Today. 1999;20:95–101. doi: 10.1016/s0167-5699(98)01397-8. [DOI] [PubMed] [Google Scholar]