Abstract
Background
Among critically ill patients in the intensive care unit (ICU), sepsis is an urgent global public health problem due to its high incidence, high mortality rate and complex pathogenesis.
Objective
This study was to evaluate the predictive value of neutrophil-to-lymphocyte ratio (NLR), and neutrophil-to-lymphocyte and platelet ratio (NLPR) in-hospital mortality in septic patients on days 1, 3 and 5 in ICU.
Methods
The data of septic patients admitted to the ICU of the Sixth Affiliated Hospital of Sun Yat-sen University from March, 2018 to July, 2019 were collected. NLR and NLPR were calculated and multivariate logistic regression analysis was performed to identify the relationship between them and in-hospital mortality, respectively. Receiver operating characteristic curve (ROC) was used to determine the efficacy and optimal cutoff value of diagnostic tests.
Results
A total of 173 septic patients were included in this analysis, including 108 cases in the survival group and 65 in the death group, with a total mortality rate of 37.6%. A multivariate logistic regression analysis showed that NLR on day 5 was independently correlated with in-hospital mortality rate (OR 1.041, 95% CI: 1.008–1.074), and Day 5 NLPR was also independently associated with in-hospital mortality rate (OR 1.020, 95% CI: 1.001–1.040). The areas under the receiver operating characteristic curve (AUC) of the NLR on days 1 and 3 was 0.513 and 0.542 respectively, and the optimal cutoff value were 23.16 and 15.48, and the AUC of the NLR on day 5 was 0.589, and the best cutoff value was 15.85. The AUC of NLPR on day 1 and 3 was 0.517 and 0.547, respectively, and the optimal cutoff value was 10.25 and 18.47. The AUC of NLPR on day 5 was the largest, 0.654, and the optimal cutoff value was 8.22. After combined NLPR on day 5 with age and sequential organ failure assessment (SOFA) scores, the AUC increase to 0.718. Among the joint predictors, the optimal cutoff value for NLPR on day 5 was 9.31.
Conclusion
We found that Day 5 NLPR and NLR were independently correlated with in-hospital mortality. Day 5 NLPR Combined with age and SOFA scores may be help predict mortality in ICU septic hospitalized patients.
Keywords: Sepsis, Neutrophil, Lymphocyte, Platelet, Mortality
Sepsis; Neutrophil; Lymphocyte; Platelet; Mortality.
1. Introduction
Among critically ill patients in the intensive care unit (ICU), sepsis is the leading cause of death [1]. Sepsis lacks specific, accurate, and efficient diagnostic tools and specific treatments. Although anti-infection, organ protection, and fluid resuscitation strategies are used clinically to achieve the purpose of treatment, the incidence of sepsis and the mortality remain high [2, 3]. There are about 31.5 million cases of sepsis worldwide each year, of which about 5.3 million septic patients die [4]. In the United States alone, there are 1 million new cases of sepsis every year [5]. In Western Asia, the incidence of sepsis in patients admitted to ICU is >41.76%, and the mortality >55.8% [6]. The mortality of sepsis is higher in developing countries such as China and India than that in developed countries [7, 8]. Because of its high incidence, high mortality and complex pathogenesis, sepsis has now become an urgent global public health problem [9].
Immune dysfunction and coagulation dysfunction accompany the whole process of the occurrence and development in sepsis. Neutrophils and lymphocytes are immune cells, which play an important role in immune response. Platelets are a key factor affecting coagulation function and inflammatory response [10]. Actually, neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are considered a simple, inexpensive, widely available inflammatory indicator, independent risk factor and prognostic predictor of sepsis [11, 12, 13, 14]. Some recent studies have used neutrophil-to-lymphocyte and platelet ratio (NLPR) to predict post-abdominal surgery, post-cardiovascular surgery, post-emergency surgery trauma, acute kidney injury and the disease progression and risk of death in coronavirus 2019 disease (COVID-19) patients in the ICU [15, 16, 17, 18]. However, there are few studies on the correlation between NLPR and sepsis.
The present study aims to evaluate the predictive value of NLR and NLPR in septic patients in the ICU on days 1, 3 and 5 in in-hospital mortality.
2. Materials and methods
2.1. Study design
Clinical data of 173 septic patients admitted to the ICU of the Sixth Affiliated Hospital of Sun Yat-sen University from March, 2018 to July, 2019 were collected. The retrospective study was approved by the Ethics Committee of the Sixth Affiliated Hospital of Sun Yat-sen University (Ethical Approval No.: 2022ZSLYEC-057).
2.2. Inclusion and exclusion criteria
The definition of sepsis is based on Sepsis-3 standard in 2016 [19]. The inclusion criteria are: (1) age ≥18 years and ≤85 years; (2) at least one acute severe organ failure associated with sepsis, and sequential organ failure assessment (SOFA) score ≥5; (3) the presence of confirmed or suspected foci of infection, meeting at least one of the following criteria: (a) pathogenic microorganism growth in the blood or sterile site, (b) the appearance of an abscess or partially infected tissue, (c) suspected infection, supported by at least one of the following: leukocytes in normal sterile sites; perforation of internal organs; imaging evidence of pneumonia with purulent discharge; and syndromes associated with high risk of infection. Exclusion criteria are: (1) solid organ or bone marrow transplantation; (2) systemic autoimmune diseases or acquired immune diseases such as Human Immunodeficiency Virus (HIV); (3) malignant blood system tumors; (4) radiotherapy or chemotherapy in the last two weeks; (5) long-term glucocorticoid or immunosuppressive agent treatment history; (6) elevated leucocyte and platelet drugs have been used in the past week; (7) any action to stop the willingness of sustaining life in an artificial way (abandoning active rescue treatment), or severe underlying illness, terminal state, or death within the next 24 h; (8) pregnant and lactating patients. As for patients who were admitted to the ICU multiple times, only their first visit was included in the study.
2.3. Data extraction
Data were collected on the patient's age, gender, history of smoking and drinking, site of infection, underlying disease, vasoactive drug use, laboratory results, Glasgow coma scale (GCS) score, acute physiology and chronic health evaluation II (APACHE II) score, SOFA score, continuous renal replacement therapy (CRRT), mechanical ventilation, hospital stay, ICU retention time. Record the patient's peripheral blood neutrophil, lymphocyte, and platelet counts for days 1, 3, and 5 to calculate the NLR and NLPR for days 1, 3 and 5. Day 1 was defined as the first 24 h of entering the ICU.
2.4. Outcome definition
The main outcome is the in-hospital mortality rate, which is defined as death during hospitalization. NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count. NLPR is calculated by multiplying NLR by 100 and dividing by the platelet count.
2.5. Statistical analysis
SPSS 25.0 software was used for data analysis. The measurement data in accordance with normal distribution were described by mean ± standard deviation (SD), and the independent sample t-test was used for comparison between groups. Non-normally distributed measurement data were presented by median (interquartile range), and comparison between groups was performed by Mann-Whitney U test. Chi-square test or Fisher's exact test were used for counting data. NLR and NLPR were included in multivariate logistic regression analysis respectively to find the relationship between them and in-hospital mortality. Receiver operating characteristic curve (ROC) was used to determine the efficacy and optimal cutoff value of diagnostic tests, and the reliability and stability of diagnostic tests were evaluated by sensitivity and specificity. For the analysis of combined variable, the predicted probability value is determined by logistic regression analysis. The ROC curve was plotted by the predicted probability value. P < 0.05 was considered as statistically significant difference.
3. Results
3.1. Patient characteristics
A total of 173 septic patients were included in this analysis, 108 cases in the survival group and 65 in the death group, with a total mortality rate of 37.6%. The baseline characteristic comparison between the survival group and the death group is shown in Table 1. The average age of the included patients was 64 years, 123 cases in men and 50 in women, and 71.1% of men. There were statistical differences in age, APACHE II score, SOFA score, length of stay in hospital, and vasoactive drug use between the survival group and the death group (P < 0.05). The death group had older age, higher APACHE II scores, SOFA scores, vasoactive drug use rates and long hospitalization. However, there were no statistical differences between the two groups in terms of gender, history of smoking and drinking, site of infection, underlying disease, GCS score, CRRT, mechanical ventilation, ICU retention time, creatinine, total bilirubin and oxygenation index.
Table 1.
Characteristics of patients enrolled in the study.
| Total | Survivor | Death | P | |
|---|---|---|---|---|
| N | 173 | 108 (62.43%) | 65 (37.57%) | |
| Age (years, median [IQR]) | 64 (55–75.5) | 62 (54–71) | 68 (60–79.5) | 0.008 |
| Sex, n (%) | 0.266 | |||
| Male | 123 | 80 (74.07%) | 43 (66.15%) | |
| Female | 50 | 28 (25.93) | 22 (33.85%) | |
| Main site of infection, n (%) | ||||
| Abdomen | 100 | 62 (57.41%) | 38 (58.46%) | 0.892 |
| Lung | 65 | 40 (37.04%) | 25 (38.46%) | 0.851 |
| Other | 8 | 6 (5.56%) | 2 (3.08%) | 0.452 |
| APACHE II, median (IQR) | 19 (15–24) | 18 (14–23.75) | 19 (16–27) | 0.046 |
| SOFA, median (IQR) | 8 (6–10) | 8 (6–10) | 9 (7–11) | 0.001 |
| GCS, median (IQR) | 13 (9–15) | 13 (9–15) | 12 (7–15) | 0.065 |
| Comorbidities, n (%) | ||||
| Hypertension | 53 | 31 (28.70%) | 22 (33.85%) | 0.477 |
| Diabetes | 30 | 15 (13.89%) | 15 (23.08%) | 0.122 |
| CAD | 13 | 6 (5.56%) | 7 (10.77%) | 0.208 |
| COPD | 16 | 9 (8.33%) | 7 (10.77%) | 0.592 |
| Stroke | 27 | 14 (12.96%) | 13 (20%) | 0.217 |
| Smoker, n (%) | 27 | 16 (14.81%) | 11 (16.92%) | 0.711 |
| Alcohol, n (%) | 14 | 11 (10.18%) | 3 (4.62%) | 0.193 |
| Hospital stay (day, median [IQR]) | 27 (17–43) | 30.5 (20–53) | 21 (12–33.5) | 0.000 |
| ICU stay (day, median [IQR]) | 6 (4–12) | 5 (4–9.75) | 7 (4–12) | 0.103 |
| Vasopressor, n (%) | 109 | 61 (56.48%) | 48 (73.85%) | 0.022 |
| CRRT, n (%) | 30 | 15 (13.89%) | 15 (23.08%) | 0.122 |
| Mechanical ventilation (day, median [IQR]) | 121 | 73 (67.59%) | 48 (73.85%) | 0.434 |
| Laboratory parameters, (day, median [IQR]) | ||||
| Creatinine (μmol/L) | 172.95 ± 192.18 | 178.78 ± 212.28 | 163.26 ± 154.15 | 0.608 |
| Total bilirubin (μmol/L) | 27.89 ± 33.72 | 24.17 ± 25.85 | 34.05 ± 43.33 | 0.062 |
| PaO2/FiO2 (mmHg) | 261.92 | 260.77 ± 121.57 | 263.82 ± 146.30 | 0.883 |
| Neutrophil (109/L, median [IQR]) | ||||
| Day 1 | 9.99 (6.42–14.52) | 9.91 (5.80–14.36) | 10.48 (7.6–14.63) | 0.378 |
| Day 3 | 8.85 (6.08–13.10) | 8.56 (5.91–12.66) | 9.69 (7.06–13.55) | 0.144 |
| Day 5 | 8.00 (5.73–13.23) | 7.23 (5.37–11.68) | 10.11 (6.86–13.66) | 0.02 |
| Lymphocyte (109/L, median [IQR]) | ||||
| Day 1 | 0.62 (0.38–1.03) | 0.57 (0.40–1.03) | 0.68 (0.33–1.08) | 0.769 |
| Day 3 | 0.83 (0.53–1.18) | 0.83 (0.55–1.16) | 0.74 (0.5–1.2) | 0.562 |
| Day 5 | 0.86 (0.58–1.21) | 0.91 (0.60–1.21) | 0.74 (0.5–1.23) | 0.19 |
| Platelet (109/L, median [IQR]) | ||||
| Day 1 | 151.0 (93.0–233.0) | 144.50 (97.25–217.23) | 168 (81.5–251) | 0.578 |
| Day 3 | 144.0 (86.0–208.0) | 142.5 (89.0–203.5) | 146 (75–218) | 0.967 |
| Day 5 | 161.0 (93.5–242.1) | 172.5 (117.75–245.03) | 134 (70–233) | 0.028 |
| NLR, median (IQR) | ||||
| Day 1 | 14.43 (9.36–24.34) | 14.66 (9.27–23.79) | 14.14 (9.43–28.06) | 0.771 |
| Day 3 | 11.03 (6.79–18.80) | 10.29 (6.55–17.37) | 11.68 (6.79–19.23) | 0.353 |
| Day 5 | 10.32 (6.15–14.51) | 9.91 (5.53–13.88) | 11.23 (6.77–19.77) | 0.049 |
| NLPR, median (IQR) | ||||
| Day 1 | 10.04 (4.95–20.95) | 9.66 (5.72–18.83) | 11.57 (4.19–26.19) | 0.716 |
| Day 3 | 7.73 (4.15–16.94) | 7.45 (4.31–14.88) | 8.51 (3.99–22.47) | 0.298 |
| Day 5 | 5.73 (3.14–13.43) | 5.16 (2.91–9.12) | 9.81 (4.23–20.32) | 0.001 |
IQR, interquartile range; SOFA, sequential organ failure assessment; APACHE II: Acute physiology and chronic health evaluation II; GCS, Glasgow coma scale; COPD, Chronic obstructive pulmonary disease; CAD, coronary artery disease; ICU, intensive care unit; PaO2/FiO2, Oxygen partial pressure/oxygen concentration; CRRT, continuous renal replacement therapy; NLR, neutrophil-to-lymphocyte ratio; NLPR, neutrophil-to-lymphocyte and platelet ratio.
PaO2/FiO2, Oxygen partial pressure/oxygen concentration; CRRT, continuous renal replacement therapy; NLR, neutrophil-to-lymphocyte ratio; NLPR, neutrophil-to-lymphocyte and platelet ratio.
3.2. Predictors of mortality
There was no statistical difference in neutrophils, lymphocytes, platelet count, NLR, NLPR on days 1 and 3 between the two groups, but neutrophils, lymphocytes, platelet counts, NLR, NLPR on day 5 are statistically different. The death group had higher neutrophil count, NLR, NLPR, and lower lymphocyte and platelet counts on day 5 (Table 1).
Therefore, we put age, gender, Day 5 NLR, APACHE II score and SOFA score in the multivariate logistic regression analysis and found that Day 5 NLR was independently correlated with the inpatient mortality (ORs 1.041, 95% CI: 1.008–1.074) (Table 2). Additionally, the age, gender, Day 5 NLPR, APACHE II score and SOFA score were included in the multivariate logistic regression analysis, and the results showed that Day 5 NLPR was independently correlated with in-hospital mortality (ORs 1.020, 95% CI: 1.001–1.040) (Table 3).
Table 2.
Multivariable logistic regressions exploring the association of NLR(d5) with in-hospital mortality.
| Factor | Odds ratio | 95% CI | P |
|---|---|---|---|
| NLR(d5) | 1.041 | 1.008–1.074 | 0.014 |
| Sex (male gender) | 1.502 | 0.728–3.098 | 0.271 |
| Age (years) | 1.033 | 1.005–1.061 | 0.019 |
| SOFA | 1.257 | 1.085–1.455 | 0.002 |
| APACHE II | 0.988 | 0.932–1.047 | 0.678 |
NLR, neutrophil-to-lymphocyte ratio; SOFA, sequential organ failure assessment; APACHE II: Acute physiology and chronic health evaluation.
Table 3.
Multivariable logistic regressions exploring the association of NLPR (d5) with in-hospital mortality.
| Factor | OR | 95% CI | P |
|---|---|---|---|
| NLPR (d5) | 1.020 | 1.001–1.040 | 0.036 |
| Sex (male gender) | 1.334 | 0.646–2.755 | 0.436 |
| Age (years) | 1.037 | 1.010–1.066 | 0.008 |
| SOFA | 1.221 | 1.053–1.416 | 0.008 |
| APACHE II | 0.987 | 0.931–1.045 | 0.646 |
NLPR, neutrophil-to-lymphocyte and platelet ratio; SOFA, sequential organ failure assessment; APACHE II: Acute physiology and chronic health evaluation; OR, odds ratio.
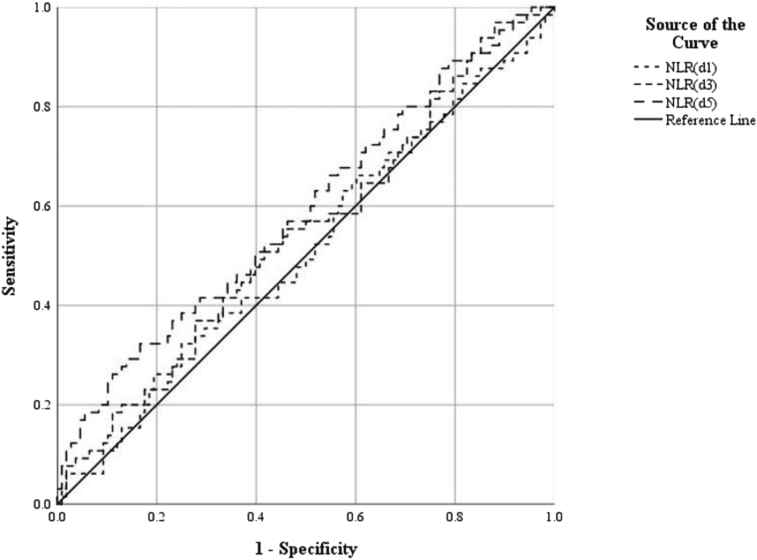
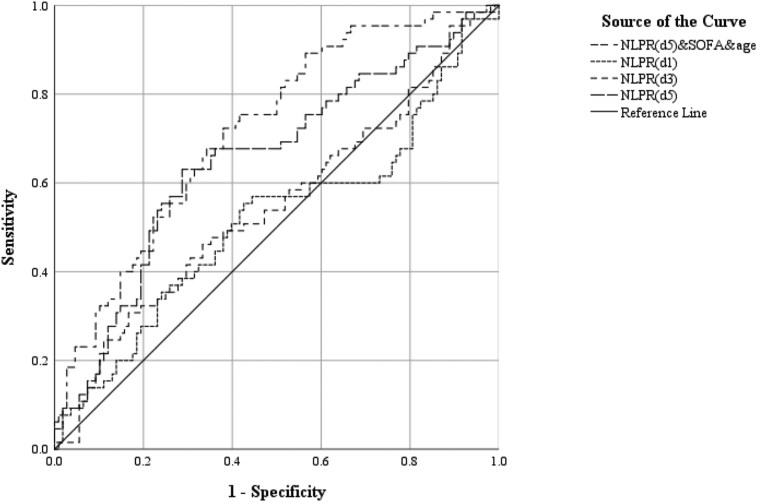
To further explore predictive value of NLR and NLPR in in-hospital mortality, we performed the ROC to determine the efficacy and optimal cutoff value of diagnostic tests. We observed that the areas under the ROC curve (AUC) of the NLR on the 1st and 3rd days were 0.513 and 0.542 respectively, and the optimal cutoff value was 23.16 and 15.48. Compared with days 1 and 3, the AUC of the NLR on the 5th day was greater, i.e., 0.589, and the optimal cutoff value was 15.85 (Table 4, Figure 1). With the progression of time, the AUC of NLPR levels predicted in-hospital mortality rate in patients with severe infections gradually increased, and their sensibility and specificity increased. Of interest, we noticed that the AUC of NLPR on days 1 and 3 were 0.517 and 0.547 respectively, and the optimal cutoff value were 10.25 and 18.47, of which the AUC of NLPR on day 5 was greater than 0.654, and the optimal cutoff value was 8.22 (Table 4, Figure 2). Therefore, with the extension of hospitalization time, the AUC of NLPR and NLR were gradually increased. Moreover, the AUC of NLPR was much higher than that of NPR at the same time point. Therefore, the results indicate that NLPR and NLR on days 5 had relevancy with in-hospital mortality in ICU septic patients. Compared with NLR, the relevancy of NLPR was higher. Therefore, the results indicate that NLPR and NLR on days 5 had relevancy with in-hospital mortality in ICU septic patients. Compared with NLR, the relevancy of NLPR was higher. After combined NLPR on day 5 with age and SOFA scores, the AUC increase to 0.718 and the sensitivity and specificity were 0.723 and 0.62 respectively (Table 4, Figure 2).
Table 4.
Predictive value of NLR and NLPR at different time points for in-hospital mortality of sepsis patients.
| Parameter | Cut off value | Specificity | Sensitivity | AUC | 95% CI | P |
|---|---|---|---|---|---|---|
| NLR(d1) | 23.159 | 0.75 | 0.323 | 0.513 | 0.424–0.603 | 0.046 |
| NLR(d3) | 15.48 | 0.72 | 0.369 | 0.542 | 0.453–0.631 | 0.045 |
| NLR(d5) | 15.85 | 0.833 | 0.323 | 0.589 | 0.501–0.678 | 0.045 |
| NLPR (d1) | 10.25 | 0.574 | 0.554 | 0.517 | 0.423–0.610 | 0.047 |
| NLPR (d3) | 18.469 | 0.833 | 0.308 | 0.547 | 0.456–0.638 | 0.046 |
| NLPR (d5) | 8.22 | 0.713 | 0.631 | 0.654 | 0.568–0.740 | 0.044 |
| NLPR (d5) & age & SOFA | 0.35 | 0.723 | 0.62 | 0.718 | 0.641–0.795 | 0.039 |
NLR, neutrophil-to-lymphocyte ratio; NLPR, neutrophil-to-lymphocyte and platelet ratio; AUC, Areas under the ROC curve; CI, confidence interval.
Figure 1.
Receiver operating characteristic analysis for the best cutoff values of NLR. NLR, neutrophil-to-lymphocyte ratio.
Figure 2.
Receiver operating characteristic analysis for the best cutoff values of NLPR. NLPR, neutrophil-to-lymphocyte and platelet ratio.
4. Discussion
Previous studies have shown that mortality are positively correlated with SOFA scores [20]. In order to avoid the impact of low mortality due to low SOFA scores, our study selected cases with a SOFA score ≥5 for retrospective analysis. In this study, we found that NLPR and NLR on days 1, 3, and 5 were correlated with in-hospital mortality of septic patients in ICU. The NLPR and NLR on day 5 had a better correlation with in-hospital mortality than those on day 1 and day 3. In addition, day 5 NLPR combined with SOFA score and age may help predict in-hospital mortality of septic patients in ICU.
It is documented that neutrophils are innate immune cells of the body, scavenging pathogenic microorganisms and activating other immune cells [12]. The main mechanism of lymphocytopenia is lymphocyte apoptosis, which is the main cause of immunosuppression [10, 21]. The duration and degree of lymphopenia induced by apoptosis are related to higher risk of infection and mortality. In addition, platelets regulate leukocyte recruitment and activation through the secretion of serotonin, which promotes the release of cytokines and chemokines, affects the activation process of lymphocytes and neutrophils, and regulates the function of monocytes, macrophages, lymphocytes and endothelial cells [22]. Single neutrophils, lymphocytes or platelets are affected by many factors in clinic, so they have little correlation with the prognosis of patients with sepsis. In addition, due to the release of a large number of inflammatory mediators and cytokines in sepsis, the body's response to infection is out of control, and the balance between pro-inflammation and anti-inflammation is broken, resulting in the increase of neutrophils, the decrease of lymphocyte and platelet production and the increase of consumption. NLR and NLPR reflect the balance between innate and adaptive immunity [23], and NLR combined with neutrophils and lymphocytes and NLPR combined with neutrophils, lymphocytes and platelets improved the prognostic correlation of sepsis, so the higher NLR and NLPR, the more severe sepsis and the higher mortality.
Many studies have shown that NLR, PLR are inflammatory indicators, independent risk factors and prognostic predictors of sepsis, which is consistent with our findings [23, 24, 25, 26, 27]. Also, Platelets have also been shown to be associated with the severity and prognosis of sepsis [28]. More than half of sepsis patients with thrombocytopenia develop during hospitalization, and septic patients with moderate to severe thrombocytopenia have higher mortality [29]. Recently, Gameiro J's study found that postoperative NLPR was independently correlated with the incidence of AKI after major abdominal surgery [15], and that NLPR at the time of admission was independently associated with in-hospital mortality rate of sepsis AKI [30]. Koo CH's study also found that high NLPR were associated with postoperative AKI incidence and 5-year mortality, and that NLPR may help predict AKI incidence and mortality in cardiovascular surgery [16]. Studies have also found that NLPR on day 7 may be a better predictor of advanced mortality for trauma patients undergoing emergency surgery, better reflecting the patient's systemic inflammatory status [17]. Additionally, it is reported that the NLPR can also be used to predict the risk of mechanical ventilation, ICU admission and in-hospital death in COVID-19 patients [18]. As far as we know, our study is the first to study the association between NLPR and in-hospital mortality of ICU septic patients. Moreover, we found that NLPR was more correlated with in-hospital mortality of sepsis in ICU than NLR.
In addition, there was a new and interesting finding that NLPR on day 5 was more correlated with the prognosis of patients with sepsis than on day 1 and 3 in the dynamic observation in ICU. In sepsis, the inflammatory response is out of control, and the balance between pro-inflammatory and anti-inflammatory is broken, resulting in an increase in neutrophils, an decrease in the production and consumption of lymphocytes and platelets [26]. Conversely, if infection is controlled, sepsis improves, neutrophils decrease, lymphocytes and platelets recover. Therefore, day 5 NLPR correlation with in-hospital mortality of sepsis in ICU was better, which may be related to neutrophils decline, the recovery of lymphocytes and platelets in patients with sepsis control in the survival group, and the reverse displayed in patients in the death group.
Mortality in sepsis and septic shock are positively correlated with SOFA scores [20], and our study found that age and SOFA scores were independently correlated with in-hospital mortality by the multivariate logistic regression analysis. So we combined Day 5 NLPR with age and SOFA scores in the multivariate logistic regression analysis. We found that Day 5 NLPR combined with age and SOFA scores were correlated with in-hospital mortality and could be help predict mortality in ICU septic hospitalized patients.
The present study has some limitations: first, the study is unicentral and only based on a small sample size; second, retrospective studies may be biased. Therefore, further multicenter prospective studies are needed to verify the effectiveness of NLPR in predicting in-hospital mortality in septic patients in the ICU.
5. Conclusion
In summary, our study found that NLPR was independently correlated with in-hospital mortality in septic patients in ICU. Day 5 NLPR correlation with in-hospital mortality of sepsis in ICU was better. Day 5 NLPR Combined with age and SOFA scores may be help predict mortality in ICU septic hospitalized patients. Nevertheless, a large multicenter prospective study is needed to verify the findings.
Declarations
Author contribution statement
Yiming Shi – conceived and designed the experiments, performed the experiments, analysed and interpreted the data, contributed reagents analysis tools or data, and wrote the paper.
Wenfeng Xie – conceived and designed the experiments, analyzed and interpreted the data, contributed reagents analysis tools or data, and wrote reviews and editing.
Chunhua Yang – analyzed and interpreted the data, contributed reagents analysis tools or data.
Lei Chen – conceived and designed the experiments.
Min Cheng – contributed reagents analysis data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to acknowledge and thank all of the authors for their hard work.
References
- 1.Gaborit B.J., Chaumette T., Chauveau M., Asquier-Khati A., Roquilly A., Boutoille D., et al. Circulating regulatory T cells expressing tumor necrosis factor receptor type 2 contribute to sepsis-induced immunosuppression in patients during septic shock. J. Infect. Dis. 2021;224(12):2160–2169. doi: 10.1093/infdis/jiab276. [DOI] [PubMed] [Google Scholar]
- 2.Zhong X., Xie L., Yang X., Liang F., Yang Y., Tong J., et al. Ethyl pyruvate protects against sepsis-associated encephalopathy through inhibiting the NLRP3 inflammasome. Mol. Med. 2020;26(1):55. doi: 10.1186/s10020-020-00181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burke J., Wood S., Hermon A., Szakmany T. Improving outcome of sepsis on the ward: introducing the 'Sepsis Six' bundle. Nurs. Crit. Care. 2019;24(1):33–39. doi: 10.1111/nicc.12358. [DOI] [PubMed] [Google Scholar]
- 4.Fleischmann C., Scherag A., Adhikari N.K., Hartog C.S., Tsaganos T., Schlattmann P., et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am. J. Respir. Crit. Care Med. 2016;193(3):259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 5.Nolan A., Weiden M.D. Trends in sepsis and infection sources in the United States. A population-based study. Ann. Am. Thorac. Soc. 2015;12(5):784. doi: 10.1513/AnnalsATS.201501-044LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baykara N., Akalin H., Arslantas M.K., Hanci V., Caglayan C., Kahveci F., et al. Epidemiology of sepsis in intensive care units in Turkey: a multicenter, point-prevalence study. Crit. Care. 2018;22(1):93. doi: 10.1186/s13054-018-2013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao X., Du B., Lu M., Wu M., Kang Y. Current epidemiology of sepsis in mainland China. Ann. Transl. Med. 2016;4(17):324. doi: 10.21037/atm.2016.08.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatterjee S., Bhattacharya M., Todi S.K. Epidemiology of adult-population sepsis in India: a single center 5 Year experience. Ind. J. Crit. Care Med. 2017;21(9):573–577. doi: 10.4103/ijccm.IJCCM_240_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleischmann-Struzek C., Goldfarb D.M., Schlattmann P., Schlapbach L.J., Reinhart K., Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir. Med. 2018;6(3):223–230. doi: 10.1016/S2213-2600(18)30063-8. [DOI] [PubMed] [Google Scholar]
- 10.Marshall J.C. Inflammation, coagulopathy, and the pathogenesis of multiple organ dysfunction syndrome. Crit. Care Med. 2001;29(7 Suppl):S99–106. doi: 10.1097/00003246-200107001-00032. [DOI] [PubMed] [Google Scholar]
- 11.Ye W., Chen X., Huang Y., Li Y., Xu Y., Liang Z., et al. The association between neutrophil-to-lymphocyte count ratio and mortality in septic patients: a retrospective analysis of the MIMIC-III database. J. Thorac. Dis. 2020;12(5):1843–1855. doi: 10.21037/jtd-20-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bu X., Zhang L., Chen P., Wu X. Relation of neutrophil-to-lymphocyte ratio to acute kidney injury in patients with sepsis and septic shock: a retrospective study. Int. Immunopharm. 2019;70:372–377. doi: 10.1016/j.intimp.2019.02.043. [DOI] [PubMed] [Google Scholar]
- 13.Piotrowski D., Saczewska-Piotrowska A., Jaroszewicz J. Boron-kaczmarska A. Lymphocyte-To-Monocyte ratio as the best simple predictor of bacterial infection in patients with liver cirrhosis. Int. J. Environ. Res. Publ. Health. 2020;17(5) doi: 10.3390/ijerph17051727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Y., Huang X., Zhang W. Platelet-to-lymphocyte ratio as a prognostic predictor of mortality for sepsis: interaction effect with disease severity-a retrospective study. BMJ Open. 2019;9(1) doi: 10.1136/bmjopen-2018-022896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gameiro J., Fonseca J.A., Dias J.M., Milho J., Rosa R., Jorge S., et al. Neutrophil, lymphocyte and platelet ratio as a predictor of postoperative acute kidney injury in major abdominal surgery. BMC Nephrol. 2018;19(1):320. doi: 10.1186/s12882-018-1073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koo C.H., Eun Jung D., Park Y.S., Bae J., Cho Y.J., Kim W.H., et al. Neutrophil, lymphocyte, and platelet counts and acute kidney injury after cardiovascular surgery. J. Cardiothorac. Vasc. Anesth. 2018;32(1):212–222. doi: 10.1053/j.jvca.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 17.Chae Y.J., Lee J., Park J.H., Han D.G., Ha E., Yi I.K. Late mortality prediction of neutrophil-to-lymphocyte and platelet ratio in patients with trauma who underwent emergency surgery: a retrospective study. J. Surg. Res. 2021;267:755–761. doi: 10.1016/j.jss.2020.11.088. [DOI] [PubMed] [Google Scholar]
- 18.Cakir Guney B., Hayiroglu M., Senocak D., Cicek V., Cinar T., Kaplan M. Evaluation of N/LP ratio as a predictor of disease progression and mortality in COVID-19 patients admitted to the intensive care unit. Medeni Med. J. 2021;36(3):241–248. doi: 10.5222/MMJ.2021.95676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauer M., Gerlach H., Vogelmann T., Preissing F., Stiefel J., Adam D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019- results from a systematic review and meta-analysis. Crit. Care. 2020;24(1):239. doi: 10.1186/s13054-020-02950-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girardot T., Rimmele T., Venet F., Monneret G. Apoptosis-induced lymphopenia in sepsis and other severe injuries. Apoptosis. 2017;22(2):295–305. doi: 10.1007/s10495-016-1325-3. [DOI] [PubMed] [Google Scholar]
- 22.Mauler M., Bode C., Duerschmied D. Platelet serotonin modulates immune functions. Hämostaseologie. 2016;36(1):11–16. doi: 10.5482/HAMO-14-11-0073. [DOI] [PubMed] [Google Scholar]
- 23.Liu S., Wang X., She F., Zhang W., Liu H., Zhao X. Effects of neutrophil-to-lymphocyte ratio combined with interleukin-6 in predicting 28-day mortality in patients with sepsis. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.639735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Djordjevic D., Rondovic G., Surbatovic M., Stanojevic I., Udovicic I., Andjelic T., et al. Neutrophil-to-Lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and mean platelet volume-to-platelet count ratio as biomarkers in critically ill and injured patients: which ratio to choose to predict outcome and nature of bacteremia? Mediat. Inflamm. 2018;2018 doi: 10.1155/2018/3758068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spoto S., Lupoi D.M., Valeriani E., Fogolari M., Locorriere L., Beretta Anguissola G., et al. Diagnostic accuracy and prognostic value of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in septic patients outside the intensive care unit. Medicina. 2021;57(8) doi: 10.3390/medicina57080811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorente L., Martin M.M., Ortiz-Lopez R., Alvarez-Castillo A., Ruiz C., Uribe L., et al. Association between neutrophil-to-lymphocyte ratio in the first seven days of sepsis and mortality. Enferm. Infecc. Microbiol. Clín. 2022;40(5):235–240. doi: 10.1016/j.eimce.2020.11.022. [DOI] [PubMed] [Google Scholar]
- 27.Ren Y., Zhang L., Xu F., Han D., Zheng S., Zhang F., et al. Risk factor analysis and nomogram for predicting in-hospital mortality in ICU patients with sepsis and lung infection. BMC Pulm. Med. 2022;22(1):17. doi: 10.1186/s12890-021-01809-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greco E., Lupia E., Bosco O., Vizio B., Montrucchio G. Platelets and multi-organ failure in sepsis. Int. J. Mol. Sci. 2017;18(10) doi: 10.3390/ijms18102200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou H., Li Z., Liang H., Yan Z. Thrombocytopenia and platelet count recovery in patients with sepsis-3: a retrospective observational study. Platelets. 2021:1–9. doi: 10.1080/09537104.2021.1970124. [DOI] [PubMed] [Google Scholar]
- 30.Gameiro J., Fonseca J.A., Jorge S., Gouveia J., Lopes J.A. Neutrophil, lymphocyte and platelet ratio as a predictor of mortality in septic-acute kidney injury patients. Nefrologia. 2020;40(4):461–468. doi: 10.1016/j.nefro.2019.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.