Abstract
The poor regenerative ability of injured tendon tissues remains a clinical challenge. However, decellularized extracellular matrix (ECM) combined with stem cells shows promise. In contrast to bovine and porcine ECM, marine-derived decellularized ECM has several advantages; it is easily obtained, poses less biological risk, and is not contraindicated on religious grounds. This study successfully fabricated decellularized tilapia fish skin (DTFS) with copious preserved collagen fibers and natural pore structures. The outer layer is smooth and dense, while the inner layer has a soft structure with a rough surface. After crosslinking with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) and N-hydroxysuccinimide (NHS), crosslinked DTFS (C-DTFS) showed improved mechanics in dry and wet conditions. In vitro, the leach liquor of crosslinked DTFS showed no cytotoxicity and promoted migration and tenonic differentiation of tendon-derived stem cells (TDSCs). Meanwhile, TDSCs seeded in the inner surface of DTFS maintained viability, differentiated, and exhibited spreading. Furthermore, cell-seeded scaffolds guided the regeneration of tendon tissue in a rat Achilles tendon defect model. Our results suggest that DTFS combined with TDSCs is a novel and promising therapeutic option for tendon tissue engineering.
Keywords: Decellularized tilapia fish skin, Tendon tissue engineering, Tendon derived stem cells, Tenonic differentiation, Achilles tendon defect
1. Introduction
Achilles tendon rupture is a sports injury commonly encountered in clinical practice [1]. Since tendon tissues are characterized by hypocellularity and a lack of nutrient supply, the regeneration of massive tendon defects remains challenging for surgeons [2]. Furthermore, during the healing process, the injured site might be covered by fibrotic scars and exhibit heterotopic ossification, which significantly affect functional recovery [[3], [4], [5]]. Aberrant repair results in poor mechanical properties and a higher risk of re-rupture. Thus, there is an urgent need for an effective therapeutic strategy. Tissue engineering is a promising alternative to autografts, allografts, xenografts, and synthetic prostheses [6,7].
Tissue engineering involves the application of scaffolds, growth factors, and seed cells [8]. For tendon defect repair, appropriate scaffolds must be used. An ideal scaffold for tendon repair should have the biomimetic structure and mechanical properties of the native tendon, and guide tendon regeneration [9,10]. Type I collagen (Col-I) is the main component of the extracellular matrix (ECM) of native tendon, accounting for ∼65–80% of dry tendon mass and 95% of the total tendon collagen [11,12]. Thus, several collagen-based decellularized scaffolds have been widely applied in tendon tissue engineering [13,14]. These scaffolds are mostly mammalian-derived, including decellularized human, porcine, bovine, and equine tendon sheets, dermis, pericardium, small intestinal submucosa, etc. [[15], [16], [17], [18], [19]]. However, these mammal-derived decellularized ECMs have limitations such as limited donor sources, the potential risk of zoonotic diseases, and ethical and religious issues [20]. By contrast, decellularized marine collagen scaffolds could overcome these disadvantages [21]. Decellularized tilapia fish skin (DTFS) has recently received considerable attention because it is easily obtained and processed, and displays excellent biosafety; moreover, its use is not contraindicated on ethical or religious grounds [20,22]. DTFS has been used successfully for burn treatment, bone regeneration, and wound healing [20,22,23]. Besides, 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide and N-hydroxysuccinimide (EDC-NHS) is one of the most common crosslinking agents for collagen materials, offering a non-cytotoxic and zero length crosslinking option, since it is highly water soluble so that it could be easily and thoroughly removed by repeated post-crosslinking rinsing with water [[24], [25], [26]]. Therefore, we investigated whether crosslinked DTFS is suitable for tendon repair or tendon tissue engineering; it merits investigation based on its biological potential and economic value.
Seed cells are also important for tissue engineering, and promote tissue regeneration. Several cell types with potential as sources for tendon repair have bene identified, including tenocytes [27], fibroblasts [28], bone marrow-derived mesenchymal stem cells (BMSCs), adipose-derived stem cells (ADSCs) [29], and tendon-derived stem cells (TDSCs) [30]. TDSCs promote more rapid and complete recovery from tendon injuries due to their high proliferative capacity and strong tenonic differentiation ability [31]. Moreover, TDSCs can be easily obtained from injured tendon tissues during surgery. Combined use of TDSCs and ECM scaffolds is an attractive strategy for tendon repair.
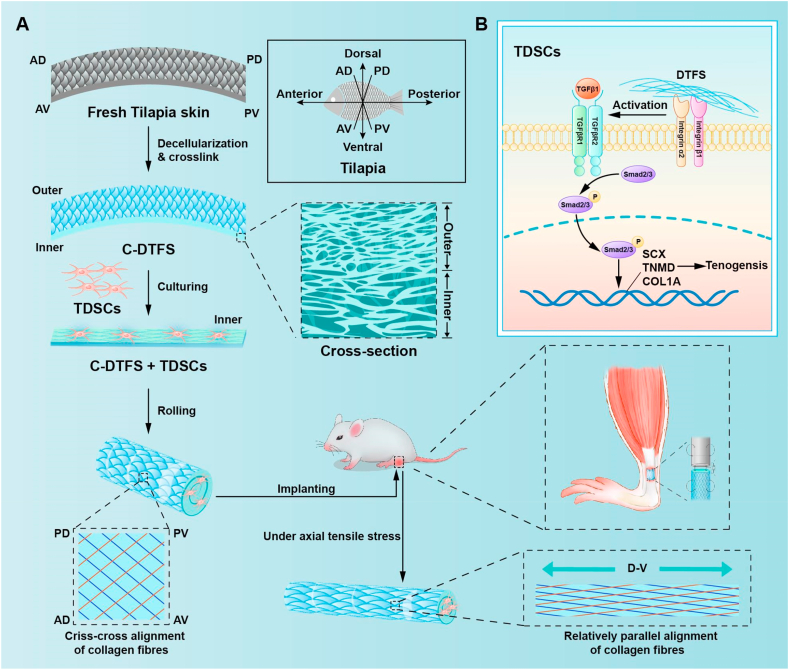
This study applied DTFS via a mild decellularization method to preserve the native porous structure and abundant collagen fibers, without immunogenic cellular components. To improve mechanical performance, we used a chemical crosslinker and examined the utility of this scaffold for tenonic differentiation of TDSCs in vitro. After that, the TDSCs loaded DTFS was determined to exhibit strong synergetic effect for the regeneration of critical size tendon defect in a rat model (Scheme 1).
Scheme 1.
Schematic illustration of DTFS fabrication and application (A), and the potential molecular mechanisms underlying DTFS-mediated tenonic differentiation (B).
2. Materials and methods
The detailed experimental methods can be found in the Supporting Information file.
3. Results
3.1. Properties of the prepared DTFS scaffolds
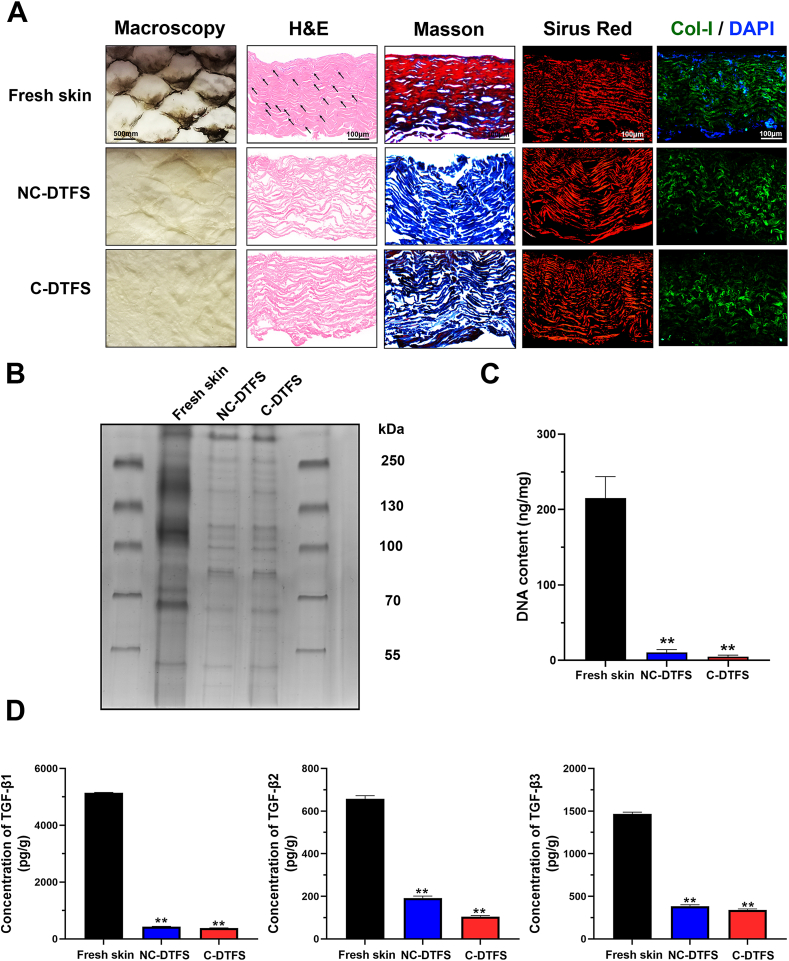
As shown in Fig. 1A, the macroscopic views demonstrated complete removal of the pigments after decellularization. Histologically, H&E and DAPI staining confirmed the absence of a nucleus in the DTFS groups. Masson's trichrome staining showed a preserved collagen structure (stained blue). Sirius Red staining and immunofluorescence demonstrated that Col-I accounted for a large proportion of the DTFS. On the other hand, the tissues of outer layers had a tight structure, while the inner layers were relatively soft. Electrophoresis showed that both non-crosslinked decellularized tilapia fish skin (NC-DTFS) and crosslinked DTFS (C-DTFS) had a clear background with limited bands, indicating that cell proteins were eliminated (Fig. 1B). However, the collagen components were preserved (α-, β-, and γ-chains of approximately 110–130, 250, and 350 kDa, respectively). Furthermore, DNA quantification (Fig. 1C) revealed that over 95% of the dsDNA was removed following the decellularization process (fresh skin, 215.25 ± 28.51 ng/mg; NC-DTFS, 10.73 ± 3.58 ng/mg; C-DTFS, 4.90 ± 2.10 ng/mg). Additionally, even after decellularizing and crosslinking process, about 8.35 ± 0.21% of TGFβ1, 29.14 ± 1.53% of TGFβ2, and 26.19 ± 1.08% of TGFβ3 were retained in DTFS, and about 7.41 ± 0.11% of TGFβ1, 15.94 ± 0.78% of TGFβ2, and 23.25 ± 0.72% of TGFβ3 retained in C-DTFS; no significant difference between above two groups. These data indicated a successful decellularization process, and that NC-DTFS and C-DTFS had retained an abundant collagen structure that was not significantly affected by crosslinking.
Fig. 1.
Decellularization of tilapia fish skin. (A) Gross views and histological staining (H&E (black arrow indicated blue stained nucleus), Masson's trichrome, Sirius Red, and immunofluorescence of Col-I and DAPI) of the skin before and after decellularization, with or without crosslinking. (B) SDS-PAGE analysis of fresh skin, NC-DTFS, and C-DTFS. (C) dsDNA contents of fresh skin, NC-DTFS, and C-DTFS. (D) TGF-β1, TGF-β2, and TGF-β3 content of fresh skin, NC-DTFS, and C-DTFS. Data are means ± SD of three replicate experiments. ∗∗P < 0.01.
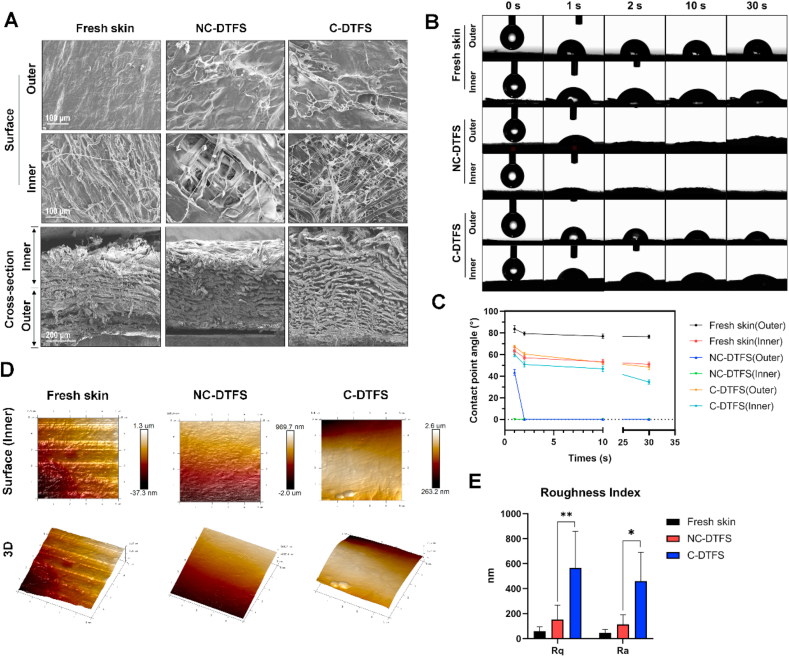
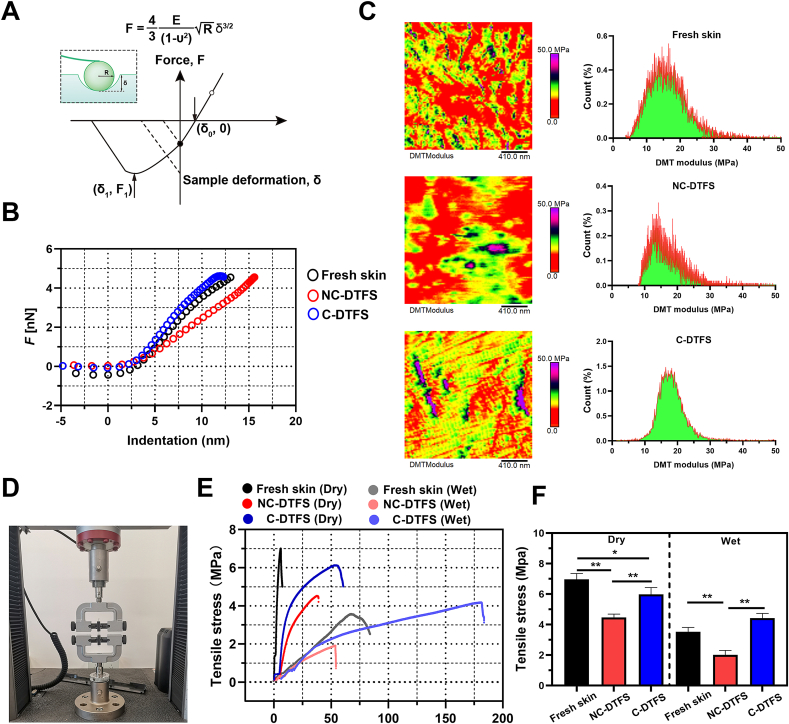
The SEM results (Fig. 2A) revealed a relatively smooth outer surface in each group; however, the inner surface exhibit rough appearance. Meanwhile, the inner surface of decellularized skins showed typical criss-cross alignment of collagen fibers, especially in C-DTFS. Besides, the cross-sectional views agreed with the histological findings; the fibers in the inner layers showed soft structure, while the outer layers displayed a dense structure. Moreover, measurement of the dynamic contact angles revealed that the decellularization process increased the wettability of the skin surface, but crosslinking helped to decreased the hydrophilicity in a moderate level. At the same time, the hydrophilicity of outer surfaces of skins is weaker than inner surface in each group (Fig. 2B and C). Since the cells were scheduled to implanted into the inner surface of skins, AFM was used to systemically evaluate its roughness, and the elastic and adhesive stress. There was a trend toward increasing roughness following decellularization, but this was not statistically significant. However, after crosslinking, the roughness improved significantly (Fig. 2D and E). By applying the nanoindentation AFM function, Young's modulus was obtained with the Hertzian model according to the force-distance curves (Fig. 3A). The force is enhanced following an increase in indentation depth, and the slope of the curve reflects the scaffold “modules” (Fig. 3B). The reconstructed map of Young's modulus indicated that the elastic moduli were decreased after decellularization, but crosslinking reversed this and improved the stiffness of the acellular scaffold (Fig. 3C). The C-DTFC sample showed a relatively homogenous distribution of Young's modulus (Fig. 3C). The order of the adhesive force of these skins was as follows: NC-DTFS > C-DTFS > fresh DTFS (Fig. S1). A universal strength testing machine was used to examine the tensile stress of the different skins (Fig. 3D–F). In the dry condition, the free skin had a maximum tensile stress of 7 MPa at 6% strain. After decellularization, the maximum strength decreased to 4.46 MPa at 38% strain. C-DTFS had a maximal tensile stress of 6.11 MPa at 55% strain. C-DTFS had a similar tensile strength but significantly improved extensibility, in comparison with fresh skins. Interestingly, in the wet condition, the strength in all groups decreased and the strain increased; C-DTFS had the highest maximum tensile stress, of 4.12 MPa at 175% strain; that of fresh skin was 1.9 MPa at 53% strain and that of NC-DTFS was 3.54 MPa at 66% strain. Our findings show that C-DTFS has greater tensile strength and extensibility in wet conditions.
Fig. 2.
DTFS properties. (A) SEM images of fresh skin, NC-DTFS, and C-DTFS. (B) Dynamic changes of contact angles in the inner and outer surfaces of various skin samples. (C) Measurement of contact angles. (D). Surfaces and 3D images of the topographies of various skins samples. (E). Average surface roughness (Ra) and root-mean-square roughness (Rq) values; the data are means ± SD of three replicate experiments. ∗P < 0.05, ∗∗P < 0.01.
Fig. 3.
Mechanical behaviors of C-DTFS. (A) Illustration and equation used to calculate Young's modulus using the Hertzian model. (B) Typical force-indentation curves of various skin samples. (C) Young's modulus of various skin samples calculated using AFM nanoindentation. (D) Tensile stress measurements. (E) Tensile stress-strain curves of various skin samples in dry and wet conditions. (F) Maximal tensile stress of various skin samples in dry and wet conditions; the data are means ± SD of three replicate experiments. ∗P < 0.05, ∗∗P < 0.01.
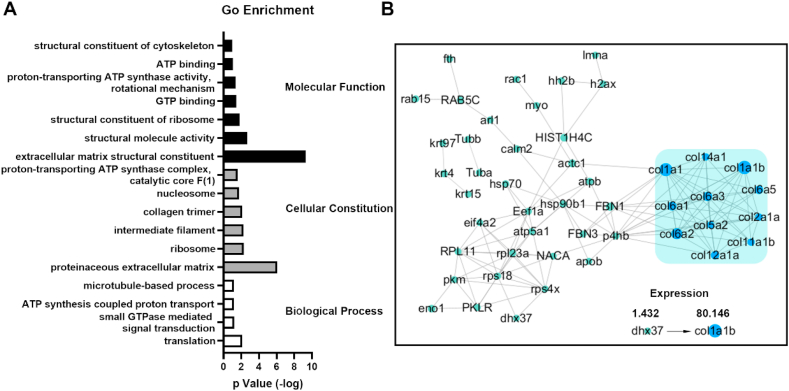
Considering the mechanical properties of the Achilles tendon (strength and extensibility both value), we used C-DTFS for following biological assessment and performed proteomics analysis to explore the protein composition. We identified 266 peptide groups and 69 proteins with ≥2 unique peptides. Fig. S2 shows the base peak chromatogram. Functional enrichment analysis provided further valuable information (Fig. 4A). Gene Ontology (GO) analysis indicated that C-DTFS contained mostly ECM structural proteins that participated in translation, energy-related activities (like ATP and GTP), and microtubule-based processes. The protein-protein interaction network plot showed that collagen (Col-1, 5, 6, 12, and 14) and fibronectin were the major components, especially the a-chain of Col-I (Fig. 4B). The proteomics analysis illustrated that the collagen-based extracellular structure performed the central functions of the C-DTFS.
Fig. 4.
LC-ESI-MS/MS-based proteomic analysis of C-DTFS. (A) GO enrichment analysis of proteins extracted from C-DTFS. (B) Protein-protein interaction network of C-DTFS.
3.2. C-DTFS promotes the tenonic differentiation of TDSCs
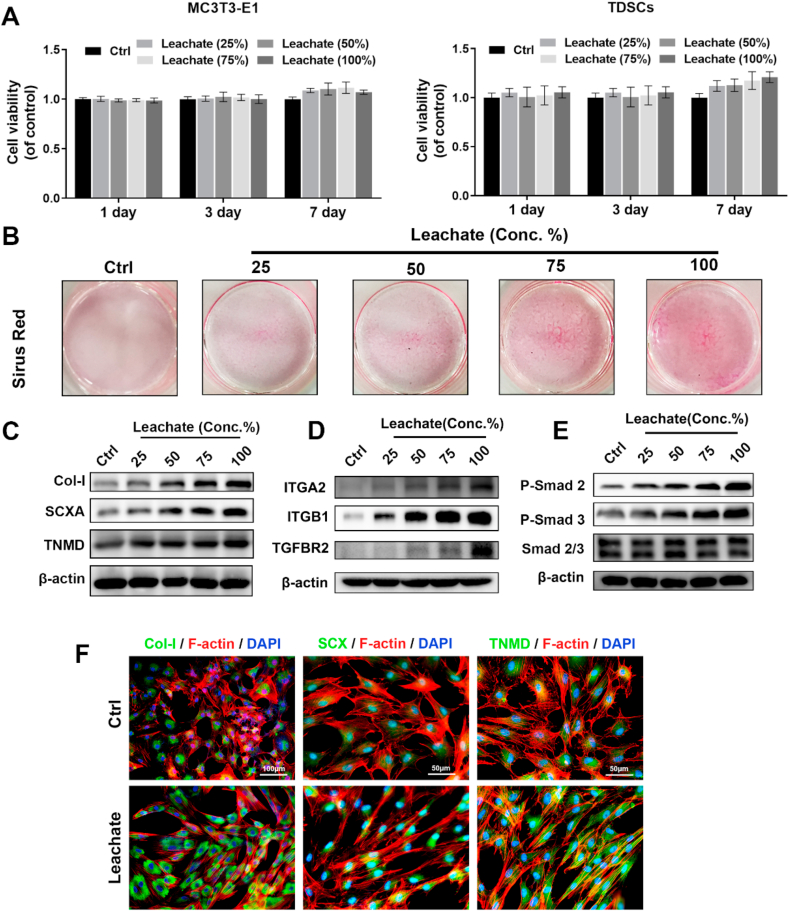
We examined the surface markers of isolated TDSCs. After incubation with the corresponding inductive culture medium, the TDSCs successfully exerted osteogenic, adipogenic, and chondrogenic differentiation abilities (Fig. S3A–B). Flow cytometry showed that the stem cell markers CD29, CD44, and CD90 were highly expressed (all >95%), while CD34 and CD45 were almost absent from isolated cells (Fig. S3C). These data strongly indicated that the isolated cells were TDSCs. The effects of the leachates of C-DTFS on TDSCs were examined. A 25–100% concentration of leachates exerted no significant cytotoxic effects on standard MC3T3-E1 cell lines or primary TDSCs (Fig. 5A). Concentration-dependent improvements in cell migration and tenonic differentiation of TDSCs were seen in the leachate treatment groups (Fig. S4 and Fig. 5B). According to western blot results, C-DTFS leachates upregulated Col-I, SCXA, and TNMD protein levels ((Fig. 5C). For potential upstream signals screening, the mRNA levels of collagen and RGD binding related integrin family and TGF receptors were detected by using qRT-PCR. The results indicated that C-DTFS leachate could enhance the expression of integrin α2 (ITGA2), integrin β1 (ITGB1), and TGFBR2 (Fig. S5). The protein level of ITGA2, ITGB1 and TGFBR2 in combined with the phosphorylation level of Smad2 and Smad3 were upregulated by C-DTFS leachate treatment in a dose-dependent manner (Fig. 5D–E). It indicated that the potential cross-talk of integrin α2/β1 and TGFβ/Smad axis contribute to the pro-tenonic effects of C-DTFS. The results of immunofluorescence staining also displayed that the 100% leachate of C-DTFS enhanced the level of Col-I, SCX and TNMD (Fig. 5F).
Fig. 5.
In vitro biocompatibility and pro-tenonic capacity of C-DTFS. (A) Cell viability of MC3T3-E1 and TDSCs in different concentrations of C-DTFS leachates on days 1, 3, and 7. (B) Sirius Red staining of TDSCs incubated with different concentrations of C-DTFS leachates in tenonic induction medium. (C–E) Col-I, SCXA, TNMD, ITGA2, ITGB1, TGFBR2, Smad 2 and Smad 3 protein levels in TDSCs treated with different concentrations of C-DTFS leachates on day 3. (F) Immunohistochemical staining of Col-I, SCXA, and TNMD (green) counterstained with TRITC-phalloidin (Red) and DAPI (blue) in TDSCs under different treatments on day 3.
3.3. Viability, morphology and functional alterations of TDSCs seeded on C-DTFS scaffolds
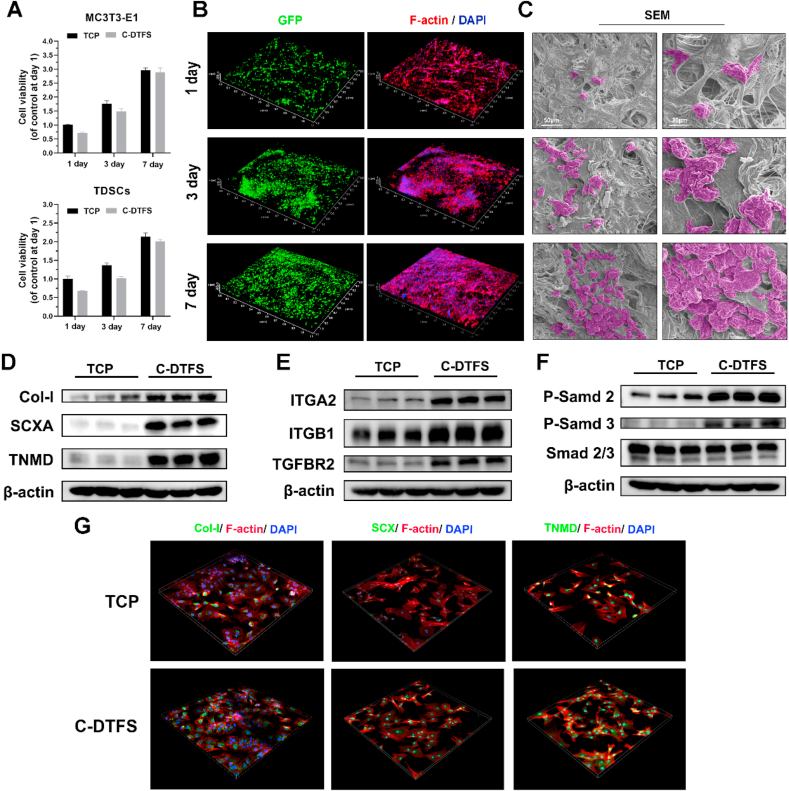
The day 1 results of CCK-8 showed about 70% implanting rates of both MC3T3-E1 and TDSCs cultured in C-DTFS comparing with commercial tissue culture plates (TCP). Both two type of cells could proliferate when co-cultured with scaffold, and the difference between C-DTFS and TCP was decreased as culture time goes on (Fig. 6A). In line with CCK-8 finding, GFP signals showed the high cell viability of TDSCs seeded in C-DTFS scaffolds (Fig. 6B). The cells successfully implanted in the scaffold and proliferated from day 1–7. F-actin staining revealed a more spreading appearance of TDSCs in day 7 (Fig. 6B). At the same time, SEM revealed the cell numbers and morphology of the TDSCs seeded in the C-DTFS (Fig. 6C); the results were consistent with those of immunofluorescence. In addition, the WB results showed that the level of tenonic markers (Col-I, SCXA, and TNMD) and the upstream signaling (integrin α2/β1 and TGFβ/Smad axis) were all upregulated in C-DTFS group in comparison with standard TCP (Fig. 6C–F). The C-DTFS induced upregulation of Col-I, SCXA, and TNMD were also verified by immunofluorescence staining (Fig. 6G).
Fig. 6.
Viability and morphology of TDSCs seeded in C-DTFS. (A) Cell viability of MC3T3-E1 and TDSCs seeded on the C-DTFS scaffolds at day 1, 3, and 7. (B) CLSM images of GFP-TDSCs and F-actin staining of cells implanted in C-DTFS on days 1, 3, and 7. (C) SEM images of TDSCs seeded in C-DTFS on days 1, 3, and 7. (D–F) Col-I, SCXA, TNMD, ITGA2, ITGB1, TGFBR2, Smad 2 and Smad 3 protein levels in TDSCs seeded onto TCP or C-DTFS on day 3. (G) Immunohistochemical staining (X200) of Col-I, SCXA, and TNMD (green) counterstained with TRITC-phalloidin (Red) and DAPI (blue) in TDSCs seeded on the C-DTFS scaffolds at day 3.
3.4. TDSCs seeded C-DTFS scaffolds promote tendon regeneration in vivo
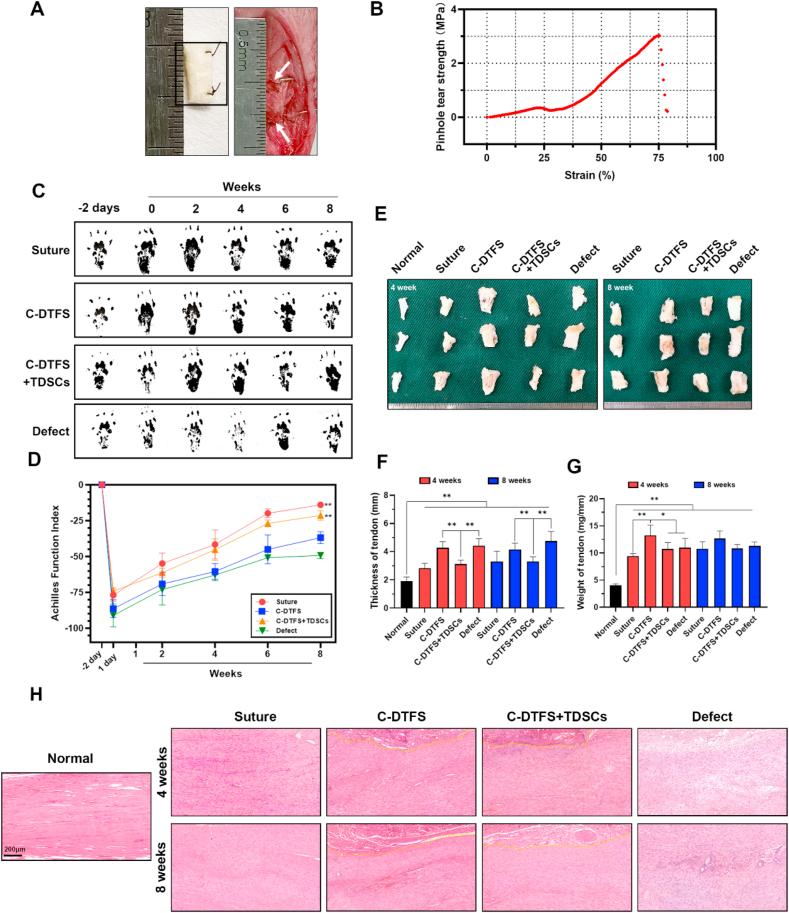
Fig. 7A depicts the rolled C-DTFS implanted into the defect site. The pinhole tear strength test indicated that C-DTFS had a strength of approximately 3 MPa, indicating robustness of the sutured tendon (Fig. 7B). The AFI test conducted 2 weeks postoperatively indicated significant functional improvements in the C-DTFS + TDSCs and autograft-sutured groups than the bare C-DTFS and defect groups. C-DTFS-implanted rats also recovered better than the negative controls, with significant differences seen at 8 weeks postoperatively (Fig. 7C and D). Gross observations revealed thickening and heaviness of tendons in all surgical groups at 4 and 8 weeks comparing with normal tendon (Fig. 7E–G); the C-DTFS + TDSCs group showed less thickening than the C-DTFS and defect groups, and was similar to the suture group, indirectly indicating that C-DTFS + TDSCs can decrease fibrous scarring (Fig. 7F). Only the bare C-DTFS group exhibited more weight in the regenerated tendon than the other three surgery groups (Fig. 7G).
Fig. 7.
TDSC-implanted C-DTFS promotes tendon defect repair. (A) Implantation of C-DTFS into the defect site. (B) Pinhole tear strength-strain curves of C-DTFS. (C–D) AFI of healed tendons following various treatments at the indicated time points. (E) Gross morphology of the healed tendon following the different treatments at 4 and 8 weeks postoperatively. (F–G) Quantification of the thickness and weight of healed tendons in the different treatment groups. (H) Representative H&E staining of regenerated tendons in the different treatment groups. All quantitative results are means ± SD of three replicate experiments. ∗P < 0.05, ∗∗P < 0.01.
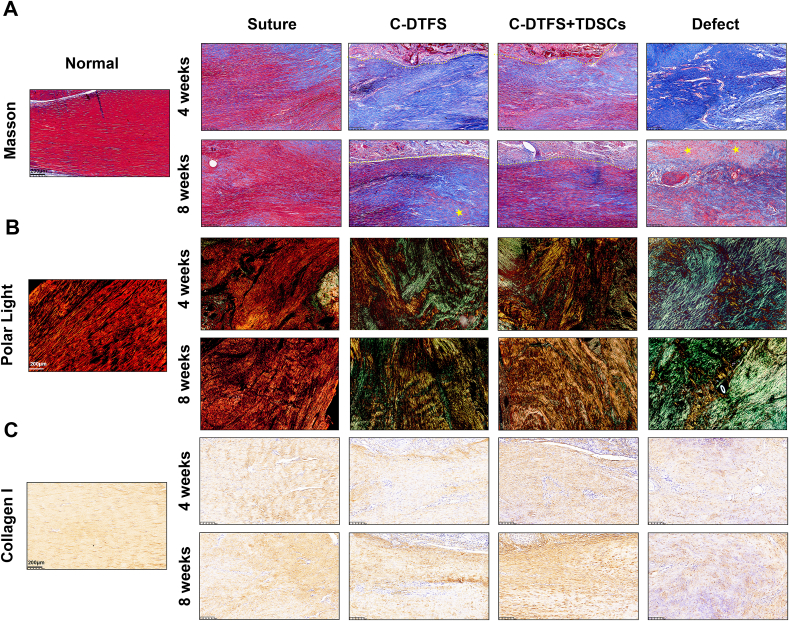
Histologically, a longitudinal alignment of collagen fibers is seen in the normal Achilles tendon. At 4 weeks postoperatively, aside from minor disorder of the ECM in the suture group, the operated groups exhibited significant losses of fiber alignment, and clear transition of regenerated tissue. Additionally, the defect group displayed obvious atrophy and disordered tendon remnants. After 8 weeks of healing, all implanted groups showed improved collagen fiber arrangements. However, the C-DTFS + TDSCs exhibited a better outcome than the bare DTFS group, with a denser ECM and better-aligned and more mature collagen fibers (Fig. 7, Fig. 8A). At 8 weeks postoperatively, some cartilaginous tissues were present in the C-DTFS and defect groups (Fig. 8A), indicating potential heterotopic ossification. The polarized light on the Sirius Red stained sections denoted regenerated collagen fibers (Fig. 8B). The red and yellow signals indicated Col-I in the normal control and suture groups. Yellow-colored collagen fibers were also present in the C-DTFS + TDSCs group, especially at 8 weeks postoperatively. In the bare C-DTFS groups, the yellow signal was relatively localized and sporadic, and the remaining tissues were characterized by green-colored fibers associated with type III collagen (the main component of scars in tendon tissues). Notably, the defect group showed green-colored fibers, indicating severe scaring. In addition, IHC staining indicated the protein levels of Col-I, SCXA, and TNMD, and showed that C-DTFS + TDSCs treatment had similar effects to autografts, with significantly higher protein levels seen compared with the bare C-DTFS and defect groups (Fig. 8C and S7A-B). Also, the IHC staining reveled higher level of α-SMA in C-DTFS and defect groups, which indirectly indicated the tendon scar formation; while the TDSCs loaded C-DTFS group showed relative lower expression, which is closed to the suture group and normal tendon (Fig. S7C).
Fig. 8.
TDSC-implanted C-DTFS promotes functional collagen fiber deposition during tendon defect repair. (A) Masson's trichrome staining of regenerated tendons in the different treatment groups. (B) Polar light images of healed tendons stained by Sirius Red in the different treatment groups. (C) IHC staining of COL-I levels in regenerated tendons in the different treatment groups.
4. Discussion
Collagen-based scaffolds are essential for tissue engineering in the musculoskeletal system, and especially for skin, bone, cartilage, tendon, and ligament reconstruction [32,33]. Marine-derived acellular collagen scaffolds are emerging as attractive alternatives to traditional mammal-derived materials because they have similar structures and functions to the ECM, lower the risk of disease transmission, and are less likely to be contraindicated on religious grounds [21]. The scale of the fishery industry has rapidly increased, and approximately 70–85% of all products generated are waste or byproducts, including skin, scales, and skeletons [22,34,35]. Converting these unused resources into products that could aid economic or environmental development is of great interest. In clinical practice, MariGen™ (an omega-3 fatty acid product), developed by an Icelandic biomedical company, is the first FDA-approved acellular fish skin-based treatment for burns and diabetic wounds with proven efficacy [36,37]. Besides, MJ. Lacqua in 2021 reported two cases by wrapped this product around repaired tendon to promote its healing and inhibit peritendinous adhesion [38]. Both patients achieved favorable outcomes and excellent functional recovery (100% according to the standard clinical scoring system). Moreover, magnetic resonance imaging revealed complete absorption of the scaffolds with no peritendinous adhesions. Thus, decellularized fish skin shows promise as a tendon rupture treatment due to its multifunctional bioactivities and biodegradability. Inspired by its clinical potential, this study utilized the skin of Nile tilapia, a more popular seafood, to fabricate DTFS and explore its utility for Achilles tendon repair.
We compared NC-DTFS and C-DTFS: electrophoresis and DNA content tests demonstrated successful decellularization, similar protein expression patterns and closed TGFβ contents (Fig. 1B–C). Histological findings and SEM observations showed that both DTFSs had relatively dense outer layers with hydrophobic surfaces, and soft inner layers with relatively hydrophilic surfaces. Based on these structural characteristics, the inner surface was used for cell seeding. The relatively dense and hydrophobic outer surface could help prevent fibrotic scarring due to infiltration and inhibit peritendinous adhesion. However, the smooth and soft surface, and suboptimal interface wettability (too high or too low), were not favorable with respect to cell adhesion in most cases [[39], [40], [41], [42]]. Hence, C-DTFS, with its rough and stiff topography and moderate hydrophilicity, was more suitable for seed cell implantation (Fig. 2, Fig. 3, Fig. 4B–C). C-DTFS was also preferred over NC-DTFS because of the demands imposed on the Achilles tendon by intense exercise. C-DTFS had higher tensile strength and better extensibility in both wet and dry conditions, which would significantly reduce the risk of re-rupture. Moreover, when the skin was pulled in the “dorsal-ventral” direction, the collagen fibers were parallel to the tensile stress and could be fully extended, hence behaving similarly to a real tendon. This is especially important for tendon tissue engineering (Scheme 1).
Collagens play vital roles in tendon development and healing, and the natural ECM environment enhances the adhesion and tenonic differentiation of stem cells [[42], [43], [44]]. Our proteomic analysis identified abundant tenonic-related collagens (Col-1, 5, 6, 12, 14) in C-DTFS [45], as well as fibronectin (containing the RGD sequence) (Fig. 4). All collagen molecules have a three alpha helix chains, each of which typically contains at least repeating amino acid sequence, such as Gly-X-Y [46]. The GFOGER sequence in Col-I is responsible for integrin receptor recognition, as well as cell adhesion and proliferation, and exhibits desirable biocompatibility for biological applications [47,48]. On the other hand, previous studies demonstrated that integrins can crosstalk with growth factors (GFs) signals to modulate cell differentiation [49,50]. Among these GFs, TGF-β/Smad signaling serves as a paradigm to understand the interaction among integrin, ECM, and GF function in tenonic differentiation [51,52]. Moreover, acellular tendon ECM can enhance integrin α2β1 expression and trigger crosstalk within the TGFβ1R/Smad axis, thereby promoting the tenonic differentiation of ADSCs [29]. Similarly, in our study the leachate of C-DTFS also promoted tenogenesis via the activation of integrin α2β1, TGFβ1R and Smad2/3, suggesting that collagen-enriched C-DTFS has promise for tendon repair. Of note, with EDC-NHS, the standard 100% concentration of crosslink degree is often defined as a precise molar ratio of 5:2:1 (EDC: NHS: COO−), which could be converted into mass ratio with 1.150:0.276:1 (EDC: NHS: Collagen) [24]. Although it demonstrated that EDC mediated crosslink would impair some integrin-specific binding at high concentration; however, using low EDC concentration (∼10% crosslink degree) did not significantly affect cell adhesion, demonstrating that most part of collagen motif could be retained [25,53]. Previous studies indicated that the stability and mechanics of the resultant scaffold would not be affected when the crosslinking degree was reduced to about 10%, and the post-processed scaffolds could be stable for over 28 days in aqueous condition [54]. This kind of collagen scaffolds crosslinked by low degree of EDC, is suitable for long-term applications. In the current study, 50 mg EDC and 20 mg NHS were used for 1 g of DTFS crosslinking. While it was reported that the collagen content in tilapia skin was about 36% so that it could be conducted that the crosslink concentration is lower than 12%, which is safe for cell adhesion and integrin binding [55]. By contrast, glutaraldehyde (GTA) and genipin are other typical crosslinker used in collagen-based products; however, both of them are non-zero length crosslinker so that they could not be removed thoroughly after crosslinking [24]. GTA possesses high cytotoxicity while genipin is biofriendly but would impart a blue color into materials, which is limited in industrial usage [24].
In clinical practice, the massive tendon defect can not be repaired by simply end-to-end suturing or using autografts due to the length or resource restriction, which commonly need the extra allografts or some biomaterials to handle this challenge. Currently, most of the commercially biological products are mammal-derived collagen products, like acellular small intestine submucosa (SIS), dermis, and pericardium [56]. However, just as we mentioned in introduction section, these mammal-derived decellularized ECMs have limitations such as limited donor sources, the potential risk of zoonotic diseases, and ethical and religious issues [20]. Whereas, the C-DTFS in our study, as a decellularized marine collagen scaffolds, could overcome these disadvantages, which owned favorable structural, mechanical, and biological properties, and is suitable for cell seeding. Although its healing effects could not reach the height of autogenous tendon grafts (suture group) according to our results, its broad resource, excellent biosecurity and pro-tenonic effects are of great value for clinical and market requirements. Nevertheless, some limitations still exist and further improvement should be proposed. First of all, the C-DTFS + TDSCs group showed better recovery than the bare C-DTFS group, which indicated that the C-DTFS is more suitable as a cell-loaded scaffold than cell-free option for treatments. While the resource of TDSCs should firstly be taken into consideration in the future, and establishing stem cell bank might solve this problem. Besides cell seeding, the naturally porous structure of C-DTFS could also allow its use as a drug carrier for additional tenonic agents like GFs, platelet-rich plasma, and other pharmacological agents, as an off-the-shelf product. Moreover, according to the in vivo histological findings, the scaffolds were not degraded in rat model until 2 months; therefore, longer observation time and large animal model should be performed in the future. Then, the degradation speed should also be adjusted by modulating the crosslinking degree. What's more, although many fish-derived antimicrobial peptides have been reported, native DTFS had limited antibacterial effects [57,58]. Coating the outer surface of C-DTFS with antibacterial material might be useful for preventing postoperative infection. Additionally, collagen fibers could be extracted to fabricate more homogenous materials via electrospinning or 3D printing. However, mechanical strength might be lost, and further chemical modification or an organic/inorganic hybrid strategy might be necessary.
5. Conclusion
We created a crosslinked acellular dermal matrix scaffold from Nile tilapia fish skin and described its properties. The results showed that C-DTFS has a natural ECM structure with abundant collagen, and possesses favorable mechanical strength, biocompatibility, and tenonic capacity. C-DTFS is a suitable scaffold for TDSC seeding and aided cell proliferation. More importantly, TDSC-implanted C-DTFS in a rat Achilles defect model promoted the orderly generation of collagen fibers, induced the expression of biological factors important for tendon repair, and decreased the risk of fibrotic scar formation and heterotopic ossification. The process described herein could easily be industrialized and shows great promise for clinical tendon repair applications.
Finding statement
This work was supported by the National Natural Science Foundation of China (82172400).
Credit statement
Zhe Liu: Conceptualization, Methodology, Investigation, Formal analysis, Writing - original draft, Visualization. Ming-Zhao Yu: Conceptualization, Methodology, Investigation, Formal analysis. Hao Peng: Methodology, Formal analysis, Conceptualization, Software. Ruo-Tao Liu: Formal analysis, Software, Validation. Thou Lim: Conceptualization, Methodology, Investigation, Visualization, Software. Chang-Qing Zhang: Conceptualization, Supervision, Project administration, Resources. Zhen-Zhong Zhu: Methodology, Writing-review & editing, Supervision, Funding acquisition. Xiao-Juan Wei: Conceptualization, Writing-review & editing, Data curation.
Ethics approval statement
The use of animals in these experiments was in accordance with the Interdisciplinary Principles and Guidelines for the Use of Animals in Research, Testing, and Education. The welfare of the experimental animals was prioritized, and all animal experiments were approved by the Animal Care Committee of Shanghai Jiao Tong University Affiliated Sixth People's Hospital at the Shanghai Jiao Tong University School of Medicine and followed the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Zhe Liu, Ming-Zhao Yu and Hao Peng contributed equally to this work.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mtbio.2022.100488.
Contributor Information
Zhen-Zhong Zhu, Email: zzz1129@gmail.com.
Xiao-Juan Wei, Email: xjweish@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Park S.H., Lee H.S., Young K.W., Seo S.G. Treatment of acute Achilles tendon rupture. Clin. Orthop. Surg. 2020;12(1):1–8. doi: 10.4055/cios.2020.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarantino D., Palermi S., Sirico F., Corrado B. Achilles tendon rupture: mechanisms of injury, Principles of rehabilitation and return to play. J. Func.t Morphol. Kinesiol. 2020;5(4):95. doi: 10.3390/jfmk5040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magnusson S.P., Agergaard A.-S., Couppé C., Svensson R.B., Warming S., Krogsgaard M.R., Kjaer M., Eliasson P. Heterotopic ossification after an Achilles tendon rupture cannot Be prevented by early functional rehabilitation: a cohort study. Clin. Orthop. Relat. Res. 2020;478(5):1101–1108. doi: 10.1097/corr.0000000000001085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voleti P.B., Buckley M.R., Soslowsky L.J. Tendon healing: repair and regeneration. Annu. Rev. Biomed. Eng. 2012;14:47–71. doi: 10.1146/annurev-bioeng-071811-150122. [DOI] [PubMed] [Google Scholar]
- 5.Docheva D., Muller S.A., Majewski M., Evans C.H. Biologics for tendon repair. Adv. Drug Deliv. Rev. 2015;84:222–239. doi: 10.1016/j.addr.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasiliadis A.V., Katakalos K. The role of scaffolds in tendon tissue engineering. J. Funct. Biomater. 2020;11(4):78. doi: 10.3390/jfb11040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y., Han Y., Wong Y.S., Fuh J.Y.H. Fibre-based scaffolding techniques for tendon tissue engineering. J. Tissue Eng. Regen. Med. 2018;12(7):1798–1821. doi: 10.1002/term.2701. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz-Alonso S., Lafuente-Merchan M., Ciriza J., Saenz-Del-Burgo L., Pedraz J.L. Tendon tissue engineering: cells, growth factors, scaffolds and production techniques. J. Contr. Release. 2021;333:448–486. doi: 10.1016/j.jconrel.2021.03.040. [DOI] [PubMed] [Google Scholar]
- 9.Ratcliffe A., Butler D.L., Dyment N.A., Cagle P.J., Jr., Proctor C.S., Ratcliffe S.S., Flatow E.L. Scaffolds for tendon and ligament repair and regeneration. Ann. Biomed. Eng. 2015;43(3):819–831. doi: 10.1007/s10439-015-1263-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim S.E., Kim J.G., Park K. Biomaterials for the treatment of tendon injury. Tissue Eng. Regen. Med. 2019;16(5):467–477. doi: 10.1007/s13770-019-00217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franchi M., Trire A., Quaranta M., Orsini E., Ottani V. Collagen structure of tendon relates to function. Sci. World J. 2007;7:404–420. doi: 10.1100/tsw.2007.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang S., Shi X., Li X., Wang J., Wang Y., Luo Y. Oriented collagen fiber membranes formed through counter-rotating extrusion and their application in tendon regeneration. Biomaterials. 2019;207:61–75. doi: 10.1016/j.biomaterials.2019.03.041. [DOI] [PubMed] [Google Scholar]
- 13.Wang S., Wang Y., Song L., Chen J., Ma Y., Chen Y., Fan S., Su M., Lin X. Decellularized tendon as a prospective scaffold for tendon repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;77:1290–1301. doi: 10.1016/j.msec.2017.03.279. [DOI] [PubMed] [Google Scholar]
- 14.Yeung D.A., Kelly N.H. The role of collagen-based biomaterials in chronic wound healing and sports medicine applications. Bioengineering (Basel) 2021;8(1):8. doi: 10.3390/bioengineering8010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbert T.W., Stewart-Akers A.M., Simmons-Byrd A., Badylak S.F. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J. Bone Joint Surg. Am. 2007;89(3):621–630. doi: 10.2106/JBJS.E.00742. [DOI] [PubMed] [Google Scholar]
- 16.Megerle K., Woon C., Kraus A., Raghavan S., Pham H., Chang J. Flexor tendon sheath engineering using decellularized porcine pericardium. Plast. Reconstr. Surg. 2016;138(4):630e–641e. doi: 10.1097/PRS.0000000000002459. [DOI] [PubMed] [Google Scholar]
- 17.Branch J.P. A tendon graft weave using an acellular dermal matrix for repair of the Achilles tendon and other foot and ankle tendons. J. Foot Ankle Surg. 2011;50(2):257–265. doi: 10.1053/j.jfas.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Anaim A.A. Use of a non-crosslinked porcine dermal matrix in repair of the Achilles and other tendons of the foot. Foot Ankle Spec. 2018 doi: 10.1177/1938640017751189. [DOI] [PubMed] [Google Scholar]
- 19.Song H., Yin Z., Wu T., Li Y., Luo X., Xu M., Duan L., Li J. Enhanced effect of tendon stem/progenitor cells combined with tendon-derived decellularized extracellular matrix on tendon regeneration. Cell Transplant. 2018;27(11):1634–1643. doi: 10.1177/0963689718805383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau C.S., Hassanbhai A., Wen F., Wang D., Chanchareonsook N., Goh B.T., Yu N., Teoh S.H. Evaluation of decellularized tilapia skin as a tissue engineering scaffold. J. Tissue Eng. Regen. Med. 2019;13(10):1779–1791. doi: 10.1002/term.2928. [DOI] [PubMed] [Google Scholar]
- 21.Liu S., Lau C.S., Liang K., Wen F., Teoh S.H. Marine collagen scaffolds in tissue engineering. Curr. Opin. Biotechnol. 2021;74:92–103. doi: 10.1016/j.copbio.2021.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Lv K., Wang L., He X., Li W., Han L., Qin S. Application of Tilapia skin acellular dermal matrix to induce acute skin wound repair in rats. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.792344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon J., Yoon D., Lee H., Lee J., Jo S., Kym D., Yim H., Hur J., Chun W., Kim G., Cho Y.S. Wound healing ability of acellular fish skin and bovine collagen grafts for split-thickness donor sites in burn patients: characterization of acellular grafts and clinical application. Int. J. Biol. Macromol. 2022;205:452–461. doi: 10.1016/j.ijbiomac.2022.02.055. [DOI] [PubMed] [Google Scholar]
- 24.Nair M., Best S.M., Cameron R.E. Crosslinking collagen constructs: achieving cellular selectivity through modifications of physical and chemical properties. Appl. Sci. 2020;10(19) doi: 10.3390/app10196911. [DOI] [Google Scholar]
- 25.Nair M., Johal R.K., Hamaia S.W., Best S.M., Cameron R.E. Tunable bioactivity and mechanics of collagen-based tissue engineering constructs: a comparison of EDC-NHS, genipin and TG2 crosslinkers. Biomaterials. 2020;254 doi: 10.1016/j.biomaterials.2020.120109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adamiak K., Sionkowska A. Current methods of collagen cross-linking: Review. Int. J. Biol. Macromol. 2020;161:550–560. doi: 10.1016/j.ijbiomac.2020.06.075. [DOI] [PubMed] [Google Scholar]
- 27.Chen J., Yu Q., Wu B., Lin Z., Pavlos N.J., Xu J., Ouyang H., Wang A., Zheng M.H. Autologous tenocyte therapy for experimental Achilles tendinopathy in a rabbit model. Tissue Eng. 2011;17(15–16):2037–2048. doi: 10.1089/ten.TEA.2010.0492. [DOI] [PubMed] [Google Scholar]
- 28.Deng D., Liu W., Xu F., Yang Y., Zhou G., Zhang W.J., Cui L., Cao Y. Engineering human neo-tendon tissue in vitro with human dermal fibroblasts under static mechanical strain. Biomaterials. 2009;30(35):6724–6730. doi: 10.1016/j.biomaterials.2009.08.054. [DOI] [PubMed] [Google Scholar]
- 29.Wang D., Pun C.C.M., Huang S., Tang T.C.M., Ho K.K.W., Rothrauff B.B., Yung P.S.H., Blocki A.M., Ker E.D.F., Tuan R.S. Tendon-derived extracellular matrix induces mesenchymal stem cell tenogenesis via an integrin/transforming growth factor-beta crosstalk-mediated mechanism. Faseb. J. 2020;34(6):8172–8186. doi: 10.1096/fj.201902377RR. [DOI] [PubMed] [Google Scholar]
- 30.Ni M., Rui Y.F., Tan Q., Liu Y., Xu L.L., Chan K.M., Wang Y., Li G. Engineered scaffold-free tendon tissue produced by tendon-derived stem cells. Biomaterials. 2013;34(8):2024–2037. doi: 10.1016/j.biomaterials.2012.11.046. [DOI] [PubMed] [Google Scholar]
- 31.Lui P.P., Chan K.M. Tendon-derived stem cells (TDSCs): from basic science to potential roles in tendon pathology and tissue engineering applications. Stem Cell Rev. Rep. 2011;7(4):883–897. doi: 10.1007/s12015-011-9276-0. [DOI] [PubMed] [Google Scholar]
- 32.Oliveira S.M., Ringshia R.A., Legeros R.Z., Clark E., Yost M.J., Terracio L., Teixeira C.C. An improved collagen scaffold for skeletal regeneration. J. Biomed. Mater. Res. 2010;94(2):371–379. doi: 10.1002/jbm.a.32694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong C., Lv Y. Application of collagen scaffold in tissue engineering: recent advances and new perspectives. Polymers. 2016;8(2):42. doi: 10.3390/polym8020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dean M.N., Shahar R. The structure-mechanics relationship and the response to load of the acellular bone of neoteleost fish: a review. J. Appl. Ichthyol. 2012;28(3):320–329. doi: 10.1111/j.1439-0426.2012.01991.x. [DOI] [Google Scholar]
- 35.Shalaby M., Agwa M., Saeed H., Khedr S.M., Morsy O., El-Demellawy M.A. Fish scale collagen preparation, characterization and its application in wound healing. J. Polym. Environ. 2019;28(1):166–178. doi: 10.1007/s10924-019-01594-w. [DOI] [Google Scholar]
- 36.Sigurjonsson G.F., Gisladottir D.H., G G. US Patents; 2013. Scaffold Material for Wound Care And/or Other Tissue Healing Applications. [Google Scholar]
- 37.Woodrow T., Chant T., Chant H. Treatment of diabetic foot wounds with acellular fish skin graft rich in omega-3: a prospective evaluation. J. Wound Care. 2019;28(2):76–80. doi: 10.12968/jowc.2019.28.2.76. [DOI] [PubMed] [Google Scholar]
- 38.Lacqua M. The use of intact fish-skin wrap in preventing adhesions during tendon repair: a report of two cases. Edorium. J. Orthop. 2021;7(100018O03ML2021) doi: 10.5348/100018o03ml2021cs. [DOI] [Google Scholar]
- 39.Dave K., Gomes V.G. Interactions at scaffold interfaces: effect of surface chemistry, structural attributes and bioaffinity. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;105 doi: 10.1016/j.msec.2019.110078. [DOI] [PubMed] [Google Scholar]
- 40.Ferrari M., Cirisano F., Morán M.C. Mammalian cell behavior on hydrophobic substrates: influence of surface properties. Colloids Interfaces. 2019;3(2):48. doi: 10.3390/colloids3020048. [DOI] [Google Scholar]
- 41.Chang H.-I., Wang Y. Cell responses to surface and architecture of tissue engineering scaffolds, regenerative medicine and tissue engineering - cells and biomaterials. InTechOpen. 2011 [Google Scholar]
- 42.Lin J., Zhou W., Han S., Bunpetch V., Zhao K., Liu C., Yin Z., Ouyang H. Cell-material interactions in tendon tissue engineering. Acta Biomater. 2018;70:1–11. doi: 10.1016/j.actbio.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 43.Banos C.C., Thomas A.H., Kuo C.K. Collagen fibrillogenesis in tendon development: current models and regulation of fibril assembly. Birth Defects Res. C Embryo Today. 2008;84(3):228–244. doi: 10.1002/bdrc.20130. [DOI] [PubMed] [Google Scholar]
- 44.Liu S.H., Yang R.S., al-Shaikh R., Lane J.M. Collagen in tendon, ligament, and bone healing. A current review. Clin. Orthop. Relat. Res. 1995;318:265–278. [PubMed] [Google Scholar]
- 45.Xu Y., Yin H., Chu J., Eglin D., Serra T., Docheva D. An anisotropic nanocomposite hydrogel guides aligned orientation and enhances tenogenesis of human tendon stem/progenitor cells. Biomater. Sci. 2021;9(4):1237–1245. doi: 10.1039/d0bm01127d. [DOI] [PubMed] [Google Scholar]
- 46.Ramshaw J.A., Shah N.K., Brodsky B. Gly-X-Y tripeptide frequencies in collagen: a context for host-guest triple-helical peptides. J. Struct. Biol. 1998;122(1–2):86–91. doi: 10.1006/jsbi.1998.3977. [DOI] [PubMed] [Google Scholar]
- 47.Knight C.G., Morton L.F., Peachey A.R., Tuckwell D.S., Farndale R.W., Barnes M.J. The collagen-binding A-domains of integrins alpha(1)beta(1) and alpha(2)beta(1) recognize the same specific amino acid sequence, GFOGER, in native (triple-helical) collagens. J. Biol. Chem. 2000;275(1):35–40. doi: 10.1074/jbc.275.1.35. [DOI] [PubMed] [Google Scholar]
- 48.Khew S.T., Zhu X.H., Tong Y.W. An integrin-specific collagen-mimetic peptide approach for optimizing Hep3B liver cell adhesion, proliferation, and cellular functions. Tissue Eng. 2007;13(10):2451–2463. doi: 10.1089/ten.2007.0063. [DOI] [PubMed] [Google Scholar]
- 49.Kim S.H., Turnbull J., Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 2011;209(2):139–151. doi: 10.1530/JOE-10-0377. [DOI] [PubMed] [Google Scholar]
- 50.Munger J.S., Sheppard D. Cross talk among TGF-β signaling pathways, integrins, and the extracellular matrix. Cold Spring Harbor Perspect. Biol. 2011;3(11) doi: 10.1101/cshperspect.a005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayashida T., Wu M.H., Pierce A., Poncelet A.C., Varga J., Schnaper H.W. MAP-kinase activity necessary for TGFbeta1-stimulated mesangial cell type I collagen expression requires adhesion-dependent phosphorylation of FAK tyrosine 397. J. Cell Sci. 2007;120(Pt 23):4230–4240. doi: 10.1242/jcs.03492. [DOI] [PubMed] [Google Scholar]
- 52.Wang D., Sun L., Zborowska E., Willson J.K., Gong J., Verraraghavan J., Brattain M.G. Control of type II transforming growth factor-beta receptor expression by integrin ligation. J. Biol. Chem. 1999;274(18):12840–12847. doi: 10.1074/jbc.274.18.12840. [DOI] [PubMed] [Google Scholar]
- 53.Bax D.V., Davidenko N., Gullberg D., Hamaia S.W., Farndale R.W., Best S.M., Cameron R.E. Fundamental insight into the effect of carbodiimide crosslinking on cellular recognition of collagen-based scaffolds. Acta Biomater. 2017;49:218–234. doi: 10.1016/j.actbio.2016.11.059. [DOI] [PubMed] [Google Scholar]
- 54.Davidenko N., Schuster C.F., Bax D.V., Raynal N., Farndale R.W., Best S.M., Cameron R.E. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 2015;25:131–142. doi: 10.1016/j.actbio.2015.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lau C.S. Nanyang Technol. Univ.; 2019. Decellularization of Fish Skin for Tissue Engineering Applications. [Google Scholar]
- 56.Wang Z., Xiang L., Lin F., Tang Y., Deng L., Cui W. A biomaterial-based hedging immune strategy for scarless tendon healing. Adv. Mater. 2022;34(19) doi: 10.1002/adma.202200789. [DOI] [PubMed] [Google Scholar]
- 57.Guimarães M.L., da Silva F.A.G., da Costa M.M., de Oliveira H.P. Coating of conducting polymer-silver nanoparticles for antibacterial protection of Nile tilapia skin xenografts. Synth. Met. 2022;287 doi: 10.1016/j.synthmet.2022.117055. [DOI] [Google Scholar]
- 58.Antonio Gomes da Silva F., Eckhart K.E., Matiuzzi da Costa M., Sydlik S.A., Pequeno de Oliveira H. Inhibition of biofilm formation induced by functional graphenic materials impregnated in Nile tilapia (Oreochromis niloticus) skin. Appl. Surf. Sci. 2022;576 doi: 10.1016/j.apsusc.2021.151768. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.