Abstract
A tissue culture bilayer system that mimics some aspects of early alveolar infection by Mycobacterium tuberculosis was developed. This model incorporates human lung epithelial type II pneumocyte (A549) (upper chamber) and endothelial cell (lower chamber) layers separated by a microporous membrane. This construction makes it possible to observe and quantify the passage of bacteria through the two layers, to observe the interaction of the bacteria with the various cell types, and to examine the basic mechanisms of immune cell recruitment to the site of infection. After 107 organisms were added to the upper chamber we microscopically observed large numbers of bacteria attached to and within the pneumocytes and we determined by viable-cell counting that a small percentage of the inoculum (0.02 to 0.43%) passed through the bilayer into the lower chamber. When peripheral blood mononuclear cells were added to the lower chamber, microscopic examination indicated a migration of the mononuclear cells through the bilayer to the apical surface, where they were seen associated with the mycobacteria on the pneumocytes. The added complexity of the bilayer system offers an opportunity to define more precisely the roles of the various lung cell types in the pathogenesis of early tuberculosis.
The recent resurgence of tuberculosis has brought new focus to the study of its pathogenesis. Worldwide, Mycobacterium tuberculosis has infected 2 billion people, and tuberculosis causes 3 million deaths each year (5). Because the efficacy of the currently available vaccine is questionable and the widely used skin test is not a valid indicator of active disease, especially in AIDS patients, better methods of prevention and diagnosis are needed (3, 5). The search for improved methods requires a better understanding of the pathogenic mechanisms used by this organism.
M. tuberculosis bacilli are inhaled into the lung, eventually reaching the alveoli, where the organisms may be ingested by alveolar macrophages. It is suspected that if not killed by the macrophages, the bacilli are able to survive, replicate intracellularly, and spread to other alveolar macrophages and to the nonactivated blood-borne macrophages attracted to the site by the released bacterial cell debris and host chemotactic factors (11). Dissemination of viable organisms from these macrophages into the lymph or circulatory system is critical to the establishment of disease (17, 18).
It is not known precisely how many alveolar macrophages the bacilli might encounter as they enter the alveolar space. It has been estimated that in an average human male there are 28,000 type I pneumocytes, 1,400 type II pneumocytes, and 50 to 100 alveolar macrophages per alveolus (7, 8, 23). Thus, the bacilli might well interact initially with epithelial cells lining this space. If the invading bacteria are able to enter these cells, not only would they be protected from the bactericidal activity of the macrophage, but they might also be able to pass through these cells into adjacent endothelial cells and thus enter the circulatory system as do other respiratory pathogens, such as Streptococcus pneumoniae (9). In vitro studies using cultured pneumocyte monolayers have shown that M. tuberculosis bacilli are able not only to enter these cells but also to multiply intracellularly in far greater numbers than those seen within cultured macrophages (1, 19). Similar studies also showed intracellular growth within cultured human lung endothelial cells (HULEC) (21a). Thus, even a few organisms inhaled into the alveolar space could potentially multiply to a much larger number before entering the bloodstream.
Current knowledge of the early events in the pathogenesis of M. tuberculosis is based on research using animal models or in vitro tissue culture monolayer models, and studies involving patients with tuberculosis have added more to our understanding of later events after infection has progressed to clinical disease. While animal models provide much useful data, even in animals that are susceptible to mycobacterial infection, the immune response is very different from that seen in humans (12, 21, 22). The fact that many of these animal models do not develop typical mycobacterial disease limits their relevance to the study of the human disease process. Alternatively, human tissue culture cell monolayers are simpler to work with, can be maintained under controlled conditions, and are more relevant to human disease. However, when infecting a human host, the bacilli must interact with a wide variety of cell types, an environment not well represented by a monolayer. We have developed an artificial tissue system incorporating epithelial and endothelial cell monolayers separated by a microporous membrane. This system allows the cell-to-cell communication and polarization that are essential for the growth and differentiation of the component cells that would occur in vivo. Using this system with human type II pneumocyte epithelial cells (A549) and HULEC we were able to observe bacterial attachment, internalization, and passage through the two cell layers into the space below. We were also able to incorporate an immunological component by adding peripheral blood mononuclear cells (PBMCs) and could then observe migration of monocytes in response to the presence of the bacilli. Strains of M. tuberculosis can be analyzed in terms of their abilities to invade and multiply within epithelial and endothelial cells, to pass through these cells, and to elicit an immune response. Determining and characterizing these traits may lead to improved prevention, intervention, and treatment strategies for infection by M. tuberculosis.
MATERIALS AND METHODS
Bacterial strains.
M. tuberculosis Erdman and Mycobacterium bovis BCG were obtained from the culture collection of the Diagnostic Mycobacteriology Section, Centers for Disease Control and Prevention, and an M. tuberculosis clinical isolate from an AIDS patient was provided by Robert Horsburgh, Grady Memorial Hospital, Atlanta, Ga. All strains were grown in Middlebrook 7H9 broth (Carr-Scarborough, Atlanta, Ga.) at 37°C in 5% CO2 shaken at 50 rpm for 7 days. Cultures were adjusted to an optical density at 600 nm of 0.5 (∼107 CFU/ml). Aliquots were frozen at −70°C, and these stocks were used for all subsequent experimental infections.
Isolation of PBMCs.
Blood from purified protein derivative-negative donors was collected in acid-citrate-dextrose anticoagulant. One milliliter of Gentran (6%) (Baxter Healthcare Corporation, Deerfield, Ill.) was added for each 10 ml of blood; this was mixed gently and incubated at 37°C for 30 to 60 min to sediment the erythrocytes. The upper layer and some erythrocytes were removed and washed by adding Hanks’ balanced salt solution (HBSS) without Ca2+ or Mg2+ and centrifuging for 10 min at 1,000 × g. For each 50 ml of blood, the pellet was resuspended in HBSS to approximately 10 ml and layered over 5 ml of Ficoll/Hypaque (Pharmacia Biotech, Uppsala, Sweden) in a 15-ml polypropylene tube. This gradient was centrifuged at 1,000 × g for 30 to 45 min. The cells collected at the interface were removed with a Pasteur pipette and washed twice with 3 volumes of HBSS. After the final wash the cells were resuspended in Iscove’s modified Dulbecco’s medium (Gibco BRL, Grand Island, N.Y.) with 10% pooled human male serum. Based on trypan blue exclusion, cells were approximately 95% viable.
Bilayer construction.
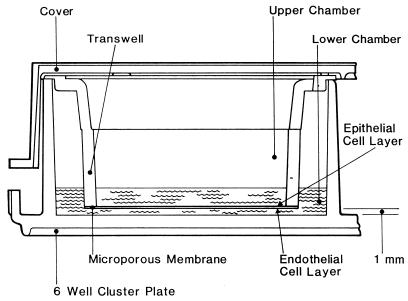
A Transwell-COL insert (Costar, Cambridge, Mass.) with 3.0-μm pores was placed in each well of a six-well tissue culture cluster plate. HULEC (14, 25a) were suspended in endothelial basal medium (EBM) (Gibco BRL) with 7% fetal bovine serum (FBS) at 105 cells/ml, and 3 ml was added to each upper chamber (above the membrane). Two milliliters of EBM plus 7% FBS (without cells) was added to the lower chamber (beneath the Transwell insert). The cells were incubated at 37°C in 5% CO2 for 7 to 8 days, allowing formation of a continuous monolayer. The medium was removed from both upper and lower chambers, and 3 ml of a suspension of A549 human pneumocyte cells (ATCC CCL 185) in minimal essential medium (Gibco BRL) with 5% FBS (105 cells/ml) was added to the upper chamber. Two milliliters of fresh EBM with 7% FBS was added to the lower chamber. At 7-day intervals all medium was removed from both chambers and replaced with fresh EBM with 7% FBS. As demonstrated by histological analysis, the bilayer was completely formed and ready for use after 15 to 20 days’ incubation of the epithelial cell layer (Fig. 1).
FIG. 1.
Bilayer system. A microporous membrane separates a monolayer of A549 lung epithelial cells on the apical surface and a monolayer of HULEC on the basal surface.
Determination of bilayer integrity.
To determine integrity of the HULEC/A549 bilayer and the extent of tight junction formation, growth medium was removed and replaced with fresh EBM, 1 ml in the upper chamber and 1 ml in the lower chamber. One milliliter of 1% blue dextran 2000 (Pharmacia Biotech) was added to each upper chamber and membranes were incubated for 2 h. Contents of each lower chamber were removed and their optical densities were determined. Negative and positive controls included membranes without cells, membranes with nonconfluent HULEC monolayers, and membranes with confluent monolayers of MDCK cells (ATCC CCL 34) known to produce tight junctions (6). To determine the effect of a bacterial infection on the integrity of the bilayer, this assay was performed 4 and 48 h after addition of M. tuberculosis or M. bovis BCG bacilli.
Bilayer infection.
Before the bilayer was infected all medium was removed, the bilayer was washed once with phosphate-buffered saline, and EBM with 10% human serum was added (1 ml in the upper chamber and 1 ml in the lower chamber). Frozen samples of bacteria were thawed and spun in a microcentrifuge for 2 min at maximum speed (∼13,000 × g); each cell pellet was resuspended in 100 μl of EBM and vortexed vigorously for 30 s. This suspension of ∼107 CFU was added to the upper chamber of each well, giving an approximate multiplicity of infection of 10:1 (bacteria to cells). For each time point two wells were infected with each strain; to one of these wells ∼5 × 106 PBMCs (one donor per experiment) were added to the lower chamber. Plates were incubated at 37°C in 5% CO2 for 4, 24, or 48 h. At each time point all contents of the lower chamber were collected and centrifuged at maximum speed for 2 min in a microcentrifuge to pellet the bacteria and cells. Pellets were resuspended in 0.1% Triton X-100 and vortexed vigorously for 30 s. Dilutions were plated on Middlebrook 7H10 medium to determine CFU. Numbers in the upper chamber were determined similarly. To determine numbers of cell- and membrane-associated bacteria, membranes were washed twice with phosphate-buffered saline before 0.5 ml of 0.1% Triton X-100 was added to each upper and lower chamber. Membranes were then scraped, removed from the plastic supports, and minced well with a sharp scalpel. Membrane fragments suspended in Triton X-100 were vortexed, and the suspension was diluted and plated for counts of viable cells. In experiments prepared for microscopy, intact membranes were fixed for 4 to 12 h in 10% neutral buffered formalin.
Histological processing.
Each Transwell insert was removed from the formalin with forceps and placed on a 47-mm-diameter 0.2-μm-pore-size Vericel (Gelman Sciences, Ann Arbor, Mich.) membrane filter. A sharp-pointed scalpel blade was used to cut the Costar membrane away from the inside of the chamber, at the same time cutting all the way through the underlying Vericel membrane. This stack was then placed on another 47-mm Vericel membrane saturated with 70% alcohol. Supported by the larger Vericel membrane the small stack was compactly rolled up with forceps. The original upper side of the Costar membrane thus reliably corresponded to its concave surfaces once it was rolled up. Single-ply cotton string was used to tie off three areas near the center of the roll. Transverse sections approximately 4 mm thick were cut with a sharp blade from the middle of the roll between and on either side of the strings. The three sections were placed on edge on a square piece of wet lens paper. The lens paper was then folded over the membrane ensembles and the wrapped specimens were chemically processed as tissue and embedded to maximize exposure of the membrane and associated cells. For the embedding process cassettes were immersed in 70% ethanol, dried, processed for 16 hours in a Histomatic (Fisher Scientific, Pittsburgh, Pa.) tissue processor, embedded in Polyfin (Triangle Biomedical Sciences, Durham, N.C.) embedding medium (after removal of strings), and sectioned at 4 μm on a Leitz 1512 microtome. Blocks were trimmed deeply enough to compensate for any retraction of the cell layer from the edges of the membranes. Sections were floated on a 40°C water bath, collected on aminosilane-coated 3-by 1-in. glass microscope slides, warmed in a 60 to 65°C paraffin oven for 20 to 30 min, and stained with acid-fast stain.
Processing for TEM.
Transwell inserts were prepared for transmission electron microscopy (TEM) by a modification of the procedure described by the manufacturer (Costar). Growth medium was removed, and membranes were fixed with 2% gluteraldehyde for 1 h at room temperature. Fixative was removed and replaced with collidine buffer. The specimen either was stored at 4°C for later processing or immediately postfixed with 1% osmium tetroxide for 45 min at 4°C. Osmium was removed and replaced with uranyl acetate. The specimen was held at 4°C overnight and then was dehydrated through a graded series of ethanol concentrations: 70, 95, 100, and 100% ethyl alcohol (EtOH) each for 10 min. Specimens then were infiltrated with complete embedding resin (LR White, medium grade) as follows: 75% EtOH, 25% resin for 60 min; 50% EtOH, 50% resin for 60 min; 25% EtOH, 75% resin for 60 min. The wells were filled with complete resin and allowed to stand overnight (16 h) at room temperature. Overnight resin was replaced with fresh resin and the dish was placed in a 60°C oven and allowed to polymerize for 72 h. After polymerization the plastic of the wells and sides of membrane inserts was removed and resin containing the filter was cut out and trimmed with a jeweler’s saw. Standard TEM protocols were followed for the remaining steps.
RESULTS
Bilayer integrity.
In the construction of the bilayer, endothelial cells were grown to confluency on the apical surface of the microporous membrane before addition of the epithelial cells. When the second layer of cells was added, the endothelial cells migrated through the 3-μm pores of the membrane and formed a continuous monolayer on the basal surface of the membrane (data not shown). This membrane appeared to have some of the functions of a basement membrane for both the epithelium and the endothelium. As the epithelial cells grew to become a confluent layer they were seen primarily as a single cell layer, consistent with what can be seen in a cross section of human lung alveoli (4). The endothelial cell layer was seen as a delicate single cell monolayer, such as would be found in a capillary wall. Determination of bilayer integrity was based on microscopic observation and the passage of blue dextran 2000 (molecular weight, 2 × 106) through the system. Two hours after addition of a 1% dextran solution to the upper chamber the optical density (600 nm) in the lower chamber of the bilayer was 0.30, compared to 1.60 in the lower chamber beneath a membrane without cells, 1.25 in the lower chamber beneath a membrane with a nonconfluent HULEC monolayer, and 0.33 in the lower chamber beneath a membrane with a monolayer of MDCK cells, which have been shown to form tight intercellular occluding junctions (6). Results of this assay on the uninfected bilayer remained consistent for as long as 3 weeks after the bilayer was first ready for use (data not shown).
Infections and bilayer passage.
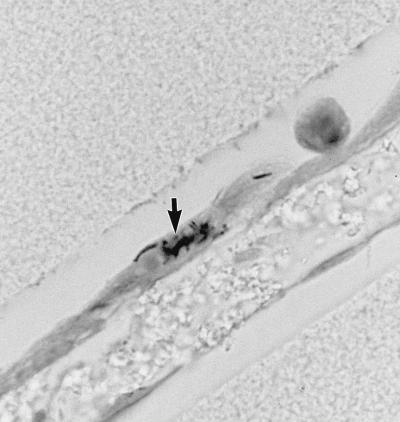
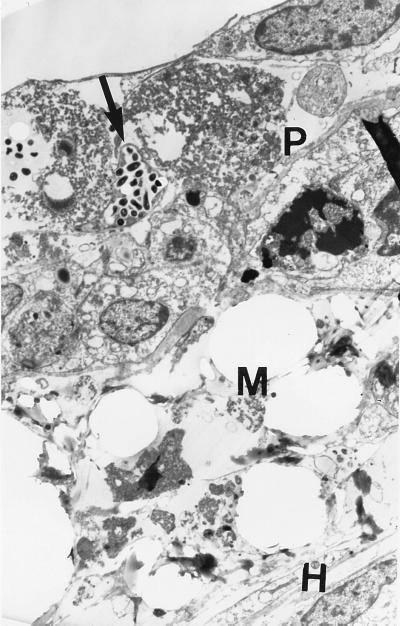
Based on light-microscopic observations, all three bacterial strains appeared to attach to the apical surface of the epithelial cells (Fig. 2). By 48 h, 30 to 40% of these pneumocytes appeared by light (data not shown) and electron (Fig. 3) microscopy to have 10 to 100 internalized bacteria. Very few bacteria associated with the endothelial cells. When blue dextran was added to the upper chamber following a 48-h infection, the optical density in the lower chamber after 2 hours was 0.60, compared to 0.30 in the lower chamber of an uninfected bilayer. This would suggest that there was damage to the host cells or to the structural integrity of the cell layers in the first 48 h after infection, allowing more of the dextran to pass through. Regardless of this potential loss of integrity, less than 0.08% of the bacteria in the upper chamber (including both bacteria free in the supernatant and bacteria associated with host cells and membranes) migrated through the cell layers to the lower chamber. This represented between 2.1 × 103 and 3.4 × 104 CFU after 48 h (Table 1). Although CFU in upper chambers infected with Erdman and the clinical isolate increased between two- and fivefold over the 48-h time course, CFU in the lower chamber did not increase. In contrast, there was a slight decline in CFU of BCG bacilli in the upper chamber between 4 and 48 h and, in the absence of PBMCs, an even greater decrease in CFU in the lower chamber.
FIG. 2.
Bilayer 24 h after infection with M. tuberculosis Erdman: Bacteria (arrow) are seen closely associated with the pneumocytes. Magnification, ×1,000.
FIG. 3.
Electron micrograph of bilayer after 48-h infection. Bacteria (arrow) are seen within pneumocytes (P) on the apical side of the membrane (M) with the HULEC layer (H) on the basal side. Magnification, ×7,000.
TABLE 1.
Passage through lung bilayer
| Culture | No. (%) of CFU after infection timea
|
|||||
|---|---|---|---|---|---|---|
| 4 h
|
48 h
|
|||||
| In upper chamber | Cell associatedb | In lower chamber | In upper chamber | Cell associatedb | In lower chamber | |
| Erdman | (4.9 ± 0.69) × 106 | (1.0 ± 0.14) × 107 | (7.3 ± 4.6) × 103 (0.05) | (2.5 ± 0.21) × 107 | (5.1 ± 0.44) × 107 | (2.1 ± 1.3) × 104 (0.03) |
| Erdman + PBMCsc | (5.8 ± 0.82) × 106 | (9.2 ± 1.3) × 106 | (4.1 ± 3.0) × 104 (0.27) | (2.6 ± 0.66) × 107 | (4.3 ± 1.0) × 107 | (3.4 ± 2.0) × 104 (0.05) |
| Clinical isolate | (2.0 ± 0.88) × 106 | (2.3 ± 1.0) × 107 | (7.5 ± 3.5) × 104 (0.30) | (3.4 ± 0.12) × 106 | (4.0 ± 0.14) × 107 | (2.2 ± 1.3) × 104 (0.05) |
| Clinical isolate + PBMCsc | (4.7 ± 2.1) × 106 | (2.0 ± 0.89) × 107 | (3.5 ± 3.3) × 104 (0.14) | (9.5 ± 0.74) × 106 | (4.5 ± 0.32) × 107 | (3.3 ± 1.6) × 104 (0.07) |
| BCG | (3.1 ± 0.62) × 106 | (1.1 ± 0.22) × 107 | (6.1 ± 4.6) × 104 (0.43) | (2.4 ± 0.24) × 106 | (9.2 ± 0.86) × 106 | (3.0 ± 2.3) × 103 (0.03) |
| BCG + PBMCsc | (3.5 ± 0.70) × 106 | (1.0 ± 0.21) × 107 | (2.4 ± 1.6) × 103 (0.02) | (2.0 ± 0.06) × 106 | (7.3 ± 0.22) × 106 | (2.1 ± 1.2) × 103 (0.02) |
Data are means ± standard errors of four independent experiments.
Includes bacteria associated with host cells and membrane.
PBMCs were added to the lower chamber at the time of infection.
At the 4-h time point, passage of M. tuberculosis Erdman was greater in the cultures in which PBMCs were added to the lower chamber. Passage of the clinical strain declined slightly, while that of M. bovis BCG bacilli decreased 25-fold. After 48 h, CFU in the lower chamber were not significantly altered by the presence of PBMCs for any of the three strains. The majority of bacteria were associated with the host cells and membrane after 48 h, ranging from 7 × 106 CFU for M. bovis BCG with PBMCs to 5 × 107 CFU for M. tuberculosis Erdman without PBMCs.
Mononuclear cell migration.
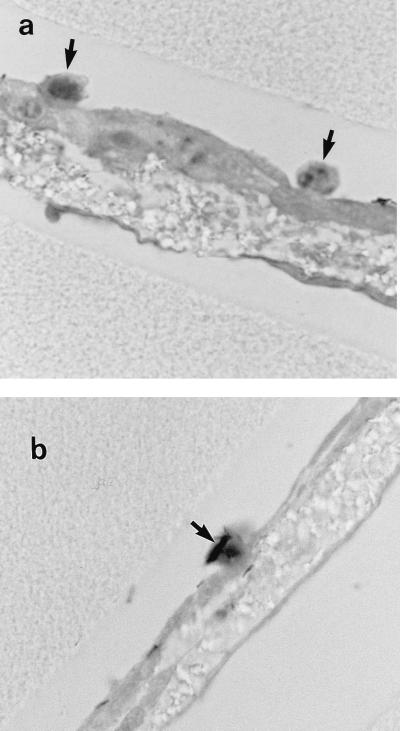
Small numbers of mononuclear cells were observed microscopically on the apical surface after 4 h following infection with all three strains. This was the result of passage of the mononuclear cells from the basal to the apical surface and was seen only when bacteria were added to the apical surface; mononuclear cells were not observed on the surfaces of uninfected bilayers. Mononuclear cells were seen within the microporous membrane in 5 to 10% of the sections observed, while in 90 to 95% of the sections the PBMCs migrated through both cell layers to the apical surface, where they were seen in close association with bacteria and pneumocytes (Fig. 4). Mononuclear cell-associated bacteria also were observed beneath the endothelial cell layer in one or two sections from each experiment (data not shown).
FIG. 4.
Bilayers 4 h (a) and 24 h (b) after infection with M. tuberculosis Erdman. Mononuclear cells (arrows) have migrated from the lower chamber to the apical surface, where they are seen in association with bacilli. Magnification, ×1,000.
DISCUSSION
The bilayer system incorporates an epithelial cell layer and an endothelial cell layer separated by a microporous membrane. This two-layer system allows the cell-to-cell communication that influences cell morphology, cell differentiation, cell orientation and polarization, and cytokine production (10). It makes possible the examination of microbial attachment, internalization, intracellular multiplication, and intra- or intercellular passage from the epithelial cell surface through the two layers and into the lower chamber. With the addition of cells of the immune system such as PBMCs, it is possible to observe the effect of a bacterial infection on the migration of these cells from the lower chamber through the endothelial cells to the epithelial cell surface. One also can observe the effect of the cells on the growth and movement of the invading microbe during the course of infection.
Perhaps it is evidence of the cell-to-cell communication possible in the bilayer that the epithelial cell layer remains, for the most part, as a single flat layer. In our previously reported Neisseria meningitidis bilayer model (2), Hec1B epithelial cells became columnar and often formed layers two or three cells deep, as might be seen in the tissue from which they were derived. In contrast, alveolar cells as observed in a cross section of the human lung are a thin monolayer, consistent with their function. The endothelial layer is also a very thin single-cell layer, as can be seen in the delicate capillary wall of the alveolus. In most of the alveolar wall, the basement membrane which supports the capillary endothelium is directly applied to the basement membrane supporting the surface epithelium; in such sites the two basement membranes are fused and the supporting tissue layer is absent. This arrangement provides an interface of minimal thickness between alveolar air and blood (4). This suggests that passage into the circulatory system would require the bacteria to pass only through two single cell layers, rather than multiple layers, making this potentially a very direct route of infection.
Although interaction with the alveolar macrophage is an important factor in the course of the disease process, this initial interaction may not be the most significant, as these resident alveolar macrophages do not bind M. tuberculosis efficiently (25). As is observed with other lung pathogens, such as S. pneumoniae (9), the invading bacilli more likely encounter pneumocytes as they first enter the alveolar space, and this may be the more important initial interaction. If some of these bacilli are internalized by the pneumocytes, this would likely offer them some protection from the bactericidal activity of the macrophages and provide an environment in which they might replicate. It has been shown that M. tuberculosis can invade A549 cell monolayers and multiply intracellularly, with numbers increasing up to sixfold in 4 days (1, 19). Electron micrographs of infected bilayers after 48 h showed bacterial association and large numbers of bacilli within pneumocytes. Thus, infection with only a few organisms could result in greater numbers that could potentially move from the pneumocytes to infect adjacent pneumocytes, eventually gaining entry into the circulatory system by passage through the endothelial layer either as free bacteria or within macrophages returning to the bloodstream.
The bilayer model facilitates the study of microbial cell association and internalization through light and electron-microscopic observation. Studies with more than 20 strains of N. meningitidis (2) showed a wide range of numbers in terms of the percentage of the inoculum able to pass through a bilayer of endometrial and microvascular endothelial cells, and these variations appeared to correlate with differences in human virulence among the strains. The two M. tuberculosis strains examined in this study pass through the bilayer after 48 h at the same low rate even in the presence of a high inoculum. Although, in the absence of PBMCs, the percentage of M. bovis BCG bacilli passing into the lower chamber after the first 4 h is slightly higher than those of the M. tuberculosis strains, there is little difference in percentages among the three strains after 48 h. Unlike the passage of N. meningitidis bacteria, these are all very small percentages of the total mycobacterial population, and since a majority of the passage occurs in the first 4 h after infection, it is likely that passage is not a major determinant of virulence. In the case of M. bovis BCG passage is inhibited by the addition of PBMCs to the lower chamber; it is possible that these bacilli may be more susceptible to phagocytosis by the mononuclear cells. Passage of the clinical isolate is inhibited to a much lesser degree. However, passage of M. tuberculosis Erdman after 4 h appears to be enhanced by the presence of PBMCs. The difference in behavior between strain Erdman and the clinical isolate in the presence of PBMCs could be due to the fact that Erdman is a laboratory strain while the recent clinical isolate has been minimally passaged on artificial media. We will observe the passage characteristics in PBMCs of additional laboratory and clinical strains to determine if this trend holds. Alternatively, chemokines produced by epithelial, endothelial, and mononuclear cells likely play a role in varying the passage rate obtained in association with PBMCs. Investigations to more clearly define these interactions are currently under way. In the M. bovis BCG infection a decrease in viability can be seen in the lower chamber between 4 and 48 h, as much as 20-fold. Since viability remains constant in tissue culture medium alone (reference 20 and data not shown), the cause of the apparent cell death has not yet been determined. However, we may not be seeing cell death but instead the effect of differences in the extent of clumping of organisms commonly seen in mycobacterial cultures. Larger or more numerous bacterial aggregates would produce a lower viable count than a dispersed single-cell suspension of the same culture.
The passage of blue dextran, a molecule with a molecular weight of 2 × 106 and an average diameter of 54 nm, through the intact bilayer is less than that observed in passage through a polarized monolayer of MDCK cells. MDCK cells have been shown to form tight intercellular junctions. The fact that there is an increase in the passage of dextran into the lower chamber following a 48-h infection with M. tuberculosis suggests that there is some cytotoxic damage to the host cells or alterations in the structural integrity of the cell layers during this time. Other research has shown significant damage to A549 pneumocyte monolayers infected with M. tuberculosis (4a, 17). Such damage may account for some of the observable tissue damage which is a hallmark of the infection process in this disease. Even with the increase in dextran passage after 48 h, passage of bacilli into the lower chamber did not increase over time. It appeared that the majority of the bacteria remained associated with the host cells and the membrane.
The bilayer model offers several directions for further study of mycobacterial pathogenesis. For example, the roles of the various chemokines in leukocyte migration and bacterial passage through the bilayer might be confirmed by blocking the activity of these cytokines with the addition of antichemokine antibodies. Following the time course of macrophage migration may suggest whether bacteria travel through the cell layers independently or are carried across by the macrophages. A very important component of the host immune response to M. tuberculosis infection is the formation of a granuloma. Since one of the places this complex of macrophages, T lymphocytes, and fibroblasts is established is within the tissues of the lung (15, 16), the best framework for an in vitro model of the granuloma may well be the complex of tissues found in the bilayer. The bilayer is obviously a simple system compared to human lung tissue. The A549 epithelial cell layer is composed of only type II pneumocytes, which make up just 5% of the alveolar surface; substituting a primary cell culture including both type I and type II cells would add necessary cellular diversity. It has been suggested that dendritic cells present in airway epithelium and lung parenchyma may play an important role in the uptake of M. tuberculosis bacilli and subsequent priming of T cells to initiate the host immune response (13). The addition of dendritic cells to this model might allow us to more clearly identify their role in this response. It would also be of interest to observe what happens as the cell support system is made more complex with the addition of a variety of extracellular matrix components. Further refinement of this model will enhance our abilities to elucidate the pathogenic mechanisms of M. tuberculosis in the human host, leading to more effective prevention and intervention strategies.
ACKNOWLEDGMENTS
We thank Jack Crawford and Tom Shinnick for their helpful discussion and their critical reviews of the manuscript and Ed Ades for technical assistance in several parts of this project.
REFERENCES
- 1.Bermudez L E, Goodman J. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect Immun. 1996;64:1400–1406. doi: 10.1128/iai.64.4.1400-1406.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birkness K A, Swisher B L, White E H, Long E G, Ewing E P, Jr, Quinn F D. A tissue culture bilayer model to study the passage of Neisseria meningitidis. Infect Immun. 1995;63:402–409. doi: 10.1128/iai.63.2.402-409.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloom B R, Fine P E M. The BCG experience: implications for future vaccines against tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C: ASM Press; 1994. pp. 531–558. [Google Scholar]
- 4.Burkitt H G, Young B, Heath J W. Wheater’s functional histology: a text and colour atlas. Edinburgh, Scotland: Churchill Livingstone; 1993. [Google Scholar]
- 4a.Castro-Garza, J. C., et. al. Unpublished data.
- 5.Centers for Disease Control and Prevention. Tuberculin skin test survey in a pediatric population with high BCG vaccination coverage—Botswana, 1996. Morbid Mortal Weekly Rep. 1997;46:846–851. [PubMed] [Google Scholar]
- 6.Cereijido M, Meza I, Martinez-Palomo A. Occluding junctions in cultured epithelial monolayers. Am J Physiol. 1981;240:C96–C102. doi: 10.1152/ajpcell.1981.240.3.C96. [DOI] [PubMed] [Google Scholar]
- 7.Crandall E D, Kim K J. Alveolar epithelial barrier properties. In: Crystal R J, West J B, editors. The lung: scientific foundations. New York, N.Y: Raven Press; 1991. pp. 273–287. [Google Scholar]
- 8.Crystal R J. Alveolar macrophages. In: Crystal R J, West J B, editors. The lung: scientific foundations. New York, N.Y: Raven Press; 1991. pp. 527–538. [Google Scholar]
- 9.Cundell D R, Tuomanen E I. Receptor specificity of adherence of Streptococcus pneumoniae to human type-II pneumocytes and vascular endothelial cells in vitro. Microb Pathog. 1994;17:361–374. doi: 10.1006/mpat.1994.1082. [DOI] [PubMed] [Google Scholar]
- 10.Dainiak N. Surface membrane-associated regulation of cell assembly, differentiation, and growth. Blood. 1991;78:264–276. [PubMed] [Google Scholar]
- 11.Dannenberg A M, Jr, Rook G A. Pathogenesis of pulmonary tuberculosis: an interplay of tissue-damaging and macrophage-activating immune responses—dual mechanisms that control bacillary multiplication. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C: ASM Press; 1994. pp. 459–483. [Google Scholar]
- 12.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson R A, Watkins S C, Flynn J L. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J Immunol. 1997;159:635–643. [PubMed] [Google Scholar]
- 14.Lawrence E C, Daneker G W, Waite-Reese P A, Lund S A, Candal F J, Bosse D C, Kuno R, Kanter K R, Ades E W. Establishment of immortalized human lung microvascular endothelial cell lines. Chest. 1995;108:1265. [Google Scholar]
- 15.Lurie M B, Abramson S, Heppleston A G. On the response of genetically resistant and susceptible rabbits to the quantitative inhalation of human type tubercle bacilli and the nature of resistance to tuberculosis. J Exp Med. 1952;95:119–137. doi: 10.1084/jem.95.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCune R M, Tompsett R. Fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. J Exp Med. 1956;104:737–762. doi: 10.1084/jem.104.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonough K A, Kress Y. Cytotoxicity for lung epithelial cells is a virulence-associated phenotype of Mycobacterium tuberculosis. Infect Immun. 1995;63:4802–4811. doi: 10.1128/iai.63.12.4802-4811.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonough K A, Kress Y, Bloom B R. Pathogenesis of tuberculosis: interaction of Mycobacterium tuberculosis with macrophages. Infect Immun. 1993;61:2763–2773. doi: 10.1128/iai.61.7.2763-2773.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta P K, King C H, White E H, Murtagh J J, Jr, Quinn F D. Comparison of in vitro models for the study of Mycobacterium tuberculosis invasion and intracellular replication. Infect Immun. 1996;64:2673–2679. doi: 10.1128/iai.64.7.2673-2679.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guérin. J Exp Med. 1994;180:1499–1509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orme I M. Immunity to mycobacteria. Curr Opin Immunol. 1993;5:497–502. doi: 10.1016/0952-7915(93)90029-r. [DOI] [PubMed] [Google Scholar]
- 21a.Quinn, F. D., et al. Unpublished data.
- 22.Rhoades E R, Cooper A M, Orme I M. Chemokine response in mice infected with Mycobacterium tuberculosis. Infect Immun. 1995;63:3871–3877. doi: 10.1128/iai.63.10.3871-3877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneeberger E E. Alveolar type I cells. In: Crystal R J, West J B, editors. The lung: scientific foundations. New York, N.Y: Raven Press; 1991. pp. 229–234. [Google Scholar]
- 24.Shinnick T M, King C H, Quinn F D. Molecular biology, virulence, and pathogenicity of mycobacteria. Am J Med Sci. 1995;309:92–98. doi: 10.1097/00000441-199502000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Stokes R W, Thorson L M, Speert D P. Nonopsonic and opsonic association of Mycobacterium tuberculosis with resident alveolar macrophages is inefficient. J Immunol. 1998;160:5514–5521. [PubMed] [Google Scholar]
- 25a.Waite-Reese, P. A., et al. Unpublished data.
- 26.Wallis R S, Ellner J J. Cytokines and tuberculosis. J Leukoc Biol. 1994;55:676–681. doi: 10.1002/jlb.55.5.676. [DOI] [PubMed] [Google Scholar]